Summary
Background
In French Polynesia, hepatitis B virus (HBV) infection appears as a major risk factor for hepatocellular carcinoma (HCC), which detection rate in the Austral archipelago is among the highest in the world. Through a nationally representative cross-sectional survey of the adult population, this study aimed at assessing the prevalence of HBV, but also hepatitis C virus (HCV), and hepatitis delta virus (HDV).
Methods
A total of 1942 blood samples from participants aged 18–69 years were tested for anti-HBc, anti-HBs, HBsAg, anti-HCV IgG, and HDV RNA. Complete genome sequencing of detected HBV strains was performed.
Findings
Among participants, 315/1834, 582/1834, 33/1834, 0/1857, and 0/33 tested positive for anti-HBc, anti-HBs, HBsAg, anti-HCV IgG, and HDV RNA, respectively. The population prevalence of HBsAg was estimated at 1.0% (95% CI: 0.6–1.7). All HBsAg carriers were born in French Polynesia before vaccination at birth became mandatory. In multivariate analyses, identified factors associated with HBsAg carriage included: the archipelago of residence (p < 0.0001), age (p < 0.0001), and education level (p = 0.0077). HBV genotypes B, C, and F were detected.
Interpretation
French Polynesia has a low endemicity level of HBV and its population may be considered at low risk for HCV and HDV infection. However, prevalence of HBsAg was found concerning in Austral (3.8%; 95% CI: 1.9–7.5) and Marquesas (6.5%; 95% CI: 3.8–11) archipelagoes. In the Austral archipelago, the presence of genotype C may account for the elevated rate of HCC. Our findings warrant more efforts to improve access to detection, prevention and care to people born before the systematic vaccination policy application, and residing in higher-risk areas, to achieve HBV elimination in French Polynesia.
Funding
Research Delegation of French Polynesia.
Keywords: Hepatitis B, Hepatitis C, Hepatitis delta, French Polynesia, Cross-sectional survey
Research in context.
Evidence before this study
Since 1995, mandatory vaccination at birth has drastically reduced the burden of hepatitis B in children from the five archipelagoes of French Polynesia. However, recent evidence has shown that hepatitis B virus (HBV) infections are still highly prevalent among the adult population of the Austral archipelago. To our knowledge, a single study investigated the presence of hepatitis delta virus (HDV) in individuals from French Polynesia with chronic HBV infection, and no evidence of co-infection was found. We also conducted a search on PubMed and MEDLINE but found no report on the prevalence of hepatitis C virus (HCV) infection in French Polynesia.
Added value of this study
This is the first nationally representative study of the prevalence of HBV, HCV, and HDV infections, evaluating the role of factors associated with infection and the distribution of HBV genotypes, among the adult population of French Polynesia. Our results confirm the impact of the systematic vaccination policy on the burden of HBV. According to our findings, French Polynesia may be considered as an area of low endemicity for HBV. However, a significant geographic heterogeneity remains, with noticeably higher prevalence rates of HBV in the Marquesas and Austral archipelagoes. We also demonstrate the presence of three different HBV genotypes. Finally, we provide evidence that the population of French Polynesia is at low risk for HCV and HDV infection.
Implications of all the available evidence
Additional efforts should be made to improve access to detection, prevention, and care for people born before the systematic vaccination policy and residing in higher-risk areas.
Introduction
The World Health Organization (WHO) estimated that viral hepatitis caused 1.1 million deaths worldwide in 2019, of which 96% were the consequence of infections from hepatitis B (HBV) and C (HCV) viruses.1 The same year, the WHO estimated that 296 million people were living with chronic HBV infection and 58 million with chronic HCV infection and that 1.5 million people were newly infected by each virus. Most viral hepatitis deaths result from chronic liver disease, including cirrhosis and liver cancer, especially hepatocellular carcinoma (HCC), with an increased risk in people with concomitant metabolic disorders including diabetes and obesity.2,3 Moreover, in HBV-infected individuals, co-infection or super-infection with hepatitis delta virus (HDV), a defective RNA virus requiring the presence of HBV surface antigen (HBsAg) for infectivity, has been associated with more rapid progression of liver disease to cirrhosis and hepatic decompensation, and an increased incidence of HCC and liver-related deaths, compared to HBV mono-infection.4 Worldwide, it has been estimated that 10–20 million people suffer from HBV/HDV co-infection.5
Treatments for chronic hepatitis B, C, and delta, as well as effective hepatitis B vaccines, are available. In 2016, WHO’s World Health Assembly (WHA) called for the global elimination of hepatitis B and C by 2030 by achieving a 90% reduction in new chronic infections, a 65% reduction in mortality, and treatment of 80% of infected people.6 In 2019, it was estimated that only 10% of people living with chronic hepatitis B, and 21% with chronic hepatitis C, were aware of their infection status, of which 22% and 62% were receiving treatment, respectively.1 The same year, an estimated 85% of all infants had received the recommended three doses of hepatitis B vaccine, and the global health sector strategy to reduce the prevalence of HBsAg to less than 1% among children younger than five years by 2020 has been met.1 Nevertheless, significant disparities exist between regions in terms of diagnosis, treatment, and vaccination.
In December 2020, WHO published a report stating that the Western Pacific Region was bearing the highest burden of viral hepatitis, with an estimated 115 million people chronically infected with HBV and 14 million with HCV, accounting for 40% of the global burden. Moreover, liver cancer was the sixth most frequent cause of death in the region, mostly attributable to chronic HBV or HCV infection.7 The prevalence of HBV infections among general population in the Western Pacific Region was estimated at 5.92% in 2019.8 A systemic review of HCV active infections reported in the region between 2016 and 2020 estimated a prevalence of 1% or less in most countries where data were available.9 The distribution of HDV infections in the region was shown to be highly irregular.10,11 Kiribati was found to bear one of the highest prevalence of HBV/HDV co-infection in the world, as shown by the finding that 40.8% of HBsAg-positive individuals had detectable HDV RNA in a serosurvey conducted in 2017.12
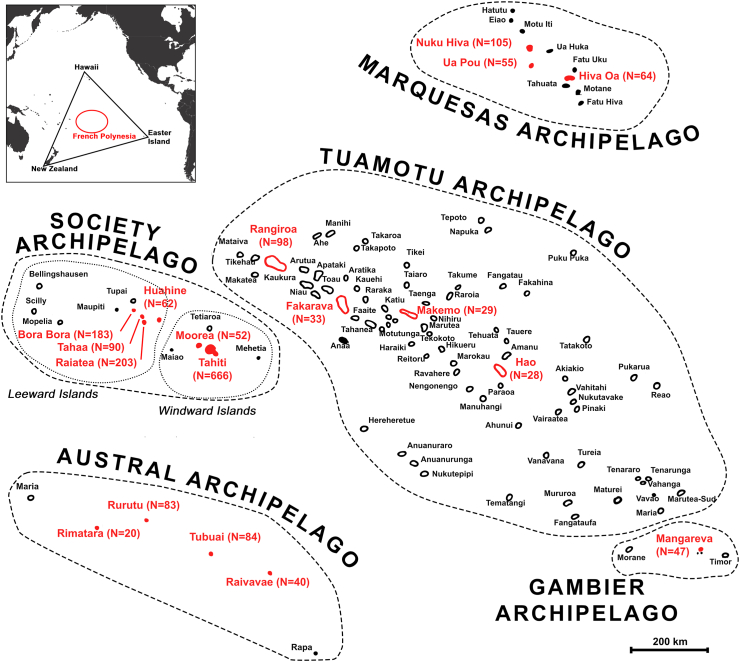
French Polynesia is a French overseas collectivity in the WHO Western Pacific Region, with about 276,000 inhabitants living in 75 different islands grouped in five distinct archipelagoes (Austral, Gambier, Marquesas, Society, and Tuamotu)13 (Fig. 1). From 1978 onwards, several serological surveys have been conducted in French Polynesia to assess the prevalence of HBV infections, indicating higher prevalence rates in the Austral archipelago.14, 15, 16, 17 In 1987, a serosurvey of HBV infection was conducted in the Austral archipelago,18 prevalence rates of HBsAg ranged from 3.1% in Tubuai to 27.0% in Rapa, with a weighted mean of 10.5% across the archipelago. Subsequently, a five-year pilot study was conducted in the same archipelago to evaluate the efficacy of different hepatitis B vaccination schedules in newborns.19 The rolling-out of a French Polynesia-wide vaccination program in newborns was then initiated on April 1st, 1992, consisting of three doses, at birth, one month, and between 6 and 12 months, followed by a booster dose at the age of 6 years. On June 8th, 1995, a systematic three-dose vaccination policy in infants was made compulsory in all archipelagoes.20,21 In 2014, a nationwide evaluation carried out among 6-year-old schoolchildren, showed a three-dose HBV vaccination coverage of 98% and a prevalence of HBsAg of 0% (95% CI: 0–0.5).22 In 2019, the screening of the entire adult population of Rapa, the most remote island of the Austral archipelago, revealed a prevalence of HBsAg of 11% in people born before 1992.23 In a retrospective study of all cases of HCC diagnosed in French Polynesia between 2008 and 2017, the detection rate of HCC reported in the Austral archipelago (43.1 new diagnosed cases per 100,000 population per year) was found to be among the highest in the world, and was mostly associated with HBV infection.23 To our knowledge only one study investigated the presence of HDV in French Polynesia, showing no cases of HDV infection among 62 chronic carriers of HBsAg residing in Tahiti and tested between 1978 and 1985.10 No published data are available regarding the prevalence of HCV in French Polynesia.
Fig. 1.
Map showing the geographical distribution of the five archipelagoes of French Polynesia (Society, Tuamotu, Gambier, Marquesas and Austral). The 18 islands selected for the serosurvey are represented in red. N indicates the number of participants recruited in each island. Inset map at upper left shows location of French Polynesia (red circle) in the Pacific Ocean.
This present study aims to fill a data gap on the current burden of HBV, HCV, and HDV across all five archipelagoes and constitutes a component of a large cross-sectional survey of adult health and its determinants.24
Methods
Study design
The present study is part of the MATAEA project, a nationally-representative cross-sectional, population-based survey conducted in French Polynesia between November 4th, 2019, and December 8th, 2021, as previously described.24 In brief, based on the population census from 2017,25 a random sample of the adult population, stratified by age (18–29, 30–44 and 45–69 years), sex (sex ratio of 1:1), and archipelago of residence, was selected (Fig. 1). The sample size (N = 2100) was calculated using the formula: n = z2 p (1-p)/d2, where z is the level of confidence set at 95% (z = 1.96), p is the expected prevalence set conservatively at 50%, and d is the precision set at 3.7%. Due to heterogeneous distribution of the population across the five archipelagoes, three geographical strata were selected: Windward Islands (Society archipelago), Leeward Islands (Society archipelago), and other archipelagoes (Tuamotu, Gambier, Marquesas and Austral), which respectively include 75%, 13% and 12% of the total population of French Polynesia. The sample size was amplified in the two least populated strata to keep sufficient precision around prevalence estimates for the health conditions under consideration in the MATAEA project. Consequently, the same sample size (N = 700) was selected for the three strata. In each stratum, for convenient reasons, the most populated islands with a health care center and an airport with regular flight rotations were selected (N = 18), and the number of participants to be recruited was proportional to the population size of the islands. In each island, households were randomly selected and visited. Among the residents of each household meeting the criteria of age and sex sought for the project, only one was invited to participate. Consenting participants were administered a questionnaire containing questions from the WHO standardized STEPS questionnaire,26 and additional questions specific to the context of French Polynesia. They were also subjected to physical measurements (height, weight, waist circumference, blood pressure, and skin pigmentation), and were taken biological samples (blood, saliva, and stool) at home by qualified nurses. Among the large amount of data and samples collected from participants for the purposes of the MATAEA project, only those allowing the assessment of the prevalence of HBV, HCV, and HDV, as well as the identification of factors related to infection, were used in the present study.
HBV and HCV serological analysis
Serological tests for HBV and HCV were performed from plasma with the ADVIA Centaur® XPT Immunoassay System analyser (Siemens Healthineers, Germany). HBsAg, total antibodies to HBV core antigen (anti-HBc), total antibodies to HBV surface antigen (anti-HBs), and Immunoglobulin G (IgG) antibodies to HCV (anti-HCV) were detected using respectively Dosage ADVIA Centaur® HbsAgII, Dosage ADVIA Centaur® HBc Total, Dosage ADVIA Centaur® anti-HBs2, and Dosage ADVIA Centaur® HCV kits, following the manufacturer’s instructions (Siemens Healthineers, Germany). According to the manufacturer, clinical specificity and sensitivity of these tests are respectively 99.9% and 100%, 99.7% and 100%, 99.8% and 99.5%, and 99.9% and 100%. Combinations of serological markers were used to determine whether participants had current HBV infection, were immune to HBV as a result of prior infection or vaccination, or were susceptible to HBV infection, as detailed in Table 1. Detection of HBsAg indicated current HBV infection, and anti-HBc evidenced HBV infection during lifetime (current or resolved). Detection of anti-HCV IgG indicated past HCV infection. Participants who tested positive for HBsAg were systematically informed of their serological status via their attending physician.
Table 1.
Interpretation of HBV serological results.
| Interpretation | Serological markers |
||
|---|---|---|---|
| HBsAg | Anti-HBs | Anti-HBc | |
| Current hepatitis B infection | + | – | +/− |
| Resolved hepatitis B infection | – | +/− | + |
| Susceptible to hepatitis B infection | – | – | – |
| Immune due to hepatitis B vaccination | – | + | – |
HBsAg: hepatitis B surface antigen; anti-HBs: antibodies to hepatitis B surface antigen; anti-HBc: antibodies to hepatitis B core antigen; +: positive; −: negative.
Interpretation of hepatitis B serological results is based on the definition from the Haute Autorité de Santé of France.27
HDV molecular detection
RNA extraction was performed from 200 μL of plasma or serum using the NucliSenS® easyMAG™ instrument (bioMérieux, France), following the manufacturer’s instructions. As previously described,11 extracted RNA was heated at 65 °C and cooled on ice. Then, reverse transcriptase polymerase chain reaction (RT-PCR) was performed on the Mastercycler X50a (Eppendorf, Germany) to amplify a 420 base pairs (bp) product including the 3′ terminal end of the HDAg coding region, using the primer set HDV-01 (5′-GATGCCATGCCGACCCGAAGAGG-3′, nucleotide positions: 885–907) and HDV-01a (5′-AGAGGCAGGATCACCGACGAAGG-3′, nucleotide positions: 1305–1283). Briefly, 5 μL of RNA were added to 20 μL of reaction mixture containing 1 μL Enzyme mix, 5 μL buffer, and 1 μL dnTPs from the QIAGEN OneStep RT-PCR kit (Qiagen, Germany), and 1.25 μL of each primer. The PCR thermocycling conditions included a reverse transcription step at 50 °C for 30 min, followed by a first denaturation step at 95 °C for 15 min, 45 cycles of amplification at 94 °C for 30 s, 62 °C for 1 min and 72 °C for 1 min, and a final extension step at 72 °C for 10 min.
Genotype characterization of HBV strains
Viral DNA extraction was performed from 200 μL of plasma using the NucliSenS® easyMAG™ instrument (bioMérieux, France), according to the manufacturer’s protocol. Then, a nested PCR was conducted on the Mastercycler X50a (Eppendorf, Germany), using previously published primers28 to amplify the complete genome of HBV, as detailed in Table 2. For the first amplification step, 5 μL of extracted DNA were added to 20 μL of reaction mixture containing 2.5 μL of 10× PCR buffer and 0.125 μL of HotStarTaq Plus DNA polymerase provided with the HotStarTaq® Plus DNA Polymerase Kit (Qiagen, Germany), 0.5 μL of dNTP, 14.38 μL of RNAse free water, and 1.25 μL of each primer. The thermocycling conditions were: 95 °C for 5 min, then 50 cycles at 94 °C for 1 min, 54 °C or 57 °C for 1 min and 72 °C for 1 min and 30 s, and finally 72 °C for 10 min. For the second amplification step, 5 μL of the product of the first amplification reaction were diluted 1:10 and added to 20 μL of reaction mixture containing 2.5 μL of 10× PCR buffer and 0.125 μL of HotStarTaq Plus DNA polymerase provided with the HotStarTaq® Plus DNA Polymerase Kit (Qiagen, Germany), 0.5 μL of dNTP, 14.38 μL of RNAse free water, and 1.25 μL of each primer. The thermocycling conditions were: 95 °C for 5 min, then 45 cycles at 94 °C for 1 min, between 50 °C and 57 °C for 1 min and 72 °C for 1 min and 30 s, and finally 72 °C for 10 min. Products of the second amplification reaction were subsequently purified using ChargeSwitch™ PCR Clean-Up Kit (Invitrogen, USA) or QIAEX II Gel Extraction Kit (Qiagen, Germany). Sequencing was performed with the 3500 series genetic analyser using reagents from the Big Dye™ Terminator V3.1 Cycle Sequencing kit (Applied Biosystems, USA) as recommended by the manufacturer, and primers described in Table 2 with an annealing temperature of 50 °C. Partial nucleotide sequences obtained for each HBV strain were cleaned and assembled using the Sequencher 5.4.6 software (Gene Codes Corporation, USA).
Table 2.
Characteristics of the primers used for the amplification and sequencing of HBV complete genomes.
| PCR primer sets |
Sequencing primers |
|||||
|---|---|---|---|---|---|---|
| Name | Sequence 5′-3′ | Position (nt) | Annealing temperature (°C) | Name | Sequence 5′-3′ | Position (nt) |
| Set 1a | 54 | |||||
| 251f | GAC TYG TGG TGG ACT TCT C | 251–269 | ||||
| 1797r | CCA ATT TMT GCY TAC AGC CTC | 1797–1777 | ||||
| Set 1ab | 54 | |||||
| 251f | GAC TYG TGG TGG ACT TCT C | 251–269 | 251f | GAC TYG TGG TGG ACT TCT C | 251–269 | |
| 1190r | TCA GCA AAY ACT YGG CA | 1190–1174 | 1190r | TCA GCA AAY ACT YGG CA | 1190–1174 | |
| Set 1bb | 54 | |||||
| 595f | CAC HTG TAT TCC CAT CCC A | 595–613 | 595f | CAC HTG TAT TCC CAT CCC A | 595–613 | |
| 1797r | CCA ATT TMT GCY TAC AGC CTC | 1797–1777 | 1190f | AYG CAA CCC CCA CTG G | 1190–1205 | |
| Set 2a | 57 | |||||
| 2300f | CCA CMW AAT GCC CCT ATC | 2300–2317 | ||||
| 654r | GSC CCA MBC CCA TAG G | 652–637 | ||||
| Set 2ab | 50 | |||||
| 2300f | CCA CMW AAT GCC CCT ATC | 2300–2317 | 2300f | CCA CMW AAT GCC CCT ATC | 2300–2317 | |
| 215r | AGR AAM ACM CCG CCT GT | 215–200 | 215r | AGR AAM ACM CCG CCT GT | 215–200 | |
| Set 2bb | 50 | |||||
| 2819f | ACC WTA TWC YTG GGA ACA A | 2819–2837 | 2819f | ACC WTA TWC YTG GGA ACA A | 2819–2837 | |
| 617r | GAY GAY GGG ATG GGA ATA CA | 617–598 | 617r | GAY GAY GGG ATG GGA ATA CA | 617–598 | |
| Set 3a | 57 | |||||
| 1859f | ACT NTT CAA GCC TCC RAG CTG | 1859–1879 | ||||
| 2835r | GTT CCC AVG WAT AWG GTG AYC C | 2835–2814 | ||||
| Set 3ab | 52 | |||||
| 1877f | CTG TGC CTT GGR TGG CTT | 1894–1877 | 1877f | CTG TGC CTT GGR TGG CTT | 1894–1877 | |
| 2835r | GTT CCC AVG WAT AWG GTG AYC C | 2835–2814 | 2835r | GTT CCC AVG WAT AWG GTG AYC C | 2835–2814 | |
| Set 4a | 57 | |||||
| 1584f | ACT TCG MBT CAC CTC TGC ACG T | 1583–1604 | ||||
| 2396r | GTC KGC GAG GYG AGG GAG TT | 2396–2377 | ||||
| Set 4ab | 57 | |||||
| 1584f | ACT TCG MBT CAC CTC TGC ACG T | 1583–1604 | 2331r | GGA AGY GTK GAY ARG ATA GGG GCA TT | 2331–2306 | |
| 2331r | GGA AGY GTK GAY ARG ATA GGG GCA TT | 2331–2306 | ||||
PCR: polymerase chain reaction; nt: nucleotide.
Primer sets used for the first amplification step.
Primer sets used for the second amplification step.
For determination of the genotype, complete genome sequences obtained in the study were compared to a set of reference sequences available for HBV genotypes A to H using the NCBI Web-based HBV Genotyping Tool (http://www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi).29 To determine the subtype, BLAST programs provided by NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) were used to identify HBV strains with known subtypes that show the highest nucleotide sequence similarity with the study strains. Study sequences were aligned with reference sequences available for HBV genotypes A to H, and with sequences showing the highest similarity with BLAST, using the multiple sequence alignment software ClustalW integrated in MEGA version 11.0.9.30 Then, a phylogenetic tree was built using the Maximum Likelihood method based on the Kimura 2-parameter model with 1000 Bootstrap replications. Complete nucleotide sequences obtained in this study have been deposited on GenBank (Accession numbers: OR250045-OR250062).
Statistical analysis
Statistical analyses were conducted using the R language (version 4.3.0).31 Characteristics of study participants were depicted as frequency counts and percentages for categorical data. Population prevalence rates for each studied condition were computed after adjusting for sampling design effects, with weights defined as the inverse probability of sampling, using the survey package.32 Inverse-probability weights were used to account for missing data. We assumed that missing data items were missing completely at random within each stratum. Overall weights were defined as the reciprocal of sampling probabilities. The main summary statistics included arithmetic means with 95% confidence intervals (CI) adjusted for sampling design effects. Factors related to the geographical origin and distribution of the population of French Polynesia (place of birth, archipelago of residence), as well as other factors previously shown to be associated with HBV infection in the literature (sex, age, vaccination, education level) were selected. The association of these factors with HBV infection was evaluated using quasi-binomial regression models. Statistical significance was set at p < 0.05.
Ethics statement
The study protocol was approved by a designated national ethical committee (CPP–OUEST III no. 19.08.60/SI CNRIPH 19.07.02.38421) and the ethical committee of French Polynesia (no. 80 CEPF-03/09/2019). Written informed consent was obtained from all participants. All data were stored and used in accordance with guidelines from the French National Commission on Data Protection and Individual Liberties.
Role of the funding source
This work was funded by the Research Delegation of French Polynesia (Convention No. 03557/MED/REC of May 29, 2019)” to cover the cost for Dr Iotefa Teiti Post-doctoral fellowship. This funding source had no role in the writing of the manuscript or the decision to submit it for publication.
Results
HCV and HBV infections during lifetime
A total of 1942 (76%) individuals aged 18–69 years agreed to participate among 2567 sampled. Valid results of serological analysis for HCV and HBV were obtained from 1857 and 1834 participants, respectively. No participants tested positive for anti-HCV IgG antibodies, whereas 315/1834 participants exhibited anti-HBc antibodies, indicating a prior exposition to HBV (Table 3). The population prevalence of HBV infection during a lifetime was estimated at 15% (95% CI: 13–17).
Table 3.
Prevalence of anti-HBc antibodies, HBsAg, and anti-HBs antibodies in the adult population of French Polynesia.
| Variable | N participants | Anti-HBc |
HBsAg |
Anti-HBs |
|||
|---|---|---|---|---|---|---|---|
| N positive | Population prevalence % [95% CI] | N positive | Population prevalence % [95% CI] | N positive | Population prevalence % [95% CI] | ||
| Total | 1834 | 315 | 15 [13–17] | 33 | 1.0 [0.6–1.7] | 582 | 32 [29–35] |
| Archipelago | |||||||
| Society (WIs) | 683 | 85 | 14 [11–17] | 4 | 0.7 [0.3–1.8] | 222 | 32 [29–36] |
| Society (LIs) | 506 | 87 | 17 [14–21] | 5 | 0.9 [0.4–2.3] | 131 | 26 [23–30] |
| Austral | 218 | 45 | 22 [17–28] | 8 | 3.8 [1.9–7.5] | 83 | 38 [32–44] |
| Marquesas | 213 | 70 | 34 [27–40] | 13 | 6.5 [3.8–11] | 73 | 34 [28–41] |
| Tuamotu | 176 | 22 | 13 [8.6–19] | 2 | 1.1 [0.3–4.4] | 59 | 34 [27–42] |
| Gambier | 38 | 6 | 16 [6.6–32] | 1 | 1.7 [0.2–12] | 14 | 38 [23–55] |
| Sex | |||||||
| Female | 967 | 144 | 13 [10–16] | 17 | 1.0 [0.5–2.0] | 295 | 31 [27–35] |
| Male | 867 | 171 | 17 [14–21] | 16 | 1.0 [0.5–2.2] | 287 | 32 [29–36] |
| Age group | |||||||
| 18–29 years | 531 | 3 | 0.4 [0.1–2.0] | 0 | 0.0 [0.0–0.0] | 200 | 34 [30–39] |
| 30–44 years | 639 | 109 | 13 [10–17] | 13 | 0.7 [0.4–1.3] | 223 | 40 [35–45] |
| 45–69 years | 664 | 203 | 27 [23–32] | 20 | 1.9 [1.0–3.8] | 159 | 23 [19–28] |
| Place of birth | |||||||
| Other country | 140 | 11 | 8.5 [4.2–17] | 0 | 0.0 [0.0–0.0] | 77 | 52 [42–62] |
| French Polynesia | 1694 | 304 | 16 [13–18] | 33 | 1.1 [0.6–1.8] | 505 | 30 [27–33] |
| Birth regarding vaccination policy | |||||||
| Born before June 8th, 1995 | 1518 | 314 | 18 [16–21] | 33 | 1.2 [0.7–2.0] | 480 | 32 [29–35] |
| Born from June 8th, 1995 | 316 | 1 | 0.1 [0.02–0.8] | 0 | 0.0 [0.0–0.0] | 102 | 33 [27–39] |
| Highest education levela | |||||||
| Elementary school | 320 | 113 | 33 [26–40] | 11 | 1.1 [0.6–2.0] | 61 | 15 [11–21] |
| Middle school | 494 | 83 | 15 [11–20] | 10 | 2.0 [0.9–4.6] | 137 | 27 [23–33] |
| Secondary school | 676 | 93 | 12 [9.5–16] | 10 | 0.8 [0.3–2.1] | 233 | 33 [29–38] |
| University | 342 | 26 | 7.3 [4.5–12] | 2 | 0.1 [0.02–0.4] | 151 | 45 [39–51] |
HBV: hepatitis B virus; anti-HBc: total antibodies to HBV core antigen; HBsAg: HBV surface antigen; anti-HBs: total antibodies to HBV surface antigen; WIs: Windward Islands; LIs: Leeward Islands; 95% CI: 95% confidence interval.
Total is not equal to 1834 because of missing data due to non-response.
An analysis of factors associated with HBV infection during lifetime is presented in Table 4. In multivariate analyses, living in the Marquesas archipelago (aOR: 3.58; 95% CI: 2.39–5.37), male sex (aOR: 1.48, 95% CI: 1.03–2.14), age (aOR: 1.05; 95% CI: 1.04–1.07), and being born in French Polynesia (aOR: 2.40, 95% CI: 1.02–5.64), were significantly associated with HBV infection during lifetime. In contrast, the risk dropped dramatically among individuals born after vaccination at birth became mandatory (aOR: 0.02; 95% CI: 0.00–0.15).
Table 4.
Risk factors associated with HBV infection during lifetime and HBV current infection in the adult population of French Polynesia.
| Variable | HBV infection during lifetime |
HBV current infection |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||||||
| OR | 95% CI | p-value a | aOR | 95% CI | p-value a | OR | 95% CI | p-value a | aOR | 95% CI | p-value a | |
| Archipelago | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||
| Society (WIs) | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Society (LIs) | 1.34 | 0.97–1.86 | 1.24 | 0.87–1.77 | 1.43 | 0.38–5.41 | 1.29 | 0.34–4.97 | ||||
| Austral | 1.77 | 1.18–2.65 | 1.40 | 0.88–2.21 | 5.97 | 1.77–20.1 | 5.93 | 1.87–18.8 | ||||
| Marquesas | 3.21 | 2.22–4.65 | 3.58 | 2.39–5.37 | 10.4 | 3.34–32.5 | 11.0 | 3.67–33.2 | ||||
| Tuamotu | 0.94 | 0.56–1.56 | 0.84 | 0.49–1.45 | 1.65 | 0.29–9.34 | 1.58 | 0.28–9.07 | ||||
| Gambier | 1.16 | 0.44–3.10 | 1.26 | 0.44–3.63 | 2.60 | 0.28–24.6 | 3.52 | 0.38–32.8 | ||||
| Sex | 0.030 | 0.036 | 0.89 | 0.88 | ||||||||
| Female | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Male | 1.45 | 1.04–2.03 | 1.48 | 1.03–2.14 | 1.08 | 0.37–3.14 | 1.09 | 0.37–3.23 | ||||
| Age (per 1 year) | 1.07 | 1.06–1.08 | <0.0001 | 1.05 | 1.04–1.07 | <0.0001 | 1.06 | 1.03–1.09 | <0.0001 | 1.06 | 1.03–1.10 | <0.0001 |
| Place of birth | 0.082 | 0.044 | NA | NA | ||||||||
| Other country | 1.00 | Reference | 1.00 | Reference | – | – | – | – | ||||
| French Polynesia | 1.98 | 0.92–4.30 | 2.40 | 1.02–5.64 | – | – | – | – | ||||
| Birth regarding vaccination policy | <0.0001 | 0.0001 | NA | NA | ||||||||
| Born before June 8th, 1995 | 1.00 | Reference | 1.00 | Reference | – | – | – | – | ||||
| Born from June 8th, 1995 | 0.01 | 0.00–0.04 | 0.02 | 0.00–0.15 | – | – | – | – | ||||
| Highest education level | <0.0001 | 0.050 | 0.0054 | 0.0077 | ||||||||
| Elementary school | 6.26 | 3.42–11.5 | 2.46 | 1.24–4.86 | 10.4 | 2.19–49.4 | 4.06 | 0.85–19.3 | ||||
| Middle school | 2.26 | 1.24–4.14 | 1.47 | 0.77–2.82 | 19.7 | 3.76–104 | 14.3 | 2.59–78.5 | ||||
| Secondary school | 1.81 | 1.00–3.28 | 1.61 | 0.88–2.97 | 7.97 | 1.44–44.1 | 7.75 | 1.35–44.6 | ||||
| University | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
HBV: hepatitis B virus; OR: odds ratio; 95% CI: 95% confidence interval; aOR: adjusted odds ratio; WIs: Windward Islands; LIs: Leeward Islands.
NA: Not applicable. This explanatory variable was excluded from the multivariate logistic regression analysis because one of its categories had a value equal to zero.
Significant p-values and aOR values are shown in bold.
p-value obtained using the Wald test.
Current HBV and HDV infections
HBsAg was detected in 33/1834 participants, corresponding to an estimated prevalence of 1.0% (95% CI: 0.6–1.7) (Table 3). All HBsAg-positive participants had detectable anti-HBc antibodies. None of them tested positive for HDV RNA. All 33 HBsAg-positive participants were born in French Polynesia before hepatitis B vaccination at birth became mandatory (Table 3), with a median age of 51 (age range: 30–68) and a balanced sex ratio (16 males vs 17 females). Of them, 13 (39%) already knew their positive status.
The analysis of factors associated with current HBV infection is shown in Table 4. Living in the Austral (aOR: 5.93; 95% CI: 1.87–18.8) and Marquesas (aOR: 11.0; 95% CI: 3.67–33.2) archipelagoes, age (aOR: 1.06; 95% CI: 1.03–1.10), and having an education below University level (middle school: aOR: 14.3; 95% CI: 2.59–78.5; secondary school: aOR: 7.75; 95% CI: 1.35–44.6), were significantly associated with current HBV infections.
Vaccination against HBV
Anti-HBs antibodies resulting from a prior natural or therapeutic immunization against HBV were found in 582/1834 participants, corresponding to a population prevalence of 32% (95% CI: 29–35) (Table 3). Among participants born from June 8th, 1995, i.e., those who theoretically benefited from an anti-hepatitis B vaccination, only 102/316 (32%) had detectable anti-HBs antibodies, while the same proportion (n = 480/1518, 32%) of participants born before the implementation of universal immunization were found anti-HBs positive. The vaccination status of participants was not documented, it was therefore not possible to estimate a vaccination coverage neither to investigate its associated factors (for instance, the correlation between the vaccination status of participants born before June 8th, 1995, and factors such as age, education level, occupation, and place of residence).
Genotype distribution of HBV strains isolated in French Polynesia
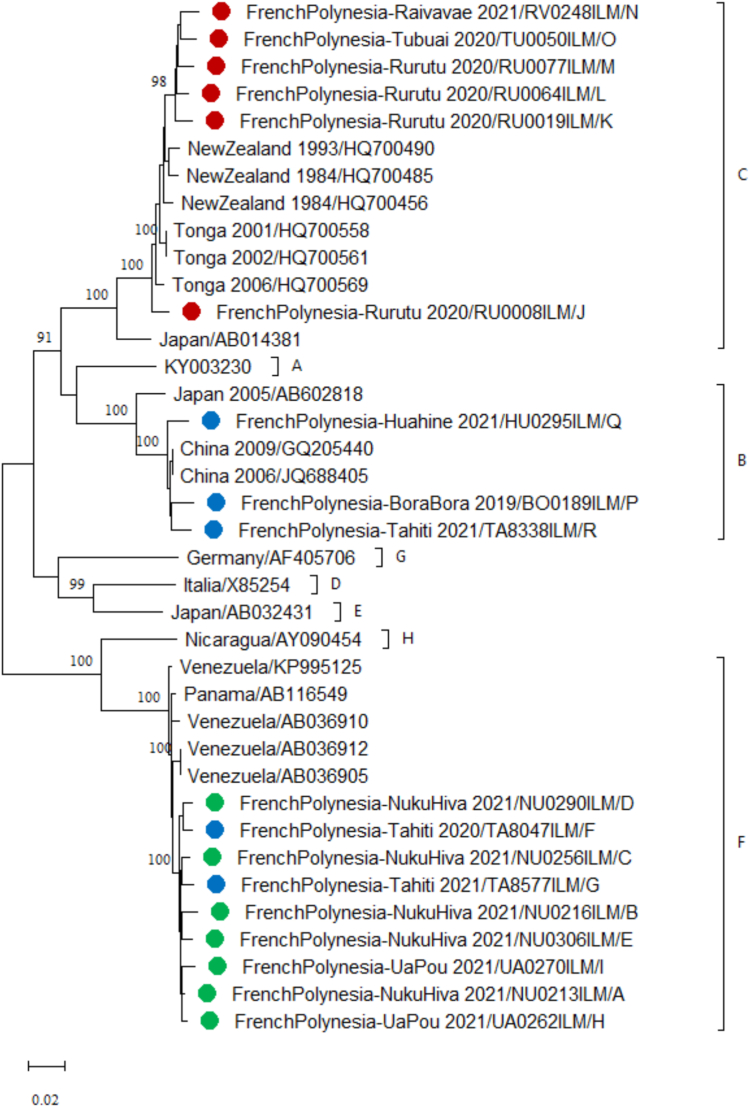
Complete genome sequences (3215 bp) of HBV strains could be obtained from 18/33 HBsAg-positive participants. Of them, respectively 9, 6 and 3 belong to genotypes F (subtype F3), C (subtype C3), and B (subtype B2). Genotype F, C, and B strains were found genetically close to strains from South America (Venezuela and Panama), Oceania (Tonga and New Zealand), and continental Asia (China), respectively (Fig. 2). Among the 9 strains of genotype F, 7 were isolated from residents of the Marquesas archipelago (Nuku Hiva and Ua Pou islands). The other 2 strains of genotype F were found in residents of the Society archipelago (Tahiti), including one whose parents originated from the Marquesas archipelago. Study strains of genotypes C and B were collected from residents of the Austral (Rurutu, Tubuai and Raivavae) and Society (Tahiti, Bora Bora, and Huahine) archipelagoes, respectively.
Fig. 2.
Phylogenetic analysis of HBV strains isolated from the study participants. The phylogenetic tree was constructed using nucleotide sequences of the complete genome of HBV (3215 bp) obtained from the study participants (N = 18) and retrieved from GenBank (N = 20). The evolutionary history was inferred by using the Maximum Likelihood method based on the Kimura 2-parameter model. The percentage of trees in which the associated taxa clustered together is shown for values over 90 next to the branches (1000 replicates). Evolutionary analyses were conducted in MEGA 11.0.9. Sequences are labeled by country_ year of collection/GenBank accession number. Sequences obtained from inhabitants of the Marquesas, Austral, and Society archipelagoes are marked with green, red, and blue circles, respectively. HBV strains belonging to the same genotype (A–H) are delimited by brackets. Scale bar indicates nucleotide substitutions per site.
Discussion
This is the first nationally representative prevalence study of HBV, HCV, and HDV infections in French Polynesia. No evidence of past HCV infections was found in the study participants, suggesting a low level of HCV circulation. The population prevalence of HBsAg carriage, estimated at 1.0% (95% CI: 0.6–1.7), corresponds to a low endemicity for HBV according to international classification.33 As no evidence of HDV infection was detected in HBsAg-positive individuals, French Polynesia may also be considered at low risk for HDV circulation.
The absence of HBsAg among individuals born after the mandatory vaccination policy, is in favour of a strong efficacy of the three-dose vaccination course,22 an interpretation consistent with conclusions from the European consensus group on hepatitis B immunity, which stated that the duration of protection among fully vaccinated children is at least 15 years.34 Only 32% of individuals born after the implementation of the mandatory anti-hepatitis B immunization still had detectable anti-HBs antibodies at the time of study enrollment. This finding is consistent with results of previous studies showing a decrease in antibody levels over time in people vaccinated during infancy.35
Previous serosurveys, conducted over the 1970–1980s in French Polynesia, had identified the Austral archipelago as the area with the highest prevalence of HBsAg carriers.14, 15, 16, 17 Our study added evidence of an increased risk for HBV infection among adults residing in the Austral archipelago, as well as the Marquesas archipelago. Individuals born in French Polynesia were found more at risk of HBV infection than participants born abroad, suggesting the dominant role of local HBV transmission over imported infections.
In our study, older age, lower education level, and being male were identified as factors significantly associated with an increased risk of HBV infection (current or resolved), as reported in other countries.36, 37, 38, 39, 40 Explanations for the age effect include a higher duration of exposure to the cumulative risk of HBV acquisition among older individuals and a lower vaccine coverage among individuals born before vaccination became compulsory. In other settings, a lower education level was shown to be associated with a lower awareness of positive status among individuals with chronic HBV infection,41 and with a lower HBV vaccination coverage rate,42 both factors also probably increasing the risk of virus transmission in French Polynesia. While males appear more exposed than females to HBV infection during lifetime, the sex ratio for current HBV infection is equivalent in French Polynesia. This finding differs from results obtained in other settings, where males were found more prone to current HBV infection than females.38, 39, 40 This discrepancy might be due to the low number of HBsAg-positive individuals detected in our study, consequently a higher sample size would be needed to identify any significant difference.
Analysis of HBV complete sequences obtained in the present study revealed the circulation of genotypes B, C, and F. All strains collected from the Austral archipelago clustered into genotype C, which is among the genotypes that carry the higher lifetime risk for cirrhosis and HCC.43 The presence of genotype C, along with a higher prevalence of HBsAg compared to the national level could potentially account for the elevated rate of HCC in the Austral archipelago.23
Our study reveals a distinct geographical distribution of the three HBV subtypes detected in French Polynesia. Subtypes B2 and C3, which are both common in the Asia–Pacific region,44 were respectively observed in the Society and Austral archipelagoes, while subtype F3, which is present in Central and South America,43 was mostly found in the Marquesas archipelago. The global distribution of HBV genotypes and subtypes was shown to be consistent with the major prehistoric modern human migrations.45 Additional investigations, particularly molecular clock analysis aiming to estimate the dates of lineage-splitting events, may help substantiate the current hypotheses concerning the history of settlements in the different islands and prehistoric contact. In this perspective, the presence in the Marquesas archipelago of subtype F3 provides support to the hypothesis of a prehistoric contact with Native Americans.46
There are some limitations to our study. First, 24% of the individuals selected for the study refused to participate. As no information was collected from these individuals, the impact on the variables analysed in the study is unknown. Second, some individuals may not have participated in the study because they were hospitalized or treated for chronic viral hepatitis, which could have underestimated the prevalence of HBV, HCV, and HDV infections. Third, due to the overlap of the study period with the COVID-19 pandemic, enrollments were interrupted several times and more than two years elapsed between the first and last inclusion. However, the time of inclusion has certainly had little impact on the prevalence of HBV, HCV and HDV, as well as on the associated risk factors. Fourth, the prevalence of HBV, HCV, and HDV infections has not been investigated in children. The absence of HBsAg detected in 6-year-old schoolchildren surveyed in 2014 suggests a low burden of HBV among children.22 Vertical transmission is considered the primary cause of HCV infection during childhood,47 but the absence of evidence of HCV infection in adults suggests that this mode of transmission is unlikely in French Polynesia. Fifth, although no evidence of past HCV infection was found in the study participants, HCV may be circulating in high-risk groups (e.g. among people who inject drugs) without diffusion in the general population. Additional screening of these high-risk groups may be undertaken to confirm the absence of HCV circulation in French Polynesia. Sixth, the analysis of factors associated with HBV infection and vaccination was limited by the data available in the questionnaire. Finally, low viral loads among the 33 HBsAg-positive participants limited full-length genome sequencing to only 18 samples.
In conclusion, our study provides evidence of the high efficacy of the vaccination policy implemented in French Polynesia against the burden of chronic HBV infection, demonstrated as a major factor associated with HCC.23 We provide evidence in favour of the absence (or very low level) of HCV and HDV transmission. Although our data show that French Polynesia may be considered an area of low endemicity for HBV, the prevalence of HBsAg carriage was found worrisome in Austral (3.8%; 95% CI: 1.9–7.5) and Marquesas (6.5%; 95% CI: 3.8–11) archipelagoes. In addition, more than 60% of the HBsAg-positive participants reported being previously unaware of their serological status. These findings warrant further efforts to improve the coverage of anti-HBV immunization, viral detection, and care, primarily in the two most affected archipelagoes and also for the elderly individuals from other archipelagoes who have escaped HBV vaccination. As a first step toward HBV elimination in French Polynesia, screening for HBsAg, anti-HBc and anti-HBs antibodies could be performed in all inhabitants of the Austral and Marquesas archipelagoes born before the mandatory vaccination policy (N ≈ 9000), except pregnant women who are already systematically tested in the first trimester of pregnancy. An estimated 500 HBsAg-positive individuals should subsequently be re-tested for HBsAg at least six months apart to confirm chronic HBV infection, and receive medical care in accordance with international recommendations,48,49 as currently performed for patients detected with HBV infection in French Polynesia. Moreover, individuals tested negative for HBsAg, anti-HBc and anti-HBs antibodies should be immediately vaccinated. The same procedure could subsequently be considered for the populations of other archipelagoes to achieve complete elimination of HBV in French Polynesia.
Contributors
AF and VMCL designed and conceptualized the study. IT, MA, and VMCL supervised the study. AT, TP, AS, LD, SO, and SL processed biological samples and performed laboratory analyses. IT, KC, VM, and YM contributed to data management. IT, VM, and PG accessed and verified the data. VM, YM, and PG performed the statistical analyses. IT and MA drafted the manuscript. PP, AF, BC, and SL provided critical revision for important intellectual content. IT, MA, and VMCL were responsible for the decision to submit the manuscript. All authors reviewed the manuscript and approved the submitted final manuscript.
Data sharing statement
Datasets generated and/or analysed in the present study are available from the corresponding author upon request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
We declare that we have no conflicts of interest.
Acknowledgements
This work was funded by the Research Delegation of French Polynesia (Convention No. 03557/MED/REC of May 29, 2019)”. We thank Sandrine Fernandes-Pellerin and Nathalie Jolly (Clinical Research Coordination Office, Institut Pasteur, Paris, France) for managing the ethical aspects of the MATAEA project. We acknowledge the municipal staff and guides on the selected islands for their support in participant recruitment. We are deeply grateful to all who willingly agreed to participate in this study.
References
- 1.World Health Organization . 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021.https://apps.who.int/iris/rest/bitstreams/1348210/retrieve Available from: [Google Scholar]
- 2.Shin H.S., Jun B.G., Yi S.W. Impact of diabetes, obesity, and dyslipidemia on the risk of hepatocellular carcinoma in patients with chronic liver diseases. Clin Mol Hepatol. 2022;28(4):773–789. doi: 10.3350/cmh.2021.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q., Tong Y., Pi B., Yu H., Lv F. Influence of metabolic risk factors on the risk of bacterial infections in hepatitis B-related cirrhosis: a 10-year cohort study. Front Med. 2022;9 doi: 10.3389/fmed.2022.847091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romeo R., Del Ninno E., Rumi M., et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136(5):1629–1638. doi: 10.1053/j.gastro.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 5.Wranke A., Pinheiro Borzacov L.M., Parana R., et al. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: the Hepatitis Delta International Network (HDIN) Liver Int. 2018;38(5):842–850. doi: 10.1111/liv.13604. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . 2016. Combating hepatitis B and C to reach elimination by 2030.https://apps.who.int/iris/rest/bitstreams/915976/retrieve Available from: [Google Scholar]
- 7.World Health Organization . 2020. Expert consultation on viral hepatitis elimination in the Western Pacific Region.https://apps.who.int/iris/bitstream/handle/10665/339977/RS-2020-GE-36-virtual-eng.pdf?sequence=1&isAllowed=y Available from: [Google Scholar]
- 8.World Health Organization Hepatitis data and statistics in the western pacific 2021. https://www.who.int/westernpacific/health-topics/hepatitis/regional-hepatitis-data Available from:
- 9.Iversen J., Wand H., Chan P.L., Le L.V., Maher L. Systematic review of hepatitis C virus prevalence in the WHO western pacific region. Viruses. 2022;14(7):1548. doi: 10.3390/v14071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimitrakakis M., Crowe S., Gust I. Prevalence of delta infection in the western Pacific region. J Med Virol. 1986;18(4):335–339. doi: 10.1002/jmv.1890180406. [DOI] [PubMed] [Google Scholar]
- 11.Han M., Littlejohn M., Yuen L., et al. Molecular epidemiology of hepatitis delta virus in the Western Pacific region. J Clin Virol. 2014;61(1):34–39. doi: 10.1016/j.jcv.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Jackson K., Tekoaua R., Holgate T., et al. Hepatitis B and D in the pacific islands of Kiribati. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104527. [DOI] [PubMed] [Google Scholar]
- 13.Institut de la statistique de la Polynésie française Référentiels–Géographie de la Polynésie française. https://www.ispf.pf/ispf/referentiels Available from:
- 14.Douglas Lewis B. Yale University School of Medicine; 1981. Hepatitis B virus infection among native inhabitants of French Polynesia : a seroepidemiologic and genetic analysis. [Google Scholar]
- 15.Parc F. ITRMLM; Papeete, French Polynesia: 1979. Le virus H.B.V en Polynésie française. [Google Scholar]
- 16.World Health Organization . 1982. Scientific group on viral hepatitis B and its related diseases.https://apps.who.int/iris/bitstream/handle/10665/208034/WP_CRP_ICP_BVM_020_eng.pdf?sequence=1&isAllowed=y Available from: [Google Scholar]
- 17.Brindle R.J., Eglin R.P., Parsons A.J., Hill A.V., Selkon J.B. HTLV-1, HIV-1, hepatitis B and hepatitis delta in the Pacific and South-East Asia: a serological survey. Epidemiol Infect. 1988;100(1):153–156. doi: 10.1017/s095026880006564x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutin J.P., Sainte Marie F.F., Cartel J.L., Cardines R., Girard M., Roux J. Prevalence of hepatitis B virus infection in the Austral archipelago, French Polynesia: identification of transmission patterns for the formulation of immunization strategies. Trans R Soc Trop Med Hyg. 1990;84(2):283–287. doi: 10.1016/0035-9203(90)90288-p. [DOI] [PubMed] [Google Scholar]
- 19.Moulia-Pelat J.P., Spiegel A., Excler J.L., et al. [Control of hepatitis B in French Polynesia with a program of systematic vaccination of newborns with the Genhevac B vaccine] Sante. 1996;6(1):11–15. [PubMed] [Google Scholar]
- 20.Gouvernement de la Polynésie Française Délibération n° 95-63 AT du 23 mai 1995 portant réglementation des vaccinations contre certaines maladies transmissibles chez l’enfant. J Officiel de la Polynésie Française. 1995;144(23):1202. [Google Scholar]
- 21.Gouvernement de la Polynésie Française Arrêté n°812 CM du 31 juillet 1995 relatif aux règles techniques des vaccinations obligatoires et recommandées. J Officiel de la Polynésie Française. 1995;144(32):1619–1622. [Google Scholar]
- 22.Patel M.K., Le Calvez E., Wannemuehler K., Ségalin J.M. Hepatitis B vaccination coverage and prevalence of hepatitis B surface antigen among children in French Polynesia, 2014. Am J Trop Med Hyg. 2016;94(6):1370–1375. doi: 10.4269/ajtmh.15-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condat B., Lascols A., Lutringer-Magnin D., Chakhtoura, Lastere S., Bronstein J. Epidemiology of hepatocellular carcinoma in French Polynesia. Bull Épidémiol Hebd. 2022;(16):280–287. [Google Scholar]
- 24.Teiti I., Aubry M., Fernandes-Pellerin S., et al. Unravelling the determinants of human health in French Polynesia: the MATAEA project. Front Epidemiol. 2023;3 doi: 10.3389/fepid.2023.1201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institut de la Statistique de la Polynésie française Recensement de la population 2017- Données détaillées–population par Géographie administrative et par Age décennal 2018. https://data.ispf.pf/bases/Recensements/2017/Donnees_detaillees.aspx Available from:
- 26.World Health Organisation Standard STEPS instrument 2020. https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps/instrument Available from:
- 27.Haute Autorité de Santé . 2011. Stratégies de dépistage biologique des hépatites virales B et C.https://www.has-sante.fr/jcms/c_1050355/fr/strategies-de-depistage-biologique-des-hepatites-virales-b-et-c Available from: [Google Scholar]
- 28.Chook J.B., Teo W.L., Ngeow Y.F., Tee K.K., Ng K.P., Mohamed R. Universal primers for detection and sequencing of hepatitis B virus genomes across genotypes A to G. J Clin Microbiol. 2015;53(6):1831–1835. doi: 10.1128/JCM.03449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozanov M., Plikat U., Chappey C., Kochergin A., Tatusova T. A web-based genotyping resource for viral sequences. Nucleic Acids Res. 2004;32(Web Server issue):W654–W659. doi: 10.1093/nar/gkh419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K., Stecher G., Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38(7):3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team . 2022. R: a language and environment for statistical computing.https://www.R-project.org/ Available from: [Google Scholar]
- 32.Lumley T. Survey: analysis of complex survey samples. 2023. http://r-survey.r-forge.r-project.org/survey/ cited R package verson 4.2. Available from:
- 33.Nguyen M.H., Wong G., Gane E., Kao J.H., Dusheiko G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin Microbiol Rev. 2020;33(2) doi: 10.1128/CMR.00046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355(9203):561–565. [PubMed] [Google Scholar]
- 35.Mahmood S., Shah K.U., Khan T.M. Immune persistence after infant hepatitis-B vaccination: a systematic review and meta-analysis. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-30512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morisco F., Stroffolini T., Lombardo F.L., et al. Prevalence of and risk factors for HBV infection in a metropolitan Southern Italian area: evidence for the effectiveness of universal Hepatitis B vaccination. Dig Liver Dis. 2017;49(11):1257–1261. doi: 10.1016/j.dld.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Nunes J.D.C., Silva D., Fonseca L.M.B., et al. Unexpected findings of hepatitis B and delta infection in northeastern Brazil: a public health alert. Ann Hepatol. 2021;22 doi: 10.1016/j.aohep.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Ataei B., Alavian S.M., Shahriari-Fard F., et al. A case-control study of risk factors for hepatitis B infection: a regional report among Isfahanian adults. J Res Med Sci. 2019;24:22. doi: 10.4103/jrms.JRMS_761_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang A.C., Geng J.H., Wang C.W., Wu D.W., Chen S.C. Sex difference in the associations among risk factors with hepatitis B and C infections in a large Taiwanese population study. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.1068078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton E.M., Rassam W., Yan Y., et al. Correlates of chronic hepatitis B virus infection in the general adult population of China: systematic review and meta-analysis. J Viral Hepat. 2023;30(6):470–488. doi: 10.1111/jvh.13816. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Zhou H., Zhang L., et al. Prevalence of chronic hepatitis B and status of HBV care among rural women who planned to conceive in China. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-12005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang Y., Bai X., Liu X., et al. Hepatitis B vaccination coverage rates and associated factors: a community-based, cross-sectional study conducted in Beijing, 2019-2020. Vaccines (Basel) 2021;9(10):1070. doi: 10.3390/vaccines9101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin C.L., Kao J.H. Natural history of acute and chronic hepatitis B: the role of HBV genotypes and mutants. Best Pract Res Clin Gastroenterol. 2017;31(3):249–255. doi: 10.1016/j.bpg.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Norder H., Couroucé A.M., Coursaget P., et al. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47(6):289–309. doi: 10.1159/000080872. [DOI] [PubMed] [Google Scholar]
- 45.Paraskevis D., Magiorkinis G., Magiorkinis E., et al. Dating the origin and dispersal of hepatitis B virus infection in humans and primates. Hepatology. 2013;57(3):908–916. doi: 10.1002/hep.26079. [DOI] [PubMed] [Google Scholar]
- 46.Ioannidis A.G., Blanco-Portillo J., Sandoval K., et al. Native American gene flow into Polynesia predating Easter Island settlement. Nature. 2020;583(7817):572–577. doi: 10.1038/s41586-020-2487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeung C.Y., Lee H.C., Chan W.T., Jiang C.B., Chang S.W., Chuang C.K. Vertical transmission of hepatitis C virus: current knowledge and perspectives. World J Hepatol. 2014;6(9):643–651. doi: 10.4254/wjh.v6.i9.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 49.Terrault N.A., Lok A.S.F., McMahon B.J., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]