Abstract
Background
The upper airways of cystic fibrosis (CF) persons are an evolutionary niche where genetically adapted bacterial strains are selected for lung infection. The microbiological studies conducted up to now on the upper airways are not easily comparable.
Methods
Using classical culture methods, we simultaneously studied the microbiological status of upper and lower airways in persons not chronically infected with P. aeruginosa. Each person had a single upper airways sampling and a concomitant lower airways sampling. Lower airways sampling was performed by oropharyngeal swab or sputum collection. Using a quasi-experimental design of study, we evaluated the performance of 2 different upper airways’ sampling methods, nasal lavage according to method described by Mainz or nasal lavage with a rhino-set. Pain was measured with appropriate scales.
Results
A total of 194 persons were enrolled in this study. Pathogenic flora was found in 128 (6.6%) of 194 upper airways samples and in 164 (84.6%) lower airways samples. A statistically significant difference between the upper airways and the lower airways was found in the isolation of S. aureus and non-fermenter gram negatives. Nasal lavage according to Mainz resulted in the isolation of more non-fermenter gramnegatives than the rhino-set (p < 0.05). No differences were found in the pain caused bythe two methods.
Conclusions
In our study population, cultures of the upper airway and lower airway differ in CF persons. In people sampled with nasal lavage according to Mainz more non-fermenter gram negatives were detected than with rhino-set. The two sampling methods were comparable with regard to the caused pain, nasal lavage according to Mainz method being quicker to perform.
1. Introduction
Cystic Fibrosis (CF) is the most common lethal genetic disease in the Caucasian population and affects more than 100,000 persons worldwide, with an incidence in Tuscany of one new person per 4000 newborns [[1], [2], [3], [4], [5]]. Over the last 30 years, advances in CF care have led to a significant increase in survival [[1], [2], [3], [4]]. Further improvements in expectancy and quality of life result from the recent introduction of cystic fibrosis transmembrane conductance regulator (CFTR) modulators in clinical practice [2,[6], [7], [8], [9], [10], [11], [12]]. This type of therapy, which is already available to a substantial proportion of persons with CF (pwCF), has led to a substantial improvement in clinical condition with an improvement of forced expiratory volume in the first second (FEV1) and nutritional status along with a reduction in sputum volume and in pulmonary exacerbations [[6], [7], [8], [9], [10], [11], [12]].
Despite the progress made, bacterial respiratory infections continue to remain the leading cause of lung damage, mortality and morbidity [[2], [3], [4]]. A relatively small number of pathogens are responsible for pulmonary infections and the prevalence of different bacterial species varies depending on the age of the pwCF [[2], [3], [4]]. At a young age Staphylococcus aureus (S. aureus) is most frequently isolated while nonfermenter gram-negatives, especially Pseudomonas aeruginosa (P. aeruginosa), are the most frequent pathogens in adults [4]. Chronic P. aeruginosa infection is associated with a progressive reduction in lung function and an unfavourable clinical course. The effects are more severe when chronic infection develops early [3,13].
The CFTR genotype also influences the upper airways (UAW), which are susceptible to infection [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]]. A correlation between the UAW and lower airways (LAW) flora has been described. P. aeruginosa strains isolated in both anatomical sites have the same genotype, and strains isolated from the UAW may have phenotypic characteristics of chronic infection (e.g. mucoid phenotype) before isolation in the LAW [[19], [20], [21], [22], [23], [24]]. This and other observations lead clinicians to believe that infection in the paranasal sinuses may precede pulmonary infection [14,15]. At the level of the paranasal sinuses, the oedema of the mucosa, the reduced concentration of oxygen and antibiotics, and the particular nutritional conditions of the bacteria provide a formidable evolutionary drive for micro-organisms [15,[21], [22], [23], [24], [25]]. UAW are considered an evolutionary niche in which genetically adapted bacterial strains are selected to infect the lung [[23], [24], [25]].
However, studies on UAW flora are difficult to compare due to the heterogeneous sampling methods used, the different ages, the heterogeneous clinical conditions and the different microbiological status of pwCF [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]].
Lacking a gold standard for UAW sampling [3], it is necessary to evaluate the performance of the various methods to enable a correct interpretation of the results and to implement careful surveillance of the LAW in order to detect initial P. aeruginosa lung infection.
The importance of the role of the UAW could pave the way for the routine microbiological monitoring of UAW and for a greater attention to this anatomical site. In a context where the increasingly widespread use of modulators leads to a substantial reduction in the number of expectorating patients, microbiological surveillance conducted at the upper airway level could be complementary to the current clinical practice of airway surveillance, classically based on sputum and oropharyngeal (OP) cultures. The main aim of our study was to assess the microbiological status of the airways in pwCF not chronically infected with P. aeruginosa. As a secondary aim we evaluated the performance of two different sampling methods, nasal lavage according to the method described by Mainz (NLM) [20] or nasal lavage with a syringe containing sterile isotonic saline solution with a specific silicone nasal adapter (rhino-set). Neither of the nasal lavage methods under comparison for microbiological sampling is described in the standard of care but both are used in clinical practice. Our a priori hypothesis was that there were no differences in the result of cultures performed on samples collected with the two nasal lavage methods. The impact of the different approaches on the pwCF was also measured for assessing the pain caused by the sampling procedures.
2. Methods
This is a single-centre, observational, prospective study. Each pwCF participating in this study had a single UAW sampling and a concomitant LAW sampling. Sampling of the lower airways was performed by oropharyngeal swab or sputum collection.
A quasi-experimental design (the allocation of pwCFs is described later) was used to evaluate the performance of two different UAW sampling methods. This design was chosen, in the context of the SARS-CoV-2 pandemic, in order to make the appointments for access to the CF Centre compatible with the provisions of the local health authorities aimed at containing the pandemic [26,27]. Throughout the duration of the study, pwCF requesting access to the Centre or with symptoms suggestive of viral infection had to be swabbed for SARS-CoV-2 within 24 h preceding their scheduled clinical check-up date [26,27]. This diagnostic pathway influenced the date of the clinical check-up. At the same time, the infections that occurred over time among healthcare personnel and their employment in other wards of the hospital, to cope with the pandemic, led to continuous reorganisation of appointment schedules.
Airway sampling was performed from December 2020 to December 2022. Persons with CF in the care of the Tuscan Regional Centre, in regular clinical follow-up (quarterly checks), treated according to the standard of care, regardless of their CFTR genotype and Elexacaftor-Tezacaftor-Ivacaftor (ETI) treatment (available in Italy since July 2021) took part in the study. Persons with CF older than 4 years in clinical stability, not chronically infected with P. aeruginosa (never or P. aeruginosa free according to the Leeds classification) were considered eligible [13].
Unstable clinical status or concomitant use of parenteral antibiotics, age under 4 years, bipulmonary transplantation and chronic P. aeruginosa infection were considered exclusion criteria. The sinonasal measurement of symptoms is outside the scope of this study.
The diagnosis of CF was in agreement with international criteria [28]. Pathogenic germs were considered to be those reported in the European CF Registry [4]. The definition of the microbiological status of the pwCF was based on the Leeds definition [13].
The quasi-experimental design was realised by allocating the pwCF to two different UAW sampling methods according to the days (date) chosen by the pwCF depending on the availability of the Centre on 4 days per week (as on the remaining days the medical staff was involved in care commitments towards patients with other types of pathology). The administrative staff who actually booked the follow-up appointments were not aware of the study.
Experienced physiotherapists performed sampling of the UAW with the nasal lavage according to Mainz method on even dates, and nasal lavage using rhino-set on odd dates. Specifically, the first method was performed by inserting 10 ml of sterile isotonic saline solution into each nostril with a 10 ml syringe, asking the pwCF to keep his head in a slightly extended position in order to facilitate the occlusion of the soft palate [20]. The lavage was collected in a sterile container.
Lavage with rhino-set was performed using a 10 ml syringe of sterile isotonic saline solution with a specific silicone nasal lavage adapter. The physiological saline was gently inserted into the right nostril by means of pressure exerted on the syringe piston and re-aspirated using the same device. This procedure was then repeated in the left nostril.
In both types of UAW sampling, the collected fluid was placed in a single container and sent to the microbiology laboratory.
Airway samples were processed according to classical microbiology methods following guidelines for CF patients (Recommendations SIFC Microbiologists Group) [29]. Isolations of pathogens from the UAW and LAW were used as outcome measures.
The pain caused by the lavage methods was assessed using special scales [[30], [31], [32]]. For pwCF in paediatric age, the Wong-Baker Faces Pain Rating Scale was used, a horizontal scale of 6 drawn faces, from smiling to crying, corresponding respectively to the absence of pain and the worst possible pain [30]. The scale provides a score between 0 and 10 and was used for individuals from 3 to 8 years of age.
A numeric pain rating scale was used for individuals over 8 years of age [31]. The pwCF was asked to mark the number indicating the intensity of their pain. The left end of the scale corresponds to 'no pain' and the right end to 'worst pain imaginable'.
The study was conducted according to the Declaration of Helsinki and in accordance with the principles of ethics and good clinical practice (E6: Good Clinical Practice: Consolidated Guideline (CPMP/ICH/135/95). Approval to conduct the present study was provided by the Paediatric Ethics Committee (CEP) of the Tuscany Region (Florence, Italy) K37 271/2020 on 10/11/2020.
3. Statistical analysis
All collected values were entered into an electronic database. Descriptive statistics for quantitative variables were performed using normal distribution tests. Differences between groups in continuous and categorical variables were evaluated using descriptive statistics, χ2 test, and Fisher's exact test. Comparisons between independent samples were performed using Student's t-test for the equality of the means or the Mann-Whitney test. The level of statistical significance was expressed as a p-value and it was considered statistically significant if p was <0.05. No interim analysis was planned.
4. Results
A total of 194 pwCF (112 males, mean ± SD age 20.3 ± 14.4 years and 82 females, mean ± SD age 17.7 ± 12.2 years) not chronically infected with P. aeruginosa were enrolled for this study. One hundred and five (54.1%) pwCF were older than 18 years and 89 (45.9%) were younger than 18 years.
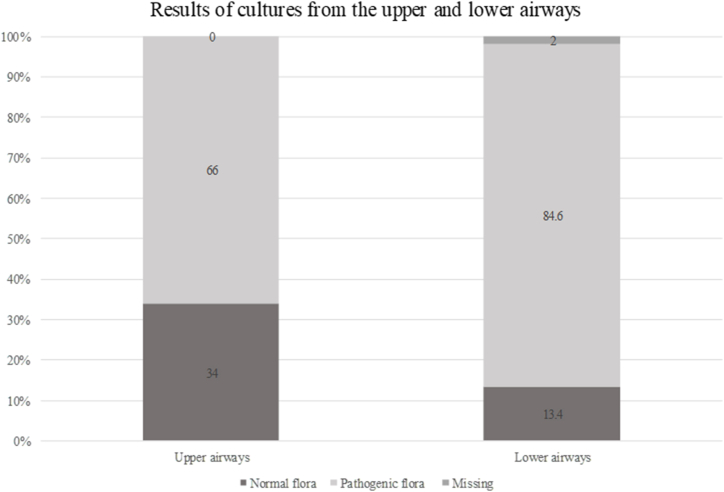
During the study period, 194 concomitant UAW and LAW respiratory tract samplings were performed. With regard to sampling from the upper tract 128 (66%) resulted in the isolation of pathogenic flora and 66 (34%) of normal flora. Sampling of the lower tract was performed in 56 (28.9%) pwCF by sputum collection, in 132 (68.0%) by oropharyngeal swabs, in 6 (3%) data were missing. One hundred and sixty-four (84.6%) of 194 samplings resulted in the isolation of pathogens, 26 (13.4%) in the detection of normal flora, 4 (2%) data missing.
Fig. 1 illustrates the results of upper and lower respiratory tract sampling. A statistically significant difference was found (p < 0.05).
Fig. 1.
Results of upper and lower airways cultures.
With regard to the UAW sampling the NLM method was performed in 88 (45.4%) pwCF and the rhino-set method in 104 (53.6%), 1 (0.5%) swabbed nasal secretions, 1 (0.5%) missing data.
Table 1 shows the demographic characteristics of the 192 pwCF and the method of UAW sampling.
Table 1.
Demographic characteristics and method of UAW sampling of 192 pwCF.
| Nasal lavage according to Mainz | Nasal lavage with rhino-set | Total | |
|---|---|---|---|
| Number (males %) | 88 (54.5%) | 104 (59.6%) | 192 (57.3%) |
| Mean age (years) | 18.1 ± 13.5 | 20.0 ± 13.1 | 19.3 ± 13.6 |
| <8 years (males %) | 16 (50%) | 11 (45.4%) | 27 (48.1%) |
| >8 years (males %) | 71 (54.9%) | 94 (61.7%) | 165 (50.5%) |
| F508del homozygous | 21 | 22 | 43 |
| F508del heterozygous | 38 | 49 | 87 |
| Other genotype | 29 | 33 | 62 |
| ETI treatment at sampling | 3 | 12 | 15 |
| Pancreatic status (insufficiency/sufficiency) | 56/32 | 67/37 | 123/69 |
| BMI kg/m2(mean ± SD) | 19.25 ± 3.76 | 20.20 ± 3.74 | 19.75 ± 3.77 |
| FEV1 % (baseline) (mean ± SD) | 87.21 ± 23.37 | 84.16 ± 23.32 | 85.53 ± 23.32 |
| Exacerbations (2021–2022) | 11 | 6 | 17 |
| Days on parenteral antibiotic treatment (mean ± SD) | 11.27 ± 5.84 | 11.33 ± 6.74 | 11.29 ± 5.96 |
Abbreviations - BMI: body mass index; ETI: Elexacaftor-Tezacaftor-Ivacaftor; FEV1: forced expiratory volume in the first second; SD: standard deviation.
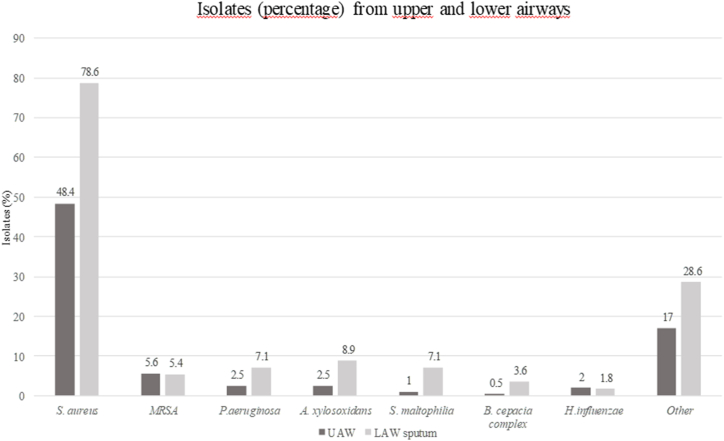
Fig. 2 illustrates in percentage the prevalence of UAW and LAW tract pathogens in pwCF not chronically infected with P. aeruginosa. A wide diversity of pathogens was detected and a statistically significant difference was found between S. aureus isolations in the upper and lower tract (p < 0.05).
Fig. 2.
Upper and lower respiratory tract flora in pwCF not chronically infected by P. aeruginosa (data non-mutually exclusive). Abbreviations - LAW: lower airways; MRSA: methicillin resistant Staphylococcus aureus; UAW: upper airways.
With regard to the isolation of nonfermenter gram-negatives, UAW sampling resulted in the isolation of 13 strains (5 strains of P. aeruginosa, 5 of A. xylosoxidans, 2 S. maltophilia and 1 Burkholderia cepacia complex), whereas sampling from LAW resulted in the isolation of 29 strains (9 P. aeruginosa, 9 A. xylosoxidans, 9 S. maltophilia and 2 B. cepacia complex). Overall, the difference observed in nonfermenter gram-negatives between UAW and LAW was statistically significant (p < 0.05). No statistically significant differences were observed in the isolations of each individual species.
In 9 pwCF in whom P. aeruginosa was isolated from the lower airways, 7 performed UAW sampling with NLM method (in 3 cases positive for P. aeruginosa) and 2 with rhino-set (negative results).
Age (mean ± SD) was 18.84 ± 13.36 years in 128 subjects with positive UAW cultures and 18.97 ± 13.09 in 165 subjects with positive LAW cultures (p = 0.93). The FEV1 values (% of predicted) were 83.83 ± 22.08 and 84.64 ± 23 (p = 0.78) in 112 (87.5%) out of 128 UAW positive patients and in 145 (87.8%) out of 165 LAW positive patients able to perform spirometry, respectively.
In 94 subjects with UAW cultures positive for S. aureus the mean ± SD age was 17.29 ± 13.36 years and that of 140 subjects with LAW cultures positive for S. aureus was 18.36 ± 12.49 (p = 0.53). The FEV1 values (% of predicted) were 88.11 ± 20.47 and 86.85 ± 21.49 (p = 0.67) in 83 (88.2%) out of 94 subjects with S. aureus positive UAW and in 125 (89.2%) out of 140 with positive LAW, respectively.
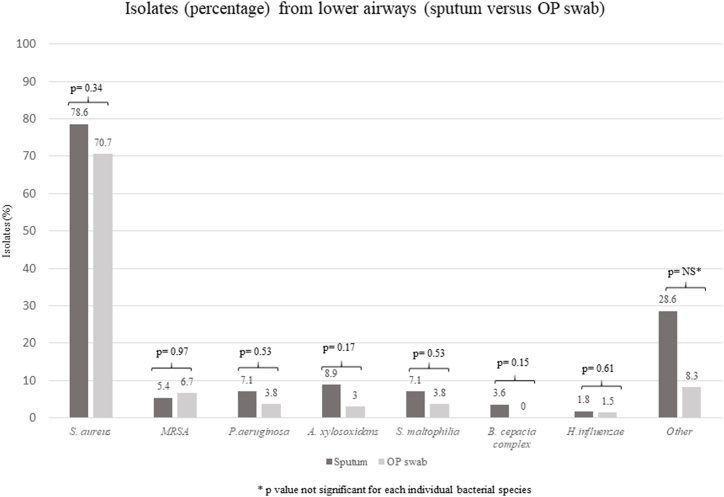
Fig. 3 illustrates the different prevalence of pathogenic bacterial species that were isolated using either sputum collection or OP swabs as sampling methods. Between the two sampling methods, no statistically significant differences were observed for any individual bacterial species considered pathogens in CF.
Fig. 3.
Prevalence of pathogenic bacterial species isolated using spontaneously expectorated sputum collection or OP swab. Abbreviations - MRSA: methicillin resistant Staphylococcus aureus; OP: oropharyngeal.
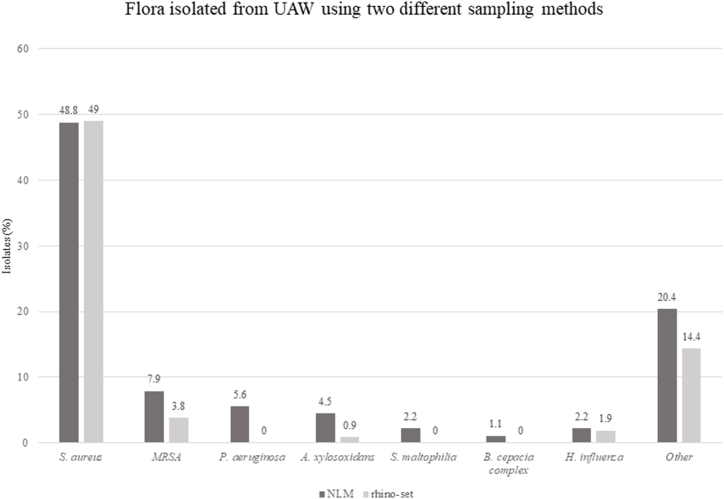
Fig. 4, which compares different nasal sampling methods, illustrates the results regarding pathogen isolation. P. aeruginosa was isolated from the UAW in 5 (5.6%) pwCF, in 3 of them concomitantly with the isolation of P. aeruginosa from the lower airways. Of 13 strains (5 P. aeruginosa, 5 A. xylosoxidans, 2 S. maltophilia and 1 B. cepacia complex) of non-fermenter gram-negatives isolated from the UAW, 12 strains were isolated using NLM sampling and only 1 using rhino-set (p < 0.05).
Fig. 4.
Flora isolated from UAW using two different sampling methods in pwCF not chronically infected by P. aeruginosa. Abbreviations - MRSA: methicillin resistant Staphylococcus aureus; NLM: nasal lavage according to Mainz.
Pain induced by the two sampling methods was assessed in 192 subjects undergoing nasal lavage.
In 13 children (3 missing data) under 8 years of age who performed the NLM method, the median score was 0 (IQR 0–2), the majority of children had no pain,4 (30%) had a score of 2. In 8 pwCF (3 missing data) using the rhino-set method, the median score was 0 (IQR 0–2.25), again most children had no pain, only 2 (18.2%) had a score of 3. We observed no statistically significant differences between the two methods (p-value: 0.96).
In 66 pwCF (5 missing data) over 8 years of age, the median score was 0 (IQR 0-0) for the NLM method, in 9 (13.6%) cases the score was between 2 and 3 and in one case the score was 7. In 91 pwCF (3 missing data) using the rhino-set method, median score was 0 (IQR 0-0), in 19 (20.8%) pwCF the score was between 1 and 4, in 2 (2.2%) the score was 5–6 and in one case the score was 8. Also in children over 8 years of age no statistically significant differences between the two methods were detected (p-value: 0.27).
Overall, both methods were well accepted, with a limited number of subjects reporting mild/moderate pain. There were no side effects associated with either sampling method.
Once the co-operation with pwCF was obtained, the time required to perform the method with rhino-set is approximately 2 min longer than with the NLM method.
5. Discussion
To our knowledge, this is one of the largest studies in the last five years aimed at assessing simultaneously the microbiological status of UAW and LAW in pwCF. In our population the prevalence of bacterial isolates from UAW and LAW differ and the microbiological study on nasal lavage does not fully represent the microbiological status at the LAW level. Moreover, in our study two different sampling methods of the UAW were also compared and we observed a significantly higher number of nonfermenter gram-negatives isolated from people undergoing NLM.
From our data the prevalence of S. aureus, both in the UAW and LAW, was higher than in other publications and this could be attributed to several factors. In comparison with other experiences the population of pwCF eligible for this study was more homogeneous, being not chronically infected with P. aeruginosa [14,19,20,22]. Moreover, the prevalence of S. aureus infection varies widely between countries [4]. It is well known that there is no evidence-based treatment for the prevention of S. aureus infection and different strategies can be adopted [33]. In our centre, as in many other centres, no antibiotic strategies were adopted over time to prevent S. aureus infection. A clinical trial is currently underway in the UK to determine the true effectiveness of prophylactic treatment [34].
In our experience data showed a statistically significant difference in the culture test results between UAW and LAW. This difference, which is more substantial for S. aureus than for the other pathogens, raises the issue of the performance of airways sampling methods. Spontaneously expectorated sputum is considered the gold standard to evaluate airways microbiology in pwCF and international guidance recommends taking respiratory samples for microbiological investigation following this method [4,35].
Much debate exists in the literature on other non-invasive sampling methods and whether OP cultures are truly representative of the LAW. In our study, although there are no statistically significant differences, the detection of bacterial species is higher using spontaneously expectorated sputum samples than OP swabs. Spontaneously expectorated sputum continues to be the recommended specimen for sampling, and OP swabs should be used if a subject cannot expectorate [35]. Our study showed that the sensitivity of OP swabs in isolating pathogens was lower than that of sputum but still higher than that of UAW. Although OP swabs are not fully representative of the flora in the lower airways, they are nevertheless widely used in clinical practice in non-expectorating CF people [4,35].
The issue of sampling methods is considered particularly important by pwCF following the introduction of highly effective CFTR modulators. With this type of therapy in fact, the quantity of secretions decreases and sputum collection, considered up to now the gold standard for microbiological analysis, appears more difficult [[6], [7], [8], [9], [10], [11], [12]]. Related to this topic, the James Lind Alliance, a non-profit initiative aimed at identifying and prioritising unanswered questions in CF, ranks the best way to diagnose a lung infection in the absence of sputum as the second of the top 10 priorities [36]. In this context, the study of UAW is easy to perform and, although not currently included in the standards of care and not carried out routinely, it could be considered useful in providing some indications on the microbiological status of pwCF.
Currently, microbiological monitoring of the UAW is not carried out regularly [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]]. The studies conducted to date on UAW microbiology are not easily interpretable due to the different sampling methods used, the different ages of pwCF and their microbiological status. Various sampling methods, including endoscopy-guided aspirate [18], could be used. However, although endoscopy sampling techniques are regarded as the most sensitive method, they are considered invasive. Nasal lavage is suggested as a non-invasive alternative [18] although culture results may not represent the complexity of the UAW flora and the sample might be contaminated with nasopharyngeal flora [24].
In our experience, using a single lavage, the microbiology of UAW cannot be used as a surrogate marker for the microbiological status of the LAW and we found a difference between the two UAW sampling methods regarding the isolation of nonfermenter gram-negative. At present, P. aeruginosa detection from the UAW is not an indication for starting early eradication treatment [3]. Microbiological monitoring of the lower airways using other methods, such as induced sputum, considered more sensitive than nasal lavage in detecting P. aeruginosa [35,37], remains important for the correct definition of microbiological status and for starting appropriate antibiotic treatment at initial P. aeruginosa isolation [4]. Whether a regular UAW monitoring to detect initial P. aeruginosa infection at this anatomical site might be useful in the future for planning eradicating treatments aimed at preventing lung infection remains a matter of debate.
In the present study, special attention was focused to pain symptoms possibly caused by the two sampling methods [20,32]. Overall, both UAW sampling methods were well accepted and we did not record any serious side effects related to the sampling methods. Analysis of the data, both in subjects over 8 years of age and in subjects under this age, showed no statistically significant differences in the perception of pain symptoms between the two sampling methods. The NLM method is preferable because of its simplicity, materials are more readily available and this procedure requires less time.
The strengths of our study were the large sample size, including both paediatric and adult pwCF, and the homogeneity of the data collected in a single centre with uniformity of sample collection and analysis methods. The findings from our study can easily be generalised to clinical practice.
The quasi-experimental study design we adopted to evaluate the UAW microbiology has obvious limitations compared to a randomised controlled trial (RCT). The limitations in access to healthcare facilities put in place by the health authorities in the context of the Sars-CoV-2 epidemic [26,27], contagions that occurred in pwCF and healthcare personnel, continuous re-scheduling of appointments, and the limitation of space and time used by healthcare personnel to conduct the study made us adopt a study design simpler than a RCT. Other limitations stem from the fact that the result of a single sample cannot be considered truly representative of the microbiological status. Furthermore, molecular typing studies on the bacterial isolates were not planned due to budget constraints. In this regard, previous molecular studies have usually shown a concordance between UAW and LAW bacterial strains in pwCF [19,20].
In conclusion, our study underlines the issues of airways sampling and emphasises both the diversity of results between UAW and LAW and the different performances of sampling methods at the level of the UAW. The analysis of a simultaneous sampling shows that the microbiological results at the level of the UAW cannot accurately represent that of LAW. Although the two UAW sampling methods used are comparable with regard to the pain caused, the NLM method is preferable due to its simpler mode of execution. Although the clinical significance and therapeutic perspectives regarding the UAW isolation of nonfermenter gram-negatives remain to be clarified, the detection of more bacterial isolates was observed in pwCF sampled with the NLM method.
Data availability
Available on request to Meyer Children's Hospital IRCCS.
CRediT authorship contribution statement
Daniela Dolce: Writing – review & editing, Writing – original draft, Investigation, Data curation, Conceptualization. Novella Ravenni: Writing – original draft, Data curation, Conceptualization. Cristina Fevola: Formal analysis, Data curation. Michela Francalanci: Data curation. Paolo Bonomi: Formal analysis. Maria Chiara Cavicchi: Investigation. Valeria Galici: Investigation. Anna Silvia Neri: Investigation. Giovanni Taccetti: Writing – review & editing, Writing – original draft, Supervision, Project administration, Data curation, Conceptualization. Vito Terlizzi: Investigation. Diletta Innocenti: Methodology, Investigation. Beatrice Ferrari: Methodology, Investigation. Chiara Bianchimani: Investigation, Data curation. Erica Camera: Methodology, Investigation. Tommaso Orioli: Methodology, Investigation. Silvia Campana: Writing – original draft, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Giovanni Taccetti reports financial support was provided by Tuscany Region. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the following medical doctors for having made this study possible:
Dr Silvia Bresci, Dr Beatrice Borchi, Dr Annalisa Cavallo, Dr Jessica Mencarini Cystic Fibrosis Unit, AOU Careggi, Florence, Italy Dr Giandomenico Maggiore, Otolaryngology, AOU Careggi, Florence, Italy. We give grateful thanks to all the physiotherapists of the Meyer Children's Hospital IRCCS, Tuscan Regional Cystic Fibrosis Centre who took part in the study (Giulia Santini, Eleonora Masi, ChiaraCastellani, Chiara Degl’Innocenti, Matteo Masolini).
References
- 1.Guo J., Garratt A., Hill A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J. Cyst. Fibros. 2022;21:456–462. doi: 10.1016/j.jcf.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Shteinberg M., Haq I.J., Polineni D., Davies J.C. Cystic fibrosis. Lancet. 2021;397(10290):2195–2211. doi: 10.1016/S0140-6736(20)32542-3. [DOI] [PubMed] [Google Scholar]
- 3.Castellani C., Duff A.J.A., Bell S.C., Heijerman H.G.M., Munck A., Ratjen F., Sermet-Gaudelus I., Southern K.W., Barben J., Flume P.A., Hodková P., Kashirskaya N., Kirszenbaum M.N., Madge S., Oxley H., Plant B., Schwarzenberg S.J., Smyth A.R., Taccetti G., Wagner T.O.F., Wolfe S.P., Drevinek P. ECFS best practice guidelines: the 2018 revision. J. Cyst. Fibros. 2018;17:153–178. doi: 10.1016/j.jcf.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 4.ECFSPR . In: van Rens J et al. Orenti A., Zolin A., Jung A., editors. 2020. Annual report. 2023. [Google Scholar]
- 5.Botti M., Terlizzi V., Francalanci M., Dolce D., Cavicchi M.C., Neri A.S., Galici V., Mergni G., Zavataro L., Centrone C., Festini F., Taccetti G. Cystic fibrosis in Tuscany: evolution of newborn screening strategies over time to the present. Ital. J. Pediatr. 2021;47(1):2. doi: 10.1186/s13052-020-00948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middleton P.G., Mall M.A., Dřevínek P., Lands L.C., McKone E.F., Polineni D., Ramsey B.W., Taylor-Cousar J.L., Tullis E., Vermeulen F., Marigowda G., McKee C.M., Moskowitz S.M., Nair N., Savage J., Simard C., Tian S., Waltz D., Xuan F., Rowe S.M., Jain R. VX17-445-102 Study Group. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heijerman H.G.M., McKone E.F., Downey D.G., Van Braeckel E., Rowe S.M., Tullis E., Mall M.A., Welter J.J., Ramsey B.W., McKee C.M., Marigowda G., Moskowitz S.M., Waltz D., Sosnay P.R., Simard C., Ahluwalia N., Xuan F., Zhang Y., Taylor-Cousar J.L., McCoy K.S. VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor-Cousar J.L., Robinson P.D., Shteinberg M., Downey D.G. CFTR modulator therapy: transforming the landscape of clinical care in cystic fibrosis. Lancet. 2023;402(10408):1171–1184. doi: 10.1016/S0140-6736(23)01609-4. [DOI] [PubMed] [Google Scholar]
- 9.Hisert K.B., Birket S.E., Clancy J.P., Downey D.G., Engelhardt J.F., Fajac I., Gray R.D., Lachowicz-Scroggins M.E., Mayer-Hamblett N., Thibodeau P., Tuggle K.L., Wainwright C.E., De Boeck K. Understanding and addressing the needs of people with cystic fibrosis in the era of CFTR modulator therapy. Lancet Respir. Med. 2023;11:916–931. doi: 10.1016/S2213-2600(23)00324-7. [DOI] [PubMed] [Google Scholar]
- 10.Graeber S.Y., Mall M.A. The future of cystic fibrosis treatment: from disease mechanisms to novel therapeutic approaches. Lancet. 2023;402(10408):1185–1198. doi: 10.1016/S0140-6736(23)01608-2. [DOI] [PubMed] [Google Scholar]
- 11.Mayer-Hamblett N., Clancy J.P., Jain R., Donaldson S.H., Fajac I., Goss C.H., Polineni D., Ratjen F., Quon B.S., Zemanick E.T., Bell S.C., Davies J.C., Jain M., Konstan M.W., Kerper N.R., LaRosa T., Mall M.A., McKone E., Pearson K., Pilewski J.M., Quittell L., Rayment J.H., Rowe S.M., Taylor-Cousar J.L., Retsch-Bogart G., Downey D.G. Advancing the pipeline of cystic fibrosis clinical trials: a new roadmap with a global trial network perspective. Lancet Respir. Med. 2023;11:932–944. doi: 10.1016/S2213-2600(23)00297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mall M.A., Brugha R., Gartner S., Legg J., Moeller A., Mondejar-Lopez P., Prais D., Pressler T., Ratjen F., Reix P., Robinson P.D., Selvadurai H., Stehling F., Ahluwalia N., Arteaga-Solis E., Bruinsma B.G., Jennings M., Moskowitz S.M., Noel S., Tian S., Weinstock T.G., Wu P., Wainwright C.E., Davies J.C. Efficacy and Safety of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 Through 11 Years of Age with Cystic Fibrosis Heterozygous for F508del and a Minimal Function Mutation: a Phase 3b, Randomized, Placebo-controlled Study. Am. J. Respir. Crit. Care Med. 2022;206:1361–1369. doi: 10.1164/rccm.202202-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee T.W., Brownlee K.G., Conway S.P., Denton M., Littlewood J.M. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J. Cyst. Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 14.Berkhout M.C., Rijntjes E., El Bouazzaoui L.H., Fokkens W.J., Brimicombe R.W., Heijerman H.G. Importance of bacteriology in upper airways of patients with Cystic Fibrosis. J. Cyst. Fibros. 2013;12:525–529. doi: 10.1016/j.jcf.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Folkesson A., Jelsbak L., Yang L., Johansen H.K., Ciofu O., Høiby N., Molin S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat. Rev. Microbiol. 2012;10:841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 16.Moss R.B. Cystic fibrosis: pathogenesis, pulmonary infection, and treatment. Clin. Infect. Dis. 1995;21:839–849. doi: 10.1093/clinids/21.4.839. [DOI] [PubMed] [Google Scholar]
- 17.Muhlebach M.S., Miller M.B., Moore C., Wedd J.P., Drake A.F., Leigh M.W. Are lower airway or throat cultures predictive of sinus bacteriology in cystic fibrosis? Pediatr. Pulmonol. 2006;41:445–451. doi: 10.1002/ppul.20396. [DOI] [PubMed] [Google Scholar]
- 18.Møller M.E., Alanin M.C., Grønhøj C., Aanæs K., Høiby N., von Buchwald C. Sinus bacteriology in patients with cystic fibrosis or primary ciliary dyskinesia: a systematic review. Am J Rhinol Allergy. 2017;31:293–298. doi: 10.2500/ajra.2017.31.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mainz J.G., Michl R., Pfister W., Beck J.F. Cystic fibrosis upper airways primary colonization with Pseudomonas aeruginosa: eradicated by sinonasal antibiotic inhalation. Am. J. Respir. Crit. Care Med. 2011;184:1089–1090. doi: 10.1164/ajrccm.184.9.1089. [DOI] [PubMed] [Google Scholar]
- 20.Mainz J.G., Naehrlich L., Schien M., Käding M., Schiller I., Mayr S., Schneider G., Wiedemann B., Wiehlmann L., Cramer N., Pfister W., Kahl B.C., Beck J.F., Tümmler B. Concordant genotype of upper and lower airways P aeruginosa and S aureus isolates in cystic fibrosis. Thorax. 2009;64:535–540. doi: 10.1136/thx.2008.104711. [DOI] [PubMed] [Google Scholar]
- 21.Johansen H.K., Aanaes K., Pressler T., Nielsen K.G., Fisker J., Skov M., Høiby N., von Buchwald C. Colonisation and infection of the paranasal sinuses in cystic fibrosis patients is accompanied by a reduced PMN response. J. Cyst. Fibros. 2012;11:525–531. doi: 10.1016/j.jcf.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Bonestroo H.J., de Winter-de Groot K.M., van der Ent C.K., Arets H.G. Upper and lower airway cultures in children with cystic fibrosis: do not neglect the upper airways. J. Cyst. Fibros. 2010;9:130–134. doi: 10.1016/j.jcf.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Aanaes K., Rickelt L.F., Johansen H.K., von Buchwald C., Pressler T., Høiby N., Jensen P.Ø. Decreased mucosal oxygen tension in the maxillary sinuses in patients with cystic fibrosis. J. Cyst. Fibros. 2011;10:114–120. doi: 10.1016/j.jcf.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Aanaes K., Johansen H.K., Skov M., Buchvald F.F., Hjuler T., Pressler T., Hoiby N., Nielsen K.G., von Buchwald C. Clinical effects of sinus surgery and adjuvant therapy in cystic fibrosis patients - can chronic lung infections be postponed? Rhinology. 2013;51:222–230. doi: 10.4193/Rhino12.207. [DOI] [PubMed] [Google Scholar]
- 25.Rau M.H., Hansen S.K., Johansen H.K., Thomsen L.E., Workman C.T., Nielsen K.F., Jelsbak L., Høiby N., Yang L., Molin S. Early adaptive developments of Pseudomonas aeruginosa after the transition from life in the environment to persistent colonization in the airways of human cystic fibrosis hosts. Environ. Microbiol. 2010;12:1643–1658. doi: 10.1111/j.1462-2920.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 26.Raccolta degli atti emanati dal Governo recanti misure urgenti in materia di contenimento e gestione dell'emergenza epidemiologica da COVID-19 https://www.gazzettaufficiale.it/attiAssociati/1/?areaNode=13 Accessed 14 October 2023.
- 27.Ordinanze Regione Toscana – Coronavirus. https://www.regione.toscana.it/-/ordinanze-della-regione-toscanahttps://www.regione.toscana.it/-/coronavirus Accessed 14 October 2023.
- 28.Farrell P.M., White T.B., Ren C.L., Hempstead S.E., Accurso F., Derichs N., Howenstine M., McColley S.A., Rock M., Rosenfeld M., Sermet-Gaudelus I., Southern K.W., Marshall B.C., Sosnay P.R. Diagnosis of cystic fibrosis: consensus guidelines from the cystic fibrosis foundation. J. Pediatr. 2017;181S:S4–S15.e1. doi: 10.1016/j.jpeds.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 29.Raccomandazioni per l’esecuzione delle indagini Microbiologiche di Campioni delle vie respiratorie di pazienti con Fibrosi Cistica. https://www.sifc.it/documento/aggiornamento-raccomandazioni-per-lesecuzione-delle-indagini-microbiologiche-di-campioni-delle-vie-respiratorie-di-pazienti-con-fibrosi-cistica/Accessed 14 October 2023.
- 30.Wong-Baker faces Foundation. https://wongbakerfaces.org/Accessed 14 October 2023.
- 31.Numeric Pain Rating Scale. https://www.physio-pedia.com/Numeric_Pain_Rating_Scale Accessed 14 October 2023.
- 32.AOPI Ospedale senza dolore (Osd) https://www.aopi.it/project/carta-dei-diritti-del-bambino-in-ospedale/Accessed 14 October 2023.
- 33.Rosenfeld M., Rayner O., Smyth A.R. Prophylactic anti-staphylococcal antibiotics for cystic fibrosis. Cochrane Database Syst. Rev. 2020 doi: 10.1002/14651858.CD001912.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CF START: A national UK trial to determine whether taking an antibiotic (flucloxacillin) every day predisposes infants with cystic fibrosis (CF) to earlier infection with a bug, Pseudomonas aeruginosa, that is resistant to treatment ISRCTN18130649 10.1186/ISRCTN18130649 Accessed 14 October 2023. [DOI]
- 35.Cystic Fibrosis Trust . second ed. 2022. Laboratory Standards for Processing Microbiological Samples from People with Cystic Fibrosis.https://www.cysticfibrosis.org.uk/sites/default/files/202301/CF%20Lab%20Standards%20FINAL.pdf [Google Scholar]
- 36.Cystic Fibrosis Refresh Top 10 priorities (priority setting in association with the James Lind Alliance). https://www.jla.nihr.ac.uk/priority-setting-partnerships/cystic-fibrosis-refresh/top-10-priorities.htm Accessed 14 October 2023.
- 37.Ronchetti K., Tame J.D., Paisey C., Thia L.P., Doull I., Howe R., Mahenthiralingam E., Forton J.T. The CF-Sputum Induction Trial (CF-SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial. Lancet Respir. Med. 2018;6:461–471. doi: 10.1016/S2213-2600(18)30171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on request to Meyer Children's Hospital IRCCS.