Abstract
Background
Berotralstat is a once-daily oral inhibitor of plasma kallikrein for the prophylaxis of hereditary angioedema (HAE) in patients ≥12 years. APeX-J aimed to evaluate the efficacy and safety of berotralstat in Japan.
Methods
APeX-J was a Phase III trial comprising 3 parts (NCT03873116). Part 1 was a randomized, placebo-controlled evaluation of berotralstat 150 or 110 mg over 24 weeks. Part 2 was a 28-week dose-blinded phase in which berotralstat-treated patients continued the same dose and placebo patients were re-randomized to berotralstat 150 or 110 mg. In Part 3, all patients remaining on study received berotralstat 150 mg in an open-label manner for up to an additional 52 weeks. The primary endpoint of Parts 2 and 3 was long-term safety and tolerability, and secondary endpoints examined effectiveness.
Results
Seventeen patients entered Part 2, and 11 continued into Part 3. Treatment-emergent adverse events (TEAEs) were reported by 14/17 patients (82.4%) in Parts 2 or 3; the most common were nasopharyngitis, abdominal pain, cystitis, influenza, and vertigo. One patient (5.9%) experienced a drug-related TEAE (Grade 4 increased hepatic enzyme). No drug-related serious TEAEs were reported. For patients who completed 26 months of treatment with berotralstat 150 mg (n = 5), mean (standard error of the mean) monthly HAE attack rates and on-demand medication use decreased from baseline by 1.15 (0.09) attacks/month and 2.8 (0.64) doses/month, respectively. Sustained improvements were also observed in patient quality of life and treatment satisfaction.
Conclusions
Long-term prophylaxis with berotralstat raised no new safety signals and was effective at reducing attacks and improving patient-reported outcomes.
Trial registration
ClinicalTrials.gov NCT03873116. Registered March 13, 2019. Retrospectively registered.
Keywords: Angioedemas, Hereditary, Berotralstat, Japan, Plasma kallikrein, Quality of life
Introduction
Hereditary angioedema (HAE) is a rare autosomal dominant disorder characterized by recurrent swelling of subcutaneous and submucosal tissues largely due to bradykinin dysregulation.1 It is most commonly caused by a deficiency in C1 inhibitor (C1-INH) but also occurs in individuals with normal C1-INH.2 In HAE due to C1-INH deficiency (HAE-C1-INH), the SERPING1 gene, which encodes C1-INH, is mutated, leading to decreased levels of C1-INH (type 1) or dysfunctional C1-INH (type 2).2 The prevalence of HAE-C1-INH is estimated to be ∼1 in 50 000 individuals;3 in Japan, this corresponds to about 2000–3000 people.4 However, the number of patients who have been diagnosed and treated in Japan is lower.4
Swelling attacks occur in various locations, including the face, larynx, abdomen, and extremities,1 and can negatively affect patients’ quality of life (QoL) and ability to perform daily activities.5, 6, 7 Onset of attacks usually occurs during the first two decades of life (mean age: 11 years), and attacks typically last 2–4 days if left untreated.8,9 Despite early onset of disease symptoms, diagnosis of HAE is often delayed.10,11
The 2021 World Allergy Organization/European Academy of Allergy and Clinical Immunology (WAO/EAACI) international guidelines for the management of HAE recommend that the goal of HAE treatment should be to achieve complete disease control and normalize patients’ lives.12,13 These guidelines state that long-term prophylaxis is currently the only way to achieve this goal and recommend subcutaneous plasma-derived C1-INH (pdC1-INH), lanadelumab, and berotralstat as first-line long-term prophylactic treatment options.12,13 In Japan, all 3 treatments are approved for this indication by the Japanese Ministry of Health, Labour and Welfare,14, 15, 16 and are recommended as first-line long-term prophylactic treatment options by the Japanese Association for Complement Research.17
Long-term HAE prophylaxis is important because it can prevent attacks, reduce disease burden, and improve patient QoL.12,13,18 However, both pdC1-INH and lanadelumab are injectable therapies,16,18 which may be burdensome for patients.18,19 Injectable therapies can lead to injection site reactions, be uncomfortable/painful to administer, and require long administration times and/or special storage conditions.
Conversely, berotralstat is a once-daily (QD), orally administered plasma kallikrein inhibitor.20 In the pivotal Phase III APeX-2 trial (NCT03485911), treatment of HAE-C1-INH patients with berotralstat led to significant reductions in HAE attack rates versus placebo.21
As there was an unmet need for targeted HAE prophylactic treatment options in Japan and APeX-2 only recruited patients in North America and Europe, a randomized Phase III trial was conducted in Japan (APeX-J: NCT03873116).21,22 In Part 1 of APeX-J, treatment of HAE-C1-INH patients with berotralstat 150 mg QD for 24 weeks significantly reduced the rate of HAE attacks compared with placebo (1.11 attacks/month [n = 7] versus 2.18 attacks/month [n = 6]; P = 0.003; based on negative binominal regression).22 Here, we report the long-term safety and effectiveness results from Parts 2 and 3 of APeX-J.
Methods
Study design
APeX-J was a randomized, double-blind, placebo-controlled, Phase III trial that evaluated the efficacy and safety of berotralstat 150 and 110 mg as an oral prophylactic treatment for patients in Japan with HAE-C1-INH.22 The clinical trial was registered on clinicaltrials.gov with identifier NCT03873116. In Part 1, patients were randomized 1:1:1 to receive placebo or berotralstat 150 or 110 mg QD, and efficacy was evaluated over 24 weeks (Supplemental Figure 1).22 All patients who continued into Part 2 received dose-blinded berotralstat treatment for an additional 28 weeks (Week 24 to Week 52). Patients who were on active berotralstat treatment in Part 1 continued the same dose in Part 2, and patients who were initially randomized to placebo were re-randomized to berotralstat 150 or 110 mg QD. In Part 3, which was added as an amendment to extend treatment for up to an additional 52 weeks, all patients were treated in an open-label manner. Based on the efficacy and safety profile of berotralstat 150 mg in Part 1 of the APeX-2 study,21 all patients on berotralstat 110 mg were transitioned to berotralstat 150 mg in Part 3 of APeX-J.
APeX-J was conducted in compliance with Good Clinical Practice, including the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines and the ethical principles of the Declaration of Helsinki. The study was also conducted in accordance with regulatory requirements set forth by the Japanese Pharmaceuticals and Medical Devices Agency and other applicable Japanese laws. Following marketing authorization of berotralstat in Japan, this study was conducted in compliance with post-marketing regulations and the sponsor changed from BioCryst Pharmaceuticals, Inc. to the marketing authorization holder, OrphanPacific, Inc. The protocol and study documents were submitted to and approved by an appropriate institutional review board (IRB)/independent ethics committee (IEC). An independent data monitoring committee reviewed safety data at least every 6 months.
Patients
Patients enrolled at 10 sites across Japan (Supplemental Table 1). Prior to study entry, patients had to sign an informed consent form written in a language in which they were fluent. Personal information was treated as strictly confidential and was not made publicly available. Patients were eligible for participation if they had a confirmed diagnosis of HAE-C1-INH, were ≥12 years, had access to and the ability to use ≥1 HAE medication for treatment of acute attacks (pdC1-INH or icatibant), and had ≥2 qualifying attacks documented during the 56-day run-in period. A full list of inclusion and exclusion criteria is in the Supplemental Appendix.
Outcomes
The primary objective of APeX-J Parts 2 and 3 was to evaluate the long-term safety and tolerability of berotralstat. Accordingly, the primary endpoints were the number and proportion of patients who had a treatment-emergent adverse event (TEAE), a treatment-emergent serious adverse event (TESAE), a Grade 3 or 4 TEAE, a Grade 3 or 4 laboratory abnormality, a drug-related TEAE consistent with transient drug rash, and a TEAE leading to discontinuation. TEAEs were assessed from informed consent through to last study visit or until the TEAE was resolved or the patient was clinically stable.
Secondary endpoints in Parts 2 and 3 aimed to examine the effectiveness of berotralstat and included the number and rate of HAE attacks, durability of response (ie, attack rate trend over time), use of on-demand medications to treat attacks, and durability in Angioedema Quality of Life Questionnaire (AE-QoL) and Treatment Satisfaction Questionnaire for Medication (TSQM) scores. The scales for both AE-QoL and TSQM range from 0 to 100 points; lower AE-QoL values indicate better QoL and higher TSQM values indicate greater satisfaction.23, 24, 25, 26
Information on scheduled visits and recording of attacks is in the Supplemental Appendix.
Statistical analyses
All patients who entered Part 2 of APeX-J and received berotralstat treatment are included in this analysis. Data are summarized by treatment group, which, because of low patient numbers, have been defined based on the treatment received at first dose in Part 1 or at first dose in Part 2 for patients originally randomized to placebo (i.e., All 150 mg group and All 110 to 150 mg group; Supplemental Figure 1).
Safety analyses focus on the long-term safety of berotralstat in Parts 2 and 3 and are presented using frequencies and percentages. TEAEs are categorized using the Medical Dictionary for Regulatory Activities version 19.1 preferred terms.
Effectiveness data are presented from baseline to 26 months of active berotralstat treatment and are analyzed descriptively using mean (SEM), median, and/or mean change from baseline values. A month is defined as 28 days. Baseline values for HAE attack rates and on-demand medication use are based on data collected during the run-in period. Baseline values for AE-QoL and TSQM are based on Day 1 assessments prior to study drug administration. For patients who received berotralstat treatment following placebo, visits are adjusted according to the date of the first dose of active treatment. Percentages of symptom-free days are presented for study parts and symptom-free durations are presented for the entire study.
As HAE attacks in Part 3 were not confirmed by an expert reviewer, all HAE attack data presented here are based on adjusted patient-reported attacks, which were computed based on a list of programmable requirements (Supplemental Appendix). Adjusted patient-reported attack rates were defined as the total number of adjusted patient-reported attacks experienced during the corresponding month.
No inferential statistics are provided in this analysis because hypothesis testing was not prespecified in the protocol for Parts 2 and 3 of APeX-J. Therefore, no P values have been included for changes from baseline values of effectiveness measures.
All statistical analyses were performed using SAS software v9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Of the 19 patients in Part 1 of APeX-J, 17 patients entered Part 2 and received berotralstat treatment (Fig. 1). Of these, 9 belonged to the All 150 mg group and 8 belonged to the All 110 to 150 mg group. Demographic and baseline characteristics were broadly similar between the 2 groups, although the All 110 to 150 mg group had a higher mean age (Table 1).
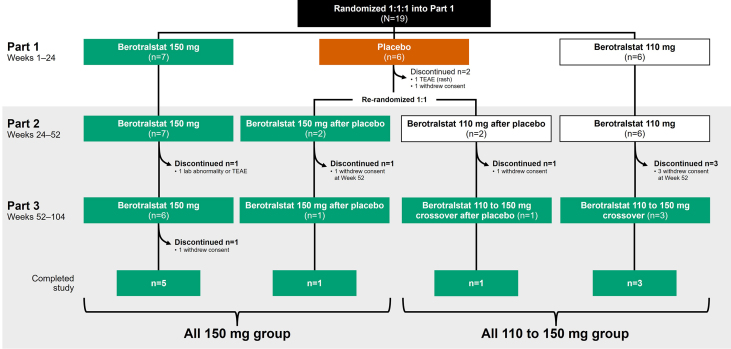
Fig. 1.
APeX-J patient disposition. Patients were considered to have completed the study if they continued study drug dosing to study Week 104. Specific reasons for withdrawal of consent were not provided. TEAE, treatment-emergent adverse event
Table 1.
Demographic and baseline characteristics of patients who entered Part 2 and Part 3 of APeX-J.
| Patients who entered Part 2 |
Patients who entered Part 3 |
|||||
|---|---|---|---|---|---|---|
| All 150 mg | All 110 to 150 mg | All patients | All 150 mg | All 110 to 150 mg | All patients | |
| n | 9 | 8 | 17 | 7 | 4 | 11 |
| Age, years | ||||||
| Mean (SD) | 39.3 (10.3) | 48.0 (13.8) | 43.4 (12.5) | 36.6 (8.9) | 51.3 (11.9) | 41.9 (12.0) |
| Range | 21–57 | 31–69 | 21–69 | 21–49 | 34–60 | 21–60 |
| Female sex, n (%) | 7 (77.8) | 7 (87.5) | 14 (82.4) | 6 (85.7) | 3 (75.0) | 9 (81.8) |
| Race, n (%) | ||||||
| Asian | 8 (88.9) | 8 (100) | 16 (94.1) | 6 (85.7) | 4 (100.0) | 10 (90.9) |
| Other | 1 (11.1) | 0 | 1 (5.9) | 1 (14.3) | 0 | 1 (9.1) |
| Mean (SD) weight at screening, kg | 62.2 (15.6) | 66.1 (10.3) | 64.0 (13.1) | 63.5 (17.1) | 66.2 (10.5) | 64.5 (14.5) |
| Mean (SD) BMI at screening, kg/m2 | 24.1 (6.5) | 25.7 (3.0) | 24.8 (5.1) | 24.8 (7.3) | 26.6 (1.7) | 25.5 (5.8) |
| Mean (SD) baseline attack ratea, attacks/month | 2.05 (0.95) | 2.75 (1.55) | 2.38 (1.28) | 1.98 (1.08) | 1.82 (0.99) | 1.92 (1.0) |
| Prior prophylactic treatmentb, n (%) | ||||||
| Any | 7 (77.8) | 6 (75.0) | 13 (76.5) | 5 (71.4) | 4 (100.0) | 9 (81.8) |
| Androgens | 3 (33.3) | 0 | 3 (17.6) | 2 (28.6) | 0 | 2 (18.2) |
| C1-INH | 1 (11.1) | 2 (25.0) | 3 (17.6) | 0 | 2 (50.0) | 2 (18.2) |
| Tranexamic acid | 5 (55.6) | 5 (62.5) | 10 (58.8) | 3 (42.9) | 3 (75.0) | 6 (54.5) |
Abbreviations: BMI, body mass index; C1-INH, C1-esterase inhibitor; SD, standard deviation.
Baseline attack rates are adjusted patient-reported attack rates.
Prior prophylactic treatments were not mutually exclusive
Patient disposition and discontinuations in Part 1 of APeX-J have already been described.22 Of the 17 patients who entered Part 2, 7 patients (41.2%) discontinued treatment before reaching Week 104. Most of these discontinuations (85.7%) occurred during Part 2 of the study or at Week 52, with one discontinuation (14.3%) occurring during Part 3. One discontinuation (14.3%) was due to a TEAE (Grade 4 increased hepatic enzyme); all others (85.7%) were attributed to patients withdrawing consent, for which specific reasons were not provided. The remaining 10 patients (58.8%) completed study drug dosing to the end of the study.
Safety
A summary of the TEAEs experienced by the 17 patients who entered Part 2 of APeX-J is shown in Table 2. Compared with Part 1, a lower percentage of these patients experienced any TEAE in Parts 2 or 3, including drug-related TEAEs and abdominal-related gastrointestinal TEAEs. As the TEAEs that occurred during Part 1 of the study have been described in detail elsewhere,22 here we focus on TEAEs that occurred from Week 24 onwards (Parts 2 and 3).
Table 2.
Summary of TEAEs experienced by patients who entered Part 2 of APeX-J.
| n (%) | TEAEs starting in Part 1 (Week 1 to Week 24) |
TEAEs starting in Parts 2 or 3 (Week 24 to Week 104) |
||
|---|---|---|---|---|
| All 150 mg | All 110 to 150 mg | All 150 mg | All 110 to 150 mg | |
| n | 9 | 8 | 9 | 8 |
| Any TEAE | 9 (100) | 8 (100) | 7 (77.8) | 7 (87.5) |
| Any drug-related TEAE | 3 (33.3) | 2 (25.0) | 1 (11.1) | 0 |
| Any TESAE | 0 | 1 (12.5) | 1 (11.1) | 1 (12.5) |
| Any drug-related TESAE | 0 | 0 | 0 | 0 |
| Any Grade 3 or 4 TEAE | 0 | 1 (12.5) | 2 (22.2) | 0 |
| Any drug-related Grade 3 or 4 TEAE | 0 | 0 | 1 (11.1) | 0 |
| Any TEAE leading to interruption of study druga | 2 (22.2) | 1 (12.5) | 0 | 0 |
| Any TEAE leading to discontinuation of study drug | 0 | 0 | 1 (11.1) | 0 |
| Any investigator-identified rashb | 2 (22.2) | 0 | 0 | 1 (12.5) |
| Any abdominal-related gastrointestinal TEAE | 4 (44.4) | 3 (37.5) | 1 (11.1) | 1 (12.5) |
| Any abdominal-related gastrointestinal TEAE leading to study drug discontinuation | 0 | 0 | 0 | 0 |
| Any drug-related abdominal-related gastrointestinal TEAE | 2 (22.2) | 2 (25.0) | 0 | 0 |
Abbreviations: TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event.
Study drug interruption is when study drug is temporarily stopped and then resumed.
Investigator-identified rash was a TEAE of special interest
The median (range) duration of exposure to berotralstat in Parts 2 and 3 was 554 (77–650) days. During this period, 14 patients (82.4%) experienced a TEAE, with the most common being nasopharyngitis, abdominal pain, cystitis, influenza, and vertigo (Table 3).
Table 3.
The most common TEAEs reported from Week 24 to Week 104.
| N (%) | All 150 mg | All 110 to 150 mg | All patients |
|---|---|---|---|
| n | 9 | 8 | 17 |
| Nasopharyngitis | 2 (22.2) | 1 (12.5) | 3 (17.6) |
| Abdominal pain | 1 (11.1) | 1 (12.5) | 2 (11.8) |
| Cystitis | 1 (11.1) | 1 (12.5) | 2 (11.8) |
| Influenza | 0 | 2 (25.0) | 2 (11.8) |
| Vertigo | 1 (11.1) | 1 (12.5) | 2 (11.8) |
The most common TEAEs are those that occurred in at least 10% of patients who entered Part 2 of APeX-J (n = 17).
Abbreviation: TEAE, treatment-emergent adverse event
One patient (5.9%) experienced a drug-related TEAE. This TEAE, Grade 4 increased hepatic enzyme in a patient from the All 150 mg group, was deemed possibly related to berotralstat. The event started during Week 34 of the study (Part 2) and caused the patient to discontinue treatment the following day. No other patient required interruption of berotralstat during Parts 2 or 3.
Three TESAEs occurred in 2 patients (11.8%) during Parts 2 and 3, none of which were deemed to be drug-related. One patient in the All 150 mg group experienced an ankle fracture and a fibula fracture and 1 patient in the All 110 to 150 mg group experienced a serious HAE event (Supplemental Appendix).
Most TEAEs were mild or moderate, with no deaths reported. Grade 3 or 4 TEAEs occurred in 2 patients (11.8%), both of whom were from the All 150 mg group. These TEAEs were 1 episode of Grade 3 dental caries, and as mentioned previously, 1 episode of Grade 4 increased hepatic enzyme (possibly drug-related).
Two patients (11.8%), 1 from each group, experienced an abdominal-related gastrointestinal TEAE, but the events were not deemed to be drug-related.
Grade 3 or 4 laboratory abnormalities occurred in 2 patients (11.8%). The patient from the All 150 mg group who experienced increased hepatic enzyme experienced elevations in alanine aminotransferase (Grade 4 [716 U/L]), aspartate aminotransferase (Grade 3 [144 U/L] and Grade 4 [289 U/L]), and glutamyl transferase (Grade 3 [189 U/L]). Another patient from the All 110 to 150 mg group experienced Grade 3 (155 U/L) glutamyl transferase elevation (not drug-related). Neither of these patients had previously been treated with androgens.
No clinically meaningful changes were observed in the vital signs of either treatment group.
Effectiveness
HAE attack rates
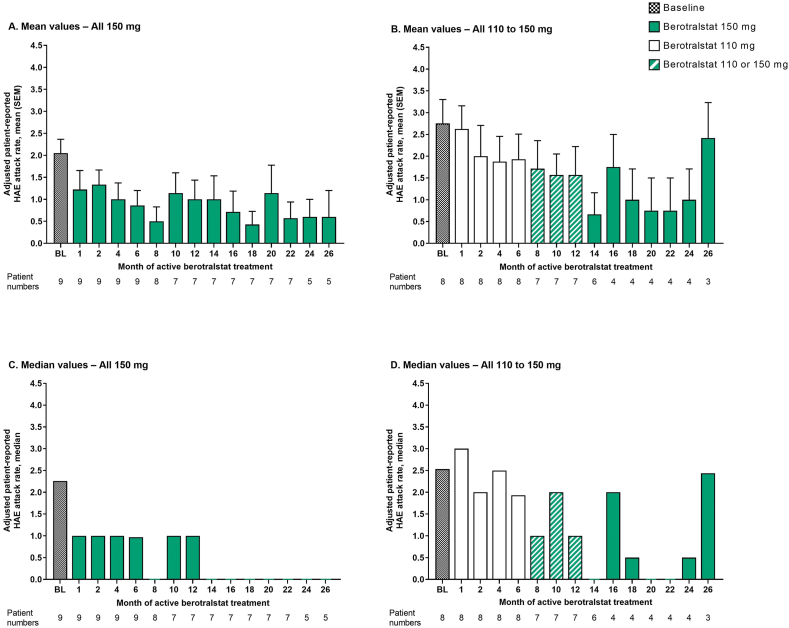
In both treatment groups, adjusted patient-reported attack rates showed a decreasing trend over time despite some fluctuations, and improvements were observed after just 1 month of berotralstat treatment (Fig. 2A and B). The mean attack rates in the All 150 mg group were generally lower than those in the All 110 to 150 mg group. For patients in the All 150 mg group who completed 26 months of active berotralstat treatment (n = 5), the mean (SEM) change in attack rate from baseline was −1.15 (0.09) attacks/month at Month 26 (mean percentage reduction: 84.8%; Supplemental Figure 2).
Fig. 2.
Adjusted patient-reported HAE attack rates from baseline to 26 months of active berotralstat treatment for patients who entered Part 2 of APeX-J (n = 17). A. Mean (SEM) values for the All 150 mg group, B. Mean (SEM) values for the All 110 to 150 mg group, C. Median values for the All 150 mg group, and D. Median values for the All 110 to 150 mg group. Baseline attack rates are based on the number of attacks that occurred during the 56-day run-in period before study entry. For patients who received berotralstat following placebo, months are adjusted according to the date of the first berotralstat dose. Two patients in the All 110 to 150 mg group who experienced attacks during the last month were not on study for the full 28 days of the month and, as such, their attack rate for this month was calculated using a denominator smaller than 28 days. BL, baseline; HAE, hereditary angioedema; SEM, standard error of the mean
Although the monthly attack rate in the All 110 to 150 mg group during the 26th month of berotralstat treatment was similar to the attack rate in this group at baseline, attack rates in the previous months were considerably lower.
Median attack rates in the All 150 mg group were ≤1 attack/month at all time points post baseline, and during the 26th month of berotralstat treatment the median rate was zero (Fig. 2C). The median rates for the All 110 to 150 mg group are shown in Fig. 2D.
Symptom-free days
Across all parts of the study, the percentage of symptom-free days was high in both treatment groups. In the All 150 mg group, the percentage remained stable (around 90%) throughout the study. Conversely, in the All 110 to 150 mg group, the percentage increased over time. The largest increase in this group was observed between Parts 2 and 3, coinciding with the switch from berotralstat 110 to 150 mg. In Part 3, both groups had a similar percentage of symptom-free days (∼90%).
In the All 150 mg group, the mean (SEM) average duration of symptom-free periods was 68.4 (23.9) days. The maximum symptom-free duration in this group was 339 days.
On-demand medication use
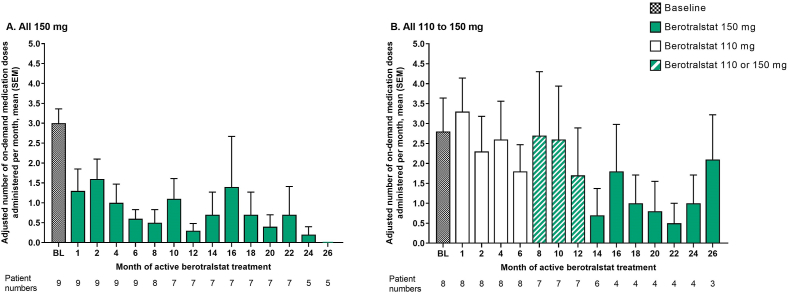
In both treatment groups, use of on-demand medication for HAE attacks tended to decrease over time, with reductions being more pronounced in the All 150 mg group (Fig. 3A and B). During the 26th month of treatment, the All 150 mg group and the All 110 to 150 mg group administered a mean (SEM) of 0.0 (0.0) and 2.1 (1.1) doses/month, respectively.
Fig. 3.
Adjusted number of on-demand HAE attack medication doses administered per month from baseline to 26 months of active berotralstat treatment for patients who entered Part 2 of APeX-J (n = 17). Mean (SEM) values for the A.All 150 mg group and B.All 110 to 150 mg group. Baseline rates are based on medication use during the run-in period before study entry and are adjusted for the length of a month. For patients who received berotralstat following placebo, months are adjusted according to the date of the first berotralstat dose. BL, baseline; HAE, hereditary angioedema; SEM, standard error of the mean
For patients in the All 150 mg group who completed 26 months of active berotralstat treatment (n = 5), the mean (SEM) change in on-demand medication use from baseline was −2.8 (0.6) doses/month at Month 26, representing a mean percentage reduction of 100% (Supplemental Figure 3).
Quality of life
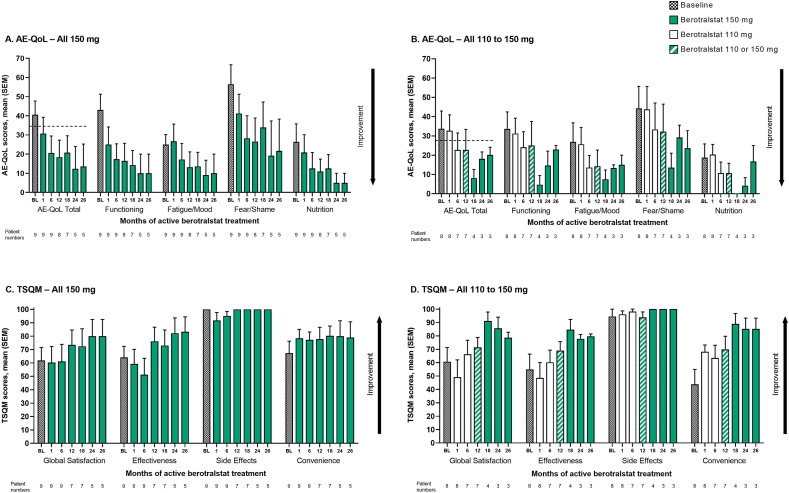
QoL (as assessed using AE-QoL) improved over time in both treatment groups (Fig. 4A and B). Although improvements were generally more pronounced and more rapid in the All 150 mg group than in the All 110 to 150 mg group, improvements versus baseline were seen across all AE-QoL domains in both groups after 26 months of berotralstat treatment.
Fig. 4.
Patient-reported outcomes from baseline to 26 months of active berotralstat treatment for patients who entered Part 2 of APeX-J (n = 17). Mean (SEM) AE-QoL and TSQM scores for the All 150 mg group (A. and C.) and the All 110 to 150 mg group (B. and D.). Baseline values represent values on Day 1 before study drug administration. For TSQM, baseline values are based on the patient's reflection of the last treatment received. For patients who received berotralstat following placebo, months are adjusted according to the date of the first berotralstat dose. The black dotted line represents the MCID for the AE-QoL Total score. AE-QoL, Angioedema Quality of Life Questionnaire; BL, baseline; MCID, minimal clinically important difference; SEM, standard error of the mean; TSQM, Treatment Satisfaction Questionnaire for Medication
In both groups, improvements in mean AE-QoL Total scores after 26 months of treatment also exceeded the minimal clinically important difference (MCID) threshold of 6 points versus baseline, indicating that improvements were clinically meaningful.
For patients in the All 150 mg group who completed 26 months of berotralstat treatment (n = 5), mean (SEM) change from baseline was −32.65 (13.78) points in the AE-QoL Total score, −40.00 (14.87) points in the Functioning domain, −16.00 (10.42) points in the Fatigue/Mood domain, −41.67 (17.33) points in the Fear/Shame domain, and −32.50 (14.58) points in the Nutrition domain (Supplemental Figure 4).
Treatment satisfaction
Overall, treatment satisfaction (as assessed using the TSQM) improved with berotralstat prophylaxis (Fig. 4C and D). In both treatment groups, improvements were observed in the Effectiveness and Convenience domains and in the overall Global Satisfaction score. For the Side Effects domain, mean scores were high at baseline (100.0 in the All 150 mg group and 94.5 in the All 110 to 150 mg group) and, despite slight fluctuations during the first 6–12 months, high scores were maintained over 26 months.
For patients in the All 150 mg group who completed 26 months of berotralstat treatment (n = 5), mean (SEM) change from baseline was +14.30 (11.95) points in the Global Satisfaction score, +15.60 (7.74) points in the Effectiveness domain, 0.00 (0.00) points in the Side Effects domain, and +15.60 (10.45) points in the Convenience domain (Supplemental Figure 5).
Discussion
In Part 1 of APeX-J, treatment with berotralstat significantly reduced the rate of HAE attacks versus placebo (P = 0.003) and demonstrated a favorable benefit-to-risk profile.22 Treatment through the 24 weeks of Part 1 was completed by 95% of the patients, with the only patient who discontinued treatment belonging to the placebo group. In Parts 2 and 3 presented here, long-term treatment of 17 patients with berotralstat raised no new safety signals compared with previously reported studies,21,27 and further reduced attack rates and on-demand medication use compared with what was observed after 6 months in Part 1.22 Additional improvements in QoL and treatment satisfaction were also observed with long-term treatment for those patients who remained on study. In Parts 2 and 3, one patient experienced drug-related increased hepatic enzyme. The study was completed by 10 patients, with most discontinuations due to withdrawal of consent. Overall, results from APeX-J are comparable with results from the APeX-2 and APeX-S studies,21,27, 28, 29 and thereby reinforce findings in an underrepresented population that was not enrolled in previous APeX studies.
No drug-related TESAE occurred during any part of APeX-J, and one berotralstat-treated patient discontinued because of a TEAE. The percentages of patients experiencing any TEAE and any drug-related TEAE with berotralstat were lower in Parts 2 and 3 than in Part 1, suggesting long-term tolerability in patients who did not discontinue treatment. Long-term tolerability is further supported by the fact that no TEAEs led to interruption of treatment in Parts 2 or 3, and TSQM scores for the Side Effects domain were consistently high from baseline to 26 months. This latter observation also suggests no worsening of side effects compared with prior treatment.
Mean HAE attack rates tended to decline over time and were consistently lower than at baseline for both treatment groups. As previously mentioned, attack rates were generally higher in the All 110 to 150 mg group than in the All 150 mg group. However, patients in the All 110 to 150 mg group had a higher mean attack rate at baseline and were also treated with a lower dose of berotralstat for 7–13 months before being transitioned to the 150 mg dose. Indeed, previous studies have shown that the 150 mg dose is more effective.21
In the All 110 to 150 mg group, the attack rate during Month 26 of berotralstat treatment was considerably higher than in the previous months, and a similar trend was observed for the other effectiveness endpoints. This may be attributed to the fact that only 3 patients were being treated in this group at this last time point and, as such, any fluctuation in the value of 1 patient could markedly affect the overall value for the group. Indeed, the attack rate of 1 patient in this group jumped from 1.00 to 3.82 attacks/month at this time point. This patient, who completed their last study visit, was on study for 22 out of 28 days of the last month and, as such, their attack rate for this month was calculated using the number of days they were on therapy during the month (ie, the rate was calculated as follows: [3 adjusted patient-reported attacks]/[22 days on study in last month] × 28 days/month). If this patient's last visit had occurred later in that month without any additional attacks, their attack rate would have been lower. Similarly, another patient in this group was also only on study for part of the last month (23 days).
In both treatment groups, reductions in attack rates were accompanied by reductions in on-demand medication use. Reductions in the rate of on-demand medication use versus baseline were more pronounced than reductions in attack rates, possibly suggesting that even though patients were still having attacks, the attacks were less severe. Although one goal of long-term prophylaxis is to achieve disease control by preventing attacks, reducing the severity of HAE attacks can also contribute to lessening the disease burden for patients and reduces costs.18,30,31
HAE is known to greatly affect patient QoL; it not only places an emotional burden on patients but can also lead to reduced function and productivity.32 For instance, a patient-reported outcome study in Japan found that 60% of patients with HAE felt that it affected their daily activities.7 Importantly, in APeX-J, long-term prophylaxis with berotralstat led to improvements (versus baseline) in all AE-QoL domains for both treatment groups, with the largest improvements observed in the Fear/Shame domain.
Additionally, improvements in treatment satisfaction were observed in both patient groups, including improvements in Global Satisfaction and in the Effectiveness and Convenience domains. Of note, whereas improvements in most effectiveness outcomes were more pronounced in the All 150 mg group than in the All 110 to 150 mg group, improvements in treatment satisfaction were similar across both groups. A survey study of 75 patients with HAE in the United States found that 19% of the patients reported skipping their medication because of the inconvenience of injections or infusions.19 In this same study, which was conducted in 2020 before the approval of berotralstat, 20% of patients reported that the administration time associated with their current prophylactic treatment had a negative impact on their lives, and most patients desired therapies with a simpler administration route.19 In fact, another survey of patients with HAE in the United States, which was conducted in 2018, found that 98% of patients on prophylactic HAE medication would have preferred an oral treatment option.33 Therefore, as an approved oral prophylactic therapy for HAE in Japan,34 berotralstat could be an important treatment option versus approved parenteral therapies.
An important limitation of APeX-J was that a small number of patients enrolled, and even fewer patients were treated through to 26 months. Despite low patient numbers in Parts 2 and 3 of APeX-J resulting in fluctuations of aggregate data and large levels of variability, the data provide insight into the long-term safety and effectiveness of berotralstat in this patient population. Furthermore, this long-term data is consistent with the long-term safety and effectiveness data from the larger patient population of APeX-2,29 suggesting that these data sets could be representative of the wider HAE population. Another limitation of this study is that results from long-term open-label extensions can be associated with selection bias because patients who respond well to treatment tend to continue. Other limitations were that attack data, despite being adjusted as described in the methods, were patient-reported rather than confirmed by an expert investigator, no placebo comparator or control arm were included in Parts 2 and 3, and no hypothesis testing was prespecified in the protocol. Furthermore, because effectiveness measures were analyzed according to the date of the first berotralstat dose, baseline values are used for comparison. This means that for patients who were on placebo in Part 1 and crossed over to berotralstat in Parts 2 and 3, the change from placebo to active berotralstat treatment was not evaluated. Additional data on long-term effectiveness could be obtained in future studies by assessing disease control using the Angioedema Control Test (AECT) and/or the Angioedema Activity Score (AAS).35,36
In patients from Japan, long-term prophylaxis with berotralstat resulted in no drug-related TESAEs and raised no new safety signals. Despite some fluctuations in HAE attack rates, sustained improvements in on-demand medication use, QoL, and treatment satisfaction provide strong evidence for the benefits of its long-term use. Altogether, these results are consistent with other berotralstat studies and suggest that berotralstat is a convenient and effective treatment option for long-term prophylaxis in patients with HAE in Japan.
Abbreviations
AAS, Angioedema Activity Score; AECT, Angioedema Control Test; AE-QoL, Angioedema Quality of Life Questionnaire; BL, baseline; BMI, body mass index; C1-INH, C1 inhibitor; HAE, hereditary angioedema; HAE-C1-INH, HAE due to C1-INH deficiency; IEC, independent ethics committee; IRB, institutional review board; MCID, minimal clinically important difference; pdC1-INH, plasma-derived C1-INH; QD, once-daily; QoL, quality of life; SD, standard deviation; SEM, standard error of the mean; TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event; TSQM, Treatment Satisfaction Questionnaire for Medication.
Funding
This study was sponsored by BioCryst Pharmaceuticals, Inc., Durham, NC, United States. The authors declare that funding from BioCryst Pharmaceuticals, Inc., Durham, NC, United States, was also provided for medical writing support, editorial assistance, and article processing charges. The sponsor was involved in designing the study and in analyzing and interpreting the data together with the other authors.
Ethics statement
The APeX-J study was designed, performed, and monitored in accordance with Good Clinical Practice guidelines according to the International Council for Harmonisation and in compliance with the Declaration of Helsinki and regulatory requirements set forth by the Japanese Pharmaceuticals and Medical Devices Agency and other applicable Japanese laws. The protocol, amendments, informed consent forms, and other relevant study documentation were approved by institutional review boards and independent ethics committees. Following marketing authorization of berotralstat in Japan, this study was conducted in compliance with post-marketing regulations. Trial registration: 23-00211r1 23-00145r1 23-00169r2.
Author contributions
MH, PC, and IO were involved in the conception and design of the study. DH, MH, TF, KK, EM, SM, YSa, YSu, and IO were study investigators responsible for the recruitment of patients and acquisition of data. DT was responsible for the statistical analyses. All authors contributed to the interpretation of the data and were involved in writing or critically reviewing the drafts of the manuscript. All authors approved the final version of the manuscript for publication.
Data availability statement
Individual participant data will not be made available.
Submission declaration
The authors declare that this manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server. The authors agree to publish this work in World Allergy Organization Journal.
Declaration of competing interest
DH received consulting fees from Takeda; payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Takeda, CSL Behring, and Torii Pharmaceutical Company Ltd; participated in a Data Safety Monitoring Board or Advisory Board for Kalvista; and acted in a leadership or fiduciary role for the Diagnostic Consortium to Advance the Ecosystem for Hereditary Angioedema. MH received consulting fees from BioCryst Pharmaceuticals, CSL Behring, Kalvista, Pharvaris, and Shire/Takeda; and received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from CSL Behring, Takeda, and Torii Pharmaceutical Company Ltd. DTJ received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from BioCryst, Takeda, CSL Behring, and Pharming; and acted in a leadership or fiduciary for the American Academy of Allergy, Asthma & Immunology. IO received honoraria as a speaker/advisor, and/or participated in advisory boards for CSL Behring, Takeda/Shire, Torii Pharmaceutical Company Ltd, and BioCryst Pharmaceuticals. PC, DTJ, DT, and BD are employees of, and own stock in, BioCryst Pharmaceuticals. DT owns stock in Amgen Inc. TF, KK, EM, SM, YSa, and YSu have nothing to disclose.
Acknowledgements
The authors would like to thank all patients, study investigators, and site staff who participated in APeX-J. The authors also thank Tony Ferrar, MSc, ISMPP CMPP™, of Porterhouse Medical Group, Reading, United Kingdom, for providing medical writing support and editorial assistance, which was funded by BioCryst Pharmaceuticals, Inc., Durham, NC, United States, in accordance with Good Publication Practice guidelines. BioCryst Pharmaceuticals, Inc., Durham, NC, United States, sponsored this study and paid for article processing charges. Statistical programming support was provided by Amy Huber, MPH, of BioCryst Pharmaceuticals, Inc.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2024.100882.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zafra H. Hereditary angioedema: a review. Wis Med J. 2022;121(1):48–53. [PubMed] [Google Scholar]
- 2.Proper S.P., Lavery W.J., Bernstein J.A. Definition and classification of hereditary angioedema. Allergy Asthma Proc. 2020;41(Suppl 1):S03–S07. doi: 10.2500/aap.2020.41.200040. [DOI] [PubMed] [Google Scholar]
- 3.Busse P.J., Christiansen S.C., Riedl M.A., et al. US HAEA medical advisory board 2020 guidelines for the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9(1):132–150.e3. doi: 10.1016/j.jaip.2020.08.046. [DOI] [PubMed] [Google Scholar]
- 4.Ohsawa I., Honda D., Hisada A., et al. Clinical features of hereditary and mast cell-mediated angioedema focusing on the differential diagnosis in Japanese patients. Intern Med. 2018;57(3):319–324. doi: 10.2169/internalmedicine.8624-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumry W.R., Castaldo A.J., Vernon M.K., Blaustein M.B., Wilson D.A., Horn P.T. The humanistic burden of hereditary angioedema: impact on health-related quality of life, productivity, and depression. Allergy Asthma Proc. 2010;31(5):407–414. doi: 10.2500/aap.2010.31.3394. [DOI] [PubMed] [Google Scholar]
- 6.Caballero T., Aygören-Pürsün E., Bygum A., et al. The humanistic burden of hereditary angioedema: results from the Burden of Illness Study in Europe. Allergy Asthma Proc. 2014;35(1):47–53. doi: 10.2500/aap.2013.34.3685. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto B., Honda D., Ohsawa I., et al. Burden of illness seen in hereditary angioedema in Japanese patients: results from a patient reported outcome survey. Intractable Rare Dis Res. 2023;12(1):35–44. doi: 10.5582/irdr.2022.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bork K., Meng G., Staubach P., Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):267–274. doi: 10.1016/j.amjmed.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 9.Azmy V., Brooks J.P., Hsu F.I. Clinical presentation of hereditary angioedema. Allergy Asthma Proc. 2020;41(Suppl 1):S18–S21. doi: 10.2500/aap.2020.41.200065. [DOI] [PubMed] [Google Scholar]
- 10.Raasch J., Glaum M.C., O'Connor M. The multifactorial impact of receiving a hereditary angioedema diagnosis. World Allergy Organ J. 2023;16(6) doi: 10.1016/j.waojou.2023.100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanichelli A., Magerl M., Longhurst H., Fabien V., Maurer M. Hereditary angioedema with C1 inhibitor deficiency: delay in diagnosis in Europe. Allergy Asthma Clin Immunol. 2013;9(1):29. doi: 10.1186/1710-1492-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurer M., Magerl M., Betschel S., et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77(7):1961–1990. doi: 10.1111/all.15214. [DOI] [PubMed] [Google Scholar]
- 13.Maurer M., Magerl M., Betschel S., et al. The international WAO/EAACI guideline for the management of hereditary angioedema - the 2021 revision and update. World Allergy Organ J. 2022;15(3) doi: 10.1016/j.waojou.2022.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HAE International. TAKHZYRO® approved in Japan. https://haei.org/takhzyro-approved-in-japan/. Accessed 19 September 2023.
- 15.HAE International. Approval of Orladeyo in Japan for prophylactic HAE treatment. https://haei.org/approval-of-orladeyo-in-japan-for-prophylactic-hae-treatment/. Accessed 19 September 2023..
- 16.HAE International. Berinert® S.C. Injection 2000 authorized in Japan. https://haei.org/berinert-s-c-injection-2000-authorized-in-japan/. Accessed 19 September 2023..
- 17.Horiuchi T., Ohsawa I., Imai M., et al. Hereditary angioedema (HAE) guidelines – revised 2023 edition. Journal of The Japanese Association for Complement Research. 2023;60(2):103–131. [Google Scholar]
- 18.Bork K., Anderson J.T., Caballero T., et al. Assessment and management of disease burden and quality of life in patients with hereditary angioedema: a consensus report. Allergy Asthma Clin Immunol. 2021;17(1):40. doi: 10.1186/s13223-021-00537-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radojicic C., Riedl M.A., Craig T.J., et al. Patient perspectives on the treatment burden of injectable medication for hereditary angioedema. Allergy Asthma Proc. 2021;42(3):S4–S10. doi: 10.2500/aap.2021.42.210025. [DOI] [PubMed] [Google Scholar]
- 20.Lee A. Berotralstat: first approval. Drugs. 2021;81(3):405–409. doi: 10.1007/s40265-021-01475-4. [DOI] [PubMed] [Google Scholar]
- 21.Zuraw B., Lumry W.R., Johnston D.T., et al. Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: a randomized, double-blind, placebo-controlled phase 3 trial. J Allergy Clin Immunol. 2021;148(1):164–172. doi: 10.1016/j.jaci.2020.10.015. e9. [DOI] [PubMed] [Google Scholar]
- 22.Ohsawa I., Honda D., Suzuki Y., et al. Oral berotralstat for the prophylaxis of hereditary angioedema attacks in patients in Japan: a phase 3 randomized trial. Allergy. 2021;76(6):1789–1799. doi: 10.1111/all.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weller K., Groffik A., Magerl M., et al. Development and construct validation of the angioedema quality of life questionnaire. Allergy. 2012;67(10):1289–1298. doi: 10.1111/all.12007. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson M.J., Sinha A., Hass S.L., et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcome. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulthanan K., Chularojanamontri L., Rujitharanawong C., Weerasubpong P., Maurer M., Weller K. Angioedema quality of life questionnaire (AE-QoL) - interpretability and sensitivity to change. Health Qual Life Outcome. 2019;17(1):160. doi: 10.1186/s12955-019-1229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller K., Magerl M., Peveling-Oberhag A., Martus P., Staubach P., Maurer M. The Angioedema Quality of Life Questionnaire (AE-QoL) - assessment of sensitivity to change and minimal clinically important difference. Allergy. 2016;71(8):1203–1209. doi: 10.1111/all.12900. [DOI] [PubMed] [Google Scholar]
- 27.Farkas H., Stobiecki M., Peter J., et al. Long-term safety and effectiveness of berotralstat for hereditary angioedema: the open-label APeX-S study. Clin Transl Allergy. 2021;11(4) doi: 10.1002/clt2.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedner H.J., Aygören-Pürsün E., Bernstein J., et al. Randomized trial of the efficacy and safety of berotralstat (BCX7353) as an oral prophylactic therapy for hereditary angioedema: results of APeX-2 through 48 weeks (part 2) J Allergy Clin Immunol Pract. 2021;9(6):2305–2314. doi: 10.1016/j.jaip.2021.03.057. e4. [DOI] [PubMed] [Google Scholar]
- 29.Kiani-Alikhan S., Gower R., Craig T., et al. Once-daily oral berotralstat for long-term prophylaxis of hereditary angioedema: the open-label extension of the APeX-2 randomized trial. J Allergy Clin Immunol Pract. 2024 doi: 10.1016/j.jaip.2023.12.019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Wilson D.A., Bork K., Shea E.P., Rentz A.M., Blaustein M.B., Pullman W.E. Economic costs associated with acute attacks and long-term management of hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104(4):314–320. doi: 10.1016/j.anai.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Nordenfelt P., Dawson S., Wahlgren C.F., Lindfors A., Mallbris L., Björkander J. Quantifying the burden of disease and perceived health state in patients with hereditary angioedema in Sweden. Allergy Asthma Proc. 2014;35(2):185–190. doi: 10.2500/aap.2014.35.3738. [DOI] [PubMed] [Google Scholar]
- 32.Lumry W.R., Settipane R.A. Hereditary angioedema: epidemiology and burden of disease. Allergy Asthma Proc. 2020;41(Suppl 1):S08–S13. doi: 10.2500/aap.2020.41.200050. [DOI] [PubMed] [Google Scholar]
- 33.Geba D., Mohd Sani J., Gascon M., Hahn R., Aggarwal K., Rosselli J. Hereditary angioedema patients would prefer newer-generation oral prophylaxis. J Drug Assess. 2021;10(1):51–56. doi: 10.1080/21556660.2020.1863699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.BioCryst. BioCryst announces approval of Japanese NHI price listing of ORLADEYO™ (berotralstat) for prophylactic treatment of hereditary angioedema. https://www.globenewswire.com/fr/news-release/2021/04/14/2209965/29446/en/BioCryst-Announces-Approval-of-Japanese-NHI-Price-Listing-of-ORLADEYO-berotralstat-for-Prophylactic-Treatment-of-Hereditary-Angioedema.html. Accessed 19 September 2023..
- 35.Kulthanan K., Chularojanamontri L., Rujitharanawong C., Weerasubpong P., Weller K., Maurer M. Angioedema activity score (AAS): a valid and reliable tool to use in Asian patients. BioMed Res Int. 2019;2019 doi: 10.1155/2019/9157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weller K., Donoso T., Magerl M., et al. Development of the Angioedema Control Test-A patient-reported outcome measure that assesses disease control in patients with recurrent angioedema. Allergy. 2020;75(5):1165–1177. doi: 10.1111/all.14144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data will not be made available.