Abstract
Introduction
Individuals with a history of smoking and a high risk of lung cancer often have a high prevalence of smoking-related comorbidities. The presence of these comorbidities might alter the benefit-to-harm ratio of lung cancer screening by influencing the risk of complications, quality of life, and competing risks of death. Nevertheless, individuals with chronic diseases are underrepresented in screening clinical trials. In this study, we use microsimulation modeling to determine the impact of chronic diseases on lung cancer benefits and harms.
Methods
We extended a validated lung cancer screening microsimulation model that comprehensively recapitulates an individual’s lung cancer development, progression, detection, follow-up, treatment, and survival. We parameterized the model to reflect the impact of chronic diseases on complications from invasive testing, quality of life, and mortality in individuals in five-year age categories between the ages of 50 and 80 years. Outcomes included life-years (LY) gained per 100,000 in patients with chronic obstructive pulmonary disease, diabetes mellitus, heart disease, and history of stroke compared with screening-eligible individuals without comorbidities.
Results
Among individuals between the ages of 50 and 54 years, we found that the presence of a comorbidity altered the LY gained from screening per 100,000 individuals depending on the comorbidity: 4296 LY with no comorbidities; 3462 LY, 3260 LY, 3031 LY, and 3257 LY with chronic obstructive pulmonary disease, heart disease, diabetes mellitus, and stroke, respectively. We observed greater reductions in LY gained in individuals with two comorbidities; we observed similar patterns for individuals between the ages of 55 and 59 years, 60 and 64 years, 65 and 69 years, 70 and 74 years, and 75 and 80 years.
Conclusions
Comorbidities reduce LY gained from screening per 100,000 compared with no comorbidities, and our results can be used by clinicians when discussing the benefits and harms of screening in their patients with comorbidities.
Keywords: Microsimulation modeling, Lung cancer screening, Comorbidity, Decision analytic model
Introduction
Individuals in the United States between the ages of 50 and 80 years who have smoked at least 20 pack-years and currently smoke or have quit within the past 15 years are eligible for lung cancer screening (LCS). The U.S. Preventive Services Task Force (USPSTF) recommends LCS for such high-risk individuals; these recommendations are supported by the results of multiple randomized controlled trials (RCTs) including the National Lung Screening Trial (NLST).1, 2, 3 The NLST was the first RCT to reveal a 20% reduction in lung cancer mortality associated with low-dose computed tomography screening.2,3 Nevertheless, it is important to note that the study population of the NLST and most RCTs consisted of relatively healthy participants, which may not be representative of the general population of current and former smokers.4 Patients with comorbidities were substantially underrepresented in the NLST. A comparison of screening-eligible smokers from a population-based study versus NLST participants found that the former group was older, had a higher prevalence of comorbidities, and had a shorter life expectancy.5 In addition, modeling studies evaluating the effectiveness of low-dose computed tomography did not fully consider the impact of comorbidities on the benefits and harms of screening. Therefore, it is unclear whether caution should be exercised when generalizing the results of NLST to the population of screening-eligible smokers, especially those with comorbidities.
Individuals at high risk of lung cancer often have a high prevalence of smoking-related comorbidities.5 Among the most common is chronic obstructive pulmonary disease (COPD), which co-occurs in approximately 35% of screening-eligible individuals.6, 7, 8 In addition, cardiovascular disease (CVD), including both ischemic heart disease and congestive heart failure, frequently co-occur, affecting approximately 30% of screening-eligible individuals.4 Other comorbidities such as stroke (11% prevalence) and diabetes mellitus (DM, approximately 25% prevalence) also frequently co-occur.5 These comorbidities may increase the risk of complications related to the workup of suspicious nodules and lead to decreased quality of life and a limited life expectancy, substantially altering the harm-benefit ratio of LCS. The high burden of comorbidities among individuals who smoke may have contributed to the cautious adoption of LCS, despite the evidence of its effectiveness.9 A study evaluating LCS implementation in eight Veterans Administration hospitals found that physicians excluded many USPSTF-eligible individuals from screening due to their comorbidities.10
At present, clinicians have no guidance to determine whether individuals with comorbidities will benefit from LCS. There has been an urgent call by the American Thoracic Society and others for research that can help direct clinical decision-making with patients who may experience different harms and benefits from LCS due to coexisting chronic illness.11,12 Exploring the nuanced relationship between lung cancer risk and screening and diagnostic and treatment-related harms that may be worsened by chronic disease and risk of death from competing causes is crucial in determining the indications for LCS. In this study, we use simulation modeling to determine the benefits and harms of individuals eligible for LCS while considering comorbidities. Simulation modeling is a comparative effectiveness technique that has been successfully used to extrapolate findings to unstudied groups, offering a complementary approach to RCTs. Modeling has been used to inform national guidelines for breast, colorectal, and lung cancer screening.13, 14, 15, 16
Methods
Simulation Model Overview
We developed our LCS simulation model by creating an improved version of a National Cancer Institute–sponsored state-transition microsimulation model that comprehensively simulates a patient’s lung cancer development, progression, detection, follow-up, treatment, and survival.17, 18, 19, 20, 21 The model was used in the development of two rounds of the USPSTF’s LCS recommendations.13,22 We developed the model using a rigorous, object-oriented design that generates the life histories of simulated patients. The model initially populates with disease-free individuals who then go through different health states according to monthly transition probabilities. In each monthly cycle, an individual may develop lung cancer, have an existing cancer grow, or develop metastases.23 The model has been extensively calibrated and validated.24,25 Although the model was comprehensive, it did not incorporate details on the simulated patient’s comorbidities and the outcomes of follow-up procedures and lung cancer treatments based on patients’ underlying health conditions. For this study, we have expanded the model to estimate the outcomes of LCS based on patients’ comorbidities. The study was determined to be exempt human research as defined by DHHS regulations by the Institutional Review Board of Icahn School of Medicine at Mount Sinai (HS-19-01319).
Model Inputs for Patients With Comorbidities
We comprehensively reviewed the literature and conducted primary analyses of a large cancer screening data set to assess factors unique to patients with comorbidities who are eligible for LCS. The newly parameterized model reflects changes to mortality (from competing risks), complications from workup, and quality of life utilities among these individuals. Parameters and data sources are listed in Table 1,26, 27, 28, 29, 30, 31 Supplementary Table 1, and Supplementary Table 2.
Table 1.
Key Input Parameters for Developing a Microsimulation Model of Lung Cancer Screening in Patients With Comorbidities
| Model Parameter | Definition | Value | Sources | |
|---|---|---|---|---|
| Complications from Lung Cancer Screening | Probability of complications from lung cancer screening | Supplementary Table 1 | Previous work using PLCO26 | |
| Mortality from Non-Lung Cancer Causes | Death rates for non-lung cancer causes by age and comorbidity | Supplementary Table 2 | Secondary data, NHIS | |
| Comorbidity | Utility | |||
|---|---|---|---|---|
| Quality of Life for Patients With Comorbidities | Expected quality of life by comorbidity | Stroke | −0.051 | 27, 28, 29, 30, 31 |
| DM | −0.044 | |||
| COPD | −0.038 | |||
| CVD | −0.0235 |
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; NHIS, National Health Interview Survey; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
Probability of Complications Due to Comorbidities
To estimate the probability of complications from LCS diagnostic evaluation, we used data from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) to identify participants with baseline questionnaire information that included risk factors and health history.26 We identified 3032 participants above 50 years old with at least a 20 pack-year smoking history and who had one or more diagnostic procedures, defined as surgical biopsy (including thoracotomy, thoracoscopy, and resection), needle biopsy (including thoracentesis), bronchoscopy (with or without biopsy), mediastinoscopy, and “other” procedure. Our primary outcomes were complications stratified by severity (major, intermediate, and minor) and comorbidity and defined as those that occurred within 14 days of needle biopsy and bronchoscopy and within 60 days of mediastinoscopy and surgical biopsy (see the definitions of severity in Supplementary Table 3).
Chronic Disease Mortality
To estimate chronic disease-attributed mortality, we conducted analyses of the National Health Interview Survey (NHIS), which is a series of annual cross-sectional national surveys that provide information on the health of the noninstitutionalized population of the United States. The sample design uses a multistage area probability design that adjusts for nonresponse and further allows for a nationally representative sampling of households and individuals, including traditionally underrepresented groups. We used the publicly available NHIS Linked Mortality File, which provides users with information from the National Death Index (NDI) for eligible NHIS respondents. We used self-reported age, sex, comorbidities, and smoking history for NHIS files from 1998 to 2018 and removed lung cancer deaths. We estimated the baseline hazard and hazard ratios using age, sex, self-reported comorbidities, and smoking history.
Quality-Adjusted Life Expectancy
We accounted for quality of life affected by comorbidities by comprehensively reviewing the literature to derive the negative impact of comorbidities with estimated utility values based on the Eq-5D administered in the National Health Measurement Study.27, 28, 29, 30, 31
Population
The model was populated with 1 million individual men and women in the following six age categories: 50 to 54, 55 to 59, 60 to 64, 65 to 69, 70 to 74, and 75 to 80 years of age. We segmented age into five-year increments, as this interval is suitable for assessing the influence of chronic diseases on quality of life and life expectancy within the screening age range of 50 to 80 years, consistent with the LCS recommendations of the USPSTF. Smoking histories for each person were simulated by the CISNET Smoking History Generator, which was developed to provide stochastic simulation of smoking history specific to age, birth cohort, and sex (more information may be found at https://cisnet.cancer.gov/lung).32, 33, 34, 35 Standardized inputs from the SHG include rate of smoking initiation, smoking intensity, and rate of smoking cessation.
Outcomes
The primary analysis focused on the 2011 USPSTF eligibility criteria of 20 pack-year smoking history and annual screening between the ages 50 and 80 years. The model was used to estimate the benefits and harms of screening in patients with comorbidities who received screening versus individuals without comorbidities. Simulated outcomes included life-years (LY) and quality-adjusted life years (QALY) gained per 100,000 individuals to compare patients with comorbidities who received screening to individuals without comorbidities who received screening. We also evaluated lung cancer deaths averted per 100,000 individuals and lifetime cumulative lung cancer mortality reduction if annual screening is initiated for individuals in a specific age range. Harms included the number of complications, including major, intermediate, and minor per 100,000 screened individuals. We also evaluated the number of biopsies. We simulated patients up to age 100 years with lone comorbidities and the presence of two comorbidities.
Results
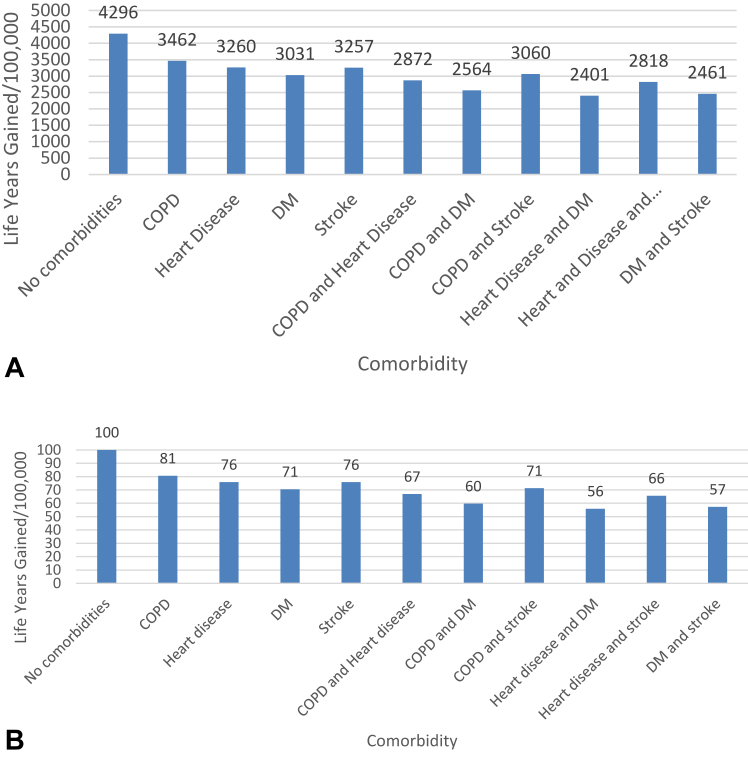
Figure 1A summarizes the life-years gained per 100,000 individuals based on presence of comorbidity relative to individuals without comorbidities; complete estimates for all age categories can be found in the Supplement. For example, per 100,000 individuals without comorbidities screened between the ages of 50 and 54 years, 4296 LY were gained, compared with individuals with COPD (3462 LY-gained), heart disease (3260 LY-gained), DM (3031 LY-gained), stroke (3257 LY-gained), COPD and heart disease (2872 LY-gained), COPD and DM (2564 LY-gained), COPD and stroke (3060 LY-gained), heart disease and DM (2401 LY-gained), heart disease and stroke (2818 LY-gained), and DM and stroke (2461 LY-gained). In Supplementary Figures 1 and 2, we summarize the effects of screening on LG-gained in all age group categories. Among individuals in the highest age category (75–80 yo), we found that screening led to 1237 LY gained per 100K screened, compared with individuals with COPD (1006 LY-gained), heart disease (957 LY-gained), DM (898 LY-gained), and stroke (941 LY-gained). We found a similar pattern of effect of comorbidities on LY-gained from screening in each age group; with screening leading to the highest LY-gained among individuals without comorbidities and declining LY-gained among individuals with comorbidities in each age group.
Figure 1.
(A) Life-years gained per 100,000 screened in individuals 50 to 54 years of age. (B) Relative life-years gained per 100,000 from screening in individuals 50 to 54 years of age. DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
In Figure 1B, we summarized these results as proportions relative to individuals without comorbidities. We found that compared with individuals without comorbidities, the presence of one or more comorbidities led to relative LY gained from screening ranging from 56% to 81%. In individuals between the ages of 75 and 80, LY gained relative to individuals with no comorbidities ranged from 28% to 81% (Supplementary Figs. 1 and 2), with similar ranges for other age groups. The estimated number of QALY gained per 100,000 due to screening by presence of one or two comorbidities followed the general pattern of LY gained (Supplementary Figs. 3 and 4).
Lung Cancer Deaths Averted and Lung Cancer Mortality Reduction
The estimated number of lung cancer deaths averted (Supplementary Table 4) per 100,000 screened individuals between the ages of 50 and 54 years was 399 (3465 and 3066 without and with screening, respectively) for individuals with no comorbidities and ranged from 334 (DM—3255 and 2921 without and with screening, respectively) to 365 (COPD—3330 and 2965 without and with screening, respectively) per 100,000 screened individuals with one comorbidity. This corresponded to a lung cancer mortality reduction ranging from 10.0% to 10.1% for individuals with one comorbidity and 10.0% for individuals with no comorbidities (Table 2). We found declining numbers of lung cancer deaths averted with increasing age, corresponding to declining lung cancer mortality reduction. For individuals between the ages of 75 and 80 years, there were 250 lung cancer deaths averted for those with no comorbidities and ranged from 207 (DM) to 232 (COPD) per 100,000 screened individuals with one comorbidity (Supplementary Table 4).
Table 2.
Lung Cancer Mortality Reduction in Individuals With One Comorbidity
| Age (y) | No Comorbidities (%) | COPD (%) | Heart Disease (%) | DM (%) | Stroke (%) |
|---|---|---|---|---|---|
| 50–54 | 9.98 | 10.03 | 10.07 | 10.09 | 10.07 |
| 55–59 | 9.34 | 9.61 | 9.61 | 9.70 | 9.61 |
| 60–65 | 9.13 | 9.18 | 9.29 | 9.34 | 9.28 |
| 65–69 | 8.54 | 8.70 | 8.75 | 8.82 | 8.75 |
| 70–74 | 6.98 | 7.11 | 7.09 | 7.19 | 7.09 |
| 75–80 | 5.33 | 5.51 | 5.41 | 5.42 | 5.54 |
DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
The lung cancer mortality reduction for individuals between the ages of 75 and 80 years was 5.3% with no comorbidities and ranged from 5.4% to 5.1% for individuals with one comorbidity. In the presence of two comorbidities (Table 3), lung cancer mortality reductions similarly declined with increasing age.
Table 3.
Lung Cancer Mortality Reduction in Individuals With Two Comorbidities
| Age (y) | COPD and Heart Disease (%) | COPD and DM (%) | COPD and Stroke (%) | Heart Disease and DM (%) | Heart Disease and Stroke (%) | DM and Stroke (%) |
|---|---|---|---|---|---|---|
| 50–54 | 10.19 | 10.26 | 10.15 | 10.25 | 10.19 | 10.25 |
| 55–59 | 9.76 | 9.77 | 9.74 | 9.78 | 9.75 | 9.74 |
| 60–65 | 9.27 | 9.27 | 9.25 | 9.39 | 9.40 | 9.43 |
| 65–69 | 8.78 | 8.84 | 8.79 | 8.86 | 8.82 | 8.86 |
| 70–74 | 7.18 | 7.16 | 7.17 | 7.18 | 7.21 | 7.17 |
| 75–80 | 5.63 | 5.51 | 5.64 | 5.63 | 5.50 | 5.35 |
DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Complications From Screening
We simulated the numbers of biopsies performed per 100,000 screened individuals (Supplementary Table 5). These analyses revealed that the presence of comorbidities led to a decrease in the number of biopsies performed, with larger decreases in the presence of two comorbidities due to a shorter life expectancy and fewer screenings and evaluations. Among individuals between the ages of 50 and 54 years, there were 862, 852, and 841 biopsies per 100,000 screened individuals in the setting of no comorbidities, COPD, and COPD and heart disease, respectively. Among individuals between the ages of 75 and 80 years, there were 248, 248, and 237 biopsies per 100,000 screened individuals in the setting of no comorbidities, COPD, and COPD and heart disease, respectively. Increasing age led to a decrease in the number of biopsies performed across all comorbidities. We used our microsimulation to estimate the occurrence of major complications per 100,000 screened individuals (Table 4). In individuals between the ages of 50 and 54 years without comorbidities, there were 44.7 major complications, and in the presence of a lone comorbidity, there were 29.8 to 45.6 major complications. In the presence of two comorbidities, there were 45.4 to 46.6 major complications per 100,000 screened individuals. In individuals between the ages of 75 and 80 years, there were 11.2 major complications per 100,000 screened individuals, and in the presence of a lone comorbidity, there were 7.5 to 11.7 major complications per 100,000 screened individuals. In the presence of two comorbidities, there were 11.4 to 12.5 major complications per 100,000 screened individuals.
Table 4.
Major Complications per 100,000
| Age (y) | No Comorbidities | COPD | Heart Disease | DM | Stroke | COPD and Heart Disease | COPD and DM | COPD and Stroke | Heart Disease and DM | Heart Disease and Stroke | DM and Stroke |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50–54 | 44.7 (<0.1%) | 45.0 (<0.1% | 45.6 (<0.1%) | 29.8 (<0.1%) | 45.3 (<0.1%) | 46.0 (<0.1%) | 46.6 (<0.1%) | 45.7 (<0.1%) | 45.5 (<0.1%) | 45.7 (<0.1%) | 45.4 (<0.1%) |
| 55–59 | 43.0 (<0.1%) | 43.1 (<0.1%) | 43.8 (<0.1%) | 28.7 (<0.1%) | 43.6 (<0.1%) | 44.2 (<0.1%) | 44.9 (<0.1%) | 44.1 (<0.1%) | 43.8 (<0.1%) | 44.1 (<0.1%) | 43.5 (<0.1%) |
| 60–65 | 32.4 (<0.1%) | 32.7 (<0.1%) | 33.5 (<0.1%) | 21.6 (<0.1%) | 33.1 (<0.1%) | 33.8 (<0.1%) | 34.2 (<0.1%) | 33.4 (<0.1%) | 33.2 (<0.1%) | 33.4 (<0.1%) | 33.2 (<0.1%) |
| 65–69 | 29.4 (<0.1%) | 29.9 (<0.1%) | 30.8 (<0.1%) | 19.5 (<0.1%) | 30.0 (<0.1%) | 31.2 (<0.1%) | 31.6 (<0.1%) | 30.6 (<0.1%) | 30.3 (<0.1%) | 30.6 (<0.1%) | 30.5 (<0.1%) |
| 70–74 | 20.4 (<0.1%) | 20.8 (<0.1%) | 21.3 (<0.1%) | 13.4 (<0.1%) | 20.7 (<0.1%) | 21.7 (<0.1%) | 22.0 (<0.1%) | 21.1 (<0.1%) | 20.9 (<0.1%) | 21.1 (<0.1%) | 21.2 (<0.1%) |
| 75–80 | 11.2 (<0.1%) | 11.6 (<0.1%) | 11.6 (<0.1%) | 7.5 (<0.1%) | 11.7 (<0.1%) | 12.2 (<0.1%) | 12.5 (<0.1%) | 11.4 (<0.1%) | 11.6 (<0.1%) | 11.8 (<0.1%) | 12.0 (<0.1%) |
DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Across all age ranges, the presence of comorbidities with the exception of DM led to increased major complications. This pattern was also observed in estimated intermediate and minor complications (Supplementary Tables 6 and 7).
Discussion
In this study, we expanded an established LCS microsimulation model to evaluate the impact of the presence of diabetes, stroke, COPD, and CVD on LCS benefits and harms. We found that in individuals with one and two comorbidities, there was a decrease in projected LY gained and a larger decrease in QALY gained per 100,000 screened compared with individuals with no comorbidity with little impact on complications from screening. The relative decrease in LY and QALY gained per screening was constant across age groups. We also found that the presence of comorbidities led to a reduced estimate of lung cancer deaths averted as the presence of comorbidities led to a shortened life expectancy. Nevertheless, this did not translate into attenuated lung cancer mortality reduction, which is a ratio of lung cancer deaths averted divided by total lung cancer deaths, as comorbidities affected both parts of this ratio. Our findings suggest that the presence of comorbidities diminishes LCS benefit in individuals with comorbidities without affecting harms.
The potential impact of comorbidities on LCS benefit and harm gained substantial attention in large part because of recommendations from the USPSTF, which advised that LCS should be discontinued if a person develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery.1 The presence of comorbidities leading to excessive harm was further explicated in the Medicare policy decision, in which individuals should be counseled on the “the impact of comorbidities.”36 These policy decisions were in advance of supporting evidence; our study is among the first to use microsimulation modeling to determine the population impact of comorbidities on LCS benefit and harm.
Systematic reviews and meta-analyses have confirmed the higher prevalence of comorbidities in the general population compared with clinical trials.4,37 The underrepresentation of individuals with comorbidities is true of LCS trials as well.38, 39, 40 Nevertheless, studies assessing the impact of these comorbidities on LCS have been limited to the complication rate. A secondary analysis of data from the NLST found that COPD increased the risk of complications from an invasive evaluation; however, a real-world investigation of Veterans found that comorbidities (with the exception of dementia) were not associated with an increase in complications.41,42 In our study, we found that the presence of chronic diseases on competing risk of mortality had the greatest impact on LCS outcomes. There is a growing recognition of the importance of accounting for competing risks of death from chronic diseases in individuals with cancer; competing risks can affect cancer mortality by reducing the probability of dying from cancer as individuals age and become more susceptible to other causes of death.43 This is an important factor despite the aggressiveness of cancer, such as in lung cancer. In one institution-based study of early stage lung cancer, the cumulative incidence of non-cancer death was higher than cancer death in the 2.5 years after surgery among people more than 65 years of age.44 In our study, we found that LY gained from screening was diminished by 20% to 30% with one comorbidity and by 40% to 45% with two comorbidities; the presence of DM led to the greatest reduction in LY and QALY gains. Multiple population-based studies have revealed that DM increases the risk of all-cause mortality and other disease-specific mortality, such as CVD.45,46
There are limitations to our study. We determined the presence of comorbidity in the PLCO using self-report and had limited ability to determine severity of disease. PLCO is also an older LCS trial and, as such, reflects a slightly different diagnostic landscape than the advances in fine-needle biopsy and minimally invasive techniques. Nevertheless, the use of this data set allowed us to include individuals with at least a 20 pack-year smoking history which other cancer screening data sets did not include. In addition, the content of NHIS surveys changed over time, so we were limited to using data sets after the year 1997 to be able to appropriately estimate the impact of our chronic diseases of interest. Nevertheless, the use of NHIS linked to mortality data enabled us to have a rich data source with cause of death. Furthermore, the NHIS establishes the presence of chronic diseases by self-report, which may have led to underreporting of certain diseases such as COPD.47 Future modeling work will assess the impact of the presence of three or more comorbidities in this population and model the potential benefits and harms of the identification of not only lung cancer but also the radiographic presence of emphysema and coronary artery calcifications.
In summary, we used a simulation model to evaluate the LY and QALY gains from screening for patients with comorbidities who are eligible for LCS. We found that the presence of comorbidities decreased LY and QALY compared with individuals with no comorbidities without affecting harms such as complications from invasive diagnostic testing. We also found that this loss was primarily due to competing risk of death from chronic disease. This was especially true for individuals with two comorbidities. Our results help to contextualize the benefits of screening for patients who are underrepresented in clinical trials, which favor the healthy and those without chronic disease. Nevertheless, additional research is needed to understand how these findings may be translated in clinical practice, as LY and QALY are chiefly used to determine health care policies.
CRediT Authorship Contribution Statement
Minal S. Kale: Had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Concept and design; Acquisition, analysis, or interpretation of data; Drafting of the manuscript; Statistical analysis; Funding; Supervision.
Keith Sigel: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content.
Arushi Arora: Acquisition, analysis, or interpretation of data; Statistical analysis.
Bart S. Ferket: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content.
Juan Wisnivesky: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content.
Chung Yin Kong: Concept and design; Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content; Statistical analysis; Supervision.
Disclosure
Dr. Wisnivesky has received consulting honorarium from Sanofi, PPD, and Banook and research grants from Sanofi, Regeneron, Axella, and Arnold Consultants. The remaining authors declare no conflict of interest.
Acknowledgments
This work was supported by the American Cancer Society (RSG 1911801) and the National Institutes of Health (R01MD014890). The funding sources had no role in the design, conduct, or analysis of the study or in the decision to submit the manuscript for publication.
Footnotes
Cite this article as: Kale MS, Sigel K, Arora A, et al. The benefits and harms of lung cancer screening in individuals with comorbidities. JTO Clin Res Rep. 2024;5:100635.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2024.100635.
Supplementary Data
References
- 1.United States Preventive Services Task Force Lung cancer: screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
- 2.Aberle D.R., Adams A.M., Berg C.D., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Koning H.J., van der Aalst C.M., de Jong P.A., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 4.Van Spall H.G., Toren A., Kiss A., Fowler R.A. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233–1240. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 5.Howard D.H., Richards T.B., Bach P.B., Kegler M.C., Berg C.J. Comorbidities, smoking status, and life expectancy among individuals eligible for lung cancer screening. Cancer. 2015;121:4341–4347. doi: 10.1002/cncr.29677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen-Heijnen M.L., Schipper R.M., Razenberg P.P., Crommelin M.A., Coebergh J.W. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer. 1998;21:105–113. doi: 10.1016/s0169-5002(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 7.Young R.P., Hopkins R.J., Christmas T., Black P.N., Metcalf P., Gamble G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 8.Young R.P., Duan F., Chiles C., et al. Airflow limitation and histology shift in the National Lung Screening Trial. The NLST-ACRIN cohort substudy. Am J Respir Crit Care Med. 2015;192:1060–1067. doi: 10.1164/rccm.201505-0894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahnd W.E., Eberth J.M. Lung cancer screening utilization: a behavioral risk factor surveillance system analysis. Am J Prev Med. 2019;57:250–255. doi: 10.1016/j.amepre.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Kinsinger L.S., Anderson C., Kim J., et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399–406. doi: 10.1001/jamainternmed.2016.9022. [DOI] [PubMed] [Google Scholar]
- 11.Rivera M.P., Tanner N.T., Silvestri G.A., et al. Incorporating coexisting chronic illness into decisions about patient selection for lung cancer screening. An official American Thoracic Society research statement. Am J Respir Crit Care Med. 2018;198:e3–e13. doi: 10.1164/rccm.201805-0986ST. [DOI] [PubMed] [Google Scholar]
- 12.Advani S., Braithwaite D. Optimizing selection of candidates for lung cancer screening: role of comorbidity, frailty and life expectancy. Transl Lung Cancer Res. 2019;8(suppl 4):S454–S459. doi: 10.21037/tlcr.2019.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meza R., Jeon J., Toumazis I., et al. Agency for Healthcare Research and Quality; Rockville, MD: 2021. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: A Collaborative Modeling Study for the U.S. Preventive Services Task Force. [PubMed] [Google Scholar]
- 14.Mandelblatt J., Cronin K., de Koning H., Miglioretti D., Schechter C., Stout N. Agency for Healthcare Research and Quality; Rockville, MD: 2015. Collaborative Modeling of U.S. Breast Cancer Screening Strategies. [Google Scholar]
- 15.Knudsen A.B., Rutter C.M., Peterse E.F.P., et al. Agency for Healthcare Research and Quality (US); Rockville, MD: 2021. Colorectal Cancer Screening: An Updated Decision Analysis for the U.S. Preventive Services Task Force. [PubMed] [Google Scholar]
- 16.Kim J.J., Burger E.A., Regan C., Sy S. Agency for Healthcare Research and Quality (US); Rockville, MD: 2018. Screening for Cervical Cancer in Primary Care: A Decision Analysis for the U.S. Preventive Services Task Force. [PubMed] [Google Scholar]
- 17.McMahon P.M., Kong C.Y., Johnson B.E., et al. Chapter 9: the MGH-HMS lung cancer policy model: tobacco control versus screening. Risk Anal. 2012;32(suppl 1):S117–S124. doi: 10.1111/j.1539-6924.2011.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon P.M., Kong C.Y., Bouzan C., et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6:1841–1848. doi: 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Criss S.D., Cao P., Bastani M., et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796–804. doi: 10.7326/M19-0322. [DOI] [PubMed] [Google Scholar]
- 20.McMahon P.M., Kong C.Y., Weinstein M.C., et al. Adopting helical CT screening for lung cancer: potential health consequences during a 15-year period. Cancer. 2008;113:3440–3449. doi: 10.1002/cncr.23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong C.Y., Lee J.M., McMahon P.M., et al. Using radiation risk models in cancer screening simulations: important assumptions and effects on outcome projections. Radiology. 2012;262:977–984. doi: 10.1148/radiol.11110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Koning H.J., Meza R., Plevritis S.K., et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:311–320. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirsadraee S., Oswal D., Alizadeh Y., Caulo A., van Beek E., Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Rad. 2012;4:128–134. doi: 10.4329/wjr.v4.i4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong C.Y., McMahon P.M., Gazelle G.S. Calibration of disease simulation model using an engineering approach. Value Health. 2009;12:521–529. doi: 10.1111/j.1524-4733.2008.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meza R., ten Haaf K., Kong C.Y., et al. Comparative analysis of 5 lung cancer natural history and screening models that reproduce outcomes of the NLST and PLCO trials. Cancer. 2014;120:1713–1724. doi: 10.1002/cncr.28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson E.M., Liu B.Y., Sigel K., Yin C., Wisnivesky J., Kale M.S. Impact of comorbidities on lung cancer screening evaluation. Clin Lung Cancer. 2022;23:402–409. doi: 10.1016/j.cllc.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanmer J., Cherepanov D., Palta M., Kaplan R.M., Feeny D., Fryback D.G. Health condition impacts in a nationally representative cross-sectional survey vary substantially by preference-based health index. Med Decis Making. 2016;36:264–274. doi: 10.1177/0272989X15599546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabin R., de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 29.Moayeri F., Hsueh Y.S., Clarke P., Hua X., Dunt D. Health state utility value in chronic obstructive pulmonary disease (COPD); the challenge of heterogeneity: a systematic review and meta-analysis. COPD. 2016;13:380–398. doi: 10.3109/15412555.2015.1092953. [DOI] [PubMed] [Google Scholar]
- 30.Morey J.R., Jiang S., Klein S., et al. Estimating long-term health utility scores and expenditures for cardiovascular disease from the medical expenditure panel survey. Circ Cardiovasc Qual Outcomes. 2021;14 doi: 10.1161/CIRCOUTCOMES.120.006769. [DOI] [PubMed] [Google Scholar]
- 31.Betts M.B., Rane P., Bergrath E., et al. Utility value estimates in cardiovascular disease and the effect of changing elicitation methods: a systematic literature review. Health Qual Life Outcomes. 2020;18:251. doi: 10.1186/s12955-020-01407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holford T.R., Meza R., Warner K.E., et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA. 2014;311:164–171. doi: 10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holford T.R., Levy D.T., McKay L.A., et al. Patterns of birth cohort-specific smoking histories, 1965–2009. Am J Prev Med. 2014;46:e31–e37. doi: 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon J., Meza R., Krapcho M., Clarke L.D., Byrne J., Levy D.T. Chapter 5: actual and counterfactual smoking prevalence rates in the U.S. population via microsimulation. Risk Anal. 2012;32(suppl 1):S51–S68. doi: 10.1111/j.1539-6924.2011.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeon J., Holford T.R., Levy D.T., et al. Smoking and lung cancer mortality in the United States from 2015 to 2065: a comparative modeling approach. Ann Intern Med. 2018;169:684–693. doi: 10.7326/M18-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen T., Chin J., Baldwin J., et al. CMS.GOV 2022. CAG-00439R: screening for lung cancer with low dose computed tomography (LDCT) https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- 37.Hanlon P., Hannigan L., Rodriguez-Perez J., et al. Representation of people with comorbidity and multimorbidity in clinical trials of novel drug therapies: an individual-level participant data analysis. BMC Med. 2019;17:201. doi: 10.1186/s12916-019-1427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Advani S., Zhang D., Tammemagi M., et al. Comorbidity profiles and lung cancer screening among older adults: U.S. behavioral risk factor surveillance system 2017–2019. Ann Am Thorac Soc. 2021;18:1886–1893. doi: 10.1513/AnnalsATS.202010-1276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almatrafi A., Thomas O., Callister M., Gabe R., Beeken R.J., Neal R. The prevalence of comorbidity in the lung cancer screening population: a systematic review and meta-analysis. J Med Screen. 2023;30:3–13. doi: 10.1177/09691413221117685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll N.M., Burnett-Hartman A.N., Joyce C.A., et al. Real-world clinical implementation of lung cancer screening-evaluating processes to improve screening guidelines-concordance. J Gen Intern Med. 2020;35:1143–1152. doi: 10.1007/s11606-019-05539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iaccarino J.M., Silvestri G.A., Wiener R.S. Patient-level trajectories and outcomes after low-dose CT screening in the national lung screening trial. Chest. 2019;156:965–971. doi: 10.1016/j.chest.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Núñez E.R., Caverly T.J., Zhang S., et al. Invasive procedures and associated complications after initial lung cancer screening in a national cohort of veterans. Chest. 2022;162:475–484. doi: 10.1016/j.chest.2022.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Glas N.A., Kiderlen M., Vandenbroucke J.P., et al. Performing survival analyses in the presence of competing risks: a clinical example in older breast cancer patients. J Natl Cancer Inst. 2015;108:djv366. doi: 10.1093/jnci/djv366. [DOI] [PubMed] [Google Scholar]
- 44.Eguchi T., Bains S., Lee M.C., et al. Impact of increasing age on cause-specific mortality and morbidity in patients with Stage I non-small-cell lung cancer: a competing risks analysis. J Clin Oncol. 2017;35:281–290. doi: 10.1200/JCO.2016.69.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L., Simon B., Shi J., Mallhi A.K., Eisen H.J. Impact of diabetes mellitus on risk of cardiovascular disease and all-cause mortality: evidence on health outcomes and antidiabetic treatment in United States adults. World J Diabetes. 2016;7:449–461. doi: 10.4239/wjd.v7.i18.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S., Wang J., Zhang B., Li X., Liu Y. Diabetes mellitus and cause-specific mortality: a population-based study. Diabetes Metab J. 2019;43:319–341. doi: 10.4093/dmj.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diab N., Gershon A.S., Sin D.D., et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198:1130–1139. doi: 10.1164/rccm.201804-0621CI. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.