Abstract
Drug-resistant epilepsy (DRE) poses a significant global challenge, impacting the well-being of patients. Anti-epileptic drugs often fail to effectively control seizures in individuals with DRE. This condition not only leads to persistent seizures but also induces neurochemical imbalances, elevating the risk of sudden unexpected death in epilepsy and comorbidities. Moreover, patients experience mood and personality alterations, educational and vocational setbacks, social isolation, and cognitive impairments. Ketogenic diet has emerged as a valuable therapeutic approach for DRE, having been utilized since 1920. Various types of ketogenic diets have demonstrated efficacy in controlling seizures. By having a multimodal mechanism of action, the ketogenic diet reduces neuronal excitability and the frequency of seizure episodes. In our narrative review, we have initially provided a concise overview of the factors contributing to drug resistance in epilepsy. Subsequently, we have discussed the different available ketogenic diets. We have reviewed the underlying mechanisms through which the ketogenic diet operates. These mechanisms encompass decreased neuronal excitability, enhanced mitochondrial function, alterations in sleep patterns, and modulation of the gut microbiome. Understanding the complex mechanisms by which this diet acts is essential as it is a rigorous diet and requires good compliance. Hence knowledge of the mechanisms may help to advance research on achieving similar therapeutic effects through other less stringent approaches.
Keywords: Ketogenic diet, Refractory epilepsy, Anti-Epileptic drugs, Neuronal excitability, Sleep, Gut microbiome, Gut–brain axis, Drug resistance
Graphical abstract
1. Introduction
Epilepsy is a condition that affects approximately 70 million individuals worldwide.1 In India, there are approximately 10 million cases of epilepsy.2 Although there are only a few incidence studies from India, the most recent one reports an age-standardized incidence rate of 27.3/100,000 per year.3 Despite the increasing number of anti-epileptic drugs (AEDs) available since the discovery of phenytoin in 1930,4 nearly one-third of individuals with epilepsy experience persistent seizures.5 Drug-resistant epilepsy (DRE) is characterized by “the failure of adequate trials of two tolerated appropriately chosen and used AED schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom".6 According to a recent meta-analysis of 38 studies,7 the incidence proportion of DRE ranges from 0.06 to 0.51, and the prevalence ranges from 0.11 to 0.58.
The ketogenic diet has emerged as a promising non-pharmacological and non-invasive approach to managing DRE. This diet is characterized by its high-fat, low-carbohydrate composition, leading to the production of ketone bodies, primarily β-hydroxybutyrate, acetoacetate, and acetone. The ketogenic diet's hypothesized mechanisms often stem from its primary effect of elevated ketone bodies, suggesting that disruptions in cellular metabolism and homeostasis play a crucial role in excessive neuronal activity seen in seizures.8 While the clinical efficacy of the ketogenic diet in reducing seizure frequency is well-documented, the precise mechanisms underlying its therapeutic action in DRE remain a subject of intense research interest. This review seeks to provide a comprehensive overview of the intricate mechanisms by which the ketogenic diet exerts its anti-seizure effects in individuals with DRE.
2. Probable causes of resistance to pharmacotherapy
Several hypotheses have been proposed to explain drug resistance in epilepsy. One hypothesis is the pharmacokinetic hypothesis, which suggests that changes in the metabolism and elimination of anti-epileptic drugs, such as overexpression of transporters like Multi Drug Resistance 1 (MDR1) or polymorphisms of cytochrome P450 (CYP) 2C9 enzyme, may lead to drug resistance.9,10 Another hypothesis is the intrinsic severity hypothesis, which postulates that the severity of the disease determines pharmacoresistance.11,12 The neural network hypothesis suggests that remodelling of neural circuits due to repeated seizures leads to abnormal circuits, which can cause drug resistance.13 The genetic variant hypothesis states that genetic variation in enzymes involved in metabolism of AEDs, receptors or ion channels targeted by AEDs can cause DRE.14,15 The target hypothesis suggests that DRE may be due to molecular targets like altered expression of GABAA receptors or changes in cellular distribution of channels.16, 17, 18 The transporter hypothesis proposes that upregulation of certain efflux transporters (P-glycoprotein) in peripheral organs reduces the amount of AED available to cross the blood–brain barrier.19,20 The epigenetic theory of DRE suggests that upregulation of certain microRNAs and manipulation of microRNAs in animal models can influence seizures.21,22 Lastly, the neuroinflammation hypothesis suggests that inflammation can increase blood–brain permeability, leading to activation of certain pathways that can increase excitability of neurons and seizures.23 Additionally, the influx of albumin due to disruption of the blood–brain barrier may lead to buffering of AEDs and drug resistance.24
3. Ketogenic diets
DRE, or drug-resistant epilepsy, can have a significant impact on patients' quality of life due to various factors. These include the side effects of anti-epileptic drugs, persistent seizures, changes in neurochemicals, an increased risk of SUDEP (Sudden Unexpected Death in Epilepsy), co-existing health conditions, alterations in mood and personality, as well as negative consequences on education, employment, and social life.25 Treatment options for DRE are limited, but among the notable ones are novel anti-epileptic drugs, vagal nerve stimulation, deep brain stimulation, epilepsy surgery, and the ketogenic diet.26 Although the ketogenic diet has been known since the 1920s to have positive effects in treating DRE, its use diminished with the advent of anti-epileptic drugs in the 1930s. However, in the 1990s, there was a resurgence in its utilization due to its effectiveness in DRE and the introduction of alternative ketogenic diets (such as the Modified Atkins Diet, Low Glycemic Index Treatment diet, and Medium Chain Triglyceride diet) that showed comparable seizure control effects,27,28 as well as better adherence compared to the traditional ketogenic diet. The ketogenic diet represents a non-pharmacological, non-invasive therapy that has been proven to be effective in treating DRE. A meta-analysis of 33 studies focusing on the efficacy of the ketogenic diet in infants with DRE demonstrated that approximately 60% of infants achieved a seizure reduction of at least 50%, with 33% becoming seizure-free.29
3.1. Types of ketogenic diet
An ideal ketogenic diet would consist solely of fats but practical considerations, including compliance and side effects, have led to various modifications with a goal to strike a balance between achieving effective ketosis and maintaining good compliance.
3.1.1. Classical ketogenic diet
The classical ketogenic diet follows a specific ratio of grams of fat to grams of protein plus carbohydrates. The commonly used ratio is 4:1, where 90% of the daily energy comes from long chain fats, while carbohydrates and proteins contribute 10%.28 Additionally, calorie intake is limited to 80–90% of the recommended daily amount for the respective age group.
Traditionally, the initiation of the classic diet involves careful monitoring and a fasting period ranging from 12 h up to a maximum of 72 h. Following the fasting phase, meals are gradually introduced in one-third caloric increments until the individual can tolerate a full-caloric meal. An alternative initiation method involves gradually increasing the fat-to-protein + carbohydrate ratio (1:1, 2:1, 3:1, 4:1) while maintaining full calorie intake over several days. This approach offers advantages as it does not require hospitalization and helps avoid the weight loss, hypoglycaemia, and acidosis associated with fasting.30
3.1.2. Medium Chain Triglyceride diet
The medium-chain triglyceride (MCT) diet was introduced by Huttenlocher et al in 1971 as an alternative to the classical ketogenic diet with long-chain triglycerides.31 MCT fats have the advantage of producing a higher amount of ketones per gram compared to the fats used in the classical ketogenic diet. Unlike the classical ketogenic diet, the MCT diet utilizes 60% of the total energy from MCT fats. This allows for a slight increase in the daily intake of dietary protein and carbohydrates for patients.32
3.1.3. Modified Atkins Diet
The Modified Atkins Diet (MAD) was developed at the Johns Hopkins Hospital to address the challenges of palatability and compliance often associated with the classic ketogenic diet. The MAD follows a 0.9:1 ketogenic ratio, which means approximately 65% of the calories come from fat. In children, the diet initially limits carbohydrates to 10 g per day, with planned increases to 15 g after one month, and further increases to 20–30 g per day based on individual seizure control and tolerance. For adults, the carbohydrate limit begins at 15 g per day and can be increased to 20–30 g after one month.33 The MAD has been reported to achieve comparable seizure control effects with fewer side effects compared to other diets (26).
3.1.4. Low Glycaemic Index Treatment diet
Among the ketogenic diets, this one is considered the least restrictive. Its main emphasis is on maintaining stable blood glucose levels by permitting the consumption of carbohydrates with a low glycaemic index, typically below 50.34
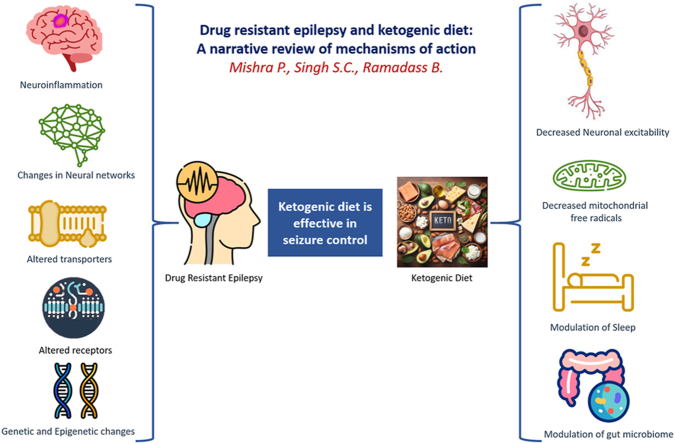
4. Mechanisms of action of ketogenic diet in epilepsy (Fig. 1)
Fig. 1.
Ketogenic diet can exert its effects by decreasing neuronal excitability and modulation of mitochondrial activity, sleep and gut microbiome. (ROS-reactive oxygen species).
The mechanisms by which this diet exerts its therapeutic effects can be categorized into four key areas: mechanisms reducing neuronal excitability, mechanisms enhancing mitochondrial function, and the mechanisms related to modulation of sleep patterns and the gut microbiome.
4.1. Decreased neuronal excitability via modulation of neurotransmitters/receptors
Ketogenic diet can reduce neuronal excitability through several mechanisms which include changes in the processes affecting neurotransmitter release, binding and synthesis, influencing the resting membrane potential and modulating inflammatory processes.
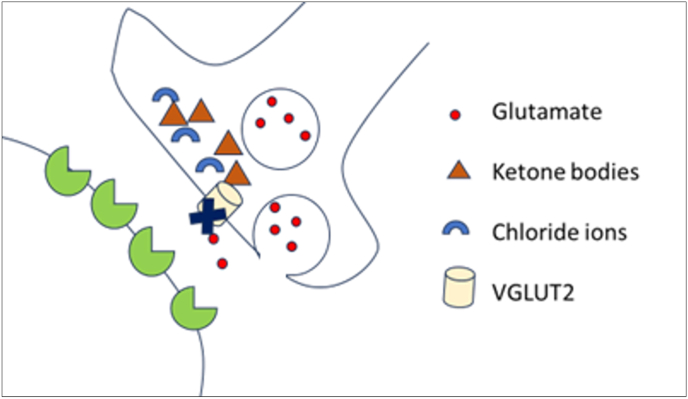
4.1.1. Inhibition of VGLUT2 (vesicular glutamate transporter)
By inhibiting VGLUT2, acetoacetate reduces the transport of glutamate into synaptic vesicles. The proper filling of these vesicles is crucial for the release of glutamate. VGLUTs play a vital role in this transport process, and their function is dependent on the concentration of chloride ions. In a study by Juge et al in 2010, it was found that chloride ions act as allosteric activators for VGLUTs, and ketone bodies, including acetoacetate, compete with chloride ions for activation of the transporter Fig. 2.35 The study further demonstrated that acetoacetate can reversibly inhibit glutamate release in vivo.
Fig. 2.
Ketone bodies compete with chloride ions to decrease uptake of glutamate by vesicular glutamate transporter (VGLUT2).
4.1.2. Enhanced glutamate recycling
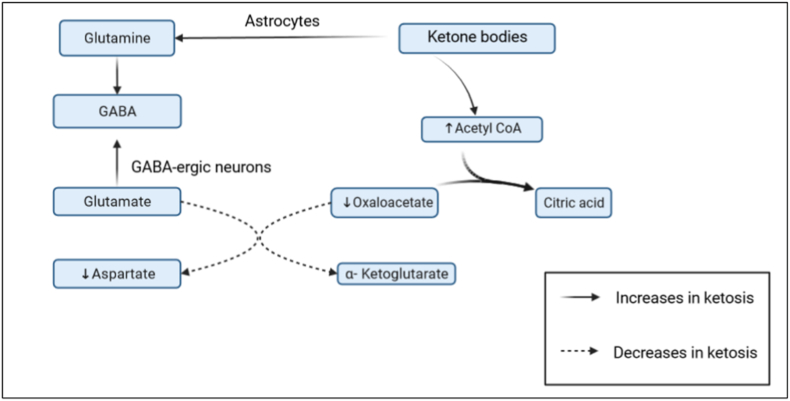
This phenomenon, also known as the anaplerotic effect, occurs when increased ketosis reduces glycolysis and promotes the utilization of non-glucose sources. Fatty acids and ketones are oxidized to fuel the tricarboxylic acid (TCA) cycle, which in turn supports the efficient recycling of glutamate.36 More efficient glutamate recycling in presence of ketone bodies leading to increased GABA synthesis.37 While the oxidation of glucose requires glycolysis to take place in the cytoplasm, ketone bodies like β-Hydroxy butyrate are oxidised in mitochondria directly to be rapidly converted to Acetyl CoA causing its concentration to increase in neurons of the brain. This increase in acetyl CoA increases the rate of production of citrate through citrate synthetase thereby decreasing the level of oxaloacetate which is the other substrate of this reaction. Low levels of oxaloacetate decrease the conversion of glutamate to aspartate (excitatory neurotransmitter) while making more glutamate available for production of GABA (inhibitory neurotransmitter).37 Moreover, ketosis leads to increase in levels of blood acetate which is utilised by the astrocytes for the production of Glutamine-another precursor of GABA (Fig. 3).
Fig. 3.
Ketone bodies exert an anaplerotic effect by diverting glutamate towards increased synthesis of GABA and thereby reducing production of excitatory neurotransmitter aspartate.
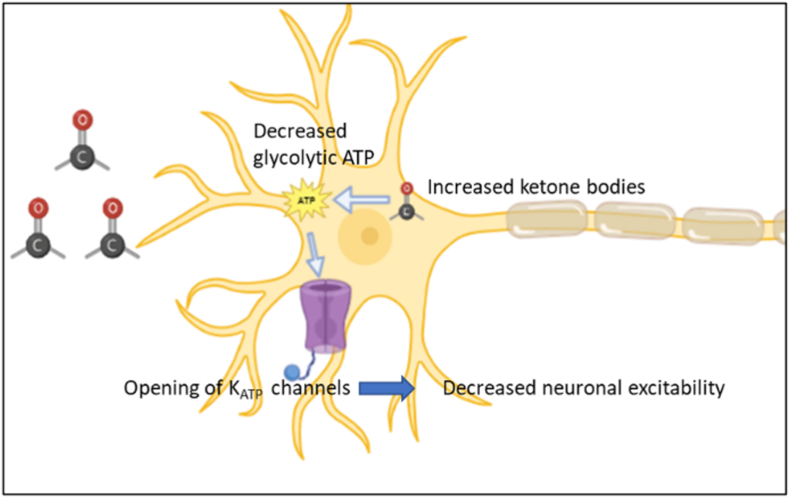
4.1.3. Alteration in membrane potential of neurons
KATP channels are metabolic sensors that regulate electrical activities.38 Decreased glycolysis due to ketosis, leads to opening of ATP sensitive K+ channels which can cause hyperpolarisation and decreased neuronal excitability (Fig. 4).39
Fig. 4.
Ketone bodies produce hyperpolarisation in neurons caused by opening of ATP sensitive K+ channels.
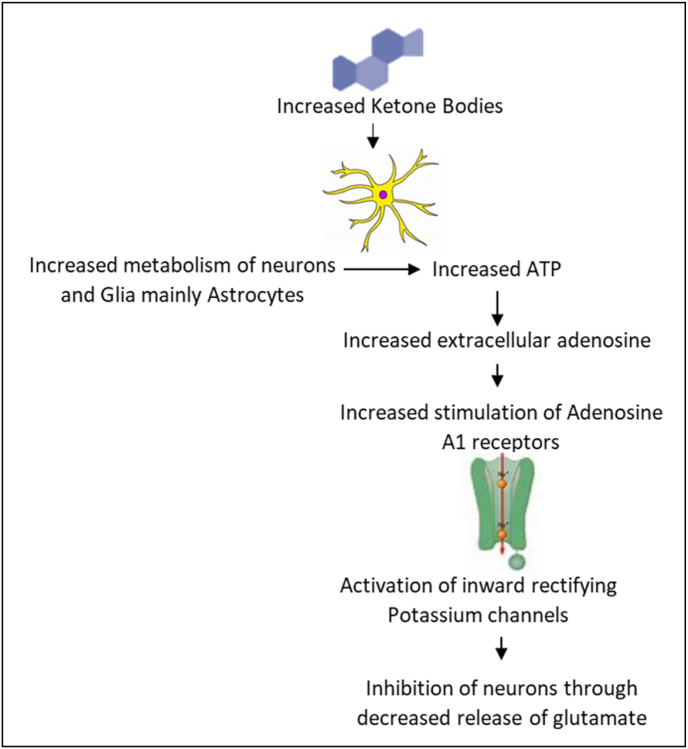
4.1.4. Increased production of adenosine
Adenosine A1 receptors have inhibitory properties and reduce neural excitability by inhibiting adenyl cyclase, activating inwardly rectifying potassium channels, and inhibiting calcium channels. As a result, the release of glutamate is decreased, leading to an anti-convulsant effect40 (Fig. 5). The basal production of endogenous adenosine occurs when neurons and astrocytes, primarily astrocytes, release ATP.41 The ketogenic diet enhances astrocyte metabolism, which in turn increases adenosine levels.42 The increased presence of extracellular adenosine, acting through A1 receptors, can produce an anti-convulsant effect.
Fig. 5.
Ketogenic diet increases metabolism of astrocytes and neurons leading to increased extracellular adenosine which activates Adenosine A1 receptors in neurons. This leads to decreased release of glutamate.
4.1.5. Production of inhibitory neurotransmitters
According to Calderon et al, the ketogenic diet has the potential to raise levels of agmatine, an inhibitory neurotransmitter that can inhibit NMDA, adrenaline, and histamine receptors.43 It has been demonstrated that the proper functioning of the norepinephrine system is crucial for the effectiveness of certain antiepileptic drugs such as Valproic acid, Phenytoin, and phenobarbital, as well as treatment approaches like Vagal nerve stimulation.44 Moreover, the ketogenic diet has been found to elevate extracellular norepinephrine levels.45
4.1.6. Anti-inflammatory effects
Recent research highlights the role of inflammatory mediators originating from peripheral and brain sources in altering neuronal excitability and potentially perpetuating a cycle of glial and neuronal activation associated with seizures.46 Ketones influence molecules related to seizures, such as β-hydroxybutyrate's activation of the G-protein coupled receptor Hydroxy-carboxylic acid receptor 2. This receptor is expressed in brain macrophage which mediates its neuroprotective effects.47 Also, NOD-like receptor protein 3(NLRP3) is an inflammasome that controls apoptotic process (caspase 1 activation) and release of IL-1β and IL-18 is inhibited by β hydroxybuterate.48
4.2. Ketogenic diet can improve mitochondrial function
The ketogenic diet has the potential to enhance mitochondrial function by promoting increased metabolic efficiency, simultaneously reducing the generation of reactive oxygen species (ROS). This mechanism serves to safeguard the brain against oxidative stress.49, 50, 51 Neuronal damage induced by rotenone may be attributed to ATP depletion and the production of ROS within the mitochondria. In a study by Kim et al in 2010, it was observed that inhibitors of mitochondrial respiratory complexes impair synaptic transmission in the hippocampus, whereas ketones offer protection against this impairment by restoring catalase activity, facilitating ATP production, and preventing the formation of reactive oxygen species.51 Stabilizing mitochondria could be a mechanism by which ketone bodies reduce seizure activity. Ketone body metabolism decreases mitochondrial free radical production, which can damage mitochondria and contribute to seizures.52 Ketone bodies also inhibit the opening of the mitochondrial permeability transition pore (mPTP), a channel that can lead to cell death.53 In addition, the ketogenic diet increases the phosphorylation of BAD, a protein involved in cell death. This prevents BAD from interacting with Bcl-2, a protein that protects cells from death.54 Ketone bodies, like β-hydroxybutyrate, interact with histone deacetylases promoting gene transcription by modifying histones, resulting in increased antioxidant expression and reduced oxidative stress.55 Ketone bodies could also increase the expression of NAD + Histone deacetylase sirtuin 3 (SIRT3) to achieve similar effects.56
4.3. Ketogenic diet may modify sleep
Sleep disturbances can present as comorbid conditions in patients with epilepsy, posing challenges to the effective management of epilepsy itself. Sleep disorders and the use of anti-epileptic medications can contribute to sleep fragmentation, ultimately lowering the threshold for seizures.57 Parents of children with epilepsy often report their children experiencing poor sleep quality, daytime sleepiness, behavioral changes, and sleep-related anxiety.58,59 Sleep disruptions can stem from alterations in sleep habits, the presence of specific sleep disorders, the interference of epileptiform activities with sleep patterns at both micro- and macro-levels, or the effects of anti-epileptic drugs.57 In children with refractory epilepsy, studies have indicated a decrease in stage 2 sleep and an increase in slow-wave sleep (SWS).60,61 Cyclic alternating patterns (CAP) are an inherent rhythm observed during non-rapid eye movement (NREM) sleep, and the ratio of CAP time to NREM sleep time serves as a physiological indicator of NREM sleep instability.62 Additionally, an increased CAP rate during sleep facilitates the occurrence of seizures in a clustered manner during sleep.63
The ketogenic diet has been documented to restore normal sleep patterns in cases of super refractory status epilepticus.64 In patients with electrical status epilepticus during slow wave sleep (ESES), who experienced a 50–74% reduction in seizures with the ketogenic diet, improvements ranging from 20 to 50% were observed in EEG abnormalities during sleep.65 However, Iacovides et al, in 2019, reported no subjective changes in sleep status after three weeks of consistent nutritional ketosis.66 In children with therapy-resistant epilepsy, the ketogenic diet led to a significant decrease in total sleep time after three months, along with increased REM sleep and reduced stage 2 sleep. Upon re-evaluation after 11 months of following the ketogenic diet, daytime sleep decreased further, while REM sleep continued to increase.67 The ventrolateral pre-optic nucleus of the hypothalamus, which contains GABA and galanin, plays a role in regulating sub-regions associated with REM control via the locus ceruleus.68 Therefore, changes induced by the ketogenic diet in GABA and galanin levels may contribute to the normalization of REM sleep.
4.4. Ketogenic diet may modulate the microbiota gut brain axis
Studies have shown that various conditions leading to ketosis, such as fasting or adopting a ketogenic diet, can result in distinct changes in the gut microbiota.69 For instance, fasting Syrian hamsters have been reported to have increased levels of the Akkermansia, Disulfvibrio, and Proteobacter genera, while Clostridia levels were reduced.70 Obese humans who fasted for a week had increased levels of Lactobacilli, Enterobacteria, and Akkermansia.71 Obese women who underwent caloric restriction for 28 days demonstrated decreased relative abundance of Proteobacteria and an increase in A. hadrus, which produces butyrate.72 In BTBR mice that mimicked autism spectrum disorder, a ketogenic diet for 10–14 days reversed the low Firmicutes to Bacteroides ratio and reduced the elevated Akkermansia muciniphila content.73 Multiple sclerosis patients on a ketogenic diet for 6 months had reduced diversity of fecal microbiota, with all groups of bacteria except Akkermansia showing decreases.74 Additionally, adults on a ketogenic diet for 4 weeks and mice on a ketogenic diet for 3 weeks had reduced levels of bifidobacteria. The KD-associated gut microbiota was also found to reduce levels of intestinal pro-inflammatory Th17 cells, as revealed by mono-colonizations and human microbiome transplantations into germ-free mice.75
The ketogenic diet has been used to treat drug-resistant epilepsy and recent research has linked the diet's beneficial effects to changes in the gut microbiome. In a study of 20 patients with drug-resistant epilepsy on a 4:1 ketogenic diet for 6 months, 10 patients showed a reduction in seizures of more than 50%, and their fecal microbial profiles revealed decreased levels of Firmicutes and increased levels of Bacteroides.76 Xie et al (2017) also reported an increase in Bacteroides and a shift towards a healthier microbiome resembling that of control subjects after just one week of ketogenic diet in 14 drug-resistant epilepsy cases.77 A comparison of the gut microbiome of drug-resistant epilepsy cases on the ketogenic diet and healthy controls not on the ketogenic diet showed a decrease in relative abundance of bifidobacteria, E. rectale, and Dialister, and an increase in E. coli.78 In patients with GLUT1 deficiency syndrome, Tagliabue et al (2016) reported a statistically significant increase in Desulfovibrio spp. after 90 days of ketogenic diet.79
A study by Olson et al (2018) investigated the role of gut microbiota in mediating the anti-seizure effects of the ketogenic diet (KD) and found that KD-fed mice showed an increased abundance of A. muciniphila, Parabacteroides, Sutterella, and Erysipelotrichaceae, as well as an increased seizure threshold (6 Hz stimulation) compared to those fed a chow diet. This effect was absent in germ-free mice and mice treated with antibiotics. The KD-fed mice also showed increased hippocampal GABA/glutamate ratios. Metabolomic analysis revealed a decrease in ketogenic gamma-glutamylated amino acids in KD-fed mice and decreased gamma-glutamyl transpeptidase activity. The seizure protection effect was also noted in chow diet-fed Specific Pathogen Free (SPF) mice treated with A. muciniphila and Parabacteroides.80 Thus, ketogenic diet may cause seizure reduction through changes in the gut microbiome which can influence the gut–brain axis.
5. Complications of ketogenic diet
The ketogenic diet, while effective for some, can bring about potential complications and side effects. At the time of initiation, some patients might experience gastrointestinal symptoms like nausea, vomiting and constipation.81 Later more serious complications like protein losing enteropathy may lead to hypoproteinaemia and may require cessation of the diet in some individuals.82 Other extraluminal complications like hepatitis, cholelithiasis and pancreatitis are also seen.83,84 Cardiac complications associated with the ketogenic diet, namely prolonged QT intervals linked to metabolic disturbances and cardiomyopathy related to selenium deficiency, are infrequent yet significant.85,86 Being a lipid rich diet, KD has been associated with dyslipidaemia and atherosclerosis. Other complications of KD include osteopenia, renal stones, decreased platelet function as well as overall poor nutritional status of the child.87, 88, 89 While concerns exist regarding potential growth retardation in children following the ketogenic diet due to reduced ghrelin and des-acyl ghrelin levels, studies on growth retardation in relation to the ketogenic diet have yielded conflicting results.90, 91, 92, 93
6. Conclusion
The efficacy of the ketogenic diet in managing drug-resistant epilepsy (DRE) is well-documented in existing literature. Nonetheless, current research exhibits limitations such as the lack of study standardization, a predominant focus on short-term outcomes in most studies, potential challenges related to patient adherence, and an ongoing quest to fully comprehend the diet's exact mechanisms of action. The mechanisms of action are thought to be diverse, including metabolic changes leading to altered neuronal excitability and neurotransmitter levels, improved mitochondrial function, reduced neuronal injury, normalization of sleep architecture, and modulation of the gut microbiome. The concept of “ketomicrobiota” suggests that the gut–brain axis is influenced by the microbiome, leading to improved outcomes in DRE. While this is a promising concept, it is still relatively new and requires further research to fully understand the role of the gut microbiome in drug resistant epilepsy. Also, future research on the ketogenic diet for drug-resistant epilepsy should focus on optimizing diet variations, exploring combination therapies, conducting long-term safety studies, evaluating quality of life impact, expanding global applicability, and establishing standardized clinical guidelines for prescription and management.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Priyadarshini Mishra: Writing – review & editing, Writing – original draft, Supervision, Resources, Conceptualization. Sajal Clarence Singh: Writing – review & editing, Resources. Balamurugan Ramadass: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- AED
Anti-Epileptic Drugs
- DRE
Drug resistant Epilepsy
- EEG
Electroencephalograph
- ESES
Electrical status epilepticus during slow wave sleep
- GABA
Gamma Amino butyric acid
- LGIT
Low Glycaemic Index Treatment
- MAD
Modified Atkins Diet
- MCT
Medium-chain triglyceride
- MDR
Multi drug resistant
- NMDA
N-methyl d-aspartate
- NREM
Non-rapid eye movement
- REM
Rapid eye movement
- ROS
Reactive oxygen species
- SUDEP
Sudden unexpected death in epilepsy
- SWS
Slow wave sleep
- TCA
Tricarboxylic acid
- VGLUT
Vesicular glutamate transporter
References
- 1.Singh A., Trevick S. The epidemiology of global epilepsy. Neurol Clin. 2016;34(4):837–847. doi: 10.1016/j.ncl.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Dixit AB, Banerjee J, Chandra PS, Tripathi M. Recent advances in epilepsy research in India. Neurol India. 65(Supplement):S83-S92. doi:10.4103/neuroindia.NI_1070_16. [DOI] [PubMed]
- 3.Banerjee T.K., Ray B.K., Das S.K., et al. A longitudinal study of epilepsy in Kolkata, India. Epilepsia. 2010;51(12):2384–2391. doi: 10.1111/j.1528-1167.2010.02740.x. [DOI] [PubMed] [Google Scholar]
- 4.Rho J.M., White H.S. Brief history of anti-seizure drug development. Epilepsia open. 2018;3(Suppl Suppl 2):114–119. doi: 10.1002/epi4.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwan P., Brodie M.J. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 6.Kwan P., Arzimanoglou A., Berg A.T., et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 7.Kalilani L., Sun X., Pelgrims B., Noack-Rink M., Villanueva V. The epidemiology of drug-resistant epilepsy: a systematic review and meta-analysis. Epilepsia. 2018;59(12):2179–2193. doi: 10.1111/epi.14596. [DOI] [PubMed] [Google Scholar]
- 8.Masino S.A., Rho J.M. Metabolism and epilepsy: ketogenic diets as a homeostatic link. Brain Res. 2019;1703:26–30. doi: 10.1016/j.brainres.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazarowski A., Czornyj L., Lubienieki F., Girardi E., Vazquez S., D'Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48(Suppl 5):140–149. doi: 10.1111/j.1528-1167.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- 10.Dagenais R., Wilby K.J., Elewa H., Ensom M.H.H. Impact of genetic polymorphisms on phenytoin pharmacokinetics and clinical outcomes in the Middle East and North Africa region. Drugs R. 2017;17(3):341–361. doi: 10.1007/s40268-017-0195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogawski MA, Johnson MR. Intrinsic severity as a determinant of antiepileptic drug refractoriness. Epilepsy Curr. 8(5):127-130. doi:10.1111/j.1535-7511.2008.00272.x. [DOI] [PMC free article] [PubMed]
- 12.Hitiris N., Mohanraj R., Norrie J., Sills G.J., Brodie M.J. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75(2–3):192–196. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Fang M., Xi Z.-Q., Wu Y., Wang X.-F. A new hypothesis of drug refractory epilepsy: neural network hypothesis. Med Hypotheses. 2011;76(6):871–876. doi: 10.1016/j.mehy.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 14.Tate S.K., Depondt C., Sisodiya S.M., et al. Genetic predictors of the maximum doses patients receive during clinical use of the anti-epileptic drugs carbamazepine and phenytoin. Proc Natl Acad Sci U S A. 2005;102(15):5507–5512. doi: 10.1073/pnas.0407346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Löscher W., Klotz U., Zimprich F., Schmidt D. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia. 2009;50(1):1–23. doi: 10.1111/j.1528-1167.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 16.Loup F., Wieser H.G., Yonekawa Y., Aguzzi A., Fritschy J.M. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J Neurosci. 2000;20(14):5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000. http://www.ncbi.nlm.nih.gov/pubmed/10884325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gastaldi M., Robaglia-Schlupp A., Massacrier A., Planells R., Cau P. mRNA coding for voltage-gated sodium channel beta2 subunit in rat central nervous system: cellular distribution and changes following kainate-induced seizures. Neurosci Lett. 1998;249(1):53–56. doi: 10.1016/s0304-3940(98)00394-2. [DOI] [PubMed] [Google Scholar]
- 18.Remy S., Gabriel S., Urban B.W., et al. A novel mechanism underlying drug resistance in chronic epilepsy. Ann Neurol. 2003;53(4):469–479. doi: 10.1002/ana.10473. [DOI] [PubMed] [Google Scholar]
- 19.Potschka H., Löscher W. In vivo evidence for P‐Glycoprotein–Mediated transport of phenytoin at the blood–brain barrier of rats. Epilepsia. 2001;42(10):1231–1240. doi: 10.1046/j.1528-1157.2001.01901.x. [DOI] [PubMed] [Google Scholar]
- 20.Marchi N., Guiso G., Rizzi M., et al. A pilot study on brain-to-plasma partition of 10,11-Dyhydro-10-hydroxy-5H-dibenzo(b,f)azepine-5-carboxamide and MDR1 brain expression in epilepsy patients not responding to oxcarbazepine. Epilepsia. 2005;46(10):1613–1619. doi: 10.1111/j.1528-1167.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller-Delaney S.F.C., Bryan K., Das S., et al. Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain. 2015;138(Pt 3):616–631. doi: 10.1093/brain/awu373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris G., Reschke C.R., Henshall D.C. Targeting microRNA-134 for seizure control and disease modification in epilepsy. EBioMedicine. 2019;45:646–654. doi: 10.1016/j.ebiom.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari C.C., Depino A.M., Prada F., et al. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004;165(5):1827–1837. doi: 10.1016/S0002-9440(10)63438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salar S., Maslarova A., Lippmann K., et al. Blood-brain barrier dysfunction can contribute to pharmacoresistance of seizures. Epilepsia. 2014;55(8):1255–1263. doi: 10.1111/epi.12713. [DOI] [PubMed] [Google Scholar]
- 25.Tang F., Hartz A.M.S., Bauer B. Drug-resistant epilepsy: Multiple hypotheses, few answers. Front Neurol. 2017;8:301. doi: 10.3389/fneur.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair D.R. Management of drug-resistant epilepsy. Continuum. 2016;22(1 Epilepsy):157–172. doi: 10.1212/CON.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 27.Sondhi V., Agarwala A., Pandey R.M., et al. Efficacy of ketogenic diet, modified Atkins diet, and low glycemic index therapy diet among children with drug-resistant epilepsy: a randomized clinical trial. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.2282. Published online August 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kossoff E.H., Zupec-Kania B.A., Rho J.M. Ketogenic diets: an update for child neurologists. J Child Neurol. 2009;24(8):979–988. doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- 29.Liu H., Yang Y., Wang Y., et al. Ketogenic diet for treatment of intractable epilepsy in adults: a meta-analysis of observational studies. Epilepsia open. 2018;3(1):9–17. doi: 10.1002/epi4.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergqvist A.G.C., Schall J.I., Gallagher P.R., Cnaan A., Stallings V.A. Fasting versus gradual initiation of the ketogenic diet: a prospective, randomized clinical trial of efficacy. Epilepsia. 2005;46(11):1810–1819. doi: 10.1111/j.1528-1167.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 31.Dahlin M.G., Beck O.M.L., Amark P.E. Plasma levels of antiepileptic drugs in children on the ketogenic diet. Pediatr Neurol. 2006;35(1):6–10. doi: 10.1016/j.pediatrneurol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Miranda M.J., Turner Z., Magrath G. Alternative diets to the classical ketogenic diet--can we be more liberal? Epilepsy Res. 2012;100(3):278–285. doi: 10.1016/j.eplepsyres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Kossoff E.H., Dorward J.L. The modified Atkins diet. Epilepsia. 2008;49(Suppl 8):37–41. doi: 10.1111/j.1528-1167.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 34.Pfeifer H.H., Lyczkowski D.A., Thiele E.A. Low glycemic index treatment: implementation and new insights into efficacy. Epilepsia. 2008;49(Suppl 8):42–45. doi: 10.1111/j.1528-1167.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 35.Juge N., Gray J.A., Omote H., et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68(1):99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogawski M.A., Löscher W., Rho J.M. Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harb Perspect Med. 2016;6(5) doi: 10.1101/cshperspect.a022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yudkoff M., Daikhin Y., Melø T.M., Nissim I., Sonnewald U., Nissim I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu Rev Nutr. 2007;27:415–430. doi: 10.1146/annurev.nutr.27.061406.093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sada N., Inoue T. Electrical control in neurons by the ketogenic diet. Front Cell Neurosci. 2018;12:208. doi: 10.3389/fncel.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yellen G. Ketone bodies, glycolysis, and KATP channels in the mechanism of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):80–82. doi: 10.1111/j.1528-1167.2008.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27(12):652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Fellin T., Pascual O., Haydon P.G. Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology. 2006;21:208–215. doi: 10.1152/physiol.00161.2005. [DOI] [PubMed] [Google Scholar]
- 42.Masino S.A., Geiger J.D. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci. 2008;31(6):273–278. doi: 10.1016/j.tins.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calderón N., Betancourt L., Hernández L., Rada P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: a microdialysis study. Neurosci Lett. 2017;642:158–162. doi: 10.1016/j.neulet.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Weinshenker D., Szot P. The role of catecholamines in seizure susceptibility: new results using genetically engineered mice. Pharmacol Ther. 2002;94(3):213–233. doi: 10.1016/s0163-7258(02)00218-8. [DOI] [PubMed] [Google Scholar]
- 45.Weinshenker D. The contribution of norepinephrine and orexigenic neuropeptides to the anticonvulsant effect of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):104–107. doi: 10.1111/j.1528-1167.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- 46.Isbrandt D. A mechanistic link between glia and neuronal excitability in acute neuroinflammation. J Physiol. 2017;595(3):603–604. doi: 10.1113/JP273252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahman M., Muhammad S., Khan M.A., et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 48.Youm Y.-H., Nguyen K.Y., Grant R.W., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D.Y., Davis L.M., Sullivan P.G., et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem. 2007;101(5):1316–1326. doi: 10.1111/j.1471-4159.2007.04483.x. [DOI] [PubMed] [Google Scholar]
- 50.Bough K.J., Rho J.M. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48(1) doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim D.Y., Vallejo J., Rho J.M. Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J Neurochem. 2010;114(1):130–141. doi: 10.1111/j.1471-4159.2010.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simeone T.A., Simeone K.A., Stafstrom C.E., Rho J.M. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology. 2018;133:233–241. doi: 10.1016/j.neuropharm.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D.Y., Simeone K.A., Simeone T.A., et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78(1):77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan Y., Demeter M.R., Ruan H., Comb M.J. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem. 2000;275(33):25865–25869. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- 55.Shimazu T., Hirschey M.D., Newman J., et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin J., Han P., Tang Z., Liu Q., Shi J. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cerebr Blood Flow Metabol. 2015;35(11):1783–1789. doi: 10.1038/jcbfm.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parisi P., Bruni O., Pia Villa M., et al. The relationship between sleep and epilepsy: the effect on cognitive functioning in children. Dev Med Child Neurol. 2010;52(9):805–810. doi: 10.1111/j.1469-8749.2010.03662.x. [DOI] [PubMed] [Google Scholar]
- 58.Cortesi F., Giannotti F., Ottaviano S. Sleep problems and daytime behavior in childhood idiopathic epilepsy. Epilepsia. 1999;40(11):1557–1565. doi: 10.1111/j.1528-1157.1999.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 59.Becker D.A., Fennell E.B., Carney P.R. Sleep disturbance in children with epilepsy. Epilepsy Behav. 2003;4(6):651–658. doi: 10.1016/j.yebeh.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Nunes M.L., Ferri R., Arzimanoglou A., Curzi L., Appel C.C., Costa da Costa J. Sleep organization in children with partial refractory epilepsy. J Child Neurol. 2003;18(11):763–766. doi: 10.1177/08830738030180110601. [DOI] [PubMed] [Google Scholar]
- 61.Bazil C.W., Walczak T.S. Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia. 1997;38(1):56–62. doi: 10.1111/j.1528-1157.1997.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 62.Terzano M.G., Parrino L. Origin and significance of the cyclic alternating pattern (CAP). REVIEW ARTICLE. Sleep Med Rev. 2000;4(1):101–123. doi: 10.1053/smrv.1999.0083. [DOI] [PubMed] [Google Scholar]
- 63.Manni R, Zambrelli E, Bellazzi R, Terzaghi M. The relationship between focal seizures and sleep: an analysis of the cyclic alternating pattern. Epilepsy Res 67(1–2):73-80. doi:10.1016/j.eplepsyres.2005.08.008. [DOI] [PubMed]
- 64.Peng P., Peng J., Yin F., et al. Ketogenic diet as a treatment for super-refractory status epilepticus in febrile infection-related epilepsy syndrome. Front Neurol. 2019;10:423. doi: 10.3389/fneur.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reyes G., Flesler S., Armeno M., et al. Ketogenic diet in patients with epileptic encephalopathy with electrical status epilepticus during slow sleep. Epilepsy Res. 2015;113:126–131. doi: 10.1016/j.eplepsyres.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Iacovides S., Goble D., Paterson B., Meiring R.M. Three consecutive weeks of nutritional ketosis has no effect on cognitive function, sleep, and mood compared with a high-carbohydrate, low-fat diet in healthy individuals: a randomized, crossover, controlled trial. Am J Clin Nutr. 2019;110(2):349–357. doi: 10.1093/ajcn/nqz073. [DOI] [PubMed] [Google Scholar]
- 67.Hallböök T., Lundgren J., Rosén I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia. 2007;48(1):59–65. doi: 10.1111/j.1528-1167.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 68.Saper C.B., Scammell T.E., Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 69.Rinninella E., Cintoni M., Raoul P., et al. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11(10) doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sonoyama K., Fujiwara R., Takemura N., et al. Response of gut microbiota to fasting and hibernation in Syrian hamsters. Appl Environ Microbiol. 2009;75(20):6451–6456. doi: 10.1128/AEM.00692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Remely M., Hippe B., Geretschlaeger I., Stegmayer S., Hoefinger I., Haslberger A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: a pilot study. Wien Klin Wochenschr. 2015;127(9–10):394–398. doi: 10.1007/s00508-015-0755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ott B., Skurk T., Hastreiter L., et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-12109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newell C., Bomhof M.R., Reimer R.A., Hittel D.S., Rho J.M., Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism. 2016;7(1):37. doi: 10.1186/s13229-016-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swidsinski A., Dörffel Y., Loening-Baucke V., et al. Reduced mass and diversity of the colonic microbiome in patients with Multiple sclerosis and their improvement with ketogenic diet. Front Microbiol. 2017;8:1141. doi: 10.3389/fmicb.2017.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ang Q.Y., Alexander M., Newman J.C., et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181(6):1263–1275.e16. doi: 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y., Zhou S., Zhou Y., Yu L., Zhang L., Wang Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018;145:163–168. doi: 10.1016/j.eplepsyres.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 77.Xie G., Zhou Q., Qiu C.-Z., et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J Gastroenterol. 2017;23(33):6164–6171. doi: 10.3748/wjg.v23.i33.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindefeldt M., Eng A., Darban H., et al. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ biofilms microbiomes. 2019;5:5. doi: 10.1038/s41522-018-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tagliabue A., Ferraris C., Uggeri F., et al. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 Deficiency Syndrome: a 3-month prospective observational study. Clin Nutr ESPEN. 2017;17:33–37. doi: 10.1016/j.clnesp.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Olson C.A., Vuong H.E., Yano J.M., Liang Q.Y., Nusbaum D.J., Hsiao E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–1741.e13. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai Q.-Y., Zhou Z.-J., Luo R., et al. Safety and tolerability of the ketogenic diet used for the treatment of refractory childhood epilepsy: a systematic review of published prospective studies. World J Pediatr. 2017;13(6):528–536. doi: 10.1007/s12519-017-0053-2. [DOI] [PubMed] [Google Scholar]
- 82.Braamskamp M.J.A.M., Dolman K.M., Tabbers M.M. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169(10):1179–1185. doi: 10.1007/s00431-010-1235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi J., Young T.L., Chartier L.B. Recurrent acute pancreatitis during a ketogenic diet-a case report and literature review. Int J Emerg Med. 2021;14(1):52. doi: 10.1186/s12245-021-00374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mori M., Kumada T., Inoue K., et al. Ketogenic diet for refractory epilepsy with MEHMO syndrome: caution for acute necrotizing pancreatitis. Brain Dev. 2021;43(6):724–728. doi: 10.1016/j.braindev.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 85.Best T.H., Franz D.N., Gilbert D.L., Nelson D.P., Epstein M.R. Cardiac complications in pediatric patients on the ketogenic diet. Neurology. 2000;54(12):2328–2330. doi: 10.1212/wnl.54.12.2328. [DOI] [PubMed] [Google Scholar]
- 86.Bergqvist A.G.C., Chee C.M., Lutchka L., Rychik J., Stallings V.A. Selenium deficiency associated with cardiomyopathy: a complication of the ketogenic diet. Epilepsia. 2003;44(4):618–620. doi: 10.1046/j.1528-1157.2003.26102.x. [DOI] [PubMed] [Google Scholar]
- 87.Sampath A., Kossoff E.H., Furth S.L., Pyzik P.L., Vining E.P.G. Kidney stones and the ketogenic diet: risk factors and prevention. J Child Neurol. 2007;22(4):375–378. doi: 10.1177/0883073807301926. [DOI] [PubMed] [Google Scholar]
- 88.Kose E., Guzel O., Arslan N. Analysis of hematological parameters in patients treated with ketogenic diet due to drug-resistant epilepsy. Neurol Sci. 2018;39(1):85–89. doi: 10.1007/s10072-017-3152-x. [DOI] [PubMed] [Google Scholar]
- 89.Armeno M., Verini A., del Pino M., et al. A prospective study on changes in nutritional status and growth following two years of ketogenic diet (KD) therapy in children with refractory epilepsy. Nutrients. 2019;11(7):1596. doi: 10.3390/nu11071596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marchiò M., Roli L., Giordano C., Trenti T., Guerra A., Biagini G. Decreased ghrelin and des-acyl ghrelin plasma levels in patients affected by pharmacoresistant epilepsy and maintained on the ketogenic diet. Clin Nutr. 2019;38(2):954–957. doi: 10.1016/j.clnu.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 91.Hsieh T.-Y., Su T.-Y., Hung K.-Y., et al. Feasibility of ketogenic diet therapy variants for refractory epilepsy in neonates to infants under 2 years old. Epilepsy Behav. 2023;146 doi: 10.1016/j.yebeh.2023.109315. [DOI] [PubMed] [Google Scholar]
- 92.Groleau V., Schall J.I., Stallings V.A., Bergqvist C.A. Long-term impact of the ketogenic diet on growth and resting energy expenditure in children with intractable epilepsy. Dev Med Child Neurol. 2014;56(9):898–904. doi: 10.1111/dmcn.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferraris C., Guglielmetti M., Pasca L., et al. Impact of the ketogenic diet on linear growth in children: a single-center retrospective analysis of 34 cases. Nutrients. 2019;11(7) doi: 10.3390/nu11071442. [DOI] [PMC free article] [PubMed] [Google Scholar]