Abstract
This study was conducted for identifying phylogenetic relationships between 15 scab-causing Streptomyces species including S. bottropensis, S. europaeiscabiei, S. scabiei, S. stelliscabiei and, other 11 Streptomyces sp. All of the strains were originally isolated from symptomatic potatoes in Erzurum Province, The Eastern Anatolia Region of Turkey. Some morphological and biochemical properties of the strains were defined in our former research. Then, 16 s rRNA regions of them were sequenced. After the sequence data assembly, phylogenetic analyzes were performed. The phylogenetic analyses revealed that the strains are involved in the same major group and, substantially similar to reference strains. Additionally, some subgroup formations were also recorded. Moreover, Repetitive element-based PCR (Rep-PCR), Enterobacterial repetitive intergenic consensus (ERIC-PCR), and BOX-PCR fingerprinting molecular typing methods were used for as molecular typing methods. According to our knowledge, this is the first report on phylogenetic relationships of scab-causing Streptomyces species from Turkey. However, the identification of most pathogenic strains remained at the species level.
Keywords: Common-scab, Phylogeny, 16S rRNA, Rep-PCR, ERIC-PCR, BOX-PCR Streptomyces
Introduction
Streptomyces are gram-positive, filamentous bacteria that form extensively branched substrate mycelium. It is also the ability to produce antibiotics or other industrially valuable secondary metabolites (Kumar et al. 2007; Elias et al. 2022) and some of the genus members are used as biocontrol agents against plant disease (Simizu et al. 2009). Some Streptomyces species can also cause a plant disease called scab, which is an important problem for potato growers worldwide (Bukhalid and Loria 1997) and the disease significantly reduces the quality of tubers (Gutierrez et al. 2022).
Different phytopathogenic Streptomyces species, which are critical hazardous effects on potatoes have been reported all over the world, especially in USA and Canada (Wanner et al. 2009), Sweden (Natsume et al. 2018), China (Liang et al. 2019), Argentina, Mexico, Finland, South Korea, Japan etc. (Shuang et al. 2022).
Scab disease affects some other crops containing beet, carrot, radish, parsnip, and sweet potato (Hill and Lazarovits 2005; Planckaert et al. 2021; Shuang et al. 2022). Moreover, scab-causing Streptomyces can damage some seedlings of monocotyledonous and dicotyledonous plants. Injuring of the plant is not necessary for symptoms to occur. Although the symptoms are generally visible on the damaged part of tubers, pathogens can be introduced from lenticels (Wanner 2009). On potato tubers, scab symptoms are variable. Superficial or raised brown spots and dark pits on the skin extending several millimeters into the potato tuber can be observed. The lesions may be small and discrete, or they may coalesce to cover larger areas of the tuber surface (Wanner 2006). Symptom type depends on plant varieties, infection time, the virulence of the pathogen, and environmental conditions.
Three marker genes, Nec1, TomA (Natsume et al. 2018), and thatxtomin synthesize (txtA, txtB) were found to be related to the pathogenicity of Streptomyces (Zhao et al. 2022). Most of the studies recommended that Nec1 and TomA genes deal with pathogenicity, but these genes are not key factors of pathogenicity (Wanner 2009; Leiminger et al. 2013). Some studies also suggest that different virulence factors may participate in the pathogenicity of Streptomyces (Lapaz et al. 2017).
It is also well known that there are plenty of methods for disease control such as cultural, chemical and biological control or resistant varieties. However, in general, commercially insignificant varieties have resistance and none of them is completely resistant (Zadina et al. 1975; Hosny et al. 2014; Sarwar et al. 2018). Moreover, the resistance of the varieties can be alterably related to strains or species of pathogens and soil properties such as pH and moisture etc. (Haynes et al. 1997). Growers generally do not harvest infected tubers and tubers left in fields serve as inoculums for further vegetation (Pavlista 1996). The infected tuber can be accepted more effective than soil inoculums in transferring pathogens. Further, infected tubers significantly transfer novel scab formations of more virulent strains (Loria 2001).
It is well known that there are difficulties arising from some reasons for the taxonomy of Streptomycetes (Hatano et al. 2003; Kim et al. 2012). Therefore, taxonomy and relationships between scab-causing Streptomyces spp. have been studied in different ways. Numerical analyses of phenotypic data, fatty acid analyses, and DNA-DNA hybridization (Bouchek-Mechice et al. 2000) are some of these. 16S rRNA gene analysis is also another method with little doubt. The method has some drawbacks like unconformity with DNA-DNA relatedness or heterogeneity among copies within a genome (Kim et al. 2012). Nevertheless, Phylogeny based on 16S rRNA gene sequences has been considered a powerful and promising tool in prokaryote systematic for elucidating phylogenetic relationships among prokaryotic organisms (Stackebrandt et al. 1997) and has been used for as well-known identification of Streptomycetes (Kreuze et al. 1999). In addition to these methods, PCR-based molecular methods have been the center of attraction of scientists. Especially, PCR-based methods of fingerprinting have beneficial role in the existence of repetitive sequences that are distributed bed throughout the genome of distinct bacterial species. For instance, Rep-PCR has been commonly exploited to assessment of the strain specific motifs provided from PCR amplification repetitive DNA fragments exist in bacterial genomes. As an alternative version of Rep-PCR is the amplification of genomic DNA situated among the ERIC-PCR sequences. These sequences are shared along the extragenic regions of the genomes of numerous bacteria (Tajima et al. 2000). On the other hand, BOX-PCR fingerprinting is useful method for typing of diverse bacterial species and it is thought as advantageous complementary instrument for epidemiological researches of members of various type of genus (Tacão et al. 2005).
Our former research showed existence of different Streptomyces species causing common scab symptoms on potato in Turkey. This study was designed to the research the relationships between 15 phytopathogenic Streptomyces spp., which belong to distinct morphologic groups via 16S rRNA, Rep-PCR, ERIC-PCR and BOX-PCR.
Materials and methods
Bacterial strains
All the strains were isolated from symptomatic potato tubers in Erzurum Province, Turkey. Identification of the strains by classical and molecular methods, and characterization of the pathogenicity island (PAI) was performed in our former research (Karagoz 2013; Karagoz and Kotan 2017). Morphological, biochemical properties, and PAI profiles of strains are presented in Table 2.
Table 2.
Properties and PAI profiles of the strains
| Strain number | Identification result | Spore color | Spore morphology | Melanoid pigment production |
Streptomycin | Penicillin | Chrystal violet | Phenol | NaCl (%) | Minimum growth of pH | Growth at 37 ºC | PAI profiles | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nec1 | tomA | txtAB | |||||||||||||||
| PYI | TYR | 5 | 6 | 7 | |||||||||||||
| KS196 | S. scabiei | G | S | + | + | – | – | – | – | – | – | – | 5,0 | + | + | + | + |
| KS176 | S. stelliscabiei | G | S | + | + | – | + | – | – | – | – | – | 5,5 | + | + | + | + |
| KS464 | S. europaeiscabiei | G | S | + | + | – | + | – | + | – | – | – | 5,0 | + | + | + | + |
| KS573 | S. bottropesnsis | G | S | + | + | – | + | – | + | + | + | – | 4,0 | + | + | + | – |
| KS613 | Streptomyces sp. | P | R | – | + | – | + | + | + | + | + | + | 4,0 | + | + | + | + |
| KS227 | Streptomyces sp. | G | R | – | + | – | + | – | + | + | + | + | 4,0 | + | + | + | + |
| KS177 | Streptomyces sp. | G | S | – | + | – | + | – | – | + | – | – | 5,0 | + | + | – | + |
| KS308 | Streptomyces sp. | G | S | + | + | – | + | – | – | – | – | – | 5,0 | + | + | + | + |
| KS541 | Streptomyces sp. | G | S | – | + | + | + | + | + | + | + | + | 4,0 | + | + | + | – |
| KS606 | Streptomyces sp. | G | R | – | + | + | + | – | + | + | + | – | 4,5 | – | + | + | + |
| KS229 | Streptomyces sp. | G | S | – | – | – | + | – | + | + | + | + | 5,0 | + | + | + | – |
| KS465 | Streptomyces sp. | G | R | – | + | – | – | – | – | – | – | – | 5,0 | + | – | + | + |
| KS525 | Streptomyces sp. | W | R | + | + | – | + | – | – | – | – | – | 4,0 | + | + | + | + |
| KS542 | Streptomyces sp. | W | R | – | + | – | + | + | + | + | + | + | 4,0 | + | + | – | + |
| KS561 | Streptomyces sp. | G | R | + | + | – | + | + | + | – | – | – | 5,0 | – | + | + | + |
Spore color; G. grey, W: white, P: pale orange. Spore morphology; S: spiral, R. rectiflexous. Phenol: 1%, Chrystal violet: 0,5 µg/ml, Streptomycin 20 µg/ml, Penicillin 10 IU/ml, + : positive, -: negative
Pathogenicity assays
Two different pathogenicity tests were performed. First, potato tuber, cv. marfona, was peeled and sterilized. Disks (2 cm2 X 0.5 cm thick) cut from tubers were situated in Petri dishes. Then, strains grown on oatmeal (OM) agar plates were cut and located upside down on the disks. And then, the pathogenicity test of Conn et al. protocol (1998) was performed on the Streptomyces species (Conn et al. 1998). Other pathogenicity tests were performed on radish seeds. Briefly, radish seeds were washed and sterilized with 5% sodium hypochlorite for 2 min. Sterilized seeds were placed on Petri plates including 1% water agar. Then, germinated seeds were dipped in bacterial spore suspension at a concentration of ~ 109 CFU / ml. Inoculated seedlings were transferred to tubes containing 1% water agar. Symptoms were evaluated after two weeks (Schaad et al. 2001). Necrosis formation and abnormal growth like dwarfing or hypertrophy are recorded as positive pathogenicity. Tests were repeated three times.
Sequencing of 16S rRNA genes
16S rRNA gene was amplified by using primers 16S1F and 16S1R. Primer pairs was given in Table 1. The reaction mixture was used according to the Wanner 2006 method. PCR was performed with an Eppendorf gradient PCR thermocycler using the following conditions: an initial denaturation at 95 °C for 5 min, 40 cycles consisting of 94 °C for 20 sn, annealing at 59 °C for 30 sn, and extension at 72 °C for 2 min. Products were run on 1.5% agarose gel. Finally, sequencing was carried out via the dideoxy-chain termination method (Intergen, C.O, Ankara, TURKEY).
Table 1.
Primers used in this study
| Primers | Reference | |
|---|---|---|
| 16S rRNA |
16S1F (5’ CATTCACGGAGAGTTTGATCC 3’) 16S1R (5’ AGAAAGGAGGTGATCCAGCC 5’) |
Wanner 2006 |
| ERIC-PCR |
ERIC 1R (5'-ATGTAAGCTCCTGGGGAT-3') ERIC 2 (5'- AAGTAAGTGACTGGGGGT GAGC-3') |
Versalovic et al. 1994 |
| REP-PCR |
REP 1R (5'-IIIICGICGICATCIGGC-3') REP 2 (5'-ICGICTTATCIGGCCTAC-3') |
Versalovic et al. 1994 |
| BOX-PCR | BOXA1R (5'-CTACGGCAAGGCGACGCTG ACG-3') | Ogutcu et al. 2009 |
ERIC, REP and BOX PCR analyses
All the isolates were also characterized by genomic fingerprinting. For this purpose; ERIC, REP and BOX primer sets were used. PCR reactions were prepared according to the former research with some modifications (Versalovic et al. 1994). The primer sets used are presented in Table 1. Briefly, the reaction mixture including; 5 ul 10X PCR buffer without MgCl2, 2,0 mM MgCl2, 0,4 mM each dNTP’s, 5U Taq DNA polymerase, 0,5 µM each primer and 50 ng template DNA was made up to 50 µl with PCR grade water. PCR was conducted with thermocycler using the following conditions: initial denaturation at 95 °C for 7 min, 30 cycles consisting of 94 °C for 1 min and annealing at 40, 40 and 55 °C for 1 min, for REP, ERIC and BOX primers, respectively; extension at 72 °C for 8 min; a final extension at 72 °C for 15 min. After the PCR, the tubes were cooled at 4 °C. Then PCR products were separated with 1,5% agarose gel and visualized.
Phylogenetic analysis
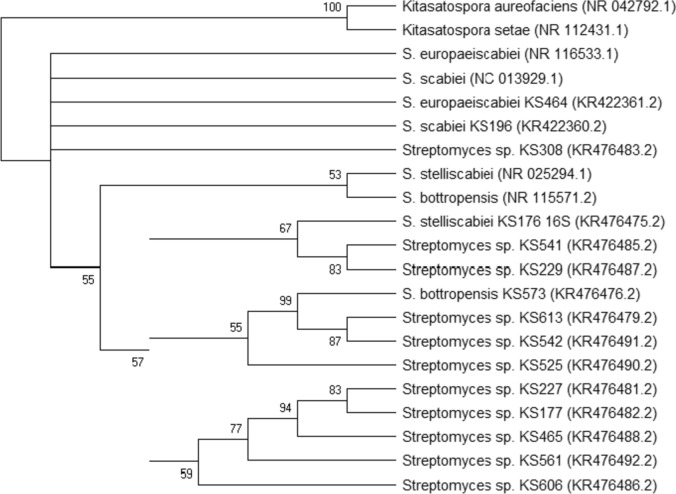
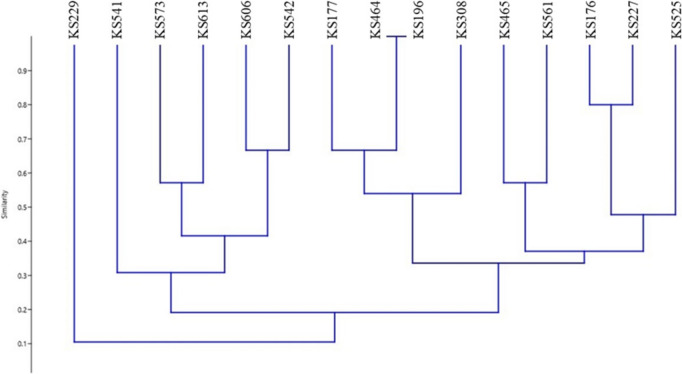
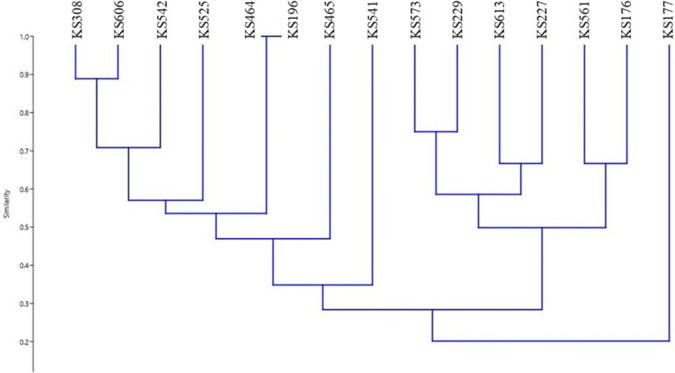
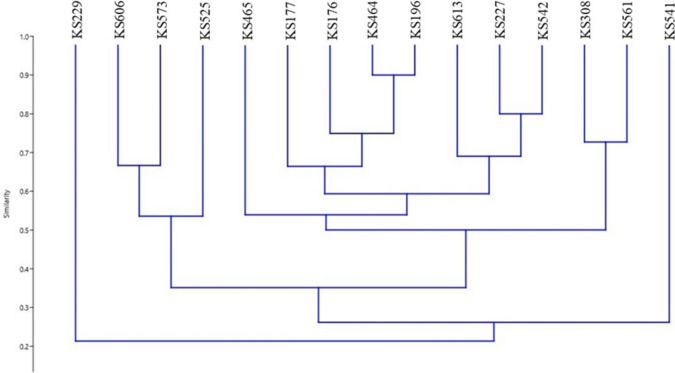
Sequences data were edited and analyzed, using the BioEdit Sequence Alignment Editor 7.0.4.1 software (Hall 1999). All sequence data obtained was confirmed by BLAST searching and was deposited in GenBank® (accession numbers are given in Fig. 1 with brackets). The evolutionary history was inferred using the Neighbor-Joining method. The bootstrap consensus tree deduced from 1000 replicates is provided to represent the evolutionary history of the taxa analyzed. The evolutionary distances were figured out using the Maximum Composite Likelihood technique. All positions including gaps and missing data were removed. There were 1399 positions in the last dataset. Evolutionary analyses were realized in MEGA 6 software (Tamura et al. 2013). 16S rRNA sequence of the reference (S. bottropensis NR_115571.2, S. europaeiscabiei NR_116533.1, S. scabiei NC_013929.1, and S. stelliscabiei NR_025294.1) and the out group (Kitasatospora aureofaciens NR_042792.1 and Kitasatospora setae NR_112431.1) strains were obtained from GenBank®. The banding patterns formed by ERIC- PCR, REP- PCR and BOX-PCR were examined by using Paleontological Statistics Software (PAST). According to the PAST software, the related dendrograms were carried out using an unweighted pair group method with arithmetic mean (UPGMA). Hammer et al. (2001) were used as a reference guideline to analyze for constructed phylogenetic trees of ERIC- PCR, REP- PCR and BOX-PCR data (Fig. 2, 3, 4).
Fig. 1.
Phylogenetic tree of scab-causing Streptomyces spp. based on 16S rRNA regions sequences. (GenBank® accession numbers are presented in brackets)
Fig. 2.
Dendrogram generated from ERIC-PCR banding pattern of 15 Streptomyces strains. The similarity analysis was performed with Bray–Curtis and UPGMA method
Fig. 3.
Dendrogram generated from REP-PCR banding pattern of 15 Streptomyces strains. The similarity analysis was performed with Bray–Curtis and UPGMA method
Fig. 4.
Dendrogram generated from BOX-PCR banding pattern of 15 Streptomyces strains. The similarity analysis was performed with Bray–Curtis and UPGMA method
Results and discussion
When the obtained data were analyzed, all the strains utilized in this study have positive pathogenicity on potato discs and radish seedlings. As well known, pathogenicity test results of tuber slice and radish seedling assays are not parallel at all times (Conn et al. 1998). For this reason, the results were confirmed by both pathogenicity tests. These tests were successfully used in different studies. Although there are exceptions, the results of the pathogenicity tests are generally parallel (Hasani and Taghavi 2014; Lapaz et al. 2017).
Morphological and biochemical test results and marker genes (Nec1, TomA, and TxtAB) in PAI of the strains were determined in our former research (Karagoz 2013). Morphological and biochemical test results (Table 2) are mostly fitted in the literature with a few exceptions. Some variations were observed like resistance to chemicals and antibiotics. Marker genes generally exist in the strains. KS229, KS541, and KS573 strains lack TxtAB, KS177 and KS542 strains lack TomA, and KS465 strain lacks Nec1 genes according to PCR results. PAI profiles of strains were presented in Table 2. According to the literature knowledge, it is reported that various pathogenic Streptomyces species can be deficient in Nec1 or TomA. Many researchers have mentioned that Nec1 and TomA genes are relevant to pathogenicity but they are not basic determinants of pathogenicity. (Lerat et al. 2009; Park et al. 2003; Leiminger et al. 2013; Dees et al. 2013). Besides, the existence of Nec1 and TomA genes was also reported in non-pathogenic strains (Wanner 2009). Production of thaxtomin was defined as the primary pathogenicity determinant of pathogenic Streptomyces species on potatoes in many studies (Wanner 2007a, b, 2009; Flores-Gonzalez et al. 2008; Leiminger et al. 2013). In some studies, however, pathogen Streptomyces species, which lack of thaxtomin production ability were also reported (Flores-Gonzalez et al. 2008). Additionally, another study screened that 17% of pathogen strains used in the study did not contain any of the marker genes. Researchers suggest that different virulence factors may participate in pathogenicity (Lapaz et al. 2017) and our findings support this approach.
For the phylogenetic analysis, 16S rRNA genes, expected ~ 1531 bp size, were cloned by PCR and 1399 bp.16S rRNA gene sequences, between positions in 50 and 1448, were assembled. Coordinated sequence data were analyzed by BLAST. All strains showed 99% similarity with the members of the genus Streptomyces. As a result of phylogenetic analyzes, two major groups were obtained. While the out-group strains, Kitasatospora aureofaciens and Kitasatospora setae, constitute the first group, all the Streptomyces species constitute the second group. Formations of some subgroups are also recorded in the second group. S. scabiei and S. europaeiscabiei were defined as closely related. Positions of S. scabiei KS196 and S. europaeiscabiei KS464 found to be very close to each other and reference strains (S. scabiei NC_013929.1 and S. europaeiscabiei NR_116533.1). S. stelliscabiei KS176 and S. bottropensis KS573 are located in closed positions which are related to reference strains (S. stelliscabiei NR_025294.1 and S. bottropensis NR_115571.2). Phylogenetic tree of scab-causing Streptomyces spp. based on 16S rRNA gene sequences are presented in Fig. 1. Phylogenetic tree derived from 16S rRNA sequence of strains generally show similarity with previous studies. While position of S. scabiei and S. europaeiscabiei were defined very close, the distance of other strains to them and each other was also recorded as similar (Bouchek-Mechiche et al. 2000; Kim et al. 2012; Park et al. 2003). According to our results, Turkish strains are generally closer to each other.
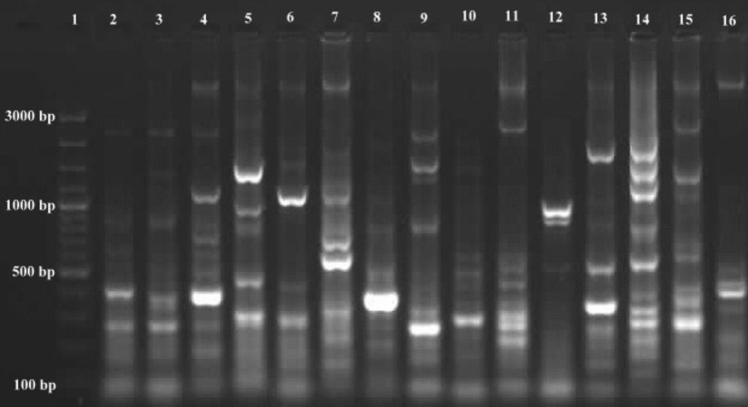
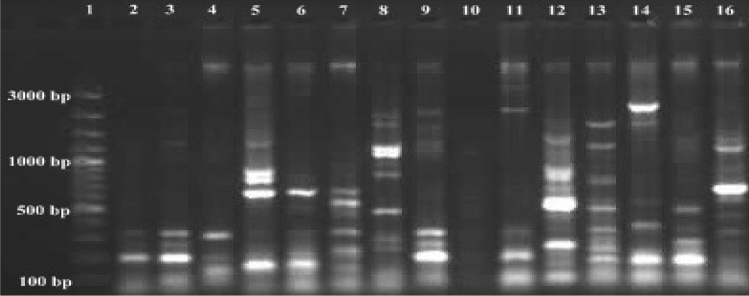
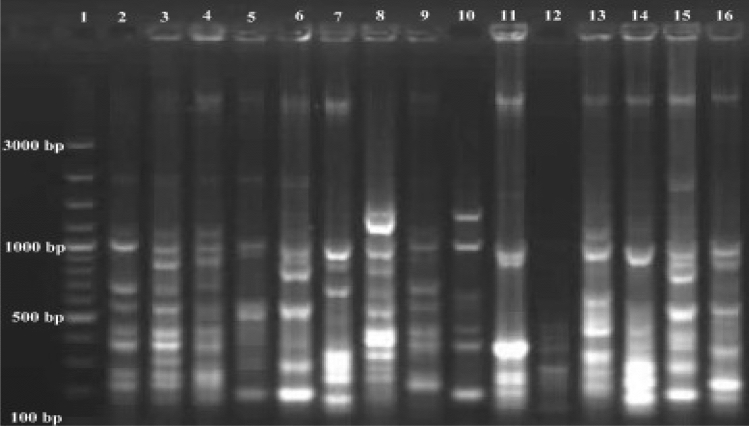
γ, α and 1435 variable regions were also analyzed. Some variations were observed. Especially γ variable regions have high-value variations in positions 174–202. α Variable region has some variations position in 974–999. It was observed that a few variations in 1435 variable regions position in 1435–1438. Variations in γ, α and 1435 regions of strains are given in Table 3. As a result of the analyzes, the γ region was shown to possess high variability potential than α and 1435 regions. Different formations generally were defined in γ region. S. scabiei is also different from S. europaeiscabiei in this region. It is known that S. scabiei and S. europaeiscabiei 16S rRNA regions very similar with just 1 bp mismatch. Mostly similar sequences with former research were detected in γ, α and 1435 regions of strains except for S. stelliscabiei KS176 and S. bottropensis KS573. Some differences were encountered in γ, α and 1435 regions of S. stelliscabiei KS176 and S. bottropensis KS573, when compared to literature (Wanner 2006). We think that some changes in 16S rRNA sequence could be possible depending on conditions (locations and climates etc.) because of their genetic variation potential. These strains were identified by classical methods (given in Table 2.) and PCR analyzes were also performed by using specific primer pairs Stel3/T2st2 and Stel3/Aci2 (Wanner 2006) for S. stelliscabiei and S. bottropensis, respectively in our former studies (Karagoz 2013; Karagoz and Kotan 2017). Specific DNA bands were observed for both S.stelliscabiei and S. bottropensis. Moreover, the ERIC primer set formed reproducible and distinct fingerprints containing 3–9 fragments between 100 and 3000 bp. REP PCR demonstrated that Streptomyces strains have different patterns with 2–8 fragments ranging from to 100 3000 bp. For BOX-PCR fingerprint showed 2–11 fragments in the size of 100-3000 bp (Figs. 5, 6, 7).
Table 3.
Genetic variations in γ, α and 1435 variable regions of the strains
| Strains | γ—variable region Position in 174–202 |
α—variable region Position in 974–999 |
1435 variable region Position in 1435–1438 |
|
|---|---|---|---|---|
| KS196 S. scabiei | (KR422360.2)* | CGACACTCTCGGGCATCCGATGAGTGTGG | ACACCGGAAACGGCCAGAGATGGTCG | GTAA |
| KS464 S. europaeiscabiei | (KR422361.2) | CAACACTCTCGGGCATCCGATGAGTGTGG | ACACCGGAAACGGCCAGAGATGGTCG | GTAA |
| KS176 S. stelliscabiei | (KR476475.2) | CTATCGCCTTGGGCATCCTT-GGTGATCG | ACACCGGAAAGCATCAGAGATGGTGC | TTGT |
| KS573 S. bottropensis | (KR476476.2) | ACACTTCTGCTCTCATGGGC-AGGGGTTA | ACACCGGAAAGCATCAGAGATGGTGC | TTGT |
| KS613 Streptomyces sp. | (KR476479.2) | ACACTTCTGCTCTCATGGGC-AGGGGTTA | ACACCGGAAAGCATCAGAGATGGTGC | TTGT |
| KS227 Streptomyces sp. | (KR476481.2) | ACACTCTGTCCCGCATGGGA-CGGGGTTA | ATACCGGAAAGCATCAGAGATGGTGC | TTGT |
| KS177 Streptomyces sp. | (KR476482.2) | ACACTCTGTCCCGCATGGGA-CGGGGTTA | ATACCGGAAAGCATCAGAGATGGTGC | TTGT |
| KS308 Streptomyces sp. | (KR476483.2) | ACACTCTCTCGGGCATGGGATGAGTGTGG | ACACCGGAAACGGCCAGAGATGGTGC | GTAA |
| KS541 Streptomyces sp. | (KR476485.2) | CTACCCGCTTGGGCATCCAA-GCGGTTCG | ACACCGGAAAGCATTAGAGATGGTGC | TTGT |
| KS606 Streptomyces sp. | (KR476486.2) | ATACTTTCCCTCTCATGGGG-GAAGGTTA | CGCCCGGAAAGCCGTAGAGATGGTGC | TTGT |
| KS229 Streptomyces sp. | (KR476487.2) | CTACGCGCTCAGGCATCTGATGCGCGTGG | ACACCGAAAAACTTTGGAGACAAGGC | TTGT |
| KS465 Streptomyces sp. | (KR476488.2) | ACACTCTGTCCCGCATGGGA-CGGGGTTA | ATACCGGAAAGCATCAGAGATGGTGC | TTGT |
| KS525 Streptomyces sp. | (KR476490.2) | ACACTGCCACGGGCATCTGT-GGTGGTTA | CGCCCGGAAAGCATCAGAGATGGTGC | TTGT |
| KS542 Streptomyces sp. | (KR476491.2) | ACACTCCTGCTCTCATGGGC-AGGGGTTA | ACACCGGAAAGCATCAGAGATGGTGC | TTGT |
| KS561 Streptomyces sp. | (KR476492.2) | ACACCGGCTTCCGCATGGGA-GCTGGTTG | ATACCGGAAAGCATTAGAGATGGTGC | TTGT |
*GenBank® accession numbers are given in brackets
Fig. 5.
ERIC-PCR band profiles of Streptomyces strains with ERIC 1R and ERIC 2 primers. Lanes 1, Marker; 2, KS196; 3, KS464; 4, KS176; 5, KS573; 6, KS613; 7, KS227; 8, KS177; 9, KS308; 10, KS541; 11, KS606; 12, KS229; 13, KS465; 14, KS525; 15, KS542; 16, KS561
Fig. 6.
REP-PCR band profiles of Streptomyces strains with REP 1R and REP 2 primers. Lanes 1, Marker; 2, KS196; 3, KS464; 4, KS176; 5, KS573; 6, KS613; 7, KS227; 8, KS177; 9, KS308; 10, KS541; 11, KS606; 12, KS229; 13, KS465; 14, KS525; 15, KS542; 16, KS561
Fig. 7.
BOX-PCR band profiles of Streptomyces strains with BOXA1R primer. Lanes 1, Marker; 2, KS196; 3, KS464; 4, KS176; 5, KS573; 6, KS613; 7, KS227; 8, KS177; 9, KS308; 10, KS541; 11, KS606; 12, KS229; 13, KS465; 14, KS525; 15, KS542; 16, KS561
Numerous methods use for determining to the molecular diversity of scab-causing Streptomyces species. ERIC-PCR, REP-PCR and BOX-PCR have unique and promising discriminatory methods and a rapid and relatively simple comparative methods, making them beneficial for procedure epidemiological studies (Bakshi et al. 2018). In this study, ERIC-PCR, REP-PCR and BOX-PCR were also used as discriminatory methods. As it is expected, the analyzes of the ERIC-PCR, Rep-PCR, and BOX-PCR data showed distinct phylogenetic patterns. All the three methods precisely demonstrated that KS196 S. scabiei and KS464 S. europaeiscabiei, closely related species, positioned and classified mutual group. On the other hand, the other strains were determined in various phylogenetic positions according to the exploited PCR methods. Among these methods, when we compared ERIC-PCR and 16S rRNA PCR results, the phylogenetic patterns have high level of similarity between each other. In both analyses, closely related strains were situated in similar positions. It was observed that Rep-PCR, and BOX-PCR methods were insufficient to locate the strains determined to be related according to ERIC-PCR and 16S rRNA PCR analyses. Considering all the data, it is thought that ERIC-PCR method may be useful in phylogenetic analyzes of Streptomyces species as an auxiliary tool.
Consequently, 15 different scab-causing Streptomyces species from Turkey were identified and analyzed based on 16S rRNA sequences. The results in the current study clearly showed that ERIC-PCR, Rep-PCR, and BOX-PCR fingerprinting molecular typing methods are useful and safe methods for the investigation of Streptomyces strains isolated from symptomatic potato tubers. According to our knowledge, this is the first report on phylogenetic analysis of scab-causing Streptomyces species in Turkey. However, most of the pathogenic strains remain to be identified at the species level.
Conclusion
According to the literature, there are numerous unknown local pathogenic microorganisms. Hence, it is important to know pathogenic isolates in soil systems to struggle and overcome to these problems for sustainable agricultural productivity. Therefore, when more pathogenic strains are identified for species level, it will be helpful for control of the various pathogenic strains in agroecosystems.
Acknowledgements
This study was conducted in Agri İbrahim Cecen University, Central Research and Application Laboratory and was supported by Agri İbrahim Cecen University, Scientific Research Council (BAP) with FEF.014.13 project number.
Author’s contributions
KK, FD, RK conceived and designed the research. KK collected the samples, KK, FD conducted the experiments. KK, RK, and BA evaluated the data. KK and BA wrote the manuscript. All the authors read and approved the manuscript.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Data availability
Sequencing data are openly available in the NCBI database.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical approval
The manuscript does not involve any animal study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bakhshi B, Afshari N, Fallah, Enterobacterial repetitive intergenic consensus (ERIC)-PCR analysis as a reliable evidence for suspected Shigella spp. outbreaks. Braz J Microbiol. 2018;49(3):529–533. doi: 10.1016/j.bjm.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencheikh M, Setti B. Characterizatıon of Streptomyces scabies isolated from common scab lesions on potato tubers by morphological, biochemical and pathogenicity tests in chlef region in western Algeria. Sci Technol. 2007;26:61–67. [Google Scholar]
- Bouchek-Mechiche K, Gardan NP, Jouan B. DNA relatedness among strains of Streptomyces pathogenic to potato in France: description of three new species, S. europaeiscabiei sp. nov, and S. stelliscabiei sp. nov associated with common scab, and S. reticuliscabiei sp nov associated with netted scab. Int J Syst Evol. 2000;50:91–99. doi: 10.1099/00207713-50-1-91. [DOI] [PubMed] [Google Scholar]
- Bukhalid RA, Loria R. Cloning and expression of a gene from Streptomyces scabies encoding a putative pathogenicity factor. J Bacteriol. 1997;179(24):7776–7783. doi: 10.1128/jb.179.24.7776-7783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn KL, Leci E, Kritzman G, Lazarovits G. A quantitative method for determining soil populations of Streptomyces and differentiating potential potato scab-inducing strains. Plant Dis. 1998;82(6):631–638. doi: 10.1094/pdis.1998.82.6.631. [DOI] [PubMed] [Google Scholar]
- Dees MW, Sletten A, Hermansen A. Isolation and characterization of Streptomyces species from potato common scab lesions in Norway. Plant Pathol. 2013;62(1):217–225. doi: 10.1111/j.1365-3059.2012.02619.x. [DOI] [Google Scholar]
- Elias F, Muddada S, Muleta D, Belachew T. Antimicrobial potential of Streptomyces spp. isolated from the rift valley regions of Ethiopia. Adv Pharmacol Sci. 2022;2022:1724906. doi: 10.1155/2022/1724906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Gonzalez R, Velasco I, Montes F. Detection and characterization of Streptomyces causing potato common scab in Western Europe. Plant Pathol. 2008;57(1):162–169. doi: 10.1111/j.1365-3059.2007.01734.x. [DOI] [Google Scholar]
- Goyer C, Faucher E, Beaulieu C. Streptomyces caviscabies sp. nov, from deep-pitted lesions in potatoes in Quebec. Canada. Int J Syst Bacteriol. 1996;46(3):635–639. doi: 10.1099/00207713-46-3-635. [DOI] [Google Scholar]
- Gutierrez J, Bakke A, Vatta M, Merrill AR. Plant natural products as antimicrobials for control of Streptomyces scabies: a causative agent of the common scab disease. Front Microbiol. 2022;12:833233. doi: 10.3389/fmicb.2021.833233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological data sequence alignment editor and analysis program for Windows 95/98 NT. Oxford: Oxford University Press; 1999. pp. 95–98. [Google Scholar]
- Hammer Q, Harper DAT, Ryan DR. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4(1):1–9. [Google Scholar]
- Hasani S, Taghavi SM. Phenotype and genotype diversity of iranian streptomyces isolates that cause potato common scab. J Plant Pathol. 2014;96:467–476. doi: 10.4454/JPP.V96I3.019. [DOI] [Google Scholar]
- Hatano K, Nishii T, Kasai H. Taxonomic re-evaluation of whorl-forming Streptomyces (formerly Streptoverticillium) species by using phenotypes, DNA-DNA hybridization and sequences of gyrB, and proposal of Streptomyces luteireticuli (ex Katoh and Aral 1957) corrig., sp. nov., nom. rev. Int J Syst Evol Microbiol. 2003;53:1519–1529. doi: 10.1099/ijs.0.02238-0. [DOI] [PubMed] [Google Scholar]
- Haynes KG, Goth RW, Young RJ. Genotype x environment interactions for resistance to common scab in tetraploid potato. Crop Sci. 1997;37(4):1163–1167. doi: 10.2135/cropsci1997.0011183X003700040023x. [DOI] [Google Scholar]
- Hill J, Lazarovits G. A mail survey of growers to estimate potato common scab prevalence and economic loss in Canada. Can J Plant Pathol. 2005;27(1):46–52. doi: 10.1080/07060660509507192. [DOI] [Google Scholar]
- Hosny M, Abo-Elyousr KAM, Asran MR, Saead FA. Chemical control of potato common scab disease under field conditions. Arch Phytopathol Plant Prot. 2014;47:2193–2199. doi: 10.1080/03235408.2013.870375. [DOI] [Google Scholar]
- Jiang HH, Meng QX, Hanson LE, Hao JJ. First report of Streptomyces stelliscabiei causing potato common scab in Michigan. Plant Dis. 2012;96(6):904–904. doi: 10.1094/pdis-02-12-0132-pdn. [DOI] [PubMed] [Google Scholar]
- Karagoz K. Identification and characterization of plant pathogenic Streptomyces species from potato fields in Erzurum province. Natural Science Institute: Ataturk University, Erzurum; 2013. p. 116. [Google Scholar]
- Karagoz K, Kotan R. Identification and characterization of some Streptomyces species isolated from symtomatic potatoes in Erzurum Province of Turkey. EAJS. 2017;3(1):27–37. [Google Scholar]
- Khodakaramian G, Zafari D, Solaimani-E-Pari MJ. Diversity of Streptomyces strains causing potato scab disease in Hamedan province and their thaxtomin production potential. Appl Entomol Phytopathol. 2011;79(1):53–69. doi: 10.22092/JAEP.2011.107233. [DOI] [Google Scholar]
- Kim KO, Shin KS, Kim MN, Shin KS, Labeda DP, et al. Reassessment of the status of Streptomyces setonii and reclassification of Streptomyces fimicarius as a later synonym of Streptomyces setonii and Streptomyces albovinaceus as a later synonym of Streptomyces globisporus based on combined 16S rRNA/gyrB gene sequence analysis. Int J Syst Evol Microbiol. 2012;62:2978–2985. doi: 10.1099/ijs.0.040287-0. [DOI] [PubMed] [Google Scholar]
- Kreuze JF, Suomalainen S, Paulin L, Valkonen JPT. Phylogenetic analysis of 16S rRNA genes and PCR analysis of the nec1 gene from Streptomyces spp. causing common scab, pitted scab, and netted scab in Finland. Phytopathology. 1999;89(6):462–469. doi: 10.1094/phyto.1999.89.6.462. [DOI] [PubMed] [Google Scholar]
- Kumar Y, Aiemsum-Ang P, Ward AC, Goodfellow M. Diversity and geographical distribution of members of the Streptomyces violaceusniger 16S rRNA gene clade detected by clade-specific PCR primers. FEMS Microbiol Ecol. 2007;62(1):54–63. doi: 10.1111/j.1574-6941.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- Lambert DH, Loria R. Streptomyces acidiscabies sp. nov. Int J Syst Bacteriol. 1989;39(4):393–396. doi: 10.1099/00207713-39-4-393. [DOI] [Google Scholar]
- Lambert DH, Loria R. Streptomyces scabies sp. Nov., nom. rev. Int J Syst Bacteriol. 1989;39(4):387–392. doi: 10.1099/00207713-39-4-387. [DOI] [Google Scholar]
- Lapaz MI, Verdier E, Pianzzola MJ. First report regarding potato scab caused by Streptomyces acidiscabies in Uruguay. Plant Dis. 2012;96(7):1064–1064. doi: 10.1094/PDIS-02-12-0203-PDN. [DOI] [PubMed] [Google Scholar]
- Lapaz MI, Huguet-Tapia JC, Siri MI, Verdier E, Loria R, Pianzzola MJ. Genotypic and phenotypic characterization of Streptomyces species causing potato common scab in Uruguay. Plant Dis. 2017;101:1362–1372. doi: 10.1094/PDIS-09-16-1348-RE. [DOI] [PubMed] [Google Scholar]
- Leiminger J, Frank M, Wenk C, Poschenrieder G, Kellermann A, Schwarzfischer A. distribution and characterization of Streptomyces species causing potato common scab in Germany. Plant Pathol. 2013;62(3):611–623. doi: 10.1111/j.1365-3059.2012.02659.x. [DOI] [Google Scholar]
- Lerat S, Simao-Beaunoır AM, Beaulieu C. Genetic and physiological determinants of Streptomyces scabies pathogenicity. Mol Plant Pathol. 2009;10:579–585. doi: 10.1111/j.1364-3703.2009.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethonen MJ, Rantala H, Kreuze JF, Bang H, Kuisma, , et al. Occurance and survival ofo potato scab pathogens (Streptomyces species) on tuber lesions: quick diagnosis on a PCR-based assay. Plant Pathol. 2004;53:280–287. doi: 10.1111/j.0032-0862.2004.01009.x. [DOI] [Google Scholar]
- Liang F, Lin R, Yao Y, Xiao Y, Zhang M, Shi C, He X, Zhou B, Wang B. Systematic Identification of pathogenic Streptomyces sp. AMCC400023 that causes common scab and genomic analysis of its pathogenicity island. Phytopathology. 2019;109:1115–1128. doi: 10.1094/PHYTO-07-18-0266-R. [DOI] [PubMed] [Google Scholar]
- Loria R. Compendium of potato diseases. St Paul, Minnesota: The American Phytopathological Society; 2001. [Google Scholar]
- Miyajima K, Tanaka F, Takeuch T, Kuninaga S. Streptomyces turgidiscabies sp. nov. Int J Syst Bacteriol. 1998;48:495–502. doi: 10.1099/00207713-48-2-495. [DOI] [PubMed] [Google Scholar]
- Natsume M, Nagagata A, Aittamaa M, Okaniwa N, Somervuo P, Fiedler HP, Kreuze JF, Rokka VM, Bang H, Kawaide H, Valkonen JPT. Phytotoxin produced by the netted scab pathogen, Streptomyces turgidiscabies strain 65, isolated in Sweden. J Gen Plant Pathol. 2018;84:108–117. doi: 10.1007/s10327-018-0765-8. [DOI] [Google Scholar]
- Ogutcu H, Adiguzel A, Gulluce M, Karadayi M, Sahin F. Molecular characterization of Rhizobium strains isolated from wild chickpeas collected from high altitudes in Erzurum-Turkey. Rom Biotechnol Lett. 2009;14(2):4294–4300. [Google Scholar]
- Park DH, Yu YM, Kim JS, Cho JM, Hur JH, Lim CK. Characterization of streptomycetes causing potato common scab in Korea. Plant Dis. 2003;87(11):1290–1296. doi: 10.1094/PDIS.2003.87.11.1290. [DOI] [PubMed] [Google Scholar]
- Pavlista AD. How important is common scab in seed potatoes? Am Potato J. 1996;73(6):275–278. doi: 10.1007/BF02849277. [DOI] [Google Scholar]
- Planckaert S, Deflandre B, de Vries AM, Ameye M, Martins JC, Audenaert K, Rigali S, Devreese Identification of novel rotihibin analogues in Streptomyces scabies, including discovery of its biosynthetic gene cluster. Microbiol Spectr. 2021;9(1):e0057121. doi: 10.1128/Spectrum.00571-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar A, Latif Z, Zhang S, Zhu J, Zechel DL, Bechthold A. Biological control of potato common scab with rare isatropolone C compound produced by plant growth promoting Streptomyces A1RT. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad NW, Jone JB, Chun W. Laboratory Guide for Identification of plant pathogenic bacteria. St. Paul, MN, USA: American Phytopathological Society; 2001. [Google Scholar]
- Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Bacteriol. 1966;16:313–340. doi: 10.1099/00207713-16-3-313. [DOI] [Google Scholar]
- Shuang M, Wang Y, Teng W, Jin G. Isolation and identification of an endophytic bacteria Bacillus sp. K-9 exhibiting biocontrol activity against potato common scab. Arch Microbiol. 2022 doi: 10.1007/s00203-022-02989-5. [DOI] [PubMed] [Google Scholar]
- Simizu M, Yazawa S, Ushijima Y. A promising strain of endophytic Streptomyces sp. for biological control of cucumber anthracnose. J Gen Plant Pathol. 2009;75:27–36. doi: 10.1007/s10327-008-0138-9. [DOI] [Google Scholar]
- Stackebrandt E, Rainey FA, Ward-Rainey NL. Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int J Syst Bacteriol. 1997;47(2):479–491. doi: 10.1099/00207713-47-2-479. [DOI] [Google Scholar]
- St-Onge R, Goyer C, Coffin R, Filion M. Genetic diversity of Streptomyces spp. causing common scab of potato in eastern Canada. Syst Appl Microbiol. 2008;31(6–8):474–484. doi: 10.1016/j.syapm.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Tacão M, Alves A, Saavedra M, Correia A. BOX-PCR is an adequate tool for typing Aeromonas spp. Antonie Van Leeuwenhoek. 2005;88:173–179. doi: 10.1007/s10482-005-3450-9. [DOI] [PubMed] [Google Scholar]
- Tajima S, Hirashita T, Yoshihara K, Bhromsiri A, Nomura M. Application of repetitive extragenic palindromic (REP)-PCR and enterobacterial repetitive intergenic consensus (ERIC)-PCR analysis to the identification and classification of Japan and Thai local isolates of Bradyrhizobium japonicum, Shinorhizobium meliloti Rhizobium Leguminosarum. Soil Sci Plant Nutr. 2000;46(1):241–247. doi: 10.1080/00380768.2000.10408779. [DOI] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites R, Wale SJ, Nelson D, Munday D, Elphinstone JG. Streptomyces turgidiscabies and S. acidiscabies: two new causal agents of common scab of potato (Solanum tuberosum) in the UK. Plant Pathol. 2010;59(4):804–804. doi: 10.1111/j.1365-3059.2009.02241.x. [DOI] [Google Scholar]
- Versalovıc J, Schneider M, De Bruijn FJ, Lupsi JR. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol. 1994;5:25–40. [Google Scholar]
- Wanner LA. A survey of genetic variation in Streptomyces isolates causing potato common scab in the United States. Phytopathology. 2006;96(12):1363–1371. doi: 10.1094/phyto-96-1363. [DOI] [PubMed] [Google Scholar]
- Wanner LA. High proportions of nonpathogenic Streptomyces are associated with common scab-resistant potato lines and less severe disease. Can J Microbiol. 2007;53(9):1062–1075. doi: 10.1139/W07-061. [DOI] [PubMed] [Google Scholar]
- Wanner LA. A new strain of Streptomyces causing common scab in potato. Plant Dis. 2007;91(4):352–359. doi: 10.1094/PDIS-91-4-0352. [DOI] [PubMed] [Google Scholar]
- Wanner LA. A patchwork of Streptomyces species isolated from potato common scab lesions in North America. Am J Pot Res. 2009;86(4):247–264. doi: 10.1007/s12230-009-9078-y. [DOI] [Google Scholar]
- Zadina J, Dobias K, Horackova V. The resistance of potato varieties of the world assortment to common scab Streptomyces scabies. Ochrana Rostlin. 1975;11(3):195–204. [Google Scholar]
- Zhao P, Liu L, Cao J, Wang Z, Zhao Y, Zhong N. Transcriptome analysis of tryptophan-induced resistance against potato common scab. Int J Mol Sci. 2022;23:8420. doi: 10.3390/ijms23158420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequencing data are openly available in the NCBI database.