Abstract
Objectives
This study assessed antibiotic prescribing patterns in primary healthcare facilities and antimicrobial resistance (AMR) profiles of commensal Escherichia coli and enterococci isolated from pregnant women and children under 5 years of age.
Materials and methods
This cross-sectional study was conducted in Lusaka and Ndola districts of Zambia. Prescription pattern data were obtained from hospital pharmacies. Identification and antimicrobial susceptibility profiles of E. coli and enterococci were determined by conventional methods, while confirmation of both pathogens and AMR genes were determined by PCR. Data were analysed using WHONET and SPSS version 25.0.
Results
Most prescribed antibiotics at the primary healthcare facilities belonged to the Access group of the WHO Access, Watch and Reserve (AWaRe) classification. All the primary healthcare facilities adhered to the AWaRe framework of ≥60% prescribed antibiotics belonging to the Access group. However, resistance was highest in the Access group of antibiotics. E. coli resistance to ampicillin ranged from 71% to 77% and to co-trimoxazole from 74% to 80%, while enterococcal resistance to tetracycline was 59%–64%. MDR was highest in E. coli (75%) isolates, while XDR was highest in enterococcal isolates (97%). The identified AMR genes in E. coli included blaCTX-M, sul2 and qnrA, while those of enterococci included erm(B), erm(C) and erm(A).
Conclusions
Resistance was highest in the prescribed WHO Access group of antibiotics. These findings highlight the need to use local susceptibility data to formulate country-specific treatment guidelines in line with WHO AWaRe classification and enforce regulations that prohibit easy access to antibiotics.
Introduction
The human gut microbiota harbours both commensals and opportunistic pathogens, which can acquire antibiotic resistance through mutation and horizontal gene transfer.1 The term ‘human gut microbiota’ describes the microbial community colonizing the human intestinal tract, which has a symbiotic relationship with its host, facilitating a balanced, mutually beneficial state.2 The gut microbiota maintains human nutrition and health by supplying nutrients and providing protection from pathogenic organisms.3 When pathogenic bacteria invade the host, the intestine’s immune system can distinguish commensal bacteria from pathogenic ones and only attack those harmful to the host.4 Equally, opportunistic pathogens carried asymptomatically in healthy individuals can also be present in the gut microbiota and only cause infections in immunocompromised hosts.1
Escherichia coli and enterococci are the most prevalent commensal bacteria that colonize the gastrointestinal tract (GIT) of humans and animals and are also known to be opportunistic pathogens.5 Both E. coli and enterococci are causative agents of community-acquired and healthcare-associated infections, with E. coli being the leading cause of bloodstream infections (BSIs) and urinary tract infections (UTIs).6,7 The outcome of infections caused by these pathogens has become difficult to treat due to the emergence of antimicrobial resistance (AMR).8 The escalating predominance of MDR organisms (MDROs) in the community and the increasing incidence of community-associated AMR infections pose a significant threat to public health.9,10 AMR in commensal bacteria could contribute to an increase in AMR among pathogenic bacteria through the horizontal transfer of resistance genes.11 This cross-transmission of AMR genes from commensal to pathogenic bacteria and vice versa may lead to community- or hospital-acquired infections caused by resistant pathogens, should such bacteria acquire additional genetic material that enables them to become pathogenic.12
Most primary healthcare facilities in low- and middle-income countries (LMICs) lack microbiology diagnostic infrastructure and capacity; hence, treatment is often empirical without microbiology guidance.13–15 Treating infections without the guidance of antimicrobial susceptibility coupled with the purchase of antibiotics without clinical indication and prescriptions worsens the emergence and spread of AMR in the community and further limits treatment options for more invasive infections.16,17 The WHO has developed a framework for antimicrobial stewardship; this tool guides antimicrobial usage and limits the selection and spread of antibiotic resistance.18 This tool emphasizes that narrow-spectrum antibiotics included in the Access group should be preferred over broad-spectrum antibiotics from the Watch and Reserve groups and encourages a target of at least 60% of total antibiotic consumption to be from the Access group.19,20 However, there have been inconsistencies in adherence to this framework due to the lack of locally generated antimicrobial susceptibility data and sustained, reliable availability of antibiotics that supports the allocation of antibiotics in the Access, Watch and Reserve (AWaRe) classification.21,22
The human gut microbiota has been identified as a reservoir of AMR genes, referred to as the gut resistome, thus there is a need to study antibiotic resistance in the human gut microbiota to characterize the resistome’s ability to contribute to the emergence of MDR opportunistic pathogens.1 Knowledge of resistance patterns and the burden of MDR among commensal bacteria could help predict the resistance profile of a subsequent clinical infection.12 Resistance genes are prevalent in the faecal E. coli strains from healthy individuals; hence, AMR surveillance programmes have highlighted the importance of assessing resistance patterns in commensal intestinal bacteria to estimate AMR trends in the communities.23,24 It is in this regard that this study sought to assess the antibiotic prescribing patterns and AMR profiles of commensal E. coli and enterococci isolated from healthy pregnant women and children ≤5 years old who accessed antenatal and under-five services at the selected primary healthcare facilities in Lusaka and Ndola districts of Zambia.
Materials and methods
Study design and site
This cross-sectional study was conducted in the Lusaka and Ndola districts of Zambia between October 2020 and January 2021. Rectal swabs were collected from pregnant women and children ≤5 years old from different communities attending antenatal and under-five care, respectively, at primary healthcare facilities in Lusaka and Ndola. Inclusion criteria were all pregnant women and children ≤5 years old whose mothers gave consent and had no history of hospital admission and/or antibiotic use in the past 30 days.
Study area in Lusaka district
Three primary healthcare facilities targeted in the Lusaka district were Chilenje and Kanyama first-level hospitals and Kalingalinga health centre. Kanyama is densely populated with approximately 169 253 inhabitants, and a population density of 5636/km2.25 Kalingalinga and Chilenje are middle-density areas with approximately 39 139 and 52 220 inhabitants, respectively, and 3771 and 4769/km2 population density, respectively.25
Study area in Ndola district
Similarly, the three primary healthcare facilities targeted in the Ndola district were Lubuto, New Masala and Mapalo health centres. Lubuto has a population of 22 915 and 7695/km2 population density, New Masala has 9059 inhabitants and 10 391/km2 population density, and Mapalo urban health centres have 37 703 inhabitants and 7769/km2.25
All six healthcare facilities acted as the first contact point for patients, offering both inpatient and outpatient services to the approximated inhabitants of their catchment area. However, cases needing specialist care were transferred to tertiary hospitals. A tertiary hospital is a hospital that provides healthcare obtained from specialists in a large hospital after referral from the providers of primary care and secondary care.
Data collection
Antibiotic prescribing patterns
Antibiotic prescribing patterns were collected from the six primary healthcare facilities using a questionnaire imbedded in the CSPro 7.6 data collection tool (https://cspro.software.informer.com/7.6/) and exported into Excel, where the antibiotics were categorized as ‘Access’, ‘Watch’ or ‘Reserve’ according to the WHO AWaRe classification of antibiotics.26
Specimen collection and processing
After obtaining consent and explaining the procedure to the participants, a sterile swab moistened in 0.85% sterile saline was used to collect a rectal swab. The swab was placed into Amie’s transport medium (Oxoid, Basingstoke, UK). Upon receipt at the Veterinary Public Health Laboratory at the University of Zambia, the swabs were immediately placed in alkaline peptone water (APW) and incubated aerobically at 35–37°C for 18–24 h before subculturing. A total of 290 rectal swabs were collected from pregnant women and children under 5 years, of which 168 were collected in Lusaka and 122 in Ndola.
Identification of E. coli
The samples were then cultured on MacConkey agar (Oxoid, Basingstoke, UK) and incubated aerobically at 35°C–37°C for 18–24 h. Bacterial growth on the plates was examined for colony morphology characteristic of E. coli. Gram stain was also performed to confirm Gram-negative morphology. Presumptive identification of E. coli was based on standard biochemical reactions while PCR was used to confirm the species identification of E. coli. This was achieved using the uidA gene, which encodes the β-glucuronidase enzyme.27 DNA was extracted using the NucliSENS Easy MAG machine (bioMérieux). PCR amplification was performed as described by Godambe et al.27 The Veriti 96 Well Thermal Cycler-Applied Biosystems (Pittsburgh, PA, USA) was used for PCR amplification. The PCR products (1/10 volume) were analysed by gel electrophoresis (Bio-Rad, Hercules, CA, USA) at 100 V for 30 min using 1.5% agarose gels (BD Difco) in 1× Tris-acetate EDTA (TAE) buffer. The gels were stained with ethidium bromide (Sigma, St. Louis, MO, USA), and the PCR products were visualized under UV light using a gel documentation machine.
Identification of Enterococcus species
The samples were cultured on blood agar (Oxoid, Basingstoke, UK) and then incubated in a 5% CO2 incubator at 35°C–37°C for 18–24 h. Bacterial growth on the plates was examined for colony morphology characteristic of enterococci. Gram stain was performed, and the Gram-positive cocci and catalase-negative isolates were then plated on bile esculin azide (BEA) agar (Oxoid, Basingstoke, UK) to identify enterococci presumptively. PCR was conducted on single colonies that turned the media black (bile aesculin-positive colonies) for genus confirmation using the tuf gene, while the sodA gene was used to speciate Enterococcus faecium and Enterococcus faecalis, respectively, as was done by Li et al.28 and Pillay et al.29
Antimicrobial susceptibility testing (AST) of E. coli and Enterococcus species
Conventional AST using the Kirby–Bauer disc diffusion method was used to determine antimicrobial susceptibility profiles of E. coli and enterococci. The following antibiotics were used. For E. coli: ampicillin 10 μg, cefoxitin 30 μg, cefotaxime 30 μg, ceftazidime 30 μg, cefepime 30 μg, imipenem 10 μg, meropenem 10 μg, co-trimoxazole 1.25 μg/23.75 μg, ciprofloxacin 5 μg, gentamicin 10 μg, amikacin 30 μg, tetracycline 30 μg, nitrofurantoin 300 μg and aztreonam 30 μg; for enterococci: high-level gentamicin 120 μg, chloramphenicol 30 μg, vancomycin 30 μg, erythromycin 15 μg, nitrofurantoin 300 μg, ampicillin 10 μg, penicillin 10 μg, ciprofloxacin 5 μg, quinupristin/dalfopristin 15 μg, tetracycline 30 μg, linezolid 30 μg. The selection of antibiotics was based on the recommendations in the CLSI guidelines.30
Determination of resistance genes using PCR
The selection of isolates for resistance-gene determination was based on the resistance profiles to the different antibiotic classes of interest. E. coli isolates that were resistant to co-trimoxazole (n = 70), ciprofloxacin (n = 40), cefotaxime (n = 17) and imipenem (n = 2) were screened for sulphamethoxazole/trimethoprim-resistance genes, plasmid-mediated quinolone-resistant (PMQR) genes, ESBL/ampC, and carbapenemase-resistance genes, respectively. The primer selection and PCR protocol were as earlier described by Farkas et al.31 Fifty-eight enterococci that were resistant to macrolides were screened for macrolide-resistance genes. The primer selection and PCR protocol were based on those by Zou et al.32
Data management and analysis
Questionnaire data were collected using the CSPro 7.6, while the AST data were entered into WHONET 2020. The data were then managed in Excel spreadsheets and exported to STATA14 for analysis. The proportion (%) of resistant, intermediate, susceptible and MDR, XDR, pan-drug resistant (PDR) isolates were estimated with WHONET analysis. MDR isolates were defined as resistance to at least one agent in three or more antibiotic classes, XDR as resistance to at least one agent in all but two or fewer antimicrobial categories (i.e. bacterial isolates remained susceptible to only one or two categories) and PDR was defined as resistance to all agents in all antimicrobial categories.33 The data on prescribing patterns were analysed using the IBM Statistical Package for Social Sciences version 25.0 and presented in tables and figures.
Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee at Eres Converge institutional review board (Ref. No. 2019-Aug-017). The regulatory approval was obtained from the National Health Research Authority (NHRA). Permission to conduct the study at the different hospitals/institutions was obtained from the provincial and district health directors of all the healthcare facilities included in the study. Written informed consent was sought and obtained from the participants before administering the oral questionnaires and collecting samples; for paediatric patients, their guardians gave consent. Only those that gave consent were included. The study participants were assured of confidentiality by not identifying them by name but by codes, age and sex. The data were secured, and the results were used for research purposes only. To ensure safety, the collection of swabs was performed by a qualified health professional.
Results
Six health facilities were enrolled in this study, three from the Lusaka district (Chilenje, Kalingalinga and Kanyama hospitals) and three from the Ndola district (Lubuto, Mapalo and Masala health centres).
Prescribing patterns in primary healthcare facilities
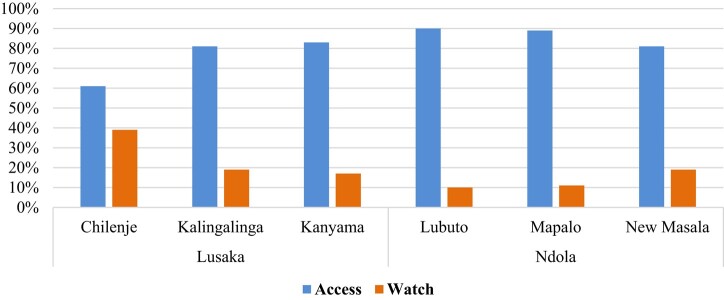
Most antibiotics prescribed in the primary healthcare facilities in the Lusaka and Ndola districts belonged to the Access group of antibiotics (Table 1). Chilenje first-level hospital had the highest ceftriaxone prescribed (Table 1). The top four most commonly prescribed antibiotics were amoxicillin, cefalexin, metronidazole and co-trimoxazole, with amoxicillin and cefalexin being the most commonly prescribed in Lusaka and Ndola primary healthcare facilities, respectively (Tables 1 and 2). Ciprofloxacin was the most commonly prescribed Watch antibiotic at all primary healthcare facilities (Tables 1 and 2). All the primary healthcare facilities adhered to the WHO AWaRe framework of having ≥60% of prescribed antibiotics belonging to the Access group of antibiotics. Prescription of the Watch group of antibiotics was below 20% in five primary healthcare facilities, with only Chilenje recording 39% (Figure 1).
Table 1.
Prescribing patterns at primary healthcare facilities in Lusaka district
| Name of antibiotics | Indication | Frequency (n) | Percent (%) | AWaRe classification |
|---|---|---|---|---|
| Amoxicillin | RTI, otitis media | 7272 | 23 | Access |
| Metronidazole | UTI, diarrhoea | 5871 | 18 | Access |
| Ciprofloxacin | UTI, PID, STI | 4955 | 15 | Watch |
| Cefalexin | RTI, UTI, pyelonephritis | 4873 | 15 | Access |
| Co-trimoxazole | Diarrhoea, prophylaxis for HIV patients | 2242 | 7 | Access |
| Cefotaxime/Ceftriaxone | RTI, UTI, sepsis | 1792 | 6 | Watch |
| Azithromycin | RTI, COVID-19-related symptoms, tonsillitis | 1469 | 5 | Watch |
| Doxycycline | UTI, STI | 1284 | 4 | Access |
| Benzathine penicillin | Tonsillitis, syphilis | 882 | 3 | Access |
| Gentamicin | STI | 751 | 2 | Access |
| Cloxacillin | RTI | 378 | 1 | Access |
| Penicillin V | URTI, tonsillitis | 289 | 1 | Access |
| Nitrofurantoin | UTI | 134 | 0 | Access |
| Chloramphenicol | Otitis media | 25 | 0 | Access |
| Ampicillin | Sepsis | 8 | 0 | Access |
| Total | — | 32 205 | 100 | — |
RTI, respiratory tract infection; URTI, upper RTI; STI, sexually transmitted infection; PID, pelvic inflammatory Disease.
Table 2.
Prescribing patterns at health primary healthcare facilities in Ndola district
| Name of antibiotic | Indication | Frequency (n) | Per cent (%) | AWaRe classification |
|---|---|---|---|---|
| Cefalexin | RTI, UTI | 7038 | 20 | Access |
| Amoxicillin | RTI | 5834 | 17 | Access |
| Metronidazole | Diarrhoea | 5558 | 16 | Access |
| Co-trimoxazole | URTI, RTI | 5403 | 16 | Access |
| Ciprofloxacin | UTI | 3182 | 9 | Watch |
| Cloxacillin | RTI, wounds, burns | 1830 | 5 | Access |
| Penicillin | Febrile illness, sepsis | 1634 | 5 | Access |
| Doxycycline | UTI | 1416 | 4 | Access |
| Azithromycin/erythromycin | RTI | 1338 | 4 | Watch |
| Gentamicin | UTI | 411 | 1 | Access |
| Chloramphenicol | Conjunctivitis | 89 | 0 | Access |
| Total | — | 34 594 | 100 | — |
Figure 1.
Antibiotic prescriptions based on AWaRe classification of the primary healthcare facilities in Lusaka and Ndola districts.
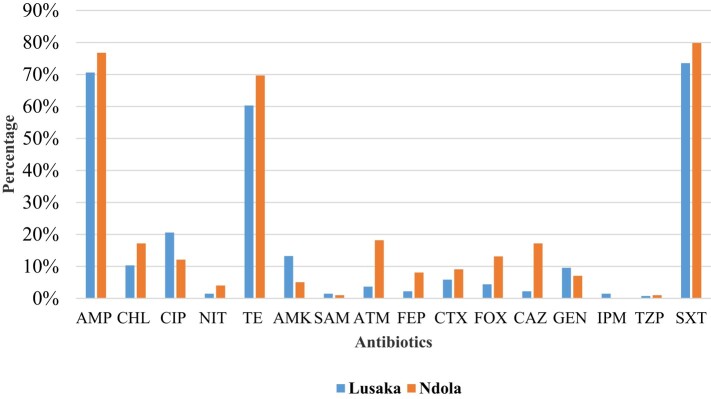
The prevalence of E. coli and Enterococcus species was 94% (272/290) and 69% (200/290), respectively. AMR in E. coli was highest in sulfamethoxazole/trimethoprim (co-trimoxazole) (76%), ampicillin (73%) and tetracycline (64%) and lowest in imipenem (1%) and piperacillin/tazobactam (1%) (Figure 2). Resistance in E. coli was generally higher in Ndola compared with Lusaka (Figure 2).
Figure 2.
Resistance profiles of E. coli from healthy individuals at primary healthcare facilities in Lusaka and Ndola districts. AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; NIT, nitrofurantoin; TE, tetracycline; AMK, amikacin; SAM, ampicillin/sulbactam; ATM, aztreonam; FEP, cefepime; CTX, cefotaxime; FOX, cefoxitin; CAZ, ceftazidime; GEN, gentamicin; IPM, imipenem; TZP, piperacillin/tazobactam; SXT, co-trimoxazole.
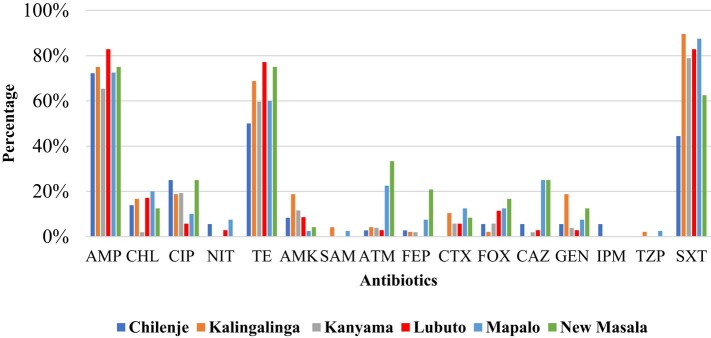
Resistance to ampicillin was noted to be high in all six different healthcare facilities. Similarly, co-trimoxazole and tetracycline resistance were high in all the facilities, with the least resistance in Chilenje. Imipenem resistance was only seen in Chilenje, while piperacillin/tazobactam and ampicillin/sulbactam resistance was recorded in Mapalo (Ndola) and Kalingalinga (Lusaka) health facilities. Resistance to other antibiotics such as chloramphenicol, ciprofloxacin, ceftriaxone, gentamicin, amikacin and aztreonam was recorded in all the primary healthcare facilities but at diverse percentages (Figure 3).
Figure 3.
Resistance profiles for E. coli from the different primary healthcare facilities in Lusaka and Ndola districts. Lusaka primary healthcare facilities—Chilenje, Kalingalinga, Kanyama; Ndola primary healthcare facilities—Lubuto, Mapalo, New Masala. AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; NIT, nitrofurantoin; TE, tetracycline; AMK, amikacin; SAM, ampicillin/sulbactam; ATM, aztreonam; FEP, cefepime; CTX, cefotaxime; FOX, cefoxitin; CAZ, ceftazidime; GEN, gentamicin; IPM, imipenem; TZP, piperacillin/tazobactam; SXT, co-trimoxazole.
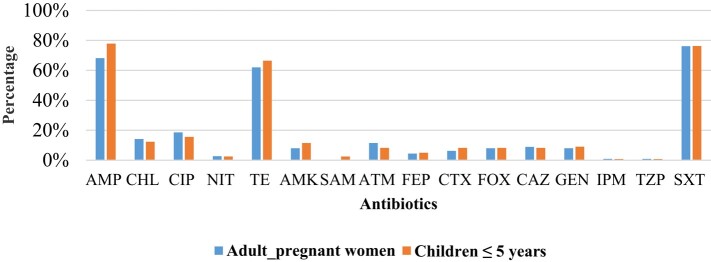
There was no difference in the occurrence of AMR in adults (pregnant women) compared with children ≤5 years old (Figure 4) for all antibiotics tested except for amikacin (P = 0.033) and co-trimoxazole (P = 0.032). Though low, resistance to antibiotics such as ceftriaxone, cefepime, piperacillin/tazobactam and amikacin, which are used to treat invasive infections in hospitalized patients, was noted in both pregnant women and children ≤5 years old (Figure 4).
Figure 4.
Resistance profiles of E. coli from healthy adults (pregnant women) and children ≤5 years old. AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; NIT, nitrofurantoin; TE, tetracycline; AMK, amikacin; SAM, ampicillin/sulbactam; ATM, aztreonam; FEP, cefepime; CTX, cefotaxime; FOX, cefoxitin; CAZ, ceftazidime; GEN, gentamicin; IPM, imipenem; TZP, piperacillin/tazobactam; SXT, co-trimoxazole.
Resistance-gene determinants in E. coli
Several resistance-gene determinants were identified (Table 3). Notably, most isolates had multiple resistance genes from the same class and/or from other antibiotic classes. The prevalence of ESBL resistance-gene determinants was 5% (14/272), co-trimoxazole 39% (70/179) and fluoroquinolones 7% (20/272).
Table 3.
Resistance-gene determinants in E. coli
| Antibiotic class | Resistance genes | Frequency, % (n) |
|---|---|---|
| β-Lactams | ||
| ESBL resistance-gene determinants | bla SHV | 48 (11) |
| bla TEM | 30 (7) | |
| bla CTX-M | 22 (5) | |
| Total | 100 (23) | |
| Folate-pathway antagonists | ||
| Sulphamethoxazole | Sul2 | 52 (43) |
| sul1 | 26 (21) | |
| Trimethoprim | dfA7 | 22 (18) |
| Total | 100 (82) | |
| Fluoroquinolones | ||
| qnrA | 36 (19) | |
| qnrS | 34 (18) | |
| qnrB | 30 (16) | |
| Total | 100 (53) | |
Enterococci from a healthy community
Among the identified enterococci, E. faecium was predominant compared with E. faecalis and the other species, which were in relatively low numbers (Table 4).
Table 4.
The distribution of Enterococcus species from the healthy individuals in the community
| Enterococcus species | Frequency (n) | Percent (%) |
|---|---|---|
| E. faecium | 93 | 46 |
| E. faecalis | 85 | 43 |
| E. gallinarum | 10 | 5 |
| E. hirae | 8 | 4 |
| E. casseliflavus | 2 | 1 |
| E. durans | 2 | 1 |
| Total | 200 | 100 |
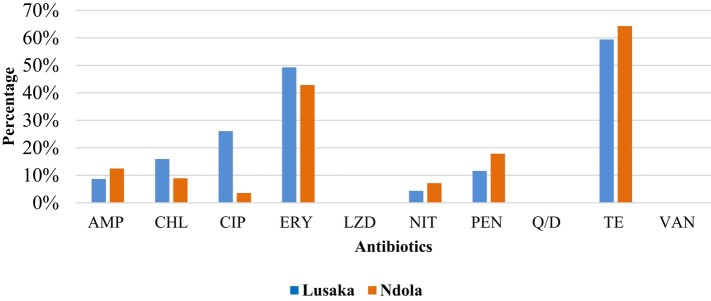
The highest resistance was observed to tetracycline, followed by erythromycin for both Lusaka and Ndola primary healthcare facilities (Figure 5). There was no resistance recorded to linezolid, quinupristin/dalfopristin and vancomycin, though reduced susceptibility (intermediate results) were as follows: linezolid (n = 31), quinupristin/dalfopristin (n = 16) and vancomycin (n = 14). These three antibiotics are used to treat invasive infections and are considered last-resort treatment options for MDR Enterococcus infections.
Figure 5.
Resistance profiles for enterococci from healthy communities in Lusaka and Ndola districts. AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; ERY, erythromycin; LZD, linezolid; NIT, nitrofurantoin, PEN, penicillin, Q/D, quinupristin/dalfopristin; TE, tetracycline; VAN, vancomycin.
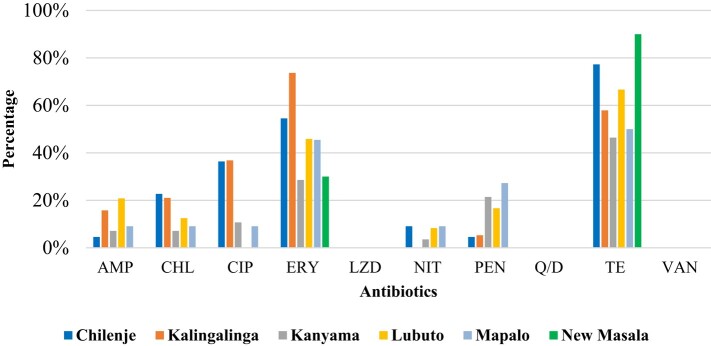
Erythromycin resistance was highest at Kalingalinga, followed by Chilenje, while tetracycline resistance was highest at New Masala, followed by Chilenje. The three primary healthcare facilities in the Lusaka district recorded the highest frequency of reduced susceptibility to linezolid, quinupristin/dalfopristin and vancomycin (Figure 6).
Figure 6.
Resistance profiles for enterococci from the different primary healthcare facilities in Lusaka and Ndola districts. AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; ERY, erythromycin; LZD, linezolid; NIT, nitrofurantoin, PEN, penicillin, Q/D, quinupristin/dalfopristin; TE, tetracycline; VAN, vancomycin.
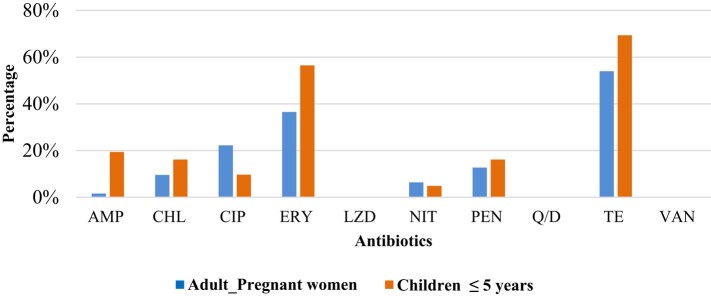
The reduced susceptibility to the three antibiotics was mostly in adult pregnant women than children ≤5 years old. Resistance to tetracycline, erythromycin, nitrofurantoin and ciprofloxacin was higher in adult pregnant women than in children ≤5 years old, while resistance to the remaining antibiotics tested was higher in children ≤5 years old than in adult pregnant women (Figure 7). In children ≤5 years old, reduced susceptibility to linezolid was found in 10, to quinupristin/dalfopristin in 9 and to vancomycin in 5.
Figure 7.
Resistance profiles in adults (pregnant women) and children ≤5 years old. AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; ERY, erythromycin; LZD, linezolid; NIT, nitrofurantoin, PEN, penicillin, Q/D, quinupristin/dalfopristin; TE, tetracycline; VAN, vancomycin.
Resistance to macrolides was 29% (58/200), and the prevalence of macrolide-resistance genes was 16% (32/200), with erm(B) (69%; 22/32) being the most prevalent, followed by erm(C) (19%; 6/32) and erm(A) (12%; 4/32).
Seventy-five per cent (153/204) of E. coli and 25% (51/204) of enterococci were classified as MDR, with Kalingalinga and Kanyama primary healthcare facilities recording the highest MDR E. coli and enterococci rates, respectively. XDR was more prevalent in enterococci isolates (97%; 29/30) compared with E. coli (3%; 1/30). Only one Enterococcus isolate was classified as PDR (Table 5).
Table 5.
Distribution of MDR, XDR and PDR in E. coli and Enterococcus isolates
| Location | Facility | Frequency, n (%) | ||
|---|---|---|---|---|
| MDR | XDR | PDR | ||
| E. coli | ||||
| Lusaka | Chilenje | 25 (16) | — | — |
| Kalingalinga | 34 (22) | — | — | |
| Kanyama | 24 (16) | 1 (100) | — | |
| Ndola | Lubuto | 28 (18) | ||
| Mapalo | 22 (15) | |||
| Masala | 20 (13) | |||
| Total (E. coli) | 153 (75) | 1 (3) | 0 | |
| Enterococci | ||||
| Lusaka | Chilenje | 11(21.5) | 8 (28) | — |
| Kalingalinga | 5 (10) | 4 (14) | — | |
| Kanyama | 13 (25) | 10 (34) | 1 (100) | |
| Ndola | Lubuto | 12 (23.5) | 4 (14) | — |
| Mapalo | 5 (10) | 2 (7) | — | |
| Masala | 5 (10) | 1(3) | ||
| Total (Enterococci) | 51 (25) | 29 (97) | 1 (100) | |
| Total (E. coli and enterococci) | 204 (100) | 30 (100) | 1 (100) | |
Discussion
This study assessed the prescribing patterns of antibiotics and AMR profiles of carriage E. coli and enterococci isolated from pregnant women and children ≤5 years old in the Lusaka and Ndola districts of Zambia. Our study found that the Access group of antibiotics, namely amoxicillin, metronidazole, cefalexin and co-trimoxazole, were the most commonly prescribed in the primary healthcare facilities, similar to findings in two districts in Cameroon that both recorded amoxicillin, co-trimoxazole and metronidazole as the most commonly prescribed.34 Penicillins were widely used in primary healthcare, with oral amoxicillin being the most commonly prescribed; comparable to our findings, a scoping review found amoxicillin to be the most commonly prescribed in East Africa, West Africa, New Zealand, Cuba and Brazil,35 and India.36 The overuse of penicillins in primary healthcare facilities could be attributed to the lack of facility-based treatment guidelines supported by local microbiology data.14
Although the prescription of antibiotics was above the WHO-recommended threshold of 30%, the choice of antibiotic classes in the primary healthcare facilities was in line with the WHO recommendations, which state that more than 60% of all prescribed antibiotics must be from the Access group.18,37 Our findings agreed with those from a study that included Ghana, Uganda, Tanzania and Zambia, where almost half of the antibiotics prescribed belonged to WHO’s Access group.38 Ceftriaxone, ciprofloxacin and erythromycin/azithromycin were the only prescribed antibiotics belonging to the WHO Watch group, with ciprofloxacin being the most commonly prescribed. Similar to our finding, a study at nine primary healthcare facilities in India found ciprofloxacin to be the most prescribed Watch antibiotic.36 Contrary to our findings, a study that assessed antibiotic overuse in primary healthcare settings revealed higher use of Watch antibiotics, mostly quinolones and cephalosporins, in China and India, with only Kenya mostly using Access antibiotics.39 A study in rural Burkina Faso that assessed the use of Watch antibiotics before hospital presentation and another study in China that assessed antibiotic prescription patterns in 48 primary healthcare facilities both found ceftriaxone to be the most commonly prescribed, followed by ciprofloxacin.40,41 The limited availability of culture and sensitivity testing (CST) to guide antibiotic choices in primary healthcare facilities results in consistently high rates of empirical prescribing.42
Overprescribing of antibiotics in primary healthcare facilities has resulted in AMR, limiting the treatment options and increasing the population carriage of resistant organisms in the community.43 Encouragingly, none of the facilities included in this study prescribed antibiotics from the WHO Reserve group, and the three antibiotics from the Watch group accounted for less than 20% in all facilities except one that also serves as a first-level hospital. This was contrary to a study by D’Arcy et al.38 that found the use of Watch antibiotics to be above 38% in all four studied countries, with Zambia recording 41% use. Again, contrary to our findings, a study in India recorded the use of Reserve antibiotics such as carbapenems and colistin, the last-resort antibiotics for treating MDR, XDR and ESBL-producing Gram-negative bacilli, respectively.39,44 Inappropriate prescribing of Watch and Reserve antibiotics outside specialist hospitals reduces their potential to tackle serious and critical infections when needed.45
High levels of E. coli resistance to ampicillin and co-trimoxazole could be attributed to selective pressure resulting from misuse and overuse of antibiotics in the community and at primary healthcare facilities.46,47 Comparable to our findings, a systematic review that analysed the prevalence of resistance to the top 10 antibiotics commonly prescribed in LMICs in commensal E. coli isolates from human sources in community settings found high resistance to ampicillin, co-trimoxazole and tetracycline.48 Studies included in this systematic review were mostly from Asia (n = 13) and Africa (n = 10).48 The use of co-trimoxazole for prophylaxis of opportunistic infections in people living with HIV (PLWHIV) might also promote selective pressure, hence the high levels of resistance noted.49 A study in southern Ethiopia revealed that co-trimoxazole prophylaxis increased the risk of resistance to co-trimoxazole, and this was statistically associated with co-resistance to penicillin.49
High antibiotic resistance rates were observed in carriage E. coli and enterococci from healthy individuals from the communities, especially to antibiotics commonly accessed without prescriptions and used as empirical treatment in primary health facilities.22,46 This agrees with the rising resistance in healthy individuals, especially children, who are potential reservoirs of antibiotic-resistant bacteria.50 Studies have linked carriage of antibiotic-resistant organisms in healthy communities to poor sanitation and infrastructure in high-density areas, environmental contamination, high prevalence of HIV in LMICs and the lack of regulations that allow access to antibiotics without an indication and prescriptions.51–55 In this study, antibiotic resistance was recorded in both high- and medium-density areas, which could be attributed to the behaviour towards antibiotic use in the community and the lack of knowledge on the effects of irrational antibiotic use.56,57
The presence of resistance genes found on mobile genetic elements such as plasmids is alarming as these can easily be transferred to other bacteria that can potentially cause invasive infections.58 Notably, ESBL resistance genes (blaCTX-M, blaSHV, blaTEM), PMQR genes (qnrA, qnrS and qnrB) and trimethoprim/sulphamethoxazole-resistance genes (sul1, sul2, dfA7) recorded in healthy individuals in this study were also found in clinical isolates causing BSIs at the University Teaching Hospital, a tertiary hospital in Lusaka, Zambia.59 A study in uMgungundlovu, South Africa, revealed a higher prevalence of ESBL-mediating MDR Gram-negative ESKAPE bacteria in faecal carriage (46%) than in clinical samples (28%), with colonization being mainly associated with a referral from district to tertiary hospitals.60
The presence of MDR and XDR in carriage E. coli and enterococci in this study further increases the chances of transmitting resistance genes to pathogenic bacteria, which might lead to increased transfer of resistant pathogens in the community and hospital settings.61 The rise in MDR/XDR pathogens causing serious illnesses limits treatment options, prolongs hospital stay, increases treatment costs and leads to poor treatment outcomes.62 Most patients who seek primary healthcare facility services present with respiratory tract infections and acute diarrhoea, which could be caused by viruses and/or be self-limiting.63 However, due to the lack of diagnostic capacity in these facilities and poor knowledge of AMR, these patients are treated with antibiotics.16,64,65 Habits such as purchasing half the course, self-medication, sharing medicines and interruption of treatment may contribute to resistance and the development of MDR/XDR in the community.56 The need to inform and educate the communities on the drivers of AMR and their effects and to implement measures that tackle inappropriate use and behavioural change cannot be overemphasized.65
This study was conducted during the COVID-19 pandemic, which could explain the high resistance to erythromycin as an attribute of self-medication and the irrational use of macrolides during the COVID-19 pandemic.66 The other limitations were the inability to speciate enterococci and screen for more resistance genes due to financial constraints. Lastly, antimicrobial prescribing data were extracted from paper-based databases, which had some limitations in that not all the files had complete data and the records were not up to date.
Conclusions
Antibiotic prescribing patterns in primary healthcare facilities adhered to the WHO AWaRe framework. However, carriage E. coli and enterococci from healthy individuals were mostly resistant to the prescribed antibiotics. These findings highlight the need to use local susceptibility data to formulate country-specific treatment guidelines in line with the WHO Aware classification. The high resistance and MDR to affordable antibiotics are a public health concern requiring urgent actions such as improving diagnostic capacity and surveillance, strengthening antimicrobial stewardship programmes, enforcing regulations that forbid easy access to antibiotics, and community AMR education and awareness.
Acknowledgements
We acknowledge the Ministry of Health, University Teaching Hospitals, the primary healthcare facilities in Lusaka and Ndola districts, the Bacteriology Unit under the Pathology and Microbiology Department and the Public Health laboratory in the Department of Disease Control in the School of Veterinary Medicine for providing a conducive working environment to enable this work.
Contributor Information
Kaunda Yamba, Department of Pathology & Microbiology, University Teaching Hospitals, Lusaka, Zambia; Department of Disease Control University of Zambia, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia; Antimicrobial Resistance Cluster, Zambia National Public Health Institute, Lusaka, Zambia.
Steward Mudenda, Department of Disease Control University of Zambia, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia; Department of Pharmacy, School of Health Sciences, University of Zambia, Lusaka, Zambia.
Evans Mpabalwani, Department of Paediatrics & Child Health, School of Medicine, University of Zambia, Lusaka, Zambia.
Geoffrey Mainda, Food and Agriculture Organization (FAO) of the United Nations, House No. 5, Chaholi, Off Addis Ababa Drive, Lusaka, Zambia; Department of Veterinary Services Central Veterinary Research Institute (CVRI), Ministry of Fisheries and Livestock, Lusaka, Zambia.
Mercy Mukuma, Department of Food Science, School of Agricultural Sciences and Nutrition, University of Zambia, Lusaka, Zambia.
Mulemba Tillika Samutela, Department of Biomedical Sciences, School of Health Sciences, University of Zambia, Lusaka, Zambia.
Chileshe Lukwesa, Department of Pathology & Microbiology, University Teaching Hospitals, Lusaka, Zambia.
Joseph Chizimu, Antimicrobial Resistance Cluster, Zambia National Public Health Institute, Lusaka, Zambia.
Ciluvya Kavimba Kaluba, Department of Disease Control University of Zambia, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia.
Matenge Mutalange, Department of Disease Control University of Zambia, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia; Department of Pathology and Microbiology, School of Medicine and Health Sciences, Mulungushi University, Livingstone, Zambia.
Roma Chilengi, Zambia National Public Health Institute, Ministry of Health, Lusaka, Zambia.
John Bwalya Muma, Department of Disease Control University of Zambia, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia.
Funding
This study was completed as part of a PhD research project. Hence, any costs associated with the study were internally funded by the authors.
Transparency declarations
All authors have declared no conflict of interest. All the authors do not have any financial interests or connections that may directly or indirectly raise concerns of bias in the work reported or the conclusions, implications or opinions made in this publication.
References
- 1. Lamberte LE, van Schaik W. Antibiotic resistance in the commensal human gut microbiota. Curr Opin Microbiol 2022; 68: 102150. 10.1016/j.mib.2022.102150 [DOI] [PubMed] [Google Scholar]
- 2. Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 2011; 10: 311–23. 10.1016/j.chom.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012; 3: 4–14. 10.4161/gmic.19320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanoue T, Umesaki Y, Honda K. Immune responses to gut microbiota-commensals and pathogens. Gut Microbes 2010; 1: 224. 10.4161/gmic.1.4.12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silva N, Igrejas G, Gonçalves A et al. Commensal gut bacteria: distribution of Enterococcus species and prevalence of Escherichia coli phylogenetic groups in animals and humans in Portugal. Ann Microbiol 2012; 62: 449–59. 10.1007/s13213-011-0308-4 [DOI] [Google Scholar]
- 6. Braz VS, Melchior K, Moreira CG. Escherichia coli as a multifaceted pathogenic and versatile bacterium. Front Cell Infect Microbiol 2020; 10: 793. 10.3389/fcimb.2020.548492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou X, Willems RJL, Friedrich AW et al. Enterococcus faecium: from microbiological insights to practical recommendations for infection control and diagnostics. Antimicrob Resist Infect Control 2020; 9: 130. 10.1186/s13756-020-00770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verway M, Brown KA, Marchand-Austin A et al. Prevalence and mortality associated with bloodstream organisms: a population-wide retrospective cohort study. J Clin Microbiol 2022; 60: e0242921. 10.1128/jcm.02429-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Duin D, Paterson DL. Multidrug resistant bacteria in the community: trends and lessons learned. Infect Dis Clin North Am 2016; 30: 377. 10.1016/j.idc.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray CJ, Ikuta KS, Sharara F et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Szmolka A, Nagy B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front Microbiol 2013; 4: 258. 10.3389/fmicb.2013.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shrestha A, Shrestha R, Koju P et al. The resistance patterns in E. coli isolates among apparently healthy adults and local drivers of antimicrobial resistance: a mixed-methods study in a suburban area of Nepal. Trop Med Infect Dis 2022; 7: 133. 10.3390/tropicalmed7070133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otaigbe II, Elikwu CJ. Drivers of inappropriate antibiotic use in low- and middle-income countries. JAC Antimicrob Resist 2023; 5: dlad062. 10.1093/jacamr/dlad062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sartelli M, Hardcastle TC, Catena F et al. Antibiotic use in low and middle-income countries and the challenges of antimicrobial resistance in surgery. Antibiotics 2020; 9: 497. 10.3390/antibiotics9080497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iskandar K, Molinier L, Hallit S et al. Surveillance of antimicrobial resistance in low- and middle-income countries: a scattered picture. Antimicrob Resist Infect Control 2021; 10: 63. 10.1186/s13756-021-00931-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control 2017; 6: 47. 10.1186/s13756-017-0208-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langford BJ, Daneman N, Diong C et al. Antibiotic susceptibility reporting and association with antibiotic prescribing: a cohort study. Clin Microbiol Infect 2021; 27: 568–75. 10.1016/j.cmi.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 18. Sharland M, Pulcini C, Harbarth S et al. Classifying antibiotics in the WHO essential medicines list for optimal use—be AWaRe. Lancet Infect Dis 2018; 18: 18–20. 10.1016/S1473-3099(17)30724-7 [DOI] [PubMed] [Google Scholar]
- 19. Hsia Y, Lee BR, Versporten A et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Heal 2019; 7: e861–71. 10.1016/S2214-109X(19)30071-3 [DOI] [PubMed] [Google Scholar]
- 20. Gandra S, Kotwani A. Need to improve availability of “access” group antibiotics and reduce the use of “watch” group antibiotics in India for optimum use of antibiotics to contain antimicrobial resistance. J Pharm Policy Pract 2019; 12: 20. 10.1186/s40545-019-0182-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adekoya I, Maraj D, Steiner L et al. Comparison of antibiotics included in national essential medicines lists of 138 countries using the WHO Access, Watch, Reserve (AWaRe) classification: a cross-sectional study. Lancet Infect Dis 2021; 21: 1429–40. 10.1016/S1473-3099(20)30854-9 [DOI] [PubMed] [Google Scholar]
- 22. Mudenda S, Chomba M, Chabalenge B et al. Antibiotic prescribing patterns in adult patients according to the WHO AWaRe classification: a multi-facility cross-sectional study in primary healthcare hospitals in Lusaka, Zambia. Pharmacol Pharm 2022; 13: 379–92. 10.4236/pp.2022.1310029 [DOI] [Google Scholar]
- 23. Li B, Zhao ZC, Wang MH et al. Antimicrobial resistance and integrons of commensal Escherichia coli strains from healthy humans in China. J Chemother 2014; 26: 190–2. 10.1179/1973947813Y.0000000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bumbangi FN, Llarena A-K, Skjerve E et al. Evidence of community-wide spread of multi-drug resistant Escherichia coli in young children in Lusaka and Ndola Districts, Zambia. Microorganisms 2022; 10: 1684. 10.3390/microorganisms10081684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Central Statistical Office Zambia, City Population . Zambia: Provinces, Major Cities & Urban Centers—Population Statistics, Maps, Charts, Weather and Web Information. 2018. http://www.citypopulation.de/en/zambia/cities/.
- 26. WHO . The WHO AWaRe (Access, Watch, Reserve Reserve) Antibiotic Book. 2022. https://www.who.int/publications/i/item/9789240062382.
- 27. Godambe LP, Bandekar J, Shashidhar R. Species specific PCR based detection of Escherichia coli from Indian foods. 3 Biotech 2017; 7: 130. 10.1007/s13205-017-0784-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li X, Xing J, Li B et al. Use of tuf as a target for sequence-based identification of gram-positive cocci of the genus Enterococcus, Streptococcus, coagulase-negative Staphylococcus, and Lactococcus. Ann Clin Microbiol Antimicrob 2012; 11: 31. 10.1186/1476-0711-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pillay S, Zishiri OT, Adeleke MA. Prevalence of virulence genes in Enterococcus species isolated from companion animals and livestock. Onderstepoort J Vet Res 2018; 85: e1–8. 10.4102/ojvr.v85i1.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirtieth Edition: M100. 2020.
- 31. Farkas A, Tarco E, Butiuc-Keul A. Antibiotic resistance profiling of pathogenic Enterobacteriaceae from Cluj-Napoca, Romania. Germs 2019; 9: 17–27. 10.18683/germs.2019.1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zou LK, Wang HN, Zeng B et al. Erythromycin resistance and virulence genes in Enterococcus faecalis from swine in China. New Microbiol 2011; 34: 73–80. https://pubmed.ncbi.nlm.nih.gov/21344149/ [PubMed] [Google Scholar]
- 33. Magiorakos AP, Srinivasan A, Carey RB et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 34. Chem ED, Anong DN, Akoachere JFKT. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. PLoS One 2018; 13: e0193353. 10.1371/journal.pone.0193353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carvalho HEF, Schneider G, dos Santos Junior AG et al. Prescription of antimicrobials in primary health care: scoping review. Open Nurs J 2021; 15: 343–50. 10.2174/1874434602115010343 [DOI] [Google Scholar]
- 36. Meena DK, Jayanthi M. Monitoring antibiotic use in public health care facilities of South Indian union territory: a step to promote rational use of antibiotics. Cureus 2021; 13: e18431. 10.7759/cureus.18431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ofori-Asenso R, Brhlikova P, Pollock AM. Prescribing indicators at primary health care centers within the WHO African region: a systematic analysis (1995-2015). BMC Public Health 2016; 16: 724. 10.1186/s12889-016-3428-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D’Arcy N, Ashiru-Oredope D, Olaoye O et al. Antibiotic prescribing patterns in Ghana, Uganda, Zambia and Tanzania hospitals: results from the global point prevalence survey (G-PPS) on antimicrobial use and stewardship interventions implemented. Antibiotics 2021; 10: 1122. 10.3390/antibiotics10091122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sulis G, Daniels B, Kwan A et al. Antibiotic overuse in the primary health care setting: a secondary data analysis of standardised patient studies from India, China and Kenya. BMJ Glob Heal 2020; 5: e003393. 10.1136/bmjgh-2020-003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valia D, Ingelbeen B, Kaboré B et al. Use of WATCH antibiotics prior to presentation to the hospital in rural Burkina Faso. Antimicrob Resist Infect Control 2022; 11: 59. 10.1186/s13756-022-01098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang J, Wang P, Wang X et al. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern Med 2014; 174: 1914–20. 10.1001/jamainternmed.2014.5214 [DOI] [PubMed] [Google Scholar]
- 42. Kalungia AC, Mukosha M, Mwila C et al. Antibiotic use and stewardship indicators in the first- and second-level hospitals in Zambia: findings and implications for the future. Antibiotics 2022; 11: 1626. 10.3390/antibiotics11111626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Costelloe C, Metcalfe C, Lovering A et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: c2096. 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 44. Harris PNA, Tambyah PA, Lye DC et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320: 984–94. 10.1001/jama.2018.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pauwels I, Versporten A, Drapier N et al. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): results from a worldwide point prevalence survey in 69 countries. J Antimicrob Chemother 2021; 76: 1614–24. 10.1093/jac/dkab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalungia AC, Burger J, Godman B et al. Non-prescription sale and dispensing of antibiotics in community pharmacies in Zambia. Expert Rev Anti Infect Ther 2016; 14: 1215–23. 10.1080/14787210.2016.1227702 [DOI] [PubMed] [Google Scholar]
- 47. Mudenda S, Nsofu E, Chisha P et al. Prescribing patterns of antibiotics according to the WHO AWaRe classification during the COVID-19 pandemic at a teaching hospital in Lusaka, Zambia: implications for strengthening of antimicrobial stewardship programmes. Pharmacoepidemiology 2023; 2: 42–53. 10.3390/pharma2010005 [DOI] [Google Scholar]
- 48. Nji E, Kazibwe J, Hambridge T et al. High prevalence of antibiotic resistance in commensal Escherichia coli from healthy human sources in community settings. Sci Rep 2021; 11: 3372. 10.1038/s41598-021-82693-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seid M, Beyene G, Alemu Y et al. Does cotrimoxazole prophylaxis in HIV patients increase the drug resistance of pneumococci? A comparative cross-sectional study in southern Ethiopia. PLoS One 2020; 15: e0243054. 10.1371/journal.pone.0243054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Messina NL, Williamson DA, Robins-Browne R et al. Risk factors for carriage of antibiotic-resistant bacteria in healthy children in the community: a systematic review. Pediatr Infect Dis J 2020; 39: 397–405. 10.1097/INF.0000000000002532 [DOI] [PubMed] [Google Scholar]
- 51. Omulo S, Lofgren ET, Lockwood S et al. Carriage of antimicrobial-resistant bacteria in a high-density informal settlement in Kenya is associated with environmental risk-factors. Antimicrob Resist Infect Control 2021; 10: 18. 10.1186/s13756-021-00886-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kalter HD, Gilman RH, Moulton LH et al. Risk factors for antibiotic-resistant Escherichia coli carriage in young children in Peru: community-based cross-sectional prevalence study. Am J Trop Med Hyg 2010; 82: 879–88. 10.4269/ajtmh.2010.09-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Olaru ID, Tacconelli E, Yeung S et al. The association between antimicrobial resistance and HIV infection: a systematic review and meta-analysis. Clin Microbiol Infect 2021; 27: 846–53. 10.1016/j.cmi.2021.03.026 [DOI] [PubMed] [Google Scholar]
- 54. Sampane-Donkor E, Badoe EV, Annan JA et al. Colonisation of antibiotic resistant bacteria in a cohort of HIV infected children in Ghana. Pan Afr Med J 2017; 26: 60. 10.11604/pamj.2017.26.60.10981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Belachew SA, Hall L, Selvey LA. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control 2021; 10: 13. 10.1186/s13756-020-00880-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cambaco O, Alonso Menendez Y, Kinsman J et al. Community knowledge and practices regarding antibiotic use in rural Mozambique: where is the starting point for prevention of antibiotic resistance? BMC Public Health 2020; 20: 1183. 10.1186/s12889-020-09243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tangcharoensathien V, Chanvatik S, Kosiyaporn H et al. Population knowledge and awareness of antibiotic use and antimicrobial resistance: results from national household survey 2019 and changes from 2017. BMC Public Health 2021; 21: 2188. 10.1186/s12889-021-12237-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hegstad K, Mikalsen T, Coque TM et al. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin Microbiol Infect 2010; 16: 541–54. 10.1111/j.1469-0691.2010.03226.x [DOI] [PubMed] [Google Scholar]
- 59. Li G, Walker MJ, De Oliveira DMP. Vancomycin resistance in Enterococcus and Staphylococcus aureus. Microorganisms 2023; 11: 24. 10.3390/microorganisms11010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Founou RC, Founou LL, Essack SY. Extended spectrum beta-lactamase mediated resistance in carriage and clinical gram-negative ESKAPE bacteria: a comparative study between a district and tertiary hospital in South Africa. Antimicrob Resist Infect Control 2018; 7: 134. 10.1186/s13756-018-0423-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Oliveira MC, Oliveira CRA, Gonçalves KV et al. Enterobacteriaceae resistant to third generation cephalosporins upon hospital admission: risk factors and clinical outcomes. Brazilian J Infect Dis 2015; 19: 239–45. 10.1016/j.bjid.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist 2019; 12: 3903–10. 10.2147/IDR.S234610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mueller AK, Matoba J, Schue JL et al. The unmeasured burden of febrile, respiratory, and diarrheal illnesses identified through active household surveillance in a low malaria transmission setting in Southern Zambia. Am J Trop Med Hyg 2022; 106: 1791–9. 10.4269/ajtmh.21-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Majumder MAA, Rahman S, Cohall D et al. Antimicrobial stewardship: fighting antimicrobial resistance and protecting global public health. Infect Drug Resist 2020; 13: 4713–38. 10.2147/IDR.S290835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sharma A, Singh A, Dar MA et al. Menace of antimicrobial resistance in LMICs: current surveillance practices and control measures to tackle hostility. J Infect Public Health 2022; 15: 172–81. 10.1016/j.jiph.2021.12.008 [DOI] [PubMed] [Google Scholar]
- 66. Shomuyiwa DO, Lucero-Prisno DE, Manirambona E et al. Curbing antimicrobial resistance in post-COVID Africa: challenges, actions and recommendations. Heal Sci Reports 2022; 5: e771. 10.1002/hsr2.771 [DOI] [PMC free article] [PubMed] [Google Scholar]