Abstract
Background
There is a lack of data concerning sexual health following open radical cystectomy (RC), especially in elderly patients and women.
Aim
To describe sexual health and its impact on general health as well as survival in patients undergoing standard open RC for the treatment of bladder cancer (BC). Due to limited data, subgroup analysis for elderly patients and women was performed.
Methods
A prospective noninterventional clinical study was performed evaluating sexual health in RC with any kind of urinary diversion due to BC with a follow-up of 12 months after RC. The study was approved by the local ethics review board (A 2021-0175) and was registered at the German Clinical Trial Register (DRKS00026255). Assessment of sexual health was done with the following validated questionnaires: EORTC QLQ-C30 (for quality of life; European Organisation for Research and Treatment of Cancer), EORTC SH22 (for sexual health), and IIEF-5 (5-item International Index of Erectile Function).
Outcomes
The standard measurements of EORTC QLQ-C30, EORTC SH22, and IIEF-5 as well as overall survival.
Results
Thirty-two patients participated in the study with a mean age of 71.5 years (SD, 9.7): 25 (78.1%) were male and 7 (21.9%) were female. Overall there is a heterogenic picture for sexual health in the study population, but sexual satisfaction is significantly higher prior to surgery while the importance of a sex life stays high and stable. Interestingly, the general health score is significantly correlated to sexual satisfaction (Pearson’s correlation; r = 0.522, P = .002) preoperatively but not following surgery: r = 0.103 (P = .665) after 3 months, r = 0.478 (P = .052) after 6 months, r = 0.276 (P = .302) after 9 months, and r = 0.337 (P = .202) after 12 months. The importance of a sex life is still essential for the patients, especially when recovering from RC; nearly the same can be reported for elderly patients. Unfortunately, the data for women are too limited to report robust results.
Clinical Implications
Evaluation, advice, and monitoring of sexual health must be integrated into clinical practice, particularly in women.
Strengths and Limitations
At least to our knowledge, this is the first systematic prospective evaluation of sexual health in patients with BC receiving RC. Due to the small sample size, there is a risk of selection bias.
Conclusion
Sexual health is important for patients with BC receiving RC, and it is an essential part of quality of life, especially in elderly patients.
Keywords: muscle-invasive bladder cancer (MIBC), radical cystectomy, quality of life, sexual health, ptient-reported outcomes (PROs)
Introduction
In accordance with 2018 GLOBOCAN data, urothelial carcinoma of the bladder (BC) is the 10th-most common malignancy worldwide, with 549 393 new cases and 200 000 cancer-related deaths. In the United States, BC represents 5% of new cancer diagnoses and is the sixth-most prevalent malignancy.1,2
Furthermore, the impact on quality of life (QoL) in patients after treatment for BC is significantly worse than in other pelvic cancers, especially for radical cystectomy (RC). In the context of QoL, oncologic outcomes must be debated as well as functional outcomes (eg, sexual problems are commonly reported).3 Unfortunately, sexual dysfunction and treatment options following RC are rarely discussed with the patients.4,5 Additionally, high-quality prospective research concerning sexual health and its impact on QoL following RC in the treatment of BC is rare and urgently needed.6–12 The data situation in special patient groups, such as elderly patients and women, is even worse.4,7,11,12 However, guidelines, particularly those of the European Association of Urology, recommend monitoring of QoL during all phases of treatment in BC.13
Consequently, we performed a prospective noninterventional clinical study evaluating sexual health in RC due to BC with a follow-up of 12 months after RC. The primary end point was the descriptive analysis of sexual health with validated questionnaires, including subgroup analysis for elderly patients aged >70 years and women. Secondary end points were the impact of sexual health on general health, QoL, and overall survival. In summary, we hypothesize that those patient subgroups have special needs and that sexual health is also an important issue for elderly people.
Methods
Development of the study and population
The study was designed according to the guidelines in the synthesis of qualitative research (ENTREQ) found on equatornetwork.org.14 Before starting the study, we obtained the approval of the local ethics review board (A 2021-0175), and the study was registered at the German Clinical Trial Register (DRKS00026255). The inclusion criteria were adult patient (age >18 years) with BC scheduled for standard open RC with any kind of urinary diversion. If the inclusion criteria were met and informed consent was obtained from the patient, there were no further exclusion criteria. All relevant patient data (eg, patient history, demographic characteristics) and all relevant clinical data were prospectively collected at the time of study inclusion. Furthermore, all patients were asked on the ward or via postal reminder to fill out the EORTC QLQ-C30 (for QoL; European Organisation for Research and Treatment of Cancer) and EORTC SH22 (for sexual health); male patients also received the 5-item International Index of Erectile Function (IIEF-5) before RC and every 3 months after RC until month 12. Additionally, the patients did not receive specific counseling about sexual health prior to surgery. Patients were included between October 2021 and July 2022.
Definitions and questionnaires
The current EORTC QLQ-C30 (version 3.0) is a well-established and validated questionnaire for the QoL of patients in oncology. It has 5 functional scales—physical, role, emotional, cognitive, and social functioning—where a higher value means higher functionality. It also has 9 symptom scales—fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties—where a higher score means a higher symptom burden. Furthermore, a global health score (GHS) can be calculated, meaning that a higher score indicates better health status.15,16
The EORTC SH22 is a validated questionnaire assessing sexual health in male and female patients and survivors of cancer. Higher values stand for more importance of the item. It has 2 multiscale items—sexual satisfaction and pain—and 11 single-item scales: importance of sex life, decreased libido, worry incontinence, fatigue, treatment effect on sexual activity, communication, insecurity with the partner, confidence of erection, feeling less masculine, dry vagina, and feeling less feminine.17,18
The IIEF-5 is a validated diagnostic tool for evaluating erectile dysfunction (ED). The 5-question short version has a sensitivity of 98% and a specificity of 88% in diagnosing ED.19
All questionnaires were analyzed according to their scoring manuals.16,18,19
Furthermore, we used the Charlson Comorbidity Index (CCI) for general health assessment only prior to RC. The CCI is a well-established and validated method of classifying comorbidity and estimating risk of death from comorbidity.20
Statistical analysis
For each numeric variable, the numeric distribution was preliminarily assessed by the Kolmogorov-Smirnov test. Descriptive statistics were made with mean and SD for normal distribution or median and IQR for nonparametric data. For parametric continuous variables, the Student t-test was used, and for parametric categorical variables, the chi-square test or Fisher’s exact test was used. Additionally, Pearson’s correlation was used. For nonparametric data (categorical and continuous), we used the Mann-Whitney U test. Kaplan-Meier plots were used to estimate the median overall survival, and univariate comparisons were performed with the log rank test. All reported P values were based on a 2-sided hypothesis. P < .05 was considered significant. All statistical calculations were performed with SPSS version 28.0 (IBM).
Results
Demographic characterization of the study population
Thirty-two patients participated in the study with a mean age of 71.5 years (SD, 9.7): 25 (78.1%) were male and 7 (21.9%) were female. Table 1 gives a detailed overview of the demographic characterization of the study population.
Table 1.
Demographic characterization of the study population (N = 32).
| Characteristic | n (%) or median (IQR) |
|---|---|
| Age, y, mean (SD) | 71.5 (9.7) |
| Gender | |
| Male | 25 (78.1) |
| Female | 7 (21.9) |
| Charlson Comorbidity Index | 6.0 (5.0–8.0) |
| ASA score | 2.0 (2.0–3.0) |
| ECOG score | 1.0 (1.0–1.0) |
| Body mass index | 23.9 (22.2–27.0) |
| Smoking history | |
| No | 17 (53.1) |
| Yes | 15 (46.9) |
| Pack-years | 50.0 (30.0–57.5) |
| Neoadjuvant chemotherapy | |
| No | 31 (96.9) |
| Yes | 1 (3.1) |
| T stage | |
| T0 | 1 (3.1) |
| T1 | 7 (21.9) |
| T2a | 1 (3.1) |
| T2b | 4 (12.5) |
| T3a | 4 (12.5) |
| T3b | 9 (28.1) |
| T4a | 6 (18.8) |
| N stage | |
| N0 | 22 (68.8) |
| N1 | 3 (9.4) |
| N2 | 7 (21.9) |
| M stage | |
| cM0 | 28 (87.5) |
| cM1 | 4 (12.5) |
| L stage | |
| L0 | 14 (43.8) |
| L1 | 18 (56.3) |
| V stage | |
| V0 | 27 (84.4) |
| V1 | 5 (15.6) |
| Pn stage | |
| Pn0 | 21 (65.6) |
| Pn1 | 11 (34.4) |
| R stage | |
| R0 | 26 (81.3) |
| R1 | 6 (18.8) |
| Grading G3/high grade | 32 (100.0) |
| Variant histology | |
| No | 27 (84.4) |
| Yes | 5 5 (15.6) |
| Coincidental prostate cancer | |
| No | 22 (68.8) |
| Yes | 10 (31.2) |
| Urinary diversion | |
| Ileal conduit | 25 (78.1) |
| Ileal neobladder | 6 (18.8) |
| Ureter skin fistula | 1 (3.1) |
| Incision-suture time, min | 265.0 (235.3–340.0) |
| Perioperative blood loss, mL | 300.0 (242.5–392.5) |
| Perioperative transfusion | |
| No | 22 (68.8) |
| Yes | 10 (31.3) |
Abbreviations: ASA, American Society of Anesthesiologists; ECOG, Eastern Cooperative Oncology Group.
General QoL
General health was assessed by the EORTC QLQ-C30. The GHS over the study period is illustrated in Figure 1. Interestingly, it stayed stable over the study period and showed no significant changes, with mean scores of 68.2 (SD, 14.2) preoperatively, 67.9 (SD, 18.3) after 3 months, 70.1 (SD, 18.9) after 6 months, 68.7 (SD, 16.8) after 9 months, and 68.8 (17.4) after 12 months. Table 2 states the detailed functional and symptom scores.
Figure 1.
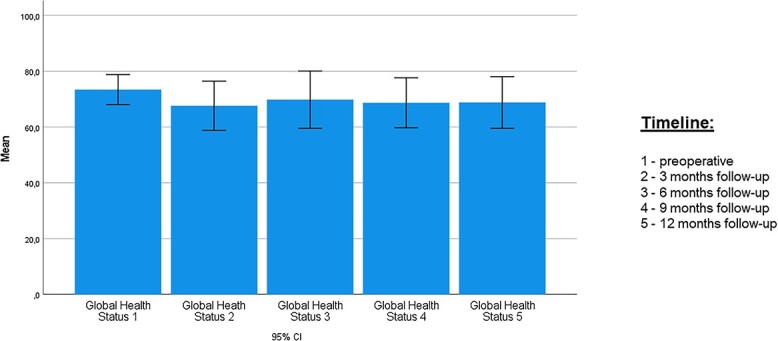
Time course of EORTC QLQ-C30 (for quality of life): global health score. Data are presented as mean (95% CI). EORTC, European Organisation for Research and Treatment of Cancer.
Table 2.
Time course of EORTC QLQ-C30 (for quality of life): functional and symptom scales.
| Mean (SD) | |||||
|---|---|---|---|---|---|
| Scale: item | Preoperative | 3 mo | 6 mo | 9 mo | 12 mo |
| Functional | |||||
| Physical | 50.4 (27.1) | 46.5 (27.7) | 55.2 (20.9) | 51.1 (26.6) | 55.2 (26.6) |
| Role | 87.6 (3.7) | 79.7 (16.1) | 79.9 (16.1) | 82.1 (12.6) | 82.8 (11.3) |
| Emotional | 85.0 (15.2) | 70.5 (32.3) | 74.5 (20.9) | 73.9 (29.2) | 81.8 (26.2) |
| Cognitive | 91.2 (14.6) | 82.5 (22.6) | 86.2 (19.8) | 86.4 (20.4) | 85.3 (24.3) |
| Social | 91.2 (14.5) | 70.9 (35.3) | 76.5 (32.8) | 82.3 (26.2) | 81.3 (25.0) |
| Symptom | |||||
| Fatigue | 17.2 (18.9) | 35.0 (34.5) | 27.4 (29.5) | 22.1 (24.0) | 22.9 (28.0) |
| Nausea and vomiting | 9.8 (18.3) | 18.3 (31.0) | 1.0 (4.1) | 3.1 (9.0) | 2.1 (5.8) |
| Pain | 11.3 (16.5) | 26.7 (34.3) | 20.6 (24.6) | 16.8 (23.7) | 16.8 (28.0) |
| Dyspnoe | 15.5 (20.7) | 16.6 (22.9) | 9.8 (19.6) | 12.4 (20.6) | 6.3 (18.2) |
| Insomnia | 23.8 (25.7) | 38.3 (39.5) | 24.4 (38.8) | 18.7 (29.7) | 16.7 (23.3) |
| Appetite loss | 14.5 (20.6) | 30.0 (38.9) | 9.8 (22.9) | 4.1 (11.3) | 4.1 (11.3) |
| Constipation | 13.5 (25.2) | 25.0 (38.8) | 19.6 (31.3) | 18.8 (32.2) | 2.1 (8.3) |
| Diarrhea | 2.1 (8.1) | 6.7 (23.2) | 5.9 (24.3) | 2.1 (8.3) | 2.1 (8.3) |
| Financial difficulties | 9.3 (15.1) | 10.0 (22.0) | 11.7 (20.2) | 12.4 (20.6) | 14.6 (24.3) |
Abbreviation: EORTC, European Organisation for Research and Treatment of Cancer.
Sexual health
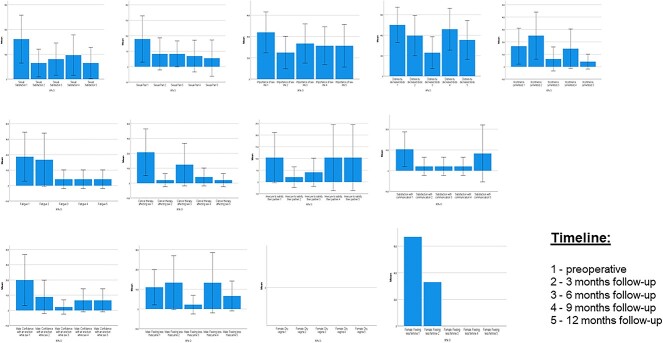
Figure 2 illustrates all scores of the EORTC SH22 in detail. Most important, the importance of a sex life stayed high and stable over the study period. Additionally, sexual satisfaction was highest prior to surgery.
Figure 2.
Time course of EORTC SH22 (for sexual health): all scores. Data are presented as mean (95% CI). EORTC, European Organisation for Research and Treatment of Cancer.
Interestingly, the GHS was significantly correlated to sexual satisfaction (r = 0.522, P = .002) preoperatively but not anymore following surgery: r = 0.103 (P = .665) after 3 months, r = 0.478 (P = .052) after 6 months, r = 0.276 (P = .302) after 9 months, and r = 0.337 (P = .202) after 12 months. Additionally, GHS was not correlated to the importance of a sex life preoperatively (r = 0.293, P = .103), after 3 months (r = 0.063, P = .791), after 6 months (r = 0.066, P = .801), and after 9 months (r = 0.201, P = .455), but it was correlated after 12 months (r = 0.571, P = .021). In this analysis the correlations were performed at the same time point (also applying for elderly patients).
In terms of comorbidities, the CCI was significantly correlated to sexual satisfaction preoperatively (r = 0.543, P = .001) but not anymore following surgery: r = 0.992 (P = .088) after 3 months, r = 0.262 (P = .310) after 6 months, r = 0.291 (P = .274) after 9 months, and r = 0.375 (P = .153) after 12 months. Furthermore, nearly the same could be described for the importance of a sex life, with a significant correlation preoperatively (r = 0.369, P = .038) but no correlation after treatment: r = 0.186 (P = .433) after 3 months, r = 0.073 (P = .782) after 6 months, r = 0.143 (P = .597) after 9 months, and r = 0.127 (P = .640) after 12 months.
Concerning the male patients, the median IIEF-5 scores were as follows: 10.0 (IQR, 4.0–17.5) preoperatively, 4.0 (IQR, 1.0–10.0) after 3 months, 5.0 (IQR, 1.0–10.0) after 6 months, 5.0 (IQR, 1.0–12.0) after 9 months, and 5.0 (IQR, 1.0–11.0) after 12 months. Before surgery, 11 of the 25 male patients (44.0%) had severe ED, and after 12 months of follow-up, 11 of the remaining 15 patients (73.3%) had severe ED. Supplementary 1 gives a detailed overview about ED during follow-up.
Subgroup analysis
Sexual health in elderly patients aged >70 years
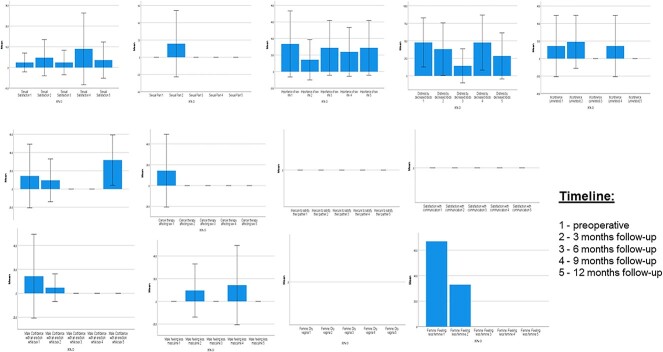
In this study population, 18 patients (56.3%) were aged >70 years. This subgroup had a median age of 79.0 years (IQR, 72.8–83.5), with 13 male (72.2%) and 5 female (27.8%) patients. Figure 3 details all scores of the EORTC SH22 of this special patient group.
Figure 3.
Subgroup analysis for patients aged >70 years: time course of EORTC SH22 (for sexual health), all scores. Data are presented as mean (95% CI). EORTC, European Organisation for Research and Treatment of Cancer.
In elderly patients, the GHS was not significantly correlated to sexual satisfaction preoperatively (r = 0.023, P = .933), after 3 months (r = 0.352, P = .353), after 6 months (r = 0.353, P = .437), after 9 months (r = 0.225, P = .627), and after 12 months (r = 0.372, P = .411). Furthermore, the GHS was not correlated with the importance of a sex life preoperatively (r = 0.069, P = .784), after 3 months (r = 0.005, P = .989), after 6 months (r = 0.422, P = .345), after 9 months (r = 0.132, P = .779), and after 12 months (r = 0.704, P = .077).
Interestingly, in elderly patients, the CCI was not significantly associated with sexual satisfaction preoperatively (r = 0.377, P = .123), after 3 months (r = 0.587, P = .097), after 6 months (r = 0.032, P = .945), after 9 months (r = 0.187, P = .688), and after 12 months (r = 0.194, P = .677). It was also not associated with the importance of a sex life preoperatively (r = 0.084, P = .741), after 3 months (r = 0.474, P = .198), after 6 months (r = 0.251, P = .587), after 9 months (r = 0.204, P = .661), and after 12 months (r = 0.251, P = .587).
Concerning the male patients, the median IIEF-5 scores were as follows: 5.0 (IQR, 1.0–8.5) preoperatively, 3.0 (IQR, 1.0–5.75) after 3 months, 2.0 (IQR, 1.0–5.0) after 6 months, 2.0 (IQR, 1.0–5.0) after 9 months, and 1.0 (IQR, 1.0–5.0) after 12 months. Before surgery, 10 of the 18 elderly male patients (55.6%) had severe ED, and after 12 months of follow-up, 7 patients were remaining and all had severe ED (100.0%).
Sexual heath in women
Seven women were included in this study (21.9%) with a median age of 79.0 years (IQR, 62.0–79.0). Unfortunately, after 3 months following RC, only 3 women completed the questionnaires, and after 6 months only 1 woman was left; thus, the sample size was too small to perform a detailed subgroup analysis.
Survival and association with sexual health
The median survival of the whole study population was 12.0 months (IQR, 4.3–12.0). Interestingly, the GHS >60 prior to treatment was associated with significantly better survival (log rank = 0.002), while sexual satisfaction (log rank = 0.229) and the importance of a sex life (log rank = 0.254) were not prior to therapy.
Discussion
We conducted a prospective study about sexual health in patients with BC receiving RC, including subgroup analysis for elderly patients and women. To the best of our knowledge, this is also one of the first detailed prospective studies addressing these issues in special patient populations. Despite the fact that our study has a small sample size and the study population is heterogenous concerning the demographic characterization, it is important because sexual health is a major part of general health and has a strong impact on QoL. Additionally, sexual satisfaction and the importance of a sex life is essential for patients, especially when they recover 12 months following RC, and it is important for elderly patients, which becomes even more urgent due to demographic development. On the other side, sexual dysfunction and treatment of sexual dysfunction are rarely discussed with patients.12 One reason for this problem might be that education of the physician about sexual dysfunction, as well as communication about these problems, is lacking.4–6 In our opinion and given our data, sexual life should be debated with elderly patients. This might need other special communication skills by the treating urologist. However, guidelines recommend timely monitoring of QoL during therapy of BC, including the discussion of specialties of the different urinary diversions and pelvic floor functioning. Sexual health is an essential part of QoL, so we should integrate these discussions and monitoring into our clinical practice.
Unfortunately, QoL data as well as data concerning the sexual health of women receiving RC are rare.12 We also report only sparse data concerning women, which is mostly due to the epidemiologic nature of the disease (BC). In this context, it must be discussed that the sexual problems of men and women are very different after RC. For example, men report ED and low libido, whereas women experience vaginal dryness, pain during sexual intercourse, and lost desire for sex because of the change in body image as well as having a stoma. These differences suggest the need for targeted or gender-specific interventions to improve QoL among survivors and their partners.12,21 Additionally, Catto et al performed one of the most detailed investigations of QoL in patients with BC. They found that sexual problems were commonly reported in men, increasing with younger age and radical treatment. One other important observation was that most women did not answer questions regarding female sexual issues, so the authors did not draw any conclusions for women and asked for further investigations. However, the authors’ conclusion of the whole study was that QoL following BC appears to be relatively independent of disease stage, treatment, and multimodal care. Issues are reported with sexual function and financial toxicity. QoL after BC is worse than after other pelvic cancers.10,12
Luckily, impaired sexual life is not associated with worse survival, while a better GHS is associated with better overall survival, which is not surprising and reveals a straightforward explanation. However, not only are oncologic outcomes important for the patient, but functional outcomes are essential as well3; thus, we must again emphasize that sexual health is an integral part of QoL, particularly for long-term survivors.
Despite some advantages of our study, such as the prospective data collection, we must assume that our analysis has some limitations. As we discussed before, there is some risk for selection bias mainly due to the small sample size. Additionally, we did not evaluate socioeconomic status, family, and partners, which might have a deep impact on QoL and especially sexual health.9 Furthermore, we did not include hormonal status or evaluation in our study, but this should be addressed in subsequent evaluations since sexual life is also hormone dependent.
In summary, sexual health is important for patients with BC receiving RC, and sexual health is an essential part of QoL. This is particularly true for elderly patients and needs to be discussed with them because RC is a safe procedure in this patient group.22 Unfortunately, data about these issues are sparse, so first, we must integrate evaluation, advice, and monitoring into clinical practice. Second, further clinical studies investigating sexual health as well as the use of intervention (eg, psychological counseling) and treatment methods are absolutely warranted to improve patient care in BC.
Last but not least, we must emphasize that women in this context are underinvestigated. Additionally, women might need special targeted questionnaires, investigations, and interventions to improve their QoL, including sexual health, following RC. In this context, von Deimling et al performed a precise narrative review about RC and urinary diversion in women. The authors came to the conclusion that pre- and postoperative counseling and support of females undergoing RC are currently insufficient regarding their expectations and experiences in terms of QoL and functional and sexual outcomes. Well-designed studies in this field are necessary to further improve the outcomes of women treated with RC, with an overarching aim to close the gender gap in managing women with BC.23 Due to our current investigation, we must strongly agree with this conclusion.
Supplementary Material
Acknowledgments
We thank all participating patients for their time and valuable discussions.
Contributor Information
Julia Nolting, Department of Urology, University Medical Center Rostock, Ernst-Heydemann-Str. 6, Rostock D-18055, Germany.
Romy Nitzsche, Department of Urology, University Medical Center Rostock, Ernst-Heydemann-Str. 6, Rostock D-18055, Germany.
Bernhard Kiss, Department of Urology, University of Bern, Inselspital, Freiburgstr. 37, Bern CH-3010, Switzerland.
Oliver W Hakenberg, Department of Urology, University Medical Center Rostock, Ernst-Heydemann-Str. 6, Rostock D-18055, Germany.
Laila Schneidewind, Department of Oncology, University Greifswald Medical Center, Ferdinand-Sauerbruchstr., Greifswald D-17475, Germany.
Author contributions
R.N., L.S., and O.W.H.: design of the study. J.N., R.N., and L.S.: data acquisition and management. L.S. and B.K.: data validation and analysis. L.S. and B.K.: writing the manuscript. All authors: critical revision of the data and the manuscript.
Funding
No external funding was received.
Conflicts of interest
All authors state that they have no conflict of interest regarding this article.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2. Degboe A, Ivanescu C, Rohay JM, Turner RR, Cella D. Validity and performance of the Functional Assessment of Cancer Therapy–Bladder (FACT-Bl) among advanced urothelial cancer patients. Support Care Cancer. 2019;27(11):4189–4198. 10.1007/s00520-019-04709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Witjes AJ, Feikama AAH. Organ-sparing strategies in muscle-invasive bladder cancer. Cancer Manag Res. 2021;13:7833–7839. 10.2147/CMAR.S294099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohamed NE, Pisipati S, Lee CT, et al. Unmet informational and supportive care needs of patients following cystectomy for bladder cancer based on age, sex, and treatment choices. Urol Oncol. 2016;34(12):531.e7–531.e14. 10.1016/j.urolonc.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 5. Mitin T, Dengina M, Chernykh M, et al. Management of muscle invasive bladder cancer with bladder preservation in Russia: a survey-based analysis of current practice and the impact of an educational workshop on clinical expertise. J Cancer Educ. 2021;36(5):1005–1013. 10.1007/s13187-020-01728-y. [DOI] [PubMed] [Google Scholar]
- 6. Rammant E, Van Wilder L, Van Hemelrijck M, et al. Health-related quality of life overview after diferent curative treatment options in muscle-invasive bladder cancer: an umbrella review. Qual Life Res. 2020;29(11):2887–2910. 10.1007/s11136-020-02544-z. [DOI] [PubMed] [Google Scholar]
- 7. Bessa A, Martin R, Häggström C, et al. Unmet needs in sexual health in bladder cancer patients: a systematic review of the evidence. BMC Urol. 2020;20(1):64. 10.1186/s12894-020-00634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asgari MA, Safarinejad MR, Shakhssalim N, Soleimani M, Shahabi A, Amini E. Quality of life after radical cystectomy for bladder cancer in men with an ileal conduit or continent urinary diversion: a comparative study. Urology Annals. 2013;5(3):190–196. 10.4103/0974-7796.115747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goossens-Laan CA, Kil PJM. Patient-reported outcomes for patients undergoing radical cystectomy: a prospective case-control study. Support Care Cancer. 2014;22(1):189–200. 10.1007/s00520-013-1946-9. [DOI] [PubMed] [Google Scholar]
- 10. Catto JWF, Downing E, Mason S, et al. Quality of life after bladder cancer: a cross-sectional survey of patient-reported outcomes. Eur Urol. 2021;79(5):621–632. 10.1016/j.eururo.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernández V, Linares Espinos E, Dunn J, et al. Oncological and functional outcomes of sexual function–preserving cystectomy compared with standard radical cystectomy in men: a systematic review. Urol Oncol. 2017;35(9):539.e17–539.e29. 10.1016/j.urolonc.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 12. Mohamed NE, Chaoprang HP, Hudson S, et al. Muscle invasive bladder cancer: examining survivors’ burden and unmet needs. J Urol. 2014;191(1):48–53. 10.1016/j.juro.2013.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. European Association of Urology (EAU) guidelines: muscle-invasive and metastatic bladder cancer. Accessed August 26, 2023. https://uroweb.org/guidelines/muscle-invasive-and-metastatic-bladder-cancer. [DOI] [PubMed]
- 14. Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12(1):181–188. 10.1186/1471-2288-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 16. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. On Behalf of the EORTC Quality of Life Group: The EORTC QLQ-C30 Scoring Manual. 3rd ed. European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 17. Oberguggenberger AS, Nagele E, Inwald EC, et al. Phase 1-3 of the cross-cultural development of an EORTC questionnaire for the assessment of sexual health in cancer patients: the EORTC SHQ-22. Cancer Med. 2018;7(3):635–645. 10.1002/cam4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greimel E, Nagele E, Lanceley A, et al. Psychometric validation of the European Organization for Research and Treatment of Cancer–Quality of Life Questionnaire Sexual Health (EORTC QLQ-SH22). Eur J Cancer. 2021;154:235–245. 10.1016/j.ejca.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 19. Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool of erectile dysfunction. Int J Impot Res. 1999;11(6):319–326. 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei E, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21. Vancavage R, Siddiqui S, Bernstein A, Avulova S. Why has sexuality research in women with bladder cancer undergoing radical cystectomy been ignored for so long? J Sex Med. 2023;20(5):580–583. 10.1093/jsxmed/qdad012. [DOI] [PubMed] [Google Scholar]
- 22. Raheem OA, Kamel MH, Leung P, et al. Radical cystectomy in the octogenarian population: a single centre experience. Curr Urol. 2011;5(4):196–201. 10.1159/000327478. [DOI] [Google Scholar]
- 23. von Deimling M, Laukhtina E, Pradere B, et al. Radical cystectomy and urinary diversion in women: techniques, outcomes, and challenges—a narrative review. Transl Androl Urol. 2022;11(11):1598–1610. 10.21037/tau-22-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.