Graphical abstract
Keywords: Chili peppers, Constant and variable temperature drying, Colors, Nutrients, Volatile compounds, GC-IMS
Highlights
-
•
Low and variable temperature drying preserved the surface color of chili peppers.
-
•
Variable temperature drying reduced the loss of total sugar, total acid, fat and capsaicin contents.
-
•
Drying led to an increase in acids, furans and sulfides, while decreasing alcohols, esters and olefins levels.
-
•
Three chili pepper varieties displayed diverse colors, nutrients and volatiles in drying process.
-
•
Variable temperature drying is a strategy for preserving the commercial value of chili peppers.
Abstract
The effects of constant and variable temperature hot-air drying methods on drying time, colors, nutrients, and volatile compounds of three chili pepper varieties were investigated in this study. Overall, the variable temperature drying could facilitate the removal of water, preserve surface color, and reduce the loss of total sugar, total acid, fat and capsaicin contents. Electronic-nose (E-nose) and gas chromatography-ion mobility spectroscopy (GC-IMS) analyses found that aldehydes, ketones, alcohols and esters contributed to the aroma of chili peppers. The drying process led to an increase in acids, furans and sulfides contents, while decreasing alcohols, esters and olefins levels. In addition, the three chili pepper varieties displayed distinct physical characteristics, drying times, chromatic values, nutrients levels and volatile profiles during dehydration. This study suggests variable temperature drying is a practical approach to reduce drying time, save costs, and maintain the commercial appeal of chili peppers.
Introduction
Chili pepper (Capsicum annuum L.) is an annual herbaceous plant in the Solanaceae family, which widely existed in North America, Southern Europe, Asia, and Africa (Baenas, Belovic, Ilic, Moreno, & Garcia-Viguera, 2019). The genus Capsicum comprises over 200 varieties, whose size, shape, flavor and sense are greatly different. To specific, the main varieties are Capsicum annuum, Capsicum frutescens, Capsicum chinense, Capsicum baccatum and Capsicum pubescens (Ananthan, Subhash, & Longvah, 2018). Due to the unique color, pungency and aroma, chili peppers are commonly employed as natural colorants and flavoring agents in the food industry (Taiti, Costa, Migliori, Comparini, Figorilli, & Mancuso, 2019). Chili peppers are abundant in health-promoting nutrients and phytochemicals such as vitamins, phenolics, flavonoids and carotenoids, which exert anti-cardiovascular diseases, relieving pain caused by arthritis, anti-aging and immunomodulatory activities (Song, Du, Ding, Yu, & Wang, 2021). Additionally, they have been found to inhibit lipid oxidation and microbial growth, as well as enhance postprandial satiety.
To our knowledge, fresh chili peppers are perishable due to their high moisture contents. Hence, there is a necessity of extending their shelf life by suitable storage methods, while minimizing the loss or damage of active ingredients and consuming the least energy in the process. Drying is one of the oldest and widely used approaches for food preservation to make products available annually. It helps preserve flavor and nutritional values of products, inhibit the proliferation of spoiling microorganisms, prolong the shelf life by removing water to the standard for safe storage, as well as reduce the weight and volume of products greatly to minimize the costs of packaging, storage and transportation (Ge et al., 2020, Zhang et al., 2019). At present, sun drying, hot-air drying, spray drying, vacuum drying, freeze-drying and solar drying are the commonly used methods for chili peppers (Deng et al., 2018, Guclu et al., 2021). As an energy-saving, clean and safe approach utilized extensively, hot-air drying is characterized by low temperature and high efficiency, whose drying parameters such as temperature, time and air velocity can be controlled easily. For instance, Hwang et al. confirmed that the combination of hot-air and hot-pump drying could improve the quality of chili peppers via up-regulating color brightness, as well as reducing tissue structure degradation, energy consumption and carcinogen generation (e.g., polycyclic aromatic hydrocarbons) in the drying process (Hwang, Kang, Kim, & Lee, 2019). However, traditional hot-air drying exhibits the disadvantages of long drying time, loss of aroma and color, occurrence of oxidation reaction, reduced rehydration capacity, and hardening of epidermis (Guclu, et al., 2021). Therefore, it is urgent to optimize the conventional hot-air drying procedure in order to shorten the drying time and reduce undesirable loss of products.
Except for the changes in the moisture contents of samples, hot-air drying also affect other components and physical properties including color, nutrients, enzymatic activity and flavor. The quality of dried chili peppers depends on the consumer acceptance that is mostly influenced by color, pungency and aroma, in particular the typical aroma of these products plays a key role in their sensory characteristics (Taiti et al., 2019). Volatile organic compounds (VOCs) have been demonstrated to be associated with the food aromas and different processing methods (Song et al., 2021, Liu et al., 2024). Thus, the identification of these compounds can provide comprehensive information about the VOCs in foods, and assist in quality control to meet consumer preferences (Xu et al., 2020). The most commonly used instrumental analysis techniques for identifying volatile compoents in foods are electronic nose (E-nose), gas chromatography-mass spectrometry (GC–MS), and GC-olfactometry-MS (GC-O-MS). Recently, gas chromatography-ion mobility spectroscopy (GC-IMS) with low detection limit has been extensively applied to investigate the VOCs of foods (Duan, Dong, Dong, & Gao, 2021). As an emerging rapid and sensitive technique, GC-IMS can provide variable injection volume without requiring any pre-treatment of samples to detect the volatile substances in liquid or solid samples (Yu, Xiang, Tan, Zhang, Shan, & Yang, 2021). For example, GC-IMS has been employed to analyze the odor of various food samples, such as olive oil (Contreras, Jurado-Campos, Arce, & Arroyo-Manzanares, 2019), pepper powder (Song, Du, Ding, Yu, & Wang, 2021), and sausage (Yu, Xiang, Tan, Zhang, Shan, & Yang, 2021).
Nowadays, some works have been performed on the hot-air drying method at constant temperature and on the volatile substances of chili pepper powder (Guclu et al., 2021, Hwang et al., 2019, Song et al., 2021), but few studies are available regarding the comparison of variable controlled-temperature and traditional constant temperature hot-air drying, as well as the differences in colors and aromas of diverse dried chili pepper varieties. Therefore, the present work aimed to explore the effects of constant (50, 60, 70, and 80 ℃) and variable temperature (80 → 60, and 80 → 70 → 60 ℃) hot-air drying procedures on the needed drying time, color parameters, nutrients and volatile compounds of three chili pepper varieties (linear, millet and bullet peppers). The obtained findings would provide a logical alternative drying method for reducing the drying time to save cost and maintaining the commercial values of chili peppers.
Materials and methods
Chemicals and materials
Three fresh chili peppers including linear, millet and bullet peppers were harvested and collected from Weinan, Shanxi Province, Kunming, Yunnan Province, and Zhoukou, Henan Province, China, separately. The fresh linear, millet and bullet peppers were labeled as XF, XMF, and ZF. While the dried linear, millet and bullet peppers were labeled as XD, XMD, and ZD. To ensure the uniformity of physical characteristics of samples, the chili peppers with the same size and maturity were carefully selected and stored at 4 ℃ until utilization. Sodium hydroxide, sodium molybdate dihydrate, methanol, anhydrous ethanol, hydrochloric acid, glucose, sodium nitrite and the internal standard 2-octanol (purity > 99 %) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Capsaicin (HPLC grade) was provided by Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Ascorbic acid was obtained from ANPEL Laboratory Technologies Inc. (Shanghai, China). All of the other reagents were of analytical grade or above.
Physical characteristics of chili peppers
A total of 30 chili peppers in each variety were randomly selected to test their physical characteristics. The distance from the top to the base of chili peppers was the length of samples, and the length of the widest part of the chili pepper body was the width of samples, which both were measured by a ruler. The peel thickness and weight of three chili pepper varieties were determined by a vernier caliper, and electronic balance, respectively. In addition, individual chili pepper was completely filled with millet, and the volume of millet was defined as the sample volume. The hardness of samples was detected by TA.XT Plus Texture Analyzer (Stable Micro System, London, UK). The force of puncturing the epidermis in five points at the middle of the chili pepper was monitored, and the maximum force was defined as the hardness value.
Hot-air drying process for chili peppers
After the removal of stem and pedicle, the chili peppers were washed with tap water and air-dried to volatilize the remaining water on the surface. The hot-air drying methods used in the present study were as follows (Table S1). One of the approaches was constant temperature drying to achieve the desired moisture contents of samples for long-term storage, which were lower than 14 %, 10 % and 10 % for linear, millet, and bullet peppers, respectively (Deng, et al., 2018). While the other one was variable temperature drying, which samples were firstly dried at a higher temperature for a certain time, followed by drying at a lower temperature until the required water contents were reached. The dynamic changes in the moisture content, surface color, nutrient substances, capsaicin content, and VOCs of these samples treated by different drying approaches were measured as described below.
Determination of moisture
The fresh and dried chili peppers were ground using a grinder and sieved to obtain sample powders. Then, 5 g fresh samples and 2 g dried samples were kept in an oven at 105 ℃ until a constant weight. The weight loss of samples was measured using an electronic balance with an accuracy of ± 0.0001 g (AUY120, SHIMADZU). Final data were expressed as g water/100 g dry weight.
Determination of surface color
The color measurement of chili peppers was conducted by the protocol as explained by a previous report with little modification (Yang, Deng, Mujumdar, Xiao, Zhang, & Kan, 2018). Briefly, the lightness (L*), redness (a*), yellowness (b*) and total color difference (ΔE) values of sample powders were monitored using a WSC-S colorimeter (Shanghai Yidian Physics Optical Instrument Co., Ltd., Shanghai, China).
Determination of nutrient substances
The protein, fat, and total sugar contents of chili peppers were determined according to the Approved Methods of AACC Method 46-10, Method 30-10, and Method 76-11, respectively (AACC, 2000). The total acid and vitamin C (VC) contents of samples were measured by pH potentiometry (Prenesti, Toso, & Berto, 2005) and UV–Vis spectrophotometry (Stevens, Buret, Garchery, Carretero, & Causse, 2006), separately.
Determination of capsaicin content
The capsaicin content of samples was determined by sodium molybdate-sodium nitrite method described formerly (Chittasupho, Thongnopkoon, Burapapisut, Charoensukkho, Shuwisitkul, & Samee, 2020). Chili pepper powders and 95 % ethanol (w:v = 1:35) were fully mixed, ultrasonicated for 45 min (60 ℃, 53 kHz), and filtered by decompression. Appropriate volume of supernatants, 2 mL of hydrochloric acid solution (0.5 mol/L), and 1 mL of sodium nitrite-sodium molybdate (0.5 mol/L sodium nitrite and 0.025 mol/L sodium molybdate mixture) were mixed in 10 mL of volumetric flask. Following 15 min of incubation and addition of sodium hydroxide solution (2 mL, 1.0 mol/L), this mixture was diluted to 10 mL with methanol and kept for 20 min to coloration. Ultimately, the absorbance was immediately read by a UV/Vis spectrophotometer (Purkinje T6-1650E, Beijing Purkinje General Instrument Co., Beijing, China) at a wavelength of 436 nm.
Identification of volatile components by E-nose analysis
In short, 5 g fresh samples and 2 g dried samples were added into the corresponding headspace vials, sealed, and incubated for 30 min at 25 ℃. The types of VOCs were analyzed by a PEN3 electronic nose (Beijing Yingsheng Hengtai Technology Co., Ltd., Beijing, China). The detection time, flow rate of carrier gas, and injection volume were 160 s, 200 mL/min, and 200 mL/min, respectively. The PEN3 system contains 10 metal oxide gas sensors, including W1C, W5S, W3C, W6S, W5C, W1S, W1W, W2S, W2W and W3S (Table S2), which can detect olfactory cross-sensitive information.
Identification of volatile components by GC-IMS analysis
The specific VOCs of three chili peppers were further detected by GC-IMS (FlavourSpec®, G.A.S. mbH, Dortmund, Germany), equipped with an MXT-5 column (15 m × 0.53 mm, 1 μm) maintained at 60 ℃. In brief, 1 g fresh samples and 0.2 g dried samples as well as 10 μL of 2-octanol (100 ppm) were added into 20 mL of headspace vials, and incubated at 40 ℃ for 15 min. The test time, flow rate of carrier gas (nitrogen), and injection volume were 20 min, 2.0 mL/min, and 500 μL, separately. The internal standard (2-octanol) was used to calculate the relative contents of VOCs in different groups. Besides, the contribution of each individual volatile compound to the overall aroma of samples was evaluated by odor activity values (OAVs): , where and are the content and corresponding odor threshold of individual VOCs, respectively. Aromatic compounds with OAVs ≥ 1 and < 1 were considered as major and minor contributors to the aroma of chili peppers, respectively.
Statistical analysis
All results were expressed as mean ± SD (n = 6) in sextuplicate. ANOVA analysis was performed by SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA), which p < 0.05 indicated significant difference. The GC-IMS data were analyzed by Laboratory Analytical Viewer (LAV) and GC × IMS Library Search (FlavourSpec®, Dortmund, Germany). The heat map analysis was performed using https://www.bioincloud.tech.
Results and discussion
Physical characteristics of chili peppers
Overall, there were significant differences in the physical characteristics among three chili pepper varieties (p < 0.05; Table S3 & Fig. S1). The linear pepper exhibited the largest volume, length and weight as well as the highest moisture content. Besides, millet pepper showed the smallest volume, width, peel thickness and weight as well as lowest moisture content, while had the largest hardness. Whereas, the bullet pepper was the shortest one with the largest width and peel thickness.
Dynamic changes in moisture content of chili peppers during different drying processes
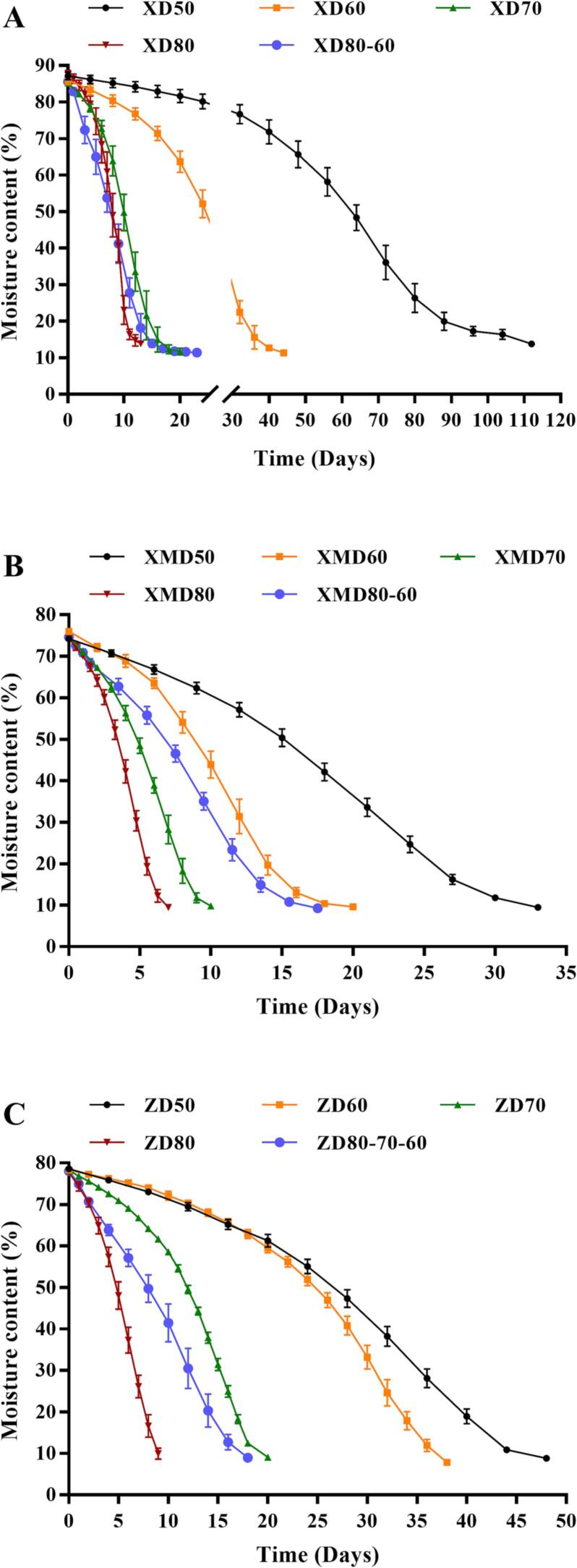
Following treated by various hot-air drying methods, the moisture contents of three chili pepper varieties all reduced with the increment of drying time, and eventually bottomed to their desired values (Fig. 1). As depicted in Fig. 1A, the needed drying time of linear pepper using the changed temperature (80 ℃→60 ℃, 17 h) was shorter than that of constant temperature drying including 60 ℃ (40 h), 70 ℃ (18 h) and 50 ℃ (112 h), but longer than that of 80 ℃ (13 h). For millet pepper, the treatment at 80 ℃ achieved the minimum drying time (7 h), which followed by 70 ℃ (10 h), 80 ℃→60 ℃ (17.5 h), 60 ℃ (20 h), 50 ℃ (33 h; Fig. 1B). With regard to bullet pepper, the required drying time at 50, 60, 70, 80 → 70 → 60 and 80 ℃ were 48, 38, 20, 18, and 9 h, respectively (Fig. 1C). The drying time using the changed temperature was ranked as follows: bullet pepper > millet pepper > linear pepper. Compared with constant temperature drying at 60 ℃, the drying time under variable temperature of linear, bullet and millet pepper were reduced by 23 h, 20 h, and 2.5 h, respectively. Obviously, the higher temperature was accompanied with shorter drying time to reach desired moisture content. This phenomenon might be explained by the fact that the higher temperature can improve the rate of water loss as well as the driving force of heat and mass transfer, leading to shorter drying time (Guo, Chen, Dong, Ju, Wu, & Lin, 2018). However, the further increment of drying time also triggers tighter crust on the chili pepper surface to cause reduced drying rate, which is the main obstacle for moisture diffusion during the dehydration process (Koç, 2020). Therefore, the hot-air drying initiated with a higher temperature (80 ℃) and altered to a lower temperature (60 ℃) can facilitate the diffusion and removal of water in the whole drying process.
Fig. 1.
The dynamic changes in moisture content of three chili pepper varieties during the different drying conditions: linear pepper (A), millet pepper (B), and bullet pepper (C). XD, XMD and ZD were represented as dried line pepper, millet pepper and bullet pepper, respectively.
Dynamic changes in chromatic values of chili peppers
Color is an important indicator to assess the quality of foods and agricultural products, which influences the consumer acceptance and commercial value. Hence, the surface color and chromatic values of three fresh and dried chili pepper varieties under different drying conditions were summarized in Figs. S2–S4 and Table 1. As shown in Fig. S1, the surface colors of fresh linear, millet and bullet peppers appeared glossy and red, and then the brightness and redness of samples increased to dark red at the drying end point. This phenomenon might be explained by the fact that heat treatment could destroy the cell structure of chili peppers and promote the release of red pigments from the matrix, thereby increasing the red color of chili peppers (Wang et al., 2020).
Table 1.
The changes in the chromatic values of three chili pepper varieties.
| Samples | Time (h) | L* | a* | b* | ΔE | a*×L* |
|---|---|---|---|---|---|---|
| XF | 0 | 28.60 ± 1.05c | 36.28 ± 1.23b | 20.37 ± 0.13d | 74.66 ± 0.17ab | 1038.25 ± 73.16d |
| XD50 | 112 | 33.51 ± 0.29a | 39.66 ± 1.78a | 31.05 ± 0.52a | 75.64 ± 0.92a | 1329.09 ± 71.21a |
| XD60 | 40 | 31.90 ± 0.25ab | 37.01 ± 1.85ab | 28.28 ± 2.51ab | 74.41 ± 2.20ab | 1180.27 ± 50.02bc |
| XD70 | 18 | 31.02 ± 1.46b | 34.55 ± 0.15bc | 23.86 ± 1.11c | 72.61 ± 1.10b | 1071.56 ± 45.84 cd |
| XD80 | 13 | 28.93 ± 0.04c | 32.29 ± 0.34c | 23.80 ± 0.24c | 73.39 ± 0.19ab | 933.99 ± 10.96d |
| XD80→60 | 3 → 14 | 33.02 ± 0.78ab | 37.51 ± 0.78ab | 27.15 ± 0.77b | 73.56 ± 0.03ab | 1238.54 ± 55.35ab |
| XMF | 0 | 30.33 ± 1.68c | 39.62 ± 0.48a | 25.21 ± 0.27c | 76.23 ± 1.01a | 1201.90 ± 81.01c |
| XMD50 | 33 | 34.49 ± 0.42a | 40.03 ± 0.06a | 32.13 ± 0.52a | 75.50 ± 0.55ab | 1380.25 ± 14.50a |
| XMD60 | 20 | 34.43 ± 1.52a | 38.36 ± 0.27b | 32.72 ± 0.91a | 74.88 ± 1.65ab | 1320.34 ± 49.07ab |
| XMD70 | 10 | 34.12 ± 0.14ab | 35.35 ± 0.37d | 23.49 ± 0.62d | 70.46 ± 0.09c | 1206.00 ± 17.79c |
| XMD80 | 7 | 31.84 ± 0.72bc | 33.88 ± 0.48e | 29.21 ± 0.56b | 73.48 ± 0.29b | 1078.91 ± 39.75d |
| XMD80→60 | 1.5 → 16 | 34.24 ± 0.09ab | 37.05 ± 0.16c | 30.25 ± 0.07b | 76.28 ± 0.35a | 1268.23 ± 2.16bc |
| ZF | 0 | 29.76 ± 0.02e | 36.00 ± 0.59b | 23.02 ± 0.53c | 74.44 ± 0.11a | 1071.19 ± 18.44d |
| ZD50 | 48 | 37.40 ± 0.95b | 39.18 ± 0.02a | 33.29 ± 1.06a | 73.36 ± 0.25a | 1465.33 ± 37.84a |
| ZD60 | 38 | 39.42 ± 0a | 33.32 ± 0c | 34.14 ± 0.02a | 69.50 ± 0.54b | 1313.47 ± 14.39b |
| ZD70 | 20 | 33.94 ± 0.2d | 33.20 ± 0.11c | 30.67 ± 0.06d | 70.38 ± 0b | 1126.82 ± 10.41c |
| ZD80 | 9 | 35.68 ± 0.08c | 32.56 ± 0.01c | 29.83 ± 2.09b | 69.94 ± 0.83b | 1161.67 ± 2.57c |
| ZD80→70→60 | 2 → 2 → 14 | 36.92 ± 0.78bc | 35.56 ± 0.97b | 29.59 ± 1.23b | 70.33 ± 1.55b | 1312.45 ± 8.17b |
XF, XMF and ZF were represented as fresh line pepper, millet pepper and bullet pepper. XD, XMD and ZD were represented as dried line pepper, millet pepper and bullet pepper, respectively. Values with different lowercase letters in each line differ significantly at p < 0.05.
As drying proceeds, the L*, a*, b* and ΔE values of the three chili pepper varieties under different conditions increased at first, then declined, and eventually reached to the maximum (Figs. S2–S4). Overall, the descending order of final chromatic values treated by the same drying method was: bullet pepper > millet pepper > linear pepper (Table 1). In contrast with fresh linear peppers, the values of L*, a*, b* and ΔE all increased at the end of XD50 drying, while the L*, a* and b* values were augmented at the end of variable temperature (XD80→60) drying accompanied by a reduced ΔE value. There were increased L* and b* values as well as declined a* and ΔE values at the end of XD60 and XD70 drying processes. In addition, the decreased values of L*, a* and ΔE as well as ascended b* value were observed at the end of XD80 drying. Among these indexes, the a* and L* values were the major parameters to evaluate the color of dried chili peppers. Thus, the a* × L* value can be used as a color degradation index for paprika, and the value larger than 700 is considered as brilliant red (Guclu et al., 2021). In our study, the a* × L* value of linear peppers through XD50 drying was the largest (1329.09 ± 71.21), followed by XD80→60 (1238.54 ± 55.35), XD60 (1180.27 ± 50.02), XD70 (1071.56 ± 45.84), and XD80 (933.99 ± 10.96). The changes of chromatic values for millet and bullet peppers generally followed the similar trends as linear peppers. Compared with fresh chili peppers, the values of L*, a*, b* and ΔE all increased at the end of XMD50 and ZD50 drying. Besides, the L*, a* and ΔE values of millet peppers were augmented at the end of variable temperature (XD80→60) drying accompanied by a reduced b* value. Whereas, the ascended L*, a* and b* values of bullet peppers as well as declined ΔE value were found at the end of variable temperature (XD80→70→60) drying. In addition, the a* × L* values of dried millet pepper samples ranged from 1078.91 ± 39.75 (XMD80) to 1380.25 ± 14.50 (XMD50). With regard to bullet peppers, the descending order of a* × L* value was: ZD50 (1465.33 ± 37.84), ZD60 (1313.47 ± 14.39), ZD80→70→60 (1312.45 ± 8.17), ZD80 (1161.67 ± 2.57), ZD70 (1126.82 ± 10.41).
The decomposition of color pigments and non-enzymatic reactions in samples may occur during dehydration process (Guo et al., 2018), thus forming dark surface at higher temperature (70 and 80 ℃) and decreasing the L* value of chili peppers. Following a short-term exposure to high temperature, the cell structure of chili peppers can be destroyed, which further promote the release of pigments from the matrix (Wang, et al., 2020), thus resulting in the increased a* and b* values. However, long-term exposure to heat will cause the loss of carotenoids via breaking its double bond conjugate system (Giuffrida et al., 2014, Maurya et al., 2018) as well as trigger fat oxidation, Maillard reaction and other browning reactions (Chetti et al., 2014, Koç, 2020), in turn declining the values of a* and b*. Our results suggested that the three chili pepper varieties treated by constant temperature drying at 50 ℃ and variable temperature drying exhibited brighter color and better sensory quality. Altogether, the variable temperature drying was a more efficient way to reduce the discoloration than constant high temperature (70 & 80 ℃), and to save more time than constant low temperature (50 & 60 ℃).
Dynamic changes in nutrient substances of chili peppers
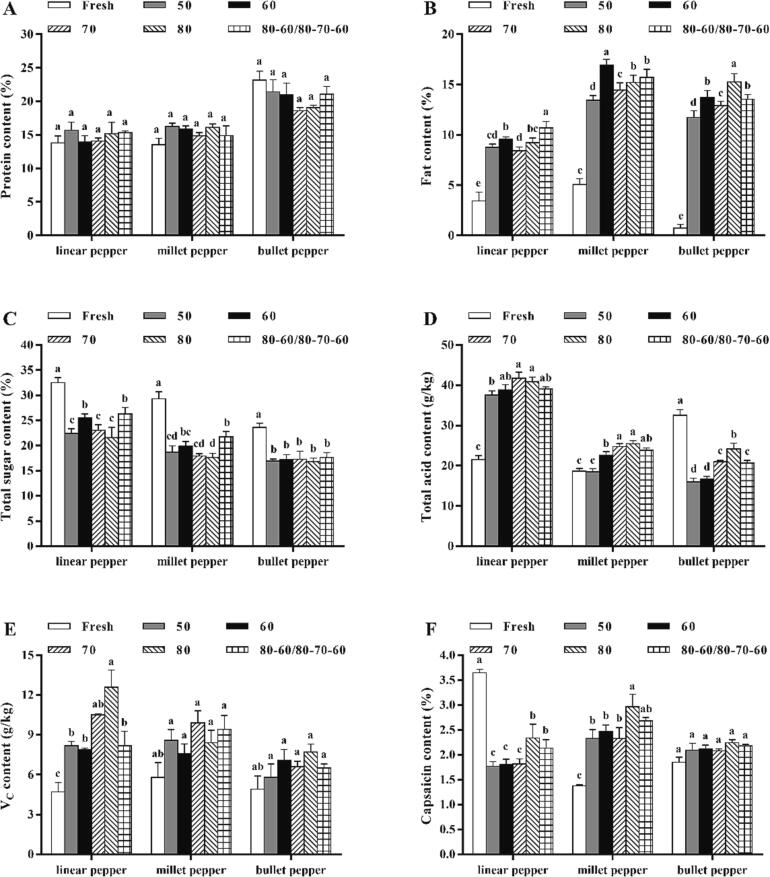
To our knowledge, different drying methods exert diverse impacts on the nutritional quality of dried chili peppers, thus suitable drying procedures can preserve its nutritional contents to the maximum. Hence, the protein, fat, total acid, total sugar, VC and capsaicin contents of three chili pepper varieties treated by different dehydration conditions were measured to obtain more information about the effects of these drying approaches on nutritional properties of samples. It can be seen in Fig. 2A that the protein contents of linear and millet peppers among various treatment groups was significantly lower than that of corresponding bullet peppers (p < 0.05). Whereas, there were no significant differences in protein contents of the same chili pepper variety after drying under different conditions (p > 0.05). Concretely, the protein contents of linear, millet and bullet peppers varied from 13.92 % ± 0.97 % to 15.70 % ± 1.22 %, from 14.85 % ± 0.50 % to 16.27 % ± 0.50 %, and from 18.61 % ± 0.48 % to 21.43 % ± 1.82 %, respectively. As depicted in Fig. 2B, the descending order of fat contents dried by different approaches was: 13.45 % ± 0.07 % – 16.96 ± 0.55 % (millet pepper), 11.73 % ± 0.07 % – 15.27 % ± 0.03 % (bullet pepper), and 8.42 % ± 0.19 % – 10.71 % ± 0.13 % (linear pepper). Among the three chili pepper varieties, the fat content was the lowest at 50 and 70 ℃, which may be due to the long-term drying time and high temperature, further causing higher levels of fat loss in samples. Whereas, the fat contents of the same pepper variety dried at 70, 80 ℃ and variable temperature were relatively higher than other conditions.
Fig. 2.
The dynamic changes in nutrient substances of three chili pepper varieties during the different drying conditions: protein (A), fat (B), total sugar (C), total acid (D), VC (E), and capsaicin contents (F). Values with different lowercase letters in each chili pepper variety differ significantly at p < 0.05.
As can be seen in Fig. 2C, the total sugar contents of linear, millet and bullet peppers dried by diverse methods varied from 21.59 % ± 2.07 % to 26.28 % ± 1.30 %, from 17.61 % ± 0.83 % to 21.73 % ± 1.04 %, and from 16.81 % ± 0.72 % to 17.61 % ± 1.01 %, separately. Because of the higher total sugar levels of linear and millet peppers, Maillard reaction can occur easily in these samples with the increment of drying time and temperature, thereby causing greater sugar loss (Koç, 2020). Among the three chili pepper varieties, their total sugar contents dried by 60 ℃ and variable controlled-temperature were obviously higher than that of other approaches. In addition, the total acid levels of dried linear, millet and bullet peppers at different drying conditions ranged from 37.55 ± 1.04 to 41.74 ± 1.49 g/kg, from 18.52 ± 0.73 to 25.45 ± 0.79 g/kg, and from 15.96 ± 0.95 to 24.16 ± 1.46 g/kg, respectively (Fig. 2D). During the whole dehydration process, the total acid contents of three chili pepper varieties all gradually increased with the prolongation of drying time, and eventually reached to the highest. For various drying methods, the total acid content of the same chili pepper variety treated by 70 and 80 ℃ were higher than that of other temperatures.
As shown in Fig. 2E, the VC contents of samples at the same drying procedure were ranked as follows: 0.79 % ± 0.07 % – 1.26 % ± 0.28 % (linear pepper), 0.76 % ± 0.13 % – 0.94 % ± 0.05 % (millet pepper) and 0.58 % ± 0.14 % – 0.77 % ± 0.01 % (bullet pepper). However, there were no significant differences in VC contents of the same chili pepper variety after drying under different conditions (p > 0.05). Interestingly, the higher VC contents of linear pepper dried at 70 and 80℃, millet pepper dried at 70 ℃ and variable controlled-temperature, as well as bullet pepper dried at 60 and 80 ℃ were found at the end of drying process. These phenomena may be explained by that the long-term dehydration time can destroy the cell structure of chili peppers and promote the release of substances from the matrix (Wang, et al., 2020), thus leading to higher VC loss of samples.
Dynamic changes in capsaicin content of chili peppers
As a kind of bioactive vanillyl amides, capsaicinoids mainly present in some chili pepper varieties. The variety, parts of the fruit, ripeness, growing and storage conditions all determine its capsaicinoids level (Giuffrida, et al., 2014). It can be seen in Fig. 2F that the capsaicin levels of dried linear, millet and bullet peppers were 1.77 % ± 0.09 % (50 ℃) – 2.34 % ± 0.28 % (80 ℃), 2.33 % ± 0.18 % (50 ℃) – 2.97 % ± 0.25 % (80 ℃), and 2.08 % ± 0.04 % (70 ℃) – 2.24 % ± 0.06 % (80 ℃), respectively. Evidently, high temperature (80 ℃) destroys the ultrastructure of samples to cause the release of phytochemicals, and the shorter drying time also inhibits the degradation of thermal sensitive nutrients such as capsaicin (Wang, et al., 2020). Moreover, the inactivation of peroxidase and the inhibition of non-enzymatic degradation reaction are responsible for the augmented capsaicin content at high temperature (Giuffrida et al., 2014, Maurya et al., 2018). During the changed temperature dehydration process, chili peppers were firstly dried at 80 ℃ to inactivate peroxidase and inhibit capsaicin degradation, and then dried at 60 ℃ for a longer time to trigger much capsaicin loss. Hence, the capsaicin levels of three dried chili pepper varieties treated by changed temperature were lower than that of constant 80 ℃ drying, but higher than that of 50 ℃. Overall, the dominant nutrients of linear pepper (total sugar and capsaicin), millet pepper (fat and VC), and bullet pepper (protein and total acid) were altered to total acid and total sugar, fat and capsaicin, as well as fat and protein, respectively. These results support the notion that diverse types of chili peppers exhibited different sensibility in the same drying procedure, thus resulting in different amount of nutrients and bioactive compounds (Loizzo, Pugliese, Bonesi, Menichini, & Tundis, 2015).
Dynamic changes in the types of VOCs in chili peppers
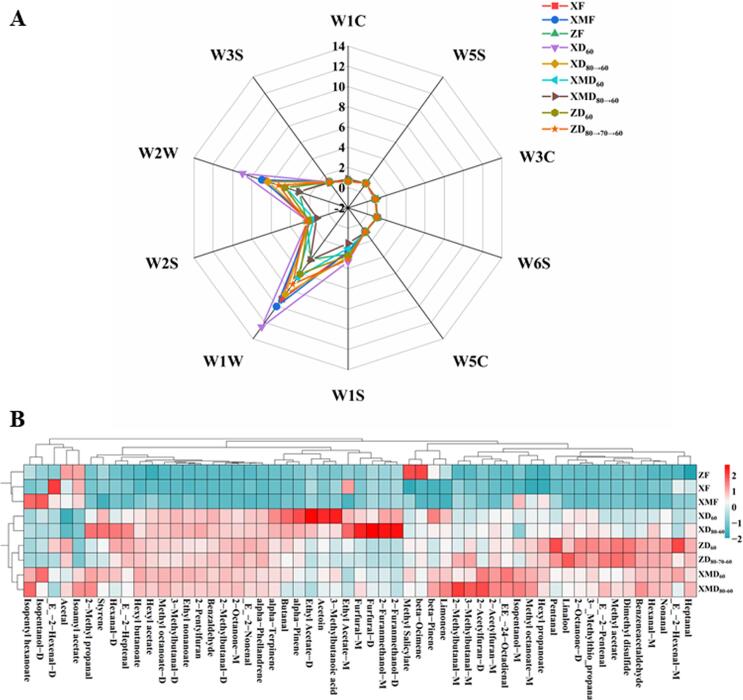
Considering that the chili peppers dried at 60 ℃ possessed shorter time than that of 50 ℃ as well as better color and higher capsaicin content than that of high temperature (70 & 80 ℃), thus 60 ℃ and variable temperature were selected as the optimal conditions to evaluate the effects of different drying methods on VOCs of samples. Evidently, slight changes in VOCs can cause differences in the responses of sensors in E-nose, which is sensitive to the aroma in samples. Therefore, E-nose was utilized to preliminarily detect the volatile composition of three chili pepper varieties treated by different drying methods. As illustrated in Fig. 3A, the sensors of W1W, W2W, W1S and W2S exhibited strong signals for VOCs of all samples, while other sensors showed no obvious responses and differences. To specific, the descending order of signals of these sensors was: linear pepper > millet pepper > bullet pepper. This phenomenon indicated that these chili peppers were abundant in methyl groups, inorganic sulfides, pyrazines, terpenes, alcohols, aldehydes, ketones, aromatic components, and organic sulfides (Table S2). Similar phenomenon was found by Liu et al. in which terpenes and alcohols mainly contributed the aroma of black, white, and green chili peppers (Liu, Zeng, Wang, Ou, Tan, & Gu, 2013). Accordingly, E-nose is an effective tool to discriminate aroma attributes in the different chili pepper varieties and drying conditions. However, the specific VOCs of these samples and their profiles were difficult to distinguish through this technique. Thus, the qualitative and quantitative analysis of volatile components among three chili pepper varieties was performed by GC-IMS analysis below.
Fig. 3.
The radar plot of E-nose (A) and the heat map between the dominant volatile compounds (OAV ≥ 1; B) in three chili pepper varieties under the different drying conditions. XF, XMF and ZF were represented as fresh line pepper, millet pepper and bullet pepper, respectively.
Dynamic changes in the profiles of VOCs in chili peppers
In this assay, the two-dimensional topographic plot combined with ion mobility spectroscopy were used to analyze the differences in aroma substances among these chili pepper samples. As shown in Fig. S5, the rows of this figure represented all of the selected signal peaks in each sample, while the columns revealed the signal peaks with the same VOC in different samples. Besides, the color represented the concentrations of substances, which blue and red indicated lower and higher contents, separately. The darker color inferred a higher level of volatile components. It can be seen that there were similar peaks and peak signal distributions in different samples, but each substance had a different peak intensity. This phenomenon might be ascribed to the fact that the same VOCs could produce different product ions such as monomers and dimers in GC-IMS, which depending on their concentrations and the analytes characterized by strong proton affinity or signal (Li, Wang, Yang, Dong, & Lin, 2020). Overall, the different chili pepper samples possessed their own characteristic and common peak regions, thus containing individual aroma compounds. Concretely, a total of 69 and 91 distinct characteristic ion peaks were detected in the fresh and dried chili peppers, respectively.
After drying at constant and variable temperature, the total amount of VOCs in the three chili pepper varieties exhibited an upward trend compared with the same type of fresh chili peppers (Fig. S6). Concretely, the total levels of volatile substances in linear pepper notably augmented by 8.6- (XD60) and 8.7-fold (XD80→60), separately, compared to the fresh sample (XF, 50789 ± 2349 µg/kg). With regard to millet pepper, the total contents of VOCs significantly increased by 6.6 (XMD60) and 6.5 folds (XMD80→60), respectively (p < 0.05), compared to the fresh sample (XMF, 63102 ± 2697 µg/kg). In terms of bullet pepper, the total abundances of VOCs remarkably changed from 47490 ± 2569 µg/kg (ZF) to 486112 ± 35891 µg/kg (ZD60) and to 471790 ± 23365 µg/kg (ZD80→70→60; p < 0.05). Obviously, the total amount of aroma substances in fresh chili peppers was ranked as follows: millet pepper > linear pepper > bullet pepper. However, no significant differences in the total contents of volatile substances between 60 ℃ and changed temperature drying were observed (p > 0.05).
To further evaluate the distribution of VOCs in different chili pepper samples, their proportions and relative contents in the total volatile substances were calculated and summarized in Fig. S6 and Table 2. The fundamental VOCs in the three fresh samples were aldehydes, ketones, alcohols, esters, olefins and acids. In contrast with the other two varieties, XF contained higher levels of aldehydes and ketones, while XMF possessed richer esters and alcohols. However, the drying process exhibited great influences on the types and abundances of volatile components. Compared with three fresh chili peppers, hot-air drying increased the levels of sulfides, furans and acids in the corresponding samples, but declined that of alcohols and esters. In addition, the relative contents of aldehydes and ketones in linear pepper decreased, while these contents increased in millet and bullet peppers. These phenomena were consistent with a previous report in which drying promoted the generation of acids, furans and aldehydes in samples via activating the degradation of terpenoids, carbohydrates and unsaturated fatty acids (Guo et al., 2018). Overall, the degradation of precursors in fresh samples and the reactions with various compounds during the whole heating process caused the significant changes in the volatile substances (Maurya, Gothandam, Ranjan, Shakya, & Pareek, 2018). Among the chili pepper varieties, dried linear and millet peppers contained richer alcohols, acids and furans, while dried bullet pepper possessed higher levels of ketones and sulfides. The relative contents of furans and aldehydes in XD80→60 sample were richer than those in XD60, but the ketones in XD80→60 were lower. Whereas, there were no significant differences in the types and contents of aroma compounds between XMD60 and XMD80→60, ZD60 and ZD80→70→60, respectively (p > 0.05).
Table 2.
The relative contents of volatile compounds in three chili pepper varieties.
| Compounds | CAS No. | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XF (%) | XMF (%) | ZF (%) | XD60 (%) | XD80→60 (%) | XMD60 (%) | XMD80→60 (%) | ZD60 (%) | ZD80→70→60 (%) | ||
| (E)-2-Nonenal | 18829–56-6 | 1.16 ± 0.05a | 0.62 ± 0.11c | 0.84 ± 0.15b | 0.44 ± 0.06d | 0.43 ± 0.06d | 0.44 ± 0.04d | 0.43 ± 0.06d | 0.48 ± 0.03d | 0.46 ± 0.04d |
| Nonanal | 124–19-6 | 0.75 ± 0.18a | 0.24 ± 0.01c | 0.23 ± 0.02c | 0.27 ± 0.03c | 0.26 ± 0.01c | 0.35 ± 0.02b | 0.35 ± 0.02b | 0.39 ± 0.02b | 0.37 ± 0.02b |
| (E)-2-Heptenal | 18829–55-5 | 0.20 ± 0.06a | 0.09 ± 0.01c | 0.09 ± 0.02c | 0.09 ± 0.01c | 0.13 ± 0.01b | 0.09 ± 0.01c | 0.09 ± 0.01c | 0.12 ± 0.02b | 0.11 ± 0.01bc |
| Heptanal | 111–71-7 | 1.63 ± 0.39a | 0.92 ± 0.26b | 0.38 ± 0.07c | 0.16 ± 0.02d | 0.21 ± 0.03 cd | 0.22 ± 0.01 cd | 0.25 ± 0.01 cd | 0.25 ± 0.01 cd | 0.25 ± 0.01 cd |
| (E)-2-Hexenal-M | 6728–26-3 | 5.72 ± 0.18a | 2.94 ± 0.19b | 2.27 ± 0.17c | 0.48 ± 0.03gh | 0.46 ± 0.03 h | 0.84 ± 0.13e | 0.61 ± 0.04 fg | 1.03 ± 0.08d | 0.70 ± 0.03f |
| (E)-2-Hexenal-D | 6728–26-3 | 6.34 ± 2.39a | 2.56 ± 0.45b | 0.50 ± 0.09c | 0.13 ± 0.02c | 0.13 ± 0.02c | 0.27 ± 0.11c | 0.12 ± 0.02c | 0.38 ± 0.06c | 0.14 ± 0.02c |
| Hexanal-M | 66–25-1 | 3.21 ± 0.20a | 1.77 ± 0.29b | 1.51 ± 0.26c | 0.74 ± 0.11e | 1.08 ± 0.06d | 1.16 ± 0.04d | 1.21 ± 0.04d | 1.22 ± 0.04d | 1.28 ± 0.05d |
| Hexanal-D | 66–25-1 | 2.08 ± 0.78a | 0.57 ± 0.24de | 0.33 ± 0.10e | 1.01 ± 0.40 cd | 2.20 ± 0.89ab | 1.66 ± 0.25bc | 1.04 ± 0.06 cd | 1.87 ± 0.11ab | 1.45 ± 0.11bc |
| Acetal | 105–57-7 | 1.99 ± 0.37b | 1.72 ± 0.27c | 2.79 ± 0.23a | 0.11 ± 0.01d | 0.13 ± 0.03d | 0.21 ± 0.01d | 0.25 ± 0.01d | 0.27 ± 0.02d | 0.26 ± 0.02d |
| (E)-2-Pentenal | 96–17-3 | 0.32 ± 0.09e | 0.29 ± 0.02e | 0.12 ± 0.02 g | 0.24 ± 0.03f | 0.39 ± 0.06d | 0.39 ± 0.02d | 0.53 ± 0.03c | 0.59 ± 0.03b | 0.67 ± 0.04a |
| 2-Methylbutanal-M | 590–86-3 | 1.72 ± 0.23a | 1.55 ± 0.09b | 1.12 ± 0.04c | 0.25 ± 0.05f | 0.26 ± 0.04f | 0.31 ± 0.02ef | 0.49 ± 0.03d | 0.26 ± 0.04f | 0.38 ± 0.01e |
| 3-Methylbutanal-M | 123–72-8 | 0.82 ± 0.08b | 1.01 ± 0.08a | 0.50 ± 0.05c | 0.19 ± 0.03 g | 0.23 ± 0.03 g | 0.28 ± 0.01f | 0.44 ± 0.02d | 0.29 ± 0.03f | 0.36 ± 0.01e |
| Butanal | 110–62-3 | 0.49 ± 0.05f | 0.79 ± 0.04de | 0.82 ± 0.08d | 1.31 ± 0.10a | 1.03 ± 0.07b | 0.83 ± 0.03d | 0.53 ± 0.03f | 0.90 ± 0.04c | 0.73 ± 0.04e |
| Pentanal | 100–52-7 | 0.18 ± 0.06de | 0.55 ± 0.08a | 0.35 ± 0.04b | 0.10 ± 0.01f | 0.13 ± 0.03f | 0.14 ± 0.01ef | 0.11 ± 0.01f | 0.25 ± 0.02c | 0.21 ± 0.04 cd |
| Benzaldehyde | 30361–28-5 | 0.13 ± 0.01d | 0.13 ± 0.01d | 0.14 ± 0.02d | 1.03 ± 0.04a | 0.94 ± 0.13bc | 0.92 ± 0.07bc | 0.97 ± 0.03ab | 0.88 ± 0.07c | 0.98 ± 0.09ab |
| (E,E)-2,4-Octadienal | 122–78-1 | 1.12 ± 0.14b | 1.82 ± 0.12a | 1.12 ± 0.08b | 0.50 ± 0.03d | 0.46 ± 0.03d | 0.77 ± 0.04c | 0.75 ± 0.05c | 0.50 ± 0.04d | 0.51 ± 0.06d |
| Benzeneacetaldehyde | 96–17-3 | ND | ND | ND | 0.25 ± 0.01e | 0.29 ± 0.02d | 0.48 ± 0.02c | 0.59 ± 0.02a | 0.54 ± 0.02b | 0.53 ± 0.01b |
| 2-Methylbutanal-D | 590–86-3 | ND | ND | ND | 7.58 ± 0.26d | 7.43 ± 0.36d | 8.16 ± 0.13a | 7.84 ± 0.18bc | 8.02 ± 0.04ab | 7.80 ± 0.17c |
| 3-Methylbutanal-D | 78–84-2 | ND | ND | ND | 5.65 ± 0.22a | 5.71 ± 0.15a | 5.78 ± 0.08a | 5.31 ± 0.09b | 5.01 ± 0.12c | 4.92 ± 0.09c |
| 2-Methyl propanal | 1576–87-0 | ND | ND | ND | 1.64 ± 0.19c | 3.58 ± 0.36a | 2.99 ± 0.20b | 3.37 ± 0.27a | 1.35 ± 0.10d | 1.18 ± 0.11d |
| Compounds | CAS No. | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XF (%) | XMF (%) | ZF (%) | XD60 (%) | XD80→60 (%) | XMD60 (%) | XMD80→60 (%) | ZD60 (%) | ZD80→70→60 (%) | ||
| Total aldehydes | 27.85 ± 3.49a | 17.57 ± 0.68f | 13.13 ± 0.55g | 22.16 ± 0.55e | 25.49 ± 0.96bc | 26.28 ± 0.58ab | 25.27 ± 0.58bc | 24.61 ± 0.34cd | 23.27 ± 0.47de | |
| 1-Hexanol-M | 111–27-3 | 2.22 ± 0.71a | 1.79 ± 0.54b | 0.65 ± 0.15c | 0.12 ± 0.01d | 0.13 ± 0.01d | 0.23 ± 0.01d | 0.30 ± 0.02d | 0.19 ± 0.02d | 0.19 ± 0.01d |
| 1-Hexanol-D | 111–27-3 | 0.72 ± 0.46a | 0.45 ± 0.29b | 0.08 ± 0.02c | 0.03 ± 0.00c | 0.03 ± 0.00c | 0.03 ± 0.00c | 0.03 ± 0.00c | 0.04 ± 0.01c | 0.03 ± 0.00c |
| 1-Pentanol-M | 71–41-0 | 1.99 ± 0.39a | 1.72 ± 0.39b | 0.74 ± 0.08c | 0.23 ± 0.02e | 0.26 ± 0.03e | 0.50 ± 0.01d | 0.51 ± 0.01d | 0.37 ± 0.01de | 0.37 ± 0.02de |
| 1-Pentanol-D | 71–41-0 | 0.31 ± 0.12a | 0.18 ± 0.06b | 0.07 ± 0.01c | 0.06 ± 0.00c | 0.05 ± 0.01c | 0.07 ± 0.01c | 0.06 ± 0.01c | 0.08 ± 0.01c | 0.07 ± 0.00c |
| Isopentanol-M | 123–51-3 | 0.68 ± 0.07c | 1.82 ± 0.29a | 1.05 ± 0.10b | 0.17 ± 0.03de | 0.11 ± 0.01e | 0.26 ± 0.01d | 0.29 ± 0.02d | 0.22 ± 0.01de | 0.18 ± 0.01de |
| Isopentanol-D | 123–51-3 | 0.17 ± 0.02bc | 0.96 ± 0.35a | 0.22 ± 0.06b | 0.04 ± 0.00cd | 0.03 ± 0.01d | 0.12 ± 0.01bcd | 0.07 ± 0.01cd | 0.04 ± 0.01cd | 0.03 ± 0.01d |
| Ethanol | 64–17-5 | 11.22 ± 1.38c | 12.87 ± 1.53b | 20.29 ± 1.07a | 4.02 ± 0.59d | 1.95 ± 0.10e | 2.72 ± 0.17e | 2.64 ± 0.37e | 2.61 ± 0.45e | 2.22 ± 0.21e |
| 2-Propanol | 67–63-0 | 3.06 ± 0.38e | 2.51 ± 0.11f | 2.98 ± 0.26e | 5.44 ± 0.34d | 5.83 ± 0.68c | 6.61 ± 0.12ab | 6.80 ± 0.22a | 6.09 ± 0.43c | 6.26 ± 0.19bc |
| Isobutanol | 78–83-1 | 0.23 ± 0.03d | 2.12 ± 0.16a | 0.63 ± 0.05b | 0.31 ± 0.01cd | 0.33 ± 0.02cd | 0.35 ± 0.02c | 0.29 ± 0.04cd | 0.27 ± 0.01cd | 0.33 ± 0.02cd |
| 4-Methylpentanol-M | 626–89-1 | 0.76 ± 0.12cd | 3.34 ± 0.56a | 1.39 ± 0.15b | 0.51 ± 0.07d | 1.51 ± 0.33b | 0.65 ± 0.02cd | 0.83 ± 0.03c | 0.24 ± 0.01e | 0.26 ± 0.02e |
| 4-Methylpentanol-D | 626–89-1 | 0.08 ± 0.01b | 1.17 ± 0.49a | 0.13 ± 0.03b | 0.05 ± 0.01b | 0.07 ± 0.01b | 0.11 ± 0.01b | 0.15 ± 0.01b | 0.04 ± 0.01b | 0.04 ± 0.01b |
| Linalool | 78–70-6 | ND | ND | ND | 0.44 ± 0.02de | 0.49 ± 0.05c | 0.46 ± 0.03cd | 0.42 ± 0.01e | 0.54 ± 0.02b | 0.85 ± 0.04a |
| 2-Methyl-1-butanol | 137–32-6 | ND | ND | ND | 0.32 ± 0.10a | 0.32 ± 0.14a | 0.11 ± 0.02b | 0.11 ± 0.01b | 0.09 ± 0.03b | 0.07 ± 0.01b |
| Total alcohols | 21.44 ± 1.58b | 28.93 ± 1.41a | 28.23 ± 1.14a | 11.75 ± 0.92cde | 11.11 ± 0.68de | 12.24 ± 0.16cd | 12.50 ± 0.40c | 10.83 ± 0.66e | 10.92 ± 0.33e | |
| Isopentyl hexanoate | 2198–61-0 | 0.78 ± 0.15d | 10.85 ± 0.71a | 4.32 ± 0.57b | 0.28 ± 0.03e | 0.40 ± 0.03e | 1.11 ± 0.04cd | 1.43 ± 0.04c | 0.30 ± 0.03e | 0.33 ± 0.04e |
| Methyl Salicylate | 119–36-8 | 1.26 ± 0.20c | 1.75 ± 0.06b | 4.42 ± 0.49a | 0.26 ± 0.03d | 0.27 ± 0.04d | 0.33 ± 0.02d | 0.31 ± 0.03d | 0.31 ± 0.02d | 0.29 ± 0.02d |
| Isoamyl acetate | 123–92-2 | 2.37 ± 0.70a | 1.75 ± 0.05b | 2.61 ± 0.22a | 0.13 ± 0.01c | 0.13 ± 0.01c | 0.23 ± 0.02c | 0.27 ± 0.02c | 0.14 ± 0.01c | 0.14 ± 0.01c |
| Ethyl Acetate-M | 141–78-6 | 3.81 ± 0.25a | 0.97 ± 0.12c | 2.00 ± 0.22b | 0.37 ± 0.01de | 0.45 ± 0.06d | 0.23 ± 0.02e | 0.26 ± 0.01e | 0.30 ± 0.04de | 0.27 ± 0.02e |
| Ethyl Acetate-D | 141–78-6 | 3.00 ± 0.20e | 1.09 ± 0.12d | 1.38 ± 0.30c | 2.44 ± 0.32b | 1.21 ± 0.12cd | 0.62 ± 0.05e | 0.31 ± 0.02g | 0.53 ± 0.08ef | 0.41 ± 0.03fg |
| Hexyl acetate | 142–92-7 | 0.20 ± 0.04b | 0.25 ± 0.03a | 0.11 ± 0.02c | 0.06 ± 0.01d | 0.07 ± 0.00d | 0.07 ± 0.01d | 0.07 ± 0.01d | 0.06 ± 0.00d | 0.06 ± 0.01d |
| Compounds | CAS No. | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XF (%) | XMF (%) | ZF (%) | XD60 (%) | XD80→60 (%) | XMD60 (%) | XMD80→60 (%) | ZD60 (%) | ZD80→70→60 (%) | ||
| Ethyl nonanoate | 123–29-5 | 0.69 ± 0.10b | 0.93 ± 0.07a | 0.77 ± 0.10b | 0.36 ± 0.03c | 0.38 ± 0.07c | 0.39 ± 0.03c | 0.36 ± 0.03c | 0.33 ± 0.03c | 0.36 ± 0.03c |
| Hexyl butanoate | 2639–63-6 | 0.70 ± 0.11b | 1.28 ± 0.13a | 0.76 ± 0.07b | 0.46 ± 0.05d | 0.47 ± 0.06d | 0.57 ± 0.05c | 0.55 ± 0.04cd | 0.52 ± 0.04cd | 0.54 ± 0.04cd |
| Methyl octanoate-M | 111–11-5 | 0.79 ± 0.05c | 3.69 ± 0.22a | 1.29 ± 0.23b | 0.52 ± 0.03d | 0.52 ± 0.03d | 0.71 ± 0.02c | 0.77 ± 0.03c | 0.62 ± 0.03cd | 0.64 ± 0.04cd |
| Methyl octanoate-D | 111–11-5 | 0.26 ± 0.03b | 0.32 ± 0.05a | 0.25 ± 0.04b | 0.13 ± 0.01c | 0.13 ± 0.01c | 0.14 ± 0.01c | 0.14 ± 0.01c | 0.13 ± 0.02c | 0.12 ± 0.01c |
| Hexyl propanoate | 2445–76-3 | 0.39 ± 0.06c | 1.71 ± 0.15a | 0.51 ± 0.07b | 0.20 ± 0.03d | 0.21 ± 0.02d | 0.25 ± 0.01d | 0.23 ± 0.03d | 0.27 ± 0.02d | 0.25 ± 0.03d |
| gamma-Butyrolactone-M | 96–48-0 | ND | ND | ND | 2.32 ± 0.13a | 2.38 ± 0.31a | 1.46 ± 0.10b | 1.20 ± 0.02c | 1.41 ± 0.18b | 1.34 ± 0.11bc |
| gamma-Butyrolactone-D | 96–48-0 | ND | ND | ND | 0.53 ± 0.08b | 0.40 ± 0.11a | 0.14 ± 0.02c | 0.15 ± 0.01c | 0.12 ± 0.02c | 0.11 ± 0.01c |
| Methyl acetate | 79–20-9 | ND | ND | ND | 2.19 ± 0.15f | 3.05 ± 0.42e | 3.54 ± 0.38d | 4.22 ± 0.32c | 6.53 ± 0.47b | 6.88 ± 0.12a |
| Total esters | 14.25 ± 1.23c | 24.59 ± 1.35a | 18.42 ± 1.39b | 10.26 ± 0.33e | 10.06 ± 0.80e | 9.78 ± 0.37e | 10.27 ± 0.36e | 11.57 ± 0.50d | 11.73 ± 0.19d | |
| Isoprene | 78–79-5 | 1.25 ± 0.12a | 0.78 ± 0.03c | 1.09 ± 0.09b | 0.25 ± 0.05de | 0.20 ± 0.056de | 0.21 ± 0.01ef | 0.29 ± 0.02d | 0.18 ± 0.03f | 0.19 ± 0.02ef |
| Camphene | 79–92-5 | 0.12 ± 0.01f | 0.12 ± 0.01f | 0.14 ± 0.01de | 0.36 ± 0.03a | 0.25 ± 0.04b | 0.16 ± 0.01cd | 0.12 ± 0.01ef | 0.17 ± 0.02c | 0.18 ± 0.01c |
| beta-Pinene | 127–91-3 | 0.13 ± 0.01e | 0.22 ± 0.01bcde | 2.00 ± 0.21a | 0.30 ± 0.03b | 0.20 ± 0.04cde | 0.24 ± 0.03bcd | 0.27 ± 0.02bc | 0.15 ± 0.02de | 0.15 ± 0.02de |
| beta-Ocimene | 237–641-2 | 0.22 ± 0.02b | 0.21 ± 0.03b | 3.22 ± 0.29a | 0.17 ± 0.02b | 0.16 ± 0.02b | 0.17 ± 0.01b | 0.17 ± 0.02b | 0.18 ± 0.01b | 0.19 ± 0.02b |
| Limonene | 138–86-3 | 0.23 ± 0.02b | 0.15 ± 0.02cd | 0.79 ± 0.08a | 0.13 ± 0.00cde | 0.11 ± 0.01e | 0.13 ± 0.02cde | 0.16 ± 0.01c | 0.11 ± 0.01e | 0.12 ± 0.01de |
| alpha-Terpinene | 99–86-5 | 0.26 ± 0.03b | 0.16 ± 0.02c | 0.29 ± 0.01a | 0.15 ± 0.01cd | 0.13 ± 0.02de | 0.12 ± 0.02e | 0.12 ± 0.01e | 0.11 ± 0.01e | 0.12 ± 0.01e |
| alpha-Phellarene | 99–83-2 | 0.21 ± 0.04bc | 0.16 ± 0.01d | 0.32 ± 0.03a | 0.22 ± 0.03bc | 0.21 ± 0.02bc | 0.20 ± 0.01c | 0.21 ± 0.01c | 0.22 ± 0.01bc | 0.24 ± 0.01b |
| Tricyclene | 508–32-7 | 0.06 ± 0.01dc | 0.08 ± 0.01d | 0.35 ± 0.04a | 0.20 ± 0.02b | 0.11 ± 0.03c | 0.07 ± 0.01d | 0.06 ± 0.00dc | 0.05 ± 0.01c | 0.05 ± 0.01c |
| Styrene | 100–42-5 | 0.24 ± 0.04b | 0.08 ± 0.01c | 0.31 ± 0.03a | 0.06 ± 0.01cde | 0.07 ± 0.02cd | 0.06 ± 0.01cde | 0.05 ± 0.00de | 0.05 ± 0.01de | 0.04 ± 0.00e |
| alpha-Pinene | 2437–95-8 | 0.09 ± 0.02g | 0.07 ± 0.01g | 0.20 ± 0.01c | 0.34 ± 0.01a | 0.23 ± 0.04b | 0.16 ± 0.01e | 0.12 ± 0.01f | 0.18 ± 0.01de | 0.18 ± 0.01cd |
| Total olefins | 2.80 ± 0.26b | 2.01 ± 0.08cd | 8.72 ± 0.67a | 2.17 ± 0.09c | 1.73 ± 0.12de | 1.51 ± 0.04ef | 1.57 ± 0.03ef | 1.39 ± 0.04f | 1.46 ± 0.03ef | |
| Compounds | CAS No. | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XF (%) | XMF (%) | ZF (%) | XD60 (%) | XD80→60 (%) | XMD60 (%) | XMD80→60 (%) | ZD60 (%) | ZD80→70→60 (%) | ||
| 3-Pentanone-M | 96–22-0 | 3.52 ± 0.25a | 2.01 ± 0.05c | 2.94 ± 0.25b | 0.50 ± 0.07f | 0.75 ± 0.09e | 0.77 ± 0.03e | 0.95 ± 0.05d | 0.53 ± 0.04f | 0.74 ± 0.12e |
| 3-Pentanone-D | 96–22-0 | 8.35 ± 0.07a | 5.75 ± 0.45b | 4.33 ± 0.36c | 0.77 ± 0.06f | 0.81 ± 0.12f | 0.82 ± 0.05f | 0.85 ± 0.06ef | 1.18 ± 0.07d | 1.08 ± 0.10de |
| 2-Propanone | 67–64-1 | 6.22 ± 0.98c | 7.70 ± 0.98b | 12.37 ± 0.63a | 2.66 ± 0.49d | 3.16 ± 1.01e | 7.25 ± 0.61e | 7.97 ± 0.54e | 9.84 ± 1.42e | 10.71 ± 0.68e |
| 2-Butanone | 78–93-3 | 0.17 ± 0.02g | 0.75 ± 0.06e | 0.50 ± 0.06f | 1.22 ± 0.07cd | 1.12 ± 0.08d | 1.30 ± 0.14c | 0.84 ± 0.06e | 1.86 ± 0.29a | 1.55 ± 0.16b |
| 2-Hexanone | 591–78-6 | 0.50 ± 0.07c | 0.61 ± 0.03b | 0.62 ± 0.05a | 0.10 ± 0.01f | 0.09 ± 0.01f | 0.12 ± 0.01ef | 0.18 ± 0.02cd | 0.15 ± 0.01de | 0.20 ± 0.01c |
| 2-Octanone-M | 111–13-7 | 2.22 ± 0.43e | 0.95 ± 0.21f | 0.60 ± 0.21g | 4.75 ± 0.24d | 4.90 ± 0.15d | 5.19 ± 0.14c | 5.50 ± 0.07b | 5.56 ± 0.09b | 5.83 ± 0.07a |
| 2-Octanone-D | 111–13-7 | 0.31 ± 0.08d | 0.17 ± 0.00d | 0.22 ± 0.02d | 6.10 ± 0.65b | 5.41 ± 0.53c | 5.68 ± 0.70bc | 5.78 ± 0.50bc | 9.09 ± 0.80a | 9.08 ± 0.24a |
| Acetoin | 513–86-0 | ND | ND | ND | 3.73 ± 0.34a | 1.68 ± 0.59b | 1.59 ± 0.08bc | 1.28 ± 0.09cd | 1.47 ± 0.10bcd | 1.22 ± 0.36d |
| Total ketones | 21.28 ± 1.75c | 17.93 ± 0.54e | 21.58 ± 0.58c | 19.87 ± 0.54d | 17.92 ± 0.74e | 22.72 ± 0.48b | 23.34 ± 0.58b | 29.70 ± 1.20a | 30.40 ± 0.56a | |
| Hexanoic acid | 142–62-1 | 0.44 ± 0.05a | 0.23 ± 0.02e | 0.28 ± 0.02d | 0.27 ± 0.01d | 0.27 ± 0.00d | 0.29 ± 0.02cd | 0.28 ± 0.02cd | 0.31 ± 0.02bc | 0.33 ± 0.00b |
| 3-Methylbutanoic acid | 503–74-2 | 0.60 ± 0.06a | 0.35 ± 0.05b | 0.37 ± 0.03b | 0.26 ± 0.02c | 0.14 ± 0.03d | 0.15 ± 0.01c | 0.12 ± 0.01de | 0.10 ± 0.01e | 0.09 ± 0.01e |
| Acetic acid-M | 64–19-7 | 1.69 ± 0.17b | 1.03 ± 0.13d | 1.92 ± 0.36a | 1.13 ± 0.11d | 1.48 ± 0.09c | 1.71 ± 0.05b | 2.08 ± 0.10a | 1.33 ± 0.12c | 1.48 ± 0.08c |
| Octanoic Acid | 124–07-2 | 0.22 ± 0.02c | 0.41 ± 0.04a | 0.26 ± 0.03b | 0.11 ± 0.01d | 0.11 ± 0.01d | 0.13 ± 0.01d | 0.13 ± 0.01d | 0.12 ± 0.01d | 0.12 ± 0.01d |
| Acetic acid-D | 64–19-7 | ND | ND | ND | 3.16 ± 0.28a | 1.59 ± 0.29c | 2.02 ± 0.12b | 1.40 ± 0.26c | 1.06 ± 0.11d | 0.92 ± 0.06d |
| Total acids | 2.95 ± 0.19d | 2.03 ± 0.20e | 2.83 ± 0.32d | 4.92 ± 0.37a | 3.60 ± 0.36c | 4.29 ± 0.18b | 4.02 ± 0.36b | 2.91 ± 0.21d | 2.94 ± 0.12d | |
| 2-Pentylfuran | 3777–69-3 | 0.17 ± 0.03ab | 0.19 ± 0.02a | 0.19 ± 0.01a | 0.16 ± 0.03bc | 0.17 ± 0.03ab | 0.17 ± 0.02ab | 0.16 ± 0.01bc | 0.14 ± 0.01c | 0.16 ± 0.01bc |
| 2-Furanmethanol-M | 98–00-0 | ND | ND | ND | 0.68 ± 0.06b | 1.18 ± 0.12a | 0.26 ± 0.03c | 0.29 ± 0.03c | 0.11 ± 0.02d | 0.14 ± 0.02d |
| 2-Furanmethanol-D | 98–00-0 | ND | ND | ND | 0.49 ± 0.09b | 0.92 ± 0.12a | 0.09 ± 0.01c | 0.07 ± 0.01c | 0.05 ± 0.00cd | 0.05 ± 0.01cd |
| 2-Acetylfuran-M | 1192–62-7 | ND | ND | ND | 1.36 ± 0.04e | 1.63 ± 0.12d | 2.25 ± 0.04b | 2.45 ± 0.06a | 1.58 ± 0.16d | 1.79 ± 0.19c |
| 2-Acetylfuran-D | 1192–62-7 | ND | ND | ND | 1.23 ± 0.17d | 1.55 ± 0.17c | 3.04 ± 0.20b | 3.64 ± 0.25a | 1.16 ± 0.31d | 1.31 ± 0.34d |
| Compounds | CAS No. | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XF (%) | XMF (%) | ZF (%) | XD60 (%) | XD80→60 (%) | XMD60 (%) | XMD80→60 (%) | ZD60 (%) | ZD80→70→60 (%) | ||
| Furfural-M | 98–01-1 | ND | ND | ND | 1.06 ± 0.06b | 2.11 ± 0.13a | 0.08 ± 0.04c | 1.03 ± 0.03b | 0.52 ± 0.04d | 0.52 ± 0.01d |
| Furfural-D | 98–01-1 | ND | ND | ND | 0.53 ± 0.04b | 1.59 ± 0.32a | 0.25 ± 0.01c | 0.34 ± 0.02c | 0.07 ± 0.01d | 0.07 ± 0.01d |
| Totall furans | 0.18 ± 0.03f | 0.19 ± 0.02f | 0.19 ± 0.01f | 5.50 ± 0.40d | 9.16 ± 0.52a | 6.85 ± 0.28c | 7.98 ± 0.34b | 3.62 ± 0.48e | 4.03 ± 0.55e | |
| 3-(Methylthio)propanal | 3268–49-3 | ND | ND | ND | 0.45 ± 0.02d | 0.46 ± 0.08cd | 0.50 ± 0.01c | 0.46 ± 0.02cd | 0.70 ± 0.06a | 0.64 ± 0.07b |
| Dimethyl disulfide | 624–92-0 | ND | ND | ND | 1.70 ± 0.16c | 1.81 ± 0.50c | 3.39 ± 0.31b | 3.71 ± 0.31b | 5.36 ± 0.33a | 5.48 ± 0.37a |
| Total sulfides | ND | ND | ND | 2.15 ± 0.16c | 2.28 ± 0.52c | 3.90 ± 0.31b | 4.17 ± 0.31b | 6.06 ± 0.28a | 6.12 ± 0.30a | |
| p-Xylene | 106–42-3 | 0.09 ± 0.02d | 0.06 ± 0.02e | 0.30 ± 0.03a | 0.10 ± 0.00cd | 0.15 ± 0.02b | 0.10 ± 0.01cd | 0.11 ± 0.01c | 0.11 ± 0.01cd | 0.11 ± 0.01c |
| Total alkanes | 0.09 ± 0.02d | 0.06 ± 0.02e | 0.30 ± 0.03a | 0.10 ± 0.00cd | 0.15 ± 0.02b | 0.10 ± 0.01cd | 0.11 ± 0.01c | 0.11 ± 0.01cd | 0.11 ± 0.01c | |
| 1 | – | 0.36 ± 0.03a | 0.12 ± 0.03b | 0.10 ± 0.01bc | 0.09 ± 0.01c | 0.10 ± 0.01bc | 0.08 ± 0.01c | 0.08 ± 0.01c | 0.10 ± 0.02c | 0.10 ± 0.01c |
| 2 | – | 1.35 ± 0.35a | 0.51 ± 0.05c | 1.06 ± 0.17b | 0.11 ± 0.01d | 0.17 ± 0.02d | 0.10 ± 0.01d | 0.10 ± 0.01d | 0.10 ± 0.01d | 0.10 ± 0.01d |
| 3 | – | 2.01 ± 0.13a | 1.02 ± 0.21b | 0.25 ± 0.05cd | 0.25 ± 0.01cd | 0.28 ± 0.02c | 0.16 ± 0.01d | 0.20 ± 0.01cd | 0.43 ± 0.05c | 0.43 ± 0.04c |
| 4 | – | 4.74 ± 0.58a | 3.89 ± 0.19c | 4.37 ± 0.17b | 0.60 ± 0.05d | 0.55 ± 0.09d | 0.36 ± 0.02d | 0.37 ± 0.01d | 0.53 ± 0.02d | 0.56 ± 0.05d |
| 5 | – | 0.18 ± 0.03c | 0.46 ± 0.11a | 0.26 ± 0.02b | 0.03 ± 0.00d | 0.03 ± 0.00d | 0.04 ± 0.00d | 0.04 ± 0.00d | 0.04 ± 0.01d | 0.04 ± 0.01d |
| 6 | – | 0.12 ± 0.01c | 0.26 ± 0.03a | 0.11 ± 0.01cd | 0.04 ± 0.01e | 0.09 ± 0.02d | 0.10 ± 0.00cd | 0.16 ± 0.01b | 0.16 ± 0.03b | 0.18 ± 0.04b |
| 7 | – | 0.14 ± 0.01b | 0.25 ± 0.04a | 0.09 ± 0.01d | 0.12 ± 0.01c | 0.06 ± 0.01e | 0.07 ± 0.01e | 0.06 ± 0.01e | 0.11 ± 0.01c | 0.11 ± 0.02c |
| 8 | – | 0.27 ± 0.06b | 0.19 ± 0.01c | 0.35 ± 0.02a | 0.37 ± 0.02a | 0.20 ± 0.03c | 0.19 ± 0.01c | 0.14 ± 0.01d | 0.14 ± 0.02d | 0.14 ± 0.01d |
| 9 | – | ND | ND | ND | 3.56 ± 0.32a | 3.76 ± 0.68a | 3.54 ± 0.15a | 3.57 ± 0.23a | 3.33 ± 0.12a | 3.70 ± 0.55a |
| 10 | – | ND | ND | ND | 4.72 ± 0.15a | 2.81 ± 0.41c | 3.39 ± 0.14b | 2.42 ± 0.25d | 1.76 ± 0.12e | 1.51 ± 0.03f |
| 11 | – | ND | ND | ND | 11.26 ± 1.26a | 10.44 ± 1.78a | 4.29 ± 0.55b | 3.63 ± 0.48b | 2.49 ± 0.84c | 2.14 ± 0.26c |
| Total unknows | 9.17 ± 0.90e | 6.70 ± 0.03f | 6.60 ± 0.28f | 21.16 ± 1.61a | 18.49 ± 1.81b | 12.33 ± 0.56c | 10.79 ± 0.47d | 9.20 ± 0.91e | 9.01 ± 0.65e | |
“ND” indicated “Not detected”. Values with different lowercase letters in each line differ significantly at p < 0.05.
As depicted in Table 2, a total of 69 and 91 VOCs were confirmed in the fresh and dried chili peppers by comparing the retention index and drift time of the corresponding standards. Specifically, three fresh chili peppers contained 16 aldehydes, 11 alcohols, 11 esters, 10 terpenes, 7 ketones, 4 acids, 1 furan, 1alkane and 8 unknown compounds, while 22 volatile substances including 4 aldehydes, 2 alcohols, 3 esters, 1 ketone, 6 furans, 1 acid, 2 sulfide and 3 unknown compounds were newly generated in the dried chili peppers. The detailed changes in different types of substances were as follows:
Aldehydes, possess a strong aroma with low thresholds, accounted for 27.85 %, 17.57 % and 13.13 % of the total volatile substances in XF, XMF and ZF, separately. The levels of (E)-2-hexenal-M/D (monomer/dimer), hexanal-M(-D), 2-methylbutanal-M, heptanal, (E)-2-nonenal, nonanal, (E)-2-heptenal in XF were significantly higher than that in XMF and ZF (p < 0.05). While richer 3-methylbutanal-M and (E,E)-2,4-octadienal, as well as acetal were found in ZF and XMF, respectively. After drying, the contents of these aldehydes in three chili pepper varieties experienced a downward trend, which was in great concordance with a previous study (Martín et al., 2017). One of the main sources of aldehydes (e.g., hexanal, octanal and nonanal) may be the oxidation of unsaturated fatty acids (Serra et al., 2014). The other aldehydes such as benzaldehyde and benzeneacetaldehyde could be obtained from Strecker degradation in Maillard reaction (Wang et al., 2020, Wen et al., 2019). For different drying methods, the contents of 2-methyl propanal in XD80→60 and XMD80→60 groups were higher than that in the corresponding XD60 and XMD60 groups. This is because the initial 80 ℃ in variable temperature drying was higher than that in constant 60 ℃ drying, thus resulting in the production of aldehydes in linear and millet peppers.
As another dominant component in chili peppers, ketones also contributed greatly to the aroma of chili peppers. The total contents of ketones in XF and ZF were higher than that of XMF, and their major ketones were 3-pentanone-M(-D) and 2-octanone-M(-D), as well as 2-propanone, respectively. Interestingly, drying for few dozens of days increased the levels of 2-octanone-M(-D), and 2-butanone as well as declined the 3-pentanone-M(-D) content in chili peppers, which were richer in dried bullet peppers. Besides, acetoin, newly produced in these samples, was richer in dried linear peppers compared to the other varieties. Altogether, heating to a certain extent was beneficial to the formation of ketones in chili peppers through lipid oxidation, Maillard reaction, and degradation of amino acids (Yang et al., 2018).
Overall, the total levels of alcohols in XMF and ZF were higher than that in XF. The characteristic alcohols in ZF and XMF were ethanol, as well as 4-methylpentanol-M(-D) and isobutanol, respectively, while that of XF was 1-pentanol, 1-hexanol and 2-propanol. Except for 2-propanol as well as newly generated linalool and 2-methyl-1-butanol, hot-air drying all significantly reduced the abundances of the other alcohols in the three chili pepper varieties, especially ethanol. Concretely, the predominant alcohols in these samples were ethanol and 2-propanol. Besides, higher contents of 2-propanol in different chili pepper varieties were produced during the variable controlled-temperature drying process compared with constant temperature (60 ℃). The diminution of alcohols may be attributed to volatilization and esterification under the exposure to heat (Guo et al., 2018). Whereas, the oxidative decomposition of fat and reduction of carbonyl compounds were the main reasons for the increments of some alcohols.
Esters were another important substance that constituted the aroma of chili peppers. In contrast with the other two varieties, XMF possessed higher contents of total esters, especially for isopentyl hexanoate, hexyl propanoate, hexyl butanoate, methyl octanoate, ethyl nonanoate and hexyl acetate, while XF and ZF contained higher levels of ethyl acetate-M(-D), and isoamyl acetate. Except for the abundant newly formed gamma-butyrolactone and methyl acetate, different drying methods all decreased the levels of the other esters in three chili pepper varieties. Among different varieties, the dominant esters in dried millet, bullet and linear peppers were isopentyl hexanoate, methyl acetate, as well as ethyl acetate and gamma-butyrolactone, respectively. To our knowledge, esters can be primarily derived from the esterification between alcohols and acids to produce floral and fruity aromas. In addition, the metabolism of substances in chili peppers during the whole dehydration process may also induce the production of esters (Guclu et al., 2021).
With regard to acid compounds, acetic acid-M was the dominant one in all of the fresh and dried chili peppers with an irritating smell. Intriguingly, hot-air drying triggered the production of acetic acid-D in these samples. Besides, chili peppers dried by changed temperature possessed richer acetic acid-M, while samples dried at constant temperature of 60 ℃ contained higher content of acetic acid-D. This phenomenon showed that drying at 60 ℃ was beneficial to the generation of acetic acid-D in the three chili pepper varieties. On the whole, the acids in chili peppers were scarcer, which could be obtained from the hydrolysis of triglycerides and phospholipids or from the lipid oxidation reaction (Yang et al., 2018).
Additionally, there were many types of olefins with lower contents in all of the samples. In comparison to the other fresh chili peppers, ZF contained higher contents of β-ocimene and β-pinene, while XF possessed higher isoprene level. During the production, the terpenoids in samples might be directly produced or transformed from initial products of sesquiterpenes or monoterpenes through dehydrogenation, oxidation and other reactions (Wen et al., 2019). Thus, the abundances of camphene and α-pinene were augmented in the dried chili peppers. However, the applied heat and energy during dehydration can result in the degradation of terpenes (Guclu, et al., 2021), which explains the diminution of isoprene concentration in the dried samples. In addition, no new types of olefins were formed in the whole drying procedures.
Sulfides also exhibited a certain contribution to the aroma of chili peppers. Overall, sulfide compounds were only detected in dried samples, including dimethyl disulfide and 3-methylthiopropional. Concretely, the dimethyl disulfide contents of bullet pepper (5.36–5.48 %) dried by different conditions were significantly higher than that of dried millet (3.39–3.71 %) and linear peppers (1.70–1.81 %). The dimethyl disulfide was associated with sulfurous aroma, which could be generated from methionine via the hydrolysis of S-methylmethionine or Streker degradation reaction (Luo et al., 2018). Besides, the 3-methylthiopropanal newly produced in the dried process mainly derived from the decomposition of sulfur-containing amino acids, which was a key aroma compound in dried chili peppers.
In terms of furans, the predominant one in fresh chili peppers was 2- pentylfuran. Whereas, 2-furanmethanol-M(-D), 2-acetylfuran-M(-D) and furfural-M(-D) were newly formed in the dried samples. Generally, the enolization and dehydration reactions of carbohydrates trigger the production of furans, thus softening the heavy odor caused by phenolic VOCs (Guo et al., 2018). Evidently, 2-acetylfuran-M(-D) with coffee, caramel and balsamic aromas has been identified in dried chili peppers (Ge, et al., 2020), which could be formed during the heating process by Maillard reaction (Kanzler, Schestkowa, Haase, & Kroh, 2017). To specific, the descending order of 2-acetylfuran-M contents was: XMD80→60 (2.45 % ± 0.06 %), XMD60 (2.25 % ± 0.04 %), ZD80→70→60 (1.79 % ± 0.19 %), XD80→60 (1.63 % ± 0.12 %), ZD60 (1.58 % ± 0.16 %), XD60 (1.36 % ± 0.04 %). As one of the most crucial furans in dried samples, furfural exhibits sweet, caramel, nutty and baked aromas, which is commonly generated through non-enzymic browning reaction at high temperature (Pham, Kityo, Buve, Hendrickx, & Van Loey, 2020). The furfural-M levels in different samples were ranked as follows: XD80→60 (2.11 % ± 0.13 %) > XD60 (1.06 % ± 0.06 %) > XMD80→60 (1.03 % ± 0.03 %) > XMD60 (0.80 % ± 0.04 %) > ZD60 (0.52 % ± 0.04 %) > ZD80→70→60 (0.52 % ± 0.01 %). Generally, chili peppers under changed temperature drying methods possessed higher levels of furans than that of the constant temperature. However, alkanes such as p-xylene with low threshold were scarcer in chili peppers, thus exerting few effects on the aroma of chili peppers. Taken together, the composition and contents of volatile components in fresh samples were obviously different from the corresponding dried samples. These diversities could be ascribed to the volatilization, degradation and formation of compounds in fresh samples during the drying period.
Calculation of OAVs of VOCs
The contribution of VOCs to the overall aroma profiles depends on their contents and odor threshold values (Rogner, Mall, & Steinhaus, 2021). Hence, OAVs for individual volatiles were calculated to further evaluate their corresponding contributions. In general, compounds with OAV ≥ 1 were considered as the key aroma components. As summarized in Table 3, the number of volatiles with OAVs greater than 1 in the XF, XMF, ZF, XD60, XD80→60, XMD60, XMD80→60, ZD60 and ZD80→70→60 groups were 33, 34, 33, 49, 51, 51, 51, 48, and 48, respectively, which played vital roles in the aroma of chili peppers.
Table 3.
The odor activity values (OAVs) of volatile compounds in three chili pepper varieties.
| Compounds | CAS No. | Description for ador A | OT (µg/kg) B | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XF | XMF | ZF | XD60 | XD80→60 | XMD60 | XMD80→60 | ZD60 | ZD80→70→60 | ||||
| Aldehydes | ||||||||||||
| (E)-2-Nonenal | 18829–56-6 | Fat, green, cucumber, citrus | 0.08 | 7351.4 | 4885.6 | 5003.4 | 26800.1 | 26,273 | 26112.5 | 25367.3 | 29076.9 | 26868.3 |
| Nonanal | 124–19-6 | Rose, fresh, orris, orange, fat | 1 | 379.9 | 152.1 | 111.2 | 1298.8 | 1274.9 | 1664 | 1678.2 | 1880.2 | 1761.6 |
| (E)-2-Heptenal | 18829–55-5 | Spicy, green, fat, fruity | 13 | 8 | 4.2 | 3.1 | 33.5 | 50.5 | 33.6 | 31.4 | 44.8 | 41.7 |
| Heptanal | 111–71-7 | Fresh, fat, green, herb, wine | 3 | 275.7 | 194.5 | 60 | 264.5 | 342.6 | 356.7 | 392.5 | 404.4 | 387.4 |
| (E)-2-Hexenal-M | 6728–26-3 | Green, banana, cheese | 17 | 170.9 | 109.2 | 63.4 | 138.6 | 134.2 | 236.4 | 170.5 | 295.2 | 193.2 |
| (E)-2-Hexenal-D | 6728–26-3 | Green, banana, fat | 17 | 189.4 | 95 | 13.9 | 36.8 | 38.5 | 76.1 | 33 | 108.2 | 37.8 |
| Hexanal-M | 66–25-1 | Green, fat, apple, green grassy, citrus | 4.5 | 362.8 | 247.9 | 159.5 | 802.5 | 1182.2 | 1233.8 | 1276.4 | 1316.1 | 1337.8 |
| Hexanal-D | 66–25-1 | Green, fat, apple, green grass, citrus | 4.5 | 234.5 | 80.2 | 34.8 | 1091 | 2402.1 | 1774.9 | 1100.9 | 2023 | 1519.7 |
| Acetal | 105–57-7 | Beet, nutty | 40 | 25.3 | 27.1 | 33.2 | 13.4 | 16.1 | 25.5 | 29.6 | 32.6 | 30.2 |
| (E)-2-Pentenal | 1576–87-0 | Spicy, apple, orange, tomato, green, fruity | 1500 | 0.1 | 0.1 | 0 | 0.8 | 1.3 | 1.2 | 1.7 | 1.9 | 2.1 |
| 2-Methylbutanal-M | 96–17-3 | Nutty, oatmeal, caramel | 1 | 872.6 | 975.1 | 533.8 | 1194.5 | 1263.7 | 1470.9 | 2315.3 | 1280.2 | 1773.1 |
| 3-Methylbutanal-M | 590–86-3 | Nutty, cocoa | 0.2 | 2070.6 | 3175.2 | 1196.8 | 4646.1 | 5591.9 | 6746.2 | 10524.2 | 6980.5 | 8463 |
| Butanal | 123–72-8 | Spicy, cocoa, green, malt, bread | 9 | 27.7 | 55.3 | 43.4 | 706.8 | 563.6 | 440.3 | 280.9 | 487.1 | 383.5 |
| Pentanal | 110–62-3 | Fruity, nutty, wine, fermented, cocoa | 12 | 7.4 | 28.7 | 14 | 39.3 | 52.2 | 54.4 | 44.6 | 100.9 | 81.1 |
| Compounds | CAS No. | Description for ador A | OT (µg/kg) B | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XF | XMF | ZF | XD60 | XD80→60 | XMD60 | XMD80→60 | ZD60 | ZD80→70→60 | ||||
| Benzaldehyde | 100–52-7 | Almond, sweet, bitter, cherry | 350 | 0.2 | 0.2 | 0.2 | 14.3 | 13.2 | 12.6 | 13.1 | 12.3 | 13.2 |
| (E,E)-2,4-Octadienal | 30361–28-5 | Green, fat, pear, muskmelon | 10 | 56.8 | 115.1 | 53.1 | 242.6 | 225.3 | 368.8 | 354.3 | 245.2 | 242.5 |
| Benzeneacetaldehyde | 122–78-1 | Floral, hyacinth, honey, cocoa | 4 | – | – | – | 304.9 | 361.9 | 581.2 | 706 | 660.8 | 629.7 |
| 2-Methylbutanal-D | 96–17-3 | Cocoa, nutty, caramel, fruity | 1 | – | – | – | 36869.2 | 36496.4 | 39,176 | 37219.4 | 38994.8 | 36794.5 |
| 3-Methylbutanal-D | 590–86-3 | Peach, fat, fruity, nutty, cocoa | 0.2 | – | – | – | 137520.1 | 140,271 | 138748.6 | 126130.6 | 121,707 | 115994.5 |
| 2-Methyl propanal | 78–84-2 | Floral | 0.1 | – | – | – | 79651.4 | 175674.5 | 143487.1 | 159918.4 | 65813.7 | 55781.1 |
| Alcohols | ||||||||||||
| 1-Hexanol-M | 111–27-3 | Green, fruity, apple | 2500 | 0.5 | 0.5 | 0.1 | 0.2 | 0.2 | 0.4 | 0.6 | 0.4 | 0.4 |
| 1-Hexanol-D | 111–27-3 | Green, fruity, apple | 2500 | 0.1 | 0.1 | 0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1-Pentanol-M | 71–41-0 | Fermented, baked, grain | 4000 | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.6 | 0.6 | 0.5 | 0.4 |
| 1-Pentanol-D | 71–41-0 | Fermented, baked, grain | 4000 | 0 | 0 | 0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Isopentanol-M | 123–51-3 | Spicy, brandy, fruity | 250 | 1.4 | 4.6 | 2 | 3.4 | 2.2 | 5 | 5.4 | 4.3 | 3.4 |
| Isopentanol-D | 123–51-3 | Spicy, brandy, fruity | 250 | 0.4 | 2.4 | 0.4 | 0.9 | 0.6 | 2.3 | 1.3 | 0.8 | 0.6 |
| Ethanol | 64–17-5 | Alcohol | 52,000 | 0.1 | 0.2 | 0.2 | 0.4 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 |
| Isobutanol | 78–83-1 | Cocoa, green, whisky | 7000 | 0 | 0.2 | 0 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Linalool | 78–70-6 | Citrus, floral, green, blueberry | 6 | – | – | – | 356.5 | 400.3 | 368.5 | 333 | 441.4 | 669.3 |
| 2-furan methanol-M | 98–00-0 | Alcohol, caramel, bread, coffee | 2000 | – | – | – | 1.6 | 2.9 | 0.6 | 0.7 | 0.3 | 0.3 |
| 2-furan methanol-D | 98–00-0 | Alcohol, caramel, bread, coffee | 2000 | – | – | – | 1.2 | 2.3 | 0.2 | 0.2 | 0.1 | 0.1 |
| 2-Methyl-1-butanol | 137–32-6 | Roasted, wine, onion, fruity | 4150 | – | – | – | 0.4 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 |
| Compounds | CAS No. | Description for ador A | OT (µg/kg) B | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XF | XMF | ZF | XD60 | XD80→60 | XMD60 | XMD80→60 | ZD60 | ZD80→70→60 | ||||
| Esters | ||||||||||||
| Isopentyl hexanoate | 2198–61-0 | Fruity, banana, apple, pineapple, green | 320 | 1.2 | 21.4 | 6.4 | 4.2 | 6.2 | 16.6 | 21.2 | 4.6 | 4.8 |
| Methyl salicylate | 119–36-8 | Holly, mint | 40 | 16 | 27.6 | 52.5 | 32.2 | 33.3 | 40.1 | 36.7 | 37.3 | 34 |
| Isoamyl acetate | 123–92-2 | Fruity, banana | 2 | 602.2 | 552 | 619.2 | 323.9 | 321.1 | 553.9 | 651.8 | 328.4 | 319.9 |
| Ethyl acetate-M | 141–78-6 | Fruity, cherry | 1000 | 1.9 | 0.6 | 1 | 1.8 | 2.2 | 1.1 | 1.2 | 1.5 | 1.3 |
| Ethyl acetate-D | 141–78-6 | Fruity, cherry | 1000 | 1.5 | 0.7 | 0.7 | 11.9 | 5.9 | 3 | 1.5 | 2.6 | 1.9 |
| Hexyl acetate | 142–92-7 | Fruity, green, apple, banana, pear | 2 | 50.2 | 79.4 | 26.4 | 151.8 | 160.5 | 162.1 | 155.6 | 153.4 | 150.4 |
| Ethyl nonanoate | 123–29-5 | Fruity, brandy, grape | 850 | 0.4 | 0.7 | 0.4 | 2.1 | 2.2 | 2.2 | 2 | 1.9 | 2 |
| Hexyl butanoate | 2639–63-6 | Green, fruity, apple | 250 | 1.4 | 3.2 | 1.4 | 8.9 | 9.1 | 11 | 10.4 | 10.1 | 10.2 |
| Methyl octanoate-M | 111–11-5 | Sweet, green, orange, fruity, fat, brandy | 200 | 2 | 11.7 | 3.1 | 12.7 | 12.8 | 17 | 18.4 | 15.2 | 15.1 |
| Methyl octanoate-D | 111–11-5 | Sweet, green, orange, fruity, fat, brandy | 200 | 0.7 | 1 | 0.6 | 3.2 | 3.2 | 3.4 | 3.3 | 3.1 | 2.9 |
| Hexyl propanoate | 2445–76-3 | Pear, green, fruity | 8 | 24.5 | 134.7 | 30.4 | 118.9 | 128.4 | 149.2 | 137.5 | 163.2 | 146.5 |
| γ -Butyrolactone-M | 96–48-0 | Creamy, fat, caramel | 20,000 | – | – | – | 0.6 | 0.6 | 0.4 | 0.3 | 0.3 | 0.3 |
| γ -Butyrolactone-D | 96–48-0 | Creamy, fat, caramel | 20,000 | – | – | – | 0.1 | 0.1 | 0 | 0 | 0 | 0 |
| Methyl acetate | 79–20-9 | Spicy, fruity, green, fresh, rum | 24,000 | – | – | – | 0.4 | 0.6 | 0.7 | 0.8 | 1.3 | 1.4 |
| Alkenes | ||||||||||||
| Compounds | CAS No. | Description for ador A | OT (µg/kg) B | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XF | XMF | ZF | XD60 | XD80→60 | XMD60 | XMD80→60 | ZD60 | ZD80→70→60 | ||||
| Camphene | 79–92-5 | Camphor, citrus, green, spicy | 1900 | 0 | 0 | 0 | 0.9 | 0.6 | 0.4 | 0.3 | 0.4 | 0.4 |
| β-Pinene | 127–91-3 | Camphor, spicy | 140 | 0.5 | 1 | 6.8 | 10.3 | 7 | 8.1 | 9.3 | 5.3 | 5.2 |
| β-Ocimene | 237–641-2 | Citrus, green, woody | 34 | 3.3 | 3.8 | 45 | 23.7 | 23.3 | 24.5 | 24 | 26 | 26.4 |
| Limonene | 138–86-3 | Lemon | 10 | 11.5 | 9.3 | 37.7 | 63.2 | 53.3 | 61.4 | 75.6 | 51.1 | 54.4 |
| α-Terpinene | 99–86-5 | Lemon, spice | 85 | 1.6 | 1.2 | 1.6 | 8.7 | 7.7 | 6.6 | 6.4 | 6.5 | 6.5 |
| α-Phellarene | 99–83-2 | Citrus, green, herb | 200 | 0.5 | 0.5 | 0.8 | 5.2 | 5.2 | 4.8 | 4.9 | 5.4 | 5.6 |
| Styrene | 100–42-5 | Balsamic, floral | 44 | 2.7 | 1.2 | 3.3 | 6.5 | 7.9 | 6.5 | 5.3 | 5.3 | 4.3 |
| α-Pinene | 2437–95-8 | – | 6 | 7.4 | 7.6 | 16.2 | 273.1 | 189.1 | 124.2 | 98.3 | 142.4 | 145.3 |
| Ketones | ||||||||||||
| 2-Propanone | 67–64-1 | Apple, pear | 450,000 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.1 | 0.1 | 0.1 |
| 2-Butanone | 78–93-3 | Fruity, camphor | 50,000 | 0 | 0 | 0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 |
| 2-Hexanone | 591–78-6 | Fruity, meaty, buttery | – | – | – | – | – | – | – | – | – | – |
| Octanone-M | 111–13-7 | Earthy, herbaceous | 50 | 22.5 | 12 | 5.7 | 461.9 | 481.1 | 498.1 | 522.5 | 540.5 | 549.6 |
| Octanone-D | 111–13-7 | Earthy, herbaceous | 50 | 3.1 | 2.1 | 2 | 593.6 | 531.7 | 545.5 | 548.9 | 883.7 | 856.4 |
| Acetoin | 513–86-0 | Creamy, fat | 55 | – | – | – | 329.8 | 150.1 | 138.4 | 110.6 | 130.2 | 104.4 |
| Acids | ||||||||||||
| Hexanoic acid | 142–62-1 | Sour, fat, cheese | 3000 | 0.1 | 0 | 0 | 0.4 | 0.4 | 0.5 | 0.4 | 0.5 | 0.5 |
| Isovaleric acid | 503–74-2 | Sour, cheese, fermented, berry | 130 | 2.3 | 1.7 | 1.3 | 9.6 | 5.3 | 5.4 | 4.3 | 3.6 | 3.4 |
| Acetic acid-M | 64–19-7 | Spicy, sour, vinegar | 22,000 | 0 | 0 | 0 | 0.2 | 0.3 | 0.4 | 0.5 | 0.3 | 0.3 |
| Octanoic acid | 124–07-2 | Fat, sour, cheese | 3000 | 0 | 0.1 | 0 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Compounds | CAS No. | Description for ador A | OT (µg/kg) B | Samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XF | XMF | ZF | XD60 | XD80→60 | XMD60 | XMD80→60 | ZD60 | ZD80→70→60 | ||||
| Acetic acid-D | 64–19-7 | Spicy, sour, vinegar | 22,000 | – | – | – | 0.7 | 0.4 | 0.4 | 0.3 | 0.2 | 0.2 |
| Furans | ||||||||||||
| 2-Pentylfuran | 3777–69-3 | Fruity, green, cooked, caramel | 6 | 14.8 | 19.7 | 14.9 | 129.8 | 142.1 | 132.8 | 125.3 | 111.6 | 123.2 |
| 2-Acetylfuran-M | 1192–62-7 | Balsamic, cocoa, caramel, nutty, baked | 10,000 | – | – | – | 0.7 | 0.8 | 1.1 | 1.2 | 0.8 | 0.8 |
| 2-Acetylfuran-D | 1192–62-7 | Balsamic, cocoa, caramel, nutty, baked | 10,000 | – | – | – | 0.6 | 0.8 | 1.5 | 1.7 | 0.6 | 0.6 |
| Furfural-M | 1998/1/1 | Bread, nutty, caramel | 3000 | – | – | – | 1.7 | 3.5 | 1.3 | 1.6 | 0.8 | 0.8 |
| 2-Methylbutanal-M | 96–17-3 | Nutty, oatmeal, caramel | 1 | 872.6 | 975.1 | 533.8 | 1194.5 | 1263.7 | 1470.9 | 2315.3 | 1280.2 | 1773.1 |
| 3-Methylbutanal-M | 590–86-3 | Nutty, cocoa | 0.2 | 2070.6 | 3175.2 | 1196.8 | 4646.1 | 5591.9 | 6746.2 | 10524.2 | 6980.5 | 8463 |
| Furfural-D | 1998/1/1 | Bread, nutty, caramel | 3000 | – | – | – | 0.9 | 2.6 | 0.4 | 0.5 | 0.1 | 0.1 |
| Sulfides | ||||||||||||
| 3-(Methylthio)propanal | 3268–49-3 | Tomato, potato, onion, egg | 0.2 | – | – | – | 10856.8 | 11392.3 | 12,055 | 11019.6 | 16906.6 | 15088.7 |
| Dimethyl disulfide | 624–92-0 | Sulphury, cabbage, creamy | 0.06 | – | – | – | 137931.5 | 148561.3 | 271560.8 | 293405.4 | 434,419 | 431223.2 |
A Odor description was obtained from www. The Good Scents Company. com. B. The threshold for volatile compounds in water were obtained from Flavor-Base software. Values with different lowercase letters in each line differ significantly at p < 0.05.
The threshold of aldehydes in these samples was low. Overall, the OAVs of aldehyde compounds in fresh (except for benzaldehyde and (E)-2-pentenal) and dried chili peppers were all greater than 1, suggesting the crucial roles of aldehydes in the chili pepper aromas. Concretely, the main components contributing to the aroma of fresh chili peppers were (E)-2-nonanal, 3-methylbutanal-M and 2-methylbutanal-M with higher OAVs (>500), which provided the aromas of fat, green grass, cucumber, citrus, nutty, caramel and cocoa for fresh chili peppers. In dried samples, (E)-2-Nonenal, nonanal, hexanal-M(-D), 2-methylbutanal-M(-D), 3-methylbutanal-M(-D), 2-methyl propanal (OAVs > 1000) were the dominant aroma substances, providing dried chili peppers with intense floral and fruity aromas. Esters also played a great role in these chili peppers. The largest contributor of esters to fresh chili peppers was isoamyl acetate (OAVs > 500) with fruity aroma, followed by hexyl propionate, hexyl acetate and methyl salicylate (16 < OAVs <140) characterized by fruity, green grass, apple, pear, holly and mint aromas. Whereas, isoamyl acetate, hexyl acetate and hexyl propionate (OAVs > 100) contributed greatly to the fruity aroma of dried chili peppers.
However, the threshold of alcohols in the fresh samples was high, indicating their small contributions to the aroma of fresh chili peppers. The only one that OAV > 1 was pentanol, exhibiting spicy, brandy and fruity aromas. Linalool (OAVs > 300), characterized by citrus, floral and blueberry aromas, was the predominant one that contributed to the aromas of the three dried chili pepper varieties, followed by pentanol. In addition, the OAV of 2-furanomethanol in the dried linear pepper was larger than 1, which brought alcohol, caramel and bread aromas to this sample.
For olefin compounds, limonene was the best one that provided lemon aroma to the fresh chili peppers, while β-ocimene with citrus aroma contributed greatly to the fresh bullet pepper. In the dried chili peppers, α-pinene was the most important olefins, followed by limonene, and β-ocimene. 2-octanone-M and acetoin, belonging to ketones, showed herbaceous, as well as creamy and fat aromas, respectively. The major acid compound was isovaleric acid (OAV > 1), which exhibited sour, cheese, fermented and berry aromas. Besides, hot-air drying significantly increased the OAVs of 2-pentylfuran in chili peppers compared to that of fresh samples (14 < OAV < 20), thus providing all samples with fruity, green grass, cooked and caramel aromas. With regard to sulfides, the predominant dimethyl disulfide and 3-methylthiopropional (OAVs > 10,000) only existed in dried chili peppers, which showed cheese, onion and egg as well as sulfur and cabbage aromas, separately.
Associations of key aroma compounds among different chili pepper varieties
A total of 55 key compounds (OAVs ≥ 1) were compared and performed into a heat map to evaluate their relationships among three chili pepper varieties under the different drying conditions. Red and blue color indicated high and low contents of aromas. As depicted in Fig. 3B, fresh and dried chili peppers were divided into two obvious clusters. In the fresh samples, XMF and XF was in a cluster. While for the dried samples, XD60 and XD80→60, ZD60 and ZD80→70→60, XMD60 and XMD80→60 was in the corresponding same cluster. This phenomenon indicated that VOCs identified in chili peppers drying at 60 ℃ were similar to that of changed temperature drying method, while volatiles in different chili pepper varieties were significantly different. It can be clearly seen that the different samples had various characteristic aromas. For fresh chili peppers, the vital aroma compounds in XF, XMF and ZF were (E)-2-hexenal-D and ethyl acetate-M, isopentyl hexanoate, isopentanol-M and isopentanol-D, as well as methyl salicylate, β-ocimene and acetal, respectively. In terms of dried chili peppers, the major VOCs in linear pepper were 2-furanmethanol-M(-D), furfural-M, ethyl acetate-M(-D), 3-methylbutanoic acid, acetoin, α-pinene, butanal and α-terpinene. Among these substances, 2-furanmethanol-M(-D) and furfural-M, can be easily formed at high temperature by Maillard reaction, were more abundant in XD80→60 group, while the contents of acetoin and α-pinene were retained higher at low temperature. Besides, the dominant aroma components in dried bullet peppers were (E)-2-hexenal-D, heptanal, dimethyl disulfide, methyl acetate, (E)-2-pentenal, 3-(methylthio) propanal, 2-octanone-D, linalool, pentanal and hexyl propanoate. Whereas, the fundamental volatiles in dried millet peppers were methyl octanoate-M, isopentanol-M, 2-acetylfuran-M(-D), limonene, 3-methylbutanal-M, 2-methylbutanal-M, (E, E)-2,4-octadienal, isopentyl hexanoate, and isopentanol-D.
PCA analysis
To further evaluate the differences and similarities among the diverse samples, principal component analysis (PCA) of VOCs in chili peppers was performed and shown in Fig. S7. The results of PCA could be explained by two first principal components, which explained 74 % of the total variance in the data set obtained from GC-IMS. The first component (PC1) and the second component (PC2) contained 61 % and 13 % of the total variance, respectively. On the whole, the aromas in fresh chili peppers were relatively similar, and XF and XMF were closer. It can be seen that all of the dried chili peppers were evidently separated from fresh samples by PC1 in the score plot, whereas dried chili peppers were distinguished by PC2. The aromas of the same dried pepper varieties under different conditions were similar, and the dried millet and bullet peppers were closer. Whereas, the dried linear pepper was farther from dried bullet and millet peppers in PC2 axis with diverse aromas. These results revealed that the characteristic volatile fingerprints of dried pepper varieties were successfully established through GC-IMS and non-targeted characteristic markers, which could be utilized as a useful tool to distinguish the chili pepper samples.
Conclusion
The present study found that the variable temperature drying was effective in facilitating the diffusion and removal of water to save more time, inhibiting the discoloration, maintaining surface color, reducing the loss of total sugar, total acid, fat, and capsicin contents in chili peppers. In terms of VOCs, aldehydes, ketones, alcohols and esters all possessed strong aromas with low thresholds, which were the important contributors to the aromas of chili peppers. Hot-air drying increased the contents of acids, furans and sulfides, while declined those of alcohols, esters and olefins. Additionally, the three varieties exhibited diverse physical characteristics, drying times, chromatic values, nutrients levels and volatile profiles during the dehydration process. The drying time under variable temperature of linear, bullet and millet pepper were reduced by 23 h, 20 h, and 2.5 h, compared with constant temperature drying at 60 ℃. In addition, the relative contents of aldehydes and ketones in linear pepper decreased, while these contents increased in millet and bullet peppers. The present research on the dynamic changes in the nutrients and volatile compounds of the three chili pepper varieties during different drying procedures would provide a guideline for controlling the aroma quality and commercial values of chili peppers, thus promoting their comprehensive utilization. However, the formation and transformation mechanism of flavor substances under different drying conditions have not been thoroughly studied, which should be elucidated in our future studies.
CRediT authorship contribution statement
Miao Liu: Data curation, Methodology, Software, Writing – original draft. Liu Hu: Formal analysis, Investigation, Methodology. Na Deng: Validation, Writing – review & editing. Yongjian Cai: Resources, Visualization. Hui Li: Formal analysis, Investigation. Bo Zhang: Software, Visualization. Jianhui Wang: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the Science and Technology Innovation Program of Hunan Province (2023RC1056), Hunan Provincial Science Fund for Distinguished Young Scholars (2021JJ10007), Research and Development Program in Key Areas of Hunan Province (2021NK2015).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101262.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- AACC. (2000). Approved methods of the American Association of Cereal Chemists. (10th ed.). In). St. Paul, MN, US: The American Association of Cereal Chemists.
- Ananthan R., Subhash K., Longvah T. Capsaicinoids, amino acid and fatty acid profiles in different fruit components of the world hottest Naga king chilli (Capsicum chinense Jacq) Food Chemistry. 2018;238:51–57. doi: 10.1016/j.foodchem.2016.12.073. [DOI] [PubMed] [Google Scholar]
- Baenas N., Belovic M., Ilic N., Moreno D.A., Garcia-Viguera C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chemistry. 2019;274:872–885. doi: 10.1016/j.foodchem.2018.09.047. [DOI] [PubMed] [Google Scholar]
- Chetti M.B., Deepa G.T., Antony R.T., Khetagoudar M.C., Uppar D.S., Navalgatti C.M. Influence of vacuum packaging and long term storage on quality of whole chilli (Capsicum annuum L.) Journal of Food Science and Technology-Mysore. 2014;51(10):2827–2832. doi: 10.1007/s13197-012-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittasupho C., Thongnopkoon T., Burapapisut S., Charoensukkho C., Shuwisitkul D., Samee W. Stability, permeation, and cytotoxicity reduction of capsicum extract nanoparticles loaded hydrogel containing wax gourd extract. Saudi Pharmaceutical Journal. 2020;28(12):1538–1547. doi: 10.1016/j.jsps.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M.D., Jurado-Campos N., Arce L., Arroyo-Manzanares N. A robustness study of calibration models for olive oil classification: Targeted and non-targeted fingerprint approaches based on GC-IMS. Food Chemistry. 2019;288:315–324. doi: 10.1016/j.foodchem.2019.02.104. [DOI] [PubMed] [Google Scholar]
- Deng L.Z., Yang X.H., Mujumdar A.S., Zhao J.H., Wang D., Zhang Q., Wang J., Gao Z.J., Xiao H.W. Red pepper (Capsicum annuum L.) drying: Effects of different drying methods on drying kinetics, physicochemical properties, antioxidant capacity, and microstructure. Drying Technology. 2018;36(8):893–907. [Google Scholar]
- Duan Z.L., Dong S.L., Dong Y.W., Gao Q.F. Geographical origin identification of two salmonid species via flavor compound analysis using headspace-gas chromatography-ion mobility spectrometry combined with electronic nose and tongue. Food Research International. 2021;145 doi: 10.1016/j.foodres.2021.110385. [DOI] [PubMed] [Google Scholar]
- Ge S., Chen Y.Y., Ding S.H., Zhou H., Jiang L.W., Yi Y.J., Deng F.M., Wang R.R. Changes in volatile flavor compounds of peppers during hot air drying process based on headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) Journal of the Science of Food and Agriculture. 2020;100(7):3087–3098. doi: 10.1002/jsfa.10341. [DOI] [PubMed] [Google Scholar]
- Giuffrida D., Dugo P., Torre G., Bignardi C., Cavazza A., Corradini C., Dugo G. Evaluation of carotenoid and capsaicinoid contents in powder of red chili peppers during one year of storage. Food Research International. 2014;65:163–170. [Google Scholar]
- Guclu G., Keser D., Kelebek H., Keskin M., Sekerli Y.E., Soysal Y., Selli S. Impact of production and drying methods on the volatile and phenolic characteristics of fresh and powdered sweet red peppers. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.128129. [DOI] [PubMed] [Google Scholar]
- Guo Y., Chen D., Dong Y.F., Ju H.P., Wu C., Lin S.Y. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried tricholoma matsutake singer by HS-GC-IMS and HS-SPME-GC-MS. Journal of Chromatography B. 2018;1099:46–55. doi: 10.1016/j.jchromb.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Hwang M.J., Kang S.J., Kim H.S., Lee K.W. Reduction of the polycyclic aromatic hydrocarbon levels in dried red peppers (Capsicum annuum L.) using heat pump-assisted drying. Food Chemistry. 2019;297 doi: 10.1016/j.foodchem.2019.124977. [DOI] [PubMed] [Google Scholar]
- Kanzler C., Schestkowa H., Haase P.T., Kroh L.W. Formation of reactive intermediates, color, and antioxidant activity in the Maillard reaction of maltose in comparison to D-glucose. Journal of Agricultural and Food Chemistry. 2017;65(40):8957–8965. doi: 10.1021/acs.jafc.7b04105. [DOI] [PubMed] [Google Scholar]
- Koç G.C. The effect of different drying techniques and microwave finish drying on the powder properties of the red pepper powder (Capsicum annuum L.) Journal of Food Science and Technology-Mysore. 2020;57(12):4576–4587. doi: 10.1007/s13197-020-04496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.R., Wang K., Yang R.W., Dong Y.F., Lin S.Y. Mechanism of aroma compounds changes from sea cucumber peptide powders (SCPPs) under different storage conditions. Food Research International. 2020;128 doi: 10.1016/j.foodres.2019.108757. [DOI] [PubMed] [Google Scholar]
- Liu H., Zeng F.K., Wang Q.H., Ou S.Y., Tan L.H., Gu F.L. The effect of cryogenic grinding and hammer milling on the flavour quality of ground pepper (Piper nigrum L.) Food Chemistry. 2013;141(4):3402–3408. doi: 10.1016/j.foodchem.2013.06.052. [DOI] [PubMed] [Google Scholar]
- Liu M., Deng N., Hou X.Y., Zhang B., Li H., Wang J.H. Characterisation of flavour profiles and microbial communities of fermented peppers with different fermentation years by combining flavouromics and metagenomics. Food Chemistry. 2024;443 doi: 10.1016/j.foodchem.2024.138550. [DOI] [PubMed] [Google Scholar]
- Loizzo M.R., Pugliese A., Bonesi M., Menichini F., Tundis R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A comparison between fresh and processed peppers. LWT-Food Science and Technology. 2015;64(2):623–631. [Google Scholar]
- Luo D.S., Pang X.L., Xu X.X., Bi S., Zhang W.T., Wu J.H. Identification of cooked off-flavor components and analysis of their formation mechanisms in melon juice during thermal processing. Journal of Agricultural and Food Chemistry. 2018;66(22):5612–5620. doi: 10.1021/acs.jafc.8b01019. [DOI] [PubMed] [Google Scholar]
- Martín A., Hernández A., Aranda E., Casquete R., Velázquez R., Bartolomé T., Cόrdoba M.G. Impact of volatile composition on the sensorial attributes of dried paprikas. Food Research International. 2017;100:691–697. doi: 10.1016/j.foodres.2017.07.068. [DOI] [PubMed] [Google Scholar]
- Maurya V.K., Gothandam K.M., Ranjan V., Shakya A., Pareek S. Effect of drying methods (microwave vacuum, freeze, hot air and sun drying) on physical, chemical and nutritional attributes of five pepper (Capsicum annuum var. annuum) cultivars. Journal of the Science of Food and Agriculture. 2018;98(9):3492–3500. doi: 10.1002/jsfa.8868. [DOI] [PubMed] [Google Scholar]
- Pham H.T.T., Kityo P., Buve C., Hendrickx M.E., Van Loey A.M. Influence of pH and composition on nonenzymatic browning of shelf-stable orange juice during storage. Journal of Agricultural and Food Chemistry. 2020;68(19):5402–5411. doi: 10.1021/acs.jafc.9b07630. [DOI] [PubMed] [Google Scholar]
- Prenesti E., Toso S., Berto S. Redox chemistry of red wine. quantification by an oscillating reaction of the overall antioxidant power as a function of the temperature. Journal of Agricultural and Food Chemistry. 2005;53(10):4220–4227. doi: 10.1021/jf048302y. [DOI] [PubMed] [Google Scholar]
- Rogner N.S., Mall V., Steinhaus M. Impact of malt extract addition on odorants in wheat bread crust and crumb. Journal of Agricultural and Food Chemistry. 2021;69(45):13586–13595. doi: 10.1021/acs.jafc.1c05638. [DOI] [PubMed] [Google Scholar]
- Serra A., Buccioni A., Rodriguez-Estrada M.T., Conte G., Cappucci A., Mele M. Fatty acid composition, oxidation status and volatile organic compounds in “Colonnata” lard from large white or cinta senese pigs as affected by curing time. Meat Science. 2014;97(4):504–512. doi: 10.1016/j.meatsci.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Song Y.T., Du B.F., Ding Z.H., Yu Y.H., Wang Y. Baked red pepper (Capsicum annuum L.) powder flavor analysis and evaluation under different exogenous Maillard reaction treatment. LWT-Food Science and Technology. 2021;139:110525. [Google Scholar]
- Stevens R., Buret M., Garchery C., Carretero Y., Causse M. Technique for rapid, small-scale analysis of vitamin C levels in fruit and application to a tomato mutant collection. Journal of Agricultural and Food Chemistry. 2006;54(17):6159–6165. doi: 10.1021/jf061241e. [DOI] [PubMed] [Google Scholar]
- Taiti C., Costa C., Migliori C.A., Comparini D., Figorilli S., Mancuso S. Correlation between volatile compounds and spiciness in domesticated and wild fresh chili peppers. Food and Bioprocess Technology. 2019;12(8):1366–1380. [Google Scholar]
- Wang H., Zhang Q., Mujumdar A.S., Fang X.M., Wang J., Pei Y.P., Wu W., Zielinska M., Xiao H.W. High-humidity hot air impingement blanching (HHAIB) efficiently inactivates enzymes, enhances extraction of phytochemicals and mitigates brown actions of chili pepper. Food Control. 2020;111 [Google Scholar]
- Wen R.X., Hu Y.Y., Zhang L., Wang Y., Chen Q., Kong B.H. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Science. 2019;156:33–43. doi: 10.1016/j.meatsci.2019.05.011. [DOI] [PubMed] [Google Scholar]
- Xu Y., Chen Y.P., Deng S.L., Li C.B., Xu X.L., Zhou G.H., Liu Y. Application of sensory evaluation, GC-ToF-MS, and E-nose to discriminate the flavor differences among five distinct parts of the Chinese blanched chicken. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109669. [DOI] [PubMed] [Google Scholar]
- Yang X.H., Deng L.Z., Mujumdar A.S., Xiao H.W., Zhang Q., Kan Z. Evolution and modeling of colour changes of red pepper (Capsicum annuum L.) during hot air drying. Journal of Food Engineering. 2018;231:101–108. [Google Scholar]
- Yu M.J., Xiang X.L., Tan H., Zhang Q., Shan Y., Yang H. Potential correlation between volatiles and microbiome of Xiang xi sausages from four different regions. Food Research International. 2021;139 doi: 10.1016/j.foodres.2020.109943. [DOI] [PubMed] [Google Scholar]
- Zhang X.L., Zhong C.S., Mujumdar A.S., Yang X.H., Deng L.Z., Wang J., Xiao H.W. Cold plasma pretreatment enhances drying kinetics and quality attributes of chili pepper (Capsicum annuum L.) Journal of Food Engineering. 2019;241:51–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.