Abstract
Background
Globally, no trial data are available on head-to-head comparison between 10 mg/kg and 25/35 mg/kg rifampicin in treating pulmonary tuberculosis during study initiation.
Methods
A multicentric, phase IIb randomized trial recruited 333 new culture-positive, drug-sensitive adult patients with pulmonary tuberculosis to compare safety and efficacy of high-dose rifampicin (R25/R35), against conventional dose (R10) given daily for 8 weeks followed by standard doses for 16 weeks. Main outcomes were treatment-emergent grade 3/4 adverse events (AEs) and time-to-culture conversion in liquid media, assessed by division of AIDS system for grading the severity of adverse events division of AIDS criteria and Kaplan-Meier methods.
Results
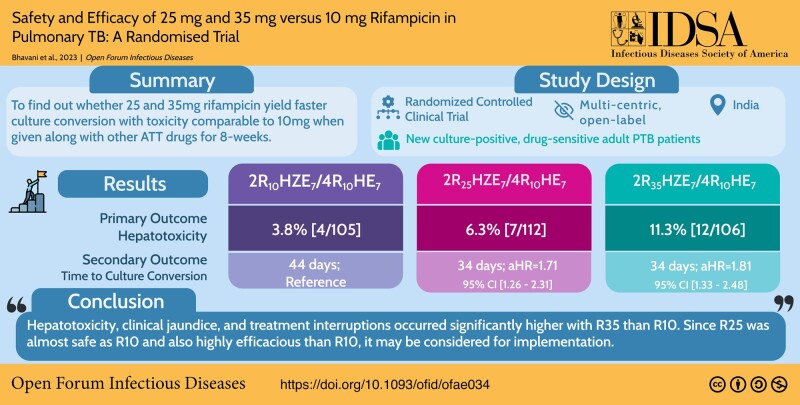
In a modified intention-to-treat population of 323 patients (R10: 105/R25: 112/R35: 106), grade 3/4 AEs were reported in 34 patients (R10: 9.5% [10/105], R25: 9.8% [11/112], R35: 12.3% [13/106]) during the intensive phase. Among 23 patients (R10: 3.8% [4/105], R25: 6.3% [7/112], R35: 11.3% [12/106]) with grade 3/4 hepatotoxicity, 15 (R10: 1.9% [2/105], R25: 3.6% [4/112], R35: 8.5% [9/106]) had grade 3/4 hyperbilirubinemia and 9 patients (R10: 1.0% [1/105], R25: 0.9% [1/112], R35: 6.6% [7/106]) developed clinical jaundice. Significant differences observed only between R10 and R35 with hepatotoxicity (P = .039), hyperbilirubinemia (P = .031), clinical jaundice (P = .032), and treatment interruption (P = .039). Eighteen serious AEs and 6 deaths (R10: 3/R25: 1/R35: 2) occurred during study period. Time to stable culture conversion in liquid media was faster in R25 (adjusted hazard ratio, 1.71; 95% confidence interval [CI], 1.26–2.31 [solid: 1.97; 95% CI, 1.46–2.67]) and R35 (1.81; 95% CI, 1.33–2.48 [solid: 2.24; 95% CI, 1.64–3.06]), than R10 (34 vs 44 days). R25 had no failure/relapse.
Conclusions
Hepatotoxicity, clinical jaundice, and treatment interruptions occurred significantly higher with R35 than R10. Because R25 was comparably safe as R10 and also highly efficacious than R10, it may be considered for implementation.
Clinical Trials Registration. CTRI/2017/12/010951.
Keywords: efficacy, high-dose, rifampicin, safety, time-to-culture-conversion
Our randomized clinical trial compared safety and efficacy of high-dose rifampicin (R25/R35) against conventional dose (R10). We found that R35 was more toxic than R10. R25 was equally safe and more efficacious than R10; hence, it may be implemented for treatment shortening of drug-susceptible pulmonary tuberculosis.
Graphical Abstract
Graphical abstract.
The standard antitubercular treatment (ATT) regimen for pulmonary tuberculosis (PTB) in India includes isoniazid, rifampicin (R), ethambutol, and pyrazinamide for the first 2 months, followed by isoniazid, rifampicin, and ethambutol for the next 4 months. Lengthy duration and adverse effects that affect drug compliance may contribute to unfavorable outcomes [1] and delay in achieving World Health Organization End TB Strategy targets. Hence, there is need for modification of existing drug regimens to achieve early bacteriologic conversion and to reduce ATT-induced toxicity. Among the few trials available with respect to a short-course regimen containing rifamycins, a trial by Dorman et al. (2021) has shown noninferiority for a 4-month rifapentine plus moxifloxacin regimen; another trial by Jindani et al. (2023) has not shown noninferiority for a 4-month high-dose rifampicin-containing regimen [2–5].
Use of high-dosage rifampicin to improve treatment outcomes appears more cost-effective than using rifapentine. Rifampicin is a potential treatment-shortening agent because dose escalation from 10 to 35 mg/kg/day is safe and leads to a rapid fall in both dormant and actively replicating forms of Mycobacterium tuberculosis and helps in faster culture conversion [6–9]. The incidence of hepatitis in patients with PTB treated with rifampicin-containing 4-drug regimen is reported to range from 2% to 8% [10–12].
Even though safety and efficacy of higher doses of rifampicin have been studied in recent years [13–18], there is no trial available that compares high-dose rifampicin (R25/R35) head to head with a conventional dose (R10-containing) regimen.
Hence, the aim of the present study is to conduct a randomized clinical trial (RCT) comparing the safety, tolerability, and efficacy of high-dose rifampicin, 25 or 35 mg/kg/day, versus 10 mg/kg/day (control arm) given daily along with other first-line drugs.
METHODS
Study Design
A multicentric, open-label, parallel arm RCT was conducted at 5 clinical trial sites across India in Chennai, New Delhi, Hyderabad, Chandigarh, and Lucknow. The respective institutional ethics committees of all study sites reviewed and approved the study protocol. The primary objective was to compare the safety and tolerability of high-dose rifampicin, 25 or 35 mg/kg/day, versus 10 mg/kg/day given daily along with other first-line drugs during the first 8 weeks of a 24-week treatment schedule of new sputum smear and culture-positive drug-sensitive patients with PTB. The secondary objectives were to (1) compare the time to culture conversion and (2) compare the proportion of patients with sputum culture conversion at week 8 and at the end of treatment between high-dose and control arms. The trial was prospectively registered in CTRI in December 2017, initiated in January 2018, and recruitment was completed by November 2019. Posttreatment 12-month follow-up was completed in May 2021.
Study Participants
New smear-positive adult patients with PTB, aged 18–60 years, were enrolled based on predefined inclusion and exclusion criteria after obtaining written informed consent from all patients. Baseline screening included sputum smear examination by fluorescence microscopy, culture by both liquid media (Mycobacteria Growth Indicator Tube [MGIT] 960) and solid media (Lowenstein-Jensen), chest radiography, hemogram, renal/liver function tests, serology for human immunodeficiency virus (HIV), hepatitis B/C, and urine pregnancy test for women. Patients who had at least 2 sputum smear samples positive for tubercle bacilli were included. Patients with history of psychiatric illness or drug abuse, patients receiving insulin, liver enzymes >2.5 upper limit of normal, alcohol dependence that could interfere with treatment adherence, seropositive for HIV/hepatitis B or C, baseline resistance to isoniazid, during screening, were excluded. Detailed eligibility criteria are available in the protocol provided in the Supplementary Data.
Patients with positive smear microscopy and confirmed to be rifampicin sensitive by X-pert M tuberculosis /rifampicin and line probe assay were randomized to study regimens. Clinical and bacteriological progress were assessed weekly for 2 months, monthly for 12 months, and every 3 months from 13 to 18 months. Two sputa were collected for smear and culture examination at every visit until 12th month, at 15th month, and at end of follow-up. MGIT drug susceptibility testing was done at baseline, week 4, month 4, and during failure/relapse.
Randomization and Stratification
Centralized randomization was done and patients were randomly allocated in the ratio of 1:1:1 to 1 of the study regimens using permuted block randomization technique. Stratification was based on involvement of lung zones ≤2 or >2 and sputum smear grading of <2+ or ≥2+. Allocated codes were concealed in sealed, opaque, sequentially numbered envelopes, prepared in advance by 2 statisticians who were not part of the study at National Institute for Research in Tuberculosis-NIRT.
Intervention
R10: Conventional-dose rifampicin (10 mg/kg/day); intensive-phase (IP): 2R(10)HZE7/continuation phase (CP): 4R(10)HE7
R25: High-dose rifampicin (25 mg/kg/day) arm: IP: 2R(25)HZE7/(CP): 4R(10)HE7
R35: High-dose rifampicin (35 mg/kg/day) arm: IP: 2R(35)HZE7/CP: 4R(10)HE7
The treatment duration was 6 months daily for all 3 arms and high-dose rifampicin was given only during IP in test regimens. Dosages of other anti-TB drugs were given per national guidelines. The drug intake was supervised except weekend doses, which were supplied for self-administration, and respective empty blister packs checked during subsequent visits.
Adverse Event Monitoring
At every visit, patients were questioned about the occurrence of adverse events (AE), and attempts were made to resolve them. AEs were graded per the division of AIDS system for grading the severity of adverse events division of AIDS AE Grading Version 2.1, July 2017 [19]. AEs and serious adverse events (SAEs) were periodically reported and causality assessment was performed by each institutional ethics committee. Because high-dose rifampicin was used in this trial, hepatotoxicity was anticipated and liver function tests was monitored weekly in the intensive phase. In case of AEs, participants were monitored for abnormal liver parameters and for clinical jaundice daily and liver function tests was repeated once every 2 or 3 days until the results reverted to normal. Treatment interruption if needed was done per American Thoracic Society guidelines. Monitoring and management of AEs including hepatotoxicity and treatment interruption [20] are detailed in annexure B of the protocol in Supplementary Data.
Study Outcomes
The modified intention-to-treat (m-ITT) population included new sputum culture-positive, drug-sensitive patients with PTB who were randomized (intention to treat) except those who had baseline isoniazid resistance and nontuberculous mycobacteria. Primary outcome was proportion of patients with treatment emergent grade 3/4 AEs in control versus 2 high-dose arms. Secondary outcome measures were (1) time to liquid/solid culture conversion from positive to negative (2) to estimate the proportion with negative sputum cultures at weeks 1, 2, 3, 4, 5, 6, 7, and 8, and at end of treatment in control versus high-dose arms. Culture conversion was defined as the time point at which 2 consecutive negative cultures were documented. TB treatment outcomes were defined per national guidelines (detailed in protocol in Supplementary Data). The laboratory staff members were blinded as to the identity of the samples that they processed.
Statistical Analysis
Assuming 5.2% [21, 22] of drug-induced AEs (severe hepatotoxic and nonhepatotoxic side effects) in the control arm and 14% in high-dose arms [17], a sample size of 99 per arm was required with 5% alpha and 90% power to detect significant difference between the control and high-dose arms. Adding a 10% loss because of death or default, the total sample size required was 109 per arm or 327 in total (sample size calculated based on method of Simon [23]).
The m-ITT population included new sputum culture-positive, drug-sensitive patients with PTB who were randomized (intention to treat) except those who had baseline isoniazid resistance and nontuberculous mycobacteria. The safety and efficacy analysis population are the same as m-ITT.
Demographic profile, factors related to clinical investigations, AEs, and bacteriological test results were summarized as frequencies and percentages, whereas multiple-group data of continuous variables were compared using 1-way analysis of variance. The stopping criteria of occurrence of >15% of grade 3/4 toxicity, in any of the treatment arms was fixed before the start of the study. Interim analysis was performed once 50% of the participants were enrolled to assess the level of grade 3/4 toxicity. The decision about continuation of the study was taken by the data and safety and monitoring board, which consists of 2 clinicians specializing in chest medicine, 1 clinician specializing in infectious diseases, 1 clinician specializing in public health, and 3 statisticians.
Primary safety analysis to compare the linear trend in toxicity (with special reference to hepatotoxicity) was performed over all 3 regimens using the Cochran-Armitage test. Secondary efficacy analysis of time to culture conversion was estimated using Kaplan-Meier method and the log-rank test was used to compare the survival distribution. To generate covariate-adjusted hazard ratio (HR), Cox proportional-hazard regression model with covariates of clinical relevance (body mass index, extent of lung lesion, and lung cavitation) was used [24]. The HR derived through this model is the measure of treatment-shortening potential exhibited by high-dose regimens. All statistical results were computed using IBM-SPSS (IBM Corp. Released 2017, Version 25.0; Armonk, NY) and Stata V15.1 (StataCorp, College Station, TX). All tests were 2-sided and statistical significance was set at P < .05.
RESULTS
Baseline Characteristics
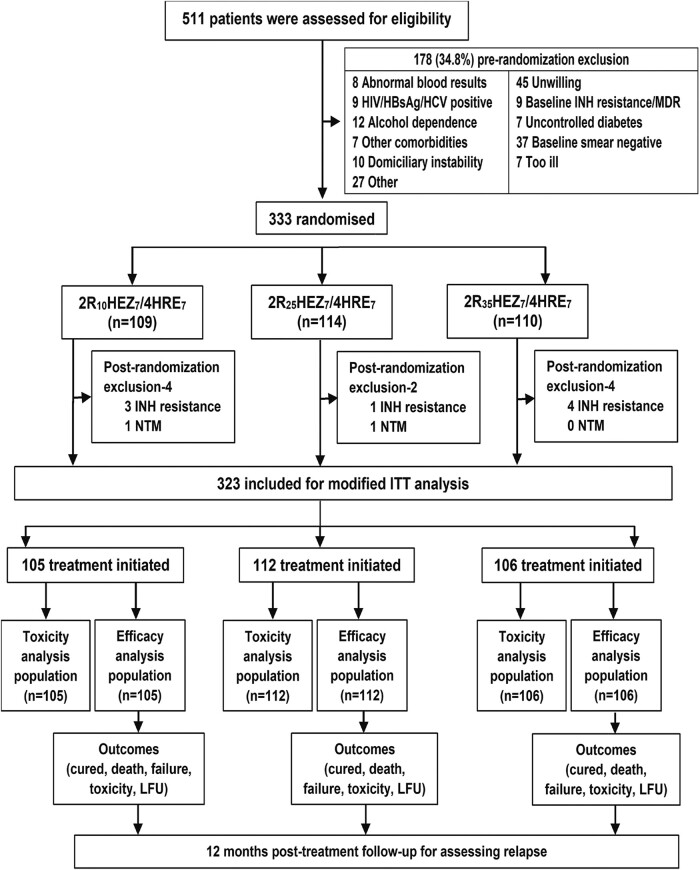
Of 511 patients screened, 333 were enrolled into the trial during the study period. A total of 109, 114, and 110 patients were randomized to treatment regimens R10, R25, and R35, respectively (Figure 1). The mean age and body mass index of study patients was 35.0 ± 12.3 years and 18.3 ± 3.1 kg/m2. A total of 77.5% had sputum smear grade of 2+/3+, 48.6% had a cavitary lesion, and 57.4% had >2 zones involved on chest radiography. The baseline characteristics of the patients were broadly similar across 3 treatment groups (Table 1, Supplementary Table 1). A total of 323 culture-confirmed patients were eligible for m-ITT, safety, and efficacy population analysis, under 3 treatment arms (R10: 105; R25: 112; R35:106), respectively (Figure 1).
Figure 1.
Randomization, and analysis of study participants. CP, continuous phase; E, ethambutol; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; H/INH, isoniazid; IP, intensive phase; LFU, lost to follow-up; LJ, Lowenstein-Jensen; MDR, multidrug resistant; MGIT BACTEC mycobacterial growth indicator tube 960; m-ITT, modified intention-to-treat; NTM, nontuberculous mycobacteria, R, rifampicin; R10 (2R10 Isoniazid, Ethambutol, Pyrizinamide [HEZ]7/4 Isoniazid, Rifampicin, Ethambutol [HRE]7), 2 months of rifampicin 10 mg/kg and HEZ given orally daily (intensive phase [IP]) followed by HRE given orally daily for 4 months (CP); R25 (2R25HEZ7/4HRE7), 2 months of rifampicin 25 mg/kg and HEZ given orally daily (IP) followed by HRE given orally daily for 4 months (CP); R35 (2R35HEZ7/4HRE7), 2 months of rifampicin 35 mg/kg and HEZ given orally daily (IP) followed by HRE given orally daily for 4 months (CP); Rx, treatment; Z, pyrazinamide.
Table 1.
Baseline Characteristics of Study Participants Categorized by Regimen
| Characteristic | Overall (N = 333) | 2R10HEZ7/4HRE7 (N = 109) | 2R25HEZ7/4HRE7 (N = 114) | 2R35HEZ7/4HRE7 (N = 110) |
|---|---|---|---|---|
| Age (y)a | 35.0 ± 12.3 | 35.9 ± 12.2 | 33.5 ± 12.3 | 35.5 ± 12.3 |
| Weight (kg)a | 47.1 ± 8.0 | 47.3 ± 7.7 | 47.2 ± 8.0 | 46.6 ± 8.4 |
| Weight bandb | ||||
| 25.1–39.9 kg | 63 (18.9) | 15 (13.8) | 26 (22.8) | 22 (20.0) |
| 40.0–54.9 kg | 213 (64.0) | 75 (68.8) | 66 (57.9) | 72 (65.5) |
| 55.0–69.9 kg | 57 (17.1) | 19 (17.4) | 22 (19.3) | 16 (14.5) |
| BMI (kg/m2)a | 18.3 ± 3.1 | 18.5 ± 3.2 | 18.3 ± 2.9 | 18.2 ± 3.2 |
| Genderb | ||||
| Male | 233 (70.0) | 76 (69.7) | 82 (71.9) | 75 (68.2) |
| Female | 100 (30.0) | 33 (30.3) | 32 (28.1) | 35 (31.8) |
| Alcohol useb | 118 (35.4) | 42 (38.5) | 37 (32.5) | 39 (35.5) |
| Tobacco smokingb | 107 (32.1) | 37 (33.9) | 34 (29.8) | 36 (32.7) |
| Diabetes mellitusb | 65 (19.5) | 20 (18.3) | 23 (20.2) | 22 (20.0) |
| Baseline smear gradeb | ||||
| Scanty/1+ | 75 (22.5) | 21 (19.3) | 30 (26.3) | 24 (21.8) |
| 2+/3+ | 258 (77.5) | 88 (80.7) | 84 (73.7) | 86 (78.2) |
| Phenotypic DSTb | ||||
| ALL SENS | 317 (95.2) | 103 (94.5) | 110 (96.5) | 104 (94.5) |
| EMB | 1 (0.3) | - | 1 (0.9) | - |
| H | 6 (1.8) | 2 (1.8) | 1 (0.9) | 3 (2.7) |
| SM | 5 (1.5) | 2 (1.8) | 1 (0.9) | 2 (1.8) |
| SM, H | 2 (0.6) | 1 (0.9) | - | 1 (0.9) |
| NTM | 2 (0.6) | 1 (0.9) | 1 (0.9) | - |
| Extent of lung lesionb | ||||
| Bilateral | 204 (61.3) | 71 (65.1) | 67 (58.8) | 66 (60.0) |
| Unilateral | 129 (38.7) | 38 (34.9) | 47 (41.2) | 44 (40.0) |
| Radiologic involvementb | ||||
| ≤2 lung zones | 142 (42.6) | 47 (43.1) | 50 (43.9) | 45 (40.9) |
| >2 lung zones | 191 (57.4) | 62 (56.9) | 64 (56.1) | 65 (59.1) |
| Cavitationb | ||||
| Present | 162 (48.6) | 56 (51.4) | 47 (41.2) | 59 (53.6) |
| Absent | 171 (51.4) | 53 (48.6) | 67 (58.8) | 51 (46.4) |
Abbreviations: 2R10HEZ7/4HRE7, 2 months of rifampicin 10 mg/kg and HEZ given orally daily (intensive phase [IP]) followed by HRE given orally daily for 4 months (CP); BMI, body mass index (weight in kilograms divided by the square of the height in meters); CP, continuous phase; 2R25HEZ7/4HRE7, 2 months of rifampicin 25 mg/kg and HEZ given orally daily (IP) followed by HRE given orally daily for 4 months (CP); 2R35HEZ7/4HRE7, 2 months of rifampicin 35 mg/kg and HEZ given orally daily (IP) followed by HRE given orally daily for 4 months (CP); DST, drug sensitivity test; E/EMB, ethambutol; H, isoniazid; IP, intensive phase; R, rifampicin; Sens, drug sensitivity; SM, streptomycin; Z, pyrazinamide.
aPlus-minus values are mean ± standard deviation.
bNumber (%).
Safety Analysis
Adverse Events
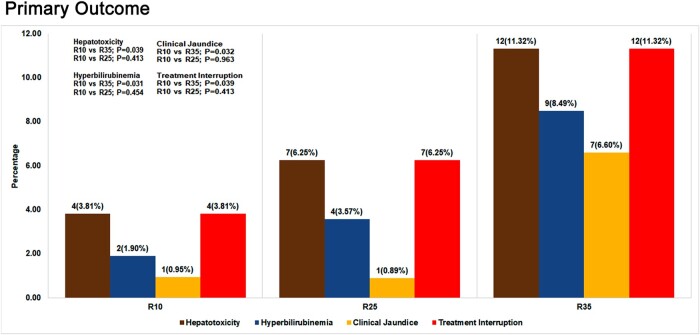
Among 323 (R10: 105; R25: 112; R35: 106) m-ITT patients, 34 (10.5%; 34/323) reported at least 1 grade 3/4 AEs (9.5% [10/105] in R10, 9.8% [11/112] in R25, 12.3% [13/106] in R35) in the intensive phase. Grade 3/4 hepatotoxicity was observed in 23 (7.1%; 23/323) patients from all 3 arms (3.8% [4/105] in R10, 6.3% [7/112] in R25, 11.3% [12/106] in R35). The difference was statistically significant only between R10 and R35 (P = .039) (Table 2; Figure 2). Sixteen patients exhibited symptomatic liver enzyme elevation (3 in R10, 4 in R25, and 9 in R35) (Table 3). Fifteen (4.6%; 15/323) patients developed grade 3/4 hyperbilirubinemia (1.9% [2/105] in R10, 3.6% [4/112] in R25, 8.5% [9/106] in R35) with statistically significant difference between R10 and R35 (P = .031), (Table 2). Clinical jaundice occurred in 1 patient each in R10 and R25 and 7 patients in R35 (R10 vs R35, P = .032) (Tables 2 and 3). A total of 23 cases (R10: 4; R25: 7; R35: 12) with grade 3 or 4 hepatotoxic AEs had treatment interruption (Figure 2; Table 2). Even though we have shown linear trend test results over all 3 regimens in Table 2, we have also undertaken analysis between R10 and R25/R35 in relation to grade 3 or 4 hepatic AEs, hyperbilirubinemia, and clinical jaundice. The results showed statistical significance among all the 3 arms (hepatotoxicity, P = .034; hyperbilirubinemia, P = .023; clinical jaundice, P = .012), thereby confirming the linear trend of toxicity. This linear trend in toxicity was also observed with more patients having supratherapeutic levels in R35. The percentage of patients with supratherapeutic levels of rifampicin in R10, R25, and R35 were 1.2%, 7.1%, and 37.0%, respectively (Table 4). The complete profile of all grade 3/4 toxicity cases are detailed in Supplementary Table 2. Only 1 case each in the R10 and R35 arms had a history of alcohol intake, clinical jaundice occurrence, and treatment interruption altogether. Grade 2 AEs were observed in 47 patients (14.6%; 47/323) from all 3 arms. Grade 2 hepatotoxicity was observed in 16 (5.0%; 16/323) patients from all 3 arms. There was no significant difference in grade 2 AEs or hepatotoxicity between the arms and none required treatment interruption. The interim analysis performed after recruitment of 50% of the participants showed safe levels of grade 3/4 toxicity and hence the data and safety and monitoring board allowed further recruitment of participants in the trial. Analysis of AEs during continuation phase showed that a total of 21 cases experienced AEs (grade 1: 20; grade 3: 1 [hyperuricemia in R10]).
Table 2.
Clinical and Laboratory Adverse Events During the Intensive Phase Graded Based on DAIDS Criteria
| Adverse Event | Overall n (%) |
2R10HEZ7 n (%) |
2R25HEZ7 n (%) |
2R35HEZ7 n (%) |
Trend Among All Regimens (P Value) |
R10 vs R25 (P Value) |
R10 vs R35 (P Value) |
R25 vs R35 (P Value) |
|---|---|---|---|---|---|---|---|---|
| Total in safety analysis | 323 | 105 | 112 | 106 | - | - | - | - |
| Patients with at least 1 AE | 237 (73.4) | 61 (58.1) | 80 (71.4) | 96 (90.6) | <.001* | .040* | <.001* | <.001* |
| Patients with at least 1 grade 2, 3, or 4 AE | 81 (25.1) | 23 (21.9) | 25 (22.3) | 33 (31.1) | .122 | .941 | .129 | .141 |
| Patients with at least 1 grade 3 or 4 AE | 34 (10.5) | 10 (9.5) | 11 (9.8) | 13 (12.3) | .516 | .941 | .523 | .565 |
| Patients with at least 1 grade 3 or 4 hepatic AE | 23 (7.1) | 4 (3.8) | 7 (6.3) | 12 (11.3) | .034* | .413 | .039* | .185 |
| Patients with hyperbilirubinemia among hepatic AE cases | 15 (4.6) | 2 (1.9) | 4 (3.6) | 9 (8.5) | .023* | .454 | .031* | .125 |
| Patients with clinical jaundice (icterus) among hepatic AE cases | 9 (2.8) | 1 (1.0) | 1 (0.9) | 7 (6.6) | .012* | .963 | .032* | .025* |
| Patients with at least 1 serious AE among total cases | 18 (5.6) | 8 (7.6) | 7 (6.3) | 3 (2.8) | .129 | .691 | .118 | .228 |
| Patients with at least 1 grade 3 or 4 AE filed for SAE | 6 (1.9) | 3 (2.9) | 1 (0.9) | 2 (1.9) | .604 | .282 | .643 | .529 |
| Number of patients with treatment interruption from hepatic AEa | 23 (7.1) | 4 (3.8) | 7 (6.3) | 12 (11.3) | .034* | .413 | .039* | .185 |
| Deaths among total cases | 3 (0.9) | 2 (1.9) | 1 (0.9) | 0 (0.0) | .149 | .524 | .153 | .330 |
| Death among hepatotoxicity cases | 0 | 0 | 0 | 0 | - | - | - | - |
Safety was assessed during the intensive phase (the time during which the patients were receiving the trial medications and up to 60 days). Adverse events were graded by the site investigators according to the DAIDS criteria.
Abbreviations: AE, adverse event; ALT, alanine transaminase; AST, aspartate aminotransferase; DAIDS, division of AIDS system for grading the severity of adverse events division of AIDS (grade 2 indicates a moderate event, grade 3 indicates a severe event, grade 4 indicates a potentially life-threatening event); E, ethambutol; H, isoniazid; IP, intensive phase; R, rifampicin; R10 (2R10HEZ7), 2 months of rifampicin 10 mg/kg and HEZ given orally daily (IP); R25 (2R25HEZ7), 2 months of rifampicin 25 mg/kg and HEZ given orally daily (IP), R35 (2R35HEZ7), 2 months of rifampicin 35 mg/kg and HEZ given orally daily (IP); SAE, serious adverse event; Z, pyrazinamide.
aProtocol criteria for treatment interruption because of hepatic AE: elevation of AST or ALT more than 3 times the upper limit of normal, with associated symptoms, or elevation of AST or ALT more than 5 times the upper limit of normal regardless of the presence of symptoms. The P value for a trend over all 3 regimens was analyzed using Cochran-Armitage trend test.
* P ≤ .05 is considered statistically significant.
Figure 2.
Grade 3 or 4 hepatic adverse events. The number of cases with hepatotoxicity (grade 3/4 liver enzymes, whichever is the higher grade), or grade 3/4 hyperbilirubinemia, or clinical jaundice or treatment interruption, between control arm (R10) and experimental arms (R25 or R35) were compared for significant differences. Trend test P value comparing R10 vs R25 and R10 vs R35 arms indicated in the diagram. P ≤ .05 is considered statistically significant.
Table 3.
Hepatotoxic and Nonhepatotoxic Regimen-wise Grade III and IV Adverse Events During the Intensive Phase
| Regimen | Grade III | Grade IV | ||||
|---|---|---|---|---|---|---|
| Clinical (N) | Laboratory (N) | Clinical and Laboratory Combined (N) | Clinical (N) | Laboratory (N) | Clinical and Laboratory Combined (N) | |
| R10 mg/kg/day (control arm) | - | Elevated liver enzyme (1)a Hyperuricemia (4)b |
Hyperuricemia with vomiting (1)b Hyperbilirubinemia, Elevated liver enzyme with nausea, vomiting, and icterus (1)a,c Elevated liver enzyme with vomiting (1)a |
- | Hyperuricemia (1)b | Hyperbilirubinemia, elevated liver enzymes with nausea and vomiting (1)a |
| R25 mg/kg/day | - | Elevated liver enzyme (1)a Hyperuricemia (3)b |
Hyperbilirubinemia, elevated liver enzyme and loss of appetite, joint pain (1)a Hyperbilirubinemia, elevated liver enzyme and nausea, vomiting and joint pain (1)a Hyperbilirubinemia, elevated liver enzyme and icterus (1)a,c |
Elevated liver enzymes (2)a | Hyperbilirubinemia, elevated liver enzyme with vomiting (1)a hyperglycemia, sweating, and giddiness (1)b |
|
| R35 mg/kg/day | Seizures (1)b | Hyperbilirubinemia, elevated liver enzyme (3)a | Hyperbilirubinemia, elevated liver enzymes and itching (1)a Hyperbilirubinemia, elevated liver enzyme and vomiting, stomach pain and Icterus (1)a,c Elevated liver enzyme with nausea and vomiting (1)a Hyperbilirubinemia, elevated liver enzyme, and icterus (1)a,c Hyperbilirubinemia, elevated liver enzyme and vomiting, rashes and icterus (1)a,c |
- | - | Elevated liver enzymes with vomiting, stomach pain and icterus (1)a,c Elevated liver enzymes with nausea, vomiting, and icterus (1)a,c Hyperbilirubinemia, elevated liver enzyme and icterus (2)a,c |
The grading of hepatotoxic and nonhepatotoxic adverse events was done as per DAIDS criteria. Grade 3/4 adverse events in intensive phase were shown because experimental doses were given only during the intensive phase. The patients were categorized based on the maximum grade of the enzymes, regardless of grading for hyperbilirubinemia.
Abbreviations: AE, adverse event; DAIDS, division of AIDS system for grading the severity of adverse events division of AIDS (grade 3 indicates a severe event, grade 4 indicates a potentially life-threatening event); E, ethambutol; H, isoniazid; R, rifampicin; R10, (2R10HEZ7/4HRE7) 2 months of rifampicin 10 mg/kg and HEZ given orally daily; R25, (2R25HEZ7/4HRE7) 2 months of rifampicin 25 mg/kg and HEZ given orally daily; R35, (2R35HEZ7/4HRE7) 2 months of rifampicin 35 mg/kg and HEZ given orally daily; ULN, upper limit of normal; Z, pyrazinamide.
Grade III vomiting: persistent vomiting resulting in orthostatic hypotension or aggressive rehydration indicated; grade III pruritus: itching causing inability to perform usual social and functional activities; grade III arthralgia: joint pain causing inability to perform usual social and functional activities; grade III seizures: new onset of 2–4 episodes of seizures; grade III serum bilirubin: 2.6–5.0× ULN; grade IV bilirubin: >5.0× ULN; grade III liver enzymes: 5.1–10.0× ULN; grade IV liver enzymes: >10.0× ULN; grade III hyperuricemia: uric acid 12.1–15.0 mg/dL; grade IV hyperuricemia: uric acid >15.0 mg/dL; grade IV hyperglycemia: glucose >500 mg/dL.
aTreatment interruption.
bNonhepatotoxic events.
cClinical jaundice (icterus).
Table 4.
Rifampicin Drug Levels in Sparse PK
| Regimen | Drug Concentration (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 2nd hour | 4th hour | |||||||
| <8 | ≥8 to ≤24a | >24 | <8 | ≥8 to ≤24a | >24 | <8 | ≥8 to ≤24a | >24 | |
| R10b (n = 85) |
85 (100) | - | - | 64 (75.3) | 20 (23.5) | 1 (1.2) | 66 (77.6) | 19 (22.4) | - |
| R25b (n = 85) |
84 (98.8) | - | 1 (1.2) | 30 (35.3) | 49 (57.6) | 6 (7.1) | 12 (14.1) | 60 (70.6) | 13 (15.3) |
| R35b (n = 81) |
79 (97.5) | 2 (2.5) | - | 20 (24.7) | 31 (38.3) | 30 (37.0) | 6 (7.4) | 38 (46.9) | 37 (45.7) |
Sparse Pharmacokinetic (PK) assay was performed for a subset of study participants (R10: 85; R25: 85; R35:81) and Cmax of rifampicin was measured using high-performance liquid chromatography at “0”' hour, second hour, and fourth hour during the end of the intensive phase.
aTherapeutic range of rifampicin: ≥8 to ≤24 µg/mL.
bNumber (percentage).
Serious Adverse Events
A total of 18 SAEs occurred that led to hospitalization, 8 in R10, 7 in R25, and 3 in R35. Reasons for hospitalization included both drug-unrelated reasons (hyperglycemia, injury, enteric fever, alcohol intake, and cardiac causes) as well as from TB disease or its treatment (drug-induced hepatitis, gastritis, vomiting, pneumothorax, hemoptysis, thrombocytopenia). Seven (4 in R10, none in R25, 3 in R35) SAEs were drug related (Supplementary Table 3). Of 4 deaths that occurred during treatment, 2 were due to a non-TB cause (acute myocardial infarction: 1 each in R25 and R10) and the other 2 were TB-related deaths (massive hemoptysis and type II respiratory failure; both in R10) (Supplementary Table 4).
Efficacy Analysis
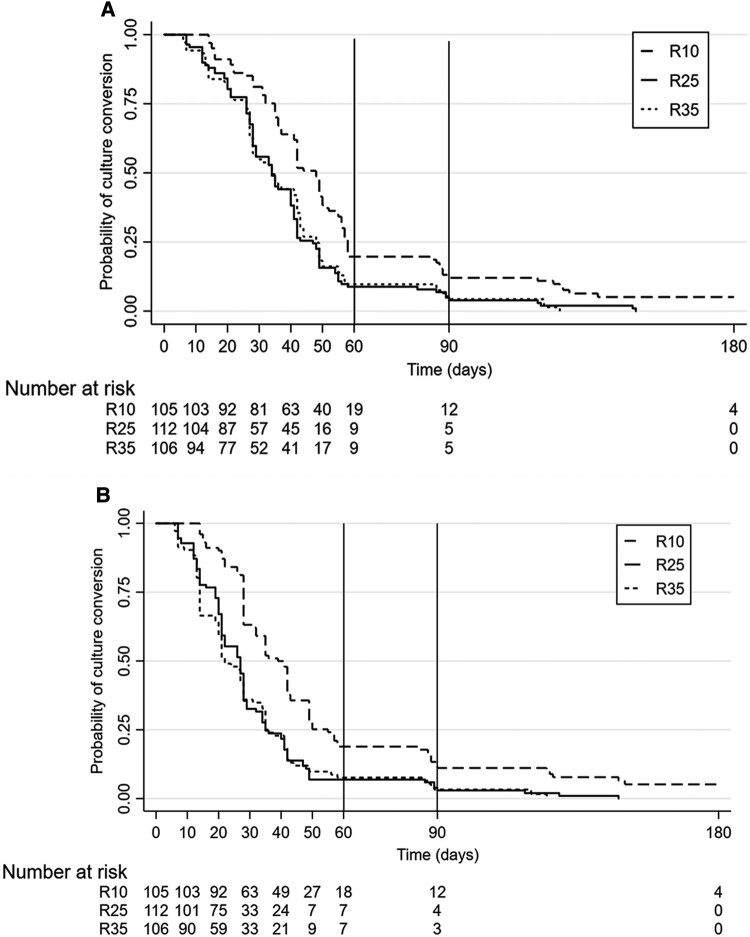
Time to stable culture conversion in liquid media was similar between R25 and R35 compared with the control arm at week 8 (Figure 3A). A similar pattern in conversion was observed with solid media (Figure 3B). Time to event data was analyzed after adjusting for clinically relevant factors such as cavity, body mass index, and extent of lung lesions [24]. Overall culture conversion at week 8 in liquid media was higher in R25 (34 days; adjusted hazard ratio [aHR]: 1.71; 95% confidence interval [CI], 1.26–2.31; P = .001; solid media: aHR: 1.97; 95% CI, 1.46–2.67; P ≤ .001) and R35 (34 days; aHR: 1.81; 95% CI, 1.33–2.48; P ≤ .001 solid media: 2.24; 95% CI, 1.64–3.06; P ≤ .001) (Table 5) than in R10 (44 days [95% CI, 40.16–47.84]) (Figure 3). The pattern of difference in aHR values between R25 and R35 was consistent during the third month (liquid media: 1.68 and 1.81; solid media: 1.94 and 2.21) (Supplementary Table 5), and until end of treatment (liquid media: 1.79 and 1.91; solid media: 2.04 and 2.35) (Table 5) with the high-dose regimen being given for 8 weeks only. Post hoc analysis (which was not planned during the start of the trial) of culture conversion between R25 and R35 did not show any statistical significance. No failures or relapses were seen in R25 arm (Supplementary Table 6).
Figure 3.
Kaplan-Meier curves for time-to-culture conversion. A, Time-to-culture conversion in liquid media-MGIT. B, Time-to-culture conversion on solid media Löwenstein-Jensen. Survival function estimated by the Kaplan-Meier method. Censoring is indicated by vertical marks. The number of patients at risk at different time points during intensive and continuation phase of treatment is presented in the graph. Solid vertical line refers to the week 8 time point (cutoff in primary analysis). Dashed vertical line refers to the week 12 time point (cutoff in post hoc analysis). Kaplan-Meier curves displaying the estimated survival probability for 3 different arms of patients after treatment given for 180 d. MGIT, Mycobacteria Growth Indicator Tube.
Table 5.
Summary of Primary Analyses of Time to Culture Conversion to 8 Weeks and End of Treatment in m-ITT Population in Liquid and Solid Media
| MGIT (Liquid Media) | |||
|---|---|---|---|
| m-ITT (N = 323) |
2R35HEZ7 (n = 106) |
2R25HEZ7 (n = 112) |
2R10HEZ7a (n = 105) |
| Week 8 | |||
| HR (95%), unadjusted | 1.65 (1.22–2.25) | 1.76 (1.30–2.37) | Reference |
| Unstratified log-rank test | 0.001 | <0.001 | |
| HR (95%), adjustedb | 1.81 (1.33–2.48) | 1.71 (1.26–2.31) | Reference |
| Stratified log-rank test | <0.001 | 0.001 | |
| End of Rx | |||
| HR (95%), unadjusted | 1.74 (1.30–2.32) | 1.81 (1.37–2.41) | Reference |
| Unstratified log-rank test | <0.001 | <0.001 | |
| HR (95%), adjustedb | 1.91 (1.41–2.58) | 1.79 (1.34–2.39) | Reference |
| Stratified log-rank test | <0.001 | <0.001 | |
| LJ (solid media) | |||
| Week 8 | |||
| HR (95%), unadjusted | 1.98 (1.46–2.68) | 1.98 (1.47–2.67) | Reference |
| Unstratified log-rank test | <0.001 | <0.001 | |
| HR (95%), adjustedb | 2.24 (1.64–3.06) | 1.97 (1.46–2.67) | Reference |
| Stratified log-rank test | <0.001 | <0.001 | |
| End of Rx | |||
| HR (95%), unadjusted | 2.08 (1.55–2.80) | 2.01 (1.51–2.68) | Reference |
| Unstratified log-rank test | <0.001 | <0.001 | |
| HR (95%), adjustedb | 2.35 (1.73–3.18) | 2.04 (1.53–2.73) | Reference |
| Stratified log-rank test | <0.001 | <0.001 | |
Abbreviations: CP, continuation phase; E, ethambutol; H/INH, isoniazid; IP, intensive phase; HR, hazard ratio; LJ, Löwenstein-Jensen; m-ITT, modified intention-to-treat; MGIT BACTEC, mycobacterial growth indicator tube 960; R, rifampicin; R10 (2R10HEZ7/4HRE7) 2 months of rifampicin 10 mg/kg and HEZ given orally daily (IP) followed by HRE given orally daily for 4 months (CP), R25 (2R25HEZ7/4HRE7), 2 months of rifampicin 25 mg/kg and HEZ given orally daily (IP) followed by HRE given orally daily for 4 months (CP); R35 (2R35HEZ7/4HRE7), 2 months of rifampicin 35 mg/kg and HEZ given orally daily (IP) followed by HRE given orally daily for 4 m (CP); Rx, treatment; Z, pyrazinamide.
aThis arm is the reference category. The m-ITT population included all the patients except those who were baseline culture–negative by MGIT and on LJ culture.
bAnalysis adjusted for factors, namely extent of lung lesion (unilateral, bilateral), cavity (present, absent), and body mass index (<18.5, ≥18.5). P ≤ .05 is considered statistically significant.
DISCUSSION
Studies worldwide have shown that high-dose rifampicin beyond rifampicin 20 mg/kg, especially R35, is safe, well-tolerated, and provides the highest adjusted HR of 2.04 with respect to time to stable culture conversion at week 8 [7, 8, 17]. The HICON-R trial is a phase IIb RCT working to address the safety, tolerability, and efficacy of an intermediary high rifampicin dose between R10 and R35, namely R25, in the treatment of drug-sensitive PTB. The reason for choosing R25 is that no clinical trial data on high-dose rifampicin R25 were available in India and globally during the start of this trial, and the expectation is that R25 could be less hepatotoxic and could yield week 8 and end-of-treatment aHR/percentage of culture conversion close to R35. Our results proved that to be true with respect to hepatotoxicity and time to culture conversion.
On comparison between R10 and the high-dose R25/R35 arms, even though the overall grade 3/4 toxicity was comparable, R35 but not R25, had significantly higher grade 3/4 hepatotoxicity, hyperbilirubinemia, clinical jaundice, and treatment interruptions. This is in contrast to findings of Boeree et al. [17], which showed that R35 had comparable level of hepatotoxicity with R10. When we analyzed the Cmax data, we found that 37% of patients in R35 showed supratherapeutic levels of rifampicin compared with only 7% in R25, whereas there is not much change in the Cmax of pyrazinamide and isoniazid. (manuscript in preparation). This is in line with Boeree et al. [7], who showed more than a proportional increase of peak concentration with increasing doses of rifampicin. This might be one of the reasons for higher hepatotoxicity observed in the R35 arm.
We analyzed the adjusted HR for time-to-culture conversion to know the ability of the experimental arms to potentially shorten the treatment duration. With our m-ITT population, the aHR in MGIT for R25 is closer to R35 from the eighth week to the end of treatment. The adjusted value of HR seen in our study at week-8 is slightly lesser than that seen with Boeree et al. [17]. This could be because of the difference in the factors used to adjust the HR. We have used well-defined clinical factors that influence the culture conversion during treatment [24]. Furthermore, our aHR at week 8 with solid media is better than MGIT-based aHR reported by Boeree et al. [17]. The aHR values calculated beyond 8 weeks have not shown any major change in our experimental arms, because 90% of our patients have got converted at eighth week time point itself. Even though the conversion rate is faster in R35, the burden of handling hepatotoxicity cases in R35 will be more in the field conditions compared with R25 and R10.
The faster culture conversion in both experimental arms seen with both liquid and solid media is similar to a scenario observed in a well-designed large trial such as ReMoxTB assessing culture conversion with new regimens based on moxifloxacin [5]. This is also reflected in our Kaplan-Meier time-to-culture conversion curve analyses from both media, where R25 showing closely tethered conversion curve pattern with R35. Also, we did not observe any significant level of grade 3/4 adverse events in the continuation phase. In line with other studies [17, 25], our current sample size used in this trial for assessing grade 3 and 4 toxicity, is adequate to detect HR of 1.8 in our well-characterized cohort. Hence, our findings on HR are of valid importance.
In comparison to the 4-month rifapentine and moxifloxacin-containing regimen recommended recently by World Health Organization guidelines for drug-sensitive PTB [2, 26], the R10 and R25 arms used in our study showed lesser percentage of overall grade 3 or higher level of toxicity, but at the same time registered significantly higher culture conversion rates during IP. Besides, the use of R25 will provide an option to reserve moxifloxacin, which is an integral component of drug-resistant TB regimen. So, our findings are in favor of R25 rather than R35 in promoting the desired treatment-shortening potential in a safer way with uncompromised efficacy. This has field implications in terms of easiness of toxicity management and an easily scalable option in high-burden countries where rifapentine use is currently limited or its safety is not well established. Our study has few limitations. The open-label design of the trial along with knowledge of effects of high-dose rifampicin might have had an impact on overreporting of AEs in high-dose arms. To avoid such an impact, the trial team was trained in such a way that all participants regardless of the study regimens were closely monitored for occurrence of AEs and were notified and managed appropriately per the protocol. The power used in the study will not be sufficient to differentiate between the experimental regimens, but only between individual experimental and the control arm. Furthermore, we have not evaluated the long-term cost efficiency of monitoring weekly liver function test in the field. The study was conducted only in Indian population and in patients without HIV.
To conclude, this is the first RCT to directly compare R10 with higher doses of rifampicin (R25 and R35) for evaluating higher grades of toxicity as well as efficacy, and it showed that in comparison with R10, R35 but not R25, had significantly higher grade 3/4 hepatotoxicity, clinical jaundice, and treatment interruptions. Because R25 had toxicity of a comparable magnitude as R10 and was also more efficacious than R10, it can be considered for implementation in the field for shortening treatment duration.
Supplementary Material
Contributor Information
Bhavani Perumal Kannabiran, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Natarajan Alangudi Palaniappan, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Tamizhselvan Manoharan, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Paul Kumaran Paramasivam, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Jitendra Kumar Saini, Department of Pulmonary Oncology, National Institute for Tuberculosis and Respiratory Diseases, New Delhi, India.
Mohammed Soheb Ansari, Department of Respiratory Medicine, Bhagwan Mahavir Medical Hospital and Research Centre, Hyderabad, India.
Lavanya Jayabal, District TB office, Greater Chennai Corporation, Chennai, India.
Ashutosh N Aggarwal, Department of Respiratory Medicine, Post Graduate Institute of Medical Research, Chandigarh, India.
Rajiv Garg, Department of Respiratory Medicine, King George's Medical University, Lucknow, India.
Balaji Subramanyam, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Deepika Thakur, Department of Respiratory Medicine, Post Graduate Institute of Medical Research, Chandigarh, India.
Shilpa Pantula, Department of Respiratory Medicine, Bhagwan Mahavir Medical Hospital and Research Centre, Hyderabad, India.
Ramesh P M, Department of Respiratory Medicine, Government Thiruvotteeswarar Hospital of Thoracic Medicine, Chennai, India.
Vijayachandar GS, Department of Respiratory Medicine, Institute of Thoracic Medicine, Chennai, India.
Saravanan Natarajan, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Radha Krishnan Ammayappan, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Bhalla Manpreet, Department of Pulmonary Oncology, National Institute for Tuberculosis and Respiratory Diseases, New Delhi, India.
Mangalambal Ganesan, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Dhanalakshmi Angamuthu, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Ponnuraja Chinnaiyan, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Manjula Singh, Division of Communicable Diseases, Indian Council of Medical Research, New Delhi, India.
Padmapriyadarsini Chandrasekaran, Department of Clinical Resarch, ICMR - National Institute for Research in Tuberculosis, Chennai, India.
Soumya Swaminathan, Former Chief Scientist, World Health Organisation, Geneva, Switzerland.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Gunasundari B, all clinic nurses, junior staff nurses, and the staff of sociobehavioral research of Chennai and Madurai units of ICMR- National Institute for Research in Tuberculosis for their efforts toward participant recruitment, retention, and management. The technical assistance and support from the bacteriology, biochemistry, and serology laboratory staff of ICMR-NIRT is highly appreciated. We would like to extend our warm gratitude to the all our project staff from collaborating sites, NTEP staff, and research participants.
This study is registered at the Clinical trial registry of India with the identifier CTRI/2017/12/010951. The corresponding author can provide, on request, individual participant data that underlie the results reported in this article or protocol after applying necessary measures to guarantee that no individual is identified or identifiable.
Author contributions. P. K. B. is the lead investigator, conceptualized and designed the trial, and prepared the original draft. A. P. N. was involved in drafting of manuscript, patient monitoring, and in providing critical comments. P. P. K. was involved in protocol writing, was site investigator, and provided critical comments. J. K. S., M. S. A., L. J., A. A., R. G., and P. M. R. were study collaborators and were in charge of project administration at their respective sites. B. S. and N. S. were in charge of bacteriological procedures and biochemical analysis for the trial, respectively. A. D. was the study social worker involved in counseling and patient retention. T. S. and C. P. R. were involved in data management and analysis. M. S. was involved in fund acquisition and periodic trial monitoring. P. P. and S. S. provided overall supervision and guidance of trial design and conduct. P. K. B., P. P., T. M., C. P. R., J. K. S., M. S. A., A. A., and R. G. had access to the data and have verified the data submitted.
Patient Consent Statement. The participant information sheet, consent forms, and case report forms used in the trial were approved by the ethics committee of all participating sites (NIRT-IEC No: 2017 015, date 14 December 2017). Written informed consent was obtained from all screened participants.
Financial support. This work was supported by India TB research Consortium of Indian Council of Medical Research (ICMR- ITRC funded project [No. 5/8/5/57/TRC/3/2018-ECD-I]).
References
- 1. Thamineni R, Peraman R, Chenniah J. Level of adherence to anti-tubercular treatment among drug-sensitive tuberculosis participants on a newly introduced daily dose regimen in South India: a cross-sectional study. Trop Med Int Health 2022; 27:1013–23. [DOI] [PubMed] [Google Scholar]
- 2. Dorman SE, Nahid P, Kurbatova EV, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med 2021; 384:1705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jindani A, Atwine D, Grint D, et al. Four-month high-dose rifampicin regimens for pulmonary tuberculosis. NEJM Evid 2023; 2:9. doi: 10.1056/EVIDoa2300054 https://evidence.nejm.org/doi/abs/10.1056/EVIDoa2300054 [DOI] [PubMed] [Google Scholar]
- 4. Jindani A, Harrison TS, Nunn AJ, et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014; 371:1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillespie SH, Crook AM, McHugh TD, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014; 371:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steingart KR, Jotblad S, Robsky K, et al. Higher-dose rifampin for the treatment of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 2011; 15:305–16. [PubMed] [Google Scholar]
- 7. Boeree MJ, Diacon AH, Dawson R, et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med 2015; 191:1058–65. [DOI] [PubMed] [Google Scholar]
- 8. Svensson EM, Svensson RJ, te Brake LHM, et al. The potential for treatment shortening with higher rifampicin doses: relating drug exposure to treatment response in patients with pulmonary tuberculosis. Clin Infect Dis 2018; 67:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seijger C, Hoefsloot W, Bergsma-de Guchteneire I, et al. High-dose rifampicin in tuberculosis: experiences from a Dutch tuberculosis centre. PLoS One 2019; 14:e0213718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parthasarathy R, Sarma GR, Janardhanam B, et al. Hepatic toxicity in South Indian patients during treatment of tuberculosis with short-course regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle 1986; 67:99–108. [DOI] [PubMed] [Google Scholar]
- 11. Wada M. Effectiveness and problems of PZA-containing 6-month regimen for the treatment of new pulmonary tuberculosis patients. Kekkaku 2001; 76:33–43. [PubMed] [Google Scholar]
- 12. Tariq S, Khan TS, Malik S, Anwar MS, Rashid A. Frequency of anti-tuberculous therapy-induced hepatotoxicity in patients and their outcome. J Ayub Med Coll Abbottabad 2009; 21:50–2. [PubMed] [Google Scholar]
- 13. Abera W, Cheneke W, Abebe G. Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis participants in Dawro Zone, South Ethiopia: a cohort study. Int J Mycobacteriol 2016; 5:14–20. [DOI] [PubMed] [Google Scholar]
- 14. Shakya R, Rao BS, Shrestha B. Incidence of hepatotoxicity due to antitubercular medicines and assessment of risk factors. Ann Pharmacother 2004; 38:1074–9. [DOI] [PubMed] [Google Scholar]
- 15. Jiang F, Yan H, Liang L, et al. Incidence and risk factors of anti-tuberculosis drug induced liver injury (DILI): large cohort study involving 4652 Chinese adult tuberculosis patients. Liver Int 2021; 41:1565–75. [DOI] [PubMed] [Google Scholar]
- 16. Chang KC, Leung CC, Yew WW, et al. Standard anti-tuberculosis treatment and hepatotoxicity: do dosing schedules matter? Eur Respir J 2007; 29:347–51. [DOI] [PubMed] [Google Scholar]
- 17. Boeree MJ, Heinrich N, Aarnoutse R, et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Velásquez GE, Brooks MB, Coit JM, et al. Efficacy and safety of high-dose rifampin in pulmonary tuberculosis. A randomized controlled trial. Am J Respir Crit Care Med 2018; 198:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DAIDS AE Grading Table Corrected Version 2.1-July 2017. Available at: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Updated July 2017. Accessed July 2017.
- 20. Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006; 174:935–52. [DOI] [PubMed] [Google Scholar]
- 21. Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med 2006; 100:1834–42. [DOI] [PubMed] [Google Scholar]
- 22. Jindani A, Borgulya G, de Patiño IW, et al. A randomised phase II trial to evaluate the toxicity of high-dose rifampicin to treat pulmonary tuberculosis. Int J Tuberc Lung Dis 2016; 20:832–8. [DOI] [PubMed] [Google Scholar]
- 23. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10:1–10. [DOI] [PubMed] [Google Scholar]
- 24. Diktanas S, Vasiliauskiene E, Polubenko K, et al. Factors associated with persistent sputum positivity at the end of the second month of tuberculosis treatment in Lithuania. Tuberc Respir Dis (Seoul) 2018; 81:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rustomjee R, Lienhardt C, Kanyok T, et al. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2008; 12:128–38. [PubMed] [Google Scholar]
- 26. World Health Organization . In: WHO consolidated guidelines on tuberculosis: Module 4: Treatment—Drug-susceptible tuberculosis treatment. Geneva; 2022; Available at: https://www.who.int/publications/i/item/9789240048126. Updated 24 May 2022. Accessed 24 May 2022. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.