Abstract
The gender role influences vulnerability to mental illness. Substance use, even critical in scale, is perceived as masculine, just like hard (over-)work, while not seeking help. With the ongoing separation between gender and sex, masculine norms become more relevant also to females’ mental health. The male depression concept highlights the role of male symptoms in affective disorders. However, the empirical evidence is still limited. Here, we use the denomination ‘masculine depression’ to open the category for female patients and tested substance use patterns, health services’ utilization, and working hours as predictors in a case–control study of 163 depressed in-patients (44% women; masculine vs. non-masculine depression according to a median split of the Male Depression Rating Scale-22) and 176 controls (51% women). We assessed higher depression severity in patients with masculine (vs. non-masculine) depression. Masculine depression (vs. non-masculine depression and vs. no depression) was predicted by more frequent and critical use of alcohol (including binge drinking), tobacco, and illicit drugs, and by longer working times. Moreover, fewer health services contacts due to mental complaints during the previous year were associated with masculine (vs. non-masculine) depression. Alarmingly, even critical substance misuse was not significantly associated with more frequent health services contacts; however, the higher the depression severity, the more contacts the patients reported. Here, we provide evidence that patients with masculine depression are highly burdened and undertreated, which applies equally to female and male patients. This study identified promising targets to establish specialized care offers.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00406-023-01567-0.
Keywords: Male depression, Masculine depression, Alcoholism, Substance use, Drug use, Nicotine, Help seeking
Introduction
Sex and gender influence the risk to develop illness. This effect is well known, e.g. for cardiovascular diseases; while sex has been shown to be more relevant for the etiopathogenesis, gender is more likely to influence disease manifestations and access to and quality of care [1, 2]. Such mechanisms also contribute to mental disorders like depression [3, 4]. For a long time, it has been assumed that females are twice as likely to develop depression than males [5]. However, applying newer and more specific screening tools in the context of research efforts on the Male Depression revealed a similarly high prevalence of depression in both sexes [6, 7]. Those screening tools are for example the Gotland Male Depression Scale (GMDS) [8], the Male Depression Risk Scale 22 (MDRS-22) [9], and the Gender-Sensitive Depression Screening (GSDS) [10, 11]. They survey so-called masculine (i.e., more externalizing symptoms) like anger, aggression, distraction, avoidance, emotional suppression, irritability, substance use, and risk-seeking behavior, which are typical for male depression [9, 12]. Interestingly, females can also be affected by the male depression subtype [13]. A greater exposure to chronic stressors [13] due to the burdens associated with pursuing a career and raising a family [14, 15] have been postulated to be reasons for masculine depression in women. With the ongoing separation between sex and gender, gender roles – independently from sex – gain greater relevance for the individual’s illness risk. Masculine norms become more and more relevant for females and could thus also be responsible for so-called male depression symptoms in females. To deliberately include females, we choose to use the term “masculine depression” instead of “male depression” in the following.
Thus far, little is known about the risk factors for masculine depression. E.g. maladaptive early childhood schemas have been suggested as predictors [16]. New findings in this area have the potential to improve care for those affected via tailored help approaches. Here we investigated whether adherence to masculine norms is an indicator for masculine depression.
“Typically masculine norms” are for example winning, emotional control, risk-taking, violence, dominance, self-reliance, and primacy of work [17]. Those masculine norms also refer to substance use [18], even critical in scale, just like hard (over-)work [19], but not seeking help [20].
Substance abuse frequently co-occurs with Major Depressive Disorder (MDD) [21–23]. Patients with substance use disorder (SUD) often develop depressive symptoms and use alcohol to reduce psychological strain [24, 25], and vice versa, patients suffering from depression often report harmful and addictive substance use. Saha et al. [26] found a six-fold elevated risk for broadly defined mood disorder and drug dependence in depressed patients. Eberhard et al. [27] describe a comorbidity of 41% of risky alcohol use and affective disorder in an emergency psychiatric inpatient population of adolescents. The link between MDD and SUD suggests a common pathophysiology. Overlapping genetic liability of alcohol use disorder (AUD) and cannabis use with depressive disorder has been reported [21, 28–30]. Common dysregulation of the neutral sphingomyelinase has also been shown to mediate parts of the SUD-depression comorbidity [31]. Alcohol exerts different effects on mice with genetically induced depression than normal control animals. It normalizes sphingolipid- and monoamine deficits in the brain [32]. This may provide a biological base for potentially beneficial effects of alcohol in the self-management of mood disorders [33]. However, the reasons for the large overlap of depression and SUD are still insufficiently understood, and the literature remains controversial. Kelly et al. [34] recently published a large national survey of persons with depressive symptoms and showed that men and emerging adults are at disproportionately higher risks of AUD and binge drinking than either women or older adults. Alarmingly, it has been shown that the prevalence of alcohol drinking among women increased in the USA between 2001/2002 and 2012/2013 [35]. We recently found an overlap of masculine depression with Cluster B personality [36]. Cluster B personality disorders (narcissistic, borderline, dissocial, and histrionic personality disorders) are characterized by impulsive, dramatic, emotionally unstable, and erratic behaviors. Patients with such a personality disorder are also at an increased risk of AUD [37].
The so-called male norm “overwork” is commonly defined as 50 or more hours per week. This is much more often found in men than in women [19] and women with children more often leave those male-dominated occupations in comparison to men or childless women [38]. The harmful impact of overwork on mental health is well known [39–41]. Although Kuroda et al. [41] found increasing job satisfaction in people who work more than 55 h per week, they also showed an impairment of workers’ mental health [41]. Interestingly, Virtanen et al. [42] showed that working more than 55 h a week is associated with an excess risk of depression and anxiety in women, but not men. Work beyond the standard time norm is associated with an increased alcohol use in men and women equally [43].
Men’s claim to be able to work and to be efficient could also be linked to the norm “not seeking help”. Depressed men's help-seeking behavior has been described as negatively affected by masculine norms [20, 44] previously. Reasons therefore could be that weakness and need for help are believed not to be masculine and help-seeking implies loss of status, loss of control and autonomy, incompetence, dependence, and damage of identity [45]. Instead, men consume alcohol, become more often “workaholics”, and have a higher risk of suicide [45].
Magovcevic et al. [46] showed that men adhering to hegemonic masculine norms are more likely to exhibit externalizing symptoms than symptoms of prototypic depression, in comparison to males not adhering to those masculine norms following stressful life events. But masculine norms become more and more important, also for females’ causes of depression and symptoms. Accordingly, a cross-sectional study with 200 pregnant women showed an association between conformity to male gender norms and nonconformity to some female gender norms and an increased risk of suffering from depression [47]. Taken together, the masculine depression is an important, yet underinvestigated field. We still lack empirical evidence on the risk factors in both sexes. To the best of our knowledge, there is no in-depth study available on how masculine depression associates with use of alcohol, tobacco, and illicit drugs and how this comorbidity is related to working hours and utilization of health services.
Aims of the study
Here, we analyzed whether masculine depression is associated with substance use per se, more frequent substance use, more critical substance use, longer working hours, and less use of health care, regardless of sex. We tested if use patterns of alcohol, tobacco, and illicit drugs and health services contacts due to physical and/or mental complaints differ between patients with masculine depression and patients with non-masculine depression (patients with high and those with low MDRS-22 scores) and whether these parameters predict MDRS-22 scores. We recruited a sex-balanced cohort of in-patients with moderate to severe depression according to the ICD-10 diagnostic criteria and applied a sex-specific median split to subclassify patients with masculine depression and patients with non-masculine depression. We also compared these groups of depressed patients with healthy control subjects.
Methods
Sample population
The data analyzed here were collected as part of the Masculine Depression Project [36, 48] aiming to gain more knowledge about the masculine depression. It was conducted as a prospective, open-label, comparative cohort study with one single data collection point per participant. From May 2017 to November 2019, we screened a total of 658 study subjects and included 170 patients and 176 healthy control subjects. Recruitment of participants was conducted by a medically trained team and took place between 7 and 10 am. Each visit lasted approximately 3–4 h and consisted of several parts. Participation requirements were: a minimum age of 18 years, a body mass index < 35 kg/m2, and written informed consent.
The patient population was recruited from the Department of Psychiatry and Psychotherapy at the Friedrich-Alexander-University Erlangen-Nürnberg (FAU) and the Clinic for Psychiatry, Addiction, Psychotherapy, and Psychosomatics at the Europakanal in Erlangen, Germany. Inclusion required an inpatient stay due to a moderate or severe depressive ICD-10 episode in one of the two clinics mentioned or depressive symptoms of a recurrent unipolar or bipolar affective disorder classified as moderate or severe according to ICD-10 [49]. Also the diagnostic criteria for depression according to DSM-5 [50] had to be fulfilled. Study recruitment had to take place during the first five days of hospitalization. Psychotic disorders led to exclusion from the study in the group of patients and in the healthy control group.
The healthy control subjects were recruited among individuals who had expressed interest in participating in studies at the University Hospital in Erlangen. They were recruited by distributing flyers and by online advertising via social platforms. Flyers were mainly distributed in Erlangen and Nuremberg. A telephone screening was conducted with those interested in the study. The inclusion and exclusion criteria were confirmed at this step. We used the exclusion criterion mentioned for the patients’ group and added the following criteria: regular intake of psychotropic drugs, a current psychiatric diagnosis according to ICD-10 (except for nicotine dependence), or a history of in-patient treatment. The control subjects received an expense allowance of 30 euros.
Evaluation of the depression symptoms, substance use parameters, and health services contacts
The MDRS-22 [9] was utilized to capture the characteristics of the masculine depression. The MDRS-22 is a self-reported scale consisting of 22 items to assess predominantly externalizing symptoms which are assumed to be typical for masculine depression. Six domains including emotional suppression, drug use, alcohol use, anger and aggression, somatic symptoms, and risk-taking were assessed. The participants evaluated the items in comparison with the previous month. Every item is rated on a scale from 0 to 7. Higher values indicate a more frequent occurrence of symptoms. We calculated means; thus, values of 0 to 7 can be achieved for the MDRS-22 score [9, 51, 52]. Due to the lack of a validated German version at the time of recruitment, the version by Rice et al. [9] was translated into German by the study team (Supplementary Table 1). We found a Cronbach's alpha of 0.828 for the patient group (0.787 for the control group), showing a good internal consistency, similar to another recently published German MDRS-22 version by Walther et al. [53].
The Beck’s Depression Inventory-II (BDI-II) [54] was used to determine depression severity. We employed the Alcohol Use Disorder Identification Test (AUDIT) in the German version [55] (scores ≥ 8 and ≥ 20 indicate zone II and IV risk levels; [56]) and a questionnaire on binge drinking behavior to obtain information about alcohol consumption [57]. The study subjects were asked to indicate on how many occasions they had consumed ≥ 5, ≥ 10, and ≥ 15 standard drinks within two hours during the last two weeks and the last 24 months. A standard drink was defined as 330 mL of beer (5% alcohol), 140 mL of wine (12% alcohol), or 70 mL of a 25% (e.g., aperitif) or 40 mL of a 40% alcohol liquor (e.g., whiskey, gin, vodka). Smoking behavior was determined using the Fagerström Test for Nicotine Dependence (FTND) [58], which includes six items. The drug intake was recorded using a short questionnaire. The last four weeks and the entire lifetime were analyzed. The following substances were inquired: cannabis, opioids, cocaine, hallucinogens, PCP, and sedatives (including hypnotics, anxiolytics, and analgesics). Finally, the participants reported how often they had contacted a physician within the last six months because of physical complaints and within the last 12 months because of psychological complaints.
Measurement of gamma-glutamyl transferase activity
The gamma-glutamyl transferase (GGT) activity was determined at the Central Laboratory of the Universitätsklinikum Erlangen, Germany (DIN EN ISO 15189 accredited).
Statistical analyses
We used sex-separated median values of the MDRS-22 to divide the sample of depressed patients into a group of 81 “patients with masculine depression” (i.e., high MDRS-22 scores) and 82 “patients with non-masculine depression” (i.e., low MDRS-22 scores). Seven study subjects with missing data for the MDRS-22 were excluded.
SPSS for Windows 27.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data and GraphPad Prism 8.4.3 (Graph Pad Software Inc., San Diego, CA, USA) to visualize the results. Variation in frequencies was tested using χ2 tests (and we report P values from two-tailed Fisher’s exact test if at least one cell failed to reach an expected value of five observations). Correlations used the Pearson method. We employed Student’s t-tests for differences in two independent groups, and the statistics were adjusted when necessary, according to the Levene’s test. We used binary regression analyses with high vs. low MDRS-22 score groups as primary dependent variable and linear regression analyses with the MDRS-22 score as dependent variable and substance use parameters, number of health services contacts, GGT, or working times as predictors. We then analyzed how these parameters predicted the secondary dependent variables, high MDRS-22 score group vs. healthy control group and low MDRS-22 score group vs. healthy control group (binary regression analyses). The regression models included sex and age and the models with high vs. low MDRS-22 score groups or the MDRS-22 score as dependent variable the BDI-II scores. Furthermore, linear regression models were computed to identify predictors of health services contacts. We report B coefficients and validated the results using bias-corrected and accelerated bootstrap (1000 resamples). A P value < 0.05 was considered significant.
Results
Cohort characteristics
Relative to patients with non-masculine depression, patients with masculine depression were younger (mean age: 36.4 vs. 45.7 years) and less often married (28% vs. 48%) (Table 1). Patients with masculine depression showed also more months of employment during the previous year (8.1 vs. 6.2), more hours of employment per week (25.0 vs. 19.1), and higher BDI-II scores (37.3 vs. 28.7). See Table 1 for further comparisons between the patients’ groups and the healthy controls. Table 2 reports the descriptive characteristics of the groups in terms of substance use, health services contacts, and GGT activity. The MDRS-22 sum score correlated with BDI-II scores in the group of patients with non-masculine depression (N = 81, r = 0.495, P < 0.001), in the control subjects (N = 174, r = 0.603, P < 0.001), and in the total sample (N = 335, r = 0.783, P < 0.001), but not significantly in patients with masculine depression (N = 80, r = 0.161, P = 0.153). The MDRS-22 sum score did not significantly correlate with age: patients with masculine depression: N = 81, r = – 0.149, P = 0.184; patients with non-masculine depression: N = 82, r = 0.049, P = 0.661; control subjects: N = 176, r = 0.060, P = 0.432; and total sample: N = 339, r = – 0.023, P = 0.670. Moreover, the MDRS-22 sum score did not significantly differ between females and males (coded “2” and “1”): patients with masculine depression: N = 81, t = 0.757, P = 0.452; patients with non-masculine depression: N = 82, t = – 0.554, P = 0.581; control subjects: N = 176, t = 1.158, P = 0.249; and total sample: N = 339, t = 1.384, P = 0.167.
Table 1.
Cohort characteristics
| Patients with masculine depression (Ntotal = 81) | Patients with non-masculine depression (Ntotal = 82) | Healthy control subjects (Ntotal = 176) | Patients with masculine vs. patients with non-masculine depression | Patients with masculine depression vs. controls | Patients with non-masculine depression vs. controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M/F | SD | N | M/F | SD | N | M/F | SD | χ2 or t | P | χ2 or t | P | χ2 or t | P | |
| % Men | 81 | 56 | 82 | 56 | 176 | 49 | 0.0 | 0.944 | 1.0 | 0.319 | 1.2 | 0.279 | |||
| Age (years) | 81 | 36.4 | 14.1 | 82 | 45.7 | 14.6 | 176 | 37.2 | 13.7 | – 4.1 | < 0.001 | – 0.4 | 0.684 | 4.5 | < 0.001 |
| Months of employment during the previous year | 77 | 8.1 | 4.9 | 76 | 6.2 | 5.6 | 173 | 8.2 | 4.9 | 2.3 | 0.023 | – 0.1 | 0.894 | – 2.7 | 0.007 |
| Hours of employment per week | 77 | 25.0 | 17.6 | 74 | 19.1 | 19.0 | 173 | 19.0 | 15.7 | 2.0 | 0.049 | 2.6 | 0.012 | 0.0 | 0.989 |
| % Living in a current partnership | 79 | 48 | 77 | 55 | 176 | 68 | 0.6 | 0.421 | 9.3 | 0.002 | 4.3 | 0.038 | |||
| % Married | 79 | 28 | 80 | 48 | 175 | 27 | 6.5 | 0.011 | 0.0 | 0.945 | 9.9 | 0.002 | |||
| % Divorced | 79 | 14 | 78 | 21 | 176 | 14 | 1.2 | 0.274 | 0.0 | 0.953 | 1.6 | 0.207 | |||
| % At School | 79 | 5 | 80 | 1 | 176 | 1 | 1.9 | 0.210 | 3.7 | 0.076 | 0.0 | 1.0 | |||
| % In Vocational training | 79 | 9 | 80 | 4 | 176 | 2 | 1.8 | 0.210 | 7.4 | 0.011 | 1.0 | 0.380 | |||
| % At university | 79 | 11 | 80 | 8 | 176 | 40 | 0.7 | 0.401 | 20.5 | < 0.001 | 27.4 | < 0.001 | |||
| % In an employment relationship | 79 | 51 | 80 | 39 | 176 | 52 | 2.3 | 0.132 | 0.0 | 0.874 | 3.7 | 0.054 | |||
| % Self-Employed | 79 | 6 | 80 | 6 | 176 | 11 | 0.0 | 1.0 | 1.6 | 0.211 | 1.6 | 0.201 | |||
| % Unemployed | 79 | 13 | 80 | 19 | 176 | 3 | 1.1 | 0.291 | 9.5 | 0.004 | 19.3 | < 0.001 | |||
| % Retired | 79 | 10 | 80 | 20 | 176 | 3 | 3.0 | 0.082 | 4.7 | 0.039 | 19.3 | < 0.001 | |||
| BDI-II score | 80 | 37.3 | 10.6 | 81 | 28.7 | 10.3 | 174 | 3.4 | 3.8 | 5.2 | < 0.001 | 27.9 | < 0.001 | 21.5 | < 0.001 |
| MDRS-22 score | 81 | 2.6 | 0.8 | 82 | 1.1 | 0.4 | 176 | 0.4 | 0.4 | 15.2 | < 0.001 | 24.2 | < 0.001 | 14.7 | < 0.001 |
The table shows the valid number of subjects analyzed (N), means (M) or relative frequencies (F), standard deviation (SD), and the results of χ2 / Fisher and Student's t-tests. BDI-II Beck’s Depression Inventory-II, MDRS-22 Male Depression Rating Scale-22. P < 0.05 in bold
Table 2.
Descriptive statistics of substance use parameters, health services contacts, and liver enzyme activity
| Patients with masculine depression (Ntotal = 81) |
Patients with non-masculine depression (Ntotal = 82) |
Healthy control subjects (Ntotal = 176) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | M/F | SD | N | M/F | SD | N | M/F | SD | |
| Alcohol | |||||||||
| AUDIT score | 80 | 9.8 | 9.2 | 80 | 2.9 | 3.1 | 176 | 4.4 | 3.1 |
| % AUDIT score (≥ 8) | 80 | 44 | 80 | 5 | 176 | 15 | |||
| % AUDIT score (≥ 20) | 80 | 20 | 80 | 1 | 176 | 0 | |||
| % Binge drinking Yes vs. No (2-week) | 63 | 35 | 70 | 4 | 167 | 31 | |||
| Binge drinking frequency (2-week; drinks per week) | 63 | 1.1 | 2.9 | 70 | 0.0 | 0.1 | 167 | 0.3 | 0.5 |
| Binge drinking severity (2-week; drinks per week) | 63 | 0.5 | 0.9 | 70 | 0.0 | 0.2 | 167 | 0.3 | 0.6 |
| % Binge drinking Yes vs. No (24-month) | 60 | 62 | 70 | 27 | 165 | 50 | |||
| Binge drinking frequency (24-month; drinks per week) | 60 | 0.5 | 1.0 | 70 | 0.0 | 0.1 | 165 | 0.1 | 0.3 |
| Binge drinking severity (24-month; drinks per week) | 60 | 1.1 | 1.1 | 70 | 0.3 | 0.6 | 165 | 0.7 | 0.9 |
| Nicotine | |||||||||
| % Cigarette smoking (yes) | 81 | 47 | 81 | 30 | 175 | 6 | |||
| FTND score | 81 | 2.0 | 2.7 | 81 | 1.0 | 2.0 | 175 | 0.1 | 0.4 |
| Drugs | |||||||||
| % Use of sedative medication (4-week) | 72 | 28 | 77 | 31 | 174 | 5 | |||
| % Use of cannabis (4-week) | 74 | 26 | 77 | 5 | 174 | 3 | |||
| % Use of stimulants (4-week) | 75 | 3 | 76 | 1 | 174 | 1 | |||
| % Use of opioids (4-week) | 75 | 3 | 76 | 3 | 174 | 1 | |||
| % Use of cocaine (4-week; yes) | 75 | 0 | 76 | 0 | 173 | 0 | |||
| % Use of hallucinogens/PCP (4-week) | 75 | 3 | 76 | 0 | 173 | 0 | |||
| % Use of sedative medication (lifetime) | 76 | 41 | 81 | 36 | 174 | 11 | |||
| % Use of cannabis (lifetime) | 77 | 53 | 80 | 18 | 174 | 18 | |||
| % Use of stimulants (lifetime) | 76 | 20 | 79 | 6 | 174 | 3 | |||
| % Use of opioids (lifetime) | 78 | 12 | 79 | 3 | 176 | 1 | |||
| % Use of cocaine (lifetime) | 78 | 9 | 79 | 1 | 175 | 2 | |||
| % Use of hallucinogens / PCP (lifetime) | 78 | 17 | 79 | 3 | 174 | 2 | |||
| Number of health services contacts | |||||||||
| Due to physical complaints (6-month) | 80 | 3.5 | 3.6 | 80 | 2.9 | 4.2 | 175 | 1.0 | 1.6 |
| Due to mental complaints (12-month) | 80 | 11.0 | 15.4 | 80 | 12.0 | 14.8 | 175 | 0.1 | 0.5 |
| GGT activity (U/L) | 81 | 33.1 | 30.1 | 82 | 28.3 | 21.8 | 176 | 25.8 | 38.7 |
The table shows the valid number of subjects analyzed (N), means (M) or relative frequencies (F) and standard deviation (SD). AUDIT Alcohol Use Disorder Identification Test, FTND Fagerström Test for Nicotine Dependence, GGT gamma-glutamyl transferase, 2-week previous 2 weeks, 4-week previous 4 weeks, 6-month previous 6 months, 12-month previous 12 months, 24-month previous 24-months
Substance use parameters
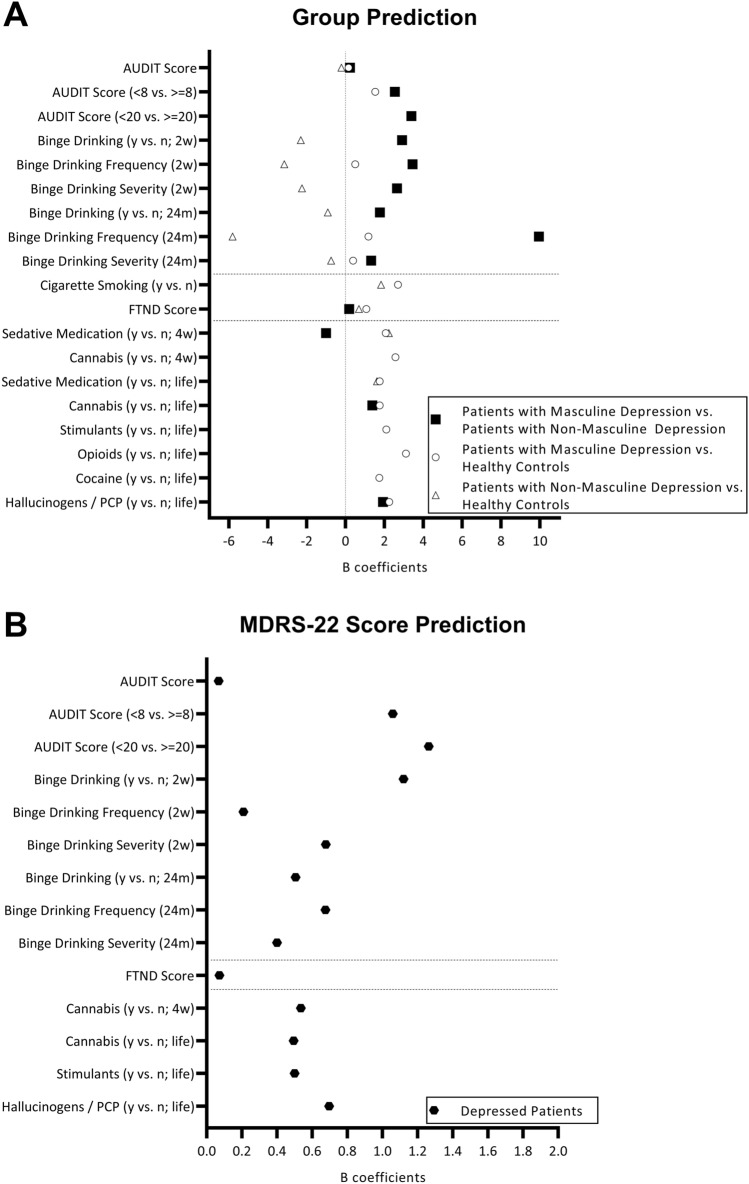
Patients with masculine depression vs. patients with non-masculine depression: The group of patients with masculine depression was predicted by higher AUDIT scores (B = 0.231, P < 0.001), an AUDIT score of at least 8 (vs. less than 8; B = 2.541, P < 0.001), an AUDIT score of at least 20 (vs. less than 20; B = 3.392, P = 0.003), binge drinking for both the 2-week and the 24-month periods (yes vs. no; B = 2.917, P < 0.001 and B = 1.771, P < 0.001), higher binge drinking frequency (2-week and 24-month periods: B = 3.454, P = 0.005 and B = 9.953, P = 0.001) and severity (2-week and 24-month periods: B = 2.658, P = 0.001 and B = 1.324, P < 0.001), higher FTND scores (B = 0.198, P = 0.012), and lifetime use of cannabis (B = 1.387, P = 0.002) and hallucinogens (B = 1.934, P = 0.024) (Fig. 1A, Supplementary Table S2). Moreover, the 4-week use of sedative medication was associated with the group of patients with non-masculine depression (B = – 0.999, P = 0.037), and there was a statistical trend for an association between smoking and the group of patients with masculine depression (B = 0.679, P = 0.072). Overall, the group of patients with masculine depression was predicted by higher BDI-II scores (B from 0.088 to 0.127, P < 0.001) and younger age (B from – 0.055 to – 0.030, P from < 0.001 to 0.037; except for the model with 24-month binge drinking severity).
Fig. 1.
The figure shows the significant and bootstrap-validated B coefficients from binary logistic regression analyses to predict group (i.e., patients with masculine depression vs. patients with non-masculine depression vs. healthy controls; A Supplementary Tables S2, S4, S5) and linear regression analyses to predict MDRS-22 scores in the group of depressed patients (B Supplementary Table S3). MDRS-22, Male Depression Rating Scale 22; 2w, previous 2 weeks; 24 m, previous 24 months; 4w, previous 4 weeks; life, lifetime; y vs. n, yes vs. no
MDRS-22 scores: We found results similar to the above mentioned comparisons between patients with masculine depression and patients with non-masculine depression. In depressed patients, higher MDRS-22 scores were related to higher AUDIT scores (B = 0.068, P < 0.001), an AUDIT score of at least 8 (vs. less than 8; B = 1.059, P < 0.001), an AUDIT score of at least 20 (vs. less than 20; B = 1.264, P < 0.001), binge drinking behavior for both the 2-week and the 24-month periods (yes vs. no; B = 1.120, P < 0.001 and B = 0.506, P = 0.001), higher binge drinking frequency (2-week and 24-month periods: B = 0.209, P < 0.001 and B = 0.676, P < 0.001) and binge drinking severity (2-week and 24-month periods: B = 0.678, P < 0.001 and B = 0.400, P < 0.001), higher FTND scores (B = 0.073, P = 0.006), use of cannabis (4-week period, B = 0.536, P = 0.007; lifetime, B = 0.495, P = 0.002), use of stimulants (lifetime, B = 0.501, P = 0.012), and use of hallucinogens (lifetime, B = 0.697, P = 0.002) (Fig. 1B, Supplementary Table S3). Overall, higher BDI-II scores (B = from 0.034 to 0.044, P < 0.001) and younger age (B = from – 0.018 to – 0.008, P from < 0.001 to 0.046; except for 24-month binge drinking severity) predicted the MDRS-22 scores.
Patients with masculine depression vs. healthy control subjects: The group of patients with masculine depression (vs. the group of healthy controls) was related to higher AUDIT scores (B = 0.163, P < 0.001), an AUDIT score of at least 8 (vs. less than 8; B = 1.533, P < 0.001), higher 2-week and 24-month binge drinking frequency (B = 0.503, P = 0.006 and B = 1.188, P = 0.002) and 24-month severity (B = 0.392, P = 0.022), smoking behavior (B = 2.704, P < 0.001), higher FTND scores (B = 1.076, P < 0.001), 4-week use of sedative medication (B = 2.077, P < 0.001) and cannabis (B = 2.574, P < 0.001) and lifetime use of sedative medication (B = 1.760, P < 0.001), cannabis (B = 1.769, P < 0.001), stimulants (B = 2.105, P < 0.001), opioids (B = 3.114, P = 0.004), cocaine (B = 1.740, P = 0.014), and hallucinogens (B = 2.258, P < 0.001) (Fig. 1A, Supplementary Table S4). These models were not significantly affected by sex or age.
Patients with non-masculine depression vs. healthy control subjects: The group of patients with non-masculine depression (vs. the group of healthy controls) was linked to lower AUDIT scores (B = – 0.201, P = 0.001), risk of binge drinking behavior (yes vs. no; 2-week and 24-month periods: B = – 2.292, P < 0.001 and B = – 0.909, P = 0.007), a lower frequency (2-week and 24-month periods: B = – 3.144, P = 0.001 and B = – 5.810, P = 0.007) and a milder severity (2-week and 24-month periods: B = – 2.235, P < 0.001 and B = – 0.732, P = 0.004) of binge drinking, smoking (B = 1.833, P < 0.001), a higher FTND score (B = 0.699, P = 0.001), and use of sedative medication (4-week period, B = 2.237, P < 0.001; lifetime, B = 1.622, P < 0.001) (Fig. 1A, Supplementary Table S5). Overall, patients with non-masculine depression were significantly predicted by higher age (B from 0.030 to 0.044, P from < 0.001 to 0.006).
Number of health services contacts
Patients with masculine depression vs. patients with non-masculine depression: A lower number of health services contacts due to mental complaints predicted the group of patients with masculine depression (B = – 0.025, P = 0.038), and there was a statistical trend for a higher GGT activity in patients with masculine depression (B = 0.015, P = 0.066). The models were again affected by BDI-II scores (B from 0.092 to 0.105, P < 0.001) and age (B from – 0.055 to – 0.051, P < 0.001) (Table 3).
Table 3.
Regression analyses to predict groups of patients with masculine depression, patients with non-masculine depression, and healthy controls and Male Depression Rating Scale 22 scores
| Health services contacts, GGT activity, and working hours | Sex | BDI-II | Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | B | Wald / T | P | B | Wald / T | P | B | Wald / T | P | B | Wald / T | P | |
| Patients with masculine depression vs. patients with non-masculine depression | |||||||||||||
| Health services contacts (Physical complaints) | 159 | 0.038 | 0.6 | 0.424 | 0.570 | 2.1 | 0.145 | 0.092 | 21.3 | < 0.001* | – 0.052 | 15.7 | < 0.001* |
| Health services contacts (Mental complaints) | 159 | – 0.025 | 4.3 | 0.038* | 0.694 | 3.0 | 0.083 | 0.105 | 23.9 | < 0.001* | – 0.051 | 15.0 | < 0.001* |
| GGT activity | 161 | 0.015 | 3.4 | 0.066 | 0.466 | 1.3 | 0.246 | 0.094 | 21.8 | < 0.001* | – 0.055 | 17.0 | < 0.001* |
| Months of employment during the previous year | 152 | 0.080 | 4.7 | 0.030* | 0.682 | 2.9 | 0.090 | 0.101 | 22.5 | < 0.001* | – 0.040 | 9.1 | 0.003* |
| Hours of employment per week | 150 | 0.027 | 5.9 | 0.015* | 0.514 | 1.6 | 0.210 | 0.114 | 24.7 | < 0.001* | – 0.042 | 9.6 | 0.002* |
| MDRS-22 scores | |||||||||||||
| Health services contacts (Physical complaints) | 159 | 0.025 | 1.5 | 0.141 | 0.296 | 2.2 | 0.032* | 0.039 | 6.5 | < 0.001* | – 0.018 | – 4.2 | < 0.001* |
| Health services contacts (Mental complaints) | 159 | – 0.012 | – 2.8 | 0.005* | 0.328 | 2.4 | 0.016* | 0.045 | 7.4 | < 0.001* | – 0.018 | – 4.0 | < 0.001* |
| GGT Activity | 161 | 0.005 | 1.9 | 0.065 | 0.251 | 1.8 | 0.075 | 0.040 | 6.7 | < 0.001* | – 0.019 | – 4.2 | < 0.001* |
| Months of employment during the previous year | 152 | 0.024 | 1.9 | 0.063 | 0.326 | 2.3 | 0.021* | 0.041 | 6.6 | < 0.001* | – 0.014 | – 2.9 | 0.004* |
| Hours of employment per week | 150 | 0.007 | 2.0 | 0.053 | 0.265 | 1.9 | 0.059 | 0.044 | 7.0 | < 0.001* | – 0.014 | – 3.0 | 0.003* |
| Patients with masculine depression vs. controls | |||||||||||||
| Health services contacts (Physical complaints) | 255 | 0.459 | 30.9 | < 0.001* | 0.417 | 1.9 | 0.173 | – 0.007 | 0.4 | 0.548 | |||
| Health services contacts (Mental complaints) | 255 | 1.599 | 26.3 | < 0.001* | 0.299 | 0.4 | 0.525 | – 0.017 | 0.9 | 0.338 | |||
| GGT activity | 257 | 0.005 | 1.6 | 0.206 | 0.194 | 0.5 | 0.483 | – 0.007 | 0.5 | 0.499 | |||
| Months of employment during the previous year | 250 | – 0.004 | 0.0 | 0.874 | 0.296 | 1.2 | 0.284 | – 0.002 | 0.0 | 0.853 | |||
| Hours of employment per week | 250 | 0.022 | 6.1 | 0.014* | 0.169 | 0.4 | 0.554 | – 0.004 | 0.2 | 0.669 | |||
| Patients with non-masculine depression vs. controls | |||||||||||||
| Health services contacts (Physical complaints) | 255 | 0.259 | 14.1 | < 0.001* | 0.080 | 0.1 | 0.788 | 0.035 | 11.0 | 0.001* | |||
| Health services contacts (Mental complaints) | 255 | 2.089 | 29.5 | < 0.001* | 0.143 | 0.1 | 0.802 | 0.043 | 5.1 | 0.024* | |||
| GGT activity | 258 | – 0.001 | 0.1 | 0.774 | 0.183 | 0.4 | 0.521 | 0.042 | 17.4 | < 0.001* | |||
| Months of employment during the previous year | 249 | – 0.074 | 7.5 | 0.006* | 0.213 | 0.5 | 0.465 | 0.035 | 12.2 | < 0.001* | |||
| Hours of employment per week | 247 | – 0.006 | 0.4 | 0.515 | 0.280 | 0.9 | 0.343 | 0.037 | 13.1 | < 0.001* | |||
The table shows the valid number of subjects analyzed (N) and the results of binary logistic regression analyses (Patients with masculine depression vs. patients with non-masculine depression, patients with masculine depression vs. controls, patients with non-masculine depression vs. controls) and linear regression analyses (MDRS-22 score). P < 0.05 in bold, *also significant in bootstrap analysis. Coding: Patients with non-masculine depression = 0 vs. patients with masculine depression = 1, controls = 0 vs. patients with masculine / non-masculine depression = 1; females = 0 vs. males = 1. GGT gamma-glutamyl transferase, MDRS-22 male depression rating scale 22
MDRS-22 scores: Similar to the above group prediction analysis, a higher MDRS-22 score was predicted by fewer previous health services contacts due to mental complaints (B = – 0.012, P = 0.005) and there was a trend for a higher GGT activity (B = 0.005, P = 0.065) (Table 3). The models were influenced by BDI-II scores (B from 0.039 to 0.045, P < 0.001), age (B from – 0.019 to – 0.018, P < 0.001), and sex (B from 0.296 to 0.328, P from 0.016 to 0.032; except for GGT activity).
Patients with masculine depression and patients with non-masculine depression vs. healthy control subjects: A higher number of health service contacts for both physical and mental complaints was associated with the groups of patients with masculine depression and patients with non-masculine depression vs. healthy control subjects (physical issues: B = 0.459, P < 0.001, B = 0.259, P < 0.001; mental issues: 1.599, P < 0.001, B = 2.089, P < 0.001). Higher age predicted the patient group with non-masculine depression (B from 0.035 to 0.043, P from < 0.001 to 0.024) (Table 3) similar to the other models.
Predictors of health care services contacts due to mental complaints: We found that higher BDI-II scores indicative of more severe depression predict the group of patients with masculine vs. the group of patients with non-masculine depression and higher MDRS-22 scores in the group of depressed patients (Supplementary Tables S2 and S3). Simultaneously, fewer health services contacts due to mental complaints also predict the group of patients with masculine vs. the group of patients with non-masculine depression and lower MDRS-22 scores (Table 3). These results suggest that patients who are more severely affected by both depression symptoms and substance use request less frequently support for mental health complaints and may thus receive less intense treatment. To further explore underlying mechanisms, we analyzed how BDI-II score and substance use parameters were related to health services contacts in the group of depressed patients (Supplementary Table S6). As expected, higher BDI-II scores and use of sedative medication (4-week, lifetime) predicted more contacts (B = 0.350, P = 0.001; B = 6.543, P = 0.023; B = 6.792, P = 0.009). In contrast, alcohol, tobacco, and illicit drug use parameters were not significantly related to the number of health services contacts due to mental complaints in the prior year.
Working hours
The sociodemographic characteristics showed significant differences in working hours (months of employment during the previous year, hours of employment per week) between the groups of patients with masculine depression, patients with non-masculine depression, and healthy control subjects (Table 1). Hence, we tested these parameters as predictors in regression analyses (Table 3) and found that more working hours for both parameters were associated with patients with masculine vs. patients with non-masculine depression (B = 0.080, P = 0.030 and B = 0.027, P = 0.015) and tended to predict the MDRS-22 score (B = 0.024, P = 0.063 and B = 0.007, P = 0.053). Relative to healthy controls, more hours of employment per week were associated with patients with masculine depression (B = 0.022, P = 0.014) and fewer months of employment during the previous year with patient with non-masculine depression (B = – 0.074, P = 0.006). Overall, higher BDI-II scores and younger age predicted the group of patients with masculine depression vs. the group of patients with non-masculine depression and MDRS-22 scores (BDI-II scores: B from 0.041 to 0.114, P < 0.001; age: B from – 0.042 to – 0.014, P from 0.002 to 0.004); younger age also predicted healthy controls vs. patients with non-masculine depression (B from 0.035 to 0.037, P < 0.001). Notably, months of employment during the previous year and hours of employment per week did not significantly predict the number of health services contacts due to mental complaints in the prior year (Supplementary Table S6).
Discussion
We investigated substance use patterns and health services contacts in masculine depression. We dichotomized a group of 163 depressed in-patients (44% women) into masculine vs. non-masculine depression (according to the Male Depression Rating Scale-22) and opened the category for female patients. We established that patients with masculine depression relative to those with non-masculine depression show more critical use patterns of alcohol, tobacco, cannabis, and hallucinogens. Simultaneously, patients with masculine depression report less frequent health services contacts due to mental complaints and less frequent use of sedatives. This finding is particularly important because patients with masculine depression are also burdened with increased depression severity. In conclusion, we show here that despite the more critical substance use patterns, patients with masculine depression are less likely to utilize health care services. This significant gap needs to be reduced in the future.
Here, patients with masculine depression showed more frequent and more problematic alcohol use than patients with non-masculine depression. This was applicable in terms of higher AUDIT scores, an AUDIT score of at least 8 (at least zone II risk level), an AUDIT score of at least 20 (zone IV risk level requesting referral to a specialist for diagnostic evaluation and treatment), as well as for binge drinking per se and higher binge drinking frequency and severity for both the 2-week and the 24-month periods. We also found a statistical trend for higher GGT activities in patients with masculine depression, which supports the self-rated higher AUDIT scores and higher binge drinking frequency and severity in this group of patients. Our validation analyses confirmed that higher MDRS-22 scores in the depressed patients were linked to more severe substance use, less health service utilization, and higher depression severity. Versus healthy control subjects, patients with masculine depression showed higher AUDIT scores and higher binge drinking frequency and severity. Interestingly, we found the opposite results for patients with non-masculine depression, i.e., a lower risk and less frequent and less severe binge drinking behavior compared to healthy controls.
Further support for this study’s findings comes from a previous investigation of the same study cohort showing more pronounced impulsive, borderline, and dissocial personality dimensions in patients with masculine depression than in patients with non-masculine depression [36]. Those personality traits are often associated with substance misuse [59]. The presence of SUD or antisocial behavior have been discussed as possible causes of externalizing behaviors [60, 61]. However, our study does not indicate whether SUD precede the masculine depression or vice versa. Future longitudinal studies are needed to elucidate which factors are causes and which ones represent consequences of the masculine depression.
Our results agree with the instrumentalization model of alcohol use. Alcohol can be instrumentalized in a way such that it is consumed in a controlled fashion. This in turn can lead to achievement of goals that would not be achievable or with a significantly higher work load without the drug. Humans and animals both use alcohol to self-manage depression and anxiety symptoms [62–64]. This may also apply to masculine depression. In particular, males reported alcohol use for self-management of masculine depression symptoms [65], which went along with a rejection of other medical treatment. Thereby, alcohol use is regarded as a “quick solution” [65].
We identified a significant gap in mental health care of patients with masculine depression. In this study, patients with masculine depression reported fewer health services contacts due to mental complaints and a lower rate of having used sedative medication during the previous 4-week period relative to those with non-masculine depression. These data suggest that patients with masculine depression have worse access to health service and/or show less help-seeking behavior. Here, higher BDI-II scores predicted more health services contacts due to mental complaints. However, the substance use parameters (except for sedative medication) failed to do so consistently.
Several reasons might account for this health care gap. A huge German survey showed that women more often consult psychiatrists or psychotherapists than men [66]. These differences may be driven by the masculine role model, which inhibits help-seeking behavior [67]. Such a mechanism might also explain this study’s findings that patients with masculine depression (both women and men) show fewer health services contacts. Further support for this assumption comes from our observation that patients with masculine depression reported more months of employment during the previous year as well as more hours of employment per week than patients with non-masculine depression. Versus healthy control subjects, patients with masculine depression even showed a higher number of hours of employment per week, while employment rates were not significantly different to the healthy controls. This finding is again in line with the masculine role model with, e.g., greater willingness to work (even to exhaustion) and higher avoidance of demonstrating weakness, e.g., in the form of a sick leave. Apparently, this also applies to women in the group of patients with masculine depression. Although this was outside the study objectives, our data indicate that the masculine depression is related to a male role model which does not allow oneself to show any weakness.
The results might also indicate that longer working hours promote externalizing symptoms of depression. Möller-Leimkühler et al. [13] assumed that masculine depression is highly prevalent in female university students as a result of exposure to chronic stressors. The combination of the higher risk for cluster B personality traits [36] and the higher consumption of alcohol identified here could lead to worse therapy outcomes [59]. This study’s data might also indicate that depressed patients are not sufficiently screened for their substance use and/or diagnosed with SUD. Future research is needed to enlighten the definite mechanisms underlying the health care gap identified here for patients with masculine depression.
The MDRS-22 correlated with the BDI-II score in the control group and the non-masculine depression group, but not significantly in patients with masculine depression. This lack of a significant correlation in patients with masculine depression is in line with the assumption that patients with masculine depression report only unreliably about typical depression symptoms. In our cohort, patients with masculine depression were younger than patients with non-masculine depression. These results agree with Kelly et al. [34] who found higher rates of comorbid suicidal ideation, AUD, and binge drinking in adults younger than 35 years than in older ones.
Strengths and limitations
Major strengths of this project include the sex-balanced and large study cohort and the inclusion of relevant influencing factors such as sex, BDI-II, and age in the statistical models. The study is limited by the associational study design, which does not allow for conclusions regarding causality or directionality. Future longitudinal research is needed. We analyzed only depressed in-patients diagnosed according to the ICD-10. Individuals with masculine depression and lower depression severity might have been missed because less help-seeking behavior is assumed in patients with masculine depression. Future studies should also investigate the effects of depression history and antidepressants on masculine depression. The here employed median split method is limited due to its dependency on the specific cohort. Certainly, future research is needed to determine how to best distinguish between patients with masculine vs. patients with non-masculine depression.
Conclusion
This study established that the masculine depression is related to more frequent and more critical use of alcohol, tobacco, and illicit drugs and simultaneously to less frequent health services contacts due to mental complaints and lower rates of use of sedative medication. Thus, we identify a significant gap in utilization of health services. Our results highlight the need for specialized and low-threshold help offers at an early stage of affective disorders related to the masculine depression.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients and control persons for their participation in this research project. We thank Dr. Andreas Ahnert for the opportunity and the support to recruit patients at the Klinik für Psychiatrie, Psychotherapie, Psychosomatik of the Klinikum am Europakanal Erlangen. We gratefully appreciate the support of Terezie Sedlinská, Lena Brückner, and Colin Rentsch in recruiting patients and control subjects. We are thankful to Katrin Ebert, Juliana Monti and Sabine Müller for technical assistance.
Author contributions
Conceived and designed the experiments: MH, CM, CW, JK, BL. Performed the experiments: MH, CM, CW, BL. Analyzed the data and wrote the paper: CZ, MH, BL. Commented on the manuscript and provided intellectual input: CM, CPM, CW, JK. The present work was performed in partial fulfillment of the requirements for obtaining the degree “Dr. med.” for MH.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by intramural grants from the University Hospital of the Friedrich-Alexander University Erlangen-Nürnberg (FAU). It was supported by the STAEDTLER-Stiftung and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 402170461 – TRR265 [68]. C.M. is an associated fellow of the research training group 2162 funded by the DFG (270949263/GRK2162/1). The funders had no role in the study design, data collection, analysis, decision to publish, or manuscript preparation.
Availability of data and material
Data are available upon request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and study registration
The Ethics Committee of the Medical Faculty of the Friedrich-Alexander University Erlangen-Nürnberg (ID 194_16 B) approved this study. It has been registered in the German Clinical Trials Register (ID DRKS00015291).
Consent to participate
All study participants provided informed consent. The study was conducted in accordance with the Declaration of Helsinki after review and approval by the Ethics Committee of the Medical Faculty of the FAU.
Footnotes
Johannes Kornhuber and Bernd Lenz authors contributed equally.
References
- 1.Arora S, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139(8):1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Den Ruijter H. Sex and Gender Matters to the Heart. Front Cardiovasc Med. 2020 doi: 10.3389/fcvm.2020.587888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, et al. Gender differences in depression: evidence from genetics. Front Genet. 2020 doi: 10.1038/s41598-020-66672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang H-J, et al. Sex differences in the genetic architecture of depression. Sci Rep. 2020;10(1):9927. doi: 10.1038/s41598-020-66672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobi F, et al. Psychische Störungen in der Allgemeinbevölkerung. Nervenarzt. 2014;85(1):77–87. doi: 10.1007/s00115-013-3961-y. [DOI] [PubMed] [Google Scholar]
- 6.Martin LA, Neighbors HW, Griffith DM. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA Psychiat. 2013;70(10):1100–1106. doi: 10.1001/jamapsychiatry.2013.1985. [DOI] [PubMed] [Google Scholar]
- 7.Rutz W, et al. Prevention of male suicides: lessons from Gotland study. The Lancet. 1995;345(8948):524. doi: 10.1016/s0140-6736(95)90622-3. [DOI] [PubMed] [Google Scholar]
- 8.Strömberg R, Backlund LG, Löfvander M. A comparison between the Beck's depression inventory and the Gotland male depression scale in detecting depression among men visiting a drop-in clinic in primary care. Nord J Psychiatry. 2010;64(4):258–264. doi: 10.3109/08039480903511407. [DOI] [PubMed] [Google Scholar]
- 9.Rice SM, et al. Development and preliminary validation of the male depression risk scale: Furthering the assessment of depression in men. J Affect Disord. 2013;151(3):950–958. doi: 10.1016/j.jad.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Möller-Leimkühler AM, Mühleck J. Konstruktion und vorläufige Validierung eines gendersensitiven Depressionsscreenings (GSDS) Psychiatr Prax. 2020;47(02):79–86. doi: 10.1055/a-1067-0241. [DOI] [PubMed] [Google Scholar]
- 11.Möller-Leimkühler AM, Jackl A, Weissbach L. Gender-sensitive depression screening (GSDS) - further validation of a new self-rating instrument. Psychiatr Prax. 2022;49(7):367–374. doi: 10.1055/a-1615-8274. [DOI] [PubMed] [Google Scholar]
- 12.Oliffe JL, et al. Faux masculinities among college men who experience depression. Health (London) 2013;17(1):75–92. doi: 10.1177/1363459312447256. [DOI] [PubMed] [Google Scholar]
- 13.Möller-Leimkühler AM, Yücel M. Male depression in females? J Affect Disord. 2010;121(1–2):22–29. doi: 10.1016/j.jad.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Grant BF, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiat. 2017;74(9):911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochlen AB, et al. Barriers in diagnosing and treating men with depression: a focus group report. Am J Mens Health. 2010;4(2):167–175. doi: 10.1177/1557988309335823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chodkiewicz J, Wydrzyński M, Talarowska J. Young's early maladaptive schemas and symptoms of male depression. Life (Basel) 2022;12(2):167. doi: 10.3390/life12020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahalik JR, et al. Development of the conformity to masculine norms inventory. Psychol Men Masculinit. 2003;4:3–25. [Google Scholar]
- 18.Hemsing N, Greaves L. Gender norms, roles and relations and cannabis-use patterns: a scoping review. Intl J Environm Res Public Health. 2020;17(3):947. doi: 10.3390/ijerph17030947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha Y, Weeden KA. Overwork and the slow convergence in the gender gap in wages. Am Sociol Rev. 2014;79(3):457–484. [Google Scholar]
- 20.Seidler ZE, et al. The role of masculinity in men's help-seeking for depression: a systematic review. Clin Psychol Rev. 2016;49:106–118. doi: 10.1016/j.cpr.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Mcugh RK, Weiss RD (2019) Alcohol use disorder and depressive disorders. Alcohol Res Current Rev 10.35946/arcr.v40.1.01 [DOI] [PMC free article] [PubMed]
- 22.Grant BF, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 23.Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20(2):173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 24.Müller CP, et al. Sex-dependent alcohol instrumentalization goals in non-addicted alcohol consumers versus patients with alcohol use disorder: longitudinal change and outcome prediction. Alcohol Clin Exp Res. 2021;45(3):577–586. doi: 10.1111/acer.14550. [DOI] [PubMed] [Google Scholar]
- 25.Müller CP, Schumann G. Drugs as instruments: a new framework for non-addictive psychoactive drug use. Behav Brain Sci. 2011;34(6):293–310. doi: 10.1017/S0140525X11000057. [DOI] [PubMed] [Google Scholar]
- 26.Saha S, et al. Comorbidity between mood and substance-related disorders: A systematic review and meta-analysis. Aust N Z J Psychiatry. 2021 doi: 10.1155/2021/5514144. [DOI] [PubMed] [Google Scholar]
- 27.Eberhard S, et al. Risky alcohol use and comorbidity in a swedish adolescent emergency psychiatric inpatient population. J Addict. 2021;2021:5514144. doi: 10.1155/2021/5514144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen AM, et al. Polygenic scores for major depressive disorder and risk of alcohol dependence. JAMA Psychiat. 2017;74(11):1153–1160. doi: 10.1001/jamapsychiatry.2017.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, et al. Genetic risk variants associated with comorbid alcohol dependence and major depression. JAMA Psychiat. 2017;74(12):1234–1241. doi: 10.1001/jamapsychiatry.2017.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgson K, et al. The genetic basis of the comorbidity between cannabis use and major depression. Addiction. 2017;112(1):113–123. doi: 10.1111/add.13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinichenko LS, et al. Neutral sphingomyelinase mediates the co-morbidity trias of alcohol abuse, major depression and bone defects. Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01304-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller CP, et al. Paradoxical antidepressant effects of alcohol are related to acid sphingomyelinase and its control of sphingolipid homeostasis. Acta Neuropathol. 2017;133(3):463–483. doi: 10.1007/s00401-016-1658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller CP, Kornhuber J. Biological evidence for paradoxical improvement of psychiatric disorder symptoms by addictive drugs. Trends Pharmacol Sci. 2017;38(6):501–502. doi: 10.1016/j.tips.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Kelly LM, Liu RT, Zajac K. Comorbid alcohol-related problems and suicidality disproportionately impact men and emerging adults among individuals with depressive symptoms. J Affect Disord. 2021;293:329–337. doi: 10.1016/j.jad.2021.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson DA, et al. Changes in alcohol consumption: United States, 2001–2002 to 2012–2013. Drug Alcohol Depend. 2015;148:56–61. doi: 10.1016/j.drugalcdep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedlinská T, et al. Male depression syndrome is characterized by pronounced cluster b personality traits. J Affect Disord. 2021;292:725–732. doi: 10.1016/j.jad.2021.05.114. [DOI] [PubMed] [Google Scholar]
- 37.Hanna Chaim C, et al. Alcohol use patterns and disorders among individuals with personality disorders in the Sao Paulo Metropolitan area. PLoS ONE. 2021;16(3):e0248403. doi: 10.1371/journal.pone.0248403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cha Y. Overwork and the persistence of gender segregation in occupations. Gend Soc. 2013;27(2):158–184. [Google Scholar]
- 39.Afonso P, Fonseca M, Pires JF. Impact of working hours on sleep and mental health. Occup Med (Lond) 2017;67(5):377–382. doi: 10.1093/occmed/kqx054. [DOI] [PubMed] [Google Scholar]
- 40.Wong K, Chan AHS, Ngan SC. The Effect of long working hours and overtime on occupational health: a meta-analysis of evidence from 1998 to 2018. Int J Environ Res Public Health. 2019 doi: 10.3390/ijerph16122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuroda S, Yamamoto I. Why do people overwork at the risk of impairing mental health? J Happiness Stud. 2019;20(5):1519–1538. [Google Scholar]
- 42.Virtanen M, et al. Long working hours and symptoms of anxiety and depression: a 5-year follow-up of the Whitehall II study. Psychol Med. 2011;41(12):2485–2494. doi: 10.1017/S0033291711000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virtanen M, et al. Long working hours and alcohol use: systematic review and meta-analysis of published studies and unpublished individual participant data. BMJ British Med J. 2015;350:g7772. doi: 10.1136/bmj.g7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staiger T, et al. Masculinity and help-seeking among men with depression: a qualitative study. Front Psychiatry. 2020 doi: 10.3389/fpsyt.2020.599039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Möller-Leimkühler AM. Barriers to help-seeking by men: a review of sociocultural and clinical literature with particular reference to depression. J Affect Disord. 2002;71(1–3):1–9. doi: 10.1016/s0165-0327(01)00379-2. [DOI] [PubMed] [Google Scholar]
- 46.Magovcevic M, Addis ME. The masculine depression scale: development and psychometric evaluation. Psychol Men Masculin. 2008;9(3):117–132. [Google Scholar]
- 47.Esteban-Gonzalo L, et al. The relationship between conformity to male and female gender norms and depression during pregnancy. Arch Womens Ment Health. 2019;22(6):809–815. doi: 10.1007/s00737-019-01003-0. [DOI] [PubMed] [Google Scholar]
- 48.von Zimmermann C, et al. Bioimpedance body measures and serum lipid levels in masculine depression. Front Psychiatry. 2022;13:794351. doi: 10.3389/fpsyt.2022.794351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO (2004) ICD-10: international statistical classification of diseases and related health problems: tenth revision. World Health Organization: Geneva [PubMed]
- 50.APA (2013) Diagnostic and statistical manual of mental disorders. Fifth Edition ed. Arlington: American Psychiatric Association
- 51.Rice SM, et al. Male-type and prototypal depression trajectories for men experiencing mental health problems. Int J Environm Res Public Health. 2020;17(19):7322. doi: 10.3390/ijerph17197322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice SM, et al. Validity of the male depression risk scale in a representative Canadian sample: sensitivity and specificity in identifying men with recent suicide attempt. J Ment Health. 2019;28(2):132–140. doi: 10.1080/09638237.2017.1417565. [DOI] [PubMed] [Google Scholar]
- 53.Walther A, et al. Male depression risk, psychological distress, and psychotherapy uptake: Validation of the German version of the male depression risk scale. J Affect Disorders Rep. 2021;4:100107. [Google Scholar]
- 54.Beck AT, Steer RA, Brown G (1996) Beck depression inventory-II. psychological assessment
- 55.Rumpf H-J et al (2003) Deutsche Version des Alcohol Use Disorder Identification Test (AUDT-G-L), in Elektronisches Handbuch zu Erhebungsinstrumenten im Suchtbereich (EHES). Glöckner-Rist A, Rist F, Küfner Mannheim H: Zentrum für Umfragen, Methoden und Analysen
- 56.Babor TF et al (2001) Audit. The alcohol use disorders identification test. Guidelines for use in primary care. Second ed. Geneva: World Health Organisation
- 57.Lenz B, et al. Low digit ratio (2D:4D) and late pubertal onset indicate prenatal hyperandrogenziation in alcohol binge drinking. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:370–378. doi: 10.1016/j.pnpbp.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 58.Schumann A et al (2003) Deutsche version des Fragebogens zur Self-Efficacy für Raucher (SER-G). in Elektronisches Handbuch zu Erhebungsinstrumenten im Suchtbereich (EHES). Glöckner-Rist A, Rist F, Küfner H: Mannheim: Zentrum für Umfragen, Methoden und Analysen
- 59.Sher KJ, Trull TJ. Substance use disorder and personality disorder. Curr Psychiatry Rep. 2002;4(1):25–29. doi: 10.1007/s11920-002-0008-7. [DOI] [PubMed] [Google Scholar]
- 60.Soe-Agnie SE, et al. Psychometric properties of the externalizing spectrum inventory: replication and extension across clinical and non-clinical samples. J Pers Assess. 2021;103(3):332–341. doi: 10.1080/00223891.2020.1753752. [DOI] [PubMed] [Google Scholar]
- 61.Durbeej N, et al. Mental health services and public safety: substance abuse outpatient visits were associated with reduced crime rates in a swedish cohort. PLoS ONE. 2015;10(9):e0137780. doi: 10.1371/journal.pone.0137780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed SH, et al. Non-pharmacological factors that determine drug use and addiction. Neurosci Biobehav Rev. 2020;110:3–27. doi: 10.1016/j.neubiorev.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Müller CP (2017) Non addictive drug use: The way forward, in The SAGE Handbook of Drugs & Alcohol Studies – Biological Approaches, Wolff K, White J, Karch S Editor. SAGE: London. p. 411–434
- 64.Müller CP. Drug instrumentalization. Behav Brain Res. 2020;390:112672. doi: 10.1016/j.bbr.2020.112672. [DOI] [PubMed] [Google Scholar]
- 65.Krumm S, et al. Men's views on depression: a systematic review and metasynthesis of qualitative research. Psychopathology. 2017;50(2):107–124. doi: 10.1159/000455256. [DOI] [PubMed] [Google Scholar]
- 66.Keil J, et al. Gender-specific differences in the utilization of health care services in an Urban population sample. Gesundheitswesen. 2020;82(3):e17–e23. doi: 10.1055/a-0820-3584. [DOI] [PubMed] [Google Scholar]
- 67.Streb J, et al. Gender-specific differences in depressive behavior among forensic psychiatric patients. Front Psychol. 2021;12:3691. doi: 10.3389/fpsyg.2021.639191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heinz A, et al. Addiction research consortium: losing and regaining control over drug intake (ReCoDe)-From trajectories to mechanisms and interventions. Addict Biol. 2020;25(2):e12866. doi: 10.1111/adb.12866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.
Not applicable.