Abstract
Over the past two decades, research on bat-associated microbes such as viruses, bacteria and fungi has dramatically increased. Here, we synthesize themes from a conference symposium focused on advances in the research of bats and their microbes, including physiological, immunological, ecological and epidemiological research that has improved our understanding of bat infection dynamics at multiple biological scales. We first present metrics for measuring individual bat responses to infection and challenges associated with using these metrics. We next discuss infection dynamics within bat populations of the same species, before introducing complexities that arise in multi-species communities of bats, humans and/or livestock. Finally, we outline critical gaps and opportunities for future interdisciplinary work on topics involving bats and their microbes.
Keywords: biomarkers, Chiroptera, disease ecology, health, stress, physiology
1. Introduction
Studies of bat-associated microbes (i.e. microorganisms detected in or isolated from bats) date back to rabies virus investigations in the early 1900s [1]. In the past two decades, following the emergence of Severe Acute Respiratory Syndrome (SARS) coronavirus (CoV) in 2003 and SARS-CoV-2 in 2019, there has been a dramatic increase in research on bat-associated microbes, including viruses, bacteria, haemosporidians and fungi [2–5]. These microbes may or may not cause disease in bats, and thus we broadly use the term ‘microbes’ rather than ‘pathogens’ throughout this paper to acknowledge that detecting microorganisms in bats is distinct from the process of determining pathogenicity [6]. Research has moved far beyond simple microbe detection in bat hosts and includes cutting-edge investigations into infection dynamics at individual, population and community scales, and One Health approaches to integrate bat ecology and health [7–11].
As part of the joint 50th North American Symposium for Bat Research and 19th International Bat Research Conference, we organized a symposium focused on advances in the research of bats and their microbes (electronic supplementary material, table S1). We invited early-career scientists to present on physiological, immunological, ecological and epidemiological investigations that have improved our understanding of bat health and infection dynamics. Building on topics discussed by our presenters, here, we review recent bat infection research at the individual, population and community scales. We first present novel approaches and metrics for measuring individual bat responses to infection and challenges associated with assessing consequences of infection. We next discuss infection dynamics within bat populations of the same species, before introducing complexities that arise in multi-species communities, including humans or livestock. Throughout, we highlight case studies from a diverse set of bat species (figure 1). We conclude by summarizing critical gaps and opportunities for future interdisciplinary work on health topics involving bats and their microbes.
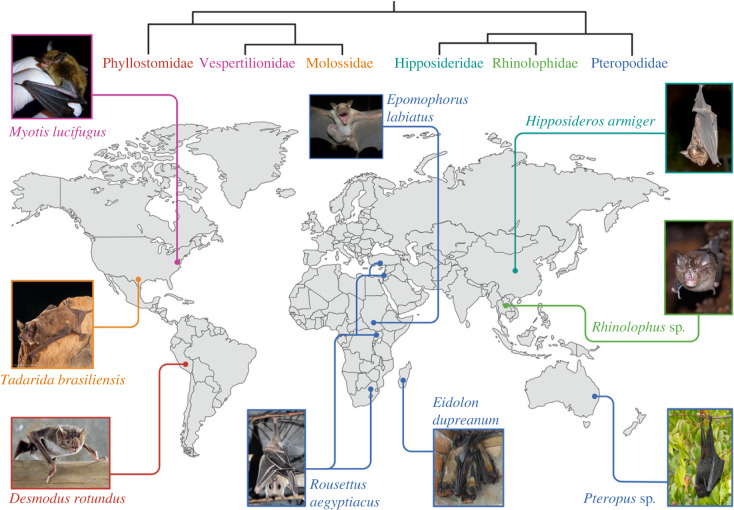
Figure 1.
Map illustrating the geographical and taxonomic diversity of bat species highlighted in case studies throughout the main text, with approximate study location and photo. Species names are coloured according to bat family, with a simplified phylogeny showing relationships between families. See electronic supplementary material for photo permissions.
2. Research at the individual scale: metrics for assessing bat responses to infection
A prevailing narrative in infectious disease research is that bats do not get ‘sick’ when infected with viruses or other microbes (with some exceptions [12]). Experimental challenges on individual bats and bat cell line infection studies have reinforced this narrative, suggesting bats may be more tolerant of viral infection than other mammals [13–15], especially for microbes for which they are the putative reservoir host. However, other studies suggest bats sometimes develop disease due to microbial infection (e.g. morbidity and mass mortality events caused by viruses, fungi and bacteria [16–18]). We submit that researchers must employ a broader set of metrics and technologies to build a more complete picture of bat responses to infection.
(a) . Physiological responses to infection
While acute responses to microbial infections may have minimal consequences for physiological status, cumulative and/or interactive effects of co-occurring or repeat infections can precipitate a cascade of detrimental physiological responses [19]. A comprehensive strategy to assess bat responses to infection should include complementary ‘snapshot’ indicators that show individual short-term reactions as well as downstream metrics that reflect prolonged physiological responses.
A reliable tool for examining the relationship between physiological status and infection status in bats is the measurement of glucocorticoid (GC) hormones. GCs (i.e. cortisol and corticosterone) are critical in regulating physiological processes (e.g. metabolism, reproduction, immunity). While short-term increases in GCs are beneficial for survival, prolonged elevated levels of GCs can downregulate immunological functioning, potentially increasing vulnerability to infection and transmission risk to other species [20,21]. Minimally invasive (e.g. blood, < 5 µl) and non-invasive (e.g. faeces, urine and fur) methods to quantify GCs are increasingly available [22,23].
Body condition can serve as a downstream indicator of the consequences of infection [24], with studies identifying associations between decreased body condition and infection status [25–27]. However, significant variation in morphology among bat species means a one-size-fits-all measure of body condition may not exist. The most widely used body condition indices (BCI) are the ratio index (body mass/forearm length) and the residual index (residuals of body mass-forearm length regression), which attempt to provide size corrections for body mass [28]. For temperate insectivorous bat species, body mass alone has been suggested as a more effective measure of body condition [24]. Regardless of the index used, it is worth noting that short-term factors affecting mass (e.g. pregnancy, food consumption, waste elimination) can alter BCI values and interpretation.
With a small amount of whole blood (< 100 µl), researchers can assess a bat's physiological status via blood chemistry parameters. For instance, handheld point-of-care blood analysers (e.g. i-STAT) were used to demonstrate that little brown bats (Myotis lucifugus) infected by Pseudogymnoascus destructans (the aetiological agent of white-nose syndrome (WNS) [29]) had depleted electrolyte levels and exhibited respiratory acidosis [30,31]. In Ethiopian epauletted fruit bats (Epomophorus labiatus), haematological and electrolyte values varied by infection intensity with the malarial parasite Hepatocystis [32,33]. Although blood chemistry analysis is promising, it is important to establish reference ranges to serve as a baseline against which measurements from infected individuals can be compared [34–36].
Blood smears, easily prepared in the field from < 5 µl of blood, are a tool by which to characterize leucocyte (white blood cell) profiles that provide a window into the immune status of individual bats [37]. Because leucocytes are energetically costly to produce and maintain, a high leucocyte count can indicate a robust cellular or inflammatory response to acute infection [38]. Neutrophils and lymphocytes are associated with the innate and adaptive immune responses, respectively [39]; therefore, the ratio of neutrophils to lymphocytes is used to measure the relative investment on each arm of the immune response and as an indication of acute infection or chronic stress [40]. As with other physiological metrics, we lack an understanding of baseline values and the interpretation of ‘abnormal’ leucocyte profiles in bats. Promising lines of work include the validation of markers for more detailed classification and study of bat lymphocyte types (e.g. T-cell subsets, B-cells, natural killer cells [41]), and studies of B- and T-cell receptors [42], the characterization of which will greatly improve our understanding of bat infection responses.
Transcriptomic approaches, in which a snapshot of expressed genes is sequenced and identified, have proven invaluable in understanding the severity of metabolic and immune consequences of infection for bats [15,43,44]. Additionally, the blood proteome contains proteins secreted from blood cells and organs, including those involved in host response to infection and immune biomarkers, and innovative proteomic tools show potential in characterizing bat immune systems and their responses to microbial infections [45]. Complemented by recent advances in genomics [46], ‘-omics’ approaches stand to further our ability to explore mechanisms by which bats interact with microbes and consequences for bat physiological status.
(b) . Behavioural responses to infection
Sickness behaviours are largely consistent across vertebrate species and include decreased movement, food consumption and social interactions [47,48]. However, few studies have focused on how bats alter their behaviour during illness. Several species (M. lucifugus; common vampire bat (Desmodus rotundus); Egyptian rousette (Rousettus aegyptiacus)) reduce overall activity levels when experiencing immune challenges (e.g. lipopolysaccharide injections) or microbial infections [49–51]. Additional behavioural changes include social isolation, temporary cessation of foraging flights, and reduced grooming, production of contact calls and food intake [49–54]. Given the diversity of bat species, data from only three species is insufficient to fully describe how bats alter their behaviours when infected.
Understanding behavioural responses to infection is also important for designing and interpreting microbe surveillance studies, given that infected individuals may be underrepresented in sampling due to a reduction or cessation of foraging [51]. Most knowledge of behavioural changes comes from work with captive bat colonies, allowing for continual monitoring of behaviours of interest. However, two studies tracked free-ranging bats [51,52], demonstrating the feasibility of observing behavioural changes in free-ranging bats in the context of infection. Ongoing technological advances will continue to expand opportunities for monitoring previously inaccessible bats. For instance, smaller on-animal trackers and batteries will enable movement studies for smaller species [55]. Automated video analysis tools, combined with thermal imaging cameras, will enable monitoring of departures from typical behavioural patterns in high bat density environments [56–58]. Identifying disruptions to typical patterns will require robust long-term baseline behavioural data for multiple species. Collaborations between disease researchers and those conducting long-term behavioural studies [59] would be especially valuable in this context; individual-scale, longitudinal infection data could be added to behavioural studies to understand changes linked with infection status.
(c) . The role of bat microbiomes in regulating infection
Much research has demonstrated the importance of host-associated microbiomes, particularly in the gastrointestinal tract (GIT), in influencing host immune function [60]. However, the extent to which the GIT microbiome affects the ability of bats to maintain or mitigate infections remains largely unknown [61]. Destabilization of gut and other symbiotic microbial communities (i.e. dysbiosis) can negatively affect an individual's immune status [62]. Experimental studies have shown that GIT microbiota transplanted from great roundleaf bats (Hipposideros armiger) into antibiotic-treated mice altered immune cell levels and conferred greater resistance and survival to H1N1 influenza infection compared with control groups, indicating the GIT microbiome can interact with and change the host immune system [63]. Additionally, lipopolysaccharide injections in R. aegyptiacus induced significant and rapid (24–48 h) changes to the composition and diversity of gut microbial communities [64].
Many questions regarding the relationship between GIT microbial communities and bat health remain, including: how do GIT microbes interact with host immune function to maintain, prevent or clear infections? To what extent do GIT microbial communities differ and influence responses to infection relative to other mammalian species, especially given rapid gut transit times in bats? How do microbial communities change naturally over time or with viral, bacterial or other active infections? Repeat sampling of individual bats will aid our ability to answer these questions and identify how dysbiosis presents in bats. Studies of bat microbial communities paired with whole-genome sequencing, transcriptomics, metabolomics and viral screening approaches will provide a holistic picture of tolerance and resistance mechanisms.
3. Research at the population scale: elucidating patterns of infection dynamics
The often-gregarious nature of bats allows researchers to examine links between population demographics (e.g. age composition, density) and population measures of infection such as prevalence and seroprevalence. Different sampling methods (e.g. cross-sectional versus longitudinal) can provide an understanding of infection at a single time point or across time scales. Data collected in the field can be used to develop and validate mechanistic models to understand how viruses are maintained in bat populations [65]. Relatedly, model-guided fieldwork approaches are useful to focus data collection on the key drivers of infection dynamics, and to maximize the power of inference during data analyses [66].
(a) . Linking population characteristics to infection dynamics
Population-scale demographics can significantly influence infection dynamics. Seasonal reproductive cycles are common across bat species and are thought to mediate population-scale infection dynamics [67–69]. During gestation, immune function is biased towards anti-inflammatory responses that are important for a successful pregnancy but can increase virus susceptibility within females [70]. These shifts are modulated by hormonal changes that trigger an anti-inflammatory polarization of immune cells [70]. Bats, which rely particularly on inflammatory innate and cellular responses for heightened viral suppression and regulation of latent infections, are expected to be heavily influenced by a gestation-induced anti-inflammatory polarization [71]. Immunological shifts could explain seasonal and sex-specific biases commonly observed in bat antibody seroprevalence [72–74], and seasonal patterns in shedding and spillover [75]. The importance of reproduction in infection dynamics remains to be investigated in a mechanistic fashion, partly due to challenges in sampling sufficient individuals per demographic or reproductive cohort for meaningful analyses.
Seasonal dynamics relating to the influx of susceptible juveniles have been studied in detail and have been associated with increased infection prevalence in populations. For example, pulses of Marburg virus infection have been noted in older juvenile R. aegyptiacus in Uganda, co-occurring with synchronous bi-annual birthing cycles [76]. The combined effects of waning maternal antibodies and immunologically naive bats roosting beneath adult bats contribute to the circulation of Marburg virus in this reservoir host [76]. Similar viral dynamics have been reported for diverse henipavirus-related viruses among R. aegyptiacus [67], and ‘amplification’ cycles for coronaviruses in multiple other species [25,68,77,78].
Roosting preferences relating to habitat type and aggregation patterns often correlate with infection dynamics [79]. Cave-roosting species typically exhibit higher rates of infection and a greater diversity of viruses than non-cave-roosting species [80]. For tree-roosting species, within-roost aggregation structures can mediate infection dynamics. For example, sparsely distributed tree stands can promote high within-tree bat densities due to limited tree availability, further promoting transmission and generation of more explosive epidemics upon virus introduction [81]. Not all species within a genus roost in the same densities. For instance, Asian Rhinolophus species linked to SARS-related coronaviruses (sarbecoviruses) roost in higher densities and with more species than European and African Rhinolophus sarbecovirus hosts [82], increasing risk of viral recombination and adaptation to new hosts.
(b) . Sampling strategies to infer population-scale infection dynamics
Biosurveillance among bat populations has traditionally been performed opportunistically and as cross-sectional studies [83]. One-time cross-sectional sampling can provide an excellent overview of microbe presence and diversity within and across host species, as well as insights into tissue tropism and routes of excretion [84,85]. Opportunistic sampling across diverse species has also led to the discovery and characterization of new microbes [86]. By contrast, repeated sampling of populations and individuals lends ecological context to infection dynamics through time. Questions regarding infection prevalence and shedding at the population scale in association with season, age cohort or reproductive phenology can be addressed, as well as long-term patterns between population demographics and infection status [87,88]. Tracking individual bats using passive integrated transponder (PIT) tags [89], tattooing [67], satellite/radio transmitters [90] or other long-term marking methods facilitates monitoring of infections or seroconversion rates. Tracking data also elucidates bat and bat-associated microbe movement between roosts (i.e. metapopulation insight) and allows estimation of population size over time. Combining host and/or parasite population genetics with infection studies also holds promise for better understanding patterns of bat dispersal and migration [91]. Future bat movement research would benefit from PIT tag data sharing (e.g. https://www.ausbats.org.au/pit-tag-register.html) to facilitate repeat detections across broad geographical areas. This would be particularly valuable for epidemiological insights into bats with long-distance migratory and dispersal behaviours.
(c) . New modelling approaches to understand viral dynamics
Multidisciplinary modelling approaches integrate theory, fieldwork and laboratory work, and allow for holistic approaches to mechanistically understand complex bat–microbe systems [66]. Empirical studies of bat infection traditionally use antibody or microbe detection in populations to construct time-series curves of active infection and exposure. While useful for hypothesis generation, integrative research is needed to identify causal drivers of dynamics, and to predict times and locations of spillover risk. Integration of age into serological time series can improve estimates of key infection parameters (e.g. R0 and force of infection) [92,93]. Age-structured serological data has been used to evaluate models of filovirus and henipavirus dynamics in Madagascar fruit bats; however, evidence of within-host variation in immunological status through time and limited model recovery of serological patterns among age classes suggests alternative dynamics may underlie viral persistence in these bat species [74]. In addition, molecular tests often contain more information than the binary presence/absence reported. As recently demonstrated with human testing data, cross-sectional viral load distributions have been used to estimate epidemic trajectories by drawing from information in cycle threshold (Ct) values from reverse transcription quantitative PCR tests [94]. This method has yet to be applied to wildlife populations and may be beneficial in cases where Ct values reflect a (probabilistic) measure of time since infection. Careful consideration of Ct values may also improve researchers' ability to determine when bats are shedding infectious viruses and estimate the risk of viral spillover [95]. Viral shedding and serology data are not regularly paired in bat–virus systems [96], though this can yield powerful insights to triangulate mechanisms of infection dynamics [97].
Sequencing and further characterization of samples positive for viral infection is necessary to understand strain diversity, identify specific molecular or phenotypic traits, and construct virus phylogenies [98]. Given the rapid evolution of viral species compared with their bat hosts, virus phylodynamics can provide insight into host movement and past transmission over the landscape [99]. Similarly, population genetic studies of bat hosts can elucidate mechanisms and pathways for present and future disease transmission [100]. Furthermore, sequencing can allow the identification of co-circulating virus strains [99]. Sequencing complete viral genomes is necessary to investigate viral recombination; incorporation of novel genes may highlight co-circulation of multiple viral families within bat populations [101]. Obtaining sequences depends on the ability to sample actively infected bats—a challenge for acute infections [88]. Phylogenetic information can thus be obtained by sampling not only the bat host but also sentinel spillover species. Sequencing viral genomes allows for the design of more inclusive molecular panels. Divergent viruses may be missed by conventional PCR [102], and while these assays are important to inform population-scale viral dynamics, they may miss nuanced virus-specific patterns in a particular bat system, especially in viral discovery efforts where a priori knowledge of viruses is missing.
4. Research at the community scale: multi-species dynamics and complexities
As with all species, bats do not exist in an ecological vacuum; thus, insights gained from individual- or population-scale studies must be re-examined within a multi-species framework. Bat infection research at the community scale involves interactions between two or more species (e.g. bats, livestock, humans) and can have great relevance to human, wildlife, agricultural and ecosystem health.
(a) . Linking host infection dynamics to spillover risk
With the large number of emerging infectious diseases reported from wildlife, often causing high morbidity and mortality, zoonotic spillover has become a great source of concern [103], and information to enable prediction and prevention of spillover is imperative. When considering spillover of microbes from wildlife to other species, there are three broad categories to consider—the reservoir species, the infectious agent and the recipient host [104]. However, these factors are not mutually exclusive and can be influenced by extrinsic variables such as climate and food availability [72,105].
Insights into bat reservoir infection dynamics and interactions with susceptible (spillover) hosts are needed to make informed risk assessments and require longitudinal research approaches [88]. Identification of spillover risk factors can be achieved by assessing infection dynamics in the reservoir host in conjunction with data on known spillover events [8]. For newly recognized viruses or those of unknown zoonotic potential, identifying possible risk factors or bridge hosts for spillover is more challenging. Closely related host species or individuals within a species may differ significantly in host proteins bound by viruses (e.g. angiotensin converting enzyme 2 (ACE2) bound by SARS-CoV-2 or dipeptidyl peptidase 4 (DPP4) bound by Middle East Respiratory Syndrome (MERS)-CoV), making predictions of susceptibility difficult [106]. In addition, a lack of expertise in bat species identification and continued changes to host and microbe taxonomy pose real challenges for standardizing analyses across temporal and spatial scales. Host–microbe datasets should specify details of bat species identification and be linked with taxonomic resources to reconcile nomenclature changes over time (e.g. https://batnames.org/). Infection dynamics can also vary across virus species and reservoir hosts, and between geographically dispersed populations of the same host species. For example, in a monoestrous R. aegyptiacus population in South Africa, peaks of henipavirus-related virus excretion occurred during the winter and were thought to be driven by concurrent waning of maternal immunity and nutritional stress [67]. Consequently, spillover risk was considered highest during winter and in plantations where bats were seeking food, thereby increasing the potential for human contact [67]. By contrast, R. aegyptiacus populations in more equatorial regions display bimodal polyoestry [107] and are subject to different climates and food availability [108], potentially altering viral excretion dynamics and the timing of peak henipavirus spillover risk.
(b) . Interfaces and behaviours promoting microbe transmission
Agricultural intensification has been linked to increased interactions and microbe spillover from bats to livestock [109,110]. For instance, the expansion of cattle farming in Latin America has allowed D. rotundus to feed almost exclusively on livestock blood, driving more frequent bat–livestock interactions [111,112]—a concern given their role in the transmission of rabies virus and potentially other zoonoses (e.g. Bartonella, Trypanosoma). Generally, bat–livestock interfaces are less studied than other wildlife–livestock interfaces in the context of infectious diseases [113]. Further surveillance is needed to detect spillover of bat microbes to livestock, given that asymptomatic infections may go unnoticed [114,115]. Beyond traditional microbe surveillance, movement trackers, proximity loggers and acoustic surveys can uncover patterns of overlapping landscape use [116], while surveys of farmers can provide insight into common bat–livestock interactions [117].
Urban habitats represent one interface where bats and people may come into contact. While a meta-analysis found that areas with intermediate and high levels of urban development were associated with lower bat habitat use [118], many species can adapt to human-dominated landscapes. Some bats use human infrastructure (e.g. tunnels, bridges, houses) as their roosting sites [119], sometimes sustaining large colony sizes near humans. These interactions can result in microbe transmission, such as with histoplasmosis, caused by inhaling Histoplasma capsulatum spores that grow on bat guano [120]. Within the flying fox (Pteropus spp.)–Hendra virus system, loss of native foraging habitat combined with planting of cultivated trees in urban and agricultural areas has brought bats into closer proximity with humans and horses, thereby increasing viral spillover risk [8,121]. More data are needed on the ways, frequency and duration in which humans and bats contact each other to improve estimates of spillover risk [122–124].
(c) . Anthropogenic disturbances and bat infection
Anthropogenic disturbances on bats are diverse and occur at different spatial scales and with varying severity (e.g. land modification, light and noise pollution, cave tourism, guano mining, hunting [125]). Changes in bat behaviour or community composition in response to these disturbances can alter infection and parasitism dynamics. Deforestation, through changes in bat community and roosting behaviour, has been linked to differences in the richness and prevalence of viruses and parasites across multiple Neotropical systems [79,126,127]. Anthropogenic disturbances might also cause physiological changes (e.g. stress-induced immune suppression) that increase susceptibility, reactivate latent infections [98] or increase shedding of infectious particles. Though other stressors such as food shortages, poor nutrition and fungal infection have been linked to greater viral seroprevalence, shedding and replication in bats [72,128,129], evidence for effects of direct anthropogenic disturbances on infection dynamics is limited. During periods of early and late reproduction, female Mexican free-tailed bats (Tadarida brasiliensis) roosting in bridges had higher rabies virus seroprevalence than those roosting in caves [130]. Other work found that T. brasiliensis roosting in bridges had lower plasma cortisol levels and ectoparasite loads compared with their cave-roosting counterparts [131]. Future research to resolve the effects of anthropogenic disturbance on bat infection will need to incorporate qualitative and quantitative metrics of disturbance [132,133] and assess multiple behavioural and physiological bat responses to these anthropogenic changes.
(d) . Novel approaches to reduce transmission of bat microbes
Culling of reservoir species has been employed in numerous wildlife systems to reduce disease transmission [134], yet culling outcomes in bats can be complex and may contribute to increased microbe transmission [135–137]. Reducing microbe spillover from free-ranging bats to other hosts requires a better understanding of infection dynamics from the individual to the community scales to effectively target control measures and interventions. Innovative ‘low-tech’ methods to prevent cross-species transmission between bats and other hosts, such as culturally tailored community outreach tools [7] and cost-effective physical barriers to transmission (e.g. bamboo skirts for Nipah virus [138]), should be integrated with landscape-level interventions such as ecological engineering to reduce contact with people and livestock [139] and vaccination of host species. Vaccination could complement or replace culling as proactive spillover risk reduction; however, vaccine delivery is challenging given large, reclusive bat populations. While oral vaccines held inside edible baits have been successfully implemented in some wildlife disease systems [140], the diets of most bat species preclude this approach. Novel approaches to vaccine distribution include the use of aerosolized spray vaccines [141], which are promising for cavity-roosting bat species in which large groups aggregate at high density. Alternatively, self-spreading vaccines exploit bat behaviours to spread vaccines from founder individuals to direct contacts (transferable vaccines) [142] or over multiple generations (transmissible vaccines) [143]. These methods are being investigated for combating vampire bat-transmitted rabies virus [11,144], and have potential utility in other bat–virus systems. Vaccines can also provide avenues for bat conservation (e.g. for bat populations threatened by WNS [145]).
5. Strengthening interdisciplinary collaboration in bat research
Historically, the bat research community has been siloed between the infectious disease and ecology/conservation disciplines, with few influential researchers bridging interdisciplinary science between these disciplines [6,146]. The emergence of WNS in the US represented one instance in which researchers came together to combat an infectious disease threatening the viability and conservation of bat populations [146]. Following the coronavirus disease (COVID)-19 pandemic, the culture of the bat research community has shifted to adopt a more integrative, interdisciplinary and collaborative approach (electronic supplementary material, figure S1). The focus on bats as sarbecovirus hosts during the pandemic had negative impacts on bats and conservation programmes [147–149] but also created an area of common concern that brought research communities together.
This momentum towards interdisciplinary collaboration in the peer-reviewed literature has been mirrored in professional networks. Global research communities joined forces to address knowledge gaps surrounding SARS-CoV-2-associated threats to bats [150,151], and facilitate regional bat One Health surveillance [152]. The International Union for Conservation of Nature (IUCN) Bat Specialist Group (https://www.iucnbsg.org/) mobilized a working group during the COVID-19 pandemic to develop guidelines for researchers, cavers, guano collectors and wildlife rehabilitators to prevent SARS-CoV-2 transmission from humans to bats, and led a zoonotic diseases science communication workshop [153]. The Global Union of Bat Diversity Networks has convened multiple networks spanning conservation to infectious diseases and initiated numerous interdisciplinary projects (https://www.gbatnet.org/interdisciplinary-projects/). The Bat Health Foundation (https://www.bathealthfoundation.org/) seeks to build a database for bat physiological parameters to inform conservation and infectious disease research. Additional interdisciplinary partnerships and projects will be critical to advance a One Health mission.
6. Conclusion
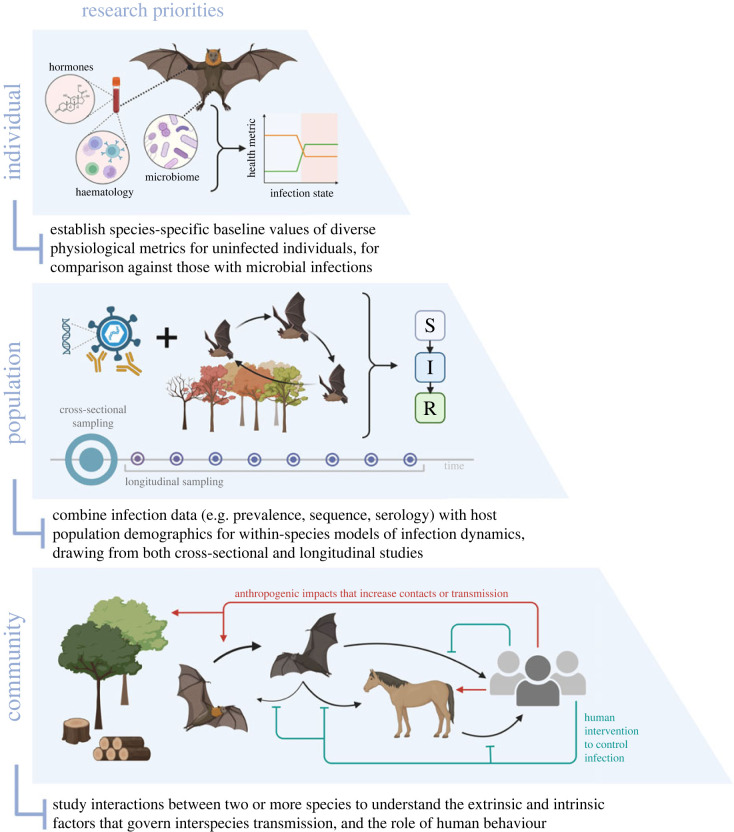
We have highlighted our current understanding of factors impacting bat–microbe interactions at individual, population and community scales, and identified future research needs (figure 2), including: (i) establishing species-specific baseline values for individual physiological biomarkers (especially in free-ranging populations) and including broad metrics of bat responses to infection, (ii) combining infection prevalence, sequence and serology data with host population ecology, physiology and phenology to create more informative models of infection dynamics, especially through the synthesis of cross-sectional and longitudinal studies, and (iii) generating an understanding of the extrinsic and intrinsic factors impacting microbe spread between species in communities, with special attention to the role of humans and environmental factors in these dynamics. In all cases, emphasis should be placed on communication and collaboration within the bat research community and across disciplines. Through integrated research, we can discover patterns and make predictions that will safeguard bats, humans and other species.
Figure 2.
Overarching research priorities for future studies on bat infection dynamics, organized at the individual, population and community scales; S, susceptible; I, infected; R, recovered.
Acknowledgements
We thank Simon Anthony for contributions to our symposium and initial manuscript discussions.
Contributor Information
Cecilia A. Sánchez, Email: sanchez@ecohealthalliance.org.
Kendra L. Phelps, Email: phelps@ecohealthalliance.org.
Kevin J. Olival, Email: olival@ecohealthalliance.org.
Data accessibility
Data to support authorship network mapping of the bat research community (described in the electronic supplementary material) are available at Zenodo: https://doi.org/10.5281/zenodo.8003910 [154].
Supplementary material is available online [155].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
C.A.S.: conceptualization, investigation, project administration, supervision, visualization, writing—original draft, writing—review and editing; K.L.P.: conceptualization, funding acquisition, investigation, project administration, supervision, writing—original draft, writing—review and editing; H.K.F.: conceptualization, writing—original draft, writing—review and editing; M.G.: conceptualization, investigation, writing—original draft, writing—review and editing; M.E.G.: conceptualization, visualization, writing—original draft, writing—review and editing; D.N.J.: investigation, writing—original draft, writing—review and editing; G.K.: writing—original draft, writing—review and editing; T.J.L.: conceptualization, investigation, writing—original draft, writing—review and editing; K.R.M.: conceptualization, investigation, writing—original draft, writing—review and editing; M.M.: conceptualization, investigation, writing—original draft, writing—review and editing; A.V.S.: conceptualization, investigation, visualization, writing—original draft, writing—review and editing; L.R.V.R.: conceptualization, investigation, writing—original draft, writing—review and editing; R.C.K.: formal analysis, investigation, visualization, writing—original draft, writing—review and editing; W.M.: conceptualization, funding acquisition, writing—original draft, writing—review and editing; D.M.R.: conceptualization, validation, writing—original draft, writing—review and editing; K.J.O.: conceptualization, funding acquisition, project administration, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases under awards U01AI151797 (C.A.S., K.J.O.), 1R01AI129822-01 (G.K.) and 5R01AI151144 (L.R.V.R., D.M.R.); the Defense Threat Reduction Agency under awards HDTRA1-17-0064 (K.L.P., K.J.O.), HDTRA1-23-1-0006 (K.L.P., K.J.O.), HDTRA1-19-1-0030 (R.C.K.) and HDTRA1-20-1-0025 (M.G., M.M., W.M.); the South African Research Chair Initiative of the Department of Science and Innovation and administered by the National Research Foundation (NRF) of South Africa (UID: 98339) (M.G., M.M., W.M.); the Defense Advanced Research Projects Agency under award D18AC00031 (G.K.); the Bill and Melinda Gates Foundation under award OPP1211841 (G.K.); the Medical Research Council under award MC_UU_12014/12 (M.E.G.); and the National Science Foundation under award 2032157 (H.K.F.). The content of the information in this manuscript does not necessarily reflect the position or policy of the US government, and no official endorsement should be inferred. Figures were created with BioRender.com.
References
- 1.Haupt H, Rehaag H. 1921. Durch Fledermäuse verbreitete seuchenhafte Tollwut unter Viehbeständen in Santa Catharina (Süd Brasilien). Zeitschrift für Infektionskrankheiten, Parasitäre Krankheiten und Hygiene der Haustiere 22, 104-127. [Google Scholar]
- 2.Wang LF, Cowled C. 2015. Bats and viruses: a new frontier of emerging infectious diseases. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- 3.Perkins SL, Schaer J. 2016. A modern menagerie of mammalian malaria. Trends Parasitol. 32, 772-782. ( 10.1016/j.pt.2016.06.001) [DOI] [PubMed] [Google Scholar]
- 4.Hoyt JR, Kilpatrick AM, Langwig KE. 2021. Ecology and impacts of white-nose syndrome on bats. Nat. Rev. Microbiol. 19, 196-210. ( 10.1038/s41579-020-00493-5) [DOI] [PubMed] [Google Scholar]
- 5.Szentivanyi T, McKee C, Jones G, Foster JT. 2023. Trends in bacterial pathogens of bats: global distribution and knowledge gaps. Transboundary Emerg. Dis. 2023, 9285855. ( 10.1155/2023/9285855) [DOI] [Google Scholar]
- 6.Weber N, et al. 2023. Robust evidence for bats as reservoir hosts is lacking in most African virus studies: a review and call to optimize sampling and conserve bats. Biol. Lett. 19, 20230358. ( 10.1098/rsbl.2023.0358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez S, et al. 2022. Living safely with bats: lessons in developing and sharing a global One Health educational resource. Global Health: Sci. Practice 10, e2200106. ( 10.9745/GHSP-D-22-00106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eby P, Peel AJ, Hoegh A, Madden W, Giles JR, Hudson PJ, Plowright RK. 2023. Pathogen spillover driven by rapid changes in bat ecology. Nature 613, 340-344. ( 10.1038/s41586-022-05506-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geldenhuys M, Ross N, Dietrich M, de Vries JL, Mortlock M, Epstein JH, Weyer J, Markotter W. 2023. Viral maintenance and excretion dynamics of coronaviruses within an Egyptian rousette fruit bat maternal colony: considerations for spillover. Sci. Rep. 13, 15829. ( 10.1038/s41598-023-42938-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicente-Santos A, Ledezma-Campos P, Rodríguez-Herrera B, Corrales-Aguilar E, Czirják G, Civitello D, Gillespie T. 2023. Disentangling effects of anthropogenic disturbance and community structure on multi-pathogen dynamics in tropical cave-dwelling bat communities. Res. Square. ( 10.21203/rs.3.rs-3073229/v2) [DOI] [Google Scholar]
- 11.Griffiths ME, Meza DK, Haydon DT, Streicker DG. 2023. Inferring the disruption of rabies circulation in vampire bat populations using a betaherpesvirus-vectored transmissible vaccine. Proc. Natl Acad. Sci. USA 120, e2216667120. ( 10.1073/pnas.2216667120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brook CE, Dobson AP. 2015. Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends Microbiol. 23, 172-180. ( 10.1016/j.tim.2014.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brook CE, et al. 2020. Accelerated viral dynamics in bat cell lines, with implications for zoonotic emergence. eLife 9, e48401. ( 10.7554/eLife.48401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno Santillán DD, et al. 2021. Large-scale genome sampling reveals unique immunity and metabolic adaptations in bats. Mol. Ecol. 30, 6449-6467. ( 10.1111/mec.16027) [DOI] [PubMed] [Google Scholar]
- 15.Guito JC, et al. 2021. Asymptomatic infection of Marburg virus reservoir bats is explained by a strategy of immunoprotective disease tolerance. Curr. Biol. 31, 257-270. ( 10.1016/j.cub.2020.10.015) [DOI] [PubMed] [Google Scholar]
- 16.Kemenesi G, et al. 2022. Isolation of infectious Lloviu virus from Schreiber's bats in Hungary. Nat. Commun. 13, 1706. ( 10.1038/s41467-022-29298-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imnadze T, et al. 2020. Identification of a novel Yersinia enterocolitica strain from bats in association with a bat die-off that occurred in Georgia (Caucasus). Microorganisms 8, 1000. ( 10.3390/microorganisms8071000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Shea TJ, Cryan PM, Hayman DTS, Plowright RK, Streicker DG. 2016. Multiple mortality events in bats: a global review. Mamm. Rev. 46, 175-190. ( 10.1111/mam.12064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seltmann A, Czirják GÁ, Courtiol A, Bernard H, Struebig MJ, Voigt CC. 2017. Habitat disturbance results in chronic stress and impaired health status in forest-dwelling paleotropical bats. Conserv. Physiol. 5, cox020. ( 10.1093/conphys/cox020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimba A, Ejima A, Ikuta K. 2021. Pleiotropic effects of glucocorticoids on the immune system in circadian rhythm and stress. Front. Immunol. 12, 706951. ( 10.3389/fimmu.2021.706951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMichael L, Edson D, Smith C, Mayer D, Smith I, Kopp S, Meers J, Field H. 2017. Physiological stress and Hendra virus in flying-foxes (Pteropus spp.), Australia. PLoS ONE 12, e0182171. ( 10.1371/journal.pone.0182171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeder DM, Widmaier EP. 2009. Hormone analysis in bats. In Ecological and behavioral methods for the study of bats (eds Kunz TH, Parsons S), pp. 554-563, 2nd edn. Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- 23.Sandoval-Herrera NI, Mastromonaco GF, Becker DJ, Simmons NB, Welch KC Jr. 2021. Inter- and intra-specific variation in hair cortisol concentrations of Neotropical bats. Conserv. Physiol. 9, coab053. ( 10.1093/conphys/coab053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez CA, Becker DJ, Teitelbaum CS, Barriga P, Brown LM, Majewska AA, Hall RJ, Altizer S. 2018. On the relationship between body condition and parasite infection in wildlife: a review and meta-analysis. Ecol. Lett. 21, 1869-1884. ( 10.1111/ele.13160) [DOI] [PubMed] [Google Scholar]
- 25.Wacharapluesadee S, et al. 2018. Longitudinal study of age-specific pattern of coronavirus infection in Lyle's flying fox (Pteropus lylei) in Thailand. Virol. J. 15, 38. ( 10.1186/s12985-018-0950-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edson D, et al. 2019. Time of year, age class and body condition predict Hendra virus infection in Australian black flying foxes (Pteropus alecto). Epidemiol. Infect. 147, e240. ( 10.1017/S0950268819001237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, Cryan PM, Wibbelt G, Blehert DS, Willis CKR. 2012. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl Acad. Sci. USA 109, 6999-7003. ( 10.1073/pnas.1200374109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuire LP, et al. 2018. Common condition indices are no more effective than body mass for estimating fat stores in insectivorous bats. J. Mammal. 99, 1065-1071. ( 10.1093/jmammal/gyy103) [DOI] [Google Scholar]
- 29.Cryan PM, Meteyer CU, Boyles JG, Blehert DS. 2010. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 8, 135. ( 10.1186/1741-7007-8-135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cryan PM, et al. 2013. Electrolyte depletion in white-nose syndrome bats. J. Wildl. Dis. 49, 398-402. ( 10.7589/2012-04-121) [DOI] [PubMed] [Google Scholar]
- 31.Verant ML, Meteyer CU, Speakman JR, Cryan PM, Lorch JM, Blehert DS. 2014. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiol. 14, 10. ( 10.1186/s12899-014-0010-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurpiers LA. 2015. Disease and biodiversity in South Sudan: exploring habitat disturbance and the health and viruses of fruit bats. Master's, Bucknell University, USA. [Google Scholar]
- 33.Ejotre I. 2015. Quantification of health and immunocompetence in the little Epauletted fruit bat (Epomophorus labiatus). Master's, Bucknell University, USA. [Google Scholar]
- 34.Bandouchova H, et al. 2020. Low seasonal variation in greater mouse-eared bat (Myotis myotis) blood parameters. PLoS ONE 15, e0234784. ( 10.1371/journal.pone.0234784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMichael L, Edson D, Mayer D, Broos A, Kopp S, Meers J, Field H. 2017. Physiologic biomarkers and Hendra virus infection in Australian black flying foxes (Pteropus alecto). J. Wildl. Dis. 53, 111-120. ( 10.7589/2016-05-100) [DOI] [PubMed] [Google Scholar]
- 36.McMichael L, Edson D, Mayer D, McLaughlin A, Goldspink L, Vidgen ME, Kopp S, Meers J, Field H. 2016. Temporal variation in physiological biomarkers in black flying-foxes (Pteropus alecto), Australia. EcoHealth 13, 49-59. ( 10.1007/s10393-016-1113-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phelps KL, Kingston T. 2018. Environmental and biological context modulates the physiological stress response of bats to human disturbance. Oecologia 188, 41-52. ( 10.1007/s00442-018-4179-2) [DOI] [PubMed] [Google Scholar]
- 38.Salvante KG. 2006. Techniques for studying integrated immune function in birds. The Auk 123, 575-586. ( 10.1093/auk/123.2.575) [DOI] [Google Scholar]
- 39.Lanier LL. 2013. Shades of grey — the blurring view of innate and adaptive immunity. Nat. Rev. Immunol. 13, 73-74. ( 10.1038/nri3389) [DOI] [PubMed] [Google Scholar]
- 40.Davis AK, Maney DL, Maerz JC. 2008. The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct. Ecol. 22, 760-772. ( 10.1111/j.1365-2435.2008.01467.x) [DOI] [Google Scholar]
- 41.Martínez Gómez JM, et al. 2016. Phenotypic and functional characterization of the major lymphocyte populations in the fruit-eating bat Pteropus alecto. Sci. Rep. 6, 37796. ( 10.1038/srep37796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou H, Li J, Zhou D, Wu Y, Wang X, Zhou J, Ma Q, Yao X, Ma L. 2023. New insights into the germline genes and CDR3 repertoire of the TCRβ chain in Chiroptera. Front. Immunol. 14, 1147859. ( 10.3389/fimmu.2023.1147859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerrard DL, Hawkinson A, Sherman T, Modahl CM, Hume G, Campbell CL, Schountz T, Frietze S. 2017. Transcriptomic signatures of Tacaribe virus-infected Jamaican fruit bats. mSphere 2, 10-128. ( 10.1128/msphere.00245-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lilley TM, et al. 2019. Resistance is futile: RNA-sequencing reveals differing responses to bat fungal pathogen in Nearctic Myotis lucifugus and Palearctic Myotis myotis. Oecologia 191, 295-309. ( 10.1007/s00442-019-04499-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vicente-Santos A, et al. 2023. Serum proteomics reveals a tolerant immune phenotype across multiple pathogen taxa in wild vampire bats. Front. Immunol. 14, 1281732. ( 10.3389/fimmu.2023.1281732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian S, Zeng J, Jiao H, Zhang D, Zhang L, Lei C, Rossiter SJ, Zhao H. 2023. Comparative analyses of bat genomes identify distinct evolution of immunity in Old World fruit bats. Sci. Adv. 9, eadd0141. ( 10.1126/sciadv.add0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123-137. ( 10.1016/S0149-7634(88)80004-6) [DOI] [PubMed] [Google Scholar]
- 48.Johnson RW. 2002. The concept of sickness behavior: a brief chronological account of four key discoveries. Vet. Immunol. Immunopathol. 87, 443-450. ( 10.1016/S0165-2427(02)00069-7) [DOI] [PubMed] [Google Scholar]
- 49.Bohn SJ, Turner JM, Warnecke L, Mayo C, Mcguire LP, Misra V, Bollinger TK, Willis CKR. 2016. Evidence of ‘sickness behaviour’ in bats with white-nose syndrome. Behaviour 153, 981-1003. ( 10.1163/1568539X-00003384) [DOI] [Google Scholar]
- 50.Stockmaier S, Bolnick DI, Page RA, Carter GG. 2018. An immune challenge reduces social grooming in vampire bats. Anim. Behav. 140, 141-149. ( 10.1016/j.anbehav.2018.04.021) [DOI] [Google Scholar]
- 51.Moreno KR, Weinberg M, Harten L, Salinas Ramos VB, Herrera M LG, Czirják GÁ, Yovel Y. 2021. Sick bats stay home alone: fruit bats practice social distancing when faced with an immunological challenge. Ann. N Y Acad. Sci. 1505, 178-190. ( 10.1111/nyas.14600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ripperger SP, Stockmaier S, Carter GG. 2020. Tracking sickness effects on social encounters via continuous proximity sensing in wild vampire bats. Behav. Ecol. 31, 1296-1302. ( 10.1093/beheco/araa111) [DOI] [Google Scholar]
- 53.Stockmaier S, Bolnick DI, Page RA, Josic D, Carter GG. 2020. Immune-challenged vampire bats produce fewer contact calls. Biol. Lett. 16, 20200272. ( 10.1098/rsbl.2020.0272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melhado G, Herrera MLG, Da Cruz-Neto AP. 2020. Bats respond to simulated bacterial infection during the active phase by reducing food intake. J. Exp. Zool. A 333, 536-542. ( 10.1002/jez.2399) [DOI] [PubMed] [Google Scholar]
- 55.O'Mara MT, Wikelski M, Dechmann DKN. 2014. 50 years of bat tracking: device attachment and future directions. Methods Ecol. Evol. 5, 311-319. ( 10.1111/2041-210X.12172) [DOI] [Google Scholar]
- 56.Bentley I, Kuczynska V, Eddington VM, Armstrong M, Kloepper LN. 2023. BatCount: A software program to count moving animals. PLoS ONE 18, e0278012. ( 10.1371/journal.pone.0278012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luxem K, Sun JJ, Bradley SP, Krishnan K, Yttri E, Zimmermann J, Pereira TD, Laubach M. 2023. Open-source tools for behavioral video analysis: setup, methods, and best practices. eLife 12, e79305. ( 10.7554/eLife.79305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayman DTS, Cryan PM, Fricker PD, Dannemiller NG. 2017. Long-term video surveillance and automated analyses reveal arousal patterns in groups of hibernating bats. Methods Ecol. Evol. 8, 1813-1821. ( 10.1111/2041-210X.12823) [DOI] [Google Scholar]
- 59.Kerth G. 2022. Long-term field studies in bat research: importance for basic and applied research questions in animal behavior. Behav. Ecol. Sociobiol. 76, 75. ( 10.1007/s00265-022-03180-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157, 121-141. ( 10.1016/j.cell.2014.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones DN, Ravelomanantsoa NAF, Yeoman CJ, Plowright RK, Brook CE. 2022. Do gastrointestinal microbiomes play a role in bats' unique viral hosting capacity? Trends Microbiol. 30, 632-642. ( 10.1016/j.tim.2021.12.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin N-R, Whon TW, Bae J-W. 2015. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496-503. ( 10.1016/j.tibtech.2015.06.011) [DOI] [PubMed] [Google Scholar]
- 63.Liu B, et al. 2022. The gut microbiota of bats confers tolerance to influenza virus (H1N1) infection in mice. Transboundary Emerg. Dis. 69, e1469. ( 10.1111/tbed.14478) [DOI] [PubMed] [Google Scholar]
- 64.Berman TS, Weinberg M, Moreno KR, Czirják GÁ, Yovel Y. 2023. In sickness and in health: the dynamics of the fruit bat gut microbiota under a bacterial antigen challenge and its association with the immune response. Front. Immunol. 14, 1152107. ( 10.3389/fimmu.2023.1152107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayman DTS, Bowen RA, Cryan PM, McCracken GF, O'Shea TJ, Peel AJ, Gilbert A, Webb CT, Wood JLN. 2013. Ecology of zoonotic infectious diseases in bats: current knowledge and future directions. Zoonoses Public Health 60, 2-21. ( 10.1111/zph.12000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Restif O, et al. 2012. Model-guided fieldwork: practical guidelines for multidisciplinary research on wildlife ecological and epidemiological dynamics. Ecol. Lett. 15, 1083-1094. ( 10.1111/j.1461-0248.2012.01836.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mortlock M, Geldenhuys M, Dietrich M, Epstein JH, Weyer J, Pawęska JT, Markotter W. 2021. Seasonal shedding patterns of diverse henipavirus-related paramyxoviruses in Egyptian rousette bats. Sci. Rep. 11, 24262. ( 10.1038/s41598-021-03641-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joffrin L, Hoarau AOG, Lagadec E, Torrontegi O, Köster M, Le Minter G, Dietrich M, Mavingui P, Lebarbenchon C. 2022. Seasonality of coronavirus shedding in tropical bats. R. Soc. Open Sci. 9, 211600. ( 10.1098/rsos.211600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montecino-Latorre D, et al. 2020. Reproduction of East-African bats may guide risk mitigation for coronavirus spillover. One Health Outlook 2, 2. ( 10.1186/s42522-019-0008-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson DP, Klein SL. 2012. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 62, 263-271. ( 10.1016/j.yhbeh.2012.02.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou P, et al. 2016. Contraction of the type I IFN locus and unusual constitutive expression of IFN-α in bats. Proc. Natl Acad. Sci. USA 113, 2696-2701. ( 10.1073/pnas.1518240113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plowright RK, Field HE, Smith C, Divljan A, Palmer C, Tabor G, Daszak P, Foley JE. 2008. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc. R. Soc. B 275, 861-869. ( 10.1098/rspb.2007.1260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baker KS, et al. 2014. Viral antibody dynamics in a chiropteran host. J. Anim. Ecol. 83, 415-428. ( 10.1111/1365-2656.12153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brook CE, et al. 2019. Disentangling serology to elucidate henipa- and filovirus transmission in Madagascar fruit bats. J. Anim. Ecol. 88, 1001-1016. ( 10.1111/1365-2656.12985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Field H, et al. 2015. Spatiotemporal aspects of Hendra virus infection in Pteropid bats (flying-foxes) in eastern Australia. PLoS ONE 10, e0144055. ( 10.1371/journal.pone.0144055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amman BR, et al. 2012. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 8, 11. ( 10.1371/journal.ppat.1002877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drexler JF, Corman VM, Wegner T, Tateno AF, Zerbinati RM, Gloza-Rausch F, Seebens A, Müller MA, Drosten C. 2011. Amplification of emerging viruses in a bat colony. Emerg. Infect. Dis. 17, 449-456. ( 10.3201/eid1703.100526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cappelle J, et al. 2021. Longitudinal monitoring in Cambodia suggests higher circulation of alpha and betacoronaviruses in juvenile and immature bats of three species. Sci. Rep. 11, 24145. ( 10.1038/s41598-021-03169-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frank HK, Mendenhall CD, Judson SD, Daily GC, Hadly EA. 2016. Anthropogenic impacts on Costa Rican bat parasitism are sex specific. Ecol. Evol. 6, 4898-4909. ( 10.1002/ece3.2245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Willoughby A, Phelps K, PREDICT Consortium, Olival K. 2017. A comparative analysis of viral richness and viral sharing in cave-roosting bats. Diversity 9, 35. ( 10.3390/d9030035) [DOI] [Google Scholar]
- 81.Lunn TJ, Peel AJ, McCallum H, Eby P, Kessler MK, Plowright RK, Restif O. 2021. Spatial dynamics of pathogen transmission in communally roosting species: impacts of changing habitats on bat-virus dynamics. J. Anim. Ecol. 90, 2609-2622. ( 10.1111/1365-2656.13566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muylaert RL, Kingston T, Luo J, Vancine MHV, Galli N, Carlson CJ, John RS, Rulli MC, Hayman DTS. 2022. Present and future distribution of bat hosts of sarbecoviruses: implications for conservation and public health. Proc. R. Soc. B 289, 20220397. ( 10.1098/rspb.2022.0397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Plowright RK, Becker DJ, McCallum H, Manlove KR. 2019. Sampling to elucidate the dynamics of infections in reservoir hosts. Phil. Trans. R. Soc. B 374, 20180336. ( 10.1098/rstb.2018.0336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edson D, et al. 2015. Routes of Hendra virus excretion in naturally-infected flying-foxes: implications for viral transmission and spillover risk. PLoS ONE 10, e0140670. ( 10.1371/journal.pone.0140670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldspink LK, Edson DW, Vidgen ME, Bingham J, Field HE, Smith CS. 2015. Natural Hendra virus infection in flying-foxes - tissue tropism and risk factors. PLoS ONE 10, e0128835. ( 10.1371/journal.pone.0128835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Geldenhuys M, Mortlock M, Epstein JH, Pawęska JT, Weyer J, Markotter W. 2021. Overview of bat and wildlife coronavirus surveillance in Africa: a framework for global investigations. Viruses 13, 936. ( 10.3390/v13050936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meza DK, et al. 2022. Ecological determinants of rabies virus dynamics in vampire bats and spillover to livestock. Proc. R. Soc. B 289, 20220860. ( 10.1098/rspb.2022.0860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Epstein JH, et al. 2020. Nipah virus dynamics in bats and implications for spillover to humans. Proc. Natl Acad. Sci. USA 117, 29190. ( 10.1073/pnas.2000429117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Harten E, Reardon T, Lumsden LF, Meyers N, Prowse TAA, Weyland J, Lawrence R. 2019. High detectability with low impact: optimizing large PIT tracking systems for cave-dwelling bats. Ecol. Evol. 9, 10 916-10 928. ( 10.1002/ece3.5482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Welbergen JA, Meade J, Field HE, Edson D, McMichael L, Shoo LP, Praszczalek J, Smith C, Martin JM. 2020. Extreme mobility of the world's largest flying mammals creates key challenges for management and conservation. BMC Biol. 18, 101. ( 10.1186/s12915-020-00829-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Streicker DG, et al. 2016. Host–pathogen evolutionary signatures reveal dynamics and future invasions of vampire bat rabies. Proc. Natl Acad. Sci. USA 113, 10 926-10 931. ( 10.1073/pnas.1606587113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilbert AT, et al. 2013. Deciphering serology to understand the ecology of infectious diseases in wildlife. EcoHealth 10, 298-313. ( 10.1007/s10393-013-0856-0) [DOI] [PubMed] [Google Scholar]
- 93.Farrington CP, Kanaan MN, Gay NJ. 2001. Estimation of the basic reproduction number for infectious diseases from age-stratified serological survey data. J. R. Stat. Soc. C 50, 251-292. ( 10.1111/1467-9876.00233) [DOI] [Google Scholar]
- 94.Hay JA, Kennedy-Shaffer L, Kanjilal S, Lennon NJ, Gabriel SB, Lipsitch M, Mina MJ. 2021. Estimating epidemiologic dynamics from cross-sectional viral load distributions. Science 373, eabh0635. ( 10.1126/science.abh0635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lunn TJ, et al. 2023. Periodic shifts in viral load increase risk of spillover from bats. bioRxiv. 556454. ( 10.1101/2023.09.06.556454) [DOI]
- 96.Gentles AD, Guth S, Rozins C, Brook CE. 2020. A review of mechanistic models of viral dynamics in bat reservoirs for zoonotic disease. Pathogens Global Health 114, 407-425. ( 10.1080/20477724.2020.1833161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glennon EE, et al. 2019. What is stirring in the reservoir? Modelling mechanisms of henipavirus circulation in fruit bat hosts. Phil. Trans. R. Soc. B 374, 20190021. ( 10.1098/rstb.2019.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Plowright RK, Peel AJ, Streicker DG, Gilbert AT, McCallum H, Wood J, Baker ML, Restif O. 2016. Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir–host populations. PLoS Neglect. Trop. Dis. 10, e0004796. ( 10.1371/journal.pntd.0004796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Streicker DG, Fallas González SL, Luconi G, Barrientos RG, Leon B. 2019. Phylodynamics reveals extinction–recolonization dynamics underpin apparently endemic vampire bat rabies in Costa Rica. Proc. R. Soc. B 286, 20191527. ( 10.1098/rspb.2019.1527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Olival KJ, et al. 2020. Population genetics of fruit bat reservoir informs the dynamics, distribution and diversity of Nipah virus. Mol. Ecol. 29, 970-985. ( 10.1111/mec.15288) [DOI] [PubMed] [Google Scholar]
- 101.Huang C, et al. 2016. A bat-derived putative cross-family recombinant coronavirus with a reovirus gene. PLoS Pathog. 12, e1005883. ( 10.1371/journal.ppat.1005883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng X, et al. 2020. Metagenomic sequencing with spiked primer enrichment for viral diagnostics and genomic surveillance. Nat. Microbiol. 5, 443-454. ( 10.1038/s41564-019-0637-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Letko M, Seifert SN, Olival KJ, Plowright RK, Munster VJ. 2020. Bat-borne virus diversity, spillover and emergence. Nat. Rev. Microbiol. 18, 461-471. ( 10.1038/s41579-020-0394-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. 2017. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 15, 502-510. ( 10.1038/nrmicro.2017.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin G, Yanez-Arenas C, Plowright RK, Chen C, Roberts B, Skerratt LF. 2018. Hendra virus spillover is a bimodal system driven by climatic factors. EcoHealth 15, 526-542. ( 10.1007/s10393-017-1309-y) [DOI] [PubMed] [Google Scholar]
- 106.Frank HK, Enard D, Boyd SD. 2022. Exceptional diversity and selection pressure on coronavirus host receptors in bats compared to other mammals. Proc. R. Soc. B 289, 20220193. ( 10.1098/rspb.2022.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lučan RK, et al. 2014. Reproductive seasonality of the Egyptian fruit bat (Rousettus aegyptiacus) at the northern limits of its distribution. J. Mammal. 95, 1036-1042. ( 10.1644/14-MAMM-A-035) [DOI] [Google Scholar]
- 108.Cumming GS, Bernard RTF. 1997. Rainfall, food abundance and timing of parturition in African bats. Oecologia 111, 309-317. ( 10.1007/s004420050240) [DOI] [PubMed] [Google Scholar]
- 109.Pulliam JRC, et al. 2012. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J. R. Soc. Interface 9, 89-101. ( 10.1098/rsif.2011.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou P, et al. 2018. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 556, 255-258. ( 10.1038/s41586-018-0010-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voigt CC, Kelm DH. 2006. Host preference of the common vampire bat (Desmodus rotundus; Chiroptera) assessed by stable isotopes. J. Mammal. 87, 1-6. ( 10.1644/05-MAMM-F-276R1.1) [DOI] [Google Scholar]
- 112.Sanchez-Gomez WS, Selem-Salas CI, Cordova-Aldana DI, Erales-Villamil JA. 2022. Common vampire bat (Desmodus rotundus) abundance and frequency of attacks to cattle in landscapes of Yucatan, Mexico. Trop. Anim. Health Prod. 54, 130. ( 10.1007/s11250-022-03122-w) [DOI] [PubMed] [Google Scholar]
- 113.Wiethoelter AK, Beltrán-Alcrudo D, Kock R, Mor SM. 2015. Global trends in infectious diseases at the wildlife–livestock interface. Proc. Natl Acad. Sci. USA 112, 9662-9667. ( 10.1073/pnas.1422741112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hayman DTS, Wang LF, Barr J, Baker KS, Suu-Ire R, Broder CC, Cunningham AA, Wood JL. 2011. Antibodies to henipavirus or henipa-like viruses in domestic pigs in Ghana, West Africa. PLoS ONE 6, 4. ( 10.1371/journal.pone.0025256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Olufemi OT, Umoh JU, Dzikwi AA, Wang L, Crameri G, Morrissy C, Barr J, Olufemi YO. 2016. Serological evidence of henipavirus among horses and pigs in Zaria and environs, Kaduna State Nigeria. Int. J. Infect. Dis. 45, 189. ( 10.1016/j.ijid.2016.02.439) [DOI] [Google Scholar]
- 116.Field HE, Smith CS, de Jong CE, Melville D, Broos A, Kung N, Thompson J, Dechmann DKN. 2016. Landscape utilisation, animal behaviour and Hendra virus risk. EcoHealth 13, 26-38. ( 10.1007/s10393-015-1066-8) [DOI] [PubMed] [Google Scholar]
- 117.Shapiro HG, Willcox AS, Tate M, Willcox EV. 2020. Can farmers and bats co-exist? Farmer attitudes, knowledge, and experiences with bats in Belize. Hum. Wildlife Interact. 14, 5-15. ( 10.26077/5wwp-sp53) [DOI] [Google Scholar]
- 118.Jung K, Threlfall CG. 2016. Urbanisation and its effects on bats—a global meta-analysis. In Bats in the Anthropocene: conservation of bats in a changing world (eds Voigt CC, Kingston T), pp. 13-33. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 119.Voigt CC, Phelps KL, Aguirre LF, Schoeman MC, Vanitharani J, Zubaid A. 2016. Bats and buildings: the conservation of synanthropic bats. In Bats in the Anthropocene: conservation of bats in a changing world (eds Voigt CC, Kingston T), pp. 427-462. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 120.Huhn GD, et al. 2005. Two outbreaks of occupationally acquired histoplasmosis: more than workers at risk. Environ. Health Perspect. 113, 585-589. ( 10.1289/ehp.7484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kessler MK, et al. 2018. Changing resource landscapes and spillover of henipaviruses. Ann. N Y Acad. Sci. 1429, 78-99. ( 10.1111/nyas.13910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sánchez CA, Li H, Phelps KL, Zambrana-Torrelio C, Wang L-F, Zhou P, Shi Z-L, Olival KJ, Daszak P. 2022. A strategy to assess spillover risk of bat SARS-related coronaviruses in Southeast Asia. Nat. Commun. 13, 4380. ( 10.1038/s41467-022-31860-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pernet O, et al. 2014. Evidence for henipavirus spillover into human populations in Africa. Nat. Commun. 5, 5342. ( 10.1038/ncomms6342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lange CE, et al. 2023. Human interactions with bats and bat coronaviruses in rural Côte d'Ivoire. One Health 16, 100569. ( 10.1016/j.onehlt.2023.100569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Voigt CC, Kingston T. 2016. Bats in the Anthropocene: conservation of bats in a changing world. Cham, Switzerland: Springer. [Google Scholar]
- 126.Loh EH, et al. 2022. Prevalence of bat viruses associated with land-use change in the Atlantic Forest, Brazil. Front. Cell. Infect. Microbiol. 12, 1717. ( 10.3389/fcimb.2022.921950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hiller T, et al. 2019. Host biology and anthropogenic factors affect hepadnavirus infection in a neotropical bat. EcoHealth 16, 82-94. ( 10.1007/s10393-018-1387-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davy CM, et al. 2018. White-nose syndrome is associated with increased replication of a naturally persisting coronaviruses in bats. Sci. Rep. 8, 15508. ( 10.1038/s41598-018-33975-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Becker DJ, Eby P, Madden W, Peel AJ, Plowright RK. 2023. Ecological conditions predict the intensity of Hendra virus excretion over space and time from bat reservoir hosts. Ecol. Lett. 26, 23-36. ( 10.1111/ele.14007) [DOI] [PubMed] [Google Scholar]
- 130.Turmelle AS, Allen LC, Jackson FR, Kunz TH, Rupprecht CE, McCracken GF. 2010. Ecology of rabies virus exposure in colonies of Brazilian free-tailed bats (Tadarida brasiliensis) at natural and man-made roosts in Texas. Vector-Borne Zoonotic Dis. 10, 165-175. ( 10.1089/vbz.2008.0163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Allen LC, Turmelle AS, Widmaier EP, Hristov NI, McCracken GF, Kunz TH. 2011. Variation in physiological stress between bridge- and cave-roosting Brazilian free-tailed bats. Conserv. Biol. 25, 374-381. ( 10.1111/j.1523-1739.2010.01624.x) [DOI] [PubMed] [Google Scholar]
- 132.Edson D, Field H, McMichael L, Jordan D, Kung N, Mayer D, Smith C. 2015. Flying-fox roost disturbance and Hendra virus spillover risk. PLoS ONE 10, e0125881. ( 10.1371/journal.pone.0125881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Phelps K, Jose R, Labonite M, Kingston T. 2018. Assemblage and species threshold responses to environmental and disturbance gradients shape bat diversity in disturbed cave landscapes. Diversity 10, 55. ( 10.3390/d10030055) [DOI] [Google Scholar]
- 134.Carter SP, et al. 2009. Options for the control of disease 2: targeting hosts. In Management of disease in wild mammals (eds Delahay RJ, Smith GC, Hutchings MR), pp. 121-146. Tokyo, Japan: Springer. [Google Scholar]
- 135.Jeong J, McCallum H. 2021. Using stochastic modeling to predict the effect of culling and colony dispersal of bats on zoonotic viral epidemics. Vector-Borne Zoonotic Dis. 21, 369-377. ( 10.1089/vbz.2020.2700) [DOI] [PubMed] [Google Scholar]
- 136.Viana M, et al. 2023. Effects of culling vampire bats on the spatial spread and spillover of rabies virus. Sci. Adv. 9, eadd7437. ( 10.1126/sciadv.add7437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Olival KJ. 2016. To cull, or not to cull, bat is the question. EcoHealth 13, 6-8. ( 10.1007/s10393-015-1075-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nahar N, Mondal UK, Hossain MJ, Khan MSU, Sultana R, Gurley ES, Luby SP. 2014. Piloting the promotion of bamboo skirt barriers to prevent Nipah virus transmission through date palm sap in Bangladesh. Global Health Promotion 21, 7-15. ( 10.1177/1757975914528249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reaser JK, Witt A, Tabor GM, Hudson PJ, Plowright RK. 2021. Ecological countermeasures for preventing zoonotic disease outbreaks: when ecological restoration is a human health imperative. Restor. Ecol. 29, e13357. ( 10.1111/rec.13357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maki J, et al. 2017. Oral vaccination of wildlife using a vaccinia–rabies-glycoprotein recombinant virus vaccine (RABORAL V-RG®): a global review. Vet. Res. 48, 57. ( 10.1186/s13567-017-0459-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumosani T, Yaghmoor S, Abdulaal WH, Barbour E. 2020. Evaluation in broilers of aerosolized nanoparticles vaccine encapsulating imuno-stimulant and antigens of avian influenza virus/Mycoplasma gallisepticum. BMC Vet. Res. 16, 319. ( 10.1186/s12917-020-02539-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bakker KM, et al. 2019. Fluorescent biomarkers demonstrate prospects for spreadable vaccines to control disease transmission in wild bats. Nat. Ecol. Evol. 3, 1697-1704. ( 10.1038/s41559-019-1032-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nuismer SL, Bull JJ. 2020. Self-disseminating vaccines to suppress zoonoses. Nat. Ecol. Evol. 4, 1168-1173. ( 10.1038/s41559-020-1254-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Griffiths ME, Bergner LM, Broos A, Meza DK, Filipe AS, Davison A, Tello C, Becker DJ, Streicker DG. 2020. Epidemiology and biology of a herpesvirus in rabies endemic vampire bat populations. Nat. Commun. 11, 5951. ( 10.1038/s41467-020-19832-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rocke TE, et al. 2019. Virally-vectored vaccine candidates against white-nose syndrome induce anti-fungal immune response in little brown bats (Myotis lucifugus). Sci. Rep. 9, 6788. ( 10.1038/s41598-019-43210-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kading RC, Kingston T. 2020. Common ground: the foundation of interdisciplinary research on bat disease emergence. PLoS Biol. 18, e3000947. ( 10.1371/journal.pbio.3000947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rocha R, et al. 2021. Bat conservation and zoonotic disease risk: a research agenda to prevent misguided persecution in the aftermath of COVID-19. Anim. Conserv. 24, 303-307. ( 10.1111/acv.12636) [DOI] [Google Scholar]
- 148.Lu M, Wang X, Ye H, Wang H, Qiu S, Zhang H, Liu Y, Luo J, Feng J. 2021. Does public fear that bats spread COVID-19 jeopardize bat conservation? Biol. Conserv. 254, 108952. ( 10.1016/j.biocon.2021.108952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ejotre I, Reeder DM, Matuschewski K, Kityo R, Schaer J. 2022. Negative perception of bats, exacerbated by the SARS-CoV-2 pandemic, may hinder bat conservation in Northern Uganda. Sustainability 14, 16924. ( 10.3390/su142416924) [DOI] [Google Scholar]
- 150.Olival KJ, et al. 2020. Possibility for reverse zoonotic transmission of SARS-CoV-2 to free-ranging wildlife: a case study of bats. PLoS Pathog. 16, e1008758. ( 10.1371/journal.ppat.1008758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Runge MC, et al. 2020. Assessing the risks posed by SARS-CoV-2 in and via North American bats—decision framing and rapid risk assessment. Report. Reston, VA; 2020. Report No: 2020-1060.
- 152.Phelps K, Hamel L, Alhmoud N, Ali S, Bilgin R, Sidamonidze K, Urushadze L, Karesh W, Olival K. 2019. Bat research networks and viral surveillance: gaps and opportunities in Western Asia. Viruses 11, 240. ( 10.3390/v11030240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shapiro JT, et al. 2021. Setting the terms for zoonotic diseases: effective communication for research, conservation, and public policy. Viruses 13, 1356. ( 10.3390/v13071356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sánchez CA, et al. 2023. Advances in understanding bat infection dynamics across biological scales. Zenodo. ( 10.5281/zenodo.8003910) [DOI] [PMC free article] [PubMed]
- 155.Sánchez CA, et al. 2024. Supplementary material from “Advances in understanding bat infection dynamics across biological scales”. Figshare. ( 10.6084/m9.figshare.c.7075588) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sánchez CA, et al. 2024. Supplementary material from “Advances in understanding bat infection dynamics across biological scales”. Figshare. ( 10.6084/m9.figshare.c.7075588) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data to support authorship network mapping of the bat research community (described in the electronic supplementary material) are available at Zenodo: https://doi.org/10.5281/zenodo.8003910 [154].
Supplementary material is available online [155].