Abstract
Aims
The aim of our project was to identify proteins associated with the extent of spinal cord injury (SCI) and subsequent long-term neurological recovery.
Methods
Through proteomic analysis, we identified proteins that are differentially expressed specifically in the acute phase of injury. We analyzed the concentrations of differentially expressed proteins in serum and the injured spinal cord segment by ELISA.
Results
Serpina3n protein expression in the injured spinal cord segment was increased 101-fold at 12 h after severe SCI and 89-fold at 12 h after mild SCI, as determined by LC‒MS/MS. In the mild and severe SCI groups, serum Serpina3n levels began to increase at 12 h and peaked at 24 h. At 12 h, 24 h and 3 d after injury, serum Serpina3n protein levels were significantly correlated with the severity of injury (12 h: r = 0.6034, P = 0.008; 24 h: r = 0.7542, P = 0.0003; 3 d: r = 0.862, P < 0.001). Serum Serpina3n levels at 2 h, 24 h and 3 d post injury were significantly correlated with long-term neurological recovery at 28 d after SCI (2 h: r = −0.5781, P = 0.012; 24 h: r = −0.5912, P = 0.0098; 3 d: r = −0.7792, P < 0.0001). Methylprednisolone treatment would decrease the serum Serpina3n levels in mice with mild and severe SCI compared with those in placebo-group mice at 12 h and 24 h after SCI. The serum Serpina3n concentration in the severe SCI group was significantly reduced on the third day after steroid treatment.
Conclusion
Taken together, these data suggest that serpina3n may be a circulating biomarker of acute SCI and may be closely associated with injury severity and long-term motor function recovery.
Keywords: Spinal cord injury, Serum Serpina3n, Recovery of motor function, Degree of damage
1. Introduction
Spinal cord injury (SCI) is one of the common causes of disability in humans, and its incidence increases significantly every year [1]. SCI is a type of severe injury with a high disability rate that seriously threatens human life and health and has a lasting impact on the health and quality of life of individuals [2]. Due to the complexity of the mechanism underlying SCI, it is difficult to quantitatively evaluate the degree of nerve injury in clinical practice, and this challenge hinders SCI research. At present, clinical assessment of SCI severity and prediction of long-term neurological function recovery are mainly based on functional examination according to the International Standard for Neurological Classification of Spinal Cord Injury (ISNCSCI) and the American Spinal Injury Association (ASIA) scale (AIS) [3]. However, in clinical settings, these assessments largely rely on doctors to make judgments on the basis of their clinical experience and are highly subjective and unreliable; therefore, their clinical application has certain limitations [4]. There is an urgent need to find other auxiliary indicators to accurately evaluate the degree of injury and clinical prognosis of patients with SCI.
In recent years, with the progress of molecular biology and genomics techniques, many studies have found that the levels of some structural proteins and inflammatory factors in cerebrospinal fluid and serum can reflect the degree of SCI [[5], [6], [7]]. In acute SCI models, Tau, S-100β, and NSE protein levels were found to be significantly increased [5,[8], [9], [10]]. The preliminary conclusion that serum NSE, serum albumin, and S-100β levels were correlated with the degree of spinal cord injury remains to be confirmed with a large sample size [[11], [12], [13], [14]]. The serum level of the astrocyte marker GFAP is associated with neurological dysfunction and spinal cord edema after traumatic SCI and can be used as a diagnostic biomarker for the occurrence and severity of SCI in the acute injury phase but has limited reliability for clinical use [15]. None of these proteins have been tested and validated as biomarkers in larger cohort studies [[16], [17], [18]].
Serpina3n, the rodent homolog of human SERPINA3 (human α-1 antichymotrypsin, α1-ACT) [5], belongs to clade A of the serine protease inhibitor (serpin) superfamily. Previous studies on Serpina3n have suggested that it is upregulated under pathological conditions such as endotoxin-induced inflammation [6], ischemia‒reperfusion injury [7], and diabetes mellitus [19]. Endotoxin (LPS) and inflammatory factors such as IL-6 [20], TNF-α [21] and IL-1β [6] can induce the expression of Serpina3n in vitro and in vivo. In recent years, Serpina3n has been regarded as a marker of reactive astrocytes and neuro inflammation [[22], [23], [24], [25]]. Reactive astrocytes play an important role in SCI pathogenesis. Large number of astrocytes is activated after SCI, and some of them proliferate rapidly to form the astrocytic scar boundary and participate in glial scar formation [26]. However, whether serum Serpina3n levels can reflect the severity of SCI and predict long-term neurological recovery has not been elucidated.
In the current study, by using Liquid Chromatography–Tandem Mass Spectrometry (LC‒MS/MS), we found that Serpina3n protein expression in the injured spinal cord segment was increased 101-fold in the severe SCI group and up to 89-fold in the mild SCI group compared with the WT group at 12 h after injury. In addition, we investigated the correlation between serum Serpina3n protein levels and the degrees of spinal cord injury and neurological function recovery and assessed the potential of serum Serpina3n as a biomarker of acute SCI. Furthermore, methylprednisolone treatment would promote neurological recovery and reduce the serum concentration of serpina3n at early time points, indicating Serpina3n might be a therapeutic target of SCI.
2. Material and methods
2.1. Animals
A total of 180 female C57BL/6 mice aged 10–12 weeks (weight: 19–22 g) [27], which were obtained from Shanghai JieSiJie Laboratory Animals Co., LTD were used in this study. The animal use protocols were approved by the Animal Care and Use Department of Minhang Hospital Affiliated with Fudan University. All animal experiments were conducted according to the guidelines of the US National Institutes of Health “Guidelines for the Use of Laboratory Animals” and Institutions of Minhang Hospital Affiliated with Fudan University.
2.2. Surgical operation
The animals were anesthetized by intraperitoneally injection with 1% pentobarbital. The lower thoracic spine was exposed at the T10/T11 level, and the lamina was removed. The MASCIS Impactor, NYU Impactor II [28] (W.M. Keck Center for Collaborative Neuroscience, Rutgers the State University of New Jersey, America) was set to one of two forces (mild SCI group: 5 g × 10 mm; severe SCI group: 5 g × 40 mm) to generate a mild or severe SCI mouse model [13]. After surgery, the mice were allowed to recover on warm blankets. One day after the operation, artificial assisted urination was performed until voluntary urination function returned.
2.3. Mouse function assessment
Two researchers blinded to the experimental groups assessed hind limb function using the Basso Mouse Scale (BMS) [29]. The scores definition for BMS was as follows: 0: No ankle movement; 1: Slight ankle movement; 2: Extensive ankle movement; 3: Plantar placing of the paw with or without weight support -OR- Occasional, frequent or consistent dorsal stepping but no plantar stepping; 4: Occasional plantar stepping; 5: Frequent or consistent plantar stepping, no coordination -OR- Frequent or consistent plantar stepping, some coordination, paws rotated at initial contact and lift off (R/R); 6: Frequent or consistent plantar stepping, some coordination, paws parallel at initial contact (P/R, P/P) -OR- Frequent or consistent plantar stepping, mostly coordinated, paws rotated at initial contact and lift off (R/R); 7: Frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and rotated at lift off (P/R)-OR- Frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and lift off (P/P), and severe trunk instability; 8: Frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and lift off (P/P), and mild trunk instability -OR- Frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and lift off (P/P), and normal trunk stability and tail down or up & down; 9: Frequent or consistent plantar stepping, mostly coordinated, paws parallel at initial contact and lift off (P/P), and normal trunk stability and tail always up. The BMS score of the lower limbs was determined by monitoring the mice for 4 min. Animals with a difference between the hind limbs of more than 2 points were excluded from the experimental analysis. Motor function was assessed 24 h after injury and weekly for 4 weeks.
2.4. Sample acquisition and processing
After treatment, the mice were sacrificed by intraperitoneally injection with excess pentobarbital sodium. Then the blood, spinal cord tissues, muscle, kidneys and spleens from all groups of mice were collected. Blood samples were collected from the mice in sterile tubes, and the blood was coagulated at room temperature for 10–20 min and centrifuged at 2–8 °C for approximately 20 min (2000–3000 rpm). The supernatant was carefully collected, and if precipitation occurred during the storage process, the serum was centrifuged again and separated for ELISA. The tissue specimens were cut into 1 g pieces and weighed, 9 ml of PBS (pH 7.2–7.4) was added, and the specimens were fully homogenized by hand or with a homogenizer. The samples were centrifuged for about 20 min (2000–3000 rpm), and the supernatant was carefully collected. One aliquot was used for analysis, and the rest of each sample was stored in a refrigerator for further research.
2.5. LC‒MS analysis
The proteins extracted from spinal cord tissues of mice in the control group or injured with mild or severe degrees of SCI for12 h were analyzed by LC‒MS/MS (n = 3 per group). In this study, LC‒MS/MS was used for quantitative label-free proteomic analysis of three groups of mouse cells [30]. For LC‒MS/MS analysis, peptides were separated by a 90 min gradient elution at a flow rate of 0.22 μl/min with a Thermo Scientific EASY-nLC 1000 HPLC system, which was directly connected to a Thermo Scientific Q Exactive mass spectrometer. The analytical column was a Thermo Scientific Acclaim PepMap RSLC column (50 μm ID, 15 cm length, C18, 2 μm, 100 Å). The precolumn was a Thermo Scientific Acclaim PepMap100 column (100 μm ID, 2 cm length, C18, 5 μm, 100 Å). Mobile phase A consisted of 0.1% formic acid, and mobile phase B consisted of acetonitrile with 0.1% formic acid. The Q Exactive mass spectrometer was operated in data-dependent acquisition mode using Xcalibur 2.2 SP1 software, and Orbitrap-based single full-scan mass spectrometry was performed (300–2000 m/z, 70,000 resolution) followed by 20 data-dependent MS/MS scans at 27% normalized collision energy (HCD). Protein identification required at least one unique or razor peptide per protein group. After extracting total proteolysis combined with high-resolution mass spectrometry and extracting tissue total protein, the proteome database was retrieved from UniProt, Mus musculus (Mouse) proteome, 54,189 proteins. And database retrieval and relative quantification were conducted using the label-free quantification (LFQ) algorithm of Maxquant software (Version 1.6.0.1) and Protein Discoverer (edition 1.4) software [31,32]. Briefly, MaxLFQ is an intensity determination and normalization procedure that is fully compatible with any separated peptide or protein before LC‒MS analysis. Given that the presence of quantifiable peptides varies from sample to sample, the algorithm uses only common peptides for pairwise ratio determination for each protein and calculates a median ratio to protect against outliers. It then determines all pairwise protein ratios and requires a minimal number of two peptide ratios for a given protein ratio to be considered valid. A least-squares analysis is then performed to reconstruct the abundance profile before rescaling the whole profile to the cumulative intensity across samples. This step preserves the total summed intensity for a protein over all samples. This procedure is repeated for all proteins, resulting in an accurate abundance profile for each protein across the samples. Perseus software was used to process the quantitative data and for biostatistics analysis.
2.6. Enzyme-linked immunosorbent assay (ELISA)
Serpina3n levels were measured using a commercially available ELISA kit (Jiangsu Meimian Industrial Co., Ltd., Cat# MM-45904 M, detection limit: 3 pg/ml-1800 pg/ml). Frozen serum samples and the protein supernatant of muscle, kidney, liver, spleen, and spinal cord were thawed and then diluted 1/5 with dilution buffer. Then the samples were loaded into ELISA plates. Testing was carried out according to the manufacturer's protocol.
2.7. Methylprednisolone administration to acute SCI model mice
A total of 24 mice were used in this study. The mice were randomly divided into 4 groups (n = 10 per group): the mild SCI + normal saline group, the mild SCI + methylprednisolone group, the severe SCI + normal saline group, and the severe SCI + methylprednisolone group; the normal saline groups were intravenously injected with normal saline (placebo) immediately after SCI, while the methylprednisolone groups were intravenously injected with methylprednisolone immediately after SCI. Methylprednisolone was administered at 30 mg/kg via the tail vein (Pfizer, USA) [33]. At days 1, 3, 7, 14, 21 and 28 after injury, the functional recovery of 5 mice from each group was measured using the BMS. Serum was obtained from mice at 12 h, 24 h, 3 d and 7 d after surgery.
2.8. Statistical analysis
Statistical analyses of the differences between different groups were performed using Two-way repeated-measures ANOVA with Bonferroni's post hoc test. The Spearman rank correlation coefficient was used to evaluate the relationship between serum Serpina3n levels and hind limb function scores. Statistical analyses were performed using GraphPad Prism (version 7). The error bars indicated the standard errors, and p values < 0.05 were considered statistically significant.
3. Results
3.1. Quantitative analysis of protein levels in the injured spinal cord segment of mice with different degrees of SCI by LC‒MS/MS
We established three experimental groups, namely, the control group, mild SCI group and severe SCI group, and there were 3 mice in each group. The injured segment of the spinal cord was harvested 12 h after injury, and differentially expressed proteins were identified by LC‒MS/MS, including Serpina3n, Ngp, Itih4, S100a9, Chil3, S100a8, and Ttr between mild SCI and control groups, and Serpina3n, Mylpf, S100a8, Ttr, Chil3, Ngp, and S100a9 between severe SCI and control groups. Interestingly, Serpina3n was the most significantly upregulated proteins in the injured spinal cord of mice with mild and severe SCI. Serpina3n protein expression in the injured spinal cord segment was increased in the mild SCI group (89.02-fold, p = 0.0003) (Table 1) and the severe SCI group (101.3-fold, p = 0.0002) (Table 2) compared with the control group. Later, we used ELISA to analyze the expression of Serpina3n in the injured spinal cord segment, and the concentration of Serpina3n protein was higher in the severe SCI group, confirming the results of LC‒MS/MS.
Table 1.
Differentially expressed proteins between mild SCI group and control group identified by nano-LC-MS/MS analysis.
| UniProt ID | Protein name | Gene name | Fold change | p-value |
|---|---|---|---|---|
| G3X8T9 | Serine protease inhibitor A3N | Serpina3n | 89.02 | 0.0003 |
| O08692 | Neutrophilic granule protein (NGP) | Ngp | 85.60 | 0.0027 |
|
A0A2K6 EDJ7 |
Inter alpha-trypsin inhibitor, heavy chain 4 | Itih4 | 74.92 | 0.0004 |
| P31725 | (S100 calcium-binding protein A9 | S100a9 | 61.64 | 0.0000 |
| O35744 | Chitinase-3-like protein 3 | Chil3 | 49.68 | 0.0000 |
| P27005 | S100 calcium-binding protein A8 | S100a8 | 47.32 | 0.0266 |
| P07309 | Transthyretin | Ttr | 40.91 | 0.0001 |
The statistical used was Mann-Whitney U Test.
Table 2.
Differentially expressed proteins between severe SCI group and control group identified by nano-LC-MS/MS analysis.
| UniProt ID | Protein name | Gene name | Fold change | p-value |
|---|---|---|---|---|
| G3X8T9 | Serine protease inhibitor A3N | Serpina3n | 101.30 | 0.0002 |
| P97457 | Myosin regulatory light chain 2, skeletal muscle isoform | Mylpf | 83.47 | 0.0000 |
| P27005 | S100 calcium-binding protein A8 | S100a8 | 81.79 | 0.0000 |
| P07309 | Transthyretin | Ttr | 61.70 | 0.0000 |
| O35744 | Chitinase-like protein 3 | Chil3 | 61.52 | 0.0000 |
| O08692 | Neutrophilic granule protein (NGP) | Ngp | 61.02 | 0.0000 |
| P31725 | S100 calcium-binding protein A9 | S100a9 | 59.81 | 0.0000 |
The statistical used was Mann-Whitney U Test.
3.2. Confirmation of Serpina3n expression after SCI
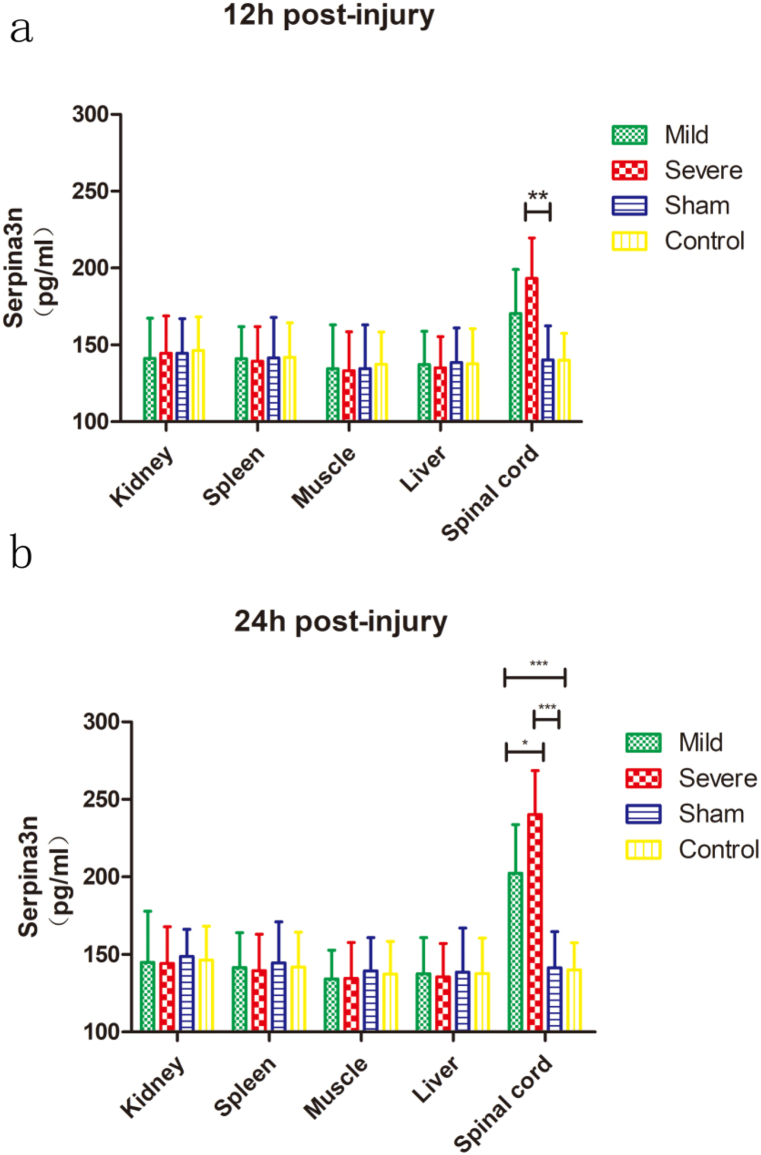
In mice, Serpina3n is mainly expressed in the muscle, kidney, liver, spleen and spinal cord. To further analyze the source of serum Serpina3n after SCI, we analyzed the content of Serpina3n in the abovementioned organs at 12 and 24 h after SCI. The results showed that at 12 h after injury, the levels of Serpina3n in the injured spinal cord segment in the mild SCI group and severe SCI group were significantly higher than those in the negative control (sham) group. There was no significant difference between the mild SCI group and the negative control group, but there was a significant difference between the severe SCI group and the negative control group (P < 0.001) (Fig. 1a). There was no difference in Serpina3n expression in other tissues or organs between groups (Fig. 1a). At 24 h after injury, both injured groups had higher Serpina3n expression than the negative control group, and the difference was statistically significant (both P < 0.0001) (Fig. 1b). There was also a significant difference between the mild SCI group and the severe SCI group (P < 0.05) (Fig. 1b).
Fig. 1.
Analysis of Serpina3n expression in the muscle, kidney, liver, spleen, and spinal cord after acute SCI.
(a, b) ELISA assay was used to measure the protein expression of Serpina3n in the muscle, kidney, liver, spleen and spinal cord at 12 h (a) or 24 h (b) after injury. Two-way repeated-measures ANOVA with Bonferroni's post hoc test. N = 6 per group. *p < 0.05, **p < 0.01, ***p < 0.001. The data are presented as the mean ± SEM.
3.3. Serum Serpina3n concentrations in mice with different degrees of SCI
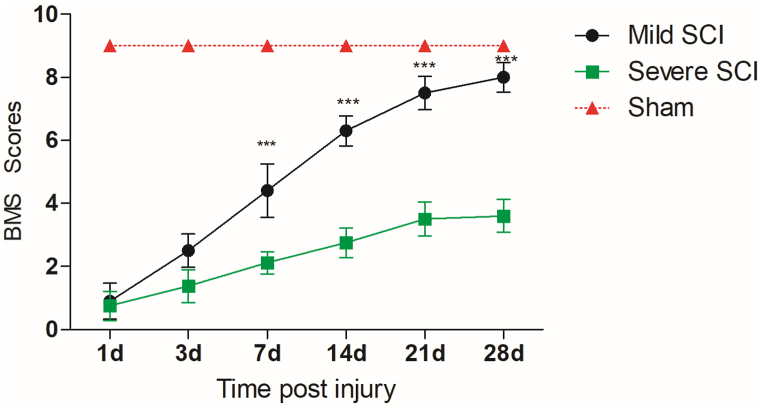
We established two models with different degrees of SCI. The BMS scores of the mild SCI group were significantly higher than those of the severe SCI group from 7 d to 28 d after injury (P < 0.001) (Fig. 2).
Fig. 2.
Long-term motor recovery occurred in the mild and severe SCI groups.
The time course of functional recovery based on BMS scores in the mild and severe SCI groups is shown (n = 10 per group). Two-way repeated-measures ANOVA with Bonferroni's post hoc test. The data are presented as the means ± SEMs. Mild SCI vs. severe SCI: *p < 0.05, **p < 0.01, ***p < 0.001.
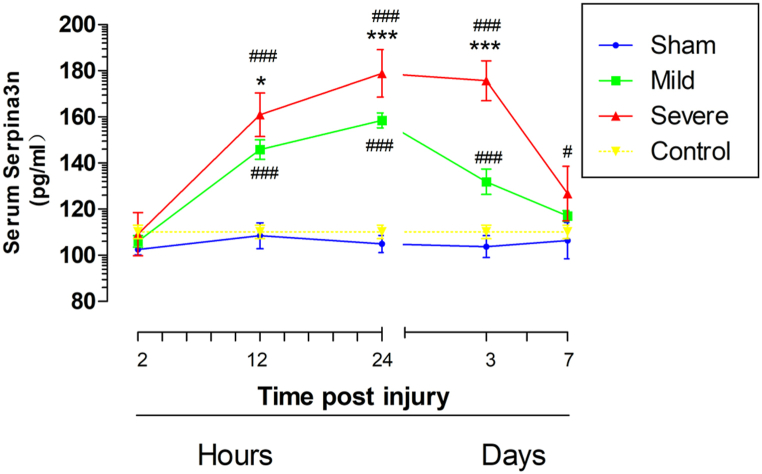
After acute SCI, Serpina3n expression increased significantly in the injured spinal cord segment, and the Serpina3n concentration in the injured segment was positively correlated with the injury degree. We therefore analyzed serum Serpina3n concentrations after acute SCI. The results revealed that at 12 h after SCI, Serpina3n levels were significantly increased in both the mild SCI group and severe SCI group (mild SCI: 145.85 ± 13.4; severe SCI: 160.91 ± 9.43, P < 0.05) compared with the control group (Fig. 3). At 24 h after injury, the Serpina3n level peaked in both groups; the Serpina3n level in the severe SCI group was 178.87 ± 10.29 pg/ml, while that in the mild SCI group was 158.54 ± 10.18 pg/ml, and there was a significant difference between the two groups (P < 0.01) (Fig. 3). The serum Serpina3n level in the severe SCI group continued to increase until 7 days after injury, at which point it was significantly higher than that in the control group. The serum Serpina3n level in the mild SCI group continued to rise until 3 days after injury, at which point there was a significant difference compared between the mild SCI group and the control and severe SCI groups (mild SCI: 131.85 ± 17.44; severe SCI: 175.69 ± 8.64, P < 0.01) (Fig. 3). Although the serum Serpina3n level of the mild SCI group was higher than that of the control group on 7 days after injury, the difference was not significant (Fig. 3).
Fig. 3.
Serum serpina3n protein levels at different time points after SCI. The serum Serpina3n level increased over the period from 12 h to 7 d in the mild SCI group; the severe SCI group exhibited higher serum serpina3n levels than the mild SCI group from 12 h to 7 d (n = 10 per group). The data are presented as the means ± SEMs. Two-way repeated-measures ANOVA with Bonferroni's post hoc test. Mild SCI vs. severe SCI: *p < 0.05, **p < 0.01, ***p < 0.001; mild SCI vs. sham or severe SCI vs. sham: #p < 0.05, ##p < 0.01, ###p < 0.001.
4. Correlation of serum Serpina3n levels at 12 h, 24 h and 3 d after SCI with the degree of injury and neurological function recovery
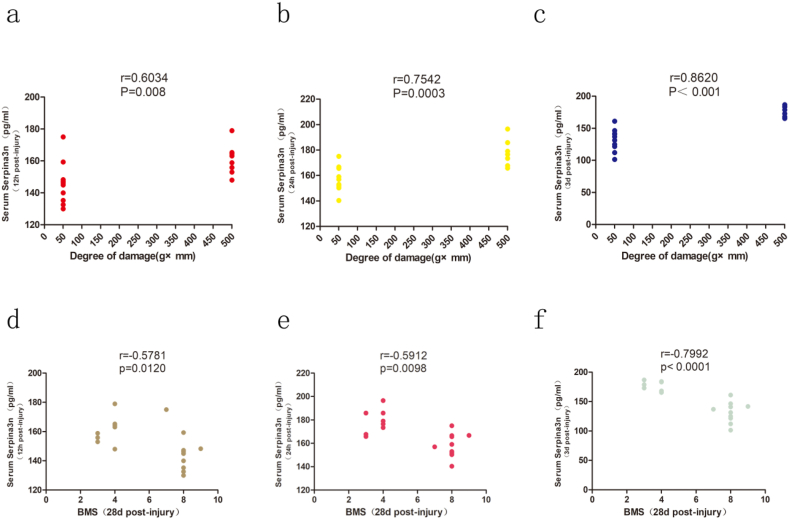
Serum Serpina3n expression was significantly affected by the degree of injury. Therefore, we analyzed the correlation between the serum Serpina3n level and the degree of injury at 12 h, 24 h and 3 d after injury. The results showed that the serum Serpina3n concentration at 12 h after injury was positively correlated with the severity of injury (r = 0.6034, P = 0.008) (Fig. 4a). The serum Serpina3n concentration at 24 h after injury correlated with the degree of injury (r = 0.7542, P = 0.0003) (Fig. 4b). There was a significant correlation between the serum Serpina3n concentration on 3 days after injury and the degree of injury (r = 0.862, P < 0.001) (Fig. 4c). The above study found a positive correlation between the serum serpina3n concentration and the degree of injury. We further analyzed the correlation between the serum serpina3n concentration and long-term recovery of motor function. The results showed that the Serpina3n concentration at 12 h after injury was negatively correlated with the BMS score at 28 days after injury (r = −0.5781, P = 0.012) (Fig. 4d). The serum Serpina3n concentration at 24 h after injury was correlated with the BMS score at 28 days after injury (r = −0.5912, P = 0.0098) (Fig. 4e). The serum Serpina3n concentration on day 3 after injury was significantly correlated with the BMS score on day 28 after injury (r = −0.7792, P < 0.0001) (Fig. 4f).
Fig. 4.
Correlation of serum Serpina3n levels with injury severity and motor function scores 28 days after injury.
(a–c) The Spearman rank correlation analyses between the serum Serpina3n protein level at 12 h (a), 24 h (b), and 3 days (c) post injury and injury degree. N = 10 per group.
(d–f) The Spearman rank correlation analyses of the serum Serpina3n protein level at 12 h (d), 24 h (e), and 3 days (f) after injury and BMS score at 28 d after injury. N = 10 per group.
4.1. The serum Serpina3n level can reflect the efficacy of glucocorticoids in the treatment of acute SCI
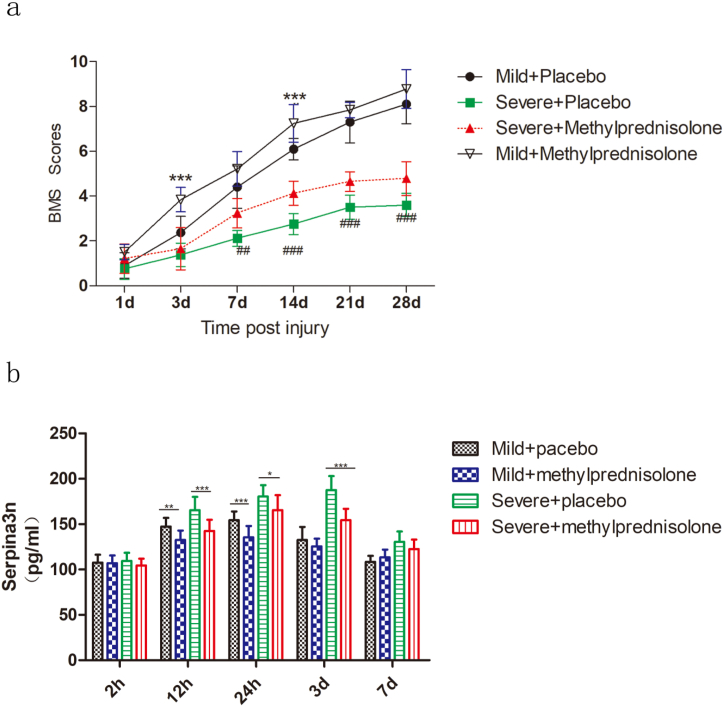
To further analyze whether the Serpina3n level could reflect the efficacy of drugs in the treatment of SCI, we treated mice with acute SCI with methylprednisolone. Previous studies have shown that methylprednisolone could alleviate inflammatory responses after spinal cord injury, protect neuronal cells, and improve long-term motor function recovery [[34], [35], [36]]. In the present study, it was found that after methylprednisolone treatment the BMS scores of mice in the mild SCI group were significantly higher than those of mice in the control group at 3 days and 14 days after injury (Fig. 5a). Although the BMS scores were also higher in the mild SCI group than those in the control group at 28 days after injury, the difference was not statistically significant. The BMS scores of the severe SCI group were significantly higher than those of the control group from 7 days to 28 days after methylprednisolone treatment, indicating better motor function recovery occurred (Fig. 5a). Further analysis of the serum Serpina3n concentration at early time points after treatment showed that the serum serpina3n concentration in methylprednisolone-treated mice in the mild and severe SCI groups was lower than placebo-treated mice in the SCI groups at 12 h and 24 h after injury, and the difference was statistically significant. The serum Serpina3n concentration was significantly lower in the severe SCI group on the third day after steroid treatment, and the difference was statistically significant. These results suggest that the treatment of acute SCI with methylprednisolone may help to promote neurological recovery and that the serum serpina3n concentration at early time points may reflect the therapeutic efficacy of methylprednisolone (Fig. 5b).
Fig. 5.
The serum serpina3n level can reflect the efficacy of methylprednisolone.
(a) BMS scores reflecting motor function after methylprednisolone and placebo treatment in mild and severe SCI model mice. N = 10 per group. Two-way repeated-measures ANOVA with Bonferroni's post hoc test. ***p < 0.001 (mild SCI + placebo vs. mild SCI + methylprednisolone); ##p < 0.01 and ###p < 0.001 (severe SCI + placebo vs. severe SCI + methylprednisolone).
(b) Measurement of serum serpina3n concentrations after methylprednisolone and placebo treatment at 2 h, 12 h, 24 h, 3 d and 7 d after injury. N = 10 per group. Two-way repeated-measures ANOVA with Bonferroni's post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001.
5. Discussion
In clinical practice, patients with SCI show significant heterogeneity in functional recovery; specifically, patients with injuries at similar spinal cord levels and injuries of similar severities may show significant differences in functional recovery [37,38]. Currently, the main clinical measure for assessing the severity of SCI and the prognosis of patients with acute SCI is AIS [39]. However, the AIS has some limitations in clinical practice [40]; these limitations mainly include the fact that ①scoring is relatively time-consuming; ②coma, sedation, intubation, or other injuries (including potential traumatic brain injury) may affect the ability of patients to under evaluation with the AIS; and ③ in the early stage after SCI, the application of the AIS is limited by spinal shock. Our study showed that serum Serpina3n concentrations at 12 h, 24 h and 3 d after injury were positively correlated with the degrees of SCI but negatively correlated with long-term motor function recovery. The correlations with the serum Serpina3n concentration on the third day after injury were more significant. Furthermore, methylprednisolone treatment would improve neurological recovery and decrease the serum level of serpina3n at early time points, indicating Serpina3n might be a therapeutic target of SCI.
Serum serpina3n is the rodent homolog of human SERPINA3 (human α1-ACT) and belongs to clade A of the serpin superfamily [5]. In relation to the nervous system, serpina3n and its human homolog α1-ACT have been studied mainly in the context of neuropathic pain [41], Alzheimer's disease (AD) [42], and hypothalamic inflammation caused by a high-fat diet [43], and these proteins are generally regarded as reactive astrocyte markers and neuro inflammation markers [[22], [23], [24], [25]]. The serum serpina3n level was shown to be elevated in steroid-dependent muscular dystrophy and type 2 diabetes [19,44]. However, its correlation with central nervous system damage or other tissue damage has not been elucidated.
Previous studies on serum markers of SCI have mainly focused on inflammatory factors (such as IL-1β, TNF-a and IL-6), metalloproteinases (such as MMP-2, MMP-8 and MMP-9), chemokines (such as MCP-1 and CXCL12D), neuronal cell-specific proteins (such as neuron-specific enolase, NSE, etc.), astrocyte-specific proteins (such as S-100 and GFAP), and oligodendrocyte-specific proteins (such as myelin basic protein (MBP)) [[8], [9], [10]]. Although findings suggest that structural proteins and inflammatory markers may be useful in assessing SCI severity and predicting neurological outcomes, the current level of evidence is generally low. Most of the potential biomarkers have only been studied in animals, and clinical studies are relatively lacking [16,45]. S100 calcium binding protein β (S100β) is a calcium-binding protein that is mainly found in astrocytes and Schwann cells and is involved in calcium homeostasis, cell proliferation, and differentiation [46]. In animal models, the S100β protein level was found to be rapidly increased in serum and cerebrospinal fluid at 6 h after injury and to continue to increase until 4 h after injury, after which its concentration in serum returned to normal levels, suggesting the promise of S100β as a diagnostic tool for injury [47]. However, there is some controversy regarding the serum S100β concentration as a biomarker indicator of injury severity. Because S100β is also present in adipocytes, chondrocytes, and melanocytes, its release in these tissues may confound the interpretation of elevated concentrations [34,35]. Our study found that although Serpina3n was mainly expressed in the muscle, liver, spleen, nervous system and testis, Serpina3n expression was only increased in the injured spinal cord segment in the early stage after SCI, and the difference was statistically significant. No significant increase in Serpina3n expression was observed in other tissues. These results suggest that the elevation of the serum serpina3n level was derived from the injured segment of the spinal cord. Our further study suggested that methylprednisolone improved long-term motor function after acute SCI, and early changes in the serum serpina3n concentration could reflect the efficacy of the drug.
Despite of Serpina3n, our study also found other proteins which were differentially expressed in the spinal cord of mice with mild or severe SCI, including Ngp, Itih4, Chil3, S100a8, Ttr, Mylpf, and S100a9 between severe SCI and control groups. Interestingly, another LC-MS/MS analysis revealed Itih4, S100a8, and Ttr were significantly up-regulated in cerebrospinal fluid derived from rats 24 h post mild or severe SCI [48]. And S100a9 inhibition was reported to protect spinal cord from inflammation and decrease the infiltration of neutrophil to the lesion [49]. Furthermore, S100a8 deficiency would alleviate the apoptosis and inflammatory response in spinal cord tissues after SCI injury [50]. Our study indicated not only S100a8 and S100a9, but also Ngp, Itih4, Chil3, Ttr, and Mylpf were increased in the spinal cord of mice with mild or severe SCI. However, nothing was known about the other proteins and the SCI injury. It's worth investigating the roles of Ngp, Itih4, Chil3, Ttr, and Mylpf in the future.
Our study has limitations. The main measure was the correlation between the serum serpina3n concentration and spontaneous motor function recovery in mice. Clinical treatments for patients with SCI are often comprehensive and include surgery, drug treatment and hyperbaric oxygen therapy. Whether the serum serpina3n concentration correlates with the recovery of motor function after other treatments remains to be studied.
6. Conclusions
Serum Serpina3n levels in the early stage after SCI are significantly correlated with the degree of injury and long-term motor function recovery. In addition, methylprednisolone treatment could improve neurological recovery and decrease the serum level of Serpina3n at early time points. Our study reveals Serpina3n may be a potential biomarker for SCI.
Role of the funding source
We are most grateful to the National Natural Science Foundation of China (NSFC; Grant No. 81772433) and the Department of Orthopedics, Minhang Hospital Affiliated to Fudan University (No. 2017MWDXK04), for providing funding support for our experiments.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Haihong Chen: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation. Liang Wu: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation. Yue Zhang: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation. Wang Ding: Resources, Methodology. Yin Xiaofan: Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Contributor Information
Haihong Chen, Email: 563287706@qq.com.
Liang Wu, Email: wuliang7860@163.com.
Yue Zhang, Email: 394116475@qq.com.
Wang Ding, Email: 18211360004@fudan.edu.cn.
Yin Xiaofan, Email: yin_xiaofan@fudan.edu.cn.
References
- 1.Collaborators G.B.D.N. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krueger H., Noonan V.K., Trenaman L.M., Joshi P., Rivers C.S. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis Inj Can. 2013;33(3):113–122. [PubMed] [Google Scholar]
- 3.Chun A., Delgado A.D., Tsai C.Y., Spielman L., Taylor K., Ramirez A., et al. An interview based approach to the anorectal portion of the International Standards of Neurological Classification of Spinal Cord injury Exam (I-A-ISNCSCI): a pilot study. Spinal Cord. 2020;58(5):553–559. doi: 10.1038/s41393-019-0399-5. [DOI] [PubMed] [Google Scholar]
- 4.Burns A.S., Lee B.S., Ditunno J.F., Jr., Tessler A. Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J. Neurotrauma. 2003;20(5):477–482. doi: 10.1089/089771503765355540. [DOI] [PubMed] [Google Scholar]
- 5.Norton E.S., Da Mesquita S., Guerrero-Cazares H. SERPINA3 in glioblastoma and Alzheimer's disease. Aging (Albany NY) 2021;13(18):21812–21813. doi: 10.18632/aging.203603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takamiya A., Takeda M., Yoshida A., Kiyama H. Expression of serine protease inhibitor 3 in ocular tissues in endotoxin-induced uveitis in rat. Invest. Ophthalmol. Vis. Sci. 2001;42(11):2427–2433. [PubMed] [Google Scholar]
- 7.Abcouwer S.F., Shanmugam S., Muthusamy A., Lin C.M., Kong D., Hager H., et al. Inflammatory resolution and vascular barrier restoration after retinal ischemia reperfusion injury. J. Neuroinflammation. 2021;18(1):186. doi: 10.1186/s12974-021-02237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albayar A.A., Roche A., Swiatkowski P., Antar S., Ouda N., Emara E., et al. Biomarkers in spinal cord injury: prognostic insights and future potentials. Front. Neurol. 2019;10:27. doi: 10.3389/fneur.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-El-Hassan H., Bsat S., Sukhon F., Assaf E.J., Mondello S., Kobeissy F., et al. Protein Degradome of spinal cord injury: biomarkers and potential therapeutic targets. Mol. Neurobiol. 2020;57(6):2702–2726. doi: 10.1007/s12035-020-01916-3. [DOI] [PubMed] [Google Scholar]
- 10.Kwon B.K., Casha S., Hurlbert R.J., Yong V.W. Inflammatory and structural biomarkers in acute traumatic spinal cord injury. Clin. Chem. Lab. Med. 2011;49(3):425–433. doi: 10.1515/CCLM.2011.068. [DOI] [PubMed] [Google Scholar]
- 11.Olivecrona M., Rodling-Wahlstrom M., Naredi S., Koskinen L.O. S-100B and neuron specific enolase are poor outcome predictors in severe traumatic brain injury treated by an intracranial pressure targeted therapy. J. Neurol. Neurosurg. Psychiatry. 2009;80(11):1241–1247. doi: 10.1136/jnnp.2008.158196. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa K., Okazaki R., Ishii K., Ueno T., Izawa N., Tanaka Y., et al. Phosphorylated neurofilament subunit NF-H as a biomarker for evaluating the severity of spinal cord injury patients, a pilot study. Spinal Cord. 2012;50(7):493–496. doi: 10.1038/sc.2011.184. [DOI] [PubMed] [Google Scholar]
- 13.Zhong G., Yang Y., Huang X., Chen J., Feng D., Wei K., et al. The serum SIRT1 protein is associated with the severity of injury and neurological recovery in mice with traumatic spinal cord injury. Neuroscience. 2021;469:103–109. doi: 10.1016/j.neuroscience.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Vo A.K., Geisler F., Grassner L., Schwab J., Whiteneck G., Jutzeler C., et al. Serum albumin as a predictor of neurological recovery after spinal cord injury: a replication study. Spinal Cord. 2021;59(3):282–290. doi: 10.1038/s41393-020-00536-x. [DOI] [PubMed] [Google Scholar]
- 15.Leister I., Altendorfer B., Maier D., Mach O., Wutte C., Grillhosl A., et al. Serum levels of glial Fibrillary acidic protein and neurofilament light protein are related to the neurological Impairment and spinal edema after traumatic spinal cord injury. J. Neurotrauma. 2021;38(24):3431–3439. doi: 10.1089/neu.2021.0264. [DOI] [PubMed] [Google Scholar]
- 16.Leister I., Haider T., Mattiassich G., Kramer J.L.K., Linde L.D., Pajalic A., et al. Biomarkers in traumatic spinal cord injury-technical and clinical considerations: a systematic review. Neurorehabilitation Neural Repair. 2020;34(2):95–110. doi: 10.1177/1545968319899920. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues L.F., Moura-Neto V., Tcls E.S. Biomarkers in spinal cord injury: from prognosis to treatment. Mol. Neurobiol. 2018;55(8):6436–6448. doi: 10.1007/s12035-017-0858-y. [DOI] [PubMed] [Google Scholar]
- 18.Leister I., Linde L.D., Vo A.K., Haider T., Mattiassich G., Grassner L., et al. Routine blood chemistry predicts functional recovery after traumatic spinal cord injury: a post hoc analysis. Neurorehabilitation Neural Repair. 2021;35(4):321–333. doi: 10.1177/1545968321992328. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi E., Unoki-Kubota H., Shimizu Y., Okamura T., Iwata W., Kajio H., et al. Proteomic analysis of serum biomarkers for prediabetes using the Long-Evans Agouti rat, a spontaneous animal model of type 2 diabetes mellitus. J Diabetes Investig. 2017;8(5):661–671. doi: 10.1111/jdi.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akbor M.M., Kurosawa N., Nakayama H., Nakatani A., Tomobe K., Chiba Y., et al. Polymorphic SERPINA3 prolongs oligomeric state of amyloid beta. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aslam M.S., Yuan L. Serpina3n: potential drug and challenges, mini review. J. Drug Target. 2020;28(4):368–378. doi: 10.1080/1061186X.2019.1693576. [DOI] [PubMed] [Google Scholar]
- 22.Sun B.B., Maranville J.C., Peters J.E., Stacey D., Staley J.R., Blackshaw J., et al. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73–79. doi: 10.1038/s41586-018-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ugbode C.I., Hirst W.D., Rattray M. Astrocytes grown in alvetex((R)) three dimensional scaffolds retain a non-reactive phenotype. Neurochem. Res. 2016;41(8):1857–1867. doi: 10.1007/s11064-016-1911-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Catts V.S., Sheedy D., McCrossin T., Kril J.J., Shannon Weickert C. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl. Psychiatry. 2016;6(12):e982. doi: 10.1038/tp.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fillman S.G., Sinclair D., Fung S.J., Webster M.J., Shannon Weickert C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl. Psychiatry. 2014;4(2):e365. doi: 10.1038/tp.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukovic D., Stojkovic M., Moreno-Manzano V., Jendelova P., Sykova E., Bhattacharya S.S., et al. Concise review: reactive astrocytes and stem cells in spinal cord injury: good guys or bad guys? Stem Cell. 2015;33(4):1036–1041. doi: 10.1002/stem.1959. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Ritzel R.M., Lei Z., Cao T., He J., Faden A.I., et al. Sexual dimorphism in neurological function after SCI is associated with disrupted neuroinflammation in both injured spinal cord and brain. Brain Behav. Immun. 2022;101:1–22. doi: 10.1016/j.bbi.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Yu H.L., Chen L.F., Duan C.X., Zhang J.Y., Li B.C. Survival and number of olfactory ensheathing cells transplanted in contused spinal cord of rats. Chin. J. Traumatol. 2010;13(6):356–361. [PubMed] [Google Scholar]
- 29.Basso D.M., Fisher L.C., Anderson A.J., Jakeman L.B., McTigue D.M., Popovich P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23(5):635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 30.Kulyyassov A., Fresnais M., Longuespee R. Targeted liquid chromatography-tandem mass spectrometry analysis of proteins: basic principles, applications, and perspectives. Proteomics. 2021;21(23–24) doi: 10.1002/pmic.202100153. [DOI] [PubMed] [Google Scholar]
- 31.Tyanova S., Temu T., Carlson A., Sinitcyn P., Mann M., Cox J. Visualization of LC-MS/MS proteomics data in MaxQuant. Proteomics. 2015;15(8):1453–1456. doi: 10.1002/pmic.201400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orsburn B.C. Proteome discoverer-A community enhanced data processing suite for protein informatics. Proteomes. 2021;9(1) doi: 10.3390/proteomes9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou H.J., Guo S.W., Zhu L., Xu X., Liu J.B. Methylprednisolone induces neuro-protective effects via the inhibition of A1 astrocyte activation in traumatic spinal cord injury mouse models. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.628917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatfield D.A., Zemlan F.P., Day D.J., Menon D.K. Discordant temporal patterns of S100beta and cleaved tau protein elevation after head injury: a pilot study. Br. J. Neurosurg. 2002;16(5):471–476. doi: 10.1080/0268869021000030285. [DOI] [PubMed] [Google Scholar]
- 35.Tsutsumi S., Ueta T., Shiba K., Yamamoto S., Takagishi K. Effects of the Second National Acute Spinal Cord Injury Study of high-dose methylprednisolone therapy on acute cervical spinal cord injury-results in spinal injuries center. Spine. 2006;31(26):2992–2996. doi: 10.1097/01.brs.0000250273.28483.5c. discussion 7. [DOI] [PubMed] [Google Scholar]
- 36.A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. N. Engl. J. Med. 1990;323(17):1207–1209. doi: 10.1056/NEJM199010253231712. [DOI] [PubMed] [Google Scholar]
- 37.Fichtenbaum J., Kirshblum S., Ruppert L., Flaum T., Spill G.R., Mukherjee D. Prognosis disclosure in spinal cord injury. Pharm. Manag. PM R. 2017;9(1):76–82. doi: 10.1016/j.pmrj.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Kramer J.L.K., Geisler F., Ramer L., Plunet W., Cragg J.J. Open access platforms in spinal cord injury: existing clinical trial data to predict and improve outcomes. Neurorehabilitation Neural Repair. 2017;31(5):399–401. doi: 10.1177/1545968316688801. [DOI] [PubMed] [Google Scholar]
- 39.Hulme C.H., Brown S.J., Fuller H.R., Riddell J., Osman A., Chowdhury J., et al. The developing landscape of diagnostic and prognostic biomarkers for spinal cord injury in cerebrospinal fluid and blood. Spinal Cord. 2017;55(2):114–125. doi: 10.1038/sc.2016.174. [DOI] [PubMed] [Google Scholar]
- 40.Kwon B.K., Streijger F., Fallah N., Noonan V.K., Belanger L.M., Ritchie L., et al. Cerebrospinal fluid biomarkers to stratify injury severity and predict outcome in human traumatic spinal cord injury. J. Neurotrauma. 2017;34(3):567–580. doi: 10.1089/neu.2016.4435. [DOI] [PubMed] [Google Scholar]
- 41.Bali K.K., Kuner R. Therapeutic potential for leukocyte elastase in chronic pain states harboring a neuropathic component. Pain. 2017;158(11):2243–2258. doi: 10.1097/j.pain.0000000000001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su L., Chen S., Zheng C., Wei H., Song X. Meta-analysis of gene expression and identification of biological regulatory mechanisms in Alzheimer's disease. Front. Neurosci. 2019;13:633. doi: 10.3389/fnins.2019.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalby M.J., Aviello G., Ross A.W., Walker A.W., Barrett P., Morgan P.J. Diet induced obesity is independent of metabolic endotoxemia and TLR4 signalling, but markedly increases hypothalamic expression of the acute phase protein. SerpinA3N. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-33928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gueugneau M., d'Hose D., Barbe C., de Barsy M., Lause P., Maiter D., et al. Increased Serpina3n release into circulation during glucocorticoid-mediated muscle atrophy. J Cachexia Sarcopenia Muscle. 2018;9(5):929–946. doi: 10.1002/jcsm.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Badhiwala J.H., Wilson J.R., Kwon B.K., Casha S., Fehlings M.G. A review of Clinical trials in spinal cord injury including biomarkers. J. Neurotrauma. 2018;35(16):1906–1917. doi: 10.1089/neu.2018.5935. [DOI] [PubMed] [Google Scholar]
- 46.Donato R., Sorci G., Riuzzi F., Arcuri C., Bianchi R., Brozzi F., et al. S100B's double life: intracellular regulator and extracellular signal. Biochim. Biophys. Acta. 2009;1793(6):1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Trolle C., Goldberg E., Linnman C. Spinal cord atrophy after spinal cord injury - a systematic review and meta-analysis. Neuroimage Clin. 2023;38 doi: 10.1016/j.nicl.2023.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lubieniecka J.M., Streijger F., Lee J.H., Stoynov N., Liu J., Mottus R., et al. Biomarkers for severity of spinal cord injury in the cerebrospinal fluid of rats. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0019247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun F., Zhang H., Huang T., Shi J., Wei T., Wang Y. S100A9 blockade improves the functional recovery after spinal cord injury via mediating neutrophil infiltration. Exp. Ther. Med. 2022;23(4):291. doi: 10.3892/etm.2022.11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y., Dong Y., Zhang Z.L., Han J., Chen F.S., Tong X.Y., et al. Fra-1 induces apoptosis and neuroinflammation by targeting S100A8 to modulate TLR4 pathways in spinal cord ischemia/reperfusion injury. Brain Pathol. 2023;33(1) doi: 10.1111/bpa.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.