Abstract
Here, we provide the first regional analysis of intact and defective HIV reservoirs within the brain. Brain tissue from both viremic and virally suppressed people with HIV (PWH) harbored HIV pol DNA in all regions tested, with lower levels present in basal ganglia and cerebellum relative to frontal white matter. Intact proviruses were primarily found in the frontal white matter but also detected in other brain regions of PWH, demonstrating frontal white matter as a major brain reservoir of intact, potentially replication competent HIV DNA that persists despite antiretroviral therapy.
HIV persistence in cellular and tissue reservoirs is the major barrier to HIV cure. During acute infection, HIV enters the brain via infiltrating T cells and monocytes, leading to viral dissemination in microglia, perivascular macrophages, astrocytes, and pericytes.1,2 We and others have also shown that viral DNA persists in the brain of virally suppressed people with HIV (PWH) on antiretroviral therapy (ART) at similar levels to those present during untreated infection.3,4 Recently, we utilized the intact proviral DNA assay (IPDA) to further characterize the brain reservoir, providing the first evidence of intact and defective HIV proviruses in the frontal white matter of PWH.3 Importantly, levels of intact proviruses did not appear to be impacted by long-term viral suppression, supporting the presence of a stable and potentially replication competent HIV reservoir in the brain. This study focused on HIV persistence in the frontal white matter due to the key role that this region plays in the pathogenesis of HIV-associated neurocognitive disorders (HAND),5 which affects ~40% of virally suppressed PWH.6 Studies using non-human primate models of HIV and earlier analyses of autopsy brain tissue from PWH with HIV-associated dementia have also reported regional differences in the size and activity of the HIV reservoir in the brain.7,8 Differences in the intact and defective HIV reservoir throughout regions of the brain may reflect more significant roles in potential central nervous system (CNS) escape or domain-specific cognitive impairment. Therefore, understanding the proviral landscape across the brain, particularly the presence of intact proviruses in virally suppressed PWH, is essential for defining the effect of HIV persistence on brain health in PWH.
Methods
Tissue Cohort
Matched fresh frozen frontal white matter, basal ganglia, and cerebellum brain tissue from PWH or HIV-seronegative individuals was provided by the National NeuroAIDS Tissue Consortium (NNTC, USA; https://nntc.org9) and processed with ethics approval (RMIT University, Australia; HREC#20843).
Quantification of HIV Reservoirs in Brain Regions
The gDNA was extracted from brain tissue using the AllPrep DNA/RNA/miRNA universal kit (QIAGEN, Hilden, Germany) for HIV pol quantification or the DNA extraction kit (Agilent, Santa Clara, CA) for the IPDA, as previously described.3 HIV pol and ribonuclease P/MRP Subunit P30 (RPP30) for total input gDNA quantification was quantified, as previously described.10 A median (interquartile range [IQR]) of 502,725 (IQR = 405,526 – 686,992) cells were screened for HIV pol DNA analysis and 510,292 (IQR = 401,101 – 610,438) screened by IPDA per tissue region per patient. Intact (Ψ+ and env+) and defective (Ψ+ or env+) HIV genomes were measured by the IPDA, as previously described.3 Median droplet shearing index (DSI) was 0.24 (IQR = 0.19–0.30), with DSI levels similar between frontal white matter tissue and other sites.
Statistics
Comparisons were made using nonparametric Mann–Whitney U (unpaired) or Wilcoxon tests (paired) using GraphPad Prism (version 9.2.0; GraphPad Software, La Jolla, CA).
Results
HIV DNA Is Present in Basal Ganglia and Cerebellum at Lower Levels than Frontal White Matter Tissue in Virally Suppressed PWH
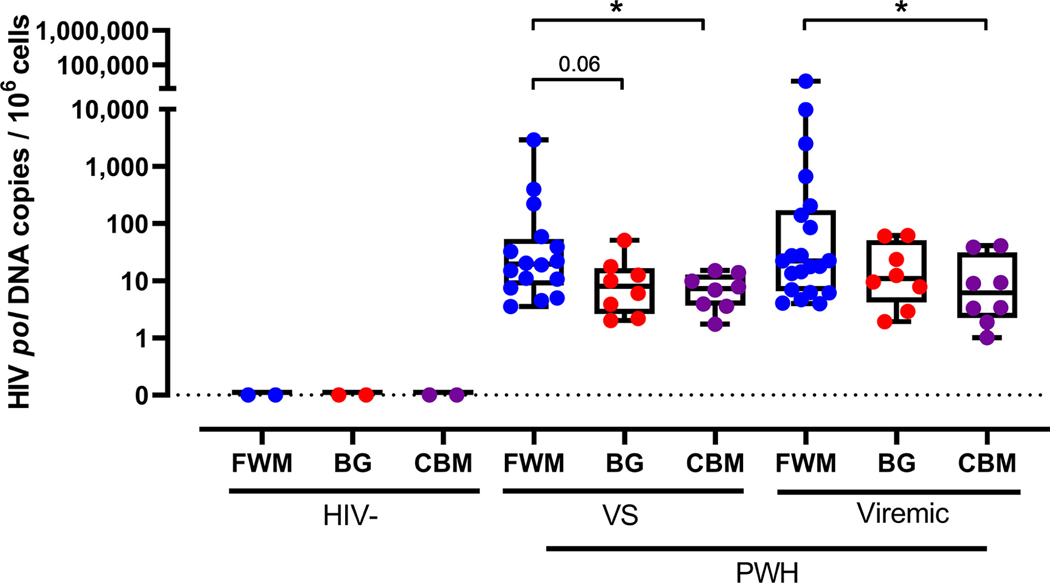
To characterize HIV persistence throughout the brain, fresh frozen frontal white matter, cerebellum, and basal ganglia tissue from virally suppressed or untreated PWH were assessed for HIV pol DNA by ddPCR (Supplementary Table S1). Viral suppression was defined as > 12 months of continuous ART treatment with undetectable viral load (one blip < 250 copies/ml was allowed > 6 months prior to sampling unless stated in Supplementary Table S1). HIV pol DNA was detected in all brain regions from all subjects (Fig 1), indicating widespread HIV persistence. HIV pol DNA was detected in the cerebellum at a lower level than in frontal white matter tissue in both viremic and virally suppressed PWH (both p < 0.05). Basal ganglia from virally suppressed PWH also showed a trend to lower levels of HIV pol DNA than in the frontal white matter ( p = 0.06). HIV DNA was not detected in HIV-seronegative controls. Levels of HIV pol DNA in each region were similar between virally suppressed and viremic PWH, indicating that long-term viral suppression did not affect the distribution of the HIV reservoir.
FIGURE 1:
Quantification of HIV pol DNA in human brain regions. HIV pol DNA was quantified in fresh frozen FWM (blue), BG (red), and CBM ( purple) tissue from VS (n = 16) and untreated PWH (viremic; n = 21) or HIV-seronegative controls (HIV-; n = 2) by ddPCR. HIV pol DNA copies standardized to 106 cell equivalents. Median and IQRs shown. Comparisons made using non-parametric Mann–Whitney U tests. *p < 0.05. BG = basal ganglia; CBM = cerebellum; ddPCR = droplet digital polymerase chain reaction; FWM = frontal white matter; IQR = interquartile range; PWH = people with HIV; VS = virally suppressed.
Frontal White Matter Is a Major Reservoir of Intact Proviral DNA Relative to Other Brain Regions in Virally Suppressed PWH
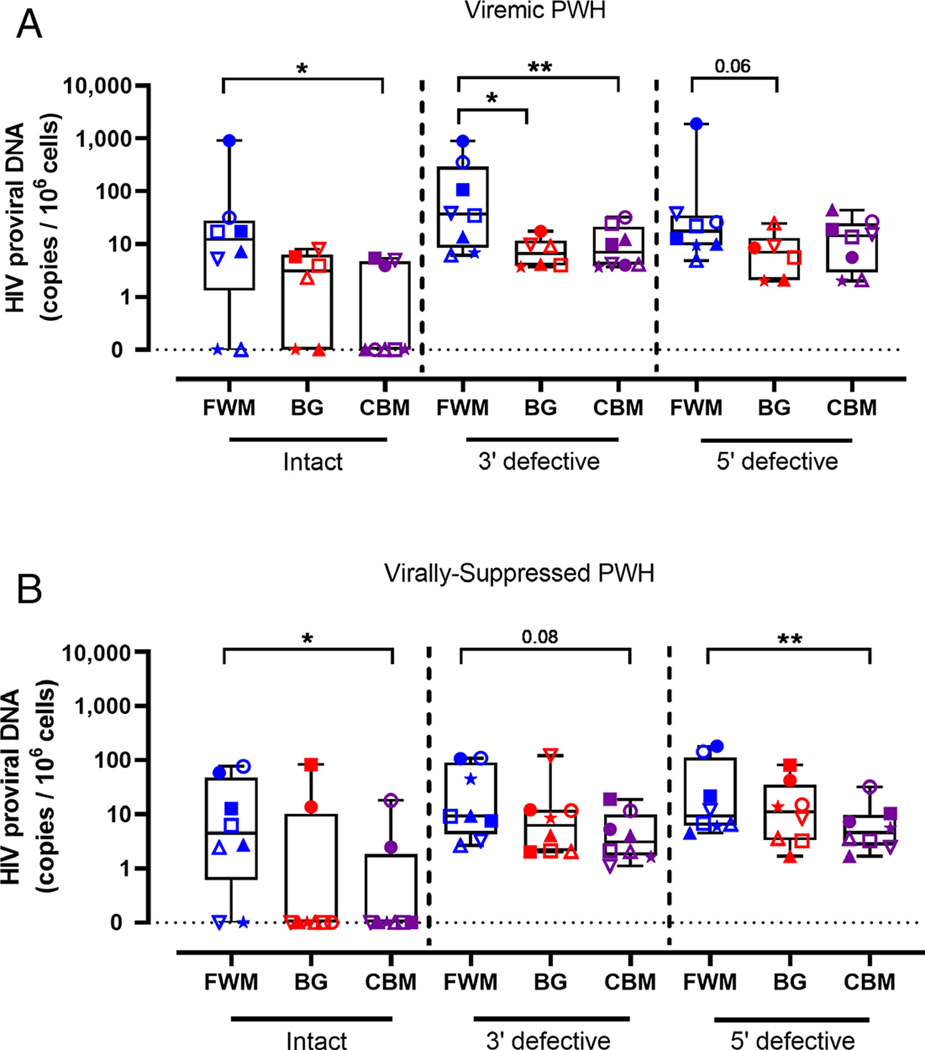
We next used the IPDA to characterize intact and defective HIV DNA across the frontal white matter, basal ganglia, and cerebellum from matched PWH. The IPDA is a multiplex droplet digital polymerase chain reaction (ddPCR) approach that utilizes fluorescent primer/probe sets targeting either HIV psi (Ψ) or HIV envelope (env) regions at alternate ends of the genome.3,11 Therefore, detection of proviral DNA expressing both fluorophores is considered intact, and potentially capable of viral replication. The IPDA was performed on viremic PWH (n = 8), virally suppressed PWH (n = 8) or HIV-seronegative controls (n = 2) where matched tissue was available. Two basal ganglia tissues were excluded from IPDA analysis due to suboptimum levels of DSI (> 0.50; Supplementary Table S1). Intact HIV proviral genomes were present in the frontal white matter in 6 of 8, cerebellum in 3 of 8 and basal ganglia tissue in 4 of 6 viremic PWH (Fig 2A). Frontal white matter tissue harbored higher levels of intact HIV DNA relative to the cerebellum (p < 0.05). Frontal white matter also had higher levels of 3′ defective HIV DNA than other brain regions in viremic PWH (both p < 0.05 relative to frontal white matter) and a trend to higher levels of 5′ defective proviral DNA in basal ganglia was observed. As expected, the majority of proviral DNA in each region were defective (either 3′ or 5′ defective proviruses; > 80% of total proviruses for all regions). No HIV DNA was detected in HIV-seronegative controls (n = 2; data not shown).
FIGURE 2:
Intact and defective proviral landscape across multiple brain regions in PWH. (A) Intact (Ψ+env+), 3′ defective (Ψ+env−), and 5′ defective (Ψ−env+) HIV proviruses were quantified in matched frozen human FWM (blue; n = 8), BG (red; n = 6), or CBM ( purple; n = 8) from viremic PWH by IPDA. Proviral copy number was standardized to 106 cells. (B) Proviral copy number of intact, 3′ defective, and 5′ defective proviral HIV DNA in FWM (n = 8), BG (n = 8), and CBM (n = 8) from virally suppressed PWH. Symbols represent individuals. Comparisons made using nonparametric paired Wilcoxon tests. *p < 0.05, **p < 0.01. BG = basal ganglia; CBM = cerebellum; FWM = frontal white matter; IPDA = intact proviral DNA assay; PWH = people with HIV; VS = virally suppressed.
Similar to findings in viremic PWH, the majority of virally suppressed PWH tested contained intact proviral DNA in the frontal white matter (6 of 8; Fig 2B). However, contrary to findings in viremic PWH, comparatively few virally suppressed PWH had detectable intact proviral HIV DNA in cerebellum (2 of 8) or basal ganglia (2 of 8), demonstrating that the frontal white matter is a more stable reservoir of intact proviral DNA following long-term viral suppression. One PWH contained intact proviral DNA in each region (see solid circle in Fig 2B). Frontal white matter also contained higher levels of intact proviral DNA than other regions (median [IQR]: frontal vs basal ganglia vs cerebellum; 4.46 [0.61–47.0] vs. 0.00 [0.00–10.3] vs. 0.00 [0.00–1.85]; see Fig 2B), indicating that of the brain regions analyzed in this study the frontal white matter is the major HIV reservoir in the brain of virally suppressed PWH.
Discussion
Our study provides the first characterization of the intact and defective proviral HIV reservoir across multiple regions of the brain of virally suppressed PWH. HIV pol DNA was detected in cerebellum, basal ganglia, and frontal white matter of virally suppressed PWH which supports widespread viral persistence throughout the brain in line with earlier studies in viremic and ART-treated PWH.4,12–14 Although these studies did not find a statistically significant difference in HIV gag DNA levels between basal ganglia and frontal white matter tissue, we find higher levels of HIV pol DNA in the frontal white matter relative to the other regions tested. These differences may relate to cohort differences including viremia status, length of ART-suppression, presence/absence of HIVE, types of ART drugs used, and use of HIV pol instead of gag as a measure of HIV DNA. Moreover, we used a stringent definition of viral suppression with individuals being suppressed for a median of 4.1 years prior to autopsy.
Importantly, frontal white matter tissue harbored the most intact HIV proviral DNA relative to the other 2 brain regions tested. Intact proviral DNA was also detected, albeit infrequently, in other regions with 2 of 8 virally suppressed PWH harboring intact proviral DNA in basal ganglia or cerebellum, respectively, with one virally suppressed PWH harboring intact proviral DNA in each region. The relatively limited number of PWH harboring intact proviral DNA in the basal ganglia and cerebellum may result from these sites containing a smaller and/or more labile HIV reservoir. Basal ganglia and cerebellum are also rich in gray matter made up of neuronal cell bodies (known to not be receptive to HIV infection) and are more permissive to ART penetration than frontal white matter.15 In contrast, there is a higher density of HIV permissive microglia in frontal white matter tissue than either basal ganglia or cerebellum,16 which may impact the capacity for HIV to infect and persist in gray matter at levels seen in the frontal white matter. It is possible that treatment regimen may impact regional distribution of intact proviral reservoirs in the brain, however, this would need to be assessed in larger studies powered to address this question. The IPDA cannot distinguish between integrated and unintegrated forms of HIV DNA, however, a recent study demonstrated that the majority of HIV DNA in the brain is integrated (n = 63).14 Whether unintegrated forms of HIV DNA are intact in the brain remains unclear.
The majority of the HIV reservoir in frontal white matter, basal ganglia, and cerebellum tissue contained defective proviruses which mimics findings from other cellular and tissue HIV reservoirs.3,11,17,18 Defective proviral DNA may be capable of producing viral proteins with potential neurotoxic effects, which may lead to ongoing immune activation and neuropathology throughout the brain.19,20
Finally, in line with our previous work and other studies in frontal white matter,3,18 no difference in the levels of HIV intact and defective proviral genomes were observed between viremic and virally suppressed PWH across all regions. Together these data support recent evidence of the persistence of an intact, potentially replication competent HIV reservoir in the brain that is not eradicated by ART.21 Therefore, future studies need to consider the impact of both intact and defective proviral DNA (and potentially RNA) throughout the brain of virally suppressed PWH on the underlying pathogenesis of HAND in PWH.
Supplementary Material
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC), Australia 1183032 (M.J.C., J.D.E., M.R., and P.R.G.) and by the National Institute On Drug Abuse of the National Institutes of Health under Award Number R21DA055489 (M.J.C., M.R., and T.A.A.). The authors gratefully acknowledge the support from the NNTC financially supported by National Institutes of Health (NIH) funding through the NIMH and NINDS Institutes (grant nos. U24MH100930, U24MH100928, U24MH100929, U24MH100931, and U24MH100925). The contents are the responsibility of the authors and do not represent the official view of the NNTC or NIH. S.R.L. is supported by an NHMRC Practitioner Fellowship. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. 75N91019D00024/HHSN261201500003I. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Open access publishing facilitated by RMIT University, as part of the Wiley - RMIT University agreement via the Council of Australian University Librarians.
S.R.L. has received investigator-initiated grant funding from Gilead, Merck, and ViiV Healthcare.
Footnotes
Potential Conflicts of Interest
She has participated as a paid member of scientific advisory boards to Abivax, Immunocore, Efsam, AbbVie, and Gilead. C.R.C. is currently employed by ViiV Healthcare. C.R.C.’s contribution to this project was completed while at RMIT University.
References
- 1.Churchill MJ, Wesselingh SL, Cowley D, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol 2009;66:253–258. [DOI] [PubMed] [Google Scholar]
- 2.Cho HJ, Bertrand L, Toborek M. Blood–brain barrier pericytes as a target for HIV-1 infection. Brain 2019;142:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cochrane CR, Angelovich TA, Byrnes SJ, et al. Intact HIV proviruses persist in the brain despite viral suppression with ART. Ann Neurol 2022;92:532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamers SL, Rose R, Maidji E, et al. HIV DNA is frequently present within pathologic tissues evaluated at autopsy from cART-treated patients with undetectable viral load. J Virol 2016;90:8968–8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melrose RJ, Tinaz S, Castelo JMB, et al. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res 2008;188:337–347. [DOI] [PubMed] [Google Scholar]
- 6.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez S, Johnson A-M, Xiang SH, et al. Persistence of SIV in the brain of SIV-infected Chinese rhesus macaques with or without antiretroviral therapy. J Neurovirol 2018;24:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L, Rua R, Ng T, et al. Evidence for predilection of macrophage infiltration patterns in the deeper midline and mesial temporal structures of the brain uniquely in patients with HIV-associated dementia. BMC Infect Dis 2009;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgello S, Gelman BB, Kozlowski PB, et al. The national NeuroAIDS tissue consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol 2001;27: 326–335. [DOI] [PubMed] [Google Scholar]
- 10.Strain MC, Lada SM, Luong T, et al. Highly precise measurement of HIV DNA by droplet digital PCR. PloS One 2013;8:e55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019;566: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sei S, Saito K, Stewart SK, et al. Increased human immunodeficiency virus (HIV) type 1 DNA content and Quinolinic acid concentration in brain tissues from patients with HIV encephalopathy. J Infect Dis 1995;172:638–647. [DOI] [PubMed] [Google Scholar]
- 13.Fujimura RK, Goodkin K, Petito CK, et al. HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. J Acquir Immune Defic Syndr Hum Retrovirol 1997;16:146–152. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira MF, Pankow A, Vollbrecht T, et al. Evaluation of archival HIV DNA in brain and lymphoid tissues. J Virol 2023;97: e0054323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara M, Bumpus NN, Ma Q, et al. Antiretroviral drug concentrations in brain tissue of adult decedents. AIDS 2020;34:1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan YL, Yuan Y, Tian L. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry 2020;25:351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruner KM, Murray AJ, Pollack RA, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med 2016;22:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabuzda D, Yin J, Misra V, et al. Intact Proviral DNA analysis of the brain viral reservoir and relationship to Neuroinflammation in people with HIV on suppressive antiretroviral therapy. Viruses 2023;15:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imamichi H, Smith M, Adelsberger JW, et al. Defective HIV-1 proviruses produce viral proteins. Proc Natl Acad Sci U S A 2020;117:3704–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack RA, Jones RB, Pertea M, et al. Defective HIV-1 proviruses are expressed and can Be recognized by cytotoxic T lymphocytes, which shape the Proviral landscape. Cell Host Microbe 2017;21:494–506.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Chaillon A, Gianella S, et al. Brain microglia serve as a persistent HIV reservoir despite durable antiretroviral therapy. J Clin Invest 2023;133:e167417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.