Abstract
Dipeptidyl-peptidase-like protein 6 (DPPX) antibody-mediated encephalitis is a rare type of autoimmune encephalitis (AE), which mainly manifests as diarrhea accompanied by weight loss, cognitive decline, epileptic seizures, and even psychiatric symptoms. Remarkably, it is also reported to be associated with tumors, predominantly B-cell lymphoma. Overall, the AE remains uncharacterized clinically and its long-term prognosis remains elusive. Herein, we report the first case of DPPX antibody-mediated AE secondary to breast cancer. Importantly, it substantially improves after aggressive immunotherapy. Our case highlights DPPX antibody-mediated AE as a paraneoplastic syndrome and discusses the pearls in its diagnosis and management.
Keywords: Dipeptidyl-peptidase–like protein 6, Autoimmune encephalitis, Breast cancer, Consciousness disorder, Seizures
1. Introduction
Encephalitis due to antibodies targeting dipeptidyl-peptidase-like protein 6 (DPPX), a potassium channel subunit, is rare and is typically characterized by a triad of weight loss, central nervous system hyperexcitability, and cognitive symptoms. The target antigen of DPPX is located on the surface of neuronal cells and causes relatively reversible neuronal dysfunction mainly through the humoral immune mechanism. Given the wide distribution of DPPX across the hippocampus, cerebellum, as well as the myenteric plexus, the clinical picture may be diverse. In addition, DPPX antibody-mediated AE is also associated with underlying malignancies, which are mainly lymphoma according to previous reports. In this case, we describe the first case of DPPX antibody-mediated AE secondary to breast cancer. We also highlight diarrhea, cognitive decline, and seizures as clues indicative of the diagnosis and emphasize early immunosuppressive treatments as critical determinants of a favorable prognosis.
2. Case presentation
A 60-year-old female patient was admitted to the hospital on October 07, 2022, with blurred visions and paroxysmal convulsions over the past two weeks. Two weeks ago, the patient experienced episodes of binocular vision loss accompanied by slurred speech, which resolved spontaneously after a few hours. Cerebral magnetic resonance imaging was not indicative of acute infarctions, leading to the preliminary diagnosis of a transient ischemic attack (TIA). Despite the initiation of dual antiplatelet therapy, the patient's symptoms progressively worsened, leading to lethargy and unresponsiveness. Two days before admission, the patient experienced acute repetitive seizures ultimately controlled with levetiracetam.
On physical examination, the patient was afebrile and displayed lethargy and slurred speech. Neck stiffness was observed. Visual agnosia, conjunctival without hyperemia and edema, cornea transparent, pupil sensitive to light reflex, crystal and vitreous body transparent, fundus normal. The muscle strength was 4 in the right limb according to the Muscle Research Council (MRC) scale, with the remaining extremities graded as 5. Bilateral Babinski signs were positive. The patient's Modified Rankin Scale score was 5, indicating severe disability.
Decades ago, the patient was diagnosed with "metastatic carcinoma with necrosis in the right occipital lobe, combined with clinical indications of breast infiltrating ductal carcinoma metastasis", then underwent surgery. Postoperative pathology: ER (−), PR (−), CerbB-2 (+++), followed by one year of Herceptin therapy. "Hydrocephalus drainage" was performed in 2017 and 2022, and postoperative drainage tubes were indwelled in bilateral ventricles. After regular review, the recovery is good, with no recurrence of the tumor. No members of the family have had similar conditions.
Based on the loss of consciousness, blurred vision, and epileptic seizures, localizations in the cerebral cortex, especially the temporal and occipital lobes were considered. Given the rapid course of deterioration, and the previous history of cancers, inflammatory and malignant etiologies were considered most likely.
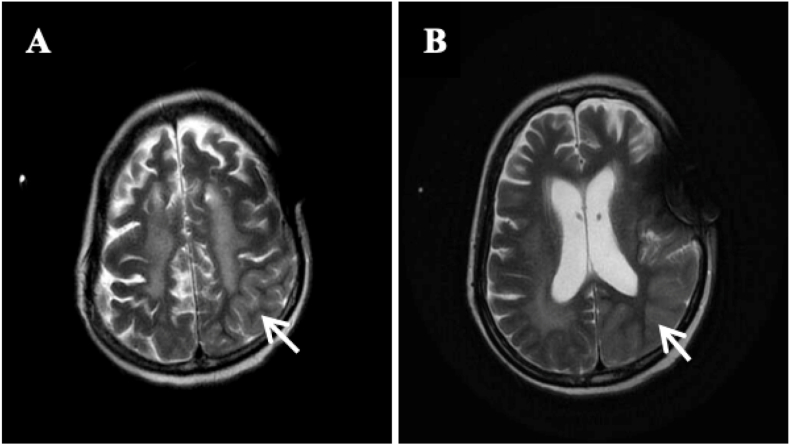
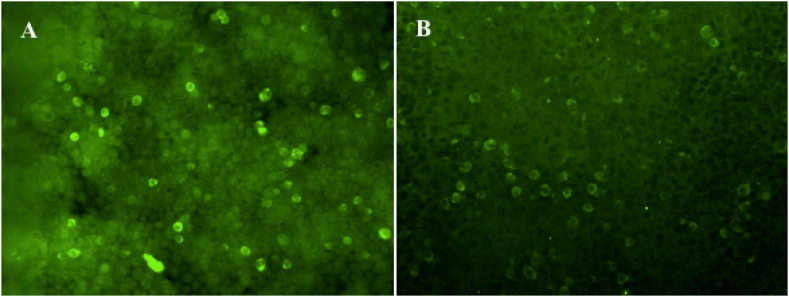
A comprehensive blood work was unremarkable. A brain MRI scan revealed swelling of the left occipital-parietal cortex (Fig. 1 (A,B)), but with substantial artifacts due to the drainage tube (not shown). A lumbar puncture was performed, and the intracranial pressure was measured to be 145 mm H2O. Cerebrospinal fluid (CSF) examination indicated an absence of nucleated cells. The CSF protein concentration was elevated (91.8 mg/dL) Other results were all negative. Autoimmune encephalitis and paraneoplastic antibodies were further screened (Fig. 2). Paired testing of AE antibodies (NMDAR, AMPA1, AMPA2, LGI1, CASPRA2, GABAB, DPPX, IgLON5) in the serum and CSF showed positive results of serum anti-DPPX antibodies at a titer of 1:32 (Fig. 3 (A)). Paraneoplastic antibodies were negative.
Fig. 1.
Magnetic resonance imaging of the brain. T2 (A) and T2 flair (B) images showing swelling of the left parietal lobe (white arrow).
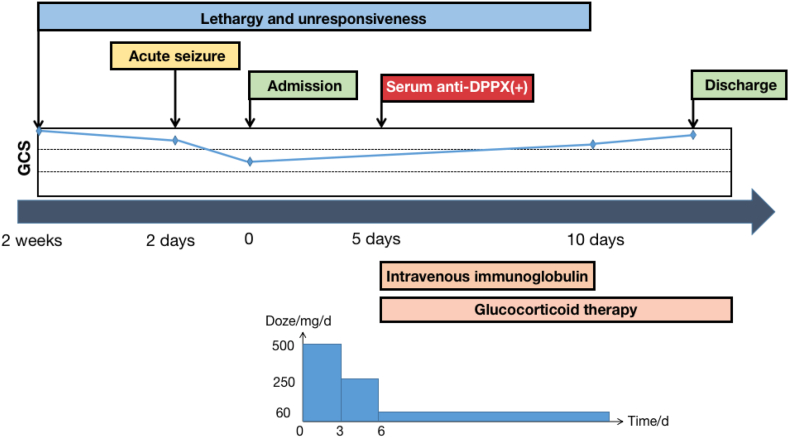
Fig. 2.
Clinic course of the patient.
Fig. 3.
Positive reaction with Anti-DPPX antibody with the patient's serum (A) (titer 1:32). The repeat serum (B) (titer 1:10) was lower after half a year follow-up visit. (Cell Based Assay, x400).
Based on altered consciousness, seizures, a history of breast cancer, cortical swelling on neuroimaging, and the presence of serum anti-DPPX antibodies, reasonable exclusion of alternative causes [1,2] (a broad differential diagnosis [3] including acute disseminated encephalomyelitis, acute hemorrhagicleukoencephalitis tumefactive multiple sclerosis, and Marburg), a primary diagnosis [1] of DPPX antibody-mediated AE is made.
The patient received intravenous injections of 0.04 g/kg/day of human immunoglobulin for 5 days. Methylprednisolone was administered at a dose of 500 mg per day, gradually reducing over a period of 3 days before transitioning to oral prednisone tablets at a dose of 60 mg/day. Levetiracetam was given at a dosage of 0.5 g twice daily.
Ten days later, there was a notable improvement in the patient's level of consciousness. Her mental status was clear and oriented, and she was able to follow simple instructions. Almost a month later, the patient could talk and walk with assistance. Two months later, the patient exhibited the ability to walk independently. Meanwhile, a cranial CT scan demonstrated an even better condition, with reduced swelling of the left occipital-parietal lobe (Fig. 1). After more than half a year, serum anti-DPPX antibody was retested at lower concentrations than before (Fig. 3 (B)).
3. Discussion
Anti-DPPX antibody-mediated encephalitis is characterized by the presence of specific antibodies targeting DPPX, predominantly IgG1 and IgG4 isotypes [4]. DPPX is a regulatory protein of the voltage-dependent potassium channels Kv4.2, which plays a crucial role in somatodendritic signal integration and attenuation of back-propagation of action potentials. The exact pathogenesis of this disease remains incompletely understood, although several studies have been conducted to shed light on it. Piepgras [5] et al. discovered that serum containing DPPX antibody can cause hyperexcitability in neurons of the enteric nervous system. The binding of DPPX antibody to excitatory and inhibitory synapse neurons in the central nervous system is mediated by the Kv4.2 potassium channel. As Kv4.2 potassium channels are extensively distributed in the central nervous system, the clinical manifestations of this disease are very complicated.
The clinical reports of DPPX antibody-mediated encephalitis are rare and display a wide range of characteristics. It primarily affects middle-aged and older individuals, with a male-to-female ratio of nearly 2:1. Obvious weight loss and diarrhea commonly occur as prodromal symptoms. The main clinical manifestations are cognitive decline, neurohyperexcitability (seizures [6], myoclonus, etc.), autonomic hyperexcitability (diarrhea, sleep disorders), and psychiatric symptoms.
Wijntjes [7] et al. reported a case of DPPX antibody-mediated encephalitis with severe itching as the primary symptom. Additionally, Deuel [8] et al. reported a case where prominent oral-buccal-lingual dyskinesia was the main symptom. Furthermore, there have been cases where patients experienced headaches and sleep disturbances [9] as manifestations of this disease. In this case, a patient initially presented with repeated blurred vision. As the condition progressed, there was a gradual decline in vision accompanied by a disturbance of consciousness and a progressive decline of consciousness, ultimately leading to lethargy. During this period, the patients also experienced two episodes of seizures, aligning with the documented cognitive decline and nerve hyperexcitability associated with encephalitis. Based on the characteristic signs observed in the patient, MRI scanning results, and the presence of DPPX antibodies, a clinical diagnosis [1] of DPPX antibody-mediated encephalitis was made.
Autoimmune antibody-mediated encephalitis with tumors has been documented in several studies [10]. For example, N-methyl-d-aspartate receptor (NMDAR) antibodies-mediated encephalitis are commonly found in young women with ovarian teratoma, often presenting severe cases. Another case is γ-amino butyric acid type B receptor (GABABR) antibody-mediated encephalitis, which occurs in approximately one-third of patients with small-cell lung cancer. Contactin-associated protein 2 (CASPR2) antibody-mediated encephalitis is associated with a few complicated tumors, mostly thymoma. α-amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor (AMPAR) antibody-mediated encephalitis has been diagnosed with more than half of patients with lung cancer or thymic cancer. Furthermore, γ-amino butyric acid type A receptor (GABAAR) antibody-mediated encephalitis is present in 40% of patients, with thymoma being the most common. Metabolic glutamate receptor 5 (mGluR5) antibody-mediated encephalitis is found in more than half of the cases with tumors, especially Hodgkin's lymphoma, and small-cell lung cancer. Recent studies have revealed that lymphoma, predominantly B-cell lymphoma, is confirmed to be the only neoplastic disease associated with DPPX antibody-mediated encephalitis and accounts for 10% of the case [1]. The presence of breast cancer in this patient has significantly contributed to our expanding understanding of this disease.
Breast cancer is the leading cause of cancer in women worldwide. The nervous system can be affected by the side effects of breast cancer treatments. Neurological syndromes associated with breast cancer encompass various conditions such as encephalitis, retinopathy, cerebellar degeneration, sensorimotor neuropathy, and stiff person syndrome. Many of these syndromes are mediated by antibodies targeting specific antigens of neurons [11]. Of note, immunosuppressive treatments can also impact the central nervous system by increasing susceptibility to opportunistic infections [12]. In the case discussed in this paper, the patient has a prior history of metastatic breast cancer and has developed secondary symptoms of DPPX antibody-mediated encephalitis and retinopathy following immunotherapy.
Mounting evidence demonstrates that there is a correlation between autoimmunity and cancer [[13], [14], [15], [16]]. Accordingly, Lu et al. have confirmed a clear bidirectional relationship exists between breast cancer and different autoimmune diseases. Specifically, women with breast cancer have higher rates of certain autoimmune conditions including rheumatoid arthritis, inflammatory bowel disease and systemic lupus erythematosus [17,18]. However, the mechanism of both is not clear, may be related to endocrine [19], still need to be further explored.
Currently, the primary treatment of DPPX antibody-mediated encephalitis involves immunotherapy, symptomatic management, and rehabilitation therapy. The recommended first-line treatment for patients newly diagnosed with AE is immunotherapy [20], including the administration of glucocorticoids, intravenous immunoglobulin therapy, and plasmapheresis. Among these treatments, glucocorticoids are the preferred first-line treatment. If patients do not respond well to initial first-line immunotherapy, the use of anti-CD20 mAb like rituximab, along with second-line immunotherapy such as intravenous cyclophosphamide, may be considered. Second-line immunotherapy has shown better long-term outcomes compared to intensive first-line immunotherapy. If patients do not show significant improvement after intensive first-line or second-line immunotherapy, long-term immunotherapy can be considered, including mycophenolate, azathioprine, and repeated rituximab. The duration of treatment should not be less than 12 months.
Following intravenous immunoglobulin and glucocorticoid bolus injections, there was a significant improvement in the patient's consciousness in this study. After a two-month follow-up, the patient could walk independently and answer simple questions well, similar to her condition before her disease onset. However, certain symptoms such as vision loss and dyslexia still require further observation during subsequent follow-up observation.
In summary, DPPX antibody-mediated encephalitis is a rare condition with atypical symptoms, often leading to potential misdiagnoses as cerebrovascular disease or other systemic disorders. Other autoimmune antibodies secondary to breast cancer such as AMPAR antibodies [21] and RI antibodies [22] have also been reported, which should be considered to make an accurate clinical diagnosis. At the same time, previous studies have only found that DPPX antibody-mediated AE is related to lymphoma, and others can not be seen. Breast cancer was present for the first time in this case, future studies need to focus on the correlation between DPPX encephalitis and other tumors. It takes some time to assess the presence of autoantibodies, so the earlier immunotherapy should be started based on clinical symptoms and ruling out infection and other causes, the more effective it will be.
Patient consent
Consent was obtained from the patient before submission.
Study funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Yijie Dai: Writing – original draft, Investigation, Formal analysis, Data curation. Yang Zheng: Writing – review & editing, Visualization, Resources, Methodology. Jiahui Zhu: Writing – review & editing, Methodology, Formal analysis. Jiao Ding: Writing – review & editing, Resources, Formal analysis. Kefan Qiu: Methodology, Funding acquisition. Bo Tang: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the patient for the kind cooperation throughout the process.
Contributor Information
Yijie Dai, Email: ddyj0807@126.com.
Yang Zheng, Email: yangzh92@zju.edu.cn.
Jiahui Zhu, Email: zhujh20210601@163.com.
Jiao Ding, Email: dingj323@126.com.
Kefan Qiu, Email: qqiukefan@163.com.
Bo Tang, Email: tangbo159@163.com.
References
- 1.Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Cellucci T., Cortese I., Dale R.C., Gelfand J.M., Geschwind M., Glaser C.A., Honnorat J., Höftberger R., Iizuka T., Irani S.R., Lancaster E., Leypoldt F., Prüss H., Rae-Grant A., Reindl M., Rosenfeld M.R., Rostásy K., Saiz A., Venkatesan A., Vincent A., Wandinger K.P., Waters P., Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016 Apr;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J., Graus F. Antibody-mediated encephalitis. N. Engl. J. Med. 2018 Mar 1;378(9):840–851. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- 3.Abboud H., Probasco J.C., Irani S., Ances B., Benavides D.R., Bradshaw M., Christo P.P., Dale R.C., Fernandez-Fournier M., Flanagan E.P., Gadoth A., George P., Grebenciucova E., Jammoul A., Lee S.T., Li Y., Matiello M., Morse A.M., Rae-Grant A., Rojas G., Rossman I., Schmitt S., Venkatesan A., Vernino S., Pittock S.J., Titulaer M.J. Autoimmune Encephalitis Alliance Clinicians Network. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J. Neurol. Neurosurg. Psychiatry. 2021 Jul;92(7):757–768. doi: 10.1136/jnnp-2020-325300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hara M., Ariño H., Petit-Pedrol M., Sabater L., Titulaer M.J., Martinez-Hernandez E., Schreurs M.W., Rosenfeld M.R., Graus F., Dalmau J. DPPX antibody-associated encephalitis: main syndrome and antibody effects. Neurology. 2017 Apr 4;88(14):1340–1348. doi: 10.1212/WNL.0000000000003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piepgras J., Höltje M., Michel K., Li Q., Otto C., Drenckhahn C., Probst C., Schemann M., Jarius S., Stöcker W., Balint B., Meinck H.M., Buchert R., Dalmau J., Ahnert-Hilger G., Ruprecht K. Anti-DPPX encephalitis: pathogenic effects of antibodies on gut and brain neurons. Neurology. 2015 Sep 8;85(10):890–897. doi: 10.1212/WNL.0000000000001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bien C.G., Holtkamp M. "Autoimmune epilepsy": encephalitis with autoantibodies for epileptologists. Epilepsy Curr. 2017 May-Jun;17(3):134–141. doi: 10.5698/1535-7511.17.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijntjes J., Bechakra M., Schreurs M.W.J., Jongen J.L.M., Koppenaal A., Titulaer M.J. Pruritus in anti-DPPX encephalitis. Neurol Neuroimmunol Neuroinflamm. 2018 Apr 2;5(3):e455. doi: 10.1212/NXI.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deuel L.M., Yu C.H., Vaughan C.L., Piquet A.L. Oro-bucco-lingual dyskinesia, weight loss, and cognitive decline in anti-DPPX antibody-mediated encephalitis. Mov. Disord. Clin. Pract. 2020 Sep 29;7(Suppl 3):S80–S82. doi: 10.1002/mdc3.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadoth A., Devine M.F., Pittock S.J., McKeon A., Tobin W.O., Gossard T.R., Cattaneo E.F.D., McCarter S.J., St Louis E.K., Sleep disturbances associated with DPPX autoantibodies: a case series, J. Neurol. 270(7) (2023 Apr 7) 3543-3552. [DOI] [PubMed]
- 10.Laurido‐Soto O., Brier M.R., Simon L.E., etal Patient characteristics and outcome associations in AMPA receptor encephalitis[J] J. Neurol. 2019;266(2):450–460. doi: 10.1007/s00415-018-9153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanous I., Dillon P. Paraneoplastic neurological complications of breast cancer. Exp. Hematol. Oncol. 2016 Oct 24;5:29. doi: 10.1186/s40164-016-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hekmatnia Y., Movahednia N., Hajhamidiasl N., Hekmat E., Hekmat A., Khademi S. A review of the neurological complications of breast cancer. J. Fam. Med. Prim. Care. 2022 Aug;11(8):4205–4214. doi: 10.4103/jfmpc.jfmpc_580_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemminki K., Liu X., Ji J., Försti A., Sundquist J., Sundquist K. Effect of autoimmune diseases on risk and survival in female cancers. Gynecol. Oncol. 2012 Oct;127(1):180–185. doi: 10.1016/j.ygyno.2012.07.100. [DOI] [PubMed] [Google Scholar]
- 14.Nayak P., Luo R., Elting L., Zhao H., Suarez-Almazor M.E. Impact of rheumatoid arthritis on the mortality of elderly patients who develop cancer: a population-based study. Arthritis Care Res. 2017 Jan;69(1):75–83. doi: 10.1002/acr.22997. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y., Ma L. Investigation of the causal relationship between breast cancer and autoimmune diseases: a bidirectional mendelian randomization study. Medicine (Baltim.) 2023 Aug 25;102(34) doi: 10.1097/MD.0000000000034612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z., Liu H., Yang Y., Zhou J., Zhao L., Chen H., Fei Y., Zhang W., Li M., Zhao Y., Zeng X., Zhang F., Yang H., Zhang X. The five major autoimmune diseases increase the risk of cancer: epidemiological data from a large-scale cohort study in China. Cancer Commun. 2022 May;42(5):435–446. doi: 10.1002/cac2.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schairer C., Pfeiffer R.M., Gadalla S.M. Autoimmune diseases and breast cancer risk by tumor hormone-receptor status among elderly women. Int. J. Cancer. 2018 Mar 15;142(6):1202–1208. doi: 10.1002/ijc.31148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W., Wang R., Wang W. Exploring the causality and pathogenesis of systemic lupus erythematosus in breast cancer based on Mendelian randomization and transcriptome data analyses. Front. Immunol. 2023 Jan 16;13 doi: 10.3389/fimmu.2022.1029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dedousis D., Zhang A.L., Vassiliou A.N., Cao S., Yammani D., Kyasaram R.K., Shanahan J.P., Keinath M.C., Hsu M.L., Fu P., Dowlati A., Montero A.J. Survival in elderly patients with breast cancer with and without autoimmune disease. Cancer Med. 2023 Jun;12(12):13086–13099. doi: 10.1002/cam4.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Titulaer M.J., Mccracken L., Gabilondo I., et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study[J] Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews E., Schmitt B., Passeri M., Mizenko C., Orjuela K., Piquet A. AMPA receptor encephalitis in a patient with metastatic breast cancer receiving palbociclib: a case report. Neurol Neuroimmunol Neuroinflamm. 2022 Jul 6;9(5) doi: 10.1212/NXI.0000000000200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho Neto E.G., Gomes M.F., Alves R.P.M., Santos A.P.D., Brum C., Santin R. Anti-Ri autoimmune encephalitis associated with breast cancer. Arq. Neuropsiquiatr. 2020 Nov;78(11):737. doi: 10.1590/0004-282X20200091. [DOI] [PubMed] [Google Scholar]