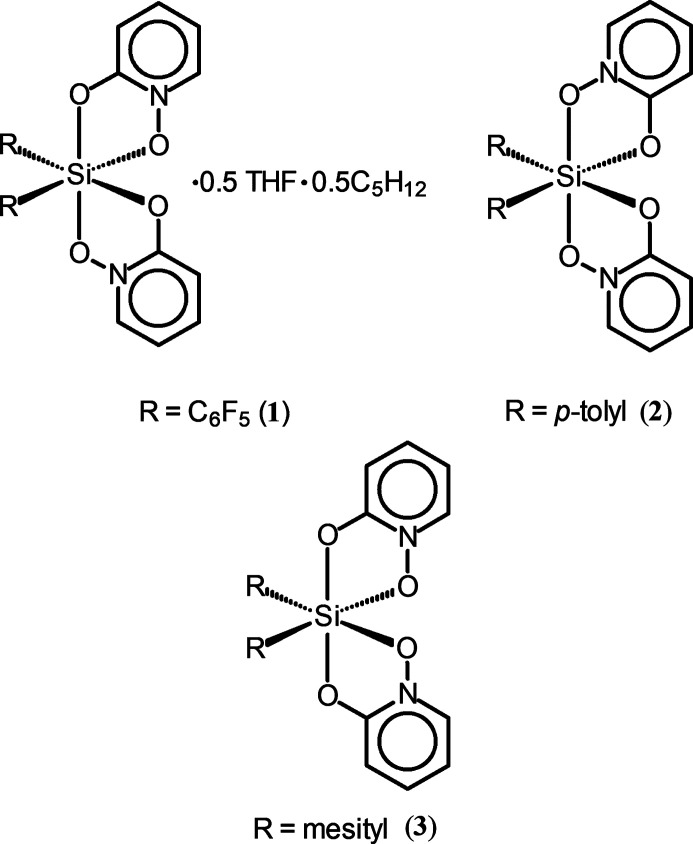
Three hexacoordinated bis(aryl)silicon(IV) complexes of 1-oxopyridin-2-one (OPO) are reported, each of which exhibit C/N site disorder in their pyridine rings. In (C6F5)2Si(OPO)2, the equal disorder ratios and solution NMR characterization together indicate the presence of a single totally asymmetric ON-trans-OC isomer. Unequal disorder ratios in p-tolyl2Si(OPO)2 and in mesityl2Si(OPO)2 indicate the presence of up to three isomers.
Keywords: crystal structure, silicon, pyridinone, pyridine N-oxide
Abstract
The neutral organosilicon(IV) complex, (C6F5)2Si(OPO)2 (OPO = 1-oxopyridin-2-one, C5H4NO2), was synthesized from (C6F5)2Si(OCH3)2 and 2 equiv. of 1-hydroxypyridin-2-one in tetrahydrofuran (THF). Single crystals grown from the diffusion of n-pentane into a THF solution were identified as a THF hemisolvate and an n-pentane hemisolvate, (C6F5)2Si(OPO)2·0.5THF·0.5C5H12 (1). p-Tolyl2Si(OPO)2 (2) and mesityl2Si(OPO)2 (3) crystallized directly from reaction mixtures of 2 equiv. of Me3Si(OPO) with p-tolyl2SiCl2 and mesityl2SiCl2, respectively, in acetonitrile. The oxygen-bonded carbon and nitrogen atoms of the OPO ligands in 1, 2, and 3 were modeled as disordered indicating co-crystallization of up to three possible diastereomers in each. Solution NMR studies support the presence of exclusively the all-cis isomer in 1 and multiple isomers in 2. Poor solubility of 3 limited its characterization in solution.
1. Chemical context
The intriguing capacity of 1-hydroxypyridin-2-one (HOPO) to dissolve silica to form [Si(OPO)3]+ in aqueous solution was reported by Weiss & Harvey in 1964 ▸. More recently, related ligand derivatives have been utilized as sequestering agents of lead and rare-earth metals, among others (Lewis & Cohen, 2004 ▸; Szigethy & Raymond, 2011 ▸; Wang, et al., 2019 ▸). In order to further study the powerful chelate effect of the OPO ligand, we have examined the solution- and solid-state structures of silicon complexes with varying organo ancillary ligands.
Previously reported hexacoordinate neutral dialkylsilicon 1-oxopyridin-2-one (OPO) complexes, R 2Si(OPO)2 [R = Me, Et, iPr; R 2 = (CH2)3], and one diaryl complex, Ph2Si(OPO)2, each exhibit co-crystallization of up to three possible isomers due, in part, to the isosteric character of the OPO ligand with the coplanar flip of itself (Kraft & Brennessel, 2014 ▸). In solution at room temperature, the dialkyl complexes exhibit only five OPO ligand resonances by NMR spectroscopy, indicating rapid interconversion of isomers that occurs with concomitant Si←OC bond dissociation. For Me2Si(OPO)2, three isomers were observed at 193 K by 1H NMR spectroscopy. In Ph2Si(OPO)2, the more electron-withdrawing phenyl groups strengthened the OPO ligand chelate interaction as given by generally shorter Si—O distances, and this resulted also in a slower interconversion between isomers relative to the alkyl derivatives (Kraft & Brennessel, 2014 ▸).
In all known R
2Si(OPO)2 complexes, the pair of Si—O bond distances trans to alkyl or aryl groups are longer than those cis. This characteristic, together with the observed C/N site disorder, highlights the underlying ambidentate character of the OPO ligand with interchangeability of canonical structures having either 2-pyridinone or N-oxide electronic forms. In contrast with the four known alkyl R
2Si(OPO)2 complexes in the crystalline state which favored primarily the ON-trans-ON isomer, the aryl derivative, Ph2Si(OPO)2, favored primarily the OC-trans-OC isomer and suggested that electron-withdrawing ancillary ligands might favor structures with primarily N-oxide forms. We report here the crystal structures and solution characterization of three additional aryl-substituted R
2Si(OPO)2 [R = C6F5 (1), p-tolyl (2), mesityl (3)] complexes.
2. Structural commentary
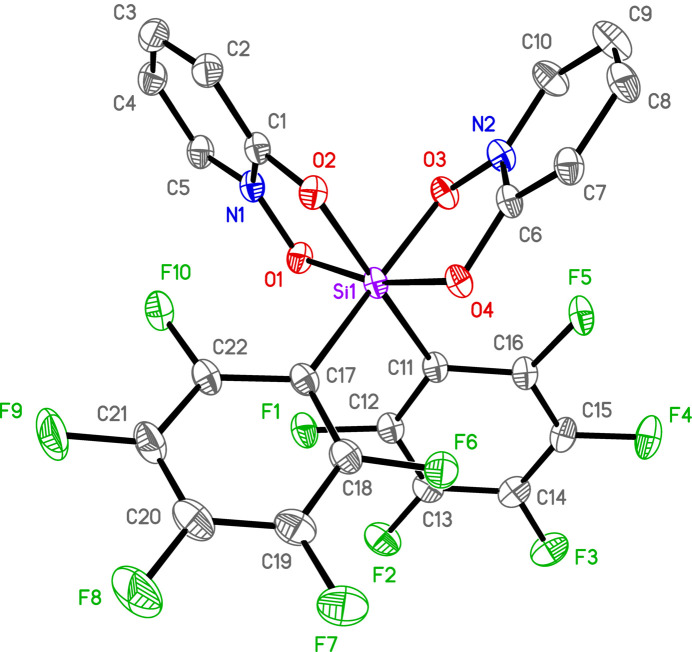
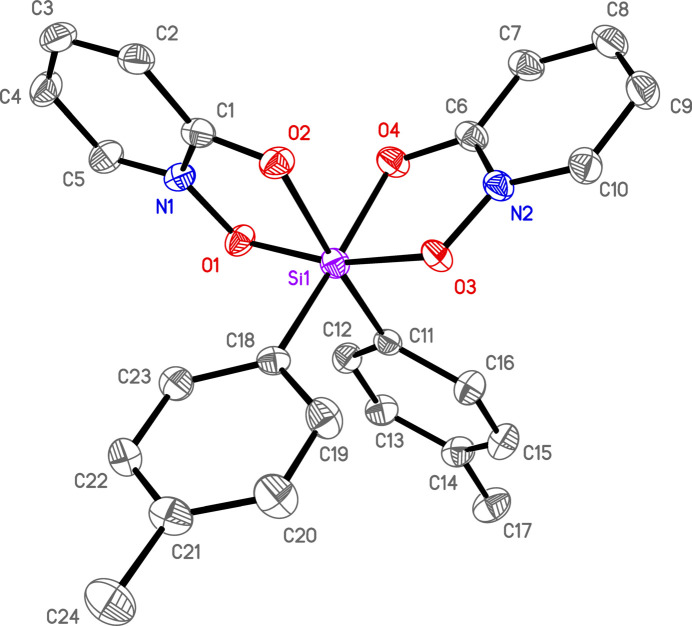
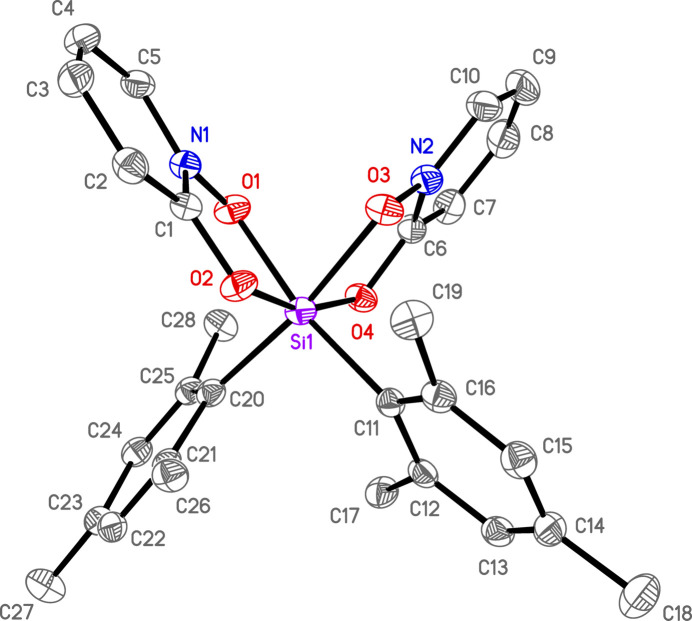
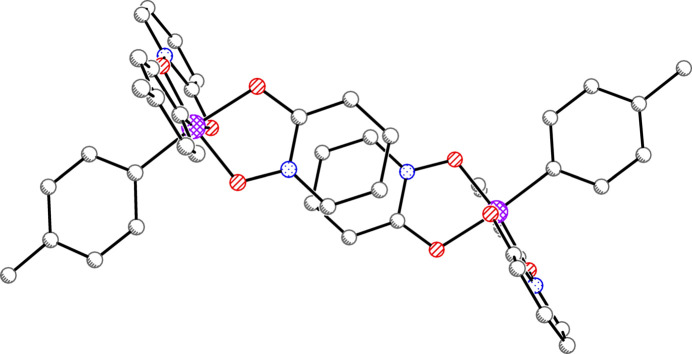
There is one silicon complex in a general position per asymmetric unit for all three structures. In 1, there are also solvents of crystallization (see Refinement). Each of the three complexes is hexacoordinate in a distorted octahedral geometry with cis-aryl groups and two chelating OPO ligands (Figs. 1 ▸–3 ▸ ▸). Selected bond lengths and angles are summarized in Tables 1 ▸, 2 ▸ and 3 ▸. In all three complexes, the oxygen-bonded C and N atoms of each pyridine ring are modeled as disordered (see Refinement), which indicates the presence of up to three possible diastereomers in each. In 1, the C1/N1 and C6/N2 disorder ratios indicate approximately equal C/N atom occupancy in both OPO ligand sites. In 2, to an uncertain degree, a larger proportion of the ON-trans-ON arrangement is indicated from the disorder ratios, and in 3, a larger proportion of the OC-trans-OC arrangement is indicated. In our previous work (Kraft & Brennessel, 2014 ▸), similarly disordered dialkyl R 2Si(OPO)2 [R = Me, Et, iPr; R 2 = (CH2)3] complexes were found to favor a larger proportion of the ON-trans-ON arrangement, whereas the more electron-withdrawing Ph2Si(OPO)2 favored a larger proportion of the OC-trans-OC arrangement. The structures of 1, 2, and 3 indicate no trend in major isomer preference with aryl/electron withdrawing ancillary ligands. As in all other R 2Si(OPO)2 complexes, the Si—O bonds trans to alkyl or aryl groups in 1–3 are consistently longer than those cis.
Figure 1.
Anisotropic displacement ellipsoid plot of 1 drawn at the 50% probability level with H atoms and solvent omitted. Only the major components of disorder are shown.
Figure 2.
Anisotropic displacement ellipsoid plot of 2 drawn at the 50% probability level with H atoms omitted. Only the major components of disorder are shown.
Figure 3.
Anisotropic displacement ellipsoid plot of 3 drawn at the 50% probability level with H atoms omitted. Only the major components of disorder are shown.
Table 1. Selected geometric parameters (Å, °) for 1 .
| Si1—O1 | 1.7910 (9) | Si1—O2 | 1.8503 (9) |
| Si1—O4 | 1.8042 (9) | Si1—C11 | 1.9559 (12) |
| Si1—O3 | 1.8480 (9) | Si1—C17 | 1.9683 (12) |
| O1—Si1—O4 | 166.74 (4) | O3—Si1—C11 | 90.16 (4) |
| O1—Si1—O3 | 86.65 (4) | O2—Si1—C11 | 175.99 (5) |
| O4—Si1—O3 | 84.60 (4) | O1—Si1—C17 | 99.44 (5) |
| O1—Si1—O2 | 85.17 (4) | O4—Si1—C17 | 88.68 (4) |
| O4—Si1—O2 | 84.53 (4) | O3—Si1—C17 | 172.66 (4) |
| O3—Si1—O2 | 87.56 (4) | O2—Si1—C17 | 88.88 (5) |
| O1—Si1—C11 | 91.41 (4) | C11—Si1—C17 | 93.75 (5) |
| O4—Si1—C11 | 98.54 (4) |
Table 2. Selected geometric parameters (Å, °) for 2 .
| Si1—O3 | 1.8093 (14) | Si1—C11 | 1.9202 (19) |
| Si1—O1 | 1.8097 (14) | Si1—O2 | 1.9290 (15) |
| Si1—O4 | 1.9179 (15) | Si1—C18 | 1.9301 (19) |
| O3—Si1—O1 | 165.96 (7) | O4—Si1—O2 | 83.28 (6) |
| O3—Si1—O4 | 83.76 (6) | C11—Si1—O2 | 171.36 (8) |
| O1—Si1—O4 | 86.24 (7) | O3—Si1—C18 | 91.04 (7) |
| O3—Si1—C11 | 98.02 (8) | O1—Si1—C18 | 97.64 (8) |
| O1—Si1—C11 | 91.68 (7) | O4—Si1—C18 | 171.40 (7) |
| O4—Si1—C11 | 89.37 (7) | C11—Si1—C18 | 98.16 (8) |
| O3—Si1—O2 | 85.72 (6) | O2—Si1—C18 | 89.53 (7) |
| O1—Si1—O2 | 83.35 (6) |
Table 3. Selected geometric parameters (Å, °) for 3 .
| Si1—O1 | 1.9291 (16) | Si1—O4 | 1.8096 (15) |
| Si1—O2 | 1.7896 (15) | Si1—C11 | 1.975 (2) |
| Si1—O3 | 1.9581 (16) | Si1—C20 | 1.955 (2) |
| O1—Si1—O3 | 80.99 (7) | O3—Si1—C11 | 88.61 (8) |
| O1—Si1—C11 | 169.59 (8) | O4—Si1—O1 | 84.30 (7) |
| O1—Si1—C20 | 90.09 (8) | O4—Si1—O3 | 81.82 (7) |
| O2—Si1—O1 | 83.25 (7) | O4—Si1—C11 | 94.57 (8) |
| O2—Si1—O3 | 84.09 (7) | O4—Si1—C20 | 95.73 (8) |
| O2—Si1—O4 | 162.48 (8) | C20—Si1—O3 | 170.93 (8) |
| O2—Si1—C11 | 95.44 (8) | C20—Si1—C11 | 100.32 (9) |
| O2—Si1—C20 | 96.57 (8) |
The 29Si NMR spectrum of 1 in DMSO-d 6 displays a single broadened resonance at −152.5 ppm, consistent with hexacoordinated silicon. Two sets of sharp OPO ligand resonances in 1:1 ratio are observed in the 13C NMR spectrum, and two sets of C6F5 ligand resonances in 1:1 ratio are observed in the 19F NMR spectrum, pointing to magnetic inequivalence of all four ligands. At 298 K, the ortho and meta 19F NMR resonances are significantly broadened, and each of the ten sharp OPO ligand 13C NMR resonances appears as a pair of closely-spaced peaks (a total of 20 peaks) separated by ≤ 0.2 ppm. Variable temperature NMR studies at 353 K show coalesced and sharpened meta 19F resonances, broadened ortho 19F resonances that approach coalescence, and 1H and 13C resonances of the OPO ligands that remain sharp. These observations are consistent with the absence of evidence of interconversion between diastereomers and the presence of two rotamers in 1:1 ratio of the totally asymmetric ON-trans-OC isomer with hindered rotation about the Si–C6F5 bonds. The absence of dynamic stereoisomerism at the observed temperatures is striking in light of that observed with all other known R 2Si(OPO)2 complexes. This may be explained by the markedly stronger chelate interaction in 1, manifested by its shorter average Si—O bond lengths (Table 1 ▸) and larger O—Si—O ‘bite’ angles [84.60 (4) and 85.17 (4)°], which are ∼1–3° larger than those of all known R 2Si(OPO)2 complexes (Kraft & Brennessel, 2014 ▸). As a result, Si←OC bond dissociation would be expected to be inhibited as observed, which has been shown as part of the mechanism of isomerization of R 2Si(OPO)2 complexes. Similarly, interconversion of fac and mer isomers in the even more strongly chelated [Si(OPO)3]+ cation is not observed for likely the same reason (Kraft et al., 2015 ▸). Bite angles in homoleptic [Si(OPO)3]+ silyl cations range from 87.0–87.4° in [Si(OPO)3]Cl·2CDCl3, [Si(OPO)3]Cl·xCH3CN, and [Si(OPO)3]·[CF3SO3]·0.5HOPO [Cambridge Structural Database (CSD; Groom et al., 2016 ▸), version 5.45, update Nov. 2023; refcodes RUTQUU, RUTRAB (Kraft et al., 2015 ▸) and QOXSIF (Tacke, Willeke, & Penka, 2001 ▸)], respectively, indicating even stronger chelate interactions in comparison with 1. The presence of only one isomer of 1 in solution is consistent with the crystallographic data having a common disorder ratio of 0.52 (2):0.48 (2) for both C1/N1 and C6/N2. The ON-trans-OC isomer and molecular superimposition of the flip of itself (i.e., a C 2 rotation about the axis bisecting the C—Si—C angle) uniquely reverses the positions of C and N atoms in all four oxygen-bonded sites, necessarily resulting in an equal disorder ratio.
The strength of the chelate interaction increases in the complexes in the order 3→2→1 as given by decreasing average Si—O bond distances and increasing O2Si bite angles (Tables 1 ▸, 2 ▸ and 3 ▸). This can be explained by the electron-withdrawing effect of the fluoroaryl groups which strengthens the interaction in 1 and the increase in steric hindrance from ortho-methyl substitution, which weakens the interaction in 3. Steric influences in 3 are further evident by the greater deviation of the trans-O—Si—O angle [162.48 (8)°] from ideal (i.e., 180°) versus those in 1 and 2 [166.74 (4) and 165.96 (7)°, respectively] and by the larger C—Si—C angle in 3 versus 2 and 1. The electron-donating p-tolyl groups of 2 appear to increase slightly the chelate strength of the OPO ligand in comparison with that in Ph2Si(OPO)2 given by the comparable Si—O bond lengths and ∼1° larger O2Si bite angles [for Ph2Si(OPO)2: Si—O = 1.9175 (4), 1.8157 (13) Å; O—Si—O = 82.47 (6)°].
For 2 in CDCl3 solution, a single set of OPO and p-tolyl ligand resonances was observed by 1H and 13C NMR spectroscopy with varying extents of broadened OPO ligand and p-tolyl peaks that sharpen further at higher temperature. These observations are consistent with stereodynamic isomerization occurring similar to that observed with Ph2Si(OPO)2 (Kraft & Brennessel, 2014 ▸). Complex 3 could not be characterized in solution due to its poor solubility.
Each O2Si chelate ring and planar OPO ligand in 1 forms a relatively large dihedral angle [9.60 (2) and 16.36 (4)°] in comparison with those of other alkyl R 2Si(OPO)2 complexes [R = Me, Et, iPr, tBu; R 2 = (CH2)3, range = 1.78–12.47°), 2 [2.41 (8) and 0.97 (9)°], and 3 [6.68 (11) and 8.41 (9)°]. Larger dihedral angles [both 21.51 (9)°] are also observed in Ph2Si(OPO)2. Unspecific crystal packing effects are likely responsible for these variations as no correlation could be found relating the magnitude of these fold angles with chelate strength or other ancillary ligand characteristics.
3. Supramolecular features
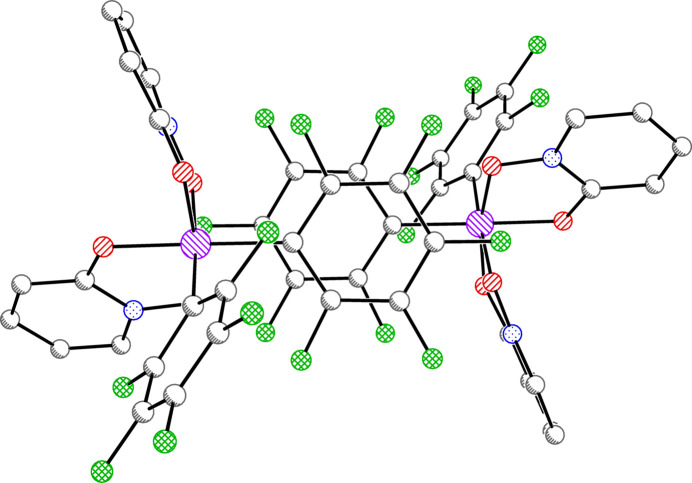
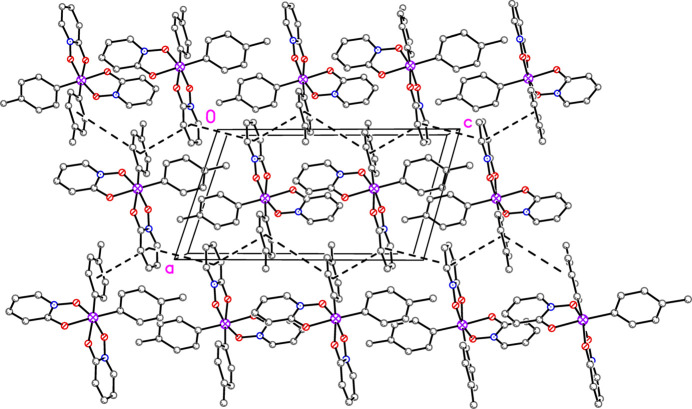
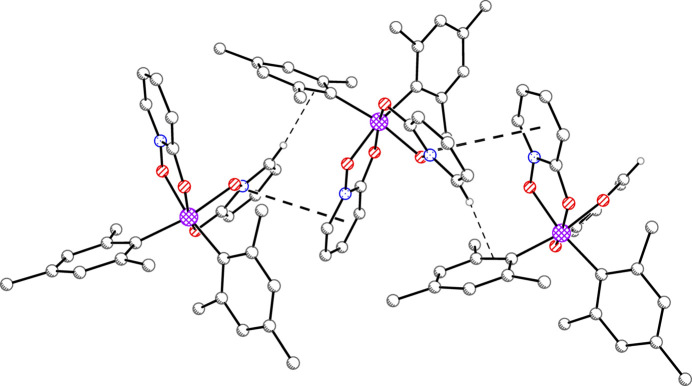
In 1 there is an offset parallel π–π interaction between ring C11–C16 from pairs of inverted molecules (Fig. 4 ▸), with a centroid–centroid distance of 3.8613 (8) Å and an interplanar distance of 3.7876 (13) Å. Further π–π interactions may have been inhibited during crystal growth by the presence of solvent. There are a few short intermolecular C—H⋯F—C(aromatic) contacts, the strongest of which are listed in Table 4 ▸. However, it should be noted that only two [C2—H2⋯F1(
 − x,
− x,
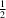 + y,
+ y,
 − z)] and C10—H10⋯F8(−1 + x, y, z)] have H⋯F distances of significance compared with the sum of the individual van der Waals radii (2.56 Å; Rowland & Taylor, 1996 ▸) and that these attractions tend to be very weak – of the order of the energies of van der Waals complexes (Howard et al., 1996 ▸).
− z)] and C10—H10⋯F8(−1 + x, y, z)] have H⋯F distances of significance compared with the sum of the individual van der Waals radii (2.56 Å; Rowland & Taylor, 1996 ▸) and that these attractions tend to be very weak – of the order of the energies of van der Waals complexes (Howard et al., 1996 ▸).
Figure 4.
Offset parallel π–π interaction between inverted pairs of molecules of 1. The second molecule is generated by the symmetry operation 1 − x, 1 − y, 1 − z. Centroid–centroid distance, 3.86 Å.
Table 4. Hydrogen-bond geometry (Å, °) for 1 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯F1i | 0.95 | 2.34 | 3.2809 (15) | 170 |
| C4—H4⋯F6ii | 0.95 | 2.68 | 3.5757 (16) | 158 |
| C5—H5⋯F4iii | 0.95 | 2.59 | 3.2997 (15) | 132 |
| C7—H7⋯F5iv | 0.95 | 2.57 | 3.2307 (14) | 127 |
| C8—H8⋯F6iv | 0.95 | 2.56 | 3.2230 (15) | 127 |
| C10—H10⋯F8v | 0.95 | 2.37 | 3.0797 (16) | 131 |
Symmetry codes: (i)
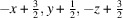 ; (ii)
; (ii)
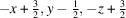 ; (iii)
; (iii)
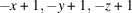 ; (iv)
; (iv)
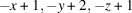 ; (v)
; (v)
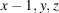 .
.
The packing of 2 features sheets of molecules parallel to the ac plane (Figs. 5 ▸ and 6 ▸). Inverted pairs of ring N1/C1–C5 alternate with inverted pairs of ring C11–C16 to form staggered, but parallel arene ring alignments along [001] (Fig. 5 ▸). The centroid–centroid distances are 3.7548 (14), 4.1725 (12), and 5.0523 (13) Å with interplanar spacings of 3.588 (2), 3.556 (3), and 3.532 (4) Å, respectively. The alignment of rings at the largest centroid-centroid distance of 5.05 Å is likely a mere consequence of a favorable packing arrangement rather than significant π–π overlap. These sheets are linked in the third dimension by pairs of offset parallel π–π interactions involving ring N2/C6–C10 (Fig. 7 ▸) with a centroid-centroid distance of 3.5067 (14) Å and an interplanar spacing of 3.350 (2) Å.
Figure 5.
Packing plot of 2 with H atoms omitted. Rows of interlocking molecules along the [001] direction create two-dimensional sheets. Centroid–centroid distances are 3.76, 4.17, and 5.05 Å, for which the smaller two may allow for offset parallel π–π interactions.
Figure 6.
Packing plot of 2 with H atoms omitted that shows the divisions between the sheets shown in Fig. 5 ▸.
Figure 7.
The sheets depicted in Figs. 5 ▸ and 6 ▸ are connected via additional π–π interactions between inverted pairs of molecules. Second molecule generated by 1 − x, −y, 1 − z. Centroid–centroid distance, 3.51 Å.
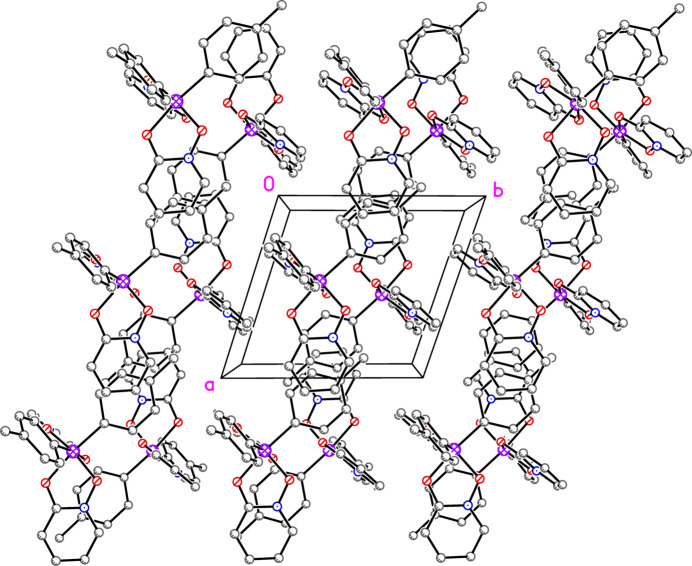
Molecules of 3 appear linked along [100] via π–π interactions between rings N1/C1–C5 and N2/C6–C10 of symmetry-equivalent molecules (Fig. 8 ▸). Although the centroid-centroid distance is short at 3.7416 (14) Å, the angle between ring planes is 23.03 (11)°, perhaps limiting the attractive force. The interplanar spacings range from 3.191 (3) to 4.268 (3) Å, with an average of 3.722 (7) Å. One C—H⋯π interaction accompanies each π–π interaction just described (Fig. 8 ▸). The distance between H and the midpoint of the C11—C16 bond is 2.50 Å, with a C—H⋯CC(midpoint) angle of 174°. The angle between the plane containing the C—H donor and that of the π-acceptor is 68.27 (7)°.
Figure 8.
Possible π–π interaction in 3 shown by thick dashes. Centroid–centroid distances, 3.74 Å. Angles between ring planes, 23°. Edge-to-face C—H⋯π interactions shown by thin dashes between H atoms and the π systems at the edge of each acceptor ring. Symmetry-equivalent molecules generated by
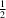 + x,
+ x,
 − y, 1 − z and
− y, 1 − z and
 + x,
+ x,
 − y, 1 − z.
− y, 1 − z.
4. Database survey
There are currently no reported structures of hexacoordinate bis(pentafluorophenyl)silicon(IV) complexes, nor other hexacoordinate dimesitylsilicon(IV) complexes. The related hexacoordinate pyrithione (OPTO) complex, (p-tolyl)2Si(OPTO)2, crystallizes with cis aryl groups and primarily with two bidentate OPTO ligands in an S-trans-S arrangement with additional disordered monodentate modes (CSD refcode DEWGAR; Tiede et al., 2022 ▸). Mesityl2Si(OPTO)2 is tetracoordinate with two monodentate κO OPTO ligands (CSD refcode DEWSUX; Tiede et al., 2022 ▸).
There are five entries of hexacoordinate R 2Si(OPO)2 [R = Me, Et, iPr, Ph; R 2 = (CH2)3] complexes containing two bidentate OPO ligands (CSD refcodes NITSAM, NITSEQ, NITSOA, NISMIN, NITSUG, respectively; Kraft & Brennessel, 2014 ▸). Also reported with two bidentate OPO ligands are monoorgano neutral hexacoordinate complexes, RSi(OPO)2 X (X = Cl, F; CSD refcodes ODEFIP, ODEFOV, ODEFUB, ODEHAJ, and ODEHEN), and cationic pentacoordinate complexes, [RSi(OPO)2]+ X − (X = Cl, trifluoromethanesulfonate; CSD refcodes ODEGAI, ODEGIQ, ODEGOW, and ODEGUC; Koch et al., 2017 ▸). Other related entries include [Si(OPO)2(μ-CH2CH2SCH2C(=O)O)]2·2CH3CN and [O(CH2)3]Si(OPO)2 (CSD refcodes UBUWET and UBUWIX, respectively; Tacke, Burschka et al., 2001 ▸). Monodentate OPO ligand complexes of any metal are limited to three organosilicon complexes: Me3Si(OPO), tBu2Si(κ 1-OPO)(κ 2-OPO), and Ph3Si(OPO)·Ph3Si(OH)·0.5C5H12 (CSD refcodes NITROZ, NITSOA, and NITRIT, respectively; Kraft & Brennessel, 2014 ▸). Upon review of a total of 70 complexes of any metal in the CSD containing the OPO ligand (Groom et al., 2016 ▸), complexes with OPO ligand/O2 M dihedral angles deviating more than 15° from coplanarity are relatively rare comprising of seven complexes of Si, V, Cu, Zn, Eu, Gd, and Th (CSD refcodes NISMIN: Kraft & Brennessel, 2014 ▸; OJEHOB: Jakusch et al., 2010 ▸; HUSHEJ: Peyroux et al., 2009 ▸; TADXAY: Puerta & Cohen, 2003 ▸; JAFZEW and JAFZIA: Tedeschi et al., 2003 ▸; BURPEJ: Casellato et al., 1983 ▸).
5. Synthesis and crystallization
(C6F5)2Si(OPO)2·0.5THF·0.5C5H12 (1): To a solution of HOPO (0.1508 g, 1.357 mmol) in ∼2 ml of THF was added a solution of (C6F5)2Si(OCH3)2 (0.2883 g, 1.025 mmol) in ∼2 ml THF. The resulting solution was stirred for two days and the solvent removed under vacuum. A portion (0.100 g) was recrystallized by vapor diffusion of n-pentane into a THF solution to yield white crystals of (C6F5)2Si(OPO)2·0.5THF·0.5C5H12. Subsequent washing of the crystals with THF and drying for 3 h under vacuum resulted in partial removal of solvents of crystallization, which analyzed as (C6F5)2Si(OPO)2·0.36C4H8O·0.11C5H12 (0.046 g, 46%) by a quantitative 1H NMR experiment and by elemental analysis. 1H NMR (DMSO-d 6, 353 K): δ 0.87 (t, pentane), 1.28 (pentane), 1.77 (THF), 3.62 (THF), 7.10 (m, 3H), 7.35 (ddd, 3 J = 8.6, 3 J = 4.5, 4 J = 1.0 Hz, 1H), 7.88 (m, 2H), 8.41 (ddd, 3 J = 10.6, 3 J = 6.6, 4 J = 1.2 Hz, 1H), 8.64 (m, 1H). 13C NMR (DMSO-d 6, 298 K): δ 13.9 (pentane), 21.7 (pentane), 25.1 (THF), 33.5 (pentane), 67.0 (THF), 112.0, 112.2, 112.2, 114.2, 114.2, 115.5, 115.5, 124.4 (br, Si—C), 132.6, 132.6, 132.7, 132.8, 136.0 (br d, 1 J C—F = 250 Hz), 138.8 (br d, 1 J C—F = 250 Hz), 138.9, 138.9, 139.8, 139.9, 147.7 (br d, 1 J C—F = 230 Hz), 154.5, 154.6, 155.4 (CO), 155.4 (CO). 19F NMR (DMSO-d 6, 298 K, referenced to α,α,α-trifluorotoluene at δ −63.73): δ −167.1 (br, m-C6F5), −166.6 (br, m-C6F5), −160.8 (m, p-C6F5), −160.5 (t, J = 21.1 Hz, p-C6F5), −136.2 (br, o-C6F5), −130.0 (br, o-C6F5), −128.8 (br, o-C6F5). 29Si NMR (DMSO-d 6, 298 K): δ −152.5 (br). Analysis calculated for (C6F5)2Si(OPO)2 0.36·C4H8O 0.11·C5H12: C, 46.72%; H, 1.98%; N, 4.55%. Found: C, 47.09%; H, 1.95%; N, 4.68%.
p-Tolyl2Si(OPO)2 (2): To a solution of Me3Si(OPO) (0.1243 g, 0.678 mmol) in 7 ml of CH3CN was added dropwise a solution of p-tolyl2SiCl2 (87.0 µL, d = 1.10 g ml−1, 0.340 mmol) in 2 ml of CH3CN at room temperature. The mixture was allowed to stand undisturbed for nine days. Decantation, washing with ∼1 ml of CH3CN, and drying under vacuum afforded 0.1132 g (75.5%) of a combination of a white powder and crystals used for structure determination. 1H NMR (CDCl3, 333 K): δ 2.24 (s, 6H), 6.61 (m, 2H), 6.82 (br d, 3 J = 7.9 Hz, 2H), 6.96 (d, 3 J = 7.8 Hz, 4H, p-tolyl), 7.38 (ddd, 3 J = 7.3, 3 J = 8.7, 4 J = 1.7 Hz, 2H), 7.53 (d, 3 J = 7.8 Hz, 4H, p-tolyl), 8.00 (br d, 3 J = 6.1 Hz, 2H). 13C NMR (CDCl3, 333 K): δ 21.4 (CH3), 111.5 (br), 113.2, 127.5, 132.4, 134.8, 135.1, 136.5 (br), 148.4 (br), 156.8 (CO). 29Si NMR (CDCl3, 333 K): δ −128.3. Analysis calculated for C24H22N2O4Si: C, 66.95; H, 5.15; N, 6.51. Found: C, 66.30; H, 5.09; N, 6.71.
Mesityl2Si(OPO)2 (3): To a filtered solution of Me3Si(OPO) (0.0904 g, 0.493 mmol) in 4 ml of CH3CN was added a filtered solution of mesityl2SiCl2 (0.0832 g, 0.247 mmol) in 4 ml of CH3CN. Colorless crystals deposited after one day at room temperature. Decantation and drying under vacuum afforded 0.0633 g (52.8%) of product that was insoluble in hot chloroform and hot acetonitrile. An attempt to dissolve 3 in DMSO-d 6 with heating resulted in dissolution with complete decomposition into unidentified products. NMR analysis of a CDCl3 solution prior to precipitation showed severely broadened indecipherable peaks. Analysis calculated for C28H30N2O4Si: C, 69.11; H, 6.21; N, 5.76. Found: C, 68.85; H, 6.16; N, 5.69.
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 5 ▸. In all three structures, both bidentate ligands are disordered with the coplanar flips of themselves. For the rings containing C1/N1 and C6/N2, respectively, the disorder ratios are 0.52 (2):0.48 (2) and 0.52 (2):0.48 (2), 0.66 (2):0.34 (2) and 0.61 (2):0.39 (2), and 0.68 (3):0.32 (3) and 0.61 (3):0.39 (3), for structures 1, 2, and 3, respectively. Due to resolution limitations, the disorder model did not include the entire ring, but was modeled by refining the occupancies of the two atoms types (C and N) at the oxygen-coordinating portions of the rings. The occupancies at each site were constrained to sum to one and additionally to sum to one C and one N atom between the two sites on each ring. The positional and anisotropic displacement parameters, respectively, at each site of disorder were constrained to be equivalent. It is understood that this type of disorder model will likely exhibit a weighted average of Si—O bond lengths, trending with the disorder ratios.
Table 5. Experimental details.
| 1 | 2 | 3 | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | C22H8F10N2O4Si·0.5C5H12·0.5C4H8O | C24H22N2O4Si | C28H30N2O4Si |
| M r | 654.52 | 430.52 | 486.63 |
| Crystal system, space group | Monoclinic, P21/n | Triclinic, P
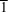
|
Orthorhombic, P212121 |
| Temperature (K) | 100 | 100 | 100 |
| a, b, c (Å) | 12.6809 (9), 12.1217 (9), 17.7335 (13) | 8.5662 (8), 8.8343 (8), 14.7801 (14) | 12.5710 (2), 12.68898 (19), 15.3580 (2) |
| α, β, γ (°) | 90, 105.7674 (15), 90 | 93.057 (2), 105.3716 (19), 106.7565 (18) | 90, 90, 90 |
| V (Å3) | 2623.3 (3) | 1022.45 (17) | 2449.80 (7) |
| Z | 4 | 2 | 4 |
| Radiation type | Mo Kα | Mo Kα | Cu Kα |
| μ (mm−1) | 0.20 | 0.15 | 1.15 |
| Crystal size (mm) | 0.40 × 0.36 × 0.14 | 0.24 × 0.24 × 0.20 | 0.09 × 0.07 × 0.06 |
| Data collection | |||
| Diffractometer | Bruker SMART APEXII CCD platform | Bruker SMART APEXII CCD platform | XtaLAB Synergy, Dualflex, HyPix |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) | Multi-scan (SADABS; Krause et al., 2015 ▸) | Multi-scan (CrysAlis PRO; Rigaku OD, 2019 ▸) |
| T min, T max | 0.694, 0.748 | 0.695, 0.746 | 0.674, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 97594, 14595, 9977 | 25883, 6239, 4231 | 22120, 5138, 4847 |
| R int | 0.043 | 0.065 | 0.048 |
| (sin θ/λ)max (Å−1) | 0.881 | 0.715 | 0.634 |
| Refinement | |||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.051, 0.163, 1.03 | 0.054, 0.149, 1.05 | 0.032, 0.079, 1.05 |
| No. of reflections | 14595 | 6239 | 5138 |
| No. of parameters | 446 | 284 | 324 |
| No. of restraints | 55 | 0 | 0 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.61, −0.58 | 1.01, −0.44 | 0.27, −0.25 |
| Absolute structure | – | – | Flack x determined using 1985 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) |
| Absolute structure parameter | – | – | −0.034 (17) |
In 1, the solvent volume contains one each of THF and n-pentane disordered over a crystallographic inversion center (0.50:0.50). Analogous bond lengths and angles in both directions along each solvent molecule were restrained to be similar. Anisotropic displacement parameters for proximal atoms were restrained to be similar.
All H atoms were placed geometrically and treated as riding atoms. Aromatic/sp 2, C–H = 0.95 Å and methylene, C–H = 0.99 Å, with U iso(H) = 1.2U eq(C). Methyl, C–H = 0.98 Å, with U iso(H) = 1.5U eq(C).
For 1 the maximum residual peak of 0.61 e− Å−3 and the deepest hole of −0.58 e− Å−3 are found 0.69 and 0.35 Å from atoms C21 and C25, respectively.
For 2 the maximum residual peak of 1.01 e− Å−3 and the deepest hole of −0.43 e− Å−3 are found 0.92 and 0.61 Å from atom Si1.
For 3 the maximum residual peak of 0.27 e− Å−3 and the deepest hole of −0.25 e− Å−3 are found 0.92 and 0.58 Å from atoms C20 and Si1, respectively.
Supplementary Material
Crystal structure: contains datablock(s) 1, 2, 3, global. DOI: 10.1107/S2056989024001543/ee2004sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989024001543/ee20041sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989024001543/ee20042sup3.hkl
Structure factors: contains datablock(s) 3. DOI: 10.1107/S2056989024001543/ee20043sup4.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors gratefully acknowledge St. John Fisher University for support, NSF MRI program award #1828310 for the purchase of an NMR spectrometer, and the University of Rochester X-ray Crystallographic Facility and associated funding from NSF MRI program award CHE-1725028.
supplementary crystallographic information
Bis[1-oxopyridin-2-olato(1-)]bis(pentafluorophenyl)silicon(IV)–tetrahydrofuran–pentane (2/1/1) (1). Crystal data
| C22H8F10N2O4Si·0.5C5H12·0.5C4H8O | F(000) = 1324 |
| Mr = 654.52 | Dx = 1.657 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.6809 (9) Å | Cell parameters from 3989 reflections |
| b = 12.1217 (9) Å | θ = 2.3–37.4° |
| c = 17.7335 (13) Å | µ = 0.20 mm−1 |
| β = 105.7674 (15)° | T = 100 K |
| V = 2623.3 (3) Å3 | Plate, colorless |
| Z = 4 | 0.40 × 0.36 × 0.14 mm |
Bis[1-oxopyridin-2-olato(1-)]bis(pentafluorophenyl)silicon(IV)–tetrahydrofuran–pentane (2/1/1) (1). Data collection
| Bruker SMART APEXII CCD platform diffractometer | 9977 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.043 |
| ω scans | θmax = 38.8°, θmin = 1.8° |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | h = −22→22 |
| Tmin = 0.694, Tmax = 0.748 | k = −21→21 |
| 97594 measured reflections | l = −30→30 |
| 14595 independent reflections |
Bis[1-oxopyridin-2-olato(1-)]bis(pentafluorophenyl)silicon(IV)–tetrahydrofuran–pentane (2/1/1) (1). Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.051 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.163 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0822P)2 + 0.9141P] where P = (Fo2 + 2Fc2)/3 |
| 14595 reflections | (Δ/σ)max = 0.001 |
| 446 parameters | Δρmax = 0.61 e Å−3 |
| 55 restraints | Δρmin = −0.58 e Å−3 |
Bis[1-oxopyridin-2-olato(1-)]bis(pentafluorophenyl)silicon(IV)–tetrahydrofuran–pentane (2/1/1) (1). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Both bidentate ligands are disordered with the coplanar flips of themselves (0.524 (16):0.476 (16) and 0.516 (15):0.484 (16) for the rings containing C1/N1 and C6/N2, respectively). Due to resolution limitations, the disorder was modeled by refining the occupancies of the two atoms types (C and N) at the oxygen-coordinating portions of the rings. The occupancies at each site were constrained to sum to one and additionally sum to one C and one N atom between the two sites on each ring. The positional and anisotropic displacement parameters,respectively, at each site of disorder were constrained to be equivalent.The solvent volume contains once each of n-pentane and tetrahydrofuran disordered over a crystallographic inversion center (0.50:0.50). Analogous bond lengths and angles in both directions along each solvent molecule were restrained to be similar. Anisotropic displacement parameters for proximal atoms were restrained to be similar. |
Bis[1-oxopyridin-2-olato(1-)]bis(pentafluorophenyl)silicon(IV)–tetrahydrofuran–pentane (2/1/1) (1). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Si1 | 0.66487 (2) | 0.71292 (2) | 0.62708 (2) | 0.01575 (6) | |
| O1 | 0.67613 (7) | 0.58885 (7) | 0.68356 (5) | 0.01829 (14) | |
| O2 | 0.69747 (7) | 0.78437 (7) | 0.72284 (5) | 0.01843 (14) | |
| O3 | 0.51949 (7) | 0.71616 (6) | 0.62790 (5) | 0.01877 (15) | |
| O4 | 0.63848 (6) | 0.84997 (7) | 0.58666 (5) | 0.01750 (14) | |
| N1 | 0.68205 (8) | 0.60779 (8) | 0.75861 (6) | 0.01816 (19) | 0.524 (16) |
| C1 | 0.69390 (9) | 0.71546 (8) | 0.78005 (6) | 0.01806 (18) | 0.524 (16) |
| N2 | 0.48096 (8) | 0.81844 (9) | 0.61909 (7) | 0.01913 (19) | 0.516 (15) |
| C6 | 0.54737 (8) | 0.89276 (8) | 0.59860 (6) | 0.01757 (18) | 0.516 (15) |
| N1' | 0.69390 (9) | 0.71546 (8) | 0.78005 (6) | 0.01806 (18) | 0.476 (16) |
| C1' | 0.68205 (8) | 0.60779 (8) | 0.75861 (6) | 0.01816 (19) | 0.476 (16) |
| N2' | 0.54737 (8) | 0.89276 (8) | 0.59860 (6) | 0.01757 (18) | 0.484 (15) |
| C6' | 0.48096 (8) | 0.81844 (9) | 0.61909 (7) | 0.01913 (19) | 0.484 (15) |
| C2 | 0.70444 (11) | 0.74796 (10) | 0.85565 (7) | 0.0224 (2) | |
| H2 | 0.713350 | 0.823669 | 0.869789 | 0.027* | |
| C3 | 0.70189 (12) | 0.66902 (12) | 0.91066 (8) | 0.0259 (2) | |
| H3 | 0.708483 | 0.689880 | 0.963383 | 0.031* | |
| C4 | 0.68956 (11) | 0.55763 (11) | 0.88890 (8) | 0.0250 (2) | |
| H4 | 0.687662 | 0.503036 | 0.926946 | 0.030* | |
| C5 | 0.68013 (10) | 0.52686 (10) | 0.81226 (7) | 0.0215 (2) | |
| H5 | 0.672500 | 0.451465 | 0.797136 | 0.026* | |
| C7 | 0.52081 (10) | 1.00284 (9) | 0.59067 (7) | 0.02030 (19) | |
| H7 | 0.569884 | 1.054923 | 0.578717 | 0.024* | |
| C8 | 0.42148 (11) | 1.03599 (10) | 0.60042 (8) | 0.0255 (2) | |
| H8 | 0.400870 | 1.111515 | 0.594537 | 0.031* | |
| C9 | 0.35103 (11) | 0.95821 (12) | 0.61899 (10) | 0.0304 (3) | |
| H9 | 0.281838 | 0.980781 | 0.624646 | 0.036* | |
| C10 | 0.38159 (10) | 0.84898 (11) | 0.62913 (9) | 0.0258 (2) | |
| H10 | 0.334779 | 0.795943 | 0.642792 | 0.031* | |
| C11 | 0.62534 (9) | 0.62938 (9) | 0.52904 (7) | 0.01742 (18) | |
| C12 | 0.68528 (9) | 0.53833 (9) | 0.51616 (7) | 0.01920 (19) | |
| F1 | 0.77010 (7) | 0.50006 (6) | 0.57439 (5) | 0.02438 (15) | |
| C13 | 0.66631 (10) | 0.48285 (10) | 0.44545 (8) | 0.0219 (2) | |
| F2 | 0.72793 (8) | 0.39565 (7) | 0.43788 (6) | 0.03029 (18) | |
| C14 | 0.58391 (11) | 0.51903 (10) | 0.38166 (7) | 0.0229 (2) | |
| F3 | 0.56808 (8) | 0.47189 (7) | 0.31140 (5) | 0.03075 (18) | |
| C15 | 0.52011 (10) | 0.60735 (10) | 0.39152 (7) | 0.0219 (2) | |
| F4 | 0.43903 (8) | 0.64323 (7) | 0.33081 (5) | 0.03056 (18) | |
| C16 | 0.54109 (9) | 0.65902 (9) | 0.46376 (7) | 0.01871 (18) | |
| F5 | 0.47407 (6) | 0.74494 (6) | 0.46711 (5) | 0.02394 (15) | |
| C17 | 0.81897 (9) | 0.73023 (9) | 0.62649 (7) | 0.01881 (18) | |
| C18 | 0.85009 (10) | 0.78010 (10) | 0.56525 (8) | 0.0225 (2) | |
| F6 | 0.77384 (7) | 0.80890 (7) | 0.49856 (5) | 0.02657 (16) | |
| C19 | 0.95760 (12) | 0.80612 (13) | 0.56729 (9) | 0.0300 (3) | |
| F7 | 0.98118 (9) | 0.85775 (10) | 0.50685 (7) | 0.0423 (2) | |
| C20 | 1.04070 (11) | 0.77953 (15) | 0.63276 (11) | 0.0349 (3) | |
| F8 | 1.14520 (8) | 0.80396 (12) | 0.63564 (8) | 0.0545 (3) | |
| C21 | 1.01591 (11) | 0.72697 (13) | 0.69452 (10) | 0.0305 (3) | |
| F9 | 1.09644 (7) | 0.70038 (10) | 0.75876 (7) | 0.0443 (3) | |
| C22 | 0.90737 (10) | 0.70317 (10) | 0.69006 (8) | 0.0221 (2) | |
| F10 | 0.89124 (6) | 0.65161 (7) | 0.75357 (5) | 0.02621 (16) | |
| C23 | 0.4695 (4) | 0.9762 (6) | 0.9416 (4) | 0.086 (2) | 0.5 |
| H23A | 0.417411 | 0.974458 | 0.973522 | 0.128* | 0.5 |
| H23B | 0.511420 | 1.045096 | 0.951452 | 0.128* | 0.5 |
| H23C | 0.519747 | 0.913386 | 0.955440 | 0.128* | 0.5 |
| C24 | 0.4109 (6) | 0.9697 (6) | 0.8603 (5) | 0.0749 (18) | 0.5 |
| H24A | 0.464327 | 0.975185 | 0.828792 | 0.090* | 0.5 |
| H24B | 0.361469 | 1.034272 | 0.847065 | 0.090* | 0.5 |
| C25 | 0.3418 (6) | 0.8635 (5) | 0.8355 (4) | 0.0589 (13) | 0.5 |
| H25A | 0.296849 | 0.849535 | 0.872273 | 0.071* | 0.5 |
| H25B | 0.291733 | 0.873160 | 0.782364 | 0.071* | 0.5 |
| C26 | 0.4188 (6) | 0.7637 (8) | 0.8360 (5) | 0.0513 (16) | 0.5 |
| H26A | 0.457846 | 0.775760 | 0.795365 | 0.062* | 0.5 |
| H26B | 0.474409 | 0.761122 | 0.887388 | 0.062* | 0.5 |
| C27 | 0.3620 (4) | 0.6549 (4) | 0.8216 (3) | 0.0601 (12) | 0.5 |
| H27A | 0.325890 | 0.640356 | 0.862907 | 0.090* | 0.5 |
| H27B | 0.415795 | 0.596735 | 0.821837 | 0.090* | 0.5 |
| H27C | 0.307168 | 0.656125 | 0.770602 | 0.090* | 0.5 |
| O5' | 0.4671 (2) | 0.8125 (3) | 0.79186 (18) | 0.0468 (6) | 0.5 |
| C23' | 0.4719 (4) | 0.9254 (4) | 0.8165 (3) | 0.0516 (10) | 0.5 |
| H23D | 0.483429 | 0.974883 | 0.775048 | 0.062* | 0.5 |
| H23E | 0.532101 | 0.936716 | 0.864779 | 0.062* | 0.5 |
| C24' | 0.3630 (5) | 0.9471 (5) | 0.8314 (4) | 0.0565 (12) | 0.5 |
| H24C | 0.368106 | 1.006523 | 0.870569 | 0.068* | 0.5 |
| H24D | 0.307096 | 0.967374 | 0.782534 | 0.068* | 0.5 |
| C25' | 0.3374 (6) | 0.8395 (5) | 0.8620 (4) | 0.0616 (14) | 0.5 |
| H25C | 0.257285 | 0.830186 | 0.852460 | 0.074* | 0.5 |
| H25D | 0.372560 | 0.833615 | 0.918975 | 0.074* | 0.5 |
| C26' | 0.3826 (7) | 0.7550 (8) | 0.8179 (6) | 0.062 (2) | 0.5 |
| H26C | 0.414208 | 0.692110 | 0.852291 | 0.075* | 0.5 |
| H26D | 0.324670 | 0.726969 | 0.772589 | 0.075* | 0.5 |
Bis[1-oxopyridin-2-olato(1-)]bis(pentafluorophenyl)silicon(IV)–tetrahydrofuran–pentane (2/1/1) (1). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Si1 | 0.01361 (12) | 0.01244 (12) | 0.01899 (14) | −0.00038 (9) | 0.00070 (10) | 0.00039 (9) |
| O1 | 0.0211 (4) | 0.0138 (3) | 0.0178 (3) | 0.0001 (3) | 0.0015 (3) | 0.0003 (3) |
| O2 | 0.0201 (3) | 0.0138 (3) | 0.0194 (3) | −0.0008 (3) | 0.0020 (3) | 0.0006 (3) |
| O3 | 0.0157 (3) | 0.0129 (3) | 0.0264 (4) | 0.0001 (2) | 0.0035 (3) | 0.0010 (3) |
| O4 | 0.0140 (3) | 0.0142 (3) | 0.0232 (4) | 0.0006 (2) | 0.0031 (3) | 0.0017 (3) |
| N1 | 0.0170 (4) | 0.0152 (4) | 0.0199 (4) | 0.0008 (3) | 0.0010 (3) | 0.0011 (3) |
| C1 | 0.0167 (4) | 0.0154 (4) | 0.0197 (4) | 0.0008 (3) | 0.0010 (3) | 0.0004 (3) |
| N2 | 0.0152 (4) | 0.0161 (4) | 0.0247 (5) | 0.0002 (3) | 0.0030 (3) | −0.0001 (3) |
| C6 | 0.0152 (4) | 0.0139 (4) | 0.0213 (4) | 0.0010 (3) | 0.0010 (3) | 0.0006 (3) |
| N1' | 0.0167 (4) | 0.0154 (4) | 0.0197 (4) | 0.0008 (3) | 0.0010 (3) | 0.0004 (3) |
| C1' | 0.0170 (4) | 0.0152 (4) | 0.0199 (4) | 0.0008 (3) | 0.0010 (3) | 0.0011 (3) |
| N2' | 0.0152 (4) | 0.0139 (4) | 0.0213 (4) | 0.0010 (3) | 0.0010 (3) | 0.0006 (3) |
| C6' | 0.0152 (4) | 0.0161 (4) | 0.0247 (5) | 0.0002 (3) | 0.0030 (3) | −0.0001 (3) |
| C2 | 0.0239 (5) | 0.0188 (4) | 0.0224 (5) | 0.0009 (4) | 0.0026 (4) | −0.0029 (4) |
| C3 | 0.0283 (6) | 0.0269 (6) | 0.0205 (5) | 0.0035 (5) | 0.0034 (4) | −0.0001 (4) |
| C4 | 0.0272 (6) | 0.0237 (5) | 0.0225 (5) | 0.0021 (4) | 0.0041 (4) | 0.0045 (4) |
| C5 | 0.0226 (5) | 0.0168 (4) | 0.0227 (5) | 0.0008 (4) | 0.0020 (4) | 0.0025 (4) |
| C7 | 0.0212 (5) | 0.0145 (4) | 0.0227 (5) | 0.0008 (3) | 0.0017 (4) | 0.0009 (3) |
| C8 | 0.0228 (5) | 0.0176 (5) | 0.0334 (6) | 0.0050 (4) | 0.0031 (5) | −0.0002 (4) |
| C9 | 0.0215 (5) | 0.0236 (5) | 0.0466 (8) | 0.0046 (4) | 0.0103 (5) | −0.0014 (5) |
| C10 | 0.0187 (5) | 0.0209 (5) | 0.0390 (7) | −0.0003 (4) | 0.0097 (5) | −0.0008 (5) |
| C11 | 0.0169 (4) | 0.0134 (4) | 0.0203 (5) | −0.0006 (3) | 0.0023 (3) | 0.0006 (3) |
| C12 | 0.0189 (4) | 0.0148 (4) | 0.0225 (5) | −0.0003 (3) | 0.0033 (4) | −0.0002 (3) |
| F1 | 0.0220 (3) | 0.0197 (3) | 0.0281 (4) | 0.0055 (3) | 0.0010 (3) | 0.0004 (3) |
| C13 | 0.0233 (5) | 0.0162 (4) | 0.0265 (5) | −0.0019 (4) | 0.0072 (4) | −0.0028 (4) |
| F2 | 0.0306 (4) | 0.0221 (3) | 0.0392 (5) | 0.0028 (3) | 0.0114 (4) | −0.0084 (3) |
| C14 | 0.0280 (6) | 0.0193 (5) | 0.0212 (5) | −0.0071 (4) | 0.0061 (4) | −0.0031 (4) |
| F3 | 0.0402 (5) | 0.0288 (4) | 0.0235 (4) | −0.0098 (3) | 0.0091 (3) | −0.0074 (3) |
| C15 | 0.0243 (5) | 0.0183 (4) | 0.0194 (5) | −0.0047 (4) | −0.0005 (4) | 0.0016 (4) |
| F4 | 0.0353 (4) | 0.0258 (4) | 0.0218 (4) | −0.0030 (3) | −0.0073 (3) | 0.0025 (3) |
| C16 | 0.0185 (4) | 0.0136 (4) | 0.0213 (5) | −0.0014 (3) | 0.0007 (4) | 0.0007 (3) |
| F5 | 0.0221 (3) | 0.0171 (3) | 0.0266 (4) | 0.0040 (2) | −0.0036 (3) | 0.0004 (3) |
| C17 | 0.0155 (4) | 0.0165 (4) | 0.0233 (5) | 0.0000 (3) | 0.0033 (4) | −0.0009 (3) |
| C18 | 0.0184 (4) | 0.0215 (5) | 0.0267 (6) | −0.0011 (4) | 0.0049 (4) | −0.0008 (4) |
| F6 | 0.0240 (4) | 0.0300 (4) | 0.0250 (4) | 0.0003 (3) | 0.0054 (3) | 0.0031 (3) |
| C19 | 0.0229 (5) | 0.0334 (7) | 0.0362 (7) | −0.0040 (5) | 0.0124 (5) | 0.0005 (5) |
| F7 | 0.0343 (5) | 0.0526 (6) | 0.0457 (6) | −0.0077 (4) | 0.0206 (4) | 0.0073 (5) |
| C20 | 0.0165 (5) | 0.0425 (8) | 0.0457 (9) | −0.0052 (5) | 0.0086 (5) | −0.0013 (7) |
| F8 | 0.0186 (4) | 0.0766 (9) | 0.0686 (8) | −0.0104 (5) | 0.0126 (5) | 0.0092 (7) |
| C21 | 0.0151 (5) | 0.0373 (7) | 0.0359 (7) | −0.0003 (5) | 0.0012 (5) | 0.0014 (6) |
| F9 | 0.0162 (4) | 0.0625 (7) | 0.0466 (6) | −0.0005 (4) | −0.0046 (4) | 0.0081 (5) |
| C22 | 0.0148 (4) | 0.0221 (5) | 0.0274 (6) | −0.0001 (4) | 0.0022 (4) | −0.0001 (4) |
| F10 | 0.0193 (3) | 0.0278 (4) | 0.0277 (4) | 0.0008 (3) | 0.0001 (3) | 0.0045 (3) |
| C23 | 0.039 (2) | 0.100 (5) | 0.120 (6) | −0.009 (3) | 0.026 (3) | −0.029 (4) |
| C24 | 0.081 (4) | 0.057 (3) | 0.095 (5) | −0.023 (3) | 0.038 (4) | −0.016 (3) |
| C25 | 0.072 (3) | 0.054 (3) | 0.060 (3) | 0.009 (2) | 0.032 (3) | 0.015 (2) |
| C26 | 0.049 (3) | 0.060 (3) | 0.050 (4) | 0.013 (3) | 0.021 (3) | 0.014 (3) |
| C27 | 0.057 (3) | 0.070 (3) | 0.053 (3) | 0.004 (2) | 0.015 (2) | −0.018 (2) |
| O5' | 0.0403 (14) | 0.0526 (16) | 0.0476 (15) | 0.0081 (12) | 0.0122 (12) | −0.0033 (12) |
| C23' | 0.042 (2) | 0.064 (3) | 0.050 (2) | −0.0106 (18) | 0.0138 (17) | −0.0147 (19) |
| C24' | 0.059 (3) | 0.047 (2) | 0.075 (3) | 0.008 (2) | 0.037 (3) | 0.001 (2) |
| C25' | 0.071 (3) | 0.051 (3) | 0.072 (4) | 0.003 (2) | 0.036 (3) | 0.001 (3) |
| C26' | 0.083 (6) | 0.053 (3) | 0.056 (4) | 0.006 (4) | 0.026 (4) | 0.009 (3) |
Bis[1-oxopyridin-2-olato(1-)]bis(pentafluorophenyl)silicon(IV)–tetrahydrofuran–pentane (2/1/1) (1). Geometric parameters (Å, º)
| Si1—O1 | 1.7910 (9) | C14—F3 | 1.3353 (15) |
| Si1—O4 | 1.8042 (9) | C14—C15 | 1.3814 (19) |
| Si1—O3 | 1.8480 (9) | C15—F4 | 1.3430 (14) |
| Si1—O2 | 1.8503 (9) | C15—C16 | 1.3853 (17) |
| Si1—C11 | 1.9559 (12) | C16—F5 | 1.3557 (14) |
| Si1—C17 | 1.9683 (12) | C17—C18 | 1.3904 (18) |
| O1—C1' | 1.3324 (14) | C17—C22 | 1.3957 (17) |
| O1—N1 | 1.3324 (14) | C18—F6 | 1.3543 (15) |
| O2—N1' | 1.3244 (14) | C18—C19 | 1.3903 (18) |
| O2—C1 | 1.3244 (14) | C19—F7 | 1.3431 (18) |
| O3—C6' | 1.3263 (13) | C19—C20 | 1.377 (2) |
| O3—N2 | 1.3263 (13) | C20—F8 | 1.3452 (17) |
| O4—N2' | 1.3348 (13) | C20—C21 | 1.375 (2) |
| O4—C6 | 1.3348 (13) | C21—F9 | 1.3462 (17) |
| N1—C1 | 1.3562 (14) | C21—C22 | 1.3874 (18) |
| N1—C5 | 1.3714 (16) | C22—F10 | 1.3513 (16) |
| C1—C2 | 1.3686 (17) | C23—C24 | 1.434 (10) |
| N2—C6 | 1.3487 (15) | C23—H23A | 0.9800 |
| N2—C10 | 1.3706 (16) | C23—H23B | 0.9800 |
| C6—C7 | 1.3740 (15) | C23—H23C | 0.9800 |
| N1'—C1' | 1.3562 (14) | C24—C25 | 1.553 (8) |
| N1'—C2 | 1.3686 (17) | C24—H24A | 0.9900 |
| C1'—C5 | 1.3714 (16) | C24—H24B | 0.9900 |
| N2'—C6' | 1.3487 (15) | C25—C26 | 1.552 (10) |
| N2'—C7 | 1.3740 (15) | C25—H25A | 0.9900 |
| C6'—C10 | 1.3706 (16) | C25—H25B | 0.9900 |
| C2—C3 | 1.3732 (19) | C26—C27 | 1.491 (10) |
| C2—H2 | 0.9500 | C26—H26A | 0.9900 |
| C3—C4 | 1.4013 (19) | C26—H26B | 0.9900 |
| C3—H3 | 0.9500 | C27—H27A | 0.9800 |
| C4—C5 | 1.3828 (19) | C27—H27B | 0.9800 |
| C4—H4 | 0.9500 | C27—H27C | 0.9800 |
| C5—H5 | 0.9500 | O5'—C23' | 1.432 (5) |
| C7—C8 | 1.3770 (18) | O5'—C26' | 1.456 (10) |
| C7—H7 | 0.9500 | C23'—C24' | 1.498 (6) |
| C8—C9 | 1.398 (2) | C23'—H23D | 0.9900 |
| C8—H8 | 0.9500 | C23'—H23E | 0.9900 |
| C9—C10 | 1.3776 (19) | C24'—C25' | 1.482 (8) |
| C9—H9 | 0.9500 | C24'—H24C | 0.9900 |
| C10—H10 | 0.9500 | C24'—H24D | 0.9900 |
| C11—C16 | 1.3923 (16) | C25'—C26' | 1.494 (11) |
| C11—C12 | 1.3937 (16) | C25'—H25C | 0.9900 |
| C12—F1 | 1.3545 (14) | C25'—H25D | 0.9900 |
| C12—C13 | 1.3851 (17) | C26'—H26C | 0.9900 |
| C13—F2 | 1.3428 (14) | C26'—H26D | 0.9900 |
| C13—C14 | 1.3861 (19) | ||
| O1—Si1—O4 | 166.74 (4) | C12—C13—C14 | 119.49 (11) |
| O1—Si1—O3 | 86.65 (4) | F3—C14—C15 | 120.33 (12) |
| O4—Si1—O3 | 84.60 (4) | F3—C14—C13 | 120.99 (12) |
| O1—Si1—O2 | 85.17 (4) | C15—C14—C13 | 118.65 (11) |
| O4—Si1—O2 | 84.53 (4) | F4—C15—C14 | 119.78 (11) |
| O3—Si1—O2 | 87.56 (4) | F4—C15—C16 | 120.51 (11) |
| O1—Si1—C11 | 91.41 (4) | C14—C15—C16 | 119.71 (11) |
| O4—Si1—C11 | 98.54 (4) | F5—C16—C15 | 115.00 (10) |
| O3—Si1—C11 | 90.16 (4) | F5—C16—C11 | 120.69 (10) |
| O2—Si1—C11 | 175.99 (5) | C15—C16—C11 | 124.29 (11) |
| O1—Si1—C17 | 99.44 (5) | C18—C17—C22 | 113.39 (11) |
| O4—Si1—C17 | 88.68 (4) | C18—C17—Si1 | 122.91 (9) |
| O3—Si1—C17 | 172.66 (4) | C22—C17—Si1 | 123.45 (9) |
| O2—Si1—C17 | 88.88 (5) | F6—C18—C19 | 115.25 (12) |
| C11—Si1—C17 | 93.75 (5) | F6—C18—C17 | 120.49 (11) |
| C1'—O1—Si1 | 112.84 (7) | C19—C18—C17 | 124.25 (12) |
| N1—O1—Si1 | 112.84 (7) | F7—C19—C20 | 119.76 (13) |
| N1'—O2—Si1 | 111.23 (7) | F7—C19—C18 | 120.96 (14) |
| C1—O2—Si1 | 111.23 (7) | C20—C19—C18 | 119.28 (13) |
| C6'—O3—Si1 | 110.81 (7) | F8—C20—C21 | 120.52 (15) |
| N2—O3—Si1 | 110.81 (7) | F8—C20—C19 | 120.04 (15) |
| N2'—O4—Si1 | 111.71 (7) | C21—C20—C19 | 119.44 (13) |
| C6—O4—Si1 | 111.71 (7) | F9—C21—C20 | 119.99 (13) |
| O1—N1—C1 | 114.69 (9) | F9—C21—C22 | 120.74 (14) |
| O1—N1—C5 | 124.26 (10) | C20—C21—C22 | 119.27 (13) |
| C1—N1—C5 | 121.02 (11) | F10—C22—C21 | 114.91 (11) |
| O2—C1—N1 | 114.61 (10) | F10—C22—C17 | 120.77 (10) |
| O2—C1—C2 | 123.66 (10) | C21—C22—C17 | 124.32 (13) |
| N1—C1—C2 | 121.70 (10) | C24—C23—H23A | 109.5 |
| O3—N2—C6 | 114.78 (9) | C24—C23—H23B | 109.5 |
| O3—N2—C10 | 124.02 (10) | H23A—C23—H23B | 109.5 |
| C6—N2—C10 | 121.20 (10) | C24—C23—H23C | 109.5 |
| O4—C6—N2 | 114.45 (9) | H23A—C23—H23C | 109.5 |
| O4—C6—C7 | 124.04 (10) | H23B—C23—H23C | 109.5 |
| N2—C6—C7 | 121.50 (10) | C23—C24—C25 | 115.3 (6) |
| O2—N1'—C1' | 114.61 (10) | C23—C24—H24A | 108.4 |
| O2—N1'—C2 | 123.66 (10) | C25—C24—H24A | 108.4 |
| C1'—N1'—C2 | 121.70 (10) | C23—C24—H24B | 108.4 |
| O1—C1'—N1' | 114.69 (9) | C25—C24—H24B | 108.4 |
| O1—C1'—C5 | 124.26 (10) | H24A—C24—H24B | 107.5 |
| N1'—C1'—C5 | 121.02 (11) | C26—C25—C24 | 109.8 (6) |
| O4—N2'—C6' | 114.45 (9) | C26—C25—H25A | 109.7 |
| O4—N2'—C7 | 124.04 (10) | C24—C25—H25A | 109.7 |
| C6'—N2'—C7 | 121.50 (10) | C26—C25—H25B | 109.7 |
| O3—C6'—N2' | 114.78 (9) | C24—C25—H25B | 109.7 |
| O3—C6'—C10 | 124.02 (10) | H25A—C25—H25B | 108.2 |
| N2'—C6'—C10 | 121.20 (10) | C27—C26—C25 | 114.5 (6) |
| N1'—C2—C3 | 118.72 (11) | C27—C26—H26A | 108.6 |
| C1—C2—C3 | 118.72 (11) | C25—C26—H26A | 108.6 |
| C1—C2—H2 | 120.6 | C27—C26—H26B | 108.6 |
| C3—C2—H2 | 120.6 | C25—C26—H26B | 108.6 |
| C2—C3—C4 | 119.88 (12) | H26A—C26—H26B | 107.6 |
| C2—C3—H3 | 120.1 | C26—C27—H27A | 109.5 |
| C4—C3—H3 | 120.1 | C26—C27—H27B | 109.5 |
| C5—C4—C3 | 120.26 (12) | H27A—C27—H27B | 109.5 |
| C5—C4—H4 | 119.9 | C26—C27—H27C | 109.5 |
| C3—C4—H4 | 119.9 | H27A—C27—H27C | 109.5 |
| C1'—C5—C4 | 118.42 (11) | H27B—C27—H27C | 109.5 |
| N1—C5—C4 | 118.42 (11) | C23'—O5'—C26' | 109.5 (5) |
| N1—C5—H5 | 120.8 | O5'—C23'—C24' | 104.9 (4) |
| C4—C5—H5 | 120.8 | O5'—C23'—H23D | 110.8 |
| N2'—C7—C8 | 118.54 (11) | C24'—C23'—H23D | 110.8 |
| C6—C7—C8 | 118.54 (11) | O5'—C23'—H23E | 110.8 |
| C6—C7—H7 | 120.7 | C24'—C23'—H23E | 110.8 |
| C8—C7—H7 | 120.7 | H23D—C23'—H23E | 108.8 |
| C7—C8—C9 | 119.84 (11) | C25'—C24'—C23' | 102.3 (4) |
| C7—C8—H8 | 120.1 | C25'—C24'—H24C | 111.3 |
| C9—C8—H8 | 120.1 | C23'—C24'—H24C | 111.3 |
| C10—C9—C8 | 120.24 (12) | C25'—C24'—H24D | 111.3 |
| C10—C9—H9 | 119.9 | C23'—C24'—H24D | 111.3 |
| C8—C9—H9 | 119.9 | H24C—C24'—H24D | 109.2 |
| C6'—C10—C9 | 118.59 (12) | C24'—C25'—C26' | 105.0 (6) |
| N2—C10—C9 | 118.59 (12) | C24'—C25'—H25C | 110.7 |
| N2—C10—H10 | 120.7 | C26'—C25'—H25C | 110.7 |
| C9—C10—H10 | 120.7 | C24'—C25'—H25D | 110.7 |
| C16—C11—C12 | 113.42 (10) | C26'—C25'—H25D | 110.7 |
| C16—C11—Si1 | 123.97 (8) | H25C—C25'—H25D | 108.8 |
| C12—C11—Si1 | 122.43 (8) | O5'—C26'—C25' | 104.9 (7) |
| F1—C12—C13 | 115.50 (10) | O5'—C26'—H26C | 110.8 |
| F1—C12—C11 | 120.11 (10) | C25'—C26'—H26C | 110.8 |
| C13—C12—C11 | 124.37 (11) | O5'—C26'—H26D | 110.8 |
| F2—C13—C12 | 120.66 (12) | C25'—C26'—H26D | 110.8 |
| F2—C13—C14 | 119.84 (11) | H26C—C26'—H26D | 108.8 |
| O4—Si1—O1—C1' | −28.5 (2) | O1—C1'—C5—C4 | 178.47 (11) |
| O3—Si1—O1—C1' | −77.23 (8) | N1'—C1'—C5—C4 | 0.59 (18) |
| O2—Si1—O1—C1' | 10.60 (8) | O1—N1—C5—C4 | 178.47 (11) |
| C11—Si1—O1—C1' | −167.31 (8) | C1—N1—C5—C4 | 0.59 (18) |
| C17—Si1—O1—C1' | 98.65 (8) | C3—C4—C5—C1' | −0.66 (19) |
| O4—Si1—O1—N1 | −28.5 (2) | C3—C4—C5—N1 | −0.66 (19) |
| O3—Si1—O1—N1 | −77.23 (8) | O4—N2'—C7—C8 | 176.47 (11) |
| O2—Si1—O1—N1 | 10.60 (8) | C6'—N2'—C7—C8 | −3.09 (18) |
| C11—Si1—O1—N1 | −167.31 (8) | O4—C6—C7—C8 | 176.47 (11) |
| C17—Si1—O1—N1 | 98.65 (8) | N2—C6—C7—C8 | −3.09 (18) |
| O1—Si1—O2—N1' | −10.44 (7) | N2'—C7—C8—C9 | 0.8 (2) |
| O4—Si1—O2—N1' | 161.20 (8) | C6—C7—C8—C9 | 0.8 (2) |
| O3—Si1—O2—N1' | 76.40 (7) | C7—C8—C9—C10 | 1.3 (2) |
| C17—Si1—O2—N1' | −110.02 (8) | O3—C6'—C10—C9 | 179.41 (13) |
| O1—Si1—O2—C1 | −10.44 (7) | N2'—C6'—C10—C9 | −1.0 (2) |
| O4—Si1—O2—C1 | 161.20 (8) | O3—N2—C10—C9 | 179.41 (13) |
| O3—Si1—O2—C1 | 76.40 (7) | C6—N2—C10—C9 | −1.0 (2) |
| C17—Si1—O2—C1 | −110.02 (8) | C8—C9—C10—C6' | −1.3 (2) |
| O1—Si1—O3—C6' | 154.54 (8) | C8—C9—C10—N2 | −1.3 (2) |
| O4—Si1—O3—C6' | −15.49 (8) | C16—C11—C12—F1 | 179.86 (10) |
| O2—Si1—O3—C6' | 69.23 (8) | Si1—C11—C12—F1 | 4.45 (15) |
| C11—Si1—O3—C6' | −114.06 (8) | C16—C11—C12—C13 | 1.43 (17) |
| O1—Si1—O3—N2 | 154.54 (8) | Si1—C11—C12—C13 | −173.97 (9) |
| O4—Si1—O3—N2 | −15.49 (8) | F1—C12—C13—F2 | 1.51 (17) |
| O2—Si1—O3—N2 | 69.23 (8) | C11—C12—C13—F2 | 180.00 (11) |
| C11—Si1—O3—N2 | −114.06 (8) | F1—C12—C13—C14 | −177.48 (11) |
| O1—Si1—O4—N2' | −31.6 (2) | C11—C12—C13—C14 | 1.01 (19) |
| O3—Si1—O4—N2' | 17.32 (7) | F2—C13—C14—F3 | −3.34 (18) |
| O2—Si1—O4—N2' | −70.74 (8) | C12—C13—C14—F3 | 175.65 (11) |
| C11—Si1—O4—N2' | 106.66 (8) | F2—C13—C14—C15 | 178.54 (11) |
| C17—Si1—O4—N2' | −159.74 (8) | C12—C13—C14—C15 | −2.46 (18) |
| O1—Si1—O4—C6 | −31.6 (2) | F3—C14—C15—F4 | 2.55 (18) |
| O3—Si1—O4—C6 | 17.32 (7) | C13—C14—C15—F4 | −179.32 (11) |
| O2—Si1—O4—C6 | −70.74 (8) | F3—C14—C15—C16 | −176.71 (11) |
| C11—Si1—O4—C6 | 106.66 (8) | C13—C14—C15—C16 | 1.41 (18) |
| C17—Si1—O4—C6 | −159.74 (8) | F4—C15—C16—F5 | 0.51 (16) |
| Si1—O1—N1—C1 | −8.78 (12) | C14—C15—C16—F5 | 179.77 (11) |
| Si1—O1—N1—C5 | 173.22 (9) | F4—C15—C16—C11 | −178.03 (11) |
| Si1—O2—C1—N1 | 8.22 (12) | C14—C15—C16—C11 | 1.23 (19) |
| Si1—O2—C1—C2 | −173.65 (9) | C12—C11—C16—F5 | 178.98 (10) |
| O1—N1—C1—O2 | 0.12 (14) | Si1—C11—C16—F5 | −5.70 (16) |
| C5—N1—C1—O2 | 178.19 (10) | C12—C11—C16—C15 | −2.56 (17) |
| O1—N1—C1—C2 | −178.04 (10) | Si1—C11—C16—C15 | 172.76 (9) |
| C5—N1—C1—C2 | 0.02 (17) | C22—C17—C18—F6 | 178.41 (11) |
| Si1—O3—N2—C6 | 10.62 (12) | Si1—C17—C18—F6 | −7.13 (16) |
| Si1—O3—N2—C10 | −169.72 (11) | C22—C17—C18—C19 | −2.95 (19) |
| Si1—O4—C6—N2 | −15.96 (12) | Si1—C17—C18—C19 | 171.50 (11) |
| Si1—O4—C6—C7 | 164.44 (9) | F6—C18—C19—F7 | 1.0 (2) |
| O3—N2—C6—O4 | 3.27 (15) | C17—C18—C19—F7 | −177.71 (13) |
| C10—N2—C6—O4 | −176.40 (11) | F6—C18—C19—C20 | −179.78 (14) |
| O3—N2—C6—C7 | −177.13 (11) | C17—C18—C19—C20 | 1.5 (2) |
| C10—N2—C6—C7 | 3.20 (18) | F7—C19—C20—F8 | −0.9 (3) |
| Si1—O2—N1'—C1' | 8.22 (12) | C18—C19—C20—F8 | 179.89 (15) |
| Si1—O2—N1'—C2 | −173.65 (9) | F7—C19—C20—C21 | 179.80 (15) |
| Si1—O1—C1'—N1' | −8.78 (12) | C18—C19—C20—C21 | 0.6 (2) |
| Si1—O1—C1'—C5 | 173.22 (9) | F8—C20—C21—F9 | 0.5 (3) |
| O2—N1'—C1'—O1 | 0.12 (14) | C19—C20—C21—F9 | 179.87 (15) |
| C2—N1'—C1'—O1 | −178.04 (10) | F8—C20—C21—C22 | 179.77 (15) |
| O2—N1'—C1'—C5 | 178.19 (10) | C19—C20—C21—C22 | −0.9 (3) |
| C2—N1'—C1'—C5 | 0.02 (17) | F9—C21—C22—F10 | −0.7 (2) |
| Si1—O4—N2'—C6' | −15.96 (12) | C20—C21—C22—F10 | −179.93 (14) |
| Si1—O4—N2'—C7 | 164.44 (9) | F9—C21—C22—C17 | 178.44 (13) |
| Si1—O3—C6'—N2' | 10.62 (12) | C20—C21—C22—C17 | −0.8 (2) |
| Si1—O3—C6'—C10 | −169.72 (11) | C18—C17—C22—F10 | −178.32 (11) |
| O4—N2'—C6'—O3 | 3.27 (15) | Si1—C17—C22—F10 | 7.26 (17) |
| C7—N2'—C6'—O3 | −177.13 (11) | C18—C17—C22—C21 | 2.58 (19) |
| O4—N2'—C6'—C10 | −176.40 (11) | Si1—C17—C22—C21 | −171.84 (11) |
| C7—N2'—C6'—C10 | 3.20 (18) | C23—C24—C25—C26 | −72.1 (8) |
| O2—N1'—C2—C3 | −178.57 (11) | C24—C25—C26—C27 | 173.7 (6) |
| C1'—N1'—C2—C3 | −0.58 (18) | C26'—O5'—C23'—C24' | −20.7 (6) |
| O2—C1—C2—C3 | −178.57 (11) | O5'—C23'—C24'—C25' | 34.3 (6) |
| N1—C1—C2—C3 | −0.58 (18) | C23'—C24'—C25'—C26' | −35.2 (7) |
| N1'—C2—C3—C4 | 0.5 (2) | C23'—O5'—C26'—C25' | −1.3 (7) |
| C1—C2—C3—C4 | 0.5 (2) | C24'—C25'—C26'—O5' | 23.2 (8) |
| C2—C3—C4—C5 | 0.1 (2) |
Bis[1-oxopyridin-2-olato(1-)]bis(pentafluorophenyl)silicon(IV)–tetrahydrofuran–pentane (2/1/1) (1). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···F1i | 0.95 | 2.34 | 3.2809 (15) | 170 |
| C3—H3···F7ii | 0.95 | 3.23 | 3.6665 (18) | 110 |
| C4—H4···F6iii | 0.95 | 2.68 | 3.5757 (16) | 158 |
| C5—H5···F4iv | 0.95 | 2.59 | 3.2997 (15) | 132 |
| C7—H7···F5v | 0.95 | 2.57 | 3.2307 (14) | 127 |
| C8—H8···F5v | 0.95 | 2.77 | 3.3319 (16) | 119 |
| C8—H8···F6v | 0.95 | 2.56 | 3.2230 (15) | 127 |
| C8—H8···F9i | 0.95 | 2.81 | 3.2507 (18) | 110 |
| C9—H9···F6v | 0.95 | 3.31 | 3.6095 (17) | 101 |
| C9—H9···F8vi | 0.95 | 2.80 | 3.2885 (18) | 113 |
| C10—H10···F2iv | 0.95 | 2.73 | 3.3522 (16) | 124 |
| C10—H10···F8vi | 0.95 | 2.37 | 3.0797 (16) | 131 |
Symmetry codes: (i) −x+3/2, y+1/2, −z+3/2; (ii) x−1/2, −y+3/2, z+1/2; (iii) −x+3/2, y−1/2, −z+3/2; (iv) −x+1, −y+1, −z+1; (v) −x+1, −y+2, −z+1; (vi) x−1, y, z.
Bis[1-oxopyridin-2-olato(1-)]bis(4-mwthylphenyl)silicon(IV) (2). Crystal data
| C24H22N2O4Si | Z = 2 |
| Mr = 430.52 | F(000) = 452 |
| Triclinic, P1 | Dx = 1.398 Mg m−3 |
| a = 8.5662 (8) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 8.8343 (8) Å | Cell parameters from 4074 reflections |
| c = 14.7801 (14) Å | θ = 2.4–30.3° |
| α = 93.057 (2)° | µ = 0.15 mm−1 |
| β = 105.3716 (19)° | T = 100 K |
| γ = 106.7565 (18)° | Block, colorless |
| V = 1022.45 (17) Å3 | 0.24 × 0.24 × 0.20 mm |
Bis[1-oxopyridin-2-olato(1-)]bis(4-mwthylphenyl)silicon(IV) (2). Data collection
| Bruker SMART APEXII CCD platform diffractometer | 4231 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.065 |
| ω scans | θmax = 30.6°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | h = −12→12 |
| Tmin = 0.695, Tmax = 0.746 | k = −12→12 |
| 25883 measured reflections | l = −21→21 |
| 6239 independent reflections |
Bis[1-oxopyridin-2-olato(1-)]bis(4-mwthylphenyl)silicon(IV) (2). Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.054 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.149 | H-atom parameters constrained |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.061P)2 + 0.4958P] where P = (Fo2 + 2Fc2)/3 |
| 6239 reflections | (Δ/σ)max = 0.001 |
| 284 parameters | Δρmax = 1.01 e Å−3 |
| 0 restraints | Δρmin = −0.43 e Å−3 |
Bis[1-oxopyridin-2-olato(1-)]bis(4-mwthylphenyl)silicon(IV) (2). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Both bidentate ligands are disordered with the coplanar flips of themselves (0.658 (19):0.342 (19) and 0.612 (19):0.388 (19) for the rings containing C1/N1 and C6/N2, respectively). Due to resolution limitations, the disorder model did not include the entire ring, but was modeled by refining the occupancies of the two atoms types (C and N) at the oxygen-coordinating portions of the rings. The occupancies at each site were constrained to sum to one and additionally to sum to one C and one N atom between the two sites on each ring. The positional and anisotropic displacement parameters, espectively, at each site of disorder were constrained to be equivalent. It is understood that this type of disorder model will likely exhibit a weighted average of Si–O bond lengths, trending with the disorder ratios. |
Bis[1-oxopyridin-2-olato(1-)]bis(4-mwthylphenyl)silicon(IV) (2). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Si1 | 0.46085 (7) | 0.32324 (6) | 0.72662 (4) | 0.01840 (13) | |
| O1 | 0.62723 (17) | 0.50446 (16) | 0.78902 (10) | 0.0207 (3) | |
| O2 | 0.64598 (17) | 0.23371 (16) | 0.75110 (10) | 0.0219 (3) | |
| O3 | 0.33449 (17) | 0.13549 (16) | 0.65212 (9) | 0.0202 (3) | |
| O4 | 0.53005 (18) | 0.37788 (16) | 0.61623 (10) | 0.0225 (3) | |
| N1 | 0.7819 (2) | 0.4865 (2) | 0.82216 (12) | 0.0200 (4) | 0.658 (19) |
| C1 | 0.7904 (2) | 0.3388 (2) | 0.80021 (13) | 0.0210 (4) | 0.658 (19) |
| N2 | 0.3498 (2) | 0.1276 (2) | 0.56448 (12) | 0.0198 (4) | 0.612 (19) |
| C6 | 0.4563 (2) | 0.2595 (2) | 0.54532 (13) | 0.0211 (4) | 0.612 (19) |
| N1' | 0.7904 (2) | 0.3388 (2) | 0.80021 (13) | 0.0210 (4) | 0.342 (19) |
| C1' | 0.7819 (2) | 0.4865 (2) | 0.82216 (12) | 0.0200 (4) | 0.342 (19) |
| N2' | 0.4563 (2) | 0.2595 (2) | 0.54532 (13) | 0.0211 (4) | 0.388 (19) |
| C6' | 0.3498 (2) | 0.1276 (2) | 0.56448 (12) | 0.0198 (4) | 0.388 (19) |
| C2 | 0.9459 (3) | 0.3113 (3) | 0.83015 (14) | 0.0241 (4) | |
| H2 | 0.954380 | 0.208720 | 0.814746 | 0.029* | |
| C3 | 1.0883 (3) | 0.4329 (3) | 0.88228 (15) | 0.0269 (4) | |
| H3 | 1.195403 | 0.414653 | 0.902786 | 0.032* | |
| C4 | 1.0749 (3) | 0.5832 (3) | 0.90496 (15) | 0.0283 (5) | |
| H4 | 1.172679 | 0.667323 | 0.941344 | 0.034* | |
| C5 | 0.9201 (3) | 0.6090 (3) | 0.87452 (15) | 0.0248 (4) | |
| H5 | 0.909370 | 0.710641 | 0.889705 | 0.030* | |
| C7 | 0.4840 (3) | 0.2623 (3) | 0.45720 (14) | 0.0248 (4) | |
| H7 | 0.558326 | 0.354501 | 0.443053 | 0.030* | |
| C8 | 0.4013 (3) | 0.1286 (3) | 0.39071 (15) | 0.0282 (5) | |
| H8 | 0.418987 | 0.128457 | 0.329907 | 0.034* | |
| C9 | 0.2919 (3) | −0.0068 (3) | 0.41123 (15) | 0.0279 (5) | |
| H9 | 0.235815 | −0.098844 | 0.364838 | 0.034* | |
| C10 | 0.2658 (3) | −0.0062 (2) | 0.49899 (14) | 0.0242 (4) | |
| H10 | 0.190706 | −0.097031 | 0.513907 | 0.029* | |
| C11 | 0.2970 (2) | 0.4356 (2) | 0.69348 (13) | 0.0176 (4) | |
| C12 | 0.3356 (3) | 0.5987 (2) | 0.72212 (14) | 0.0232 (4) | |
| H12 | 0.448901 | 0.656954 | 0.758615 | 0.028* | |
| C13 | 0.2159 (3) | 0.6806 (3) | 0.69979 (15) | 0.0252 (4) | |
| H13 | 0.249201 | 0.791922 | 0.720466 | 0.030* | |
| C14 | 0.0474 (3) | 0.5988 (3) | 0.64712 (15) | 0.0256 (4) | |
| C15 | 0.0053 (3) | 0.4365 (3) | 0.61821 (16) | 0.0280 (5) | |
| H15 | −0.108304 | 0.378531 | 0.582028 | 0.034* | |
| C16 | 0.1265 (3) | 0.3562 (3) | 0.64118 (15) | 0.0255 (4) | |
| H16 | 0.092432 | 0.244689 | 0.620812 | 0.031* | |
| C17 | −0.0844 (3) | 0.6841 (3) | 0.62348 (18) | 0.0337 (5) | |
| H17A | −0.124820 | 0.680593 | 0.554615 | 0.051* | |
| H17B | −0.033391 | 0.795498 | 0.653631 | 0.051* | |
| H17C | −0.180549 | 0.631563 | 0.646813 | 0.051* | |
| C18 | 0.4015 (2) | 0.2402 (2) | 0.83540 (13) | 0.0176 (4) | |
| C19 | 0.2635 (3) | 0.1029 (2) | 0.82682 (15) | 0.0261 (4) | |
| H19 | 0.195061 | 0.050060 | 0.765313 | 0.031* | |
| C20 | 0.2234 (3) | 0.0412 (3) | 0.90568 (16) | 0.0303 (5) | |
| H20 | 0.128409 | −0.052066 | 0.896705 | 0.036* | |
| C21 | 0.3202 (3) | 0.1141 (3) | 0.99752 (15) | 0.0266 (4) | |
| C22 | 0.4531 (3) | 0.2532 (3) | 1.00683 (15) | 0.0275 (4) | |
| H22 | 0.518803 | 0.308042 | 1.068349 | 0.033* | |
| C23 | 0.4923 (3) | 0.3146 (3) | 0.92768 (15) | 0.0255 (4) | |
| H23 | 0.584245 | 0.410556 | 0.936991 | 0.031* | |
| C24 | 0.2834 (3) | 0.0407 (3) | 1.08269 (17) | 0.0371 (6) | |
| H24A | 0.163999 | −0.026642 | 1.065461 | 0.056* | |
| H24B | 0.304562 | 0.125643 | 1.134065 | 0.056* | |
| H24C | 0.357891 | −0.024422 | 1.103770 | 0.056* |
Bis[1-oxopyridin-2-olato(1-)]bis(4-mwthylphenyl)silicon(IV) (2). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Si1 | 0.0199 (3) | 0.0163 (3) | 0.0186 (3) | 0.00566 (19) | 0.0056 (2) | 0.00027 (19) |
| O1 | 0.0170 (6) | 0.0180 (7) | 0.0257 (7) | 0.0070 (5) | 0.0028 (5) | −0.0002 (5) |
| O2 | 0.0197 (7) | 0.0190 (7) | 0.0274 (7) | 0.0076 (5) | 0.0068 (6) | −0.0006 (5) |
| O3 | 0.0252 (7) | 0.0200 (7) | 0.0156 (6) | 0.0062 (5) | 0.0078 (5) | −0.0001 (5) |
| O4 | 0.0261 (7) | 0.0206 (7) | 0.0214 (7) | 0.0058 (6) | 0.0100 (6) | 0.0003 (5) |
| N1 | 0.0170 (8) | 0.0218 (9) | 0.0224 (9) | 0.0065 (7) | 0.0069 (7) | 0.0041 (7) |
| C1 | 0.0227 (9) | 0.0231 (9) | 0.0210 (9) | 0.0097 (7) | 0.0096 (7) | 0.0051 (7) |
| N2 | 0.0224 (9) | 0.0228 (9) | 0.0167 (8) | 0.0121 (7) | 0.0049 (7) | 0.0007 (6) |
| C6 | 0.0263 (10) | 0.0222 (9) | 0.0192 (9) | 0.0126 (8) | 0.0082 (7) | 0.0039 (7) |
| N1' | 0.0227 (9) | 0.0231 (9) | 0.0210 (9) | 0.0097 (7) | 0.0096 (7) | 0.0051 (7) |
| C1' | 0.0170 (8) | 0.0218 (9) | 0.0224 (9) | 0.0065 (7) | 0.0069 (7) | 0.0041 (7) |
| N2' | 0.0263 (10) | 0.0222 (9) | 0.0192 (9) | 0.0126 (8) | 0.0082 (7) | 0.0039 (7) |
| C6' | 0.0224 (9) | 0.0228 (9) | 0.0167 (8) | 0.0121 (7) | 0.0049 (7) | 0.0007 (6) |
| C2 | 0.0265 (10) | 0.0287 (11) | 0.0232 (10) | 0.0142 (8) | 0.0110 (8) | 0.0058 (8) |
| C3 | 0.0208 (10) | 0.0371 (12) | 0.0285 (11) | 0.0144 (9) | 0.0101 (8) | 0.0092 (9) |
| C4 | 0.0174 (9) | 0.0336 (12) | 0.0286 (11) | 0.0032 (8) | 0.0036 (8) | 0.0040 (9) |
| C5 | 0.0213 (10) | 0.0219 (10) | 0.0304 (11) | 0.0055 (8) | 0.0074 (8) | 0.0045 (8) |
| C7 | 0.0297 (11) | 0.0307 (11) | 0.0208 (9) | 0.0167 (9) | 0.0100 (8) | 0.0074 (8) |
| C8 | 0.0335 (12) | 0.0360 (12) | 0.0205 (10) | 0.0194 (10) | 0.0082 (9) | 0.0014 (8) |
| C9 | 0.0303 (11) | 0.0290 (11) | 0.0230 (10) | 0.0117 (9) | 0.0044 (8) | −0.0044 (8) |
| C10 | 0.0249 (10) | 0.0224 (10) | 0.0249 (10) | 0.0104 (8) | 0.0042 (8) | −0.0021 (8) |
| C11 | 0.0187 (9) | 0.0202 (9) | 0.0158 (8) | 0.0071 (7) | 0.0068 (7) | 0.0037 (7) |
| C12 | 0.0210 (9) | 0.0243 (10) | 0.0236 (10) | 0.0074 (8) | 0.0052 (8) | 0.0023 (8) |
| C13 | 0.0261 (10) | 0.0230 (10) | 0.0286 (11) | 0.0101 (8) | 0.0090 (8) | 0.0045 (8) |
| C14 | 0.0239 (10) | 0.0301 (11) | 0.0271 (10) | 0.0131 (9) | 0.0082 (8) | 0.0106 (8) |
| C15 | 0.0218 (10) | 0.0271 (11) | 0.0301 (11) | 0.0045 (8) | 0.0031 (8) | 0.0044 (8) |
| C16 | 0.0214 (10) | 0.0242 (10) | 0.0272 (10) | 0.0041 (8) | 0.0048 (8) | 0.0019 (8) |
| C17 | 0.0275 (11) | 0.0358 (13) | 0.0419 (13) | 0.0156 (10) | 0.0090 (10) | 0.0146 (10) |
| C18 | 0.0191 (9) | 0.0148 (8) | 0.0218 (9) | 0.0079 (7) | 0.0082 (7) | 0.0021 (7) |
| C19 | 0.0327 (11) | 0.0190 (10) | 0.0236 (10) | 0.0050 (8) | 0.0074 (8) | 0.0001 (8) |
| C20 | 0.0385 (12) | 0.0195 (10) | 0.0298 (11) | 0.0016 (9) | 0.0131 (10) | 0.0041 (8) |
| C21 | 0.0346 (12) | 0.0251 (10) | 0.0276 (10) | 0.0147 (9) | 0.0141 (9) | 0.0103 (8) |
| C22 | 0.0271 (11) | 0.0342 (12) | 0.0200 (10) | 0.0100 (9) | 0.0050 (8) | 0.0025 (8) |
| C23 | 0.0232 (10) | 0.0275 (11) | 0.0234 (10) | 0.0045 (8) | 0.0075 (8) | 0.0008 (8) |
| C24 | 0.0485 (15) | 0.0360 (13) | 0.0327 (12) | 0.0147 (11) | 0.0184 (11) | 0.0140 (10) |
Bis[1-oxopyridin-2-olato(1-)]bis(4-mwthylphenyl)silicon(IV) (2). Geometric parameters (Å, º)
| Si1—O3 | 1.8093 (14) | C7—H7 | 0.9500 |
| Si1—O1 | 1.8097 (14) | C8—C9 | 1.395 (3) |
| Si1—O4 | 1.9179 (15) | C8—H8 | 0.9500 |
| Si1—C11 | 1.9202 (19) | C9—C10 | 1.373 (3) |
| Si1—O2 | 1.9290 (15) | C9—H9 | 0.9500 |
| Si1—C18 | 1.9301 (19) | C10—H10 | 0.9500 |
| O1—C1' | 1.344 (2) | C11—C12 | 1.396 (3) |
| O1—N1 | 1.344 (2) | C11—C16 | 1.406 (3) |
| O2—N1' | 1.307 (2) | C12—C13 | 1.399 (3) |
| O2—C1 | 1.307 (2) | C12—H12 | 0.9500 |
| O3—C6' | 1.336 (2) | C13—C14 | 1.398 (3) |
| O3—N2 | 1.336 (2) | C13—H13 | 0.9500 |
| O4—N2' | 1.320 (2) | C14—C15 | 1.387 (3) |
| O4—C6 | 1.320 (2) | C14—C17 | 1.508 (3) |
| N1—C1 | 1.356 (3) | C15—C16 | 1.400 (3) |
| N1—C5 | 1.363 (3) | C15—H15 | 0.9500 |
| C1—C2 | 1.384 (3) | C16—H16 | 0.9500 |
| N2—C6 | 1.353 (3) | C17—H17A | 0.9800 |
| N2—C10 | 1.367 (3) | C17—H17B | 0.9800 |
| C6—C7 | 1.384 (3) | C17—H17C | 0.9800 |
| N1'—C1' | 1.356 (3) | C18—C23 | 1.395 (3) |
| N1'—C2 | 1.384 (3) | C18—C19 | 1.401 (3) |
| C1'—C5 | 1.363 (3) | C19—C20 | 1.395 (3) |
| N2'—C6' | 1.353 (3) | C19—H19 | 0.9500 |
| N2'—C7 | 1.384 (3) | C20—C21 | 1.395 (3) |
| C6'—C10 | 1.367 (3) | C20—H20 | 0.9500 |
| C2—C3 | 1.376 (3) | C21—C22 | 1.385 (3) |
| C2—H2 | 0.9500 | C21—C24 | 1.511 (3) |
| C3—C4 | 1.396 (3) | C22—C23 | 1.395 (3) |
| C3—H3 | 0.9500 | C22—H22 | 0.9500 |
| C4—C5 | 1.372 (3) | C23—H23 | 0.9500 |
| C4—H4 | 0.9500 | C24—H24A | 0.9800 |
| C5—H5 | 0.9500 | C24—H24B | 0.9800 |
| C7—C8 | 1.374 (3) | C24—H24C | 0.9800 |
| O3—Si1—O1 | 165.96 (7) | C8—C7—N2' | 118.4 (2) |
| O3—Si1—O4 | 83.76 (6) | C8—C7—H7 | 120.8 |
| O1—Si1—O4 | 86.24 (7) | C6—C7—H7 | 120.8 |
| O3—Si1—C11 | 98.02 (8) | C7—C8—C9 | 120.9 (2) |
| O1—Si1—C11 | 91.68 (7) | C7—C8—H8 | 119.5 |
| O4—Si1—C11 | 89.37 (7) | C9—C8—H8 | 119.5 |
| O3—Si1—O2 | 85.72 (6) | C10—C9—C8 | 119.52 (19) |
| O1—Si1—O2 | 83.35 (6) | C10—C9—H9 | 120.2 |
| O4—Si1—O2 | 83.28 (6) | C8—C9—H9 | 120.2 |
| C11—Si1—O2 | 171.36 (8) | C6'—C10—C9 | 118.8 (2) |
| O3—Si1—C18 | 91.04 (7) | N2—C10—C9 | 118.8 (2) |
| O1—Si1—C18 | 97.64 (8) | N2—C10—H10 | 120.6 |
| O4—Si1—C18 | 171.40 (7) | C9—C10—H10 | 120.6 |
| C11—Si1—C18 | 98.16 (8) | C12—C11—C16 | 115.36 (18) |
| O2—Si1—C18 | 89.53 (7) | C12—C11—Si1 | 123.04 (15) |
| C1'—O1—Si1 | 114.21 (11) | C16—C11—Si1 | 121.55 (15) |
| N1—O1—Si1 | 114.21 (11) | C11—C12—C13 | 123.31 (19) |
| N1'—O2—Si1 | 111.83 (12) | C11—C12—H12 | 118.3 |
| C1—O2—Si1 | 111.83 (12) | C13—C12—H12 | 118.3 |
| C6'—O3—Si1 | 114.16 (12) | C14—C13—C12 | 120.1 (2) |
| N2—O3—Si1 | 114.16 (12) | C14—C13—H13 | 119.9 |
| N2'—O4—Si1 | 111.43 (12) | C12—C13—H13 | 119.9 |
| C6—O4—Si1 | 111.43 (12) | C15—C14—C13 | 117.77 (19) |
| O1—N1—C1 | 115.44 (16) | C15—C14—C17 | 121.2 (2) |
| O1—N1—C5 | 121.98 (17) | C13—C14—C17 | 121.0 (2) |
| C1—N1—C5 | 122.57 (17) | C14—C15—C16 | 121.4 (2) |
| O2—C1—N1 | 115.09 (16) | C14—C15—H15 | 119.3 |
| O2—C1—C2 | 125.89 (18) | C16—C15—H15 | 119.3 |
| N1—C1—C2 | 119.02 (18) | C15—C16—C11 | 122.0 (2) |
| O3—N2—C6 | 115.62 (16) | C15—C16—H16 | 119.0 |
| O3—N2—C10 | 122.21 (18) | C11—C16—H16 | 119.0 |
| C6—N2—C10 | 122.16 (17) | C14—C17—H17A | 109.5 |
| O4—C6—N2 | 115.03 (16) | C14—C17—H17B | 109.5 |
| O4—C6—C7 | 124.77 (18) | H17A—C17—H17B | 109.5 |
| N2—C6—C7 | 120.18 (18) | C14—C17—H17C | 109.5 |
| O2—N1'—C1' | 115.09 (16) | H17A—C17—H17C | 109.5 |
| O2—N1'—C2 | 125.89 (18) | H17B—C17—H17C | 109.5 |
| C1'—N1'—C2 | 119.02 (18) | C23—C18—C19 | 115.71 (18) |
| O1—C1'—N1' | 115.44 (16) | C23—C18—Si1 | 121.98 (15) |
| O1—C1'—C5 | 121.98 (17) | C19—C18—Si1 | 122.31 (15) |
| N1'—C1'—C5 | 122.57 (17) | C20—C19—C18 | 122.1 (2) |
| O4—N2'—C6' | 115.03 (16) | C20—C19—H19 | 118.9 |
| O4—N2'—C7 | 124.77 (18) | C18—C19—H19 | 118.9 |
| C6'—N2'—C7 | 120.18 (18) | C19—C20—C21 | 121.1 (2) |
| O3—C6'—N2' | 115.62 (16) | C19—C20—H20 | 119.4 |
| O3—C6'—C10 | 122.21 (18) | C21—C20—H20 | 119.4 |
| N2'—C6'—C10 | 122.16 (17) | C22—C21—C20 | 117.22 (19) |
| C3—C2—C1 | 119.8 (2) | C22—C21—C24 | 121.8 (2) |
| C3—C2—N1' | 119.8 (2) | C20—C21—C24 | 120.9 (2) |
| C3—C2—H2 | 120.1 | C21—C22—C23 | 121.3 (2) |
| C1—C2—H2 | 120.1 | C21—C22—H22 | 119.3 |
| C2—C3—C4 | 119.81 (19) | C23—C22—H22 | 119.3 |
| C2—C3—H3 | 120.1 | C18—C23—C22 | 122.4 (2) |
| C4—C3—H3 | 120.1 | C18—C23—H23 | 118.8 |
| C5—C4—C3 | 119.8 (2) | C22—C23—H23 | 118.8 |
| C5—C4—H4 | 120.1 | C21—C24—H24A | 109.5 |
| C3—C4—H4 | 120.1 | C21—C24—H24B | 109.5 |
| C1'—C5—C4 | 119.0 (2) | H24A—C24—H24B | 109.5 |
| N1—C5—C4 | 119.0 (2) | C21—C24—H24C | 109.5 |
| N1—C5—H5 | 120.5 | H24A—C24—H24C | 109.5 |
| C4—C5—H5 | 120.5 | H24B—C24—H24C | 109.5 |
| C8—C7—C6 | 118.4 (2) | ||
| O3—Si1—O1—C1' | −41.7 (3) | C7—N2'—C6'—O3 | 178.59 (16) |
| O4—Si1—O1—C1' | −86.26 (13) | O4—N2'—C6'—C10 | −178.92 (17) |
| C11—Si1—O1—C1' | −175.52 (13) | C7—N2'—C6'—C10 | −0.2 (3) |
| O2—Si1—O1—C1' | −2.61 (12) | O2—C1—C2—C3 | −179.47 (18) |
| C18—Si1—O1—C1' | 86.02 (13) | N1—C1—C2—C3 | 0.7 (3) |
| O3—Si1—O1—N1 | −41.7 (3) | O2—N1'—C2—C3 | −179.47 (18) |
| O4—Si1—O1—N1 | −86.26 (13) | C1'—N1'—C2—C3 | 0.7 (3) |
| C11—Si1—O1—N1 | −175.52 (13) | C1—C2—C3—C4 | 0.2 (3) |
| O2—Si1—O1—N1 | −2.61 (12) | N1'—C2—C3—C4 | 0.2 (3) |
| C18—Si1—O1—N1 | 86.02 (13) | C2—C3—C4—C5 | −0.5 (3) |
| O1—Si1—O3—C6' | −44.8 (3) | O1—C1'—C5—C4 | −179.03 (18) |
| O4—Si1—O3—C6' | −0.04 (12) | N1'—C1'—C5—C4 | 1.2 (3) |
| C11—Si1—O3—C6' | 88.43 (13) | O1—N1—C5—C4 | −179.03 (18) |
| O2—Si1—O3—C6' | −83.73 (12) | C1—N1—C5—C4 | 1.2 (3) |
| C18—Si1—O3—C6' | −173.18 (13) | C3—C4—C5—C1' | −0.2 (3) |
| O1—Si1—O3—N2 | −44.8 (3) | C3—C4—C5—N1 | −0.2 (3) |
| O4—Si1—O3—N2 | −0.04 (12) | O4—C6—C7—C8 | 178.41 (18) |
| C11—Si1—O3—N2 | 88.43 (13) | N2—C6—C7—C8 | −0.1 (3) |
| O2—Si1—O3—N2 | −83.73 (12) | O4—N2'—C7—C8 | 178.41 (18) |
| C18—Si1—O3—N2 | −173.18 (13) | C6'—N2'—C7—C8 | −0.1 (3) |
| Si1—O1—N1—C1 | 2.8 (2) | C6—C7—C8—C9 | 0.1 (3) |
| Si1—O1—N1—C5 | −176.97 (15) | N2'—C7—C8—C9 | 0.1 (3) |
| Si1—O2—C1—N1 | −1.0 (2) | C7—C8—C9—C10 | 0.3 (3) |
| Si1—O2—C1—C2 | 179.15 (16) | O3—C6'—C10—C9 | −178.11 (18) |
| O1—N1—C1—O2 | −1.1 (2) | N2'—C6'—C10—C9 | 0.6 (3) |
| C5—N1—C1—O2 | 178.70 (17) | O3—N2—C10—C9 | −178.11 (18) |
| O1—N1—C1—C2 | 178.76 (17) | C6—N2—C10—C9 | 0.6 (3) |
| C5—N1—C1—C2 | −1.5 (3) | C8—C9—C10—C6' | −0.7 (3) |
| Si1—O3—N2—C6 | 0.1 (2) | C8—C9—C10—N2 | −0.7 (3) |
| Si1—O3—N2—C10 | 178.91 (14) | C16—C11—C12—C13 | −1.1 (3) |
| Si1—O4—C6—N2 | 0.1 (2) | Si1—C11—C12—C13 | −178.69 (16) |
| Si1—O4—C6—C7 | −178.56 (15) | C11—C12—C13—C14 | 0.7 (3) |
| O3—N2—C6—O4 | −0.1 (2) | C12—C13—C14—C15 | −0.3 (3) |
| C10—N2—C6—O4 | −178.92 (17) | C12—C13—C14—C17 | 179.1 (2) |
| O3—N2—C6—C7 | 178.59 (16) | C13—C14—C15—C16 | 0.3 (3) |
| C10—N2—C6—C7 | −0.2 (3) | C17—C14—C15—C16 | −179.0 (2) |
| Si1—O2—N1'—C1' | −1.0 (2) | C14—C15—C16—C11 | −0.8 (3) |
| Si1—O2—N1'—C2 | 179.15 (16) | C12—C11—C16—C15 | 1.1 (3) |
| Si1—O1—C1'—N1' | 2.8 (2) | Si1—C11—C16—C15 | 178.77 (16) |
| Si1—O1—C1'—C5 | −176.97 (15) | C23—C18—C19—C20 | −2.3 (3) |
| O2—N1'—C1'—O1 | −1.1 (2) | Si1—C18—C19—C20 | 178.54 (17) |
| C2—N1'—C1'—O1 | 178.76 (16) | C18—C19—C20—C21 | −0.2 (3) |
| O2—N1'—C1'—C5 | 178.70 (17) | C19—C20—C21—C22 | 2.5 (3) |
| C2—N1'—C1'—C5 | −1.5 (3) | C19—C20—C21—C24 | −175.9 (2) |
| Si1—O4—N2'—C6' | 0.1 (2) | C20—C21—C22—C23 | −2.4 (3) |
| Si1—O4—N2'—C7 | −178.56 (15) | C24—C21—C22—C23 | 176.0 (2) |
| Si1—O3—C6'—N2' | 0.1 (2) | C19—C18—C23—C22 | 2.5 (3) |
| Si1—O3—C6'—C10 | 178.91 (14) | Si1—C18—C23—C22 | −178.35 (16) |
| O4—N2'—C6'—O3 | −0.1 (2) | C21—C22—C23—C18 | −0.2 (3) |
Dimesitylbis[1-oxopyridin-2-olato(1-)]silicon(IV) (3). Crystal data
| C28H30N2O4Si | Dx = 1.319 Mg m−3 |
| Mr = 486.63 | Cu Kα radiation, λ = 1.54184 Å |
| Orthorhombic, P212121 | Cell parameters from 11827 reflections |
| a = 12.5710 (2) Å | θ = 4.5–76.9° |
| b = 12.68898 (19) Å | µ = 1.15 mm−1 |
| c = 15.3580 (2) Å | T = 100 K |
| V = 2449.80 (7) Å3 | Block, colourless |
| Z = 4 | 0.09 × 0.07 × 0.06 mm |
| F(000) = 1032 |
Dimesitylbis[1-oxopyridin-2-olato(1-)]silicon(IV) (3). Data collection
| XtaLAB Synergy, Dualflex, HyPix diffractometer | 5138 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Cu) X-ray Source | 4847 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.048 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 77.7°, θmin = 4.5° |
| ω scans | h = −15→13 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2019) | k = −15→13 |
| Tmin = 0.674, Tmax = 1.000 | l = −19→19 |
| 22120 measured reflections |
Dimesitylbis[1-oxopyridin-2-olato(1-)]silicon(IV) (3). Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.032 | H-atom parameters constrained |
| wR(F2) = 0.079 | w = 1/[σ2(Fo2) + (0.0363P)2 + 0.5221P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max < 0.001 |
| 5138 reflections | Δρmax = 0.27 e Å−3 |
| 324 parameters | Δρmin = −0.25 e Å−3 |
| 0 restraints | Absolute structure: Flack x determined using 1985 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| Primary atom site location: dual | Absolute structure parameter: −0.034 (17) |
Dimesitylbis[1-oxopyridin-2-olato(1-)]silicon(IV) (3). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Both bidentate ligands are disordered with the coplanar flips of themselves (0.68 (3):0.32 (3) and 0.61 (3):0.39 (3) for the rings containing C1/N1 and C6/N2, respectively). Due to resolution limitations, the disorder was modeled by refining the occupancies of the two atoms types (C and N) at the oxygen-coordinating portions of the rings. The occupancies at each site were constrained to sum to one and additionally sum to one C and one N atom between the two sites on each ring. The positional and anisotropic displacement parameters,respectively, at each site of disorder were constrained to be equivalent. It is understood that this type of disorder model will likely exhibit a weighted average of Si–O bond lengths, trending with the disorder ratios. |
Dimesitylbis[1-oxopyridin-2-olato(1-)]silicon(IV) (3). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Si1 | 0.74087 (5) | 0.70602 (4) | 0.61168 (4) | 0.01803 (13) | |
| O1 | 0.85697 (13) | 0.71920 (12) | 0.53026 (10) | 0.0221 (3) | |
| O2 | 0.74861 (14) | 0.56957 (11) | 0.58288 (10) | 0.0214 (3) | |
| O3 | 0.65878 (13) | 0.71836 (12) | 0.50381 (10) | 0.0234 (3) | |
| O4 | 0.73653 (13) | 0.84835 (11) | 0.60520 (10) | 0.0211 (3) | |
| N1 | 0.87289 (15) | 0.62870 (15) | 0.48904 (12) | 0.0189 (4) | 0.69 (3) |
| N2 | 0.65746 (17) | 0.81659 (16) | 0.47604 (13) | 0.0220 (5) | 0.62 (3) |
| C1 | 0.81438 (17) | 0.54657 (16) | 0.51912 (13) | 0.0188 (5) | 0.69 (3) |
| C2 | 0.8272 (2) | 0.44746 (17) | 0.48429 (15) | 0.0231 (5) | |
| H2A | 0.787040 | 0.389558 | 0.505675 | 0.028* | 0.69 (3) |
| H2B | 0.787040 | 0.389558 | 0.505675 | 0.028* | 0.31 (3) |
| C3 | 0.8994 (2) | 0.4339 (2) | 0.41775 (17) | 0.0285 (5) | |
| H3 | 0.909972 | 0.365897 | 0.393401 | 0.034* | |
| C4 | 0.9572 (2) | 0.5195 (2) | 0.38596 (16) | 0.0282 (5) | |
| H4 | 1.006388 | 0.509933 | 0.339691 | 0.034* | |
| C5 | 0.94261 (18) | 0.61737 (19) | 0.42175 (15) | 0.0228 (4) | |
| H5A | 0.980582 | 0.676479 | 0.399952 | 0.027* | 0.69 (3) |
| H5B | 0.980582 | 0.676479 | 0.399952 | 0.027* | 0.31 (3) |
| C6 | 0.70096 (17) | 0.88806 (16) | 0.53139 (14) | 0.0207 (5) | 0.62 (3) |
| C7 | 0.7032 (2) | 0.99372 (18) | 0.51074 (17) | 0.0276 (5) | |
| H7A | 0.735764 | 1.043126 | 0.548819 | 0.033* | 0.62 (3) |
| H7B | 0.735764 | 1.043126 | 0.548819 | 0.033* | 0.38 (3) |
| C8 | 0.6575 (2) | 1.0263 (2) | 0.43398 (19) | 0.0350 (6) | |
| H8 | 0.657349 | 1.098958 | 0.418960 | 0.042* | |
| C9 | 0.6110 (2) | 0.9523 (2) | 0.37793 (18) | 0.0368 (6) | |
| H9 | 0.578834 | 0.974765 | 0.325137 | 0.044* | |
| C10 | 0.6121 (2) | 0.8479 (2) | 0.39921 (16) | 0.0297 (5) | |
| H10 | 0.581545 | 0.797277 | 0.361044 | 0.036* | 0.62 (3) |
| H10A | 0.581545 | 0.797277 | 0.361044 | 0.036* | 0.38 (3) |
| C11 | 0.60545 (17) | 0.69383 (16) | 0.67607 (13) | 0.0181 (4) | |
| C12 | 0.58361 (19) | 0.76268 (17) | 0.74716 (14) | 0.0205 (4) | |
| C13 | 0.48283 (19) | 0.76687 (19) | 0.78479 (14) | 0.0234 (5) | |
| H13 | 0.471010 | 0.814780 | 0.831333 | 0.028* | |
| C14 | 0.39920 (19) | 0.70421 (19) | 0.75726 (15) | 0.0253 (5) | |
| C15 | 0.42009 (19) | 0.63418 (18) | 0.68986 (16) | 0.0240 (5) | |
| H15 | 0.364703 | 0.589014 | 0.670402 | 0.029* | |
| C16 | 0.51939 (18) | 0.62777 (17) | 0.64974 (14) | 0.0211 (4) | |
| C17 | 0.6651 (2) | 0.83405 (19) | 0.79019 (15) | 0.0250 (5) | |
| H17A | 0.716867 | 0.791058 | 0.822061 | 0.038* | |
| H17B | 0.629070 | 0.881774 | 0.830772 | 0.038* | |
| H17C | 0.701983 | 0.875357 | 0.745519 | 0.038* | |
| C18 | 0.2900 (2) | 0.7130 (2) | 0.7976 (2) | 0.0367 (6) | |
| H18A | 0.296947 | 0.733539 | 0.858907 | 0.055* | |
| H18B | 0.253707 | 0.644859 | 0.793814 | 0.055* | |
| H18C | 0.248598 | 0.766439 | 0.766425 | 0.055* | |
| C19 | 0.5262 (2) | 0.54616 (18) | 0.57768 (16) | 0.0258 (5) | |
| H19A | 0.556781 | 0.578532 | 0.525422 | 0.039* | |
| H19B | 0.454824 | 0.519629 | 0.564472 | 0.039* | |
| H19C | 0.571523 | 0.487629 | 0.596638 | 0.039* | |
| C20 | 0.84221 (17) | 0.70007 (16) | 0.70814 (13) | 0.0187 (4) | |
| C21 | 0.83877 (18) | 0.61468 (17) | 0.76842 (14) | 0.0194 (4) | |
| C22 | 0.90158 (19) | 0.61500 (17) | 0.84337 (15) | 0.0218 (4) | |
| H22 | 0.895115 | 0.558090 | 0.883212 | 0.026* | |
| C23 | 0.97317 (18) | 0.69527 (18) | 0.86195 (14) | 0.0229 (4) | |
| C24 | 0.98178 (18) | 0.77565 (19) | 0.80091 (15) | 0.0222 (4) | |
| H24 | 1.032215 | 0.830141 | 0.810637 | 0.027* | |
| C25 | 0.91924 (17) | 0.77949 (18) | 0.72584 (14) | 0.0198 (4) | |
| C26 | 0.77207 (19) | 0.51561 (17) | 0.75647 (15) | 0.0226 (5) | |
| H26A | 0.699209 | 0.535362 | 0.740715 | 0.034* | |
| H26B | 0.771184 | 0.475396 | 0.810947 | 0.034* | |
| H26C | 0.802884 | 0.472274 | 0.710061 | 0.034* | |
| C27 | 1.0393 (2) | 0.6943 (2) | 0.94376 (16) | 0.0335 (6) | |
| H27A | 0.997851 | 0.723939 | 0.991945 | 0.050* | |
| H27B | 1.103527 | 0.736728 | 0.934811 | 0.050* | |
| H27C | 1.059530 | 0.621677 | 0.957731 | 0.050* | |
| C28 | 0.9418 (2) | 0.87384 (18) | 0.66723 (16) | 0.0252 (5) | |
| H28A | 0.934572 | 0.852709 | 0.606139 | 0.038* | |
| H28B | 1.014281 | 0.899187 | 0.677740 | 0.038* | |
| H28C | 0.890940 | 0.930276 | 0.680135 | 0.038* | |
| C1' | 0.87289 (15) | 0.62870 (15) | 0.48904 (12) | 0.0189 (4) | 0.31 (3) |
| N1' | 0.81438 (17) | 0.54657 (16) | 0.51912 (13) | 0.0188 (5) | 0.31 (3) |
| C6' | 0.65746 (17) | 0.81659 (16) | 0.47604 (13) | 0.0220 (5) | 0.38 (3) |
| N2' | 0.70096 (17) | 0.88806 (16) | 0.53139 (14) | 0.0207 (5) | 0.38 (3) |
Dimesitylbis[1-oxopyridin-2-olato(1-)]silicon(IV) (3). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Si1 | 0.0229 (3) | 0.0143 (2) | 0.0169 (3) | −0.0010 (2) | 0.0020 (2) | −0.0006 (2) |
| O1 | 0.0290 (8) | 0.0170 (7) | 0.0204 (7) | −0.0031 (7) | 0.0052 (6) | −0.0021 (6) |
| O2 | 0.0268 (8) | 0.0173 (6) | 0.0203 (7) | −0.0016 (6) | 0.0064 (7) | −0.0020 (5) |
| O3 | 0.0298 (8) | 0.0212 (7) | 0.0193 (7) | −0.0037 (7) | −0.0015 (6) | 0.0004 (6) |
| O4 | 0.0277 (8) | 0.0169 (6) | 0.0188 (7) | −0.0001 (6) | −0.0014 (7) | 0.0020 (6) |
| N1 | 0.0214 (10) | 0.0185 (9) | 0.0169 (9) | −0.0006 (7) | −0.0006 (7) | 0.0000 (7) |
| N2 | 0.0218 (10) | 0.0240 (10) | 0.0202 (9) | −0.0007 (8) | 0.0016 (8) | 0.0022 (8) |
| C1 | 0.0206 (10) | 0.0207 (10) | 0.0150 (9) | 0.0002 (8) | −0.0008 (8) | 0.0006 (8) |
| C2 | 0.0282 (12) | 0.0185 (10) | 0.0226 (10) | 0.0010 (9) | −0.0038 (9) | −0.0003 (8) |
| C3 | 0.0327 (13) | 0.0271 (11) | 0.0256 (11) | 0.0085 (10) | −0.0041 (10) | −0.0088 (9) |
| C4 | 0.0259 (12) | 0.0384 (12) | 0.0204 (11) | 0.0056 (10) | 0.0030 (10) | −0.0043 (10) |
| C5 | 0.0207 (10) | 0.0312 (11) | 0.0165 (10) | −0.0019 (9) | 0.0002 (9) | 0.0029 (9) |
| C6 | 0.0198 (10) | 0.0216 (10) | 0.0208 (10) | 0.0005 (8) | 0.0014 (8) | 0.0033 (8) |
| C7 | 0.0265 (12) | 0.0221 (11) | 0.0341 (13) | 0.0003 (9) | 0.0049 (10) | 0.0044 (10) |
| C8 | 0.0319 (13) | 0.0321 (13) | 0.0412 (14) | 0.0058 (11) | 0.0074 (12) | 0.0170 (11) |
| C9 | 0.0332 (14) | 0.0477 (15) | 0.0295 (13) | 0.0075 (12) | 0.0003 (11) | 0.0172 (12) |
| C10 | 0.0256 (12) | 0.0430 (14) | 0.0204 (11) | −0.0024 (10) | 0.0001 (9) | 0.0031 (10) |
| C11 | 0.0202 (10) | 0.0169 (10) | 0.0172 (9) | 0.0027 (8) | −0.0003 (8) | 0.0007 (8) |
| C12 | 0.0257 (11) | 0.0185 (10) | 0.0171 (10) | 0.0051 (8) | −0.0025 (8) | 0.0025 (8) |
| C13 | 0.0300 (12) | 0.0222 (11) | 0.0181 (10) | 0.0072 (9) | 0.0005 (9) | 0.0005 (8) |
| C14 | 0.0241 (11) | 0.0255 (11) | 0.0261 (11) | 0.0051 (10) | 0.0037 (9) | 0.0066 (9) |
| C15 | 0.0246 (11) | 0.0213 (11) | 0.0260 (12) | −0.0012 (9) | −0.0015 (9) | 0.0030 (9) |
| C16 | 0.0251 (11) | 0.0177 (10) | 0.0204 (11) | −0.0004 (9) | −0.0004 (9) | 0.0027 (8) |
| C17 | 0.0270 (12) | 0.0266 (11) | 0.0216 (10) | 0.0020 (9) | 0.0004 (9) | −0.0076 (9) |
| C18 | 0.0287 (12) | 0.0378 (14) | 0.0436 (15) | 0.0028 (12) | 0.0109 (11) | 0.0019 (13) |
| C19 | 0.0280 (12) | 0.0222 (11) | 0.0273 (11) | −0.0060 (9) | 0.0010 (10) | −0.0035 (9) |
| C20 | 0.0216 (10) | 0.0158 (9) | 0.0186 (9) | 0.0015 (9) | 0.0034 (8) | −0.0011 (8) |
| C21 | 0.0205 (10) | 0.0166 (9) | 0.0210 (10) | 0.0026 (8) | 0.0056 (8) | −0.0005 (8) |
| C22 | 0.0249 (11) | 0.0182 (10) | 0.0223 (11) | 0.0044 (9) | 0.0037 (9) | 0.0023 (8) |
| C23 | 0.0235 (11) | 0.0237 (11) | 0.0215 (10) | 0.0026 (9) | 0.0029 (8) | 0.0003 (9) |
| C24 | 0.0225 (11) | 0.0202 (10) | 0.0237 (10) | −0.0018 (9) | 0.0035 (8) | −0.0030 (9) |
| C25 | 0.0220 (10) | 0.0163 (10) | 0.0211 (10) | 0.0008 (8) | 0.0040 (8) | −0.0007 (8) |
| C26 | 0.0276 (12) | 0.0168 (10) | 0.0232 (11) | −0.0015 (9) | 0.0029 (9) | 0.0027 (8) |
| C27 | 0.0375 (13) | 0.0348 (13) | 0.0281 (12) | −0.0056 (12) | −0.0054 (11) | 0.0063 (11) |
| C28 | 0.0287 (12) | 0.0208 (11) | 0.0260 (12) | −0.0055 (9) | −0.0026 (10) | 0.0025 (9) |
| C1' | 0.0214 (10) | 0.0185 (9) | 0.0169 (9) | −0.0006 (7) | −0.0006 (7) | 0.0000 (7) |
| N1' | 0.0206 (10) | 0.0207 (10) | 0.0150 (9) | 0.0002 (8) | −0.0008 (8) | 0.0006 (8) |
| C6' | 0.0218 (10) | 0.0240 (10) | 0.0202 (9) | −0.0007 (8) | 0.0016 (8) | 0.0022 (8) |
| N2' | 0.0198 (10) | 0.0216 (10) | 0.0208 (10) | 0.0005 (8) | 0.0014 (8) | 0.0033 (8) |
Dimesitylbis[1-oxopyridin-2-olato(1-)]silicon(IV) (3). Geometric parameters (Å, º)
| Si1—O1 | 1.9291 (16) | C11—C12 | 1.425 (3) |
| Si1—O2 | 1.7896 (15) | C11—C16 | 1.427 (3) |
| Si1—O3 | 1.9581 (16) | C12—C13 | 1.394 (3) |
| Si1—O4 | 1.8096 (15) | C12—C17 | 1.519 (3) |
| Si1—C11 | 1.975 (2) | C13—H13 | 0.9500 |
| Si1—C20 | 1.955 (2) | C13—C14 | 1.384 (4) |
| O1—N1 | 1.326 (2) | C14—C15 | 1.389 (4) |
| O1—C1' | 1.326 (2) | C14—C18 | 1.510 (3) |
| O2—C1 | 1.314 (3) | C15—H15 | 0.9500 |
| O2—N1' | 1.314 (3) | C15—C16 | 1.395 (3) |
| O3—N2 | 1.317 (3) | C16—C19 | 1.518 (3) |
| O3—C6' | 1.317 (3) | C17—H17A | 0.9800 |
| O4—C6 | 1.319 (3) | C17—H17B | 0.9800 |
| O4—N2' | 1.319 (3) | C17—H17C | 0.9800 |
| N1—C1 | 1.357 (3) | C18—H18A | 0.9800 |
| N1—C5 | 1.363 (3) | C18—H18B | 0.9800 |
| N2—C6 | 1.358 (3) | C18—H18C | 0.9800 |
| N2—C10 | 1.369 (3) | C19—H19A | 0.9800 |
| C1—C2 | 1.376 (3) | C19—H19B | 0.9800 |
| C2—H2A | 0.9500 | C19—H19C | 0.9800 |
| C2—H2B | 0.9500 | C20—C21 | 1.426 (3) |
| C2—C3 | 1.378 (4) | C20—C25 | 1.424 (3) |
| C2—N1' | 1.376 (3) | C21—C22 | 1.396 (3) |
| C3—H3 | 0.9500 | C21—C26 | 1.522 (3) |
| C3—C4 | 1.394 (4) | C22—H22 | 0.9500 |
| C4—H4 | 0.9500 | C22—C23 | 1.389 (3) |
| C4—C5 | 1.371 (3) | C23—C24 | 1.390 (3) |
| C5—H5A | 0.9500 | C23—C27 | 1.507 (3) |
| C5—H5B | 0.9500 | C24—H24 | 0.9500 |
| C5—C1' | 1.363 (3) | C24—C25 | 1.396 (3) |
| C6—C7 | 1.378 (3) | C25—C28 | 1.524 (3) |
| C7—H7A | 0.9500 | C26—H26A | 0.9800 |
| C7—H7B | 0.9500 | C26—H26B | 0.9800 |
| C7—C8 | 1.375 (4) | C26—H26C | 0.9800 |
| C7—N2' | 1.378 (3) | C27—H27A | 0.9800 |
| C8—H8 | 0.9500 | C27—H27B | 0.9800 |
| C8—C9 | 1.402 (4) | C27—H27C | 0.9800 |
| C9—H9 | 0.9500 | C28—H28A | 0.9800 |
| C9—C10 | 1.365 (4) | C28—H28B | 0.9800 |
| C10—H10 | 0.9500 | C28—H28C | 0.9800 |
| C10—H10A | 0.9500 | C1'—N1' | 1.357 (3) |
| C10—C6' | 1.369 (3) | C6'—N2' | 1.358 (3) |
| O1—Si1—O3 | 80.99 (7) | C14—C13—C12 | 122.8 (2) |
| O1—Si1—C11 | 169.59 (8) | C14—C13—H13 | 118.6 |
| O1—Si1—C20 | 90.09 (8) | C13—C14—C15 | 116.8 (2) |
| O2—Si1—O1 | 83.25 (7) | C13—C14—C18 | 121.5 (2) |
| O2—Si1—O3 | 84.09 (7) | C15—C14—C18 | 121.7 (2) |
| O2—Si1—O4 | 162.48 (8) | C14—C15—H15 | 118.8 |
| O2—Si1—C11 | 95.44 (8) | C14—C15—C16 | 122.4 (2) |
| O2—Si1—C20 | 96.57 (8) | C16—C15—H15 | 118.8 |
| O3—Si1—C11 | 88.61 (8) | C11—C16—C19 | 124.4 (2) |
| O4—Si1—O1 | 84.30 (7) | C15—C16—C11 | 121.3 (2) |
| O4—Si1—O3 | 81.82 (7) | C15—C16—C19 | 114.4 (2) |
| O4—Si1—C11 | 94.57 (8) | C12—C17—H17A | 109.5 |
| O4—Si1—C20 | 95.73 (8) | C12—C17—H17B | 109.5 |
| C20—Si1—O3 | 170.93 (8) | C12—C17—H17C | 109.5 |
| C20—Si1—C11 | 100.32 (9) | H17A—C17—H17B | 109.5 |
| N1—O1—Si1 | 110.40 (13) | H17A—C17—H17C | 109.5 |
| C1'—O1—Si1 | 110.40 (13) | H17B—C17—H17C | 109.5 |
| C1—O2—Si1 | 115.67 (13) | C14—C18—H18A | 109.5 |
| N1'—O2—Si1 | 115.67 (13) | C14—C18—H18B | 109.5 |
| N2—O3—Si1 | 110.86 (13) | C14—C18—H18C | 109.5 |
| C6'—O3—Si1 | 110.86 (13) | H18A—C18—H18B | 109.5 |
| C6—O4—Si1 | 116.03 (13) | H18A—C18—H18C | 109.5 |
| N2'—O4—Si1 | 116.03 (13) | H18B—C18—H18C | 109.5 |
| O1—N1—C1 | 114.88 (18) | C16—C19—H19A | 109.5 |
| O1—N1—C5 | 123.39 (19) | C16—C19—H19B | 109.5 |
| C1—N1—C5 | 121.73 (19) | C16—C19—H19C | 109.5 |
| O3—N2—C6 | 115.09 (18) | H19A—C19—H19B | 109.5 |
| O3—N2—C10 | 123.9 (2) | H19A—C19—H19C | 109.5 |
| C6—N2—C10 | 120.9 (2) | H19B—C19—H19C | 109.5 |
| O2—C1—N1 | 115.10 (18) | C21—C20—Si1 | 120.11 (16) |
| O2—C1—C2 | 124.5 (2) | C25—C20—Si1 | 124.08 (16) |
| N1—C1—C2 | 120.4 (2) | C25—C20—C21 | 115.75 (19) |
| C1—C2—H2A | 120.7 | C20—C21—C26 | 124.5 (2) |
| C1—C2—C3 | 118.6 (2) | C22—C21—C20 | 121.1 (2) |
| C3—C2—H2A | 120.7 | C22—C21—C26 | 114.42 (19) |
| C3—C2—H2B | 120.7 | C21—C22—H22 | 118.7 |
| N1'—C2—H2B | 120.7 | C23—C22—C21 | 122.6 (2) |
| N1'—C2—C3 | 118.6 (2) | C23—C22—H22 | 118.7 |
| C2—C3—H3 | 119.8 | C22—C23—C24 | 116.8 (2) |
| C2—C3—C4 | 120.4 (2) | C22—C23—C27 | 121.5 (2) |
| C4—C3—H3 | 119.8 | C24—C23—C27 | 121.7 (2) |
| C3—C4—H4 | 120.1 | C23—C24—H24 | 118.7 |
| C5—C4—C3 | 119.7 (2) | C23—C24—C25 | 122.6 (2) |
| C5—C4—H4 | 120.1 | C25—C24—H24 | 118.7 |
| N1—C5—C4 | 119.0 (2) | C20—C25—C28 | 124.7 (2) |
| N1—C5—H5A | 120.5 | C24—C25—C20 | 121.1 (2) |
| C4—C5—H5A | 120.5 | C24—C25—C28 | 114.2 (2) |
| C4—C5—H5B | 120.5 | C21—C26—H26A | 109.5 |
| C1'—C5—C4 | 119.0 (2) | C21—C26—H26B | 109.5 |
| C1'—C5—H5B | 120.5 | C21—C26—H26C | 109.5 |
| O4—C6—N2 | 114.80 (18) | H26A—C26—H26B | 109.5 |
| O4—C6—C7 | 124.2 (2) | H26A—C26—H26C | 109.5 |
| N2—C6—C7 | 120.9 (2) | H26B—C26—H26C | 109.5 |
| C6—C7—H7A | 120.6 | C23—C27—H27A | 109.5 |
| C8—C7—C6 | 118.8 (2) | C23—C27—H27B | 109.5 |
| C8—C7—H7A | 120.6 | C23—C27—H27C | 109.5 |
| C8—C7—H7B | 120.6 | H27A—C27—H27B | 109.5 |
| C8—C7—N2' | 118.8 (2) | H27A—C27—H27C | 109.5 |
| N2'—C7—H7B | 120.6 | H27B—C27—H27C | 109.5 |
| C7—C8—H8 | 120.0 | C25—C28—H28A | 109.5 |
| C7—C8—C9 | 119.9 (2) | C25—C28—H28B | 109.5 |
| C9—C8—H8 | 120.0 | C25—C28—H28C | 109.5 |
| C8—C9—H9 | 120.0 | H28A—C28—H28B | 109.5 |
| C10—C9—C8 | 120.0 (2) | H28A—C28—H28C | 109.5 |
| C10—C9—H9 | 120.0 | H28B—C28—H28C | 109.5 |
| N2—C10—H10 | 120.3 | O1—C1'—C5 | 123.39 (19) |
| C9—C10—N2 | 119.4 (2) | O1—C1'—N1' | 114.88 (18) |
| C9—C10—H10 | 120.3 | N1'—C1'—C5 | 121.73 (19) |
| C9—C10—H10A | 120.3 | O2—N1'—C2 | 124.5 (2) |
| C9—C10—C6' | 119.4 (2) | O2—N1'—C1' | 115.10 (18) |
| C6'—C10—H10A | 120.3 | C1'—N1'—C2 | 120.4 (2) |
| C12—C11—Si1 | 120.12 (16) | O3—C6'—C10 | 123.9 (2) |
| C12—C11—C16 | 115.5 (2) | O3—C6'—N2' | 115.09 (18) |
| C16—C11—Si1 | 123.89 (16) | N2'—C6'—C10 | 120.9 (2) |
| C11—C12—C17 | 124.7 (2) | O4—N2'—C7 | 124.2 (2) |
| C13—C12—C11 | 121.1 (2) | O4—N2'—C6' | 114.80 (18) |
| C13—C12—C17 | 114.21 (19) | C6'—N2'—C7 | 120.9 (2) |
| C12—C13—H13 | 118.6 | ||
| Si1—O1—N1—C1 | −7.0 (2) | C3—C4—C5—N1 | 1.1 (4) |
| Si1—O1—N1—C5 | 173.30 (17) | C3—C4—C5—C1' | 1.1 (4) |
| Si1—O1—C1'—C5 | 173.30 (17) | C4—C5—C1'—O1 | 177.0 (2) |
| Si1—O1—C1'—N1' | −7.0 (2) | C4—C5—C1'—N1' | −2.8 (3) |
| Si1—O2—C1—N1 | 4.9 (2) | C5—N1—C1—O2 | −178.44 (19) |
| Si1—O2—C1—C2 | −176.23 (18) | C5—N1—C1—C2 | 2.6 (3) |
| Si1—O2—N1'—C2 | −176.23 (18) | C5—C1'—N1'—O2 | −178.44 (19) |
| Si1—O2—N1'—C1' | 4.9 (2) | C5—C1'—N1'—C2 | 2.6 (3) |
| Si1—O3—N2—C6 | −7.2 (2) | C6—N2—C10—C9 | 0.3 (4) |
| Si1—O3—N2—C10 | 175.59 (18) | C6—C7—C8—C9 | −0.9 (4) |
| Si1—O3—C6'—C10 | 175.59 (18) | C7—C8—C9—C10 | −0.6 (4) |
| Si1—O3—C6'—N2' | −7.2 (2) | C8—C7—N2'—O4 | −175.9 (2) |
| Si1—O4—C6—N2 | 9.7 (2) | C8—C7—N2'—C6' | 2.0 (4) |
| Si1—O4—C6—C7 | −172.30 (18) | C8—C9—C10—N2 | 0.9 (4) |
| Si1—O4—N2'—C7 | −172.30 (18) | C8—C9—C10—C6' | 0.9 (4) |
| Si1—O4—N2'—C6' | 9.7 (2) | C9—C10—C6'—O3 | 177.3 (2) |
| Si1—C11—C12—C13 | 169.91 (16) | C9—C10—C6'—N2' | 0.3 (4) |
| Si1—C11—C12—C17 | −12.0 (3) | C10—N2—C6—O4 | 176.3 (2) |
| Si1—C11—C16—C15 | −170.09 (17) | C10—N2—C6—C7 | −1.8 (3) |
| Si1—C11—C16—C19 | 9.9 (3) | C10—C6'—N2'—O4 | 176.3 (2) |
| Si1—C20—C21—C22 | −172.32 (16) | C10—C6'—N2'—C7 | −1.8 (3) |
| Si1—C20—C21—C26 | 10.1 (3) | C11—Si1—O2—C1 | 162.73 (15) |
| Si1—C20—C25—C24 | 173.31 (16) | C11—Si1—O2—N1' | 162.73 (15) |
| Si1—C20—C25—C28 | −6.9 (3) | C11—Si1—O4—C6 | −98.55 (16) |
| O1—Si1—O2—C1 | −6.88 (15) | C11—Si1—O4—N2' | −98.55 (16) |
| O1—Si1—O2—N1' | −6.88 (15) | C11—C12—C13—C14 | 1.2 (3) |
| O1—Si1—O4—C6 | 71.06 (15) | C12—C11—C16—C15 | 2.2 (3) |
| O1—Si1—O4—N2' | 71.06 (15) | C12—C11—C16—C19 | −177.9 (2) |
| O1—N1—C1—O2 | 1.8 (3) | C12—C13—C14—C15 | 0.9 (3) |
| O1—N1—C1—C2 | −177.11 (19) | C12—C13—C14—C18 | −178.1 (2) |
| O1—N1—C5—C4 | 177.0 (2) | C13—C14—C15—C16 | −1.4 (3) |
| O1—C1'—N1'—O2 | 1.8 (3) | C14—C15—C16—C11 | −0.2 (3) |
| O1—C1'—N1'—C2 | −177.11 (19) | C14—C15—C16—C19 | 179.8 (2) |
| O2—Si1—O4—C6 | 26.2 (4) | C16—C11—C12—C13 | −2.7 (3) |
| O2—Si1—O4—N2' | 26.2 (4) | C16—C11—C12—C17 | 175.4 (2) |
| O2—C1—C2—C3 | −179.6 (2) | C17—C12—C13—C14 | −177.1 (2) |
| O3—Si1—O2—C1 | 74.71 (15) | C18—C14—C15—C16 | 177.6 (2) |
| O3—Si1—O2—N1' | 74.71 (15) | C20—Si1—O2—C1 | −96.19 (16) |
| O3—Si1—O4—C6 | −10.62 (15) | C20—Si1—O2—N1' | −96.19 (16) |
| O3—Si1—O4—N2' | −10.62 (15) | C20—Si1—O4—C6 | 160.58 (15) |
| O3—N2—C6—O4 | −1.0 (3) | C20—Si1—O4—N2' | 160.58 (15) |
| O3—N2—C6—C7 | −179.0 (2) | C20—C21—C22—C23 | −2.5 (3) |
| O3—N2—C10—C9 | 177.3 (2) | C21—C20—C25—C24 | −3.9 (3) |
| O3—C6'—N2'—O4 | −1.0 (3) | C21—C20—C25—C28 | 175.9 (2) |
| O3—C6'—N2'—C7 | −179.0 (2) | C21—C22—C23—C24 | −1.4 (3) |
| O4—Si1—O2—C1 | 38.1 (4) | C21—C22—C23—C27 | 179.4 (2) |
| O4—Si1—O2—N1' | 38.1 (4) | C22—C23—C24—C25 | 2.6 (3) |
| O4—C6—C7—C8 | −175.9 (2) | C23—C24—C25—C20 | 0.2 (3) |
| N1—C1—C2—C3 | −0.8 (3) | C23—C24—C25—C28 | −179.6 (2) |
| N2—C6—C7—C8 | 2.0 (4) | C25—C20—C21—C22 | 5.0 (3) |
| C1—N1—C5—C4 | −2.8 (3) | C25—C20—C21—C26 | −172.6 (2) |
| C1—C2—C3—C4 | −0.8 (4) | C26—C21—C22—C23 | 175.3 (2) |
| C2—C3—C4—C5 | 0.6 (4) | C27—C23—C24—C25 | −178.2 (2) |
| C3—C2—N1'—O2 | −179.6 (2) | N1'—C2—C3—C4 | −0.8 (4) |
| C3—C2—N1'—C1' | −0.8 (3) | N2'—C7—C8—C9 | −0.9 (4) |
Funding Statement
Funding for this research was provided by: National Science Foundation (grant No. CHE-1828310; grant No. CHE-1725028).
References
- Bruker (2013). SAINT. Bruker AXS, Inc., Madison, Wisconsin, USA.
- Bruker (2016). APEX3. Bruker AXS, Inc., Madison, Wisconsin, USA.
- Casellato, U., Vigato, P. A., Tamburini, S., Vidali, M. & Graziani, R. (1983). Inorg. Chim. Acta, 69, 77–82.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Howard, J. A. K., Hoy, V. J., O’Hagan, D. & Smith, G. T. (1996). Tetrahedron, 52, 12613–12622.
- Jakusch, T., Dean, A., Oncsik, T., Bényei, A. C., Di Marco, V. & Kiss, T. (2010). Dalton Trans. 39, 212–220. [DOI] [PubMed]
- Koch, J. G., Brennessel, W. W. & Kraft, B. M. (2017). Organometallics, 36, 594–604.
- Kraft, B. M. & Brennessel, W. W. (2014). Organometallics, 33, 158–171.
- Kraft, B. M., Brennessel, W. W., Ryan, A. E. & Benjamin, C. K. (2015). Acta Cryst. E71, 1531–1535. [DOI] [PMC free article] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Lewis, J. A. & Cohen, S. M. (2004). Inorg. Chem. 43, 6534–6536. [DOI] [PubMed]
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Peyroux, E., Ghattas, W., Hardré, R., Giorgi, M., Faure, B., Simaan, A. J., Belle, C. & Réglier, M. (2009). Inorg. Chem. 48, 10874–10876. [DOI] [PubMed]
- Puerta, D. T. & Cohen, S. M. (2003). Inorg. Chem. 42, 3423–3430. [DOI] [PubMed]
- Rigaku OD (2019). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Rowland, R. S. & Taylor, R. (1996). J. Phys. Chem. 100, 7384–7391.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Szigethy, G. & Raymond, K. N. (2011). J. Am. Chem. Soc. 133, 7942–7956. [DOI] [PubMed]
- Tacke, R., Burschka, C., Willeke, M. & Willeke, R. (2001). Eur. J. Inorg. Chem. pp. 1671–1674.
- Tacke, R., Willeke, M. & Penka, M. (2001). Z. Anorg. Allg. Chem. 627, 1236–1240.
- Tedeschi, C., Azéma, J., Gornitzka, H., Tisnès, P. & Picard, C. (2003). Dalton Trans. pp. 1738–1745.
- Tiede, E. R., Heckman, M. T., Brennessel, W. W. & Kraft, B. M. (2022). Organometallics, 41, 3522–3537.
- Wang, X., Dai, X., Shi, C., Wan, J., Silver, M. A., Zhang, L., Chen, L., Yi, X., Chen, B., Zhang, D., Yang, K., Diwu, J., Wang, J., Xu, Y., Zhou, R., Chai, Z. & Wang, S. (2019). Nat. Commun. 10, article No. 2570. https://www.nature.com/articles/s41467-019-10276-z [DOI] [PMC free article] [PubMed]
- Weiss, A. & Harvey, D. R. (1964). Angew. Chem. Int. Ed. Engl. 3, 698–699.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1, 2, 3, global. DOI: 10.1107/S2056989024001543/ee2004sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989024001543/ee20041sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989024001543/ee20042sup3.hkl
Structure factors: contains datablock(s) 3. DOI: 10.1107/S2056989024001543/ee20043sup4.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report