Abstract
Boron neutron capture therapy (BNCT) is a treatment method that focuses on improving the cure rate of patients with cancer who are difficult to treat using traditional clinical methods. By utilizing the high neutron absorption cross-section of boron, material rich in boron inside tumor cells can absorb neutrons and release high-energy ions, thereby destroying tumor cells. Owing to the short range of alpha particles, this method can precisely target tumor cells while minimizing the inflicted damage to the surrounding normal tissues, making it a potentially advantageous method for treating tumors. Globally, institutions have progressed in registered clinical trials of BNCT for multiple body parts. This review summarized the current achievements in registered clinical trials, Investigator-initiated clinical trials, aimed to integrate the latest clinical research literature on BNCT and to shed light on future study directions.
Keywords: Boron neutron capture therapy (BNCT), cancer, clinical trial, radiotherapy
Introduction
Recently, tumors have begun to represent a significant health challenge for humans. Numerous treatment options have been proposed for tumors; however, traditional clinical treatment methods often have limitations. Surgery can effectively remove large volumes of tumors but cannot eliminate scattered tumor cells. Radiotherapy often causes significant collateral damage to other tissues in the target area, and chemotherapy has a considerable impact on the overall condition of patients. Thus, Boron Neutron Capture Therapy (BNCT), leveraging the difference in drug absorption between tumors and healthy tissues, has certain potential therapeutic advantages.
H.J. Taylor discovered that boron-10 nuclei can capture neutrons, causing them to decay into helium-4 nuclei (alpha particles) and lithium-7 ions 1935 [1]. This reaction was recommended as a potentially advantageous method for treating tumors by G.L. Locher in 1936, based on the hypothesis that if boron can selectively accumulate in tumor tissue and absorb thermal neutrons, the tumor tissue will be destroyed by the high-energy ions produced by the reaction [2].
BNCT relies on the following reaction:
10B + n → 7Li + α + γ
The boron-10 atoms absorb neutrons and become unstable, splitting into two high energetic particles: a helium nucleus (α) and a lithium-7 (7Li) nucleus. The reaction also produces gamma rays (γ). These particles deliver high energy within an exceptionally brief pathway (<10 μm), comparable to a single cell’s diameter. The efficacy of BNCT relies on the intracellular localization of 10B, ensuring selective destruction by high-LET radiation of only 10B-containing cells. The targeted delivery of 10B by agents to tumor cells enables neutron beams to selectively eliminate boron-containing tumor cells while preserving surrounding normal tissue [3] (Figure 1).
Figure 1.
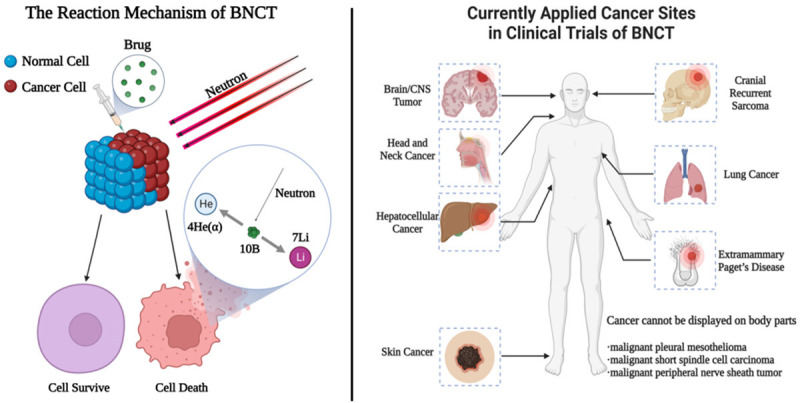
The left part is the reaction principle of Boron Neutron Capture Therapy. The right part is the body parts which have been undergoing BNCT clinical trials for several cancer types. Created with BioRender.com.
Based on the reaction formula mentioned above, BNCT has been progressively applied to various diseases such as glioblastoma multiforme (GBM), head and neck cancer, and malignant melanoma (MM). Clinical trials of BNCT have been conducted in countries such as the United States, Japan, and Europe to ascertain its safety and efficacy. Some of these trials have yielded promising outcomes. To date, pharmacokinetic investigations of BNCT have substantiated the safety profile associated with prevailing drug dosages. Moreover, no documented instances have indicated the induction of uncontrollable acute radiation injuries surpassing Grade 3 severity in patients subjected to BNCT.
Based on the statistical analysis, as of the present moment, Japan and the United States remain the two countries with the highest enrollment of patients in BNCT. This review presents a statistical overview of the patient enrollment data from clinical trials registered worldwide (Figure 2).
Figure 2.
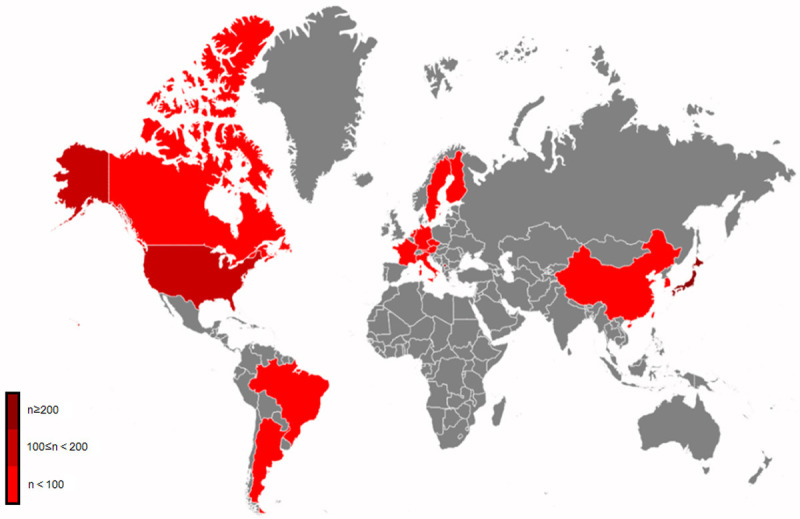
The red parts are countries and regions where BNCT clinical trials have been reported, separating in color depths by the number of cases they announced officially.
However, Japan remains the only country that has permitted the clinical use on BNCT since 2020 [4], while institutions from other countries only permitted BNCT in clinical trials.
In this review we provide detailed information on currently registered BNCT clinical trials.
Brain and central nervous system tumor
Brain and central nervous system (CNS) tumors remain significant and challenging disease types that afflict humanity. In 2020, brain/CNS tumors accounted for 308,102 new cases and caused 251,329 deaths [5]. Research of BNCT for brain/CNS tumors are currently mainly focused on malignant glioma and malignant meningioma. Several phase I/II clinical trials have been conducted by researchers of different countries. Some are still undergoing (Table 1).
Table 1.
Clinical trials of BNCT in brain and central nervous system tumor
| Time | Cancer Type | Trial ID | Phase | Case | Drug | Maximum Dose of Radiation | Outcomes (OS1/MST2) | Citation |
|---|---|---|---|---|---|---|---|---|
| Registered Clinical Trials | ||||||||
| 1999-2001 | sGB3 | NCT00115453 | I/II | 18 | 290-400 mg BPA4/kg | 61.0 Gy(W)5 | 1y OS rate: 61% | [14] |
| 2001-2009 | GB6 | NCT00115440 | I/II | 3 | 290 mg BPA/kg | 29.0 Gy(W) | 3m OS rate: 100% | [14] |
| 2002-2003 | GBM7 | NCT00004015 | I | N/A | 100 mg BSH8/kg | N/A | N/A | [17-19] |
| 2002-2005 | GBM | NCT00039572 | I/II | 21 | 350 mg BPA/kg | / | N/A | [20] |
| 2002-2006 | GBM | NCT00974987 | II | 21 | BSH## 200 mg BPA/kg | 13.0 Gy-Eq9 | MST: 15.6 months | [29] |
| 2007-2013 | Glioma | NCT01233492 | I | 2 | 350 mg BPA/kg | / | N/A | [21] |
| 2009-2018 | GB | UMIN000002385 | II | 45# | BPA## | 13.0 Gy-Eq | N/A | [22] |
| 2014-2016 | Glioma | UMIN000013419 | I/II | 50# | BPA## | 13.0 Gy-Eq | N/A | [23] |
| 2019-2020 | GBM | jRCTs051180218 | II | 4 | BPA## | 13.0 Gy-Eq | OS rate: 75% | [24] |
| 2019- | MG10 | jRCT2051190044 | II | 46# | 400 mg BPA/kg | 7.5 Gy-Eq | N/A | [38] |
| 2022- | Glioma | NCT05737212 | I/II | 39# | 500 mg BPA/kg | 13.0 Gy-Eq | N/A | [30] |
| 2022- | BM11 | ChiCTR2200066473 | I/II | 6# | 500-750 mg BPA/kg | N/A | N/A | [40] |
| Investigator-initiated Clinical Trials | ||||||||
| 1951-1961 | GBM | N/A | I/II | 28 | ~200 mg 10B borax/kg | 10.0 Gy-Eq | N/A | [10] |
| 1968-1996 | Glioma | N/A | I/II | 149 | BSH## | N/A | 2y OS: 12% (GB), 56% (AA12), 62% (low grade astrocytoma) | [11] |
| MST: 21.3 months (GB), 60.4 months (AA), 55.6 months (low grade astrocytoma) | ||||||||
| 1994-1999 | GBM | N/A | I/II | 53 | BPA## | N/A | MST: 12.8 months | [12] |
| 1996-1999 | GBM | N/A | I/II | 20 | BPA## | N/A | MST: 11.1 months | [12] |
| 1997-2002 | GBM | N/A | I/II | 29 | 100 mg BSH/kg | N/A | MST: 10.4-13.2 months | [13] |
| 1998-2007 | GB | N/A | I/II | 15 | 5 g BSH/body | N/A | MST: 25.7 months | [25] |
| 250 mg BPA/kg | ||||||||
| 1998-2008 | GB | N/A | I/II | 23 | 100 mg BSH/kg | 59.7 Gy(W) | MST: 19.5 months; 1y OS: 60.6%; 2y OS: 37.9% | [26] |
| 250 mg BPA/kg | ||||||||
| 1999-2002 | Glioma | N/A | I/II | 9 | 100 mg BSH/kg | 15.9 Gy | MST: 23.2 months (GB), 25.9 months (AA) | [27] |
| 2000-2002 | GBM | N/A | I | 5 | 100 mg BSH/kg | 14.2 Gy-Eq | N/A | [15] |
| 2001-2005 | GB | N/A | I/II | 42 | 900 mg BPA/kg | 15.5 Gy(W) | MST: 17.7 months (nGBM13), 22.2 months (rGBM14) | [16] |
| 2002-2007 | Glioma | N/A | I/II | 22 | 100 mg BSH/kg | 13.0 Gy-Eq | MST6: 10.8 months | [28] |
| 500-700 BPA/kg | ||||||||
| 2020-2022 | MG | N/A | II | 4 | 500 mg BPA/kg | 29.4 Gy-Eq | N/A | [39] |
OS: overall survival;
MST: median survival time;
sGB: supratentorial glioblastoma;
BPA: boronophenylalanine;
Gy(W): the absorbed dose of radiation considering biological effects;
GB: glioblastoma;
GBM: glioblastoma multiforme;
BSH: sodium borocaptate;
Gy-Eq: Gray Equivalent;
MG: meningioma;
BM: brain malignancy;
AA: anaplastic astrocytoma;
nGBM: none-recurrent GBM;
rGBM: recurrent GBM;
Number of patients panned to recruit in the registered information for ongoing trials or trials not yet updated after its predicted completion date.
The drug dosage is not reflected in the literature.
Gliomas are a common type of brain tumor that originates from glial cells. The current standard of care for primary GBMs involves feasible resection followed by radiotherapy and temozolomide chemotherapy [6,7]. However, there is currently no standard treatment for recurrent GBM (rGBM). The treatment options include repeated surgeries and re-irradiation therapy with a median survival time ranges from 6 to 17 months [8]. Although surgery is still the preferred method for tumor debulking and obtaining tissue samples for pathology, research has shown that boron compounds have versatile antineoplastic functions and may hold promise for the treatment of GBMs [9].
During the initial trial phases, before the implementation of a global clinical trial registration system, a considerable number of researchers initiated their trials with the endorsement of ethics committees from local medical institutions.
From 1951 to 1961, the Brookhaven National Laboratory raised a clinical trial, utilizing 10B-enriched borax, recruited 28 patients with brain cancers including GBM and other types of brain carcinoma. Due to the results, the method as utilized offered no advantage over standard methods of therapy already available [10].
With the development of sodium borocaptate (BSH), Nakagawa et al. posted a notable result of their trials between 1968-1996, including 149 patients with several types of brain cancers. The overall respond rate for glioma after BNCT had increased to 64% and a much longer median survival time (MST) than the early results [11]. However, the result was controversial due to the absence of some core data in the article they published.
Between 1994-1999 and 1996-1999, two clinical trials were proceeded in America reported a result of MST of 12.8 months and 11.1 months separately, with the usage of boronophenylalanine (BPA) [12].
Europe medical institutions also started their clinical research. From 1997, a clinical trial was settled in Germany, recruited 29 patients, injected with BSH, and then received neutron radiation from reactors. The trial finally gave an MST of 10.4-13.2 months [13].
The first two registered trials were conducted at the Helsinki University Central Hospital in Finland. From May 1999 to December 2001, a cohort of 18 patients diagnosed with supratentorial GB underwent surgery but did not receive standard radiotherapy or chemotherapy before being treated with boronophenylalanine (BPA)-based BNCT, as part of a prospective clinical trial including phase I/II (NCT00115453). BPA-fructose was administered via a 2-hour administration at dosages ranging from 290 to 400 mg/kg before exposing neutron irradiation, which was administered as a single fraction from two fields. Overall, the treatment was well tolerated, and no patient died during the first few months after BNCT. The estimated 1-year overall survival (OS) rate was 61% [14].
In a separate trial (NCT00115440), three patients who had experienced recurring or progressing GBM after undergoing surgery and traditional cranial radiotherapy of 50-60 Gy were subjected to BPA-based BNCT utilizing a BPA dosage of 290 mg/kg. All three patients were able to tolerate brain re-irradiation with BNCT, with no dead case reported within the first 3 months of treatment [14].
In Czech, a phase I/II trial was established from 2000, including 9 patients with GBM. Eventually 5 of them were recognized suitable for BNCT. The trial presented good tolerance for patients receiving BNCT on its certain condition and no clinical deterioration was observed during or after BNCT [15].
Between 2001 and 2005, according to a Swedish clinical trial using BPA on 42 patients, the MST of none recurrent glioblastoma multiform (nGBM) was 17.7 months and 22.2 months of rGBM [16].
A phase I clinical trial (NCT00004015), also known as protocol 11961, was organized by the European Organization for Research and Treatment of Cancer (EORTC) and conducted in six countries: Austria, Canada, France, Germany, Italy, and the Netherlands. This trial confirmed that normal brain tissue does not readily absorb the boron compound sodium borocaptate (BSH) [17], and that the protocol is applicable at a dose of 100 mg BSH/kg infused at a rate of 1 mg/kg/min [18,19]. In addition, a German trial reported a potential relationship between the occurrence of cerebral radiological changes and BNCT [13].
A clinical trial (NCT00039572) was conducted in Massachusetts, United States, to recruit patients with GBM. The trial included 24 patients (21 diagnosed with GBM) and presented an open two-compartment model to predict blood 10B concentrations after intravenous infusion of the l-p-boronophenylalanine-fructose complex (BPA-F) in humans. The model successfully predicted the average pharmacokinetic response in a group of participants [20].
In Birmingham, UK, a phase I clinical trial (NCT01233492) was terminated after recruiting only two patients due to a similar result with Helsinki. The trial reported that the peak 10B concentration in the blood was 28.1 mg/ml at a total BPA dose of 350 mg/kg and no serious clinical problems was observed. This finding aligns with the prior observations post by the Helsinki group regarding BPA-F [21].
Beside two pending trials (UMIN000002385/UMIN000013419) raised by Japanese researchers from 2009 to 2018 [22,23]. From 2019 to 2020, Miyatake et al. conducted a trial (jRCTs051180218) which also terminated by unstable reactor in Kyoto, eventually including 4 patients with GBM. The result presented that the BNCT and bevacizumab could prolong the cases with recurrent GBM, showing no evidence of brain radiation necrosis [24].
Japanese medical institutions restarted their BNCT clinical trials since 1999. Four unregistered trials and one registered has been posted. The trial conducted during 1998-2007, 1998-2008, 1999-2002 and 2002-2007 showed their MST of 25.7 months, 19.5 months, 23.2 months and 10.8 months, including 15, 23, 9 and 22 volunteers with brain cancers, utilizing different medication regimens and radiation doses [25-28].
Kawabata et al. conducted a phase II trial (NCT00974987) in Osaka, Japan, in which they treated 21 patients with malignant glioma using a combination of BSH and BPA along with fractionated X-ray irradiation. After undergoing BNCT treatment, the patients had a median survival time (MST) of 15.6 months after their initial diagnosis. This MST was significantly greater than that of historical controls who received treatment consisting of surgical resection followed by XRT and chemotherapy [29].
Moreover, Gachon University Gil Medical Center, Korea, is now recruiting patients with recurrent high-grade glioma, rGBM, recurrent anaplastic astrocytoma and recurrent anaplastic oligodendroglioma for a new phase I/IIa clinical trial (NCT05737212). The trial tends to use BPA and DMX101 combined with different radiation doses to cure patients with recurrent gliomas and attempts to explore the adequate radiation dose level of BNCT based on confirmation of the maximum tolerated dose of BNCT. The estimated completion time is December 2024 [30].
Although most registered trials worldwide have either been completed or terminated, research on BNCT remains lacking. Miyatake et al. conducted a study using reactor based BNCT to treat 58 recently diagnosed GBMs and 68 recurrent malignant gliomas, 52 of which were GBMs. In the group of patients with newly diagnosed GBM who underwent BNCT, the MST was 21.1 months, and the survival rate in 2 years was 45.5% [31]. A study also used recursive portioning analysis (RPA) to evaluate the effect of BNCT on recurrent gliomas [32]. The results showed that BNCT prolonged the survival of patients in every RPA class, particularly those in the poor RPA classes [33].
In Taipei Veterans General Hospital, Taiwan, meanwhile, 34 patients with malignant brain cancers had been treated with BNCT for their life emergency (approved by the institutional review board of hospital, Approval number: #2020-11-002B). The posted result presented a 29% 1-year OS rate and a 16% 1-year RFS rate, and patients with astrocytoma and glioblastoma were notably exhibited a more pronounced therapeutic response [34].
Brain radiation necrosis (BRN) is a common complication of BNCT for recurrent glioma cases [35]. In the research conducted by Kawabata et al., 25 patients were recruited of whom: 14 were classified as pGBM and 11 as non-pGBM. The patients received BNCT along with the addition of bevacizumab from June 2013 to February 2019. The median OS and progression-free survival (PFS) time for all patients after BNCT were 24.7 months (95% confidence interval (CI): 11.4-73.6) and 12.1 months (95% CI: 8.0-15.1), respectively. The 1-year survival rates for pGBM and non-pGBM were 63.5% (95% CI: 33.1-83.0) and 81.8% (95% CI: 44.7-95.1), respectively. Magnetic resonance imaging (MRI) was conducted during bevacizumab treatment, and no cases of pseudoprogression or radiation necrosis were detected [36].
Meningiomas are the most diagnosed primary tumors of the central nervous system. The majority of meningiomas are diagnosed as benign with favorable prognosis, and only a small portion of patients are diagnosed with malignancy, requiring treatment options including observation, symptomatic control, surgery, hydroxyurea administration, and radiotherapy. The 10-year recurrence rate after surgery varies between 10% and 100% depending on the extent of resection, while the 5-year tumor control rate with radiation therapy ranges from 85% to 100% [37]. However, in cases where surgery is not an option and the patient has already received the maximum dose of radiotherapy, BNCT has shown promise as a critical salvage treatment.
Clinical trials, particularly those on meningiomas, are rare. Miyatake launched a phase II clinical trial (jRCT2051190044) for recurrent and refractory high-grade meningiomas (WHO Grade 2, 3) in September 2019, and has now stopped recruiting [38].
According to the trial, Miyatake et al. used SPM-011 with reactor-based BNCT for 46 recurrent and refractory high-grade meningiomas. Although some patients were later lost due to systemic metastasis and intracranial distant recurrence outside the neutron irradiation facility, all 46 patients initially responded well to BNCT with significant shrinkage of the mass [31].
Lan TL et al. in Taiwan reported four cases of recurrent meningiomas treated with BNCT. The mean ratio of boron-containing drug uptake in tumor tissues to that in normal tissues was 4.125, and the average tumor dose via BNCT was 29.414 GyE. Of the three patients in this cohort, the mean PFS was 14.7 months, and the response rate to BNCT for recurrent atypical meningiomas was 67% [39].
In Xiamen, China, the first accelerator based BNCT center has completed construction. The trial (ChiCTR2200066473) was approved by Chinese Clinical Trial Registry [40]. Until January 2023, the center has reported to treat 12 patients with GBM or head and neck cancer, with the first 3 patients showing a notable tumor shrinkage.
Head and neck cancer
Until 2020, head and neck cancer were ranked as the seventh most prevalent cancer globally, accounting for 660,000 newly diagnosed cases and 325,000 fatalities [5]. Patients diagnosed with stage I or II disease account for approximately 30-40% of cases and can be cured using surgery or definitive radiotherapy alone [41]. According to reports, a combined surgical approach involving oral surgery and radiation therapy may potentially elevate the 3-year progression-free survival rate to a maximum of 95% [42]. However, locally advanced disease poses a significant risk of local recurrence (15%-40%) and distant metastasis, with an overall poor prognosis and less than 50% 5-year OS [43]. The use of multimodal approaches has helped improve cure rates and preserve the function and quality of life over the past two decades [44]. The rise of BNCT treatment as a viable approach in recent decades has piqued public interest. To date, eight clinical trials have been registered (Table 2).
Table 2.
Clinical trials of BNCT in head and neck cancer
| Time | Cancer Type | Trial ID | Phase | Case | Drug | Maximum Dose of Radiation | Outcomes (Response Rate/OS1/PFS2/MST3/) | Citation |
|---|---|---|---|---|---|---|---|---|
| Registered Clinical Trials | ||||||||
| 2003-2007 | SCCHN4 | NCT00062348 | I | 6 | 50 mg BSH5/kg | / | N/A | [46] |
| 100 mg BPA6/kg | ||||||||
| 2003-2007 | HNC7 | jRCTs061180067 | I/II | 10 | 500 mg BPA/kg | 20.0 Gy(W)8 | Response rate: 90% | [49] |
| 2003-2012 | r/lHNC9 | NCT00114790 | I/II | 29 | 400 mg BPA/kg | 10.0 Gy(W) | Response rate: 76%; 2y OS rate: 30%; 2y PFS rate: 20%; PFS time: 7.5 months | [47] |
| 2009-2013 | r/lHNC | NCT00927147 | I/II | 12 | 400 mg BPA/kg | 10.0 Gy(W) | Response rate: 100%. | [48] |
| 2010-2015 | r/lHNC | NCT01173172 | I/II | 17 | 400 mg BPA/kg | 20.0 Gy-Eq10 | Response rate: 71%; 2y OS rate: 47% | [54] |
| 2013-2019 | rHNC11 | NCT02004795 | I/II | 9 | 400 mg BPA/kg | 18.0 Gy(W) | N/A | [56] |
| 2016-2018 | r/lHNC | jRCT2080224571 | II | 21 | 400 mg BPA/kg | 20.0 Gy-Eq | 3m response rate: 71.4%; 2y OS rate: 58% (rSCC12); 100% (r/la-nSCC13) | [51] |
| 2019-2021 | HNC | jRCTs051180160 | II | 7 | BPA## | N/A | Response rate: 85.7% | [52] |
| 2019-2022 | HNC | jRCTs031180302 | N/A | 14 | BPA## | N/A | N/A | [53] |
| 2021- | SCCHN | UMIN000044118 | II | 120# | 400 mg BPA/kg | N/A | N/A | [57] |
| 2022- | HNC | ChiCTR2200066473 | I/II | 6# | 500-750 mg BPA/kg | N/A | N/A | [40] |
| Investigator-initiated Clinical Trials | ||||||||
| 2001-2007 | a/rHNC14 | N/A | I/II | 62 | 5 g BSH/body | 12.0 Gy-Eq | MST: 10.1 months; 1y OS: 43.1%, 2y OS: 24.2% | [45] |
| 250-500 mg BPA/kg | ||||||||
OS: overall survival;
PFS: progression-free survival;
MST: median survival time;
SCCHN: squamous cell carcinoma of head and neck;
BSH: sodium borocaptate; r/lHNC: recurrent/locally head and neck cancer;
BPA: boronophenylalanine;
HNC: head and neck cancer;
Gy(W): the absorbed dose of radiation considering biological effects;
rHNC: recurrent head and neck cancer;
Gy-Eq: Gray Equivalent;
rHNC: recurrent head and neck cacer;
rSCC: recurrent squamous cell carcinoma;
r/la-nSCC: recurrent/locally advanced non-squamous cell carcinoma;
a/rHNC: advanced/recurrent head and neck cancer.
Number of patients panned to recruit in the registered information for ongoing trials or trials not yet updated after its predicted completion date.
The drug dosage is not reflected in the literature.
Between 2001 and 2007, a series of phase I/II clinical trials approved by the Ethical Review Board of each medical institute in Japan reported recruiting 62 participants with Head and Neck Cancers. Following treatment with BSH and BPA prior to neutron radiation, their MST was reported as 10.1 months, accompanied by a 43.2% 1-year overall survival (OS) rate and a 24.2% 2-year OS rate [45].
The initial registered trial (NCT00062348) was performed in Essen, Germany, and six patients with advanced squamous cell carcinoma of the head and neck (SCCHN) were recruited. The patients received an injection of BSH or BPA, and the researchers evaluated the proportion of 10B in both the tumor and normal tissues. According to Witting A et al., BPA and BSH can efficiently deliver 10B to SCCHN, resulting in successful BNCT treatment. The researchers discovered that the most critical organs at risk were the mucosa and skin [46].
Kankaanranta L et al. reported a trial conducted in Helsinki, Finland, from May 1999 to January 2012, involving 29 patients with head and neck cancer (NCT00114790). In this phase I/II trial, patients underwent surgery and conventional fractionated photon irradiation before undergoing BNCT twice. The trial demonstrated that 76% of the patients responded to BNCT, 21% experienced tumor growth stabilization for 5.1 to 20.3 months, and only 3% showed progression. The median PFS time was found to be 7.5 months. The 2-year PFS and OS rates were 20% and 30%, respectively, with 27% of the patients surviving for 2 years without locoregional recurrence. The toxicity level was considered acceptable [47].
In another phase I/II trial (NCT00927147) conducted by the same research group, 12 more patients with locally advanced (rT3, rT4, or rN2) head and neck cancer that had recurred and was inoperable were treated with BNCT. The trial ended in 2013, with 83% of patients showing a positive response to treatment and 17% experiencing tumor growth stabilization for 5.5 to 7.6 months. The median duration of response was 12.1 months, demonstrating that BNCT exhibits effectiveness and a favorable safety profile for treating inoperable, locally advanced head and neck carcinomas, even with those occur recurrence at previously irradiated sites [48].
A phase I/II clinical trial was conducted at Kawasaki Medical School Hospital (KMS), where Aihara T et al. recruited 20 patients between October 2003 and September 2007 (jRCTs061180067). The trial included 10 patients with recurrent squamous cell carcinoma (SCC), seven patients with locally advanced non-squamous cell carcinoma without malignant melanoma (non-SCC), and three patients with newly diagnosed non-SCC. The total dose of administered BPA, which was the L-enantiomer with over 95% 10B enrichment, was 500 mg/kg body weight (B.W.), with a planned control dose of over 20 Gy-Eq for the tumor and a maximum dose of 15 Gy-Eq for the normal tissue surrounding the tumor. As per the study, 11 patients experienced complete remission and seven patients showed partial remission at the irradiated site, resulting in an effective rate of 90% [49]. Furthermore, the study concluded that BNCT has the potential to cure salivary gland carcinoma and does not induce any significant adverse effects. BNCT can be utilized regardless of prior treatment for the primary tumor [50].
In addition to the trials conducted in Germany and Finland, a research group in Japan has also conducted clinical trials on BNCT. Hirose K et al. presented a trial (jRCT2080224571) of BNCT without reactors due to the development of a cyclotron-based epithermal neutron source (C-BENS). The study included 21 patients with eight recurrent SCC (rSCC) and 13 with recurrent/locally advanced non-SCC (r/la-nSCC). The response rate (95% CI on both sides) was 42.9% (21.8%-66.0%) on day 30, 57.1% (34.0%-78.2%) on day 60, and 71.4% (47.8%-88.7%) on day 90. Patients with R-SCC exhibited a 2-year OS rate of 58%, whereas those with R/LA-nSCC showed a survival rate of 100% [51].
From 2019 to 2021, researchers from Nara Medical University conducted a trial, aiming at establishing the protocol for BNCT on HNC patients. Ending with 7 participants and a 85.7% effective rate [52].
The other trial (jRCTs031180302) enrolled 14 participants and evaluated 16 lesions, including those in the parotid gland, orbit, external auditory canal, oral cavity, nasopharynx, hypopharynx, larynx, retropharyngeal lymph nodes, level II lymph nodes, and parapharyngeal space. This study aimed to assess the efficacy of 18F-FBPA-PET and to extend its indications for BNCT. The study found that the SUVmax of 18F-FBPA was 4.5 ± 1.1 for the cervical field of view (FOV) and 3.4 ± 0.8 for the trunk FOV. The tumor-to-normal (T/N) tissue ratio was 3.6 ± 0.8 for the cervical FOV. The tumor-to-blood ratio by ascending aorta was 3.0 ± 0.8, and the target-to-background (T/B) ratio by left ventricle was 2.8 ± 0.8 [53].
In Taiwan, a clinical trial (NCT01173172) was conducted using an open-pool reactor for BNCT in patients with locally recurrent head and neck cancers previously treated with radiation. The phase I/II trial was conducted between 2010 and 2015 and included 17 patients who underwent BNCT with BPA (400 mg/kg) administered in two phases at a prescribed dose of 12-35 Gy Eq. Of these patients, six exhibited a complete response and six exhibited a partial response, with a 2-year OS rate of 47%. This study showed that fractionated BNCT administered at 30-day intervals with adaptive planning is effective and safe [54]. Wang LW et al. recommended the use of different setups for treating superficial and deep-seated tumors, with patient collimators being useful for treating superficial tumors and direct irradiation being the preferred option for deep-seated tumors [55].
Another registered trial (NCT02004795) conducted by Wang LW et al. focused on combining BNCT with image-guided intensity-modulated radiotherapy (IG-IMRT). The trial analyzed nine participants who received both treatments and found that the BNCT+IMRT plan had a significantly better conformity index for gross tumor volume (GTV) than the BNCT-alone plan (P = 0.003). This improvement was particularly notable for tumors >100 cm3, indicating that combining BNCT with IG-IMRT can improve homogeneity and conformity in the treatment of larger tumors [56].
By 2023, two other trials (UMIN000044118, ChiCTR2200066473) established by Japanese and Chinese has been registered for HNC and BNCT [40,57]. Until now, no further trial results have been published.
Skin cancer
Melanoma, a skin cancer caused by melanocyte malignancy, accounts for 1.7% of the global cancer incidence and 0.6% of cancer-related deaths [41]. The incidence of melanoma is still increasing dramatically in developed countries, especially in fair-skinned countries [58]. For instance, in America, even with the help of novel therapies including immune checkpoint inhibitors and targeted therapies [59], patients diagnosed MM often have unfavorable prognoses. Over the last century, clinical trials focusing on Malignant Melanoma (MM) have been conducted worldwide (Table 3).
Table 3.
Clinical trials of BNCT in skin cancer
| Time | Cancer Type | Trial ID | Phase | Case | Drug | Maximum Dose of Radiation | Outcomes (Response rate/CR1/PR2/OS3/PFS4) | Citation |
|---|---|---|---|---|---|---|---|---|
| Registered Clinical Trials | ||||||||
| 1996- | MM5 | NCT00002781 | I | 15# | BPA6,## | N/A | N/A | [63] |
| 2002-2004 | MM | NCT00059800 | II | 36# | BPA## | N/A | N/A | [64] |
| 2002-2005 | MM | NCT00039572 | I/II | 1 | 350 mg BPA/kg | / | N/A | [20] |
| 2013- | MM | NCT02759536 | I/II | 30# | 350 mg BPA/kg | 20.0 (RBE)Gy7 | N/A | [69] |
| 2014-2018 | Skin Malignancy | UMIN000013101 | II | 20 | BPA## | N/A | N/A | [70] |
| 2017-2020 | Skin Malignancy | jRCTs061180066 | I/II | 3 | 200 mg BPA/kg | 15.0 Gy-Eq8 | Response rate: 100% | [71] |
| 2019-2022 | MM/AS9 | NCT04293289 | I | 10 | 400 mg BPA/kg | N/A | N/A | [72] |
| 2022- | Solid Tumor | NCT05538676 | N/A | 10# | BPA## | N/A | N/A | [74] |
| 2022- | AS | NCT05601232 | II | 10# | 400 mg BPA/kg | N/A | N/A | [73] |
| Investigator-initiated Clinical Trials | ||||||||
| 1987-2001 | MM | N/A | I/II | 22 | 272 mg BPA/body | N/A | CR: 73% | [61] |
| 1994-1996 | MM | N/A | I/II | 4 | BPA## | N/A | CR: 25%, PR: 75% | [62] |
| 2002- | MM | N/A | II | 4 | 160 mg BPA/kg | 15.0 Gy-Eq | PR: 100% | [65] |
| 2003-2007 | MM | N/A | I/II | 8 | 14 g BPA/m2 | 16.5 (RBE)Gy | OS rate: 69.3% | [66,67] |
| 2003-2014 | MM | N/A | II | 8 | 160 mg BPA/kg | 15.0 Gy-Eq | CR: 75%, PR: 25% | [65] |
| 2019 | CCS10 | N/A | I/II | 1 | 500 mg BPA/kg | 30.0 Gy-Eq | PFS time: 1 year | [75] |
CR: complete remission;
PR: partial remission;
OS: overall survival;
PFS: progression-free survival;
MM: malignant melanoma;
BPA: boronophenylalanine;
(RBE)Gy: Radiation Biological Effectiveness in Gray;
Gy-Eq: Gray Equivalent;
AS: angiosarcoma;
CCS: clear cell sarcoma of tendons and aponeuroses.
Number of patients panned to recruit in the registered information for ongoing trials or trials not yet updated after its predicted completion date.
The drug dosage is not reflected in the literature.
The treatment approach for MM varies depending on the patient’s clinical stage and the location of the primary lesion. For advanced melanoma, chemotherapy, targeted therapy, and immunotherapy are commonly chosen treatment options. Surgical excision is the primary treatment method for localized melanoma [60]. Recently, BNCT has also been emerging as a potential treatment for localized (advanced) melanoma. Several clinical trials are currently underway.
In Kobe, Japan, Yutaka conducted world is first clinical trial of BNCT on treating MM in 1987. Although no registered information was posted, the phase I/II trial recruited 22 patients with MM, announcing a 73% of complete response rate (CR) by the end of 2001 [61].
Meanwhile, MIT called its first clinical trial on MM between 1994 and 1996. The trial only contained 4 patients, leading a result of 25% CR and 75% partial response rate (PR) [62]. In addition to the pharmacokinetic model for the concentration of 10B in blood presented by the trial (NCT00039572) carried out by Kiger WS 3rd et al. at the Massachusetts Institute of Technology (MIT) [20], American institutes conducted two other clinical trials for patients with stage 3 MM. The Boston University School of Medicine has planned to recruit 15 patients for a phase I trial (NCT00002781) since 1996 to receive BPA-F for biodistribution studies [63]. MIT also started another phase II trial (NCT00059800) for patients with MM to assess clinical response, acute/late dermal reactions, and pharmacokinetics from 2002 to 2004 [64].
Another unregistered trial was conducted by Essen University in Germany from 2002 with no reachable final reports. Until now, the trial only has 4 patients included, showing all patients undergoing treatments have partial response or stabilized lesions [65].
From October 2003 to June 2007, the Argentine Atomic Energy Commission (CNEA) and the Oncology Institute Angel H. Roffo (IOAHR) collaborated to conduct a phase I/II clinical trial that aimed to treat eight patients with multiple subcutaneous skin metastases of melanoma. The trial was authorized by the National Agency of Drugs, Food, and Technology (ANMAT #3976) and the Nuclear Regulatory Agency (ARN #21190) [66]. Each participant was administered an infusion containing 14 gr/m2 of BPA, followed by exposure of the targeted area to a mixed thermal-epithermal neutron beam in the RA-6 reactor. The results showed an OS rate of 69.3%, with 30% of the assessed areas showing ulcerations. These findings indicate that the toxicity of the treatment was acceptable [67]. Additionally, a biodistribution study conducted by Liberman SJ et al. found that nodular metastatic melanomas exhibit lower boron uptake [68].
From 2003 to 2014, Hiratsuka J et al. organized a clinical trial obtaining from the Kawasaki Medical School and the Kyoto University Medical and Ethics Committee. 8 patients with primary lesions were invited to accept BNCT, indicating an 88% overall control (without recurrence) [65].
In 2013, the Third Xiangya Hospital of Central South University in Hunan, China launched a phase I/II clinical trial (NCT02759536) to examine the practicality of the world’s first in-hospital neutron irradiator (IHNI). The trial sought to enroll 30 patients who had melanoma and at least one solid tumor with a size of 1 cm or more. Participants were intravenously infused with BPA-F solution at a dose of 350 mg/kg over 90 minutes, and subsequently exposed to neutron irradiation with the IHNI. In 2014, Yong Z et al. documented their first cases, which consisted of patients diagnosed with MM on their left foot with two lesions that were confirmed through clinical and histopathological analyses. After BNCT, only grade 2 acute radiation injuries were observed within the first 4 weeks. The injury healed, and no late radiation injury was detected during the 2-year follow-up period. These findings suggest that BNCT combined with IHNI is a viable treatment option [69].
Other than the trial (UMIN000013101) charging by Hiratsuka J, aiming at organizing protocols for skin malignancy [70], Kamitani conducted a phase I/II trial (jRCTs061180066) for skin malignancies from November 2017 to June 2020, enrolling three patients with recurrent skin malignancies who did not receive surgical treatment or were rejected by patients. The participants were intravenously administered the BPA-F complex (200 mg/kg. BW) for 2.5 to 3 hours before irradiation showed a 100% tumor control rate (CR+PR) without any patients presenting adverse events over Grade 3, indicating the effectiveness of BNCT against recurrent cancers and malignancies [71].
Moreover, the National Cancer Center Hospital in Tokyo, Japan, just finished its enrollment of patients for a phase I trial (NCT04293289). The trial aimed to evaluate the safety and efficacy of CICS-1 and SPM-011 for BNCT and recruited 10 patients with histopathologically diagnosed primary MM or angiosarcoma. The patients were administered intravenous SPM-011 at 200 mg/kg/h for 2 h before neutron irradiation and at 100 mg/kg/h during neutron irradiation. To date, no similar results have been reported [72]. Meanwhile, the phase II trial (NCT05601232) of CICS-1 and SPM-011 for unresectable angiosarcomas has opened recruitment and is planning to take in 10 patients and treat them with the same protocol as the phase I trial, and will focus on the response rate for primary outcomes [73].
A clinical trial (NCT05538676) is underway at Tongji Hospital in Hubei, China, to assess the effectiveness of fluoride-labeled boronophenylalanine (F-BPA) as a tracer for solid tumors. This study enrolled 10 patients with various solid tumors, including, but not limited to, recurrent head and neck cancer, glioma, pancreatic cancer, and osteosarcoma. Xiaohua Z et al. used positron emission tomography (PET) to calculate the TBR of a lesion and evaluated the biological activity of tumors. They also measured the maximum standard uptake value (SUVmax) of the region of interest (ROI) in the PET/CT images [74].
Except from MM, Fujimoto T reported 1 case of clear cell sarcoma of tendons and aponeuroses (CCS) treated by BNCT in 2019. The patient remained no symptoms or metastasis after 1 year and was survived until the publishing date [75].
Other cancers
Metastasis of cancer is commonly viewed as the terminal phase of a degenerative process; as cancer cells migrate to other parts of the body, conventional treatments such as surgery may become less effective. Established therapies such as radiosurgery and chemotherapy have been proven effective in treating metastatic cancers. However, with the emergence of new treatments such as BNCT, which enriches tumors with 10B compounds, cancers from other systems of human are receiving increased attention from the public (Table 4).
Table 4.
Clinical trials of BNCT in other cancer
| Time | Cancer Type | Trial ID | Phase | Case | Drug | Maximum Dose of Radiation | Outcomes (SD1/PFS2/CR3) | Citation |
|---|---|---|---|---|---|---|---|---|
| Registered Clinical Trials | ||||||||
| 1997-2006 | Metastatic Melanoma | NCT00085059 | II | 4 | 100 mg BSH4/kg | 60.0 muSV5/h | N/A | [76] |
| 2002-2005 | Metastatic Melanoma | NCT00039572 | I/II | 2 | 350 mg BPA6/kg | N/A | N/A | [20,78] |
| 2003-2007 | Metastatic Hepatoma | NCT00062348 | I | 6 | 600 mg BSH/kg | / | N/A | [79,80] |
| 200 mg BPA/kg | ||||||||
| 2019-2020 | AS7 | jRCTs051180217 | II | 2 | BPA## | N/A | N/A | [90] |
| 2019-2020 | rBC8 | jRCTs051180219 | II | 1 | BPA## | N/A | N/A | [91] |
| 2022- | rBC | jRCTs031220371 | 0 | 5# | BPA## | N/A | N/A | [92] |
| Investigator-initiated Clinical Trials | ||||||||
| 2005 | HCC9 | N/A | I/II | 1 | 1 g BSH/body | 2.0 Gy-Eq10 | SD time: 1 month | [84] |
| 250 mg BPA/kg | ||||||||
| 2005-2006 | MPM11/MSSpCC12 | N/A | I/II | 2 | 250-500 mg BPA/kg | 44.7 Gy-Eq | PFS time: 3-6 months | [81] |
| 2005-2014 | EMPD13 | N/A | I/II | 4 | 200 mg BPA/kg | 25.0 Gy-Eq | 6m CR: 100% | [86] |
| 2006 | MPM | N/A | I/II | 3 | 500 mg BPA/kg | 39.5 Gy-Eq | N/A | [82] |
| 2009 | MPM/Hepatoma | N/A | I/II | 10 | BPA## | 5.0 Gy-Eq | N/A | [83] |
| 2011 | HCC | N/A | I/II | 1 | 300 mg BSH/body | 5.0 Gy-Eq | SD time: 3 months | [85] |
| 2012 | EMPD | N/A | I/II | 2 | BPA## | N/A | 1y CR: 100% | [87] |
| 2014 | Sarcomas | N/A | I/II | 1 | 500 mg BPA/kg | 13.0 Gy-Eq | N/A | [88] |
| 2015 | MPNST14 | N/A | I/II | 1 | 500 mg BPA/kg | 24.3 Gy-Eq | SD time: 2 years | [89] |
SD: stable disease;
PFS: progression-free survival;
CR: complete remission;
BSH: sodium borocaptate;
uSV: microsievert;
BPA: boronophenylalanine;
AS: angiosarcoma;
rBC: refractory breast cancer;
HCC: hepatocellular carcinoma;
Gy-Eq: Gray Equivalent;
MPM: malignant pleural mesothelioma;
MSSpCC: malignant short spindle cell carcinoma;
EMPD: Extramammary Paget’s Disease;
MPNST: malignant peripheral nerve sheath tumor.
Number of patients panned to recruit in the registered information for ongoing trials or trials not yet updated after its predicted completion date.
The drug dosage is not reflected in the literature.
A phase II trial (NCT00085059) was conducted by Wittig A et al. in Essen University, Germany, in 1997, to assess a treatment for patients with melanoma that had metastasized to the brain, skin, or soft tissues of the head, neck, or extremities [76]. Unfortunately, the trial was terminated with unknown reason in 2006 after enrolling only four patients, presenting a well applicable protocol design. A side experiment conducted during the trial indicated that the absorbed dose from the activated isotopes in the irradiated volume did not significantly contribute to the absorbed dose in the target volume (<1%), which ensured the safety of the staff members [77].
In 2002, a phase I/II trial (NCT00039572) was initiated to evaluate the efficacy of intravenous (IV) BPA-F for treating patients with melanoma that had spread to the brain. The interventional trial involved supplying IV BPA-F to participants for over 90 min before administering cranial neutron capture therapy (NCT), with the aim of determining the maximum tolerated dose of cranial NCT and evaluating the clinical response to treatment [20,78].
Patients with liver metastases secondary to colorectal adenocarcinoma were included in another phase I trial (NCT00062348) that aimed to assess boron uptake ratio and toxicity in tumor and normal tissues [79]. Six patients were treated with BSH or BPA for BNCT. According to Wittig A et al., after comparing the 10B ratio in the liver, blood, tumor, and metastases, BPA was found to be suitable for extracorporeal irradiation of the liver with BNCT, while BSH was not recommended [80].
During 2005 to 2006, Kyoto University Research Reactor Institute (KURRI) treated 2 patients with malignant pleural mesothelioma (MPM) and malignant short spindle cell carcinoma (MSSpCC) by BPA separately. According to the trial, both 2 patients showed tumors regressed or remained stable in size for 3-6 months following two rounds of BNCT [81]. Also in 2006, KURRI reported 3 patients with MPM treated by BNCT with no further results posted [82]. In 2009, Kyoto University claimed a clinical trial aiming at comparing the effect of accelerator based BNCT (AB-BNCT) and reactor-based BNCT (RB-BNCT). Assembling data from 4 MPM patients and 6 liver cancer patients, the trial showed that the AB-BNCT outbalance RB-BNCT when curing tumors in deep parts [83].
Researchers from Tokyo and Osaka submitted 2 cases of hepatocellular carcinoma (HCC) treated with BNCT separately in 2005 and 2011. However, the lesions of both two patients remained unchanged in a short time after the treatment of BSH and BPA [84,85].
From 2005 to 2014, 6 patients with Extramammary Paget’s Disease (EMPD) were treated by BNCT. During the trial, the curative effect was notable, all patients’ symptoms ware completely relieved within 1 year after BPA administration and radiotherapy [86,87].
In 2014, Futamura et al. from Osaka Medical Collage reported one patient with recurrent skull osteosarcoma induced by radiation, which was considered radioresistant and inoperable. The case was administered 500 mg/kg of BPA intravenously for 3.2 hours, after with the neutron irradiation less than 13 Gy-Eq. The tumor size reduced with no radiation damage except hair loss in 2 months after BNCT, showing a possible way to cure inoperable recurrent osteosarcoma [88].
Also in Osaka, 2015, Masayoshi I et al. treated a patient suffering malignant peripheral nerve sheath tumors (MPNST) with BPA. The trial was approved by institutional review board. One year after the BNCT, the chest computed tomography scan showed a 25% shrinkage of the tumor, and the disease was stabilized for 24 months [89].
During 2019 and 2020, Miyatake et al. planned to establish a protocol in the trial (jRCTs051180217) for inoperable and recurrent angiosarcoma (AS) patients. However, due to the unstable status, only 2 patients were recruited and showed a transient local controlment on the tumors [90].
By 2020, 1 patient with refractory breast cancer (rBC) received BNCT treatment in a clinical trial (jRCTs051180219) in Osaka. Before termination in 2020, the treatment presented a transient local controlment on the tumor, showing a probable direction for curing rBC [91].
From 2022, Kurosaki H et al. in Edogawa Hospital, Japan raised a clinical trial (jRCTs031220371) for BNCT on rBC patients. The phase 0 trial is now still recruiting participants [92].
Discussion
BNCT is an emerging therapeutic approach that has undergone several clinical trials worldwide and has shown promising results in terms of drug uptake, efficacy, and toxicity. Although BNCT has been approved for clinical treatment in some countries and regions, registered clinical trials are still limited to phase I/II, and more phase II/III trials are needed to evaluate the clinical response and safety of BNCT in tumors.
Additionally, despite the progress made by BNCT in the treatment of brain and central nervous system tumors, head and neck cancer, skin cancer, and some other types of cancers, the high selectivity of boron compounds for tumors provides potential development opportunities for other cancers. The data was collected from clinicaltrials.gov; chictr.org.cn; rctportal.niph.go.jp and Pubmed. So far, BNCT has undergone limited experimentation in patients with extramammary Paget’s disease and liver cancer. According to the BNCT Training Workshop hold by IAEA in July 2023, pulmonary tumors has also been selected as target cancer for BNCT and has been undergoing several cases’ treatments. Unfortunately, no evidence has been found. Some of treatment data in the workshop also lacks a resource. Several trials for BNCT on breast cancer have been registered, but none of them have reported their progress for now. Recently, the clinical trial for recurrent breast cancer is announced to start in June 2023 in Egagawa Hospital, Japan. Further clinical studies are still warranted to expand the indications of BNCT.
In recent years, significant advances have been made in BNCT equipment. The early development of BNCT for GBM relied on the production of neutron beams generated by nuclear reactors. The excessive costs associated with acquiring and maintaining this equipment remained an obvious barrier to establishing BNCT centers. However, in many countries, proton-accelerator-based BNCT is gradually replacing the previously used nuclear-reactor-based BNCT. The increasing miniaturization of equipment has made it more convenient and efficient for patients to undergo BNCT.
Conclusion
BNCT has undergone multiple early phase clinical trials worldwide. Although more extensive clinical trials are still needed, existing results have demonstrated that this is a promising new cancer treatment with acceptable toxicity. With the advancement in pharmaceutical research and the continuous evolution of radiation devices, BNCT holds the potential to emerge as a novel and formidable medical modality, comparable to traditional surgical interventions, chemotherapy, radiotherapy, and immunotherapy.
Acknowledgements
This research was funded by the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant No. 21K09371) and Kobayashi Foundation. We thank Hideo Ueki, Shunai Li, Ai Ueda and Jie Chen at Okayama University for their technical guidance.
Disclosure of conflict of interest
None.
References
- 1.Taylor H, Goldhaber M. Detection of nuclear disintegration in a photographic emulsion. Nature. 1935;135:341–341. [Google Scholar]
- 2.GL L. Biological effects and therapeutic possibilities of neutrons. Am J Roentgenol. 1936;36:1–13. [Google Scholar]
- 3.Dymova MA, Taskaev SY, Richter VA, Kuligina EV. Boron neutron capture therapy: current status and future perspectives. Cancer Commun (Lond) 2020;40:406–421. doi: 10.1002/cac2.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. https://www.mhlw.go.jp/content/12404000/000629561.pdf.
- 5.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M, Taphoorn MJB, von Deimling A, Westphal M, Soffietti R, Reifenberger G, Wick W. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. 2020;70:299–312. doi: 10.3322/caac.21613. [DOI] [PubMed] [Google Scholar]
- 8.Minniti G, Niyazi M, Alongi F, Navarria P, Belka C. Current status and recent advances in reirradiation of glioblastoma. Radiat Oncol. 2021;16:36. doi: 10.1186/s13014-021-01767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altinoz MA, Topcu G, Elmaci İ. Boron’s neurophysiological effects and tumoricidal activity on glioblastoma cells with implications for clinical treatment. Int J Neurosci. 2019;129:963–977. doi: 10.1080/00207454.2019.1595618. [DOI] [PubMed] [Google Scholar]
- 10.Slatkin DN. A history of boron neutron capture therapy of brain tumours. Postulation of a brain radiation dose tolerance limit. Brain. 1991;114:1609–1629. doi: 10.1093/brain/114.4.1609. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa Y, Hatanaka H. Boron neutron capture therapy. Clinical brain tumor studies. J Neurooncol. 1997;33:105–115. doi: 10.1023/a:1005781517624. [DOI] [PubMed] [Google Scholar]
- 12.Coderre JA, Turcotte JC, Riley KJ, Binns PJ, Harling OK, Kiger WS 3rd. Boron neutron capture therapy: cellular targeting of high linear energy transfer radiation. Technol Cancer Res Treat. 2003;2:355–375. doi: 10.1177/153303460300200502. [DOI] [PubMed] [Google Scholar]
- 13.Vos MJ, Turowski B, Zanella FE, Paquis P, Siefert A, Hideghéty K, Haselsberger K, Grochulla F, Postma TJ, Wittig A, Heimans JJ, Slotman BJ, Vandertop WP, Sauerwein W. Radiologic findings in patients treated with boron neutron capture therapy for glioblastoma multiforme within EORTC trial 11961. Int J Radiat Oncol Biol Phys. 2005;61:392–399. doi: 10.1016/j.ijrobp.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Joensuu H, Kankaanranta L, Seppälä T, Auterinen I, Kallio M, Kulvik M, Laakso J, Vähätalo J, Kortesniemi M, Kotiluoto P, Serén T, Karila J, Brander A, Järviluoma E, Ryynänen P, Paetau A, Ruokonen I, Minn H, Tenhunen M, Jääskeläinen J, Färkkilä M, Savolainen S. Boron neutron capture therapy of brain tumors: clinical trials at the Finnish facility using boronophenylalanine. J Neurooncol. 2003;62:123–134. doi: 10.1007/BF02699939. [DOI] [PubMed] [Google Scholar]
- 15.Barth RF, Vicente MG, Harling OK, Kiger WS 3rd, Riley KJ, Binns PJ, Wagner FM, Suzuki M, Aihara T, Kato I, Kawabata S. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capala J, Stenstam BH, Sköld K, Munck af Rosenschöld P, Giusti V, Persson C, Wallin E, Brun A, Franzen L, Carlsson J, Salford L, Ceberg C, Persson B, Pellettieri L, Henriksson R. Boron neutron capture therapy for glioblastoma multiforme: clinical studies in Sweden. J Neurooncol. 2003;62:135–144. doi: 10.1007/BF02699940. [DOI] [PubMed] [Google Scholar]
- 17.Verbakel WF, Sauerwein W, Hideghety K, Stecher-Rasmussen F. Boron concentrations in brain during boron neutron capture therapy: in vivo measurements from the phase I trial EORTC 11961 using a gamma-ray telescope. Int J Radiat Oncol Biol Phys. 2003;55:743–756. doi: 10.1016/s0360-3016(02)04392-4. [DOI] [PubMed] [Google Scholar]
- 18.Hideghéty K, Sauerwein W, Haselsberger K, Grochulla F, Fankhauser H, Moss R, Huiskamp R, Gabel D, de Vries M. Postoperative treatment of glioblastoma with BNCT at the petten irradiation facility (EORTC Protocol 11961) Strahlenther Onkol. 1999;175(Suppl 2):111–114. doi: 10.1007/BF03038907. [DOI] [PubMed] [Google Scholar]
- 19.Hideghéty K, Sauerwein W, Wittig A, Götz C, Paquis P, Grochulla F, Haselsberger K, Wolbers J, Moss R, Huiskamp R, Fankhauser H, de Vries M, Gabel D. Tissue uptake of BSH in patients with glioblastoma in the EORTC 11961 phase I BNCT trial. J Neurooncol. 2003;62:145–156. doi: 10.1007/BF02699941. [DOI] [PubMed] [Google Scholar]
- 20.Kiger WS 3rd, Palmer MR, Riley KJ, Zamenhof RG, Busse PM. A pharmacokinetic model for the concentration of 10B in blood after boronophenylalanine-fructose administration in humans. Radiat Res. 2001;155:611–618. doi: 10.1667/0033-7587(2001)155[0611:apmftc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Cruickshank GS, Ngoga D, Detta A, Green S, James ND, Wojnecki C, Doran J, Hardie J, Chester M, Graham N, Ghani Z, Halbert G, Elliot M, Ford S, Braithwaite R, Sheehan TM, Vickerman J, Lockyer N, Steinfeldt H, Croswell G, Chopra A, Sugar R, Boddy A. A cancer research UK pharmacokinetic study of BPA-mannitol in patients with high grade glioma to optimise uptake parameters for clinical trials of BNCT. Appl Radiat Isot. 2009;67(Suppl):S31–S33. doi: 10.1016/j.apradiso.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 22. https://rctportal.niph.go.jp/s/detail/um?trial_id=UMIN000002385.
- 23. https://rctportal.niph.go.jp/s/detail/um?trial_id=UMIN000013419.
- 24. https://jrct.niph.go.jp/latest-detail/jRCTs051180218.
- 25.Yamamoto T, Nakai K, Kageji T, Kumada H, Endo K, Matsuda M, Shibata Y, Matsumura A. Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother Oncol. 2009;91:80–84. doi: 10.1016/j.radonc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Kageji T, Mizobuchi Y, Nagahiro S, Nakagawa Y, Kumada H. Long-survivors of glioblatoma treated with boron neutron capture therapy (BNCT) Appl Radiat Isot. 2011;69:1800–1802. doi: 10.1016/j.apradiso.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Matsumura A, Nakai K, Shibata Y, Endo K, Sakurai F, Kishi T, Kumada H, Yamamoto K, Torii Y. Current clinical results of the Tsukuba BNCT trial. Appl Radiat Isot. 2004;61:1089–1093. doi: 10.1016/j.apradiso.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Miyatake S, Kawabata S, Yokoyama K, Kuroiwa T, Michiue H, Sakurai Y, Kumada H, Suzuki M, Maruhashi A, Kirihata M, Ono K. Survival benefit of boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol. 2009;91:199–206. doi: 10.1007/s11060-008-9699-x. [DOI] [PubMed] [Google Scholar]
- 29.Kawabata S, Miyatake S, Hiramatsu R, Hirota Y, Miyata S, Takekita Y, Kuroiwa T, Kirihata M, Sakurai Y, Maruhashi A, Ono K. Phase II clinical study of boron neutron capture therapy combined with X-ray radiotherapy/temozolomide in patients with newly diagnosed glioblastoma multiforme-study design and current status report. Appl Radiat Isot. 2011;69:1796–1799. doi: 10.1016/j.apradiso.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 30. https://clinicaltrials.gov/study/NCT05737212.
- 31.Miyatake SI, Wanibuchi M, Hu N, Ono K. Boron neutron capture therapy for malignant brain tumors. J Neurooncol. 2020;149:1–11. doi: 10.1007/s11060-020-03586-6. [DOI] [PubMed] [Google Scholar]
- 32.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J. Clin. Oncol. 2007;25:2601–6. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyatake S, Kawabata S, Nonoguchi N, Yokoyama K, Kuroiwa T, Matsui H, Ono K. Pseudoprogression in boron neutron capture therapy for malignant gliomas and meningiomas. Neuro Oncol. 2009;11:430–436. doi: 10.1215/15228517-2008-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YW, Lee YY, Lin CF, Pan PS, Chen JK, Wang CW, Hsu SM, Kuo YC, Lan TL, Hsu SPC, Liang ML, Chen RH, Chang FC, Wu CC, Lin SC, Liang HK, Lee JC, Chen SK, Liu HM, Peir JJ, Lin KH, Huang WS, Chen KH, Kang YM, Liou SC, Wang CC, Pai PC, Li CW, Chiek DQS, Wong TT, Chiou SH, Chao Y, Tanaka H, Chou FI, Ono K. Salvage boron neutron capture therapy for malignant brain tumor patients in compliance with emergency and compassionate use: evaluation of 34 cases in Taiwan. Biology (Basel) 2021;10:334. doi: 10.3390/biology10040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyatake S, Furuse M, Kawabata S, Maruyama T, Kumabe T, Kuroiwa T, Ono K. Bevacizumab treatment of symptomatic pseudoprogression after boron neutron capture therapy for recurrent malignant gliomas. Report of 2 cases. Neuro Oncol. 2013;15:650–655. doi: 10.1093/neuonc/not020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuse M, Kawabata S, Wanibuchi M, Shiba H, Takeuchi K, Kondo N, Tanaka H, Sakurai Y, Suzuki M, Ono K, Miyatake SI. Boron neutron capture therapy and add-on bevacizumab in patients with recurrent malignant glioma. Jpn J Clin Oncol. 2022;52:433–440. doi: 10.1093/jjco/hyac004. [DOI] [PubMed] [Google Scholar]
- 37.Apra C, Peyre M, Kalamarides M. Current treatment options for meningioma. Expert Rev Neurother. 2018;18:241–249. doi: 10.1080/14737175.2018.1429920. [DOI] [PubMed] [Google Scholar]
- 38. https://jrct.niph.go.jp/latest-detail/jRCT2051190044.
- 39.Lan TL, Lin CF, Lee YY, Lin KH, Chang FC, Lin SC, Lee JC, Chou FI, Peir JJ, Liu HM, Mu PF, Chen YW. Advances in boron neutron capture therapy (BNCT) for recurrent intracranial meningioma. Int J Mol Sci. 2023;24:4978. doi: 10.3390/ijms24054978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. https://www.chictr.org.cn/showproj.html?proj=181032.
- 41.Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Eisele DW, Fenton M, Foote RL, Galloway T, Gillison ML, Haddad RI, Hicks WL, Hitchcock YJ, Jimeno A, Leizman D, Maghami E, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rocco JW, Rodriguez CP, Shah JP, Weber RS, Weinstein G, Witek M, Worden F, Yom SS, Zhen W, Burns JL, Darlow SD. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:873–898. doi: 10.6004/jnccn.2020.0031. [DOI] [PubMed] [Google Scholar]
- 42.Verma A, Burtness B. Top advances of the year: head and neck cancer. Cancer. 2023;129:1308–1312. doi: 10.1002/cncr.34654. [DOI] [PubMed] [Google Scholar]
- 43.Bozec A, Culié D, Poissonnet G, Dassonville O. Current role of primary surgical treatment in patients with head and neck squamous cell carcinoma. Curr Opin Oncol. 2019;31:138–145. doi: 10.1097/CCO.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 44.Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021;398:2289–2299. doi: 10.1016/S0140-6736(21)01550-6. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki M, Kato I, Aihara T, Hiratsuka J, Yoshimura K, Niimi M, Kimura Y, Ariyoshi Y, Haginomori S, Sakurai Y, Kinashi Y, Masunaga S, Fukushima M, Ono K, Maruhashi A. Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancer. J Radiat Res. 2014;55:146–153. doi: 10.1093/jrr/rrt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wittig A, Collette L, Appelman K, Bührmann S, Jäckel MC, Jöckel KH, Schmid KW, Ortmann U, Moss R, Sauerwein WAG. EORTC trial 11001: distribution of two 10B-compounds in patients with squamous cell carcinoma of head and neck, a translational research/phase 1 trial. J Cell Mol Med. 2009;13:1653–1665. doi: 10.1111/j.1582-4934.2009.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J, Salli E, Kortesniemi M, Uusi-Simola J, Välimäki P, Mäkitie A, Seppänen M, Minn H, Revitzer H, Kouri M, Kotiluoto P, Seren T, Auterinen I, Savolainen S, Joensuu H. Boron neutron capture therapy in the treatment of locally recurred head-andneck cancer: final analysis of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2012;82:e67–e75. doi: 10.1016/j.ijrobp.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 48.Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J, Salli E, Kortesniemi M, Uusi-Simola J, Mäkitie A, Seppänen M, Minn H, Kotiluoto P, Auterinen I, Savolainen S, Kouri M, Joensuu H. Boron neutron capture therapy in the treatment of locally recurred head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:475–482. doi: 10.1016/j.ijrobp.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 49.Aihara T, Morita N. BNCT for advanced or recurrent head and neck cancer. Neutron Capture Therapy: Principles and Applications. Springer; 2012. pp. 417–424. [Google Scholar]
- 50.Aihara T, Morita N, Kamitani N, Kumada H, Ono K, Hiratsuka J, Harada T. Boron neutron capture therapy for advanced salivary gland carcinoma in head and neck. Int J Clin Oncol. 2014;19:437–444. doi: 10.1007/s10147-013-0580-3. [DOI] [PubMed] [Google Scholar]
- 51.Hirose K, Konno A, Hiratsuka J, Yoshimoto S, Kato T, Ono K, Otsuki N, Hatazawa J, Tanaka H, Takayama K, Wada H, Suzuki M, Sato M, Yamaguchi H, Seto I, Ueki Y, Iketani S, Imai S, Nakamura T, Ono T, Endo H, Azami Y, Kikuchi Y, Murakami M, Takai Y. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): an open-label phase II trial. Radiother Oncol. 2021;155:182–187. doi: 10.1016/j.radonc.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 52. https://jrct.niph.go.jp/latest-detail/jRCTs051180160.
- 53. https://jrct.niph.go.jp/latest-detail/jRCTs031180302.
- 54.Wang LW, Chen YW, Ho CY, Hsueh Liu YW, Chou FI, Liu YH, Liu HM, Peir JJ, Jiang SH, Chang CW, Liu CS, Lin KH, Wang SJ, Chu PY, Lo WL, Kao SY, Yen SH. Fractionated boron neutron capture therapy in locally recurrent head and neck cancer: a prospective phase I/II trial. Int J Radiat Oncol Biol Phys. 2016;95:396–403. doi: 10.1016/j.ijrobp.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 55.Liu YW, Chang CT, Yeh LY, Wang LW, Lin TY. BNCT treatment planning for superficial and deep-seated tumors: experience from clinical trial of recurrent head and neck cancer at THOR. Appl Radiat Isot. 2015;106:121–124. doi: 10.1016/j.apradiso.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Lee JC, Chuang KS, Hsueh Liu YW, Lin TY, Teng YC, Wang LW. A comparison of dose distributions in gross tumor volume between boron neutron capture therapy alone and combined boron neutron capture therapy plus intensity modulation radiation therapy for head and neck cancer. PLoS One. 2019;14:e0210626. doi: 10.1371/journal.pone.0210626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. https://rctportal.niph.go.jp/s/detail/um?trial_id=UMIN000044118.
- 58.Saginala K, Barsouk A, Aluru JS, Rawla P, Barsouk A. Epidemiology of melanoma. Med Sci (Basel) 2021;9:63. doi: 10.3390/medsci9040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berk-Krauss J, Stein JA, Weber J, Polsky D, Geller AC. New systematic therapies and trends in cutaneous melanoma deaths among US whites, 1986-2016. Am J Public Health. 2020;110:731–733. doi: 10.2105/AJPH.2020.305567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20:1366–1379. doi: 10.1080/15384047.2019.1640032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mishima Y, Honda C, Ichihashi M, Obara H, Hiratsuka J, Fukuda H, Karashima H, Kobayashi T, Kanda K, Yoshino K. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10Bcompound. Lancet. 1989;2:388–389. doi: 10.1016/s0140-6736(89)90567-9. [DOI] [PubMed] [Google Scholar]
- 62.Busse P. Clinical follow-up of patients with melanoma of the extremity treated in a phase I boron neutron capture therapy protocol. Advances in Neutron Capture Therapy. 1997:60–64. [Google Scholar]
- 63. https://clinicaltrials.gov/ct2/show/NCT00002781.
- 64. https://clinicaltrials.gov/ct2/show/NCT00059800.
- 65.Hiratsuka J, Kamitani N, Tanaka R, Tokiya R, Yoden E, Sakurai Y, Suzuki M. Long-term outcome of cutaneous melanoma patients treated with boron neutron capture therapy (BNCT) J Radiat Res. 2020;61:945–951. doi: 10.1093/jrr/rraa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.González SJ, Bonomi MR, Santa Cruz GA, Blaumann HR, Calzetta Larrieu OA, Menéndez P, Jiménez Rebagliati R, Longhino J, Feld DB, Dagrosa MA, Argerich C, Castiglia SG, Batistoni DA, Liberman SJ, Roth BM. First BNCT treatment of a skin melanoma in Argentina: dosimetric analysis and clinical outcome. Appl Radiat Isot. 2004;61:1101–1105. doi: 10.1016/j.apradiso.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 67.Menéndez PR, Roth BM, Pereira MD, Casal MR, González SJ, Feld DB, Santa Cruz GA, Kessler J, Longhino J, Blaumann H, Jiménez Rebagliati R, Calzetta Larrieu OA, Fernández C, Nievas SI, Liberman SJ. BNCT for skin melanoma in extremities: updated Argentine clinical results. Appl Radiat Isot. 2009;67(Suppl):S50–S53. doi: 10.1016/j.apradiso.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Liberman SJ, Dagrosa A, Jiménez Rebagliati RA, Bonomi MR, Roth BM, Turjanski L, Castiglia SI, Gonzalez SJ, Menendez PR, Cabrini R, Roberti MJ, Batistoni DA. Biodistribution studies of boronophenylalanine-fructose in melanoma and brain tumor patients in Argentina. Appl Radiat Isot. 2004;61:1095–1100. doi: 10.1016/j.apradiso.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 69.Yong Z, Song Z, Zhou Y, Liu T, Zhang Z, Zhao Y, Chen Y, Jin C, Chen X, Lu J, Han R, Li P, Sun X, Wang G, Shi G, Zhu S. Boron neutron capture therapy for malignant melanoma: first clinical case report in China. Chin J Cancer Res. 2016;28:634–640. doi: 10.21147/j.issn.1000-9604.2016.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. https://rctportal.niph.go.jp/s/detail/um?trial_id=UMIN000013101#.
- 71. https://jrct.niph.go.jp/latest-detail/jRCTs061180066.
- 72. https://jrct.niph.go.jp/latest-detail/jRCT1080224974.
- 73. https://jrct.niph.go.jp/latest-detail/jRCT2031220410.
- 74. https://clinicaltrials.gov/ct2/show/NCT05538676.
- 75.Fujimoto T, Suzuki M, Sudo T, Fujita I, Sakuma T, Sakurai Y, Hirose T, Morishita M, Takata T, Tamari Y, Tanaka H, Andoh T, Kawamoto T, Hara H, Fukase N, Kawakami Y, Shigemoto R, Matsumoto T, Ichikawa H, Ono K, Kuroda R, Akisue T. Boron neutron capture therapy for clear cell sarcoma. Appl Radiat Isot. 2020;166:109324. doi: 10.1016/j.apradiso.2020.109324. [DOI] [PubMed] [Google Scholar]
- 76. https://clinicaltrials.gov/ct2/show/NCT00085059.
- 77.Wittig A, Moss RL, Stecher-Rasmussen F, Appelman K, Rassow J, Roca A, Sauerwein W. Neutron activation of patients following boron neutron capture therapy of brain tumors at the high flux reactor (HFR) Petten (EORTC Trials 11961 and 11011) Strahlenther Onkol. 2005;181:774–82. doi: 10.1007/s00066-005-1433-4. [DOI] [PubMed] [Google Scholar]
- 78. https://clinicaltrials.gov/ct2/show/NCT00039572.
- 79. https://clinicaltrials.gov/ct2/show/NCT00062348.
- 80.Wittig A, Malago M, Collette L, Huiskamp R, Bührmann S, Nievaart V, Kaiser GM, Jöckel KH, Schmid KW, Ortmann U, Sauerwein WA. Uptake of two 10B-compounds in liver metastases of colorectal adenocarcinoma for extracorporeal irradiation with boron neutron capture therapy (EORTC Trial 11001) Int J Cancer. 2008;122:1164–1171. doi: 10.1002/ijc.23224. [DOI] [PubMed] [Google Scholar]
- 81.Suzuki M, Endo K, Satoh H, Sakurai Y, Kumada H, Kimura H, Masunaga S, Kinashi Y, Nagata K, Maruhashi A, Ono K. A novel concept of treatment of diffuse or multiple pleural tumors by boron neutron capture therapy (BNCT) Radiother Oncol. 2008;88:192–195. doi: 10.1016/j.radonc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki M, Sakurai Y, Masunaga S, Kinashi Y, Nagata K, Maruhashi A, Ono K. Feasibility of boron neutron capture therapy (BNCT) for malignant pleural mesothelioma from a viewpoint of dose distribution analysis. Int J Radiat Oncol Biol Phys. 2006;66:1584–1589. doi: 10.1016/j.ijrobp.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki M, Tanaka H, Sakurai Y, Kashino G, Yong L, Masunaga S, Kinashi Y, Mitsumoto T, Yajima S, Tsutsui H, Sato T, Maruhashi A, Ono K. Impact of accelerator-based boron neutron capture therapy (AB-BNCT) on the treatment of multiple liver tumors and malignant pleural mesothelioma. Radiother Oncol. 2009;92:89–95. doi: 10.1016/j.radonc.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki M, Sakurai Y, Hagiwara S, Masunaga S, Kinashi Y, Nagata K, Maruhashi A, Kudo M, Ono K. First attempt of boron neutron capture therapy (BNCT) for hepatocellular carcinoma. Jpn J Clin Oncol. 2007;37:376–381. doi: 10.1093/jjco/hym039. [DOI] [PubMed] [Google Scholar]
- 85.Yanagie H, Higashi S, Seguchi K, Ikushima I, Fujihara M, Nonaka Y, Oyama K, Maruyama S, Hatae R, Suzuki M, Masunaga S, Kinashi T, Sakurai Y, Tanaka H, Kondo N, Narabayashi M, Kajiyama T, Maruhashi A, Ono K, Nakajima J, Ono M, Takahashi H, Eriguchi M. Pilot clinical study of boron neutron capture therapy for recurrent hepatic cancer involving the intraarterial injection of a 10BSH-containing WOW emulsion. Appl Radiat Isot. 2014;88:32–37. doi: 10.1016/j.apradiso.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 86.Hiratsuka J, Kamitani N, Tanaka R, Yoden E, Tokiya R, Suzuki M, Barth RF, Ono K. Boron neutron capture therapy for vulvar melanoma and genital extramammary Paget’s disease with curative responses. Cancer Commun (Lond) 2018;38:38. doi: 10.1186/s40880-018-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Makino E, Sasaoka S, Aihara T, Sakurai Y, Maruhashi A, Ono K, Fujimoto W, Hiratsuka J. 1013 the first clinical trial of boron neutron capture therapy using 10B-para-boronophenylalanine for treating extramammary Paget’s disease. European Journal of Cancer. 2012;48:S244–S245. [Google Scholar]
- 88.Futamura G, Kawabata S, Siba H, Kuroiwa T, Suzuki M, Kondo N, Ono K, Sakurai Y, Tanaka M, Todo T. A case of radiation-induced osteosarcoma treated effectively by boron neutron capture therapy. Radiat Oncol. 2014;9:237. doi: 10.1186/s13014-014-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inoue M, Lee CM, Ono K, Suzuki M, Tokunaga T, Sawa Y, Okumura M. Clinical effectiveness of boron neutron capture therapy for a recurrent malignant peripheral nerve sheath tumor in the mediastinum. J Thorac Oncol. 2010;5:2037–2038. doi: 10.1097/JTO.0b013e3181f1cd86. [DOI] [PubMed] [Google Scholar]
- 90. https://jrct.niph.go.jp/latest-detail/jRCTs051180217.
- 91. https://jrct.niph.go.jp/latest-detail/jRCTs051180219.
- 92. https://jrct.niph.go.jp/latest-detail/jRCTs031220371.


