Abstract
Objective
The objective of this study was to investigate the genetic diversity, population structure and whole-genome selection signatures of Luxi cattle to reveal its genomic characteristics in terms of meat and carcass traits, skeletal muscle development, body size, and other traits.
Methods
To further analyze the genomic characteristics of Luxi cattle, this study sequenced the whole-genome of 16 individuals from the core conservation farm in Shandong region, and collected 174 published genomes of cattle for conjoint analysis. Furthermore, three different statistics (pi, Fst, and XP-EHH) were used to detect potential positive selection signatures related to selection in Luxi cattle. Moreover, gene ontology and Kyoto encyclopedia of genes and genomes pathway enrichment analyses were performed to reveal the potential biological function of candidate genes harbored in selected regions.
Results
The results showed that Luxi cattle had high genomic diversity and low inbreeding levels. Using three complementary methods (pi, Fst, and XP-EHH) to detect the signatures of selection in the Luxi cattle genome, there were 2,941, 2,221 and 1,304 potentially selected genes identified, respectively. Furthermore, there were 45 genes annotated in common overlapping genomic regions covered 0.723 Mb, including PLAG1 zinc finger (PLAG1), dedicator of cytokinesis 3 (DOCK3), ephrin A2 (EFNA2), DAZ associated protein 1 (DAZAP1), Ral GTPase activating protein catalytic subunit alpha 1 (RALGAPA1), mediator complex subunit 13 (MED13), and decaprenyl diphosphate synthase subunit 2 (PDSS2), most of which were enriched in pathways related to muscle growth and differentiation and immunity.
Conclusion
In this study, we provided a series of genes associated with important economic traits were found in positive selection regions, and a scientific basis for the scientific conservation and genetic improvement of Luxi cattle.
Keywords: Luxi Cattle, Genetic Diversity, Selection Signature, Whole-genome Sequencing
INTRODUCTION
Domestic cattle can not only provide meat, skin, hair, and other living materials, but also the main source of livestock power for farming and transportation, which gives them a pivotal role in world agricultural [1,2]. According to different morphological characteristics and living habits, domestic cattle can be divided into two subspecies, humpless taurine (Bos taurus) and humped indicine (Bos indicus) [3]. Bos taurus are mainly distributed in the whole Eurasian continent, North Africa, and West Africa, while Bos indicus are mainly distributed in the Indian subcontinent and Southeast Asia, but also in the Middle East and Southern Africa, and the Americas [4]. Among them, native breeds have higher genetic diversity compared with commercial breeds, which reflects in their ability to adapt the local conditions and used as an important genetic resource for cattle breeding and improvement [5]. However, with the rapid development of urbanization and other factors, the variety and quantity of indigenous breeds are significantly reduced, and the risk of their disappearance is increasing. Therefore, it is of significance to determine the genetic diversity characteristics and optimize the managements of indigenous breeds.
For the last decades, molecular markers have been widely used in animal breeding programs and several different types of markers are available [6]. However, their application has been limited in the past due to low density, high labor intensity, high technical requirements, and high cost of large-scale analysis. The advent of Whole-genome sequencing (WGS) technology has led to an exponential increase in the number of genetic variants found in a single experiment. Especially, in recent years, with the development of sequencing technology and the reduction of sequencing cost, WGS technology has greatly promoted the mining of high-throughput genetic variants which have been applied to identify the genetic diversity, population structure, candidate genes for major economic traits, and the screening of molecular markers [7]. At present, a wide range of systematic WGS studies especially in the indigenous breeds have been carried out in pigs [8], cattle [9], sheep [10], and other livestock, and many remarkable scientific achievements have been achieved.
Luxi cattle (LUX), as one of the important indigenous breeds mainly distributed in Shandong province of China, are an important genetic resource noted for its large body size, fine-quality meat, and good strength for draft [11]. Over the past 30 years, LUX has been intensively bred for beef, leading to genetic improvement in production traits. In 2022, the results of Ge et al [12] showed the values of average carcass weight and slaughter rate in LUX were much higher than those in the past, and Luxi beef was rich in amino acids and had a high content of essential amino acids. Genetic diversity is an important component of biological diversity and a key factor for species adaptation to the varied environments or artificial selection [13]. Changes in genomic structural features caused by artificial or natural selections are called selection signals. These signals are closely related to the breeding direction of animals and the adaptation mechanism of domestication. Therefore, the detection of selection signals is of great significance for the genetic improvement of livestock and poultry, which helps to mine the genes related to the economic traits of animals, understand the potential genetic bases of the formation of traits, and analyze the phenotypic differences among populations or breeds. Up to date, however, the genomic diversity and the genetic basis of prominent characteristics of LUX are still unknown.
In this study, at the whole-genome level, single nucleotide polymorphisms (SNPs) were identified and annotated in LUX based on sequencing data of a total of 190 individuals and searched for selection signatures and candidate genes in LUX by three methods (pi, Fst, and XP-EHH) were analyzed. These results will help us to understand the process of breed formation and provide a theoretical basis for understanding the genetic mechanism of LUX breed-specific characteristics.
MATERIALS AND METHODS
Animal care
All experiments were conducted in accordance with the Institutional Animal Care and Use Committee guidelines (IACUC No. SY202306004) under currently approved protocols at Jilin University.
Sample collection, DNA extraction and whole-genome sequencing
Sixteen unrelated adult LUX individuals were collected according to the pedigree information from Hongxiang Animal Husbandry Co., Ltd in Juancheng County, Heze City, Shandong Province of China. The genomic DNA was extracted with TIANamp blood DNA kit from TIANGEN Biotech (Beijing, China) Co., Ltd. After extraction, the 1.0% agarose gel was used to detect the quality of genomic DNA fragments, and the Nanodrop ND-2000 was used to detect the purity of DNA samples (OD260/OD280 = 1.8–2.0). A paired-end library was constructed for each individual and the DNA was subjected to a 2×150 bp model using DNBSEQ-T7 platform at the Novogene Bioinformatics, Beijing, China. Furthermore, the genomic data of 174 individuals from 15 breeds available in the SRA database were downloaded, including Luxi (LUX, n = 6), Angus (ANG, n = 20), Shorthorn (SHO, n = 17), Mishima (MIS, n = 8), Kazakh (KAZ, n = 10), Mongolian (MON, n = 13), Hanwoo (HAN, n = 20), Tibetan (TIB, n = 11), Chaidamu (CHA, n = 8), Lingnan (LIN, n = 8), Wenling (WEN, n = 11), Wenshan (WES, n = 7), Xiangxi (XIA, n = 14), Zhoushan (ZHO, n = 6), and Brahman (BRA, n = 15). In total, the data from 190 individuals were used for the subsequent analysis (Supplementary Table S1).
Reads mapping, single nucleotide polymorphism calling and annotation
Clean reads from all 190 cattle were mapped against the Bos taurus reference genome (ARS-UCD1.2) using the BWA-MEM (v0.7.13) with ‘bwa mem’ parameters, which resulted in removing low-quality alignments and discarded low-quality sites [14]. The Picard tool was used to mark potential duplicate reads (REMOVE_DUPLICATES). Variant calling was performed by Genome Analysis Toolkit (GATK v4.1.4) and the module of “HaplotypeCaller”, “GenotypeGVCFs” and “SelectVariants” was performed for downstream SNP calling [15]. All autosomal biallelic SNPs were filtered using the module “Variant Filtration” of GATK to obtain high-quality SNPs based on the following criteria: ‘QD<2.0 || FS>60.0 || MQ<40.0 || SOR >3.0 || MQRankSum <–12.5 || ReadPosRankSum <–8.0’. Finally, a total of 78,727,342 autosomal biallelic SNPs in 190 samples were identified in the genome through GATK’s official website recommendation process.
In addition, the distribution and functional annotation of the autosomal SNPs of LUX were carried out using SnpEff (v5.1) based on the reference assembly (ARS-UCD1.2), and the deleterious mutations were identified in our population based on the sorting intolerant from tolerant (SIFT v5.2.2) scores using the Variant Effect Predictor tool (VEP v.91.3) with default parameter settings. The mutations with SIFT less than 0.05 were adjudged as deleterious to protein function and then mapped to their location in the protein-coding genes.
Genetic diversity analysis and linkage disequilibrium
Genetic diversity was assessed based on the SNPs filtered using PLINK software (v1.9) with parameters ‘--geno 0.05 --maf 0.03 --mind 0.1’. The expected heterozygosity (HE) and observed heterozygosity (HO) were estimated using the ‘--hardy’ option, and minor allele frequency (MAF) was estimated using the ‘--freq’ option implemented in the PLINK (v1.9). Further, the nucleotide diversity (pi) was estimated using VCFtools with the parameters ‘--window-pi 50,000 --window-pi-step 25,000’. By using the PLINK (v1.9), the homozygosity inbreeding coefficient (FHOM) was calculated using the ‘--het’ option, and the runs of homozygosity (ROHs) were measured using the ‘--homozyg’ option [16]. The runs of homozygosity-based inbreeding coefficient (FROH) were calculated for each animal as FROH = ∑LROH/LGENOME, where ∑LROH is the length of all ROH longer and LGENOME is the length of the genome covered by SNPs, which is 2,489,370,000 bp in our data [17]. The degree of linkage disequilibrium decay (LD) for each breed was measured by taking the correlation coefficients (r2) of pairwise SNPs using PopLDdecay with default parameters.
Population structure and phylogenetic analysis
After pruning in PLINK (v1.9) with the parameter ‘--indep-pair-wise 50 25 0.2’, a set of SNPs in high-level pair-wise LD were removed to reduce the SNP dependency [16]. This option removed one of each pair of SNPs with a pairwise r2>0.2 within a window of 50 SNPs and shifted the window by a step size of 25 SNPs, and the remaining 2,618,449 SNPs in approximate LD were extracted from the subset of 30,041,713 SNPs and used for population structure analysis. Principal component analysis (PCA) was performed by the GCTA (v1.92.3) with an option ‘--grm’ to discern genetic relationships among breeds [18]. The graphical representation of PCA was depicted using the plot function in R (v3.6.1). In addition, population structure analysis was carried out with genetic clusters K ranging from 2 to 7 using the ADMIXTURE (v1.3) software with the parameters ‘admixture --cv’ to estimate ancestral populations among our dataset, and the graphical representation of the ADMIXTURE results was generated using R (v3.6.1) [19]. Based on the pairwise genetic distance matrix using PLINK (v.1.9), the model of neighbor-joining (NJ) tree used for phylogenetic reconstruction was constructed using MEGA (v7.0), and was visualized using iTOL [20].
Analysis and identification of selective sweeps
To detect potential genomic regions related to selection in LUX, three different statistics were used in this study. The nucleotide diversity (pi) and the fixation index (Fst) were estimated based on a sliding window with 50 kb and 25 kb step using VCFtools [21], and the parameters are ‘--window-pi 500,000 --window-pi-step 25,000’ and ‘--fst-window-size 50,000 --fst-window-step 25,000’, respectively. Furthermore, haplotype phasing was performed using BEAGLE (v5.2), and the cross-population extended haplotype homozygosity (XP-EHH) using selscan (v1.1) was also performed to detect the positive selection signatures between LUX and Angus [22]. The average of standardized XP-EHH scores for each 50 kb region were chosen for XP-EHH selection scans. Then, the windows of the top 5% score of the three methods are selected for the next analysis. To make the results more reliable, the overlapping of genomic regions identified by at least two methods was determined as potential candidate regions of selection using BEDTools (v2.30.0) with the parameters ‘intersect’ and was annotated based on ARS-UCD1.2 by BioMart tool (http://asia.ensembl.org/index.html) [10]. To reveal the potential biological function of candidate genes harbored in selected regions, gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analyses were performed using the web-based platform KEGG Orthology Based Annotation System (KOBAS v3.0) [23]. The enriched GO terms and pathways were selected stringently with an adjusted probability (p<0.05) and were used for further analysis in this study. Moreover, the quantitative trait locus (QTL) was downloaded from the Cattle QTLdb and used to identify the overlapping regions associated with the most plausible trait-associated selective signatures [24]. The number and function of genes in candidate regions were determined after annotation.
RESULTS
Sequencing and SNPs calling
A total of 4,055,161,272 clean reads were generated after genome sequencing in 16 LUX samples and the detailed information on sequencing data was shown in Supplementary Table S2. The clean reads were aligned to the reference genome (ARS-UCD1.2) with an average depth of 13.51×. Further, we annotated 32,462,568 biallelic SNPs that were discovered in LUX, and the average genome-wide nucleotide diversity within the LUX was 13.04 SNPs/kb. The frequency of SNPs on each chromosome was different, and those on chromosome 27 were the highest (15.03 SNPs/kb). Among these SNPs, functional annotation of the polymorphic sites revealed that the vast majority of SNPs existed in the intergenic region (21.537%) and intronic region (66.158%) (Supplementary Table S3). Furthermore, 2,151 stop gain, 298 stop lost, 381 start lost, 1,401 splice donor and 1,075 splice acceptor variants were detected. According to the SIFT scoring, 30,203 missense SNPs were classified as likely deleterious to protein function (SIFT score <0.05) which will highlight the genetic variants having a potential influence on the function of the protein encoded.
Genetic diversity analysis and linkage disequilibrium
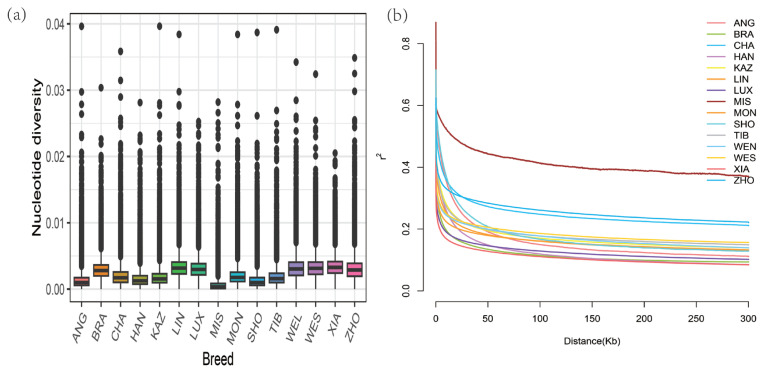
In total, 30,041,713 common autosomal biallelic SNPs were obtained in all breeds after filtering. To elucidate the genomic characteristics, the nucleotide diversity (pi) was calculated, and the result indicated that the pi values ranged from 0.00063 to 0.00333 (Supplementary Table S4). Among them, breeds belonging to indicine (followed by XIA, LIN, WES, and WEN) had the highest nucleotide diversity, and the value pi of LUX cattle was higher than that in most taurine breeds but lower than that indicine breeds (Figure 1a). Among all groups in this study, the HO of LUX (0.2556) was higher than all breeds, and the HE of LUX (0.2482) was between BRA (0.2316) and WEN (0.2494), with relatively higher heterozygosity. Overall, these results indicated high genetic diversity in LUX. It can be observed from the figure that the LD pattern of each variety is different in subpopulations, and the LD level of indicine breeds was lower than that of taurine breeds which is consistent with previous studies (Figure 1b). The LD attenuation distance of BRA population is smaller than most breeds, which may be implied the highest genetic diversity. At distances between markers (>50 kb), XIA and BRA had the lowest LD level, followed by LUX. Additionally, two inbreeding coefficients were calculated based on the genomic data of all tested individuals. As shown in Supplementary Table S4, the results showed that the FHOM (−0.0298) in LUX was very close to zero. In addition, LUX has the lowest FROH (0.1765) compared to all other breeds. This result indicated the existing breeding programs effectively avoid inbreeding.
Figure 1.
Genetic diversity among 190 individuals from 15 populations. (a) Box plots of the nucleotide diversity for each group. The black line in the boxplot is the median line and the outside points are outliers. (b) Decay of linkage disequilibrium on cattle autosomes was estimated from each breed. Different colored lines represented different breeds. ANG, Angus; BRA, Brahman; CHA, Chaidamu; HAN, Hanwoo; KAZ, Kazakh; LIN, Lingnan; LUX, Luxi; MIS, Mishima_Ushi; MON, Mongolian; SHO, Shorthorn; TIB, Tibetan; WEN, Wenling; WES, Wenshan; XIA, Xiangxi; ZHO, Zhoushan.
Population structure and admixture analysis
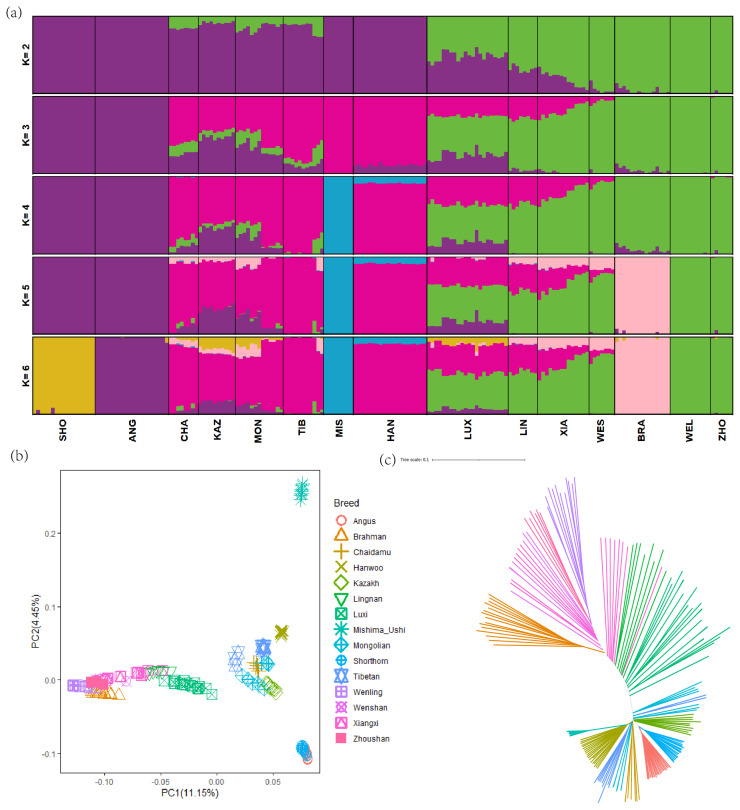
To investigate the genetic relationships among LUX and other cattle breeds, a total of 2,618,449 autosomal SNPs which generated from the pruning of pair-wise LD for further analysis. The analysis was run with K ranging from 2 to 7 to reflect the genetic background of the 15 breeds, with the lowest cross-validation error value (0.3959) being 6 (Figure 2a). There was a clear pattern of geographical distribution among these cattle breeds, which is consistent with the previous studies [25]. When K = 2, LUX showed mixed ancestral component: Bos taurus and Bos indicus ancestry; and when K = 3, LUX showed genetic heterogeneity with shared genome ancestry with East Asian taurine (HAN and MIS), European taurine (SHO and ANG) and Chinese indicine (WEL and ZHO) genetic background (Figure 2a). Furthermore, the genetic relationships among the 15 cattle breeds revealed using PCA are shown in Figure 2. In PCA analysis, PC1 divided these breeds into Bos taurus and Bos indicus and can explain 11.15% of the total variations (Figure 2b). In particular, PC1 obviously separated LUX from other breeds, which had the greatest explanatory power. The NJ tree indicated that LUX was clustered together with the LIN which was consistent with PCA and ADMIXTURE results (Figure 2c).
Figure 2.
Population structure of Luxi cattle and its relationship compared with other breeds. (a) Model-based clustering among different breeds using ADMIXTURE (K = 2 to 6). (b) the results of principal component analysis. (c) Neighbor-joining tree of the relationships among breeds (190 individuals in total).
Genome-wide scanning for selection signatures
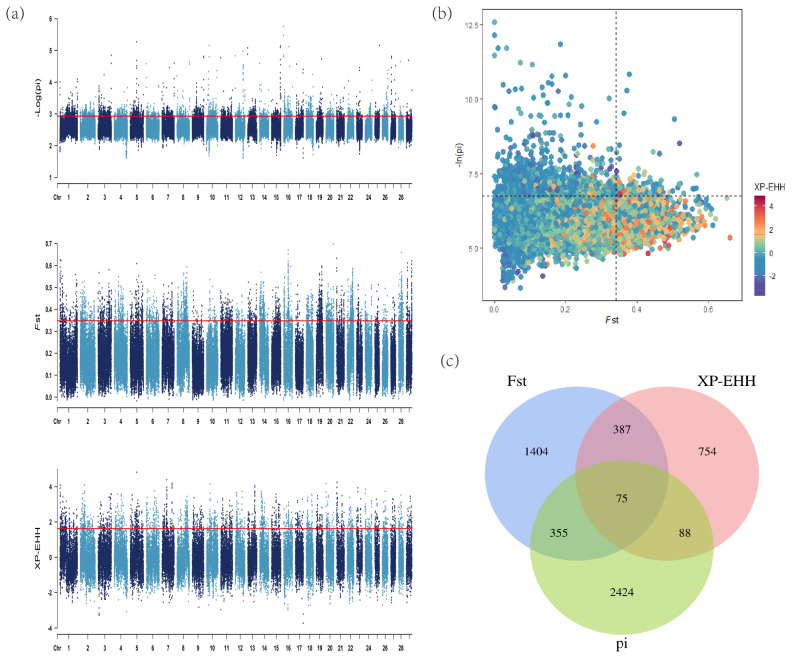
In the selection signal analyses, pi, Fst and XP-EHH methods (top 5%) were performed to identify the candidate regions and genes. There were 6,017 regions containing 2,941 genes, 4,804 regions containing 2,221 genes and 1,920 regions containing 1,304 genes identified, respectively. The detailed information including the particular candidate regions under selection were identified by different methods and shown in Figure 3a and Supplementary Table S5–S7. Conjoint analysis of the signatures of pi, Fst, and XP-EHH are shown in Figure 3b. Of these, 75 overlapped genes were detected by all three methods (Figure 3c). Furthermore, among the candidate genes identified by at least two methods, several genes with overlapped regions were strongly selected in LUX and related to muscle development (dedicator of cytokinesis 3 [DOCK3]) [26], production traits (Ral GTPase activating protein catalytic subunit alpha 1 [RALGAPA1] and DAZ associated protein 1 [DAZAP1]) [27,28], body length (PLAG1 zinc finger [PLAG1]) [29], marbling-related (myosin binding protein C1 [MYBPC1]) [30] (Table 1).
Figure 3.
Analysis of the signatures of positive selection in the genome of Luxi cattle. Red line displays the threshold levels of 5%. (a) Manhattan plot of selective sweeps in Luxi cattle. Alternating colors distinguish markers on neighboring chromosomes. (b) Conjoint analysis of the signatures of pi, Fst, and XP-EHH of Luxi cattle. (c) Venn diagram showing the genes overlap among pi, Fst, and XP-EHH significant selection region.
Table 1.
Potential selected genes associated with important economic traits in Luxi cattle
| Chr | Candidate genes | Methods | Traits |
|---|---|---|---|
| 22 | Dedicator of cytokinesis 3, DOCK3 | pi, Fst, XP-EHH | Muscle development |
| 14 | PLAG1 zinc finger, PLAG1 | pi, Fst, XP-EHH | Body length |
| 21 | Ral GTPase activating protein cat-alytic subunit alpha 1, RALGAPA1 | pi, Fst, XP-EHH | Growth |
| 8 | Insulin like 6, INSL6 | pi, Fst, XP-EHH | Reproduction |
| 12 | Caudal type Homeobox 2, CDX2 | pi, Fst | Embryo development |
| 20 | Growth hormone receptor, GHR | Fst, XP-EHH | Growth and carcass |
| 18 | Cyclin dependent kinase 10, CDK10 | pi, Fst | Immune system |
| 5 | Myosin binding protein C1, MYBPC1 | pi, Fst, XP-EHH | Marbling-related |
| 23 | KH RNA binding domain con-taining, signal transduction asso-ciated 2, KHDRBS2 | pi, XP-EHH | Reproduction |
After integration, 45 common regions within 45 candidate genes were detected in all three methods, indicating that these genes can be considered as potential candidates for positive selection in LUX (Supplementary Table S8). These genes were further enriched in KEGG pathways and GO terms to gain a better understanding of their functions and signaling pathways (Supplementary Table S9). In KEGG analysis, 14 significant enriched pathways were obtained, including the oxidative phosphorylation pathway (bta00190; p = 0.00109), the adipocytokine signaling pathway (bta04920; p = 0.00495), and the ubiquitin mediated proteolysis pathway (bta04120; p = 0.01726), which might be related to immunity and fat metabolism in LUX. Furthermore, a total of 169 significantly enriched GO terms with p-value <0.05 were observed, such as regulation of lipid metabolic process (GO:0019216; p = 0.03343), embryonic skeletal system development (GO:0048706; p = 0.04025), and bone remodeling (GO:0046849; p = 0.02102), which are related to metabolism and protein synthesis and skeletal muscle development.
Moreover, the QTL database was used to identify which genetic markers of candidate regions are most correlated with quantitative traits. The results showed that a significant region might overlap with several QTLs associated with different traits, 271 QTLs were located within or overlapping with these 45 candidate regions (Supplementary Table S10). Simultaneously, most of the candidate regions detected in the present study contain several QTLs for economically important traits, such as carcass traits, milk traits, and body weight traits. Among 45 candidate regions, 91.11% (41 candidate regions) were related to 65 QTLs for production traits, and 95.56% (43 candidate regions) were related to 61 meat and carcass traits suggesting the strong selection for growth and meat performance traits of LUX. Notably, 88.89% (40 candidate regions) were associated with 59 QTLs for milk traits, suggesting the potential for selection of milk traits during the breeding of LUX.
DISCUSSION
Initially, LUX was a dual purpose cattle breed that was used as a draft animal and for beef production and an important part of a farm’s assets [31]. Based on the whole-genome sequencing data, this study conducted an overall evaluation of the genetic diversity and population structure of LUX, which is helpful to accurately understand the genetic diversity and breeding situation of current LUX. Compared with microsatellite markers, genome-wide SNP markers can more objectively reflect genetic differences between individuals and are increasingly used to analyze the genetic diversity of populations [32]. Genetic diversity is a key factor for population survival and evolution, and based on the analysis of the whole genome sequencing results, we can develop a rational breeding program to reduce inbreeding and maintain the genetic diversity of LUX [33]. In this study, the HO and HE of LUX were 0.2556 and 0.2482 respectively, and it was found that HO was greater than HE which indicates that the genetic characteristics of LUX may have high genetic diversity among the 15 cattle populations. In population structure analysis, the ADMIXTURE analysis showed that LUX was located between taurine cattle and indicine cattle, which was also consistent with the situation of hybrid breeds in central China, such as Bohai-black, Qinchuan and Jiaxian cattle while the ratio of ancestral component is different [34-36]. Furthermore, the NJ tree showed LUX were separated from other cattle breeds, and the PCA result provides similar results to the above mentioned. Based on the study of autosomal SNPs, results showed that LUX have the second highest genetic diversity, probably due to being a cross between the Bos taurus and Bos indicus. In addition, different populations of the same species have large differences in LD decay rates due to different genetic backgrounds, and domestication selection will lead to a decrease in population genetic diversity and an increase in loci linkage. Interestingly, LUX had the lowest LD levels than other commercial varieties and higher than that of XIA and HAN cattle, which may be due to the lower selection intensity experienced by the LUX herd and there are still many breeding strategies to choose from in the future.
With the improvement of whole-genome sequencing technology, the biological basic research on the formation of important traits and the demand for improving the efficiency of livestock breeding are gradually increasing [37]. The “1,000 Bull Genome” project has greatly met people’s demand for functional gene mining of important economic traits and provided a theoretical basis and technical support for the development of efficient cattle breeding technology [38]. However, the current sequencing objects are mainly commercial varieties, and limited attention is paid to the genomic diversity and whole-genome scanning footprint of positive selection characteristics of indigenous varieties [39]. This study lays a foundation for the study of genetic diversity and genetic characteristics of other indigenous cattle in China in the future.
To improve detection efficiency and reduce false positives, we used pi, Fst and XP-EHH methods to identify plausible positive selection regions associated with important economic traits in LUX, and if a gene is clearly detected by at least two methods, then it will be used as a candidate gene for further analysis. When comparing the selection signatures of LUX with ANG cattle, 45 genes were identified by all three methods, indicating that these genes are strongly selected in LUX. Among them, a series of genes in LUX with strong signals for regulating skeletal muscle regeneration to varying degrees (DOCK3 and mediator complex subunit 13 [MED13]) have also been reported [26,40]. Furthermore, we also identified several genes related to production and growth traits (RALGAPA1 and DAZAP1) [27,28]. INSL6 is associated with mammalian reproduction, nutrient metabolism and immune response [41]. PLAG1 in candidate regions is associated with the body weight/height in animals including cattle, pigs, horses, and so on [42–44]. In addition, most of the annotated genes among genomic regions are uncharacterized symbols, and their biological importance remains to be elucidated. Enrichment analysis is used for identifying the likelihood of biological processes which understand the function and complex pathways of candidate genes highly related to the biological phenomena under study. In this study, GO terms (e.g., regulation of lipid metabolic process, embryonic skeletal system development, and bone remodeling) were enriched, involving metabolism and protein synthesis and skeletal muscle development related genes, such as ephrin A2 (EFNA2) and golgi membrane protein 1 (GOLM1) [45]. Most of KEGG analysis are concentrated on immunity and fat metabolism pathways, further study of LUX is needed to understand the role of genetic variants resistant to disease and strong reproductive performance traits.
Due to the change of breeding direction from draft-beef dual-purpose to beef cattle in LUX, some measures focusing on improving its defects such as late body maturity and low daily gain have been taken by crossing with some commercial breed such as Simmental. For purebred LUX, we should further strengthen the management of breeding areas, take effective measures to prevent the number of purebred cattle from decreasing, and avoid blind hybridization.
CONCLUSION
In conclusion, this study not only provides novel insights into LUX genomic diversity, phylogenetic relationship, and selection sweep, but also provides theoretical bases regarding the genetic mechanism underlying LUX characteristics and molecular breeding strategies of the composite cattle clades in the future. In addition, a series of candidate genes were identified that may be important for body size, skeletal muscle development traits, and production of meat and milk traits of this breed. These results will give an extensive reference for other important indigenous beef cattle and contribute to understanding the genetic mechanism behind artificial selection in the future.
ACKNOWLEDGMENTS
The authors thank Jishan Liu for providing constructive suggestions for this paper.
Footnotes
AUTHOR CONTRIBUTIONS
Investigation and writing—original draft, M.H.; conceptualization and methodologyL.S.; investigation, visualization, and validation, W.Y.; resources and funding acquisition, F.L; supervision, project administration, and funding acquisition, S.Y.; All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
This study was supported by the National Key Research and Development Program of China (no. 2023YFE0106100).
SUPPLEMENTARY MATERIAL
Supplementary file is available from: https://doi.org/10.5713/ab.23.0304
Supplementary Table S1. Summary of 174 cattle sample information
Supplementary Table S2. Summary of sequencing data
Supplementary Table S3. Distribution of SNPs identified in LUX annotated by SnpEff
Supplementary Table S4. Genetic diversity of the 15 cattle breeds used in this study
Supplementary Table S5. A summary of genes from pi in LUX
Supplementary Table S6. A summary of genes from Fst in LUX
Supplementary Table S7. A summary of genes from XP-EHH in LUX
Supplementary Table S8. A summary of genes overlapped by pi, Fst and XP-EHH methods in common regions
Supplementary Table S9. GO and KEGG enrichment analysis of LUX candidate genes by three methods
Supplementary Table S10. QTLs overlapped with candidate selected regions
REFERENCES
- 1.Qanbari S, Pausch H, Jansen S, et al. Classic selective sweeps revealed by massive sequencing in cattle. PLoS Genet. 2014;10:e1004148. doi: 10.1371/journal.pgen.1004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal N, Liu X, Yang T, et al. Genomic variants identified from whole-genome resequencing of indicine cattle breeds from Pakistan. PLoS One. 2019;14:e0215065. doi: 10.1371/journal.pone.0215065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Gao X, Zhang Y, et al. Genome-wide assessment of genetic diversity and population structure insights into admixture and introgression in Chinese indigenous cattle. BMC Genet. 2018;19:114. doi: 10.1186/s12863-018-0705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Bovine Hapmap Consortium. Gibbs RA, Taylor JF, et al. Genome-wide survey of SNP variation uncovers the genetic structure of cattle breeds. Science. 2009;324:528–32. doi: 10.1126/science.1167936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho IC, Park HB, Ahn JS, et al. A functional regulatory variant of MYH3 influences muscle fiber-type composition and intramuscular fat content in pigs. PLoS Genet. 2019;15:e1008279. doi: 10.1371/journal.pgen.1008279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matukumalli LK, Lawley CT, Schnabel RD, et al. Development and characterization of a high density SNP genotyping assay for cattle. PLoS One. 2009;4:e5350. doi: 10.1371/journal.pone.0005350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Wang F, Gao G, et al. Genome-wide association study of body weight traits in inner mongolia cashmere goats. Front Vet Sci. 2021;8:752746. doi: 10.3389/fvets.2021.752746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Wei S, Li H, et al. Genome-wide genetic structure and differentially selected regions among Landrace, Erhualian, and Meishan pigs using specific-locus amplified fragment sequencing. Sci Rep. 2017;7:10063. doi: 10.1038/s41598-017-09969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Sun H, Lai W, et al. Genome-wide re-sequencing reveals population structure and genetic diversity of Bohai Black cattle. Anim Genet. 2022;53:133–6. doi: 10.1111/age.13155. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Bai C, Shi L, et al. Detection of selection signatures in South African Mutton Merino sheep using whole-genome sequencing data. Anim Genet. 2022;53:224–9. doi: 10.1111/age.13173. [DOI] [PubMed] [Google Scholar]
- 11.Mao Y, Chang H, Yang Z, et al. Genetic structure and differentiation of three Chinese indigenous cattle populations. Biochem Genet. 2007;45:195–209. doi: 10.1007/s10528-006-9061-y. [DOI] [PubMed] [Google Scholar]
- 12.Ge FLH, Li J, et al. Analysis of growth and slaughter performances and meat quality of Luxi cattle. Shandong Agric Sci. 2022;54:112–20. [Google Scholar]
- 13.Groeneveld LF, Lenstra JA, Eding H, et al. Genetic diversity in farm animals--a review. Anim Genet. 2010;41(Suppl 1):6–31. doi: 10.1111/j.1365-2052.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Addo S, Klingel S, Hinrichs D, Thaller G. Runs of Homozygosity and NetView analyses provide new insight into the genome-wide diversity and admixture of three German cattle breeds. PLoS One. 2019;14:e0225847. doi: 10.1371/journal.pone.0225847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–64. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan CL, Lin J, Huang YY, et al. Population genomics reveals that natural variation in PRDM16 contributes to cold tolerance in domestic cattle. Zool Res. 2022;43:275–84. doi: 10.24272/j.issn.2095-8137.2021.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browning SR, Browning BL. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet. 2007;81:1084–97. doi: 10.1086/521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bu D, Luo H, Huo P, et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49:W317–25. doi: 10.1093/nar/gkab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu ZL, Park CA, Reecy JM. Building a livestock genetic and genomic information knowledgebase through integrative developments of Animal QTLdb and CorrDB. Nucleic Acids Res. 2019;47:D701–10. doi: 10.1093/nar/gky1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N, Cai Y, Chen Q, et al. Whole-genome resequencing reveals world-wide ancestry and adaptive introgression events of domesticated cattle in East Asia. Nat Commun. 2018;9:2337. doi: 10.1038/s41467-018-04737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samani A, Karuppasamy M, English KG, et al. DOCK3 regulates normal skeletal muscle regeneration and glucose metabolism. bioRxiv. 2023 doi: 10.1101/2023.02.22.529576. 02.22.529576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun T, Huang GY, Wang ZH, et al. Selection signatures of Fuzhong Buffalo based on whole-genome sequences. BMC Genomics. 2020;21:674. doi: 10.1186/s12864-020-07095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith RWP, Anderson RC, Smith JWS, Brook M, Richardson WA, Gray NK. DAZAP1, an RNA-binding protein required for development and spermatogenesis, can regulate mRNA translation. RNA. 2011;17:1282–95. doi: 10.1261/rna.2717711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y, Xu L, Chen Y, et al. Genome-wide association study reveals the PLAG1 gene for knuckle, biceps and shank weight in simmental beef cattle. PLoS One. 2016;11:e0168316. doi: 10.1371/journal.pone.0168316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Wang M, Li Q, et al. Transcriptome profiling of longissimus lumborum in Holstein bulls and steers with different beef qualities. PLoS One. 2020;15:e0235218. doi: 10.1371/journal.pone.0235218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, Mu Y, Zhang D, et al. Determination of microbiological characteristics in the digestive tract of different ruminant species. Microbiologyopen. 2019;8:e00769. doi: 10.1002/mbo3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito R, Kondo NI, Nemoto Y, et al. Genetic population structure of wild boars (Sus scrofa) in fukushima prefecture. Animals (Basel) 2022;12:491. doi: 10.3390/ani12040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Y, Tan T, Li Z, et al. Identification of selection signatures and loci associated with important economic traits in Yunan Black and Huainan pigs. Genes (Basel) 2023;14:655. doi: 10.3390/genes14030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma X, Cheng H, Liu Y, et al. Assessing Genomic diversity and selective pressures in bohai black cattle using whole-genome sequencing data. Animals (Basel) 2022;12:665. doi: 10.3390/ani12050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu X, Yang Y, Zhang Y, et al. The relationship between myofiber characteristics and meat quality of Chinese Qinchuan and Luxi cattle. Anim Biosci. 2021;34:743–50. doi: 10.5713/ajas.20.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia X, Zhang S, Zhang H, et al. Assessing genomic diversity and signatures of selection in Jiaxian Red cattle using whole-genome sequencing data. BMC Genomics. 2021;22:43. doi: 10.1186/s12864-020-07340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng T, Li P, Li L, Zhang Q. Research advances in and prospects of ornamental plant genomics. Hortic Res. 2021;8:65. doi: 10.1038/s41438-021-00499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwane AA, Schnabel RD, Hoff J, et al. Genome-Wide SNP Discovery in Indigenous Cattle Breeds of South Africa. Front Genet. 2019;10:273. doi: 10.3389/fgene.2019.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiao R, Zhang M, Zhang B, et al. Population genetic structure analysis and identification of backfat thickness loci of Chinese synthetic Yunan pigs. Front Genet. 2022;13:1039838. doi: 10.3389/fgene.2022.1039838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amoasii L, Holland W, Sanchez-Ortiz E, et al. A MED13-dependent skeletal muscle gene program controls systemic glucose homeostasis and hepatic metabolism. Genes Dev. 2016;30:434–46. doi: 10.1101/gad.273128.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rehman MS, Hassan FU, Rehman ZU, et al. Comparative genomic characterization of relaxin peptide family in cattle and buffalo. Biomed Res Int. 2022;2022:1581714. doi: 10.1155/2022/1581714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saatchi M, Schnabel RD, Taylor JF, Garrick DJ. Large-effect pleiotropic or closely linked QTL segregate within and across ten US cattle breeds. BMC Genomics. 2014;15:442. doi: 10.1186/1471-2164-15-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bongiorni S, Mancini G, Chillemi G, Pariset L, Valentini A. Identification of a short region on chromosome 6 affecting direct calving ease in Piedmontese cattle breed. PLoS One. 2012;7:e50137. doi: 10.1371/journal.pone.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyko AR, Brooks SA, Behan-Braman A, et al. Genomic analysis establishes correlation between growth and laryngeal neuropathy in Thoroughbreds. BMC Genomics. 2014;15:259. doi: 10.1186/1471-2164-15-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagaraj M, Horing M, Ahonen MA, et al. GOLM1 depletion modifies cellular sphingolipid metabolism and adversely affects cell growth. J Lipid Res. 2022;63:100259. doi: 10.1016/j.jlr.2022.100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Summary of 174 cattle sample information
Supplementary Table S2. Summary of sequencing data
Supplementary Table S3. Distribution of SNPs identified in LUX annotated by SnpEff
Supplementary Table S4. Genetic diversity of the 15 cattle breeds used in this study
Supplementary Table S5. A summary of genes from pi in LUX
Supplementary Table S6. A summary of genes from Fst in LUX
Supplementary Table S7. A summary of genes from XP-EHH in LUX
Supplementary Table S8. A summary of genes overlapped by pi, Fst and XP-EHH methods in common regions
Supplementary Table S9. GO and KEGG enrichment analysis of LUX candidate genes by three methods
Supplementary Table S10. QTLs overlapped with candidate selected regions