Abstract
Since the Final Rule regarding transplantation was published in 1999, organ distribution policies have been implemented to reduce geographic disparity. While a recent change in liver allocation, termed acuity circles, eliminated the donor service area as a unit of distribution to decrease the geographic disparity of waitlisted patients to liver transplantation, recently published results highlight the complexity of addressing geographic disparity. From geographic variation in donor supply, as well as liver disease burden and differing MELD scores of candidates and MELD scores necessary to receive a liver transplantation, to the urban-rural disparity in specialty care access, to neighborhood deprivation (community measure of socioeconomic status) in liver transplant access, addressing disparities of access will require a multi-pronged approach at the patient, transplant center, and national-level. Herein we review the current knowledge of these disparities – from variation in larger (regional) to smaller (census tract or zip code) levels to the common etiologies of liver disease that are particularly affected by these geographic boundaries. Geographic disparity in liver transplant access must balance the limited organ supply with the growing demand. We must identify patient-level factors that contribute to their geographic disparity and incorporate these findings at the transplant center-level to develop targeted interventions. We must simultaneously work at the national level to standardize and share patient data (including socioeconomic status and geographic social deprivation indices) to better understand the factors that contribute to geographic disparity. The complex interplay between organ distribution policy, referral patterns and variable waitlisting practices with the proportion of high MELD patients and differences in potential donor supply, must all be considered to create a national policy strategy to address the inequities in the system.
INTRODUCTION
The incidence of chronic liver disease (CLD) and rate of CLD-related hospitalizations continue to increase in the U.S., particularly in relation to nonalcoholic fatty liver disease (NAFLD) and alcohol-related liver disease (ALD).1,2 For patients who develop end stage liver disease (ELSD), the treatment of choice remains liver transplantation (LT), a lifesaving option for which disparities in access have persisted. In 1998, the Final Rule directed that organ allocation policies must not be based on LT candidates’ place of residence.3 Prior to 2013, organs were first distributed at the donor service area (DSA) level, which resulted in wide variation in median MELD scores at transplant (MMaT) across DSAs.4 Share 35 policy allowed broader sharing within Organ Procurement and Transplantation Network (OPTN) regions with the goal of reducing waitlist mortality for patients with the highest MELD scores.5 Post-Share 35, although this policy led to an increase in organ offers for those with allocation MELD ≥ 35, there was an overall decrease in organ acceptance rates with center and regional variations.6
In 2018, the United Network for Organ Sharing (UNOS) board of directors approved a distribution policy, termed ‘acuity circles’ (AC) that eliminated DSAs and instead used concentric circles centered around a donor hospital; this finally went into effect in 2020.7 UNOS’s two-year post-AC report shows increase in the number of LTs, especially among waitlisted candidates with MELD score of ≥ 29 and status 1 patients. At the same time, there has been a decrease in the number of waitlist removal for death or being too sick for transplant. Despite this, the variance in MMaT did not decrease as much as had been predicted.8 Furthermore, preliminary findings suggest that there might be center and regional variations in deceased donor LT (DDLTs) even post-AC, with regions 2, 7, and 9 not seeing an increase in DDLTs within the same allocation MELD category, and five out of twelve centers in region 5 accounting for the largest increase in DDLTs.4
The number of organ donors in the U.S. has been increasing over the last few years through the use of extended criteria donors (ECDs).9 As a result of the opioid epidemic, there has been an increase in the number of hepatitis C positive donors.10,11 Despite this, there are regional variations in both hepatitis C positive donors,12 and utilization of these organs in hepatitis C negative recipients.13 The organ supply problem is further exacerbated by the underutilization of donation after circulatory death (DCD) livers, which contribute to a relatively low number of the overall LT activity in the U.S. (6.1%) compared to other countries (Netherlands at 49.7%, Belgium at 42.3%, Switzerland at 26.7%, Spain at 26.0%, and U.K at 20.8%).14 For instance, from 2010 to 2020, there were 18,197 DCD livers offered, of which 38.1% (6,940) were procured and 27.1% (4,928) were transplanted.14 The utilization rate (livers transplanted over livers offered) in the U.S. is far below that of other countries – Belgium (74.2%), France (69.4%), Italy (68.1%), and Switzerland (61.8%) to name a few.14
The differences in DCD utilization can partly be accounted for by differences in liver perfusion utilization and donor selection criteria.14 The discard rate of deceased donor livers (recovered but not transplanted) is expected to increase from 22.0% to 56.0% from 2010 to 2030.15 In 2018, there were 1685 potential grafts eligible for procurement.16 While the introduction of machine perfusion has increased utilization of marginal organs through salvage transplantation of organs that would have otherwise been discarded,9 less than 5.0% of DCD livers are perfused using machine perfusion in the U.S., a drastically smaller proportion compared to other countries who perfuse 20.0–100.0% of DCD livers.14 In addition, countries with established perfusion protocols have higher rates of DCD liver utilization, with more than twice as many DCD liver offers being accepted.17 The benefits of liver perfusion are vast, including transplantation of 50.0 – 71.0% of discarded livers,16,18 improvement in cold19,20 and donor warm ischemic time,21 reducing ischemic cholangiopathy,22 and 94% 1-year overall graft survival.23 In the U.S., liver perfusion has the potential to increase the number of transplantable livers from high-risk donors,16,24 and address long cold ischemic time that might result from longer distance travel between centers.25
Studies suggest that the number of potential deceased donors in the U.S. are higher than the actual number of donors, with gaps ranging from 4,50026 to 31,000.27 This is further compounded by the variation in organ recovery by organ procurement organizations (OPOs), with some OPOs recovering 78.0% more donors than others, irrespective of hospital types and DSAs.28 Some attribute this variation in organ recovery on hospital referrals of ventilated patients to OPOs while others attribute it to center acceptance practices.29,30 A retrospective study across 2 DSAs covered by 2 OPOs found that only 26.0% of potential donors actually become donors, failure of hospital to refer ventilated patients to OPOs had a minor role in donor procurement variation, and center acceptance practices did not explain the differences in OPO performance.31 At some OPOs, 30.0% of donors fall under the subjective category of ‘noneligible’ ‘non-ideal group,’ which comprises older and DCD donors.32,33 As such, there have been recent calls to improve objectivity around donor selection in order to promote the utilization of all donors.34,35 In 2020, the Centers for Medicare and Medicaid Services (CMS) modified OPO performance metrics, with centers performing below the median in their donation area risking decertification. Early evidence showed an increase in the number of deceased donor recoveries, especially in older and DCD donors, with a decrease in the overall variation between OPOs.36
As OPO metrices are being evaluated and ECDs are being increasingly utilized, we must be cognizant of cost. LT, as the only lifesaving procedure for ESLD, has annual expenditure of $700 million annually in the U.S.,37 with earlier studies showing a 17.0% increase in direct hospital cost and 30.0% increase in indirect OPO cost for ECDs.38 MELD-based organ allocation and use of high-risk donors has been associated with longer hospital stay, higher overall complication rates, and thus higher cost.39,40 A more recent study found higher cost for DCDs per organ transplanted compared to donation after brain death (DBDs).41 A study in the Netherlands found that patients receiving DCD livers had higher rates of complications requiring intervention, acquiring higher costs than those receiving DBD livers.42 As liver perfusion allows us to transplant previously discarded livers and livers from high-risk donors, it remains to be seen the cumulative impact it will have on post-transplant cost. Currently, at a viability rate of 55.0%, the median cost to perform machine perfusion is $15,400 and the cost to identify one transplantable liver is $28,000, which is $6000 higher than the estimated monthly Medicare cost of providing care for a MELD 30 patient.16 A Canadian study found that the mean cost of transplant for perfused livers was $456,455 (versus $519,222 for standard cold solution), associated with greater Quality-Adjusted Life Years (QALYs; 3.48 vs. 3.17, respectively), decreased waitlist and mortality rate.43
Post-Share 35 gives us some insight on the potential costs associated with increased travel that might result from perfused livers. With broader regional sharing, Share 35 led to transplantation of sicker patients and increased distance that livers traveled.44 A study of 9 OPOs, found an increase in the number of imported and exported organs post-Share 35, with associated increase in average cost of 6.3% and 54.0%, respectively.45 Under acuity circles, a single transplant institution study (composed of 2 transplant centers in two different DSAs) found higher acceptance and transplantation of DCD donors post-AC. While there was no difference in percentage of donors requiring flights or distance traveled between pre- and post-AC period, the percentage of import donors (donors outside of recipient’s DSA) significantly increased. Post-AC, there was a 16.0% total increased cost per accepted donor and 55.0% increased cost per declined donor, which was attributed to import, acquisition, and charter flight fees.46 A single center study found that charter flight utilization for liver donor procurement led to significantly higher carbon emission compared to carbon emission from passenger car with 8.6% of the flights taken being within driving distance.47
Despite ECDs, machine perfusion technologies and OPO performance metrics, graft supplies continue to be limited. In addition, these efforts only affect waitlisted patients. While race, socioeconomic, and sex-based disparities play significant roles in LT access,3–11 growing evidence suggest geospatial factors beyond organ procurement and distribution might play an important and more upstream role. The purpose of this review is to highlight the geographic variation in LT access, from larger (regional) to smaller (census tract or zip code) levels, as well as the common etiologies of liver disease, that might contribute to geographic variation in LT access.
GEOGRAPHIC DISPARITIES BY REGION AND NEIGHBORHOOD
Hepatocellular Carcinoma (HCC) and policy changes to improve disparities
HCC patients comprise a significant subset of those being evaluated to undergo LT with geographic variations in HCC incidence and LT access. According to a study of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER), although the overall incidence of HCC has been declining since 2015, it has continued to rise in 26 states, correlating directly with state-level obesity and indirectly with state-level physical activity.48 Moreover, while urban communities account for 85.0% of cases diagnosed nationally,49 rural communities have an estimated average annual increase of 5.7% cases.49 At a more local level, neighborhood socioeconomic factors and access to health care played a larger role in HCC incidence than individual age, sex, and race.50 On an even more granular level, disparities in HCC incidence and stage of HCC at diagnosis exist at the zip code level, with one study showing significant “hotspot” areas with a high density of late-stage HCC and an attributable risk of 43.0% within these hotspots.51
HCC patients derive significant benefit from LT but have significant risk of waitlist dropout that is not reflected by their MELD score. As such, in 2002, HCC patients started receiving MELD exception points. This inadvertently led to increased waitlist removal for non-HCC patients by giving HCC-patients a significant advantage.52 In response, a six-month wait period before awarding MELD exception points and a MELD exception cap were enacted nationally, which led to improved LT access for non-HCC patients.53,54 However, because of the geographic differences in MELD score necessary to get a transplant, there have been unequal access to LT for HCC patients across regions, whereby OPTN regions with long wait times (e.g. regions 1, 5, and 9) have higher rates of waitlist drop out, often related to tumor progression or liver-related death.55 Furthermore, LT rates are significantly different between short and long wait-time regions,56 with patients in the West being two times less likely to undergo LT than patients in the Midwest.57 Mortality also varied, with one study showing lower death rates in regions 3, 6, and 1058 and another study showing mortality rates ranging from 21.6% in region 4 to 42.0% in region 2.59
To address the over prioritization of HCC patients for LT,54 OPTN introduced a policy change to cap the first HCC exception score to MMAT within DSA minus 3 points (MMaT-3), with early results suggesting a 22.0% decrease in LT for HCC patients, especially in low and medium MELD regions.60 These differences by region stem from geographic variation in MELD score needed to receive LT.61 Although AC was predicted to decrease this geographic variation in MMaT, early studies suggest that AC has not accomplished this goal.62,63 As we currently stand, for HCC patients within Milan Criteria with the same MELD exception points, there continues to be geographic variation in LT rates as a result of patients being listed in areas with very different MMaT,
Impact of Urban-Rural Area and Distance from LT Center on Waitlisting and Transplantation
Approximately 14.0% of the U.S. population live in rural areas,64 with most transplant centers located in urban areas.65 In fact, living > 150 miles from an LT center has been associated with 20.0% increased risk of overall mortality.66 This is due to the fact that patients from rural regions are 29.0% less likely to undergo LT evaluation,67 14.0% less likely to get waitlisted, and 20.0% less likely to receive LT than patients from urban areas.65,68 These urban-rural differences are irrespective of OPTN regions.68 The urban-rural LT disparity is more nuanced than just distance and specialty care location, with a patient’s socioeconomic status (SES) playing a mitigating or contributing role in overcoming these barriers. OPTN policies restrict the distance a graft can travel, but do not restrict patients from being evaluated or listed at any transplant center.69 An OPTN study of 104,914 waitlisted patients found that 2.8% were waitlisted at an LT center >500 miles from their residence, and 68.0% of that group received LT at a distant center.70 Patients who reside in higher income neighborhoods have higher education, non-Medicaid insurance,70 and are more likely to travel to other DSAs and regions, increasing their LT likelihood by up to 74.0%. These inter-DSA travelers tend to have compensated liver disease, low MELD,71 and originate from long wait time and high MELD regions.72 As expected, multiple DSA listing is associated with higher LT rates than single DSA listing (83.0% vs 36.0%) at a lower MELD (25 vs 32).72
A Role for Community and Neighborhood Deprivation in Addressing Geographic Disparity
An individual’s community can impact educational achievement, occupational prospects, and income opportunities, which can all directly affect health.73 Several area-based measures of SES exist, including at the level of county, zip-code, or census-tract. Neighborhood deprivation indices incorporate socioeconomic indicators such as housing stability, income/poverty, education, and employment to generate a composite index that captures the economic milieu of a particular neighborhood.74,75 Community health scores, a composite of community health and environmental risk, have been used as well with worse scores associated with rurality, increased distance to LT center and increased waitlist mortality.76
In particular, neighborhood deprivation has been associated with several health outcomes including all-cause mortality,77 cardiovascular mortality,78 and cancer mortality.79–81 It is associated with increased risk of HCC,82 with HCC cases within hotspots being more likely to be associated with racial and ethnic minorities, foreign-born individuals, and patients with Medicaid.51 A study of Canadian administrative health data of 38,700 decompensated cirrhotic and/or HCC patients found living in a low resource neighborhood associated with 45.0% lower odds of receiving LT compared to living in a high resource neighborhood.83 A single center retrospective study of 3,454 referred patients found that only a quarter were waitlisted for LT, with patients from more deprived communities being 44.0% less likely to get waitlisted.
Several studies have used neighborhood deprivation indices to assess pediatric LT from access to post-transplant outcomes. Pre-LT, increasing neighborhood deprivation was associated with higher MELD/PELD84 and lower odds of receiving living donor LT (LDLT).84,85 Compared to White patients, Black and Hispanic/Latinx patients from more deprived communities had decreased hazard of receiving LDLT.85 Post-LT, where immunosuppressive therapy is instrumental for optimal graft function, patients residing in more deprived neighborhoods had decreased rate of medical adherence. Post-LT, increasing neighborhood deprivation was associated with graft failure and death after LT.84,86 Neighborhood deprivation was also associated with single-parent household, caregivers with less educational attainment, and increased barriers to medication.87 To summarize, the findings from these studies provide a compelling argument that contextual poverty impacts waitlisting, LT rates, and post-transplant outcomes.
GEOGRAPHIC DISPARITIES BY LIVER DISEASE ETIOLOGY
Geographic variation in hospitalization rates and in-hospital mortality by liver disease etiology might be an important contributor to geographic disparity in LT access. Whereas hospitalization rates and in-hospital mortality due to chronic hepatitis B (HBV) and chronic hepatitis C (HCV) have decreased between 2007 and 2016, hospitalization rates and in-hospital mortality due to alcoholic liver disease (ALD) and NAFLD have increased.1,88,89 By 2030, prediction models estimate that deaths associated with NAFLD will increase by 178.0%.90 Geographic variation in hospitalizations for cirrhosis have also been identified, with hospitalization rates in the South at 37.9% compared to 19.9% in the Midwest; this variation extends beyond regions to urban-rural differences, with hospitalization rates in urban areas at 91.3% compared to 8.7% in rural areas.89
HCV
County-level mortality related to HCV has decreased since 2005, but there remains significant heterogeneity in the West, Southwest, Appalachia, and northern Florida.91 High HCV-related mortality continues to be associated with neighborhood deprivation, educational attainment, and non-English language.92 Effective treatment of HCV with direct-acting antivirals (DAAs) has led to an overall decrease in HCV as an indication for LT.93 Thus, widespread access to DAAs has the potential to reduce HCV-related morbidity, progression to ESLD requiring LT, and mortality. Currently, rural residents are less likely to receive DAA treatment than urban residents.94–96 Solutions to address these urban-rural differences in DAA treatment include the use of telehealth to educate rural primary care providers about prescribing DAAs.95 Yet, even among patients with high sustained virological response, unemployment and low educational attainment were associated with severe liver fibrosis.97 Addressing these disparities in DAA treatment could help decrease the LT indication for HCV.93
Nonalcoholic fatty liver disease (NAFLD)/Nonalcoholic steatohepatitis (NASH)
NAFLD/NASH is the fastest growing cause of HCC in LT candidates and recipients98 and is expected to surpass ALD as the leading indication for LT in all patients within the next few years.99 There is geographic variation in the burden of NASH/NAFLD with Western regions having the highest rate of hospitalization (90.3/100,000) and Northeast regions having the lowest rate (67.0/100,000) of hospitalization; in addition, there is urban-rural variation in in-hospital mortality with higher rates in urban versus rural centers.100
It is predicted that NASH-related HCC will increase in conjunction with the obesity pandemic, and by 2030, 49.0% of the U.S. population is projected to have obesity.101,102 Contextual poverty has been independently associated with increased risk of obesity103,104 and diabetes,104,105 with living in more deprived neighborhoods being associated with increased odds of obesity106–108 and diabetes.105 Given these associations with diabetes and obesity, it is reasonable to expect that NAFLD might impact patients from more deprived communities at higher rates than patients from less deprived households.109
Alcohol-associated liver disease (ALD)
ALD has been increasing in incidence in recent years, with one study demonstrating a 43.0% increase in alcohol-associated cirrhosis between 2009 and 2015.110 At the same time, the proportion of waitlisted patients with ALD increased from 22.0% in 2014 to 40.0% in 2019.111 There are significant center and regional variations in LT for both chronic ALD and medically-refractory severe alcohol-associated hepatitis (AH).112 While there has been a 5-fold increase in LT for AH between 2014–2019, there are variations in LT by OPTN regions and centers, with only 3 transplant centers accounting for 50.0–90.0% of LT within each region.113 These variations are partly due to differing criteria for LT across center, despite similar post-transplant outcomes.114
INTERNATIONAL TRENDS
Like the U.S., international geographic disparities in LT exist, making it possible to compare the effectiveness of global policies while accounting for the unique challenges of each population and healthcare system.
LT policies accounting for geographic boundaries have led to unequal results. In Canada, the Multi-Organ Transplant Program began providing LT services in 1985, which were suspended in 2001 due to staffing issues, and restarted in 2004. Since the reactivation, significant geographic differences were observed among potential LT candidates. Interestingly, the incidence of liver disease requiring LT varied significantly by provinces but had similar waitlisting rate.115 In Korea, organ allocation policies initially divided the country into three regions of unequal population density, which contributed to geographic disparity. To rectify this, policies of broader organ sharing based on MELD scores were implemented.116
The etiology of liver disease and indication for transplant also vary significantly between regions and countries. In France, the incidence of alcohol-related HCC is higher than HCV-related HCC, but varies between regions.117 Similarly, whereas HCC and NAFLD are the most common indications for LT in the US, in Canada it was ALD (20.5%), followed by HCC (16.6%), and then HCV (14.0%).115 In the United Kingdom (UK), 42.0% of patients on the liver transplant waiting list had ALD or viral hepatitis as the etiology of their cirrhosis.118
There have been urban-rural differences in LT access. A study of LT between 2000–2013 in Taiwan found that similar to the U.S., lower rates of LT were seen in satellite (prevalence rate ratio PRR 0.6) and rural (PRR 0.8) areas compared to urban areas.119 In addition, neighborhood poverty is associated with lower likelihood of LT.120 Similarly in Canada and Brazil, neighborhood poverty is associated with lower rates of LT.83,115,118,119
Challenges to increasing LT access include creating a more robust transplant network as well as meeting the personnel requirement to staff these networks.118 Naturally, the donor pool within any given country also impacts access to LT. For instance, in Brazil, 88% of population was unwilling to become an organ donor.118 Within Europe, there are highly variable donor rates, with Spain having the highest donor rate.121 Overall, these international trends reinforce that etiology of liver disease, donor characteristics, and transplant network features likely play a role in transplant geographic disparities.
WHAT CAN BE DONE TO ADDRESS EXISTING GEOGRAPHIC DISPARITIES?
From geographic variation in liver disease burden and MELD scores at transplantation, to the urban-rural disparity in specialty care access, to neighborhood deprivation in LT access, addressing geographic disparity will require a multi-pronged approach at the patient, transplant center, and national-levels. For example, higher neighborhood deprivation is associated with increased odds of obesity106–108 and diabetes.105 As such, it is reasonable to expect NASH/NAFLD, a growing indication for LT,99 to follow a similar trend. While the association between neighborhood deprivation and NASH/NAFLD needs further studies, community clinic outreach efforts to address diabetes and obesity, especially in more deprived communities, could potentially address the impending geographic variation in NASH/NAFLD incidence. Currently, patients residing far from transplant centers68,122 have lower rates of LT. One potential solution would be to provide low SES patients with reliable transportation. A pilot study is currently underway at University of Southern California to address distance/transportation barriers by using their existing Lyft Health rideshare to trial a needs-based app-support program for low SES patients. Telehealth is another tool to address lack of transportation, and has been associated with decreased time from referral to evaluation and waitlisting.123 To achieve its full potential, telehealth must first overcome the “digital divide”124 that exists, with patients living in low SES communities being less likely to access high-quality internet, which is a necessary tool for telehealth.125
Studies showing that patients from more deprived communities are less likely to receive LT,83 with only a quarter getting waitlisted for LT,67 suggest that neighborhood deprivation could be used as a measure of SES to identify patients at increased risk of dropping out during LT evaluation. One potential solution would be incorporating neighborhood deprivation indices in LT evaluation, allowing transplant centers to identify patients who might benefit from additional support in the form of social workers, case managers, and patient navigators. These support personnel could be charged with identifying the specific barriers to LT (financial/economical, educational, cultural, social) that patients from more deprived communities face. While further research is needed to elucidate these factors, one important step would be mandatory SES data collection on all patients referred for LT, and their fate relative to listing and reasons for non-listing. An even more upstream step would be mandatory data collection on all ESLD patients, which will not only enable us to understand liver disease burden but gain insight into referral patterns. Current efforts to elucidate LT access have utilized listing to death ratio126 and liver waitlisting ratio,127 with each of these efforts serving as proxies for waitlist access. In addition to understanding the barriers along the LT cascade, complete data on ESLD patients could potentially allow us to increase referrals by incentivizing primary care providers and gastroenterologists. This would be similar to what currently exists for kidney referral, whereby the CMS ESRD Treatment Choices (ETC) and Kidney Care Choices (KCC) Model incentivize kidney referral, improving access to transplantation.128
CONCLUSION
UNOS/OPTN organ allocation policies have sought to reduce geographic disparity in organ allocation, but these distribution policies can only impact patients once they are waitlisted. Our review highlights several patient-level disparities in LT access, which partly result from variation in the burden of liver disease and proportion of patients with high MELD scores across regions and DSAs. Furthermore, most transplant centers are in urban areas, contributing to the urban-rural divide in LT access. Current policies do not account for patient-level SES factors – factors that disproportionately affect patients with low SES. As such, addressing geographic disparity will require a multi-pronged approach at the patient, transplant center, and national level. At the patient-level, it will require the standardized use of robust SES measures to identify patients who might benefit from additional support to overcome LT barriers. At the transplant-center level, it will require concerted effort by centers to identify and address patient-level geographic disparities that appear to be intimately interwoven with SES. At the national-level, allocation policies should consider patient-level SES factors in balancing transplant MELD acuity and volume with graft availability.
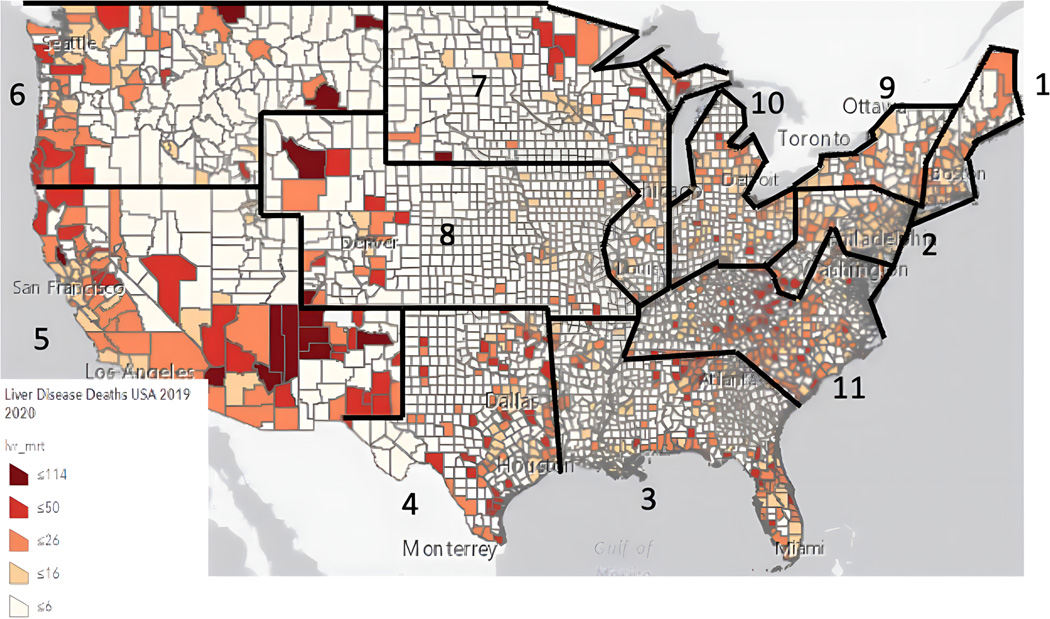
Figure 1:
Mortality by County (per 100,000 persons) and UNOS Regions
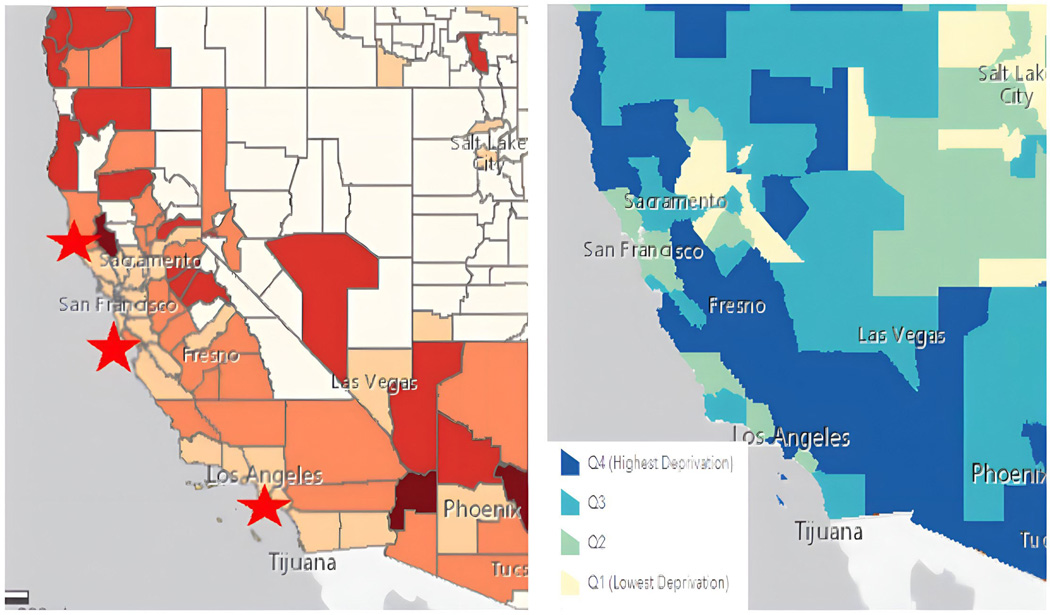
Figure 2:
Mortality by County (per 100,000 persons)
Bottom Left: Transplant Centers
Bottom Right: Neighborhood Deprivation
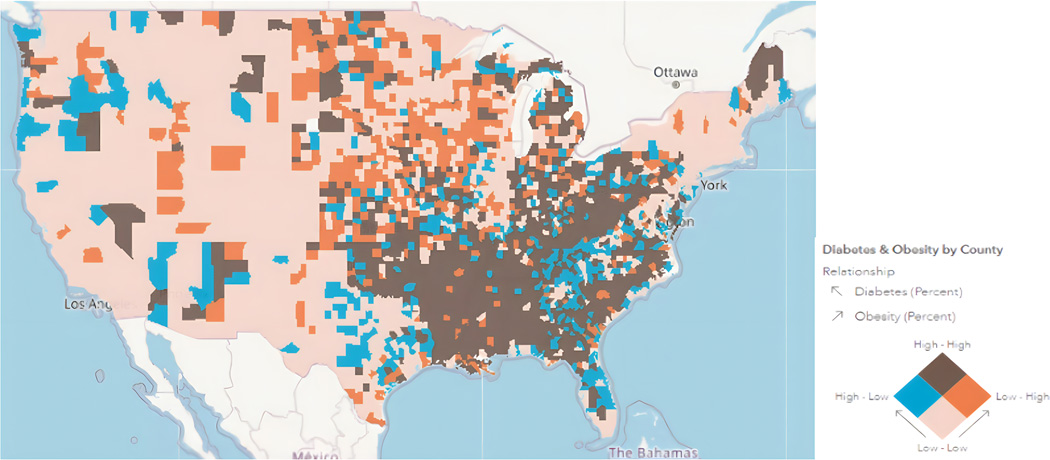
Figure 3:
The Future of NAFLD? Trends of Diabetes & Obesity by County
GRANTS AND FINANCIAL SUPPORT:
M.Y. is a National Clinical Scholars Fellow and is supported by that organization for her funding. S.I.W is supported through K23DK132454 funding.
DISCLOSURE:
Sharad Wadhwani K award: “Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK132454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
ABBREVIATIONS:
- AC
Acuity Circles
- AH
Alcohol-associated hepatitis
- ALD
Alcohol-related liver disease
- aMELD
Allocation MELD
- HBV
Chronic hepatitis B
- HCV
Chronic hepatitis C
- CLD
Chronic liver disease
- DDLTs
Deceased donor LT
- DAAs
Direct-acting antivirals
- DSAs
Donor service areas
- ELSD
End stage liver disease
- ETC
ESRD Treatment Choices
- HHS
Health and Human Services
- HCC
Hepatocellular Carcinoma
- ICU
Intensive care unit
- KCC
Kidney Care Choices
- LT
Liver transplantation
- MMaT
MELD scores at transplant
- MMaT-3
MMAT within DSA minus 3 points
- MELD
Model for End-Stage Liver Disease
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- OPOs
Organ procurement organizations
- OPTN
Organ Procurement and Transplantation Network
- PELD
Pediatric End-Stage Liver Disease
- SES
Socioeconomic status
- SEER
Surveillance, Epidemiology and End Results
- UNOS
United Network for Organ Sharing
- UK
United Kingdom
- U.S.
United States
REFERENCES
- 1.Hirode G, Saab S, Wong RJ. Trends in the Burden of Chronic Liver Disease Among Hospitalized US Adults. JAMA Netw Open 2020; 3(4): e201997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D, Perumpail BJ, Alshuwaykh O, Dennis BB, Cholankeril G, Ahmed A. Changing Trends in Etiology-Based Hospitalizations with End-Stage Liver Disease in the United States from 2016 to 2019. Liver Int 2022. [DOI] [PubMed] [Google Scholar]
- 3.OPTN Final Rule, 42 C.F.R. Section 121.8. [Google Scholar]
- 4.Burton AM, Goldberg DS. Center-level and region-level variations in liver transplantation practices following acuity circles policy change. Am J Transplant 2022. [DOI] [PubMed] [Google Scholar]
- 5.Nagai S, Chau LC, Schilke RE, et al. Effects of Allocating Livers for Transplantation Based on Model for End-Stage Liver Disease-Sodium Scores on Patient Outcomes. Gastroenterology 2018; 155(5): 1451–62.e3. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg DS, Levine M, Karp S, Gilroy R, Abt PL. Share 35 changes in center-level liver acceptance practices. Liver Transpl 2017; 23(5): 604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Realizing the Promise of Equity in the Organ Transplantation System. Washington, DC: The National Academies Press; 2022. [PubMed] [Google Scholar]
- 8.Weiss SF J. Two Year Monitoring Report of Liver and Intestine Acuity Circle Allocation Removal of DSA and Region as Units of Allocation. https://optn.transplant.hrsa.gov/media/k5yi4jvl/data_report_liver_full_2yrallocation_20220805_final_508_compliant.pdf, 2022. [Google Scholar]
- 9.Bodzin AS, Baker TB. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transplantation 2018; 24(10): 1470–5. [DOI] [PubMed] [Google Scholar]
- 10.Foster GR, Irving WL, Cheung MC, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. Journal of hepatology 2016; 64(6): 1224–31. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017; 152(5): 1090–9. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da BL, Ezaz G, Kushner T, et al. Donor Characteristics and Regional Differences in the Utilization of HCV-Positive Donors in Liver Transplantation. JAMA Netw Open 2020; 3(12): e2027551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter TG, Aronsohn A, Reddy KG, Charlton M. Liver Transplantation of HCV-viremic Donors Into HCV-negative Recipients in the United States: Increasing Frequency With Profound Geographic Variation. Transplantation 2021; 105(6): 1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 15.Orman ES, Mayorga ME, Wheeler SB, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transplantation 2015; 21(8): 1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raigani S, De Vries RJ, Carroll C, et al. Viability testing of discarded livers with normothermic machine perfusion: alleviating the organ shortage outweighs the cost. Clinical transplantation 2020; 34(11): e14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Carlis R, Schlegel A, Frassoni S, et al. How to preserve liver grafts from circulatory death with long warm ischemia? A retrospective Italian cohort study with normothermic regional perfusion and hypothermic oxygenated perfusion. Transplantation 2021; 105(11): 2385–96. [DOI] [PubMed] [Google Scholar]
- 18.Mergental H, Laing R, Kirkham A, et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun 11 (1): 2939. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Leeuwen OB, Brüggenwirth IM, de Kleine RH, et al. Machine perfusion of donation after circulatory death liver and lungs before combined liver-lung transplantation. Transplantation direct 2021; 7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller M, Kalisvaart M, Joanne OR, et al. Hypothermic oxygenated liver perfusion (HOPE) prevents tumor recurrence in liver transplantation from donation after circulatory death. Annals of Surgery 2020; 272(5): 759–65. [DOI] [PubMed] [Google Scholar]
- 21.Schlegel A, van Reeven M, Croome K, et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. Journal of Hepatology 2022; 76(2): 371–82. [DOI] [PubMed] [Google Scholar]
- 22.Watson CJ, Hunt F, Messer S, et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. American Journal of Transplantation 2019; 19(6): 1745–58. [DOI] [PubMed] [Google Scholar]
- 23.van Leeuwen OB, Bodewes SB, Lantinga VA, et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am J Transplant 2022; 22(6): 1658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Leeuwen OB, de Vries Y, Fujiyoshi M, et al. Transplantation of high-risk donor livers after ex situ resuscitation and assessment using combined hypo-and normothermic machine perfusion: a prospective clinical trial. Annals of surgery 2019; 270(5): 906–14. [DOI] [PubMed] [Google Scholar]
- 25.Eden J, Da Silva RS, Cortes-Cerisuelo M, et al. Utilization of livers donated after circulatory death for transplantation - An international comparison. J Hepatol 2023. [DOI] [PubMed] [Google Scholar]
- 26.Sheehy E, Conrad SL, Brigham LE, et al. Estimating the number of potential organ donors in the United States. New England Journal of Medicine 2003; 349(7): 667–74. [DOI] [PubMed] [Google Scholar]
- 27.Klassen D, Edwards L, Stewart D, Glazier A, Orlowski J, Berg C. The OPTN deceased donor potential study: implications for policy and practice. American Journal of Transplantation 2016; 16(6): 1707–14. [DOI] [PubMed] [Google Scholar]
- 28.Lynch RJ, Doby BL, Goldberg DS, Lee KJ, Cimeno A, Karp SJ. Procurement characteristics of high- and low-performing OPOs as seen in OPTN/SRTR data. American Journal of Transplantation 2022; 22(2): 455–63. [DOI] [PubMed] [Google Scholar]
- 29.Aubert O, Reese PP, Audry B, et al. Disparities in acceptance of deceased donor kidneys between the United States and France and estimated effects of increased US acceptance. JAMA internal medicine 2019; 179(10): 1365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor K, Glazier A. OPO performance improvement and increasing organ transplantation: Metrics are necessary but not sufficient. Elsevier; 2021. p. 2325–6. [DOI] [PubMed] [Google Scholar]
- 31.Johnson W, Kraft K, Chotai P, et al. Variability in Organ Procurement Organization Performance by Individual Hospital in the United States. JAMA Surg 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croome KP, Lee DD, Keaveny AP, Taner CB. Noneligible Donors as a Strategy to Decrease the Organ Shortage. Am J Transplant 2017; 17(6): 1649–55. [DOI] [PubMed] [Google Scholar]
- 33.Sonnenberg EM, Hsu JY, Reese PP, Goldberg DS, Abt PL. Wide Variation in the Percentage of Donation After Circulatory Death Donors Across Donor Service Areas: A Potential Target for Improvement. Transplantation 2020; 104(8): 1668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannon RM, Jones CM, Davis EG, Franklin GA, Gupta M, Shah MB. Patterns of geographic variability in mortality and eligible deaths between organ procurement organizations. Am J Transplant 2019; 19(10): 2756–63. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg D, Kallan MJ, Fu L, et al. Changing Metrics of Organ Procurement Organization Performance in Order to Increase Organ Donation Rates in the United States. Am J Transplant 2017; 17(12): 3183–92. [DOI] [PubMed] [Google Scholar]
- 36.Doby BL, Ross-Driscoll K, Shuck M, Wadsworth M, Durand CM, Lynch RJ. Public discourse and policy change: Absence of harm from increased oversight and transparency in OPO performance. Am J Transplant 2021; 21(8): 2646–52. [DOI] [PubMed] [Google Scholar]
- 37.Axelrod D, Gheorghian A, Schnitzler M, et al. The economic implications of broader sharing of liver allografts. American journal of transplantation 2011; 11(4): 798–807. [DOI] [PubMed] [Google Scholar]
- 38.Jacobbi LM, McBride VA, Etheredge EE, et al. THE RISKS, BENEFITS, AND COSTS OF EXPANDING DONOR CRITERIA: A Collaborative Prospective Three-Year Study: 1. Transplantation 1995; 60(12): 1491–6. [DOI] [PubMed] [Google Scholar]
- 39.Axelrod DA, Koffron AJ, Baker T, et al. The economic impact of MELD on liver transplant centers. American journal of transplantation 2005; 5(9): 2297–301. [DOI] [PubMed] [Google Scholar]
- 40.Olthoff KM, Brown RS Jr, Delmonico FL, et al. Summary report of a national conference: evolving concepts in liver allocation in the MELD and PELD era. Liver Transplantation 2004; 10(S10): A6–A22. [DOI] [PubMed] [Google Scholar]
- 41.Lindemann J, Dageforde LA, Vachharajani N, et al. Cost evaluation of a donation after cardiac death program: how cost per organ compares to other donor types. Journal of the American College of Surgeons 2018; 226(5): 909–16. [DOI] [PubMed] [Google Scholar]
- 42.van der Hilst CS, IJtsma AJ, Bottema JT, et al. The price of donation after cardiac death in liver transplantation: a prospective cost-effectiveness study. Transplant International 2013; 26(4): 411–8. [DOI] [PubMed] [Google Scholar]
- 43.Webb AN, Lester EL, Shapiro AMJ, Eurich DT, Bigam DL. Cost-utility analysis of normothermic machine perfusion compared to static cold storage in liver transplantation in the Canadian setting. American Journal of Transplantation 2022; 22(2): 541–51. [DOI] [PubMed] [Google Scholar]
- 44.Berumen J, Misel M, Vodkin I, Halldorson JB, Mekeel KL, Hemming A. The effects of Share 35 on the cost of liver transplantation. Clin Transplant 2017; 31(5). [DOI] [PubMed] [Google Scholar]
- 45.Fernandez H, Weber J, Barnes K, Wright L, Levy M. Financial Impact of Liver Sharing and Organ Procurement Organizations’ Experience With Share 35: Implications for National Broader Sharing. Am J Transplant 2016; 16(1): 287–91. [DOI] [PubMed] [Google Scholar]
- 46.Wall AE, da Graca B, Asrani SK, et al. Cost Analysis of Liver Acquisition Fees Before and After Acuity Circle Policy Implementation. JAMA Surg 2021; 156(11): 1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wall AE, Borries T, Reddy V, Asrani SK, Testa G, Trotter J. The carbon footprint of organ acquisition in the United States. Am J Transplant 2022; 22(12): 3184–5. [DOI] [PubMed] [Google Scholar]
- 48.Lee YT, Wang JJ, Luu M, et al. State-Level HCC Incidence and Association With Obesity and Physical Activity in the United States. Hepatology 2021; 74(3): 1384–94. [DOI] [PubMed] [Google Scholar]
- 49.Zhou K, Gainey CS, Dodge JL, et al. Diverging Incidence Trends for Hepatocellular Carcinoma in Rural and Urban Settings in the United States. Clin Gastroenterol Hepatol 2022; 20(5): 1180–5 e2. [DOI] [PubMed] [Google Scholar]
- 50.Major JM, Sargent JD, Graubard BI, et al. Local geographic variation in chronic liver disease and hepatocellular carcinoma: contributions of socioeconomic deprivation, alcohol retail outlets, and lifestyle. Ann Epidemiol 2014; 24(2): 104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou K, Thompson LK, Liu L, Terrault NA, Cockburn MG. Geographic hotspot detection for late-stage hepatocellular carcinoma: novel approach to cancer control. Cancer Causes Control 2022; 33(5): 701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl 2012; 18(4): 434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishaque T, Massie AB, Bowring MG, et al. Liver transplantation and waitlist mortality for HCC and non-HCC candidates following the 2015 HCC exception policy change. Am J Transplant 2019; 19(2): 564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagai S, Kitajima T, Yeddula S, et al. Effect of Mandatory 6-Month Waiting Period on Waitlist and Transplant Outcomes in Patients With Hepatocellular Carcinoma. Hepatology 2020; 72(6): 2051–62. [DOI] [PubMed] [Google Scholar]
- 55.Mehta N, Dodge JL, Hirose R, Roberts JP, Yao FY. Increasing Liver Transplantation Wait-List Dropout for Hepatocellular Carcinoma With Widening Geographical Disparities: Implications for Organ Allocation. Liver Transpl 2018; 24(10): 1346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brondfield MN, Dodge JL, Hirose R, Heimbach J, Yao FY, Mehta N. Unfair Advantages for Hepatocellular Carcinoma Patients Listed for Liver Transplant in Short-Wait Regions Following 2015 Hepatocellular Carcinoma Policy Change. Liver Transpl 2020; 26(5): 662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sobotka LA, Hinton A, Conteh LF. Disparities in the treatment of hepatocellular carcinoma based on geographical region are decreasing. J Gastroenterol Hepatol 2019; 34(3): 575–9. [DOI] [PubMed] [Google Scholar]
- 58.Spaggiari M, Okoye O, Tulla K, et al. Geographic Disparities in Liver Allocation and Distribution in the United States: Where Are We Now? Transplant Proc 2019; 51(10): 3205–12. [DOI] [PubMed] [Google Scholar]
- 59.Rana A, Riaz IB, Gruessner AC, Gruessner RW. Geographic inequity results in disparate mortality: a multivariate intent-to-treat analysis of liver transplant data. Clin Transplant 2015; 29(6): 484–91. [DOI] [PubMed] [Google Scholar]
- 60.Shaikh A, Goli K, Rich NE, et al. Early Impact of MMaT-3 Policy on Liver Transplant Waitlist Outcomes for Hepatocellular Carcinoma. Transplant Direct 2022; 8(5): e1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Croome KP, Lee DD, Burns JM, Keaveny AP, Taner CB. Intraregional model for end-stage liver disease score variation in liver transplantation: Disparity in our own backyard. Liver Transpl 2018; 24(4): 488–96. [DOI] [PubMed] [Google Scholar]
- 62.Zendel A, Watkins R, Moon AM, Gerber DA, Barritt ASt, Desai CS. Changing opportunities for liver transplant for patients with hepatocellular carcinoma. Clin Transplant 2022; 36(5): e14609. [DOI] [PubMed] [Google Scholar]
- 63.Wey A, Noreen S, Gentry S, et al. The Effect of Acuity Circles on Deceased Donor Transplant and Offer Rates Across Model for End-Stage Liver Disease Scores and Exception Statuses. Liver Transpl 2022; 28(3): 363–75. [DOI] [PubMed] [Google Scholar]
- 64.Weeks WB, Kazis LE, Shen Y, et al. Differences in health-related quality of life in rural and urban veterans. American Journal of Public Health 2004; 94(10): 1762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of Solid-Organ Wait-listing, Transplantation, and Survival Among Residents of Rural and Urban Areas. JAMA 2008; 299(2): 202–7. [DOI] [PubMed] [Google Scholar]
- 66.Goldberg DS, Newcomb C, Gilroy R, et al. Increased Distance to a Liver Transplant Center Is Associated With Higher Mortality for Patients With Chronic Liver Failure. Clin Gastroenterol Hepatol 2017; 15(6): 958–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohamed KA, Ghabril M, Desai A, et al. Neighborhood poverty is associated with failure to be waitlisted and death during liver transplantation evaluation. Liver Transpl 2022; 28(9): 1441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA 2014; 311(12): 1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dzebisashvili N, Massie AB, Lentine KL, et al. Following the Organ Supply: Assessing the Benefit of Inter-DSA Travel in Liver Transplantation. Transplantation 2013; 95(2): 361–71. [DOI] [PubMed] [Google Scholar]
- 70.Kwong AJ, Mannalithara A, Heimbach J, Prentice MA, Kim WR. Migration of Patients for Liver Transplantation and Waitlist Outcomes. Clin Gastroenterol Hepatol 2019; 17(11): 2347–55.e5. [DOI] [PubMed] [Google Scholar]
- 71.Vagefi PA, Feng S, Dodge JL, Markmann JF, Roberts JP. Multiple Listings as a Reflection of Geographic Disparity in Liver Transplantation. Journal of the American College of Surgeons 2014; 219(3): 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cholankeril G, Yoo ER, Perumpail RB, Younossi ZM, Ahmed A. Disparities in Liver Transplantation Resulting From Variations in Regional Donor Supply and Multiple Listing Practices. Clinical Gastroenterology and Hepatology 2017; 15(2): 313–5. [DOI] [PubMed] [Google Scholar]
- 73.Robert SA. Socioeconomic position and health: the independent contribution of community socioeconomic context. Annual review of sociology 1999: 489–516. [Google Scholar]
- 74.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. American journal of public health 2003; 93(7): 1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health 2006; 83(6): 1041–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross K, Patzer RE, Goldberg DS, Lynch RJ. Sociodemographic Determinants of Waitlist and Posttransplant Survival Among End-Stage Liver Disease Patients. Am J Transplant 2017; 17(11): 2879–89. [DOI] [PubMed] [Google Scholar]
- 77.Singh GK, Siahpush M. Widening socioeconomic inequalities in US life expectancy, 1980–2000. International journal of epidemiology 2006; 35(4): 969–79. [DOI] [PubMed] [Google Scholar]
- 78.Singh GK, Siahpush M. Increasing inequalities in all-cause and cardiovascular mortality among US adults aged 25–64 years by area socioeconomic status, 1969–1998. International Journal of Epidemiology 2002; 31(3): 600–13. [DOI] [PubMed] [Google Scholar]
- 79.Singh GK, Miller BA, Hankey BF. Changing area socioeconomic patterns in US cancer mortality, 1950–1998: part II—lung and colorectal cancers. Journal of the National Cancer Institute 2002; 94(12): 916–25. [DOI] [PubMed] [Google Scholar]
- 80.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part I—all cancers and lung cancer and part II—colorectal, prostate, breast, and cervical cancers. Journal of cancer epidemiology 2011; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in US incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer 2004; 101(5): 1051–7. [DOI] [PubMed] [Google Scholar]
- 82.Danos D, Leonardi C, Gilliland A, et al. Increased Risk of Hepatocellular Carcinoma Associated With Neighborhood Concentrated Disadvantage. Front Oncol 2018; 8: 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flemming JA, Muaddi H, Djerboua M, Neves P, Sapisochin G, Selzner N. Association between social determinants of health and rates of liver transplantation in individuals with cirrhosis. Hepatology 2022. [DOI] [PubMed] [Google Scholar]
- 84.Wadhwani SI, Beck AF, Bucuvalas J, Gottlieb L, Kotagal U, Lai JC. Neighborhood socioeconomic deprivation is associated with worse patient and graft survival following pediatric liver transplantation. Am J Transplant 2020; 20(6): 1597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wadhwani SI, Ge J, Gottlieb L, et al. Racial/ethnic disparities in wait-list outcomes are only partly explained by socioeconomic deprivation among children awaiting liver transplantation. Hepatology 2022; 75(1): 115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wadhwani SI, Huang CY, Gottlieb L, et al. Center variation in long-term outcomes for socioeconomically deprived children. Am J Transplant 2021; 21(9): 3123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wadhwani SI, Bucuvalas JC, Brokamp C, et al. Association Between Neighborhood-level Socioeconomic Deprivation and the Medication Level Variability Index for Children Following Liver Transplantation. Transplantation 2020; 104(11): 2346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shirazi F, Singal AK, Wong RJ. Alcohol-associated Cirrhosis and Alcoholic Hepatitis Hospitalization Trends in the United States. J Clin Gastroenterol 2021; 55(2): 174–9. [DOI] [PubMed] [Google Scholar]
- 89.Mellinger JL, Richardson CR, Mathur AK, Volk ML. Variation among United States hospitals in inpatient mortality for cirrhosis. Clin Gastroenterol Hepatol 2015; 13(3): 577–84; quiz e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018; 67(1): 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hall EW, Schillie S, Vaughan AS, et al. County-Level Variation in Hepatitis C Virus Mortality and Trends in the United States, 2005–2017. Hepatology 2021; 74(2): 582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ford MM, Desai PS, Maduro G, Laraque F. Neighborhood Inequalities in Hepatitis C Mortality: Spatial and Temporal Patterns and Associated Factors. J Urban Health 2017; 94(5): 746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parrish NF, Feurer ID, Matsuoka LK, Rega SA, Perri R, Alexopoulos SP. The Changing Face of Liver Transplantation in the United States: The Effect of HCV Antiviral Eras on Transplantation Trends and Outcomes. Transplant Direct 2019; 5(3): e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Njei B, Esserman D, Krishnan S, et al. Regional and Rural-Urban Differences in the Use of Direct-acting Antiviral Agents for Hepatitis C Virus: The Veteran Birth Cohort. Med Care 2019; 57(4): 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Du P, Wang X, Kong L, Jung J. Can Telementoring Reduce Urban-Rural Disparities in Utilization of Direct-Acting Antiviral Agents? Telemed J E Health 2021; 27(5): 488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du P, Wang X, Kong L, Riley T 3rd, Jung J. Changing Urban-Rural Disparities in the Utilization of Direct-Acting Antiviral Agents for Hepatitis C in U.S. Medicare Patients, 2014–2017. Am J Prev Med 2021; 60(2): 285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carrieri P, Carrat F, Di Beo V, et al. Severe liver fibrosis in the HCV cure era: Major effects of social vulnerability, diabetes, and unhealthy behaviors. JHEP Rep 2022; 4(6): 100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 2019; 17(4): 748–55.e3. [DOI] [PubMed] [Google Scholar]
- 99.Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. The American journal of gastroenterology 2018; 113(11): 1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adejumo AC, Samuel GO, Adegbala OM, et al. Prevalence, trends, outcomes, and disparities in hospitalizations for nonalcoholic fatty liver disease in the United States. Ann Gastroenterol 2019; 32(5): 504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med 2019; 381(25): 2440–50. [DOI] [PubMed] [Google Scholar]
- 102.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 2013; (131): 1–8. [PubMed] [Google Scholar]
- 103.Black JL, Macinko J. Neighborhoods and obesity. Nutr Rev 2008; 66(1): 2–20. [DOI] [PubMed] [Google Scholar]
- 104.Hu MD, Lawrence KG, Bodkin MR, Kwok RK, Engel LS, Sandler DP. Neighborhood Deprivation, Obesity, and Diabetes in Residents of the US Gulf Coast. Am J Epidemiol 2021; 190(2): 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal Associations Between Neighborhood Physical and Social Environments and Incident Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Intern Med 2015; 175(8): 1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Janssen I, Boyce WF, Simpson K, Pickett W. Influence of individual- and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. Am J Clin Nutr 2006; 83(1): 139–45. [DOI] [PubMed] [Google Scholar]
- 107.Mobley LR, Root ED, Finkelstein EA, Khavjou O, Farris RP, Will JC. Environment, obesity, and cardiovascular disease risk in low-income women. Am J Prev Med 2006; 30(4): 327–32. [DOI] [PubMed] [Google Scholar]
- 108.van Lenthe FJ, Mackenbach JP. Neighbourhood deprivation and overweight: the GLOBE study. Int J Obes Relat Metab Disord 2002; 26(2): 234–40. [DOI] [PubMed] [Google Scholar]
- 109.Tutunchi H, Saghafi-Asl M, Ebrahimi-Mameghani M, Ostadrahimi A. Food Insecurity and Lipid Profile Abnormalities Are Associated with an Increased Risk of Nonalcoholic Fatty Liver Disease (NAFLD): A Case-Control Study. Ecol Food Nutr 2021; 60(4): 508–24. [DOI] [PubMed] [Google Scholar]
- 110.Mellinger JL, Shedden K, Winder GS, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology 2018; 68(3): 872–82. [DOI] [PubMed] [Google Scholar]
- 111.Singal AK, Mathurin P. Diagnosis and Treatment of Alcohol-Associated Liver Disease: A Review. Jama 2021; 326(2): 165–76. [DOI] [PubMed] [Google Scholar]
- 112.Shawcross DL, O’Grady JG. The 6-month abstinence rule in liver transplantation. Lancet 2010; 376(9737): 216–7. [DOI] [PubMed] [Google Scholar]
- 113.Cotter TG, Sandıkçı B, Paul S, et al. Liver transplantation for alcoholic hepatitis in the United States: Excellent outcomes with profound temporal and geographic variation in frequency. Am J Transplant 2021; 21(3): 1039–55. [DOI] [PubMed] [Google Scholar]
- 114.Marot A, Dubois M, Trépo E, Moreno C, Deltenre P. Liver transplantation for alcoholic hepatitis: A systematic review with meta-analysis. PLoS One 2018; 13(1): e0190823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Renfrew PD, Molinari M. Are there geographical disparities in access to liver transplantation in Atlantic Canada? Can J Gastroenterol 2012; 26(10): 705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee J, Lee JG, Jung I, Joo DJ, Kim SI, Kim MS. Development of a Korean Liver Allocation System using Model for End Stage Liver Disease Scores: A Nationwide, Multicenter study. Sci Rep 2019; 9(1): 7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Costentin CE, Sogni P, Falissard B, et al. Geographical Disparities of Outcomes of Hepatocellular Carcinoma in France: The Heavier Burden of Alcohol Compared to Hepatitis C. Dig Dis Sci 2020; 65(1): 301–11. [DOI] [PubMed] [Google Scholar]
- 118.Bittencourt PL, Farias AQ, Couto CA. Liver Transplantation in Brazil. Liver Transpl 2016; 22(9): 1254–8. [DOI] [PubMed] [Google Scholar]
- 119.Wen PH, Lu CL, Strong C, et al. Demographic and Urbanization Disparities of Liver Transplantation in Taiwan. Int J Environ Res Public Health 2018; 15(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu CC, Lu CL, Notobroto HB, Tsai CC, Wen PH, Li CY. Individual and neighborhood socioeconomic status in the prediction of liver transplantation among patients with liver disease: A population-based cohort study in Taiwan. Medicine (Baltimore) 2019; 98(11): e14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rudge CJ, Fuggle SV, Burbidge KM. Geographic disparities in access to organ transplantation in the United Kingdom. Transplantation 2003; 76(9): 1395–8. [DOI] [PubMed] [Google Scholar]
- 122.Goldberg DS, French B, Forde KA, et al. Association of Distance From a Transplant Center With Access to Waitlist Placement, Receipt of Liver Transplantation, and Survival Among US Veterans. JAMA 2014; 311(12): 1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.John BV, Love E, Dahman B, et al. Use of Telehealth Expedites Evaluation and Listing of Patients Referred for Liver Transplantation. Clin Gastroenterol Hepatol 2020; 18(8): 1822–30.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wadhwani SI, Lai JC. The digital determinants of liver disease. Hepatology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Henson JB, Wegermann K, Patel YA, Wilder JM, Muir AJ. Access to technology to support telehealth in areas without specialty care for liver disease. Hepatology 2022. [DOI] [PubMed] [Google Scholar]
- 126.Rosenblatt R, Wahid N, Halazun KJ, et al. Black Patients Have Unequal Access to Listing for Liver Transplantation in the United States. Hepatology 2021; 74(3): 1523–32. [DOI] [PubMed] [Google Scholar]
- 127.Mathur AK, Ashby VB, Fuller DS, et al. Variation in access to the liver transplant waiting list in the United States. Transplantation 2014; 98(1): 94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mazumdar S, Blackwell K, Duvall T, Boyer G, Missett L. Centers for Medicare & Medicaid Services’ Models to Improve Late-stage Chronic Kidney Disease and End-stage Renal Disease Care: Leveraging Nephrology Payment Policy to Achieve Value. Advances in Chronic Kidney Disease 2022; 29(1): 24–9. [DOI] [PubMed] [Google Scholar]