Abstract
The negative effect of advanced female age on fertility and offspring health is well understood. In comparison, much less is known about the implications of male age on fertility, with many studies showing conflicting results. Nevertheless, increasing evidence suggests that advanced paternal age has negative effects on sperm parameters, reproductive success, and offspring health. Herein, we summarize the current body of knowledge on this controversial topic, with the belief that this review will serve as a resource for the clinicians providing fertility counseling to couples with older male partners.
Keywords: Male infertility, andrology, paternal age
It is widely accepted that female fertility decreases with advancing age (1). Women are born with a finite number of oocytes that cannot be replaced, and there is a steady and continuous decline in the ovarian reserve after birth. In contrast, men generally retain the ability to generate a limitless number of sperm over their lifetime, and seemingly have no age-related time limit on reproductive potential. Indeed, clinical cases of older men even above 90 years of age fathering biological children have been reported (2). Societal trends in the industrialized world have led to couples having children at older ages (3–5). These include educational and career-related commitments, desire for financial security, increased life expectancy, and increased use of contraceptives (1, 6). Over the past 4 decades, the number of fathers aged <30 years has decreased by >27%, whereas the number of fathers aged 30–34 years increased by 15%, and the number of fathers aged 35–49 years increased by 52%–63% (7).
Significant research advancements have led to a better understanding of the effect of “advanced paternal age” (APA) on fertility and offspring health, as well as possible underlying mechanisms. The purpose of this article is to provide a comprehensive review of the topic, with a focus on the following: the effect of APA on semen analysis and sperm quality; the relationship between APA and offspring health; the association between APA and pregnancy success rates with both natural and assisted reproduction; potential mechanisms underlying paternal age-related problems; and potential treatments. A thorough understanding of these issues is critical for the clinician providing fertility counseling to couples with older male partners.
EFFECT OF PATERNAL AGE ON SEMEN ANALYSIS AND SPERM QUALITY
Semen analysis is the cornerstone of the evaluation of the infertile male (1,7). Numerous studies have examined the relationship between paternal age and key sperm parameters such as count, motility, concentration, and percent normal morphology (5). Overall, the results are conflicting. Many studies have reported statistically significant age-related declines in the aforementioned parameters, whereas others have reported no changes or rarely, even improvements in some parameters with age (8–16). However, these investigations were often limited in their study design, e.g., most of the studies did not include semen samples of fertile men (i.e., controls). Moreover, additional confounding factors, such as female partner age, were not accounted for in most studies. To better define the effect of paternal age on sperm parameters, Johnson et al. (17) performed a comprehensive meta-analysis using 90 studies that were published through the end of 2013. They concluded that increasing paternal age negatively affected nearly all semen analysis parameters (aside from sperm concentration). In other words, APA was associated with decreases in semen volume, total sperm count, sperm (progressive) motility, and percent sperm with normal morphology as well as an increase in DNA fragmentation (Table 1). However, the observed effect sizes were uniformly small, with the greatest changes noted in progressive motility (r = −0.200) and DNA fragmentation (r = −0.209).
Table 1.
Relationship of advanced paternal age on semen parameters in the 2015 meta-analysis (in bold) and subsequently published studies.
| Year | Authors | Study design | # patients | Volume | Count | Concentration | Motility | Morphology | Vitality | DFI |
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | Johnson et al.(17) | Meta-analysis | 93,839 | ↓ | ↓ | – | ↓ | ↓ | N/A | ↑ |
| 2016 | Yeste et al.(18) | Case-control study | 71 | N/A | N/A | N/A | ↓ | N/A | N/A | N/A |
| 2018 | Veron et al.(19) | Cross sectional study | 11,706 | ↓ | ↓ | – | ↓ | – | ↓ | N/A |
| 2018 | Lai et al.(20) | Retrospective cohort study | 3,549 | ↓ | – | ↑ | ↓ | – | N/A | N/A |
| 2019 | Colasante et al.(6) | Retrospective cohort study | 3,124 | ↓ | – | – | ↓ | – | N/A | ↑ |
| 2019 | Albani et al.(21) | Prospective cohort study | 89 | N/A | N/A | N/A | N/A | N/A | N/A | ↑ |
| 2019 | Rosiak-Gill(22) | Cross sectional study | 1,124 | ↓ | – | – | – | ↓ | N/A | ↑ |
| 2019 | Vinnakota et al.(23) | Cross sectional study | 1,316 | N/A | N/A | N/A | N/A | N/A | N/A | ↑ |
| 2020 | Pino et al.(9) | Cross sectional study | 2,681 | ↓ | ↓ | ↓ | ↓ | – | N/A | ↑ |
| 2020 | Guo et al.(24) | Cross sectional study | 654 | N/A | N/A | N/A | N/A | N/A | N/A | ↑ |
| 2020 | Lu et al.(25) | Cross sectional study | 1,790 | N/A | N/A | N/A | N/A | N/A | N/A | ↑ |
| 2020 | Evenson et al.(26) | Case-control study | 25,445 | N/A | N/A | N/A | N/A | N/A | N/A | ↑ |
| 2020 | Salmon-Divon et al.(27) | Cross sectional study | 12,188 | ↓ | ↓ | – | ↓ | – | N/A | N/A |
| 2021 | Zhang et al.(28) | Cross sectional study | 5,114 | N/A | N/A | N/A | N/A | N/A | N/A | ↑ |
| 2021 | Collodel et al.(29) | Cross sectional study | 1,294 | – | N/A | ↓ | ↓ | N/A | ↓ | N/A |
| 2021 | Gao et al.(30) | Cross sectional study | 18,441 | ↓ | ↓ | ↑ | ↓ | – | N/A | ↑ |
| 2021 | Rubes et al.(31) | Cross sectional study | 150 | N/A | N/A | N/A | N/A | N/A | ↓ | ↑ |
| 2021 | Demirkol et al.(8) | Cross sectional study | 500 | – | – | – | ↓ | ↓ | ↓ | N/A |
↑ = increase with age; ↓ = decrease with age; ─ = no correlation; N/A = not assessed.
Following up on the work of Johnson et al. (17), we conducted a literature search of newer articles (2014– present), and after filtering out reviews, identified 17 additional English language studies assessing APA and various semen analysis parameters (6, 8, 9, 18–31) (Table 1). Direct comparisons were difficult because of the heterogeneity in patient populations, study designs, and reporting of data. Overall, the most consistent findings included APA’s negative correlation with semen volume (7 of 9 studies) and motility (9 of 10 studies) as well as positive correlation with DNA fragmentation (11 of 11 studies). However, improved standardization of study protocols is necessary to fully define age-related changes in sperm parameters.
RELATIONSHIP BETWEEN PATERNAL AGE AND NATURAL (UNASSISTED) PREGNANCY
Advanced paternal age increases the risk of natural (unassisted) reproductive failure in 2 important areas: infertility and miscarriage (32). Numerous studies have shown that APA is associated with a longer time to pregnancy and subfecundity (33–35). In one study, Dunson et al. (36) found that 18%–28% of men aged 35–40 years were unable to achieve natural pregnancy with their partners within 12 cycles (36). Pasqualotto et al. (37) showed that the probability of conception in >12 months doubled from 8% to 15% when comparing <25 men to >35 men. In another study, Louis et al. (33) demonstrated that APA, lack of biological children, and lack of health insurance were the 3 most important issues correlated with time to pregnancy >12 months.
Regarding miscarriage, several studies found that APA significantly increased the risk of miscarriage (38–41). In a 2020 meta-analysis, Du Fosse et al. (39) investigated the association between APA and spontaneous abortion. They chose the group of men aged 25–29 years as a reference and evaluated pooled risk estimates for miscarriage among older age groups. These risks were 1.04, 1.15, 1.23, and 1.43 for the age groups 30–34, 35–39, 40–44, and ≥45 years, respectively. Finally, they concluded that APA correlated with the risk of spontaneous miscarriage. Similarly, Belloc et al. (40) demonstrated that APA was an independent risk factor for miscarriage in natural pregnancy. de La Rochebrochard and Thonneau (41) analyzed 19 articles and found that the risk of infertility and miscarriage was high when men were over 40 years of age. In addition, the influence of male age was more pronounced when the woman had reproductive risk factors, such as being older than 35 years or having a low ovarian reserve (41). Thus, APA is strongly linked with a high risk of not only infertility but also for pregnancy loss after natural conception.
RELATIONSHIP BETWEEN PATERNAL AGE AND ASSISTED REPRODUCTIVE TECHNIQUES
The profound effects of maternal age on assisted reproductive technique (ART) outcomes have been widely studied. Pregnancy and fertility rates after ART are significantly lower in women >40 years of age than the women in younger age groups (42). On the other hand, the influence of APA on ART outcomes is highly controversial (Table 2). Although many studies found that APA has a negative independent effect on the success rates of ART (43–49), others found this to be insignificant (3, 14, 50–52).
Table 2.
Summary of studies evaluating the impact of paternal age on ART outcome
| Authors & Year | Country | Number of IVF Cycles | ART method | Effect of paternal age on ART success rate | Paternal age Cutoff (years) |
|---|---|---|---|---|---|
| Horta, F., B. Vollenhoven, et al. (2019). | Australia | 2425 | IVF/ISCI | Worsening of clinical outcomes with increasing male age, with a significantly worse live birth and clinical pregnancy outcomes | >50 |
| Vogiatzi, P., A. Pouliakis, et al. (2021). | Greece | 339 | ICSI | Advanced male age was a negative predictive factor for biochemical pregnancy, clinical pregnancy, and live birth | >50 |
| Cheung, S., A. Parrella, et al. (2019). | USA | 157 | IVF/ICSI | Fertilization rate was lowest in the >55 age group. Clinical pregnancy rate was highest in the 25–30 age group (81.0%) but was absent in the 50–55 and >55 age groups. Rate of pregnancy loss was highest in the 51–55 age group. | >50 |
| Plastira, K., R. Angelopoulou, et al. (2007). | Greece | 78 | ICSI | Advanced paternal age does not have a significantly increased risk of producing offspring with chromosomal abnormalities compared to younger infertile men. No relationship between paternal age and ICSI outcomes of OA patients was observed. | >35 |
| de La Rochebrochard, E., J. de Mouzon, et al. (2006 | France | 1938 | ICSI | The odds ratio of failure to conceive for paternal age >40 years was 2.00 when the woman was 35–37 years old, 2.03 for age 38–40 years, and 5.74 for age 41 years and over. | >40 |
| Abdel Raheem, A., N. Rushwan, et al. (2013 | United Kingdom | 137 | ICSI | In men with azoospermia, paternal age did not influence the ICSI outcome. | NA |
| Bartolacci, A., L. Pagliardini, et al. (2018). | Italy | 1266 | ICSI | Paternal age did not affect top quality blastocyst formation rate nor ongoing pregnancy rate. | NA |
As for intrauterine insemination, most studies found that APA negatively affected pregnancy rates (40, 44). Belloc et al. (40) showed that APA was associated with low pregnancy rates and high spontaneous miscarriage rates with intrauterine insemination, independent of maternal age (40).
In in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI), the impact of paternal age on fertility and pregnancy rates remains unclear (3). A recent analysis of 2,425 IVF/ICSI cycles suggests that clinical pregnancy outcomes and fertility rates decrease in men aged >50 years compared with men aged <40 years (45). A study by Cheung et al. (46) included the ART outcomes of 113 men. Men aged 25–30 years had fertilization rates of 87.7%, which decreased to 46.0% among men aged >55 years. In addition, the 25–30-year-old group peaked in clinical pregnancy rates at 80.0%, whereas no pregnancies were reported among the >55-year-old age group (46). Similarly, Frattarelli et al. found that paternal age >50 years had a large negative impact on pregnancy outcome and percentage of blastocyst formation after controlling for female age in 1,023 oocyte donation cycles (53). As noted by most of the studies, the decrease in sperm fertilization rate starts between the ages of 45 and 50 years (47). This decline may be due to a decrease in sperm parameters, an increase in sperm DNA fragmentation, and alterations in sperm genetic and epigenetic characteristics (53). On the other hand, the results of other studies contradict the aforementioned data (51, 52, 54, 55). In a study by Bartolacciet al. (54), 1,266 ICSI cycles were evaluated. After controlling for maternal age, the investigators found that APA did not affect the percentage of high-quality blastocyst formation or the rate of sustained pregnancies (54). In another study, Tsai et al. (52) examined the effect of APA on the outcome of 184 ICSI cycles and concluded that it did not affect the fertility and pregnancy rate after adjusting for female age. However, this study only included men aged 31–51 years and did not include men aged >51 years.
Overall, the controversial results of studies on the effect of paternal age on ART outcomes may be because of several issues. First, some studies did not control for the confounding factor of maternal age which is highly correlated with paternal age (3). Second, a number of environmental factors such as alcohol consumption, smoking, and obesity should be considered as they could affect ART outcomes (3, 56, 57). Finally, there is no global consensus on what should be considered APA (55). Although a couple of studies selected the cutoff of >40 years old (48), the results of studies that included males with >50 years old showed a more negative impact on ART success rate (45, 58).
Sperm donation is an efficient solution in older males with severe oligospermia or azoospermia which can be divided into non-identified (anonymous) or directed (known) sperm donation (59). Many studies showed the promising outcome of this technique (60–63). Allen et al reviewed the results and complications of ARTs using donor sperm compared with partner sperm in a meta-analysis of 37 studies. They concluded that there was no difference in the risk of complications such as abortion, preterm labor, and low birth weight in these 2 groups. However, they found a mildly increased risk of hypertensive disease during pregnancy and a small for gestational age in the sperm donation group (63). Besides ethical issues of sperm donation which have been discussed in several studies, determining optimal criteria for sperm donation is really challenging (64, 65). Most studies focused on good physical and psychological health as well as the minimum World Health Organization criteria for normal semen quality (59, 66). Age between 18 and 35 years old is ideal for sperm donation since the age of 40 and more affects sperm motility (67). Also, donors should follow a healthy lifestyle and not have genetic and infectious diseases which may deteriorate the quantity and quality of sperm (68).
THE RELATIONSHIP BETWEEN PATERNAL AGE, REACTIVE OXYGEN SPECIES, AND SPERM DNA DAMAGE
A complex interplay of genetic and biochemical processes underlie male infertility. Given the high prevalence of male infertility, understanding these mechanisms is essential to integrating effective precision medicine strategies when clinically managing men with infertility and their families (69). One promising area of research is understanding how increased paternal age, and the biochemical processes therein, impact germline genetic integrity and male fertility. Here, we summarize research investigating the relationship between paternal aging, oxidative stress, and genetic instability, and how these factors contribute to male infertility and poor pregnancy outcomes after ART procedures.
We previously discussed several studies that report increased sperm DNA fragmentation with paternal age. Given that elevated degrees of DNA fragmentation mirror reduced fertilization, cleavage, blastulation, and successful ART-mediated pregnancy rates, understanding the mechanism of germline genetic instability is essential. One mechanism to explain the relationship between DNA fragmentation and age is oxidative stress, generated by the accumulation of reactive oxygen species (ROS) in spermatozoa (70) (Fig. 1A). During fertilization, ROS normally facilitates capacitation and the acrosome reaction (71, 72). Paradoxically, excessive ROS accumulation to pathologic levels impairs sperm function and fragments sperm DNA (73). Furthermore, ROS can alter the chemical composition of guanine into 8-oxoguanine, which complements adenine rather than cytosine (71), resulting in a G>T point mutation (Fig. 1B). Dysregulation in sperm chromatin packaging, specifically age-related aberrant chromatin decondensation, has been hypothesized as a potential mechanism that renders sperm DNA vulnerable to ROS-induced damage (4, 74).
Figure 1 -.
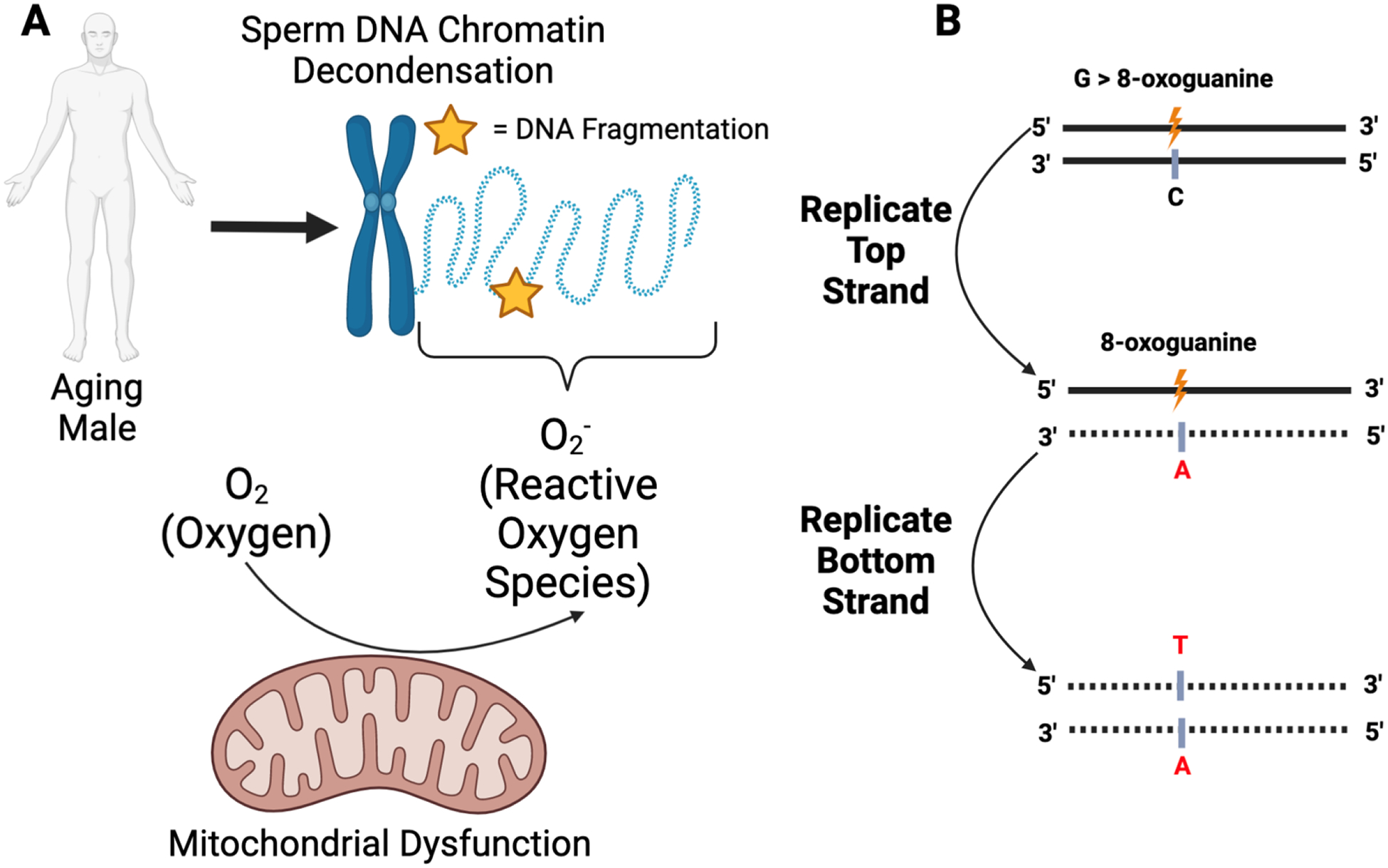
Schematic diagram of oxidative-stress induced DNA damage and mutation
(A) Sperm DNA chromatin decondensation, as a function of increased paternal age, has been hypothesized as a mechanism underlying increased DNA fragmentation rates. Specifically, sperm DNA decondensation exposes the DNA to reactive oxygen species (ROS) generated from several sources, including mitochondrial dysfunction. These ROS can induce DNA damage in the form of double- or single-stranded breaks (yellow star). (B) Oxidative stress can result in the conversion of the guanine nucleotide to 8-oxoguanine (orange). During subsequent rounds of replication, if left unrepaired, 8-oxoguanine will complement adenine rather than cytosine. Further replication of the strand harboring adenine (red, middle panel) will result in a complementary strand harboring a thymine nucleotide at the site formerly occupied by a guanine nucleotide (red, bottom panel). Figure made with biorender.
Oxidative stress, as it relates to male infertility, can originate in either the spermatozoa itself or the seminal fluid. Within the spermatozoa, mitochondrial dysfunction and lipid peroxidation of the sperm’s plasma membrane phospholipid bilayer can contribute to the overabundance of ROS (75–77). Additionally, seminal fluid leukocytes can produce 1,000-fold more ROS than spermatozoa, especially after infection (78). Antioxidant mechanisms are required to maintain an appropriate balance of ROS and optimize sperm function. For example, the enzymatic activity of superoxide dismutase and glutathione peroxidase in semen is essential to limit ROS levels and prevent oxidative stress in sperm (79, 80). Smith et al. (81) functionally investigated the role of thioredoxin redox proteins (Txdnc2 and Txdnc3) in mice to show that these genes limited oxidative stress (P<.01) and protected sperm DNA from fragmentation. Nikitaras et al. (82) further found that exogenous administration of 5uM of Idebenone, a free radical scavenger, reduced superoxide ROS concentrations in human sperm by approximately 20% and improved blastocyst implantation rates in mice from 18% (no idebenone) to 35% (P<.01). Although the relationship between increased paternal age and sperm-related antioxidant capacity has yet to be elucidated, these findings point to ROS’s clinically relevant role in sperm DNA instability and, ultimately, male infertility (71, 83, 84).
To date, however, investigations into the link between ROS levels and APA report discrepant findings (70). Cocuzza et al. (83) examined 98 fertile men seeking vasectomy to observe a positive correlation in semen ROS levels with age (r = 0.20, P=.04). Building off this finding, the investigators reported that men ≥40 years had significantly elevated seminal ROS levels relative to men <40 years (P=.0009). To study the oxidative stress in the context of fertility status, Cocuzza et al. (83) further compared ROS levels to men with infertility falling in both age groups and reported significantly elevated ROS levels in men with impaired fertility. Similarly, Vaughan et al. (85) performed a large-scale retrospective study of nearly 17,000 semen samples from men seeking infertility treatment. They found a linear increase in oxidative stress with increased paternal age (weighted linear trend P<.001). In line with the hypothesis that oxidative stress destabilizes sperm DNA, these investigators also observed a significant concomitant trend in DNA fragmentation with age across the cohort (weighted linear trend P<.001). Conversely, Alshahrani et al. (12) examined 472 men with infertility and did not observe elevated ROS levels in men >40 years of age compared with those younger than 40 years of age, although a significant relationship between DNA fragmentation and increased paternal age was observed. As described in their article, one possible reason for this discrepancy is that the cohort in the study by Alshahrani et al. comprised men seeking fertility treatment at a tertiary hospital and thus may not be representative of the general fertile and even infertile population. Nevertheless, given the increasingly established role of ROS in inducing sperm DNA damage, male infertility, and poor pregnancy outcomes, understanding risk factors for increased germline oxidative stress can inform improved clinical management strategies for men infertility and their families (86).
AN ARRAY OF COMPLEX, AGE-DEPENDENT MECHANISMS CONTRIBUTE TO MALE INFERTILITY
Beyond ROS-induced DNA damage, several age-related mechanisms can contribute to male infertility. These include: the accumulation of male germline de novo mutations; epigenetic modifications in the sperm methylation landscape; and transcriptomic and proteomic alterations in the male germline.
Germline de novo Mutations Accumulate with Paternal Age
Human male gametes differ from female gametes in that spermatogonial stem cells constantly divide throughout the lifetime of the testis-bearing individual. Estimates from Crow in 2000 suggest that sperm in a 20-year-old male would undergo 150 rounds of cell division, whereas sperm in a 40-year-old male would undergo 610 rounds of cell division (87). Therefore, along with dysfunctional DNA repair mechanisms and exposure to exogenous mutagens, male germ cells are uniquely susceptible to the accumulation of de novo mutations (DNMs) in an age-dependent manner via DNA replication errors (88). The patterns and rates of male germline DNMs are conventionally examined through genome sequencing of parent-child trios (89, 90) or multigenerational pedigrees (91). These studies reproducibly estimate the male germline mutation rate at approximately 0.9×10−8 (i.e., the probability of a mutation occurring at a given site) and report an accumulation of approximately 1.5 germline DNMs/year. Although germline mutations may underlie human evolution and drive human diseases, their contribution to male infertility remains unknown (92–94). To this end, our group is currently testing the hypothesis that men with infertility harbor elevated germline and somatic mutation rates, because of the transmission of impaired DNA repair and replication mechanisms, which could explain the association of male infertility with poor individual and familial somatic health.
The Sperm Methylation Landscape in Clinically Relevant Genes Changes with Increased Paternal Age
In addition to chromatin decondensation, age-associated alterations in sperm DNA methylation have been associated with reduced fertility and impaired embryonic development (95). Work from our group in 2015 devised predictive hierarchic clustering models to distinguish men with and without infertility using genome-wide sperm DNA methylation data with 82% sensitivity and 99% positive predictive value. Further, our model leveraged sperm methylation data to identify the samples with successful and unsuccessful embryo-genesis events after IVF with >94% positive predictive value (96). To characterize the effect of male age on sperm methylation, Oluwayiose et al. (38) examined sperm DNA methylation profiles in 47 males between the ages of 21–45 years seeking fertility treatment. After adjusting for body mass index and smoking, the investigators discovered 19 CpG hypermethylated sites significantly associated with APA. Furthermore, a multivariate linear regression identified 1,146 differentially methylated regions significantly associated with male age, which primarily impacted genes involved in pathways associated with embryonic development, organ morphogenesis, and neuronal development. Similarly, Atsem et al. (97) identified a trend of decreased FOXK1 methylation, a gene implicated in autism, in the sperm of aged men as well as the cord blood of their children diagnosed with autism (correlation coefficient = −0.35 and −.20, respectively) (98). Given the transmissibility of clinically relevant epigenetic markers, there exists a clinical need to further characterize genes linked to age-dependent sperm DNA methylation events (99, 100).
AGE-RELATED TRANSCRIPTOMIC AND PROTEOMIC ALTERATIONS ARE ASSOCIATED WITH MALE INFERTILITY
Given the heterogeneous nature of male infertility, additional “omic” approaches beyond genomic and epigenetic approaches are needed to ameliorate the high prevalence of idiopathic male infertility cases (101, 102). To this end, Cheung et al. (46) performed bulk RNA sequencing on spermatozoa-derived RNA from 8 fertile and 5 infertile males to identify 86 differentially expressed genes. Critically, the investigators further noted that decreased expression of DNA repair and apoptosis-modulating genes were associated with increased age and DNA fragmentation. Regarding proteomics, Panner-Selvam et al. (103) studied the proteomic landscape of 8 normozoospermic fertile and 9 normozoospermic men with infertility. Their analysis revealed 162 differentially expressed proteins in men with infertility, including ANXA2 (2.03 fold change, P=.0243), SPA17 (0.37 fold change, P=.0205), and SERPINA5 (0.32 fold change, P=.0073), all 3 of which are key proteins in spermatogenesis and sperm function. Adding to the controversial relationship between oxidative stress in the aging, infertile male, the investigators report an insignificant overexpression of PRDX2 (peroxiredoxin antioxidant protein, P=.3258), in men with infertility, perhaps as a response to the increased oxidative stress experienced in the reproductive tract of men with infertility. Additional research efforts using large fertile and infertile cohorts are needed to elucidate the relationship between APA, proteomic changes, and male infertility.
EFFECT OF PATERNAL AGE ON OFFSPRING HEALTH
The impact of APA on offspring health was first documented in 1955 through a genetic disorder, namely, achondroplasia, which is the most common cause of skeletal dysplasia and dwarfism resulting from dysfunctional osteogenesis (5, 104). Since then, numerous investigations have associated APA with genetically heterogeneous conditions that are distinct in etiology and development (Table 3) (105). Here, we will focus on the relationship between APA and poor offspring health in specific disease contexts such as malignancies, chromosomal aneuploidies and aberrations, congenital disorders, autosomal dominant Mendelian diseases, and neurocognitive disorders.
Table 3:
Effects of paternal aging on various disorders with distribution of relative risks.
| Disorder Category | Disorder | Relative risk(s) | References |
|---|---|---|---|
| Cancer | Acute lymphoblastic leukemia | 1.49 (1) – 1.97 (5) | (1,5,105) |
| Breast cancer | 1.06 (5,107) – 1.6 (1,106) | (1,5,105–107) | |
| Central nervous system tumor | 1.12 (5) – 1.69 (106) | (5,106,107) | |
| Early onset prostate cancer | 1.7 (5) | (5) | |
| Non-Hodgkin’s lymphoma | 1.59 (1) | (1) | |
| Chromosomal | Chromosome 1 disomy | p-value 0.01, mean .11%, range .05% - .18%(110) | (110) |
| Klinefelter syndrome | 1.6 (1,5) | (1,5) | |
| Trisomy 21 (Down syndrome) | 1.28 (70) | (1,5,70,105,111) | |
| XX disomy | R2 0.04, p-value 0.360 (109,110) | (70,109,110) | |
| XY disomy | Risk increase: 56.6%, z = 2.79, p = 0.005 (106) R2 0.28, p-value 0.008(110) |
(5,104,106,108–110) | |
| YY disomy | Pearson correlation coefficient, r = 0.584, p-value < 0.02 (109) R2 0.28, p-value 0.008 (110) |
(70,109,110) | |
| Congenital | Anencephaly | 1.31 (104) | (104) |
| Aniridia | 3.7 (112) | (112) | |
| Atrial septal defect | 1.95 (5,104) – 2.7 (107) | (5,104,107) | |
| Cleft palate | 1.07 (70) 1.02 risk increase for each paternal year (70) |
(70) | |
| Diaphragmatic hernia | 1.04 risk increase for each paternal year (70) | (70) | |
| Neural tube defect | 1.6 (104) | (104) | |
| Patent ductus arteriosus | 1.5 (107) | (5,107) | |
| Right ventricular outflow tract obstruction | 1.03 risk increase for each paternal year (70) | (70) | |
| Situs inversus | 4.46 (110) | (110) | |
| Ventricular septal defect | 1.2 (5,107) | (5,104,107) | |
| Genetic | Achondroplasia | 1.66 – 7.89 (1,70,104,106) | (1,5,40,70,104–107,110–112,120) |
| Apert syndrome | 9.5 (5,70,106) | (1,5,32,40,70,105–107,120) | |
| Bilateral retinoblastoma | 3 (106) – 5 (5) | (5,106) | |
| Crouzon syndrome | 3.7 (1,106) | (1,5,70,106,107) | |
| Marfan syndrome | – | (32,105,107,111) | |
| MEN2A | Effect seen (1) | (1,70,107) | |
| MEN2B | Effect seen (1) | (1,5,70,107) | |
| Neurofibromatosis | 3.7 (5) | (5) | |
| Osteogenesis imperfecta | 2.5 (5) | (5,105,111) | |
| Pfeiffer syndrome | 6 (1,106) | (1,5,70,106,107) | |
| Polycystic kidney disease | 3.7 (112) | (112) | |
| Thanatophoric dysplasia | 3.18 (5) | (5) | |
| Tuberous sclerosis | 3.70 (112) | (112) | |
| Waardenburg syndrome | – | (32) | |
| Neuro-cognitive | Alzheimer’s disease | Potential association between AD and paternal age for patients without a major AD gene | (107) |
| Autism spectrum disorder | 2.21 (1) – 5.75 (70,105,111) | (1,40,70,104–107,111) | |
| Bipolar disorder | 1.37 (104) – 3.45 (104) | (1,104,105,107) | |
| Schizophrenia | 1.66 (104) – 2.96 (70) | (1,5,40,70,104–107,110,111) | |
| Psychosis | 2.42 (110) | (110) | |
| Obstetric & Perinatal | Low birth weight | 1.19 (104) – 1.7 (1) | (1,104) |
| Low APGAR score | 1.13 (1) | (1) | |
| Miscarriage | 2.33 (32) – 6.73 (110) | (32,106,110) | |
| Stillbirth | 1.12 (107) – 1.33 (1) | (1,5,32,104,106,107) |
– = no risk identified.
Several studies have suggested an association between APA and increased rates of childhood cancer. Among these malignancies, the most common diagnosis is acute lymphoblastic leukemia (1, 5, 106, 107). Indeed, Kovac et al. (1) reported that the relative risk for a child to develop acute lymphoblastic leukemia is as high as 1.5 for fathers aged ≥35 years. Other childhood malignancies linked to APA include central nervous system tumors (5, 107, 108), breast cancer (with Kuhnert and Nieschlag (5) also found an association between paternal aging and early death from breast cancer), early onset (<65 years) prostate cancer, and non-Hodgkin’s lymphoma (1). In contrast, Paul et al. (108) described a negative (i.e., protective) relationship between paternal age and rates of colon and thyroid cancer. The mechanisms underlying these observed positive and negative associations remain unknown.
Although extensive literature highlights the effect of increased maternal age on elevated rates of chromosomal aneuploidy (most commonly with trisomy 21), less research has been conducted with regards to paternal aging (1, 5, 106, 107). In 2000, Asada et al. (109) used flupressense in situ hybridizatipn to analyze sperm chromosomal nondisjunction in the context of paternal age. They identified a significant increase in XY disomy in men aged >39 years with idiopathic infertility compared with healthy donors aged <25 years. These results contrasted with an earlier study that reported YY disomy as the only nondisjunction event to exhibit a paternal effect (110). Subsequent studies and reviews established a consistent finding that paternal aging did indeed have an effect on chromosomal aneuploidies, especially sex chromosome disomies (i.e., XX, YY, and XY) as well as trisomy 21 (5, 104, 107, 35). In particular, Sharma et al. (104) noted that on an average, 10% of sperm cells of healthy male populations possessed chromosomal aneuploidies; however, this value increased with paternal age. Additionally, the strongest associations between paternal aging and increased rates of chromosomal aneuploidies occurred when maternal age was >35 years, with Kuhnert and Nieschlag commenting that the paternal effect in aged couples could no longer be neglected (5, 104, 107, 35).
To build on these findings, several studies examined the relationship between APA, chromosomal aneuploidies, and nondisjunction-mediated conditions. For example, the frequency of XY spermatozoa increased with age in fathers of offspring with Klinefelter syndrome (5, 104, 107, 35). In contrast, Ramasamy et al. (106) commented how the frequency of XY spermatozoa is increased in older men compared with younger men, and yet, this did not translate to an increased risk of having an affected child for older men (5, 104, 35). To explain this observation, the investigators hypothesized a possible mechanism by which germ cells self-correct their aneuploidy, thereby protecting against the transmission of XY spermatozoa which would result in Klinefelter males. For trisomy 21 (Down syndrome), Kovac et al. (1) and Stewart et al. (112) both concluded that a significant association existed with APA only when the female partner was >35 years of age. No association was observed with younger female partners; however, Stewart et al. (111) further described a strong association if the female partner was >40 years of age, noting that the incidence of Down syndrome seemed to be related to sperm approximately 50% of the time. Of note, Kovac et al. (1) also observed a significantly increased risk of trisomy 21 with younger fathers aged 20–24 as compared with fathers aged 25–29. Such bimodal distributions in relation to paternal aging have been documented in other studies, primarily with a focus on obstetric outcomes (1).
Multiple congenital cardiac defects, including atrial septal defects, ventricular septal defects, and patent ductus arteriosus have been associated with APA (5, 70, 104, 108). Furthermore, Humm et al. (70) identified associations between APA and multifactorial congenital defects, including cleft palate, diaphragmatic hernia, and right ventricular outflow tract obstruction. In their reviews, Sharma et al. described the associations existing between paternal aging and anencephaly, whereas Kovac et al. (1) could not corroborate their finding (104). However, associations for the former lacked statistical significance and only existed when paternal age was >45 years (104). Combined with a low sample size of 186 total anencephalus cases, further studies are required to demonstrate any increased risk for anencephaly resulting from paternal aging. Additionally, Kovac et al. (1) discussed that their analysis of paternal age and anencephaly was limited as they were missing 55% of paternal age records on these cases.
An increase in germline de novo mutation burden, primarily manifesting as single base-pair mutations, is strongly associated with APA and directly implicated in offspring health (1, 5, 107). Ramasamy et al. (106) described how mutation accumulation and frequency is theoretically higher in males compared with females, as a 70-year-old male would have had approximately 1,300 rounds of spermatogonial mitotic division. Transmission of mutations that accumulate throughout spermatogonial stem cell division can contribute to many autosomal dominant disorders, including achondroplasia, Apert syndrome, Crouzon syndrome, Pfeiffer syndrome, MEN2A syndrome, and MEN2B syndrome. This association is especially true for paternal age effect genes, such as FGFR2, FGFR3, and RET, which drive spermatogenesis and are responsible for the pathogenesis of human diseases (1, 32, 107, 108). However, these reviews noted that age-related nucleotide substitution rates in FGFR3 were not high enough to fully explain the exponentially increased rates of achondroplasia associated with APA. Such a finding suggests additional undiscovered mechanisms in older fathers that may influence the incidence of autosomal dominant disorders in offspring.
The age-dependent accumulation of male germline DNMs is also implicated in neurocognitive disorders. Sharma et al. (104) reported a twofold increase in risk for schizophrenia in offspring of men ≥45 years, increasing to a three-fold increase in risk for men >50 years (104). Even higher magnitudes of risk elevations for autism spectrum disorders have been noted, ranging from 3.3 to 5.75 for paternal groups aged >45 years and >50 years, respectively. Bipolar disorder has demonstrated significant associations with APA; however, it does so to a lesser degree compared with schizophrenia and autism, with a relative risk of 1.37 (1). Although some studies (107, 111) have found associations between APA and Alzheimer’s disease, others have failed to do so (5).
Several studies have identified the associations between APA and adverse birth outcomes, including preterm birth, low birth weight, stillbirth, and miscarriage (1, 32, 104, 107). Sharma et al. (104) summarized associations between paternal aging and preterm birth (with odds ratios ranging from 1.3 to 2.1) and low birth weight (with odds ratios ranging from 1.2 to 1.9). As low birth weight has been identified as the leading cause of infant mortality in the United States (104), such associations should require considerable attention during family counseling. For offspring of older fathers, stillbirth has been found to have odds ratios ranging from 1.22 to 1.48 (5, 32, 70, 108).
When assessing how APA influences the risk of adverse birth outcomes, there has been a predominant focus on how APA affects reproductive function through variables such as paternal DNA integrity, sperm quality, and hormonal changes. Although these factors do change with APA and play a critical role in affecting offspring health, the topic of overall paternal health and its relation to adverse birth outcomes has been less scrutinized. In 2020, Kasman et al. (113) demonstrated that fathers possessing most or all components of metabolic syndrome exhibited significantly high risks for preterm birth, low birth weight, and neonatal intensive care unit stay, even after adjusting for maternal age and comorbidities. The risk of developing metabolic syndrome or its components (i.e., hypertension, hyperlipidemia, diabetes mellitus, and obesity) and other chronic disorders undoubtedly increases with APA. Thus, the effects of APA on offspring health may be much more widely distributed than currently understood, impacting beyond just paternal reproductive function and necessitating further research.
POSSIBLE TREATMENTS
As men age, physiologic changes can impair the fertility potential. These include conditions such as benign prostatic hyperplasia, erectile dysfunction, and hypogonadism (decreasing testosterone and increasing follicle-stimulating hormone) (40). Additionally, men are susceptible to a variety of disease processes as they age, and some of them can impair fertility as well. These include, but are not limited to, infections (e.g., prostatitis, epididymitis), cancer and associated treatments (e.g., prostate cancer, testicular cancer), diabetes, vitamin deficiency, and obesity. Finally, medications commonly used by older men, such as exogenous testosterone (for testosterone deficiency) and alpha blockers (for benign prostatic hyperplasia) can cause impairment in fertility through mechanisms such as inhibition of spermatogenesis and retrograde ejaculation. These pathologies and environmental factors can contribute to the age-related decline in male fertility. Fortunately, many of these factors can be fully or at least partially ameliorated through specific interventions, such as weight loss, antimicrobial therapy, medical and surgical treatments for erectile dysfunction, and cessation of fertility impairing medications (Table 4) (40). Even for conditions that cannot be reversed, there exist treatment options that can improve a man’s reproductive potential, such as a-adrenergic agonists for diabetes-related retrograde ejaculation and sperm extraction procedures for men with a history of prostatectomy.
Table 4.
Types of interventions available for older males with various etiologies of infertility.
| Lifestyle modifications (e.g. weight loss) | Optimize health and minimize toxins |
| Antioxidant rich foods | Reduce oxidative stress-related damage |
| Medical management | |
| Oral PDE5 inhibitors, ICI | Enhance erectile function |
| Antimicrobials | Treat infection to improve sperm parameters |
| Antioxidants | Reduce oxidative stress-related damage |
| Alpha adrenergic agonists | Treat retrograde ejaculation |
| Surgery | |
| Varicocelectomy | Optimize sperm production |
| Penile prosthesis | Allow penetrative sex in men with ED |
| Sperm extraction | Allow for assisted reproduction in men with oligo/azoospermia, elevated DNA fragmentation index |
One pathology that bears special mention is varicocele, which is the most common reversible cause of male infertility. The abnormal dilation of the pampiniform plexus in the spermatic cord is thought to elevate testicular temperature, which subsequently increases oxidative stress within the testis and reduces the fertility potential (114, 115). The commonly quoted prevalence of varicocele is 15% in the general population; however, it has been shown that the prevalence of varicocele increases with age. Levinger et al. (116) reported that the prevalence of varicocele increases by approximately 10% per decade of life, ranging from 18% in men aged 30–39 years to 75% in men aged 80–89 years. Clearly, physical examination of an older male for the presence of clinically palpable varicoceles is critical, as varicocelectomy is the sole surgical treatment option that can enhance and optimize sperm production.
Additionally, there have been many studies that explored the utility of antioxidant supplementation as a measure to combat oxidative stress. These include agents such as vitamin C, vitamin E, carotenoids, selenium, zinc, folic acid, N-acetyl cysteine, L-carnitine, polyunsaturated fatty acids, and coenzyme Q (40, 117, 118). Overall findings suggest that antioxidant therapy can improve semen parameters and pregnancy outcomes (40). As older men seem to have higher levels of oxidative stress and resultant sperm DNA fragmentation, these treatments are particularly noteworthy. However, consistent conclusions are difficult to obtain as study designs lack uniformity. With that said, Li et al. (119) have performed a meta-analysis of 23 randomized controlled trials focusing on the effects of various antioxidants on sperm parameters and pregnancy rates in idiopathic male infertility. They found the strongest evidence for L-carnitine (improvement in sperm motility and morphology) and omega-3 fatty acids (improvement in sperm concentration). Unfortunately, none of the treatments significantly affected actual pregnancy rates. Finally, there is some evidence that for men with sperm DNA fragmentation index >30%, the use of testicular sperm for ICSI (vs. ejaculated sperm) results in high pregnancy and live birth rates (114).
CONCLUSIONS
It has become increasingly clear that APA, just like advanced maternal age, is associated with reduced fertility and poor health effects in offspring. Significant research efforts have uncovered some of the genetic, environmental, and disease factors contributing to age-related male infertility. This progress has, in turn, led to the development of strategies to improve or restore fertility in older men, such as the hormonal treatment of hypogonadism and the use of antioxidant therapy to counteract ROS-induced DNA damage. These advancements in clinical management strategies for men with infertility are highly relevant in the modern age, where because of multiple socioeconomic factors, many couples (and especially men) are choosing to have children at older ages compared with previous generations. Clinicians are encouraged to familiarize themselves with the issues discussed within this article to facilitate fertility counseling for couples with older male partners.
Figure 2 -.
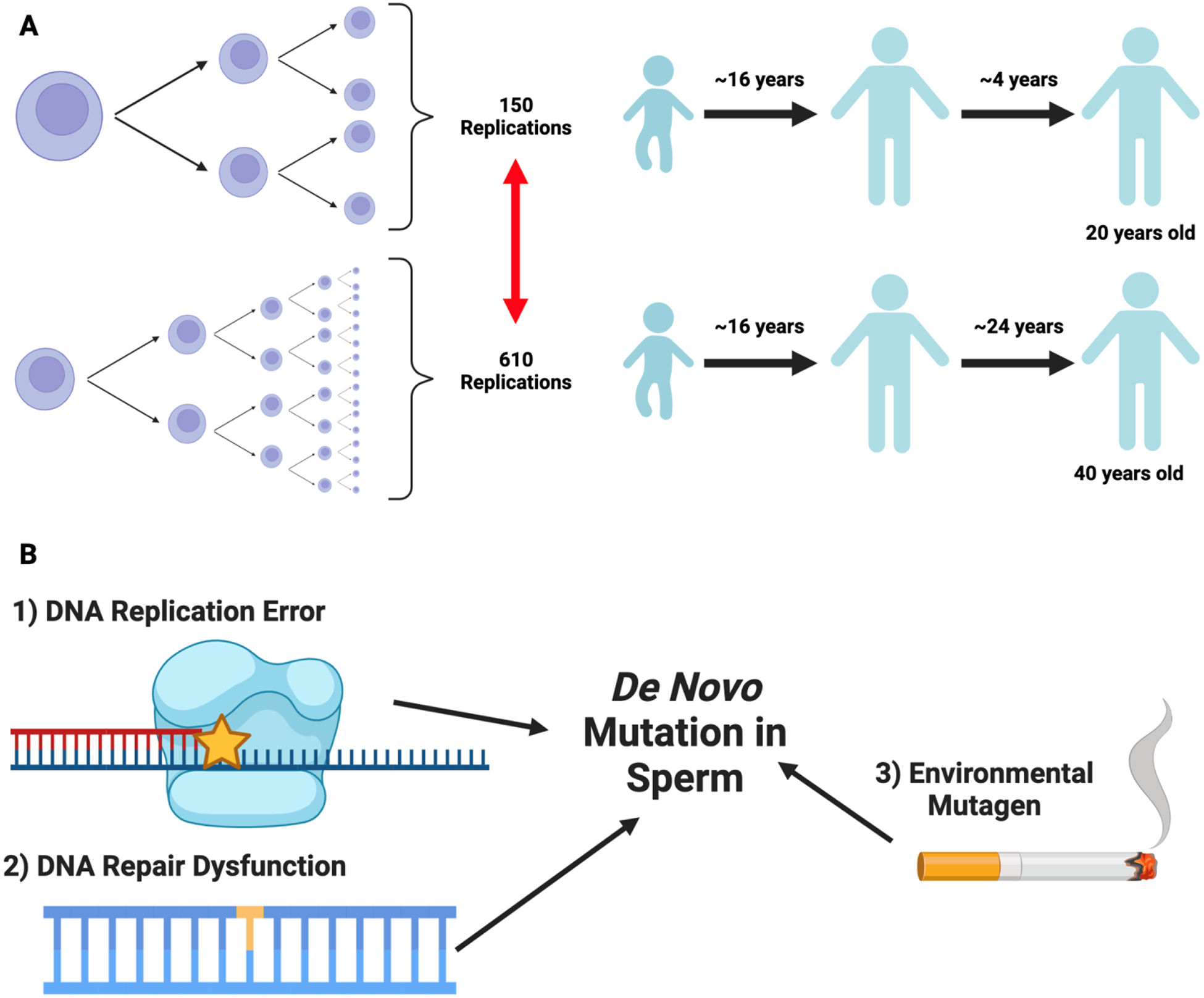
Source of male germline de novo mutations
(A) We provide a schematic diagram of James Crow’s estimates(87) which describe the number of cell divisions mature sperm would undergo as a function of male age. Here, we aim to highlight the years elapsed post-puberty (after roughly 16 years), which contributes to the non-linear increase in spermatogonial stem cell divisions by age 20 and 40. (B) As discussed in Cioppi et al.(88), several processes can introduce male germline de novo mutations, including dysfunctional DNA repair mechanisms, environmental mutagens such as smoking, and DNA replication error - the latter of which is related to age-dependent differences in spermatogonial stem cell divisions. Figure made with biorender.
Footnotes
M.J. has nothing to disclose. J.K. has nothing to disclose. M.G. has nothing to disclose. VY has nothing to disclose. H.A.F. has nothing to disclose. J.M.H. has nothing to disclose.
REFERENCES
- 1.Kovac JR, Addai J, Smith RP, Coward RM, Lamb DJ, Lipshultz LI. The effects of advanced paternal age on fertility. Asian J Androl 2013;15:723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schill WB. Fertility and sexual life of men after their forties and in older age. Asian J Androl 2001;3:1–7. [PubMed] [Google Scholar]
- 3.Bertoncelli Tanaka M, Agarwal A, Esteves SC. Paternal age and assisted reproductive technology: problem solver or trouble maker? Panminerva Med 2019;61:138–51. [DOI] [PubMed] [Google Scholar]
- 4.Kaarouch I, Bouamoud N, Madkour A, Louanjli N, Saadani B, Assou S, et al. Paternal age: negative impact on sperm genome decays and IVF outcomes after 40 years. Mol Reprod Dev 2018;85:271–80. [DOI] [PubMed] [Google Scholar]
- 5.Kuhnert B, Nieschlag E. Reproductive functions of the ageing male. Hum Reprod Update 2004;10:327–39. [DOI] [PubMed] [Google Scholar]
- 6.Colasante A, Minasi MG, Scarselli F, Casciani V, Zazzaro V, Ruberti A, et al. The aging male: relationship between male age, sperm quality and sperm DNA damage in an unselected population of 3124 men attending the fertility centre for the first time. Arch Ital Urol Androl 2019;90:254–9. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg ML, Meldrum D. Effects of age on fertility and sexual function. Fertil Steril 2017;107:301–4. [DOI] [PubMed] [Google Scholar]
- 8.Demirkol MK, Barut O, Dogan NT, Hamarat MB, Resim S. At what age threshold does the decline in semen parameters begin? J Coll Physicians Surg Pak 2021;31:4–7. [DOI] [PubMed] [Google Scholar]
- 9.Pino V, Sanz A, Valdés N, Crosby J, Mackenna A. The effects of aging on semen parameters and sperm DNA fragmentation. JBRA Assist Reprod 2020;24:82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condorelli RA, La Vignera S, Barbagallo F, Alamo A, Mongioi LM, Cannarella R, et al. Bio-functional sperm parameters: does age matter? Front Endocrinol 2020;11:558374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiegs AW, Landis J, Garrido N, Scott RT Jr, Hotaling JM. Total motile sperm count trend over time: evaluation of semen analyses from 119,972 men from subfertile couples. Urology 2019;132:109–16. [DOI] [PubMed] [Google Scholar]
- 12.Alshahrani S, Agarwal A, Assidi M, Abuzenadah AM, Durairajanayagam D, Ayaz A, et al. Men with infertility older than 40 years are at higher risk of sperm DNA damage. Reprod Biol Endocrinol 2014;12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das M, Al-Hathal N, San-Gabriel M, Phillips S, Kadoch IJ, Bissonnette F, et al. High prevalence of isolated sperm DNA damage in men with infertility with advanced paternal age. J Assist Reprod Genet 2013;30: 843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumtepe Y, Yakin K, Kahraman S, Sertyel S, Vanlioglu F, Cengiz S, et al. Male age is not an independent factor to affect the outcome of assisted reproductive techniques. Int J Androl 2003;26:161–5. [DOI] [PubMed] [Google Scholar]
- 15.Brahem S, Mehdi M, Elghezal H, Saad A. The effects of male aging on semen quality, sperm DNA fragmentation and chromosomal abnormalities in an infertile population. J Assist Reprod Genet 2011;28:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause W, Habermann B. No change with age in semen volume, sperm count and sperm motility in individual men consulting an infertility clinic. Urol Int 2000;64:139–42. [DOI] [PubMed] [Google Scholar]
- 17.Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev 2015;19:22–33. [DOI] [PubMed] [Google Scholar]
- 18.Yeste M, Jones C, Amdani SN, Yelumalai S, Mounce G, da Silva SJM, et al. Does advancing male age influence the expression levels and localisation patterns of phospholipase C zeta (PLCζ) in human sperm? Sci Rep 2016; 6:27543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verón GL, Tissera AD, Bello R, Beltramone F, Estofan G, Molina RI, et al. Impact of age, clinical conditions, and lifestyle on routine semen parameters and sperm kinematics. Fertil Steril 2018;110:68–75.e4. [DOI] [PubMed] [Google Scholar]
- 20.Lai SF, Li RH, Yeung WS, Ng EH. Effect of paternal age on semen parameters and live birth rate of in-vitro fertilisation treatment: a retrospective analysis. HK Med J 2018;24:444–50. [DOI] [PubMed] [Google Scholar]
- 21.Albani E, Castellano S, Gurrieri B, Arruzzolo L, Negri L, Borroni EM, et al. Male age: negative impact on sperm DNA fragmentation. Aging 2019; 11:2749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosiak-Gill A, Gill K, Jakubik J, Fraczek M, Patorski L, Gaczarzewicz D, et al. Age-related changes in human sperm DNA integrity. Aging 2019;11: 5399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinnakota C, Cree L, Peek J, Morbeck DE. Incidence of high sperm DNA fragmentation in a targeted population of subfertile men. Syst Biol Reprod Med 2019;65:451–7. [DOI] [PubMed] [Google Scholar]
- 24.Guo LY, Zhou H, Liu M, Li Q, Sun XF. Male age is more critical to sperm DNA integrity than routine semen parameters in Chinese infertile males. Andrologia 2020;52:e13449. [DOI] [PubMed] [Google Scholar]
- 25.Lu R, Chen X, Yu W, Jiang F, Zhou X, Xu Y, et al. Analysis of age-associated alternation of SCSA sperm DNA fragmentation index and semen characteristics of 1790 subfertile males in China. J Clin Lab Anal 2020;34:e23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evenson DP, Djira G, Kasperson K, Christianson J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil Steril 2020;114:311–20. [DOI] [PubMed] [Google Scholar]
- 27.Salmon-Divon M, Shrem G, Balayla J, Nehushtan T, Volodarsky-Perel A, Steiner N, et al. An age-based sperm nomogram: the McGill reference guide. Hum Reprod 2020;35:2213–25. [DOI] [PubMed] [Google Scholar]
- 28.Zhang F, Li J, Liang Z, Wu J, Li L, Chen C, et al. Sperm DNA fragmentation and male fertility: a retrospective study of 5114 men attending a reproductive center. J Assist Reprod Genet 2021;38:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collodel G, Ferretti F, Masini M, Gualtieri G, Moretti E. Influence of age on sperm characteristics evaluated by light and electron microscopies. Sci Rep 2021;11:4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J, Yuan R, Yang S, Wang Y, Huang Y, Yan L, et al. Age-related changes in human conventional semen parameters and sperm chromatin structure assay-defined sperm DNA/chromatin integrity. Reprod Biomed Online 2021;42:973–82. [DOI] [PubMed] [Google Scholar]
- 31.Rubes J, Sipek J, Kopecka V, Musilova P, Vozdova M, Prinosilova P, et al. The effects of age on DNA fragmentation, the condensation of chromatin and conventional semen parameters in healthy nonsmoking men exposed to traffic air pollution. Health Sci Rep 2021;4:e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de La Rochebrochard E, Mcelreavey K, Thonneau P. Paternal age over 40 years: the “amber light” in the reproductive life of men? J Androl 2003; 24:459–65. [DOI] [PubMed] [Google Scholar]
- 33.Louis JF, Thoma ME, Sørensen DN, McLain AC, King RB, Sundaram R, et al. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology 2013;1:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutsaerts MAQ, Groen H, Huiting HG, Kuchenbecker WKH, Sauer PJJ, Land JA, et al. The influence of maternal and paternal factors on time to pregnancy–a Dutch population-based birth-cohort study: the GECKO Drenthe study. Hum Reprod 2012;27:583–93. [DOI] [PubMed] [Google Scholar]
- 35.Auger J, Jouannet P. Age and male fertility: biological factors. Rev Epidemiol S Publ 2005;53:25–35. [PubMed] [Google Scholar]
- 36.Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004;103:51–6. [DOI] [PubMed] [Google Scholar]
- 37.Pasqualotto FF, Borges Junior E, Pasqualotto EB. The male biological clock is ticking: a review of the literature. Sao Paulo Med J 2008;126:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oluwayiose OA, Wu H, Saddiki H, Whitcomb BW, Balzer LB, Brandon N, et al. Sperm DNA methylation mediates the association of male age on reproductive outcomes among couples undergoing infertility treatment. Sci Rep 2021;11:3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.du Fossé NA, van der Hoorn MLP, van Lith JMM, le Cessie S, Lashley EELO. Advanced paternal age is associated with an increased risk of spontaneous miscarriage: a systematic review and meta-analysis. Hum Reprod Update 2020;26:650–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belloc S, Hazout A, Zini A, Merviel P, Cabry R, Chahine H, et al. How to overcome male infertility after 40: Influence of paternal age on fertility. Maturitas 2014;78:22–9. [DOI] [PubMed] [Google Scholar]
- 41.de La Rochebrochard E, Thonneau P. Paternal age: are the risks of infecundity and miscarriage higher when the man is aged 40 years or over? Rev Epidemiol S Publ 2005;53. Special No 2:2S47–55. [PubMed] [Google Scholar]
- 42.Hanson BM, Kaser DJ, Franasiak JM. Male infertility and the future of in vitro fertilization. Urol Clin North Am 2020;47:257–70. [DOI] [PubMed] [Google Scholar]
- 43.Vogiatzi P, Pouliakis A, Sakellariou M, Athanasiou A, Athanasiou A, Colaghis A, et al. Male age and progressive sperm motility are critical factors affecting embryological and clinical outcomes in oocyte donor ICSI cycles. Reprod Sci 2021;15:15. [DOI] [PubMed] [Google Scholar]
- 44.Starosta A, Gordon CE, Hornstein MD. Predictive factors for intrauterine insemination outcomes: a review. Fertil Res Pract 2020;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horta F, Vollenhoven B, Healey M, Busija L, Catt S, Temple-Smith P. Male ageing is negatively associated with the chance of live birth in IVF/ICSI cycles for idiopathic infertility. Hum Reprod 2019;34:2523–32. [DOI] [PubMed] [Google Scholar]
- 46.Cheung S, Parrella A, Rosenwaks Z, Palermo GD. Genetic and epigenetic profiling of the infertile male. PLoS ONE 2019;14:e0214275. Available at: 10.1371/journal.pone.0214275. Accessed July 2, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Jonge C, Barratt CLR. The present crisis in male reproductive health: an urgent need for a political, social, and research roadmap. Andrology 2019; 7:762–8. Available at: 10.1111/andr.12673. Accessed July 2, 2022. [DOI] [PubMed] [Google Scholar]
- 48.Plastira K, Angelopoulou R, Mantas D, Msaouel P, Lyrakou S, Plastiras A, et al. The effects of age on the incidence of aneuploidy rates in spermatozoa of oligoasthenozoospermic patients and its relationship with ICSI outcome. Int J Androl 2007;30:65–72. [DOI] [PubMed] [Google Scholar]
- 49.de La Rochebrochard E, de Mouzon J, Thepot F, Thonneau P. Fathers over 40 and increased failure to conceive: the lessons of in vitro fertilization in France. Fertil Steril 2006;85:1420–4. Available at: 10.1016/j.fertnstert.2005.11.040. Accessed July 2, 2022. [DOI] [PubMed] [Google Scholar]
- 50.Park YS, Lee SH, Lim CK, Choi HW, An JH, Park CW, et al. Paternal age as an independent factor does not affect embryo quality and pregnancy outcomes of testicular sperm extraction-intracytoplasmic sperm injection in azoospermia. Andrologia 2018;50. Available at: 10.1111/and.12864. Accessed July 2, 2022. [DOI] [PubMed] [Google Scholar]
- 51.Meijerink AM, Ramos L, Fleischer K, Veltman JA, Hendriks JC, Braat DD. Influence of paternal age on ongoing pregnancy rate at eight weeks’ gestation in assisted reproduction. Reprod Biomed Online 2016;32:96–103. Available at: 10.1016/j.rbmo.2015.09.017. Accessed July 2, 2022. [DOI] [PubMed] [Google Scholar]
- 52.Tsai YR, Lan KC, Kung FT, Lin PY, Chiang HJ, Lin YJ, et al. The effect of advanced paternal age on the outcomes of assisted reproductive techniques among patients with azoospermia using cryopreserved testicular spermatozoa. Taiwan J Obstet Gynecol 2013;52:351–5. [DOI] [PubMed] [Google Scholar]
- 53.Frattarelli JL, Miller KA, Miller BT, Elkind-Hirsch K, Scott RT Jr. Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril 2008; 90:97–103. Available at: 10.1016/j.fertnstert.2007.06.009. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 54.Bartolacci A, Pagliardini L, Makieva S, Salonia A, Papaleo E, Vigano P. Abnormal sperm concentration and motility as well as advanced paternal age compromise early embryonic development but not pregnancy outcomes: a retrospective study of 1266 ICSI cycles. J Assist Reprod Genet 2018;35:1897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdel Raheem A, Rushwan N, Garaffa G, Zacharakis E, Doshi A, Heath C, et al. Factors influencing intracytoplasmic sperm injection (ICSI) outcome in men with azoospermia. BJU Int 2013;112:258–64. Available at: 10.1111/j.1464-410x.2012.11714.x. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 56.Oostingh EC, Koster MPH, van Dijk MR, Willemsen SP, Broekmans FJM, Hoek A, et al. First effective mHealth nutrition and lifestyle coaching program for subfertile couples undergoing in vitro fertilization treatment: a single-blinded multicenter randomized controlled trial. Fertil Steril 2020; 114:945–54. Available at: 10.1016/j.fertnstert.2020.04.051. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 57.Bellver J BMI and miscarriage after IVF. Curr Opin Obstet Gynecol 2022;34: 114–21. [DOI] [PubMed] [Google Scholar]
- 58.Brincat D, Catania S, Wismayer PS, Calleja-Agius J. Male factors in ART outcome prediction. Gynecol Endocrinol 2015;31:169–75. Available at: 10.3109/09513590.2014.984678. [DOI] [PubMed] [Google Scholar]
- 59.Practice Committee of the American Society for Reproductive Medicine and the Practice Committee for the Society for Assisted Reproductive Technology. Electronic address: ASRM@asrm.org and the Practice Committee for the Society for Assisted Reproductive Technology. Guidance regarding gamete and embryo donation. Fertil Steril 2021;115:1395–410. Available at: 10.1016/j.fertnstert.2021.01.045. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 60.Blázquez A, García D, Rodríguez A, Vassena R, Figueras F, Vernaeve V. Is oocyte donation a risk factor for preeclampsia? A systematic review and meta-analysis. J Assist Reprod Genet 2016;33:855–63. Available at: 10.1007/s10815-016-0701-9. Accessed July 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bu Z, Xiong Y, Wang K, Sun Y. Risk factors for ectopic pregnancy in assisted reproductive technology: a 6-year, single-center study. Fertil Steril 2016; 106:90–4. Available at: 10.1016/j.fertnstert.2016.02.035. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 62.Adams DH, Clark RA, Davies MJ, de Lacey S. Update on: a meta-analysis of sperm donation offspring health outcomes – 2018 update. J Dev Orig Health Dis 2018;9:561–2. Available at: 10.1017/s2040174418000272. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 63.Allen CP, Marconi N, McLernon DJ, Bhattacharya S, Maheshwari A. Outcomes of pregnancies using donor sperm compared with those using partner sperm: systematic review and meta-analysis. Hum Reprod Update 2021;27:190–211. [DOI] [PubMed] [Google Scholar]
- 64.Marshall LA. Ethical and legal issues in the use of related donors for therapeutic insemination. Urol Clin North Am 2002;29:855–61. [DOI] [PubMed] [Google Scholar]
- 65.Robertson JA. Ethical and legal issues in human embryo donation. Fertil Steril 1995;64:885–94. [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization (WHO). WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Cambridge University Press; 1999. Available at: https://play.google.com/store/books/details?id=dEfWhZZcC0AC. Accessed July 5, 2022. [Google Scholar]
- 67.U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for industry: eligibility determination for donors of human cells, tissues, and cellular and tissue-based products (HCT/Ps). 2007. Available at: https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Eligibility-Determination-for-Donors-of-Human-Cells–Tissues–and-Cellular-and-Tissue-Based-Products–Guidance-for-Industry.pdf. Accessed July 5, 2022.
- 68.Green C Current good tissue practice basics for human cells, tissues, and cellular and tissue-based products. J GXP Compliance 2009;13. Available at: https://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=15525791&AN=40210837&h=hT2INRW1w7JaLs0UVLE%2B6Pv%2FsgnZNyJaRFgKvklVJ92PZlpVzDIB94Mjh%2FfSJJb7MzYZLyAnzab7gifXL3xeow%3D%3D&crl=c. Accessed July 5, 2022. [Google Scholar]
- 69.Kunisaki J, Quinlan A, Aston KI, Hotaling J. Integrating precision medicine into the standard of care for male infertility: what will it take? Eur Urol 2022;82:339–40. Available at: 10.1016/j.eururo.2022.06.015. Accessed July 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Humm KC, Sakkas D. Role of increased male age in IVF and egg donation: is sperm DNA fragmentation responsible? Fertil Steril 2013;99:30–6. [DOI] [PubMed] [Google Scholar]
- 71.Evans EPP, Scholten JTM, Mzyk A, Reyes-San-Martin C, Llumbet AE, Hamoh T, et al. Male subfertility and oxidative stress. Redox Biol 2021; 46:102071. Available at: 10.1016/j.redox.2021.102071. Accessed July 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leisegang K, Henkel R, Agarwal A. Redox regulation of fertility in aging male and the role of antioxidants: a savior or stressor. Curr Pharm Des 2017;23:4438–50. [DOI] [PubMed] [Google Scholar]
- 73.Angelopoulou R, Lavranos G, Manolakou P. ROS in the aging male: model diseases with ROS-related pathophysiology. Reprod Toxicol 2009;28:167–71. Available at: https://www.sciencedirect.com/science/article/pii/S0890623809000902. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 74.Petersen CG, Mauri AL, Vagnini LD, Renzi A, Petersen B, Mattila M, et al. The effects of male age on sperm DNA damage: an evaluation of 2,178 semen samples. JBRA Assist Reprod 2018;22:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed 2016;14:231–40. [PMC free article] [PubMed] [Google Scholar]
- 76.Treulen F, Uribe P, Boguen R, Villegas JV. Mitochondrial permeability transition increases reactive oxygen species production and induces DNA fragmentation in human spermatozoa. Hum Reprod [Internet] 2015;30:767–76. Available from: 10.1093/humrep/dev015. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 77.Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol [Internet] 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl 2011;13:43–52. Available at: 10.1038/aja.2010.76. Accessed July 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan L, Liu J, Wu S, Zhang S, Ji G, Gu A. Seminal superoxide dismutase activity and its relationship with semen quality and SOD gene polymorphism. J Assist Reprod Genet 2014;31:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: an evidence based review. Int J Reprod Biomed 2016;14:729–36. [PMC free article] [PubMed] [Google Scholar]
- 81.Smith TB, Baker MA, Connaughton HS, Habenicht U, Aitken RJ. Functional deletion of Txndc2 and Txndc3 increases the susceptibility of spermatozoa to age-related oxidative stress. Free Radic Biol Med 2013;65:872–81. [DOI] [PubMed] [Google Scholar]
- 82.Nikitaras V, Zander-Fox D, McPherson NO. Improving sperm oxidative stress and embryo quality in advanced paternal age using idebenone in vitro-A proof-of-concept study. Antioxidants (Basel) 2021;10:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cocuzza M, Athayde KS, Agarwal A, Sharma R, Pagani R, Lucon AM, et al. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology 2008;71:490–4. [DOI] [PubMed] [Google Scholar]
- 84.Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol 2017;14:470–85. Available at: 10.1038/nrurol.2017.69. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 85.Vaughan DA, Tirado E, Garcia D, Datta V, Sakkas D. DNA fragmentation of sperm: a radical examination of the contribution of oxidative stress and age in 16 945 semen samples. Hum Reprod 2020;35:2188–96. [DOI] [PubMed] [Google Scholar]
- 86.Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online 2003;7:65–70. [DOI] [PubMed] [Google Scholar]
- 87.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet 2000;1:40–7. [DOI] [PubMed] [Google Scholar]
- 88.Cioppi F, Casamonti E, Krausz C. Age-dependent de novo mutations during spermatogenesis and their consequences. Adv Exp Med Biol 2019; 1166:29–46. [DOI] [PubMed] [Google Scholar]
- 89.Jónsson H, Sulem P, Kehr B, Kristmundsdottir S, Zink F, Hjartarson E, et al. Parental influence on human germline de novo mutations in 1,548 trios from Iceland. Nature 2017;549:519–22. [DOI] [PubMed] [Google Scholar]
- 90.Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012;488:471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sasani TA, Pedersen BS, Gao Z, Baird L, Przeworski M, Jorde LB, et al. Large, three-generation human families reveal post-zygotic mosaicism and variability in germline mutation accumulation. eLife 2019;8:e46922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuen RKC, Merico D, Cao H, Pellecchia G, Alipanahi B, Thiruvahindrapuram B, et al. Genome-wide characteristics of de novo mutations in autism. NPJ Genom Med 2016;1. 160271–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Howrigan DP, Rose SA, Samocha KE, Fromer M, Cerrato F, Chen WJ, et al. Exome sequencing in schizophrenia-affected parent-offspring trios reveals risk conferred by protein-coding de novo mutations. Nat Neurosci 2020; 23:185–93. Available at: 10.1038/s41593-019-0564-3. Accessed July 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goriely A, Wilkie AOM. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet 2012;90:175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jenkins TG, Aston KI, James ER, Carrell DT. Sperm epigenetics in the study of male fertility, offspring health, and potential clinical applications. Syst Biol Reprod Med 2017;63:69–76. Available at: 10.1080/19396368.2016.1274791. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 96.Aston KI, Uren PJ, Jenkins TG, Horsager A, Cairns BR, Smith AD, et al. Aberrant sperm DNA methylation predicts male fertility status and embryo quality. Fertil Steril 2015;104:1388. 97.e1s–5. Available at: 10.1016/j.fertnstert.2015.08.019. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 97.Atsem S, Reichenbach J, Potabattula R, Dittrich M, Nava C, Depienne C, et al. Paternal age effects on sperm FOXK1 and KCNA7 methylation and transmission into the next generation. Hum Mol Genet 2016;25:4996–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bacon C, Schneider M, Le Magueresse C, Froehlich H, Sticht C, Gluch C, et al. Brain-specific Foxp1 deletion impairs neuronal development and causes autistic-like behaviour. Mol Psychiatry 2015;20:632–9. Available at: 10.1038/mp.2014.116. Accessed July 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Milekic MH, Xin Y, O’Donnell A, Kumar KK, Bradley-Moore M, Malaspina D, et al. Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol Psychiatry 2015;20:995–1001. Available at: 10.1038/mp.2014.84. Accessed July 5, 2022. [DOI] [PubMed] [Google Scholar]
- 100.Jenkins TG, Aston KI, Carrell DT. Sperm epigenetics and aging. Transl Androl Urol 2018;7(Suppl 3):S328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Omolaoye TS, Omolaoye VA, Kandasamy RK, Hachim MY, Du Plessis SS. Omics and male infertility: highlighting the application of transcriptomic data. Life (Basel) 2022;12:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agharezaee N, Hashemi M, Shahani M, Gilany K. Male infertility, precision medicine and systems proteomics. J Reprod Infertil 2018;19:185–92. [PMC free article] [PubMed] [Google Scholar]
- 103.Panner Selvam MK, Agarwal A, Pushparaj PN, Baskaran S, Bendou H. Sperm proteome analysis and identification of fertility-associated bio-markers in unexplained male infertility. Genes (Basel) 2019;10:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol 2015; 13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Winkle T, Rosenbusch B, Gagsteiger F, Paiss T, Zoller N. The correlation between male age, sperm quality and sperm DNA fragmentation in 320 men attending a fertility center. J Assist Reprod Genet 2009;26:41–6. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2649333/pdf/10815_2008_Article_9277.pdf. Accessed July 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crosnoe LE, Kim ED. Impact of age on male fertility. Curr Opin Obstet Gynecol 2013;25:181–5. [DOI] [PubMed] [Google Scholar]
- 107.Ramasamy R, Chiba K, Butler P, Lamb DJ. Male biological clock: a critical analysis of advanced paternal age. Fertil Steril 2015;103:1402–6. Available at: https://www.fertstert.org/article/S0015-0282(15)00210-1/pdf. Accessed July 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paul C, Robaire B. Ageing of the male germ line. Nat Rev Urol 2013;10: 227–34. [DOI] [PubMed] [Google Scholar]
- 109.Asada H, Sueoka K, Hashiba T, Kuroshima M, Kobayashi N, Yoshimura Y. The effects of age and abnormal sperm count on the nondisjunction of spermatozoa. J Assist Reprod Genet 2000;17:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martin RH. Genetics of human sperm. J Assist Reprod Genet 1998;15: 240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stewart AF, Kim ED. Fertility concerns for the aging male. Urology 2011;78: 496–9. [DOI] [PubMed] [Google Scholar]
- 112.Murray MJ, Meacham RB. The effect of age on male reproductive function. World J Urol 1993;11:137–40. [DOI] [PubMed] [Google Scholar]
- 113.Kasman AM, Zhang CA, Li S, Stevenson DK, Shaw GM, Eisenberg ML. Association of preconception paternal health on perinatal outcomes: analysis of U.S. claims data. Fertil Steril 2020;113:947–54. [DOI] [PubMed] [Google Scholar]
- 114.Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398–405. [DOI] [PubMed] [Google Scholar]
- 115.Alsaikhan B, Alrabeeah K, Delouya G, Zini A. Epidemiology of varicocele. Asian J Androl 2016;18:179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Levinger U, Gornish M, Gat Y, Bachar GN. Is varicocele prevalence increasing with age? Andrologia 2007;39:77–80. [DOI] [PubMed] [Google Scholar]
- 117.Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev 2011;1:CD007411. [DOI] [PubMed] [Google Scholar]
- 118.Adewoyin M, Ibrahim M, Roszaman R, Isa MLM, Alewi NAM, Rafa AAA, et al. Male infertility: the effect of natural antioxidants and phytocom-pounds on seminal oxidative stress. Diseases 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li KP, Yang XS, Wu T. The effect of antioxidants on sperm quality parameters and pregnancy rates for idiopathic male infertility: a network meta-analysis of randomized controlled trials. Front Endocrinol (Laussane) 2022;13:810242. Available at: 10.3389/fendo.2022.810242. Accessed July 5, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]


