Abstract
Sepsis-induced myocardial dysfunction (SIMD) has become one of the most lethal complications of sepsis, while the treatment was limited by a shortage of pertinent drugs. Epigallocatechin-3-gallate (EGCG) is the highest content of active substances in green tea, and its application in cardiovascular diseases has broad prospects. This study was conducted to test the hypothesis that EGCG was able to inhibit lipopolysaccharide (LPS) induced myocardial dysfunction and investigate the underlying molecular mechanisms. The cardiac systolic function was assessed by echocardiography. The cardiomyocyte apoptosis was determined by TUNEL staining. The expression of inflammatory factors and apoptosis-related protein, cardiac markers were examined by Western Blot and qRT-PCR. EGCG effectively improve LPS-induced cardiac function damage, enhance left ventricular systolic function, and restore myocardial cell vitality. It can effectively inhibit the upregulation of TLR4 expression induced by LPS and inhibit IκB α/NF- κB/p65 signaling pathway, thereby inhibiting cardiomyocyte apoptosis and improving myocarditis. In conclusion, EGCG protects against SIMD through anti-inflammatory and anti-apoptosis effects; it was mediated by the inhibition of the TLR4/NF-κB signal pathway. Our results demonstrated that EGCG might be a possible medicine for SIMD prevention and treatment.
Keywords: Apoptosis, Epigallocatechin-3-gallate, Inflammation, Myocardial dysfunction, Lipopolysaccharide
1. Introduction
Sepsis is a clinical syndrome defined by a systemic response to infection. It is a systemic inflammatory response syndrome (SIRS), which is caused by the imbalance of host response to infection and can evolve into multiple organ dysfunction [1]. For clinical operationalization, organ dysfunction can be represented by an increase in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score of 2 points or more, which is associated with an in-hospital mortality greater than 10% [2]. Despite the incidence and mortality of sepsis have declined a lot worldwide since 1990, it remains a major cause of global deaths and brings serious burden in areas with poor sanitation conditions [3]. Furthermore, clinical trials of therapeutics have failed to obtain promising results [4].
Sepsis-induced myocardial dysfunction (SIMD) is the main cause of mortality in hospitalized patients for the lack of consistent diagnostic criteria and effective therapeutics. Patients usually manifested ventricular dilation, reduced ventricular contractility, and bilateral ventricular dysfunction with their response to volume infusion reduced [5]. The potential pathophysiology of SIMD consists of various pathways, including the release of circulating myocardial depressant substances, the release of nitric oxide and reactive oxygen species, the downregulation of adrenergic pathways, mitochondrial dysfunction, abnormalities in calcium handling, coronary microvascular perturbation, and the downregulation of genes encoding sarcomeric and mitochondrial proteins [6]. Myocardial dysfunction is associated with poor prognosis for people with sepsis and results in mortality [7]. The measurement of cardiac biomarkers can reflect systolic or diastolic myocardial dysfunction, but whether it is related to inflammatory cytokines remains controversial [8,9].
The toll-like receptors 4 (TLR4) is a transmembrane protein located on the surface of the cell membrane, which participates in a variety of immune and inflammatory reactions. After lipopolysaccharide (LPS) stimulation, the activation of TLR4 in the myocardium was upregulated, and the serum protein levels of tumor necrosis factor-α (TNF-α) and cardiac troponin T (cTnT) were significantly increased, with myocardial histopathological changes and cardiac function inhibited [10]. The silence of TLR4 suppressed inflammation and apoptosis to alleviate LPS-induced damage through TLR4/MyD88/nuclear factor-kappa B (NF-κB) signal pathway [11]. NF-κB is activated by a variety of etiologies known to cause sepsis. Whether in animal models of septic shock or in human subjects with sepsis, NF-κB activity increased markedly in each organ studied. With the rising level of NF- κB activity, the mortality increased and the clinical outcome got worse [12]. Potential strategies for the treatment of SIMD may have the capability of reducing inflammatory reactions and inhibiting the TLR4/NF-κB signal pathway.
The utilization of green tea can date back to 3000 years ago, and it has been one of the most popular beverages around the world. The role and action of tea plants have been changed for clinical translation, with more well-designed trials to develop drugs and avoid toxicity [13]. Previous studies have suggested that green tea has the potential to prevent the development of a variety of diseases, including diabetes, hypertension, cardiovascular diseases, and cancer [13,14]. Our recent studies showed that moderate consumption of green tea could reduce the risk of coronary heart disease and stroke [15,16]. The active components of green tea are composed mainly of its most abundant tea polyphenols, epigallocatechin-3-gallate (EGCG), which has the effects of anti-inflammatory, antioxidant, antifibrotic, anti-remodelation, and tissue-protective properties [17]. Recent study by Li et al. [18] suggested that EGCG possesses cardiomyocyte-protective action in reducing the LPS-induced inflammatory response due to the inhibition of the phosphorylation of Akt and ERK signaling molecules. However, to the best of our knowledge, few studies have investigated the effect of EGCG on SIMD. Therefore, the aim of the present study was to use both molecular biology and pathophysiology approaches in vivo and in vitro for further investigation on the protection mechanism of EGCG and provide latent therapeutic targets for SIMD.
2. Materials and methods
2.1. Animals
Male C57BL/6 mice were purchased from the Experimental Animal Center of Nanjing Medical University (Nanjing, China). They were randomly classified into four groups (n = 6 each): (1) Control group; (2) LPS group; (3) EGCG group; and (4) LPS + EGCG group. The control group was injected with 0.9% saline + sodium carboxymethylcellulose. EGCG group and LPS + EGCG group were injected intraperitoneally with 200 mg/kg EGCG (Sigma-Aldrich, St. Louis, USA, Cat. NO. M5250). Five days later, to establish the animal model of sepsis-induced cardiac dysfunction, the LPS group and LPS + EGCG group were injected through tail vein with 15 mg/kg LPS (Sigma-Aldrich, St. Louis, USA, Cat. NO. L2880). The cardiac function was measured 24 h after LPS treatment. And then the myocardium was harvested for further examination.
2.2. Echocardiography
The cardiac systolic function was assessed by echocardiography 24 h after LPS administration. Mice were anesthetized with Isoflurane (1.5–2%) and then placed in recumbent figure. Echocardiographic images were recorded using a Vevo 2100 instrument (Visual Sonics Inc, Toronto, Ontario, Canada) equipped with a 30 MHz central frequency scan head. The left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF) were calculated to assess cardiac function.
2.3. Histological analysis
24 h after LPS administration, myocardial tissues of various groups were collected and immediately fixed with 4% paraformaldehyde at room temperature for 48 h. The samples were then embedded with paraffin, sectioned transversely into 4 μm thickness and deparaffinized with xylene. Finally, the slides were stained with hematoxylin and eosin and viewed by a light microscope at 400 × magnification for histological analysis. The cardiomyocyte cross-sectional areas were assessed by measuring the circumferential length of the cardiomyocyte using Image J software.
2.4. Cell culture and treatment
H9c2 cells purchased from Procell Life Science&Technology were cultured in a DMEM medium (Gibco, USA) that contained 10% fetal bovine serum (Gibco, USA) with double antibodies (penicillin 100U/ml and streptomycin 100 mg/ml). The cells were cultured in a 5% CO2 and 37 °C constant-temperature incubator. When the cells covered about 90% of the extent of the Petri dish, they were digested and passaged with 0.25% trypsin (Gibco, USA). The cells were divided into 4 groups: (1) Control group; (2) LPS group (1 μg/mL, 12 h); (3) EGCG group (25 μM, 1 h); and (4) LPS + EGCG group (25 μM EGCG pretreatment for 1 h, followed by LPS treatment for 12 h).
2.5. Cytotoxicity assay
To detect the cytotoxicity of EGCG (0, 10, 25, 50, 75 and 100 μM) and LPS (0, 0.25, 0.5, 1.0, 1.5 and 2.0 μg/ml), after treating H9c2 cells with EGCG or LPS at different concentrations for 24 h, add the detection reagent according to the cell counting kit-8 (CCK-8) assay instructions, and measure the absorbance value at 450 nm with an enzyme marker to calculate the cell activity.
2.6. TUNEL staining
The cardiomyocyte apoptosis was determined by TUNEL staining according to the manufacturer's instructions. Briefly, Paraformaldehyde (4%) and Triton X-100 (0.5%) were used to fix and permeabilize cells, respectively, for 20 min. After three times of washing with PBS. Then, TUNEL staining was performed using a TUNEL Apoptosis Detection Kit according to the manufacturer's instructions (Yeasen, Shanghai, China). Adding TdT Enzyme and incubated at 37 °C for 60 min in the wet box. After rinsed with PBS for four times, the slides were incubated with DAPI at room temperature for 10 min to detect the nuclei in the darkness. The formula for calculating the percentage of apoptotic nuclei was the total number of nuclei divided by the total number of TUNEL-positive nuclei.
2.7. 7.Quantitative reverse transcription polymerase chain reaction assay (qRT-PCR)
Total RNAs were extracted using Trizol reagent (Life Technologies, USA) according to the manufacturer's instructions. The extracted RNAs were transcribed into cDNA by using iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Real‐time polymerase chain reaction was carried out by using SYBR Green qPCR Master Mix (Bio-Rad). Relative fold changes of interleukin-1β (IL‐1β), IL‐6, TNF‐α, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and cTnT were normalized to β‐actin and calculated on the basis of the 2−ΔΔCt method.
3. Western blot analysis
Briefy, cells were washed with PBS and lysed with lysis buffer on ice for 30 min. The total cell protein concentration was detected using the BCA Protein Assay Kit (Thermo Fisher, Waltham, MA, USA). The total protein (20 μg) was separated using SDS-PAGE (Invitrogen) and transferred to a PVDF membrane (Roche). The membrane was blocked with 5% bovine serum albumin (0.1%) in TBS-Tween and incubated against the required antibody. The primary antibodies Bax (1:1000;Cell Signaling Technolog, USA), Bcl-2 (1:1000;Cell Signaling Technolog, USA), β-actin (1:10000; Proteintech Group, Wuhan, China), IL-6 (1:1000; Proteintech Group, Wuhan, China), IL-1β (1:1000; Proteintech Group, Wuhan, China), TNF-α (1:1000; Proteintech Group, Wuhan, China), TLR4 (1:1000; Cell Signaling Technolog, USA), pP65 (1:1000; Abcam, USA), P65 (1:1000; Proteintech, shanghai, China), p-IκBα (1:1000; Abcam, USA), IκBα (1:1000; Abmart, Shanghai, China) and secondary antibody (Santa Cruz) were used. Bands were visualized using an ECL Chemiluminescence Kit (Thermo Fisher) and analyzed using ImageJ software (National Institutes of Health).
3.1. Statistical analysis
All data were analyzed with GraphPad Prism 8 software (GraphPad Software, CA) and expressed as mean ± SD. Comparisons among multiple groups were performed by one-way ANOVA followed by Bonferroni's correction. P < 0.05 was considered statistically significant. All experimental procedures were approved by the ethical animal committees of Nanjing Medical University and were performed in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals.
4. Results
4.1. Effect of EGCG and LPS on the cell viability of H9c2 cells
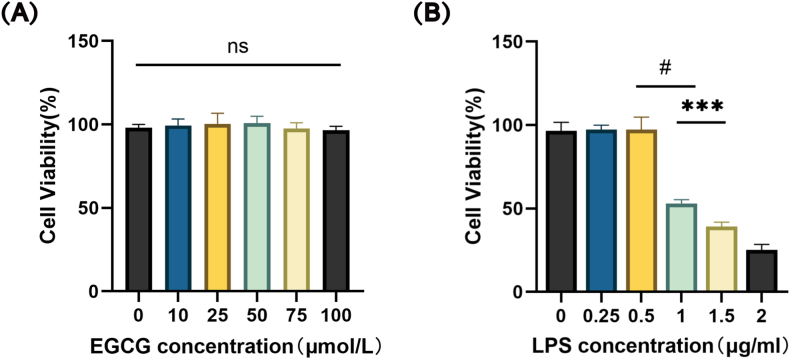
We performed a CCK-8 assay to assess the cell viability. As shown in Fig. 1A, EGCG of all concentrations did not display any cellular toxicity against the H9c2 cells; we chose 25 μM EGCG for subsequent experiments. The viability of H9c2 cells markedly declined with the LPS at concentrations over 1 μg/mL (Fig. 1B). Therefore, we chose a concentration of 1 μg/mL LPS stimulation in the following studies.
Fig. 1.
Effects of EGCG and LPS on the viability of H9c2 cells. H9c2 cells were treated with different concentrations of EGCG (0, 10, 25, 50, 75 and 100 μM) (A) and LPS (0, 0.25, 0.5, 1.0, 1.5 and 2.0 μg/ml) (B) for 24 h. Cell viability was measured using CCK-8, n = 5. Data represent the mean ± S.D. of five independent experiments. Statistical analysis was performed with one-way ANOVA followed by Bonferroni's correction. #P < 0.0001 vs. LPS 0.5 μg/ml; ***P < 0.001 vs. LPS 1.5 μg/ml.
4.2. EGCG reduces cardiomyocyte injury and inflammatory cell infiltration in vivo
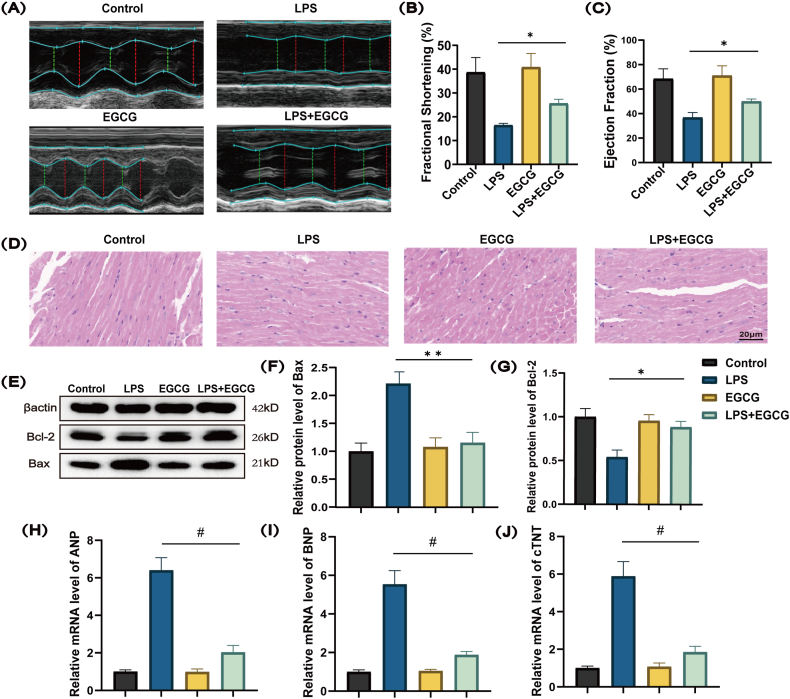
To assess the effect of EGCG on myocardial dysfunction in mice with LPS-induced sepsis, we performed echocardiography and pathological H & E staining. The echocardiography results suggested that pretreatment of EGCG significantly increased the LVFS and LVEF (Fig. 2A, B and 2C). The results of H & E staining showed that cardiomyocytes were swollen and enlarged in the LPS group. The pretreatment of EGCG brought smaller cardiomyocytes and less amounts of infiltrated lymphocytes (Fig. 2D). These results indicated that EGCG improved left ventricular systolic function and alleviated cardiomyocytes swelling induced by the infiltration of lymphocytes, as well as myocardial fibre fracture. Western blot analysis showed that pretreatment with EGCG ameliorated LPS induced apoptosis by upregulating the expression of anti-apoptotic Bcl-2 protein and down-regulating the expression of pro-apoptotic Bax protein in the myocardial tissues (Fig. 2E, F and 2G; Supplementary Fig. 1). It was also found that the intervention of EGCG could significantly decrease the level of cardiac injury markers such as ANP, BNP, and cTnT (Fig. 2H, I and 2J).
Fig. 2.
Effects of EGCG on LPS induced cardiac dysfunction and myocardial injury in mice.
Male C57BL/6 mice (n = 24) were randomly classified into four groups. Male C57BL/6 mice (n = 6) were pretreated with EGCG (200 mg/kg/d) for 5 days, then stimulated with LPS (15 mg/kg) for 24 h. (A) Representative echocardiographic images showed EGCG improves the inhibitory effect of LPS on heart function. (B, C) EGCG improves the inhibitory effect of LPS on LVFS and LVEF. (D) EGCG improves LPS induced myocardial tissue damage assessed by H & E staining. (E, F and G) Western blotting analysis was carried out to evaluate the levels of pro-apoptotic Bax protein and anti-apoptotic Bcl-2 protein in the myocardial tissues (Supplementary Fig. 1). (H, I and J) RT-qPCR analysis of BNP, ANP and cTNT mRNA expression in the myocardial tissues. Data represent the mean ± S.D. of three independent experiments. Statistical analysis was performed with one-way ANOVA followed by Bonferroni's correction. *P < 0.05, **P < 0.01, #P < 0.0001.
4.3. EGCG attenuates LPS‐induced cardiomyocytes inflammation
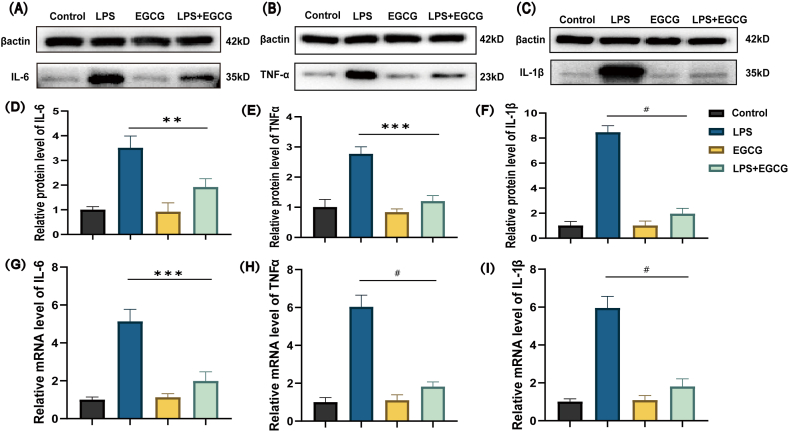
To detect the anti-inflammation effects of EGCG on the LPS‐induced H9c2 cells, the cells were treated with LPS alone or in combination with EGCG. The results in Fig. 3 (A - I) revealed that proinflammatory cytokines IL-6, TNF-α and IL-1β were highly expressed in LPS‐treated cells compared with the control group and the EGCG group, while the level of cytokines mRNA and protein were much lower in the EGCG + LPS group (Supplementary Fig. 2). These results suggested that EGCG alleviated inflammatory response in the LPS‐induced H9c2 cells.
Fig. 3.
EGCG down-regulated the expression of proinflammatory cytokine induced by LPS.
H9c2 were pretreated with EGCG (25 μM) for 1 h, then stimulated with LPS (1 μg/ml) for 12 h (A, B, C, D, E and F) Western blotting analysis was carried out to evaluate the levels of IL-6, TNF-α and IL-1β, n = 3 (Supplementary Fig. 2). (G, H and I) RT-qPCR analysis was carried out to evaluate the mRNA expression of IL-6, TNF-α and IL-1β, n = 6. Data represent the mean ± S.D. of three independent experiments. Statistical analysis was performed with one-way ANOVA followed by Bonferroni's correction. **P < 0.01, ***P < 0.001, #P < 0.0001.
4.4. EGCG suppresses LPS-induced cardiomyocytes apoptosis
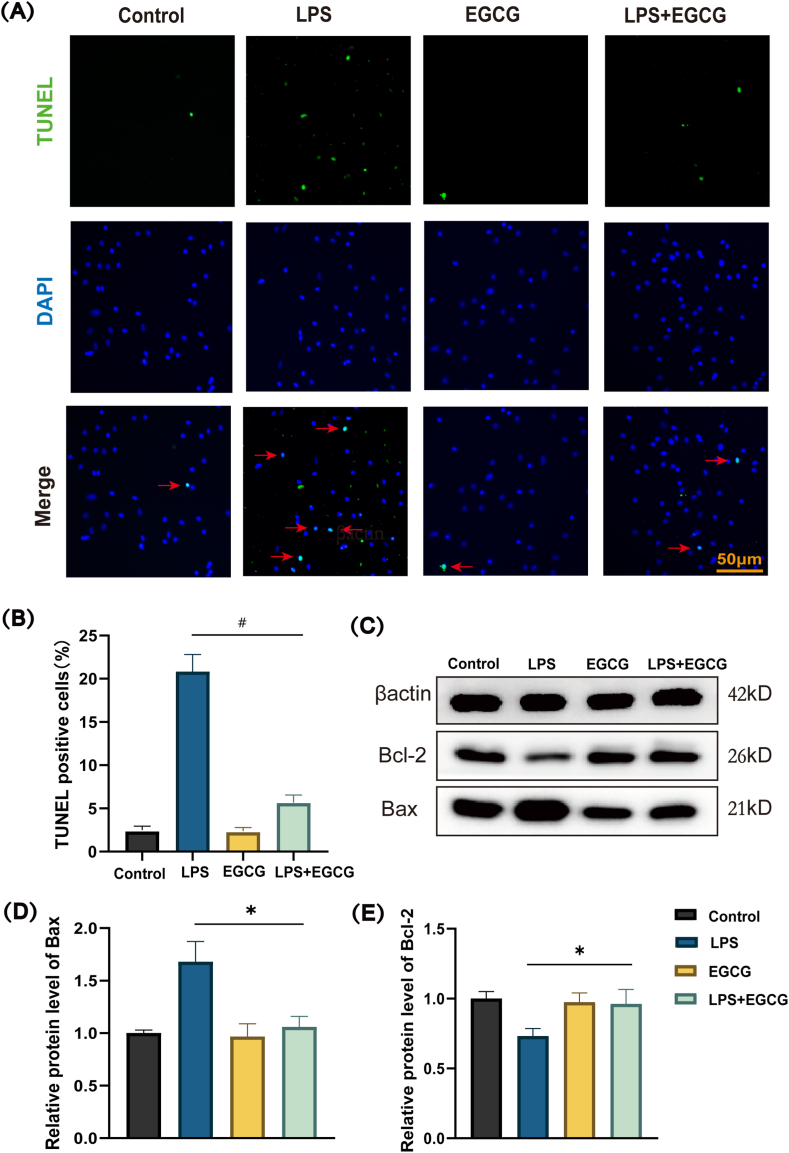
Sustained inflammation has been proved to be one of the influence factors that can contribute to the apoptosis of cardiomyocytes. As shown in Fig. 4A a prominent increased percentage of TUNEL-positive nuclei was observed in the LPS-treatment group after 12 h, whereas EGCG treatment significantly reduced the percentage of TUNEL-positive cells. The cell counting results showed that the number of TUNEL positive nuclei in LPS group was over 20%, while that in the control group and the EGCG group was less than 2% (Fig. 4B). The change of apoptosis related protein Bcl-2 and Bax suggested that treatment with LPS for 12 h sharply upregulated the expression of Bax but downregulated the expression of Bcl-2. Nevertheless, pretreatment with EGCG increased the expression of anti-apoptotic Bcl-2 protein and decreased the expression of pro-apoptotic Bax protein, which means that EGCG could significantly inhibit the apoptosis of H9c2 cells induced by LPS stimulation (Fig. 4C, D and 4E; Supplementary Fig. 3).
Fig. 4.
Effect of EGCG on LPS-induced apoptosis generation in H9c2.
H9c2 were pretreated with EGCG (25 μM) for 1 h, then stimulated with LPS (1 μg/ml) for 12 h. (A) TUNEL analysis for H9c2 cells. Green, TUNEL-positive nuclei; blue, DAPI-stained nuclei. Scale bars = 50 μm, n = 3. (B) Quantitative results of TUNEL positive cells. (C, D and E) Western blotting analysis was carried out to evaluate the levels of pro-apoptotic Bax protein and anti-apoptotic Bcl-2 protein, n = 3 (Supplementary Fig. 3). Data represent the mean ± S.D. of three independent experiments. Statistical analysis was performed with one-way ANOVA followed by Bonferroni's correction. *P < 0.05, #P < 0.0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
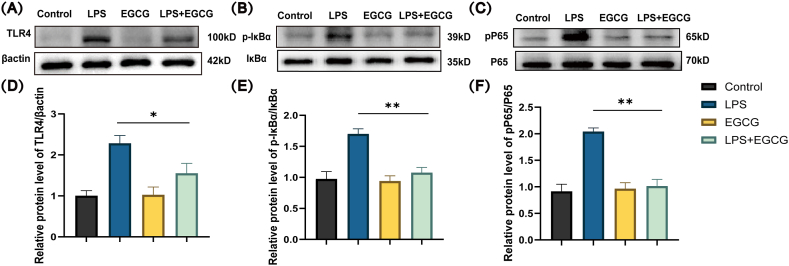
4.5. Effects of EGCG on TLR4/NF-κB signaling pathway in LPS-induced cardiomyocytes
Western blotting was used to detect the expression of TLR4/NF-κB signaling pathway-related proteins. As shown in Fig. 5 (A - F), the expression levels of TLR4, pP65/P65, and p-IκBα/IκBα in the LPS group was upregulated, which suggested the activation of the TLR4/NF-κB signaling pathway. With EGCG treatment beforehand, the expression levels of the pathway-related protein were markedly decreased (Supplementary Fig. 4). Thus, EGCG possibly inhibited the activation of NF-κB signal pathway in H9c2 cells.
Fig. 5.
Effect of EGCG on LPS-induced TLR4/NF-κB activation in H9c2 cells.
H9c2 were pretreated with EGCG (25 μM) for 1 h, then stimulated with LPS (1 μg/ml) for 12 h. Western blotting analysis was carried out to evaluate the levels of TLR4, β-actin (A, D), phosphorylated IκBα, total IκBα (B, E), phosphorylated P65, and total P65 (C, F), n = 3 (Supplementary Fig. 4). Data represent the mean ± S.D. of three independent experiments. Statistical analysis was performed with one-way ANOVA followed by Bonferroni's correction. *P < 0.05, **P < 0.01.
5. Discussion
Sepsis is defined as life-threatening organ dysfunction caused by the dysregulated host response to infection. Myocardial dysfunction induced by sepsis is gradually considered as a potential complication of septic shock, and it is characterized as reversible left ventricular systolic dysfunction. In previous studies, the incidence rate of SIMD varies from 18% to 40% in septic shock patients, while the presence of SIMD significantly increases the mortality of patients to 70%–90% [19]. The pathogenesis of SIMD is ambitious, depending on the existing evidence, it can be roughly considered as the result of the interaction of multiple factors including inflammation, metabolism and neuroimmune regulation [20], and the pathophysiology can be summarized as direct myocardial depression, mitochondrial dysfunction and impaired myocardial circulation [21]. In spite of serious mortality and incidence rate, no specific drugs have been developed for sepsis. The endoxemia model is often established to simulate the hyperinflammatory state similar to early sepsis. This model has been used in many published articles; it classically uses LPS to activate the immune system, triggering high inflammation, microcirculation disorders, and death [[22], [23], [24]].
EGCG is the principal constituent of biologically active polyphenols in green tea. It can interact with cell surface receptors, intracellular signal pathways, and nuclear transcription factors. EGCG has a variable effect of anti-inflammatory, antioxidant, anti-fibrosis, anti-remodeling and tissue protection, and possesses the potential to treat cancer, nerve, cardiovascular, respiratory, and metabolic disorders [17]. We have investigated the relevant mechanisms of EGCG in preventing and treating cardiovascular diseases for several years. Our previous studies demonstrated that EGCG promotes atherosclerotic plaque stability by decreasing the expression of plaque instability-mediating cytokines [25,26].
A recent study using network pharmacology reveals that EGCG may lessen myocardial damage in septic cardiomyopathy by reducing the expression of six target proteins (IL-6, TNF-α, Caspase3, Mitogen-activated protein kinase 3, AKT1, and vascular endothelial growth factor) [27]. Another study reported that EGCG possesses cardiomyocyte-protective action in reducing the LPS-induced inflammatory response due to the inhibition of the phosphorylation of Akt and ERK signaling molecules in vitro [18]. However, the role of EGCG in protecting against SIMD remains ambitious and lacks of experimental validation. In the present study, EGCG was demonstrated to exert a protective effect in septic cardiomyopathy rats possibly by anti-inflammatory effects via inhibition of the TLR4/NF-κB signal pathway. It was also found that the intervention of EGCG could significantly decrease the level of cardiac injury markers such as ANP, BNP, and cTnT. In addition, the results of HE staining and echocardiography could reflect the mitigation of myocardial injury in vivo. Furthermore, our tunnel staining and western-blot results provide evidence that pretreatment of EGCG significantly reduces LPS-induced cardiomyocytes apoptosis. Thus, the current research added value to previous studies.
NF-κB has long been considered a prototypical proinflammatory signaling pathway [28]. To understand the potential mechanisms for EGCG, we confirmed in H9c2 cells that a predominant signal transduction pathway by which LPS-induced cardiomyocytes is through the inhibition of NF-κB activation. In the present study, Western blot analytical results showed that EGCG significantly suppressed LPS-induced phosphorylated-P65. In addition, we found that EGCG inhibited LPS-induced phosphorylation of IκBα. Our previous study has shown that EGCG significantly inhibits NF-κB transcriptional activity in human umbilical vein endothelial cells (HUVECs) [29]. Also we have reported that EGCG can regulate the production of inflammatory cytokines via inhibiting the activation of NF-κB pathway in LPS-induced macrophages [25]. These are compatible with our present study suggesting one mechanism of action for EGCG on H9c2 cells is the inhibition of the NF-κB pathway following LPS stimulation.
Previous studies have shown that in the early stage of sepsis, the upstream of the NF-κB signal pathway, TLR4 is activated under the stimulation of inflammation and endotoxins [20]. Activation of TLR4 during sepsis promotes nuclear NF-κB translocation, which accelerates the release of septic myocardial injury-related pro-inflammatory cytokines [22,30]. In our study, EGCG not only reduced the upregulation of TLR4, but also alleviated the increases of the protein and mRNA expression of IL-6, TNF-a and IL-1β in LPS-induced H9c2 cells. These inflammatory factors play key roles in the pathophysiology of SIMD [30]. Therefore, it is speculated that the inhibition of TLR4/NF-κB signal pathway from EGCG may be a key point to reducing myocardial injury.
Taken together, the present data demonstrate that EGCG could suppress SIMD through anti-inflammatory and anti-apoptosis effects via inhibition of the TLR4/NF-κB signal pathway. Our results suggest that EGCG, a major polyphenol of green tea, might be a possible medicine for SIMD prevention and treatment.
Funding sources
This work was supported by grants from the National Natural Science Foundation of China (No 81703213), the Natural Science Youth Foundation of Jiangsu Province of China (No. BK20151034) to Dr. Z.-M. Wang.
Animal research ethical approval
The use of laboratory animals in this study was approved by the Animal Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (IACUC-1601038-4).
Data availability statement
Data included in article/supp. material/referenced in article.
CRediT authorship contribution statement
Bei Chen: Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ya-Fei Li: Visualization, Validation, Resources, Methodology, Funding acquisition, Conceptualization. Zhang Fang: Software, Methodology, Formal analysis, Data curation. Wen-Yi Cai: Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. Zhi-Qiang Tian: Validation, Methodology, Investigation, Formal analysis, Data curation. Dianfu Li: Writing – review & editing, Supervision, Software, Resources, Formal analysis. Ze-Mu Wang: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27163.
Contributor Information
Dianfu Li, Email: doctorldf@163.com.
Ze-Mu Wang, Email: zemu.wang@njmu.edu.cn.
Appendix B. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Potz B.A., Sellke F.W., Abid M.R. Endothelial ROS and impaired myocardial oxygen consumption in sepsis-induced cardiac dysfunction. J Intensive Crit Care. 2016;2(1):20. doi: 10.21767/2471-8505.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.L., Angus D.C. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., Fleischmann-Struzek C., Machado F.R., Reinhart K.K., Rowan K., Seymour C.W., Watson R.S., West T.E., Marinho F., Hay S.I., Lozano R., Lopez A.D., Angus D.C., Murray C.J.L., Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y.Y., Ning B.T. Signaling pathways and intervention therapies in sepsis. Signal Transduct. Targeted Ther. 2021;6(1):407. doi: 10.1038/s41392-021-00816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin L., Derwall M., Al Zoubi S., Zechendorf E., Reuter D.A., Thiemermann C., Schuerholz T. The septic heart: current understanding of molecular mechanisms and clinical implications. Chest. 2019;155(2):427–437. doi: 10.1016/j.chest.2018.08.1037. [DOI] [PubMed] [Google Scholar]
- 6.Hollenberg S.M., Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 2021;18(6):424–434. doi: 10.1038/s41569-020-00492-2. [DOI] [PubMed] [Google Scholar]
- 7.Merx M.W., Weber C. Sepsis and the heart. Circulation. 2007;116(7):793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 8.Hobai I.A., Edgecomb J., LaBarge K., Colucci W.S. Dysregulation of intracellular calcium transporters in animal models of sepsis-induced cardiomyopathy. Shock. 2015;43(1):3–15. doi: 10.1097/SHK.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landesberg G., Levin P.D., Gilon D., Goodman S., Georgieva M., Weissman C., Jaffe A.S., Sprung C.L., Barak V. Myocardial dysfunction in severe sepsis and septic shock: No correlation with inflammatory cytokines in real-life clinical setting. Chest. 2015;148(1):93–102. doi: 10.1378/chest.14-2259. [DOI] [PubMed] [Google Scholar]
- 10.Chang C., Hu L., Sun S., Song Y., Liu S., Wang J., Li P. Regulatory role of the TLR4/JNK signaling pathway in sepsis-induced myocardial dysfunction. Mol. Med. Rep. 2021;23(5):334. doi: 10.3892/mmr.2021.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S.N., Tan Y., Xiao X.C., Li Q., Wu Q., Peng Y.Y., Ren J., Dong M.L. Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol. Sin. 2021;42(10):1610–1619. doi: 10.1038/s41401-020-00597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S.F., Malik A.B. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290(4):L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 13.Brimson J.M., Prasanth M.I., Kumaree K.K., Thitilertdecha P., Malar D.S., Tencomnao T., Prasansuklab A. Tea plant (Camellia sinensis): a current update on use in diabetes, obesity, and cardiovascular disease. Nutrients. 2022;15(1):37. doi: 10.3390/nu15010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farhan M. Green tea Catechins: nature's way of preventing and treating cancer. Int. J. Mol. Sci. 2022;23(18) doi: 10.3390/ijms231810713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z.M., Zhao D., Wang H., Wang Q.M., Zhou B., Wang L.S. Green tea consumption and the risk of coronary heart disease: a systematic review and meta-analysis of cohort studies. Nutr. Metabol. Cardiovasc. Dis. 2023;33(4):715–723. doi: 10.1016/j.numecd.2023.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z.M., Chen B., Zhou B., Zhao D., Wang L.S. Green tea consumption and the risk of stroke: a systematic review and meta-analysis of cohort studies. Nutrition. 2023;107 doi: 10.1016/j.nut.2022.111936. [DOI] [PubMed] [Google Scholar]
- 17.Mokra D., Joskova M., Mokry J. Therapeutic effects of green tea polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. Int. J. Mol. Sci. 2022;24(1):340. doi: 10.3390/ijms24010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z.H., Shi Z., Tang S., Yao H.P., Lin X., Wu F. Epigallocatechin-3-gallate ameliorates LPS-induced inflammation by inhibiting the phosphorylation of Akt and ERK signaling molecules in rat H9c2 cells. Exp. Ther. Med. 2020;20(2):1621–1629. doi: 10.3892/etm.2020.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravikumar N., Sayed M.A., Poonsuph C.J., Sehgal R., Shirke M.M., Harky A. Septic cardiomyopathy: from basics to management choices. Curr. Probl. Cardiol. 2021;46(4) doi: 10.1016/j.cpcardiol.2020.100767. [DOI] [PubMed] [Google Scholar]
- 20.Lv X., Wang H. Pathophysiology of sepsis-induced myocardial dysfunction. Mil Med Res. 2016;3:30. doi: 10.1186/s40779-016-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habimana R., Choi I., Cho H.J., Kim D., Lee K., Jeong I. Sepsis-induced cardiac dysfunction: a review of pathophysiology. Acute Crit Care. 2020;35(2):57–66. doi: 10.4266/acc.2020.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H., Liu Q., Liu X., Jin J. Berberine attenuates septic cardiomyopathy by inhibiting TLR4/NF-κB signalling in rats. Pharm. Biol. 2021;59(1):121–128. doi: 10.1080/13880209.2021.1877736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo M., Yan D., Sun Q., Tao J., Xu L., Sun H., Zhao H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J. Cell. Biochem. 2020;121(4):2994–3004. doi: 10.1002/jcb.29556. [DOI] [PubMed] [Google Scholar]
- 24.Dickson K., Lehmann C. Inflammatory response to different toxins in experimental sepsis models. Int. J. Mol. Sci. 2019;20(18):4341. doi: 10.3390/ijms20184341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y.F., Wang H., Fan Y., Shi H.J., Wang Q.M., Chen B.R., Khurwolah M.R., Long Q.Q., Wang S.B., Wang Z.M., Wang L.S. Epigallocatechin-3-Gallate inhibits matrix metalloproteinase-9 and monocyte chemotactic protein-1 expression through the 67-κDa Laminin receptor and the TLR4/MAPK/NF-κB signalling pathway in lipopolysaccharide-induced macrophages. Cell. Physiol. Biochem. 2017;43(3):926–936. doi: 10.1159/000481643. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q., Zhang J., Li Y., Shi H., Wang H., Chen B., Wang F., Wang Z., Yang Z., Wang L. Green tea polyphenol epigallocatechin-3-gallate increases atherosclerotic plaque stability in apolipoprotein E-deficient mice fed a high-fat diet. Kardiol. Pol. 2018;76(8):1263–1270. doi: 10.5603/KP.a2018.0114. [DOI] [PubMed] [Google Scholar]
- 27.Wu J., Wang Z., Xu S., Fu Y., Gao Y., Wu Z., Yu Y., Yuan Y., Zhou L., Li P. Analysis of the role and mechanism of EGCG in septic cardiomyopathy based on network pharmacology. PeerJ. 2022;10 doi: 10.7717/peerj.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspect. Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z.M., Gao W., Wang H., Zhao D., Nie Z.L., Shi J.Q., Zhao S., Lu X., Wang L.S., Yang Z.J. Green tea polyphenol epigallocatechin-3-gallate inhibits TNF-α-induced production of monocyte chemoattractant protein-1 in human umbilical vein endothelial cells. Cell. Physiol. Biochem. 2014;33(5):1349–1358. doi: 10.1159/000358702. [DOI] [PubMed] [Google Scholar]
- 30.Bi C.F., Liu J., Yang L.S., Zhang J.F. Research progress on the mechanism of sepsis induced myocardial injury. J. Inflamm. Res. 2022;15:4275–4290. doi: 10.2147/JIR.S374117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.