Abstract
Background
Antimicrobial resistance (AMR) is an urgent global health concern, especially in countries facing instability or conflicts, with compromised healthcare systems. Médecins Sans Frontières (MSF) established an acute trauma hospital in Aden, Yemen, treating mainly war-wounded civilians, and implemented an antimicrobial stewardship (AMS) programme. This study aimed to describe clinical characteristics and identify antibiotic susceptibility patterns representative of patients treated with antibiotics.
Methods
Retrospective cross-sectional study using routinely collected data from all patients treated with antibiotics in the MSF-Aden Acute Trauma hospital between January 2018 and June 2021. Routine clinical data from patients’ files was entered into an AMS electronic database and microbiological data were entered into WHONET. Both databases were imported and merged in REDCap and analysed using RStudio.
Results
Three hundred and sixty-three of 481 (75%) included patients were injured by violence-related trauma. Most were men aged 19–45 years (n = 331; 68.8%). In total, 598 infections were diagnosed and treated. MDR organisms were identified in 362 (60.5%) infections in 311 (65%) patients. Skin and soft-tissue infections (SSTIs) (n = 143; 24%) were the most common, followed by osteomyelitis (n = 125; 21%) and intra-abdominal-infections (IAIs) (n = 116; 19%), and 111 (19%) secondary bloodstream infections were identified. Escherichia coli was the most frequently identified pathogen, causing IAI (n = 87; 28%) and SSTI (n = 43; 16%), while Staphylococcus aureus caused mainly osteomyelitis (n = 84; 19%). Most Gram-negatives were ESBL producers, including E. coli (n = 193; 81.4%), Klebsiella pneumoniae (n = 72; 77.4%) and Enterobacter cloacae (n = 39; 50%) while most S. aureus were methicillin resistant (n = 93; 72.6%).
Conclusions
High rates of MDR were found. This information will facilitate a comprehensive review of the empirical antibiotic treatment guidelines.
Introduction
Antimicrobial resistance (AMR) is a global health emergency that is growing at an alarming rate. AMR-related infections are currently responsible for 700 000 deaths annually, and this number is projected to escalate to 10 million deaths in 2050.1,2 Low- and middle-income countries (LMICs) carry a disproportionate burden of AMR infections compared with high-income countries. The lack of AMR surveillance and antimicrobial stewardship (AMS), under-resourced laboratories, irrational antibiotic prescription practices and limited infection and prevention control (IPC) measures compound the emergence of AMR and increase the risk of mortality in the population.3,4
The emergence and spread of AMR, such as in MRSA, isolates resistant to third-generation cephalosporins, including ESBL-producing pathogens, and other patterns of resistance to the first- and second-line antibiotics in the Middle East region, particularly in conflict-affected areas, is a growing concern.5 Such countries are confronted with a high number of war-related injuries that require advanced medical care, strict IPC measures and necessitate appropriate and curative antibiotic therapy to manage the risk of AMR infections.6 Despite the limited number of studies conducted in conflict-affected countries, the available evidence suggests a high prevalence of AMR. Studies from Iraq, Syria and Yemen have reported high levels of AMR among commonly used antibiotics.5–7 One study focused on Yemeni children aged 1 to 15 years treated for otitis media, and another study examined adults in Aden; both showed widespread resistance to commonly used antibiotics.8,9 Another study conducted among adults in Sana’a reported high resistance patterns for common bacterial isolates in ophthalmic patients.10 High rates of antibiotic misuse are reported in Yemen, with self-medication estimated to be as high as 78%.11 This may be a contributing factor to the observed resistance, which is among the highest in the region.7,9 Furthermore, a systematic review on AMR in Yemen highlighted the lack of reliable data and the urgent need for increased surveillance and monitoring of AMR.5
Médecins Sans Frontières (MSF), an international, independent medical humanitarian organization, established a trauma hospital in Aden in 2012, which treats war-related injuries for both soldiers and civilians. In 2017, an AMS programme was introduced to ensure proper antibiotic use and safety. A national Yemeni doctor received appropriate training to implement the programme based on MSF protocols and international standards then received frequent coaching and support from infectious disease referents regionally and in the headquarters. MSF-Aden now has an effective programme in place, including daily feedback on laboratory results and regular audits of prescribing practices. The hospital introduced an empirical treatment guideline in 2018, but it has not been updated since. This study aims to describe the clinical characteristics of patients and their infection diagnoses treated with antibiotics between January 2018 and June 2021 to update the empirical antibiotic treatment guidelines with up-to-date local antibiogram data.
Materials and methods
Settings
The MSF Aden Acute Trauma Hospital
MSFs Aden hospital, opened in 2012, treats war-related injuries regardless of whether the patient is a soldier or civilian. It has an emergency room, an ICU and an outpatient department, with a total of 81 beds. A microbiology laboratory and two isolation wards for MDR infections were added in 2017. Over 50 000 patients have been treated. An AMS programme and IPC measures were implemented to ensure proper antibiotic use and safety.
Study design and population
This is a retrospective cross-sectional study using routinely collected data. The study included all patients who were admitted and treated with antibiotics for different types of infections at the MSF Aden Acute Trauma hospital between January 2018 and June 2021.
Identification of bacterial isolates and antibiotic susceptibility testing (AST)
Samples of blood and urine were taken from patients who showed signs of infection as part of a sepsis screening protocol. Blood cultures were taken routinely for patients with severe infections, including intra-abdominal infections, pneumonia, necrotizing fasciitis, line infections, septic shock and unexplained continuous fever. Urine cultures were collected from patients with symptoms of urinary tract infections (UTIs). In cases where the source of infection was unknown, both blood and urine cultures were taken. Intraoperative samples were also taken from infected wounds after washout and debridement procedures. All sample collections were performed with strict sterile techniques in accordance with MSF microbiology sample collection standard operating procedures (SOPs). A clinical microbiology laboratory technician performed culture and AST. Main culture media were blood CNA (colistin and nalidixic acid), chocolate PVX (PolyViteX supplement) and MacConkey and CHROMagars. Species were identified through manual flowcharts (API Gallery System).
Antibiotic susceptibilities were determined by manual disc diffusion (Bio-Rad and Oxoid), concentration gradient test and broth microdilution test (Liofilchem). They were interpreted using the EUCAST guideline.12 An isolate was defined as MDR if it showed non-susceptibility to at least one agent in ≥3 antimicrobial categories. The diagnosis of wound infection was based on clinical signs of infection with or without confirmatory positive microbiological culture.
Data collection and analysis
The routinely gathered hospital data, including clinical and demographic characteristics, were collected by the dedicated AMS doctor and entered into a REDCap 12.2.0 software dedicated study database. Culture and sensitivity results were obtained from the WHONET database;13 WHONET data for the patients who were not treated by antibiotics were excluded from this study analysis. Descriptive statistics were used to characterize patients’ demographic and clinical data. Pathogen identification and antibiotic susceptibility were described based on the EUCAST guidelines.12 Stratified analysis was used to assess the difference in outcomes, and Fisher’s exact tests were used for categorical variables and parametric or non-parametric tests were used for numerical variables. The data were analysed using RStudio v.1.2.5.033 statistical software.
Ethics
The study protocol was approved by the Ethical Research Committee of the Faculty of Medicine and Health Sciences in Aden, Yemen (Research code: REC-106-2021) and was exempted by the MSF-OCP medical director, mandated by the MSF Ethics Review Board.
Results
Baseline characteristics and prevalence of MDR infections
The study included a total of 481 patients. Most patients were male (88%) and in the 19–45 age group (68.8%). Violence-related trauma was the leading cause of admission (75%), mainly due to gunshots (64%). Penetrating wounds were the most frequent nature of trauma (46%). The abdomen (47%) and lower limb (44%) were the most commonly affected injury sites. Three hundred and-eleven (65%) patients were diagnosed with at least one MDR infection, while the remaining 170 (35%) had non-MDR infections, including those with no conclusive bacterial growth results or were not sampled (Table 1).
Table 1.
Demographic and clinical characteristics of antibiotic-treated patients by MDR status at the MSF Aden Acute Trauma Hospital, Yemen, January 2018—June 2021
| Characteristic | Total N = 481 | Non-MDR-infected patients, N = 170 (35%)a | MDR-infected patients N = 311 (65%) | P value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 423 (88) | 147 (86) | 276 (89) | 0.5 |
| Female | 58 (12) | 23 (14) | 35 (11) | |
| Age group, years, n (%) | ||||
| 1–18 | 100 (21) | 38 (22) | 62 (20) | 0.2 |
| 19–25 | 161 (33.5) | 64 (38) | 97 (31) | |
| 26–45 | 170 (35.3) | 57 (33) | 113 (36) | |
| 46–65 | 44 (9) | 10 (6) | 34 (11) | |
| 66–95 | 6 (1.2) | 1 (1) | 5 (2) | |
| Year of injury, n (%) | ||||
| 2018 | 148 (31) | 51 (30) | 97 (31) | 0.2 |
| 2019 | 141 (29) | 44 (26) | 97 (31) | |
| 2020 | 143 (30) | 51 (30) | 92 (30) | |
| 2021 | 49 (10) | 24 (14) | 25 (8) | |
| Violence-related trauma, n (%) | 363 (75) | 109 (64) | 254 (82) | <0.001 |
| Cause of admission, n (%) | ||||
| Gunshot | 306 (64) | 95 (56) | 211 (68) | 0.003 |
| Road traffic accident | 79 (16) | 41 (24) | 38 (12) | <0.001 |
| Bomb/mine explosion | 59 (12) | 12 (7) | 47 (15) | 0.012 |
| Infection | 13 (3) | 11 (6.5) | 2 (1) | <0.001 |
| Other | 24 (5) | 11 (6.5) | 13 (4) | 0.3 |
| Number of injury sites, n (%) | ||||
| One | 333 (69) | 118 (69) | 215 (69) | 0.9 |
| Two | 87 (18) | 32 (19) | 55 (18) | |
| Three or more | 61 (13) | 20 (12) | 41 (13) | |
| Injury site, n (%) | ||||
| Abdomen | 228 (47) | 71 (42) | 157 (50) | 0.067 |
| Lower limb | 213 (44) | 75 (44) | 138 (44) | >0.9 |
| Thorax | 77 (16) | 36 (21) | 41 (13) | 0.022 |
| Upper limb | 51 (11) | 23 (14) | 28 (9) | 0.12 |
| Pelvis | 49 (10) | 16 (9.4) | 33 (11) | 0.7 |
| Head/neck | 13 (2.7) | 4 (2.4) | 9 (2.9) | >0.9 |
| Nature of trauma, n (%) | ||||
| Penetrating wound | 221 (46) | 64 (38) | 157 (50) | 0.007 |
| Open fracture | 190 (40) | 64 (38) | 126 (41) | 0.5 |
| Soft tissue | 68 (14) | 27 (16) | 41 (13) | 0.4 |
| Blunt | 31 (6.4) | 16 (9.4) | 15 (4.8) | 0.05 |
| Closed fracture | 27 (5.6) | 14 (8.3) | 13 (4.2) | 0.065 |
| Traumatic amputation | 23 (4.8) | 4 (2.4) | 19 (6.1) | 0.065 |
| Vascular | 21 (4.4) | 5 (2.9) | 16 (5.1) | 0.3 |
| Number of diagnosed infections, n (%) | ||||
| One | 390 (81) | 151 (89) | 239 (77) | 0.005 |
| Two | 72 (15) | 16 (9) | 56 (18) | |
| Three or more | 19 (4.0) | 3 (2) | 16 (5) |
Numbers of injury sites and nature of trauma add up to more than 481 because of polytraumatic injuries that can involve more than one site and nature of injure in one patient.
aFifty-seven patients had no growth from their samples, or no samples were taken.
Patients with violence-related trauma had a significantly higher proportion of MDR infections (82%) compared with those without violence-related trauma (64%) (P < 0.001). The proportion of MDR infections was significantly higher in patients with gunshot injuries (68%) compared with those with non-gunshot injuries (32%) (P = 0.003). Patients with MDR infections were more likely to have multiple infections diagnosed (23% versus 11%) (P = 0.005). In addition, patients with penetrating wounds and abdominal injuries were more likely to have MDR infections compared with other types of trauma and injury sites (P = 0.007 and P = 0.067, respectively) (Table 1).
Infection diagnoses and isolated bacteria
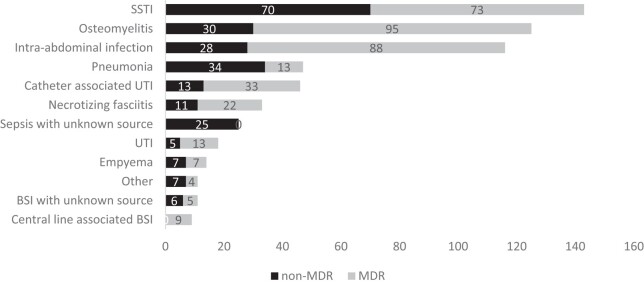
The analysis of the 598 infections revealed that skin and soft-tissue infection (SSTI) (n = 143; 24%) was the most common infection diagnosis, followed by osteomyelitis (n = 125; 21%) and intra-abdominal infection (IAI) (n = 116; 19%). MDR infections (n = 362; 60.5%) were more common than non-MDR infections (n = 236; 39.5%) in most infection diagnoses, except for blood stream infection (BSI) with unknown source (n = 5; 45%) and pneumonia (n = 13; 28%) (Figure 1).
Figure 1.
Frequency of 598 infections by diagnosis and MDR status at the MSF Aden Acute Trauma Hospital, Yemen, January 2018—June 2021.
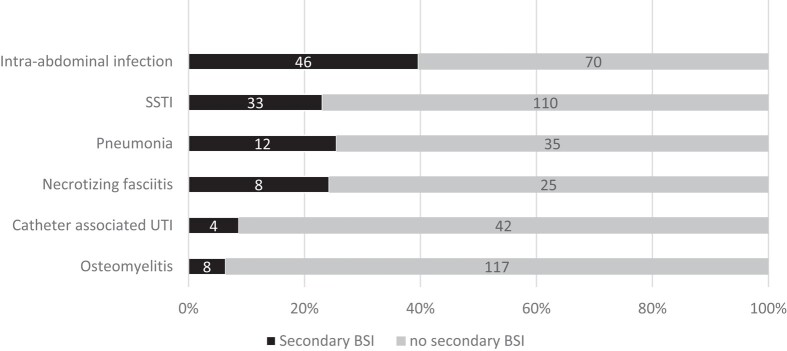
From the same 598 infections, 111 (19%) were also diagnosed with secondary BSI, (n = 60; 54%) confirmed through matched positive growth in the peripheral blood sample and primary source samples, while others (n = 51; 46%) had positive peripheral blood samples only with clinically diagnosed and a highly suspected primary source. The most common infections associated with secondary BSI were IAI (n = 46; 41%) and SSTI (n = 33; 30%). In contrast, osteomyelitis and catheter-associated UTI (CAUTI) had lower incidence of secondary BSI (≤8 cases) (Figure 2).
Figure 2.
Incidence of 111 BSIs by infection diagnosis at the MSF Aden Acute Trauma Hospital, Yemen, January 2018—June 2021.
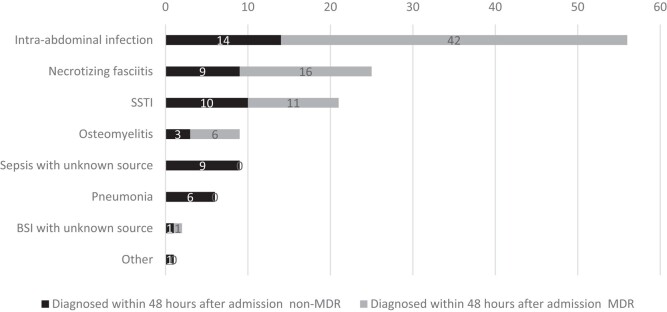
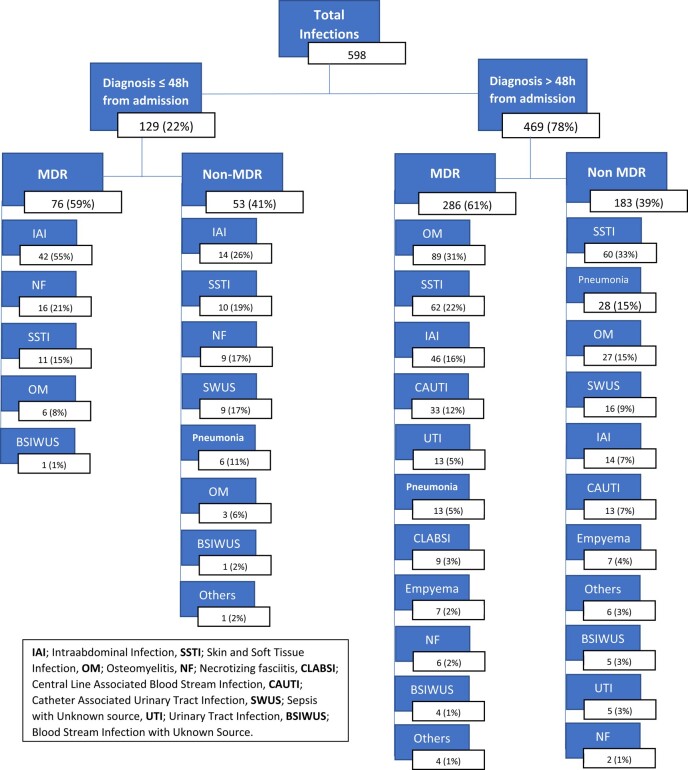
Overall, 129 (22%) infections were diagnosed within 48 h of admission; 59% of these were caused by MDR organisms. The most common diagnoses were IAI (n = 56; 43%), followed by necrotizing fasciitis (n = 25; 19%) and SSTI (n = 21; 16%) (Figures 3 and 4).
Figure 3.
Frequency of the types of the 129 infections diagnosed within 48 h of admission by MDR status at the MSF Aden Acute Trauma Hospital, Yemen, January 2018—June 2021.
Figure 4.
Distribution of the 598 infections diagnosed according to time of diagnosis and MDR status at the MSF Aden Acute Trauma Hospital, Yemen, January 2018—June 2021.
Of the 598 infection diagnoses, 1249 bacterial isolates comprised 52 different species. The most common isolates were Escherichia coli (n = 238; 19%), followed by Enterococcus faecalis (n = 154; 12%) and Staphylococcus aureus (n = 129; 10%). The distribution of bacterial species responsible for these infections varied widely among different infection diagnoses (Table 2). For example, E. coli was the most common bacterial species responsible for causing IAI (n = 87; 28%), SSTI (n = 43; 16%), necrotizing fasciitis (n = 18; 26%), CAUTI (n = 29; 44%) and UTI (n = 9; 53%), while E. faecalis was mostly isolated from patients diagnosed with IAI (n = 52; 17%) and osteomyelitis (n = 50; 12%). S. aureus was mainly isolated from those with osteomyelitis (n = 84; 19%).
Table 2.
Distribution of 1249 bacterial isolates by infection diagnoses at the MSF Aden Acute Trauma Hospital, Yemen, January 2018—June 2021
| Osteomyelitis (N = 432) |
IAI (N = 312) |
SSTI (N = 263) |
Necrotic zing fasciitis (N = 70) |
CAUTI (N = 66) |
Pneumonia (N = 30) |
Empyema (N = 23) |
UTI (N = 17) |
BSI with unknown source (N = 13) |
Central line-associated BSI (N = 10) |
Other (N = 13) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli, n (%) | 41 (10) | 87 (28) | 43 (16) | 18 (26) | 29 (44) | 2 (7) | 3 (13) | 9 (53) | 3 (23) | 1(10) | 2 (15) |
| E. faecalis, n (%) | 50 (12) | 52 (17) | 28 (11) | 13 (19) | 2 (3) | — | 3 (13) | — | 3 (23) | — | 3 (23) |
| S. aureus, n (%) | 84 (19) | 4 (1) | 28 (11) | 1 (1.5) | 3 (4) | 1 (3) | 2 (9) | — | 1 (8) | 3(30) | 2 (15) |
| P. aeruginosa, n (%) | 44 (10) | 17 (5) | 31 (12) | 3 (4.5) | 6 (9) | 4 (13) | 3 (13) | — | — | — | 1 (8) |
| K. pneumoniae, n (%) | 24 (6) | 28 (9) | 17 (6) | 10 (14) | 5 (8) | 2 (7) | 3 (13) | 2 (12) | 1 (8) | — | 1 (8) |
| P. mirabilis, n (%) | 27 (6) | 27 (9) | 14 (5) | 4 (6) | 7 (11) | 3 (10) | 3 (13) | 2 (12) | — | — | — |
| A. baumannii, n (%) | 9 (2) | 25 (8) | 27 (10) | 4 (6) | 1 (1.5) | 11 (37) | 2 (9) | — | 3 (23) | 5(50) | — |
| E. cloacae, n (%) | 31 (7) | 11 (3) | 21 (8) | 8 (11) | 4 (6) | — | — | 2 (12) | — | 1(10) | 2 (15) |
| Streptococcus pyogenes, n (%) | 24 (6) | — | 7 (3) | 1 (1) | — | 1 (3) | — | — | — | — | — |
| E. faecium, n (%) | 3 (1) | 15 (5) | 7 (3) | 3 (4) | — | — | — | 1 (6) | 1 (8) | — | — |
| CoNS, n (%) | 11 (2) | 9 (3) | 4 (1) | — | 1 (1.5) | 1 (3) | 2 (9) | 1 (6) | — | — | 1 (8) |
| Other, n (%) | 84 (19) | 37 (12) | 36 (14) | 5 (7) | 8 (12) | 5 (17) | 2 (9) | — | 1 (8) | — | 1 (8) |
Percentages in columns may not sum to exactly 100% due to rounding.
Antibiotic susceptibility and resistance mechanisms
Ampicillin showed low effectiveness against Enterobacterales, with only 3.4% of E. coli, 1.3% of Enterobacter cloacae and 1.1% of Klebsiella pneumoniae being susceptible. Ceftriaxone also showed low effectiveness, with susceptibility rates of only 14.8% for E. coli and 19.4% for K. pneumoniae. Piperacillin/tazobactam, ertapenem and amikacin were the most effective antibiotics, with high percentages of susceptible isolates, ranging from 67.7% to 100% (Table 3).
Table 3.
Antibiotic susceptibility of the most common isolates from different sites (blood, urine, bone, tissue and fluid aspirates) and causing different types of infections in the patients treated with antibiotics at the MSF Aden Acute Trauma Hospital, Aden, Yemen, January 2018–June 2021
| No. of isolates tested (% susceptible)a | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Amoxicillin/clavulanic acid | Piperacillin/tazobactam | Cefoxitin | Ceftazidime | Ceftriaxone | Cefepime | Aztreonam | Ertapenem | Imipenem | Amikacin | Gentamicin | Tobramycin | Ciprofloxacin | Levofloxacin | Trimethoprim/sulfamethoxazole | Clindamycin | Rifampicin | Vancomycin | Tigecycline | Colistin | |
| Enterobacterales | |||||||||||||||||||||
| E. coli | 237 (3.4) | 237 (57) | 237 (78.5) | 237 (84.4) | 235 (15.7) | 237 (14.8) | 237 (15.6) | 237 (14.8) | 226 (93.4) | — | 237 (97) | 237 (51.5) | 237 (45.1) | 237 (37.1) | 44 (34.1) | 237 (36.7) | — | — | — | — | — |
| K. pneumoniae | 93 (1.1) | 93 (55.9) | 93 (67.7) | 93 (92.5) | 93 (29) | 93 (19.4) | 93 (20.4) | 93 (23.9) | 93 (96.8) | — | 93 (98.9) | 93 (44.1) | 93 (37.6) | 93 (36.6) | — | 93 (31.2) | — | — | — | — | — |
| E. cloacae | 77 (1.3) | 78 (16.7) | 77 (74) | 78 (17.9) | 77 (42.9) | 78 (44.9) | 78 (47.4) | 78 (43.6) | 69 (98.6) | — | 78 (98.7) | 78 (46.2) | 77 (48.1) | 78 (50) | — | 78 (53.8) | — | — | — | — | — |
| P. mirabilis | 86 (48.8) | 86 (94.2) | 86 (97.7) | 86 (98.8) | 86 (72.1) | 86 (66.3) | 86 (65.1) | 86 (72.1) | 82 (100) | 86 (75.6) | 86 (70.9) | 86 (67.4) | 86 (68.6) | — | 86 (55.8) | — | — | — | — | — | |
| b Gram-negative | |||||||||||||||||||||
| P. aeruginosa | — | — | 106 (86.8) | — | 106 (83) | — | 106 (85.8) | 106 (0.9) | — | 106 (92.5) | 106 (89.6) | 106 (84) | 106 (84) | 106 (91.5) | — | — | — | — | — | — | — |
| A. baumannii | — | — | — | — | — | — | — | — | — | 87 (3.5) | 87 (39.1) | 87 (2.3) | 84 (4.8) | 87 (12.6) | — | — | — | — | — | — | 49 (100) |
| Gram-positive | |||||||||||||||||||||
| S. aureus | — | — | — | 128 (27.4) | — | — | — | — | — | — | 128 (97.7) | 128 (93.8) | 128 (93) | 128 (85.9) | — | 128 (99.2) | 128 (85.9) | 127 (97.6) | 90 (100) | — | — |
| E. faecalis | 151 (94) | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 151 (100) | — | — |
| E. faecium | 30 (13.3) | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 30 (100) | — | — |
| Streptococcus pyogenes | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 32 (100) | — | — | — | — |
aAST results are only displayed for results found for 30 isolates or more of the same bacterial species.
bNon-fermenting Gram-negative bacteria.
Pseudomonas aeruginosa had low susceptibility to aztreonam, with only 0.9% of isolates being susceptible. Other antibiotics such as piperacillin/tazobactam, ceftazidime, cefepime and imipenem showed high effectiveness against this bacterial species, with susceptible isolates ranging from 83% to 92.5%. Moreover, imipenem, gentamicin, tobramycin and ciprofloxacin showed low effectiveness against Acinetobacter baumannii isolates, susceptibility ranging from 2.3% to 12.6%, while it was 100% susceptible to colistin. S. aureus showed high susceptibility to most of the tested antibiotics, except for cefoxitin (27.3%). E. faecalis and Enterococcus faecium showed 100% susceptibility to vancomycin, but ampicillin was more effective against E. faecalis (94%) compared with E. faecium (13.3%).
Table 4 shows that S. aureus had a high rate of resistance to methicillin (72.6% of isolates). E. coli had the highest percentage of ESBL-producing isolates, at 81.4%, followed by K. pneumoniae with 77.4% and E. cloacae with 50%. A. baumannii showed the highest percentage of carbapenem-resistant isolates at 96.5%. P. aeruginosa had a low percentage of carbapenem-resistant isolates, at 7.5%; however, it had a 15% rate of ceftazidime-resistant isolates.
Table 4.
Resistance patterns of the most commonly isolated bacteria from patients treated with antibiotics at the MSF Aden Acute Trauma Hospital, Yemen, January 2018–June 2021
| Pathogen | Total (n) | MRSA, n (%) | ESBL, n (%) | Carbapenem resistant, n (%) | PARC, n (%) |
|---|---|---|---|---|---|
| S. aureus | 128 | 93 (72.6) | __ | __ | __ |
| E. coli | 237 | __ | 193 (81.4) | 6 (2.5) | __ |
| K. pneumoniae | 93 | __ | 72 (77.4) | 3 (3.1) | __ |
| E. cloacae | 78 | __ | 39 (50) | 2 (2.5) | __ |
| P. mirabilis | 86 | __ | 29 (33.7) | __ | __ |
| A. baumannii | 87 | __ | __ | 84 (96.5) | __ |
| P. aeruginosa | 106 | __ | __ | 8 (7.5) | 16 (15) |
PARC, P. aeruginosa resistant to ceftazidime.
Discussion
To our knowledge, this study is the first to provide a detailed description of infections and antimicrobial resistance patterns among trauma patients in Yemen. The study found a high prevalence of MDR infections among patients treated with antibiotics for conflict-related injuries, with 65% of patients affected. The most common infections were SSTIs, followed by osteomyelitis, IAIs and BSIs. MDR organisms were identified in 60.5% of infections, while violence-related trauma, particularly gunshot injuries, were strongly associated with MDR infections. Patients with MDR infections were also more likely to have multiple infection diagnoses.
During the study period, Yemen was experiencing intense fighting, which led to a significant rise in casualties and civilian injuries, primarily caused by shootings, bombings and other armed violence against civilians.14 Consequently, most trauma patients treated at the MSF Aden Acute Trauma Hospital were victims of war-related injuries, mainly affecting the young male population. The mechanism and types of injuries observed, predominantly characterized by gunshots and penetrating wounds, were similar to other conflict settings reported in the Middle East, including Syria, Iraq and Lebanon.15–17 Recent armed conflicts are marked by higher usage of warfare technology that correlate with more severe war injuries. This also leads to a high risk of mortality, and complications, as well as increased costs of treatment.18 Injuries sustained during armed conflicts are frequently complex, with a high risk of contamination due to foreign objects. This often leads to severe complications, resulting in a significant number of tissue infections, bone infections and BSIs,19 as was also observed in our study.
Secondary BSI represented 19% of all treated infections and the main source of secondary BSI (41%) was IAI in our trauma centre. Interestingly, this was different from a prospective study done in a level one trauma centre in Jai Prakash Narayan Apex Trauma Centre (JPNATC), India, where ventilator-associated pneumonia was the most common source.20 As we found in our study, IAI and BSI were also among the three infectious diagnoses dominating the global burdens associated with AMR in a global systemic analysis done in 2019.21
In our study, we found that E. coli was the most frequently isolated bacterium (19%) among patients treated for underlying infections, followed by E. faecalis (12%), S. aureus (10%), P. aeruginosa (8.7%) and K. pneumoniae (7.4%). We were unable to compare our results with previous studies of trauma patients only in Yemen, but a recent study conducted in Aden including a mix of patients from multiple hospitals and medical laboratories showed different findings, as here Staphylococcus spp. was the most commonly isolated bacteria (41.7%), followed by E. coli (39.8%), Pseudomonas spp. (8.9%) and K. pneumoniae (4.36%).9 The difference in the most commonly found organisms between our study and the previously mentioned study could be due to the type of patients, injuries, diagnoses and samples studied. The other study included multiple centres, mixed patients with wounds, and pus superficial swabs, while our study focused on conflict-related traumatic injuries and analysed deep tissue and bone samples in one centre. These factors, particularly sample type, may have influenced the prevalence of certain organisms in each study.22 Another study among Yemeni patients treated for osteomyelitis in an MSF reconstructive surgery hospital in Amman, Jordan showed that infections were mainly caused by S. aureus.23 Other comparisons with conflict settings in the Middle East showed some similarities with our findings, although the prevalence of the isolated bacteria was highly influenced by the study methodologies, sample size and study population.24–26
In Palestine, among patients with postoperative surgical site infections (SSIs), findings showed prevalence of E. coli (56.7%), S. aureus (30%), Klebsiella spp. (6.7%) and A. baumannii (3.3%),24 and another study from Iraq but with a smaller sample size of patients (n = 174) compared with our study, showed that S. aureus (48.2%) was the most common isolated bacterium, followed by Enterobacterales including Proteus mirabilis, E.coli, E. cloacae and K. pneumoniae and P. aeruginosa together representing 35.9%.25 Moreover, in a surgery hospital in Lebanon, treating acute and chronic war-related trauma patients, predominantly from the Syrian war, where S. aureus (49.1%) was the most commonly isolated bacterium, followed by Enterobacterales (28.5%) and P. aeruginosa (13.2%) but only from bone and tissue samples.26
MRSA infections are a public health concern as hospital-acquired MRSA infection rates have slowly increased over the last 25 years and find their way into the community.27 However, other studies show that community-acquired MRSA and hospital-acquired MRSA possess different and specific virulence factors and toxins.28
The prevalence of MRSA across conflict regions is generally high,29 with a range of MRSA from all S. aureus isolates of 72.6% in our hospital to 95.4% in Mosel, Iraq25 and 48.5% in one of Lebanon’s studies.26
As observed in our study, S. aureus and particularly MRSA was mainly identified from bone samples as a major pathogen for osteomyelitis in the trauma centre in Lebanon.26
ESBL-producing organisms are another emerging challenge, as they causes nosocomial and community-acquired infections.30 In our study, we observed a high rate of ESBL-producing Enterobacterales, with E. coli (81.4%), K. pneumoniae (77.4%) and E. cloacae (50%) being the most common. This was also seen in the Mosel study,25 where even higher rates of ESBL-producing Enterobacterales were reported in Jordan with E. cloacae (88.2%), whereas, ESBL production in E. coli was much lower (10.8%).31 Iran showed similarities concerning E. coli (89.8%) and K. pneumoniae (72.1%) but had other results with A. baumannii (84.2%) and P. aeruginosa (83.8%).32 Carbapenems are usually recognized as the drug of choice to treat severe infections caused by ESBL-producing pathogens.30 However, their overuse has already led to the emergence of resistance, necessitating the exploration of carbapenem-sparing strategies and effective agents against MDR pathogens.30 Piperacillin/tazobactam and cefoxitin have been reviewed as potential carbapenem-sparing agents for mild to moderate infections caused by ESBL-producing pathogens,33,34 but their efficacy varies in severe infections such as IAIs, bone infections and BSIs. Here, the optimal efficacy of its usage is still unclear and may be dependent on in vitro susceptibility, MICs and/or the site and severity of the infection.33–36 In our study, some ESBL-producing Enterobacterales showed a relatively high-rate prevalence of susceptibility to them in vitro, although their potential use in our hospital might be limited due to the lack of an MIC-reporting method in our laboratory, and the site or severity and/or complications of the infections we deal with in our acute surgical trauma setting.33–36
Fluoroquinolones and trimethoprim/sulfamethoxazole are other carbapenem-sparing and de-escalating agents that could be the best choice for an IV-to-oral switch therapy for infections caused by ESBL-producing pathogens.35 However, in our study, these MDR infections showed low susceptibility to treatment with these agents, leading to longer hospitalization stays and more pressure on carbapenem use, especially for infections that required long-term antibiotic therapy, such as bone infections. The most common carbapenem-resistant Enterobacterales showed low reported prevalence rates (2.5%–3.1%) in our study, similar to findings in Haiti (2.6%).29 However, carbapenem resistance in P. aeruginosa showed a totally different prevalence, with high rates in Haiti (26.9%), Palestine (47.6%), lower rates in Iraq (12.4%), and much lower rates in our hospital (7.5%). These variations in P. aeruginosa findings might need further in-depth analysis to explore relevant correlated and risk factors.
MDR A. baumannii has been globally recognized as an emerging nosocomial pathogen.37–39 It has been identified with high rates among war-wounded patients in different conflict zones, including the Middle East region40 Carbapenem-resistant A. baumannii was found at a very high rate (96.5%) in our study, which is very similar to other studies from conflict-affected settings in the region (78%), but also in non-conflict settings (69%–75%).5 Despite high susceptibility (100%) of MDR A. baumannii to colistin in our study, colistin is a drug with an unfavourable side-effect profile, and pharmacokinetics that limit its effect in some sites of infection.41,42 Therefore, creating access to safe and efficacious new therapeutics, like novel new-generation cephalosporins, is an urgent need in contexts with high rates of MDR A. baumannii infections.43
Lastly, in a 2019 systemic analysis of the AMR global burden, E. coli, S. aureus, K. pneumoniae and A. baumannii were among six leading pathogens responsible for more than 250 000 deaths attributed to AMR; this may support the results of high-prevalence AMR patterns seen among these pathogens in this study, as shown in Table 4.21
AMR is a significant public health challenge in Yemen,44 although its actual extent is unknown. Available data are very limited due to the lack of access to equipped microbiological laboratories to monitor the patterns of AMR across the country.45 In 2018, the MSF hospital adapted its empirical antibiotic treatment based on a 1 year review of the microbiology hospital data. As a result of this review, many of the usual first-line antibiotics were replaced by second- and third-line antibiotics considering the high level of AMR seen among the patients treated that year. For instance, the guide suggested vancomycin and carbapenem as the first choice for sepsis/septic shock syndrome empirical treatment after taking blood and other possible samples and pending microbiology results. Our practice is guided by the principle of ‘Start smart, then focus’. This means that when sepsis is suspected, we endeavour to identify and sample the likely source, initiating empirical treatment based on the recommended guidelines for that suspected source. In instances where the source of sepsis is not immediately apparent, a diagnosis of sepsis with an unknown source is made, pending results from a comprehensive septic screen targeting all possible and accessible sources (such as blood, lines, urine, lungs, etc.). For these patients, vancomycin and meropenem may be started empirically. However, these treatments are tailored as soon as possible, based on microbiology laboratory results and/or the clinical evaluation of the patient, which may reveal a more obvious source of infection.
Concerning nurturing an evaluation of the empirical antibiotic guideline, our study findings do support the existing MSF empirical treatment guidelines that were implemented in 2018 and confirm the need for second-line antibiotics such as vancomycin and a carbapenem for empirical therapy of sepsis and septic shock from most common infections seen, like IAI and SSTI.
Ensuring access to reliable microbiology, these and other affordable quality-assured second-line antibiotics, and anticipating the need for, and ensuring affordable access to third-line antibiotics as rates of carbapenemases increase, is essential. Rigorous IPC efforts and AMS must be expanded to address these high rates of MDR.
This study has limitations that should be noted. Firstly, it was conducted in one hospital in Aden and only included trauma patients who met specific admission criteria. This means that the findings may not be generalizable to other trauma patients in Yemen, and certain traumas, such as head injuries, were excluded. Additionally, the study relied on routinely collected data, which may not be completely reliable or available. However, missing or erroneous data were checked and corrected by the principal investigators. Another limitation is that the microbiology laboratory was unable to confirm carbapenem mechanisms of resistance, which would have provided valuable insights into the risk of AMR. Despite these limitations, the study had strengths, such as a large sample size over multiple years and high-quality laboratory data. The study also provided insights into the AMR patterns underlying the most common infectious complications in trauma patients in Yemen.
Conclusions
The prevalence of AMR among patients with acute trauma injuries in Aden, Yemen is alarmingly high and underscores the urgent need for context-specific antibiotic treatment guidelines, applicable IPC strategies, access to reliable microbiological diagnostics, and clinical and microbiological surveillance. Overall, the study highlights the need for a collaborative effort to improve AMS, IPC preventive measures, and source control by appropriate surgical management, in order to combat the spread of MDR infections in conflict-affected areas.
Acknowledgements
The authors want to thank all those who supported the study in the field and MSF-Yemen mission in different periods and in particular Conor Bowman, Nada Malou, Omar Al-Nagda, Arwa Mansori, James Philip, Ayokunnu Raji, Michael Adeyemi Lawal, Uche Daniel, Meurant Remi, and Pilar Garcia-Vello.
Contributor Information
Hussein Almehdar, Médecins Sans Frontières—Operational Centre Paris (MSFOCP), Acute Trauma Hospital, Aden, Yemen.
Nagwan Yousef, Médecins Sans Frontières—Operational Centre Paris (MSFOCP), Acute Trauma Hospital, Aden, Yemen.
Wilma van den Boogaard, Médecins Sans Frontières—Operational Centre Brussels, Medical Department, Luxembourg Operational Research (LuxOR) Unit, Luxembourg City, Luxembourg.
Amna Haider, Department of Epidemiology and Training, Epicentre, Dubai, United Arab Emirates.
Rupa Kanapathipillai, Médecins Sans Frontières—Operational Centre Paris, Medical Department, Paris, France.
Emad Al-Hodiani, Médecins Sans Frontières—Operational Centre Paris (MSFOCP), Acute Trauma Hospital, Aden, Yemen.
Evgenia Zelikova, Médecins Sans Frontières—Operational Centre Paris, Medical Department, Paris, France.
Waddah G Moh’d, Médecins Sans Frontières—Operational Centre Paris (MSFOCP), Acute Trauma Hospital, Aden, Yemen.
Justine Michel, Médecins Sans Frontières—Operational Centre Paris, Medical Department, Paris, France.
Rami Malaeb, Department of Epidemiology and Training, Epicentre, Dubai, United Arab Emirates.
Funding
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Medécins Sans Frontières (MSF/Doctors Without Borders). The specific SORT IT programme which resulted in this publication was organised by MSF specifically for Antimicrobial Resistance research.
Transparency declarations
None to declare.
References
- 1. Interagency Coordination Group on Antimicrobial Resistance . No time to wait: securing the future from drug-resistant infections. Report to the Secretary-General of the United Nations. 2019. https://www.who.int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.pdf.
- 2. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations the review on antimicrobial resistance. 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 3. Gandra S, Alvarez-Uria G, Turner P et al. Antimicrobial resistance surveillance in low- and middle-income countries: progress and challenges in eight south Asian and southeast Asian countries. Clin Microbiol Rev 2020; 33: e00048-19. 10.1128/CMR.00048-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shallal A, Lahoud C, Zervos M et al. Antibiotic stewardship in disaster situations: lessons learned in Lebanon. Antibiotics (Basel) 2022; 11: 560. 10.3390/antibiotics11050560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Truppa C, Abo-Shehada MN. Antimicrobial resistance among GLASS pathogens in conflict and non-conflict affected settings in the Middle East: a systematic review. BMC Infect Dis 2020; 20: 936. 10.1186/s12879-020-05503-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Devi S. AMR in the Middle East: “a perfect storm”. Lancet 2019; 394: 1311–2. 10.1016/S0140-6736(19)32306-2 [DOI] [PubMed] [Google Scholar]
- 7. Kanapathipillai R, Malou N, Hopman J et al. Antibiotic resistance in conflict settings: lessons learned in the Middle East. JAC Antimicrob Resist 2019; 1: dlz002. 10.1093/jacamr/dlz002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bin Mohanna MA Bahannan AA. Bacterial profile and antibiogram of otitis media among children in Yemen. J Ayub Med Coll Abbottabad 2016; 28: 480–3. https://jamc.ayubmed.edu.pk/jamc/index.php/jamc/article/view/630/397. [PubMed] [Google Scholar]
- 9. Badulla WFS, Alshakka M, Mohamed Ibrahim MI. Antimicrobial resistance profiles for different isolates in Aden, Yemen: a cross-sectional study in a resource-poor setting. Biomed Res Int 2020; 2020: 1810290. 10.1155/2020/1810290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al-Eryani SA, Alshamahi EYA, Al-Shamahy HA et al. Bacterial conjunctivitis of adults: causes and ophthalmic antibiotic resistance patterns for the common bacterial isolates. Univers J Pharm Res 2021; 6: i1.535. 10.22270/ujpr.v6i1.535 [DOI] [Google Scholar]
- 11. Belkina T, Al Warafi A, Hussein Eltom E et al. Antibiotic use and knowledge in the community of Yemen, Saudi Arabia, and Uzbekistan. J Infect Dev Ctries 2014; 8: 424–9. 10.3855/jidc.3866 [DOI] [PubMed] [Google Scholar]
- 12. EUCAST . Breakpoint tables for interpretation of MICs and zone diameters, version 10.0. 2020. https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents.
- 13. Agarwal A, Kapila K, Kumar S. WHONET software for the surveillance of antimicrobial susceptibility. Med J Armed Forces India 2009; 65: 264–6. 10.1016/S0377-1237(09)80020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campaign Against Arms Trade. The war on Yemen’s civilians. 2022. https://caat.org.uk/homepage/stop-arming-saudi-arabia/the-war-on-yemens-civilians/.
- 15. Petersen K, Riddle MS, Danko JR et al. Trauma-related infections in battlefield casualties from Iraq. Ann Surg 2007; 245: 803–11. 10.1097/01.sla.0000251707.32332.c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nassoura Z, Hajj H, Dajani O et al. Trauma management in a war zone: the Lebanese war experience. J Trauma 1991; 31: 1596–9. 10.1097/00005373-199112000-00005 [DOI] [PubMed] [Google Scholar]
- 17. Duramaz A, Bilgili MG, Bayram B et al. Orthopedic trauma surgery and hospital cost analysis in refugees; the effect of the Syrian civil war. Int Orthop 2017; 41: 877–84. 10.1007/s00264-016-3378-x [DOI] [PubMed] [Google Scholar]
- 18. Bektaş YE, Özmanevra R, Polat B et al. Orthopedic treatment, complications, and cost analysis of 67 soldiers injured in a three-month period. Jt Dis Relat Surg 2020; 31: 102–8. 10.5606/ehc.2020.71808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yun HC, Blyth DM, Murray CK. Infectious complications after battlefield injuries: epidemiology, prevention, and treatment. Curr Trauma Rep 2017; 3: 315–23. 10.1007/s40719-017-0102-2 [DOI] [Google Scholar]
- 20. Mathur P, Varghese P, Tak V et al. Epidemiology of blood stream infections at a level-1 trauma care center of India. J Lab Physicians 2014; 6: 22–7. 10.4103/0974-2727.129086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray CJL, Ikuta KS, Sharara F et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Esposito S, De Simone G, Gioia R et al. Deep tissue biopsy vs. superficial swab culture, including microbial loading determination, in the microbiological assessment of skin and soft tissue infections (SSTIs). J Chemother 2017; 29: 154–8. 10.1080/1120009X.2016.1205309 [DOI] [PubMed] [Google Scholar]
- 23. Fily F, Ronat JB, Malou N et al. Post-traumatic osteomyelitis in Middle East war-wounded civilians: resistance to first-line antibiotics in selected bacteria over the decade 2006–2016. BMC Infect Dis 2019; 19: 103. 10.1186/s12879-019-3741-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adwan G, Hasan N, Sabra I et al. Detection of bacterial pathogens in surgical site infections and their antibiotic sensitivity profile. Int J Med Res Health Sci 2016; 5: 75–82. https://staff-old.najah.edu/sites/default/files/Detection%20of%20bacterial%20pathogens%20in%20surgical%20site%20infections%20and%20their%20antibiotic.pdf [Google Scholar]
- 25. M’Aiber S, Maamari K, Williams A et al. The challenge of antibiotic resistance in post-war Mosul, Iraq: an analysis of 20 months microbiological samples from a tertiary orthopaedic care centre. J Glob Antimicrob Resist 2022; 30: 311–8. 10.1016/j.jgar.2022.06.022 [DOI] [PubMed] [Google Scholar]
- 26. Yaacoub S, Truppa C, Pedersen TI et al. Antibiotic resistance among bacteria isolated from war-wounded patients at the Weapon Traumatology Training Center of the International Committee of the Red Cross from 2016 to 2019: a secondary analysis of WHONET surveillance data. BMC Infect Dis 2022; 22: 257. 10.1186/s12879-022-07253-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lodise TP, McKinnon PS. Burden of methicillin-resistant Staphylococcus aureus: focus on clinical and economic outcomes. Pharmacotherapy 2007; 27: 1001–12. 10.1592/phco.27.7.1001 [DOI] [PubMed] [Google Scholar]
- 28. Tsouklidis N, Kumar R, Heindl SE et al. Understanding the fight against resistance: hospital-acquired methicillin-resistant Staphylococcus aureus vs. community-acquired methicillin-resistant Staphylococcus aureus. Cureus 2020; 12: e8867. 10.7759/cureus.8867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Açma A, Williams A, Repetto E et al. Prevalence of MDR bacteria in an acute trauma hospital in Port-au-Prince, Haiti: a retrospective analysis from 2012 to 2018. JAC Antimicrob Resist 2021; 3: dlab140. 10.1093/jacamr/dlab140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paterson DL. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs). Clin Microbiol Infect 2000; 6: 460–3. 10.1046/j.1469-0691.2000.00107.x [DOI] [PubMed] [Google Scholar]
- 31. Batchoun RG, Swedan SF, Shurman AM et al. Extended spectrum β-lactamases among gram-negative bacterial isolates from clinical specimens in three major hospitals in northern Jordan. Int J Microbiol 2009; 2009: 513874. 10.1155/2009/513874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leylabadlo HE, Pourlak T, Bialvaei AZ et al. Extended-spectrum beta-lactamase producing Gram negative bacteria in Iran: a review. Afr J Infect Dis 2017; 11: 39–53. 10.21010/ajid.v11i2.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Nekidy WS, Abdelsalam MM, Nusair AR et al. Is cefoxitin a carbapenem sparing agent in the management of urinary tract infections caused by ESBL producing Enterobacterales? Hosp Pharm 2022 Aug; 57: 568–74. 10.1177/00185787211066460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pilmis B, Jullien V, Tabah A et al. Piperacillin-tazobactam as alternative to carbapenems for ICU patients. Ann Intensive Care 2017; 7: 113. 10.1186/s13613-017-0334-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karaiskos I, Giamarellou H. Carbapenem-sparing strategies for ESBL producers: when and how. Antibiotics (Basel) 2020; 9: 61. 10.3390/antibiotics9020061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kernéis S, Valade S, Geri G et al. Cefoxitin as a carbapenem-sparing antibiotic for infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Dis (Lond) 2015; 47: 789–95. 10.3109/23744235.2015.1062133 [DOI] [PubMed] [Google Scholar]
- 37. Yadav SK, Bhujel R, Hamal P et al. Burden of multidrug-resistant Acinetobacter baumannii infection in hospitalized patients in a tertiary care hospital of Nepal. Infect Drug Resist 2020; 3: 725–32. 10.2147/IDR.S239514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perez F, Hujer AM, Hujer KM et al. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2007; 51: 3471–84. 10.1128/AAC.01464-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wong D, Nielsen TB, Bonomo RA et al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 2017; 30: 409–47. 10.1128/CMR.00058-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scott P, Deye G, Srinivasan A et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis 2007; 44: 1577–84. 10.1086/518170 [DOI] [PubMed] [Google Scholar]
- 41. El-Sayed Ahmed MAE, Zhong LL, Shen C et al. Colistin and its role in the era of antibiotic resistance: an extended review (2000–2019). Emerg Microbes Infect 2020; 9: 868–85. 10.1080/22221751.2020.1754133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grégoire N, Aranzana-Climent V, Magréault S et al. Clinical pharmacokinetics and pharmacodynamics of colistin. Clin Pharmacokinet 2017; 56: 1441–60. 10.1007/s40262-017-0561-1 [DOI] [PubMed] [Google Scholar]
- 43. Isler B, Doi Y, Bonomo RA et al. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2018; 63: e01110-18. 10.1128/AAC.01110-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. New York Times. Will the next superbug come from Yemen? 2018. https://www.nytimes.com/2018/04/14/opinion/sunday/yemen-antibiotic-resistance-disease.html.
- 45. Orubu ESF, Al-Dheeb N, Ching C et al. Assessing antimicrobial resistance, utilization, and stewardship in Yemen: an exploratory mixed-methods study. Am J Trop Med Hyg 2021; 105: 1404–12. 10.4269/ajtmh.21-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]