Abstract
Systemic inflammation and reciprocal organ interactions are associated with the pathophysiology of heart failure with preserved ejection fraction (HFpEF). However, the clinical value, especially the diagnositc prediction power of inflammation and extra-cardiac organ dysfunction for HfpEF is not explored. In this cross-sectional study, 1808 hospitalized patients from January 2014 to June 2022 in ChiHFpEF cohort were totally enrolled according to inclusion and exclusion criteria. A diagnostic model with markers from routine blood test as well as liver and renal dysfunction for HFpEF was developed using data from ChiHFpEF-cohort by logistic regression and assessed by receiver operating characteristic curve (ROC) and Brier score. Then, the model was validated by the tenfold cross-validation and presented as nomogram and a web-based online risk calculator as well. Multivariate and LASSO regression analysis revealed that age, hemoglobin, neutrophil to lymphocyte ratio, AST/ALT ratio, creatinine, uric acid, atrial fibrillation, and pulmonary hypertension were associated with HFpEF. The predictive model exhibited reasonably accurate discrimination (ROC, 0.753, 95% CI 0.732–0.772) and calibration (Brier score was 0.200). Subsequent internal validation showed good discrimination and calibration (AUC = 0.750, Brier score was 0.202). In additoin to participating in pathophysiology of HFpEF, inflammation and multi-organ interactions have diagnostic prediction value for HFpEF. Screening and optimizing biomarkers of inflammation and multi-organ interactions stand for a new field to improve noninvasive diagnostic tool for HFpEF.
Keywords: Heart failure preserved ejection fraction, Inflammation, Organ interaction, Diagnostic prediction model, Nomogram
Subject terms: Biomarkers, Interventional cardiology, Cardiovascular diseases, Heart failure
Introduction
Heart failure (HF) is the end-stage syndrome of diverse heart diseases. It is a rapidly growing and life-threatening public health problem as the aging of global population. The prevalence of HF is more than 64.3 million people around the world1, and approximately 50% of patients with HF have heart failure preserved ejection fraction (HFpEF)2. There are heterogeneities in both etiologies and phenotypes of HFpEF3. Thus far, the treatment of HFpEF still remains challenging. The DELIVER trial found that the sodium-glucose cotransporter 2 inhibitor dapagliflozin could reduce the risk of cardiovascular death and worsening of HF4. The EMPEROR-preserved study also found that treatment with the sodium-glucose cotransporter 2 inhibitor empagliflozin reduced the composite end point of cardiovascular death and hospitalization for HF by 21% in patients with HFpEF5.
The gold standard for diagnosis of HFpEF is right heart catheterization (RHC) followed by invasive exercise testing6. Considering the medical cost and invasiveness, RHC is not universally available. Recently, scoring systems have been developed to improve the diagnostic accuracy for HFpEF, and increasing evidences suggested invasive or non-invasive exercise testings in those with at least intermediate HFpEF probability7. The H2FPEF and HFA–PEFF noninvasive scoring systems for the diagnostic workup of HFpEF have been reported8,9, which are considered as reliable methods in diagnosis and treatment of cardiovascular diseases (CVDs)10. The H2FPEF score enables discrimination of HFpEF from noncardiac causes of dyspnea, relying on clinical characteristics (age > 60 years, obesity, atrial fibrillation, treatment with ≥ 2 antihypertensives) and echocardiographic measurements (E/E’ ratio > 9, pulmonary artery systolic pressure (PASP) > 35 mmHg)8. The HFA–PEFF score provides a new stepwise diagnostic process, including pre-test assessment, data from echocardiography and natriuretic peptides, and functional testings9.
Currently, systemic inflammation-induced microvascular endothelial dysfunction is deemed as one of the major contributors for the pathogenesis and progression of HFpEF11,12. Clusters of inflammation proteins were reported to mediate the coupling of comorbidity burden, right ventricular dysfunction and poor outcomes of HFpEF by increasing cardiomyocyte passive tension and aggravating aberrant myocardial collagen deposition13,14. In addition, local inflammation could lead the hypophosphorylation of giant sarcomeric protein titin by reducing nitric oxide and cyclic guanosine monophosphate availability, and increase myocardial stiffness and worsen diastolic function15. The neutrophil to lymphocyte ratio (NLR), a sensitive inflammatory marker, has gained recognition correlated with poor outcomes in CVDs16, and a significant elevation of NLR was observed in patients with HFpEF17. In addition, elevated uric acid (UA) has been considered as a marker of inflammatory cytokine activation18. It is common that patients with HFpEF have hyperuricemia, and several studies have revealed the correlation of elevated UA level with adverse outcomes of HFpEF19–21. Moreover, renal dysfunction22 and liver diseases23,24, were clinical co-morbidities for HFpEF. However, the clinical value, especially the diagnositc prediction power of inflammation and extra-cardiac organ dysfunction for HFpEF is not explored. We proposed a new diagnostic scoring systems with inflammatory markers and markers for extra-cardiac organ dysfunction in the study.
Materials and methods
Study design, data source
ChiHFpEF cohort is a prospective Study of HFpEF in Chinese Han patients with documented CVDs in Nanjing First Hospital, Nanjing Medical University (NCT05278026)25. In this study, 2967 patients in ChiHFpEF cohort were totally enrolled. According to inclusion and exclusion criteria, 1808 hospitalized patients from January 2014 to June 2022 with full recordings of routine blood test, liver and renal function test were included in the final analysis. This study was approved by the institutional research ethics committee of Nanjing First Hospital, Nanjing Medical University (KY20211011-04). This study was performed in accordance with the Declaration of Helsinki, and all patients signed the informed consent.
Participants
Inclusion criteria
Patients aged > 18.
Patients with at least one following cardiovascular comorbidity: coronary heart disease, or hypertension, or diabetes mellitus.
LVEF ≥ 50%, determined by Simpson’s biplane method.
Exclusion criteria
Patients declined the N-Terminal pro B-type natriuretic peptide (NT-pro BNP) test.
Patients declined echocardiography or with LVEF < 50%.
Patients had severe hepatic impairment, including alanine aminotransferase (ALT) > 140U/L, or aspartate aminotransferase (AST) > 140U/L.
Patients refused to sign informed consent.
Patients with arrhythmia causing aberrant hemodynamics, chronic obstructive pulmonary disease, sleep apnea, aortic dissection, peripheral vascular diseases, pericardial diseases, myocarditis, hypertrophic cardiomyopathy, severe valvular heart diseases, cardiovascular neurosis, amyloidosis, costochondritis, shock, thyroid diseases and infectious diseases.
Predictor variables
The candidate variables were extracted based on already published literature and expert consensus. We collected the data on demographics, and comorbidities from medical record. The following laboratory parameters were obtained: white blood cell count (WBC count), lymphocyte count, monocyte count, neutrophil count, eosinophil count, basophil count, hemoglobin (Hb), ALT, AST, blood urea nitrogen (BUN), creatinine (Cr), UA, total-cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), Apolipoproteins A1 (Apo A1), Apolipoproteins B (Apo B), and lipoprotein A (Lpa). The following indices of echocardiography were collected: left atrium diameter (LAD), left ventricular diameter in diastole (LVDd), left ventricular diameter in systole (LVDs), end diastolic thickness of posterior left ventricular wall (LVPWD), interventricular septal thickness (IVSD), fractional shortening (FS), stroke volume (SV), LVEF, the maximum early transmitral flow velocity (Peak E velocity), the maximum early transmitral flow velocity in atrial systole (Peak A velocity), myocardial tissue velocity measured atthe septal and/or lateral mitral annulus (Peak E' velocity), the maximum myocardial tissue velocity measured at the mitral annulus in atrial systole (Peak A' velocity), E/A ratio, E'/A' ratio, and E/E' ratio. The laboratory tests, including routine blood test, renal and liver function tests, and NT-pro BNP level were measured on the next morning after admission. The echocardiography was performed by trained and qualified sonographers, and the quality of echocardiographic images was controlled by the Cardiovascular Imaging Laboratory of Nanjing First Hospital, Nanjing Medical University. The coronary heart disease was defined by coronary artery stenosis ≥ 50% on percutaneous coronary angiography or CT coronary imaging. The hypertension was defined by a systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg measured three times on non-same day. The diabetes mellitus was defined as fasting plasma glucose ≥ 7.0 mmol/L, or random blood glucose ≥ 11.0 mmol/L, or a hemoglobin A1c ≥ 6.5 mg/dL. The NLR was defined as the ratio of neutrophil count to lymphocyte count. The AST/ALT ratio was defined as the ratio of AST to ALT. The atrial fibrillation (AF) was diagnosed by clinical history and electrocardiograph, and pulmonary hypertension (PH) was evaluated by PASP from the echocardiography26,27.
Outcomes
HFpEF was diagnosed independently by two expert cardiologists according to the combinations of clinical manifestation, echocardiography and the NT-pro BNP, and any disagreement would be discussed. The diagnosis of HFpEF was made by the following criteria6:
Patients with orthopnoea, paroxysmal nocturnal dyspnoea, fatigue, pulmonary rales, bilateral ankle oedema, or other clinical signs and symptoms.
The LVEF ≥ 50%.
NT-pro BNP ≥ 125 pg/mL with sinus rhythm and ≥ 450 pg/mL with AF.
To fulfil at least one following additional criterion revealing cardiac functional and structural alterations: LAD > 40 mm; E/E’ ≥ 13, E’/A’ < 1.
Handling of missing data
Of all 44 candidate variables, 31 variables contained missing values. It was not randomly occurred for the missing values of peak velocity in the echocardiography because there were the patients with AF included28. Therefore, we dismissed these missing values of peak velocity. The residual missing values ranged from 1 (0.06%) missing value for age to 169 (9.35%) missing values for Apo B. In previous studies, missing values were imputed by applying multiple imputation8,29. Cummings’ study suggested that multiple imputation might be not superior to other interpolation methods when the proportion of missing data was less than 10%30. Hence, for these low missing values, we imputed them via the EM algorithm to minimize the bias in our study.
Statistical analysis
Variable selection and development of model
Continuous variables were reported as mean and SD or median and interquartile ranges according to their distribution, and categorical variables were summarized as the proportions within each category. First, univariate regression analysis was performed to screen the significant candidate predictors for HFpEF using SPSS software (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Continuous variables were compared using the t-test or Mann–Whitney U test, and categorical variables were compared using the Chi-squared test or Fisher's exact test. A p-value of less than 0.05 was considered statistically significant. Second, based on significant candidate predictors obtaining from univariate analysis, multivariate and least absolute shrinkage and selection operator (LASSO) regression analyses were applied, respectively, and the intersection was taken for screening optimal predictors for HFpEF. The stepwise forwards method was performed in multivariate regression analysis by using SPSS. The LASSO regression analysis was performed by using “glmnet” package in R software (version 4.1.2; http://www.r-project.org/). Third, based on these optimal predictors, the final model was refitting by using a logistic regression model.
Evaluation and validation of model
At the stage of model evaluation, the predictive ability for patients with HFpEF was calculated. The discrimination of model was evaluated by the area under the curve (AUC)31. Receiver operating characteristic curve (ROC) was plotted by using MedCalc software, and AUC and cut-off points for sensitivity or specificity levels were obtained as well. The calibration of model was assessed by the Brier score31. The calibration curve was plotted by using R “rms” package. The potential multicollinarity of model was diagnosed using the variance inflation factor (VIF) by the R “car” package32. Internal validation of model was performed using tenfold cross-validation. The discrimination and calibration of internal validation were determined by the mean of AUC and Brier score. The tenfold cross-validated calibration plot was used to exam graphically the model calibration. In order to evaluate the importance of each predictor in terms of prediction for HFpEF, the new regression model was refitted by removing any one predictor in the original model. Then, the predictive ability of each new model was calculated. The Delong method and likelihood ratio test were applied for comparing predictive ability, and fitting ability between original model and each new model, respectively. In addition, a nomogram was generated using R “rms” package, and a web-based online risk calculator using the R “shiny” package was designed as well. Figure 1 showed the flowchart of predictive model construction.
Figure 1.

The flowchart of predictive model construction.
Results
Patient characteristics
Figure 2 showed the flowchart of patient inclusion. A total of 1808 patients were analyzed in our study according to inclusion and exclusion criteria. Of these, 47.23% patients were HFpEF. Clinical characteristics of the HFpEF group and the non- HFpEF control were summarized in Table 1.
Figure 2.
The flowchart of patient inclusion.
Table 1.
Clinical characteristics of HFpEF vs. non-HFpEF patients.
| Variables | Total subjects (n = 1808) | non-HFpEF patients (n = 954) | HFpEF patients (n = 854) | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, yrs | 68 ± 11 | 65 ± 11 | 71 ± 11 | < 0.001 |
| Female, n (%) | 738 (40.8) | 360 (37.7) | 378 (44.3) | 0.005 |
| AF, n (%) | 253 (14.0) | 47 (4.9) | 206 (24.1) | < 0.001 |
| BMI, kg/m^2 | 27.44 ± 5.09 | 27.32 ± 4.95 | 27.59 ± 5.25 | 0.273 |
| Smoking, n (%) | 559 (30.9) | 340 (35.6) | 219 (25.6) | < 0.001 |
| Alcohol habit, n (%) | 432 (23.9) | 270 (28.3) | 162 (19.0) | < 0.001 |
| Hypertension, n (%) | 1424 (78.8) | 726 (76.1) | 698 (81.7) | 0.003 |
| Coronary heart diseases, n (%) | 1297 (71.7) | 684 (71.7) | 613 (71.8) | 0.969 |
| Diabetes mellitus, n (%) | 575 (31.8) | 279 (29.3) | 296 (34.7) | 0.014 |
| PH, n (%) | 314 (17.4) | 95 (10.0) | 219 (25.6) | < 0.001 |
| Laboratory Characteristics | ||||
| NT-proBNP, pg/ml | 256 (100,594) | 50 (20,162) | 630 (407,1394) | < 0.001 |
| WBC count, 10^9/L | 6.50 ± 1.98 | 6.53 ± 1.87 | 6.46 ± 2.09 | 0.419 |
| Lymphocyte count, 10^9/L | 1.66 ± 0.63 | 1.75 ± 0.63 | 1.56 ± 0.62 | < 0.001 |
| Monocyte count, 10^9/L | 0.44 ± 0.19 | 0.44 ± 0.18 | 0.44 ± 0.20 | 0.735 |
| Neutrophil count, 10^9/L | 4.23 ± 1.73 | 4.17 ± 1.61 | 4.29 ± 1.85 | 0.134 |
| Eosinophil count, 10^9/L | 0.10 (0.06,0.17) | 0.10 (0.06,0.17) | 0.10 (0.05,0.17) | 0.22 |
| Basophil count, 10^9/L | 0.03 (0.02,0.04) | 0.03 (0.02,0.05) | 0.03 (0.02,0.04) | < 0.001 |
| NLR | 2.99 ± 2.41 | 2.71 ± 1.72 | 3.30 ± 2.98 | < 0.001 |
| Hb, g/L | 130 ± 19 | 134 ± 16 | 125 ± 21 | < 0.001 |
| AST/ALT | 1.21 ± 0.74 | 1.10 ± 0.47 | 1.34 ± 0.93 | < 0.001 |
| BUN, mmol/L | 5.8 (4.8,7.2) | 5.6 (4.7,6.6) | 6.2 (4.9,8.3) | < 0.001 |
| Cr, umol/L | 74 (61,89) | 71 (59,83) | 78 (64,101) | < 0.001 |
| UA, umol/L | 340 ± 110 | 325 ± 101 | 357 ± 116 | < 0.001 |
| Total-cholesterol, mmol/L | 3.95 ± 1.14 | 3.94 ± 1.04 | 3.95 ± 1.25 | 0.817 |
| Triglycerides, mmol/L | 1.62 ± 1.03 | 1.71 ± 1.11 | 1.51 ± 0.93 | < 0.001 |
| HDL-C, mmol/L | 1.04 ± 0.25 | 1.04 ± 0.25 | 1.04 ± 0.25 | 0.885 |
| LDL-C, mmol/L | 2.23 ± 0.91 | 2.21 ± 0.79 | 2.26 ± 1.02 | 0.223 |
| Apo A1, g/L | 1.25 ± 0.23 | 1.27 ± 0.22 | 1.24 ± 0.23 | 0.033 |
| Apo B, g/L | 0.75 ± 0.25 | 0.75 ± 0.23 | 0.76 ± 0.28 | 0.894 |
| Lpa, mg/L | 178 (88,332) | 162 (83,307) | 200 (95,369) | < 0.001 |
| Echocardiographic results | ||||
| LAD, mm | 42 ± 6 | 40 ± 5 | 44 ± 7 | < 0.001 |
| LVDd, mm | 49 ± 4 | 48 ± 4 | 49 ± 5 | < 0.001 |
| LVDs, mm | 32 ± 3 | 31 ± 3 | 32 ± 4 | < 0.001 |
| LVPWD, mm | 9 ± 1 | 9 ± 1 | 10 ± 1 | < 0.001 |
| IVSD, mm | 10 ± 2 | 10 ± 2 | 11 ± 2 | < 0.001 |
| FS, % | 35 ± 3 | 35 ± 2 | 34 ± 3 | < 0.001 |
| SV, ml | 71 ± 15 | 70 ± 14 | 71 ± 16 | 0.14 |
| LVEF, % | 63 ± 3 | 64 ± 3 | 63 ± 4 | < 0.001 |
| Peak E velocity, cm/s | 69 ± 19 | 69 ± 17 | 70 ± 21 | 0.161 |
| Peak A velocity, cm/s | 89 ± 19 | 87 ± 18 | 91 ± 21 | < 0.001 |
| Peak E' velocity, cm/s | 6.0 ± 1.7 | 6.2 ± 1.7 | 5.7 ± 1.6 | < 0.001 |
| Peak A' velocity, cm/s | 9.7 ± 2.0 | 9.9 ± 1.9 | 9.5 ± 2.1 | 0.001 |
| E/A ratio | 0.81 ± 0.30 | 0.82 ± 0.29 | 0.80 ± 0.32 | 0.397 |
| E'/A' ratio | 0.64 ± 0.20 | 0.64 ± 0.19 | 0.64 ± 0.21 | 0.811 |
| E/E' ratio | 12.35 ± 4.15 | 11.79 ± 3.64 | 13.14 ± 4.66 | < 0.001 |
AF, atrial fibrillation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Apo A1, Apolipoproteins A1; Apo B, Apolipoproteins B; BUN, blood urea nitrogen; Cr, creatinine; FS, fractional shortening; Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; IVSD, interventricular septal thickness; LDL-C, low-density lipoprotein cholesterol; Lpa, lipoprotein A; LAD, left atrial diameter; LVDd, left ventricular diameter in diastole; LVDs, left ventricular diameter in systole; LVPWD, End diastolic thickness of posterior left ventricular wall; LVEF, left ventricular ejection fraction; NT-proBNP, N-Terminal pro B-type natriuretic peptide; NLR, neutrophil to lymphocyte ratio; PH, pulmonary hypertension; Peak E, the maximum early transmitral flow velocity; Peak A, the maximum early transmitral flow velocity in atrial systole; Peak E', myocardial tissue velocity measured atthe septal and/or lateral mitral annulus; Peak A', the maximum myocardial tissue velocity measured at the mitral annulus in atrial systole; RBC, red blood cell; SV, stroke volume; UA, uric acid; WBC, white blood cell.
Model development and presentation
We initially conducted univariate analysis to screen potential predictors for HFpEF in the 44 variables (p < 0.05). The univariate analysis revealed statistical differences in 8 variables in demographics (age, gender, AF, smoking, alcohol habit, hypertension, diabetes mellitus, and PH), 12 variables in laboratory parameters (NT-pro BNP, lymphocyte count, basophil count, NLR, Hb, AST/ALT ratio, BUN, Cr, UA, triglycerides, Apo A1 and Lpa), and 11 variables in echocardiographic results (LAD, LVDd, LVDs, LVPWD, IVSD, FS, LVEF, Peak A velocity, Peak E' velocity, Peak A' velocity, and E/E' ratio).
Next, echocardiographic indices and NT-pro BNP were part of the clinical diagnostic criteria for HFpEF. First, echocardiography and NT-pro BNP are not routinely performed in patients outside department of cardiology. Moreover, they are structural and functional alterations resultant from HFpEF other than causes or risks of HFpEF incidence. We excluded echocardiographic indices and NT-pro BNP when developing this prediction model. Then, 19 variables were selected as candidate predictors, including age, gender, AF, smoking, alcohol habit, hypertension, diabetes mellitus, PH, lymphocyte count, basophil count, NLR, Hb, AST/ALT ratio, BUN, Cr, UA, triglycerides, Apo A1, and Lpa. By multivariate regression analysis, 9 variables were selected as significant predictors of HFpEF (Table 2). By LASSO regression analysis, 9 variables were selected, including Age, L, Hb, NLR, AST/ALT, Cr, UA, AF, PH. After taking the intersection, 8 variables were obtained, namely optimal predictors for HFpEF, and a final model was built using the logistic regression model (Table 3). In this model, AF was an independent predictor with a 4.7-fold increased risk for HFpEF (OR, 4.70; 95% CI 3.32–6.77; p < 0.001).
Table 2.
Multivariable analyses of candidate predictors for HFpEF.
| Variables | OR | OR 95% CI | p-value |
|---|---|---|---|
| Age, yrs | 1.02 | (1.01,1.03) | < 0.001 |
| Hb, g/L | 0.985 | (0.98,0.99) | < 0.001 |
| NLR | 1.082 | (1.02,1.14) | 0.006 |
| AST/ALT | 1.619 | (1.30,2.02) | < 0.001 |
| BUN, mmol/L | 0.985 | (0.97,1.00) | 0.052 |
| Cr, umol/L | 1.003 | (1.00,1.01) | < 0.001 |
| UA, umol/L | 1.003 | (1.00,1.01) | < 0.001 |
| AF, n(%) | 4.718 | (3.30,6.74) | < 0.001 |
| PH, n(%) | 1.837 | (1.37,2.47) | < 0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AF, atrial fibrillation; BUN, blood urea nitrogen; Cr, creatinine; Hb, Hemoglobin; NLR, neutrophil to lymphocyte ratio; PH, pulmonary hypertension; UA, uric acid.
Table 3.
The predictors of HFpEF.
| Variables | OR | OR 95% CI | p-value |
|---|---|---|---|
| Age, yrs | 1.02 | 1.010,1.031 | < 0.001 |
| Hb, g/L | 0.98 | 0.978,0.991 | < 0.001 |
| NLR | 1.07 | 1.017,1.134 | 0.014 |
| AST/ALT | 1.58 | 1.277,1.979 | < 0.001 |
| Cr, umol/L | 1.00 | 1.001,1.005 | < 0.001 |
| UA, umol/L | 1.00 | 1.000,1.003 | 0.004 |
| AF, n(%) | 4.70 | 3.319,6.765 | < 0.001 |
| PH, n(%) | 1.82 | 1.360,2.449 | < 0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AF, atrial fibrillation; Cr, creatinine; Hb, Hemoglobin; NLR, neutrophil to lymphocyte ratio; PH, pulmonary hypertension; UA, uric acid.
Model evaluation and validation
No significant multicollinearity was observed for any variables included in our model (Table 4). The potential linear relationship between each variable and HFpEF was assessed by the spline function (Fig. 3). There was a positive linear relationship between age, NLR, UA, AST/ALT ratio, Cr and HFpEF, and there was an inversely linear relationship between Hb and HFpEF.
Table 4.
The multicollinearity of variables in the model.
| Variables | Multicollinearity |
|---|---|
| Age, yrs | 1.15 |
| Hb, g/L | 1.19 |
| NLR | 1.03 |
| AST/ALT | 1.10 |
| Cr, umol/L | 1.14 |
| UA, umol/L | 1.08 |
| AF, n(%) | 1.06 |
| PH, n(%) | 1.06 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AF, atrial fibrillation; Cr, creatinine; Hb, Hemoglobin; NLR, neutrophil to lymphocyte ratio; PH, pulmonary hypertension; UA, uric acid.
Figure 3.
Linear relationship diagram (A, B, C, D, E and F). (A) Linear relationship between age and HFpEF. (B) Linear relationship between Hb and HFpEF. (C) Linear relationship between NLR and HFpEF. (D) Linear relationship between AST/ALT ratio and HFpEF. (E) Linear relationship between Cr and HFpEF. (F) Linear relationship between UA and HFpEF.
The ROC of this model was 0.753 (95% CI 0.732–0.772) (Fig. 4), indicating the satisfactory discrimination. The Youden index, sensitivity, and specificity were 0.396, 63.35% and 76.21%, respectively. The optimal cutoff of predicted probability was 0.451. The patient was considered to have HFpEF when the predicted probability was greater than 0.451. The Brier score was 0.200, and the calibration plots showed favorable consistency between the prediction of the model and actual observations (Fig. 5). The internal validation showed remarkable discrimination and calibration (AUC = 0.750, Brier score = 0.202) (Table 5). The tenfold cross-validated calibration plot with good stability and accuracy was shown in Fig. 6.
Figure 4.
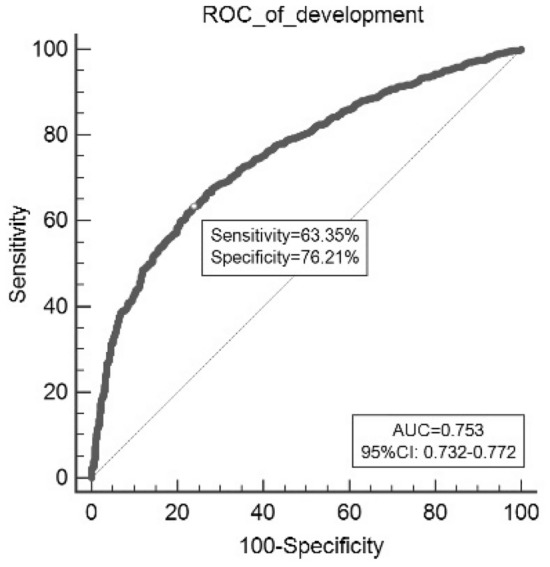
Receiver operating characteristic curves (ROC) of model in the development data.
Figure 5.
Calibration plot for model-predicted probability of HFpEF in the development data.
Table 5.
The diagnostic performance of model.
| Variables | AUC | Brier score |
|---|---|---|
| Development cohort | 0.753 | 0.200 |
| Internal validation | 0.750 | 0.202 |
Figure 6.
Tenfold cross-validated calibration plot.
We rebuilt the model by removing any one predictor, and calculated the predictive ability of each new model (Model 2-Model 9). The ROC of different models was presented as Fig. 7. We found the additive effects of AF, AST/ALT ratio and Cr on predictive of model by Z test (Table 6). In addition, through likelihood ratio test, the original model showed a higher goodness of fit compared with each new model (Table 7). The prediction nomogram for HFpEF was constructed, comprising age, Hb, NLR, AST/ALT ratio, Cr, UA, AF, and PH (Fig. 8). The weighted score was assigned to each of the independent predictors, and a higher score calculated from the sum of the assigned points for each predictor corresponding to a higher probability of HFpEF. In this nomogram, the highest total score was 100 points, and the scale of predicted value of HFpEF ranged from 0.1 to 0.9. Moreover, we designed a web-based online risk calculator, and made it freely available to all user (https://rose619.shinyapps.io/dynnomapp/). After entering related variables in the interface, we can get participant’s probability of HFpEF.
Figure 7.

The ROC of different models.
Table 6.
The comparison of performance in different models.
| Model | Age | Hb | NLR | AST/ALT | Cr | UA | AF | PH | AUC | Z | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | + | + | + | + | + | + | + | + | 0.753 | ||
| Model 2 | – | + | + | + | + | + | + | + | 0.745 | 2.100 | 0.036 |
| Model 3 | + | – | + | + | + | + | + | + | 0.746 | 1.774 | 0.076 |
| Model 4 | + | + | – | + | + | + | + | + | 0.749 | 1.583 | 0.114 |
| Model 5 | + | + | + | – | + | + | + | + | 0.744 | 2.547 | 0.011 |
| Model 6 | + | + | + | + | – | + | + | + | 0.746 | 3.233 | 0.001 |
| Model 7 | + | + | + | + | + | – | + | + | 0.750 | 1.069 | 0.285 |
| Model 8 | + | + | + | + | + | + | – | + | 0.726 | 4.892 | < 0.001 |
| Model 9 | + | + | + | + | + | + | + | – | 0.748 | 1.531 | 0.126 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AF, atrial fibrillation; Cr, creatinine; Hb, Hemoglobin; NLR, neutrophil to lymphocyte ratio; PH, pulmonary hypertension; UA, uric acid.
Table 7.
The likelihood ratio test for different models.
| Model | Age | Hb | NLR | AST/ALT | Cr | UA | AF | PH | χ2 | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | + | + | + | + | + | + | + | + | ||
| Model 2 | – | + | + | + | + | + | + | + | 14.88 | < 0.001 |
| Model 3 | + | – | + | + | + | + | + | + | 21.94 | < 0.001 |
| Model 4 | + | + | – | + | + | + | + | + | 7.19 | 0.007 |
| Model 5 | + | + | + | – | + | + | + | + | 17.98 | < 0.001 |
| Model 6 | + | + | + | + | – | + | + | + | 19.28 | < 0.001 |
| Model 7 | + | + | + | + | + | – | + | + | 8.54 | 0.003 |
| Model 8 | + | + | + | + | + | + | – | + | 84.66 | < 0.001 |
| Model 9 | + | + | + | + | + | + | + | – | 16.25 | < 0.001 |
Hb, Hemoglobin; NLR, neutrophil to lymphocyte ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, creatinine; UA, uric acid; AF, atrial fibrillation; PH, pulmonary hypertension.
Figure 8.
The nomogram of the model for predicting the probability of HFpEF.
Discussion
In this study, we developed and validated a novel diagnostic prediction model for HFpEF using the clinical data from ChiHFpEF cohort. The model was built with eight predictors of HFpEF, including age, Hb, NLR, AST/ALT ratio, Cr, UA, AF, and PH. This model showed excellent discrimination, calibration, and well internal validation. Of note, to emphasize the importance of inflammation and organ interaction in the pathogenesis of HFpEF, this model incorporated the inflammatory markers and markers for liver and renal function. We also excluded clinical diagnostic criteria for HFpEF including echocardiographic indices and NT-pro BNP because they were used in the adjudication of HFpEF diagnosis. Therefore, this new score can be applicable in hospital departments without results of echocardiography and natriuretic peptides examination comparing with previous diagnostic scoring systems for HFpEF.
HFpEF was considered as a syndrome recapitulating the proinflammatory state14. In cardiac specimens of both HFpEF animals and patients, it was observed that inflammatory cell infiltration was associated with cardiac inflammation and fibrotic damages33–35. The proinflammatory molecules could lead to cardiomyocyte hypertrophy and passive stiffness through increasing oxidative stress and reducing nitric oxide bioavailability, and it could also cause cardiac interstitial fibrosis by triggering abnormal extracellular matrix conversion14,36,37. In the mouse model, the interaction between inflammation and mitochondrial hyperacetylation was thought to be a key driver in HFpEF pathogenesis, which could be ameliorated by enhancing β-hydroxybutyrate abundance38. In this study, we found that NLR was independently associated with the HFpEF, consistent to the report that NLR might be useful to stratify the risk of patients hospitalized with HFpEF39. Moreover, previous studies have revealed elevated UA could lead to inflammation and oxidative stress in vascular endothelial cells20. UA was an independent predictor of adverse outcomes in patients with HFpEF19–21,40, and lowering UA might have a beneficial effect on the prognosis of patients with hyperuricemia and HFpEF20. In this study, we found the elevation of UA was a diagnostic predictor for HFpEF, suggesting that UA may be a relevant target for clinical prevention and treatment in HFpEF. Therefore, introducing these inflammatory markers into the diagnostic model may improve the prediction efficiency, and facilitate the identification of patients at different risk for HFpEF.
HFpEF was generally recognized as a disease of elderly, with the majority of patients age > 65 years41. In the patients with HFpEF, the PH was highly prevalent42. The pulmonary venous congested passively in the setting of elevated left atrium pressure, and pulmonary arterial vessels constricted or remodeled, resulting in the post-capillary PH, or the combined pre-capillary and post-capillary PH42,43. PH was considered as a critical prognostic factor for HFpEF42. Our study indicated that PH was one of predictors of HFpEF, suggesting the improvement of pulmonary vascular function may be a potential therapeutic target for HFpEF. In addition, we found AF was a major predictor for HFpEF, and Reddy’s study also demonstrated that AF increased significantly risk for HFpEF8,44. AF could directly cause the development of HFpEF by remodeling left atrium, impairing atrial function, and aggravating atrial fibrosis45. Considering catheter ablation plays an important role in the treatment of AF, further studies are needed to explore whether catheter ablation could reduce the incidence of HFpEF in the future.
A heart-liver axis, that was drafted by inflammatory reactants from the heart and the liver, placed a new spotlight on the crosstalk of organs in disease development23,46. In the phenomapping analysis of HFpEF, one of phenogroups demonstrated high proinflammatory biomarkers including tumor necrosis factor-alpha-mediated inflammation, liver fibrosis, and tissue remodeling47. Chirinos’ study indicated these biomarker clusters consisting of fibrosis/tissue remodeling, inflammation, and liver fibrosis were correlated with the outcomes of HFpEF48. In addition, AST/ALT ratio was used as one of predictors of liver fibrosis49, and a prospective observational study demonstrated that the non-alcoholic fatty liver disease fibrosis score, based on AST/ALT ratio, platelet counts, and albumin, was associated with higher all-cause mortality in HFpEF patients50. We found the AST/ALT ratio was a predictor for HFpEF, suggesting the correlation between liver fibrosis and HFpEF. Recently, Lusis' group found that a liver-derived protein, coagulation factor XI, could protect against diastolic dysfunction and decrease the cardiac fibrosis and inflammation, which was associated with the activity of SMAD pathway51. Therefore, identifying the mediator of liver-heart cross-talk and focusing on heart-liver axis may stand for the new target for the treatment of HFpEF.
The bidirectional interaction between the failing heart and kidneys was described as the cardiorenal syndrome52. Electrolyte dysregulation and fluid retention were common in patients with HFpEF, and renal insufficiency was thought as the main reason53. Previous studies indicated that renal insufficiency was a major predictor of outcome for HFpEF53,54, and we also found elevated Cr was associated with increasing risk of HFpEF. There were overlapping pathophysiological changes including systemic inflammation, oxidative stress, arterial stiffening, and endothelial dysfunction between HFpEF and renal insufficiency53,55. These pathways contribute to cardiac diastolic dysfunction, nephron loss, nephron compensatory hypertrophy and hyperfiltration of the remaining nephrons53. Therefore, focusing on the alleviation of factors provoking renal injury and slowing the progression of renal dysfunction may be helpful to reduce the risk of HFpEF.
Anemia was considered as an independent risk factor for the pathogenesis and development of HFpEF56,57. Similarly, we found a negative linear relationship between Hb with HFpEF. Anemia could reduce oxygen-carrying capacity of the blood, and increase the myocardial work, leading to the disbalance between myocardial oxygen demand and supply57. The deficiency of iron was the commonest contributing factor to anemia in HF patients58. In a double-blind randomized controlled trial, the HF patients with iron deficiency had significant improvements in functional capacity and quality of life when given intravenously ferric carboxymaltose59. Therefore, improving the Hb for patients with anemia might reduce the risk of HFpEF.
Several limitations should be considered. First, this study was conducted in only single heart center, and the sample size was relatively small. Owing to the study population was only patients with coronary heart disease, or hypertension, or diabetes mellitus in hospital, selection bias was inevitable in this study. Second, although the model presented favorable discrimination and calibration ability in the development data and internal validation, the validation with Chinese Han population or with existing data sets or in other centers was not performed, which may limit the generalizability of model.
Conclusions
In this study, we developed and validated a novel, simple model with inflammatory markers and markers for liver and renal dysfunction to predict the probability of HFpEF in patients with CVDs. This diagnostic model could improve the clinical prediction efficiency and facilitate the identification of patients at different risk for HFpEF.
Author contributions
J.X.Z. contributed to the design this study. Y.Y.X., Z.L., L.D.W., and Y.S. conducted data collection. R.Z., and Z.Y.L. participated in the analysis and interpretation of data. R.Z. wrote this manuscript. Z.Y.L. and J.X.Z. revised this manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Funding
This research was founded by the National Natural Science Foundation of China (NSFC) grant [No. 81970309, No. 81700398].
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhi-Yu Ling, Email: lingzhiyu@cqmu.edu.cn.
Jun-Xia Zhang, Email: zhangjunshia@njmu.edu.cn.
References
- 1.Collaborators., G. D. a. I. I. a. P. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet392, 1789–1858. 10.1016/s0140-6736(18)32279-7 (2018). [DOI] [PMC free article] [PubMed]
- 2.van Riet E, et al. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016;18:242–252. doi: 10.1002/ejhf.483. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020;17:559–573. doi: 10.1038/s41569-020-0363-2. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SD, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 5.Anker SD, et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chron ic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Associ ation (HFA) of the ESC. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 7.Pugliese NR, et al. Predicting the transition to and progression of heart failure with preserved ejection fraction: A weighted risk score using bio-humoural, cardiopulmonary, and echocardiographic stress testing. Eur. J. Prev. Cardiol. 2021;28:1650–1661. doi: 10.1093/eurjpc/zwaa129. [DOI] [PubMed] [Google Scholar]
- 8.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pieske B, et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur. Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 10.Tadic M, Cuspidi C, Calicchio F, Grassi G, Mancia G. Diagnostic algorithm for HFpEF: How much is the recent consensus applicable in clinical practice? Heart Fail. Rev. 2021;26:1485–1493. doi: 10.1007/s10741-020-09966-4. [DOI] [PubMed] [Google Scholar]
- 11.Samson R, Le Jemtel T. Therapeutic stalemate in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2021;10:e021120. doi: 10.1161/jaha.121.021120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, et al. Mimicking metabolic disturbance in establishing animal models of heart failure with preserved ejection fraction. Front. Physiol. 2022;13:879214. doi: 10.3389/fphys.2022.879214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders-van Wijk S, et al. Proteomic evaluation of the comorbidity-inflammation paradigm in heart failure with preserved ejection fraction: Results From the PROMIS-HFpEF study. Circulation. 2020;142:2029–2044. doi: 10.1161/circulationaha.120.045810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra S, Kass D. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2021;18:400–423. doi: 10.1038/s41569-020-00480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pugliese NR, et al. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: Implications for future interventions. Cardiovasc. Res. 2023;118:3536–3555. doi: 10.1093/cvr/cvac133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silberman S, et al. Neutrophil-lymphocyte ratio: Prognostic impact in heart surgery. Early outcomes and late survival. Ann. Thorac. Surg. 2018;105:581–586. doi: 10.1016/j.athoracsur.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 17.Bai B, et al. High neutrophil to lymphocyte ratio and its gene signatures correlate with diastolic dysfunction in heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 2021;8:614757. doi: 10.3389/fcvm.2021.614757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandurino-Mirizzi A, et al. Elevated serum uric acid is associated with a greater inflammatory response and with short- and long-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Nutr. Metab. Cardiovasc. Dis. NMCD. 2021;31:608–614. doi: 10.1016/j.numecd.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Selvaraj S, et al. Serum uric acid, influence of sacubitril-valsartan, and cardiovascular outcomes in heart failure with preserved ejection fraction: PARAGON-HF. Eur. J. Heart Fail. 2020;22:2093–2101. doi: 10.1002/ejhf.1984. [DOI] [PubMed] [Google Scholar]
- 20.Nishino M, et al. Lowering uric acid may improve prognosis in patients with hyperuricemia and heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2022;11:e026301. doi: 10.1161/jaha.122.026301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrosio G, et al. Serum uric acid and outcomes in patients with chronic heart failure through the whole spectrum of ejection fraction phenotypes: Analysis of the ESC-EORP Heart Failure Long-Term (HFLT) Registry. Eur. J. Intern. Med. 2021;89:65–75. doi: 10.1016/j.ejim.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Ananthram MG, Gottlieb SS. Renal dysfunction and heart failure with preserved ejection fraction. Heart Fail. Clin. 2021;17:357–367. doi: 10.1016/j.hfc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Salah HM, et al. Relationship of nonalcoholic fatty liver disease and heart failure with preserved ejection fraction. JACC Basic Transl. Sci. 2021;6:918–932. doi: 10.1016/j.jacbts.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Xu M, Chen T, Zhou Y. Correlation between liver stiffness and diastolic function, left ventricular hypertrophy, and right cardiac function in patients with ejection fraction preserved heart failure. Front. Cardiovasc. Med. 2021;8:748173. doi: 10.3389/fcvm.2021.748173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, et al. Disparate clinical characteristics and prognosis of HFpEF versus HFrEF phenotype of diabetic cardiomyopathy. J. Clin. Med. 2023 doi: 10.3390/jcm12041565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galiè N, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 27.Carpio AM, et al. Unrecognized pulmonary arterial hypertension in hospitalized patients. Int. J. Cardiovasc. Imaging. 2021;37:1237–1243. doi: 10.1007/s10554-020-02108-9. [DOI] [PubMed] [Google Scholar]
- 28.Nagueh SF, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echoca rdiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Sturdza AE, et al. Nomogram predicting overall survival in patients with locally advanced cervical cancer treated with radiochemotherapy including image-guided brachytherapy: A retro-EMBRACE study. Int. J. Radiat. Oncol. Biol. Phys. 2021;111:168–177. doi: 10.1016/j.ijrobp.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Cummings P. Missing data and multiple imputation. JAMA Pediatr. 2013;167:656–661. doi: 10.1001/jamapediatrics.2013.1329. [DOI] [PubMed] [Google Scholar]
- 31.Alba A, et al. Discrimination and calibration of clinical prediction models: Users' guides to the medical literature. JAMA. 2017;318:1377–1384. doi: 10.1001/jama.2017.12126. [DOI] [PubMed] [Google Scholar]
- 32.Kim JH. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019;72:558–569. doi: 10.4097/kja.19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai B, et al. Seipin knockout mice develop heart failure with preserved ejection fraction. JACC Basic Transl. Sci. 2019;4:924–937. doi: 10.1016/j.jacbts.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suetomi T, et al. Inflammation and NLRP3 Inflammasome Activation Initiated in Response to Pressure Overload by Ca(2+)/Calmodulin-Dependent Protein Kinase II δ Signaling in Cardiomyocytes Are Essential for Adverse Cardiac Remodeling. Circulation. 2018;138:2530–2544. doi: 10.1161/circulationaha.118.034621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westermann D, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 36.Paulus W, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 37.Frangogiannis NG. Cardiac fibrosis. Cardiovasc. Res. 2021;117:1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng Y, et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ. Res. 2021;128:232–245. doi: 10.1161/circresaha.120.317933. [DOI] [PubMed] [Google Scholar]
- 39.Boralkar K, et al. Value of neutrophil to lymphocyte ratio and its trajectory in patients hospitalized with acute heart failure and preserved ejection fraction. Am. J. Cardiol. 2020;125:229–235. doi: 10.1016/j.amjcard.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Carnicelli AP, et al. Elevated uric acid prevalence and clinical outcomes in patients with heart failure with preserved ejection fraction: Insights from RELAX. Am. J. Med. 2020;133:e716–e721. doi: 10.1016/j.amjmed.2020.03.054. [DOI] [PubMed] [Google Scholar]
- 41.Tromp J, et al. Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2019;74:601–612. doi: 10.1016/j.jacc.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 42.Dixon DD, Trivedi A, Shah SJ. Combined post- and pre-capillary pulmonary hypertension in heart failure with preserved ejection fraction. Heart Fail. Rev. 2016;21:285–297. doi: 10.1007/s10741-015-9523-6. [DOI] [PubMed] [Google Scholar]
- 43.Thenappan T, Prins KW, Cogswell R, Shah SJ. Pulmonary hypertension secondary to heart failure with preserved ejection fraction. Can. J. Cardiol. 2015;31:430–439. doi: 10.1016/j.cjca.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 44.Reddy Y, Obokata M, Gersh B, Borlaug B. High prevalence of occult heart failure with preserved ejection fraction among patients with atrial fibrillation and dyspnea. Circulation. 2018;137:534–535. doi: 10.1161/circulationaha.117.030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotecha D, et al. Heart failure with preserved ejection fraction and atrial fibrillation: Vicious twins. J. Am. Coll. Cardiol. 2016;68:2217–2228. doi: 10.1016/j.jacc.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 46.Correale M, et al. Liver disease and heart failure: Back and forth. Eur. J. Intern. Med. 2018;48:25–34. doi: 10.1016/j.ejim.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Cohen JB, et al. Clinical phenogroups in heart failure with preserved ejection fraction: Detailed phenotypes, prognosis, and response to spironolactone. JACC. Heart Fail. 2020;8:172–184. doi: 10.1016/j.jchf.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chirinos JA, et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2020;75:1281–1295. doi: 10.1016/j.jacc.2019.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angulo P, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology (Baltimore, Md.) 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 50.Yoshihisa A, et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. 2018;5:262–270. doi: 10.1002/ehf2.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y, et al. Liver-heart cross-talk mediated by coagulation factor XI protects against heart failure. Science (New York, N. Y.) 2022;377:1399–1406. doi: 10.1126/science.abn0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138:929–944. doi: 10.1161/circulationaha.117.028814. [DOI] [PubMed] [Google Scholar]
- 53.Shah KS, Fang JC. Is heart failure with preserved ejection fraction a kidney disorder? Curr. Hypertens. Rep. 2019;21:86. doi: 10.1007/s11906-019-0993-0. [DOI] [PubMed] [Google Scholar]
- 54.Kanjanahattakij N, et al. High right ventricular stroke work index is associated with worse kidney function in patients with heart failure with preserved ejection fraction. Cardiorenal. Med. 2018;8:123–129. doi: 10.1159/000486629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katz DH, Burns JA, Aguilar FG, Beussink L, Shah SJ. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC. Heart Fail. 2014;2:586–596. doi: 10.1016/j.jchf.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okuno K, et al. Effective blood hemoglobin level to predict prognosis in heart failure with preserved left ventricular ejection fraction: Results of the Japanese heart failure syndrome with preserved ejection fraction registry. Heart Vessel. 2019;34:1168–1177. doi: 10.1007/s00380-019-01349-6. [DOI] [PubMed] [Google Scholar]
- 57.van de Wouw J, et al. Chronic kidney disease as a risk factor for heart failure with preserved ejection fraction: A focus on microcirculatory factors and therapeutic targets. Front. Physiol. 2019;10:1108. doi: 10.3389/fphys.2019.01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chopra VK, Anker SD. Anaemia, iron deficiency and heart failure in 2020: Facts and numbers. ESC Heart Fail. 2020;7:2007–2011. doi: 10.1002/ehf2.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anker SD, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: An individual patient data meta-analysis. Eur. J. Heart Fail. 2018;20:125–133. doi: 10.1002/ejhf.823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.







