Abstract
The emergence of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected many countries throughout the world. As urgency is a necessity, most efforts have focused on identifying small molecule drugs that can be repurposed for use as anti-SARS-CoV-2 agents. Although several drug candidates have been identified using in silico method and in vitro studies, most of these drugs require the support of in vivo data before they can be considered for clinical trials. Several drugs are considered promising therapeutic agents for COVID-19. In addition to the direct-acting antiviral drugs, supportive therapies including traditional Chinese medicine, immunotherapies, immunomodulators, and nutritional therapy could contribute a major role in treating COVID-19 patients. Some of these drugs have already been included in the treatment guidelines, recommendations, and standard operating procedures. In this article, we comprehensively review the approved and potential therapeutic drugs, immune cells-based therapies, immunomodulatory agents/drugs, herbs and plant metabolites, nutritional and dietary for COVID-19.
Keywords: Drug, viral inhibitor, immunotherapeutic, supportive therapy, nutrition
Introduction
The newly emerged novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19), is associated with significant global health problems. The most common clinical manifestations of COVID-19 are dry cough, fever, and fatigue [1]. Compared with diseases caused by other highly pathogenic human coronaviruses, COVID-19 has a higher transmission but less severe pathogenesis [2]. It also disproportionately affects the elderly people and causes a severe form of the disease and higher mortality mainly because elderly have a weak immune system and multiple age-related co-morbidities like hypertension, diabetes, chronic renal disorder, and chronic obstructive pulmonary disease [1].
Being a pandemic virus posing high global threats and challenges, rapid advancements have been made to understand the SARS-CoV-2 and COVID-19 from various aspects viz., molecular virology, genome sequencing, cellular and molecular pathways, bioinformatics, pathology, immunopathogenesis, immunobiology, which altogether are helping in identifying potential points of therapeutic interventions, developing vaccines and drugs against COVID-19 [3-7]. Despite the extensive efforts made to develop effective vaccines, drugs, immunotherapeutics, and therapeutic agents for SARS-CoV-2, several of these candidates require further trials and validation before they can be made commercially available, and for this purpose, clinical trials are underway [8-15]. Several of the available options have shown promising results in in vitro studies, and currently, high efforts are being made for generating appropriate supporting data from the ongoing clinical trials to find out effective drugs and therapeutic regimens against SARS-CoV-2 [16]. In the early outbreak stages, several therapeutic agents were used in combination to manage clinical cases of SARS-CoV-2 infection. In addition to supportive therapy involving nebulization, oxygen therapy, the management of fluid conservation in pneumonic lungs, and broad-spectrum antibiotics to prevent the possibility of secondary bacterial infection, antiviral medicines, such as lopinavir/ritonavir, and umifenovir (arbidol) were also administered [17, 18]. In several countries, including China, France, Italy, and Spain, the COVID-19 patients are already being given lopinavir-ritonavir, ribavirin, interferon (IFN), chloroquine, hydroxychloroquine, azithromycin, remdesivir, favipiravir, corticosteroids, and convalescent plasma on the sole basis of the in vitro efficacy of these therapies against SARS-CoV-2 [19].
The major strategies that can be used to control or prevent COVID-19 include vaccines, monoclonal antibodies, IFN therapies, peptides, oligonucleotide-based therapies, and small-molecule drugs. Given the time pressure, current research has predominately focused on the repurposing existing antiviral drugs that are already approved or are in the developmental stage to treat other viral diseases [17, 20]. Several treatment options have been proposed for the clinical management of SARS-CoV-2 infection, like remdesivir, lopinavir/ritonavir, neuraminidase inhibitors, nucleoside analogs, arbidol, peptides such as EK1, RNA synthesis inhibitors, and traditional Chinese medicine (TCM) (ShuFengJieDu capsule and lianhuaqingwen capsule) [21]. Clinical trials in various regions of the world are in progress to assess the efficacy and safety profile of many drugs for curing COVID-19 [22, 23]. Corticosteroid has been found to save lives from COVID-19 in critically ill patients [24]. Additionally, other therapeutic modalities that can harness the benefits of the defense system of the body's immunity as well as boost immune system are being exploited for their effectiveness against SARS-CoV-2, practical utility in treating COVID-19 patients, and improved outcome of COVID-19. These include immune cells-based therapies (NK cells and T cells), immunomodulatory agents/drugs, monoclonal antibodies, cytokines, IFNs, Toll-like receptors (TLRs) based therapy, stem cell therapy, traditional Chinese medicines, herbs and plant metabolites, and nutritional and dietary approaches [8, 17, 25-33].
This review highlights progress and advances being made on identifying various potent drug candidates, viral inhibitors, immune cells-based therapies, immunomodulatory agents/drugs, herbs and plant metabolites, nutritional and dietary approaches for countering COVID-19 that possess potential to be used as a monotherapy or in combination with other therapeutic agents.
Drug targets against SARS-CoV-2
The therapeutic agents used for treating SARS-CoV-2 infections can be categorized into three main groups depending upon the mechanism of action: (1) blocking SARS-CoV-2 entry into the host cell (Figure 1); (2) blocking viral replication and reduce its ability to survive within the host cell (Figure 2); and (3) inhibiting the exaggerated host immune response (Figure 3). Of the different types of therapeutic agents available, drugs directly targeting SARS-CoV-2 may be the most effective [34]. The repurposing of existing drugs is an important strategy with major appeal in the current situation owing to the need for rapid development of specific new drugs for SARS-CoV-2. Therapeutic agents targeting adhesion and viral entry, endocytosis, replication, protein proteases, and cytokine storms can be effective for COVID-19 management [35]. Fusion inhibitors are drugs that inhibit the fusion of the virus to host cells, and therefore, inhibit entry into the host cells under attack.
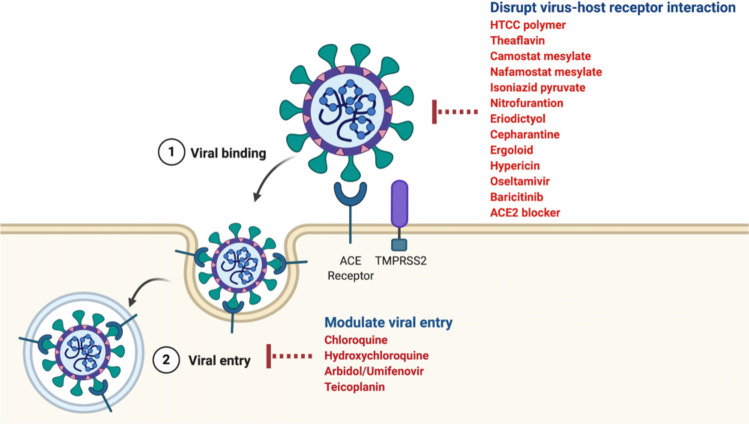
Figure 1.
Viral entry inhibitors. The virus enters target cells through binding to human angiotensin-converting enzyme 2 (ACE2) receptor. The antiviral drugs target this viral binding and viral entry preventing the virus entering the cells and therefore block viral replication inside the host cells. Some potential drugs are given in the red. These drugs work through inhibiting interaction of SAR-CoV-2 protein with the ACE2 receptor or disrupting endocytosis.
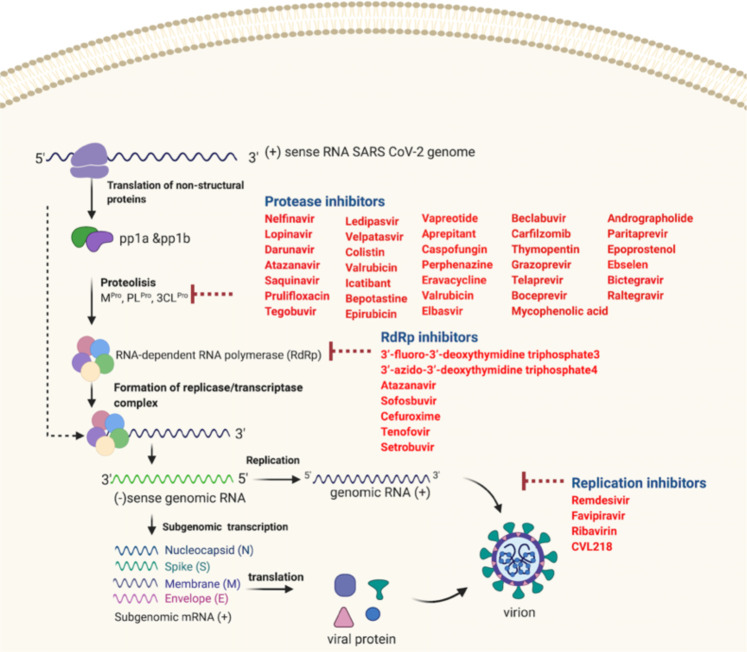
Figure 2.
Viral replication inhibitors. Following entry of the virus into the host cell, the viral RNA is translated and replicated to produce viral genome and viral protein. Viral genome and viral proteins are assembled in the cytoplasm to form virion progeny. Virions are then released from the infected cell through exocytosis. The viral replication inhibitors block viral replication inside the host cells and thereby reducing viral multiplication. These drugs include protease inhibitors, RNA-dependent RNA polymerase inhibitors, and RNA replication inhibitors and the names of the potential drugs within each group are given in red.
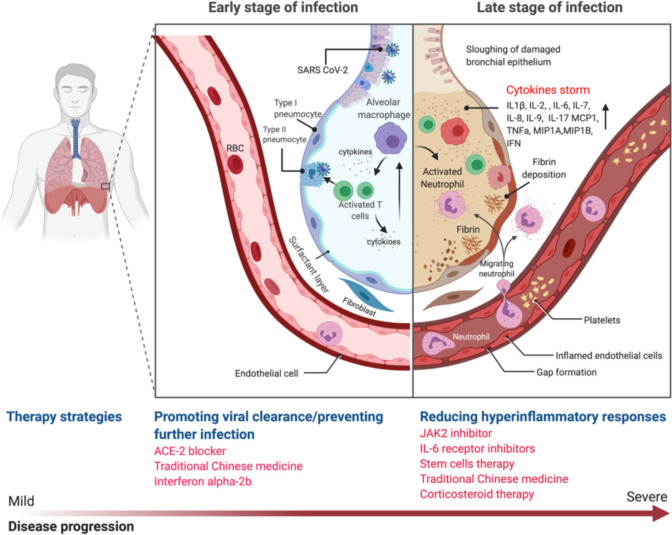
Figure 3.
Host immune response modulators. In the early stage of infection, SARS-CoV-2 virus enters alveoli and infects type II pneumocytes. In response to the viral infection, the pneumocytes produce type I interferon (IFN). Macrophages also identify the virus and produce cytokines such as Interleukin 1 (IL-1), IL-6, IL-8, IL-12 and tumor necrosis factors (TNF). Macrophages as antigen presenting cells activate the helper and cytotoxic T cells. The activated T cells produce cytokines and attack infected cells. In this period, therapeutic strategies to boost immune response can be applied, for example IFN-α-2b treatment. At the later stage, the pro-inflammatory cytokines recruit more immune cells, such as monocytes and neutrophils, which in turn produce more cytokines that result in a condition known as “cytokine storm”. The severe inflammation damages the lung leading to fibrin deposition and fluid leakage. In this period, therapeutic strategies to reduce the inflammatory response are applicable.
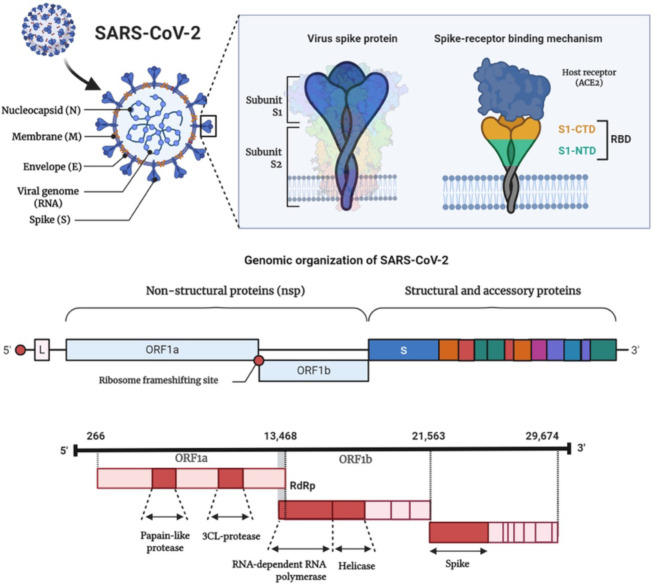
The major drug targets identified in SARS-CoV-2 are RNA-dependent RNA polymerase (RdRp), main protease (Mpro), or 3C-like protease (3CLpro), papain-like protease (PLpro), Nsp13 helicase, 2′-O-ribose methyltransferase (2′-O-MTase), and spike glycoprotein (Figure 4) [17, 36-38]. Cyclophilin A (CypA) is a highly conserved protein that is essential for replication in several coronaviruses, such as CoV-NL63, SARS-CoV, and CoV-229E, and may, therefore, be regarded as a potential antiviral drug target for SARS-CoV-2 [39]. A novel entry route, via the CD147 receptor on the host cells, was recently proposed for SARS-CoV-2 [40, 41]. Therefore, drugs that can directly interfere either with the spike protein-CD147 interaction and/or the expression of CD147 may potentially impede the viral invasion [40]. Computational drug discovery methods are ideal for the current situation as they are faster than high-throughput screening and can be used for the preliminary screening of potential drug candidates [37]. Drug repurposing (drug reprofiling or drug re-tasking) has several advantages, including reduced risk of failing the toxicity and safety tests, reduced project costs, and a much shorter time for drug development [36, 42].
Figure 4.
The genomic organization of SARS-CoV-2 along with the major drug targets that can be utilized for developing SARS-CoV-2 specific therapeutics.
The human ACE2 is the receptor of SARS-CoV-2 [43]. The successful SARS-CoV-2 entry into the host cell depends upon the attachment of spike protein receptor-binding domain (RBD) with the cellular ACE2 receptor. Therefore, therapeutic compounds blocking SARS-CoV-2 binding to ACE2 can prevent its entry into the host cells and therefore serve as a potent antiviral drugs [44]. The virus enters host cells through the process of endocytosis; proteins such as PIKfyve, two-pore calcium channel protein (TPC2), and cathepsin L play a major role in this process. Hence, these components may be potential targets against which vaccines and therapeutics can be developed [43]. The 3CLpro sequence is conserved in SARS-CoV-2 and was found to share great similarity with 3CLpro of bat SARS-like coronavirus. It also shared 99.02% sequence similarity with the 3CLpro of SARS-CoV, but the substrate-binding site of SARS-CoV-2 3CLpro had some key differences owing to point mutations in its sequence [45]. The molecular docking study of SARS-CoV-2 RdRp identified that anti-polymerase drugs like ribavirin, remdesivir, galidesivir, tenofovir, and sofosbuvir, may possess therapeutic potential for SARS-CoV-2 [46]. The selection of suitable therapeutic drugs should be performed only after assessing their efficacy and safety in pre-clinical (in vitro and in vivo) and clinical studies [34]. Among the different therapeutic agents evaluated for managing COVID-19, the majority relies on their anti-inflammatory, antiviral, and immunomodulatory activities to counter COVID-19. The most common therapeutic agents that are registered for COVID-19 clinical trials are antivirals, hydroxychloroquine, monoclonal antibodies, and drugs modulating the renin-angiotensin system [47]. The chemical structures of major therapeutic drugs for SARS-CoV-2 are illustrated in Figure 5.
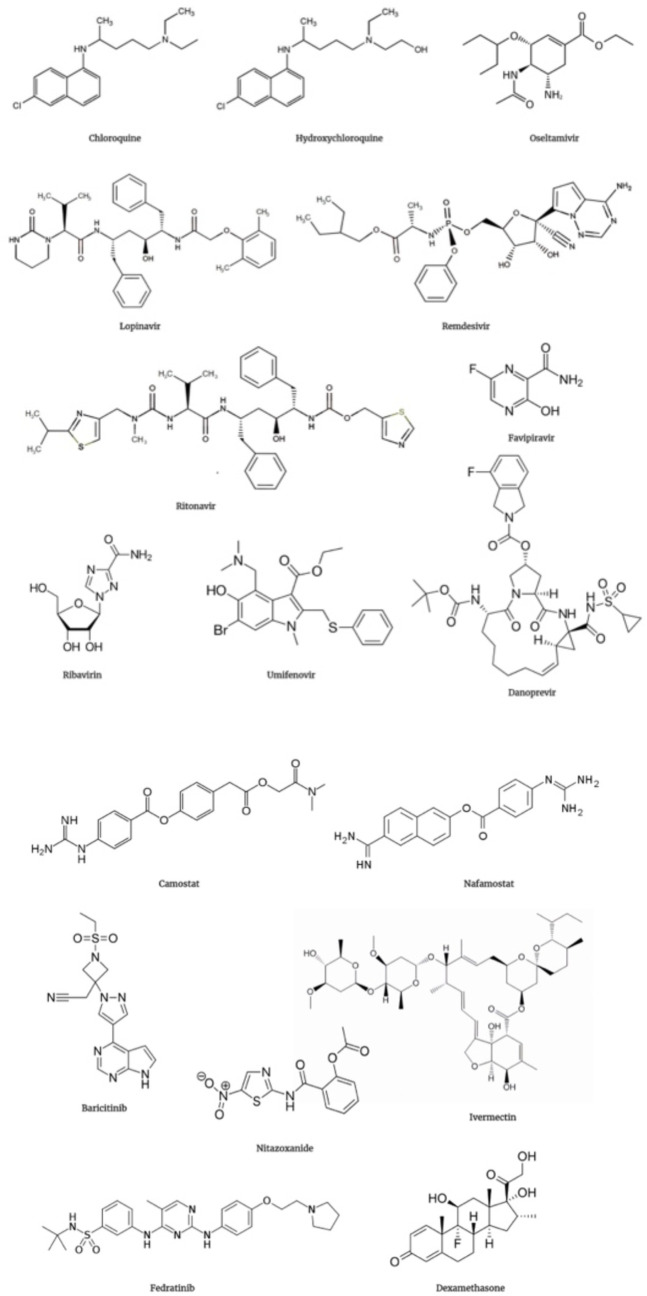
Figure 5.
· Chemical structure of some major therapeutic drugs that have been evaluated against SARS-CoV-2: chloroquine, hydroxychloroquine, oseltamivir, lopinavir, remdesivir, titonavir, favipiravir, tibavitin, umifenovir, danoprevir, camostat, nafamostat, baricitinib, nitazoxanide, ivermectin, fedratinib, and dexamethasone.
Pharmacologic agents against SARS-CoV-2
Oseltamivir
Antiviral drugs in routine use, such as oseltamivir (neuraminidase inhibitor), acyclovir, and ganciclovir, may not be effective against SARS-CoV-2 [33]. Despite the lack of confirmatory evidence of their clinical efficacy, neuraminidase inhibitors, like oseltamivir have been applied widely for treating suspected cases of COVID-19 in China [21]. In a recent review, oseltamivir has been found to be the third most common drug used in COVID-19 treatment [48]. It has been used in mild, moderate, and severe cases in some countries [44, 48]. Oseltamivir is a broad-spectrum antiviral drug that has been approved for the treatment of influenza. After the exclusion of influenza in a patient suspected to have COVID-19, oseltamivir has no role in managing COVID-19 [49].
Oseltamivir is a nucleoside analog that hinders viral neuraminidase to prevent the release of viruses from the host cells, which limits the spread of the virus in the respiratory system [23]. However, this mechanism may not be useful in SARS-CoV-2 as it lacks neuraminidase [50]. Oseltamivir has been used along with chloroquine and favipiravir for the assessment of antiviral efficacy in clinical trials. Computational methods were used to analyze the efficacy of the three-drug combination therapy of oseltamivir, lopinavir, and ritonavir. This drug combination was found to be highly effective against SARS-CoV-2 protease [51]. Nevertheless, additional studies are warranted to confirm these findings. A randomized clinical trial using a combination of hydroxychloroquine, oseltamivir, and azithromycin has been conducted (ClinicalTrials.gov identifier: NCT04338698) of which patients were treated with hydroxychloroquine phosphate/sulfate orally dosed at 200 mg thrice daily day for 5 days along with oseltamivir at 75 mg twice daily and azithromycin initially at 500 mg on day 1, followed by 250 mg twice daily during days 2-5. In silico analysis was performed to identify potential candidate therapeutics from the N-substituted Oseltamivir derivatives that can inhibit main protease of SARS-CoV-2 [52]. The findings indicate that some of these molecules studied had better inhibitory activity against SARS-CoV-2 main protease than chloroquine and hydroxychloroquine.
Lopinavir-ritonavir and nirmatrelvir-ritonavir combination
Protease inhibitors like lopinavir, ritonavir, and saquinavir have been used for human immunodeficiency virus (HIV) treatment. Similarly, an in-silico approach was used to evaluate the antiviral potential of these inhibitors for the main protease of SARS-CoV-2 (Figure 2). This study identified a strong interaction between the main protease and the HIV inhibitors lopinavir, ritonavir, and saquinavir [53]. Lopinavir is the second most commonly used drug for curing COVID-19 patients [48]. It is currently being used for treating critical and serious patients [48]. The combination therapy of lopinavir (400 mg) and ritonavir (100 mg) was administered twice daily for 14 days in COVID-19 patients [54]. However, another study used the same combination at a dose rate of 500 mg orally for 3–14 days when it is was used for treating COVID-19 [55]. This study noted no mortality, reduced time for negativity on testing, and reduced hospital stay [55]; however, the study did not observe any significant difference in clinical improvement and fatality rate in patients treated with lopinavir-ritonavir or standard therapy [54]. Decreased viral noted in patients’ needs further elaboration [56]. Others have also reported good recovery, reduced stay period, and decrease in mortality due to lopinavir-ritonavir therapy in COVID-19 patients [57,58].
The Central Drugs Standard Control Organization (CDSCO) has recently permitted the restricted therapeutic use of lopinavir/ritonavir combination in patients with COVID-19 symptoms in India based on the positive results obtained from treating severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) as well as those from preliminary docking analyses [59-62]. A randomized, controlled trial conducted among the hospitalized adult patients (n=199) with confirmed SARS-CoV-2 infection (ChiCTR2000029308) revealed no benefit from lopinavir-ritonavir treatment compared with that from the standard care [54].
Ritonavir is used in combination with other protease inhibitors such as lopinavir to take advantage of its ability to inhibit cytochrome P450 3A4 (CYP3A4), thus increasing the plasma concentration of lopinavir [63]. The ability of ritonavir to interfere with the major hepatic drug-metabolizing enzymes can lead to several clinically important interactions between the drugs. The drug-drug interaction with ritonavir-boosted therapy is important especially in the case of cardiac medications. Therefore, cardiac medications such as ranolazine, sildenafil, dronedarone, simvastatin, and colchicine, should not be used with lopinavir-ritonavir combination to ensure better patient care [63]. Ritonavir use is also associated with retinal toxicity and impairment. Long-term ritonavir treatment may induce retinal pigment epithelium changes resulting in retinal impairment in COVID-19 patients [64]. Similarly, the utilization of lopinavir/ritonavir in severely ill patients affected with COVID-19 may result in hepatotoxicity. It was found that treatment with lopinavir-ritonavir was associated with increased incidence of jaundice or elevation of total bilirubin in critically ill patients [65]. Therefore, it is advised that the liver function in such cases should be closely monitored.
Recently, combination of nirmatrelvir-ritonavir (known as Paxlovid®) already approved for COVID-19 treatment by the US FDA [66].
Remdesivir (GS-5734)
Remdesivir (GS-5734™) is useful as a broad-spectrum antiviral drug. After it is metabolized to GS-441524 within the body, it inhibits replication of human endemic and zoonotic deltacoronaviruses, SARS-CoV and MERS-CoV. In vitro studies demonstrated that remdesivir has viral activity against the Ebola virus [67, 68], murine hepatitis virus [69], the Junin virus, and the Lassa fever virus [68], and viruses from other genera, such as pneumo-, bunya-, and flaviviruses [70], as well as Bat-CoVs and human-CoVs, such as SARS-CoV and MERS-CoV [71-73]. Some in vitro studies showed that combination therapy of remdesivir and IFN-β had better protective effects in mice compared with the combined therapy of lopinavir, ritonavir, and IFN-β against MERS-CoV [54].
In vivo studies revealed that remdesivir exhibited antiviral activity for the Ebola virus in rhesus monkeys [68], the Nipah virus in African green monkeys [74], MERS-CoV in mice [75] and rhesus monkeys [76], and SARS-CoV in mice [71]. Remdesivir, a monophosphoramidate prodrug of an adenosine C–nucleoside that acts as an adenosine analog [77], exerts antiviral activity by adversely affecting viral RNA production, causing the termination of nascent viral RNA copies. This interrupts the function of enzymes, such as viral RdRp and viral exonuclease; hence, after treatment with this drug, the number of viral RNA molecules gradually diminishes owing to the disturbance in proofreading [78]. GS-441524 is efficacious and has been proposed for treating cats with feline infectious peritonitis caused by feline coronavirus (FCoV) [79].
Remdesivir targets viral replication and can therefore be used to prevent the progression of COVID-19 from asymptomatic, mild, or moderate cases to the more severe form of the disease [80]. The first COVID-19 case in USA was treated with remdesivir based on disease severity and the therapy progressed without any side effect [81]. The first randomized, double-blind, placebo-controlled clinical trial revealed that faster clinical improvement was observed compared with that in the placebo group; however, a statistically significant difference was not obtained [82]. One of the reasons for early study termination was the occurrence of adverse effects, and therefore, the study did not have a large enough sample size, leading to insufficient evidence to confirm the efficacy of remdesivir [83]. However, in another double-blind, randomized, placebo-controlled trial of intravenous remdesivir therapy in adult COVID-19 patients, improved recovery, reduced time stay (11 days), and decreased mortality (7.1%) was noted in remdesivir treated group indicating beneficial use compared to the placebo group [84]. Further fewer individuals (21.2%) showed side effects as compared to the placebo group (27.0%) [84]. Several randomized, double-blind, placebo-controlled clinical trials that are ongoing to assess the safety and efficacy of remdesivir are expected to have more than 500 COVID-19 patients (NCT04292730, NCT04292899, NCT04315948, NCT04280705, NCT04321616).
Another study assessed the clinical efficacy of remdesivir in COVID-19 patients under mechanical ventilation. Among the 51 patients analyzed, 25 were treated with remdesivir. The findings from the study indicate that the mortality rate in COVID-19 patients under mechanicalventilation is high and the use of remdesivir resulted in better survival in such patients [85]. Remdesivir has previously demonstrated clinical usefulness in severe COVID-19 patients [84, 86]. However, its efficacy has not been widely studied in patients with a moderate form of the disease. Therefore, a randomized, open-label trial was conducted among the hospitalized patients found SARS-CoV-2 positive and showing moderate COVID-19 pneumonia [87]. The patients received either a 10-day course of intravenous remdesivir, a 5-day course of intravenous remdesivir, or standard care. The 5-day course was associated with a significant difference in the clinical profile than standard care. However, this difference was of insignificant clinical importance [87]. At this stage, very limited information is available on the clinical pharmacokinetics and drug-drug interactions (DDIs) of remdesivir. The combination therapy involving remdesivir and P-glycoprotein inhibitors such as azithromycin, hydroxychloroquine, cyclosporine, and amiodarone leads to increased intrahepatocellular concentration resulting in hepatocellular toxicity associated with DDIs [88].
A recent clinical evaluation study of COVID-19 patients used remdesivir at a dose of 200 mg intravenous on day 1, following 100 mg for 9 days. Of the 53 patients, 36 (68%) showed clinical improvement [89]. The antiviral efficacy and cardiotoxicity evaluation study revealed that remdesivir exhibited almost 60-fold higher antiviral activity in cardiomyocytes-derived from human pluripotent stem cells as compared to the Vero E6 cells while inducing moderate levels of cardiotoxicity [90]. The study also identified a potential risk of QT prolongation with a higher concentration of remdesivir compared to the estimated peak plasma concentration indicating a potential for cardiotoxicity. A recently study showed that the early use of injectable remdesivir is very effective to prevent hospitalizations [91].
Recently, remdesivir (Veklury®) is approved for the treatment of COVID-19 in adults and pediatric patients by the US FDA [66].
Favipiravir
Favipiravir, a purine nucleoside, is a guanine analog that may exhibit potential activity against SARS-CoV-2 through the inhibition of RdRp. It causes disturbance in viral RNA synthesis [80,92]. Favipiravir acts as a chain terminator at the viral RNA incorporation site, thereby reducing the viral load [93]. As a prodrug, favipiravir requires metabolic activation in the body via ribosylation and phosphorylation and is then converted to the triphosphate form (favipiravir-RTP) [94]. It is used against influenza A and B. In Japan, it has started to be used in trials on COVID-19 [95]. In addition to the therapeutic use in influenza, favipiravir has revealed a broad spectrum in vitro antiviral potential against life-threatening RNA viruses, like the rabies, Ebola, and the Lassa virus [93]. It has also revealed in vitro antiviral activity for SARS-CoV-2 , however requiring a slightly higher dose than the remdesivir and chloroquine [96]. In silico studies conducted to throw light upon the underlying molecular interactions between favipiravir and the RdRp of coronaviruses revealed that the active form of favipiravir, F-RTP binds the active sites of coronavirus RdRp. In addition to that, F-RTP is also bound to the replicated RNA terminus suggesting a distinct mechanism of action against coronaviruses as compared to influenza [97].
Cai et al. [98] evaluated favipiravir (initial dose 1600 mg orally twice daily, followed by 600 mg twice daily up to day 14) against lopinavir/ritonavir (400/100 mg twice daily for 14 days) along with IFN-α by aerosol inhalation (5×106 U twice daily). Shorter viral clearance, better resolution of lung pathology, and fewer side effects were noted in favipiravir treated patients than those in other groups. Multiple clinical trials have already been registered in China for evaluating the therapeutic potential of favipiravir alone and in combination with tocilizumab and chloroquine in COVID-19 patients [94]. In a clinical trial of 200 patients conducted in China, Watanabe et al. [99] found that COVID-19 patients who received favipiravir tested negative in a relatively short period (4 days) compared with patients not receiving this drug (11 days). Hence, favipiravir may be an ideal drug for compassionate use in COVID-19 patients because of its potential to hinder the RdRp of SARS-CoV-2 [94].
The comparative efficacy of favipiravir and hydroxychloroquine with(out) azithromycin was studied in SARS-CoV-2-infected Syrian hamsters. Although, treatment with a low dose of favipiravir or hydroxychloroquine with(out) azithromycin was not associated with much reduction in virus titers. However, high doses favipiravir therapy was associated with a reduction in infectious virus titers in the lungs that improved lung histopathology findings [100]. Although favipiravir demonstrated antiviral activity at non-toxic doses against SARS-CoV-2 in the small animal models, further studies are warranted to confirm a similar efficacy in humans.
Ribavirin
Ribavirin is a guanosine analog that acts as an inhibitor of RNA synthesis [80]. It is an antiviral drug that has been previously used for the treatment of several viruses, such as the respiratory syncytial virus and hepatitis C virus [101]. Ribavirin binds to the SARS-CoV-2 RNA-dependent RNA polymerase and may therefore be effective against COVID-19 [46]. It has been proven in vitro anti-SARS-CoV-2 actions and was used in SARS and MERS [102]. It has been used in combination with recombinant IFN, and with plasma or antibodies from convalescent patients for treating COVID-19 patients [103].
In a retrospective cohort study, ribavirin therapy was compared with supportive therapy alone in laboratory-confirmed COVID-19 patients. Among the 115 patients studied, 44 received intravenous ribavirin, and 71 received only supportive therapy (control group). The findings from the study indicate that treatment with ribavirin is not associated with improvement in mortality rate as well as negative conversion time for SARS-CoV-2 test suggesting lack of clinical benefits [104]. Ribavirin has also been studied as a combination therapy along with sofosbuvir and daclatasvir in hospitalized adults suffering from moderate COVID-19. The intervention group in this randomized controlled trial (IRCT20200328046886N1) was treated with a combination of 400 mg sofosbuvir, 60 mg daclatasvir, and 1200 mg ribavirin. Although the combination therapy showed a better recovery rate and lower death rates, being a small trial with few patients (24 patients each), the authors have reported an imbalance in the baseline characteristics thereby preventing us from reaching a conclusion [105].
In another clinical trial, ribavirin therapy was compared with the combination therapy with sofosbuvir/daclatasvir in patients with severe COVID-19 illness. The result indicates that combination therapy with sofosbuvir/daclatasvir was associated with a reduced duration of hospital stay (5 days) as compared to ribavirin therapy (9 days). In addition to that, combination therapy was associated with a reduced mortality rate (6%) as compared to ribavirin group (33%) [106]. Therefore, the better recovery rate and lower death rates associated with sofosbuvir/daclatasvir/ribavirin combination therapy can be attributed to the effect of sofosbuvir/daclatasvir alone [105].
Arbidol
Arbidol is an indole-derivative developed by Russia to treat of respiratory viral infections such as influenza [107]. Arbidol and its derivative, arbidol mesylate, may possess potential in vitro antiviral activity against SARS-CoV given their direct antiviral activity in the early stages of viral replication [108]. Arbidol hydrochloride was also found to be effective in treating influenza infection owing to its ability to suppress viral propagation and to modulate inflammatory cytokine expression in in vitro and in vivo studies [109]. The mechanism of action involves the inhibition of virus-mediated fusion, which blocks the entry of the virus into target cells (Figure 1) [107]. Arbidol mesylate was found to possess almost five times stronger antiviral activity than its parent compound in in vitro studies [108]. Arbidol has potential against SARS-CoV-2 as it can block trimerization of the spike glycoprotein which is essential for binding, entry, and fusion of the virus to host cell [110].
Although arbidol is widely used for treating patients with COVID-19 and was still used by several countries, the desired recovery was not achieved [111]. It was believed that Arbidol inhibits the S glycoprotein of SARS-CoV-2 due to the close similarity between hemagglutinin and spike proteins [111]. Several analogues of arbidol that are designed by scaffold morphing and developed using structure-based formulating approaches were found to possess a superior therapeutic profile as compared to the parent compound [112]. However, not all the active compounds of arbidol interact with the active sites in each protein and were inactive against spike glycoprotein [111]. This might be the reason why arbidol does not exhibit the desired activity during clinical studies.
A preliminary study conducted in confirmed COVID-19 patients suggests that treatment with arbidol improved the discharge rate and decreased the mortality rate compared with patients that did not receive arbidol treatment [1]. In a retrospective cohort study conducted in patients with laboratory-confirmed COVID-19, combination therapy using arbidol and lopinavir/ritonavir treatment was found to be linked with favorable clinical responses compared with that after treatment with lopinavir/ritonavir alone [113]. Both these studies had a limited sample size and the findings require verification in a randomized controlled clinical trial.
Danoprevir
Danoprevir is an antiviral drug used in China for treating patients with chronic hepatitis C owing to its ability to inhibit hepatitis C virus protease (HCV protease inhibitor/NS3/4A). Ritonavir, an HIV protease (CYP3A4 inhibitor), enhances the plasma concentration of danoprevir. The replication cycle of SARS-CoV-2 chymotrypsin-like protease exerts a critical role in both viral transcription and replication. It is documented that the chymotrypsin-like protease (3CLpro) associated with SARS-CoV-2 has some structural resemblance to HCV and HIV proteases and the use of danoprevir as a protease inhibitor along with ritonavir may enhance the therapeutic activity against COVID-19 (Figure 2). Studies using a combination of danoprevir and ritonavir, the repurposed drug for treating COVID-19, showed a significant decrease in viral replication after 4–12 days, improved CT images by efficiently reducing the ground glass opacities (GGO) and patchy lesions in the lungs, and causing a gradual reduction in the viral nucleic acid count as shown by RT-PCR [114]. The findings suggested that this combination can be used as a safe, well-tolerated, and efficient treatment protocol [115]. Recently a clinical trial conducted on the use of danoprevir in COVID-19 cases showed its therapeutic potential. It helped in regaining normal body temperature, improved respiratory symptoms, resolved lung lesions, and negative results on two consecutive RT-PCRs [116]. Danoprevir is safe and well-tolerated in all patients [116]. The therapeutic potential of danoprevir-ritonavir was evaluated in a small open-label study (100 mg danoprevir with 100 mg ritonavir twice a day for up to 14 days) (NCT04291729). The available data indicate that the combination of danoprevir-ritonavir is very much efficacious, safe, and well-tolerated by patients with COVID-19. This combination has to be administered during the early period of the viral infection to be effective for patients with milder symptoms [114].
Camostat mesylate and nafamostat mesylate
Serine protease inhibitors such as camostat mesylate target the fusion step of the viral entry into host cells. Entry of SARS-CoV-2 into host cells is facilitated via ACE2 receptor and/or transmembrane serine protease 2 (TMPRSS2) receptors. Camostat mesylate can function as a TMPRSS2 inhibitor that blocks cell surface fusion by downregulating the expression of the spike protein of SARS-CoV-2, thereby blocking the cellular entry of virus [117, 118]. In a previous study, commercially available camostat mesylate was found to block SARS-CoV-2 entry into human bronchial epithelial cells [119], and it has also been demonstrated that clinically proven protease inhibitors, such as E-64d (a cysteine protease inhibitor) and camostat mesylate can proficiently block SARS-CoV-2 from binding to TMPRSS2 [120, 121].
Nafamostat mesylate, another serine protease inhibitor, has been shown to have superior inhibitory activity (15-fold higher than that of camostat mesylate) with respect to preventing SARS-CoV-2 entry into host cells. Moreover, it has a better safety profile, and therefore, may be a preferable alternative to camostat mesylate [120]. Nafamostat mesylate also has an additional advantage in that it is used for treating disseminated intravascular coagulation (DIC), and hence might prove useful for therapeutic purposes in COVID-19 patients who manifest DIC with enhanced fibrinolysis [122].
Nafamostat mesylate has been used to treat critically ill COVID-19 patients in combination with favipiravir. The results of the case series indicate that the combination therapy aided in reducing the mortality rate [123]. Nafamostat mesylate when used in combination with favipiravir may block virus entry as well as subsequent replication in the host cell. In addition to directly targeting the virus entry, nafamostat mesylate also inhibits intravascular coagulopathy, therefore can be considered beneficial for COVID-19 patients [123]. However, serious adverse events, such as CNS and bleeding complications that are associated with nafamostat mesylate therapy and its combination with favipiravir require careful consideration in the case of COVID-19 patients [124]. This is important as both the drugs (nafamostat mesylate and favipiravir) were developed and used primarily in Japan.
Baricitinib
The entry of SARS-CoV-2 into the host cells is achieved via receptor-mediated endocytosis, a phenomenon regulated by AP2-associated protein kinase 1 (AAK1). Accordingly, interference of AAK1 has been found to inhibit viral entry, and the process of intracellular viral assembly [125]. Baricitinib is a Janus kinase inhibitor (JAK) that can inhibit AAK1 [126], and can thus be used for blocking SARS-CoV-2 entry into a host cell. Moreover, it can inhibit the inflammatory response associated with infection [126].
Baricitinib has been previously used in patients with rheumatoid arthritis, atopic dermatitis, and active systemic lupus erythematosus with impressive efficacy and safety records [127]. Therefore, baricitinib can be used to block SARS-CoV-2 entry into the pneumocytes and to prevent cytokine storm in SARS-CoV-2 patients [127, 128]. The role of baricitinib was assessed in patients treated with high-dose corticosteroids using an observational study. The addition of baricitinib into the treatment regime was found to improve pulmonary function in COVID-19 patients with moderate to severe pneumonia as compared to corticosteroids alone [128]. Furthermore, the immunologic and virologic efficacy of baricitinib was studied in SARS-CoV-2 non-primate model (rhesus macaque model). Treatment with baricitinib was found to be associated with limited lung pathology, reduced immune activation, and decreased neutrophil infiltration into the lungs. In addition to that, there was a suppression in the production of cytokines and chemokines by alveolar macrophages indicating beneficial anti-inflammatory activity that can be used for treating COVID-19 [129]. The use of baricitinib is also associated with important adverse reactions such as abnormal blood routine and elevated liver enzymes [127]. However, it is currently unknown whether the adverse reactions will occur when used as a therapeutic for COVID-19 due to the short course of treatment. Therefore, baricitinib should be administered cautiously in patients with the above risk factors. Further investigations are needed to assess the safety of baricitinib in pregnant women and patients with renal insufficiency [127].
Ruxolitinib and fedratinib, two other JAK inhibitors that are very much related to baricitinib, inhibit clathrin-mediated endocytosis but only at higher doses, therefore may not be effectual at tolerable doses [130]. JAK-STAT signal inhibition by baricitinib, a selective JAK1, and JAK2 inhibitor, results in impairment of IFN-mediated antiviral response, thus having prospects to prevent SARS-CoV-2 infection [131]. However, one major factor that limits the use of baricitinib in COVID-19 patients is the occurrence of adverse side effects, such as lymphocytopenia, neutropenia, and viral reactivation. Its use would thus be detrimental for COVID-19 patients, who already manifest lower absolute lymphocyte count, as it is likely to augment the incidence of co-infection [132]. Further trials are therefore mandatory to establish whether baricitinib therapy can have any clinical utility in the treatment of COVID-19. Synergistic potential has been noted between remdesivir and baricitinib when used as combination therapy in COVID-19 patients [130, 131].
Recently, baricitinib (Olumiant®) is approved for treatment of COVID-19 in hospitalized adults patients [66].
Teicoplanin
Teicoplanin is a glycopeptide antibiotic that has been used routinely for treating bacterial infections caused by staphylococci [133]. This compound was previously found to inhibit the cellular entry of viruses such as Ebola, MERS-CoV, and SARS-CoV [134]. Teicoplanin specifically inhibits the activity of cathepsin L and consequently blocks viral entry into cells. Hence, this glycopeptide compound has potent inhibitory activity against cathepsin L-dependent viruses [135]. Teicoplanin is found to be the most effective drug with an IC50 value of approximately 1.5 µM [136]. Studies have also identified a relatively high affinity between Teicoplanin and SARS-CoV-2 3CLpro indicating good interaction. Teicoplanin possesses about 10-20-fold more protease inhibition activity than drugs such as hydroxychloroquine, chloroquine, lopinavir, azithromycin, and atazanavir making it a more promising therapeutic candidate for COVID-19 [136]. Treatment with teicoplanin will therefore decrease the proteolytic activity of SARS-CoV-2 3CLpro. Since 3CLpro plays an irreplaceable function in the processing of viral polyproteins, it can be confirmed that the anti-SARS-CoV-2 potential of teicoplanin is mediated via the inhibition of viral replication.
Teicoplanin derivatives, such as telavancin, dalbavancin, and oritavancin, also possess similar inhibitory activity against Ebola, MERS-CoV, and SARS-CoV viruses [134]. A preliminary study on SARS-CoV-2 reflects that the compound may be effective against the virus by preventing viral entry (Figure 1) [135]. Further investigations are required to evaluate the antiviral potential of teicoplanin against SARS-CoV-2. It has been recommended in complicated infections of SARS-CoV-2 including those with Staphylococcus aureus and has shown improvement in clinical cases including in critical cases [137, 138].
Chloroquine and hydroxychloroquine
Chloroquine and hydroxychloroquine are two aminoquinoline drugs with similar modes of action that are routinely used in treating malaria and autoimmune conditions. The two drugs differ structurally in that hydroxychloroquine possesses a hydroxyl group at the end of a side chain in which an N-ethyl group is substituted by a β-hydroxylated group [139]. Of the two, hydroxychloroquine is generally more preferable, owing to its superior safety profile and lower toxicity after long-term use [140]. Given its antiviral and anti-inflammatory activities, chloroquine-based treatment is believed to offer a prospective strategy for managing COVID-19-induced pneumonia [141]. It has been used in mild, moderate, and severe COVID-19 cases [48]. There are more than 80 trials registered trials with chloroquine and hydroxychloroquine-based treatment in COVID-19 patients with some showing good results and some having raised concerns [142, 143]. The pharmacological activities of chloroquine and hydroxychloroquine against COVID-19 were recently evaluated in in vitro studies employing SARS-CoV-2-infected Vero cells, the findings of which have indicated that hydroxychloroquine has a superior in vitro inhibitory potential against SARS-CoV-2 [144]. Combination therapy has also been found useful to reduce viral load in COVID-19 patients [145].
COVID-19 patients were administered 600 mg of hydroxychloroquine daily, which caused a substantial reduction of viral load and eventually helped to gain complete recovery [145]. In order to ensure more reliable results, it has been recommended to administer a loading dose of hydroxychloroquine along with a maintenance dose [146]. However, the sample size upon which these findings were based was notably very small, and thus the possibility of misinterpretation cannot be discounted. Although hydroxychloroquine sulfate tablets are already registered by the FDA for treating diseases such as malaria, rheumatoid arthritis, and lupus erythematosus [147], hydroxychloroquine is yet to receive FDA approval for use as a therapeutic agent in managing COVID-19. Nevertheless, it has also been found that the addition of azithromycin to the hydroxychloroquine protocol has a reinforcing effect, which enhances the efficiency of viral elimination [145]. The synergistic effect of hydroxychloroquine and azithromycin combination against SARS-CoV-2 has been further validated based on in vitro evaluations [125]. Moreover, the concentrations of hydroxychloroquine and azithromycin that are required when used in combination to achieve in vitro viral inhibition can be replicated in vivo, both in serum and pulmonary tissues [125]. The beneficial effects of azithromycin in COVID-19 patients are suspected to be mediated via its interference with ligand-CD147 receptor interactions, thereby reducing the viral load [40].
With respect to the efficacy of chloroquine, it is suspected that this drug may interfere with ACE2 receptor glycosylation, thereby limiting the SARS-CoV-2 binding to target host cells. It is also conceivable that chloroquine suppresses the biosynthesis of sialic acid receptors necessary for the cell surface binding of SARS-CoV-2. If, however, some viral particles do succeed in binding, chloroquine can modulate acidification of endosomes, thus inhibiting the development of autophagosomes (Figure 1). Furthermore, based on a reduction in cellular mitogen-activated protein (MAP) kinase activation, it is conjectured that chloroquine might also inhibit viral replication or can modify M protein maturation and hinder virion assembly and budding. Finally, chloroquine may act indirectly via dropping the pro-inflammatory cytokines production and/or by potentiating anti-SARS-CoV-2 CD8+ T-cells.
Recent reports have demonstrated that, along with remdesivir, chloroquine can be used for inhibiting SARS-CoV-2 in vitro, thus the use of these drugs for treating COVID-19 patients has accordingly been advocated [148, 149].
Overall, it appears that chloroquine functions as a hurdling molecule at various steps in the life cycle of SARS-CoV-2. It is presumed that the drug initially prevents the binding of SARS-CoV-2 to its target cells by hindering the glycosylation of the ACE2 receptor, and can further prevent the biosynthesis of sialic acid receptors on the surface of cells that are required for the binding of the SARS-CoV-2. If, however, the virus succeeds in breaching this first line of defense, and owing to being bound to the cell surface gains access to the cell interior, then chloroquine may mobilize a second line of defense by altering the acidification of endosomes, thereby contributing to the inhibition of the autophagosome formation. Moreover, chloroquine can also affect virion assembly and budding by modulating M protein maturation [139]. Despite these attributes, however, studies have revealed that hydroxychloroquine is a more potent preventive and therapeutic alternative as a repurposed drug. Nevertheless, compared to the spike protein of SARS-CoV-2 , chloroquine, or preferably hydroxychloroquine, can bind with higher affinity to gangliosides and sialic acids present on the surface of the host cell, thereby blocking potential virus binding sites on cells of the upper and lower respiratory tract [150].
However, even though the initial results of treatment using chloroquine and hydroxychloroquine appear promising, the scientific community has voiced several concerns regarding the immediate implementation of these two drugs in the management of COVID-19 based solely on results obtained from the preliminary studies. Our understanding of COVID-19 pathogenesis is still rudimentary, and therefore at present, it cannot be confidently guaranteed that administration of chloroquine/hydroxychloroquine to COVID-19 patients would not provoke an adverse immune response [151]. Given that the recommended clinical dosage and course of treatment using chloroquine phosphate in COVID-19 patients are larger than those used for the treatment of malaria [152], close monitoring of patients who are under treatment with chloroquine phosphate would be necessary to detect any signs of adverse reactions.
Despite the in vitro findings confirming the ability of chloroquine to inhibit SARS-CoV-2 replication [144, 153], and the fact that the drug has also shown significant in vitro activity against several viruses, there is still limited evidence as to its benefits in animal models [153]. The in vivo potential of chloroquine against SARS has previously been evaluated using a SARS-CoV-2 replication model in mice, but this failed to establish any inhibitory effect on viral replication [154]. Hence, further large-scale clinical trials are required before including these drugs in the therapeutic guidelines. Recently, the prophylactic role of chloroquine and hydroxychloroquine has been demonstrated in the absence of any risk of resistance [155]. Thus, although chloroquine and hydroxychloroquine are being enthusiastically promoted as two of the most promising drugs for combatting COVID-19, there have been relatively few clinical trials conducted and recoveries reported, and thus rigorous assessments of adverse effects, such as QT prolongation, ventricular arrhythmias, and other cardiac toxicities, and well as deaths, are necessary before giving final approval for their usage [156].
Both chloroquine and hydroxychloroquine can cause retinal toxicity in case of prolonged use and this is well documented during the long-term management of lupus erythematosus and other rheumatoid diseases [157, 158]. However, the proposed doses of chloroquine and hydroxychloroquine for treating COVID-19 are 4-5 times higher, duration of treatment is shot. Therefore, may not be a concern for the physician [158]. Further surveillance is required to identify the gastrointestinal, cardiologic, and neuropsychiatric side-effects that might be exhibited in COVID-19 patients treated with hydroxychloroquine. Considering this the clinical trials should evaluate the long-term effects of chloroquine and hydroxychloroquine treatment in COVID-19 patients, such as anxiety, sleeplessness, cardiomyopathy, muscle weakness, and gastrointestinal disorders [159]. Being a lysosomotropic agent, chloroquine and hydroxychloroquine may also further worsen acute kidney injury and other organ failures owing to their capability to elevate lysosomal pH and to inhibit autophagy. Therefore, chloroquine should be considered as a double-edged sword that slows the infection in the early stages but may potentiate tissue damage in the later stages by inhibiting autophagy [160]. Even though initial evidence pointed towards the therapeutic use of hydroxychloroquine in COVID-19 patients, current evidence are not supportive of this fact. In an observational study conducted on COVID-19 patients who were hospitalized, administration of hydroxychloroquine was not found to be associated with any clinical benefits [161]. However, this cannot be considered as conclusive evidence and requires randomized, clinically controlled trials.
Though there have been quite progressive in understanding and evaluating the safety of chloroquine and hydroxychloroquine in COVID-19 treatment with a rise in utilization and increased global demand resulting in a shortage of supply on one hand however concerns including increased mortality in some trials has resulted in the stoppage of trials halfway on the other hand which need to be taken care of in future also [162]. Fatal cardiomyopathy, severe hypoglycemia with loss of consciousness, QT interval prolongation, severe cutaneous reactions, and irreversible retinal damage are some of the adverse toxic effects of these drugs. Hydroxychloroquine has garnered great interest as a repurposed drug for treating COVID-19. However, the extreme enthusiasm related to the use of hydroxychloroquine can be linked to politicization rather than a science-based approach. This is the same enthusiasm that is responsible for continuing scientific investigations surrounding the use of hydroxychloroquine in COVID-19 patients [163]. Among the 300 hydroxychloroquine clinical trials registered, less than 50% have recruited any patients, and the majority of them failed to achieve the intended sample size. Yet, the investigators failed to prove the therapeutic potential of hydroxychloroquine against COVID-19 [163].
Ivermectin
Ivermectin is an endectocide antiparasitic drug that is routinely used in veterinary science. Previous reports have found that ivermectin exhibits antiviral activities against influenza [164], HIV [165], dengue viruses [166], West Nile virus [167], and Venezuelan equine encephalitis virus [168]. The broad-spectrum antiviral activity exhibited by ivermectin against several animal and human viruses are mediated via targeting major components/processes like importin α/β-mediated nuclear transport, nuclear import of UL42, NS3 helicase, and nuclear localization signal-mediated nuclear import of Cap [169]. Recently, the findings of an in vitro study, which revealed that ivermectin reduced viral load by approximately 5000-fold in a cell culture system, have indicated that this drug may also be useful antiviral for treating SARS-CoV-2 [170].
It has been demonstrated that ivermectin inhibits the nuclear import of host and viral proteins [171], inhibits RNA viruses by inhibiting viral replication [170]. However, although the nuclear transport inhibitory activity of ivermectin can prove effective against SARS-CoV-2 [170], there are concerns that the in vitro inhibitory concentrations of ivermectin may not be reproducible in humans [172], owing to the potential toxicity of this drug [173]. Ivermectin has been revealed to be a potent SARS-CoV-2 inhibitor, with an IC50 value of approximately 2 µM under in vitro conditions; however, achieving such a dose clinically in human beings is evidently difficult [170, 174]. The IC50 concentration of the ivermectin reported in the in vitro study is almost >35x higher as compared to the maximum plasma concentration that can be achieved by delivering an oral dose of ivermectin that is both approved and safe for human use [175].
The treatment of seriously ill COVID-19 patients with standard doses of ivermectin was not associated with better clinical and microbiological outcomes than the patients receiving standard care [176]. Therefore, further randomized trials should include a separate arm for high-dose ivermectin therapy to assess the safety and efficacy of ivermectin against SARS-CoV-2. However, studies conducted in patients with acute myeloid leukemia have reported that high doses of ivermectin are safe when administered as a treatment regimen for refractory acute myeloid leukemia for prolonged periods in pediatric patients [177].
Accordingly, the in vivo potential of ivermectin, which is yet to be elucidated, may disclose other facets of therapy based on this drug. A further major concern associated with ivermectin therapy concerns its co-administration with lopinavir/ritonavir and darunavir/cobicistat, which are considered to be potent inhibitors of cytochrome P450 3A4, a component of the main metabolic pathway upon which ivermectin acts. Such co-administration would thus increase the systemic concentrations of ivermectin, and thereby enhance the likelihood of toxicity [173]. Nevertheless, it has also been suggested that a therapy based on the combination of ivermectin and hydroxychloroquine might have a beneficial synergistic result, given that hydroxychloroquine would function as a first-level barrier that inhibits viral entry into the host cell, whereas ivermectin would inhibit viral replication within the cell, thereby strengthening the antiviral activity [178]. Considering the paucity of relevant data, however, it is at present too early to enable an adequate assessment of the therapeutic efficacy of ivermectin against SARS-CoV-2 infection, and therefore further clinical trials are necessary to be conducted. In clinical trial ivermectin and nitazoxanide were used in combination for treating COVID-19 patients (NCT04360356).
Nitazoxanide
Nitazoxanide is an FDA-approved antiparasitic drug that possesses broad-spectrum antiviral potential against as influenza, coronaviruses, hepatitis B virus, hepatitis C virus, and other viruses [179]. The preliminary findings from the in vitro studies indicate that reported that nitazoxanide inhibits SARS-CoV-2 (SARS-CoV-2/Wuhan/WIV04/20192) in Vero E6 cells at a low-micromolar concentration indicating potent antiviral activity [180]. In addition to the direct antiviral action, nitazoxanide can suppress the synthesis of pro-inflammatory cytokines, including IL-6, and thus can be used for managing COVID-19-induced cytokine storm [179]. Nitazoxanide is presently being assessed in a clinical trial (NCT04341493) as a combination therapy along with hydroxychloroquine in COVID-19 patients with underlying risk conditions (hypertension, diabetes mellitus, and morbid obesity) for poor prognosis [181]. The patients receiving combination therapy will be given 500 mg nitazoxanide orally every 6 hours along with food, for a period of seven days.
One of the major advantages of nitazoxanide as compared to other repurposed drugs is the high ratio of maximum plasma concentration (Cmax) to the effective concentration to inhibit 50% replication, roughly equal to 14:1 for SARS-CoV-2. This high ratio is attained after a single day treatment with nitazoxanide (500 mg twice daily) [149]. In addition to that, physiologically-based pharmacokinetic (PBPK) modelling was used to analyze the optimal nitazoxanide doses that are sufficient for maintaining plasma and lung tizoxanide (a major circulating metabolite of nitazoxanide) exposures beyond the reported 90% effective concentration (EC90) against SARS-CoV-2 [182]. The findings indicate that it is possible to achieve effective concentrations of tizoxanide in plasma and lungs using established safe doses of nitazoxanide and this drug can be potentially used for treating COVID-19. Another important advantage of nitazoxanide therapy is the low overall cost of treatment. It is estimated that nitazoxanide can be manufactured as a generic drug at the cost of $1.41 for a 14-day treatment course given at a dose of 500 mg BD and at a cost of $4.08 when given at a higher dose of 1100 mg three times daily [149]. Nitazoxanide can also be applied in combination with azithromycin for the early management of COVID-19 and can even replace hydroxychloroquine/azithromycin combination due to the superior safety profile of this combination [183].
Molnupiravir
Molnupiravir is a nucleoside analog that inhibits the replication of SARS-CoV-2. It is lethally mutagenic against RNA of SARS-CoV-2 [184]. Recently, molnupiravir (Lagevrio®) is EUA approved for the treatment of COVID-19 in adults and pediatric patients by the US FDA [66].
The potential therapeutic drugs that might have anti-SARS-CoV-2 activity based on the results obtained from in vitro studies, virtual screening, or in silico studies are presented in Table 1.
Table 1. Compounds with possible anti-SARS-CoV-2 activity based on in vitro studies, virtual screening, or in silico studies.
| Drug | Drug target/drug class | Mechanism | Reference(s) | |
|---|---|---|---|---|
| Nelfinavir | SARS-CoV-2 main protease (Mpro) | Inhibition of the viral protease enzyme | [185] | |
| HTCC polymer a | Spike protein | Blocks interaction of spike protein with cellular receptor | [186] | |
| Theaflavin | Receptor binding domain (RBD) | Binds to the RBD of spike protein preventing viral entry | [187] | |
| Dipyridamole | SARS-CoV-2 Mpro | Inhibitor of Mpro and NF-κB signaling pathway | [188] | |
| Niclosamide | SKP2 | Inhibition of SKP2 activity thereby enhancing autophagy and reduces replication | [189, 190] | |
| Ciclesonide | Viral riboendonuclease NSP15 | Blocks replication of SARS-CoV-2 (direct-acting antiviral) and its anti-inflammatory activity | [190, 191] | |
| EIDD-2801 b | Ribonucleoside analog | Lethal mutagenesis causes the accumulation of deleterious transition mutations in the viral RNA | [192, 193] | |
| 3’-fluoro-3’-deoxythymidine triphosphate c | RNA-dependent RNA polymerase (RdRp) | Gets incorporated into SARS-CoV RdRp and terminates further polymerase extension. | [194] | |
| 3’-azido-3’-deoxythymidine triphosphate d | RdRp | Gets incorporated into SARS-CoV RdRp and terminates further polymerase extension. | [194] | |
| CVL218 | N-terminal domain of nucleocapsid (N) protein | Inhibits SARS-CoV-2 replication (antiviral) and suppress the CpG-induced IL-6 production (anti-inflammatory) | [195] | |
| Atazanavir | RdRp, Helicase, 3'-to-5' exonuclease , 2'-O-ribose methyltransferase, and endoRNAse | Inhibits the subunits of SARS-CoV-2 replication complex thereby inhibiting viral replication | [196] | |
| Prulifloxacin | Viral main protease (Mpro) | Inhibits viral replication and proliferation | [197] | |
| Tegobuvir | Viral main protease (Mpro) | Inhibits viral replication and proliferation | [197] | |
| Bictegravir | Viral main protease (Mpro) and 2′-O-ribose methyltransferase (2′-O-MTase) | Inhibits viral replication and proliferation | [36, 197] | |
| Sofosbuvir | RNA-dependent RNA polymerase (RdRp) | Binds to RdRp and inhibits further RNA chain extension thereby halting RNA replication. | [198] | |
| Camostat mesylate | Transmembrane serine protease family member II (TMPRSS2) | Blocks SARS-CoV-2 entry into the host cell | [120] | |
| Velpatasvir | 3C-like protease (3CLpro) | Inhibits viral replication and proliferation | [199] | |
| Ledipasvir | 3C-like protease (3CLpro) | Inhibits viral replication and proliferation | [199] | |
| Colistin | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Valrubicin | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Icatibant | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Bepotastine | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Epirubicin | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Epoprostenol | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Vapreotide | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Aprepitant | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Caspofungin | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Perphenazine | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [200] | |
| Selamectin | Unknown | Unknown | [201] | |
| Mefloquine hydrochloride | Unknown | Unknown | [201] | |
| Eravacycline | SARS-CoV-2 protease | Inhibits SARS-CoV-2 replication | [202] | |
| Valrubicin | SARS-CoV-2 protease | Inhibits SARS-CoV-2 replication | [202] | |
| Elbasvir | SARS-CoV-2 protease | Inhibits SARS-CoV-2 replication | [202] | |
| Ebselen | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [203] | |
| Saquinavir | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [204] | |
| Beclabuvir | SARS-CoV-2 Mpro | Inhibits SARS-CoV-2 replication | [204] | |
| Isoniazid pyruvate | Spike protein-ACE2 receptor complex | Limits binding of SARS-CoV-2 spike protein with ACE2 receptor | [205] | |
| Nitrofurantoin | Spike protein-ACE2 receptor complex | Limits binding of SARS-CoV-2 spike protein with ACE2 receptor | [205] | |
| Eriodictyol | Spike protein-ACE2 receptor complex | Limits binding of the SARS-CoV-2 spike protein with ACE2 receptor | [205] | |
| Cepharanthine | Virus spike protein host recognition domain | Disrupts the host-virus interactions | [205] | |
| Ergoloid | Virus spike protein host recognition domain | Disrupts the host-virus interactions | [205] | |
| Hypericin | Virus spike protein host recognition domain | Disrupts the host-virus interactions | [205] | |
| Carfilzomib | SARS-CoV-2 3C-like proteinase (3CLpro) | Inhibits SARS-CoV-2 replication | [206] | |
| Thymopentin | SARS-CoV-2 3CLpro | Inhibits SARS-CoV-2 replication | [206] | |
| Ivermectin | Impα/β1 heterodimer | Destabilizes Impα/β1 heterodimer and prevents its binding to the viral protein thereby preventing it from entering the nucleus leading to more efficient antiviral response | [170] | |
| Grazoprevir | SARS-CoV-2 papain-like protease (PLpro) | Inhibits SARS-CoV-2 replication | [207] | |
| Telaprevir | SARS-CoV-2 PLpro | Inhibits SARS-CoV-2 replication | [207] | |
| Boceprevir | SARS-CoV-2 PLpro | Inhibits SARS-CoV-2 replication | [207] | |
| Mycophenolic acid | SARS-CoV-2 PLpro | Inhibits SARS-CoV-2 replication | [207] | |
| Cyclosporin A | Cyclophilin A (CypA) | CypA inhibitor (CypA is essential for replication) | [39] | |
| Cefuroxime | RdRp | Inhibits SARS-CoV-2 RdRp | [208] | |
| Tenofovir | RdRp | Inhibits SARS-CoV-2 RdRp | [208] | |
| Setrobuvir | RdRp | Inhibits SARS-CoV-2 RdRp | [208] | |
| Andrographolide | SARS-CoV-2 main protease (Mpro) | Inhibits SARS-CoV-2 replication | [209] | |
| Dolutegravir | 2′-O-ribose methyltransferase (2′-O-MTase) | Inhibits 2′-O-MTase (Inhibiting methylation of ribose 2′-O position of first and second nucleotide of viral mRNA, which sequesters it from host immune system) | [36] | |
| Paritaprevir | SARS-CoV-2 3CLpro | Inhibits SARS-CoV-2 replication | [36] | |
| Raltegravir | SARS-CoV-2 3CLpro | Inhibits SARS-CoV-2 replication | [36] | |
| GC373 | SARS-CoV-2 3CLpro | Inhibits SARS-CoV-2 replication | [210] | |
| GC376 | SARS-CoV-2 3CLpro | Inhibits SARS-CoV-2 replication | [210] | |
| Boceprevir | SARS-CoV-2 3CLpro | Inhibits SARS-CoV-2 replication | [211] | |
| Tretinoin | SARS-CoV-2 E protein ion channel | Inhibitors of ion channels formed by SARS-CoV-2 E protein and virus assembly Inhibitor | [212] | |
| Sovaprevir | SARS-CoV-2 receptor-binding domain (RBD) | Binds to RBD of spike protein, prevents viral entry | [213] | |
| Elbasvir | SARS-CoV-2 RBD | Binds to RBD of spike protein, prevents viral entry | [213] | |
| Grazoprevir | SARS-CoV-2 RBD | Binds to RBD of spike protein, prevents viral entry | [213] | |
| Hesperidin | SARS-CoV-2 RBD | Binds to RBD of spike protein, prevents viral entry | [213] | |
| Pamaqueside | SARS-CoV-2 RBD | Binds to RBD of spike protein, prevents viral entry | [213] | |
| Diosmin | SARS-CoV-2 RBD | Binds to RBD of spike protein, prevents viral entry | [213] | |
| Sitogluside | SARS-CoV-2 RBD | Binds to RBD of spike protein, prevents viral entry | [213] |
aN-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride, bβ-d-N4-Hydroxycytidine (NHC), cActive triphosphate forms of alovudine, dActive triphosphate forms of azidothymidine
Immunotherapeutics and immunomodulatory
Convalescent plasma
Convalescent plasma (CP) is another antibody-based immunotherapeutic strategy with proven safety records and is currently being used to manage the COVID-19 pandemic [214]. Although CP is an ancient therapeutic technique, it can provide immediate protection and complete cure from COVID-19 [215]. CP is collected from patients previously infected with SARS-CoV-2 and recovered, resulting in the development of specific neutralizing antibodies [216]. Treatment with CP can facilitate early recovery, reduce viral load, minimize disease severity, and mortality [214,217]. However, plasma therapy might also be associated with complications, such as allergic reactions, anaphylaxis, transfusion-induced acute lung injury, pulmonary oedema, hemolytic transfusion reactions, and antibody-dependent enhancement (ADE) [214, 215].
CP is one of the passive immunization strategies used for managing COVID-19 long before its efficacy was established using randomized clinical trials [216]. The CONFIDENT trial (NCT04558476) is an -open-label two-arm randomized superiority trial conducted to determine CP's effectiveness in mechanically ventilated patients. The study that is planned to include 500 adults will analyze CP's ability to reduce the mortality and viral load of SARS-CoV-2 in COVID-19 patients [216]. Immunosuppressed patients are the major important patients that are highly benefited by CP therapy. The patients with hematologic malignancy and those who have undergone organ transplantation appear at higher risk for COVID-19 mortality. Therefore, CP offers a passive immunization method that can prevent COVID-19 in immunocompromised patients [218]. The presence of antibodies in convalescent plasma exhibits their therapeutic potentialities via different mechanisms. In one pathway, binding of antibodies to viral pathogens directly neutralizes its infectivity, whereas other antibody-assisted ways, including phagocytosis, antibody-mediated cell cytotoxicity, and complement activation might also drive its therapeutic effects [219]. Binding of non-neutralizing antibodies to the given pathogens without interfering with their replication ability is also considered responsible for preventing and increasing recovery [220, 221]. The administration of passive antibodies offers only a short-range approach to confer instantaneous immune responses to vulnerable individuals, particularly in the case of emerging disease, i.e., COVID-19. On the other hand, vaccination and recovered plasma products may constitute a long-term therapeutic choice [222].
Several studies have revealed a shorter hospital stay along with a low mortality rate in persons after treatment with convalescent plasma compared to non-treated patients [223-225]. In 2014, WHO has recommended using convalescent plasma as a realistic therapy for Ebola disease recovered patients. In a non-randomized prospective study conducted in Kuwait that involved 135 patients with moderate or severe COVID-19, treatment with CP was associated with a higher rate of clinical improvement than the control group that received standard treatment [226]. In another study, treatment with CP was found to reduce the intensive care unit (ICU) stay and the requirement for mechanical ventilation support than the control group [227]. The concentration of neutralizing antibodies varies among the convalescent patients. This difference will impact the overall efficacy of CP transfusion and therefore requires quantification to standardize the therapy [228]. It was found that women and younger donors have only measurable quantities of neutralizing antibodies, while high antibody titers were observed in older male donors recovered from previous SARS-CoV-2 infection [229]. Although the neutralizing potential of human CP can be measured using different methods, surrogate virus neutralization assay can be considered a better choice as it is a rapid technique that requires only the lowest biosafety level. However, the surrogate virus neutralization assay sometimes over valuates the low neutralizing plasma [228]. Many studies have demonstrated the use of convalescent plasma in China's current pandemic for the treatment of COVID-19 patients [82, 219, 230-232]. In a pilot-scale study, Duan and coworkers collected convalescent plasma with a titer of neutralizing antibodies at or above 1:640 dilution [233]. Administration of the collected plasma showed no adverse consequences in the ten seriously ill COVID-19 patients. All the recipients displayed a notable improvement in clinical symptoms, like fever, cough, chest pain, and short breath along with radiological pulmonic improvement in 1 to 3 days of administration. The outcomes were also corroborated in another study, where transfusion of convalescent plasma in five COVID-19 patients also showed improved clinical symptoms, like eliminating mechanical aeration, reduced virus burden, clinical steadiness and increased oxygen concentration [82]. These results recommend the broader implementation of convalescent plasma as a safe therapy for reducing viral load and improving clinical status [234]. However, the executions of additional randomized trials are necessitated to substantiate the potential effectiveness and safety of convalescent plasma [234, 235].
SARS-CoV-2-specific antibody titers were lower in asymptomatic patients and those patients who were tested 60 days after the onset of symptoms. However, the hospitalized patients with COVID-19 had high titers of neutralizing antibodies with a strong potential to neutralize the active SARS-CoV-2 virus. Therefore, convalescent plasma donors should be screened to confirm high RBD antibody titers [236]. The efficacy and clinical outcome of CP therapy is largely reported based on observational data, and very limited data are available from adequately powered RCTs [237]. A study reported the finding obtained from an RCT (NCT04383535) conducted to assess the efficacy of CP therapy in hospitalized adults with severe COVID-19 pneumonia [237]. However, they could not identify any significant difference in clinical status or overall mortality between CP treatment and placebo groups [237]. Although several RCTs are underway to establish the efficacy of CP therapy in managing COVID-19, it is challenging to conclude soon. This can be attributed to the high methodological variabilities in inclusion criteria, donor selection, outcomes, times of transfusion, dosage, and the concentration of neutralizing antibodies [238].
The safety of CP transfusion was evaluated in the sample containing 20,000 patients with COVID-19. The study reported a very low incidence of serious adverse events, such as cardiac events ∼3%), thromboembolic or thrombotic events (<1%), and transfusion reactions (<1%) [239]. Among the reported adverse effects, the vast majority was found unrelated to the CP transfusion. The success of CP therapy depends on the availability and accessibility to suitable plasma donors. However, for CP therapy to be successful, the ratio of recovered cases to plasma donors should be enough to provide rapid accessibility [240]. The efficacy of CP therapy mainly depends upon several factors, such as the volume of transfusion, time of administration, and neutralizing antibody titers [241]. Transfusion with CP will be more beneficial, especially if performed within the first 20 days of infection [227, 239]. However, the optimal dose must be estimated that is both safe and efficient in patients with COVID-19 with well-designed randomized clinical studies.
Natural killer (NK) cell-based therapy
NK cells are specialized large granular lymphocytes characterized by their unique ability to kill tumor and virally infected cells and they express the CD56 molecule on the surface [242]. It is different from the B cells and T cells of the adaptive immune system. Furthermore, NK cells belong to the innate immune system which does not have the T cell receptor on the surface and they can act without any MHC molecule mediated antigen recognition [243]. Due to their unique ability to kill the virus infected cells, they are proposed as a possible therapeutic approach to counter COVID-19. In COVID-19 patients, a reduction in the number of NK cells, impairment of their functional activity such as IFN-γ production, increased expression of inhibitory receptors (e.g., NKG2A) have been observed, which resulted in decreased clearance of virus-infected cells and excessive tissue-damaging inflammation. It has been suggested restoration of NK cell function could bring a balance in the immune response for effective elimination of SARS-CoV-2 infection [244]. NK cell therapy is being tried for treating COVID-19 in many clinical trials. An allogeneic, off-the-shelf, cryopreserved NK cell therapy made by Celularity (CYNK-001) is approved by the FDA for clinical testing in COVID-19 patients. The phase-I trial (NCT04365101) is aimed to evaluate the occurrence and severity of toxicity in mild, non-ICU COVID-19 patients (n=14) following injection of NK cells. The phase-II trial (72 patients) is planned to understand the efficacy of NK cell therapy compared with standard of care as a comparator at a 1:1 allocation [33].
A recent report displayed the strong activation of different subsets of NK cells in patients with COVID-19 using 28-color flow cytometry [245]. The study revealed that specific NK cell immunotypes can be linked with disease severity. Furthermore, the increased expression of NKG2C, Ksp37, and perforin, indicates an increase in the number of adaptive NK cells that are circulating in patients with severe disease [245]. Immunological characterization of 71 COVID-19 patients showed a sharp decrease in CD16+ CD56+ NK cells that are circulating in the peripheral blood of all the infected patients, however, the decrease was highly significant in severely ill patients [246]. The study also identified the major role played by CD16+ CD56+ NK cells in the inflammation and cytokine storm. The dysregulated NK cells along with other immune cells lead to the induction of inflammatory cascade which lead to exaggerated inflammation which is one of the hall marks of COVID-19 severity. Ex vivo manipulated NK cells for the desired immunomodulated function can be beneficial for managing COVID-19 patients. CAR-NK cell therapy using off-the-shelf human umbilical cord blood derived NK cells expressing NKG2D and ACE2 CARs (NCT04324996) is currently being tested as Phase I/II study in early stage COVID-19 patients who are within 14 days of acquiring infection. This trial is a complex five-arm study compared the effectiveness of various CAR-NK products including NK cells, NKG2D CAR-NK cells, NK cells secreting IL-15, NKG2D-ACE2 CAR-NK, and ACE2 CAR-NK cells to understand the safety and efficacy [33].
In another study, the safety and efficacy of NK cells for treating patients with COVID-19 has been evaluated in combination with standard therapy, which showed an improvement in the clinical parameters and decreases in adverse events including a decrease in time of negative test, improvement in CD4+/CD8+ counts, decrease in pathological lesions in lungs, and decrease in mortality. Preliminary evidence suggested the modulation of NK cell activity can be beneficial for managing COVID-19. The application of NK cells and the NK cell modulating therapeutic compounds such as Imiquimod can use for managing COVID-19 following the clinical testing through randomized control studies (NCT04280224) [32].
T cell-based therapy
The T cells are a major component of the adaptive immune system which recognizes the antigen using the specific T cell receptor when presented through MHC molecules of antigen-presenting cells. The T cells are comprised of two major populations: CD4 helper T cells and CD8 cytotoxic T cells. T cell-based therapies have the potential to modulate the immune response against SARS-CoV-2 and against the excessive immune response. T cell-based therapies using the regulatory T cells (CD4+CD25+FoxP3) can balance the immune response in COVID-19 and avert the pathological tissue damage associated with excessive immune activation and aggravated inflammation [247]. The T cell therapies using activated T cells, tumor-infiltrating lymphocytes, virus-specific T cells, regulatory T cells have been used in several clinical trials and their safety and efficacy profile is also well understood [28, 248]. The virus-specific CD8 T cells (CD8) derived from donors had shown good results in immune-compromised patients infected with the virus [249, 250]. Adoptive T cell therapy using SARS-CoV-2-specific T cells is reported to have beneficial effects in severely diseased patients with COVID-19 (NCT04351659) [250].
The SARS-CoV-2-specific and HLA-matched cytotoxic T cells isolated from recovering COVID-19 patients can be used for treating severely ill patients [251]. The majority of the COVID-19 positive convalescent patients have SARS-CoV-2-specific CD8 T cells in their body and the proportion of SARS-CoV-2 S glycoprotein -reactive CD4+ T cells was found to be very high in severe cases (83%) compared to asymptomatic cases (35%) [252]. It has been shown that HLA-E-restricted CD8 T cell immunotherapy can benefit the COVID-19 patients by eliminating virus-infected cells and hampering the spread to new cells. It can also reduce the excessive inflammatory response and tissue damage caused by SARS-CoV-2 infection. These HLA E restricted CD8 T cells are ideal for therapy as they will not be rejected by the recipient. This approach can be very quick and cost effective if a large number of these cells could be recovered from convalescent COVID-19 donors and then stored and used in severely affected individuals as an off the shelf product [250].
Chimeric antigen receptor (CAR) T cell therapy
Chimeric antigen receptor T-cell (CAR-T) based therapy is a novel mode of T cell therapy that offers cure among patients suffering from relapsed/refractory (R/R) malignant diseases such as acute lymphoid leukemia and diffuse large B cell lymphoma [253, 254]. Two CAR T-cell products axicabtagene ciloleucel (Brand name: Yescarta) and tisagenlecleucel (Brand name: Kymriah) were approved by US-FDA for treating patients with a specific type of cancer [28, 255]. In the case of CART-cell therapy, the T cells are genetically engineered to provide antigen specificity without any need for antigen-presenting cells. In this mode of therapy, the blood T cells are recovered from a patient and manipulated in the laboratory to introduce the CAR gene which provides a synthetic receptor for recognizing a specific antigen. CAR gene is a combination of the single-chain fragment variable (ScFV) sequence of the monoclonal antibody, a transmembrane domain, and the intracytoplasmic signaling domain of the CD3 zeta chain. The engineered CAR T cells are transplanted to the patients through an intravenous infusion [256-258]. The CAR T cell therapy has been found useful for treating chronic viral infections like hepatitis B and HIV [259].
Researchers at Duke-NUS Medical School in Singapore have been exploring the use of CAR T cells for treating patients with COVID-19 [260]. There is a need for the development of CAR T-cell specific against the virus surface antigen and conduct validation studies before their use as a potential therapeutic strategy for COVID-19 [261]. However, the CAR T-cell therapy may be highly challenging during the COVID-19 pandemic given the highly complex manufacturing process. Several clinical and technological factors such as the severity of the illness and manufacturing timeline need to be taken into consideration before developing a CAR-T cell therapy for COVID-19. The toxicity issues such as cytokine release syndrome, neurotoxicity, and lymphopenia associated with CAR T cell need to be addressed, particularly while applying this therapy for COVID-19 patients [28, 262-264].
Monoclonal antibodies-based immunotherapeutics
Monoclonal antibodies (mAbs) are specific and minimize adverse effects of convalescent plasma or purified immunoglobulins [265]. mAbs are well proven biological molecules widely used for treating cancers and immunological disorders, and their use for treating infectious diseases such as COVID-19 is gaining importance [214, 265]. mAbs such as bevacizumab, sarilumab, adalimumab, camrelizumab, eculizumab, mepolizumab, nivolimumab, and tocilizumab are being evaluated for their application as a drug for treating COVID-19 [214]. The key molecule on SARS-CoV-2 for targeting is the RBD of the S1 subunit in the spike glycoprotein. As the RBD binds to the ACH2 receptor on the cells which mediate the virus entry into the host cell, blocking RBD using mAbs will be highly specific and effective in neutralizing the virus and prevent it from entering into the cell to establish the infection [266, 267].
mAbs can be used as a prophylactic drug to prevent the infection in high individuals such as health care workers and contacts of laboratory-confirmed cases. It can also be used as a therapeutic drug to shorten the course of infection and protect uninfected cells exposed to SARS-CoV-2 [268]. Several SARS-CoV-specific mAbs have been developed and are in different stages of validation [266, 269]. The fully human mAb 47D11 that binds to the conserved epitope on the spike protein RBD is shown to cross-neutralize both SARS-CoV-2 and SARS-CoV [268, 269]. The 47D11 mAb neutralizes the SARS-CoV-2 by targeting the communal epitope on the spike protein, which can be potentially used to prevent and treat COVID-19 [269]. Another human mAb CR3014 that binds to the S1 subunit of spike protein has been shown to prevent virus shedding as well as the associated lung pathology in SARS-COV infected ferrets [270]. This mAb was able to neutralize the SARS-CoV-2 at an effective concentration of 23.5 µg/ml and therefore can be utilized as a potential therapeutic agent either as monotherapy or in combination with other potent SARS-COV-specific neutralizing antibodies for treating COVID-19 [266]. A novel mAb, CR3022, was found to neutralize the viruses that escape the CR3014 binding without any competition [270].
The two neutralizing mAbs, COV2-2196 and COV2-2130 have shown to bind with the non-overlapping sites of spike protein and neutralizes the SARS-CoV-2 virus. These two mAbs are also shown to act synergistically and their functional efficacy was proven in SARS-CoV-2 infection using mouse models. Passive transfer of these antibodies either alone or in combination can reduce the viral burden as well as the levels of lung inflammation in mice and protected the rhesus macaques from SARS-CoV-2 infection [271].
A humanized anti-vascular endothelial growth factor (VEGF), Bevacizumab, has been shown to benefit COVID-19 patients by reducing inflammation and edema (NCT04275414; NCT04305106). Sarilumab, a human mAb that can bind to the IL-6 receptor is currently being evaluated for managing COVID-19 patients (NCT04315298; NCT04327388). The granulocyte macrophage-colony stimulating factor (GM-CSF) targeting mAb Gimsilumab is also being evaluated for the protective effect on hyper inflammation and lung injury [272]. There are some limitations such as cost, the scale of production, duration of treatment, and side effects in monoclonal antibody-based therapy [273]. However, novel methods of large-scale production in transgenic plants and the animal can overcome some of these limitations [273]. Application of nanobodies, which are antigen-binding domains, found in camelid species containing only heavy chain and no light chain for their application in COVID-19 [274]. They have several advantages such as compact structure, lower molecular weight, smallest active antigen-binding fragments, better stability, and better penetration/bioavailability inside the cells [274].
US FDA has approved the use of some mAbs through an EUA regulation including bebtelovimab, tixagevimab-cilgavimab combination (Evusheld®), casirivimab-imdevimab (REGEN-COV), bamlanivimab-etesevimab combination and sotroviman [66]. However, recently WHO advised against antibody treatments (casirivimab-imdevimab and sotroviman) for COVID-19 patients [275].
Cytokine therapy
The cytokines play a major role in the pathobiology and pathogenesis of COVID-19, in the form of cytokine storms that lead to excessive inflammation and tissue injury. Of the different cytokines, some are beneficial, and some are harmful. In COVID-19, the cytokines such as type-I INF, and IL-7 are beneficial, whereas other cytokines such as IL-1β, IL-6, and TNF-α are detrimental, particularly during the cytokine storm. Two patterns of cytokine dysregulation are reported in a severe form of COVID-19, which are the IL-1β-driven macrophage activation syndrome and IL-6-driven immune dysregulation. Thus, the therapeutic approaches to stimulate or suppress the immune response and use of cytokines or anti-cytokines are still unclear. However, the inhibitors of IL-6 have been found useful for managing COVID-19 [276]. It is also shown that patients requiring ICU admission had a very high level of certain cytokines than those who did not require ICU admission [277]. Other studies also showed the proinflammatory cytokines were increased in patients infected with a severe form of the disease and particularly, the level of cytokines such as IL-6 and IL-8 correlated with the severity of COVID-19 [278, 279].
Corticosteroids can be used to suppress the cytokine storm by suppressing the immune system. The use of corticosteroids has been incorporated in managing COVID-19 patients and it also reduces the excessive inflammation and tissue damage mediated through the highly activated immune system. The anti-IL6 receptor antibody, tocilizumab is also shown to be beneficial for managing the overwhelming COVID-19 associated cytokine release syndrome by blocking the IL-6 [280-282].
IL-6 receptor inhibitors
Tocilizumab is an anti-IL-6-receptor mAb that can inhibit the IL-6 signaling. It is currently used for treating rheumatoid arthritis [283]. Tocilizumab can competitively bind to the membrane-bound as well as the soluble IL-6 receptors, thereby preventing IL-6 from interacting with its receptor [284]. It has been reported that this drug can improve clinical symptoms and inhibit the progression of disease in patients with COVID-19 [285], and intravenously administered tocilizumab is currently being evaluated as a therapeutic strategy for managing COVID-19. Clinical trials testing a humanized monoclonal antibody that targets IL-6 have already been initiated in China [80]. In addition, several clinical trials are currently assessing the efficacy of tocilizumab against SARS-CoV-2 infection [286]. However, given that tocilizumab treatment may be correlated with medication-related osteonecrosis of the jaw, further investigations are required to analyze the potential adverse effects of this treatment before considering to be used for managing COVID-19 [287], including assessments of the risk of secondary bacterial infection [288]. Besides minimizing inflammatory storms by blocking IL-6 receptors it also helps in improving clinical conditions including body temperature and respiratory rate in patients with COVID-19 [289].
Recently, tocilizumab (Actemra®) is approved for the treatment for hospitalized adult patients with COVID-19 [66].
JAK2 inhibitors
Fedratinib is a specific JAK2 inhibitor already approved by the FDA for its use in treating myeloproliferative neoplasms and shown to influence the TH17 cell cytokines production [290]. The pathogenesis of COVID-19 involves a hyperinflammatory stage/cytokine storm that is characterized by an increase in the serum levels of cytokines and chemokines such as IL-1β, IL-2, IL-7, IL-8, IL-9, IL-10, IL-17, granulocyte macrophage-colony stimulating factor (GM-CSF), granulocyte-colony stimulating factor (G-CSF), IFN-γ, TNF-α, IFN-inducible protein 10 (IP10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1 alpha (MIP-1A), and macrophage inflammatory protein 1 beta (MIP-1B) (Figure 3) [291]. Given that some of these cytokines are involved in TH17-type responses, a specific JAK2 inhibitor such as fedratinib can be used to suppress the production of specific cytokines from TH17 cells [290]. Hence, this drug may prevent the deleterious effects of the cytokine storm in SARS-CoV-2 infected patients, and thus warrants further evaluation.
Interferons (IFNs)
The coronaviruses fail to induce strong type I IFN response in the infected host [292] and the bat coronaviruses have adapted to replicate under poor IFN response in bats [293]. Thus, a strong induction of a type I IFN response is a therapeutic strategy for treating COVID-19 patients. Numerous clinical trials have been undertaken for investigating the use of type I IFNs [33]. Studies have demonstrated that high-dose IFN, either used as a monotherapy or in combination with other agents, restricted the in vitro replication of both SARS-CoV and MERS-CoV [294-297] and was associated with better disease outcome [298, 299]. Therefore, IFN therapy can be further evaluated to be used in patients with COVID-19 [288]. Atomized inhalation of IFN-α along with other antivirals has been recommended [300]. The recommended dose is 5×106 units in sterile injection water per administration for adults, twice a day [301]. Two forms of IFN-α are in use, nebulized IFN-α and IFN-α2b spray [302]. In combination with remdesivir, lopinavir-ritonavir and IFN-α2b are indicated as a treatment for COVID-19 [303, 304].
A recently triple antiviral combination involving IFN beta-1b, ribavirin, and lopinavirritonavir was used for treating patients with COVID-19. The combination was found to be safe and has superior efficacy than the lopinavir-ritonavir therapy in circumventing symptoms and reducing the viral shedding duration as well as the hospital stay in mild to moderate cases [305]. Type III IFNs such as IFN-λ can be an alternative to type I IFNs, as they have low toxicity and less vulnerable to mediate immunopathology and still have antiviral activity. IFN-λ is also a potent activator of NK cells and macrophages to bring antiviral activity indirectly [306].
Toll-like receptors (TLRs)-based therapy
In the search for new therapeutic molecules to tackle SARS-CoV-2, TLRs are gaining interest. TLRs are a family of thirteen conserved transmembrane receptors (immune sensor proteins) expressed on the surface of cells like dendritic cells, monocytes, respiratory epithelium cells, and pneumonocytes [307, 308]. Viral genome and proteins in form of TLRs ligands stimulate the maturation of dendritic cells to activate adaptive immune responses to trigger the release of cytokines and chemokines.
TLR signaling is an important connecting link between innate and adaptive immunity as various TLRs play diverse roles [309]. TLR 2 is essential for the recognition of structural and non-structural viral proteins to induce inflammatory cytokines. Similarly, TLR -3, TLR-4, and TLR7/8 are responsible for the detection of viral nucleic acid to stimulate signaling via the release of cytokines to obtain optimum antiviral immune response [310]. Toll-like receptors in the form of pattern recognition receptors (PRRs) actively identify viral RNA to trigger the innate immune response in the early phase of infection [311, 312]. These TLRs act as PRRs can recognize specific PAMPs present in the SARS-CoV-2 virus. As a result of PRR-PAMP interaction and in response to this recognition, TLR elicits innate and acquired immune response in the early phase of respiratory infection by modulating the release of cytokines. Hence, TLRs should be explored as a therapeutic molecule or as an adjuvant in vaccine development to confer protective immunity against respiratory infections like SARS-CoV-2 [313].
A marked decrease in a number of B cells, T cells, and NK cells has been reported in patients with COVID-19. Similarly, immune dysfunction is also observed as lymphopenia, the elevated profile of inflammatory cytokines like TNF-α, IL-2R, and IL-6. Uncontrolled immune response in form of cytokine storm/hyper inflammation is the main factor that contributes to the immunopathogenesis of COVID-19. This is characterized by systemic inflammation and marked pulmonary immunopathological syndrome including pneumonia, pulmonary fibrosis, acute lung injury, multiple organ failure, heart tissue damage, acute kidney dysfunction, atrophy of the spleen, lymph node atrophy, and ARDS [314-316]. SARS-CoV-2 strikes the innate immune system of the body to cause infection via TLRs such as TLRs 3, 7, and 8 [317, 318]. Also, as soon as SARS-CoV-2 enters into the lungs, TLRs 3, 4, 7, 8, 9 recognize viral RNA and bind to the viral PAMPs to produce and release the signals to promote a molecular cascade of innate immune responses and to activate acquired immunity [319-321].
By active immunomodulation, TLR-5 acts as an effective therapeutic molecule to activate an innate immune response against SARS-CoV-2 and as an adjuvant in SARS-CoV-2 vaccines [322]. Targeting TLR-5 can be an innovative therapeutic strategy and through immunomodulation. TLR5 may reduce the occurrence of cytokine storm to prevent acute tissue damage in patients with COVID-19 [323, 324]. Bioinformatics encouraged the practice of docking in the development of the SARS-CoV-2 vaccine. Docking is an in-silico procedure to predict the probability of binding of SARS-CoV-2 specific vaccine candidates to TLRs under in vivo conditions. Studies demonstrated that an epitope-based peptide vaccine against SARS-CoV-2 was docked effectively with TLR5 and it enhanced the binding affinity. TLR2, TLR3, TLR4, and TLR9 were tested by molecular docking with the help of the ClusPro 2.0 protein-protein docking server [325, 326]. In another study, TLR5-coronavirus spike protein S1 subunit was used to make recombinant subunit vaccines against SARS-CoV-2 [313, 327]. TLR7 distinguishes single-stranded viral RNA and hence can play an important role against SARS-CoV-2 [31]. Likewise, the activation and contribution of TLR9 in COVID-19 immunopathology is also investigated. TLR-9 is generally expressed over nasal mucosa, dendritic cells, B cells, T lymphocytes, NK cells, monocytes, macrophages, bone marrow, neutrophils, alveolar epithelial cells in the lungs, megakaryocytes, and platelets [328]. A higher level of TLR-9 expression and its ligands are potential biomarkers in predicting the severe outcome COVID-19 in patients.
Imiquimod, commonly referred to as IQ is a member of the imidazoquinolines family. IQ is an immune-stimulator activating TLR 7 and can be utilized to augment both the innate and adaptive arms of immunity, specifically cell-mediated immunity, and has been clinically approved to be a therapeutic option for managing COVID 19 [317, 329]. IQ showed antiviral properties by modulating the expression and production of various cytokines. Researchers through various preclinical and clinical trials have confirmed strong antiviral and antitumor properties of IQ by inducing the expression of several cytokines such as IFN-α, IL-6, IL-12, TNF-α, and nitric oxide to further stimulate T-lymphocytes, B-lymphocytes, macrophages, NK-cells, and Langerhans cells to enhance the antigen presentation to T-lymphocytes. IQ indirectly activates the production of cytokine IFN-γ and T-helper type 1 (Th1) cells while hampers the expression of Th2 cytokines such as IL4 and IL5. Literature reported the therapeutic potential of IQ against COVID-19 especially in the early phase of infection and hence can be tested, validated, and used as a SARS-CoV-2-specific drug [32, 330].
Other therapeutic strategies
ACE2 inhibitors/angiotensin receptor blockers
Researchers have demonstrated that for its attachment and entry into cells, the SARS-CoV-2 virus has to bind with the host cell surface ACE2 receptors via the S1 domain of its spike protein (Figure 1), and these observations have prompted investigations designed to identify alternative antiviral therapies. Biologically, ACE2 is a mono-carboxypeptidase that is present in two forms, one of which is the rarely detected free circulatory form, and the other is the more commonly encountered cell-anchored ACE2, which is expressed over the entire surfaces of cells in the gastrointestinal tract and kidneys, and occasionally lung cells such as type 2 pneumocytes [331].
The two types of ACE2 differ structurally with respect to the presence of a transmembrane domain in the cell-anchored ACE2 that is absent in free circulatory ACE2. Thus, while the SARS-CoV-2 is unable to bind to the soluble ACE2, by specifically binding to the extracellular domain of the cell-anchored ACE2 receptor, the virus can enter the host cell by traversing the plasma membrane. The results of in vitro studies performed on monkey kidney and Vero-E6 cell lines have accordingly indicated that competitive use of the soluble form of ACE2 can serve to inhibit SARS-CoV-2 replication. Furthermore, it has been demonstrated in vitro that the use of recombinant human soluble ACE2 protein (ACE2 fused with the Fc portion of immunoglobulin) facilitated neutralization of the SARS-CoV-2 virus. Consequently, exploiting the recombinant soluble ACE2 protein may represent a viable therapeutic option that could be used to limit SARS-CoV-2 infections, and accordingly should be further examined, standardized, and validated [332].
ACE2 inhibitors (ACEIs) and ACE-R blockers (ARBs) may have a biphasic effect for treating patients with COVID-19 [333]. In a Chinese study involving 1,099 patients with COVID-19 who were co-affected with single/multiple conditions (coronary artery disease, hypertension, chronic renal disease, or diabetes), and who were provided with intravenous mixtures of ACEIs and ARBs, found that these drugs promoted an indirect increase in the numbers of ACE2 receptors in the circulation. Hence, given that SARS-CoV-2 spike proteins utilize host cell ACE2 receptors for cellular binding if treated with such drugs, elderly people with an existing underlying condition would have a heightened risk of getting infected with SARS-CoV-2. Accordingly, patients with pre-existing disorders should take appropriate preventive measures and avoid any situation where they are liable to encounter COVID-19 patients or become infected with SARS-CoV-2 [334,335].
Corticosteroid therapy
A proportion of the individuals suffering from COVID-19 progress to more fatal forms of the disease characterized by the sudden development of ARDS [336, 337], which is associated with a cytokine storm, accompanied by an exaggerated inflammatory response and cytokine-related lung injury that finally results in the rapid progression of pneumonia (Figure 3) [44, 338]. The use of corticosteroids for ARDS is still a matter of contention, and hence caution should be exercised if systemic glucocorticoids are to be administered for treating COVID-19 [339]. Corticosteroids have been the most commonly used drugs in COVID-19 patients [48]. The current guidelines recommend the use of methylprednisolone in those patients with COVID-19 having a severe illness or rapid disease progression [301]. The existing literature suggests that even though significant improvement is not observed in critically ill COVID-19 patients treated with corticosteroids, patients might gain certain benefits from a low-dose corticosteroid treatment based on clinical experience [339]. The dose of methylprednisolone that can be used daily varies depending on the disease severity, but should not exceed 2 mg/kg/day [301]. According to the recent guidelines, glucocorticoid use in patients with COVID-19 pneumonia should be undertaken with extreme caution and dosages has to be determined on a case-by-case basis [339].
The high mortality and morbidity rates associated with COVID-19 can be attributed due to the autoimmune destruction of lungs following the release of a storm of pro-inflammatory cytokines. Dexamethasone is an FDA-approved synthetic corticosteroid with broad-spectrum immunosuppression activity and has a longer duration of action than cortisone [340]. Dexamethasone has been identified as the first drug to save lives from COVID-19 [10, 24]. The preliminary findings from the RECOVERY clinical trial are promising. The findings indicate that the use of dexamethasone in critically ill patients could enhance the rate of survival [341]. The RECOVERY clinical trial is an open-label trial that compares several potential therapeutic agents in hospitalized COVID-19 patients. One of the arms in this trial involves the treatment with oral or intravenous dexamethasone (6 mg once daily) for a maximum period of 10 days. The findings from this trial that studied 2104 patients who received dexamethasone indicate that the treatment with dexamethasone lowered 28-day mortality in patients who received either oxygen alone or invasive mechanical ventilation as compared to the patients receiving usual care [342]. Although dexamethasone has several physiological pathways, the benefits of this wonder drug in COVID-19 are likely due to the potent immunosuppressive properties [341]. In addition to the suppression of pro-inflammatory cytokine production, dexamethasone might also inhibit the protective function of T cells and B cells mediated antibody production that leads to increased plasma viral load. Therefore, the use of dexamethasone during the recovery phase will result in the persistence of the virus and further prevents the body from generating protective antibodies [340].
In a retrospective investigation, it was found that the use of corticosteroids in both critical and severe patients with COVID-19 results in lower mortality. However, the use of corticosteroids may result in side effects that require careful monitoring [343]. Some scientists continue to oppose the use of corticosteroids/dexamethasone in the clinical management of COVID-19, which may be attributable to the fact that both acute lung injury and ARDS are essentially caused by host immune responses. Hence, the use of corticosteroids not only suppresses inflammation in the lung but also suppresses the host immune response thereby affecting pathogen clearance [344]. It has also been pointed out that clinical data supporting the benefits of corticosteroid use in treating respiratory viral infections are conspicuously lacking [344]. However, this claim has been rejected because these inferences have been made based on observational studies, and that inconclusive clinical evidence should not be considered as a reason for excluding corticosteroids from the treatment guidelines for COVID-19 [338]. A previous study conducted on critically ill patients with COVID-19 has proven that the appropriate corticosteroid use can shorten the hospitalization period and reduce the mortality rate without causing any secondary infection [115]. Hence, it can be concluded that a low dose of corticosteroids administered for a short period (not more than 7 days), in conjunction with the monitoring of adverse drug reactions, could be helpful for treating critically ill patients with COVID-19 [339].
Stem cell therapy
Mesenchymal stem cells (MSCs) have an immunomodulatory activity that can be utilized to treat SARS-CoV-2 infected patients [92, 345]. Hence, MSCs therapy has the potential to preventing the cytokine storm associated with COVID-19 infection, thereby reducing the mortality and morbidity of person with this disease (Figure 3). Among the different types of MSCs that are widely used, umbilical cord MSCs are believed to have considerable potential to be used as a therapeutic agent for managing critically ill patients with COVID-19 [345]. Stem cell therapy can be considered an effective and safe therapeutic option that can be used to reduce the severity of critical illnesses. The use of these cells represents an entirely different biological approach to traditional drug-based therapies that has a wide application for treating COVID-19-induced pneumonia [92]. Since the stem cells are injected via an intravenous route, they can be targeted to sites in the lungs, thereby maximizing the therapeutic effect against COVID-19 [345]. The Celltex Therapeutics Corporation (CELLTEX) has already begun preliminary discussions with the FDA regarding the possibility of using MSCs as a therapeutic agent against COVID-19 [346]. Findings from preliminary studies indicate that the intravenous implantation of MSCs is associated with significant improvements in the clinical symptoms, as well as pulmonary function in patients with COVID-19 [347]. Hence, further evaluations are warranted to gain a clearer indication of the benefits associated with the intravenous transplantation of MSCs in seriously ill COVID-19 patients with pneumonia.
Stem cell therapy is associated with certain limitations such as patient biocompatibility and the possibility of cross-infection. Such limitations can be eliminated by modulated the stem cells with nanoconjugates. Therefore, stem cell-based nanoconjugates will offer a great therapeutic strategy to manage COVID-19 and related diseases such as virus-induced pneumonia [348]. MSCs therapy will help to ameliorate the cytokine release syndrome (cytokine storm) and thereby protect the alveolar epithelial cells. The therapeutic activity of MSCs is mediated via secreting several factors that have a broad activity spectrum [349].
MSCs can be used to suppress overactive immune responses and can also promote tissue repair and regeneration. MSCs also have immune-modulatory potential by influencing various immune effector cells, such as dendritic cells, T cells, and NK cells in the body. They are also hypoimmunogenic as they lack MHC-II molecules and co-stimulatory molecules on the surface. These characteristics allows the use of allogenic MSC safely for treating various disease conditions [350]. This feature also allows the use of MSC as off shelf product. MSCs were used for treating various disease conditions, such as osteoarthritis, multiple sclerosis, and graft versus host disease in different clinical studies [351], The immunomodulatory potential of MSCs might be highly beneficial for a patient exhibiting severe forms of SARS-CoV-2 infection mainly because of the impaired adaptive immune system and excessive inflammation.
Currently, there are around 27 clinical trials are ongoing worldwide testing the use of MSC and their derivative for treating COVID-19 [17, 352]. A clinical study was conducted to understand the use of MSC for the treatment of COVID-19 patients. In this study, seven patients with COVID-19 pneumonia were enrolled and the clinical outcomes were assessed for 14 days after MSC injection. The results showed a significant improvement of cure of all the seven patients without exhibiting any adverse effects. The clinical condition of all patients was markedly improved within 2 days following MSC transplantation and all of them were discharged within 10 days after treatment. The treatment also increased the peripheral lymphocytes count while decreasing the over-activated cytokine-secreting immune cells T cells and NK cells and increasing the regulatory DC. The treatment also decreased the C-reactive protein and the level of TNF-α and increased the IL-10 in the MSC treatment group than the placebo control group. This showed that the intravenous administration of MSC in COVID-19 patients with severe pneumonia was safe and effective [353].
A systematic review to explore the use of cell therapy for treating COVID-19 patients identified a relevant study in which 117 patients were administered with allogeneic MSC either intravenously or intratracheally and followed for 14 days to 5 years [354]. Though the outcome showed a favorable trend but did not reach statistical significance. There were no adverse events in these studies, however, the observed mild adverse events resolved spontaneously and the other observed findings include improved radiographic findings, inflammatory biomarker levels, and pulmonary function [354]. that there were it can attenuative the respiratory failure by shifting the immune response from a Th1 to a Th2 type, reducing inflammatory cytokines, and cellular activities. However, there was no statistically significant benefit have been observed in these studies and it could be due to the highly variable patient selection criteria [355]. Being a host-directed therapeutic strategy, mesenchymal stem cells may offer therapeutic benefits in COVID-19 patients and can be also evaluated for treating severe cases of MERS-CoV infection that exhibits higher mortality rates of up to 34 % in humans [356]. Extracellular vesicles including the exosomes from mesenchymal stromal cells might be used in the treatments of COVID-19 patients and requires further evaluation and validation. This mode of therapy can be used for reducing cytokine storm as well as to exert regenerative effects [357]. The usefulness of mesenchymal stem cells protecting patients against severe COVID-19 pneumonia-induced acute lung injury or acute respiratory disease syndrome by preventing the overactivation of immune system and promoting regeneration in the lung microenvironment have been confirmed [345, 358]. However, variation in the route and dose of administration, characterization and source of MSC and limited patient population are limitations of these studies that need to be addressed in future clinical studies. The data presented in many of these studies were also either anecdotal or from incomplete, poorly controlled investigations and the MSC based treatment approach need to be investigated further in rationally designed and controlled clinical studies [17].
Traditional Chinese medicine
Traditional Chinese medicine (TCM) is a branch of medicine that originated in China and is anticipated to play a prominent role in COVID-19 management [359]. Evaluations of the antiviral and anti-inflammatory activities of the traditional medicinal formula Lianhuaqingwen (Figure 3) in Vero E6 cells have identified potent in vitro inhibitory activity against SARS-CoV-2 replication. The formula has also been found to reduce pro-inflammatory cytokines' production, indicating its potent anti-inflammatory activity [360]. A study has identified that the constituent components such as rhein, forsythoside A, forsythoside I, and neochlorogenic acid present in the Lianhuaqingwen capsule contribute to the clinical activity via an inhibitory effect on ACE2 [361]. It has also been established those early interventions using traditional Chinese medicines in clinical cases can improve the cure rate, delay disease progression, reduce mortality, and shortens the course of the disease [359]. Given these promising observations, further investigations using randomized trials are required to evaluate the therapeutic potential of traditional Chinese medicine for managing COVID-19.
Combining Western and TCM is another promising therapeutic strategy that has already been applied for treating COVID-19 [362]. Chinese traditional medicine has been popularized as a cure for SARS-CoV, including SARS-CoV-2. Some patented herbal drugs, including Huoxiang Zhengqi capsules, Lianhua Qingwen capsules, and Radix isatidis granula have been recommended as therapeutic options [363]. Two TCM ‘Yu Ping Feng San’ and ‘Sang Ju Yin’ have been used to investigate their effects on the defense system of host cells. They appear to improve the host defense ability by altering T cells [364]. In another report, supplementary therapy TCM conferred significant effects in symptom appearance, thus shortening viral infection duration [365]. These favorable impacts of TCM were also substantiated by laboratory-based in-vitro experiments [366]. Though the actual mechanisms of these herbals are not known, future use involves discovering specific, and active components optimizing its structure and function and evolving in vitro and in vivo functions [363, 367].
The orally administered liquid Shuanghuanglian is a patented TCM that has the potential to cure patients with COVID-19 when used as an adjunct to western medicines [362]. Some of these traditional Chinese medicinal preparations are based on herbal plants [180, 368] and have previously been used in more than 50% of SARS cases [368], improving body temperature, cough, and dyspnea, resolving pulmonary infiltration, and improving the quality of life of SARS patients [368]. TCMs have an antiviral, anti-inflammatory, and immunoregulatory activity mediated by multiple components via multiple pathways. Some major active constituents such as kaempferol, baicalein, luteolin, Quercetin, isorhamnetin, wogonin, and naringenin present in the Chinese medicine targets ACE2 and 3CL protein thereby producing therapeutic effects in patients with COVID-19 [369]. Hence, Chinese authorities are actively encouraging the integration of Chinese traditional medicine with conventional therapies for the treatment of SARS and SARS-like diseases. Generally, over 30 different TCM formulations have been utilized in Wuhan to combat the COVID-19. About 121 registered TCM protocols for COVID-19 have been recognized from the Chinese Clinical Trial Registry. All these TCM formulations are utilized according to the extent of infection [370]. Traditional Indian medicine has also prospected for curing COVID-19 [13]. Medicinal plants having antiviral activities have been enumerated and suggested as a possible cure for COVID-19 [13].
TCM can be administered by several routes. Some of which include external fumigation, wearing of the sachet, and use of moxibustion. The volatile components of TCM can help to prevent and treat COVID-19 [367]. Keguan-1 is a new TCM-based drug derived from three different formulae, Sangju drink, Yinqiao powder, and Sanren Decoction [371]. Keguan-1 was developed explicitly for suppressing and treating COVID-19 induced ARDS by targeting the host. RCT was conducted to evaluate the therapeutic potential of Keguan-1 in managing ARDS associated with COVID-19 (NCT 04251871). The trials' findings suggest that Keguan-1-based TCM therapy is a safe and superior method to suppress the growth of ARDS in patients with COVID-19 compared to the standard treatment [371].
However, further multi-center, large scale randomized controlled trials can help confirm the clinical efficacy and safety of TCM. The effectiveness of TCM as an auxiliary therapeutic strategy for managing COVID-19 was evaluated through a systematic analysis of RCTs. The systematic review could identify ten RCTs that contain a total of 1,285 patients and found that TCM can help reduce disease progression and improve the symptoms in patients with COVID-19 [372]. Although TCM along with Iranian and Indian medicine suggest the utilization of some herbs for rehabilitation, and treatment of COVID-19, the adverse effects, safety and clinical investigations of these medicines remain unknown and should be observed in more detail [373, 374].
Herbs and plant metabolites
Herbal medicine is another therapeutic option for managing COVID-19 [375-378]. Herbs and plant metabolites have shown a promising role in treating and preventing COVID-19 (Figure 6) [379-382]. However, it is important to research and screen valuable medicinal products on the clinical efficacy and safety for COVID-19 treatment [383-385]. They may contain ingredients (e.g., lycorine, psoralidin, quercetin, silvestrol, caffeic acid, isobavachalcone, tryptanthrin, ouabain, scutellarein, myricetin, homoharringtonine, tylophorine, saikosaponin B2, and 7-methoxycryptopleurine) having inhibitory effects on coronaviruses including SARS-CoV-2 [379,386-388]. They may affect any of the above-discussed mechanisms. Chloroquine phosphate, a drug that has been widely studied for treating COVID-19 is the structural analogue of quinine that is extracted from the cinchona tree bark [389]. Besides antiviral effects, it also modulates immune response [363]. Diammonium glycyrrhizinate, an extract of liquorice (Glycyrrhiza glabra) roots has also shown a potential cure for COVID-19 in association with vitamin C (ChiCTR2000029768). Curcumin inhibits proteases in viruses and has prospects for use against SARS-CoV-2 [386].
Figure 6.
The major natural constituents present in herbal plants that can target and inhibit pathways and proteins of SARS-CoV-2 such as spike protein, RdRp, 3CLpro, PLpro, and helicase. inhibit pathways and proteins of SARS-CoV-2 such as spike protein, RdRp, 3CLpro, PLpro, and helicase.
Purified compounds or crude extract from various herbs or medicinal plants, such as Astragalus membranaceus, Agastache rugosa, Artemisia annua, Cassia alata, Ecklonia cava, Gymnema sylvestre, Mollugo cerviana, Houttuynia cordata, Glycyrrhizae uralensis, Lycoris radiata, Lindera aggregate, Pyrrosia lingua, Polygonum multiflorum, Tinospora cordifolia, Saposhnikoviae divaricate and others have displayed potential to inhibit SARS-CoV Mpro [390-392]. Almost 13 compounds have been found potentially effective against SARS-CoV-2, and 125 Chinese herbs have been found to contain two or more of these compounds [380]. Kaempferol, luteolin-7-glucoside, quercetin, dimethoxy curcumin, apigenin-7-glucoside, naringenin, oleuropein, catechin, curcumin, and epicatechin-gallate containing plants have shown better Mpro inhibitory potential [392].
Terpenoids and cannabinoids have also shown anti-SARS-CoV-2 potential on molecular docking [393]. Curcumin and terpenoids can also inhibit members of the SARS family [394]. Terpenoids (from neem plant, Azadirachta indica) and curcumin have effectively regulated ARDS in experimental animals through inhibition of the NFκB and related pathways [395, 396]. On analyzing antiviral activity of plant secondary metabolites, a study reported eight active metabolites with curcumin being the most potent [386]. However, the medicinal use of these phytoconstituents needs to be evaluated. Herbal medicines lack adverse effects and are safe [375,376] and they could augment the chemical therapeutics when used in combination therapy [375,376]. Traditional medicines have also been tried in curing COVID-19 [21, 397].
The naturally occurring bioactive molecules present in the herbs and vegetables possess potent antiviral properties with minimal side effects that require further investigation [398-401]. These compounds can act on different targets of coronavirus, such as spike protein (baicalin, emodin), viral replicating enzymes, such as RdRp (sotetsuflavone), PLpro (cryptotanshinone), 3CLpro (iguesterin), and helicase (silvestrol). It is reported that various kinds of terpenoids exhibit marked inhibitory effects in viral replication. Likewise, alkaloid compounds, including emetine, lycorine, and homoharringtonine have demonstrated potent anti-coronavirus properties [402].
The dietary flavonols have the ability to target 3CLpro, RdRp, PLpro, and spike protein of SARS-CoV-2 and can also inhibit the interaction between ACE2 receptor and spike protein thereby affecting the viral entry [399, 403]. Similarly, benzisothiazolinones and synthetic flavonoids are also screened to identify potential inhibitors of SARS-CoV-2 Mpro using in silico methods. Among the short-listed compounds, TF-9, a thioflavonol compound, was identified as the potent inhibitor of SARS-CoV-2 [398]. The flavonoid-based biomedicines include linebacker equivir, caflanone, myricetin, and hesperetin [404]. Recently, a study examined a flavonoid compound library to securitize their inhibitory activities against SARS-CoV 3CLpro [405]. Among the compounds, pectolinarin, herbacetin, and rhoifolin displayed significant inhibition potential against 3CLpro with IC50 values of 37.78 µM, 33.17 µM, and 27.45 µM, respectively. Moreover, the compounds helichrysetin, isobavachalcone, herbacetin, and quercetin-3-b-D-glucoside were also capable of inhibiting MERS-CoV 3CLpro with IC50 values of 67.04 µM, 35.85 µM, 40.59 µM, and 37.03 µM, respectively [405]. Therefore, the synthetic flavonoid analogues such as thioflavonols might have the potential to inhibit SARS-CoV-2 replication.
Herbal medicine can be used during the various phases of COVID-19 disease, including before and aftercare [375, 376, 383, 384]. Over-the-counter herbal medicines are often prescribed for patients with COVID-19 having mild clinical symptoms. Therefore, herbal formulae consumption can be recommended during the medical observation period of COVID-19 [375, 376]. Herbal medicine has broad utility in managing COVID-19 to improve the immune response [402]. Several medicinal plants such as licorice (Glycyrrhiza glabra), ginger (Zingiber officinale), garlic (Allium sativum), green tea (Camellia sinensis), black cumin (Nigella sativa), Hypericum perforatum, and Scutellaria baicalensis have beneficial effects. The natural constituents present in these medicinal plants such as terpenoids can inhibit viral replication. In addition to that, alkaloids such as lycorine, homoharringtonine, and emetine possess a strong anti-coronavirus activity [402].
The recent trends suggest that plant-based substances possess broad-spectrum therapeutic potential that can be harnessed for managing COVID-19. Traditional medicinal plants have several advantages, one being less toxic and is always associated with minimal side effects. Several phytochemicals such as lycorine, hypericin, silvestrol, myricetin, emodin, tylophorine, mycophenolate mofetil, ouabain, and scutellarein have exhibited inhibitory activity against human coronaviruses and therefore can be beneficial against SARS-CoV-2 [366]. The extracts from eggplant skin (Solanum melongena L.) have exhibited antiviral, anti-inflammatory, and antioxidant activity. Similarly, white mulberry bark (Morus alba) has therapeutic benefits and can be used for managing pulmonary fever, cough, and oedema associated with respiratory virus infection [385]. Molecular docking studies have also suggested that the plants such as Curcuma Longa, Piper longum, Phaseolus vulgaris, Ocimum gratissimum, Artemisia absinthium, Syzygium aromaticum, and Inula helenium contain phytochemical compounds that could effectively bind to Mpro and ACE2 and therefore act as inhibitors [406].
Licorice plant (Glycyrrhiza glabra)
Glycyrrhizin also called glycyrrhizic acid (GLR) is the natural product that is extracted from the root of licorice plant (Glycyrrhiza glabra). It is a drug that has been previously used for treating liver diseases, including viral hepatitis and other inflammatory diseases like atopic dermatitis [382]. GLR has potent immuno-active and anti-inflammatory activity. The anti-inflammatory activity of GLR is mediated via TLR4 antagonism [407]. In addition to that, GLR has also exhibited potent antiviral activities against a number of viruses during the in vitro studies. It has shown antiviral activity against human immunodeficiency virus (HIV-1), hepatitis viruses A, B, and C [382, 408, 409].
Glycyrrhetinic acid is the primary active metabolite of GLR. GLR can prevent the entry of CoV into the host cell by inducing cholesterol-dependent disorganization of the lipid rafts [382]. GLR or its active metabolite glycyrrhetinic acid might also act directly by reducing the TMPRSS2 expression that has an important role in the virus uptake thereby affecting SARS-CoV-2 transmission [407]. In addition to that, GLR can also trap the high mobility group box 1 (HMGB1) protein thereby blocking the functions of alarmin [382]. Therefore, GLR possesses the potential to reduce the severity of COVID-19 infection mainly via a two stages process; by blocking the viral entry into the host cell and exhibits an ACE2 independent anti-inflammatory activity [407]. Other pharmacological activities of GLR, including the binding with ACE2, inhibiting intracellular ROS accumulation, downregulating proinflammatory cytokines, inhibiting thrombin, and inducing endogenous IFN might enhance the overall therapeutic utility in patients with COVID-19 [410].
Therefore, GLR requires further evaluation as a candidate therapeutic for SARS-CoV-2 and can be used as a monotherapy or in combination with other drugs. As an early effort, the in-silico approach was used to identify the active molecule of licorice with activity against the different SARS-CoV-2 protein targets (spike protein and non-structural protein 15 -Nsp15) [409]. Based on the observed binding energy and interactions, GLR was found to bind the spike glycoprotein, thereby blocking SARS-CoV-2 from entering the host cell while glyasperin A exhibited affinity towards Nsp15 endoribonuclease. Therefore, both glyasperin A and GLR could be considered useful against COVID-19. A novel combination was proposed that contains vitamin C, curcumin, and GLR for managing CoV infection. System biology tools were used to analyze and evaluate the potential of this combination to modulate immune and inflammatory pathways [361]. The result indicates that the combination of vitamin C, curcumin, and GLR can act on several targets associated with immune and inflammatory responses indicating the therapeutic potential for managing the cytokine storm of COVID-19.
The compounds derived from garlic (Allium sativum) possess the potential to decrease proinflammatory cytokine expression in addition to the immunomodulatory activity [411] It can be used to reverse the majority of the immune system dysfunctions that are predominantly observed in COVID-19 patients. Therefore, A. sativum might have a beneficial effect that can be used for managing COVID-19 as supportive therapy and preventive measure by boosting the immune system cells [412]. The active substances present in the garlic essential oil, mainly organosulfur compounds, were evaluated for their ability to inhibit host ACE2 using molecular docking techniques [413]. These organosulfur compounds that constitute 99.4% of the garlic essential oil were found to interact with ACE2 protein and the SARS-CoV-2 Mpro (PDB6LU7). The findings indicate that garlic essential oil is a storehouse of antiviral compounds that can prevent the entry of CoVs into the human body [413].
Guduchi (Tinospora cordifolia)
The SARS-CoV Mpro (also called 3CLpro) plays an important role in disease progression that is mediated by processing the polyproteins that are required for viral replication [414]. The phytochemical constituents of Guduchi (Tinospora cordifolia) were screened for their affinity to target the active site of SARS-CoV-2 3CLpro using in silico analysis. Among the compounds screened, xanosporic acid, tinosponone, tembetarine, cardiofolioside B, and berberine had significant docking scores. The further evaluation confirmed tinosponone as a potent inhibitor of SARS-CoV-2 3CLpro [414]. However, in vitro and in vivo studies are warranted to confirm the therapeutic utility in COVID-19.
Ashwagandha (Withania somnifera)
Similarly, another widely used medicinal plant is Ashwagandha (Withania somnifera) and the constituents of this plant, withanolides were screened for its potential to target SARS-CoV-2 3CLpro [415]. Among the evaluated constituents, withanoside II, withanoside IV, withanoside V, and witoindoside IX exhibited the highest docking energy. Further analysis identified withanoside V as a potential inhibitor of SARS-CoV-2 Mpro and can be further used to combat COVID-19 [415]. The herbal constituents of Ashwagandha possess potent antiviral, antioxidant, anti-inflammatory, and immunomodulatory activity. Molecular docking studies have identified withanoside X and quercetin glucoside as the major constituents of W. somnifera that can interact with SARS-CoV-2 proteins and can act as S and N protein inhibitor [416].
Green tea (Camellia sinensis L.)
The polyphenols obtained from green tea (Camellia sinensis L.) have already exhibited antiviral activity against several RNA viruses. Among the eight polyphenols evaluated, epicatechin gallate, epigallocatechin gallate, and gallocatechin-3-gallate were found to strongly interact with catalytic residues of SARS-CoV-2 Mpro [417]. Therefore, these three polyphenols can be used as SARS-CoV-2 Mpro inhibitors and will act as promising drug candidates for treating patients with COVID-19.
Turmeric (Curcuma longa L.)
Turmeric (Curcuma longa L.) is a widely used herb in Ayurveda, Unani, Siddha, and other traditional medicine systems owing to its broad-spectrum medicinal properties. Curcumin is the major curcumoid present in turmeric that possess antimicrobial, anti-inflammatory, antioxidant, hypoglycaemic, chemosensitizing, and chemopreventive properties [361]. Turmeric derivatives have exhibited therapeutic benefits in Influenza A infection by regulating the immune response [418]. Therefore, further studies can be taken up to evaluate the efficacy of turmeric derivatives in managing SARS-CoV-2 infection. A study reported the inhibitory activities of Curcuma and Citrus spp. on the growth and proliferation of infection [419]. Therefore, isolation of hesperidin from these plants might be utilized to develop anti-SARS-CoV-2 drugs as a potential remedy for COVID-19.
In silico screening was performed to identify the potent herbal compounds present in C. longa that can inhibit the SARS-CoV-2 Mpro [420]. The two compounds, C1 (1E,6E)-1,2,6,7-tetrahydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione) and C2 (4Z,6E)-1,5-dihydroxy-1,7-bis(4-hydroxyphenyl)hepta-4,6-dien-3-one exhibited strong binding with the catalytic site of SARS-CoV-2 Mpro indicating potential inhibitory activity [420]. The two polyphenols, Catechin and Curcumin, were found to possess dual binding affinity (binds to SARS-CoV-2 spike protein and ACE2). Although catechin binds with a greater affinity, curcumin can bind directly to the RBD of SARS-CoV-2 spike protein [421]. The findings from the computational study indicate the therapeutic potential of the above two polyphenols for managing COVID-19. The bioavailability of curcumin and other herbal constituents obtained from medicinal plants can be further enhanced using advanced technologies such as liposomes, nanoparticles, micelles, phospholipid complexes, and other adjuvants [422].
Nutritional and dietary approach
Nutrition plays a significant role in the treatment and prevention of numerous diseases [421, 423-426]. Plant-derived foods augmented the number of beneficial bacteria in the intestine, which are highly useful and constitute 85% of the immune system. Using abundant water, micronutrients, minerals (zinc and magnesium), vitamins C, D, and E rich food, omega-3 fatty acids, docosahexaenoic acid, herbs, a healthier lifestyle can strengthen the health status and might overcome the SARS-CoV-2 infection [427]. Several reports have documented the preventive effects of bioflavonoid quercetin and antioxidant glutathione against COVID-19 [428, 429]. In COVID-19 also recommendations have been put forward regarding the quality and quantity of food. WHO recommends eating fresh and unprocessed foods, drinking enough water every day, eating moderate amounts of fat and oil, eating less salt and sugar, and avoiding eating out [430]. UNICEF has also provided healthy tips and hygienic recommendations including keeping up fruit and vegetable intake, swapping in healthy dried or canned alternatives when fresh produce is not available, limiting high processed foods, and doing the cooking and eating fun and meaningful part of your family routine [431]. Hygienic guidelines include washing hands, using separate chopping boards, proper cooking, avoiding expiry of perishable items, proper disposal, and using clean utensils.
There are nutritional recommendations for people under quarantine [432]. Carbohydrate-rich foods like banana, almonds, cherries, and oats contain melatonin and serotonin help in lowering stress. Similarly, protein-rich foods like milk and milk products containing sleep-inducing amino acid tryptophan lowering stress besides regulating satiety and calorie centre [432]. Macronutrients and micronutrients (vitamin E, C, A, D) help maintain immunity and prevent oxidative stress [432]. Foods, fruits, and vegetables rich in these nutrients can prove beneficial in preventing and curing COVID-19. Diets containing minerals, including zinc, are vital for health and immunity and inhibit the synthesis of viral genetic material [432]. Our normal diet contains several natural antioxidants that are required for the growth and proper functioning of the body. These natural antioxidants include capsaicin, sesamin, ellagic acid, epicatechin, and galangin. In silico analysis has identified that these natural antioxidants have the potential to bind to the catalytic site of Mpro enzyme. Therefore, the natural antioxidants present in our diet can act as potential candidate drugs for treating COVID-19 [433].
Zinc is a vital micronutrient involved in the synthesis of DNA and cell replication. It also regulates adaptive and innate immune responses, cell-cell communication, and synthesis of immune cells [434, 435]. The in vitro inhibitory potential exhibited by zinc (Zn) is mediated by different mechanisms. Zn deficiency will be associated with an increased risk for severe SARS-CoV-2 infection in the susceptible population. Therefore, the potential utility of Zn supplementation (combined with an ionophore) has to be further studied using RCT in patients with COVID-19 [436]. Magnesium is also an essential mineral and electrolyte that build up our immune system by increasing lymphocytes and NK cells. It contributes to the hemoglobin for carrying oxygen from the lungs to the whole body, thus helping to COVID-19-infected individuals. Moreover, magnesium is a vital energy source for the proper functioning of our body's cells [437].
Vitamin C (ascorbic acid) has a vital role in regulating the function of the immune system. In addition to that, vitamin C also modulates stress response and has exhibited promising results in critically ill patients [438-442]. Intravenous vitamin C therapy is considered an alternative therapeutic strategy for managing COVID-19 due to the direct antiviral activity, antioxidant properties, and immunomodulatory potential [442]. Therefore, intravenous vitamin C therapy might be beneficial in managing cytokine storm associated with ARDS in COVID-19. Quercetin, a flavonoid that possesses antiviral properties, has been widely studied [441]. Therefore, the combination of quercetin and vitamin C might produce a synergistic antiviral action due to the overlapping antiviral mechanism that can be utilized for managing COVID-19 [441, 443].
Similarly, vitamin D supplementation at high-doses might impart potential benefits in the susceptible population, decreasing the severity of COVID-19 and risk of mortality [444, 445]. Vitamin D can reduce pro-inflammatory cytokine concentration and lower the rate of viral replication via inducing defensins and cathelicidins [445]. Reports evidenced that the supplementation of vitamin D reduced the COVID-19 risk in winter when 25-hydroxyvitamin D (25(OH)D) level is decreased. Therefore, intaking vitamin D might decrease COVID-19 and other related infectious diseases [26, 445, 446].
D receptors are expressed in many immune cells, which affect their function through ligand binding. Notably, vitamin D, as such, dramatically influences immune responses. For instance, it differentiates monocytes from macrophages, improving their killing ability, stimulates cytokines production; and is involved in antigen presentation. Moreover, vitamin D metabolites play a role in regulating the synthesis of specific antimicrobial peptides that directly destroy pathogenic microorganisms and halt infection [447, 448]. Consumption of vitamin D at larger doses for a week followed by a smaller dose will ensure rapid restoration of vitamin D levels [444, 445]. The consumption of vitamin D as adjuvant therapy is believed to improve the clinical status as well as prognosis of patients with COVID-19 [444, 445]. Thus, it is reasonable to suggest vitamin D supplementation in subpopulations with unfavorable COVID-19 outcomes and vitamin D deficiency, as well as, in SARS-CoV-2-infected individuals to supply the optimal concentrations of 25(OH)D3 as rapidly as possible [449].
Considering the food security during the COVID-19 pandemic, UNSCN has initiated a resource list on food systems and nutrition responses [450]. A study has also provided measures for ensuring nutritional safety during the COVID-19 pandemic [424]. Better nutrition can help in minimizing severity and mortality [424]. It improves the quality of life, boosts immunity, prevents infection, or minimizes severity, and helps in post-infection recovery [424, 451]. Nutritional deficiency can predispose to COVID-19 by lowering immunity, increasing the risk of infection to SARS-CoV-2, and aggravate severity or complications [452]. Malnutrition weakens the patient already in the deprived state and significantly lengthens recovery times [453].
Conclusion and future prospects
COVID-19 has spread across the globe at a rapid pace overthrowing our control and preventive strategies due to efficient human-to-human transmission. This extremely high transmission rate along with different types of disease manifestation such as asymptomatic, mild, and severe forms have not been observed in our previous interaction with novel coronaviruses such as SARS-CoV and MERS-CoV. Considering the present situation, the only way by which we can slow down the spread of this virus is through global vaccination programs or by the natural development of herd immunity. However, both of these scenarios seem to be farfetched and are not achievable in the near future. Therefore, we have to direct our efforts to identify and repurpose drugs that are having already established safety profile. At the time of writing this manuscript, several preclinical and clinical studies of potential antiviral and immunomodulatory drugs are being conducted to identify an efficacious and safe therapeutic agent that can be used to manage the ongoing pandemic. At present, most reports that suggest therapeutic efficacy are single-group intervention studies and lack a control group. Though the randomized control studies are a globally accepted standard for testing any novel therapeutic drug, it is time-consuming and requires ethical consideration to exclude patients receiving a lifesaving drug by enrolling in the control group. At the same time, in the absence of a control group, it is very difficult to reach a conclusion regarding the therapeutic efficacy of the administered drug. The differentiation of drug-associated adverse effects from the disease manifestations is also more challenging and often may lead to misinterpretation.
Immunotherapies are a novel mode of antiviral treatment. Although adoptive T cell, modified CAR T cells, and NK cell-based therapies have been found safe and effective in cancer patients, their true anti-viral potential need to be fully understood through large randomized controlled studies. Some factors such as cost, time, and prevention of excessive immune activation also need to be considered. Type-1 and III IFN are attractive antiviral agents that can be combined with other antiviral drugs to achieve the synergistic effect. CAR T cell therapy studies in cancer patients are shown to induce cytokine release syndrome similar to SARS-CoV-2 infection. Mesenchymal stem cell-based immunomodulatory therapy also showed a benefit to the COVID-19 patient. Other modes of immunotherapies include cytokines, TRL agonists, and monoclonal antibodies. At present, the therapeutic monoclonal antibodies with virus-neutralizing potential are the most promising practical approach that can be produced on a large scale, and their application for treating COVID-19 patients can be scaled up for treating a large number of patients during the ongoing pandemic situation. While applying these therapies for treating COVID-19 patients, excessive caution needs to be implemented. The main aim of immunotherapeutic treatments for COVID-19 patients should avoid the risk of excessive activation and inflammation that could damage host tissues and aggravate the SARS-CoV-2 mediated pathology.
Over the past several months, several therapeutic agents such as oseltamivir, remdesivir, ribavirin, favipiravir, danoprevir, teicoplanin, arbidol, chloroquine, hydroxychloroquine, ivermectin, baricitinib, camostat mesylate, nafamostat mesylate, nitazoxanide, lopinavir, ritonavir, and several others have been evaluated for treating COVID-19 patients. The approaches used to evaluate the efficacy and safety of drugs under the current clinical scenario varied from case reports to randomized control trials. To evaluate a therapeutic drug against SARS-CoV-2, randomized multi-center clinical trials should be conducted in a large population. Several therapeutic drug candidates have exhibited potential anti-SARS-CoV-2 activity in both in silico analyses and in vitro studies. However, this does not guarantee that such agents can be used as a sole therapeutic agent to treat COVID-19.
Supportive therapy also plays a major role in deciding the outcome of COVID-19 treatment. The inclusion of suitable supportive therapies can further improve the chance of patient survival thereby reducing the mortality rate. Preliminary reports suggest that supportive therapeutic strategies such as corticosteroid therapy, traditional Chinese medicine, immunomodulatory agents, stem cell therapy, JAK2 inhibitors, IL-6 receptor inhibitors, and nutritional therapy can further improve the clinical outcome COVID-19. However, further systematic studies are warranted before jumping to conclusions. The findings obtained from the largest randomized control trial conducted in the UK is a promising one since the therapeutic use of dexamethasone has been found to reduce COVID-19–associated death. Immune dysregulation and hyper inflammation phase contribute to a greater part of the pathogenesis associated with SARS-CoV-2 infection. Therefore, attaining a deeper understanding of the cellular, and molecular factors that contribute to the high virulence capacity of SARS-CoV-2 will help us to target key components of replication and infection process through the development of therapeutic drugs against it. The important role of herbal products with broad-spectrum anti-viral, anti-inflammatory, and immune system boosting activities has also been revealed during the ongoing SARS-CoV-2 infection. This approach also requires further understanding of the mechanism of action, standard dosage, frequency of administration, quality assurance, safety, and efficacy through randomized control studies. This will have a huge impact on low-and middle-income countries as a cost-effective prevention and control measure that can be scaled up easily.
Currently, our understanding of the molecular mechanism and pathogenesis involved in COVID-19 infection is limited and lacks clarity. Thus, unexpected interactions may be overlooked in the haste to develop therapeutic modalities against COVID-19. Hence, a thorough analysis of scientific evidence must be required to avoid any unnecessary misinterpretation of data to avoid any premature conclusions that may have serious long-term consequences. It is necessary to evaluate all drug candidates at our disposal through a series of rigorous in vivo and in vitro preclinical studies, as well as large-scale randomized controlled clinical trials before their inclusion into therapeutic recommendations, guidelines, and standard operating procedures.
Acknowledgments
All the authors acknowledge and thank their respective institutes and universities. The figures are created using BioRender.com. All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
Ethics approval
Not required.
Conflict of interest
All the authors declare that there are no conflicts of interest.
Funding
This study received no external funding.
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
How to cite
Sharun K, Tiwari R, Yatoo MI, et al. A comprehensive review on pharmacologic agents, immunotherapies and supportive therapeutics for COVID-19. Narra J 2022; 2 (3): e92 -http://doi.org/10.52225/narra.v2i3.92.
References
- 1.Wang Z, Yang B, Li Q, et al. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020; 71(15): 769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhama K, Sharun K, Tiwari R, et al. Coronavirus disease 2019 – COVID-19. Clin Microbiol Rev 2020; 24;33(4):e00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee SK, Saha S, Munoz MNM. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front Mol Biosci 2020; 7:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussman JP. Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention. Front Pharmacol 2020; 11:1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omolo CA, Soni N, Fasiku VO, et al. Update on therapeutic approaches and emerging therapies for SARS-CoV-2 virus. Eur J Pharmacol 2020; 883:173348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frediansyah A, Tiwari R, Sharun K, et al. Antivirals for COVID-19: a critical review. Clinical Epidemiol Global Health 2021;9:90-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsenstein S, Herbert JA, McNamara PS, et al. COVID-19: Immunology and treatment options. Clin Immunol 2020; 215:108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrage DR, Koushesh S, Sofat N. Immunomodulatory drugs in the management of SARS-CoV-2. Front Immunol 2020; 11:1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha RP, Sharma AR, Singh MK, et al. Repurposing drugs, ongoing vaccine, and new therapeutic development initiatives against COVID-19. Front Pharmacol 2020; 11:1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharun K, Tiwari R, Dhama J, et al. Dexamethasone to combat cytokine storm in COVID-19: Clinical trials and preliminary evidence. Int J Surg 2020;82:179-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh TU, Parida S, Lingaraju MC, et al. Drug repurposing approach to fight COVID-19. Pharmacol Rep 2020; 72(6):1479-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabaan AA, Al-Ahmed SH, Sah R, et al. SARS-CoV-2/COVID-19 and advances in developing potential therapeutics and vaccines to counter this emerging pandemic. Ann Clin Microbiol Antimicrob 2020; 19(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vellingiri B, Jayaramayya K, Iyer M, et al. COVID-19: A promising cure for the global panic. Sci Total Environ 2020; 725:138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah B, Modi P, Sagar SR. In silico studies on therapeutic agents for COVID-19: Drug repurposing approach. Life Sci 2020; 252:117652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yatoo I, Hamid Z, Parray OR, et al. COVID-19 -Recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines. Hum Vaccin Immunother 2020; 16(12):2891-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhama K, Sharun K, Tiwari R, et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother 2020b:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury M, Rocco PRM, Phinney DG, et al. Cell-based therapies for coronavirus disease 2019: proper clinical investigations are essential. Cytotherapy 2020; 22(11):602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Chen X, Lu Y, et al. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends 2020. b; 14(1):64-68. [DOI] [PubMed] [Google Scholar]
- 19.Kalil AC. Treating COVID-19—off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 2020; 323(19):1897-1898. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Serradilla M, Risco C, Pacheco B. Drug repurposing for new, efficient, broad spectrum antivirals. Virus Res 2019; 264:22-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends 2020; 14(1):69-71. [DOI] [PubMed] [Google Scholar]
- 22.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 2020; 14(1):58-60. [DOI] [PubMed] [Google Scholar]
- 23.Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica 2020; 44:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature 2020; 582(7813):469. [DOI] [PubMed] [Google Scholar]
- 25.Infusino F, Marazzato M, Mancone M, et al. Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 Infection: A scoping review. Nutrients 2020; 12(6):1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panyod S, Ho CT, Sheen LY. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J Tradit Complement Med 2020; 10(4):420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alijotas-Reig J, Esteve-Valverde E, Belizna C, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun Rev 2020; 19(7):102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachanova V, Bishop MR, Dahi P, et al. Chimeric antigen receptor T cell therapy during the COVID-19 pandemic. Biol Blood Marrow Transplant 2020; 26(7):1239-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury M, Cuenca J, Cruz FF, et al. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J 2020; 55(6): 2000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nile SH, Nile A, Qiu J, et al. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 2020; 53:66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onofrio L, Caraglia M, Facchini G, et al. Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci OA 2020; 6(8):FSO605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulas K, Farsalinos K, Zanidis C. Activation of TLR7 and Innate immunity as an efficient method against covid-19 pandemic: Imiquimod as a potential therapy. Front Immunol 2020; 11:1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Market M, Angka L, Martel AB, et al. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front Immunol 2020; 11:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Zhou Y, Zhang M, et al. Updated approaches against SARS-CoV-2. Antimicrobial agents and chemotherapy 2020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magro G. SARS-CoV-2 and COVID-19: What are our options? Where should we focus our attention on to find new drugs and strategies? Magro G. SARS-CoV-2 and COVID-19: What are our options? Where should we focus our attention on to find new drugs and strategies?. Travel Med Infect Dis 2020;37:101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan RJ, Jha RK, Amera GM, et al. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2'-O-ribose methyltransferase. J Biomol Struct Dyn 2021;39(8):2679-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirza MU, Froeyen M. Structural elucidation of SARS-CoV-2 vital proteins: Computational methods reveal potential drug candidates against main protease, Nsp12 polymerase and Nsp13 helicase. J Pharm Anal 2020; 10(4):320-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rismanbaf A. Potential treatments for COVID-19; a narrative literature review. Rismanbaf A. Potential Treatments for COVID-19; a Narrative Literature Review. Arch Acad Emerg Med 2020;8(1):e29. [PMC free article] [PubMed] [Google Scholar]
- 39.Tian L, Liu W, Sun L. [Role of cyclophilin A during coronavirus replication and the antiviral activities of its inhibitors]. Sheng Wu Gong Cheng Xue Bao 2020; 36(4):605-611. [DOI] [PubMed] [Google Scholar]
- 40.Ulrich H, Pillat MM. CD147 as a Target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep 2020;16(3):434-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang K, Chen W, Zhou Y-S, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019; 18(1):41-58. [DOI] [PubMed] [Google Scholar]
- 43.Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020; 11(1):1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang X, Pearce R, Zhang Y. De novo design of protein peptides to block association of the SARS-CoV-2 spike protein with human ACE2. Aging 2020, 12(12):11263-11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qamar MT Ul, Alqahtani SM, Alamri MA, et al. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal 2020; 2020;10(4):313-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life sciences 2020:253:117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babaei F, Mirzababaei M, Nassiri-Asl M, et al. Review of registered clinical trials for the treatment of COVID-19. Drug Dev Res 2020;84(4):474-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobaiqy M, Qashqary M, Al-Dahery S, et al. Therapeutic management of COVID-19 patients: a systematic review. Infect Prev Pract 2020;2(3):100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A Review JAMA 2020;323(18):1824-1836. [DOI] [PubMed] [Google Scholar]
- 50.Anonymous . Some drugs for COVID-19. Med Lett Drugs Ther 2020; 62(1595):49-50. [PubMed] [Google Scholar]
- 51.Muralidharan N, Sakthivel R, Velmurugan D, et al. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. J Biomol Struct Dyn 2020; 39(7):2673-2678. [DOI] [PubMed] [Google Scholar]
- 52.Belhassan A, Chtita S, Zaki H, et al. Molecular docking analysis of N-substituted oseltamivir derivatives with the SARS-CoV-2 main protease. Bioinformation 2020; 16(5):404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortega JT, Serrano ML, Pujol FH, et al. Unrevealing sequence and structural features of novel coronavirus using in silico approaches: The main protease as molecular target. EXCLI J 2020; 19:400-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382(19):1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen H, Zhang Z, Wang L, et al. First clinical study using hcv protease inhibitor danoprevir to treat naive and experienced covid-19 patients. Medicine (Baltimore) 2020;99(48):e23357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim J, Jeon S, Shin HY, et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of covid-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci 2020; 35(6):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020; 323(15):1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Du F, Cao XJ, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in patients out of Wuhan from China: a case control study. BMC Infect Dis. 2021;21(1):207. Published 2021 Feb 24. doi:10.1186/s12879-021-05897-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhatnagar T, Murhekar MV, Soneja M, et al. Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: Protocol for restricted public health emergency use. Indian J Med Res 2020; 151(2):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials 2018; 19(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park SY, Lee JS, Son JS, et al. Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect 2019; 101(1):42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004; 59(3):252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agarwal S, Agarwal SK. Lopinavir-ritonavir in SARS-CoV-2 Infection and drug-drug interactions with cardioactive medications. Cardiovasc Drugs Ther 2021;35(3):427-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cozzupoli GM, Savastano MC, Falsini B, et al. Possible retinal impairment secondary to ritonavir use in SARS-CoV-2 patients: A narrative systematic review. Journal Ophthalmol 2020; 2020:5350494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levy C, Lassailly G, Parmentier E, et al. Caution with the use of lopinavir/ritonavir in severely ill patients for the treatment of SARS-CoV-2: A report of severe jaundice. Am J Gastroenterol 2020;115(10):1716-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.. FDA . Coronavirus (COVID-19) drugs. 2022. Available from: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs. Accessed: 4 December 2022. [Google Scholar]
- 67.Warren T, Jordan R, Lo M, et al. Nucleotide prodrug GS-5734 is a broad-spectrum filovirus inhibitor that provides complete therapeutic protection against the development of Ebola virus disease (EVD) in infected non-human primates. Open Forum Infectious Diseases: 2015: 2: LB-2. [Google Scholar]
- 68.Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016; 531(7594):381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 2018; 9(2):e00221-00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lo MK, Jordan R, Arvey A, et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep 2017; 7:43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017; 9(396):eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown AJ, Won JJ, Graham RL, et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res 2019; 169:104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis 2020; 34:101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo MK, Feldmann F, Gary JM, et al. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci Transl Med 2019; 11(494):eaau9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020; 11(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci 2020; 117(12):6771-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko WC, Rolain JM, Lee NY, et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents 2020; 55(4):105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agostini ML, Andres EL, Sims AC, et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018; 9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pedersen NC, Perron M, Bannasch M, et al. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J Feline Med Surg 2019; 21(4):271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harrison C. Coronavirus puts drug repurposing on the fast track. Nature Biotechnol 2020; 38(4):379-381. [DOI] [PubMed] [Google Scholar]
- 81.Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020; 382(10):929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020; 323(16):1582-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Norrie JD. Remdesivir for COVID-19: challenges of underpowered studies. Lancet 2020;395: P1525-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the Treatment of Covid-19 - Preliminary Report. Reply. N Engl J Med 2020; 383(10):994.. [DOI] [PubMed] [Google Scholar]
- 85.Pasquini Z, Montalti R, Temperoni C, et al. Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU. J Antimicrob Chemother 2020; 75(11):3359-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - Final Report. N Engl J Med 2020; 383(19):1813-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020; 324(11):1048-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yalcin N, Demirkan K. COVID-19 and remdesivir in pediatric patients: the invisible part of the iceberg. Pediatr Res 2020; 89(6):1326-1327. [DOI] [PubMed] [Google Scholar]
- 89.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. New Engl J Med 2020; 382(24):2327-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi SW, Shin JS, Park S-J, et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antiviral Res 2020; 184:104955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med 2022; 386(4):305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mifsud EJ, Hayden FG, Hurt AC. Antivirals targeting the polymerase complex of influenza viruses. Antiviral Res 2019; 169:104545. [DOI] [PubMed] [Google Scholar]
- 93.Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther 2020; 209:107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther 2020; 108(2):242-247. [DOI] [PubMed] [Google Scholar]
- 95.Maxmen A. More than 80 clinical trials launch to test coronavirus treatments. Nature 2020; 578(7795):347. [DOI] [PubMed] [Google Scholar]
- 96.Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother 2020; 75(7):2013-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sada M, Saraya T, Ishii H, et al. Detailed molecular interactions of favipiravir with SARS-CoV-2, SARS-CoV, MERS- CoV, and Influenza Virus polymerases in silico. Microorganisms 2020; 8(10):1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai Q, Yang M, Liu D, et al. Experimental treatment with Favipiravir for COVID-19: An open-label control study. Engineering (Beijing) 2020; 6(10):1192-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watanabe S, Chan M, Suzuki W, et al. China says Japan-developed drug Avigan works against coronavirus. Positive reception by Chinese government contrasts with reservations in Japan. 2020 Available from: https://asianikkeicom/Business/Pharmaceuticals/China-says-Japan-developed-drug-Avigan-works-against-coronavirus2. Accessed: 2 May 2020. [Google Scholar]
- 100.Kaptein SJF, Jacobs S, Langendries L, et al. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci U S A 2020; 117(43):26955-26965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jean SS, Lee PI, Hsueh PR. Treatment options for COVID-19: The reality and challenges. J Microbiol Immunol Infect 2020; 53(3):436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khalili JS, Zhu H, Mak NSA, et al. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J Med Virol 2020; 92(7):740-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yuan J, Zou R, Zeng L, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res 2020; 69(6):599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tong S, Su Y, Yu Y, et al. Ribavirin therapy for severe COVID-19: a retrospective cohort study. Int J Antimicrob Agents 2020; 56(3):106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abbaspour Kasgari H, Moradi S, Shabani AM, et al. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J Antimicrob Chemother 2020; 75(11):3373-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eslami G, Mousaviasl S, Radmanesh E, et al. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. J Antimicrob Chemother 2020; 75(11):3366-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blaising J, Polyak SJ, Pécheur E-I. Arbidol as a broad-spectrum antiviral: an update. Antiviral research 2014; 107:84-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khamitov RA, Loginova S, Shchukina VN, et al. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol 2008; 53(4):9-13. [PubMed] [Google Scholar]
- 109.Wang Y, Ding Y, Yang C, et al. Inhibition of the infectivity and inflammatory response of influenza virus by arbidol hydrochloride in vitro and in vivo (mice and ferret). Biomed Pharmacother 2017; 91:393-401. [DOI] [PubMed] [Google Scholar]
- 110.Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int J Antimicrob Agents 2020; 56(2):105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aktas A, Tuzun B, Taskin AH, et al. How do arbidol and its analogs inhibit the SARS-CoV-2? Bratisl Lek Listy 2020; 121(10):705-711. [DOI] [PubMed] [Google Scholar]
- 112.Choudhary S, Silakari O. Scaffold morphing of arbidol (umifenovir) in search of multi-targeting therapy halting the interaction of SARS-CoV-2 with ACE2 and other proteases involved in COVID-19. Virus Res 2020; 289:198146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect 2020; 81(1):e1-e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alvi MM, Sivasankaran S, Singh M. Pharmacological and non-pharmacological efforts at prevention, mitigation, and treatment for COVID-19. J Drug Target 2020; 28(7-8):742-754. [DOI] [PubMed] [Google Scholar]
- 115.Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 2006; 129(6):1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen H, Zhang Z, Wang L, et al. First clinical study using HCV protease inhibitor danoprevir to treat COVID-19 patients. Medicine 2020; 99(48):e23357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gasmi A, Noor S, Tippairote T, et al. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol 2020:108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matsuyama S, Nao N, Shirato K, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A 2020; 117(13):7001-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawase M, Shirato K, van der Hoek L, et al. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol 2012; 86(12):6537-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoffmann M, Schroeder S, Kleine-Weber H, et al. Nafamostat mesylate blocks activation of SARS-CoV-2: New treatment option for COVID-19. Antimicrob Agents Chemother 2020; 64(6):e00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181(2):271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Asakura H, Ogawa H. Potential of heparin and nafamostat combination therapy for COVID-19. J Thromb Haemost 2020; 18(6):1521-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Doi K, Ikeda M, Hayase N, et al. Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with COVID-19: a case series. Crit Care 2020; 24(1):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hifumi T, Isokawa S, Otani N, et al. Adverse events associated with nafamostat mesylate and favipiravir treatment in COVID-19 patients. Crit Care 2020; 24(1):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andreani J, Le Bideau M, Duflot I, et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog 2020; 145:104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England) 2020; 395(10223):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang X, Zhang Y, Qiao W, et al. Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19. Int Immunopharmacol 2020; 86:106749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, et al. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford) 2020; 60(1):399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoang TN, Pino M, Boddapati AK, et al. Baricitinib treatment resolves lower airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 2020; 184(2):460-475.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020; 20(4):400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Favalli EG, Biggioggero M, Maioli G, et al. Baricitinib for COVID-19: a suitable treatment? Lancet Infect Dis 2020; 20(9):1012-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Praveen D, Chowdary PR, Aanandhi MV. Baricitinib-a januase kinase inhibitor-not an ideal option for management of COVID-19. Int J Antimicrob Agents 2020; 55(5):105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baron SA, Devaux C, Colson P, et al. Teicoplanin: an alternative drug for the treatment of coronavirus COVID-19. Int J Antimicrob Agents 2020; 105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou N, Pan T, Zhang J, et al. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola Virus, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV). J Biol Chem 2016; 291(17):9218-9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ceccarelli G, Alessandri F, Oliva A, et al. The role of teicoplanin in the treatment of SARS-CoV-2 infection: A retrospective study in critically ill COVID-19 patients (Tei-COVID study). J Med Virol. 2021;93(7):4319-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tripathi PK, Upadhyay S, Singh M, et al. Screening and evaluation of approved drugs as inhibitors of main protease of SARS-CoV-2. Int J Biol Macromol 2020; 164:2622-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ceccarelli G, Alessandri F, d'Ettorre G, et al. Is teicoplanin a complementary treatment option for COVID-19? The question remains. Int J Antimicrob Agents 2020; 56(2):106029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323(16):1574-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Devaux CA, Rolain JM, Colson P, et al. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents 2020;15:105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020; 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci trends 2020; 14(1):72-73. [DOI] [PubMed] [Google Scholar]
- 142.Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ 2020; 369:m1432. [DOI] [PubMed] [Google Scholar]
- 143.Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med 2020; 1(1):114-127.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; 71(15):732-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020; 56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146.Colson P, Rolain J-M, Lagier J-C, et al. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents 2020; 55:105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mahase E. COVID-19: six million doses of hydroxychloroquine donated to US despite lack of evidence. BMJ 2020;368:m1166. [DOI] [PubMed] [Google Scholar]
- 148.Hu TY, Frieman M, Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol 2020; 15(4):247-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pepperrell T, Pilkington V, Owen A, et al. Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19. J Virus Erad 2020; 6(2):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fantini J, Di Scala C, Chahinian H, et al. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents 2020; 55(5):105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guastalegname M, Vallone A. Could chloroquine/hydroxychloroquine be harmful in Coronavirus disease 2019 (COVID-19) treatment? Clin Infect Dis 2020; 71(15):888-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Duan YJ, Liu Q, Zhao SQ, et al. The trial of chloroquine in the treatment of COVID-19 and its research progress in forensic toxicology. Fa Yi Xue Za Zhi 2020; 36(2):157-163. [DOI] [PubMed] [Google Scholar]
- 153.Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res 2020; 177:104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barnard DL, Day CW, Bailey K, et al. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir Chem Chemother 2006; 17(5):275-284. [DOI] [PubMed] [Google Scholar]
- 155.Principi N, Esposito S. Chloroquine or hydroxychloroquine for prophylaxis of COVID-19. Lancet Infect Dis 2020; 20(10):1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yazdany J, Kim AHJ. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med 2020; 172(11):754-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Leung LS, Neal JW, Wakelee HA, et al. Rapid onset of retinal toxicity from high-dose hydroxychloroquine given for cancer therapy. Am J Ophthalmol 2015; 160(4):799-805 e791. [DOI] [PubMed] [Google Scholar]
- 158.Marmor MF. COVID-19 and chloroquine/hydroxychloroquine: Is there ophthalmological concern? Am J Ophthalmol 2020; 216:A1-A2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gevers S, Kwa MSG, Wijnans E, et al. Safety considerations for chloroquine and hydroxychloroquine in the treatment of COVID-19. Clin Microbiol Infect 2020; 26(9):1276-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Edelstein CL, Venkatachalam MA, Dong Z. Autophagy inhibition by chloroquine and hydroxychloroquine could adversely affect acute kidney injury and other organ injury in critically ill patients with COVID-19. Kidney Int 2020; 98(1):234-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med 2020; 382(25):2411-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A randomized clinical trial. JAMA Netw Open 2020; 3(4):e208857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee Z, Rayner CR, Forrest JI, et al. The Rise and fall of hydroxychloroquine for the treatment and prevention of COVID-19. Am J Trop Med Hyg 2020; 104(1):35-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gotz V, Magar L, Dornfeld D, Giese S, Pohlmann A, Hoper D, et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep 2016; 6:23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wagstaff K, Sivakumaran H, Heaton S, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J 2012; 443(3):851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tay MYF, Fraser JE, Chan WKK, et al. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir Res 2013; 99(3):301-306. [DOI] [PubMed] [Google Scholar]
- 167.Yang SNY, Atkinson SC, Wang C, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir Res 2020; 177:104760. [DOI] [PubMed] [Google Scholar]
- 168.Lundberg L, Pinkham C, Baer A, et al. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan equine encephalitis virus replication. Antivir Res 2013; 100(3):662-672. [DOI] [PubMed] [Google Scholar]
- 169.Sharun K, Dhama K, Patel SK, et al. Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19. Ann Clin Microbiol Antimicrob 2020; 19(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Caly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020; 178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van der Watt PJ, Chi A, Stelma T, et al. Targeting the nuclear import receptor Kpnβ1 as an anticancer therapeutic. Mol Cancer Ther 2016; 15(4):560-573. [DOI] [PubMed] [Google Scholar]
- 172.Momekov G, Momekova D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view. Biotechnol Biotechnol Equip 2020; 34(1):469-474. [Google Scholar]
- 173.Chaccour C, Hammann F, Ramon-Garcia S, et al. Ivermectin and novel coronavirus disease (COVID-19): Keeping rigor in times of urgency. Am J Trop Med Hyg 2020; 102(6):1156-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bray M, Rayner C, Noel F, et al. Ivermectin and COVID-19: A report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors' responses. Antiviral Res 2020:104805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schmith VD, Zhou J, Lohmer LRL. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin Pharmacol Ther 2020; 108(4):762-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Camprubi D, Almuedo-Riera A, Marti-Soler H, et al. Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients. PLoS One 2020; 15(11):e0242184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.de Castro CG, Jr., Gregianin LJ, Burger JA. Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection. Leuk Lymphoma 2020;61(10):2536-2537. [DOI] [PubMed] [Google Scholar]
- 178.Patri A, Fabbrocini G. Hydroxychloroquine and ivermectin: A synergistic combination for COVID-19 chemoprophylaxis and treatment? J Am Acad Dermatol 2020; 82(6):e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mahmoud DB, Shitu Z, Mostafa A. Drug repurposing of nitazoxanide: can it be an effective therapy for COVID-19? J Genet Eng Biotechnol 2020; 18(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30(3):269-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Calderon JM, Zeron HM, Padmanabhan S. Treatment with Hydroxychloroquine vs Hydroxychloroquine + Nitazoxanide in COVID-19 patients with risk factors for poor prognosis: A structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21(1):504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rajoli RKR, Pertinez H, Arshad U, et al. Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis. Br J Clin Pharmacol 2020; 87(4):2078-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kelleni MT. Nitazoxanide/azithromycin combination for COVID-19: A suggested new protocol for early management. Pharmacol Res 2020; 157:104874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Masyeni S, Iqhrammullah M, Frediansyah A, et al. Molnupiravir: A lethal mutagenic drug against rapidly mutating severe acute respiratory syndrome coronavirus 2-A narrative review. J Med Virol 2022; 94(7):3006-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ohashi H, Watashi K, Saso W, et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience. 2021;24(4):102367. 10.1016/j.isci.2021.102367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Milewska A, Chi Y, Szczepanski A, et al. HTCC as a highly effective polymeric inhibitor of SARS-CoV-2 and MERS-CoV. J Virol 2021; 95(4):e01622-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chitsike L, Duerksen-Hughes P. Keep out! SARS-CoV-2 entry inhibitors: their role and utility as COVID-19 therapeutics. Virol J. 2021 Jul 23;18(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li Z, Wu C, Li Y, et al. Free energy perturbation–based large-scale virtual screening for effective drug discovery against COVID-19. Int J High Perform Comput Appl. 2022;10943420221117797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gassen NC, Niemeyer D, Muth D, et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat Commun 2019; 10(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jeon S, Ko M, Lee J, et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother 2020; 64(7):e00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Matsuyama S, Kawase M, Nao N, et al. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. J Virol 2020; 95(1):e01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Agostini ML, Pruijssers AJ, Chappell JD, et al. Small-Molecule antiviral beta-d-N (4)-Hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol 2019; 93(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 2020; 12(541):eabb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chien M, Anderson TK, Jockusch S, et al. Nucleotide analogues as inhibitors of viral polymerases. J Proteome Res 2020; 19(11):4690-4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ge Y, Tian T, Huang S, et al. A data-driven drug repositioning framework discovered a potential therapeutic agent targeting COVID-19. Signal Transduct Target Ther 2020; 6(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Beck BR, Shin B, Choi Y, et al. Predicting commercially available antiviral drugs that may act on the novel coronavirus (2019-nCoV), Wuhan, China through a drug-target interaction deep learning model. Comput Struct Biotechnol J 2020; 18:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Macip G, Garcia-Segura P, Mestres-Truyol J, et al. A Review of the Current Landscape of SARS-CoV-2 Main Protease Inhibitors: Have We Hit the Bullseye Yet? Int J Mol Sci. 2021;23(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ju J, Li X, Kumar S, et al. Nucleotide Analogues as Inhibitors of SARS-CoV Polymerase. Pharmacol Res Perspect 2020; 8(6):e00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen YW, Yiu CB, Wong KY. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL (pro)) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Res 2020; 9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu X, Wang XJ. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genomics 2020; 47(2):119-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fan HH, Wang LQ, Liu WL, et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin Med J (Engl) 2020; 133(9):1051-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang J. Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J Chem Inf Model 2020; 60(6):3277-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jin Z, Du X, Xu Y, et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature 2020; 582(7811):289-293. [DOI] [PubMed] [Google Scholar]
- 204.Talluri S. Virtual screening based prediction of potential drugs for COVID-19. Comb Chem High Throughput Screen. 2021;24(5):716-728. [DOI] [PubMed] [Google Scholar]
- 205.Smith M, Smith JC. Repurposing therapeutics for covid-19: Supercomputer-based docking to the sars-cov-2 viral spike protein and viral spike protein-human ace2 interface. ChemRxiv 2020: PPR114930. [Google Scholar]
- 206.Wang Q, Zhao Y, Chen X, et al. Virtual screening of approved clinic drugs with main protease (3CLpro) reveals potential inhibitory effects on SARS-CoV-2. J Biomol Struct Dyn 2020; 40(2):685-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ran XH, Zhu JW, Chen YY, et al. Papain-like protease of SARS-CoV-2 inhibits RLR signaling in a deubiquitination-dependent and deubiquitination-independent manner. Front Immunol. 2022;13:947272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Elfiky AA. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J Biomol Struct Dyn 2020:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Enmozhi SK, Raja K, Sebastine I, et al. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach. J Biomol Struct Dyn 2021; 39(9):3092-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vuong W, Khan MB, Fischer C, et al. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat Commun 2020; 11(1):4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fu L, Ye F, Feng Y, et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat Commun 2020; 11(1):4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dey D, Borkotoky S, Banerjee M. In silico identification of Tretinoin as a SARS-CoV-2 envelope (E) protein ion channel inhibitor. Comput Biol Med 2020; 127:104063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Behloul N, Baha S, Guo Y, et al. In silico identification of strong binders of the SARS-CoV-2 receptor-binding domain. Eur J Pharmacol 2020:173701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sharun K, Tiwari R, Iqbal Yatoo M, et al. Antibody-based immunotherapeutics and use of convalescent plasma to counter COVID-19: advances and prospects. Expert Opin Biol Ther 2020; 20(9):1033-1046. [DOI] [PubMed] [Google Scholar]
- 215.Dassarma B, Tripathy S, Matsabisa M. Emergence of ancient convalescent plasma (CP) Therapy: to manage COVID-19 pandemic. Transfus Clin Biol 2020; 28(1):123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Misset B, Hoste E, Donneau AF, et al. A multicenter randomized trial to assess the efficacy of CONvalescent plasma therapy in patients with invasive COVID-19 and acute respiratory failure treated with mechanical ventilation: the CONFIDENT trial protocol. BMC Pulm Med 2020; 20(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Meher BR, Padhy BM, Das S, et al. Effectiveness of convalescent plasma therapy in the treatment of moderate to severe COVID 19 patients: A systematic review and meta-analysis. J Assoc Physicians India 2020; 68(12):35-43. [PubMed] [Google Scholar]
- 218.Fung M, Nambiar A, Pandey S, et al. Treatment of immunocompromised COVID-19 patients with convalescent plasma. Transpl Infect Dis 2021; 23(2):e13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 2020; 130(6):2757-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gunn BM, Yu W-H, Karim MM, et al. A role for Fc function in therapeutic monoclonal antibody-mediated protection against Ebola virus. Cell Host Microbe 2018; 24(2):221-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Van Erp EA, Luytjes W, Ferwerda G, et al. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Frontiers in immunology 2019; 10:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev 2020; 5(5):CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005; 24(1):44-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lai ST. Treatment of severe acute respiratory syndrome. Eur J Clin Microbiol Infect Dis 2005; 24(9):583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Soo YOY, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect 2004; 10(7):676-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Alsharidah S, Ayed M, Ameen RM, et al. COVID-19 convalescent plasma treatment of moderate and severe cases of SARS-CoV-2 infection: A multicenter interventional study. Int J Infect Dis 2020; 103:439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Altuntas F, Ata N, Yigenoglu TN, et al. Convalescent plasma therapy in patients with COVID-19. Transfus Apher Sci 2020; 60(1): 102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.von Rhein C, Scholz T, Henss L, et al. Comparison of potency assays to assess SARS-CoV-2 neutralizing antibody capacity in COVID-19 convalescent plasma. J Virol Methods 2020; 288:114031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mehew J, Johnson R, Roberts D, et al. Convalescent plasma for COVID-19: male gender, older age and hospitalisation associated with high neutralising antibody levels, England, 22 April to 12 May 2020. Eurosurveill 2020; 25(45):2001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 2020; 117(17):9490-9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Harvala H, Mehew J, Robb ML, et al. Convalescent plasma treatment for SARS-CoV-2 infection: analysis of the first 436 donors in England, 22 April to 12 May 2020. Eurosurveill 2020; 25(28):2001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hegerova L, Gooley TA, Sweerus KA, et al. Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood 2020; 136(6):759-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Duan K, Liu B, Li C, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. PNAS 2020; 117 (17) 9490-9496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chen L, Xiong J, Bao L, et al. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 2020; 20(4):398-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis 2020; 7(3):ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Terpos E, Politou M, Sergentanis TN, et al. Anti–SARS-CoV-2 antibody responses in convalescent plasma donors are increased in hospitalized patients; subanalyses of a phase 2 clinical study. Microorganisms 2020; 8(12):1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med 2021; 384(7):619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rojas M, Anaya J-M. Why will it never be known if convalescent plasma is effective for COVID-19. J Transl Autoimmun 2020; 3:100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc 2020; 95(9):1888-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Samad N, Sodunke TE, Al Banna H, et al. Convalescent plasma therapy for management of COVID-19: Perspectives and Deployment in the current global pandemic. Risk Manag Healthc Policy 2020; 13:2707-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wood EM, Estcourt LJ, McQuilten Z. How should we use convalescent plasma therapies for COVID-19? Blood 2020.; 137(12):1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Vitale M, Cantoni C, Della Chiesa M, et al. An historical overview: The discovery of how NK cells can kill enemies, recruit defense troops, and more. Front Immunol 2019; 10:1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Caligiuri MA. Human natural killer cells. Blood 2008; 112(3):461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.van Eeden C, Khan L, Osman MS, et al. Natural killer cell dysfunction and its role in COVID-19. Int J Mol Sci 2020; 21(17):6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Manickam C, Sugawara S, Reeves RK. Friends or foes? The knowns and unknowns of natural killer cell biology in COVID-19 and other coronaviruses in July 2020. PLoS Pathog 2020; 16(8):e1008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wu Y, Huang X, Sun J, et al. Clinical characteristics and immune injury mechanisms in 71 patients with COVID-19. mSphere 2020; 5(4):e00362-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Stephen-Victor E, Das M, Karnam A, et al. Potential of regulatory T-cell-based therapies in the management of severe COVID-19. Eur Respir J 2020; 56(3):2002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hu Y, Tan Su Yin E, Yang Y, et al. CAR T-cell treatment during the COVID-19 pandemic: Management strategies and challenges. Curr Res Transl Med 2020; 68(3):111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med 2006; 12(10):1160-1166. [DOI] [PubMed] [Google Scholar]
- 250.Caccamo N, Sullivan LC, Brooks AG, et al. Harnessing HLA-E-restricted CD8 T lymphocytes for adoptive cell therapy of patients with severe COVID-19. Br J Haematol 2020; 190(4):e185-e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181(7):1489-1501 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Braun J, Loyal L, Frentsch M, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020; 587(7833):270-274. [DOI] [PubMed] [Google Scholar]
- 253.Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol 2020; 21(3):e168-e178. [DOI] [PubMed] [Google Scholar]
- 254.Elsallab M, Levine BL, Wayne AS, et al. CAR T-cell product performance in haematological malignancies before and after marketing authorisation. Lancet Oncol 2020; 21(2):e104-e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Prasad V. Immunotherapy: Tisagenlecleucel - the first approved CAR-T-cell therapy: implications for payers and policy makers. Nat Rev Clin Oncol 2018; 15(1):11-12. [DOI] [PubMed] [Google Scholar]
- 256.Yoon S, Eom GH. Chimeric Antigen receptor T cell therapy: A novel modality for immune modulation. Chonnam Med J 2020; 56(1):6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol 2019; 10:2040620719841581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Titov A, Valiullina A, Zmievskaya E, et al. Advancing CAR T-cell therapy for solid tumors: Lessons learned from lymphoma treatment. Cancers (Basel) 2020; 12(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Seif M, Einsele H, Loffler J. CAR T Cells Beyond Cancer: Hope for Immunomodulatory Therapy of Infectious Diseases. Front Immunol 2019; 10:2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liu A. Scientists explore using CAR-T and other engineered immune cells to target COVID-19. Fierce Biotect 2020. 2020. Available from: https://www.fiercebiotech.com/research/scientists-explore-using-engineered-t-cells-to-target-covid-19. Accessed: 23 April 2020.
- 261.Bishop MR. Optimizing administration of CAR T-cell therapy during the COVID-19 pandemic. Clin Adv Hematol Oncol 2020; 18(7):400-403. [PubMed] [Google Scholar]
- 262.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 264.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018; 378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Shanmugaraj B, Siriwattananon K, Wangkanont K, et al. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol 2020; 38(1):10-18. [DOI] [PubMed] [Google Scholar]
- 266.Tian X, Li C, Huang A, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect 2020; 9(1):382-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 2020; 12(3):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kumar V, Jung YS, Liang PH. Anti-SARS coronavirus agents: a patent review (2008 - present). Expert Opin Ther Pat 2013; 23(10):1337-1348. [DOI] [PubMed] [Google Scholar]
- 269.Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 2020; 11(1):2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.ter Meulen J, van den Brink EN, Poon LL, et al. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med 2006; 3(7):e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020; 584(7821):443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Basu M. US begins clinical trial of an artificial antibody for Covid-19 treatment. 2020. Available from: https://theprint.in/health/us-begins-clinical-trial-of-an-artificial-antibody-for-covid-19-treatment/402978/. Accessed: 16 April 2020. [Google Scholar]
- 273.Jahanshahlu L, Rezaei N. Monoclonal antibody as a potential anti-COVID-19. Biomed Pharmacother 2020; 129:110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yu X, Xu Q, Wu Y, et al. Nanobodies derived from Camelids represent versatile biomolecules for biomedical applications. Biomater Sci 2020; 8(13):3559-3573. [DOI] [PubMed] [Google Scholar]
- 275.Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ 2020; 370:m3379. [DOI] [PubMed] [Google Scholar]
- 276.Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev 2020; 19(7):102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Miao Y, Fan L, Li JY. Potential treatments for COVID-19 related cytokine storm - beyond corticosteroids. Front Immunol 2020; 11:1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sun Y, Dong Y, Wang L, et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: The Beijing experience. J Autoimmun 2020; 112:102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature 2020; 583(7816):437-440. [DOI] [PubMed] [Google Scholar]
- 280.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020; 117(20):10970-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol 2020; 92(7):814-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhang C, Wu Z, Li JW, et al. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 2020; 55(5):105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Nishimoto N, Kishimoto T, Yoshizaki K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann Rheum Dis 2000; 59(suppl 1):i21-i27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rubbert-Roth A, Furst DE, Nebesky JM, et al. A review of recent advances using tocilizumab in the treatment of rheumatic diseases. Rheumatol Ther 2018; 5(1):21-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Xu X HM, Li T, et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc Natl Acad Sci U S A 2020; 117(20):10970-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Clinical Trials. 2020 Available from https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=tocilizumab&cntry=&state=&city=&dist=. Acesssed: 14 April 2020. [Google Scholar]
- 287.Bennardo F, Buffone C, Giudice A. New therapeutic opportunities for COVID-19 patients with Tocilizumab: Possible correlation of interleukin-6 receptor inhibitors with osteonecrosis of the jaws. Oral Oncol 2020; 106:104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Keam S, Megawati D, Patel S, et al. Immunopathology and immunotherapeutic strategies in SARS-CoV-2 infection. Rev Medical Virol 2020; 30(5): e2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med 2020; 18(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 2020; 53(3):368-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016; 19(2):181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Xie J, Li Y, Shen X, et al. Dampened STING-dependent interferon activation in bats. Cell Host Microbe 2018; 23(3):297-301 e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cinatl J, Morgenstern B, Bauer G, et al. Treatment of SARS with human interferons. The Lancet 2003; 362(9380):293-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Falzarano D, de Wit E, Martellaro C, et al. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep 2013; 3(1):1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ströher U, DiCaro A, Li Y, et al. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-α. J Infect Dis 2004; 189(7):1164-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zielecki F, Weber M, Eickmann M, et al. Human Cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. J Virol 2013; 87(9):5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Loutfy MR, Blatt LM, Siminovitch KA, et al. Interferon Alfacon-1 plus corticosteroids in severe acute respiratory syndrome: A preliminary study. JAMA 2003; 290(24):3222-3228. [DOI] [PubMed] [Google Scholar]
- 299.Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 2014; 14(11):1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wang C, Rademaker M, Baker C, et al. COVID-19 and the use of immunomodulatory and biologic agents for severe cutaneous disease: An Australian/New Zealand consensus statement. Australas J Dermatol 2020; 61(3):210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020. a;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Shen K, Yang Y, Wang T, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts'consensus statement. World J Pediatr 2020; 6(3):223-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Habibzadeh P, Stoneman EK. The novel coronavirus: A bird's eye view. Int J Occup Environ Med 2020; 11(2):65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.WHO . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. 2020. Available from: https://apps.who.int/iris/handle/10665/330893. Accessed: 31 January 2020.
- 305.Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020; 395(10238):1695-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity 2019; 50(4):907-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Jackson S, Candia AF, Delaney S, et al. First-in-human study with the inhaled TLR9 oligonucleotide agonist AZD1419 results in interferon responses in the lung, and is safe and well-tolerated. Clin Pharmacol Ther 2018; 104(2):335-345. [DOI] [PubMed] [Google Scholar]
- 308.Anwar MA, Shah M, Kim J, et al. Recent clinical trends in Toll-like receptor targeting therapeutics. Med Res Rev 2019; 39(3):1053-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol 2005; 560:11-18. [DOI] [PubMed] [Google Scholar]
- 310.Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol 2014; 426(6):1246-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Totura AL, Whitmore A, Agnihothram S, et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio 2015; 6(3):e00638-00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mukherjee S, Huda S, Sinha Babu SP. Toll-like receptor polymorphism in host immune response to infectious diseases: A review. Scand J Immunol 2019; 90(1):e12771. [DOI] [PubMed] [Google Scholar]
- 313.Chakraborty C, Sharma AR, Bhattacharya M, et al. Consider TLR5 for new therapeutic development against COVID-19. J Med Virol 2020; 92(11):2314-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Li X, Geng M, Peng Y, et al. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 2020; 10(2):102-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Liang Y, Wang ML, Chien CS, et al. Highlight of Immune Pathogenic Response and Hematopathologic Effect in SARS-CoV, MERS-CoV, and SARS-Cov-2 Infection. Front Immunol 2020; 11:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020; 38(1):1-9. [DOI] [PubMed] [Google Scholar]
- 317.Angelopoulou A, Alexandris N, Konstantinou E, et al. Imiquimod - A toll like receptor 7 agonist - Is an ideal option for management of COVID 19. Environ Res 2020; 188:109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Englmeier L. A theory on SARS-COV-2 susceptibility: reduced TLR7-activity as a mechanistic link between men, obese and elderly. J Biol Regul Homeost Agents 2020; 34(3):1125-1129. [DOI] [PubMed] [Google Scholar]
- 319.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008; 133(2):235-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID- 19) outbreak - an update on the status. Mil Med Res 2020; 7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kikkert M. Innate immune evasion by human respiratory RNA viruses. J Innate Immun 2020; 12(1):4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Patra R, Chandra Das N, Mukherjee S. Targeting human TLRs to combat COVID-19: A solution? J Med Virol 2021; 93(2):615-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395(10229):1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect 2020; 80(6):607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol 2020; 92(10):2105-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Oladipo EK, Ajayi AF, Ariyo OE, et al. Exploration of surface glycoprotein to design multi-epitope vaccine for the prevention of Covid-19. Inform Med Unlocked 2020; 21:100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Bhattacharya M, Sharma AR, Patra P, et al. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J Med Virol 2020; 92(6):618-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Fransson M, Benson M, Erjefalt JS, et al. Expression of Toll-like receptor 9 in nose, peripheral blood and bone marrow during symptomatic allergic rhinitis. Respir Res 2007; 8(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Chosidow O, Dummer R. Imiquimod: mode of action and therapeutic potential. Acta Derm Venereol Suppl (Stockh) 2003(214):8-11. [DOI] [PubMed] [Google Scholar]
- 330.Skinner RB, Jr. Imiquimod as an immune response modulator in infectious conditions. Postgrad Med 2002; 112(6 Suppl Using):8-16. [DOI] [PubMed] [Google Scholar]
- 331.Serfozo P, Wysocki J, Gulua G, et al. Ang II (angiotensin ii) conversion to angiotensin-(1-7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension 2020; 75(1):173-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 2020; 134(5):543-545. [DOI] [PubMed] [Google Scholar]
- 333.Sommerstein R, Kochen MM, Messerli FH, et al. Coronavirus disease 2019 (COVID-19): Do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc 2020; 9(7):e016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med 2020; 27(3):taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens 2020; 38(5):781-782. [DOI] [PubMed] [Google Scholar]
- 336.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020; 34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID-19): A literature review. J Infect Public Health 2020; 13(5):667-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Shang L, Zhao J, Hu Y, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020; 395(10225):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zhou W, Liu Y, Tian D, et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther 2020; 5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Theoharides TC, Conti P. Dexamethasone for COVID-19? Not so fast. J Biol Regul Homeost Agents 2020; 34(3):1241-1243. [DOI] [PubMed] [Google Scholar]
- 341.Cain DW, Cidlowski JA. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat Rev Immunol 2020; 20(10):587-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - Preliminary report. N Engl J Med 2020; 384(8):693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Chen Q, Song Y, Wang L, et al. Corticosteroids treatment in severe patients with COVID-19: a propensity score matching study. Expert Rev Respir Med 2020; 15(4):543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020; 395(10223):473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Atluri S, Manchikanti L, Hirsch JA. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically Ill COVID-19 patients: The case for compassionate use. Pain Physician 2020; 23(2):E71-E83. [PubMed] [Google Scholar]
- 346.CELLTEX . Celltex Plans to Launch Program to Use Mesenchymal Stem Cells for COVID-19-related Symptoms Under an Existing Regulatory Study. 2020. Available at: https://wwwbiospacecom/article/releases/celltex-plans-to-launch-program-to-use-mesenchymal-stem-cells-for-covid-19-related-symptoms-under-an-existing-regulatory-study/ Accessed: 29 March 2020.
- 347.Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 2020; 11(2):216-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Desai D, Shende P. Nanoconjugates-based stem cell therapy for the management of COVID-19. Stem Cell Rev Rep 2020; 17(1):231-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Li Z, Niu S, Guo B, et al. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif 2020; 53(12):e12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Amorin B, Alegretti AP, Valim V, et al. Mesenchymal stem cell therapy and acute graft-versus-host disease: a review. Hum Cell 2014; 27(4):137-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sato K, Ozaki K, Mori M, et al. Mesenchymal stromal cells for graft-versus-host disease: Basic aspects and clinical outcomes. J Clin Exp Hematop 2010; 50(2):79-89. [DOI] [PubMed] [Google Scholar]
- 352.Xiao K, Hou F, Huang X, et al. Mesenchymal stem cells: current clinical progress in ARDS and COVID-19. Stem Cell Res Ther 2020; 11(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 2020; 11(2):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Qu W, Wang Z, Hare JM, et al. Cell-based therapy to reduce mortality from COVID-19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl Med 2020; 9(9):1007-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Iacob S, Iacob DG. SARS-coV-2 treatment approaches: numerous options, no certainty for a versatile virus. Front Pharmacol 2020; 11:1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zumla A, Wang FS, Ippolito G, et al. Reducing mortality and morbidity in patients with severe COVID-19 disease by advancing ongoing trials of mesenchymal stromal (stem) Cell (MSC) therapy - Achieving global consensus and visibility for cellular host-directed therapies. Int J Infect Dis 2020; 96:431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Börger V, Weiss DJ, Anderson JD, et al. ISEV and ISCT statement on EVs from MSCs and other cells: Considerations for potential therapeutic agents to suppress COVID-19. Cytotherapy 2020; 22(9):482-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Al-Khawaga S, Abdelalim EM. Potential application of mesenchymal stem cells and their exosomes in lung injury: an emerging therapeutic option for COVID-19 patients. Stem Cell Res Ther 2020; 11(1):1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res 2020; 155:104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res 2020; 156:104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Chen X, Wu Y, Chen C, et al. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm Sin B 2020; 11(1):222-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ni L, Zhou L, Zhou M, et al. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19 in Wuhan. Front Med 2020; 14(2):210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Anonymous . Redeploying plant defences. Nat Plants 2020; 6(3):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Poon PMK, Wong CK, Fung KP, et al. Immunomodulatory effects of a traditional Chinese medicine with potential antiviral activity: a self-control study. Am J Chin Med 2006; 34(01):13-21. [DOI] [PubMed] [Google Scholar]
- 365.Hsu C-H, Hwang K-C, Chao C-L, et al. Can herbal medicine assist against avian flu? Learning from the experience of using supplementary treatment with Chinese medicine on SARS or SARS-like infectious disease in 2003. J Altern Complement Med 2006; 12(6):505-506. [DOI] [PubMed] [Google Scholar]
- 366.Jahan I, Onay A. Potentials of plant-based substance to inhabit and probable cure for the COVID-19. Turk J Biol 2020; 44(3):228-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Zhang X-r, Li T-n, Ren Y-y, et al. The important role of volatile components from a traditional chinese medicine dayuan-yin against the COVID-19 pandemic. Front Pharmacol 2020; 11:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Li Y, Liu X, Guo L, et al. Traditional Chinese herbal medicine for treating novel coronavirus (COVID-19) pneumonia: protocol for a systematic review and meta-analysis. Syst Rev 2020; 9(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Huang YF, Bai C, He F, et al. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19). Pharmacol Res 2020; 158:104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lee DYW, Li QY, Liu J, et al. Traditional Chinese herbal medicine at the forefront battle against COVID-19: Clinical experience and scientific basis. Phytomedicine 2020; 80:153337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Wang JB, Wang ZX, Jing J, et al. Exploring an integrative therapy for treating COVID-19: A randomized controlled trial. Chin J Integr Med 2020; 26(9):648-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zhou L-p, Wang J, Xie R-h, et al. The effects of traditional Chinese medicine as an auxiliary treatment for COVID-19: A systematic review and meta-analysis. J Altern Complement Med 2020; 27(3):225-237. [DOI] [PubMed] [Google Scholar]
- 373.Mirzaie A, Halaji M, Dehkordi FS, et al. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19). Complement Ther Clin Pract 2020; 40:101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Xiong X, Wang P, Su K, et al. Chinese herbal medicine for coronavirus disease 2019: A systematic review and meta-analysis. Pharmacol Res 2020; 160:105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ang L, Lee HW, Kim A, et al. Herbal medicine for the management of COVID-19 during the medical observation period: A review of guidelines. Integr Med Res 2020; 9(3):100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Ang L, Song E, Lee HW, et al. Herbal Medicine for the Treatment of Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Med 2020; 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Alagawany MM, Attia YA, Farag MR, et al. The strategy of boosting the immune system under CoViD-19 pandemic. Front Vet Sci 2020; 7:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Fatima S, Haider N, Alam MA, et al. Herbal approach for the management of C0VID-19: an overview. Drug Metab Pers Ther 2020; 20200150. [DOI] [PubMed] [Google Scholar]
- 379.Islam MT, Sarkar C, El-Kersh DM, et al. Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phytother Res 2020; 34(10):2471-2492. [DOI] [PubMed] [Google Scholar]
- 380.Zhang Y, Li Y, Wang X, et al. Herbal plants coordinate COVID-19 in multiple dimensions - An insight analysis for clinically applied remedies. Int J Med Sci 2020; 17(18):3125-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Safa O, Hassaniazad M, Farashahinejad M, et al. Effects of Ginger on clinical manifestations and paraclinical features of patients with Severe Acute Respiratory Syndrome due to COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials 2020; 21(1):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Bailly C, Vergoten G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol Ther 2020; 214:107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Divya M, Vijayakumar S, Chen J, et al. South Indian medicinal plants can combat deadly viruses along with COVID-19? - A review. Microb Pathog 2020; 148:104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Divya M, Vijayakumar S, Chen J, et al. A review of South Indian medicinal plant has the ability to combat against deadly viruses along with COVID-19? Microb Pathog 2020; 148:104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Deng JG, Hou XT, Zhang TJ, et al. Carry forward advantages of traditional medicines in prevention and control of outbreak of COVID-19 pandemic. Chin Herb Med 2020; 12(3):207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Mohammadi N, Shaghaghi N. Inhibitory effect of eight secondary metabolites from conventional medicinal plants on COVID_19 virus protease by molecular docking analysis. Chemrxiv 2020; 11987475:v1. [Google Scholar]
- 387.Mani JS, Johnson JB, Steel JC, et al. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res 2020; 284:197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Shree P, Mishra P, Selvaraj C, et al. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)–a molecular docking study. J Biomol Struct Dyn 2020; 40(1):190-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Pahan P, Pahan K. Smooth or risky revisit of an old malaria drug for COVID-19? J Neuroimmune Pharmacol 2020; 15(2):174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Shoaib A, Azmi L, Shukla I, et al. Properties of ethno-medicinal plants and their bioactives-possible use for prevention and treatment of COVID-19: A review. Curr Pharm Des 2020; 26 (13). [DOI] [PubMed] [Google Scholar]
- 391.Adhikari B, Marasini BP, Rayamajhee B, et al. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother Res 2020; 35(3):1298-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Khaerunnisa S, Kurniawan H, Awaluddin R, et al. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints 2020, 2020030226. [Google Scholar]
- 393.Shaghaghi N. Molecular docking study of novel COVID-19 protease with low risk terpenoides compounds of plants. ChemRxiv 2020: 11935722.v1. [Google Scholar]
- 394.Wen CC, Kuo YH, Jan JT, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem 2007; 50(17):4087-4095. [DOI] [PubMed] [Google Scholar]
- 395.Avasarala S, Zhang F, Liu G, et al. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS One 2013; 8(2):e57285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Pooladanda V, Thatikonda S, Bale S, et al. Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-alpha mediated NF-kappaB and HDAC-3 nuclear translocation. Cell Death Dis 2019; 10(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Wu R, Wang L, Kuo HD, et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep 2020:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Batool F, Mughal EU, Zia K, et al. Synthetic flavonoids as potential antiviral agents against SARS-CoV-2 main protease. J Biomol Struct Dyn 2020; 40(8):3777-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Mouffouk C, Mouffouk S, Mouffouk S, et al. Flavonols as potential antiviral drugs targeting SARS-CoV-2 proteases (3CLpro and PLpro), spike protein, RNA-dependent RNA polymerase (RdRp) and angiotensin-converting enzyme II receptor (ACE2). Eur J Pharmacol 2020; 891:173759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Elsayed Y, Khan NA. Immunity-boosting spices and the novel coronavirus. ACS Chem Neurosci 2020; 11(12):1696-1698. [DOI] [PubMed] [Google Scholar]
- 401.Mohan S, Elhassan Taha MM, Makeen HA, et al. Bioactive natural antivirals: An updated review of the available plants and isolated molecules. Molecules 2020; 25(21):4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Boozari M, Hosseinzadeh H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother Res 2020; 35(2):864-876. [DOI] [PubMed] [Google Scholar]
- 403.Verma S, Twilley D, Esmear T, et al. Anti-SARS-CoV Natural products with the potential to inhibit SARS-CoV-2 (COVID-19). Front Pharmacol 2020; 11:561334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Ngwa W, Kumar R, Thompson D, et al. Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules 2020; 25(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Jo S, Kim S, Shin DH, et al. Inhibition of SARS-CoV 3CL protease by flavonoids. J J Enzyme Inhib Med Chem 2020; 35(1):145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Joshi T, Joshi T, Sharma P, et al. In silico screening of natural compounds against COVID-19 by targeting Mpro and ACE2 using molecular docking. Eur Rev Med Pharmacol Sci 2020; 24(8):4529-4536. [DOI] [PubMed] [Google Scholar]
- 407.Murck H. Symptomatic Protective action of glycyrrhizin (licorice) in covid-19 infection? Front Immunol 2020; 11:1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Sinha SK, Prasad SK, Islam MA, et al. Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: a pharmacoinformatics study. J Biomol Struct Dyn 2020; 39(13):4686-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Sinha SK, Prasad SK, Islam MA, et al. Potential leads from liquorice against SARS-CoV-2 main protease using molecular docking simulation studies. Comb Chem High Throughput Screen 2020; 24(4):591-597. [DOI] [PubMed] [Google Scholar]
- 410.Luo P, Liu D, Li J. Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int J Antimicrob Agents 2020; 55(6):105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Donma MM, Donma O. The effects of Allium sativum on immunity within the scope of COVID-19 infection. Med Hypotheses 2020; 144:109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Shahzad F, Anderson D, Najafzadeh M. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients 2020; 12(9):2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Thuy BTP, My TTA, Hai NTT, et al. Investigation into SARS-CoV-2 Resistance of Compounds in Garlic Essential Oil. ACS Omega 2020; 5(14):8312-8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Krupanidhi S, Abraham Peele K, Venkateswarulu TC, et al. Screening of phytochemical compounds of Tinospora cordifolia for their inhibitory activity on SARS-CoV-2: an in silico study. J Biomol Struct Dyn 2020; 39(15):5799-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Tripathi MK, Singh P, Sharma S, et al. Identification of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor. J Biomol Struct Dyn 2020; 39(15):5668-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Chikhale RV, Gurav SS, Patil RB, et al. Sars-cov-2 host entry and replication inhibitors from Indian ginseng: an in- silico approach. J Biomol Struct Dyn 2020. 39(12):4510-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Ghosh R, Chakraborty A, Biswas A, et al. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors - an in silico docking and molecular dynamics simulation study. J Biomol Struct Dyn 2020. 39(12):4362-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Gupta R, Misra A. Contentious issues and evolving concepts in the clinical presentation and management of patients with COVID-19 infectionwith reference to use of therapeutic and other drugs used in Co-morbid diseases (Hypertension, diabetes etc). Diabetes Metab Syndr 2020; 14(3):251-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Utomo RY, Meiyanto E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. 2020. Preprints 2020, 2020030214
- 420.Gupta S, Singh AK, Kushwaha PP, et al. Identification of potential natural inhibitors of SARS-CoV2 main protease by molecular docking and simulation studies. J Biomol Struct Dyn 2020; 39(12):4334-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Jena AB, Kanungo N, Nayak V, et al. Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational studies. Sci Rep. 2021 Jan 21;11(1):2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Gupta H, Gupta M, Bhargava S. Potential use of turmeric in COVID-19. Clin Exp Dermatol 2020; 45(7):902-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Childs CE, Calder PC, Miles EA. Diet and immune function. Nutrients 2019; 11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Mehta S. Nutritional status and COVID-19: an opportunity for lasting change? Clin Med (Lond) 2020; 20(3):270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Galanakis CM. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods 2020; 9(4):523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Jaggers GK, Watkins BA, Rodriguez RL. COVID-19: repositioning nutrition research for the next pandemic. Nutr Res 2020; 81:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Calder PC, Carr AC, Gombart AF, et al. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020; 12(4):1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Arshad MS, Khan U, Sadiq A, et al. Coronavirus disease (COVID-19) and immunity booster green foods: A mini review. Food Sci Nutr 2020; 8(8):3971-3976. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 429.Beigmohammadi MT, Bitarafan S, Hoseindokht A, et al. Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: a structured summary of a study protocol for a randomized controlled trial. Trials 2020; 21(1):614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.WHO . Nutrition: Nutrition advice for adults during the COVID-19 outbreak. 2020. Available from: https://www.emro.who.int/nutrition/covid-19/nutrition-advice-for-adults-during-the-covid-19-outbreak.html#:∼:text=Eat%20moderate%20amounts%20of%20fat%20and%20oil&text=Choose%20white%20meat%20(e.g.%20poultry, Avoid%20industrially%20produced%20trans%20fats. Accessed: 31 May 2020. [Google Scholar]
- 431.UNICEF . Easy, affordable and healthy eating tips during the coronavirus disease (COVID-19) outbreak. 2020. Available from: https://wwwuniceforg/coronavirus/easy-affordable-and-healthy-eating-tips-during-coronavirus-disease-covid-19-outbreak. Accessed: 31 May 2020.
- 432.Muscogiuri G, Barrea L, Savastano S, et al. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr 2020; 74(6):850-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Pandey AK, Verma S. An in-silico evaluation of dietary components for structural inhibition of SARS-Cov-2 main protease. J Biomol Struct Dyn 2020; 40(1):136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients 2017; 9(12):1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Fuhrman J. Immunity benefits of zinc as we age. 2020. Available from: https://www.verywellhealth.com/surprising-immunity-benefits-of-zinc-047431#:∼:text=Studies%20involving%20older%20people%20supplementing,markers%20 compared%20to%20younger%20adults. Accessed: 31 May 2020.
- 436.Brewer J, Gomez Marti JL, Brufsky A. Potential interventions for SARS-CoV-2 infections: Zinc showing promise. J Med Virol 2020; 93(3):1201-1203. [DOI] [PubMed] [Google Scholar]
- 437.Sanderson S. Immune System Defence with Vitamin C and Magnesium. 2020. Available from: https://www.elektramagnesium.com.au/immune-system-defence-with-vitamin-c-and-magnesium/. Accesssed: 31 May 2020.
- 438.Hoang BX, Shaw G, Fang W, et al. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist 2020; 23:256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Baladia E, Pizarro AB, Ortiz-Munoz L, et al. Vitamin C for COVID-19: A living systematic review. Medwave 2020; 20(6):e7978. [DOI] [PubMed] [Google Scholar]
- 440.Schloss J, Lauche R, Harnett J, et al. Efficacy and safety of vitamin C in the management of acute respiratory infection and disease: A rapid review. Adv Integr Med 2020; 7(4):187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Biancatelli RML Colunga, Berrill M, Catravas JD, et al. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 Related Disease (COVID-19). Front Immunol 2020; 11:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.de Melo AF, Homem-de-Mello M. High-dose intravenous vitamin C may help in cytokine storm in severe SARS-CoV-2 infection. Crit Care 2020; 24(1):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Simonson W. Vitamin C and coronavirus. Geriatr Nurs 2020; 41(3):331-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Ebadi M, Montano-Loza AJ. Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutr 2020; 74(6):856-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020; 12(4):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Xu Y, Baylink DJ, Chen C-S, et al. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. Journal of translational medicine 2020; 18(1):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 2009; 4(9):1151-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015; 7(6):4240-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Siuka D, Pfeifer M, Pinter B. Vitamin D Supplementation During the COVID-19 Pandemic. Mayo Clin Proc 2020; 95(8):1804-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.UNSCN . The COVID-19 pandemic is disrupting people's food environments: a resource list on Food Systems and Nutrition responses. 2020. Available from: https://wwwunscnorg/en/news-events/recent-news?idnews=2039. Accessed: 20 June 2020. [Google Scholar]
- 451.Romano L, Bilotta F, Dauri M, et al. Short Report - Medical nutrition therapy for critically ill patients with COVID-19. Eur Rev Med Pharmacol Sci 2020; 24(7):4035-4039. [DOI] [PubMed] [Google Scholar]
- 452.Dunn CG, Kenney E, Fleischhacker SE, et al. Feeding low-income children during the COVID-19 pandemic. N Engl J Med 2020; 382(18):e40. [DOI] [PubMed] [Google Scholar]
- 453.Ferrara F, De Rosa F, Vitiello A. The central role of clinical nutrition in COVID-19 patients during and after hospitalization in intensive care unit. SN Compr Clin Med 2020; 2(8):1064-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.