Abstract
This review summarizes the recent advancements in alternating current (AC)-driven electroorganic synthesis since 2021 and discusses the reactivities AC electrolysis provides to achieve new and unique organic transformations.
Keywords: Electroorganic synthesis, Direct current, Alternating current, Reaction selectivity
Graphical Abstract

1. Introduction
Most electroorganic syntheses reported to date were carried out using direct current (DC) electrolysis, where electrons flow in one direction.[1] Alternating current (AC) electrolysis is an underdeveloped electroorganic synthesis technique involving the periodic change in the direction of electric current.[2–4] While there have been some early explorations of AC electrolysis for electrosynthesis, as summarized in our previous review,[5] insufficient mechanistic understanding and a lack of convenient methods to identify optimal AC conditions have hindered its development. Here, we summarize the AC-driven organic transformations developed since 2021 and discuss the unique properties of AC electrolysis that made the new reactions possible in this mini-review.
2. Recently developed AC-driven organic transformations
Hilt et al. demonstrated the acyl nitroso Diels–Alder reaction of 1,3-dienes with hydroxamic acids by utilizing AC to afford the desired product of 1,2-oxazine (Scheme 1A).[6] The Luo group achieved selective one-electron amine oxidation by applying an optimal AC frequency. This led to a higher selectivity towards arylation products and a suppressed overoxidation pathway to form cyanation products (Scheme 1B).[7] Later, the same group reported the reversible conversion of redox-labile functional groups such as quinone and aryl thioethers under AC conditions to achieve functional group-tolerant difunctionalization (Scheme 1C).[8] In the same year, they utilized the AC frequency to achieve selective D-labelling at α-amino C(sp3)−H site of amines in the presence of a thiol hydrogen atom transfer catalyst and D2O(Scheme 1D).[9] Moreover, the Lei group addressed the issue of Ag deposition on electrode under DC electrolysis, by using an asymmetric AC waveform to afford the C−H phosphorylation of alkynes, alkenes, and (hetero)arenes (Scheme 1E).[10] They also developed a C−O/O−H cross-metathesis reaction of 4-alkoxy anilines with alcohols (Scheme 1F)[11] and synthesis of N-S coupled product from amine and thiol(Scheme 1G)[12]. Meanwhile, the Baran group developed a rapid alternating polarity method or rAP (equivalent to AC electrolysis using a square waveform) for chemoselective reduction of carbonyl compounds in the presence of multiple redox-active functionalities (Scheme 1H)[13] and Kolbe reaction to form saturated C−C bonds straight from carboxylic acids (Scheme 1I).[14] They also demonstrated metal-free electrochemical decarboxylation of alkyl carboxylic acids for C−C coupling to generate olefins using rAP(Scheme 1J)[15] and chemoselective hetero(arene) electroreduction by simply modifying the AC waveform (Scheme 1K).[16] Hibino and his team converted methane to methanol in humidified conditions using AC frequency that hinders catalyst degradation (Scheme 1L).[17] He et. al. utilized the pulse technique to combine both heterogeneous and homogeneous catalysts to achieve a one-pot two-step cascade transformation: the conversion of NO2− to ammonia followed by Chan-Lam coupling of amine and aryl boronic acid to yield the desired aryl amine (Scheme 1M).[18] The Semenov group demonstrated AC electrolysis could provide stirring-free electrosynthesis up to 50-mmol scale with optimal frequency depending upon the reactor size for various organic reactions[19]. All these newly developed AC electrolysis methods exhibited yields and/or product selectivity that were better than their DC counterparts (Scheme 1).
Scheme 1.

Schematic of the AC-driven electroorganic transformations developed since 2021.
3. Unique properties of AC electrolysis behind the newly developed reactions
In the following section, we will discuss the distinctive properties of AC electrolysis that have enabled the development of new reactions in Scheme 1 using a few specific examples.
3.1. Timing electro/chemical reactions by AC frequency
AC electrolysis is known to mitigate overoxidation/reduction of substrates, but its mechanism has been elusive. In 2022, the Luo group revealed that the ability of AC electrolysis to time chemical reactions is one mechanism behind the mitigated overoxidation.[7] They used amine functionalization as a model system, in which amines can undergo one or two-electron oxidation to form an arylation product via a radical-radical coupling or a cyanation product via nucleophilic addition, respectively (Fig. 1A). They observed the arylation product is more favored than the cyanation product under AC conditions, opposite to the selectivity under the DC conditions. The mechanism is illustrated in Fig. 1B. Under DC electrolysis, amine 1 is first oxidized to radical cation 3. Then, the deprotonation of 3 occurs in an oxidizing environment, promoting further oxidation to iminium cation 5 and CN− addition. By altering the voltage polarity, the deprotonation of 3 could occur in a reducing environment to generate only amino radical 4 instead of iminium cation 5. This mechanism is supported by cyclic voltammetric (CV) analysis of amine oxidation with and without a base in Fig. 1C. At a slow scan rate of 0.02 V/s, two-electron oxidation of amine predominates as evidenced by i1 = 2io. In contrast, a shorter oxidation time at a high scan rate of 5 V/s could outpace the deprotonation of 3, favoring one electron oxidation as evidenced by i1 = io. Consequently, at an optimal AC frequency, one electron oxidation of amine generates the radical cation 3 at a positive pulse, which undergoes deprotonation to yield amino radical at the negative half-cycle, followed by coupling with arene radical anion formed at a negative pulse to selectively produce arylation product 1a. Perfect timing of the deprotonation of amine cation radical 3 by AC frequency is essential to controlling the amine oxidation level.
Fig. 1. Timing electro/chemical reactions by AC frequency.

(A)-(B) The one-electron and two-electron pathways of amine oxidation that produce cyanation and arylated products. (C) Cyclic voltammograms (CV) of 1 in the presence and absence of base at 0.02 (top) and 5 V/s (bottom) and their equivalent AC frequencies showing the degree of oxidation shifts from two-electron oxidation to one-electron oxidation according to the peak current comparison (i0 vs i1). Adapted with permission from[7], copyright (2022) by American Chemical Society. (D) AC-driven heterodifunctionalization and functional group tolerance of the AC method compared to the DC conditions. (E) AC electrolysis provides a redox-neutral environment where the redox-active functional groups can undergo reversible redox conversion, overcoming the potential window-limited functional group compatibility. (F) CV of aryl thioether at different scan rates showing the reversibility of thioether redox chemistry improves at higher scan rates. Adapted with permission from[8], copyright (2023) by American Chemical Society. (G) Illustration showing reaction outcome difference by applying DC and rAP. (H) Proposed mechanism and (I) CV of piperidine 7 and PivOH at 50 and 400 mV/s. Adapted with permission from[13], copyright (2021) by American Chemical Society.
Later, the Luo group reported an additional mechanism contributing to AC electrolysis’s ability to mitigate overoxidation/reduction. They demonstrated AC-driven chlorotrifluoromethylation well tolerates redox-labile functional groups such as aryl thioether, quinone, and unprotected pyrrole, which has redox potential within the reaction potential window (Fig. 1D).[8] They found that the oscillating redox environment of AC electrolysis enables reversible conversion of these functional groups after their initial oxidation or reduction, resulting in improved functional group tolerance (Fig. 1E). This mechanism is supported by their scan-rate-dependent CV analysis. The CVs of aryl thioether in Fig. 1F show the reduction peak at ~ −1.8 V, which is related to regenerating aryl thioether from its oxidized form, increases at high scan rates, indicating less irreversible oxidation of the aryl thioether. The anodic and cathodic peak ratio (ia/ic) that characterizes the extent of thioether regeneration was improved from ~11 at 0.1 V/s to ~2 at 40 V/s. They estimated the maximum product yield from the ia/ic values at different AC frequencies and found an excellent agreement with the experimental yield at various frequencies. This agreement confirms that the improved functional group tolerance is due to the perfect timing of the electrochemical regeneration of aryl thioethers under AC conditions.
Meanwhile, the Baran group found AC electrolysis can also be used to time electrochemical reactions to minimize undesired overoxidation.[13] They reported an impressive chemoselective reduction of carbonyl compounds such as 7 with multiple redox-active sites using rAP (Fig. 1G). In their reaction design, the carbonyl reduction occurs at the cathodic phase. A sacrificial reagent, pivalic acid, is consumed during the anodic phase. They observed two possible products under rAP conditions: hemiaminal 8a at shorter pulse durations (15–20 Hz) and fully reduced lactam 8b at longer pulse durations (2.5–10 Hz). In comparison, the prevalence of Shono oxidation in DC electrolysis leads to a complex mixture. The proposed mechanism is that under DC conditions, the oxidation of pivalic acid and the piperidine functional group of 7 would occur, causing oxidative decomposition (Fig. 1H). However, under AC conditions, the available oxidation time is shortened. Because of the slower Shono oxidation kinetics of piperidines than the oxidative decarboxylation kinetics of pivalic acid, fewer substrates would undergo the undesired Shono oxidation, leading to an improved yield toward the carbonyl reduction products. This mechanism is supported by the CV analysis for substrate 7 and pivalic acid (Fig. 2I). At a slow scan rate of 50 mV/s, both oxidation peaks of substrate 7 and pivalic acid are apparent between 1.4 and 1.8 V, whereas the only oxidation peak of pivalic acid is distinct in this potential window at 400 mV/s. This result indicates that the oxidation of substrate 7 is limited by the reaction kinetics at high scan rates (equivalent to high frequency), but the oxidation of pivalic acid is not.
Fig. 2. Controlling solution pH by AC electrolysis.
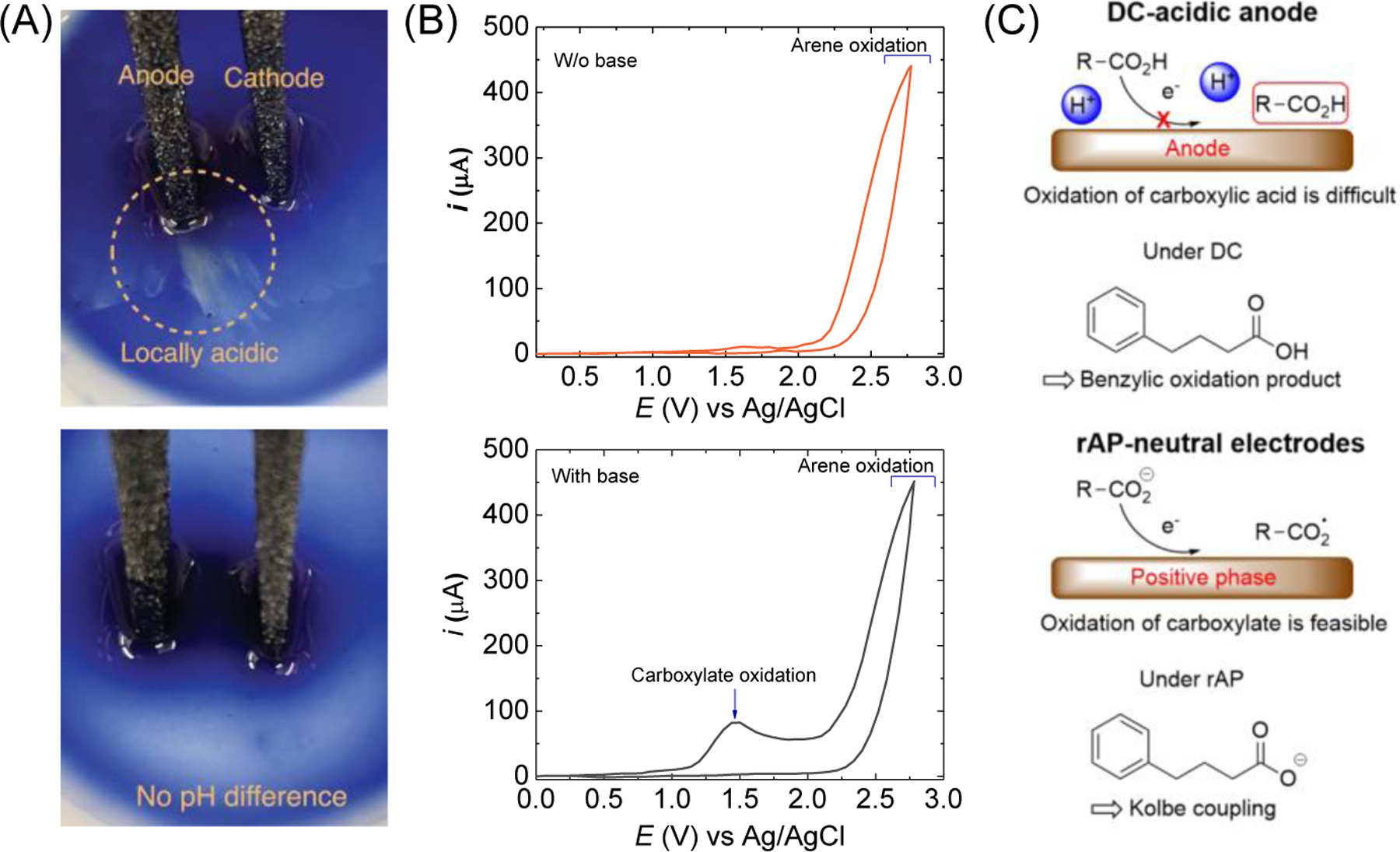
(A) Photographs of reaction pH change under DC (top) and rAP condition (bottom) using bromophenol blue as the pH indicator. (B) Comparison of reaction mechanisms under DC and rAP. (C) Cyclic voltammograms of carboxylic acid with or without a base (Me4N·OH). Adapted from[14], copyright (2023) American Association for the Advancement of Science.
The ability to time chemical steps addresses the overoxidation/reduction problem of DC electrolysis and is crucial to yielding the desired product during AC electrolysis. For example, the Luo group demonstrated electrochemical hydrogen isotope exchange (HIE) at α-amino C(sp3)–H sites of tetrahydroisoquinolines (THIQ) and pyrrolidines, employing a thiol hydrogen atom transfer (HAT) catalyst and deuterium source D2O.[9] This HIE reaction proceeds via the oxidation of amine to amino radical cation followed by two chemical steps, deprotonation, and HAT, to yield the D-labelled amine. Based on the deprotonation and HAT rates, they predicted that (i) THIQs would require a significantly lower frequency than pyrrolidines because of the slower HIE at the α-amino C(sp3)–H site of THIQ and (ii) at higher frequencies, the insufficient HAT reaction due to the limited reaction time would result in dimerization of amino radicals. Both are confirmed by the AC-frequency-dependent product distribution analysis.
3.2. Modulating chemical species concentration in solution
The Kolbe reaction is a simple method for creating Csp3–Csp3 bonds using carboxylic acid through electrochemical oxidative decarboxylation. However, due to the harsh oxidative conditions, this coupling reaction often encounters issues with low chemoselectivity and functional group tolerability. One reason is that the electrogenerated protons from the oxidation of the substrate, water, or solvent, decrease the pH at the anode’s surface (Fig. 2A). Protonated carboxylates have high overpotential, falling into the same potential window for other chemical functional groups’ oxidation. The Baran group found that rAP could prevent the accumulation of electrogenerated protons near the electrode surface (Fig. 2A).[14] This change in pH influences the oxidation potential of carboxylic acid substrates during the Kolbe reaction. As shown in Fig. 2B, the CVs of arene in the absence and presence of a base (Me4N·OH) reveals that (i) arene oxidation occurs at 2.0 V without base and (ii) another oxidation peak corresponding to the alkyl carboxylate oxidation appeared at 1.3V with base. Therefore, arene oxidation would occur in DC electrolysis, whereas the deprotonated form of carboxylic acid is preferably oxidized under rAP, resulting in selective decarboxylation and the Kolbe dimer formation. (Fig. 2C). The Baran group used the same principle to achieve decarboxylative olefination of unactivated carboxylic acids via a carbocation intermediate[15] and the chemoselective electroreduction of (hetero)arenes by circumventing the transesterification byproduct formation in a high pH environment.[16]
AC electrolysis can also modulate the concentration and oxidation states of catalytic metal ions in a reaction solution. Lei et al.[10] developed an asymmetric-waveform AC electrolysis method for Ag-catalyzed C−H phosphorylation in an undivided cell using carbon and Pt electrodes. They found that AC electrolysis prevented the removal of catalytic Ag ions from the reaction mixture by cathodic deposition because Ag is repeatedly deposited and stripped off. The waveform shape, frequency, and duty ratio were decisive in accomplishing the optimal yield. The optimal condition is that the Pt electrode acts as a cathode and the carbon electrode as an anode for a longer time than their opposite role. The difference between carbon and Pt electrodes is that Ag is oxidized predominantly to Ag(II) at the Pt one but to Ag(I) at the carbon one. Ag(II) activates phosphonyl compounds to give an Ag-phosphonyl intermediate and the final coupling products. A long cathodic time at the Pt electrode enabled the effective deposition of Ag, which later oxidized by two electrons to the active Ag(II) ions.
3.3. Promoting effective reaction of intermediates
In our previous review paper,[5] we discussed the ability of AC electrolysis to promote the effective reactions of electrogenerated intermediates. A similar approach has been used for the C−O/O−H cross-metathesis of 4-alkoxy anilines with alcohols by Wang et. al.[11] In their reaction, 4-alkoxy aniline oxidation generates an N-centered intermediate, which reacts with alcohol and generates a less stable cross-metathesis intermediate. Then, reversing electrode polarity helps for instant reduction of this intermediate to deliver 4-alkoxy aniline. Similarly, the same group applied the AC approach to cross-coupling thiophenols/thiols and amines.[12] Disulfides are reduced to thiyl radical and thiolate anion at a negative pulse. The thiyl radical is then coupled with an amine radical formed at a positive pulse, generating the desired N−S coupled product by avoiding the thiol overoxidation found in DC electrolysis.
4. Conclusion and outlook
This review summarized the recent progress of AC electroorganic synthesis and discussed the unique properties of AC electrolysis that have enabled the newly found reactivities, including timing electro/chemical reactions by AC frequency, modulating the concentrations of chemical species in solution such as pH and catalytic metal ions, and promoting effective reaction of intermediates. Since our last minireview in 2021,[5] the community has made significant progress in addressing the major challenge of AC electrolysis in identifying and understanding the optimal conditions. CV analysis has provided useful information to understand the mechanism and guide the rational design of AC conditions. The design-of-experiments approach has also been demonstrated to be efficient in finding the optimal conditions by Hilt et al.[6] However, CV analysis is limited by the information it can provide regarding the identity of reaction intermediates. Recent developments in operando mass spectrometry could address it. As shown in Fig. 3A, Wan et al.[20] developed a time-resolved operando electrochemical mass spectrometry (EC-MS) platform to detect intermediates of microsecond lifetime by conducting EC reactions at the nano-electrospray ionization tip. This platform has provided direct experimental evidence to support the proposed mechanisms for previously realized electrochemical C−C homocoupling,[21] N−S cross-coupling,[12] and C−O/O−H cross-metathesis of arylamines.[11] Fig. 3B shows the MS-detected N, N-dimethylaniline (DMA) radicals and their dimerization product, N, N, N’, N’-tetramethylbenzidine (TMB), as a function of time under DC and AC conditions. The formation of TMB under DC conditions is quicker than in AC conditions, resulting in its accumulation and formation of the overoxidized product TMB●+. In parallel, Xu et. al.[22] developed a nano-ESI MS platform integrated with a bipolar electrode to identify short-lived intermediates in the AC-driven C−O/O−H cross-metathesis reaction. Using bipolar electrodes can avoid the complexity of physically connecting an electrode to a power supply. These studies have shown operando EC-MS could provide rich information about the reaction intermediates, potentially used for rapid screening of optimal AC conditions.
Fig. 3.

(A) Schematic diagram elucidating the underlying mechanism that benefits AC electrolysis by time-resolved mapping of short-lived reactive intermediates using time-resolved operando mass spectrometry. (B)Electrochemical oxidative homo-coupling of DMA. Real-time recording of the ion intensity changes under (top) DC and (bottom) AC conditions. Adapted from[20], copyright (2023) Wiley-VCH. (C) Product selectivity changes as a function of AC pulse duration during electrochemical CO2 reduction (CO2RR). (D) The catalytic Cu species on an electrode surface under AC and DC conditions and their effect on CO adsorption and C2+ product selectivity during CO2RR. Adapted from[23], copyright (2023) by American Chemical Society.
Another underdeveloped but exciting direction in AC electrolysis for organic synthesis is heterogeneous catalysis systems. Waveform-modulated heterogeneous electrocatalysis has recently attracted significant attention in electrochemical CO2 reduction (CO2RR).[24] CO2RR can produce various C2+ and C1 products, including ethylene, ethanol, formic acid, carbon monoxide, etc., but C2+ products are more valuable and thus preferred. The Cuenya group has found that pulsed electrolysis is beneficial to maintaining both Cu(0) and Cu(I) species on the electrode surface, delivering ethanol with high selectivity.[25] Similarly, Li et al.[23] observed an improved C2+ product selectivity in pulsed CO2RR (Fig. 3C). Their time-resolved Raman spectroscopic analysis shows the presence of CuxO on the electrode surface facilitates the adsorption of CO, which impacts the C2+ selectivity (Fig. 3D). These studies indicate the potential of using AC electrolysis to modulate the oxidation states of heterogeneous electrocatalysts for achieving unique reactivities in organic synthesis.
Highlights.
The unique activities provided by AC electrolysis arise from its ability to time chemical steps, modulate chemical species concentration in solution, and promote the effective reaction of short-lived intermediates.
Operando electrochemical mass spectrometry enables direct identification of unstable reaction intermediates, providing new opportunities for understanding and developing AC electrosynthesis methods.
AC-modulated heterogeneous electrocatalysts could be explored for electroorganic synthesis.
Acknowledgments
N. B., S. R., A. H., and L.L. gratefully acknowledge the support from NIH 1R35 GM142590. R.M. and L.L. acknowledge the support from NSF CHE- 2247057.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Papers of particular interest, published within the period of review, have been highlighted as: ** of outstanding interest.
- 1.Yan M, Kawamata Y, Baran PS: Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem Rev 2017, 117:13230–13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilt G, Jamshidi M, Fastie C: Applications of alternating current/alternating potential electrolysis in organic synthesis. Synth 2022, 54:4661–4672. [Google Scholar]
- 3.Zeng L, Wang J, Wang D, Yi H, Lei A: Comprehensive comparisons between directing and alternating current electrolysis in organic synthesis. Angew Chem Int Ed 2023, 62:e202309620. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigo S, Um C, Mixdorf JC, Gunasekera D, Nguyen HM, Luo L: Alternating current electrolysis for organic electrosynthesis: trifluoromethylation of (hetero)arenes. Org Lett 2020, 22:6719–6723. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigo S, Gunasekera D, Mahajan JP, Luo L: Alternating current electrolysis for organic synthesis. Curr Opin Electrochem 2021, 28:100712–100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fährmann J, Hilt G: Alternating current electrolysis as efficient tool for the direct electrochemical oxidation of hydroxamic acids for acyl nitroso Diels–Alder reactions. Angew Chem Int Ed 2021, 60:20313–20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunasekera D, Mahajan JP, Wanzi Y, Rodrigo S, Liu W, Tan T, Luo L: Controlling one- or two-electron oxidation for selective amine functionalization by alternating current frequency. J Am Chem Soc 2022, 144:9874–9882. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This work reported a mechanism behind the controlled amine oxidation by AC frequency and found an electrochemical descriptor from cyclic voltammetry studies to predict the optimal AC frequency for various amine substrates.
- 8.Rodrigo S, Hazra A, Mahajan JP, Nguyen HM, Luo L: Overcoming the potential window-limited functional group compatibility by alternating current electrolysis. J Am Chem Soc 2023, 145:21851–21859. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This work shows that the reversible conversion of redox-labile functional groups under AC conditions is responsible for the improved functional group tolerance.
- 9.Behera N, Gunasekera D, Mahajan JP, Frimpong J, Liu ZF, Luo L: Electrochemical hydrogen isotope exchange of amines controlled by alternating current frequency. Faraday Discuss 2023, 247:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng L, Jiao Y, Yan W, Wu Y, Wang S, Wang P, Wang D, Yang Q, Wang J, Zhang H, et al. : Asymmetric-waveform alternating current-promoted silver catalysis for C–H phosphorylation. Nat Synth 2023, 2:172–181. [Google Scholar]; ** This work shows AC electrolysis modulates the oxidation potential of caboxylic acid substrate by controlling the local pH environment to achieve the desired Kolbe coupling products.
- 11.Wang D, Jiang T, Wan H, Chen Z, Qi J, Yang A, Huang Z, Yuan Y, Lei A: Alternating current electrolysis enabled formal C-O/O-H cross-metathesis of 4-alkoxy anilines with alcohols. Angew Chem Int Ed 2022, 61:e202201543. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Y, Qi J-C, Wang D-X, Chen Z, Wan H, Zhu J-Y, Yi H, Chowdhury AD, Lei A: Radical–radical cross-coupling assisted N–S bond formation using alternating current protocol. CCS Chem 2022, 4:2674–2685. [Google Scholar]
- 13.Kawamata Y, Hayashi K, Carlson E, Shaji S, Waldmann D, Simmons BJ, Edwards JT, Zapf CW, Saito M, Baran PS: Chemoselective electrosynthesis using rapid alternating polarity. J Am Chem Soc 2021, 143:16580–16588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hioki Y, Costantini M, Griffin J, Harper KC, Merini MP, Nissl B, Kawamata Y, Baran PS: Overcoming the limitations of Kolbe coupling with waveform-controlled electrosynthesis. Science 2023, 380:81–87. [DOI] [PubMed] [Google Scholar]; ** This work shows AC electrolysis modulates the oxidation potential of caboxylic acid substrate by controlling the local pH environment to achieve the desired Kolbe coupling products.
- 15.Garrido-Castro AF, Hioki Y, Kusumoto Y, Hayashi K, Griffin J, Harper KC, Kawamata Y, Baran PS: Scalable electrochemical decarboxylative olefination driven by alternating polarity. Angew Chem Int Ed 2023, 62:e202309157. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi K, Griffin J, Harper KC, Kawamata Y, Baran PS: Chemoselective (hetero)arene electroreduction enabled by rapid alternating polarity. J Am Chem Soc 2022, 144:5762–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibino T, Kobayashi K, Nagao M, Zhou D, Chen S, Yamamoto Y: Alternating current electrolysis for individual synthesis of methanol and ethane from methane in a thermo-electrochemical cell. ACS Catal 2023. 13:8890–8901. [Google Scholar]
- 18.He M, Wu Y, Li R, Wang Y, Liu C, Zhang B: Aqueous pulsed electrochemistry promotes C-N bond formation via a one-pot cascade approach. Nat Commun 2023, 14:5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bortnikov EO, Smith BS, Volochnyuk DM, Semenov SN: Stirring-free scalable electrosynthesis enabled by alternating current. Chem Eur J 2023, 29:e202203825. [DOI] [PubMed] [Google Scholar]
- 20.Wan Q, Chen K, Dong X, Ruan X, Yi H, Chen S: Elucidating the underlying reactivities of alternating current electrosynthesis by time-resolved mapping of short-lived reactive intermediates. Angew Chem Int Ed 2023, 62:e202306460. [DOI] [PubMed] [Google Scholar]
- 21.Brown TA, Chen H, Zare RN: Detection of the short-lived radical cation intermediate in the electrooxidation of N,N-dimethylaniline by mass spectrometry. Angew Chem Int Ed 2015, 54:11183–11185. [DOI] [PubMed] [Google Scholar]
- 22.Xi XJ, Hu J, Chen HY, Xu JJ: Rapid identification of the short-lived intermediates in alternating-current electrolysis by mass spectrometry. Chem Commun 2022, 58:10233–10236. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Wang L, Wang T, Sun L, Yang W: Steering the dynamics of reaction intermediates and catalyst surface during electrochemical pulsed CO2 reduction for enhanced C2+ selectivity. J Am Chem Soc 2023, 145:20655–20664. [DOI] [PubMed] [Google Scholar]
- 24.Casebolt R, Levine K, Suntivich J, Hanrath T: Pulse check: potential opportunities in pulsed electrochemical CO2 reduction. Joule 2021, 5:1987–2026. [Google Scholar]
- 25.Arán-Ais RM, Scholten F, Kunze S, Rizo R, Roldan Cuenya B: The role of in situ generated morphological motifs and Cu(I) species in C2+ product selectivity during CO2 pulsed electroreduction. Nat Energy 2020, 5:317–325. [Google Scholar]


