Abstract
Infectious threats to humans are continuously emerging. The 2022 worldwide monkeypox outbreak is the latest of these threats with the virus rapidly spreading to 106 countries by the end of September 2022. The burden of the ongoing monkeypox outbreak is manifested by 68,000 cumulative confirmed cases and 26 deaths. Although monkeypox is usually a self-limited disease, patients can suffer from extremely painful skin lesions and complications can occur with reported mortalities. The antigenic similarity between the smallpox virus (variola virus) and monkeypox virus can be utilized to prevent monkeypox using smallpox vaccines; treatment is also based on antivirals initially designed to treat smallpox. However, further studies are needed to fully decipher the immune response to monkeypox virus and the immune evasion mechanisms. In this review we provide an up-to-date discussion of the current state of knowledge regarding monkeypox virus with a special focus on innate immune response, immune evasion mechanisms and vaccination against the virus.
Keywords: Monkeypox virus, MPXV, outbreak, zoonosis, emerging viral infectious disease
Introduction
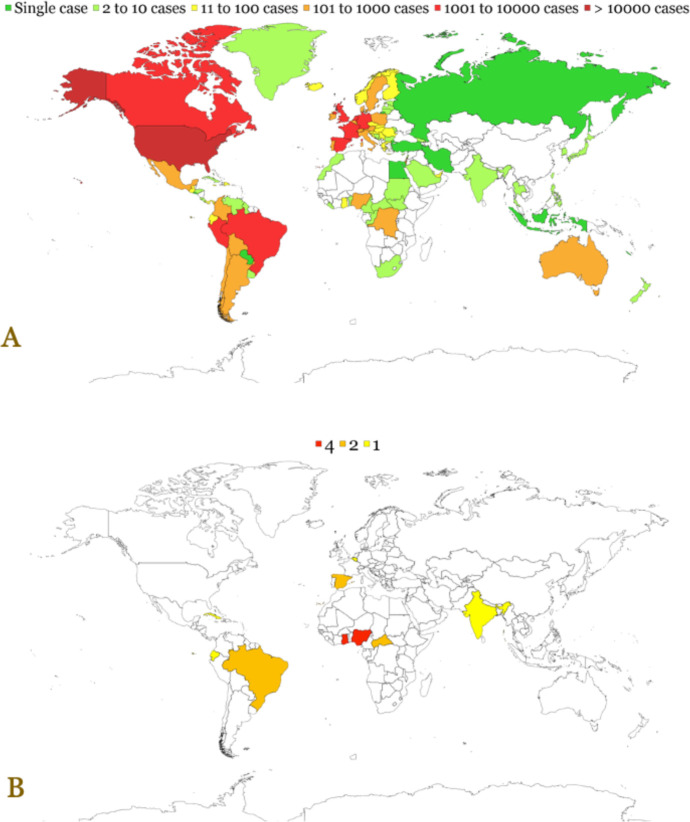
The ongoing multi-country monkeypox outbreak has been declared a Public Health Emergency of International Concern by World Health Organization (WHO) in July 2022 [1]. This is the result of the unprecedented rate and pattern of transmission of the causative agent, monkeypox virus (MPXV), with more than 68,000 cases detected in 106 countries worldwide by the end of September 2022 [2]. The distribution of confirmed monkeypox cases and deaths are presented in Figure 1.
Figure 1.
Global distribution of confirmed cases (A) and deaths (B) of monkeypox. The data were retrieved from OurWorldInData.org as of 12 September 2022 [5].
The virus was first identified as a human pathogen in 1970, with subsequent detection of cases in West and Central Africa attributed mostly to cross-species transmission from yet to be identified definitive reservoir of the virus in animals [3]. The initial endemic outbreak in Africa was dominated by two clades with distinctive features in terms of epidemiology and clinical features [3]. Infrequent outbreaks appeared in the US and the UK as a result of travel to endemic areas or import of animals from these regions [4].
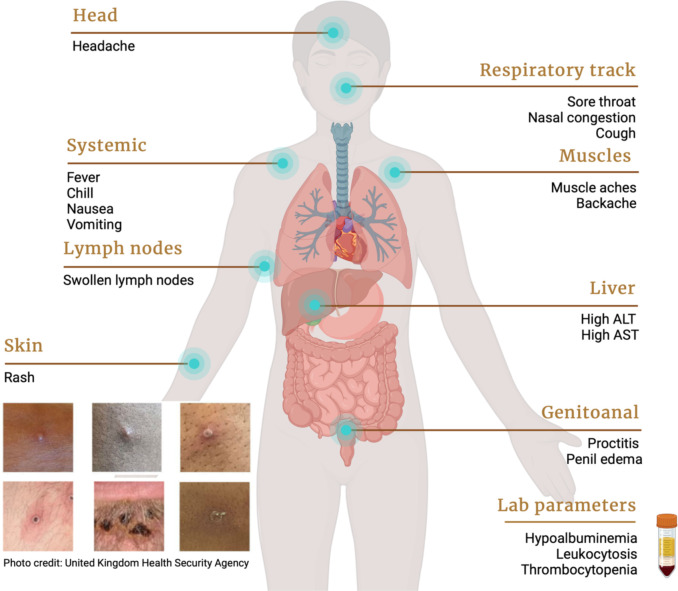
Prior to the 2022 worldwide monkeypox outbreak, human-to-human transmission was limited to household contacts [6]. Clinical features were dominated by a prodromal phase of non-specific symptoms including fever, chills, nausea, headache and cough, followed by lymphadenopathy and skin signs of macules developing into papules, pustules and scabs (Figure 2).
Figure 2.
Clinical and laboratory features of monkeypox in humans.
The case-fatality ratio was estimated at 0-13% depending on the different clades [3, 7]. This pattern of infection has recently changed, with significant human-to-human transmission. Currently the most-at-risk group are men who have sex with men (MSM), including male homosexuals and bisexuals [8-11], with growing evidence that transmission occurs via skin-to-skin contact during sexual encounters [10]. Moreover, a plethora of unusual signs and symptoms, such as proctitis, tonsillitis, and paraphimosis related to penile edema, have been reported among monkeypox patients [10, 12, 13]. Cases have also been identified among children and women, highlighting the potential for further community spread [10, 14].
The management and prevention of monkeypox is based on measures initially designed to protect against smallpox; of note, smallpox is the only infectious disease to have been eradicated from humans through public health measures [15]. Although monkeypox is generally self-limiting, and management has largely been supportive, several antivirals are potential treatment options including (1) cidofovir, (2) tecovirimat and (3) brincidofovir [16-18].
The prevention efforts to tackle the ongoing monkeypox outbreak rely heavily on promoting education and knowledge regarding the virus. [19-23]. Vaccination of at-risk groups will be a mainstay measure for prevention [24, 25]. Fortunately vaccinia-based smallpox vaccines have continued to be developed due to on-going concerns over possible re-emergence of smallpox in relation of bioterrorism [26].
These vaccines include the first-generation NYCBH strain of vaccinia virus, the secondgeneration virus culture-based vaccines (ACAM2000) and the third generation vaccine which is a live-attenuated non-replicating strain of vaccinia virus (Jynneos) [25]. Intradermal vaccination is being considered for the ongoing monkeypox outbreak [27].
Vaccination against smallpox has been shown to achieve 85% effectiveness to prevent MPXV infection [28]. Our current understanding of the protective immunologic mechanisms of smallpox vaccination is limited. It is suggested that the immune response mechanisms revolve around the production of high titers of neutralizing antibody and the levels of memory CD8+ T cells [29-31].
Due to the limited literature on MPXV, we aim to provide a general overview regarding the preventive measures to control MPXV spread with a special focus on immune response and vaccination role.
Monkeypox and its origin
The MPXV was first identified in 1958, when smallpox-like vesiculopustular lesions were observed on imported Java macaques residing in captivity [32]. For unknown reasons, the monkeys suddenly developed a rash and a fever [32]. The virus was isolated at the Statens Serum Institut in Copenhagen, Denmark [32]. Several years later, reports of similar outbreaks emerged, including in a Rotterdam zoo [33]. It was thought that the virus first infected giant anteaters in South America, and then spread to monkeys and apes. Two cases of a smallpox-like disease were reported in the 1970s from the Democratic Republic of the Congo and from Liberia; these cases led to the identification of the MPXV [33].
In the 1970s, human disease was reported in the rainforests of several countries in central and western Africa, where it had circulated for more than forty years [34-36]. In 2003, the United States reported the first case of the disease outside of Africa, after receiving a shipment of exotic animals from Ghana containing a Gambian giant rat, three dormice, and two rope squirrels [37]. After imported mammals were shipped to different states, the infectious disease spread rapidly among domestic animals [38, 39]; 72 confirmed or suspected cases of monkeypox were reported between May and July 2003 in Wisconsin, Illinois, and Indiana [40]. Since then, the disease has been reported in multiple regions of the globe, including Israel, Singapore and the United Kingdom in 2018 [41-43], the United States in 2021, and other nations in 2022 [44-46]. While all previous outbreaks were traceable to endemic regions in Africa, the lack of any known epidemiological links to either central or western Africa, indicating the possibility of a semi-endemic equilibrium, is what makes the recent global outbreak of 2022 so intriguing [47]. The question of whether or not the virus could be passed on through sexual contact became also an important question to consider [48].
The natural reservoir of MPXV remains undetermined. African rope squirrels (Funisciurus sp.) [49] and wild-living sooty mangabeys (Cercocebus atys) [50] are both considered as potential natural viral reservoirs, but monkeys (Macaca fascicularis) have been ruled out [51, 52].
Taxonomy, virology, and genome
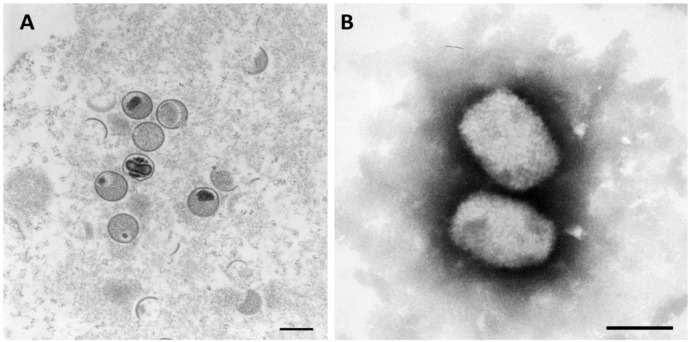
The MPXV is related to other members of the Orthopoxvirus genus both genetically and antigenically, with open reading frames (ORFs) sharing greater than 90% sequence identity [53]. Thus, MPXV is classified as a member of the Orthopoxvirus genus. In addition, this genus also includes the volepox, variola, and vaccinia viruses, as well as taterapox, skunkpox, ectromelia, raccoonpox, cowpox, camelpox, Abatino macacapox, horsepox, and akhmeta viruses. At the higher levels of classification, MPXV is placed in the subfamily of Chordopoxvirinae, the family of Poxviridae, the order of Chitovirales, the phylum of Nucleocytovoricota, and the kingdom of viruses [54, 55]. MPXV has a 197 kilobase pair (kb) DNA genome that is linear and double-stranded (ds). Its 197 kb linear DNA genome contains nearly 190 non-overlapping ORFs, each of which is more than 60 amino acid residues long [56, 57]. The core of the MPXV is characterized by a large brick or mulberry shape and contains a DNA core in the shape of a dumbbell [58] as shown in Figure 3. The core is surrounded by an envelope [58]. The diameter ranges from about 140 to 260 nm, and the length ranges from about 220 to 450 nm [59, 60].
Figure 3.
Monkeypox virus under transmission electron microscope. (A) utilizing ultrathin stained sections; Bar = 200 nm, (B) utilizing negative stained; Bar = 200 nm. *mature: oval-shaped virus particles, immature: crescents and spherical shape. Source: Robert Koch Institute (https://www.rki.de) 4 September 2022 [61].
Monkeypox is a zoonosis that spreads from animals to humans, with several rodent species [62] and non-human primates being identified as susceptible hosts [63, 64]. Human-to-human transmission can also occur, primarily through close contact with an infected person's skin lesions or bodily fluids [65, 66]. Monkeypox was endemic in West and Central Africa, but it has recently spread to Europe and several other countries, including the United States. It causes symptoms that are very similar to those of traditional smallpox, albeit with less severe clinical manifestations [67]. To date, two genetically distinct clades of MPXV have been identified; the Central African or Congo Basin (CB) clade, now known as clade I, and the West African (WA) clade, now known as clade II. Both clades exhibit similar clinical and pathological features [68, 69]. The case fatality rates for both clades are distinct; a systematic review of 28 peer-reviewed articles and ten reports prior to the current multi-country outbreak found that clade I had a rate of 10.6% and clade II a rate of 3.6% [70]. The Congo Basin clade has historically caused more severe disease and was thought to be more transmissible [71]. An analysis of the genomes of the two clades revealed that the less virulent clade II MPXV strain lacks ten thousand base pairs [72].
The inverted terminal repeat region of the human MPXV genome contains at least four known ORFs. Therefore, the identification of the MPXV was made possible through the use of the Sanger sequencing method on the gene fragment of F3L (F3L-F290 ‘CTCATTGAT-TTTTCGCGGGAT’ and F3L-500-Rv ‘TTACAACGCAATCGATACATG’); N3R (MPXV_E5R_F “ATGTTGATATTAATAATCGTATTGTGGT’ and MPXV_E5R_R “AAAGTCAATGCAC-TCTTAAAGATTCTCAA”); F4L (F4L_F ‘CGTTGGAAAACGTG AGTCCGG’ and F4L_R ‘ATT-GGCGTTTTTTGCAGCCAG’); and E5R (N3R-F319 ‘AACAACCGTCCTACAATTAAACAACA’ and N3R-500-Rv ‘TAATGCAGATATAATA TCTTT’) [58, 73-77]. In addition, human MPXV isolates obtained during this outbreak in Europe all belong to the same clade, implying that they came from a single source [78]. These isolated viruses are very similar to those isolated in Nigeria, the United Kingdom, Singapore, and Israel during 2017-2018 [35, 41-43, 79]. Compared to mutation rates of RNA viruses, Orthopoxviruses have significantly lower rates [80]. This is due to the fact that their viral DNA polymerase possesses an activity known as 3′-5′ proofreading exonuclease [81]. The essential genes for virus replication are encoded in the highly conserved central region of Orthopoxvirus genomes. In contrast, the two terminal regions of the virus are highly variable and may exhibit deletions as well as sequence rearrangements [82]. These variable terminal regions contain the vast majority of the genes that control virulence as well as host range [82]. Genes of double-stranded DNA viruses can be duplicated or deleted through the process of recombination, which enables the virus to adapt to environmental pressures as well as changes in the host [83]. A large genomic rearrangement in a MPXV sequence from a 2022 case in the state of Minnesota (MN), USA has been most recently detected [85]. Screening of WGS data of 206 U.S. MPXV samples found seven (3.4%) sequenced genomes containing similar abnormal read coverage profiles that suggested putative large deletions or genomic rearrangements [85]. In particular, three MPXV genomes contained deletions ranging from 2.3 to 15 kb and four genomes contained more complex rearrangements [85]. All samples were positive using VAC1 and Clade II (West African)-specific MPXV diagnostic tests; however, the possibility exists that similar rearrangements result in viruses in which the target of a PCR diagnostic test is deleted [85]. Continuing genomic analysis during the current outbreak is warranted.
Innate immunity evasion by monkeypox virus
The innate immune system utilizes pattern recognition receptors (PRRs) to detect invading pathogen-associated molecular patterns (PAMPs) and initiate an immune response cascade. Against viral infections, innate immunity plays an integral role in (1) sensing viral patterns as the first line of defense and (2) mediating a robust adaptive immune response via activation of cytokines, chemokines, and antiviral type I interferons (IFNs). In the context of MPXV, a dsDNA virus whose replication occurs exclusively in the cytoplasm within viral factories, it is interesting to see how cytosolic viral DNA PRRs interact with MPXV-derived PAMPs, and how the virus itself manages to protect the self-sufficient ≈197kb genome from innate sensing.
Antiviral innate sensing starts from the intracellular DNA sensors, which include Toll-like receptor (TLR) and cyclic GMP-AMP synthase (cGAS). Cascade will then be initiated by activating adaptor proteins, mainly the stimulator of interferon genes (STING), to aptly produce pro-inflammatory molecules and antiviral IFNs. DNA viruses generally evade innate detection by hiding their DNA from cytosolic PRRs or abrogating either the PRRs or downstream signaling cascade. With ≈190 non-overlapping ORFs, poxviruses possess some of the most extensive arsenals of viral proteins capable of antagonizing the host immune response. Nevertheless, most data on innate response have been gathered from other members of the Poxviridae family, for instance, vaccinia virus (VACV) and myxoma virus (MYXV) [84].
TLR9 is the only DNA sensor out of the 10 TLRs in humans which recognizes CpG motifs in dsDNA commonly found in viral genomes, although CpG-rich region does not seem to be detected in, for example, the modified vaccinia virus Ankara (MVA) genome [85]. The importance of TLR9 against poxviruses nonetheless has been chiefly explored in mousepox-causing ectromelia virus (ECTV), whose infection becomes up to a 100-fold more pathogenic in TLR9-/-, or its adaptor protein MYD88-/-, mice relative to wild type, while TLR2-/-, TLR4-/- and TLR7-/- mice survive [86]. It has been elegantly shown that the TLR9-MyD88-interferon regulatory factor 7 (IRF7) pathway in CD11c+ cells is imperative for local type I IFN response after ECTV infection, in conjunction with the cGAS-STING-IRF7/nuclear factor κB (NF-κB) pathway in CD11b+ Gr1+ monocytes [87]. Similarly, TLR9 has also been implicated in both recombinant fowlpox virus and myxoma virus (MYXV) host responses via the MyD88-dependent pathway [88, 89].
TLR recognitions may be broader than its canonical ligands, which explains why several TLRs other than TLR9 have also been implicated in poxvirus sensing. TLR3-/- mice had higher protection against VACV with a reduction in IL-6, tumor necrosis factor alpha (TNF-α), and pro-inflammatory chemokines, but knockout of its downstream adapter, Toll/interleukin-1 receptor (TIR)-domain-containing adaptor inducing IFN (TRIF), caused mice to be more susceptible to infection [90]. Subsequently, TRIF-dependent TLR4-/- mice were found to have higher VACV replication and mortality, thus establishing TLR4 as a mediator of innate protection against VACV [91]. As adjuvant, TLR3 agonist poly(I:C) also improve postexposure vaccination efficacy of MVA against ECTV, even higher than TLR9 agonist CpG ODNs [92].
Several poxviruses’ proteins possessing Bcl2-like-fold have been shown to antagonize the TLR3 and TLR4 pathway [93]. VACV A52R and A46R share similarities with the TIR domain, and the former disrupt TLR cascade by associating with interleukin-1 receptor-associated kinase 2 (IRAK2) and TNF receptor-associated factor 6 (TRAF6), while the latter associates with TRIF to inhibit IRF3 activation [94-96]. B14 also inhibits NF-κB induction by TLR3 by preventing inhibitor of NF-κB kinase β (IKKβ) phosphorylation [97].
cGAS is a universal cytosolic DNA sensor that catalyzes 2'3’-cyclic GMP-AMP (cGAMP) from ATP and GTP upon target DNA binding, which results in the binding of a second messenger cGAMP to the adaptor protein STING. The latter recruits TBK1 to phosphorylate IRF3 and relocate it into the nucleus to induce antiviral IFN while also activating NF-κB. While TLR9 may be regarded as having a relatively minor role in specialized immune cells, the cGAS-STING pathway is deemed to be vital in the response against poxviruses in many cell types. In cGAS-/- mice, VACV infection is 100% lethal without sufficient type I IFN production [98, 99]. Together with TLR9, cGAS is also imperative in ECTV sensing, subsequent production of type I IFN and cytokines, and viral replication control [100]. In modified VACV Ankara strain (MVA) infection, IFN production in conventional dendritic cells is solely dependent on the cGAS-STING, and not MDA5, MAVS, or even the TLR9-MyD88 pathway. Such findings indicate the importance of the cGAS-STING pathway in the MVA-based VARV vaccine, which has been long known to prevent MPXV infection effectively [101].
Poxvirus virulence factors can facilitate evasion of cGAS detection upstream of STING or STING activation itself. Seminal papers have described how VACV F17, known to dysregulate mammalian target of rapamycin (mTOR) by sequestering mTOR regulators Raptor/Rictor to the Golgi, blunts interferon-stimulated gene (ISG) responses via mTOR-dependent cGAS degradation while also enhancing poxviral protein production [102, 103]. More virulent strains of VACV are also capable of disrupting cGAS and blocking STING dimerization via virulence factors that are not limited to the DNA sensing inhibitor C16 [104]. The recently discovered poxvirus immune nucleases or poxins are enzymes capable of cleaving cGAMP and restrict STING-dependent signaling, of which VACV B2 has been noted as an example. Poxin-encoding genes are conserved among poxviruses but seem to be inactive in VARV and MVA [105]. Numerous other PRRs play a varying role in antigen sensing and antiviral activity against poxviruses, including other DNA sensors (e.g., interferon-γ inducible protein 16 [IFI16]), RNA sensors (e.g., retinoic acid-inducible gene I [RIG-I]-like receptors [RLR]), and inflammasomes and NOD-like receptors (NLR, e.g., NLR family pyrin domain containing 3 [NLRP3]). Thorough reviews are available [106-108].
Cross-reactive immunity by vaccination
Routine vaccination against smallpox was discontinued by the WHO following the last community acquired case in the world in 1978. Nevertheless, 350 million doses of smallpox vaccines have been stockpiled by the WHO and seven countries in the event of reemergence of smallpox and for the potential use in a monkeypox outbreak [109, 110]. Two currently licensed vaccines for smallpox (and monkeypox) are ACAM2000 (Sanofi Pasteur®), a plaque-purified clone of Dryvax vaccine cultured serum-free in Vero cells, and JYNNEOS (or MVA-BN, Bavarian Nordic®), a live attenuated vaccine derived from MVA. Specific historical reviews for both vaccines are available [111-113].
To understand the capability of cross-reactive immunity, it is interesting to compare the genomic differences between MPXV and VARV, and to identify whether smallpox vaccines might be able to provide an MPXV-specific immune response. Genomic conservation between both viruses may reach 96% in the central regions but may be less, 83-94%, in the terminal regions [72, 114]. While a small proportion of differences are found in virulence-related genes, most of these variations stem from open reading frames (ORFs) whose functions remain unclear. The following virulence genes are full-length in VARV but absent or truncated in MPXV: C3L (complement inhibitor, truncated in clade 1 but absent in clade 2), C10L (IL-1β antagonist), E3L (IFN resistance), K3L (inhibitor of antiviral protein kinase PKR), and BR-211 (group 3 ortholog of ANK/F-box genes). On the other hand, these full-length virulence genes found in MPXV are absent or truncated in VARV: BR-203 (apoptosis inhibitor), A44L (hydroxysteroid dehydrogenase), B10R (T4 ortholog, apoptosis inhibitor and ER resident). Another well-known virulence protein in orthopoxviruses, BR-209 (IL-1β binding protein), appears fragmented in MPXV yet absent in VARV. The TNF receptor homolog CrmB exists in two copies in MPXV inverted terminal repeats but only one in VARV, while CrmC, -D, and -E are truncated in MPXV but absent in VARV [115, 116]. Concerning cross-reactive immunity, differences in gene and protein expression may correlate with epitope variation between MPXV and VARV or VACV. T cell epitope mapping showed that at least 71% of VACV T cell epitopes are shared with both MPXV clade 1 and MPXV-2022, thus indicating possible cross-reactivity between the MVA vaccines and MPXV [117]. Nevertheless, experimental testing on this in silico prediction is warranted.
Prevention of monkeypox and its impact
Viral transmission occurs through close contact with respiratory secretions, skin lesions of an infected person or recently contaminated objects (fomites), or from contact with the blood, bodily fluids, or cutaneous or mucosal lesions of an infected animal, or ingestion of undercooked meat. Respiratory droplets, lesions on the skin or mucous membranes, blood, or bodily fluids all contain the virus. Mother-to-child transfer through the placenta or acquisition during or after birth has been reported [118].
During an outbreak of monkeypox, practicing hand hygiene, close contact and droplet precaution are essential public health measures. However, if aerosol-generating procedures are conducted, it is advised to take additional airborne precautions. Maintaining a distance of at least 1 meter from suspected individuals, as well as wearing a well-fitted mask and disposable gloves, is recommended. Virus from skin lesions remain transmissible until all lesions have dried, crusted, fallen off, and a new layer of skin has developed underneath; this can take 2-4 weeks. It is important to be aware that the MPXV may remain in body fluids even after all skin lesions have healed. The WHO recommends that sexually active patients use condoms for 12 weeks following recovery for both receptive and injective sexual activity [8]. Despite the lack of conclusive evidence of infectivity, viral DNA persists in the semen of infected patients for at least 9 days after the onset of symptoms [119].
Early case detection, patient isolation and contact tracing are essential to control human-to-human spread. Vaccination has also been introduced to control the current global outbreak, mainly located in the Americas and Europe. The U.S. has rolled out a vaccination strategy, using the JYNNEOS vaccine, in June 2022; over 300,000 doses have been distributed to date, mainly among males aged 25-39 years [9]. Similarily the United Kingdom has implemented a program to offer men at risk, health-care workers and close contacts of people infected with the virus, access to the vaccine. A vaccine program has been implemented in other European countries. At the end of August, the global trend of reported cases has begun to fall [120].
According to WHO's vaccine and immunization guidance, mass vaccination is currently not required, based on risks/benefits and limited vaccine supply. Both primary preventive vaccination (PPV) and post-exposure preventive vaccination (PEPV) are advised, depending on the risk of exposure. PPV is indicated for certain populations, such as persons with multiple sex partners, healthcare and laboratory personnel. PPV is also recommended for those who are at high risk of developing severe disease, including children, pregnant women, and immunocompromised patients. For PEPV, it is recommended for medium to high risk contact within 4 days, and up to 14 days, in the absence of symptoms [11]. A person who has been exposed to the monkeypox virus should be monitored for symptoms for 21 days after the last exposure [12].
Second- and third-generation vaccines are being advised for preventive vaccinations. Minimally replicating vaccine (LC16) and non-replicating vaccine (MVA-BN) are considered for children and pregnant people instead of the replicating vaccinia-based ACAM2000, which is contraindicated in severe immune deficiency patients. LC16 has been licensed for use in children in Japan, while MVA-BN is for people >18 years in most countries. The approval has been based on safety and efficacy studies in humans, with normal health or with underlying diseases, and in animal models. However, no clinical studies have been performed in pregnant women or children. Clinical trials, mostly with MVA-BN, have revealed good immunogenicity without serious safety concerns [13, 121-124], but good data on the effectiveness of preventing monkeypox remains limited. Practice-based vaccine research using standard protocols and data collection is encouraged in monkeypox control and prevention.
Conclusions and future perspectives
The worldwide dissemination of MPXV necessitates comprehensive understanding of this new public health threat. Although the current transmission network of the virus largely involves men who have sex with men, the occurrence of infection among women and children requires vigilant public health control efforts to prevent further community spread of the virus. Future research is recommended to elucidate the evolutionary history of the virus spill-over event(s) that contributed to the current outbreak. Furthermore, phylodynamic analyses can help to understand the transmission dynamics of MPXV. Focus on comprehensive understanding of the innate and adaptive immune responses directed specifically towards MPXV is also needed. This will help to design efficacious and safe vaccines that can aid to control the infection in endemic regions and to mitigate the spread of the virus implicated in the ongoing outbreak.
Acknowledgments
The authors acknowledge their respective universities/institutes/organizations.
Ethics approval
Not required.
Conflict of interest
All the authors declare that there are no conflicts of interest.
Funding
This study received no external funding.
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
How to cite
Ophinni Y, Frediansyah A, Sirinam S, et al. Monkeypox: Immune response, vaccination and preventive efforts. Narra J 2022; 2 (3): e90 - http://doi.org/10.52225/narra.v2i3.90.
References
- 1.WHO . Director-General's statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox. Accessed: 23 July 2022. [Google Scholar]
- 2.CDC . Monkeypox outbreak global map. Available from: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html. Accessed: 23 July 2022. [Google Scholar]
- 3.Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl Trop Dis 2022; 16(2):e0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sklenovska N, Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health 2018; 6:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu E, Dattani S, Ritchie H, et al. Monkeypox: Cumulative confirmed cases, by date of confirmation, Sept 9, 2022. Available from: https://ourworldindataorg/monkeypox. Accessed: 9 September 2022. [Google Scholar]
- 6.Nolen LD, Osadebe L, Katomba J, et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis 2016; 22(6):1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis 2014; 58(2):260-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vusirikala A, Charles H, Balasegaram S, et al. Epidemiology of early monkeypox virus transmission in sexual networks of gay and bisexual men, England, 2022. Emerg Infect Dis 2022; 28(10): 2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivancos R, Anderson C, Blomquist P, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill 2022; 27(22):2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet 2022;400(10353):661-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan CN, Whitehill F, Doty JB, et al. Environmental persistence of monkeypox virus on surfaces in household of person with travel-associated infection, Dallas, Texas, USA, 2021. Emerg Infect Dis 2022; 28(10): 1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Nicolas-Ruanes B, Vivancos MJ, Azcarraga-Llobet C, et al. Monkeypox virus case with maculopapular exanthem and proctitis during the Spanish outbreak in 2022. JEADV 2022; 36(8):e658-e660. [DOI] [PubMed] [Google Scholar]
- 13.Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ 2022; 378:e072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tutu van Furth AM, van der Kuip M, van Els AL, et al. Paediatric monkeypox patient with unknown source of infection, the Netherlands, June 2022. Euro Surveill 2022; 27(29):2200552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen E, Abubakar I, Ihekweazu C, et al. Monkeypox - Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis 2019; 78:78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegrist EA, Sassine J. Antivirals with activity against monkeypox: a clinically oriented review. Clin Infect Dis 2022:ciac622. 10.1093/cid/ciac622 (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy MW. Therapeutic strategies to address monkeypox. Expert Rev Anti Infect Ther 2022;20(10):1249-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harapan H, Ophinni Y, Megawati D, et al. Monkeypox: A comprehensive review. Viruses 2022; 14(10):2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harapan H, Setiawan AM, Yufika A, et al. Knowledge of human monkeypox viral infection among general practitioners: a cross-sectional study in Indonesia. Pathog Global Health 2020; 114(2):68-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harapan H, Setiawan AM, Yufika A, et al. Confidence in managing human monkeypox cases in Asia: A cross-sectional survey among general practitioners in Indonesia. Acta Tropica 2020; 206:105450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallam M, Al-Mahzoum K, Dardas LA, et al. Knowledge of human monkeypox and its relation to conspiracy beliefs among students in Jordanian health schools: filling the knowledge gap on emerging zoonotic viruses. Medicina 2022; 58(7): 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alsanafi M, Al-Mahzoum K, Sallam M. Monkeypox Knowledge and confidence in diagnosis and management with evaluation of emerging virus infection conspiracies among health professionals in Kuwait. Pathogens 2022; 11(9):994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riccò M, Ferraro P, Camisa V, et al. When a neglected tropical disease goes global: knowledge, attitudes and practices of italian physicians towards monkeypox, preliminary results. Trop Med Infect Dis 2022; 7(7):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Rio C, Malani PN. Update on the monkeypox outbreak. JAMA 2022;328(10):921-922. [DOI] [PubMed] [Google Scholar]
- 25.Gruber MF. Current status of monkeypox vaccines. NPJ Vaccines 2022; 7(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucey DR, Breman JG, Henderson DA. Smallpox and bioterrorism. Beyond Anthrax 2008:17-54. [Google Scholar]
- 27.Brooks JT, Marks P, Goldstein RH, et al. Intradermal vaccination for monkeypox — benefits for individual and public health. N Engl J Med 2022;387(13):1151-1153. [DOI] [PubMed] [Google Scholar]
- 28.Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLOS Negl Trop Dis 2019; 13(10):e0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panchanathan V, Chaudhri G, Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J Virol 2006; 80(13):6333-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karem KL, Reynolds M, Hughes C, et al. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin Vaccine Immunol 2007; 14(10):1318-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammarlund E, Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med 2003; 9(9):1131-1137. [DOI] [PubMed] [Google Scholar]
- 32.Pv Magnus, Andersen EK, Petersen KB, et al. A pox-like disease in cynomolgus monkeys. APMIS 1959; 46(2):156-176. [Google Scholar]
- 33.Dumbell K, Smith GL. Smallpox and monkeypox viruses (Poxviridae); 1999.
- 34.Durski KN, McCollum AM, Nakazawa Y, et al. Emergence of monkeypox—west and central Africa, 1970–2017. MMWR 2018; 67(10):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yinka-Ogunleye A, Aruna O, Ogoina D, et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg Infect Dis 2018; 24(6):1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis 2019; 19(8):872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larkin MJTLID. Monkeypox spreads as US public-health system plays catch-up. Lancet Infect Dis 2003; 3(8):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CDC Update: Multistate outbreak of monkeypox: Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR 2003; 52(24):561-564. [Google Scholar]
- 39.Reynolds MG, Davidson WB, Curns AT, et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg Infect Dis 2007; 13(9):1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ligon BL. Monkeypox: A review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis 2004; 15(4):280-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erez N, Achdout H, Milrot E, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis 2019; 25(5):980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill 2018; 23(38):1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng OT, Lee V, Marimuthu K, et al. A case of imported monkeypox in Singapore. Lancet Onfect Dis 2019; 19(11):1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hraib M, Jouni S, Albitar MM, et al. The outbreak of monkeypox 2022: An overview. Medicine 2022:104069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill 2022; 27(24):2200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahase E. Monkeypox: What do we know about the outbreaks in Europe and North America? BMJ 2022; 377:o1274. [DOI] [PubMed] [Google Scholar]
- 47.Bankuru SV, Kossol S, Hou W, et al. A game-theoretic model of Monkeypox to assess vaccination strategies. Economics 2020; 8:e9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kupferschmidt KJS. Why monkeypox is mostly hitting men who have sex with men. Science 2022; 376(6600):1364-1365. [DOI] [PubMed] [Google Scholar]
- 49.Falendysz EA, Lopera JG, Doty JB, et al. Characterization of monkeypox virus infection in African rope squirrels (Funisciurus sp.). PLOS Negl Trop Dis 2017; 11(8):e0005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radonić A, Metzger S, Dabrowski PW, et al. Fatal monkeypox in wild-living sooty mangabey, Cote d'Ivoire, 2012. Emerg Infect Dis 2014; 20(6):1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaucha GM, Jahrling PB, Geisbert TW, et al. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab Invest 2001; 81(12):1581-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez-Morales AJ, Lopardo GJP. Monkeypox: another sexually transmitted infection? Pathogens 2022; 11(7):713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shchelkunov S, Totmenin A, Safronov P, et al. Analysis of the monkeypox virus genome. Virology 2002; 297(2):172-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fatima N, Mandava KJAoM, Surgery. Monkeypox-a menacing challenge or an endemic? Ann Med Surg 2022; 79:103979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: A contemporary review for healthcare professionals. Open Forum Infect Dis 2022: ofac310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendrickson RC, Wang C, Hatcher EL, et al. Orthopoxvirus genome evolution: the role of gene loss. Viruses 2010; 2(9):1933-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shchelkunov SN, Totmenin AV, Babkin IV, et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett 2001; 509(l):66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J-W, Lee M, Shin H, et al. Isolation and identification of monkeypox virus MPXV-ROK-P1-2022 from the first case in the Republic of Korea. Osong Public Health Res Perspect 2022. Aug;13(4):308-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Essbauer S, Pfeffer M, Meyer HJVm. Zoonotic poxviruses. Vet Microbiol 2010; 140(3-4):229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damon IK. Poxviruses. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML and Warnock DW. Manual of Clinical Microbiology. Wiley Online Library; 2011;1647-1658. [Google Scholar]
- 61.Robert Koch Institute . Monkeypox virus. Available from: https://www.rki.de/EN/Content/infections/Diagnostics/NatRefCentresConsultantLab/CONSULAB/EM-images/EM_Tab_Monkeypox-viruses_en.html. Accessed: 23 July 2022.
- 62.Gomez-Lucia E. Monkeypox: some keys to understand this emerging disease. Animals 2022; 12(17):2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parker S, Nuara A, Buller RML, et al. Human monkeypox: an emerging zoonotic disease. Future Microbiol 2007;2(1):17-34 [DOI] [PubMed] [Google Scholar]
- 64.Haddad N. The presumed receptivity and susceptibility to monkeypox of European animal species. Infectious Disease Now 2022; 52(5):294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grant R, Nguyen L-BL, Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ 2020; 98(9):638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet 2022;400(10353):658-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs 2022;82(9):957-963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell RC, Drewes RC, Channing A, et al. Overseas dispersal of Hyperolius reed frogs from Central Africa to the oceanic islands of São Tomé and Príncipe. J Biogeogr 2015; 42(1):65-75. [Google Scholar]
- 69.Berthet N, Descorps-Declère S, Besombes C, et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci Rep 2021; 11(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl Trop Dis 2022; 16(2):e0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA 2022; 328(2):139-140. [DOI] [PubMed] [Google Scholar]
- 72.Chen N, Li G, Liszewski MK, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005; 340(1):46-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X, Zhu Z, Miao Q, et al. Monkeypox–A danger approaching Asia. Biosci Trends 2022;16(4):245-248 [DOI] [PubMed] [Google Scholar]
- 74.Douglass N, Dumbell KJJov. Independent evolution of monkeypox and variola viruses. J Virol 1992; 66(12):7565-7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Douglass N, Dumbell K. DNA sequence variation as a clue to the phylogenesis of orthopoxviruses. J Gen Virol 1996; 77(5):947-951. [DOI] [PubMed] [Google Scholar]
- 76.Kulesh DA, Loveless BM, Norwood D, et al. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan® assays on the Roche LightCycler. Lab Invest 2004; 84(9):1200-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCraith S, Holtzman T, Moss B, et al. Genome-wide analysis of vaccinia virus protein–protein interactions. PNAS 2000; 97(9):4879-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giorgi FM, Pozzobon D, Di Meglio A, et al. Genomic analysis of the recent monkeypox outbreak. Preprint 2022. 10.1101/2022.06.01.494368. [DOI] [Google Scholar]
- 79.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis 2019; 19(8):872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Babkin IV, Babkina IN, Tikunova NV. An update of orthopoxvirus molecular evolution. Viruses 2022;14(2):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gammon DB, Gowrishankar B, Duraffour S, et al. Vaccinia virus–encoded ribonucleotide reductase subunits are differentially required for replication and pathogenesis. PLOS Pathog 2010; 6(7):e1000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wittek RJ. Organization and expression of the poxvirus genome. Experientia 1982; 38(3):285-297. [DOI] [PubMed] [Google Scholar]
- 83.Roth JR, Andersson DI. Poxvirus use a “gene accordion” to tune out host defenses. Cell 2012; 150(4):671-672. [DOI] [PubMed] [Google Scholar]
- 84.Yu H, Bruneau RC, Brennan G, et al. Battle Royale: innate recognition of poxviruses and viral immune evasion. Biomedicines 2021; 9(7):765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peng C, Wyatt LS, Glushakow-Smith SG, et al. Zinc-finger antiviral protein (ZAP) is a restriction factor for replication of modified vaccinia virus Ankara (MVA) in human cells. PLOS Pathog 2020; 16(8):e1008845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samuelsson C, Hausmann J, Lauterbach H, et al. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J Clin Invest 2008; 118(5):1776-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu R-H, Wong EB, Rubio D, et al. Sequential Activation of two pathogen-sensing pathways required for type i interferon expression and resistance to an acute DNA virus infection. Immunity 2015; 43(6):1148-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lousberg EL, Diener KR, Fraser CK, et al. Antigen-specific T-cell responses to a recombinant fowlpox virus are dependent on MyD88 and interleukin-18 and independent of Toll-like receptor 7 (TLR7)- and TLR9-mediated innate immune recognition. J Virol 2011; 85(7):3385-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dai P, Cao H, Merghoub T, et al. Myxoma virus induces type I interferon production in murine plasmacytoid dendritic cells via a TLR9/MyD88-, IRF5/IRF7-, and IFNAR-dependent pathway. J Virol 2011; 85(20):10814-10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hutchens M, Luker KE, Sottile P, et al. TLR3 increases disease morbidity and mortality from vaccinia infection. J Immunol 2008; 180(1):483-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hutchens MA, Luker KE, Sonstein J, et al. Protective effect of Toll-like receptor 4 in pulmonary vaccinia infection. PLOS Pathog 2008; 4(9):e1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Israely T, Melamed S, Achdout H, et al. TLR3 and TLR9 agonists improve postexposure vaccination efficacy of live smallpox vaccines. PLoS One 2014; 9(10):e110545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Graham SC, Bahar MW, Cooray S, et al. Vaccinia virus proteins A52 and B14 share a Bcl-2–Like fold but have evolved to inhibit NF-κB rather than apoptosis. PLOS Pathog 2008; 4(8):e1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bowie A, Kiss-Toth E, Symons JA, et al. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. PNAS 2000; 97(18):10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harte MT, Haga IR, Maloney G, et al. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J Exp Med 2003; 197(3):343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stack J, Haga IR, Schröder M, et al. Vaccinia virus protein A46R targets multiple Toll-like–interleukin-1 receptor adaptors and contributes to virulence. J Exp Med 2005; 201(6):1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen RAJ, Ryzhakov G, Cooray S, et al. Inhibition of IkappaB kinase by vaccinia virus virulence factor B14. PLOS Pathog 2008; 4(2):e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X-D, Wu J, Gao D, et al. Pivotal Roles of cGAS-cGAMP Signaling in Antiviral defense and immune adjuvant effects. Science 2013; 341(6152):1390-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schoggins JW, MacDuff DA, Imanaka N, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 2014; 505(7485):691-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng W-Y, He X-B, Jia H-J, et al. The cGas-Sting signaling pathway is required for the innate immune response against Ectromelia virus. Front Immunol 2018; 9:1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stittelaar KJ, van Amerongen G, Kondova I, et al. Modified vaccinia virus ankara protects macaques against respiratory challenge with monkeypox virus. J Virol 2005; 79(12):7845-7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meade N, Furey C, Li H, et al. Poxviruses Evade cytosolic sensing through disruption of an mTORC1-mTORC2 regulatory circuit. Cell 2018; 174(5):1143-1157.e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meade N, King M, Munger J, et al. mTOR Dysregulation by vaccinia virus F17 controls multiple processes with varying roles in infection. J Virol 2019; 93(15):e00784-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Georgana I, Sumner RP, Towers GJ, et al. Virulent poxviruses inhibit DNA sensing by preventing STING activation. J Virol 2018; 92(10):e02145-02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eaglesham JB, Pan Y, Kupper TS, et al. Viral and metazoan poxins are cGAMP-specific nucleases that restrict cGAS-STING signalling. Nature 2019; 566(7743):259-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith SA, Kotwa GJ. Immune response to poxvirus infections in various animals. Crit Rev Microbiol 2002; 28(3):149-185. [DOI] [PubMed] [Google Scholar]
- 107.Lu Y, Zhang L. DNA-Sensing Antiviral Innate Immunity in Poxvirus Infection. Front Immunol 2020; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seet BT, Johnston JB, Brunetti CR, et al. Poxviruses and immune evasion. Annu Rev Immunol 2003;21:377-423. [DOI] [PubMed] [Google Scholar]
- 109.WHO . Operational framework for the deployment of the WHO smallpox vaccine emergency stockpile in response to a smallpox event. Available from: https://apps.who.int/iris/handle/10665/259574. Accessed: 23 July 2022. [Google Scholar]
- 110.FDA . Smallpox Preparedness and Response Updates from FDA. FDA. Available from: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/smallpox-preparedness-and-response-updates-fda. Accessed: 23 July 2022. [Google Scholar]
- 111.Belongia EA, Naleway AL. Smallpox vaccine: the good, the bad, and the ugly. Clin Med Res 2003; 1(2):87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gruber MF. Current status of monkeypox vaccines. NPJ Vaccines 2022; 7(1):1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.. Rizk JG, Lippi G, Henry BM, et al. Prevention and Treatment of monkeypox. Drugs 2022; 82(9):957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shchelkunov SN, Totmenin AV, Babkin IV, et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett 2001; 509(1):66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev 2008; 225:96-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bratke KA, McLysaght A, Rothenburg S. A survey of host range genes in poxvirus genomes. Infect Genet Evol 2013; 14:406-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahmed SF, Sohail MS, Quadeer AA, et al. Vaccinia-virus-based vaccines are expected to elicit highly cross-reactive immunity to the 2022 monkeypox virus. Viruses 2022; 14(9):1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis 2017; 216(7):824-828. [DOI] [PubMed] [Google Scholar]
- 119.Antinori A, Mazzotta V, Vita S, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill 2022; 27(22): pii=2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.WHO . Monkeypox Outbreak: Global Trends. Available from: https://worldhealthorg.shinyapps.io/mpx_global/#2_Global_situation_update. Accessed: 23 July 2022. [Google Scholar]
- 121.Greenberg RN, Hay CM, Stapleton JT, et al. A Randomized, double-blind, placebo-controlled phase ii trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One 2016; 11(6):e0157335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase ii study to evaluate safety and immunogenicity of mva smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One 2015; 10(10):e0138348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Overton ET, Lawrence SJ, Stapleton JT, et al. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine 2020; 38(11):2600-2607. [DOI] [PubMed] [Google Scholar]
- 124.Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia ankara as a vaccine against smallpox. N Engl J Med 2019; 381(20):1897-1908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.