Abstract
Cochlear implant surgeries have become increasingly common in India, leading to a rise in complications such as cochlear implant exposure. To address this issue, we present a novel technique involving a single incision dual cover using the temporoparietal fascial flap (TPFF) and skin flap to give durable cover for exposed cochlear implants.
Materials and Methods A retrospective study was conducted between December 2019 and December 2022 on patients who underwent the dual flap procedure for exposed cochlear implants.
Results The average defect size was 2 × 2 cm, and the average length of hospital stay was 10 days. Fourteen skin flaps were closed primarily, while two required skin grafting for donor site closure. At the time of discharge, all wounds showed successful healing with intact skin coverage over the cochlear implant device site. The average follow-up period was 12 months, during which two patients had donor site scar alopecia, while others had adequate hair growth masking the scar. All patients consistently used their cochlear implants.
Conclusion Our single-incision, dual cover TPFF + skin flap technique offers a reliable and innovative solution for managing exposed cochlear implants. With successful implant salvage and favorable postoperative outcomes, this approach demonstrates the versatility and reliability of the TPFF as an excellent option for reconstructive surgeons dealing with cochlear implant complications.
Keywords: exposed cochlear implant, single incision, temporoparietal fascial flap, dual cover, cochlear implant salvage
Introduction
Cochlear implant surgeries in India have grown exponentially with a reduction in cost and support from the government. With a proportional increase in the number of surgeries, we have witnessed a rise in the number of complications, especially wound dehiscence and exposure of the cochlear implant. 1 2 3 Our center has a high volume of cochlear implant surgeries performed yearly using the Veria (nonmastoidectomy transcanal approach) technique. Infection of the implant is an inevitable consequence of exposure, and early flap cover can decrease the chances of infection and salvage the implant. The temporoparietal fascial flap (TPFF) is a well-known pedicled flap based on the superficial temporal vessels. In 1898, Brown described it for auricular reconstruction and Golovine used it to repair the defect following orbital exenteration. It is a dependable option for the reconstructive surgeon due to its pliability, large surface area, lack of bulk, pedicle length, robust blood supply, and versatility (e.g., ability to accept skin grafts on both sides). 4 Components of the TPFF include the superficial temporal artery (STA), superficial temporal vein, and fascial layer that is 2 to 3 mm thick. The pedicled TPFF has been used for repair or augmentation of the external ear, temporal bone, middle ear, lateral and anterior skull base, orbit/maxilla, face, nasopharynx, oral cavity, and oropharynx, and can be harvested as a microvascular free flap. Given its proximity and reliable blood supply, the TPFF can cover the tissue defect over an exposed cochlear implant. Traditional coverage techniques use a two-incision approach, one for the TPFF and one for the skin flap, which has disadvantages. We describe our technique of a single-incision, dual cover (TPFF + skin) temporoparietal flap cover and its follow-up for exposed cochlear implants.
Materials and Methods
A retrospective study was conducted in a tertiary care center from December 2019 to December 2022. Institutional ethical committee approval was obtained. All patients who were referred to our department with exposed cochlear implants were included in our study. All the patients underwent reconstruction utilizing a single incision for a dual-layered cover (TPFF and skin). They were followed up postprocedure for a minimum of 12 months.
Surgical Technique
Preoperatively the STA was located, and its branches were traced using a handheld Doppler ( Fig. 1a ). The course of the facial nerve was marked to avoid injury to the frontal branch according to standard anatomical landmarks (line of Pitanguy). The existing wound was debrided, and a thorough wound wash was given. The incision was planned in such a way that the same could be utilized for both harvesting the TPFF and raising the local rotation flap ( Fig. 2 ). The incision was made by extending from the superior edge of the defect.
Fig. 1.
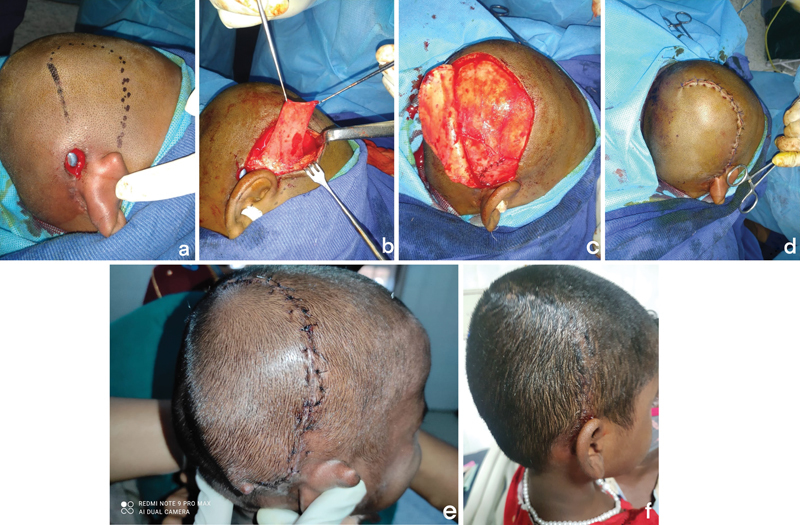
( a ) Preoperative marking showing incision ( thick line ) for rotation flap and a dotted line showing temporoparietal fascial (TPF) flap marking. ( b ) The TPF flap was raised through the same incision. ( c ) Flap transposed onto the implant and sutured as a snuggly fitting pocket. ( d ) The traditional rotation flap was raised along the same incision. ( e ) Immediate post-op. ( f ) Late post-op.
Fig. 2.
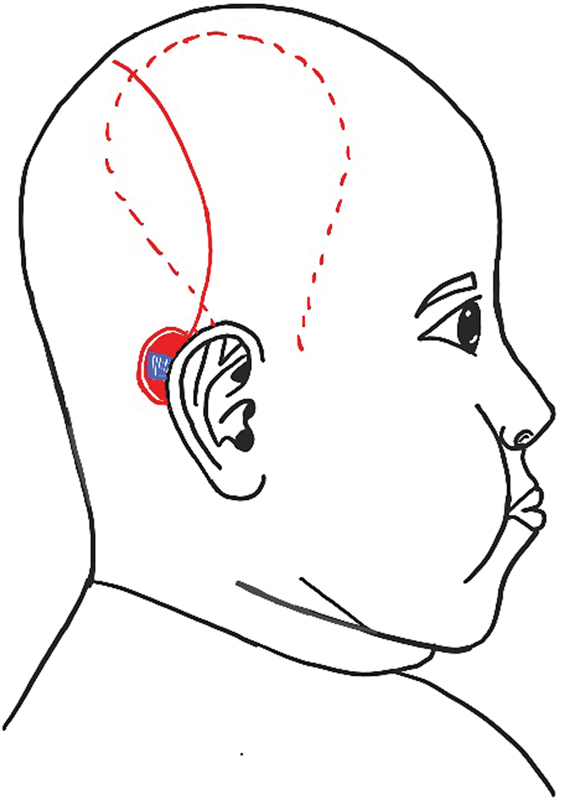
Single incision for rotation flap and Temporoparietal fascial flap harvest. The red line indicates incision for flap harvest and red dotted line indicating the extent of temporoparietal fascial [TPF] flap.
The anterior skin flap was raised and retracted for harvest of the TPFF ( Fig. 1b ). Care was taken to stay in a plane just superficial to the TPF, where the superficial temporal vessels lie. The overlying skin was separated from the TPF by developing a plane between the subcutaneous fat and the TPF. Care was taken to avoid injury to the hair follicles visible in the overlying skin flap to prevent alopecia. Dissection was not carried anteriorly to the temporal hairline to avoid injury to the facial nerve. Once the anterior and posterior branches of the STA were identified and an adequate area of fascia was liberated, the edges of the flap were marked and incised. The distal branches of the STA and associated veins were ligated. The flap was freed from the underlying deep temporal fascia, then rotated to the site of the defect. The arc of rotation and length of the flap were maximized by carefully dissecting and narrowing the pedicle inferiorly down to the level of the root of the helix. The flap was then rotated into its final location, ensuring adequate coverage of the cochlear implant and electrodes. If hemostasis was required, bipolar electrocautery and hemostatic agents were utilized judiciously. The TPFF was secured in place using simple, interrupted, dissolvable sutures ( Fig. 1c ). The rotation flap was raised and rotated onto the defect. The wound was closed in two layers ( Fig. 1d ). In patients with larger defects, a back-cut was given to facilitate wound closure, and the donor area was covered with a split-thickness skin graft (STSG). The wound was gently compressed with a mastoid dressing. A small Segmuller drain was placed under the flap prior to wound closure, which was removed after 24 hours. All the patients received antibiotics postoperatively based on available culture results or for general skin organism prophylaxis. Later, the patients were followed up for a minimum of 12 months and were monitored for wound dehiscence, implant re-exposure, and scar alopecia.
Case Illustrations
Case 1
A 3-year-old girl who was 1 month post-cochlear implant was referred to us with a defect size of 2 × 1 cm in the superior aspect of the surgical scar exposing the implant ( Table 1 ). Audiometry evaluation was done preoperatively to check the position of the electrodes. The preoperative cultures were negative. The preoperative markings showing incision for rotation flap ( thick line ) and TPFF extent ( dotted line ) are shown in Fig. 1a . The TPFF was raised through the same incision after anterior undermining ( Fig. 1b ), and the flap was transposed onto the implant and sutured as a snuggly fitting pocket ( Fig. 1c ). The traditional rotation flap was raised along the same incision and rotated onto the defect ( Fig. 1d ). Electrode position was again confirmed intraoperatively. Immediate and 6-month follow-up of the patient showed a well-settled flap with no wound dehiscence. ( Fig. 1e, f )
Table 1. Demographic details of the patients, defect size, type of flap used and complications.
| Sl. no. | Age (y) | Sex | Size of defect | Local flap used | Complications |
|---|---|---|---|---|---|
| 1 | 3 | F | 2 × 1 cm | Rotation flap | Nil |
| 2 | 5 | M | 4 × 3 cm | Transposition flap with STSG | Nil |
| 3 | 2 | F | 1.5 × 1.9 cm | Rotation flap | Nil |
| 4 | 3 | F | 1 × 3 cm | Rotation flap | Nil |
| 5 | 3 | F | 1.7 × 1.5 cm | Rotation flap | Nil |
| 6 | 5 | M | 2.5 × 3 cm | Rotation flap | Nil |
| 7 | 2 | F | 2 × 1 cm | Rotation flap | Nil |
| 8 | 4 | M | 2.3 × 1.7 cm | Rotation flap | Nil |
| 9 | 2 | F | 1.9 × 2.2 cm | Rotation flap | Nil |
| 10 | 3 | F | 2.1 × 3 cm | Rotation flap | Hematoma (evacuated) |
| 11 | 3 | M | 1.4 × 2.7 cm | Rotation flap | Nil |
| 12 | 5 | M | 3 × 3 cm | Transposition flap with STSG | Nil |
| 13 | 2 | F | 1 × 3 cm | Rotation flap | Nil |
| 14 | 2 | F | 2 × 1 cm | Rotation flap | Nil |
| 15 | 3 | M | 1.9 × 3 cm | Rotation flap | Nil |
| 16 | 4 | M | 2 × 1 cm | Rotation flap | Nil |
Abbreviation: STSG, split-thickness skin graft.
Case 2
A 5-year-old boy who was 3 months post-cochlear implant was referred with a defect size of 4 × 3 cm in the center of the surgical scar ( Table 1 ). Audiometry evaluation was done preoperatively to check the position of the electrodes. The pre-op cultures were negative. The same procedure was performed in this case ( Fig. 3a–c ). The scalp skin was raised along the same incision and rotated onto the defect ( Fig. 3d ). As the defect was larger, the rotation flap was converted into the transposition flap and the flap donor site was covered with split skin graft. The electrode position was confirmed intraoperatively. Both immediate post-op and 6-month follow-up of the patient showed a well-settled flap and graft ( Fig. 3e, f ).
Fig. 3.
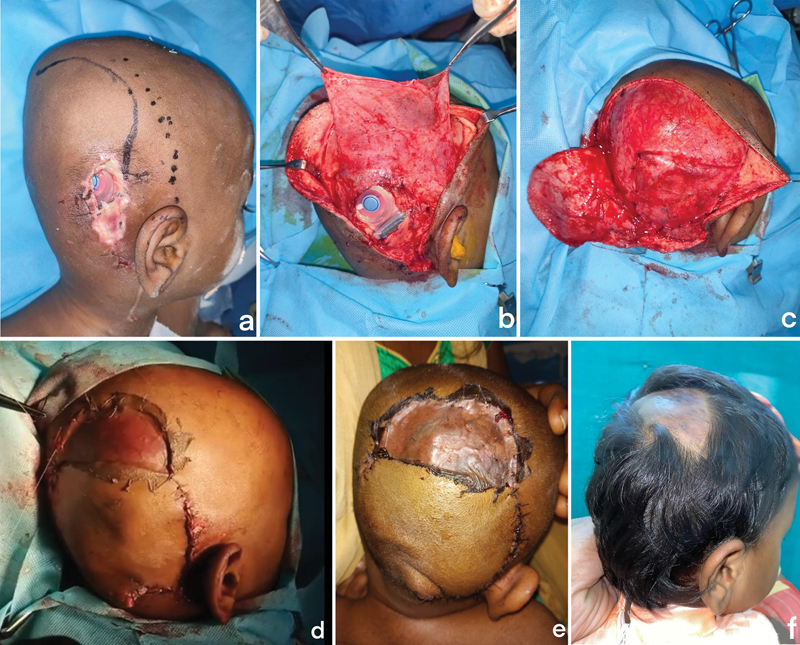
( a ) Preoperative marking showing incision ( thick line ) for rotation flap and a dotted line showing the temporoparietal fascial (TPF) flap marking. ( b ) The TPF flap was raised through the same incision. ( c ) Flap transposed onto the implant and sutured as a snuggly fitting pocket. ( d ) The traditional rotation flap was raised along the same incision with split-thickness skin graft (STSG) for donor area. ( e ) Immediate post-op. ( f ) Late post-op.
Results
Each patient described in this series met the standard cochlear implantation (CI) candidacy. The study was conducted on patients operated on between December 2019 and December 2022. All the patients had undergone cochlear implant surgery on their right side. The incision for the initial cochlear device implantation was a “lazy-S” style and was consistent for every case. Sixteen patients (9 females and 7 males) underwent a dual flap (TPFF + skin flap) procedure as part of a treatment for the exposed cochlear implant. The age of the patients ranged from 1 to 5 years, with the average age being 3 years. All the patients underwent single-stage reconstruction. The average defect size was 2 × 2 cm, with the smallest defect being 2 × 1 cm and the largest defect being 4 × 3 cm. The average length of hospital stay was 10 days. Only one complication (small donor site hematoma) required an intervention (bedside drainage without sequelae). Out of 16 patients, 14 patients' donor sites were closed primarily, and 2 required STSG cover for the donor site. All the patients were treated with perioperative antibiotics according to culture and sensitivity. At the time of discharge, all wounds had healed with intact skin coverage over the cochlear implant device site. The average follow-up period was 12 months (range, 6–24 months). Two patients ( n = 2) had a donor site scar alopecia. All other patients who had primary closure of the donor site had adequate hair growth masking the scar. All the patients are consistent users of their cochlear implants.
Discussion
CI is one of the most efficient surgical options for the management of patients suffering from severe to profound bilateral sensorineural hearing loss since its introduction in 1972. With the advent of government-sponsored programs increasing implant accessibility for a larger population, CIs have been increasingly used in thousands of patients, which increased the incidence of their complications and, subsequently, the number of revision surgeries. The incidence of postoperative complications ranges broadly from 1.7 to 10% of cases. 2 3 And the incidence of the infection of the cochlear implant is stated to be 1.08 to 8.2%. 2 5 6 However, the CI safety profile is not in question; it is still considered a safe procedure. Moreover, CIs have relatively long survival rates, estimated to be 91.9% in a 10-year period.
CI complications are generally classified as minor or major. Minor complications only require conservative management, whereas major complications necessitate revision surgeries or hospitalization for medical treatment. The possible complications include device extrusion, overlying skin necrosis, wound dehiscence, and local or systematic infections. Children aged 1 to 2 years are at a higher risk of repeated infections. Notably, CI exposure is the most common complication reported, with an incidence that reaches up to 5.4%. The causes attributed to cochlear implant exposure are poor placement of the initial incision, infections, skin flap necrosis, and external pressure from patients lying on the implant; internal pressure from the implant due to its sheer bulk can also cause pressure necrosis of the skin, particularly in thin-skinned individuals. 1 7
Exposed implant causes significant morbidity for the child ranging from soft-tissue infection to osteomyelitis of underlying bone. Implant extrusion, malfunction, and need for removal can all be addressed with a well-vascularized stable skin cover of the implant. 8 Mobilizing the adjacent scalp in the form of advancement, rotation, or transposition flap to close the wound appears to be the straightforward solution, but it often fails. So there is the need to provide an additional vascularized fascial cover that provides a secure pocket and long-term cover. 9 The galea aponeurotica, the layer adjacent to the implant, is relatively nonadherent, and there is no subgaleal vascular plexus. Hence, the scalp flaps do not bring in the much-needed vascularity to the site of infection, although the scalp flaps are highly vascular at the skin margins. This fact explains the delayed wound dehiscence as the result of latent infection, even if the flaps initially healed well. The dual cover of TPFF and local skin flap seeks to address this problem. The versatility of TPF in the coverage of exposed cochlear implants has been reported in several case series. 10 11 12
In 1999, Beckenstein et al 13 first reported salvage of an exposed cochlear implant in a 4-year-old boy by covering it with a TPFF. A “ Y ”-shaped incision was used to harvest the temporoparietal fascia ( Fig. 4 ), and the posterior limb of “Y” was in continuity with the original scar used to insert the implant. The TPFF was brought down to cover the implant and the original wound was debrided and closed primarily over the TPFF. This created a bridge of skin tissue between the Y limbs. Should this skin bridge be too thin, it can result in compromised vascularity and skin necrosis.
Fig. 4.
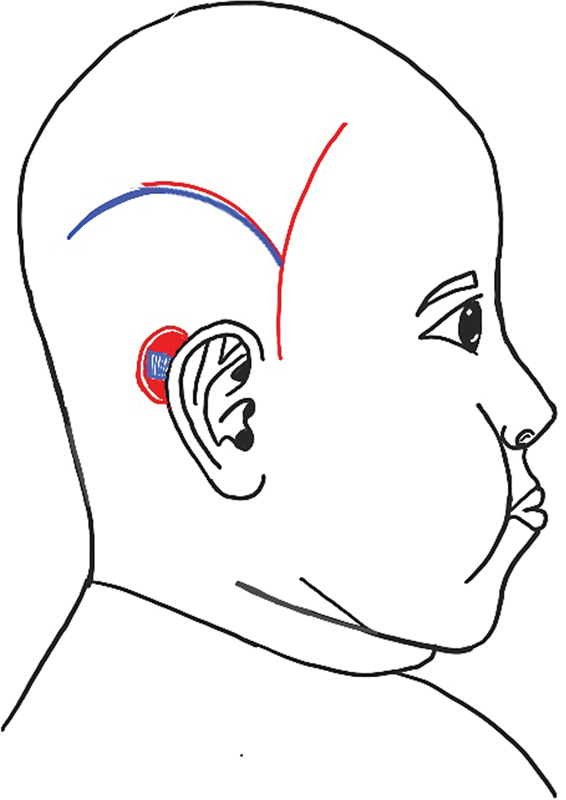
Beckenstein et al's “ Y ”-shaped incision. The red line indicates incision for flap harvest and the blue line indicates the previous scar resulting from an incision used for insertion of the cochlear implant.
Hariharan et al, 9 in their case series on coverage of exposed cochlear implants, found a dual cover with fascia and skin to be the most robust cover in preventing recurrences. However, in their series, they harvested the TPF through an anterior incision independent of the skin rotation flap ( Fig. 5 ).
Fig. 5.
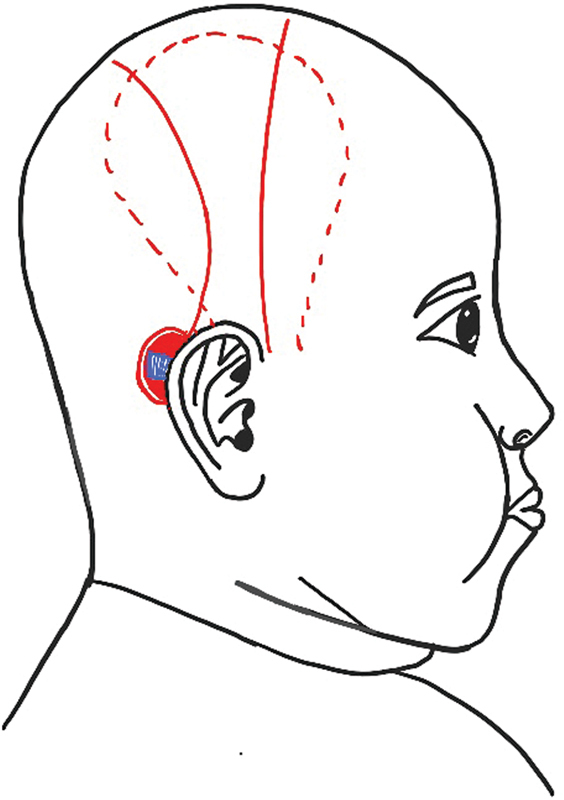
Separate incisions for temporoparietal fascial (TPF) and rotation flap. The red line indicates the incision for flap harvest and the red dotted line indicates the extent of the TPF flap.
We sought to address this problem by harvesting the TPF through the incision placed for the rotation flap extending from the superior aspect of the wound ( Fig. 2 ). We found that undermining of the anterior skin flap afforded excellent visibility and ease of harvesting the TPF without the need for another incision.
Karimnejad et al, 4 in their study, reported two cases where the exposed cochlear implant was covered with TPF. The TPF in both cases was harvested through the old postauricular incisions, which were then extended into a rotation flap. During a 20-month follow-up, they did not note any long-term complications. However, in both cases, the implant could not be salvaged due to methicillin-resistant Staphylococcus aureus (MRSA) infection. In our study, however, all implants were salvaged, and none required reimplantation.
Conclusion
The versatility and reliability of the pedicled TPFF have been well described in the literature. We believe that its ease of harvest, proximity, and well-vascularized nature lend itself well to provide a stable implant pocket where skin cover alone will not suffice. Our study shows that this can be achieved with minimal morbidity using single incision to harvest both flaps.
Conflict of Interest None declared.
Previous Presentation
This paper was presented at TANPAPS (Tamil Nadu and Pondicherry Association of Plastic Surgeons) Annual conference, Pondicherry, June 2022.
References
- 1.Ramos A, Charlone R, de Miguel I et al. Complications in cochlear implantation. Acta Otorrinolaringol Esp. 2006;57(03):122–125. doi: 10.1016/s0001-6519(06)78675-7. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham C D, III, Slattery W H, III, Luxford W M. Postoperative infection in cochlear implant patients. Otolaryngol Head Neck Surg. 2004;131(01):109–114. doi: 10.1016/j.otohns.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Geraghty M, Fagan P, Moisidis E. Management of cochlear implant device extrusion: case series and literature review. J Laryngol Otol. 2014;128 02:S55–S58. doi: 10.1017/S002221511300323X. [DOI] [PubMed] [Google Scholar]
- 4.Karimnejad K, Akhter A S, Walen S G, Mikulec A A. The temporoparietal fascia flap for coverage of cochlear reimplantation following extrusion. Int J Pediatr Otorhinolaryngol. 2017;94:64–67. doi: 10.1016/j.ijporl.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Leach J, Kruger P, Roland P. Rescuing the imperiled cochlear implant: a report of four cases. Otol Neurotol. 2005;26(01):27–33. doi: 10.1097/00129492-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Gluth M B, Singh R, Atlas M D. Prevention and management of cochlear implant infections. Cochlear Implants Int. 2011;12(04):223–227. doi: 10.1179/146701011X12950038111576. [DOI] [PubMed] [Google Scholar]
- 7.Parkins C W, Metzinger S E, Marks H W, Lyons G D. Management of late extrusions of cochlear implants. Am J Otol. 1998;19(06):768–773. [PubMed] [Google Scholar]
- 8.Arab K, Altamimi L, Al-Otaibi H, Kattan A, Gelidan A G. Salvaging exposed cochlear implants. Plast Reconstr Surg Glob Open. 2021;9(10):e3899. doi: 10.1097/GOX.0000000000003899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hariharan N C, Muthukumar R, Sridhar R, Shankari B, Valarmathy V S. Ideal flap cover for the salvage of exposed/infected cochlear implants: a case series and literature review. Indian J Otolaryngol Head Neck Surg. 2020;72(03):292–296. doi: 10.1007/s12070-019-01764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonhard L, Roche J, Wieland A, Pyle G M. The temporoparietal fascia flap is an effective strategy for cochlear implant wound coverage. Ann Otol Rhinol Laryngol. 2020;129(02):135–141. doi: 10.1177/0003489419877429. [DOI] [PubMed] [Google Scholar]
- 11.Seo B F, Park S W, Han H H, Moon S H, Oh D Y, Rhie J W. Salvaging the exposed cochlear implant. J Craniofac Surg. 2015;26(08):e749–e752. doi: 10.1097/SCS.0000000000002259. [DOI] [PubMed] [Google Scholar]
- 12.Jaquet Y, Higgins K M, Enepekides D J. The temporoparietal fascia flap: a versatile tool in head and neck reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2011;19(04):235–241. doi: 10.1097/MOO.0b013e328347f87a. [DOI] [PubMed] [Google Scholar]
- 13.Beckenstein M S, Steenerson R L, Elliott L F, Hartrampf C R., Jr Use of a superficial temporal fascia flap for coverage of an exposed cochlear implant. Otolaryngol Head Neck Surg. 1999;120(06):940–942. doi: 10.1016/S0194-5998(99)70343-8. [DOI] [PubMed] [Google Scholar]


