Abstract
Proteins of the Ras superfamily, Ras, Rac, Rho, and Cdc42, control the remodelling of the cortical actin cytoskeleton following growth factor stimulation. A major regulator of Ras, Ras-GAP, contains several structural motifs, including an SH3 domain and two SH2 domains, and there is evidence that they harbor a signalling function. We have previously described a monoclonal antibody to the SH3 domain of Ras-GAP which blocks Ras signalling in Xenopus oocytes. We now show that microinjection of this antibody into Swiss 3T3 cells prevents the formation of actin stress fibers stimulated by growth factors or activated Ras, but not membrane ruffling. This inhibition is bypassed by coinjection of activated Rho, suggesting that the Ras-GAP SH3 domain is necessary for endogenous Rho activation. In agreement, the antibody blocks lysophosphatidic acid-induced neurite retraction in differentiated PC12 cells. Furthermore, we demonstrate that microinjection of full-length Ras-GAP triggers stress fiber polymerization in fibroblasts in an SH3-dependent manner, strongly suggesting an effector function besides its role as a Ras downregulator. These results support the idea that Ras-GAP connects the Ras and Rho pathways and, therefore, regulates the actin cytoskeleton through a mechanism which probably does not involve p190 Rho-GAP.
Ras is the prototype of a superfamily of highly conserved proteins. The family can be divided into several subgroups, Ras, Rho, Rab, ARF (ADP ribosylation factor), Sar, Ran, and Rad, each of which performs essential cellular functions. Thus, while Ras proteins have a determinant role in cell growth, differentiation, and malignant transformation, Rho proteins control the formation of actin-based cytoskeletal structures, as well as growth regulation, and Rab proteins participate in intracellular vesicular transport and secretion (4, 51). In addition, Rho and Rab proteins have specific roles in cells of the immune system (8). Ras-like proteins are molecular switches whose activity is controlled by their bound nucleotide, with the GTP form being the active form competent for cellular signalling and with the GDP-bound form being inactive. They are subjected to tight control by regulatory proteins. Activation is brought about by guanine nucleotide exchange factors (GEFs) that favor nucleotide release and GTP loading following exposure of cells to growth factors. Deactivation is ensured by GTPase-activating proteins (GAPs) which greatly speed up GTP hydrolysis. In general, several GEFs and GAPs can regulate the activity of a single GTPase (3).
Microinjection of activated Ras, Cdc42, Rac, or Rho proteins induces polymerization of cortical actin, from a preexisting pool of soluble actin present in resting fibroblasts, into particular structures. It has been established that Cdc42 causes the formation of filopodia (23), while Rac generates lamellipodia and membrane ruffles (44), and that Rho controls stress fiber assembly (43). Furthermore, the use of dominant negative mutants of each protein has unraveled complex connections between them. It was found that activated Cdc42 induces not only filopodia, but also lamellipodia and ruffles through subsequent activation of endogenous Rac (23). Likewise, activated Rac can also promote stress fiber formation, because it can stimulate Rho activity (36, 44). Ras branches in this pathway upstream of Rac and stimulates ruffling through a Rac-dependent mechanism (44). These rearrangements are coupled to the clustering of integrins at focal contacts (36), which are sites of cell attachment to the extracellular matrix.
Ras-GAP is a major regulator of cellular Ras activity. The carboxy-terminal half of the protein contains the catalytic domain, which binds Ras-GTP and accelerates GTP hydrolysis (54). In the amino-terminal region lies a Src homology 3 (SH3) domain flanked by two SH2 domains which mediate interactions with signalling proteins (i.e., p190 Rho-GAP, Src, and p62), a pleckstrin homology domain, and a stretch of amino acids involved in calcium-regulated binding to phospholipids, which mediate interactions with the plasma membrane (see reference 53 for review). First thought of merely as a downregulator of Ras (22, 37, 58, 59), its role turned out to be more complex, and it is now established that Ras-GAP also mediates some of the biological effects of Ras and, therefore, has some intrinsic effector function (1, 10, 53). Some data are consistent with the fact that these effects are relayed via the NH2-terminus part of Ras-GAP and, more specifically, that its SH3 domain could be involved. Thus, overexpression of a truncated mutant Ras-GAP N terminus was found to be a potent suppressor of Ras-induced transformation (9), whereas overexpression of the N terminus or of the isolated SH3 domain blocked carbachol-dependent transformation of NIH 3T3 cells expressing muscarinic receptors (28, 57). We have previously shown that microinjection into Xenopus oocytes of a monoclonal antibody (MAb 200) (32) against human Ras-GAP that specifically recognizes the SH3 domain was able to inhibit Ras-stimulated, but not progesterone-stimulated, maturation (11), induction of c-mos expression, and subsequent activation of p34cdc2 (41).
In other respects, several lines of evidence indicate a connection between Ras-GAP and the cytoskeleton. It has been reported that Rat-2 cells overexpressing the Ras-GAP N terminus exhibit an abnormal network of actin fibers and reduced adhesive capacities (30) and that overexpression of full-length Ras-GAP abolished the chemotactic response of fibroblasts to platelet-derived growth factor (PDGF) (26). More recently, the abnormal vascular phenotype displayed by Ras-GAP knockout mouse embryos was found to be consistent with defective migration of endothelial cells (18).
A connection between Ras-GAP and the G proteins of the Rho family could explain the effects of Ras-GAP on cytoskeletal organization mentioned above. This connection should involve the N-terminus domain of Ras-GAP. Here we report that injection of the anti-Ras-GAP SH3 antibody MAb 200 into quiescent Swiss 3T3 cells inhibited stress fiber formation in response to stimulation by several growth factors, but did not block stress fibers which formed following injection of activated Rho. In pheochromocytoma PC12 cells, MAb 200 appeared to decrease endogenous Rho activity and facilitate Ras-induced neurite outgrowth in the absence of nerve growth factor (NGF); it also blocked neurite retraction stimulated by lysophosphatidic acid (LPA) in differentiated cells. These effects were reproduced by a truncated mutant Ras-GAP N terminus. In addition, we found that Ras-GAP per se had a signalling function and was able to cause actin polymerization into stress fibers in Swiss 3T3 cells. Since this activity required the SH3 domain, our results altogether indicate that the SH3 domain of Ras-GAP is necessary for Rho activation and further establish a molecular basis for the connection between the Ras and Rho signalling pathways in mammalian cells. Moreover, we found that the effect of MAb 200 cannot be explained by a competition with p190 Rho-GAP for binding to Ras-GAP.
MATERIALS AND METHODS
Cell culture and microinjection.
Rat PC12 pheochromocytoma cells were cultured in RPMI 1640 medium supplemented with 10% horse serum and 5% fetal calf serum (FCS). Cells were differentiated for 3 days in a medium containing 1/10 total serum plus 50 ng of NGF per ml. For microinjection, 2 × 104 cells per ml were seeded onto squared coverslips (CELLocate) precoated with poly-l-lysine (10 μg/ml) 2 to 3 days before injection. NGF-differentiated cells were stimulated with 10 μM LPA (Sigma) 2 h after microinjection. Cells were returned to the incubator after injection and regularly checked for morphological changes. Both the low-density seeding and the squaring allowed the precise identification and follow-up of microinjected cells.
Swiss 3T3 cells were routinely grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS. For cytoskeleton studies, cells were seeded onto CELLocate coverslips and allowed to grow to confluence in complete medium. To obtain quiescent cells, they were then starved in DMEM containing 0.2% NaHCO3 and 0.005% FCS for 48 h. After microinjection, cells were incubated for the appropriate time at 37°C before growth factor stimulation or fixation. For the DNA synthesis assay, subconfluent cells were starved for 24 h in 0.1% FCS and, after injection, incubated for 40 h in the presence of a 1/1,000 bromodeoxyuridine (BrdU)-fluorodeoxyuridine (FdU) mixture (Amersham) before fixation. When specified in the text, this medium was supplemented with 100 μM LPA or 10% FCS. Microinjection was performed with an Eppendorf transjector 5246 and an Eppendorf micromanipulator 5171. Successful injection was determined visually at the time of injection and by immunofluorescence on fixed cells.
Immunofluorescence microscopy.
For actin, phosphotyrosine, and protein staining, cells were washed in phosphate-buffered saline for 5 min (or in phosphate-buffered saline plus 1 mM Na4VO3 for phosphotyrosine staining), fixed in 4% formaldehyde for 15 min, permeabilized in 0.2% Triton X-100 for 5 min, and incubated in Power Block (BioGenex) for 10 min. Actin filaments were visualized by incubation with 0.5-μg/ml fluorescein isothiocyanate (FITC)-labelled phalloidin (Sigma). Phosphotyrosine residues were detected with a polyclonal antiphosphotyrosine antibody (Zymed) revealed with an FITC-labelled antirabbit F(ab′)2. Injected glutathione S-transferase (GST) fusion proteins were detected with a MAb to GST (given by J. Grassi, Centre d’Etudes de Saclay, Gif-sur-Yvette, France), followed by Texas red-labelled antimouse F(ab′)2. MAb 200 or the control mouse immunoglobulin G (IgG) injected was detected with Texas red-labelled antimouse F(ab′)2; Y13-259-injected cells were detected with Texas red-labelled antirat F(ab′)2. For BrdU staining, cells were fixed in 90% ethanol–5% acetic acid for 30 min, permeabilized in 0.1% Tween 20 for 5 min, and subjected to DNase I digestion (2 μg/ml) in 50 mM Tris (pH 7.5)–10 mM MgCl2–10 mM MnCl2 for 1 h. They were stained with a rat antibody to BrdU (Valbiotech), followed by FITC-coupled antirat F(ab′)2. All secondary antibodies were supplied by Jackson Immunoresearch. In each experiment, cells were double stained to detect both injected cells and the particular response under study (cytoskeleton, focal complexes, or DNA synthesis).
Antibodies and recombinant proteins for microinjection.
The MAb to Ras-GAP SH3, MAb 200, was purified from ascitic fluids (SpiBio, Massy, France) by caprylic acid precipitation. Rat anti-Ras Y13-259 hybridoma cells were grown in DMEM supplemented with 10% FCS (Hyclone), 1 mM sodium pyruvate, 2 mM glutamine, and 100 U of streptomycin per ml. Y13-259 was purified from culture supernatants by using protein G-Sepharose (Pharmacia). The vector (pGEX-2T) expressing mutant RhoAV14 cDNA was given by A. Hall. The recombinant GST-RhoA fusion protein was purified by affinity chromatography on glutathione-agarose, and GST was removed by thrombin digestion, as described previously (44). pGEX-3X-p120 GAP was obtained by inserting human Ras-GAP cDNA digested from a pSV2 vector (47) into the EcoRI-BamHI sites of pGEX-3X. GAPSH2-SH3-SH2 (amino acids 182 to 442) was obtained and cloned into pGEX-2T as described previously (11). GST fusion proteins were purified by affinity chromatography (11), and Ha-RasK12 was purified by anion-exchange chromatography (42).
RESULTS
Anti-Ras-GAP SH3 antibody inhibits growth factor-induced stress fiber formation, but not membrane ruffling.
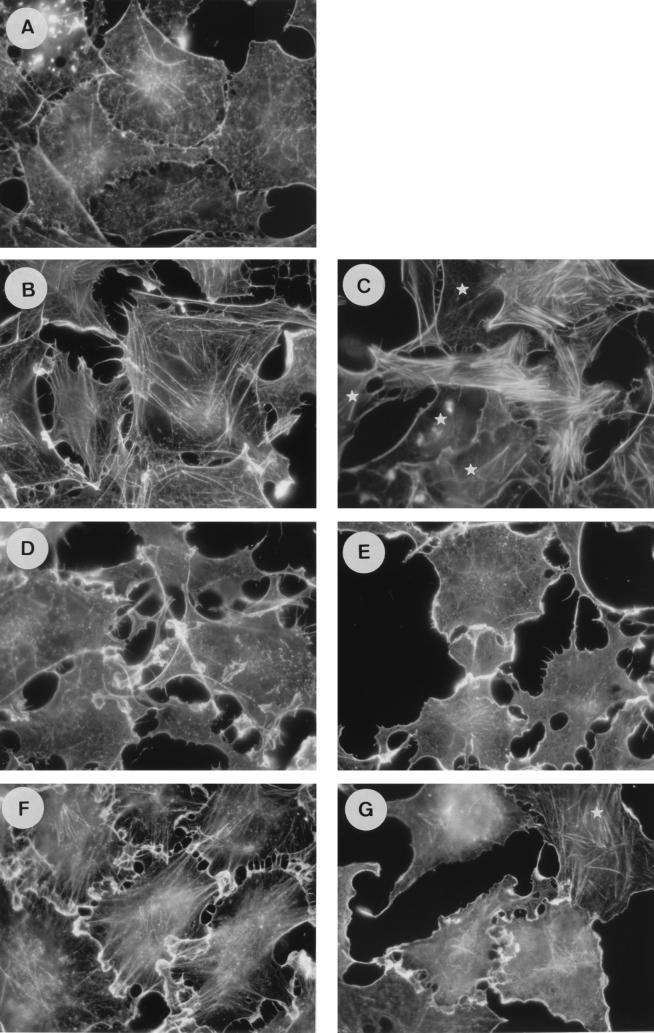
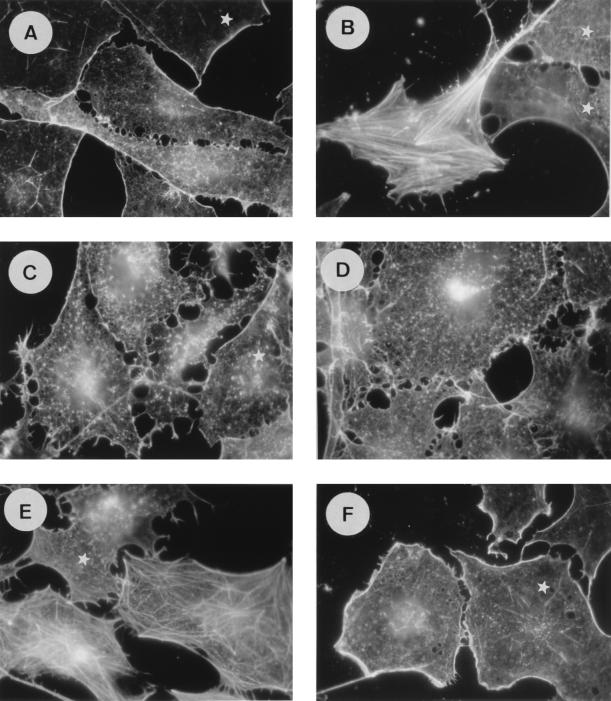
It has previously been shown that oncogenic Ras promotes cytoskeletal rearrangements in Swiss 3T3 cells. On the other hand, a growing body of evidence shows that Ras-GAP exerts some effector functions requiring the N-terminal part of the protein. To assess whether the N terminus of Ras-GAP is required downstream of Ras in this pathway, we investigated the impact of the anti-Ras-GAP SH3 antibody MAb 200 on actin stress fiber polymerization and membrane ruffling stimulated by activated Ras (Ha-RasK12) in quiescent cells. Two to three hours after injection of Ras plus an irrelevant IgG, cells exhibited membrane ruffles at their periphery, which were progressively replaced by a dense stress fiber network (Fig. 1B). After 5 h, only a very few membrane ruffles were left (Fig. 1C). These structures were absent from noninjected quiescent cells (Fig. 1A). In cells where MAb 200 was coinjected with Ras, membrane ruffles developed with the same kinetics as in control cells (Fig. 1D). In contrast, the presence of the anti-GAP SH3 antibody almost completely prevented actin polymerization into stress fibers, and the ruffles persisted for more than 5 h (Fig. 1E). As a control, the Ras-neutralizing antibody Y13-259 (14) blocked both ruffle and stress fiber formation stimulated by Ras (not shown).
FIG. 1.
MAb 200, an anti-Ras-GAP SH3 antibody, inhibits Ras- and PDGF-induced stress fiber formation, but not membrane ruffles, in Swiss 3T3 cells. Actin structures are shown in serum-deprived cells, quiescent and uninjected (A) and 3 h (B) or 5 h (C) after coinjection of Ha-RasK12 (1 mg/ml) with a control mouse IgG (8 mg/ml) and 3 h (D) or 5 h (E) after coinjection of Ha-RasK12 (1 mg/ml) with MAb 200 (8 mg/ml). (F and G) Cells were stimulated for 30 min with 3 ng of PDGF per ml 2 h after injection of a control IgG or MAb 200, respectively. Actin filaments were detected with FITC-conjugated phalloidin. The white stars indicate noninjected cells.
Growth factors, such as PDGF, trigger cytoskeletal modifications similar to those promoted by Ras that are membrane ruffles and stress fibers. These PDGF-induced rearrangements are not affected by the presence of the neutralizing Y13-259 anti-Ras antibody (14), suggesting that Ras activation was not essential for these responses (44) and that an alternative pathway may exist. To test whether MAb 200 could also inhibit stress fiber formation when it was stimulated by PDGF, cells were exposed to this growth factor for 30 min. In contrast with the results obtained with Y13-259, MAb 200-microinjected cells (Fig. 1G) exhibited membrane ruffles but had a lot fewer stress fibers than control cells (Fig. 1F). This result shows that the involvement of the SH3 domain of Ras-GAP in this response overrides the Ras signalling cascade. Ras- and PDGF-induced membrane ruffling in Swiss 3T3 cells is dependent on Rac activation, since it is blocked by a dominant inhibitory Rac mutant (44). Because activation of Rac in turn activates Rho, which leads to stress fiber formation, our results suggest that MAb 200 inhibits Rho, but not Rac, activity.
Ras-GAP SH3 domain is involved in Rho activation in response to growth factors.
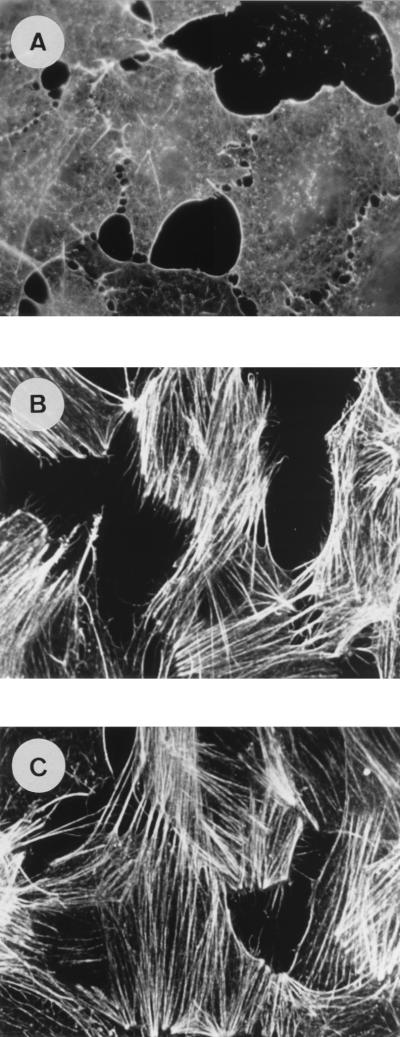
To determine if the anti-GAP SH3 antibody can indeed interfere with Rho signalling, quiescent cells (Fig. 2A) were microinjected with MAb 200 or a control IgG and then exposed to serum or LPA for 10 or 30 min. LPA activates Rho, as witnessed by actin polymerization into fibers (43). This was reproduced in control cells, where a network of fibers was readily observed after 10 min of LPA stimulation (compare Fig. 2B and A) or serum (not shown). In contrast, under the same conditions, no actin cables were seen, or if there were any, they were few and very thin, in MAb 200-injected cells (Fig. 2C). The number of stress fibers per cell and their thickness, as carefully estimated visually, were inversely correlated to the amount of antibody injected (not shown). After 30 min of serum stimulation, the inhibition of stress fiber formation produced by MAb 200 was partially relieved, whereas it was maintained in the case of LPA (data not shown). Interestingly, injection of MAb 200 per se provoked a complete disappearance of the few stress fibers that remained in some quiescent cells (not shown), suggesting that it can also block the low basal level of Rho activation which is left under these conditions (19). No inhibition of stress fiber polymerization following LPA stimulation was observed after injection of the Ras-neutralizing antibody Y13-259 (Fig. 2D), indicating that Rho induces actin polymerization independently of Ras.
FIG. 2.
Inhibition of LPA-induced stress fibers by MAb 200 but not by Y13-259. Actin filaments are shown in serum-starved Swiss 3T3 cells without injection (A) or stimulated with 0.115 μM LPA for 10 min 2 h after injection of a control mouse IgG (B), MAb 200 (C), or Y13-259 (D). Proteins were injected at a concentration of 8 mg/ml. Actin filaments were detected with FITC-conjugated phalloidin. All cells in each field have been injected.
Focal adhesions are multimolecular complexes containing tyrosine-phosphorylated proteins, which allow attachment of actin cables to the cell walls. Their assembly was shown to be regulated by Rho (36). By immunofluorescence with an antiphosphotyrosine antibody, we studied focal adhesions which formed in response to serum or LPA. In quiescent Swiss 3T3 cells, focal complexes are very few and sparse (Fig. 3A), which correlates with the low abundance of stress fibers. As reported before (43), following addition of LPA or serum, numerous adhesion plaques become visible and are evenly distributed at the cell periphery (not shown). Injection of a fragment of Ras-GAP which contains the isolated SH2-SH3-SH2 domains (amino acids 182 to 442) fused to GST resulted in a large reduction (50 to 80%) in the number of focal adhesions counted in each cell (Fig. 3D), while GST alone had no inhibitory effect (Fig. 3B). Moreover, the complexes formed in control cells seemed morphologically distinct from the few that assembled in the presence of GST-GAPSH2-SH3-SH2, the latter being much smaller and less elongated. It is possible that these complexes are Rac- or Cdc42-regulated complexes (36). Identical results were obtained with MAb 200 (not shown). Staining of cells injected either with MAb 200 or with GST-GAPSH2-SH3-SH2 revealed a peculiar notched-cell contour that was not observed in control cells (compare Fig. 3E and C). This effect could result from disrupted adhesion of the cells due to the incomplete response to LPA or serum in the presence of the antibody or, alternatively, the formation of nonfunctional focal complexes.
FIG. 3.
Inhibition of LPA-induced focal complex assembly by GST-GAPSH2-SH3-SH2. Focal complexes are shown in serum-deprived Swiss 3T3 cells without injection or stimulation (A) and in cells stimulated for 10 min with 0.115 μM LPA 2 h after injection of GST (B) or GST-GAPSH2-SH3-SH2 (D). (C and E) Injected cells corresponding to those in panels B and D, respectively. Proteins were injected at 1.5 mg/ml. Cells were double stained with an antiphosphotyrosine antibody, revealed by an FITC-labelled secondary antibody to detect focal complexes (left panel), and with an anti-GST antibody, revealed by a Texas red-labelled secondary antibody to detect injected cells (right panel). The white star indicates noninjected cells.
Ras-GAP SH3 domain is implicated in Rho regulation.
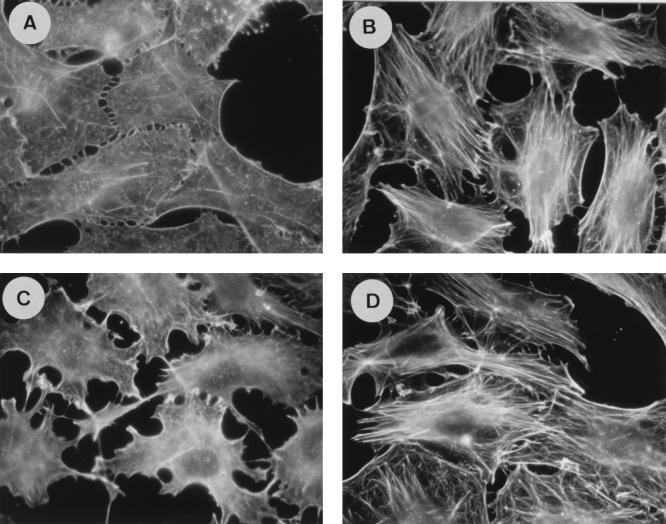
Taken together, these results strongly suggest that the NH2-terminal region of Ras-GAP, through its SH3 domain, could behave as a suppressor of the Rho pathway. To determine whether the activity of Ras-GAP on this pathway is exerted upstream or downstream from Rho, we tested if MAb 200 could inhibit stress fibers induced by a constitutively activated Rho mutant. Serum-starved cells were simultaneously injected with RhoAV14 and MAb 200. Quiescent cells are shown in Fig. 4A. A few minutes after RhoAV14 injection, actin stress fibers were readily detectable, and no difference could be observed between control (Fig. 4B) and MAb 200-injected cells (Fig. 4C), even after a 2-h period. Identical results were obtained when MAb 200 was injected 2 h before Rho (not shown). The ability of activated Rho to bypass the inhibition of stress fiber assembly produced by MAb 200 suggests that the regulatory role of Ras-GAP is exerted upstream from Rho.
FIG. 4.
MAb 200 does not inhibit RhoAV14-induced stress fibers in Swiss 3T3 cells. The photographs show phalloidin staining of uninjected cells (A) and cells 2 h after coinjection of RhoAV14 with a control IgG (B) or with MAb 200 (C). RhoAV14 was injected at a concentration of 0.3 mg/ml, and the antibodies were injected at a concentration of 8 mg/ml. F-actin was detected with FITC-conjugated phalloidin. All cells in each field have been injected.
Full-length Ras-GAP induces the formation of stress fibers.
Having established that overexpression of the NH2-terminal part of Ras-GAP or inhibition of its SH3 domain downregulates the Rho pathway, we asked whether full-length Ras-GAP had any effect by itself on the cytoskeleton. Strikingly, microinjection of full-length Ras-GAP (fused to GST) in serum-deprived cells induced the appearance of stress fibers by 4 h (Fig. 5B), whereas no actin polymerization was observed in control cells injected with GST (Fig. 5A). Typically, Ras-GAP induced this response in over 60% of the injected cells. The stress fiber-inducing activity of Ras-GAP requires functional Rho, as shown by the fact that it was blocked when the C3 exotoxin from Clostridium botulinum, a specific Rho inhibitor (40), was coinjected with Ras-GAP (compare Fig. 5D with B and C). We had previously determined the appropriate concentration of toxin as that necessary to fully inhibit stress fiber formation stimulated by serum in quiescent cells (not shown). At that time (4 h after injection), the cells injected with C3 plus GST had a normal morphology and were viable (Fig. 5C). Interestingly, the effect of Ras-GAP on the cytoskeleton was independent of Ras because it was not inhibited (Fig. 5E) by coinjection of the anti-Ras antibody Y13-259 (14). Finally, we showed that the effect of Ras-GAP was totally abolished when MAb 200 and Ras-GAP (Fig. 5F) or when GST-GAPSH2-SH3-SH2 and Ras-GAP (not shown) were coinjected; in contrast, different antibodies directed against the COOH-terminus part of Ras-GAP had no inhibitory effect (data not shown).
FIG. 5.
Full-length Ras-GAP induces stress fiber polymerization through its SH3 domain and requires Rho but not Ras activity. Actin structures are shown in serum-deprived Swiss 3T3 cells 4 h after microinjection of GST (A), GST-Ras-GAP plus a control mouse IgG (B), GST plus C3 toxin (C), GST-Ras-GAP plus C3 toxin (D), GST-Ras-GAP plus Y13-259 (E), and GST-Ras-GAP plus MAb 200 (F). GST and GST-Ras-GAP were injected at a concentration of 1.5 mg/ml, C3 toxin was injected at 70 μg/ml, and MAb 200, the control IgG, and Y13-259 were injected at 8 mg/ml. F-actin was detected with FITC-conjugated phalloidin. The white stars indicate noninjected cells.
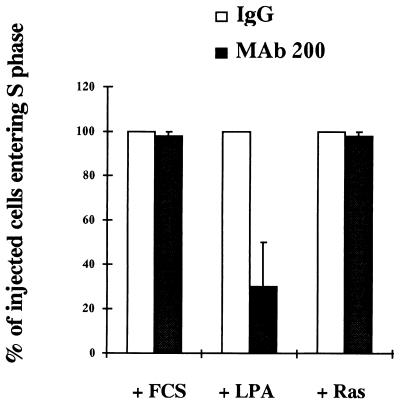
Ras-GAP SH3 domain is required for LPA-induced DNA synthesis.
Besides their role in regulating the cytoskeleton, Rho GTPases are involved in other responses to growth factors, such as promotion of S-phase entry (38). Given that Rho-mediated cytoskeletal rearrangements are dependent on the Ras-GAP SH3 domain, it was of interest to determine if this domain was implicated in other events controlled by Rho. Quiescent cells injected with MAb 200 were tested for their ability to incorporate BrdU when subjected to serum or LPA stimulation. LPA induction of DNA synthesis was inhibited by 30 to 50% by MAb 200 with respect to control IgG-injected cells (Fig. 6), whereas serum was able to induce DNA replication in 95% of both control and MAb 200-injected cells (Fig. 6). S-phase entry promoted by activated Ras was also not modified by MAb 200. Since we previously showed that MAb 200 was able to block stress fibers but not membrane ruffles induced by Ras, these data further demonstrate that the Ras-GAP SH3 domain is not required for all Ras-induced events. This finding is in agreement with the idea that serum stimulates progression into S phase through multiple pathways, including Ras (33) and Cdc42 (38), the latter of which may not be susceptible to our anti-Ras-GAP antibody. Altogether, our results show that the inhibitory effect of MAb 200 on the Rho signalling pathway is not restricted to the cytoskeleton but affects other downstream signals transduced by Rho.
FIG. 6.
Effect of MAb 200 on DNA synthesis induced by FCS, LPA, and Ras. For Ras induction of DNA synthesis, MAb 200 (8 mg/ml) or the control IgG (8 mg/ml) was coinjected with Ha-RasK12 (2 mg/ml) in serum-deprived cells. After injection, cells were incubated in the presence of BrdU-FdU for 40 h. For LPA or FCS induction of S-phase entry, cells were injected with MAb 200 or the control IgG and incubated in the presence of 100 μM LPA or 10% FCS and BrdU-FdU for 40 h. Cells were then processed for anti-BrdU staining as described in Materials and Methods. The percentage of MAb 200-injected cells which incorporated BrdU is compared to that of control IgG-injected cells, which was taken as 100% for each condition of stimulation. These control values represent 98% of the injected cells in the case of FCS stimulation, 80 to 60% in the case of LPA stimulation, and 50% in the case of Ras injection. The results are the average ± standard error of three experiments.
Ras-GAP inhibits Ha-RasK12-induced PC12 differentiation.
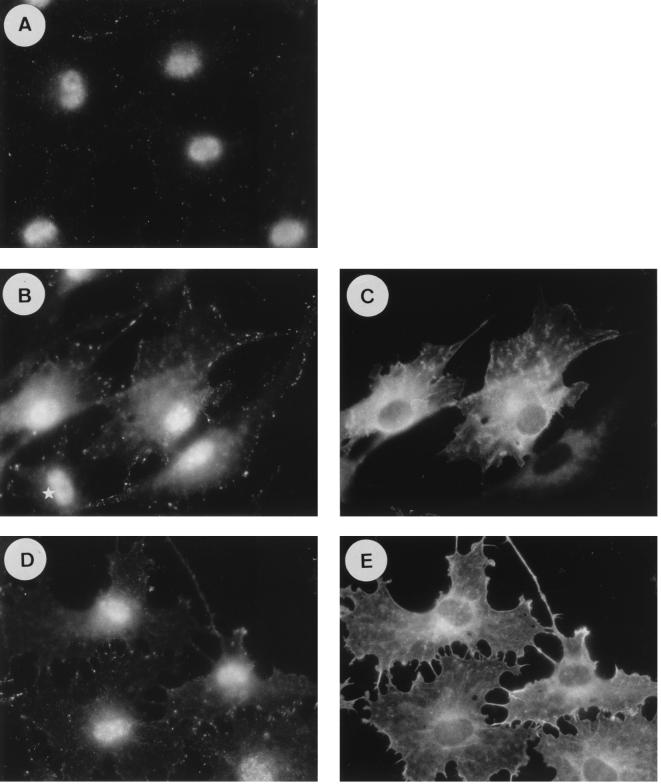
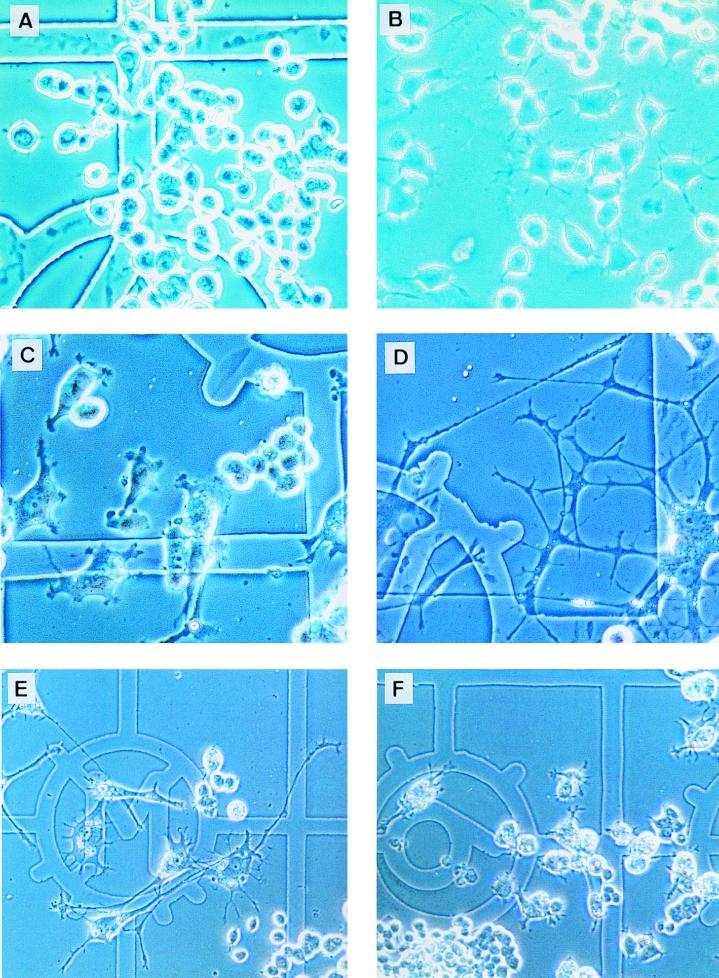
The study of the regulation of the cytoskeleton in rat PC12 pheochromocytoma cells has revealed a crucial role for small G proteins. Indeed, PC12 cells have been shown to differentiate into a neuron-like phenotype following NGF or Ras stimulation by modifying their actin cytoskeleton and extending long neurites (2). Moreover, cell differentiation has also been obtained by specific Rho inhibition (25, 35). Since our data demonstrate an impact of Ras-GAP, through its SH3 domain, on cytoskeletal reorganization controlled by Rho independently of Ras in fibroblasts, we asked whether Ras-GAP would have a similar function in PC12 cells. In a first set of experiments, PC12 cells were injected with MAb 200, GST-GAPSH2-SH3-SH2, or GST-Ras-GAP, in the presence or absence of activated Ras (Ha-RasK12), and observed for several days. Four days after injection, cells injected with MAb 200 alone (Fig. 7B) or GST-GAPSH2-SH3-SH2 (not shown) exhibited small neurites that were not seen in control cells injected with an irrelevant IgG (Fig. 7A). Cells having received MAb 200 and activated Ras (not shown) or GST-GAPSH2-SH3-SH2 and activated Ras (Fig. 7D) exhibited by 2 days postinjection a fully differentiated phenotype which they retained for more than 6 days. In contrast, at that time, Ras-plus-GST-injected cells had almost completely lost their differentiated morphology (Fig. 7C). On the other hand, a strong inhibition of Ras-stimulated differentiation was observed at 4 days when GST-Ras-GAP was coinjected with activated Ras (Fig. 7F), while GST coinjected with Ras had no effect on differentiation under the same conditions (Fig. 7E). GST-Ras-GAP on its own did not induce any morphological changes in PC12 cells (not shown). Because Ha-RasK12 cannot be deactivated by Ras-GAP (3), the inhibition of PC12 cell differentiation is unlikely to result from Ras inactivation, but could rather be the consequence of the activation, by Ras-GAP, of another pathway which prevents differentiation, such as the Rho pathway.
FIG. 7.
Effect of MAb 200, GST-GAPSH2-SH3-SH2, and GST-Ras-GAP on the morphology of PC12 cells. PC12 cells are shown 3 days after injection of a control mouse IgG (A) or MAb 200 (B). Note the presence of short neurites in MAb 200-injected cells, which are absent in control cells. In panel C are shown cells 6 days after coinjection of Ha-RasK12 with GST, while cells injected with Ha-RasK12 and GST-GAPSH2-SH3-SH2 are shown in panel D. Note the difference in neurite length between panels C and D. Cells coinjected with Ha-RasK12 plus GST are fully differentiated after 4 days (E), whereas those injected with Ha-RasK12 plus GST-Ras-GAP are not (F). The antibodies were injected at a concentration of 8 mg/ml, Ha-RasK12 was injected at 1.5 mg/ml, and GST, GST-GAPSH2-SH3-SH2, and GST-Ras-GAP were injected at 2 mg/ml. The cell clumps in panels E (bottom right corner) and F (bottom left corner) were not injected; in the other fields, all cells have been injected or stem from injected cells.
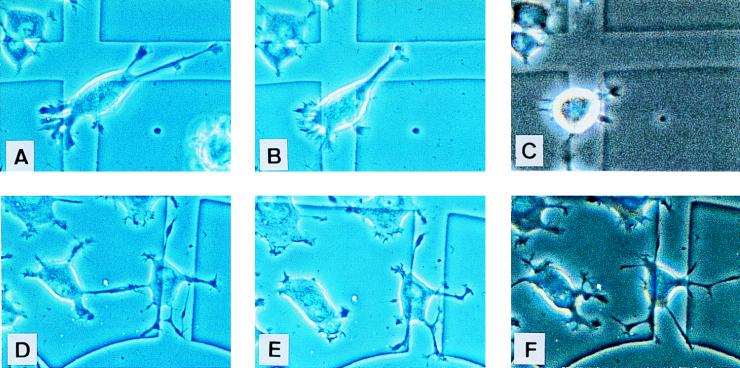
In a second set of experiments, NGF-differentiated cells were injected with a control IgG (Fig. 8A) or with MAb 200 (Fig. 8D) and then stimulated with LPA. After 30 min, most neurites had retracted in control cells, and cells started to detach from the tissue culture dish (Fig. 8B). This effect was even more pronounced after a couple of hours (Fig. 8C). In MAb 200-injected cells, neurites had also partially retracted by 30 min (Fig. 8E), but this phenomenon was only transient, because they extended back to their initial length by 2 h (Fig. 8F). Furthermore, these cells were no longer sensitive to the effect of LPA. Given that LPA-stimulated activation of Rho induces neurite retraction in NGF-differentiated cells (52), our data suggest that the presence of MAb 200 prevents Rho from being activated by LPA, possibly by preventing Ras-GAP from activating Rho. Altogether, these results show that the Ras-GAP SH3 domain is not necessary for Ras to differentiate PC12 cells, but that Ras-GAP is implicated in the control of differentiation, probably by regulating the level of activity of endogenous Rho.
FIG. 8.
MAb 200 protects NGF-differentiated PC12 cells from LPA-induced neurite retraction and cell rounding. IgG- or MAb 200-injected cells (upper and lower panels, respectively) are shown 2 h postinjection without addition (A and D) or 30 min (B and E) or 3 h (C and F) after stimulation with 10 μM LPA. All cells in these fields have been injected.
DISCUSSION
This study, initially focused on the role of the Ras-GAP SH3 domain in cytoskeletal changes stimulated by growth factors in quiescent Swiss 3T3 fibroblasts, led us to show that Ras-GAP microinjection promotes stress fiber assembly independently of any additional stimulus and, of particular interest, independently of cellular Ras. In addition, Ras-GAP promotes stress fiber formation in Swiss 3T3 cells, possibly via activation of Rho, since this effect is blocked by the C3 exotoxin, a specific Rho inhibitor. We propose that Ras-GAP-induced cytoskeletal reorganization is mediated by its SH3 domain, because the specific anti-GAP SH3 antibody, MAb 200, impedes this phenomenon. Moreover, this antibody blocked LPA-induced neurite retraction in differentiated PC12 cells, a response known to be controlled by Rho. That the inhibition of actin polymerization into stress cables is bypassed by coinjection of activated Rho further indicates that MAb 200 suppresses activation of endogenous Rho stimulated by different growth factors, but has no impact downstream of Rho. MAb 200 did not cross-react with Rho, ruling out the possibility that it might act by direct binding to Rho itself (not shown).
Several lines of evidence indicate that the effects of MAb 200 are highly specific and are not the result of a stress caused by microinjection of a high concentration of IgG into cells weakened by serum starvation. First of all, an identical concentration of an irrelevant antibody had no effect. Second, MAb 200 recognized a single band of 120 kDa corresponding to Ras-GAP on a blot of extract from Swiss 3T3 and PC12 cells; in addition, the recombinant Ras-GAP SH3 domain was the only one recognized by the antibody among those tested, including Src, Fyn, Lck, Grb-2, phospholipase Cγ, Abl, Nck, and Crk (data not shown). Moreover, the inhibitory effect of MAb 200 on growth factor-stimulated stress fiber assembly disappeared when the antibody was incubated with the Ras-GAP SH3 domain prior to injection; no other SH3 domain was capable of relieving this effect (not shown). Third, MAb 200 blocked only a defined subset of cellular responses to growth factors, such as stress fiber and focal adhesion assembly or LPA-induced DNA synthesis in fibroblasts, as well as LPA-induced neurite retraction in neuron-like cells, but it did not alter membrane ruffling, DNA replication stimulated by serum, or differentiation promoted by oncogenic Ras in PC12 cells. Finally, the effects of the anti-GAP SH3 antibody were reproduced by a truncation mutant of Ras-GAP consisting of the SH2 and SH3 domains. It is interesting that MAb 200 only partially blocked LPA-stimulated DNA synthesis; this can be explained by the fact that this response involves additional Rho-independent activities like that of Ras, which is known to be activated by LPA via a pertussis toxin-dependent mechanism (55), and the mitogen-activated protein kinase cascade (20).
We have shown for the first time that Ras-GAP can have a proper effector function independently of Ras, because its overexpression produces a Rho-like phenotype in resting fibroblasts, while in association with oncogenic Ras, it considerably slows down morphological differentiation of PC12 cells. Constructs of Ras-GAP encompassing the SH2 and SH3 domains have proved to be a valuable tool for investigating its function, and their use has been extensively reported in the literature (9, 10, 28, 30, 31). Similar to our finding that MAb 200 did not inhibit Ras-induced membrane ruffling, it was recently reported that GAPSH2-SH3 domain did not alter this response in porcine endothelial cells (46). GST-GAPSH2-SH3-SH2 as well as MAb 200 were able to slightly differentiate PC12 cells, probably via a molecular mechanism that does not involve Ras. This was clearly demonstrated in fibroblasts in which Ras-GAP does not require Ras activity to induce actin polymerization. Our finding that MAb 200, as well as GST-GAPSH2-SH3-SH2, greatly potentiated the effect of oncogenic Ras in prolonging cell differentiation is in accordance with the finding that PC12 cells overexpressing the Ras-GAP N terminus differentiated to a greater extent than control cells in response to NGF and extended longer neurites (34). This effect could be explained by a concomitant inhibition of the Rho pathway due to Ras-GAP inactivation, by the anti-Ras-GAP SH3 antibody or by competition with the inhibitory construct GST-GAPSH2-SH3-SH2, and activation of the Ras pathway by oncogenic Ras, both able to promote PC12 differentiation. Our experiments with full-length Ras-GAP in these cells also support this notion: the inhibition of Ras-induced differentiation probably results from Rho activation caused by Ras-GAP. Nevertheless, we cannot exclude the hypothesis of a competition between Ras-GAP and another Ras effector which is important for differentiation—Raf1, for example (56).
That Ras-GAP by itself triggers reorganization of the cytoskeleton independently of Ras was rather unexpected. Previous studies have shown that microinjection of n-chimaerin, a GAP for Rac1 and Cdc42, also promotes morphological changes such as filopodia, lamellipodia, and a decrease in the number of stress fibers coupled to a redistribution of vinculin to the ends of the newly formed actin structures (24). This effect does not require GAP activity but depends on the ability of n-chimaerin to bind Rac1 and Cdc42, while the isolated GAP domain was ineffective (24). Our results are somewhat different, in that Ras-GAP seems to promote cytoskeletal changes in a Ras-independent fashion. Indeed, stress fiber assembly induced by Ras-GAP was not blocked by injection of the neutralizing antibody Y13-259, which has been shown to prevent Ras-GAP from binding to Ras (42). This is also consistent with the very low level of active GTP-bound Ras which must be left in Swiss 3T3 cells after serum deprivation. Moreover, Y13-259 does not block stress fiber formation in response to LPA, whereas the anti-GAP SH3 antibody MAb 200 does, which indicates that Ras-GAP is not solely an effector of Ras, but can exert additional functions independently. The ability of Ras-GAP to rearrange the actin cytoskeleton depends on the SH3 domain, because it was inhibited by MAb 200 but not by antibodies directed against the carboxy-terminal part of the protein, ruling out the possibility that the inhibitory effect of MAb 200 is due to the sequestering of Ras-GAP to an abnormal cellular location or to Ras-GAP degradation. Finally, Ras-GAP-induced actin polymerization into stress fibers results from the subsequent activation of Rho, since it was inhibited by the C3 toxin.
Our results demonstrate that the Ras-GAP SH3 domain is required for Rho activation. Theoretically, Rho activation can be achieved via either stimulation of Rho guanine nucleotide exchange factors (GEFs), inhibition of Rho-GAP activity, or a combination of both. Two interpretations, consistent with our findings, can be put forward. In the first scenario, stimulation of quiescent cells with serum or LPA would lead to the association of Ras-GAP with a protein required for Rho activation, such as a Rho-GEF, that would only be active when bound to Ras-GAP. MAb 200 would displace from Ras-GAP, and thereby inactivate, the Rho activator. For its part, the truncated version of Ras-GAP, Ras-GAPSH2-SH3-SH2, would compete with endogenous Ras-GAP for binding to the Rho activator, but would be unable to stimulate it and, therefore, would block stress fiber assembly.
A second hypothesis, in which stimulation of quiescent cells with serum or LPA would lead to the association of Ras-GAP with a Rho inhibitor, such as Rho-GDI (13, 16) or p190 Rho-GAP (5, 12, 45, 48, 49), is also possible. This association would suppress the function of the inhibitor, thereby allowing GTP loading on Rho to take place. It is currently not known if, besides p190 and p190 B (6), other Rho-GAPs can associate with Ras-GAP, but it is worth noting that several of them contain a proline-rich sequence (27). In this case, MAb 200 would release the inhibitor from Ras-GAP and prevent Rho from being activated, while Ras-GAPSH2-SH3-SH2 would compete with Ras-GAP for binding to the inhibitor but would form unstable complexes that release the inhibitor. However, our results strongly suggest that this inhibitor is unlikely to be p190 Rho-GAP, because MAb 200 did not displace p190 from Ras-GAP, nor did it increase their association. Western blots probed with an anti-p190 antibody, or with an anti-Ras-GAP antibody, revealed that p190 came down with Ras-GAP when it was precipitated with the anti-SH3 domain antibody MAb 200 or with an antibody against the COOH-terminal domain, 15F8 (39) (data not shown). This result is not surprising, since it has been established that tyrosine-phosphorylated p190 binds the SH2 domains of Ras-GAP (5, 21). It remains possible, however, that the effect of MAb 200 or Ras-GAPSH2-SH3-SH2 on p190 might be more subtle, such as, for instance, modulation of the Rho-GAP activity of p190 when bound to Ras-GAP.
In any case, the assumption that the Rho regulatory protein, be it activator or inhibitor, binds directly to the Ras-GAP SH3 domain implies that it contains either a consensus proline-rich sequence (29) or, alternatively, a WW domain (50). As far as we know, none of the Rho-GEFs or Rho GDP dissociation inhibitors (GDIs) described to date have been reported to have such motifs. It may indicate that the interaction with Ras-GAP is indirect and requires an intermediate partner. Nevertheless, it does not preclude the existence of a new Rho-GEF or Rho-GDI not yet identified and able to bind SH3 domains, given that there are already known GEFs for Ras and Rap1, i.e., Sos (7) and C3G (17), respectively, which bind the SH3 domains of the Grb2 and Crk adapter proteins respectively. Interestingly, Gebbink et al. have described a new protein, p116 Rip, that could fit with this hypothesis (15). p116 Rip is a Rho-interacting protein whose overexpression in neuroblastoma N1E-115 cells causes cell flattening and neurite outgrowth in the presence of serum which contains LPA. This protein is thus a suppressor of Rho. Importantly, this protein is a putative SH3 binding protein with proline-rich sequences which are necessary for induction of morphological differentiation (15).
Overall, the results presented here provide a clue to how Rho gets activated by growth factors and shed new light on a putative effector function for Ras-GAP in regulating cytoskeletal organization.
ACKNOWLEDGMENTS
We are grateful to A. Hall for the Rho expression plasmid and J. Grassi for providing antibodies. We thank J. Vassy and D. Schoevaert (Laboratoire d’Analyse d’Image et Pathologie Cellulaire, Hôpital Saint Louis, Paris, France) for confocal analysis of the cytoskeleton in some experiments. Thanks go to F. Risbec and S. Bouvier for plasmids and recombinant proteins and M. Kenigsberg and F. Parker for helpful advice. We also acknowledge A. Ridley for critical reading of the manuscript.
REFERENCES
- 1.Abdellatif M, Schneider M D. An effector-like function of Ras GTPase-activating protein predominates in cardiac muscle cells. J Biol Chem. 1997;272:525–533. doi: 10.1074/jbc.272.1.525. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Sagi D, Feramisco J R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- 3.Boguski M S, McCormick F. Proteins regulating Ras and its relative. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 4.Bokoch G M, Der C J. Emerging concepts in the Ras superfamily of GTP-binding proteins. FASEB J. 1993;7:750–759. doi: 10.1096/fasebj.7.9.8330683. [DOI] [PubMed] [Google Scholar]
- 5.Bryant S S, Briggs S, Smithgall T E, Martin G A, McCormick F, Chang J H, Parsons S J, Jove R. Two SH2 domains of p120 Ras GTPase-activating protein bind synergistically to tyrosine phosphorylated p190 Rho GTPase-activating protein. J Biol Chem. 1995;270:17947–17952. doi: 10.1074/jbc.270.30.17947. [DOI] [PubMed] [Google Scholar]
- 6.Burbelo P D, Miyamoto S, Utani A, Brill S, Yamada K M, Hall A, Yamada Y. p190-B, a new member of the Rho GAP family, and Rho are induced to cluster after integrin cross-linking. J Biol Chem. 1995;270:30919–30926. doi: 10.1074/jbc.270.52.30919. [DOI] [PubMed] [Google Scholar]
- 7.Chardin P, Camonis J H, Gale N W, Van Aelst L, Schlessinger J, Wigler M H, Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to Grb2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- 8.Chavrier P, Gorvel J P, Bertoglio J. An immunologist’s look at the Rho and Rab GTP-binding proteins. Immunol Today. 1993;14:440–444. doi: 10.1016/0167-5699(93)90247-I. [DOI] [PubMed] [Google Scholar]
- 9.Clark G J, Quilliam L A, Hisaka M M, Der C J. Differential antagonism of Ras biological activity by catalytic Src homology domains of Ras GTPase activating protein. Proc Natl Acad Sci USA. 1993;90:4887–4891. doi: 10.1073/pnas.90.11.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark G J, Westwick J K, Der C J. p120 GAP modulates Ras activation of jun kinases and transformation. J Biol Chem. 1997;272:1677–1681. doi: 10.1074/jbc.272.3.1677. [DOI] [PubMed] [Google Scholar]
- 11.Duchesne M, Schweighoffer F, Parker F, Clerc F, Frobert Y, Thang M N, Tocqué B. Identification of the SH3 domain of GAP as an essential sequence for Ras-GAP-mediated signaling. Science. 1993;259:525–528. doi: 10.1126/science.7678707. [DOI] [PubMed] [Google Scholar]
- 12.Ellis C, Moran M, McCormick F, Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990;343:377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- 13.Fukumoto Y, Kaibuchi K, Hori Y, Fujioka H, Araki S, Ueda T, Kikuchi A, Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990;5:1321–1328. [PubMed] [Google Scholar]
- 14.Furth M E, Davis L J, Fleurdelys B, Scolnick E M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982;43:294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebbink M F B G, Kranenburg O, Poland M, van Horck F P G, Houssa B, Moolenaar W H. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosser Y Q, Nomanbhoy T K, Aghazadeh B, Manor D, Combs C, Cerione R A, Rosen M K. C-terminal binding domain of Rho GDP-dissociation inhibitor directs N-terminal inhibitory peptide to GTPases. Nature. 1997;387:814–819. doi: 10.1038/42961. [DOI] [PubMed] [Google Scholar]
- 17.Gotoh T, Hattori S, Nakamura S, Kitayama H, Noda M, Takai Y, Kaibuchi K, Matsui H, Hatase O, Takahashi H, Kurata T, Matsuda M. Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotide-releasing factor C3G. Mol Cell Biol. 1995;15:6746–6753. doi: 10.1128/mcb.15.12.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henkemeyer M, Rossi D J, Holmyard D P, Puri M C, Mbamalu G, Harpal K, Shih T S, Jacks T, Pawson T. Vascular system defects and neuronal apoptosis in mice lacking Ras-GTPase-activating protein. Nature. 1995;377:695–701. doi: 10.1038/377695a0. [DOI] [PubMed] [Google Scholar]
- 19.Hotchin N A, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howe L R, Marshall C J. Lysophosphatidic acid stimulates mitogen-activated protein kinase activation via a G-protein-coupled pathway requiring p21ras and p74raf-1. J Biol Chem. 1993;268:20717–20720. [PubMed] [Google Scholar]
- 21.Hu K Q, Settleman J. Tandem SH2 binding sites mediate the RasGAP-RhoGAP interaction: a conformational mechanism for SH3 domain regulation. EMBO J. 1997;16:473–483. doi: 10.1093/emboj/16.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang D C S, Marshall C J, Hancock J F. Plasma membrane-targeted ras GTPase-activating protein is a potent suppressor of p21ras function. Mol Cell Biol. 1993;13:2420–2431. doi: 10.1128/mcb.13.4.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozma R, Ahmed S, Best A, Lim L. The GTPase-activating protein n-chimaerin cooperates with Rac1 and Cdc42Hs to induce the formation of lamellipodia and filopodia. Mol Cell Biol. 1996;16:5069–5080. doi: 10.1128/mcb.16.9.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kundra V, Anand-Apte B, Feig L A, Zetter B R. The chemotactic response to PDGF-BB: evidence of a role for Ras. J Cell Biol. 1995;130:725–731. doi: 10.1083/jcb.130.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamarche N, Hall A. GAPs for rho-related GTPases. Trends Genet. 1994;10:436–440. doi: 10.1016/0168-9525(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 28.Mattingly R R, Sorisky A, Brann M R, Macara I G. Muscarinic receptors transform NIH 3T3 cells through a Ras-dependent signalling pathway inhibited by the Ras-GTPase-activating protein SH3 domain. Mol Cell Biol. 1994;14:7943–7952. doi: 10.1128/mcb.14.12.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer B J, Eck M J. Minding your p’s and q’s. Curr Biol. 1995;5:364–367. doi: 10.1016/s0960-9822(95)00073-x. [DOI] [PubMed] [Google Scholar]
- 30.McGlade J, Brunkhors B, Anderson D, Mbamalu G, Settleman J, Dedhar S, Rozakis-Adcock M, Chen L B, Pawson T. The N-terminal region of GAP regulates cytoskeletal structure and cell adhesion. EMBO J. 1993;12:3073–3081. doi: 10.1002/j.1460-2075.1993.tb05976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medema R H, de Laat W L, Martin G A, McCormick F, Bos J L. GTPase-activating protein SH2-SH3 domains induce gene expression in a Ras-dependent fashion. Mol Cell Biol. 1992;12:3425–3430. doi: 10.1128/mcb.12.8.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollat P, Zhang G Y, Frobert Y, Zhang Y H, Fournier A, Grassi J, Thang M N. Non-neutralizing monoclonal antibodies against Ras GTPase-activating protein: production, characterization and use in an enzyme immunometric assay. Bio/Technology. 1992;10:1151–1155. doi: 10.1038/nbt1092-1151. [DOI] [PubMed] [Google Scholar]
- 33.Mulcahy L S, Smith M R, Stacey D W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH3T3 cells. Nature. 1985;313:241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- 34.Nakata H, Watanabe Y. Proliferation and differentiation of PC12 cells were affected by p21ras GTPase activating proteins and its deletion mutant proteins. Biochem Biophys Res Commun. 1996;218:538–543. doi: 10.1006/bbrc.1996.0096. [DOI] [PubMed] [Google Scholar]
- 35.Nishiki T, Narumiya S, Morii N, Yamamoto M, Fujiwara M, Kamata Y, Sakaguchi G, Kozaki S. ADP-ribosylation of the rho/rac proteins induces growth inhibition, neurite outgrowth and acetylcholine esterase in cultured PC12 cells. Biochem Biophys Res Commun. 1990;167:265–272. doi: 10.1016/0006-291x(90)91760-p. [DOI] [PubMed] [Google Scholar]
- 36.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lammelipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 37.Nori M, Vogel U S, Gibbs J B, Weber M J. Inhibition of v-src-induced transformation by a GTPase-activating protein. Mol Cell Biol. 1991;11:2812–2818. doi: 10.1128/mcb.11.5.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson M F, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 39.Parker F, Maurier F, Delumeau I, Duchesne M, Faucher D, Debussche L, Dugue A, Schweighoffer F, Tocque B. A Ras-GTPase-activating protein SH3 domain-binding protein. Mol Cell Biol. 1996;16:2561–2569. doi: 10.1128/mcb.16.6.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paterson H, Self A J, Garrett M D, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pomerance M, Thang M N, Tocque B, Pierre M. The Ras-GTPase-activating protein SH3 domain is required for Cdc2 activation and Mos induction by oncogenic Ras in Xenopus oocytes independently of mitogen-activated protein kinase activation. Mol Cell Biol. 1996;16:3179–3186. doi: 10.1128/mcb.16.6.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rey I, Soubigou P, Debussche L, David C, Morgat A, Bost P E, Mayaux J F, Tocque B. Antibodies to synthetic peptide from the residue 33 to 42 domain of c-Ha-ras p21 block reconstitution of the protein with different effectors. Mol Cell Biol. 1989;9:3904–3910. doi: 10.1128/mcb.9.9.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 44.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 45.Ridley A J, Self A J, Kasmi F, Paterson H F, Hall A, Marshall C J, Ellis C. Rho family GTPase activating proteins p190, bcr and rho-GAP show distinct specificities in vitro and in vivo. EMBO J. 1993;12:5151–5160. doi: 10.1002/j.1460-2075.1993.tb06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A J, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 47.Schweighoffer F, Barlat I, Chevallier-Multon M C, Tocqué B. Implication of GAP in Ras-dependent transactivation of a polyoma enhancer sequence. Science. 1992;256:825–827. doi: 10.1126/science.1317056. [DOI] [PubMed] [Google Scholar]
- 48.Settleman J, Narasimhan V, Foster L C, Weinberg R A. Molecular cloning of cDNAs encoding the GAP-associated protein p190: implications for a signaling pathway from Ras to the nucleus. Cell. 1992;69:539–549. doi: 10.1016/0092-8674(92)90454-k. [DOI] [PubMed] [Google Scholar]
- 49.Settleman J, Albright C F, Foster L C, Weinberg R A. Association between GTPase activators for Rho and Ras families. Nature. 1992;359:153–154. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- 50.Sudol M. The WW module competes with the SH3 domain? Trends Biochem Sci. 1996;21:161–163. [PubMed] [Google Scholar]
- 51.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 52.Tigyi G, Fischer D J, Sebök A, Yang C, Dyer D L, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and rho. J Neurochem. 1996;66:537–548. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- 53.Tocqué B, Delumeau I, Parker F, Maurier F, Multon M C, Schweighoffer F. Ras-GTPase activating protein (GAP): a putative effector for Ras. Cell Signalling. 1997;9:153–158. doi: 10.1016/s0898-6568(96)00135-0. [DOI] [PubMed] [Google Scholar]
- 54.Trahey M, McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987;238:542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- 55.vanCorven E J, Hordijk P L, Medema R H, Bos J L, Moolenaar W H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci USA. 1993;90:1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood K W, Qi H, D’Arcangelo G, Armstrong R C, Roberts T M, Halegoua S. The cytoplasmic raf oncogene induces a neuronal phenotype in PC12 cells: a potential role for cellular raf kinases in neuronal growth factor signal transduction. Proc Natl Acad Sci USA. 1993;90:5016–5020. doi: 10.1073/pnas.90.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu N, McCormick F, Gutkind J S. The non-catalytic domain of ras-GAP inhibits transformation induced by G protein coupled receptors. Oncogene. 1994;9:597–601. [PubMed] [Google Scholar]
- 58.Yao R, Cooper G M. Regulation of the Ras signaling pathway by GTPase-activating protein in PC12 cells. Oncogene. 1995;11:1607–1614. [PubMed] [Google Scholar]
- 59.Zhang K, DeClue J E, Vass W C, Papageorge A G, McCormick F, Lowy D R. Suppression of c-ras transformation by GTPase-activating protein. Nature. 1990;346:754–756. doi: 10.1038/346754a0. [DOI] [PubMed] [Google Scholar]