Abstract
Background and Objective
Thymic epithelial tumors are relatively rare; thus, mostly retrospective studies and a few prospective randomized controlled trials have been conducted on the treatment and the biomarkers, with no standard therapy established. Indications for extended thymectomy, robot-assisted thoracic surgery, and multidisciplinary treatment are controversial. Here, we considered the prospects of surgical treatment and the possibility of immune checkpoint inhibitor (ICI) treatment for thymic epithelial tumors.
Methods
This is a narrative review; PubMed was searched using a set of keywords related to thymoma and its proposed treatments over the last 5 years.
Key Content and Findings
Thymic epithelial tumors are associated with autoimmune diseases. They are relatively rare, and their pathology remains unclear. Therefore, accumulating more case reports is important. Surgical resection is generally considered for both diagnosis and treatment. If the tumor has a strong tendency to invade surrounding areas, such as thymic carcinoma/thymoma, the diagnosis may be confirmed preoperatively by needle biopsy, and induction chemotherapy may be selected. Surgical resection is the most effective treatment, and complete resection is important. In cases where complete resection is difficult, multidisciplinary treatment is performed. Although there are various obstacles, using ICIs may prove effective for treatment both as preoperative and postoperative chemotherapy in the future, as shown for other cancers. Programmed cell death-ligand 1 (PD-L1) is an immunoinhibitory molecule that suppresses T cells activation, leading to tumor progression. Overexpression of PD-L1 in some cancers is associated with poor clinical outcomes. However, the role of PD-L1 expression as a prognostic factor remains controversial. Therefore, various biomarkers other than PD-L1 have been identified.
Conclusions
We reviewed the latest treatments for thymic epithelial tumors. If new therapeutic agents such as ICIs and molecular-targeted drugs are developed, this review suggests that surgery will become more important not only as therapy but also as part of multidisciplinary treatment that includes tissue collection.
Keywords: Extended thymectomy, immune checkpoint inhibitor (ICI), multidisciplinary treatment, surgery, thymoma
Introduction
Background
Thymic epithelial tumors are uncommon tumors that originate from the epithelial cells of the thymus, and the relatively rare diseases mainly occur in the anterior mediastinum and undergo malignant progression owing to recurrence and distant metastasis (1-3). These tumors include thymoma, thymic carcinoma, and neuroendocrine tumors. Generally, surgery is performed for both diagnostic and therapeutic purposes. Complete surgical resection is considered the best prognostic factor for treatment, and multidisciplinary treatment is selected in cases in which complete resection is not possible (4).
Tumors have a mechanism to suppress human immune activation, and programmed cell death-ligand 1 (PD-L1) expression is one such suppressive mechanism. Moreover, there is increasing evidence relating PD-L1 and immune checkpoint inhibitor (ICI) in thymic epithelial tumors. PD-L1 expression and ICI therapeutic efficacy are not necessarily correlated, and the surrounding tumor immune microenvironment also influences therapeutic efficacy. In recent years, resistance to ICI treatment has been reported (5,6), and in addition, it is also necessary to search for new biomarkers for thymic epithelial tumors.
Rationale and knowledge gap
The surgical treatment of thymic epithelial tumors generally involves complete resection. This disease is relatively rare, and most of the literature reporting on surgical methods and novel anticancer agents is retrospective, with only a few prospective randomized controlled trials. Although the major guidelines and thoracic societies suggest extended thymectomy as the preferred surgical procedure for thymoma, some papers suggest reduction surgery (7,8).
The surgical methods for early-stage thymic epithelial tumors include tumor resection, thymothymectomy, and extended thymothymectomy; the choice of surgical method is left to the discretion of each institution.
The effectiveness of the minimally invasive surgical approach against thymic epithelial tumors is also gradually being proven (9-11). Extended thymectomy, which was previously performed through midline sternotomy, can now be performed with minimal invasiveness using video-assisted thoracic surgery (VATS) and robot-assisted thoracic surgery (RATS) (11).
First, the indications for extended thymectomy remain controversial. This procedure is performed for thymomas accompanied by myasthenia gravis (MG) to reduce the risk of postoperative MG. However, some studies have suggested extended thymectomy regardless of the presence or absence of MG, because in addition to the presence of microthymoma in the ectopic thymus, thymoma resection alone or thymectomy is not helpful in the treatment of MG (12,13).
Second, the indications for RATS are debatable. RATS is a more precise and delicate surgical technique that uses three-dimensional (3D) spatial images and an arm with a wide range of motion that rotates 360°. Even in a narrow space within the mediastinum, a minimally invasive surgical approach can be performed by taking advantage of the wide range of motion.
Finally, multidisciplinary treatment needs improvement. Despite improvements in surgical techniques and the development of new anticancer drugs such as ICIs, the treatment of thymic epithelial tumors is not well established and has not changed to any great extent in recent years. Therefore, reviewing the current treatment methods and developing better ones is necessary. We investigated and reviewed the latest literature regarding thymic epithelial tumors and surgical treatment.
Objective
In this review, we report the latest surgical treatments for thymic epithelial tumors. With the advent of new therapeutic agents such as the ICIs, the position of surgical treatment is changing, and the significance of surgery not only in treatment but also in tissue diagnosis may become important. We present this article in accordance with the Narrative Review reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-453/rc).
Methods
We conducted a non-systematic review using PubMed as the primary source. We searched relevant literature on August 30, 2023. The search string was as follows: (“Thymoma”) AND (“Surgery”) AND ((“Multidisciplinary Treatment” OR “ICI” OR “PD-L1” OR “RATS” OR “Extended Thymectomy”)) NOT (“Review”) AND (y_5 [Filter]). Consequently, 86 documents were retrieved. The exclusion criteria included: (I) literature that we could not obtain from nonmember journals, (II) articles unrelated to thymomas, such as those on thymectomy for non-thymomatous MG, (III) articles not written in English or Japanese, and (IV) editorial comment articles. Finally, 69 studies were reviewed (Figure 1). Furthermore, we added literature related to thymic epithelial tumors published within the past year and reviewed the latest findings. We present an overview of the search strategy summary in Table 1.
Figure 1.
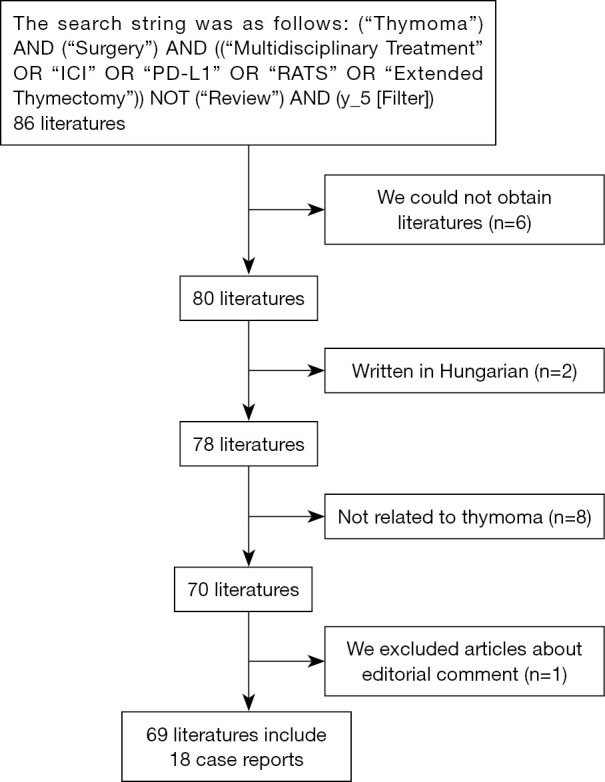
Inclusion and exclusion criteria.
Table 1. Search strategy summary.
| Items | Specification |
|---|---|
| Date of search | August 30, 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | Thymoma, surgery, multidisciplinary treatment, ICI, PD-L1, RATS, extended thymectomy |
| Timeframe | Past 5 years |
| Inclusion and exclusion criteria | We targeted literature written in English or Japanese that were searchable on PubMed. Literature written in languages other than English or Japanese was excluded. In addition, review literature was excluded regardless of language |
| Selection process | Y.N. independently selected the literature. The selection process was checked by M.I. and J.Y. |
| Any additional considerations, if applicable | We excluded the literature that we could not obtain from non-member journals |
ICI, immune checkpoint inhibitor; RATS, robot-assisted thoracic surgery; PD-L1, programmed cell death-ligand 1.
Thymic epithelial tumors
Thymic epithelial tumors are associated with autoimmune diseases. In addition to MG, this disease may be complicated by various autoimmune diseases or may be accompanied by paraneoplastic symptoms (14-17). Thymic epithelial tumors are relatively rare, and their pathology remains unclear. Therefore, accumulating more case reports is important. The case reports confirmed in this survey are summarized in Table 2 (14-31). As shown in Table 2, many institutions choose extended thymectomy for thymic epithelial tumors with MG, other autoimmune diseases, and paraneoplastic symptoms. There have been two reports of paraneoplastic symptoms’ relapse following extended thymectomy. In Case 3, polymyalgia rheumatica symptoms relapsed due to thymoma recurrence (16), and in Case 18, exacerbation of MG symptoms was observed post-surgery with symptoms improving following eculizumab administration (31). Case 2 required treatment with oral prednisolone because the postoperative symptoms showed partial remission (15). In the two reported cases of thymic cancer, extended thymectomy was performed; however, in Case 10, MG symptoms did not improve post-surgery. Therefore, administration of pyridostigmine was necessary (23). Further, in Case 15, the Lambert-Eaton myasthenic syndrome did not improve, and anticholinesterase treatment was required (28). In cases with paraneoplastic symptoms, additional treatment is required after surgery, and multidisciplinary treatment may control paraneoplastic symptoms (15-18,21,23,25,31).
Table 2. List of case reports.
| Case | Age (years) | Gender | CT size (cm) | Diagnosis | Surgical method | Content |
|---|---|---|---|---|---|---|
| 1 | 60 | Female | 12.5 | Type A thymoma | Total thymectomy | Thymoma associated with pancytopenia and Good’s syndrome |
| 2 | 68 | Female | 5.4 | Type B1 thymoma | Extended thymectomy | Thymoma-induced pure red cell aplasia |
| 3 | 68 | Male | 8.5 | Type A thymoma | Extended thymectomy | Atypical type A thymoma variant manifesting polymyalgia rheumatica |
| 4 | 26 | Female | 6 | Type B3 thymoma | Extended thymectomy | Thymoma-related stiff-person syndrome |
| 5 | 60 | Female | 6 | Type AB thymoma | Extended thymectomy | Autoimmune alopecia areata due to thymoma |
| 6 | 71 | Male | – | Type A thymoma | Thoracoscopic tumor biopsy | Neoplastic cardiac tamponade |
| 7 | 49 | Male | 6 | Type B2 thymoma | Extended thymectomy | Combined thymoma and a multilocular thymic cyst discovered due to chest pain |
| 8 | 44 | Male | 11 | Type B2 thymoma | Extended thymectomy | Thymoma exhibiting spontaneous regression with developing MG |
| 9 | – | – | – | Recurrence thymoma | – | The addition of L-carnitine to the immunotherapy rechallenge regimen effectively relieved and prevented the reoccurrence of MG |
| 10 | 63 | Male | 2.3 | Type B3 thymoma | Extended thymectomy | Multiple thymoma were possible malignant transformation |
| 1.4 | Squamous cell carcinoma | Thymic carcinoma should be considered in the differential diagnosis | ||||
| 0.6 | Squamous cell carcinoma | Extended thymectomy should be the treatment of choice for minimize the chance of recurrence | ||||
| 11 | 66 | Male | 6 | Type A thymoma | CT-guided needle biopsy | If surgery is challenging, anticoagulants might be considered before chemotherapy to prevent thrombus formation |
| 12 | 72 | Male | 2 | Type AB thymoma | Extended thymectomy | Extremely rare case of thymoma with raised levels of CA 19-9 |
| 13 | 55 | Female | – | Type B3 thymoma | Extended thymectomy | Robotic surgery might expand the indications for minimally invasive thymectomy |
| 14 | 63 | Female | 5.6 | Type B2 thymoma | Thymectomy | AIH should be carefully considered in thymoma patients with liver dysfunction |
| 15 | 71 | Male | 2.7 | Thymic small cell carcinoma | Extended thymectomy | After surgery the patient’s symptoms had not improved |
| Anticholinesterase treatment alleviated his symptoms | ||||||
| 16 | 71 | Male | 3.8 | MTWLS | Extended thymectomy | MTWLS is a very rare type of thymoma, which could be best treated by surgical resection |
| 17 | 78 | Female | – | Thymoma (WHO type B2 > B3) | Local resection | Isolated local resection of ectopic thymoma may be enough for controlling MG especially in elderly patients |
| 18 | 62 | Female | 4 | Type B2 thymoma | Extended thymectomy | Efficacy of eculizumab for postoperative exacerbation of thymoma-associated MG |
MG, myasthenia gravis; AIH, autoimmune hepatitis; MTWLS, micronodular thymoma with lymphoid stroma; WHO, World Health Organization.
Most thymic epithelial tumors (87.6%) occur in the anterior and superior mediastinum (1). If the tumor has a strong tendency to invade surrounding areas, such as thymic cancer, the diagnosis may be confirmed preoperatively by needle biopsy, and induction chemotherapy may be chosen.
Surgical resection is the most effective treatment, and complete resection is important. Although chemotherapy has not been established, multidisciplinary treatment is performed in cases in which complete resection is difficult, and developing effective therapeutic agents for thymic epithelial tumors is necessary (4).
For stage I thymomas, some facilities treat the tumor as an anterior mediastinal tumor and perform complete surgery by removing the tumor for both diagnosis and treatment. However, in patients with pathological stage I thymoma without MG, those who underwent thymothymectomy exhibited no difference in their postoperative complication rate (13.3% vs. 12.5%), 30-day mortality rate (2.6% vs. 6.3%), or postoperative hospital stay compared with those who underwent simple thymomectomy (7). Some reports revealed that the recurrence rate was significantly lower with thymothymectomy, and the postoperative morbidity rate did not increase (thymothymectomy group vs. simple thymomectomy group: 5-year overall survival rate, 89% vs. 55%, 5-year recurrence-free rate, 96% vs. 79%) (7). However, the extent of resection for stage I thymoma remains controversial, as it is unclear how much of the surrounding fat tissue should be removed to influence prognosis.
Extended thymectomy
Patients with MG with thymoma have significantly more frequent and severe postoperative complications than patients with MG without thymoma (patients with thymoma with MG: patients with MG without thymoma, 18.4%: 3.9%) (32). For thymomas associated with MG, extended thymectomy is selected to reduce the risk of postoperative MG.
Factors that improve MG symptoms include age (33,34) and early surgical intervention (33,35). Older age (36,37) and advanced-stage thymoma (36-38) have been identified as poor prognostic factors for postoperative MG symptoms. Most older patients (80%) who undergo thymectomy within 1 year after MG onset have good long-term outcomes (33). Thymectomy may be an option for older patients if surgery is performed early following disease onset. Incomplete resection and young age have been identified as risk factors for tumor recurrence (37).
However, extensive pericardial adipose tissue resection may not be necessary for all patients with MG undergoing extended thymectomy (39). Previous studies have suggested that extended thymectomy for thymic epithelial tumors is not associated with MG (12,13). This is based on the development of robotic surgical technology, and extended thymectomy can now be performed in a less invasive manner. Extended thymectomy may be an option for early-stage thymic epithelial tumors without MG if it is a minimally invasive, low-risk operation. The effectiveness of extended thymectomy for early thymic epithelial tumors without MG is controversial, as it is unclear whether the benefits outweigh the invasiveness of surgery.
Minimally invasive surgery
In addition to thoracoscopic surgery, the usefulness of robotic surgery has been reported in recent years (29,36-40). Robotic surgery uses 3D image construction and an arm with a 360° range of motion, making it effective for approaching narrow spaces within the mediastinum. Robot-assisted thoracoscopic thymectomy is advantageous because it reduces the total volume of postoperative drainage and shortens the hospital stay (41). Robotic resection is safe and feasible even for thymic epithelial tumors up to 10 cm in size (42-44). However, owing to the lack of tactile and force feedback, safety has not been established for tumors with vascular invasion that require advanced techniques such as combined vascular resection and artificial vascular replacement.
Various approaches to VATS and RATS are available. Unilateral or bilateral intrathoracic approaches are often used; however, reports of subxiphoid (45-48), transcervical (49), and inframammary approaches exist (50), which are less invasive. Uniportal VATS thymectomy may be effective if no tumor invasion into the adjacent vessels [for example, the superior vena cava (SVC) and brachiocephalic vein] is observed on computed tomography or if there is no history of radiation exposure to the mediastinum (51). The advantages and disadvantages of each surgical approach are summarized in Table 3.
Table 3. Advantages and disadvantages of each surgical approach.
| Approach | Advantages | Disadvantages |
|---|---|---|
| Subxiphoid | Easy identification of bilateral phrenic nerves | Deep perception and limited forceps movement |
| Good view of the thymus in the cervical region | Not suitable for tumors with vascular invasion | |
| Transcervical | Low cost | Not suitable for thymoma cases |
| Not suitable for cases where the neck cannot be extended | ||
| Inframammary | Low cost | Not suitable for tumors with vascular invasion |
| Direct incision possible | ||
| Median sternotomy | Directly palpable and provides a wide surgical field of view | Extension of hospitalization period |
| Applicable even if you have a history of radiation therapy | Increased bleeding | |
| Combined resection of blood vessels is possible | Big scar |
However, the cohort follow-up period was too short in that study; therefore, the impact on oncological outcomes remains unclear. Multimodal treatment is commonly used for advanced thymic epithelial tumors. Surgical treatment has been reported to be effective, with complete resection being the most effective; and debulking surgery may also have a survival effect (52). VATS or RATS may be selected in cases of pericardial invasion or pleural dissemination; however, if SVC or aortic invasion is present, artificial blood vessel replacement or angioplasty is required, and open-heart surgery is indicated. However, open-heart surgery is associated with a high incidence of postoperative complications and prolonged hospital stay (53).
Cases of thymic cancer or distant metastasis are considered poor prognostic factors (52); nevertheless, surgery is considered an effective treatment option. In clinical stage III advanced thymic cancer, extended thymectomy with vascular resection may significantly improve overall survival and prognosis compared with no surgical intervention (operation subgroup vs. non-operation subgroup: overall survival, 48 vs. 26 months; distant metastasis-free survival, 47 vs. 18 months) (54).
Multidisciplinary treatment
Because a tumor size of >5 cm is a prognostic predictor in addition to the World Health Organization and tumor-node-metastasis (TNM) classifications, multidisciplinary treatment should be developed for patients with thymic epithelial tumors with large diameters (55). Multidisciplinary treatment for advanced thymic epithelial tumors is considered effective (2,4), and aggressive multidisciplinary treatment, including postoperative radiotherapy (PORT) and chemotherapy, is effective in patients with surgically resected thymoma with lymph node metastasis and may improve survival (2). In addition, extrapleural pneumonectomy (EPP) following induction chemotherapy in patients with thymoma and pleural dissemination is associated with a low recurrence rate, and young patients with good cardiopulmonary function and well-controlled MG may be good candidates (4).
Chemotherapy for thymic epithelial tumors comprises the use of platinum drugs, anthracyclines, and taxanes, and molecular target drugs and ICIs may be second-line drug options; however, a lack of randomized controlled trials is an issue for both (56).
Recently, ICI therapy has been used for thymic epithelial tumors, and although there are some reports indicating that ICI is effective, the incidence of immune-related adverse events is high (3).
To date, dozens of biomarkers associated with checkpoint inhibitors have been identified (57). PD-L1 is one of them.
PD-L1 is typically expressed in 23–92% of thymomas (57,58) and 36–80% of thymic carcinomas (57,59). PD-L1 expression levels are determined by the histological type of thymic epithelial tumor (58,60-62), Masaoka Koga classification (48,58,60), 18F-FDG accumulation (60,63), and presence or absence of MG (58). Thymic carcinoma has a “hot” immune structure exhibiting abundant PD-L1 expression and high tumor-infiltrating lymphocytes (TILs) density (59). PD-L1 expression following induction therapy in thymic carcinoma is significantly higher than that before induction therapy. PD-L1 expression may be upregulated during epithelial-mesenchymal transition (EMT), and anti-programmed cell death 1/PD-L1 immunity therapy may be a reliable treatment in combination with chemotherapy (64).
Widespread PD-L1 expression in thymic epithelial tumors is associated with poor prognosis (62). However, patients with PD-L1-positive thymomas do not have a significantly worse prognosis than those with PD-L1-negative tumors (60). The role of PD-L1 expression as a prognostic factor is uncertain. It will be important to study more cases in the future.
Immunotherapy shows significant tumor-selective therapeutic effects, but most patients show only a transient response, making it important to understand the mechanisms underlying resistance (5). In addition to PD-L1, various tumor microenvironments are involved.
Twenty-five percent of thymic cancers are positive for epidermal growth factor receptor (EGFR) mutations (65). Other parameters that can be used as biomarkers for thymic epithelial tumors are summarized in Table 4 (57,64-74).
Table 4. Biomarkers of thymic epithelial tumors.
| Methods | Thymoma | Thymic carcinoma | Thymic epithelial tumor |
|---|---|---|---|
| Genetic mutation | ZNF721, PABPC1, GTF2I | EGFR, ZNF429, BAP1, ABI1, BCL9L, CHEK2 | – |
| Gene expression | – | – | CLDN4, FGF7, FGF10 |
| Immunohistochemistry | – | CD70, CD8+, CD20+, CD204+, TIIC | SOX9, MAGE-A, NY-ESO-1, MAGE-C1, SAGE, GAGE7 |
| qRT-PCR analysis | – | – | XLOC_003810 |
| Serum concentration | – | – | HSP90α |
EGFR, epidermal growth factor receptor; qRT-PCR, quantitative real-time polymerase chain reaction; HSP90α, heat shock protein 90α.
In the future, ICI treatment may prove effective for thymic epithelial tumors as preoperative and postoperative chemotherapy, as has been shown for other cancers; although various obstacles exist. First, thymic epithelial tumors are relatively rare and collecting cases is challenging. Prospective trials are also difficult to conduct. ICI use can cause adverse events related to autoimmune diseases, and individuals with a predisposition to autoimmune diseases are at particular risk. Thymic epithelial tumors are frequently associated with autoimmune diseases. Therefore, testing for autoimmune diseases before administering ICIs is necessary. The prognosis of thymic epithelial tumors improves if complete resection is possible; however, as surgery may become impossible due to the autoimmune disease, using ICIs as preoperative induction therapy is risky, and consideration should be given to the disadvantages that may arise. In some cases, ICI use is effective against thymic epithelial tumors, and we believe that ICI administration as a multidisciplinary treatment option is effective if appropriate cases are selected.
The prognosis for unresectable advanced thymic epithelial tumors may improve by performing selective postoperative treatment following debulking surgery, which also collects sufficient tissue for measuring PD-L1 and biomarkers. Developing ICIs and molecular-targeted drugs may expand the indications for weight-loss surgery.
Thymic cancer often advances before surgery, and ICI administration may be considered a preoperative induction therapy for cases in which complete resection is difficult.
Perioperative management of thymic epithelial tumor complicated by MG
Thymomas with large tumor sizes, partial thymectomy, histopathology of Type A or Type AB, and older patients are considered to have a higher risk of postoperative MG recurrence (75). Furthermore, in patients with thymomatous MG undergoing thymectomy, being aged 42 years or older and Masaoka-Koga stage > I are associated with poor prognosis (36). In such cases where the risk of postoperative MG recurrence is expected to be high, we believe that postoperative management in an intensive care unit is necessary to enable prompt response in the event of MG recurrence. Measurement of anti-acetylcholine receptor (AChR) antibodies (nmol/L) is required to evaluate MG before and after surgery (75).
Strength of this review, and limitations
In this review, to the best of our knowledge, 56.5% (39 of 69 cases) of the literature was retrospective, accounting for more than half of the manuscripts reviewed. Only 5.8% (4 out of 69 cases) of the literature was prospective, and there were six cases in which it was unclear whether the study was retrospective or prospective.
The strength of this review is that it summarizes the latest treatment options for thymic epithelial tumors. In particular, the possibility of multidisciplinary treatment, including ICI administration, has been mentioned. In addition, by compiling the case reports in a table, possible comorbidities that may occur with thymic epithelial tumors have been listed, which will aid in more careful treatment management.
Retrospective single-center studies are uniform in surgical techniques, facility equipment, and measurement methods. Understanding the limitations of each manuscript that comprised this literature review and comprehensively evaluating the content will contribute to determining future treatment strategies.
The limitations of this review include the inability to retrieve all searched articles and the exclusion of articles in languages other than English and Japanese, which may introduce bias. In addition, because the search was conducted only through PubMed, there is a bias in the search method used.
This report has reviewed the literature and found it to be heterogeneous in how the results were defined and measured. In addition, the keyword search may have biased the selected literature, perhaps overrepresenting the positive results. A further consideration is that the review may not have evaluated the content correctly owing to differences in the outcome definitions and/or the measurement methods.
Conclusions
We reviewed the latest treatments for thymic epithelial tumors. When a disease is associated with a thymic epithelial tumor, extended thymectomy may be considered regardless of the presence or absence of MG. Furthermore, if the technology of VATS and RATS improves and a less invasive approach becomes possible, the indications for extended thymectomy may expand. Finally, if new therapeutic agents, such as ICIs and molecular-targeted drugs are developed, we believe that surgery will become more important not only as therapy but also as part of multidisciplinary treatment that includes tissue collection.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Editage (www.editage.com) for the English language editing.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-453/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-453/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-453/coif). J.Y. served as the principal investigator for Insmed Inc. and Janssen Pharmaceutical. The other authors have no conflicts of interest to declare.
References
- 1.Ruan H, Liu B, Yang X, et al. Analysis of Pulmonary Function in Thymoma Subjects: A 20-Year Retrospective Cohort Study. Thorac Cardiovasc Surg 2023;71:425-31. 10.1055/s-0042-1749320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang F, Dai J, Lou X, et al. Prognostic factors and role of postoperative radiotherapy in surgically resected thymomas. JTCVS Open 2023;14:561-80. 10.1016/j.xjon.2023.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tartarone A, Lerose R, Lettini AR, et al. Current Treatment Approaches for Thymic Epithelial Tumors. Life (Basel) 2023;13:1170 . 10.3390/life13051170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura S, Kawaguchi K, Fukui T, et al. Multimodality therapy for thymoma patients with pleural dissemination. Gen Thorac Cardiovasc Surg 2019;67:524-9. 10.1007/s11748-018-01054-7 [DOI] [PubMed] [Google Scholar]
- 5.Tichet M, Wullschleger S, Chryplewicz A, et al. Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8(+) T cells and reprogramming macrophages. Immunity 2023;56:162-179.e6. 10.1016/j.immuni.2022.12.006 [DOI] [PubMed] [Google Scholar]
- 6.Parvez A, Choudhary F, Mudgal P, et al. PD-1 and PD-L1: architects of immune symphony and immunotherapy breakthroughs in cancer treatment. Front Immunol 2023;14:1296341 . 10.3389/fimmu.2023.1296341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerrera F, Falcoz PE, Moser B, et al. Thymomectomy plus total thymectomy versus simple thymomectomy for early-stage thymoma without myasthenia gravis: a European Society of Thoracic Surgeons Thymic Working Group Study. Eur J Cardiothorac Surg 2021;60:881-7. 10.1093/ejcts/ezab224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucchi M, Aprile V. Surgery for thymomas: is less worthwhile? A clear answer from the European experience. Eur J Cardiothorac Surg 2021;60:888-9. 10.1093/ejcts/ezab293 [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Zhang L, Tang W, et al. Non-intubated uniportal subxiphoid thoracoscopic extended thymectomy for thymoma associated with myasthenia gravis. World J Surg Oncol 2021;19:342 . 10.1186/s12957-021-02430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba Y, Miyajima M, Takase Y, et al. Robot-assisted and video-assisted thoracoscopic surgery for thymoma: comparison of the perioperative outcomes using inverse probability of treatment weighting method. Gland Surg 2022;11:1287-300. 10.21037/gs-22-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Akkawi AI, Eckardt J. Comparison of surgical outcomes after robotic assisted thoracic surgery, video-assisted thoracic surgery and open resection of thymoma. Mediastinum 2021;5:11 . 10.21037/med-20-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Yu L, Ke J. Pathological Features and Prognosis of Thymoma With or Without Myasthenia Gravis. Front Surg 2022;9:726673 . 10.3389/fsurg.2022.726673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aprile V, Korasidis S, Bacchin D, et al. Thymectomy in Myasthenic Patients With Thymoma: Killing Two Birds With One Stone. Ann Thorac Surg 2021;112:1782-9. 10.1016/j.athoracsur.2020.12.010 [DOI] [PubMed] [Google Scholar]
- 14.Hsu DS, Wilde SA, Velotta JB. Thymoma associated with severe pancytopenia and Good's syndrome: case report. AME Case Rep 2021;5:22 . 10.21037/acr-21-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagh AN, Bhagvat SR, Bhandarwar AH, et al. Minimally invasive extended thymectomy for thymoma associated with pure red cell aplasia. Indian J Thorac Cardiovasc Surg 2022;38:199-203. 10.1007/s12055-021-01273-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanagiya M, Hamaya H, Matsuzaki H, et al. Atypical Type A Thymoma Variant Manifesting Polymyalgia Rheumatica. Ann Thorac Surg 2020;110:e253-5. 10.1016/j.athoracsur.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 17.Sasaki A, Kato T, Ujiie H, et al. Thymoma-Related Stiff-Person Syndrome with Successfully Treated by Surgery. Ann Thorac Cardiovasc Surg 2022;28:448-52. 10.5761/atcs.cr.21-00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito Y, Yazawa T, Nagashima T, et al. Autoimmune alopecia areata due to thymoma without myasthenia gravis: a case report. Surg Case Rep 2023;9:68 . 10.1186/s40792-023-01655-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takashima M, Kagawa K, Sawada T, et al. Type A thymoma: a rare cause of neoplastic cardiac tamponade with long-term survival. BMC Pulm Med 2022;22:242 . 10.1186/s12890-022-02034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasuda K, Kidokoro Y, Makishima K, et al. A rare case of combined thymoma and a multilocular thymic cyst discovered due to chest pain. Surg Case Rep 2021;7:158 . 10.1186/s40792-021-01243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishina K, Suzuki M, Nakamura A, et al. Thymoma exhibiting spontaneous regression with developing myasthenia gravis: A case report. Thorac Cancer 2022;13:1533-6. 10.1111/1759-7714.14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao W, Wu L, Jin S, et al. Rechallenge of immune checkpoint inhibitors in a case with adverse events inducing myasthenia gravis. J Immunother Cancer 2022;10:e005970 . 10.1136/jitc-2022-005970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai CP, Lin CM, Yeh YC, et al. De novo thymic carcinoma or malignant transformation: a myasthenic patient presented with multiple mediastinal tumours. Respirol Case Rep 2020;8:e00629 . 10.1002/rcr2.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asami-Noyama M, Furuya-Kondo T, Suetake R, et al. Invasive thymoma extending to the right atrium with superior vena cava syndrome presenting massive intracardiac thrombosis immediately after the start of chemotherapy: an autopsy case report. Int Cancer Conf J 2022;11:158-63. 10.1007/s13691-022-00541-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohashi T, Kawago M, Hirai Y, et al. A rare case of thymoma with CA 19-9 production. Respirol Case Rep 2021;9:e0844 . 10.1002/rcr2.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Zheng Y, Chen LQ, et al. Robotic resection of a thymoma behind the left innominate vein. Interact Cardiovasc Thorac Surg 2019;29:813-5. 10.1093/icvts/ivz165 [DOI] [PubMed] [Google Scholar]
- 27.Nishimura T, Tsunezuka H, Miyata N, et al. Autoimmune hepatitis during preoperative chemotherapy in a patient with thymoma. Interact Cardiovasc Thorac Surg 2019;29:635-7. 10.1093/icvts/ivz138 [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto S, Hayasaka K, Suzuki K, et al. Thymic Small Cell Carcinoma Associated With Lambert-Eaton Myasthenic Syndrome. Ann Thorac Surg 2020;109:e347-8. 10.1016/j.athoracsur.2019.08.080 [DOI] [PubMed] [Google Scholar]
- 29.Kang MK, Kang DK, Hwang YH, et al. Surgical treatment of micronodular thymoma with lymphoid stroma. Respirol Case Rep 2020;8:e00548 . 10.1002/rcr2.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekiguchi T, Yoshida K, Minamihara Y, et al. Late-onset myasthenia gravis successfully treated with local resection of cervical ectopic thymoma. J Clin Neurosci 2020;73:321-3. 10.1016/j.jocn.2019.12.055 [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi E, Kajiyama Y, Ando K, et al. The efficacy of eculizumab against post-thymectomy exacerbations in thymoma associated myasthenia gravis (MG). Rinsho Shinkeigaku 2022;62:277-80. 10.5692/clinicalneurol.cn-001682 [DOI] [PubMed] [Google Scholar]
- 32.Marcuse F, Hoeijmakers JGJ, Hochstenbag M, et al. Outcomes after robotic thymectomy in nonthymomatous versus thymomatous patients with acetylcholine-receptor-antibody-associated myasthenia gravis. Neuromuscul Disord 2023;33:417-24. 10.1016/j.nmd.2023.03.005 [DOI] [PubMed] [Google Scholar]
- 33.Otsuka R, Ueda K, Tanaka T, et al. Who will benefit from thymectomy for myasthenia gravis? Is there any role for this procedure in elderly patients? Ann Transl Med 2019;7:4 . 10.21037/atm.2018.11.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brascia D, Lucchi M, Aprile V, et al. Thymectomy in severe (Myasthenia Gravis Foundation of America classes IV-V) generalized myasthenia gravis: is the game really worth the candle? Eur J Cardiothorac Surg 2023;63:ezad179 . 10.1093/ejcts/ezad179 [DOI] [PubMed] [Google Scholar]
- 35.Na KJ, Hyun K, Kang CH, et al. Predictors of post-thymectomy long-term neurological remission in thymomatous myasthenia gravis: an analysis from a multi-institutional database. Eur J Cardiothorac Surg 2020;57:867-73. 10.1093/ejcts/ezz334 [DOI] [PubMed] [Google Scholar]
- 36.Haoshuai Z, Jianyong Z, Lei Y, et al. Factors affecting improvement of neurologic status evaluated by Quantitative Myasthenia Gravis Score for patients with thymomatous myasthenia gravis after extended thymectomy. J Transl Med 2021;19:413 . 10.1186/s12967-021-03082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian W, Sun Y, Wu Q, et al. Surgical outcomes of 215 patients with thymic epithelial tumors: A single-center experience. Thorac Cancer 2020;11:1840-7. 10.1111/1759-7714.13464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato T, Kawaguchi K, Fukui T, et al. Risk Factors for the Exacerbation of Myasthenic Symptoms After Surgical Therapy for Myasthenia Gravis and Thymoma. Semin Thorac Cardiovasc Surg 2020;32:378-85. 10.1053/j.semtcvs.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 39.Okuda K, Hattori H, Yokota K, et al. Examination on the necessity of pericardial fat tissue resection in extended thymectomy for myasthenia gravis. Gland Surg 2021;10:2438-44. 10.21037/gs-21-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen K, Zhang X, Jin R, et al. Robot-assisted thoracoscopic surgery for mediastinal masses: a single-institution experience. J Thorac Dis 2020;12:105-13. 10.21037/jtd.2019.08.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Şehitogullari A, Nasır A, Anbar R, et al. Comparison of perioperative outcomes of videothoracoscopy and robotic surgical techniques in thymoma. Asian J Surg 2020;43:244-50. 10.1016/j.asjsur.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 42.Azenha LF, Deckarm R, Minervini F, et al. Robotic vs. Transsternal Thymectomy: A Single Center Experience over 10 Years. J Clin Med 2021;10:4991 . 10.3390/jcm10214991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alqudah O, Purmessur R, Hogan J, et al. Robotic resection of anterior mediastinal masses >10 cm: A case series. Mediastinum 2023;7:29 . 10.21037/med-22-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bongiolatti S, Salvicchi A, Puzhlyiakov V, et al. Long-term outcomes of robot-assisted radical thymectomy for large thymomas: A propensity matched analysis. Int J Med Robot 2022;18:e2439 . 10.1002/rcs.2439 [DOI] [PubMed] [Google Scholar]
- 45.Yoshida M, Yuasa M, Kondo K, et al. Evaluation of extended thymectomy approaches based on residual fat tissue. Interact Cardiovasc Thorac Surg 2021;32:250-5. 10.1093/icvts/ivaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asaf BB, Puri HV, Bishnoi S, et al. Subxiphoid robotic extended thymectomy - The first Indian report. J Minim Access Surg 2020;16:360-3. 10.4103/jmas.JMAS_34_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu H, Liu Z, Yao X, et al. Neurological outcomes of extended thymectomy for thymomatous myasthenia gravis: Subxiphoid vs. trans-sternal approaches. Front Surg 2022;9:973954 . 10.3389/fsurg.2022.973954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Ma Q, Wang X, et al. Subxiphoid and subcostal thoracoscopic surgical approach for thymectomy. Surg Endosc 2021;35:5239-46. 10.1007/s00464-020-08022-4 [DOI] [PubMed] [Google Scholar]
- 49.Sholtis C, Teymourtash M, Berry M, et al. Transcervical Thymectomy Is the Most Cost-Effective Surgical Approach in Myasthenia Gravis. Ann Thorac Surg 2020;109:1705-12. 10.1016/j.athoracsur.2020.01.047 [DOI] [PubMed] [Google Scholar]
- 50.Ferreira R, Junqueira N, Rodrigues M, et al. Inframammary approach for addressing anterior mediastinal tumours: initial experience. J Thorac Dis 2020;12:2077-81. 10.21037/jtd-19-3310b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Refai M, Gonzalez-Rivas D, Guiducci GM, et al. Uniportal video-assisted thoracoscopic thymectomy: the glove-port with carbon dioxide insufflation. Gland Surg 2020;9:879-85. 10.21037/gs-19-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi W, Tian H. The role of surgery in advanced thymic tumors: A retrospective cohort study. Front Oncol 2022;12:1073641 . 10.3389/fonc.2022.1073641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petroncini M, Solli P, Brandolini J, et al. Early Postoperative Results after Thymectomy for Thymic Cancer: A Single-Institution Experience. World J Surg 2023;47:1978-85. 10.1007/s00268-023-06996-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L, Zhang J, Wang G, et al. Extended thymectomy with blood vessel resection and reconstruction improves therapeutic outcome for clinical stage III thymic carcinoma patients: a real-world research. J Cardiothorac Surg 2020;15:267 . 10.1186/s13019-020-01316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakai T, Aokage K, Miyoshi T, et al. Tumor size exceeding 5 cm as a valid prognostic factor in all stages of thymic epithelial tumors. Surg Today 2023;53:42-50. 10.1007/s00595-022-02530-7 [DOI] [PubMed] [Google Scholar]
- 56.Kurokawa K, Shukuya T, Greenstein RA, et al. Genomic characterization of thymic epithelial tumors in a real-world dataset. ESMO Open 2023;8:101627 . 10.1016/j.esmoop.2023.101627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang W, Chen S, Cheng X, et al. Characteristics of genomic mutations and signaling pathway alterations in thymic epithelial tumors. Ann Transl Med 2021;9:1659 . 10.21037/atm-21-5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song JS, Kim D, Kwon JH, et al. Clinicopathologic Significance and Immunogenomic Analysis of Programmed Death-Ligand 1 (PD-L1) and Programmed Death 1 (PD-1) Expression in Thymic Epithelial Tumors. Front Oncol 2019;9:1055 . 10.3389/fonc.2019.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bocchialini G, Schiefer AI, Müllauer L, et al. Tumour immune microenvironment in resected thymic carcinomas as a predictor of clinical outcome. Br J Cancer 2022;127:1162-71. 10.1038/s41416-022-01875-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hakiri S, Fukui T, Mori S, et al. Clinicopathologic Features of Thymoma With the Expression of Programmed Death Ligand 1. Ann Thorac Surg 2019;107:418-24. 10.1016/j.athoracsur.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 61.Wei YF, Chu CY, Chang CC, et al. Different pattern of PD-L1, IDO, and FOXP3 Tregs expression with survival in thymoma and thymic carcinoma. Lung Cancer 2018;125:35-42. 10.1016/j.lungcan.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 62.Ishihara S, Okada S, Ogi H, et al. Programmed death-ligand 1 expression profiling in thymic epithelial cell tumors: Clinicopathological features and quantitative digital image analyses. Lung Cancer 2020;145:40-7. 10.1016/j.lungcan.2020.04.038 [DOI] [PubMed] [Google Scholar]
- 63.Imai H, Kaira K, Hashimoto K, et al. Tumor immunity is related to (18) F-FDG uptake in thymic epithelial tumor. Cancer Med 2021;10:6317-26. 10.1002/cam4.4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Funaki S, Shintani Y, Fukui E, et al. The prognostic impact of programmed cell death 1 and its ligand and the correlation with epithelial-mesenchymal transition in thymic carcinoma. Cancer Med 2019;8:216-26. 10.1002/cam4.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sato J, Kitano S, Motoi N, et al. CD20(+) tumor-infiltrating immune cells and CD204(+) M2 macrophages are associated with prognosis in thymic carcinoma. Cancer Sci 2020;111:1921-32. 10.1111/cas.14409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tahara I, Oishi N, Mochizuki K, et al. Identification of Recurrent TERT Promoter Mutations in Intrathyroid Thymic Carcinomas. Endocr Pathol 2020;31:274-82. 10.1007/s12022-020-09635-0 [DOI] [PubMed] [Google Scholar]
- 67.Hu B, Niu L, Jiang Z, et al. LncRNA XLOC_003810 promotes T cell activation and inhibits PD-1/PD-L1 expression in patients with myasthenia gravis-related thymoma. Scand J Immunol 2020;92:e12886 . 10.1111/sji.12886 [DOI] [PubMed] [Google Scholar]
- 68.Yuan X, Huang L, Luo W, et al. Diagnostic and Prognostic Significances of SOX9 in Thymic Epithelial Tumor. Front Oncol 2021;11:708735 . 10.3389/fonc.2021.708735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kashima J, Hishima T, Okuma Y, et al. CD70 in Thymic Squamous Cell Carcinoma: Potential Diagnostic Markers and Immunotherapeutic Targets. Front Oncol 2021;11:808396 . 10.3389/fonc.2021.808396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang N, Liu L, Huang C, et al. Transcriptomic and Mutational Analysis Discovering Distinct Molecular Characteristics Among Chinese Thymic Epithelial Tumor Patients. Front Oncol 2021;11:647512 . 10.3389/fonc.2021.647512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thanner J, Bekos C, Veraar C, et al. Heat shock protein 90α in thymic epithelial tumors and non-thymomatous myasthenia gravis. Oncoimmunology 2020;9:1756130 . 10.1080/2162402X.2020.1756130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang W, Wu CH, Sun QL, et al. Novel Tumor-Specific Antigens for Immunotherapy Identified From Multi-omics Profiling in Thymic Carcinomas. Front Immunol 2021;12:748820 . 10.3389/fimmu.2021.748820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakane T, Murase T, Okuda K, et al. Expression of cancer testis antigens in thymic epithelial tumors. Pathol Int 2021;71:471-9. 10.1111/pin.13103 [DOI] [PubMed] [Google Scholar]
- 74.Pardini E, Cucchiara F, Palumbo S, et al. Somatic mutations of thymic epithelial tumors with myasthenia gravis. Front Oncol 2023;13:1224491 . 10.3389/fonc.2023.1224491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim A, Choi SJ, Kang CH, et al. Risk factors for developing post-thymectomy myasthenia gravis in patients with thymoma. Muscle Nerve 2021;63:531-7. 10.1002/mus.27169 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


