Abstract
The aim of this study was to present current research trends on the synergistic use of radiotherapy and immunotherapy (IRT) for cancer treatment. On March 1, 2023, we conducted a literature search for IRT papers using the Web of Science database. We extracted information and constructed two databases – the Core Database (CD) with 864 papers and Generalized Database (GD) with 6344 papers. A bibliometric analysis was performed to provide insights into the research landscape, to identify emerging trends and highly cited papers and journals in the field of IRT. The CD contained 864 papers that were collectively cited 31,818 times. Prominent journals in this area included the New England Journal of Medicine, Lancet Oncology, and the Journal of Clinical Oncology. Corresponding authors from the USA contributed the most publications. In recent years, lung cancer, melanoma, stereotactic radiotherapy, immune checkpoint inhibitors, and the tumor microenvironment emerged as hot research areas. This bibliometric analysis presented quantitative insights into research concerning IRT and proposed potential avenues for further exploration. Moreover, researchers can use our findings to select appropriate journals for publication or identify prospective collaborators. In summary, this bibliometric analysis provides a comprehensive overview of the historical progression and recent advancements in IRT research that may serve as inspiration for future investigations.
Keywords: Bibliometric analysis, Cancer, PD1/PDL1, Radiotherapy, Pembrolizumab, Nivolumab, Immunotherapy, SABR
1. Introduction
Given the suboptimal efficacy of available therapeutic agents for malignant tumors, combination strategies rather than monotherapies could achieve better results due to synergistic effects between different treatments. The rationale and feasibility of combining radiation therapy (RT) with systemic immunotherapies, especially immune checkpoint inhibitors (ICI), have been gradually confirmed in recent years.
RT can enhance tumor-specific immunity, which should result in immune-mediated control of lesions within and surrounding the irradiated sites [[1], [2], [3], [4], [5]]. RT promotes immune recognition by uncovering or releasing previously hidden tumor antigens and upregulating the expression of the major histocompatibility complex I (MHC I) [6]. Subsequent immunostimulatory compounds and “danger signals” released from the tumor prompt the maturation of antigen-presenting cells and subsequent T cell priming and clonal expansion in draining lymph nodes [1,[7], [8], [9]]. Conventional fractionated RT (low dose) normalizes the tumor vasculature, facilitating the trafficking of immune cells into the microenvironment [10,11]. However, radiation doses exceeding 10 Gy/fraction can damage microvessels, resulting in reduced blood perfusion [12]. Furthermore, RT reduces tumor burden by inducing direct death of tumor cells, thus facilitating anti-tumor immunity.
Localized radiation can also activate systemic antitumor immunity, resulting in the destruction of lesions outside the irradiated sites that share some of the same antigens as the radiated tumors. This phenomenon is called the abscopal effect [13]. Although abscopal effects have been identified primarily in animal models and have been described in some case reports, these phenomena have inspired researchers to identify ways to increase systemic antitumor immunity elicited by RT in an attempt to improve prognosis even in patients with metastatic cancer.
ICIs are the hottest form of immunotherapy introduced in recent years and have great potential to synergize with radiotherapy to treat tumors. Treatment with the PD-1 antibody can overcome the suppression of T cells caused by the overexpression of PD-L1 in tumor cells after RT in a Kras-driven mouse model of non-small-cell lung cancer (NSCLC) [14]. Up-regulation of PD-L1 expression by tumor cells after chemoradiation has also been observed in melanoma and glioblastoma cells [15]. Preclinical studies have provided evidence that the combination of radiation with ICIs can effectively stimulate systemic antitumor immunity, highlighting the rationale for the combination of immunotherapy and RT in the clinical setting [1]. Clinical trials have further demonstrated the synergistic effectiveness of combining RT with ICIs in producing significant and long-lasting clinical responses in solid tumors, particularly in patients with lung cancer and melanoma [[16], [17], [18], [19]].
The synergistic use of RT and immunotherapy, also known as immunoradiotherapy (IRT), has shown great potential to enhance the efficacy of cancer treatments. This novel approach to cancer care has garnered significant attention from researchers and clinicians alike since the early 2010s, with a multitude of studies being published each year. Despite the growing body of research on IRT, the complexity and volume of literature can be overwhelming for researchers trying to keep up with the latest trends and advancements in the field. Thus, there is a pressing need for a comprehensive analysis that extracts essential information and provides a clear overview of the current state of IRT research. Such an analysis should not only summarize the key breakthroughs and milestones achieved thus far but should also identify the current focus of research and point to potential future directions. By providing a clear and concise summary of the latest developments in IRT, this analysis can serve as an invaluable resource for researchers and clinicians alike, who wish to stay abreast of the latest findings and trends and will ultimately contribute to the improvement of cancer treatment outcomes. However, IRT involves many subfields, including different cancer types, different immunotherapeutic approaches, and synergistic mechanisms. A traditional review or systematic review approach would be difficult to summarize the research trends and status of each subfield from the thousands of available published papers, and would not effectively reflect the panorama of the whole research field or point out important research directions in the future.
Bibliometric analysis, as a scientific method, is highly suitable for evaluating an entire academic field that encompasses a vast array of publications [20]. This method involves quantitative analysis of knowledge-based data derived from various sources, providing an objective overview of the research landscape, emerging trends, and hot topics within a specific area of study [21]. In doing so, bibliometric analysis offers a unique and comprehensive perspective that can assist researchers in understanding the progress in their field, as well as guide them in defining their own research paths [22]. Moreover, the information derived from a bibliometric analysis can be invaluable in helping researchers identify potential collaborators or suitable journals for publication. This can be particularly useful for researchers looking to expand their network and spread their findings to a wider audience. By conducting a bibliometric analysis, researchers can better understand research trends and patterns related to a specific topic, such as IRT, rather than solely focusing on RT or immunotherapy alone, as previous analyses have done. In addition, bibliometric analysis can naturally divide a large number of papers into different subfields, and the analysis and discussion of these subfields may help researchers to establish a more efficient and systematic knowledge structure of the entire academic discipline. Therefore, it is clear that embarking on a comprehensive bibliometric analysis of IRT is not only beneficial, but necessary to gain a comprehensive understanding of the field. This will not only help researchers to build on existing knowledge but may also identify potential areas for further exploration and research, ultimately contributing to the advancement of the field as a whole.
The present bibliometric analysis provides a detailed evaluation of original research articles on clinical IRT that have been published from 2010 to 2022. The primary objective of this study is to provide a comprehensive overview of the field, identify significant research trends, present notable advancements, and identify the most recent research focus areas. Specifically, this study aims to summarize the research trends and status of IRT for major cancers and point out important future research directions. This in-depth analysis can significantly aid researchers in identifying key publications, top-tier journals, and potential collaborators, thereby fostering a more robust and collaborative research environment. Furthermore, this analysis can also serve as a stimulus for the development of additional studies, as it highlights areas that may require further exploration and research. By providing a thorough understanding of the current state of clinical IRT, this analysis can ultimately contribute to the growth and development of the field.
2. Materials and methods
2.1. Database and publication search strategy
The Web of Science Core Collection (WoSCC) is a widely used bibliometric database [[23], [24], [25]]. Its document type labels are known for their greater precision compared to other databases like Scopus [26]. The Science Citation Index Expanded (SCIE), being a robust and comprehensive subset of the WoSCC, was the ideal choice for our research due to its wide-ranging coverage of scientific disciplines and its ability to provide us with a wealth of valuable data. For this bibliometric analysis, we specifically chose the SCIE database to search for relevant literature.
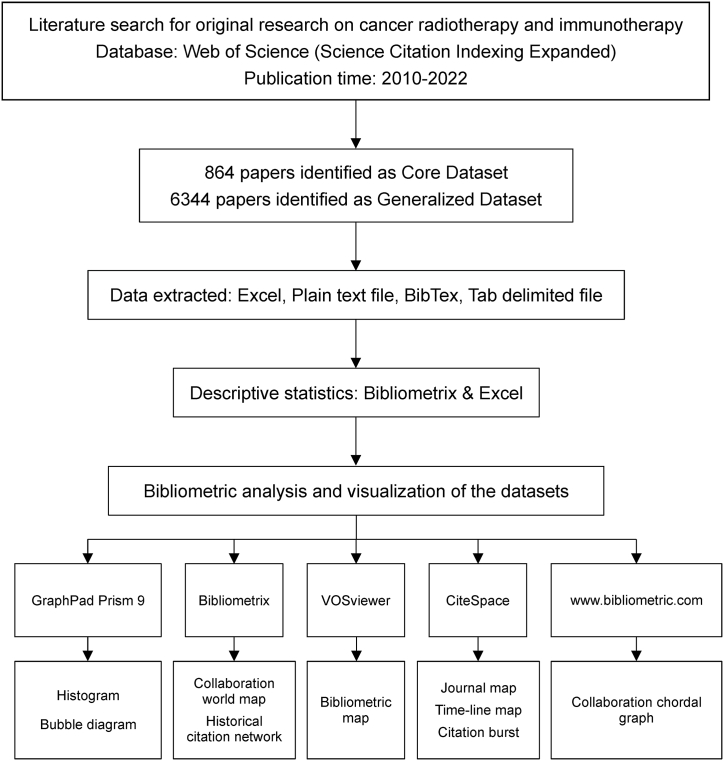
Fig. 1 presents the step-by-step workflow involved in this comprehensive study. On March 1, 2023, we defined a systematic search strategy for the WoSCC (Science Citation Index Expanded) database from 2010 to 2022 to identify literature related to cancer IRT. Our search strategy incorporated cancer-related keywords combined with keywords associated with RT and immunotherapy found in the titles or abstracts of the articles. Subsequently, two distinct datasets were constructed based on this rigorous search strategy: the core dataset (CD) and the generalized dataset (GD). The CD included all keywords that appeared exclusively in article titles to provide a more focused and precise collection of literature. In contrast, the GD included keywords that appeared in titles or abstracts, thereby broadening the scope of our research, and encompassing a wider range of relevant studies. Moreover, the search strategy was designed expressly to exclude articles that were not original research as much as possible.
Fig. 1.
The workflow of the present study.
The primary benefit of using the CD is the enhanced specificity it offers as each article that is included in the CD must have the keywords present in its title. This approach ensures that articles are more likely to directly address the research question. Thus, researchers using the CD can be more confident that the articles they are analyzing are truly relevant to their research topic. Conversely, the GD offers higher sensitivity as it includes articles that have keywords present in their abstracts, even if those keywords are not necessarily in the article's title. This broader approach allows for a more comprehensive analysis of the literature, as it captures articles that might have been overlooked when using a focused dataset. Thus, the GD can help researchers identify articles that, while not directly relevant to their research question, may still offer valuable insights or perspectives. By using both datasets for analysis, researchers can gain a more complete understanding of the literature. This can help address the limitations of each dataset, as it allows for a more balanced approach to research. The focused data set can be used for in-depth analysis, providing a detailed understanding of a specific topic. In contrast, the global dataset can be used for exploratory analysis, helping researchers identify new areas of interest or unexpected connections between articles. The use of both datasets can provide a more comprehensive and nuanced understanding of the literature, leading to more informed research conclusions.
It should be noted that the selection of the dataset relies on the particular research inquiry and analysis objectives. To ensure the sensitivity and specificity, multiple tests and adjustments were conducted. The detailed search strategy is outlined in the Supplementary Material S1.
2.2. Statistical analysis
For statistical analysis purposes, we used Microsoft Office Excel 2019 (Microsoft, Redmond WA) to perform common statistical analysis and generate tables. GraphPad Prism 9 software (Dotmatics San Diego CA) was used to create figures. We also conducted a comprehensive science mapping analysis for bibliometric purposes [27]. Bibliometric analysis and data visualization were conducted using Bibliometrix for R software (v4.1.2). In this study, VOSviewer (Leiden University Netherlands), a software tool that constructs easily interpretable bibliometric maps, was also used [28]. We employed VOSviewer (v1.6.17) to create bibliographic maps representing journals, countries/regions involved in collaborations or co-authorships within publications' author lists or affiliations respectively; as well as keywords associated with these articles. To enhance network presentation quality in VOSviewer maps by merging synonyms and different derivatives of keywords/countries/co-authors together into single entities where appropriate; a customized VOSviewer thesaurus file was created specifically for this purpose. We also standardized writing conventions by capitalizing certain letters since words default to lowercase format in VOSviewer networks. To visualize the collaboration between countries and regions, an online platform (https://bibliometric.com) was utilized. CiteSpace software (v6.1. R2) was employed to present keywords and references with significant citation bursts, visualize timeline maps of co-cited references and keywords. The articles were classified by searching for specific cancers and therapies in titles and abstracts to illustrate the research trends.
3. Results
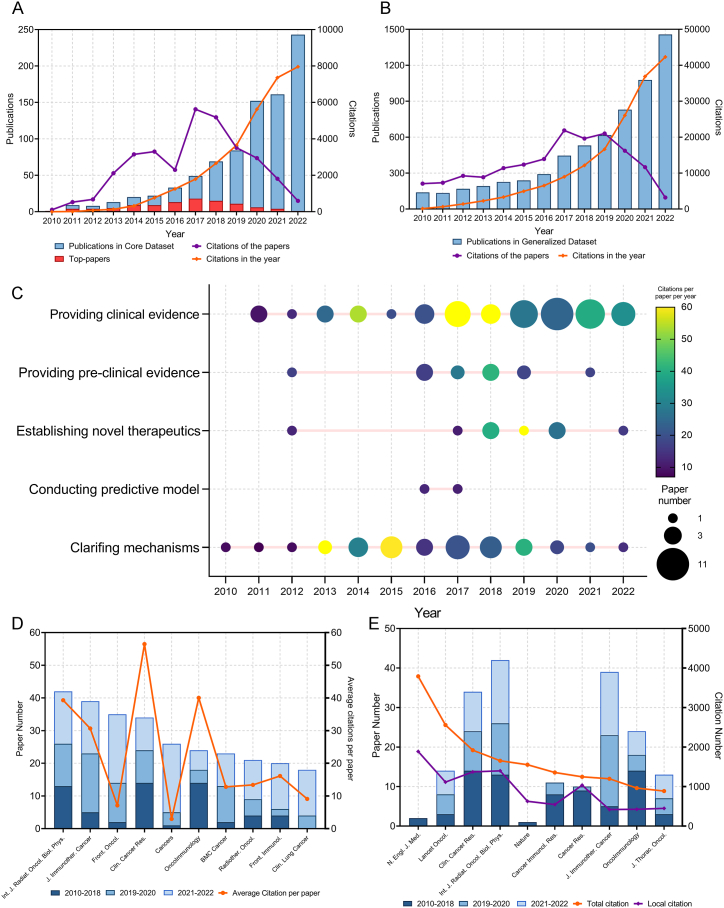
In total, 864 articles directly associated with cancer IRT were identified by an extensive literature search (Fig. 2A). These articles were included in the CD. A review of the published literature revealed a consistent upward trend in the number of publications in recent years, with a notable surge in publications after 2015. Remarkably, the number of publications witnessed a near two-fold increase from 2019 to 2020. These articles collectively received a total number of citations (TC) of 31,818, averaging approximately nine citations per article. Interestingly, despite the relatively small number of publications (49 articles) in 2015, they garnered a substantial TC of 5620. This highlights the importance and impact of these articles on the field of cancer IRT. To gain further insight into the citation relationships among these influential articles, we constructed a historical citation map, as illustrated in Supplementary Fig. S1.
Fig. 2.
(A) Publication and citation number from 2010 to 2022 of the papers in Core Dataset. The purple line indicates the total citations of papers published each year. The orange line indicates the total citations of all papers each year. The red bars indicate the number of top 100 most cited papers. (B) Publication and citation number from 2010 to 2022 of the papers in Generalized Dataset. The purple line indicates the total citations of papers published each year. The orange line indicates the total citations of all papers each year. (C) The time-distribution of the highly influenced original articles. The node size represents the paper number, and the color represents the average citations per paper per year. (D) Paper numbers and average citations per paper of the top-10 productive journals. (E) Top-10 journals with the most citations per paper per year. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Additionally, we conducted an in-depth analysis of the bibliographic map among the highly co-cited references within the CD papers (Supplementary Fig. S2). This network provides valuable information on the interconnectedness of the various research works and the overall growth of the field. The dataset also incorporated the GD, which comprised 6344 papers that experienced a rapid annual growth (Fig. 2B). These papers amassed a TC of 163,674, with an average median count of eight. This indicates that not only are the number of publications directly related to cancer IRT growing at a rapid rate, but also that the field is gaining significant attention and recognition in the broader research community.
The CD contained a collection of articles that were ranked based on the number of times they were cited. We identified the top 100 papers, which accounted for 69.4% of all citations in the CD and had a TC of 22,071 (Supplementary Table S1). The median number of citations for these top papers was 118.5 (range: 63–2302). Table 1 lists the top 10 most cited articles. Among them, 4 were important clinical trials (No.1,3,4,7), 1 was preclinical study (No.9), and the others were basic studies. The highly cited clinical trials demonstrated the efficacy and safety of ICIs (durvalumab, ipilimumab, pembrolizumab) after radiotherapy or chemoradiotherapy for patients with lung cancer or prostate cancer. The preclinical trial reported that a mechanism of abscopal effect is radiation-induced exposure of immunogenic mutations to the immune system. The basic studies revealed a synergy between radiotherapy and immunotherapy from different perspectives. We also identified and listed the top papers from the GD in Supplementary Table S2. This table provides an additional perspective on the most influential research work in the field, offering a comprehensive view of the academic landscape.
Table 1.
The 10 most cited papers of Core Dataset in IRT from 2010 to 2022.
| Rank | Title | Corresponding Author |
Journal | Year | Total citations | Average citations per year (rank) |
|---|---|---|---|---|---|---|
| 1 | Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer | Antonia SJ | N. Engl. J. Med. | 2017 | 2302 | 383.67 (1) |
| 2 | Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer | Minn AJ | Nature | 2015 | 1551 | 193.88 (3) |
| 3 | Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC | Antonia SJ | N. Engl. J. Med. | 2018 | 1488 | 297.6 (2) |
| 4 | Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomized, double-blind, phase 3 trial | Kwon ED | Lancet Oncol. | 2014 | 1017 | 113 (4) |
| 5 | Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade | Dovedi SJ | Cancer Res. | 2014 | 746 | 82.89 (12) |
| 6 | Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS(+)/M1 Phenotype that Orchestrates Effective T Cell Immunotherapy | Huber PE | Cancer Cell | 2013 | 636 | 63.6 (17) |
| 7 | Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial | Lee P | Lancet Oncol. | 2017 | 621 | 103.5 (5) |
| 8 | Anti-PD-1 Blockade and Stereotactic Radiation Produce Long-Term Survival in Mice With Intracranial Gliomas | Lim M | Int. J. Radiat. Oncol. Biol. Phys. | 2013 | 597 | 59.7 (18) |
| 9 | Radiotherapy induces responses of lung cancer to CTLA-4 blockade | Formenti SC; Demaria S | Nat. Med. | 2018 | 435 | 87 (7) |
| 10 | Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen | Drake CG | Cancer Immunol. Res. | 2015 | 424 | 53 (20) |
IRT, immunotherapy plus radiotherapy.
The authors identified a total of 119 highly influenced original articles in CD to evaluate their contribution to the field. These papers had the top-100 TC or average citations per year. Among them, 60 provided clinical evidence, 35 clarified mechanisms, and the others provided pre-clinical evidence, established novel therapeutics, or conducted predictive models. A time-distribution of these papers were visualized as Fig. 2C. Basic studies were initially of high interest; however, in recent years, clinical studies, which provided high-quality evidence, were more influential.
3.1. Journals
In total, 238 journals published articles were assigned to the CD. The International Journal of Radiation Oncology, Biology, Physics had the highest number of publications in CD with 42 articles, followed by the Journal for Immunotherapy of Cancer with 39 articles and Frontiers in Oncology with 35 articles (Fig. 2D). The top three journals covered a wide range of topics related to cancer research. Among the ten journals with the highest number of articles in CD, Clinical Cancer Research stood out as it had the highest average citations per article (CPP, 56.47), average citations per article per year (CPY, 11.66), and the impact factor (13.8), indicating both productivity and influence (Table 2). It is interesting to note that, while some journals were highly productive, they did not always have the highest citation indexes. This suggests that innovative papers, regardless of the journal in which they are published, can be highly regarded, and cited.
Table 2.
The top 10 productive journals of Core Dataset in IRT from 2010 to 2022.
| Journals | Paper number | Total citation | Citation per paper | Citation per paper per year | Local citationa | Paper number in GD | IF (2021) |
|---|---|---|---|---|---|---|---|
| Int. J. Radiat. Oncol. Biol. Phys. | 42 | 1651 | 39.31 | 8 | 1397 | 126 | 8.01 |
| J. Immunother. Cancer | 39 | 1196 | 30.67 | 8.19 | 418 | 148 | 12.47 |
| Front. Oncol. | 35 | 249 | 7.11 | 2.31 | 357 | 173 | 5.74 |
| Clin. Cancer Res. | 34 | 1920 | 56.47 | 11.66 | 1371 | 138 | 13.8 |
| Cancers | 26 | 77 | 2.96 | 1.36 | 163 | 162 | 6.58 |
| OncoImmunology | 24 | 961 | 40.04 | 7.62 | 424 | 95 | 7.72 |
| BMC Cancer | 23 | 294 | 12.78 | 3.48 | 114 | 92 | 4.64 |
| Radiother. Oncol. | 21 | 281 | 13.38 | 4.32 | 355 | 59 | 6.9 |
| Front. Immunol. | 20 | 322 | 16.1 | 3.54 | 223 | 96 | 8.79 |
| Clin. Lung Cancer | 18 | 164 | 9.11 | 3.63 | 124 | 39 | 4.84 |
IRT, immunotherapy plus radiotherapy. GD, Generalized Dataset.
Citation number in Core Dataset.
The top ten journals with the highest CPY demonstrated a notable deviation from the most productive ones, as illustrated in Fig. 2E and in Table 3. Lancet Oncology was the most prolific as it published 14 influential articles in this specific field. The New England Journal of Medicine outperformed all other journals in multiple parameters, including TC (3790), CPP (1895), CPY (340.6), and local citations (the number of citations in CD, LC) (1888). Although the New England Journal of Medicine had a relatively modest contribution with only two articles in the CD, these papers comprised a substantial portion (11.9%) of all citations received within this domain. Furthermore, the Journal of Clinical Oncology earned a high LC (1816), which indicated it had a substantial influence on cancer IRT.
Table 3.
The top 10 journals with highest citations per paper per year of Core Dataset in IRT from 2010 to 2022.b
| Journals | Paper number | Total citation | Citation per paper | Citation per paper per year | Local citationb | Paper number in GD | Citation in GD | IF (2021) |
|---|---|---|---|---|---|---|---|---|
| N. Engl. J. Med. | 2 | 3790 | 1895.00 | 340.64 | 1888 | 5 | 10,045 | 176.08 |
| Nat. Biomed. Eng | 2 | 350 | 175.00 | 41.40 | 22 | 5 | 223 | 29.23 |
| J. Clin. Oncol. | 6 | 697 | 116.17 | 41.33 | 1816 | 35 | 13,220 | 50.72 |
| Lancet Oncol. | 14 | 2558 | 182.71 | 35.76 | 1112 | 29 | 5811 | 54.43 |
| Adv. Mater. | 4 | 477 | 119.25 | 34.96 | 40 | 20 | 1445 | 32.09 |
| Cancer Discov. | 3 | 432 | 144.00 | 34.45 | 130 | 7 | 1025 | 38.27 |
| JAMA Oncol. | 5 | 580 | 116.00 | 32.73 | 458 | 14 | 1650 | 33.01 |
| Cancer Res. | 7 | 1245 | 177.86 | 23.59 | 1033 | 49 | 8040 | 13.31 |
| J. Thorac. Oncol. | 13 | 886 | 68.15 | 23.46 | 447 | 27 | 2209 | 20.12 |
| Ann. Oncol. | 5 | 687 | 137.40 | 20.89 | 557 | 21 | 3824 | 51.77 |
IRT, immunotherapy plus radiotherapy. GD, Generalized Dataset.
aOnly journals with more than one paper were included.
Citation number in Core Dataset.
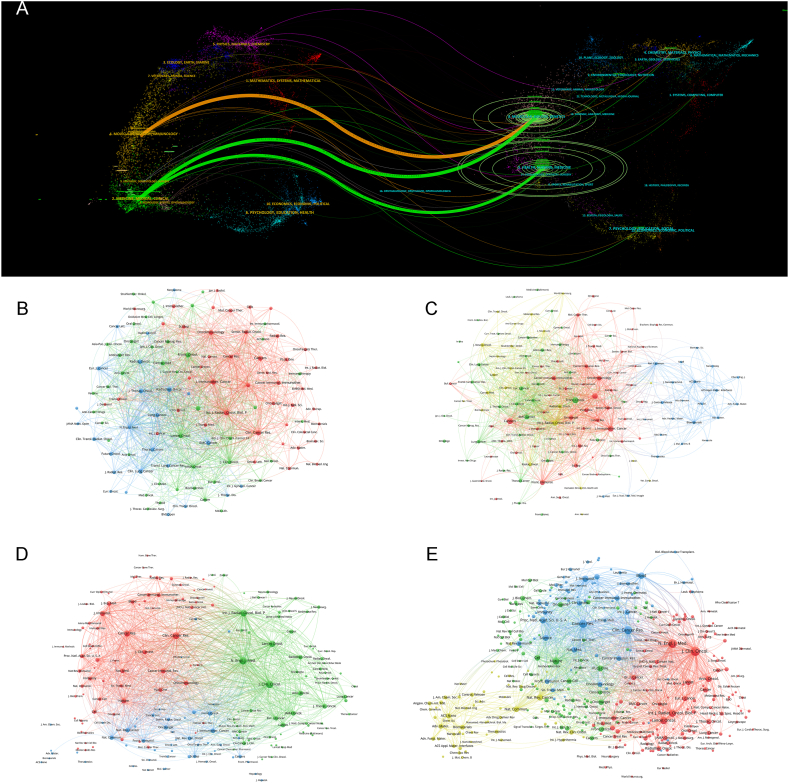
To present a more comprehensive understanding of the academic landscape, we performed a dual representation map overlay, depicting the distribution of academic disciplines and the citations in CD (Fig. 3A). This was followed by the creation of bibliographic coupling networks for journals within CD and GD, which were conducted separately (Fig. 3B–E). Through these analyses, our objective was to uncover the underlying patterns and relationships within the field and to identify the most influential journals and articles. This information can be invaluable for researchers seeking to publish their work in the most impactful journals, as well as for those seeking to stay abreast of the latest developments in their field of interest.
Fig. 3.
(A) The dual-map overlay of journal categories. The left nodes represent citing journals, and the right nodes represent cited journals. The curves represent the citation relationship. (B) Bibliographic coupling of journals with at least two papers in Core Dataset. (C) Bibliographic coupling of journals with at least ten papers in Generalized Dataset. (D) Network visualization of journals with at least 20 total link strength in Core Dataset. (E) Network visualization of journals with at least 100 total link strength in Generalized Dataset. The circle size represents the number of papers. The breadth of the curves represents the link strength. The journals in the same color are of similar research areas. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Countries/regions
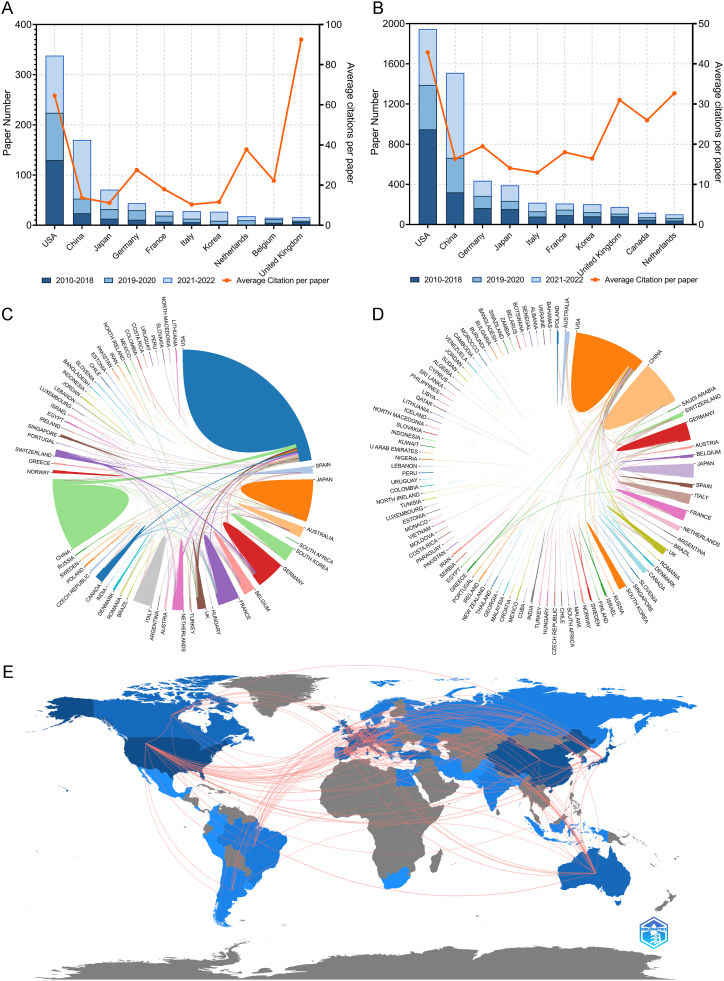
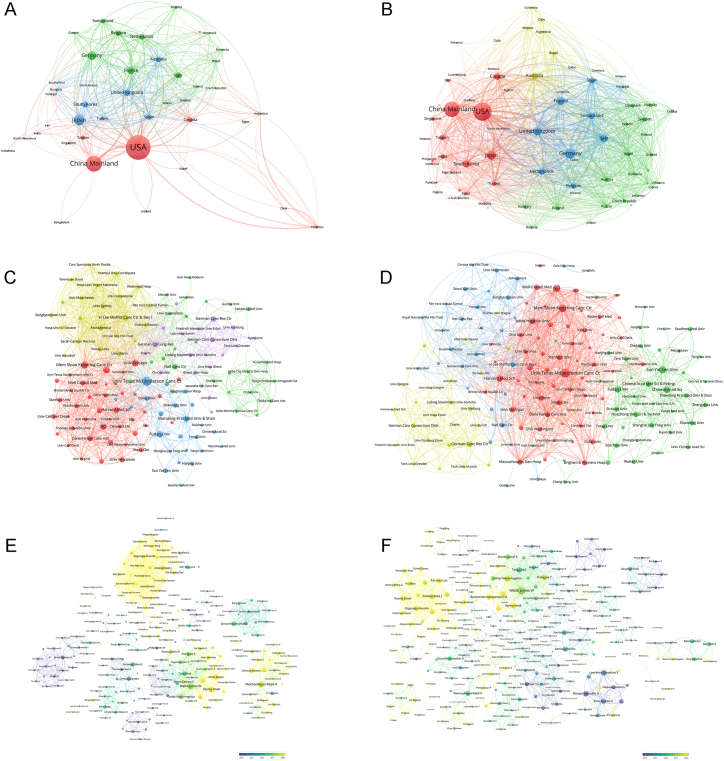
The countries or regions from which researchers contributed to the articles within CD totaled 56, while the corresponding authors were based in 36 of these countries or regions. The United States emerged as the leading contributor with 339 publications included in the CD, followed by China with 171 papers and Japan with 75 articles (Table 4 and Fig. 4A). Papers authored by individuals affiliated with institutions in the United States received the highest number of citations at a staggering count of 21,890, significantly surpassing other countries or regions. In particular, papers authored by corresponding authors from the United Kingdom also garnered a high average number of citations at approximately 92.50 per paper. Most studies originated from single-country collaborations; however, international collaboration was more prevalent among European nations compared to others. Korea exhibited the lowest rate of multi-country collaborations among highly productive countries. A total of 106 countries or regions contributed to research on CD topics. Fig. 4B presents an overview of the most prolific countries with regard to publications related to GD topics. Chordal graphs were used to demonstrate the cooperation among different countries and regions in the CD and GD (Fig. 4C and D), while a global collaborative map showcased the collaboration between countries/regions in the CD papers (Fig. 4E). The United States emerged as the most active collaborator with numerous countries and regions in IRT. Additionally, network visualization maps were used to depict collaborative relationships among countries and regions in both the CD and GD papers (Fig. 5A and B).
Table 4.
The top 10 productive countries of corresponding authorsa of Core Dataset in IRT from 2010 to 2022.
| Countries | Paper number | Percentage (N/864) | Multiple-country paper rateb | Total citation | Citation per paper | Paper number in GD | Citation of papers in GD |
|---|---|---|---|---|---|---|---|
| USA | 339 | 39.24% | 22.10% | 21,890 | 64.57 | 1963 | 84,108 |
| China | 171 | 19.79% | 12.90% | 2337 | 13.67 | 1553 | 25,361 |
| Japan | 75 | 8.68% | 13.30% | 834 | 11.12 | 402 | 5647 |
| Germany | 44 | 5.09% | 34.10% | 1212 | 27.55 | 444 | 8645 |
| France | 28 | 3.24% | 32.10% | 503 | 17.96 | 212 | 3814 |
| Italy | 28 | 3.24% | 14.30% | 290 | 10.36 | 218 | 2818 |
| Korea | 27 | 3.13% | 7.40% | 313 | 11.59 | 205 | 3368 |
| Netherlands | 19 | 2.20% | 47.40% | 718 | 37.79 | 105 | 3431 |
| Belgium | 16 | 1.85% | 43.80% | 355 | 22.19 | 54 | 1254 |
| United Kingdom | 16 | 1.85% | 56.30% | 1480 | 92.50 | 177 | 5485 |
IRT, immunotherapy plus radiotherapy. GD, Generalized Dataset.
Only the first corresponding authors of the papers were analyzed.
Percentage of multiple-country top-papers among all papers of a country.
Fig. 4.
(A) Paper number and average citations of most productive corresponding authors' countries in Core Dataset. (B) Paper number and average citations of most productive corresponding authors' countries in Generalized Dataset. (C) Chordal graphs of international collaboration base on papers in Core Dataset. (D) Chordal graphs of international collaboration base on papers in Generalized Dataset. (E) Visualization world map of publications and collaboration relationship.
Fig. 5.
(A) Network visualization of countries/regions in Core Dataset. (B) Network visualization of countries/regions in Generalized Dataset. (C) Network visualization of institutions with at least 5 papers in Core Dataset. (D) Network visualization of institutions with at least 20 papers in Generalized Dataset. (E) Network visualization of authors with at least 2 papers and 100 citations in Core Dataset. (F) Network visualization of authors with at least 5 papers and 200 citations in Generalized Dataset. The circle size represents the number of papers. The breadth of the curves represents the connection strength. The countries/regions and institutions in the same color have stronger collaboration with each other. The node colors of the authors represent the average publication year. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Institutions
In total, 1731 institutions made contributions to the research papers included in the CD. The University of Texas MD Anderson Cancer Center demonstrated its prowess by publishing an impressive 181 papers (Table 5). Among the top 15 most productive institutions, a majority of 10 were based in the United States. China and Korea, in contrast, had 4 and 1 institution respectively, in the top rank. Remarkably, the research publications from China and Korea were published later than those from their American counterparts.
Table 5.
| The top 15 institutions with the most papers of Core Dataset in IRT from 2010 to 2022.
| Institutions | Country | Paper numbera | Percentage (N/864, %) | Average publication year | Paper number in GD | Percentage of GD (N/6344, %) |
|---|---|---|---|---|---|---|
| Univ Texas Md Anderson Canc Ctr | USA | 181 | 20.95% | 2020.7 | 672 | 10.59% |
| Univ Chicago | USA | 100 | 11.57% | 2020.6 | 221 | 3.48% |
| Johns Hopkins Univ | USA | 86 | 9.95% | 2020.1 | 265 | 4.18% |
| H Lee Moffitt Canc Ctr and Res Inst | USA | 78 | 9.03% | 2020.9 | 197 | 3.11% |
| Fudan Univ | China | 71 | 8.22% | 2021.3 | 329 | 5.19% |
| Mem Sloan Kettering Canc Ctr | USA | 69 | 7.99% | 2020.5 | 381 | 6.01% |
| Univ Penn | USA | 63 | 7.29% | 2020.2 | 240 | 3.78% |
| Harvard Med Sch | USA | 60 | 6.94% | 2020.8 | 256 | 4.04% |
| Emory Univ | USA | 56 | 6.48% | 2020.8 | 134 | 2.11% |
| Univ Wisconsin | USA | 56 | 6.48% | 2021.1 | 167 | 2.63% |
| China Med Univ | China | 54 | 6.25% | 2021.6 | 178 | 2.81% |
| Sichuan Univ | China | 51 | 5.90% | 2021.6 | 243 | 3.83% |
| Sun Yat Sen Univ | China | 50 | 5.79% | 2020.8 | 489 | 7.71% |
| Sungkyunkwan Univ | Korea | 43 | 4.98% | 2022.0 | 172 | 2.71% |
| Mayo Clin | USA | 42 | 4.86% | 2020.6 | 171 | 2.70% |
IRT, immunotherapy plus radiotherapy. GD, Generalized Dataset.
All papers were included, without limitation of corresponding author's institutions.
To gain a better understanding of the collaborative relationships within these institutions, we constructed collaboration networks and conducted cluster analyses for institutions associated with papers in both the CD and GD (Fig. 5C and D). The results revealed that most establishments tended to prefer domestic collaborations over international ones.
However, it was interesting to observe that institutions that excelled in research within their respective countries often engaged in fruitful international collaborations. This suggests that while domestic collaborations may be the preferred choice for most institutions, international collaborations can play a crucial role in fostering innovative research and driving scientific advancements.
3.4. Authors
The CD and GD datasets consisted of papers authored by more than tens of thousands of researchers who made contributions to their respective fields. The most prolific corresponding author in both datasets was Welsh JW, who co-authored 15 papers in the CD and 24 papers in the GD (Table 6). The 15 papers in CD focused on mechanisms and clinical studies which related with abscopal response, while the other 9 papers exclusive to the GD were indirectly related to abscopal response or IRT. Intriguingly, Antonia SJ, despite publishing only two papers as a corresponding author within this domain, garnered the highest number of citations with a count of 3790, which was the highest in both CD and GD. Both papers proved the importance of durvalumab after chemoradiotherapy in stage III NSCLC. Although only contributing 5 papers in the CD as corresponding author, Demaria S was the second most prolific author and was cited as the corresponding author in GD (17 papers, 2580 citations). Demaria S made great contributions in molecular mechanisms of the synergy of radiotherapy and immunotherapy.
Table 6.
The top 10 productive and cited corresponding authorsa of Core Dataset in IRT from 2010 to 2022.
| Most productive corresponding author | Paper number | Total citation | Average citations per paper | Average publication year | Most cited corresponding author | Paper number | Total citation | Average citations per paper | Average publication year |
|---|---|---|---|---|---|---|---|---|---|
| Welsh JW | 15 | 744 | 49.6 | 2019.3 | Antonia SJ | 2 | 3790 | 1895.0 | 2017.5 |
| Schoenfeld JD | 8 | 416 | 52.0 | 2018.6 | Minn AJ | 2 | 1583 | 791.5 | 2016.0 |
| Karam SD | 6 | 243 | 40.5 | 2019.5 | Kwon ED | 2 | 1278 | 639.0 | 2014.5 |
| Lin WB | 6 | 596 | 99.3 | 2020.3 | Lim M | 3 | 1045 | 348.3 | 2014.7 |
| Sundahl N | 5 | 132 | 26.4 | 2018.8 | Dovedi SJ | 2 | 946 | 473.0 | 2015.5 |
| Morris ZS | 5 | 95 | 19.0 | 2019.8 | Welsh JW | 15 | 744 | 49.6 | 2019.3 |
| Kasmann L | 5 | 34 | 6.8 | 2020.6 | Huber PE | 1 | 636 | 636.0 | 2013.0 |
| Demaria S | 5 | 556 | 111.2 | 2015.8 | Lee P | 1 | 621 | 621.0 | 2017.0 |
| Drake CG | 4 | 620 | 155.0 | 2015.5 | Drake CG | 4 | 620 | 155.0 | 2015.5 |
| Chmura SJ | 4 | 359 | 89.8 | 2020.3 | Lin WB | 6 | 596 | 99.3 | 2020.3 |
IRT, immunotherapy plus radiotherapy.
Only the first corresponding authors of the papers were analyzed.
To gain further insight into the collaboration patterns of these researchers, we constructed collaboration networks and clustering analyses on co-authors affiliated with CD and GD publications (Fig. 5E and F). The results revealed an interesting trend in which Chinese and Japanese authors had a clear preference for establishing stable collaborations within their home countries. This trend could be attributed to the cultural, linguistic, and geographical similarities that these authors share, which foster a more conducive environment for research collaboration.
3.5. Keywords
The study employed a comprehensive approach to identify popular keywords in the field of cancer IRT. First, by considering the terms selected by the authors and the keyword suggestions generated by the WoSCC, a list of potential keywords was compiled. This list was then subjected to a thorough analysis to shortlist the most influential keywords with citation bursts. This analysis was conducted by examining the CD and GD (Supplementary Figs. S3 and S4).
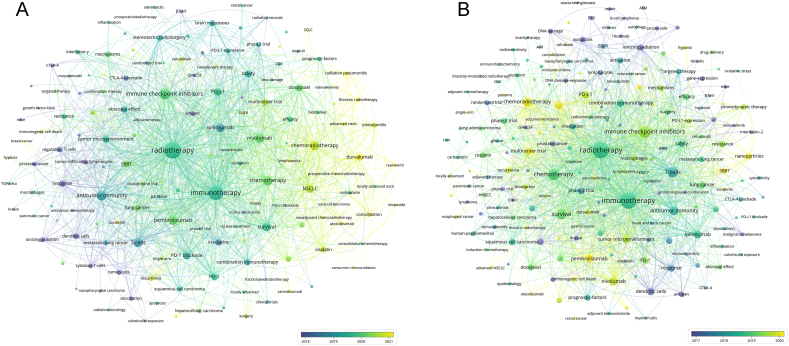
To gain a deeper understanding of the interconnectedness of these keywords, networks were constructed to visualize the co-occurrence and citations of these keywords in the CD and GD. As shown in Fig. 6A and B, these networks helped to identify the central keywords that played a crucial role in shaping the research in this field.
Fig. 6.
(A) Network visualization of keywords that occurred at least 5 times in Core Dataset. (B) Network visualization of keywords that occurred at least 30 times in Generalized Dataset. The circle size represents the number of papers. The breadth of the curves represents the connection strength. The node color represents the average publication year. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The list of notable keywords included those that were frequently mentioned in the literature, such as “nivolumab,” “pembrolizumab,” “abscopal effect,” “ipilimumab,” “PD-L1,” “tumor microenvironment,” and “durvalumab.” These keywords were found to be consistently relevant across various studies and research papers.
Recent additions to the keyword list included terms like “SCLC,” “neoadjuvant therapy,” “hypofractionated radiotherapy,” “oligoprogression,” “hypoxia,” acquired-resistance,” “immunogenic cell death,” and “SBRT.” These terms reflected the evolving landscape of cancer IRT research and highlighted emerging trends and areas of focus in this field.
3.6. Research trends
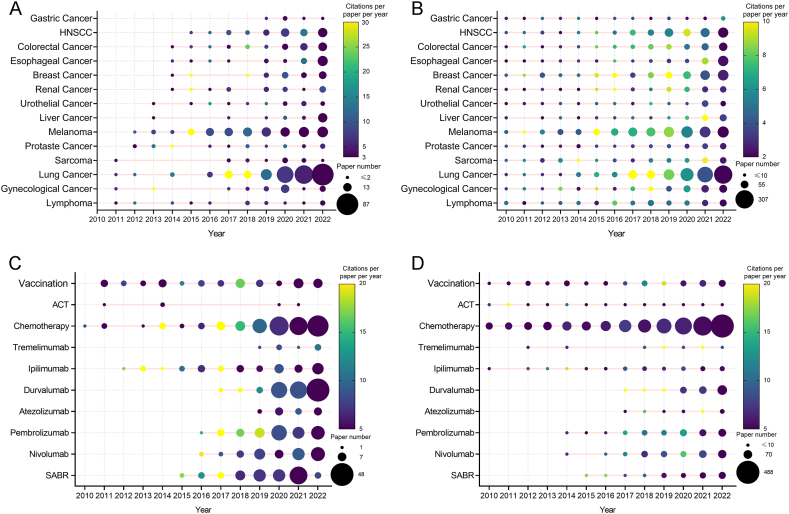
The detailed findings displayed in Fig. 7A and B provide a comprehensive overview of the number of publications and CPY related to various types of cancer in the CD. Research on IRT for lung cancer, lymphoma, melanoma, and prostate cancer began earlier than other types of cancer, indicating the significance of these cancers in the field. Interestingly, there has been a notable increase in articles associated with lung cancer and melanoma since 2016, suggesting that these years have seen significant advances and breakthroughs in research. Additionally, specific years have witnessed a high CPP for particular types of cancers, implying that important breakthroughs and significant progress were reported during those periods.
Fig. 7.
(A) Publication number and citations per paper per year of different cancers in Core Dataset. The node size represents the paper number, and the color represents the average citations per paper per year. (B) Publication number and citations per paper per year of different cancers in Generalized Dataset. The node size represents the paper number, and the color represents the average citations per paper per year. (C) The publication number and average publication year of different treatment modalities in Core Dataset. The node size represents the paper number, and the color represents the average citations per paper per year. (D) The publication number and average publication year of different treatment modalities in Generalized Dataset. The node size represents the paper number, and the color represents the average citations per paper per year. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The analysis focused on the variation in the treatment modalities employed in the studies, delving into the specific methods used and their respective frequencies. The results of this analysis are intricately detailed, as shown in Fig. 7C and D. A close inspection of the data revealed distinct research trends in both the CD and GD. In the CD, durvalumab emerged as a popular treatment option in recent years, with a significant increase in the number of articles published on anti-PD-1 antibodies and stereotactic ablative RT (SABR) since 2017. This increase in popularity was particularly pronounced compared to more traditional chemotherapy, which, interestingly, still maintained a strong presence in both datasets.
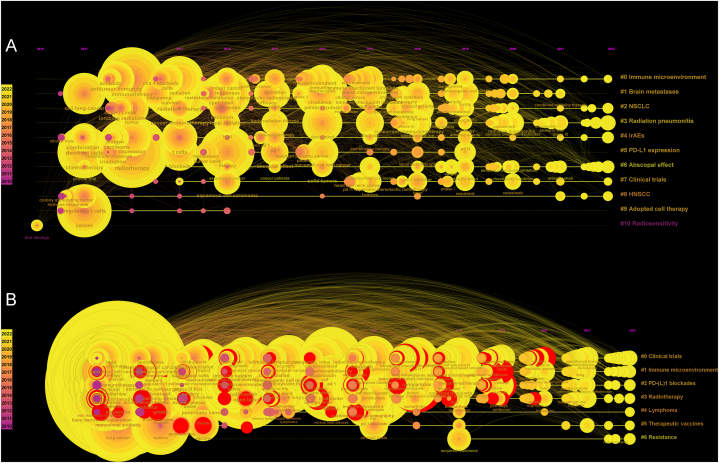
Moving on to the timeline views of the changes in the keywords cited in the IRT (Fig. 8A and B), it was observed that the keywords were clustered into different groups. These groups represented the diverse areas of research that are currently garnering attention and activity in the field. Current research hotspots included the “immune microenvironment,” the “abscopal effect,” “clinical trials,” and “radiotherapy.” Each of these topics has seen a surge in research activity, reflecting the evolving interests and priorities of researchers in the field.
Fig. 8.
(A) The timeline view for co-cited keywords in Core Dataset. (B) The timeline view for co-cited keywords in Generalized Dataset. The node size represents the citation number of the keyword. The curves between the nodes indicated co-citation relationships.
4. Discussion
IRT involves the synergistic application of RT and immunotherapy regimens, which are not necessarily synchronized [29]. RT includes stereotactic ablative RT (SABR), moderate hypofractionated RT (mHFRT), or conventionally fractionated RT (CFRT). IRT refers mainly to treatment with CTLA-4, PD-1, or PD-L1 inhibitors, which are currently widely used in clinical practice. This combination can activate a systemic immune response against tumors to induce abscopal effects, delay tumor progression, prolong survival, and even cure cancer.
4.1. Published clinical evidence for the effectiveness and feasibility of radio-immunotherapy
4.1.1. Lung cancer
Among the various types of cancer investigated in IRT studies, NSCLC has been extensively studied and documented. The combination of CFRT and immunity has shown strong evidence in the treatment of NSCLC, particularly based on findings from the PACIFIC trial regimen: CRT + consolidative PD-L1 antibody. In this trial, patients with stage III NSCLC who received one year of durvalumab treatment after definitive concurrent chemotherapy and RT (cCRT) experienced significant improvements compared to those who received a placebo. These improvements were observed in both overall survival (OS) and progression-free survival (PFS), resulting in a risk ratio of 0.68 [30]. However, further analysis revealed that the OS did not improve for tumors with less than 1% expression of PD-L1 protein [31]. Preliminary results from the ongoing PACIFIC-R study showed similar outcomes as the PACIFIC trial, with patients with stage III NSCLC who received durvalumab after cCRT and sequential CRT (sCRT) experiencing a median PFS duration of 21.7 months. Notably, patients with PD-L1 expression equal to or greater than 1% had longer PFS durations compared to those with less than 1% expression [32]. Another clinical trial known as the GEMSTONE-301 study modified the treatment approach used in the PACIFIC trial by replacing durvalumab with sugemalimab and allowing cCRT and sCRT prior to sugemalimab consolidation therapy [33]. The results demonstrated that sugemalimab is an effective consolidation therapy for patients diagnosed with unresectable LA-NSCLC who do not experience disease progression following cCRT or sCRT [33].
Currently, few studies have evaluated the use of IRT for early lung cancer. A phase II trial compared the effectiveness of durvalumab alone versus durvalumab combined with stereotactic ablative radiotherapy (SABR) in operable patients with early-stage NSCLC. The results revealed a significant difference in pathological response rates between both groups, with 6.7% in the durvalumab group and 53.3% in the IRT group [34]. Currently, there are ongoing studies investigating the potential advantages of incorporating ICIs into SABR treatment for early-stage NSCLC. A recent phase II trial (NCT03110978) compared the effectiveness of SABR alone versus its combination with immunotherapy for patients with early-stage or isolated recurrent node-negative NSCLC involving lung parenchyma. Encouragingly, the results demonstrated that I-SABR significantly enhanced event-free survival at 4 years when compared to standard treatment, achieving a rate of 77% as opposed to 53%, while maintaining acceptable levels of toxicity [35].
A secondary analysis of the KEYNOTE-001 trial suggested that prior RT was related to improved PFS and OS, and provided early evidence for the synergetic effects between immunotherapy and RT in stage IV NSCLC [36]. Preliminary findings from phase II trials and retrospective studies have showed efficacy of IRT in stage IV NSCLC: A pooled analysis evaluating pembrolizumab alone versus in combination with RT for metastatic NSCLC found improved responses and outcomes when adding RT to pembrolizumab immunotherapy [17]. The PEMBRO-RT trial compared pembrolizumab with or without single site SABR, the objective response rate (ORR) at 12 weeks, median PFS (mPFS), and median OS (mOS) were significantly improved by IRT (iSABR) [37]. Contrary to the findings of the PACIFIC trial, subgroup analyses revealed that patients with PD-L1-negative tumors derived the most significant benefit from incorporating RT. A retrospective study involving 95 individuals diagnosed with advanced NSCLC demonstrated that those who received nivolumab in combination with RT (including SABR, mHFRT, or CFRT) experienced a mPFS of 6.3 months and a mOS of 11.9 months [38].
Few data have been published on the efficacy of IRT in small cell lung cancer (SCLC), despite the high tumor mutational burden and the efficacy of PD-L1 antibodies in extensive-stage SCLC. Ongoing trials are combining RT with ICI for extensive-stage SCLC and limited-stage SCLC [39].
4.1.2. Melanoma
There is a lack of robust prospective data regarding the effectiveness of IRT in melanoma. In a prospective cohort study involving 25 advanced melanoma patients with progressive disease despite PD-1 blockade, simultaneous treatment with RT (brain SABR or mHFRT) and anti-PD-1 therapy resulted in observed outcomes such as complete response (CR, 20%), partial response (PR, 19%), and stable disease (SD, 12%) for non-radiated lesions. These findings suggest the occurrence of an abscopal effect [40]. A systematic review examined the incidence of the abscopal effect among 451 metastatic melanoma patients from 16 studies that combined RT with ipilimumab. The reported median rates for abscopal effect and OS were found to be 26.5% and 19 months respectively, with a median overall toxicity grade ≥3 being recorded at18.3%. Notably, better clinical outcomes were observed in patients receiving higher doses per fraction (>3 Gy) along with ipilimumab [41].
4.1.3. Metastatic castrate-resistant prostate cancer
Considerable evidence supports the use of IRT in individuals diagnosed with metastatic castrate-resistant prostate cancer (mCRPC). In a phase III clinical trial named CA184-043, a total of 799 mCRPC patients previously treated with docetaxel underwent RT for their bone metastases, followed by either ipilimumab or a placebo [42,43]. All participants received SABR, where a single dose of 8 Gy was administered to address 1 to 5 metastatic sites. The findings demonstrated that IRT led to enhanced OS rates at different timepoints. Another prospective phase II study called ICE-PAC enrolled 31 mCRPC patients and investigated the combination of avelumab alongside SABR treatment involving one or two disease sites receiving a single dose of 20 Gy 5 days prior to the initial and second doses of avelumab [44]. The combination treatment showed a disease control rate of 48% and a median OS of 14 months. While it is unclear whether this combination provided any additional benefits compared to avelumab alone, certain factors such as baseline androgen receptor mutations or MYC gain (indicating a more aggressive phenotype) and high levels of circulating tumor DNA at baseline (suggesting higher tumor burden) may have been linked to poorer outcomes. A retrospective study also found that patients who exhibited higher levels of PSA or a greater number of bone metastases, more genetic mutations, and previous exposure to chemotherapy showed decreased rates of PSA response following the administration of pembrolizumab with or without SABR [45].
4.1.4. Renal cell carcinoma
There is no conclusive evidence supporting the efficacy of IRT in renal cell carcinoma (RCC). A phase II study (NIVES) did not provide evidence supporting the additional benefit of SABR in metastatic RCC (mRCC) treated with nivolumab [46]. The first infusion of nivolumab was administered 7 days before SABR (30 Gy in 3 fractions). The ORR in non-irradiated lesions was 17%. The results were consistent with previous groups of patients who received treatment solely with nivolumab. A prospective phase I/II trial (RAPPORT) included patients with oligometastatic renal tumors (1–5 oligometastases) [47,48]. Total metastatic SABR (20 Gy in one fraction or 30 Gy in ten fractions), followed by pembrolizumab monotherapy. For 30 evaluable patients, treatments were well tolerated with encouraging ORR, PFS, and OS, and the effects warrant further investigation [47,48].
4.1.5. Urothelial cancer
There is still no conclusive evidence supporting IRT for the treatment of urothelial cancer and SABR combined with ICI seems infeasible for lesions that reside in hollow viscus for safety reasons. A study was performed on a group of 98 patients who were diagnosed with advanced urothelial cancer and received pembrolizumab [49]. Seventeen patients who had previously received RT therapies (in 9 patients with definitive intention, 5 as neoadjuvant therapy before definitive surgery, 4 received definitive RT, and the other 8 with palliative intent) to the primary tumors before initiating pembrolizumab therapy had a higher OS rate (77% vs 50% at 1 year) and higher ORR (65% vs 19%) compared with the non-RT group [49].
In a clinical trial involving 10 patients, the combination of palliative RT with durvalumab was well-tolerated. No abscopal effect or changes in the growth rate of tumors outside the targeted area were observed” [50]. The phase I PLUMMB trial tested pembrolizumab beginning 2 weeks before SABR (36 Gy in 6 fractions) in local advanced or metastatic bladder cancer [51]. This trial was stopped because of 1 grade 4 rectal perforation and 3 grade 3 urinary toxicities.
4.1.6. Esophageal cancer
The benefits of IRT in esophageal cancer have not been confirmed. A neoadjuvant chemoradiotherapy (CRT) regimen, in combination with atezolizumab, was administered to 40 patients diagnosed with resectable esophageal adenocarcinoma in a phase II trial known as PERFECT [52]. Six patients encountered immune-related adverse effects. The rate of achieving a complete pathological response (pCR) was 25%, which did not show any disparity when compared to a group that had been matched based on historical propensity scores. A total of 20 patients diagnosed with esophageal squamous cell carcinoma were enrolled in the PALACE-1 trial [53]. Receiving pembrolizumab before surgery in combination with neoadjuvant CRT yielded similar results as observed in the PERFECT trial. The rate of complete pathological response was unexpectedly higher at 56%. Adverse events (AEs) of grade 3 or above were experienced by 13 patients (65%), primarily lymphopenia. A case of esophageal hemorrhage resulting in fatality raised concerns about the safety and feasibility of using RT for hollow viscus lesions. To further validate the effectiveness and safety of IRT in treating esophageal cancer, a phase III study is necessary, which should involve careful patient selection and meticulous planning for RT.
4.1.7. Breast cancer
Research in testing IRT in breast cancer is preliminary. A trial provided inspiring support for the strategy of combining IRT with HER2 antibody in patients with HER2-positive breast cancer. The efficacy of IRT in triple-negative breast cancer (TNBC) is uncertain. One trial evaluated outcomes after concurrent brain RT and CTLA-4 antibody tremelimumab ± trastuzumab, in 20 patients with brain metastases negative or positive for HER2 [54]. Tremelimumab plus brain RT overcame previous resistance to trastuzumab in a patient with heavily treated HER2-positive, resulting in PR with T cell activation evidence, despite the low non-central nervous system DCR. In the TONIC trial, immune induction with RT (SABR 8Gy*3) in metastatic TNBC (mTNBC) was evaluated in an attempt to improve sensitivity to nivolumab. However, this trial failed to demonstrate the immune induction property of RT in mTNBC [55]. On the contrary, a phase II trial showed promising results. Concurrent pembrolizumab and SBRT were tested in 17 patients with refractory mTNBC, and yielded an ORR of 17.6% [56]. This result was superior to the ORR in cohort A of KEYNOTE-086 trial [57]. The efficacy of IRT in patients with (HR+)/HER2-negative metastatic breast cancer was evaluated in a phase II trial [58]. Patients received pembrolizumab 2–7 days before palliative RT. Since the ORR was 0% in the first eight enrolled patients, the trial was discontinued.
4.1.8. Head and neck squamous cell carcinoma
There is currently few evidence to confirm the effectiveness of IRT, whether in locally advanced or late-stage metastatic head and neck squamous cell carcinoma (HNSCC), although combining ICI with chemoradiotherapy was found to be safe. For locally advanced HNSCC, a phase III trial of 697 patients found no benefit in the combination of avelumab plus standard of care chemoradiotherapy (100 mg/m2 weekly cisplatin, 70 Gy in 35 fractions) followed by maintenance therapy with avelumab for 12 months compared to placebo with minimal increase in toxicity [59]. A phase Ib study treated 59 patients with locally advanced HNSCC with the combination of concurrent pembrolizumab plus standard of care chemoradiotherapy followed by pembrolizumab [60]. The trial has shown that the approach is safe and feasible. A phase III trial KEYNOTE-412 study was launched and is currently ongoing. A randomized phase II trial compared nivolumab alone or combined with SABR (9 Gy*3) for one metastatic lesion in 62 patients with metastatic HNSCC with the primary endpoint of ORR in nonirradiated lesions [61]. No improvement in response was found or with any evidence of an abscopal effect.
4.1.9. Colorectal cancer
Since patients with colorectal cancer with microsatellite stable (MSS) and microsatellite instability-high (MSI-H) lead to different responses to immunotherapy, the clinical developments of IRT should be considered separately based on the MSI status. Currently, several clinical trials are focusing on the neoadjuvant treatment of MSS for patients with locally advanced rectal cancer (LARC). The phase II AVANA trial tested the efficacy and safety of adding avelumab to neoadjuvant chemoradiation for locally advanced MMR-proficient rectal cancer [62,63]. The first interim analysis revealed a pCR rate of 25% and a major pathologic response (MPR) rate of 50% with none of the AEs secondary to the use of avelumab. In contrast, a phase II clinical study investigating the efficacy of administering six cycles of FOLFOX after long-course chemoradiation demonstrated a 37% rate of achieving pCR [64]. A different phase II randomized study was conducted to evaluate the potential enhancement of neoadjuvant rectal (NAR) score by incorporating pembrolizumab into neoadjuvant chemoradiotherapy [65]. The pCR rate was not improved by adding pembrolizumab, although the long-term survival data have not been published. The Grade 3–4 adverse event rate was increased in the pembrolizumab group. Another phase I/II trial enrolled patients with LARC who were treated with neoadjuvant chemoradiotherapy, followed by 5 cycles of nivolumab and subsequent TME 12 weeks after the last dose of chemoradiotherapy, The pCR rate was 30% in MSS patients and the toxicities were tolerable [66]. A high pCR rate (60%) was observed in 5 MSI-H patients, indicating that nivolumab is more effective in this subgroup. Another phase II single-arm trial of LARC, 27 patients received short-course preoperative RT followed by 1–2 cycles of subsequent chemotherapy plus camrelizumab (PD-1 antibody) before TME [67]. The pCR rate was 46.2% in the pMMR subgroup and 100% (1/1) in the dMMR subgroup. All of the reported immune-related AEs were below grade 3.
For metastatic MSS colorectal patients, a single arm, non-randomized phase II trial enrolled 40 metastatic MSS CRC patients [68]. Concurrent SABR (8 Gy*3) was administered every other day or every 2 days, on the second cycle of ipilimumab and nivolumab. Immunotherapy-related AEs ≥ grade 3 were reported in 70% of patients, among which 1 patient experienced grade 5 pneumonitis. The DCR was 25% with an ORR of 10% by intention-to-treat (ITT) analysis.
4.1.10. Pancreatic cancer
MMR-proficient pancreatic cancer (PDAC) is considered refractory to immunotherapy due to its aggressive biology, poor immunogenicity, and immunosuppressive microenvironment [69,70].
A phase II trial tested the combination of radiation, ipilimumab and nivolumab in 25 patients with MSS metastatic PDAC [68]. The DCR was 20% and the ORR was 12% by ITT analysis. Immunotherapy related AEs of Grade ≥3 were reported in 56% of patients with one grade 5 hepatic encephalopathy possibly related to treatment. Higher numbers of natural killer (NK) cells and expression of HERVK repeat RNA in pretreatment biopsies were observed in patients with disease control, providing suggestions for future biomarker research. A phase I study evaluated the safety of treatment with ICI with SABR in patients with metastatic PDAC as a second-line treatment [71]. The patients were divided into four groups to receive durvalumab/durvalumab plus tremelimumab in combination with SABR (8Gy*1 or 5Gy*5). The acute safety profile was acceptable with 3 grade 4 lymphopenia. ORR was 5.1%. The survival data were unsurprisingly disappointing with OS less than 4.2 months. Because of the aggressiveness of the heavily treated late stage PDAC, immunotherapy may not have had time to manifest its efficacy.
A phase II randomized trial enrolled 170 patients with locally advanced pancreatic cancer after surgical resection harboring a mutated KRAS and who were PD-L1-positive [72]. The patients were assigned to receive SBRT (35–40 Gy in five fractions) plus double ICIs (pembrolizumab and trametinib) or SBRT plus gemcitabine. The median OS was 14.9 months with SBRT plus double ICIs group versus 12.8 months with SBRT plus gemcitabine group (HR: 0.69; p = 0.021). Nineteen (22%) participants reported serious AEs in the SBRT plus double ICIs group and 12 (14%) in the SBRT plus gemcitabine group. Treatment-related deaths did not occur. Phase 3 trials are needed to confirm the efficacy and safety of combining SBRT plus pembrolizumab and trametinib.
4.1.11. High-grade glioma
Two preliminary phase I trials confirmed the safety of SABR with ICI in the treatment of recurrent high-grade glioma, and thus further investigation of SABR with ICI in high-grade gliomas is warranted. A phase I study enrolled 32 patients with recurrent high-grade gliomas, triple therapy of pembrolizumab concurrent with SABR (30 Gy in 5 fractions) and bevacizumab was well-tolerated with a grade 3 elevation of aspartate aminotransferase leading to discontinuation of treatment [73]. In the bevacizumab naïve cohort, ORR was 83%, mOS and mPFS were 13.45 months and 7.92 months, respectively. In the bevacizumab resistant cohort, ORR was 62%, mOS and mPFS were 9.3 months and 6.54 months, respectively. The STERIMGLI Phase I trial enrolled 6 patients with recurrent glioblastoma, they received SABR (8 Gy*3) followed by durvalumab until disease progression or for up to 12 months [74]. This combination was well tolerated with 1grade 3 immune-related vestibular neuritis reported. The mPFS and mOS were 2.3 and 16.7 months, respectively.
Two phase III clinical trials investigating the efficacy of combining PD-1 antibodies with standard RT (CFRT) ± temozolomide have yielded disappointing results in patients with untreated glioblastoma. In the CheckMate 498 and CheckMate 548 trials, adding nivolumab to standard RT ± temozolomide did not improve the survival of glioblastoma. The CheckMate 498 study evaluated the efficacy of nivolumab + RT compared to temozolomide + RT in newly diagnosed glioblastoma with an unmethylated MGMT promoter [75]. The study did not meet the primary endpoint of improved OS. The CheckMate 548 study evaluated RT + temozolomide combined with nivolumab or placebo in untreated glioblastoma patients with methylated MGMT promoter. The OS did not improve by adding nivolumab [76]. No new safety concerns were observed.
4.2. The central focus of clinical research on immunoradiotherapy
What defines a successful clinical trial? What demonstrates the effectiveness of a particular treatment? How are the most suitable patients for the particular treatment selected? The central focus of clinical research should not be a particular treatment but rather the patients. It is important to find an optimal and suitable treatment mode for each type of patient. IRT, as an important treatment modality, plays a key role in the treatment mode and should be continuously improved while balancing the toxicity and quality of life.
4.2.1. Optimizing immunoradiotherapy to overcome immunosuppression are the major steps of the cancer-immunity cycle
4.2.1.1. Overturning the competitive of forces between the host and tumor
Only when the tumor is weak and immune system is strong can we ensure victory in the battle against the tumor. Tumor debulking and maintaining a relatively healthy immune system are the conditions we need to achieve. RT acts as a double-edged sword in this process and should be carefully manipulated [77].
4.2.2. Tumor debulking
Increased tumor burden is correlated with decreased efficacy of PD-1 immunotherapy [78]. The goal of tumor debulking is to reduce tumor burden (the total number of tumor cells). The reduction of tumor burden brings two additional benefits: reducing the release of immunosuppressive substances and reducing tumor clones with poor immunogenicity.
RT is the most important and widely used modality in tumor debulking. Other local treatments include surgical resection, cryotherapy, and radiofrequency ablation.
Some evidence supports tumor debulking. Local consolidative therapy (LCT) with RT or surgery led to significantly demonstrated survival in oligometastatic NSCLC in a 2 phase II trial [79,80]. LCT with SABR only in patients with oligometastatic cancers enrolled in the SABR-COMET phase II trial led to improved OS of the SABR group compared to the control group [81]. However, three (4.5%) of the 66 patients in the SABR group experienced treatment-related death. A systematic review of LCT plus systemic therapy versus systemic therapy alone for metastatic NSCLC showed that LCT may improve the prognosis of metastatic NSCLC with acceptable safety profile [82].
The prospective pre-injection phase of the EXTEND basket trial that evaluated the efficacy of LCT (radiation, surgical resection, cryotherapy, radiofrequency ablation) for solid oligometastatic tumors demonstrated encouraging prognosis, and low rates of severe toxicity [5,83].
How to define an ideal tumor reduction effect in the context of immunotherapy? This question remains unanswered and needs further exploration.
4.3. Local consolidative therapy plus immunotherapy is a promising treatment mode
Patients with locally advanced or oligometastatic disease may be a unique population that benefit the most from LCT plus immunotherapy [84]. The reasons for the benefit of patients with oligometastasis from LCT + immunotherapy are as follows: (i) The tumor burden is relatively low, making it easy to eliminate all visible tumors and (ii) the biological properties of the oligometastatic tumor itself are less aggressive.
The PACIFIC trial demonstrated the effectiveness of LCT plus immunotherapy in locally advanced NSCLC [85].
For polymetastatic cancer patients whose tumor burden is beyond oligometastatic disease, it is not currently known whether RT-based LCT may benefit this patient population, and induction systemic therapy involving chemotherapy remains the standard of care. For more rapidly progressive and bulky disease, induction chemotherapy can provide a more immediate response and a biological time frame for the disease to reveal its nature, which could help avoid unnecessarily local definitive therapy. It is not realistic to administer RT or other local treatments to all lesions due to the risk of toxicity. Multimodality treatment strategy with safety concerns might be feasible: targeted SABR to one or a few lesions is safe for high dose radiation to promote immune recognition, with other ablative local treatments to some suitable lesions, followed by low-dose radiation to the remaining lesions for stromal modulation may be feasible [1,11,86].
Multisite SABR (not for all metastases) combined with ICIs showed preliminary efficacy and good tolerance in a phase I study [87]. Patients with advanced solid tumors progressing on standard treatment received SBRT (2–4 metastases) followed by pembrolizumab, the treatment was well tolerated with acceptable toxicity. Out-of-field response of nonirradiated metastases was 13.5% which correlated with interferon-γ-associated gene expression in post-SBRT tumor biopsy specimens.
4.4. Maintaining the quantity and quality of lymphocytes
Besides the immunostimulatory properties of RT, standard fractionation and large radiation fields are also immunosuppressive and can induce systemic lymphopenia [88,89]. Lymphopenia is associated with lower immune-mediated systemic effects and a poorer prognosis in patients with lung cancer who receive combined immunotherapy and RT [86,89,90].
The radiation dose, fractionation schedule, and radiation volume should be carefully formulated according to the individual context of each patient to avoid severe lymphopenia. To mitigate radiation-induced lymphopenia, hypofractionated radiation, reduction of the traditional clinical target volume, sparing healthy regional lymph nodes, where the T cell priming and activation takes place, and large blood vessels are worth exploring in the modern era of immunotherapy and technological improvements in targeting and delivery of RT [91,92].
Clinical trials evaluating the effectiveness and safety of immunopotentiators such as thymosin alpha 1 in the treatment of RT-related lymphopenia are worth trying. Furthermore, the combination of anti- PD-L1 and anti-CTLA-4 therapies should enhance T cell priming and activation (cancer-immunity cycle step 3) [93,94].
4.5. Patient stratification based on host and tumor status
There are three aspects that must be evaluated to stratify suitable patients with solid tumors for IRT.
4.5.1. Evaluation of tumor burden and tumor aggressiveness
For the assessment of tumor burden, the clinical stage is the traditional semiquantitative indicator of tumor burden. In addition, stage IV tumors can be further stratified into oligometastatic disease and polymetastatic disease. However, these indicators are not suitable in the scenario of tumor debulking treatments where quantitative indicators are needed. The circulating tumor DNA fraction and the tumor burden score (TBS) are promising indicators of tumor burden. The TBS was first derived from the tumor burden assessment of liver metastasis of colon cancer, and it was found that TBS was more predictive of prognosis than the maximum diameter or number of liver metastasis [95]. Subsequently, the TBS was found to be predictive of prognosis to liver cancer and pancreas neuroendocrine tumors [96,97]. Circulating tumor DNA fraction (ctDNAf) has been utilized to monitor the tumor burden dynamic of NSCLC patients who received therapies targeting druggable mutations [98]. The clinical feasibility of ctDNA for tumor burden monitoring in NSCLC patients without druggable mutations or other solid tumors remains unknown and is worthy further exploration [99].
The assessment of tumor aggressiveness involves the evaluation of organs affected by tumor metastases. The number of lesions and tumor growth rate (TGR) are traditional approaches used to assess the aggressiveness of cancer. Cancer cell aneuploidy state and its cellular consequence-- genome instability can serve as adaptive mechanisms of survival under stressful conditions such as chemotherapy and manifest more aggressive phenotype [100]. The copy number instability score (CNI) can be determined using cell-free DNA (cfDNA). Alterations in the CNI score have the potential to serve as an early indicator of treatment response to various systemic therapies for cancer [99]. Chromosomal instability (CIN) is a hallmark of human cancer and is associated with therapeutic resistance and tumor aggressiveness [101,102].
The existence of abnormal chromosome numbers has various effects on cellular processes, such as genome instability, metabolic changes, and proteotoxic stress. It is worth noting that this condition is highly prevalent in cancer patients and is associated with poor prognosis and resistance to chemotherapy. Chemotherapy plays a crucial role in treating most cancer patients; however, drug resistance can lead to treatment failure.
4.5.2. Evaluation of host immunity
The absolute lymphocyte count (ALC) is a clinically well standardized indicator of host immunity. As mentioned above, lymphopenia is associated with abscopal responses and a poorer prognosis in patients with lung cancer receiving IRT [86,89,90].
Characterizing T cell function and strength can offer more precise ways to stratify immune function [86]. Preliminary explorations on biomarkers to assess host immunity have been performed, although further exploratory studies are needed. T-cell receptor sequencing and peripheral blood T lymphocyte classification by flow cytometry can identify markers of T-cell activation, proliferation, and clonal expansion [5]. The combined positivity scores of PD-L1 (CPS) and the IFNγ signature in tumor biopsy samples have been associated with therapeutic benefit [52].
4.5.3. Evaluation of tumor immune immunogenicity
Unique tumor neoantigens that result from aberrations in genes are the basis of immune recognition. But not all gene aberrations result in good immunogenicity. Prediction of neoantigen quantity and quality can be obtained by bioinformatics analyses of data derived from whole-exome sequencing (WES) and/or RNA sequencing (RNA-Seq) of cancer tissue. The burden of neoantigens is not an ideal surrogate for tumor immunogenicity. It is the quality of the neoantigen, but not the quantity, that determines immunogenicity [[103], [104], [105], [106], [107]].
A low immunogenicity associated with canonical EGFR mutation (exon 19 deletion/L858R mutation/EGFR/T790 M mutation) or ALK rearrangement positive NSCLC has been reported [108], although uncommon EGFR mutations are associated with better immunogenicity [109]. Tumors with genetic instability often respond poorly to immunotherapy, despite the fact that genomic instability is a driver of tumor immunogenicity, as those tumors have developed mechanisms to escape immune surveillance [110,111]. Pancreatic cancer is characterized by recurrent copy number alterations which lead to low neoantigen and poor immunogenicity. Nonetheless, some tumors can be characterized by recurrent mutations, such as in melanoma and lung adenocarcinoma, which to date have been the most responsive to immunotherapy [70,112].
RT promotes tumor neoantigen release from tumor cells for recognition by antigen-presenting cells (APCs), but there is no evidence that RT can create neoantigen. Essentially, RT cannot alter the immunogenicity of tumors but can only promote immune recognition. Tumors of low immunogenicity may not benefit from IRT alone. Combining IRT with poly-ADP-ribose polymerase inhibitors (PARP) is a possible solution for low immunogenic tumors, as PARP inhibitors have been found to increase tumor neoantigen expression and show synergy with immunotherapy in preclinical and early clinical studies [113]. However, the potential toxicity caused by the combination of three treatments is a concern and needs further research.
4.6. Promoting immune recognition
Immune recognition comprises the first two steps in the cancer-immunity cycle: release of neoantigen (step 1) and presentation of neoantigen by APC (step 2). RT has been shown to cause immunogenic death of tumor cells [4]. Cryoablation on exposure to conventional chemotherapeutics, such as cetuximab and trastuzumab have been found to induce the release of neoantigens of tumor cells [[114], [115], [116]]. The multidisciplinary collaboration of these modalities with IRT is a concept worth investigating in the future.
To maximize the role of RT in promoting immune recognition, one should carefully consider the dose, fractionation, radiation field, and RT technique based on the individual context of each patient. Preclinical studies have found that when the radiation dose per fraction is beyond 10–12 Gy, the high radiation doses can induce DNA exonuclease Trex1 which attenuates immunogenicity by degrading DNA that accumulates in the cytosol resulted in poor synergy with ICIs [117]. Another experiment tested the immune effects of four radiation dose per fraction in a mice melanoma model. Per fraction dose of 7.5 Gy and 10 Gy but not 5 Gy resulted in the best tumor immunity, while doses above 15 Gy increased the fraction of splenic regulatory T (Treg) cells, which are immune-suppressive [118].
Granulocyte macrophage colony stimulating factor (GM-CSF) can enhance the presentation of neoantigens due to its ability to promote dendric cell maturation. A phase II trial enrolled patients with chemotherapy-resistant solid tumors, GM-CSF in combination with IRT (PD-1 inhibitor and radiotherapy) proved to have acceptable toxicity [119].
At tumor draining lymph nodes (TDLN) the presentation of neoantigens occurs plays a pivotal role in systemic antitumor immunity during IRT [120,121]. Currently, positron emission tomography (PET) imaging and mediastinal nodal staging can help to identify metastatic lymph nodes more accurately, and treatments such as immunotherapies are more effective. It is important to reconsider the application of conventional prophylactic regional lymph node irradiation when there is no sign of lymph node metastasis and to choose more prudent target delineation to preserve healthy regional lymph nodes for anti-tumor immunity.
4.6.1. Facilitating T-cell trafficking and infiltration to tumors
An abnormal vasculature and immunosuppressive microenvironment are the two obstacles in the trafficking and infiltration of T cells into tumors (cancer-immunity cycle steps 4–5). Preclinical and clinical evidence suggest that low-dose RT can overcome an immunosuppressive microenvironment of tumor by reducing TGF-β levels, promoting M1 macrophage polarization, facilitating NK cell infiltration, stimulating helper T cells, and depleting myeloid-derived suppressor cells and other immunosuppressive cells [10,11,86,[122], [123], [124]].
The abnormal vasculature of solid tumors that is mainly driven by VEGF signaling eventually forms an immunosuppressive microenvironment with hypoxia and acidosis, high interstitial fluid pressure [125,126]. Anti-VEGF treatments can normalize blood vessels and thus transform the immunosuppressive tumor microenvironment by increasing immune cell infiltration and oxygen supply in the tumor [127]. A low dose of anti-VEGF antibody, such as bevacizumab, showed a better effect on vascular normalization and reprogramming of the tumor microenvironment and may synergize with immunotherapy [125,128,129].
Tregs in the tumor microenvironment highly express CTLA-4 and suppress the function of tumor-reactive T cells responsible for the elimination of cancer cells. Anti-CTLA-4 therapy can deplete intratumoral Tregs through antibody-dependent cellular cytotoxicity and phagocytosis [94].
4.6.2. Removal obstacles during recognition and killing of cancer cells
Recognition of cancer cells by T cells (cancer-immunity cycle step 6) involves the tumor cell downregulation of the expression of MHC I expression to avoid recognition by T cells. RT can up-regulate the expression of the MHC I in tumor cells to overcome this obstacle [[130], [131], [132]].
Killing of cancer cells (cancer-immunity cycle step 7) occurs following the engagement of the immune checkpoint PD-1/PD-L1 axis, which leads to exhaustion of T cells in the phase of immune killing. PD-1/PD-L1 blockade has provided dramatic survival benefits to patients by un-braking of the final step of the cancer-immunity cycle [133]. Inhibition of other immune checkpoints such as LAG-3, immunoglobulin and ITIM domains (TIGIT), T-cell immunoglobulin mucin-3 (TIM-3), and Indoleamine 2, 3-dioxygenase-1 (IDO1) promote the function of cytotoxic T lymphocytes, according to preclinical and preliminary clinical research [[134], [135], [136]].
4.7. Consideration of toxicity
Toxicity remains a critical issue in combination therapies. Given the long half-lives of ICIs, RT conducted after the last dose of ICIs is still considered as combination therapy [137]. For example, it would require 3 months for durvalumab to be cleared from the system. Therefore, RT administered after the recent cessation of checkpoint blockade may be an effect, when given in combination.
Pneumonitis is an overlapping toxicity between immunotherapy and thoracic radiation. PD-1 inhibitors were found to have higher incidence of any grade (3.6% vs. 1.3%) and grade 3–4 (1.1% vs. 0.4%) pneumonitis than PD-L1 inhibitors based on a meta-analysis of 19 trial of NSCLC [138]. The incidence of pneumonitis is higher when the PD-1/PD-L1 inhibitor is combined with chemotherapy, RT, or other ICIs [139]. In a retrospective study including 326 locally advanced NSCLC patients who received chemoradiotherapy or chemoradiotherapy followed by ICI, grade ≥2 treatment-related pulmonary AEs (TRPAEs) were almost doubled in the latter group [140]. Higher number of ICI cycles was also associated with more grade ≥2 TRPAE. However, patients who received >12 cycles of ICI had better OS and PFS than patients who received ≤12 cycles of ICI. The mean lung dose (MLD) was a strong predictor of TRPAE for patients with CRT only, but not for patients with CRTI. These phenomena remind us that shortening the course of ICI or lowering MLD to reduce lung AEs is not a wise strategy.
The SABR-COMET trial enrolled patients with oligometastatic cancer and treated all metastatic lesions with SABR in the SOC plus SABR group. Although the researchers have carefully planned all the SABR with strict dose constraints and peer review, the grade 5 toxicity rate related to SABR reached 4.5%, which is higher than that reported in other studies [141]. CNS toxicities are rare but severe, and have been reported when ICIs are combined with CNS RT [[142], [143], [144]]. A grade 5 esophageal hemorrhage was reported in a trial evaluating the outcome after preoperative pembrolizumab combined with conventional chemoradiotherapy for 20 patients with resectable esophageal squamous cell carcinoma (ESCC) [53]. A grade 4 rectal perforation was reported in a phase I trial that tested pembrolizumab plus SABR in bladder cancer [51]. High-dose radiation for lesions that reside in the hollow viscus can be at risk of perforation or massive hemorrhage, and low-dose radiation aimed at regulating stroma immunity is more feasible. Further studies are needed to find the most immunogenic SABR doses to be used in combination with immunotherapy. Modern radiation modalities have created conditions to reduce the dose of organs at risk (OARs), to adaptively replan, to reduce the PTV, and to perform CTV-omitted RT, and elective nodal RT [91,145,146]. The safety and efficacy of CTV-omitted RT and elective nodal RT merits future studies.
The potential of pre-radiotherapy levels of the sialylated carbohydrate antigen KL-6 in predicting pneumonitis risk was observed in a study involving 27 patients who underwent palliative thoracic RT followed by PD-1 or PD-L1 inhibitors [147]. Further extensive research is required to investigate predictive biomarkers for pneumonitis in the context of IRT.
4.8. Research trends and hotspots
This comprehensive bibliometric analysis offers a detailed and quantitative overview of the current research trends, status, and foci in the field of solid tumor intensity-modulated RT (IMRT). In contrast to other review methods, such as systematic literature reviews or meta-analyses, this study uses a novel approach specifically designed for analyzing IMRT. The research trends were meticulously examined and provide valuable insights into the patterns and directions of research in this field.
This study analyzed research trends. The findings reveal that over the past decade, researchers have primarily concentrated on investigating the combination of RT with ICIs, including PD-1/PD-L1 inhibitors and CTLA-4 inhibitors. Notably, NSCLC and metastatic castrate-resistant prostate cancer have emerged as the most extensively studied solid tumors within this domain. Additionally, recent attention has been directed towards exploring neoadjuvant IRT strategies, combining immunotherapy with stereotactic ablative RT (ISABR), as well as investigating the potential benefits of multisite RT combined with immunotherapy.
The current evidence from IRT research can be classified as follows.
-
(i)
Strong evidence. The strongest evidence supporting the feasibility of IRT comes from the PACIFIC regiment for locally advanced NSCLC: concurrent chemoradiotherapy + consolidative durvalumab. Multisite SABR followed by ipilimumab demonstrated significant OS of metastatic castrate-resistant prostate cancer.
-
(ii)
Moderate evidence. Modifications of the PACIFIC regime have been tested in trials on locally advanced NSCLC, and these modifications have been shown to be effective and safe. Phase I or II studies have been launched that evaluate the addition of an ICI to SABR for early-stage NSCLC and preliminary results are encouraging. Phase II trials and retrospective studies have preliminary showed the efficacy of ICI in combination with RT (mainly SBRT) in stage IV NSCLC.
-
(iii)
Weak evidence. There have been preliminary trials, but there is still no conclusive evidence on the efficacy of ICI combined with RT (mostly SABR) in melanoma, renal cell carcinoma, urothelial cancer, breast cancer, pancreatic cancer, and high-grade glioma. The PACIFIC regimen (i.e., combination of conventional chemoradiation with ICI) was preliminarily evaluated in trials on esophageal cancer and locally advanced HNSCC, although one phase III trial failed to demonstrate the efficacy of avelumab plus chemoradiotherapy in patients with HNSCC. Several phase II trials have focused on the neoadjuvant treatment of MSS in patients with locally advanced rectal cancer and showed promising pCR rate. Overall, acceptable toxicities were present in most existing studies and some encouraging efficacies of IRT have been achieved. Additional phase III studies with prudent patient selection and a careful RT plan are required.
Current research hotspots include: (i) neoadjuvant IRT; (ii) immunotherapy plus stereotactic ablative radiotherapy (ISABR); (iii) multisite RT plus immunotherapy; and (iv) evaluation of the tumor immune microenvironment and immune–resistance mechanisms.
Future research should focus on the following areas: (i) immunotherapy after tumor debulking based on multisite RT in the context of multidisciplinary collaboration; (ii) immunotherapy in combination with CTV-omitting radiation, sparing of unaffected regional lymph nodes and large blood vessels for the purpose of protecting immune function; and (iii) fundamental research and developing innovative therapies to overcome resistance.
4.9. Limitations
This bibliometric analysis had a few limitations. First, the inclusion of papers published between 2010 and 2022 may have excluded earlier publications. Given that most important advances in IRT were published after 2010, this would not have influenced our findings. Second, it was very difficult to design a search strategy which could identify all relevant publications while excluding all irrelevant publications. The authors used two search strategies to construct the CD and GD to present reliable results. The publications included in the CD were directly related to IRT, while the GD contained almost all relevant publications, which allowed to obtain credible evidence by comparing the results from the two databases. Third, it is important to note that citation numbers can be influenced by various factors and may not accurately represent publication or journal influence. To mitigate the impact of publication time on analysis, average citations per year were also considered. Fourthly, the large amount of publication available for analysis made it impractical to read every paper thoroughly and evaluate subareas comprehensively. Therefore, an evaluation of subarea development was conducted along with discussions on important and emerging advances to present research trends and status more effectively. Fifthly, this study focused specifically on clinical IRT as reflected in the search strategy used; thus, some essential basic research studies might have been overlooked intentionally to maintain result reliability without making the article excessively lengthy. Finally, the literature search solely relied on the Web of Science database which might introduce selection bias and analytical errors by omitting papers not included in this database.
5. Conclusions
To the best of our knowledge, the present study represents the first bibliometric analysis on IRT. There is a pressing need to enhance the efficacy and applicability of IRT by improving its treatment modality. The advancement in RT technology and philosophy should be better adapted to meet the demands of immunotherapy. Multisite RT with high-dose and low-dose radiation may be more effective when combined with multidisciplinary treatments for locally consolidative therapies, and may lead to an increased degree of immune activation and improved disease control. This study offers a thorough examination of the research field of IRT, encompassing its historical progression and current areas of focus that may serve as inspiration for future investigations. Additionally, these discoveries can aid researchers in identifying suitable publication outlets and fostering collaborative partnerships.
Funding
This study was granted by the Youth Project of the Natural Science Foundation of Shandong Province of China under the grant number ZR2023QH461.
Data availability statement
The datasets generated for this study may be obtained from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Yanhao Liu: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Xu Jiang: Supervision, Writing – review & editing. Yujuan Wu: Supervision, Writing – review & editing. Haiming Yu: Conceptualization, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The author Yanhao Liu would like to thank his wife, Mrs. Yujie Zhang, and cat, Mr. Milk Cap, for their constant companionship and support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27103.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
Supplementary Fig. S1.
Historical direct citation network among the publications in Core Dataset.
Supplementary Fig. S2.
Citation relationship network of the publications in Core Dataset with the strongest link strength.
Supplementary Fig. S3.
Top 25 keywords with the strongest citation bursts in Core Dataset on immunotherapy plus radiotherapy.
Supplementary Fig. S4.
Top 50 keywords with the strongest citation bursts in Generalized Dataset on immunotherapy plus radiotherapy.
References
- 1.Brooks E.D., Chang J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019;16:123–135. doi: 10.1038/s41571-018-0119-7. [DOI] [PubMed] [Google Scholar]
- 2.Demaria S., Golden E.B., Formenti S.C. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 3.Grass G.D., Krishna N., Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer. 2016;40:10–24. doi: 10.1016/j.currproblcancer.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein M.B., Krishnan S., Hodge J.W., Chang J.Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach. Nat. Rev. Clin. Oncol. 2016;13:516–524. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang C., Sherry A.D., Haymaker C., Bathala T., Liu S., Fellman B., et al. Addition of metastasis-directed therapy to intermittent hormone therapy for oligometastatic prostate cancer: the EXTEND phase 2 randomized clinical trial. JAMA Oncol. 2023;9:825–834. doi: 10.1001/jamaoncol.2023.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallegos C.E., Michelin S., Dubner D., Carosella E.D. Immunomodulation of classical and non-classical HLA molecules by ionizing radiation. Cell. Immunol. 2016;303:16–23. doi: 10.1016/j.cellimm.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Hemphill W.O., Simpson S.R., Liu M., Salsbury F.R., Jr., Hollis T., Grayson J.M., et al. TREX1 as a novel immunotherapeutic target. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.660184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond J.M., Vanpouille-Box C., Spada S., Rudqvist N.P., Chapman J.R., Ueberheide B.M., et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol. Res. 2018;6:910–920. doi: 10.1158/2326-6066.CIR-17-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanpouille-Box C., Alard A., Aryankalayil M.J., Sarfraz Y., Diamond J.M., Schneider R.J., et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017;8 doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon H., Chen D., Ramapriyan R., Verma V., Barsoumian H.B., Cushman T.R., et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J. Immunother. Cancer. 2019;7:237. doi: 10.1186/s40425-019-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barsoumian H.B., Ramapriyan R., Younes A.I., Caetano M.S., Menon H., Comeaux N.I., et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J. Immunother. Cancer. 2020:8. doi: 10.1136/jitc-2020-000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellevik T., Martinez-Zubiaurre I. Radiotherapy and the tumor stroma: the importance of dose and fractionation. Front. Oncol. 2014;4:1. doi: 10.3389/fonc.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaria S., Ng B., Devitt M.L., Babb J.S., Kawashima N., Liebes L., et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Herter-Sprie G.S., Koyama S., Korideck H., Hai J., Deng J., Li Y.Y., et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight. 2016;1 doi: 10.1172/jci.insight.87415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derer A., Spiljar M., Bäumler M., Hecht M., Fietkau R., Frey B., et al. Chemoradiation increases PD-L1 expression in certain melanoma and glioblastoma cells. Front. Immunol. 2016;7:610. doi: 10.3389/fimmu.2016.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spigel D.R., Faivre-Finn C., Gray J.E., Vicente D., Planchard D., Paz-Ares L., et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. 2022;40:1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theelen W., Chen D., Verma V., Hobbs B.P., Peulen H., Aerts J., et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir. Med. 2021;9:467–475. doi: 10.1016/S2213-2600(20)30391-X. [DOI] [PubMed] [Google Scholar]
- 18.Koller K.M., Mackley H.B., Liu J., Wagner H., Talamo G., Schell T.D., et al. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol. Ther. 2017;18:36–42. doi: 10.1080/15384047.2016.1264543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saiag P., Molinier R., Roger A., Boru B., Otmezguine Y., Otz J., et al. Efficacy of large use of combined hypofractionated radiotherapy in a cohort of anti-PD-1 monotherapy-treated melanoma patients. Cancers. 2022:14. doi: 10.3390/cancers14174069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donthu N., Kumar S., Mukherjee D., Pandey N., Lim W.M. How to conduct a bibliometric analysis: an overview and guidelines. J. Bus. Res. 2021;133:285–296. doi: 10.1016/j.jbusres.2021.04.070. [DOI] [Google Scholar]
- 21.Liu Y., Li J., Cheng X., Zhang X. Bibliometric analysis of the top-cited publications and research trends for stereotactic body radiotherapy. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.795568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., Jiang S., Lin Y., Yu H., Yu L., Zhang X. Research landscape and trends of lung cancer radiotherapy A bibliometric analysis. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1066557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Xu Y., Cheng X., Lin Y., Jiang S., Yu H., et al. Research trends and most influential clinical studies on anti-PD1/PDL1 immunotherapy for cancers: a bibliometric analysis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.862084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Cheng X., Han X., Cheng X., Jiang S., Lin Y., et al. Global research landscape and trends of lung cancer immunotherapy: a bibliometric analysis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1032747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Yu L., Liang Y., Cheng X., Jiang S., Yu H., et al. Research landscape and trends of melanoma immunotherapy: a bibliometric analysis. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1024179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeung A.W.K. Comparison between Scopus, Web of science, PubMed and publishers for mislabelled review papers. Curr. Sci. India. 2019;116:1909–1914. doi: 10.1186/s13104-016-2026-2. [DOI] [Google Scholar]
- 27.Massimo A., Corrado C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J. Informetrics. 2017;11:959–975. doi: 10.1016/j.joi.2017.08.007. [DOI] [Google Scholar]
- 28.van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colciago R.R., Fischetti I., Giandini C., La Rocca E., Rancati T.T., Rejas Mateo A., et al. Overview of the synergistic use of radiotherapy and immunotherapy in cancer treatment: current challenges and scopes of improvement. Expert Rev. Anticancer Ther. 2023;23:135–145. doi: 10.1080/14737140.2023.2173175. [DOI] [PubMed] [Google Scholar]
- 30.Antonia S.J., Balmanoukian A., Brahmer J., Ou S.I., Hellmann M.D., Kim S.W., et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J. Thorac. Oncol. 2019;14:1794–1806. doi: 10.1016/j.jtho.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Paz-Ares L., Spira A., Raben D., Planchard D., Cho B.C., Özgüroğlu M., et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. 2020;31:798–806. doi: 10.1016/j.annonc.2020.03.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girard N., Bar J., Garrido P., Garassino M.C., McDonald F., Mornex F., et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J. Thorac. Oncol. 2023;18:181–193. doi: 10.1016/j.jtho.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q., Chen M., Jiang O., Pan Y., Hu D., Lin Q., et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23:209–219. doi: 10.1016/S1470-2045(21)00630-6. [DOI] [PubMed] [Google Scholar]
- 34.Altorki N.K., McGraw T.E., Borczuk A.C., Saxena A., Port J.L., Stiles B.M., et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 2021;22:824–835. doi: 10.1016/S1470-2045(21)00149-2. [DOI] [PubMed] [Google Scholar]
- 35.Chang J.Y., Lin S.H., Dong W., Liao Z., Gandhi S.J., Gay C.M., et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet. 2023 doi: 10.1016/S0140-6736(23)01384-3. S0140-6736(23)01384-01383 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaverdian N., Lisberg A.E., Bornazyan K., Veruttipong D., Goldman J.W., Formenti S.C., et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theelen W., Peulen H., Lalezari F., van der Noort V., de Vries J.F., Aerts J., et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bassanelli M., Ricciuti B., Giannarelli D., Cecere F.L., Roberto M., Giacinti S., et al. Systemic effect of radiotherapy before or after nivolumab in lung cancer: an observational, retrospective, multicenter study. Tumori. 2022;108:250–257. doi: 10.1177/03008916211004733. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh K., Dickstein D.R., Runnels J., Lehrer E.J., Rosenzweig K., Hirsch F.R., et al. Radiotherapy and immunotherapy in lung cancer. Biomedicines. 2023:11. doi: 10.3390/biomedicines11061642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roger A., Finet A., Boru B., Beauchet A., Mazeron J.J., Otzmeguine Y., et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. OncoImmunology. 2018;7 doi: 10.1080/2162402X.2018.1442166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chicas-Sett R., Morales-Orue I., Rodriguez-Abreu D., Lara-Jimenez P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: a systematic review. Clin. Transl. Radiat. Oncol. 2018;9:5–11. doi: 10.1016/j.ctro.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon E.D., Drake C.G., Scher H.I., Fizazi K., Bossi A., van den Eertwegh A.J., et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fizazi K., Drake C.G., Beer T.M., Kwon E.D., Scher H.I., Gerritsen W.R., et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur. Urol. 2020;78:822–830. doi: 10.1016/j.eururo.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwan E.M., Spain L., Anton A., Gan C.L., Garrett L., Chang D., et al. Avelumab combined with stereotactic ablative body radiotherapy in metastatic castration-resistant prostate cancer: the phase 2 ICE-PAC clinical trial. Eur. Urol. 2022;81:253–262. doi: 10.1016/j.eururo.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Higa J., Wilenius K., Savino S., Larsen C., Scholz M., Vogelzang N. Real world experience with pembrolizumab in recurrent or advanced prostate cancer. Clin. Genitourin. Cancer. 2020;18:e397–e401. doi: 10.1016/j.clgc.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Masini C., Iotti C., De Giorgi U., Bellia R.S., Buti S., Salaroli F., et al. Nivolumab in combination with stereotactic body radiotherapy in pretreated patients with metastatic renal cell carcinoma. Results of the phase II NIVES study. Eur. Urol. 2022;81:274–282. doi: 10.1016/j.eururo.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Pryor D., Bressel M., Lawrentschuk N., Tran B., Mooi J., Lewin J., et al. A phase I/II study of stereotactic radiotherapy and pembrolizumab for oligometastatic renal tumours (RAPPORT): clinical trial protocol. Contemp. Clin. Trials Commun. 2021;21 doi: 10.1016/j.conctc.2021.100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siva S., Bressel M., Wood S.T., Shaw M.G., Loi S., Sandhu S.K., et al. Stereotactic radiotherapy and short-course pembrolizumab for oligometastatic renal cell carcinoma-the RAPPORT trial. Eur. Urol. 2022;81:364–372. doi: 10.1016/j.eururo.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Fukushima H., Kijima T., Fukuda S., Moriyama S., Uehara S., Yasuda Y., et al. Impact of radiotherapy to the primary tumor on the efficacy of pembrolizumab for patients with advanced urothelial cancer: a preliminary study. Cancer Med. 2020;9:8355–8363. doi: 10.1002/cam4.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levy A., Massard C., Soria J.C., Deutsch E. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: single centre subset analysis from a phase 1/2 trial. Eur. J. Cancer. 2016;68:156–162. doi: 10.1016/j.ejca.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Tree A.C., Jones K., Hafeez S., Sharabiani M., Harrington K.J., Lalondrelle S., et al. Dose-limiting urinary toxicity with pembrolizumab combined with weekly hypofractionated radiation therapy in bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018;101:1168–1171. doi: 10.1016/j.ijrobp.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 52.van den Ende T., de Clercq N.C., van Berge Henegouwen M.I., Gisbertz S.S., Geijsen E.D., Verhoeven R., et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: a single-arm phase II feasibility trial (PERFECT) Clin. Cancer Res. 2021;27:3351–3359. doi: 10.1158/1078-0432.CCR-20-4443. [DOI] [PubMed] [Google Scholar]
- 53.Li C., Zhao S., Zheng Y., Han Y., Chen X., Cheng Z., et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1) Eur. J. Cancer. 2021;144:232–241. doi: 10.1016/j.ejca.2020.11.039. [DOI] [PubMed] [Google Scholar]
- 54.Page D.B., Beal K., Linch S.N., Spinelli K.J., Rodine M., Halpenny D., et al. Brain radiotherapy, tremelimumab-mediated CTLA-4-directed blockade +/- trastuzumab in patients with breast cancer brain metastases. NPJ Breast Cancer. 2022;8:50. doi: 10.1038/s41523-022-00404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voorwerk L., Slagter M., Horlings H.M., Sikorska K., van de Vijver K.K., de Maaker M., et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 2019;25:920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 56.Ho A.Y., Barker C.A., Arnold B.B., Powell S.N., Hu Z.I., Gucalp A., et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126:850–860. doi: 10.1002/cncr.32599. [DOI] [PubMed] [Google Scholar]
- 57.Adams S., Schmid P., Rugo H.S., Winer E.P., Loirat D., Awada A., et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019;30:397–404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
- 58.Barroso-Sousa R., Krop I.E., Trippa L., Tan-Wasielewski Z., Li T., Osmani W., et al. A phase II study of pembrolizumab in combination with palliative radiotherapy for hormone receptor-positive metastatic breast cancer. Clin. Breast Cancer. 2020;20:238–245. doi: 10.1016/j.clbc.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Lee N.Y., Ferris R.L., Psyrri A., Haddad R.I., Tahara M., Bourhis J., et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450–462. doi: 10.1016/S1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 60.Powell S.F., Gold K.A., Gitau M.M., Sumey C.J., Lohr M.M., McGraw S.C., et al. Safety and efficacy of pembrolizumab with chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: a phase IB study. J. Clin. Oncol. 2020;38:2427–2437. doi: 10.1200/JCO.19.03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McBride S., Sherman E., Tsai C.J., Baxi S., Aghalar J., Eng J., et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 2021;39:30–37. doi: 10.1200/JCO.20.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shamseddine A., Zeidan Y.H., El Husseini Z., Kreidieh M., Al Darazi M., Turfa R., et al. Efficacy and safety-in analysis of short-course radiation followed by mFOLFOX-6 plus avelumab for locally advanced rectal adenocarcinoma. Radiat. Oncol. 2020;15:233. doi: 10.1186/s13014-020-01673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shamseddine A., Zeidan Y.H., Kreidieh M., Khalifeh I., Turfa R., Kattan J., et al. Short-course radiation followed by mFOLFOX-6 plus avelumab for locally-advanced rectal adenocarcinoma. BMC Cancer. 2020;20:831. doi: 10.1186/s12885-020-07333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marco M.R., Zhou L., Patil S., Marcet J.E., Varma M.G., Oommen S., et al. Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis. Colon Rectum. 2018;61:1146–1155. doi: 10.1097/DCR.0000000000001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahma O.E., Yothers G., Hong T.S., Russell M.M., You Y.N., Parker W., et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol. 2021;7:1225–1230. doi: 10.1001/jamaoncol.2021.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bando H., Tsukada Y., Inamori K., Togashi Y., Koyama S., Kotani D., et al. Preoperative chemoradiotherapy plus nivolumab before surgery in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Clin. Cancer Res. 2022;28:1136–1146. doi: 10.1158/1078-0432.CCR-21-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin Z., Cai M., Zhang P., Li G., Liu T., Li X., et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parikh A.R., Szabolcs A., Allen J.N., Clark J.W., Wo J.Y., Raabe M., et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat. Can. (Ott.) 2021;2:1124–1135. doi: 10.1038/s43018-021-00269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huber M., Brehm C.U., Gress T.M., Buchholz M., Alashkar Alhamwe B., von Strandmann E.P., et al. The immune microenvironment in pancreatic cancer. Int. J. Mol. Sci. 2020;21:7307. doi: 10.3390/ijms21197307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torphy R.J., Schulick R.D., Zhu Y. Understanding the immune landscape and tumor microenvironment of pancreatic cancer to improve immunotherapy. Mol. Carcinog. 2020;59:775–782. doi: 10.1002/mc.23179. [DOI] [PubMed] [Google Scholar]
- 71.Xie C., Duffy A.G., Brar G., Fioravanti S., Mabry-Hrones D., Walker M., et al. Immune checkpoint blockade in combination with stereotactic body radiotherapy in patients with metastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2020;26:2318–2326. doi: 10.1158/1078-0432.CCR-19-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu X., Cao Y., Liu W., Ju X., Zhao X., Jiang L., et al. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2021;22:1093–1102. doi: 10.1016/S1470-2045(21)00286-2. [DOI] [PubMed] [Google Scholar]
- 73.Sahebjam S., Forsyth P.A., Tran N.D., Arrington J.A., Macaulay R., Etame A.B., et al. Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade gliomas: results from a phase I study. Neuro Oncol. 2021;23:677–686. doi: 10.1093/neuonc/noaa260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pouessel D., Ken S., Gouaze-Andersson V., Piram L., Mervoyer A., Larrieu-Ciron D., et al. Hypofractionated stereotactic Re-irradiation and anti-PDL1 durvalumab combination in recurrent glioblastoma: STERIMGLI phase I results. Oncol. 2023:oyad095. doi: 10.1093/oncolo/oyad095. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Omuro A., Brandes A.A., Carpentier A.F., Idbaih A., Reardon D.A., Cloughesy T., et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase III trial. Neuro Oncol. 2023;25:123–134. doi: 10.1093/neuonc/noac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim M., Weller M., Idbaih A., Steinbach J., Finocchiaro G., Raval R.R., et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 2022;24:1935–1949. doi: 10.1093/neuonc/noac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 78.Huang A.C., Postow M.A., Orlowski R.J., Mick R., Bengsch B., Manne S., et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomez D.R., Tang C., Zhang J., Blumenschein G.R., Jr., Hernandez M., Lee J.J., et al. Local consolidative therapy vs. Maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 2019;37:1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomez D.R., Blumenschein G.R., Jr., Lee J.J., Hernandez M., Ye R., Camidge D.R., et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 82.Wu Y., Verma V., Liang F., Lin Q., Zhou Z., Wang Z., et al. Local consolidative therapy versus systemic therapy alone for metastatic non-small cell lung cancer: a systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2022;114:635–644. doi: 10.1016/j.ijrobp.2022.02.023. [DOI] [PubMed] [Google Scholar]
- 83.Sherry A.D., Bathala T.K., Liu S., Fellman B.M., Chun S.G., Jasani N., et al. Definitive local consolidative therapy for oligometastatic solid tumors: results from the lead-in phase of the randomized basket trial EXTEND. Int. J. Radiat. Oncol. Biol. Phys. 2022;114:910–918. doi: 10.1016/j.ijrobp.2022.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sezen D., Verma V., He K., Abana C.O., Barsoumian H., Ning M.S., et al. Considerations for clinical trials testing radiotherapy combined with immunotherapy for metastatic disease. Semin. Radiat. Oncol. 2021;31:217–226. doi: 10.1016/j.semradonc.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 85.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 86.Patel R.R., Verma V., Barsoumian H.B., Ning M.S., Chun S.G., Tang C., et al. Use of multi-site radiation therapy for systemic disease control. Int. J. Radiat. Oncol. Biol. Phys. 2021;109:352–364. doi: 10.1016/j.ijrobp.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luke J.J., Lemons J.M., Karrison T.G., Pitroda S.P., Melotek J.M., Zha Y., et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J. Clin. Oncol. 2018;36:1611–1618. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venkatesulu B.P., Mallick S., Lin S.H., Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit. Rev. Oncol. Hematol. 2018;123:42–51. doi: 10.1016/j.critrevonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Chen D., Verma V., Patel R.R., Barsoumian H.B., Cortez M.A., Welsh J.W. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int. J. Radiat. Oncol. Biol. Phys. 2020;108:196–203. doi: 10.1016/j.ijrobp.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 90.Chen D., Patel R.R., Verma V., Ramapriyan R., Barsoumian H.B., Cortez M.A., et al. Interaction between lymphopenia, radiotherapy technique, dosimetry, and survival outcomes in lung cancer patients receiving combined immunotherapy and radiotherapy. Radiother. Oncol. 2020;150:114–120. doi: 10.1016/j.radonc.2020.05.051. [DOI] [PubMed] [Google Scholar]
- 91.Verma V., Chang J.Y. Could the clinical target volume be omitted for radiotherapy of locally advanced non-small cell lung cancer in the modern era. Transl. Lung Cancer Res. 2021;10:5–8. doi: 10.21037/tlcr-2020-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mireștean C.C., Iancu R.I., Iancu D.T. Immunotherapy and radiotherapy as an antitumoral long-range weapon-A partnership with unsolved challenges: dose, fractionation, volumes, therapeutic sequence. Curr. Oncol. 2022;29:7388–7395. doi: 10.3390/curroncol29100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mayoux M., Roller A., Pulko V., Sammicheli S., Chen S., Sum E., et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aav7431. [pii] [DOI] [PubMed] [Google Scholar]
- 94.Hong M., Maleki Vareki S. Addressing the elephant in the immunotherapy room: effector T-cell priming versus depletion of regulatory T-cells by anti-CTLA-4 therapy. Cancers. 2022;14:1580. doi: 10.3390/cancers14061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sasaki K., Morioka D., Conci S., Margonis G.A., Sawada Y., Ruzzenente A., et al. The tumor burden score: a new "Metro-ticket" prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann. Surg. 2018;267:132–141. doi: 10.1097/SLA.0000000000002064. [DOI] [PubMed] [Google Scholar]
- 96.Ho S.Y., Liu P.H., Hsu C.Y., Ko C.C., Huang Y.H., Su C.W., et al. Tumor burden score as a new prognostic marker for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J. Gastroenterol. Hepatol. 2021;36:3196–3203. doi: 10.1111/jgh.15593. [DOI] [PubMed] [Google Scholar]
- 97.Dong D.H., Zhang X.F., Lopez-Aguiar A.G., Poultsides G., Makris E., Rocha F., et al. Tumor burden score predicts tumor recurrence of non-functional pancreatic neuroendocrine tumors after curative resection. HPB (Oxford) 2020;22:1149–1157. doi: 10.1016/j.hpb.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ryu W.K., Oh S., Lim J.H., Lee S.J., Shin H.T., Ryu J.S. Monitoring circulating tumor DNA in untreated non-small-cell lung cancer patients. Int. J. Mol. Sci. 2022;23:9527. doi: 10.3390/ijms23179527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oellerich M., Schütz E., Beck J., Walson P.D. Circulating cell-free DNA-diagnostic and prognostic applications in personalized cancer therapy. Ther. Drug Monit. 2019;41:115–120. doi: 10.1097/FTD.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 100.Ippolito M.R., Martis V., Martin S., Tijhuis A.E., Hong C., Wardenaar R., et al. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev. Cell. 2021;56:2440–2454.e6. doi: 10.1016/j.devcel.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 101.Bakhoum S.F., Cantley L.C. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell. 2018;174:1347–1360. doi: 10.1016/j.cell.2018.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drews R.M., Hernando B., Tarabichi M., Haase K., Lesluyes T., Smith P.S., et al. A pan-cancer compendium of chromosomal instability. Nature. 2022;606:976–983. doi: 10.1038/s41586-022-04789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balachandran V.P., Łuksza M., Zhao J.N., Makarov V., Moral J.A., Remark R., et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Sousa E., Lérias J.R., Beltran A., Paraschoudi G., Condeço C., Kamiki J., et al. Targeting neoepitopes to treat solid malignancies: immunosurgery. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.592031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang J., Caruso F.P., Sa J.K., Justesen S., Nam D.H., Sims P., et al. The combination of neoantigen quality and T lymphocyte infiltrates identifies glioblastomas with the longest survival. Commun. Biol. 2019;2:135. doi: 10.1038/s42003-019-0369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ren Y., Cherukuri Y., Wickland D.P., Sarangi V., Tian S., Carter J.M., et al. HLA class-I and class-II restricted neoantigen loads predict overall survival in breast cancer. OncoImmunology. 2020;9 doi: 10.1080/2162402X.2020.1744947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi Y., Jing B., Xi R. Comprehensive analysis of neoantigens derived from structural variation across whole genomes from 2528 tumors. Genome Biol. 2023;24:169. doi: 10.1186/s13059-023-03005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dantoing E., Piton N., Salaün M., Thiberville L., Guisier F. Anti-PD1/PD-L1 immunotherapy for non-small cell lung cancer with actionable oncogenic driver mutations. Int. J. Mol. Sci. 2021;22:6288. doi: 10.3390/ijms22126288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamada T., Hirai S., Katayama Y., Yoshimura A., Shiotsu S., Watanabe S., et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer. Cancer Med. 2019;8:1521–1529. doi: 10.1002/cam4.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aguadé-Gorgorió G., Solé R. Genetic instability as a driver for immune surveillance. J. Immunother. Cancer. 2019;7:345. doi: 10.1186/s40425-019-0795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Talens F., Van Vugt M. Inflammatory signaling in genomically instable cancers. Cell Cycle. 2019;18:1830–1848. doi: 10.1080/15384101.2019.1638192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McGrail D.J., Federico L., Li Y., Dai H., Lu Y., Mills G.B., et al. Multi-omics analysis reveals neoantigen-independent immune cell infiltration in copy-number driven cancers. Nat. Commun. 2018;9:1317. doi: 10.1038/s41467-018-03730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Catalano M., Francesco Iannone L., Cosso F., Generali D., Mini E., Roviello G. Combining inhibition of immune checkpoints and PARP: rationale and perspectives in cancer treatment. Expert Opin. Ther. Targets. 2022;26:923–936. doi: 10.1080/14728222.2022.2158813. [DOI] [PubMed] [Google Scholar]
- 114.Yakkala C., Denys A., Kandalaft L., Duran R. Cryoablation and immunotherapy of cancer. Curr. Opin. Biotechnol. 2020;65:60–64. doi: 10.1016/j.copbio.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 115.Fucikova J., Kepp O., Kasikova L., Petroni G., Yamazaki T., Liu P., et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11:1013. doi: 10.1038/s41419-020-03221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varadan V., Gilmore H., Miskimen K.L., Tuck D., Parsai S., Awadallah A., et al. Immune signatures following single dose trastuzumab predict pathologic response to PreoperativeTrastuzumab and chemotherapy in HER2-positive early breast cancer. Clin. Cancer Res. 2016;22:3249–3259. doi: 10.1158/1078-0432.CCR-15-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vanpouille-Box C., Formenti S.C., Demaria S. TREX1 dictates the immune fate of irradiated cancer cells. OncoImmunology. 2017;6 doi: 10.1080/2162402X.2017.1339857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schaue D., Ratikan J.A., Iwamoto K.S., McBride W.H. Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kong Y., Zhao X., Xu M., Pan J., Ma Y., Zou L., et al. PD-1 inhibitor combined with radiotherapy and GM-CSF (PRaG) in patients with metastatic solid tumors: an open-label phase II study. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.952066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Z., Yu Z., Chen D., Verma V., Yuan C., Wang M., et al. Pivotal roles of tumor-draining lymph nodes in the abscopal effects from combined immunotherapy and radiotherapy. Cancer Commun. 2022;42:971–986. doi: 10.1002/cac2.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.du Bois H., Heim T.A., Lund A.W. Tumor-draining lymph nodes: at the crossroads of metastasis and immunity. Sci. Immunol. 2021:6. doi: 10.1126/sciimmunol.abg3551. eabg3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aryankalayil M.J., Makinde A.Y., Gameiro S.R., Hodge J.W., Rivera-Solis P.P., Palayoor S.T., et al. Defining molecular signature of pro-immunogenic radiotherapy targets in human prostate cancer cells. Radiat. Res. 2014;182:139–148. doi: 10.1667/RR13731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Menon H., Ramapriyan R., Cushman T.R., Verma V., Kim H.H., Schoenhals J.E., et al. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front. Immunol. 2019;10:193. doi: 10.3389/fimmu.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R.R., et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Elebiyo T.C., Rotimi D., Evbuomwan I.O., Maimako R.F., Iyobhebhe M., Ojo O.A., et al. Reassessing vascular endothelial growth factor (VEGF) in anti-angiogenic cancer therapy. Cancer Treat Res. Commun. 2022;32 doi: 10.1016/j.ctarc.2022.100620. [DOI] [PubMed] [Google Scholar]
- 126.Ren S., Xiong X., You H., Shen J., Zhou P. The combination of immune checkpoint blockade and angiogenesis inhibitors in the treatment of advanced non-small cell lung cancer. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.689132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Winkler F., Kozin S.V., Tong R.T., Chae S.S., Booth M.F., Garkavtsev I., et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 128.Schiffmann L.M., Brunold M., Liwschitz M., Goede V., Loges S., Wroblewski M., et al. A combination of low-dose bevacizumab and imatinib enhances vascular normalisation without inducing extracellular matrix deposition. Br. J. Cancer. 2017;116:600–608. doi: 10.1038/bjc.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.von Baumgarten L., Brucker D., Tirniceru A., Kienast Y., Grau S., Burgold S., et al. Bevacizumab has differential and dose-dependent effects on glioma blood vessels and tumor cells. Clin. Cancer Res. 2011;17:6192–6205. doi: 10.1158/1078-0432.CCR-10-1868. [DOI] [PubMed] [Google Scholar]
- 130.Zeng H., Zhang W., Gong Y., Xie C. Radiotherapy activates autophagy to increase CD8(+) T cell infiltration by modulating major histocompatibility complex class-I expression in non-small cell lung cancer. J. Int. Med. Res. 2019;47:3818–3830. doi: 10.1177/0300060519855595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hauser S.H., Calorini L., Wazer D.E., Gattoni-Celli S. Radiation-enhanced expression of major histocompatibility complex class I antigen H-2Db in B16 melanoma cells. Cancer Res. 1993;53:1952–1955. [PubMed] [Google Scholar]
- 132.Santin A.D., Hermonat P.L., Hiserodt J.C., Chiriva-Internati M., Woodliff J., Theus J.W., et al. Effects of irradiation on the expression of major histocompatibility complex class I antigen and adhesion costimulation molecules ICAM-1 in human cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 1997;39:737–742. doi: 10.1016/s0360-3016(97)00372-6. [DOI] [PubMed] [Google Scholar]
- 133.Gaikwad S., Agrawal M.Y., Kaushik I., Ramachandran S., Srivastava S.K. Immune checkpoint proteins: signaling mechanisms and molecular interactions in cancer immunotherapy. Semin. Cancer Biol. 2022;86:137–150. doi: 10.1016/j.semcancer.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 134.Patel T.D., Vanteddu V.G., Sweta B. Current trends in immuno-oncology. Cardiovasc. Hematol. Agents Med. Chem. 2023;21:96–107. doi: 10.2174/1871525720666220829142225. [DOI] [PubMed] [Google Scholar]
- 135.Rousseau A., Parisi C., Barlesi F. Anti-TIGIT therapies for solid tumors: a systematic review. ESMO Open. 2023;8 doi: 10.1016/j.esmoop.2023.101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sauer N., Szlasa W., Jonderko L., Oślizło M., Kunachowicz D., Kulbacka J., et al. LAG-3 as a potent target for novel anticancer therapies of a wide range of tumors. Int. J. Mol. Sci. 2022;23:9958. doi: 10.3390/ijms23179958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gill S., Nowak A.K., Bowyer S., Endersby R., Ebert M.A., Cook A. Clinical evidence for synergy between immunotherapy and radiotherapy (SITAR) J. Med. Imaging Radiat. Oncol. 2022;66:881–895. doi: 10.1111/1754-9485.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khunger M., Rakshit S., Pasupuleti V., Hernandez A.V., Mazzone P., Stevenson J., et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 139.Yin J., Wu Y., Yang X., Gan L., Xue J. Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy in non-small-cell lung cancer: occurrence and mechanism. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.830631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu T., Wu L., Gandhi S., Jing W., Nguyen Q.N., Chen A., et al. Treatment-related pulmonary adverse events induced by chemoradiation and Durvalumab affect survival in locally advanced non-small cell lung cancer. Radiother. Oncol. 2022;176:149–156. doi: 10.1016/j.radonc.2022.10.002. [DOI] [PubMed] [Google Scholar]
- 141.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J. Clin. Oncol. 2020;38:2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guénolé M., Lucia F., Bourbonne V., Dissaux G., Reygagne E., Goasduff G., et al. Impact of concomitant systemic treatments on toxicity and intracerebral response after stereotactic radiotherapy for brain metastases. BMC Cancer. 2020;20:991. doi: 10.1186/s12885-020-07491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang V.A., Simpson D.R., Daniels G.A., Piccioni D.E. Infliximab for treatment-refractory transverse myelitis following immune therapy and radiation. J. Immunother. Cancer. 2018;6:153. doi: 10.1186/s40425-018-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Di Stefano A.L., Savatovsky J., Feuvret L., Villa C., Reina V., Pha M., et al. CNS inflammatory disorder after concurrent radiotherapy-temozolomide and nivolumab in a glioblastoma patient. Neuro Oncol. 2019;21:139–141. doi: 10.1093/neuonc/noy168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang J.Y. When constrained by constraints: thinking outside of the box in both technology and biology. Int. J. Radiat. Oncol. Biol. Phys. 2021;110:266–267. doi: 10.1016/j.ijrobp.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 146.Zou L., Chu L., Xia F., Zhou L., Yang X., Ni J., et al. Is clinical target volume necessary?-a failure pattern analysis in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy using intensity-modulated radiotherapy technique. Transl. Lung Cancer Res. 2020;9:1986–1995. doi: 10.21037/tlcr-20-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saito S., Abe T., Iino M., Aoshika T., Ryuno Y., Ohta T., et al. Incidence and risk factors for pneumonitis among patients with lung cancer who received immune checkpoint inhibitors after palliative thoracic radiotherapy. J. Radiat. Res. 2021;62:669–675. doi: 10.1093/jrr/rrab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study may be obtained from the corresponding author upon reasonable request.