Abstract
Objective
To confirm that the simplified insole does not affect the gait speed and to identify objective sensor-based gait parameters that correlate strongly with existing clinical gait assessment scales.
Methods
Ten participants with gait impairment due to hemiplegic stroke were enrolled in this study. Pairs of insoles with four pressure sensors on each side were manufactured and placed in each shoe. Data were extracted during the 10-Meter Walk Test. Several sensor-derived parameters (for example stance time, heel_on-to-toe_peak time, and toe_peak pressure) were calculated and correlated with gait speed and lower extremity Fugl-Meyer (F-M) score.
Results
The insole pressure sensor did not affect gait, as indicated by a strong correlation (ρ=0.988) and high agreement (ICC=0.924) between the gait speeds with and without the insole. The parameters that correlated most strongly with highest β coefficients against the clinical measures were stance time of the non-hemiplegic leg (β=-0.87 with F-M and β=-0.95 with gait speed) and heel_on-to-toe_peak time of the non-hemiplegic leg (β=-0.86 with F-M and -0.94 with gait speed).
Conclusion
Stance time of the non-hemiparetic leg correlates most strongly with clinical measures and can be assessed using a non-obtrusive insole pressure sensor that does not affect gait function. These results suggest that an insole pressure sensor, which is applicable in a home environment, may be useful as a clinical endpoint in post-stroke gait therapy trials.
Keywords: Stroke, Gait analysis, Insole pressure sensor, Outcome measure
GRAPHICAL ABSTRACT
INTRODUCTION
Gait impairment is a common consequence of stroke. In the United States alone, an estimated 795,000 people suffer from stroke every year, and more than 80% of survivors experience gait impairment after stroke [1,2]. In order to test new rehabilitation therapies that may improve gait, it is important to have an objective measure of a patient’s gait. The gold standard for gait assessment is camera-based motion analysis in gait laboratories [3]. However, the relative complexity of the setup and the sparsity of gait laboratories in outpatient clinics makes gait analysis in a dedicated gait laboratory impractical for large-scale clinical trials. Another limitation of testing gait in a laboratory is the artificial environment used. As an alternative to formal gait testing in gait laboratories, trials have opted to use clinic-based assessments, such as the 10-Meter Walk Test (10MWT), as their study endpoint [4]. While these tests are more practical than those obtained in gait laboratories, they are more limited in scope because they produce only a single dimension temporal score, they still require a trained observer, and they are obtained in a clinical setting that may not reflect the patient’s gait during everyday life [5].
Assessing gait using sensors that can be worn during everyday life could prove more clinically relevant by providing multidimensional objective and quantitative outcome data without the complexities and time involved with laboratory or clinic-based testing. Recent studies have investigated the use of accelerometers, gyro sensors, and pressure sensors to assess dynamic balance during standing and walking and to assess the walking strategies of persons with stroke [6-11]. The parameters analyzed in such studies provide quantitative data such as asymmetry, kinematic characteristics, and gait performance. However, the clinical relevance of these parameters has not been verified.
To address these limitations, we designed, fabricated, and tested an insole pressure sensor to assess gait. The goal of this study was to confirm that the simplified insole does not affect the gait speed and to identify objective insole sensor-based gait parameters that correlate strongly with existing clinical gait assessment scales.
METHODS
Insole pressure sensor system
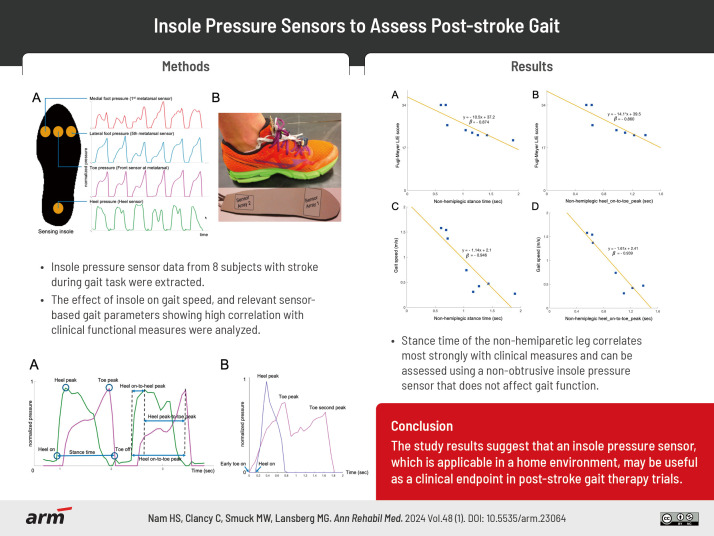
Pairs of insoles with four pressure sensors were manufactured. The sensors were positioned at the plantar aspect of the 1st metatarsal head (medial pressure), 3rd metatarsal head (toe pressure), 5th metatarsal head (lateral pressure), and the calcaneal tuberosity (heel pressure, Fig. 1A). The pressure sensor data were acquired every 60 ms (approximately 16.7 Hz) with a timestamp and normalized to a 0 to 1 scale. The beginning and end of the data acquisition were controlled using a button switch. Sensor data from the four sensors on each insole were time-synchronized, but the data were not time-synchronized between insoles. Thin insoles and simple pressure sensor arrays were selected to simplify the device and minimize its effect on gait. Multiple insoles were created to ensure proper fit and placement of the sensors across a range of foot sizes for both male and female. Fig. 1B shows a person wearing a shoe with the insole pressure sensor system.
Fig. 1.
(A) Schematic image of the 4 insole sensors and their example data. (B) A photo of a person wearing the shoe with the insole pressure sensor system is shown.
Protocol
This study was approved by the Institutional Review Board of Stanford University (IRB No. 32540), and informed consent was obtained from all participants. Stroke patients were screened for this study in the Ambulatory Stroke Clinic of the Neurology Department of Stanford University. Participants were eligible for the study if they were determined to have gait impairment secondary to stroke by the attending neurology clinician, but were able to ambulate without the assistance of a person. The use of a cane, walker, and/or ankle-foot orthosis was permitted. At baseline, demographic data including age, sex, height and weight, hemiplegic side, and the National Institutes of Health Stroke Scale (NIHSS) and lower extremity Fugl-Meyer (F-M) scores were obtained. The F-M assessment was performed by a physical therapist for the lower extremities using the standard protocol at the time of enrollment to the study [12]. The participants performed the 10MWT for three trials with and three trials without instrumented insoles [13]. Participants were instructed to walk at their normal comfortable speed and were informed that they could stop or rest at any time. To mitigate the effects of fatigue on determining whether the device itself affected gait, the order of performing the 10MWT with or without the device was first randomized. Gait speed was measured manually in all trials, following the standard scoring procedures of the 10MWT.
Sensor-derived parameters
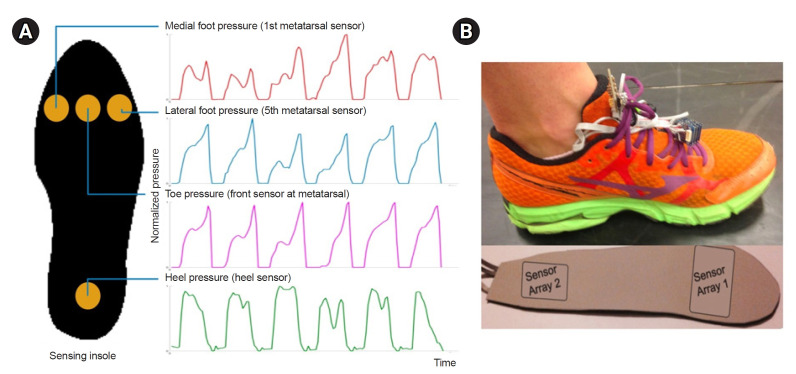
Based on the pressure data from the four sensors, more than 10 consecutive effective steps were extracted (average of approximately 12 steps), and the timestamps of heel_on, heel_peak, toe_peak, and toe_off were determined. From these timestamps, six gait parameters were calculated for both the non-hemiplegic and hemiplegic lower extremities: (1) stance time, (2) heel_on-to-heel_peak time, (3) heel_on-to-toe_peak time, (4) heel_peak-to-toe_peak time, (5) toe_peak pressure before normalization, and (6) stance ratio, calculated as non-hemiplegic stance time divided by hemiplegic stance time (Fig. 2A).
Fig. 2.
(A) Definitions of main parameters in this study are visually shown based on toe and heel pressure sensor data. (B) An example of toe and heel pressure sensor data pattern in a participant with severe ankle spasticity.
Statistical analysis
A Spearman’s rank correlation analysis, linear regression, and inter-rate correlation coefficient (ICC) calculation were performed between gait speed without the device and gait speed with the device to assess the impact of the device on gait. Spearman’s rank correlation coefficients were calculated between the sensor-derived parameters and two clinical measures (lower extremity F-M score and gait speed). Based on the correlation analysis, univariate linear regression analyses were performed for each variable. IBM SPSS 25.0 (IBM Corp.) was used for all statistical analyses. p-value was set at p<0.05.
RESULTS
Participants
Ten participants with stroke were enrolled in this study. In two participants, the right sensor malfunctioned and did not collect sufficient data for analysis, leaving eight participants for the analyses. These subjects consisted of 4 participants with left hemiplegia and 4 with right hemiplegia. The mean age was 61±15 years, and there were 6 male and 2 female. Assistive devices such as a cane, walker, and ankle-foot orthosis were used by four participants during gait testing. The demographic data of the participants are presented in Table 1. Disability levels among the participants ranged from moderate to no significant disability, with NIHSS scores ranging from 1 to 7 and lower extremity F-M scores ranging from 20 to 34.
Table 1.
Demographic data of the study subjects
| Variable | Value (n=8) |
|---|---|
| Age (yr) | 61±15 |
| Sex (male/female) | 6/2 |
| Height (m) | 1.72±0.13 |
| Weight (kg) | 74.4±20.4 |
| Hemiplegic side (left/right) | 4/4 |
| Duration since stroke onset (yr) | 2.95±2.39 |
| NIHSS score | 4.5±2.3 |
| Lower extremity F-M score | 25.6±5.45 |
| No. of subjects with assistive device | |
| Cane | 2 |
| Ankle-foot-orthosis | 1 |
| Walker | 1 |
| None | 4 |
Values are presented as mean±standard deviation or number only.
NIHSS, National Institutes of Health Stroke Scale; F-M, Fugl-Meyer.
Feasibility data (gait speed with vs. without insole pressure sensor)
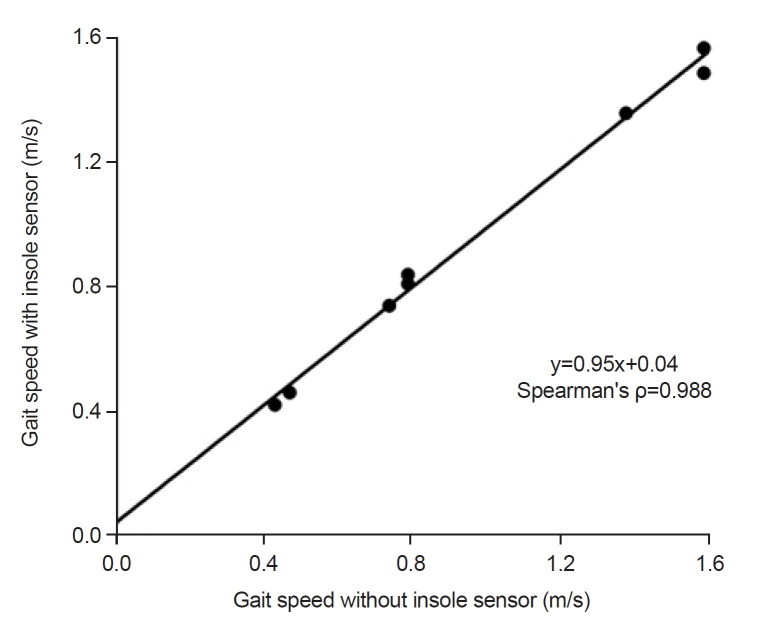
Gait speed while wearing shoes with the insole pressure sensors inside the shoes and gait speed without the insole pressure sensors showed a strong linear relationship (ρ=0.988, p<0.001) with a slope near 1 (β=0.95) and near-zero y-intercept (0.04), with an ICC of 0.924 (p=0.002), showing high agreement of the independently measured speeds. This suggests minimal influence of the insole pressure sensor on gait speed and that it is appropriate to use for gait measurements (Fig. 3).
Fig. 3.
Gait speed without insole sensor vs. gait speed with insole sensor are plotted for all 8 study subjects, which show strong linear relationship (ρ=0.988, p<0.001) with an inter-rater correlation coefficient of 0.924 (p=0.002).
Correlation analysis and univariate linear regression of sensor data and clinical measures
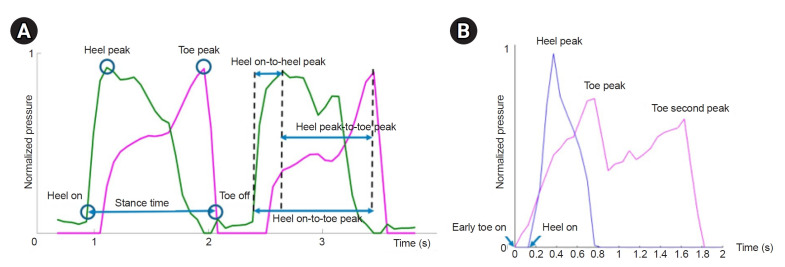
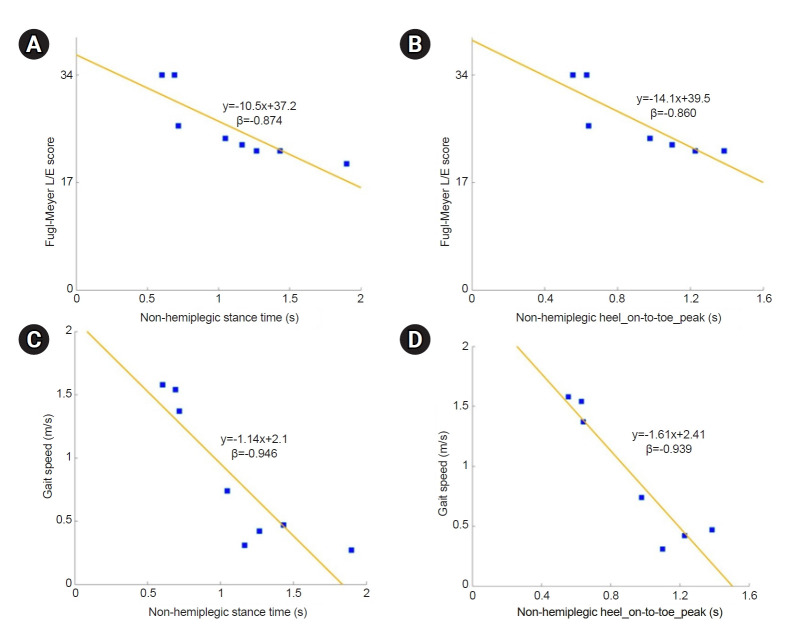
The Spearman’s correlation coefficients and β coefficients of univariate linear regression model between sensor-based parameters and clinical measures (lower extremity F-M score and gait speed) are listed in Table 2. The sensor-based data that correlated most strongly with highest β coefficient with the lower extremity F-M score were stance time of the non-hemiplegic leg (ρ=-0.99, β=-0.87), followed by heel_on-to-toe_peak time of the non-hemiplegic leg (ρ=-0.75, β=-0.86) and heel_on-to-heel_peak time of the non-hemiplegic leg (ρ=-0.78, β=-0.78). The same three parameters also showed highest β coefficient with gait speed (β=-0.95 for non-hemiplegic stance time; β=-0.94 for non-hemiplegic heel_on-to-toe_peak time; β=-0.84 for non-hemiplegic heel_on-to-heel_peak time). Among the toe_peak pressure parameters, hemiplegic toe_peak pressure showed significant correlation with gait speed (ρ=-0.79, β=0.71). Stance ratio did not show significant correlation with the clinical measures. Univariate linear regression results with highest β coefficients are shown in Fig. 4. Sensor-based parameters with high β coefficients were chosen because higher absolute value of β suggests higher prediction.
Table 2.
Mean values of the sensor-based parameters and their relationship between lower extremity F-M score and gait speed
| Gait parameter | Mean±SD (s) | Lower extremity F-M score | Gait speed (m/s) | ||
|---|---|---|---|---|---|
| ρ (p)a) | β (p)b) | ρ (p)a) | β (p)b) | ||
| Hemi_stance time | 0.97±0.36 | -0.99 (<0.01*) | -0.74 (0.03*) | -0.91 (<0.01*) | -0.76 (0.03*) |
| Hemi_heel_on-to-heel_peak time | 0.24±0.20 | -0.57 (0.14) | -0.66 (0.08) | -0.69 (0.06) | -0.71 (0.05) |
| Hemi_heel_on-to-toe_peak time | 0.73±0.28 | -0.84 (0.01*) | -0.72 (0.04*) | -0.76 (0.03*) | -0.70 (0.06) |
| Hemi_heel_peak-to-toe_peak time | 0.49±0.36 | -0.59 (0.12) | -0.23 (0.59) | -0.38 (0.35) | -0.18 (0.68) |
| Hemi_toe_peak pressure | 309.8±138.1d) | 0.66 (0.07) | 0.50 (0.21) | 0.79 (0.02*) | 0.71 (0.05*) |
| Non-hemi_stance time | 1.09±0.40 | -0.99 (<0.01*) | -0.87 (<0.01*) | -0.91 (<0.01*) | -0.95 (<0.01*) |
| Non-hemi_heel_on-to-heel_peak time | 0.29±0.27 | -0.78 (0.02*) | -0.78 (0.02*) | -0.79 (0.02*) | -0.84 (<0.01*) |
| Non-hemi_heel_on-to-toe_peak time | 0.95±0.31 | -0.75 (0.03*) | -0.86 (0.01*) | -0.67 (0.07) | -0.94 (<0.01*) |
| Non-hemi_heel_peak-to-toe_peak time | 0.67±0.22 | -0.24 (0.57) | -0.72 (0.07) | -0.17 (0.69) | -0.80 (0.03*) |
| Non-hemi_toe_peak pressure | 424.6±120.4d) | -0.24 (0.61) | -0.15 (0.75) | 0.11 (0.82) | 0.02 (0.96) |
| Stance ratioc) | 1.12±0.12 | -0.37 (0.36) | -0.50 (0.20) | -0.38 (0.35) | -0.59 (0.13) |
SD, standard deviation; F-M, Fugl-Meyer.
a)Spearman’s correlation analysis (ρ indicates coefficient of Spearman’s correlation).
b)Univariate linear regression analysis (β indicates beta coefficient of linear regression model).
c)Stance ratio=non-hemi_stance time/hemi_stance time (no dimension).
d)Presented in sensor value recorded from the insole pressure sensor.
*p<0.05 were considered statistically significant.
Fig. 4.
Univariate linear regression analyses with non-hemiplegic stance time and non-hemiplegic heel_on-to-toe_peak time as independent variables, and lower extremity Fugl-Meyer score (A, B) and gait speed (C, D) as dependent variables are shown. All results indicate significant negative linear relationships. L/E, lower extremity.
DISCUSSION
This study demonstrated that insoles with pressure sensors can be used to evaluate gait function in patients with stroke. The insole pressure sensor did not alter the subjects’ natural walking speeds (Fig. 3). Several of the sensor-based measures correlated strongly with gait speed and lower extremity sensorimotor impairment; stance time of the non-hemiplegic foot was the sensor-based measure that correlated most strongly with these clinical outcome measures. In addition to the stance time of the non-hemiplegic limb, the heel_on-to-toe_peak time of the non-hemiplegic foot also demonstrated good correlations with lower extremity F-M and gait speed. This is intuitive because the heel_on-to-toe_peak time is closely related to the stance time; heel_on-to-toe_peak time equals the stance time minus the short period from toe_peak-to-toe_off (Fig. 2A).
Several systems have been developed for the gait evaluation of various neurological and musculoskeletal diseases [14]. Camera-based gait analysis systems provide detailed, multifaceted, and accurate information but require abundant space, sufficient manpower, time, and costs. These limitations are particularly burdensome when repeated measurements are required, such as is often the case in a longitudinal follow-up study of gait function after stroke. Inertial measurement unit (IMU)-based sensor systems that can measure step time or stride time exist; however, these systems may not be accurate in patients with stroke who often have abnormal gait patterns. We aimed to address these limitations by developing a pressure-based sensor system that can automatically detect and analyze discrete and easily identifiable events such as heel strike and toe push-off [15,16]. The pressure sensor insole system developed and tested in this study consists of a simple pair of insoles that are highly portable and can be used in laboratory, clinic, or home settings.
In this study, we used two clinical measures as a reference for each subject’s functional status: lower extremity F-M score and gait speed. The F-M score is the most widely used and verified functional evaluation tool in stroke rehabilitation [12]. Gait speed is known to best describe general physical performance, including walking performance, and is a patient-centered outcome measure, as it affects patients’ quality of life [14]. Among the different sensor-based measures, this study found that the stance time of the non-hemiplegic limb had the highest correlation and predictivity with both clinical measures: lower-extremity F-M score and gait speed (Table 2). The non-hemiplegic stance time demonstrated highest β coefficient (-0.87) in linear regression model with the clinical measures (Fig. 4), in which the absolute value is near 1. This supports the potential use of the parameter in a clinical setting because a certain extent of change in the non-hemiplegic stance time may predict meaningful change in the F-M score or gait speed as well. This finding is consistent with previous studies that reported that a longer stance time of the unaffected limb correlates with worse functional outcomes assessed with the Berg Balance Scale and gait speed [17,18]. Our findings are also consistent with the common clinical knowledge that hemiplegic gait exhibits a relatively short stance time of the hemiplegic limb and a long stance time of the non-hemiplegic limb as a result of a prolonged swing phase of the hemiplegic side [15].
Parameters indicative of gait asymmetry have been investigated in previous studies, and it has been suggested that the stance ratio between the non-hemiplegic and hemiplegic sides may be a useful clinical outcome parameter [11,19-22]. In our study, this parameter did not show a significant correlation with clinical measures (Table 2). Also, the ratio of peak pressure of each sensor did not show significant correlation with clinical measures (data not shown). While same-side sensors were time synchronized for this study, they were not synchronized between sides, which may have influenced the symmetry measures. Pressure asymmetry has also been suggested to be a clinically meaningful parameter [21]. We confirmed this in our study as we showed that decreased toe_peak pressure of the hemiplegic side was correlated with slower gait speed (ρ=0.79, Table 2). This is explained by weight shifting to the non-hemiplegic side and impaired push-off function in the forefoot area of the impaired limb during walking in patients with a hemiplegic gait [15,23].
In most of the cases when the ankle spasticity is not too severe, the pressure data followed similar patterns as shown in Fig. 2A. However, in the cases with severe ankle spasticity and equinovarus deformity, it showed relatively early toe onset, short duration of heel pressure, and significant double peak of toe pressure: first on initial contact due to insufficient heel strike, and second on push off phase (Fig. 2B). In some steps the first peak was higher than the second peak. This pattern supports the study results that the heel_on-to-heel_peak time average is similar in both sides, but the heel_on-to-toe_peak time is apparently shorter in the hemiplegic side.
This study has some limitations. First, the number of participants was too small to generalize the results to the entire stroke population, and larger follow-up studies are necessary to validate our results. However, some of the sensor-based gait parameters correlated strongly with the clinical outcome measures, and the results were significant despite the small number of participants. Second, the timestamps of the left and right insole pressure sensors were not synchronized. Further investigations focusing on the pressure relationship between the two limbs may provide additional clinically relevant information. Third, the sensor did not provide accurate pressure data especially in abnormal gait patterns. There are similar issues when using IMU sensors for stroke gait analysis, especially when the system is intended to be unobtrusive and simple; usually accuracy and practical clinical feasibility is in a trade-off relationship [24]. Therefore, we aimed to extract a simple sensor-based parameter highly correlating with clinical measures.
In conclusion, the stance time and heel_on-to-toe_peak time of the non-hemiplegic foot were the most indicative of gait function in stroke subjects. Insole pressure sensors may be useful by providing clinicians and researchers with simple objective outcome measures, which correlates with clinical measures, that could be assessed in an unobtrusive way during short gait testing in clinics or in the patient’s home environment during routine activities.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING INFORMATION
None.
AUTHOR CONTRIBUTION
Conceptualization: Lansberg MG. Methodology: Clancy C, Lansberg MG. Formal analysis: Nam HS, Smuck M, Lansberg MG. Visualization: Nam HS, Lansberg MG. Writing – original draft: Nam HS, Smuck M, Lansberg MG. Writing – review and editing: Nam HS, Clancy C, Smuck M, Lansberg MG. Approval of final manuscript: all authors.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. Erratum in: Circulation 2017;135:e646. Erratum in: Circulation 2017;136:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, et al. Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke. 2005;36:e100–43. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 3.Geiger M, Supiot A, Pradon D, Do MC, Zory R, Roche N. Minimal detectable change of kinematic and spatiotemporal parameters in patients with chronic stroke across three sessions of gait analysis. Hum Mov Sci. 2019;64:101–7. doi: 10.1016/j.humov.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ. Assessing walking speed in clinical research: a systematic review. J Eval Clin Pract. 2008;14:552–62. doi: 10.1111/j.1365-2753.2007.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagano K, Hori H, Muramatsu K. A comparison of at-home walking and 10-meter walking test parameters of individuals with post-stroke hemiparesis. J Phys Ther Sci. 2015;27:357–9. doi: 10.1589/jpts.27.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergamini E, Iosa M, Belluscio V, Morone G, Tramontano M, Vannozzi G. Multi-sensor assessment of dynamic balance during gait in patients with subacute stroke. J Biomech. 2017;61:208–15. doi: 10.1016/j.jbiomech.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 7.David V, Forjan M, Martinek J, Kotzian S, Jagos H, Rafolt D. Evaluating wearable multimodal sensor insoles for motion-pattern measurements in stroke rehabilitation - a pilot study. IEEE Int Conf Rehabil Robot. 2017;2017:1543–8. doi: 10.1109/ICORR.2017.8009467. [DOI] [PubMed] [Google Scholar]
- 8.Munoz-Organero M, Parker J, Powell L, Mawson S. Assessing walking strategies using insole pressure sensors for stroke survivors. Sensors (Basel) 2016;16:1631. doi: 10.3390/s16101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howell AM, Kobayashi T, Chou TR, Daly W, Orendurff M, Bamberg SJ. A laboratory insole for analysis of sensor placement to determine ground reaction force and ankle moment in patients with stroke. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:6394–7. doi: 10.1109/EMBC.2012.6347457. [DOI] [PubMed] [Google Scholar]
- 10.Sungkarat S, Fisher BE, Kovindha A. Efficacy of an insole shoe wedge and augmented pressure sensor for gait training in individuals with stroke: a randomized controlled trial. Clin Rehabil. 2011;25:360–9. doi: 10.1177/0269215510386125. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Smuck M, Legault C, Ith MA, Muaremi A, Aminian K. Simple gait symmetry measures based on foot angular velocity: analysis in post stroke patients. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:5442–5. doi: 10.1109/EMBC.2018.8513585. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan KJ, Tilson JK, Cen SY, Rose DK, Hershberg J, Correa A, et al. Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke. 2011;42:427–32. doi: 10.1161/STROKEAHA.110.592766. [DOI] [PubMed] [Google Scholar]
- 13.Watson MJ. Refining the ten-metre walking test for use with neurologically impaired people. Physiotherapy. 2002;88:386–97. [Google Scholar]
- 14.Lindemann U. Spatiotemporal gait analysis of older persons in clinical practice and research: which parameters are relevant? Z Gerontol Geriatr. 2020;53:171–8. doi: 10.1007/s00391-019-01520-8. [DOI] [PubMed] [Google Scholar]
- 15.Balaban B, Tok F. Gait disturbances in patients with stroke. PM R. 2014;6:635–42. doi: 10.1016/j.pmrj.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Meyer P, Fulk GD, Sazonov ES. Automatic detection of temporal gait parameters in poststroke individuals. IEEE Trans Inf Technol Biomed. 2011;15:594–601. doi: 10.1109/TITB.2011.2112773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzik A, Drużbicki M, Przysada G, Kwolek A, Brzozowska-Magoń A, Wolan-Nieroda A. Analysis of consistency between temporospatial gait parameters and gait assessment with the use of Wisconsin Gait Scale in post-stroke patients. Neurol Neurochir Pol. 2017;51:60–65. doi: 10.1016/j.pjnns.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 18.van Meulen FB, Weenk D, Buurke JH, van Beijnum BJ, Veltink PH. Ambulatory assessment of walking balance after stroke using instrumented shoes. J Neuroeng Rehabil. 2016;13:48. doi: 10.1186/s12984-016-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Błażkiewicz M, Wiszomirska I, Wit A. Comparison of four methods of calculating the symmetry of spatial-temporal parameters of gait. Acta Bioeng Biomech. 2014;16:29–35. [PubMed] [Google Scholar]
- 20.Nigg S, Vienneau J, Maurer C, Nigg BM. Development of a symmetry index using discrete variables. Gait Posture. 2013;38:115–9. doi: 10.1016/j.gaitpost.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Rozanski GM, Wong JS, Inness EL, Patterson KK, Mansfield A. Longitudinal change in spatiotemporal gait symmetry after discharge from inpatient stroke rehabilitation. Disabil Rehabil. 2020;42:705–11. doi: 10.1080/09638288.2018.1508508. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Smuck M, Legault C, Ith MA, Muaremi A, Aminian K. Gait symmetry assessment with a low back 3D accelerometer in post-stroke patients. Sensors (Basel) 2018;18:3322. doi: 10.3390/s18103322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGinley JL, Morris ME, Greenwood KM, Goldie PA, Olney SJ. Accuracy of clinical observations of push-off during gait after stroke. Arch Phys Med Rehabil. 2006;87:779–85. doi: 10.1016/j.apmr.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Nam HS, Lee WH, Seo HG, Smuck MW, Kim S. Evaluation of motion segment size as a new sensor-based functional outcome measure in stroke rehabilitation. J Int Med Res. 2022;50:3000605221122750. doi: 10.1177/03000605221122750. [DOI] [PMC free article] [PubMed] [Google Scholar]