Abstract
Rewards are a broad category of stimuli inducing approach behavior to aid survival. Extensive evidence from animal research has shown that wanting (the motivation to pursue a reward) and liking (the pleasure associated with its consumption) are mostly regulated by dopaminergic and opioidergic activity in dedicated brain areas. However, less is known about the neuroanatomy of dopaminergic and opioidergic regulation of reward processing in humans, especially when considering different types of rewards (i.e., social and nonsocial). To fill this gap of knowledge, we combined dopaminergic and opioidergic antagonism (via amisulpride and naltrexone administration) with functional neuroimaging to investigate the neurochemical and neuroanatomical bases of wanting and liking of matched nonsocial (food) and social (interpersonal touch) rewards, using a randomized, between‐subject, placebo‐controlled, double‐blind design. While no drug effect was observed at the behavioral level, brain activity was modulated by the administered compounds. In particular, opioid antagonism, compared to placebo, reduced activity in the medial orbitofrontal cortex during consumption of the most valued social and nonsocial rewards. Dopamine antagonism, however, had no clear effects on brain activity in response to reward anticipation. These findings provide insights into the neurobiology of human reward processing and suggest a similar opioidergic regulation of the neural responses to social and nonsocial reward consumption.
Keywords: dopamine, fMRI, food, opioids, reward, social touch
Rewards are fundamental for survival but the brain mechanisms underlying human reward processing are not completely understood, especially for social rewards. In this study, we combined pharmacology and functional magnetic resonance imaging (fMRI) to examine the neurochemical and neuroanatomical bases of wanting and liking of matched nonsocial (food) and social (interpersonal touch) rewards. We showed that opioid antagonism reduces liking‐related activity in the medial orbitofrontal cortex when receiving food and touch, suggesting a common opioidergic mechanism underlying the neural representation of the hedonic value of social and nonsocial rewards.

1. INTRODUCTION
Rewards are powerful drivers of human behavior. They guide our motivation and choices, on which our wellbeing and survival depend. An influential theoretical account of reward identifies two main dissociable reward‐related components relying on distinct neural substrates: wanting (or incentive salience), that is, the motivation to pursue a reward, and liking, that is, the hedonic aspect associated with its consumption (Berridge & Kringelbach, 2015; Berridge & Robinson, 2003). Thirty years of animal research (Berridge & Kringelbach, 2015; Berridge & Valenstein, 1991; Peciña et al., 2006; Peciña & Smith, 2010) provided evidence in support of this theoretical model, characterizing the wanting‐liking dissociation in terms of their respective neuroanatomical and neurochemical substrates. These studies showed that while dopaminergic activity in the midbrain and striatum directly subserves wanting during reward anticipation, opioidergic activity in the nucleus accumbens (NAc) regulates specifically the hedonic reactions (liking) linked to reward consumption and reward motivation. Compared to animal research, human studies examining both the neurochemistry and neuroanatomy of wanting and liking are limited and focused mainly on nonsocial rewards, particularly money and food. In this study, we combined pharmacology and neuroimaging to systematically examine the wanting‐liking dissociation both at the neurochemical and neuroanatomical levels in primary social (interpersonal touch) and nonsocial (food) rewards.
Previous human neuroimaging studies provided evidence for the presence of an “extended common currency schema” (Ruff & Fehr, 2014) in the brain (Gu et al., 2019; Izuma et al., 2008; Lin et al., 2012; Liu et al., 2011; Sescousse et al., 2013; Wake & Izuma, 2017). According to this model, reward magnitude is processed in the same reward‐related brain structures, such as the striatum or the ventromedial prefrontal cortex (vmPFC), independently of reward type. However, differences in the neural responses to social and nonsocial rewards have also been documented, suggesting, for instance, reward‐specific representations in the orbitofrontal cortex (OFC) (Sescousse et al., 2010). These findings point to the existence of a dedicated neural circuitry for the encoding of social reward values (social valuation specific schema) (Ruff & Fehr, 2014), which may be selectively impaired in conditions characterized by deficits in social reward processing, like autism (Chevallier et al., 2012). Furthermore, it was proposed that while domain‐general neural circuits underlie reward anticipation (Gu et al., 2019), modality‐specific ones may be involved during reward consumption (Rademacher et al., 2010). The incongruity of the reported results may stem from various methodological shortcomings, such as assessing reward anticipation but not consumption or employing social and nonsocial stimuli, which are not matched in primacy and magnitude.
Regarding neurochemistry, most prior pharmacological research investigating the role of opioids and dopamine in wanting and liking focused on food and monetary rewards (see Meier et al., 2021; Webber et al., 2020 for recent reviews). Using a paradigm tailored to investigate anticipation and consumption of well‐matched social (touch) and nonsocial (food) primary rewards (Chiappini et al., 2022), we recently observed partially different effects of dopamine and opioid antagonism on behavioral (effort) and physiological (facial electromyography) correlates of wanting and liking (Korb, Götzendorfer, et al., 2020). Consistent with animal studies, we showed that opioid antagonism modulates reward anticipation and consumption, while dopaminergic antagonism only affects reward anticipation. However, the reduction in wanting following dopamine and opioid antagonism was more robust or limited to the anticipation of food rewards. Crucially, how such neurochemical modulation is implemented at the neuroanatomical level is still an open question.
By combining pharmacology with functional neuroimaging, we aimed to fill this knowledge gap and provide novel insights on the human neurochemical and neuroanatomical bases of the motivational and hedonic aspects of social and nonsocial reward processing. In a randomized, double‐blind, placebo‐controlled, between‐subjects design, 89 participants received either the highly selective D2/D3 dopamine receptor antagonist amisulpride, the nonselective opioid receptor antagonist naltrexone, or a placebo. Drug effects on behavioral and neural responses to anticipation and consumption of food and social touch were assessed on a trial‐by‐trial basis, using implicit (physical effort) and explicit (ratings of wanting and liking) measures (Chiappini et al., 2022) (Figure 1). Food and touch stimuli were carefully matched in terms of magnitude (similar levels of wanting and liking, as previously shown; Korb, Götzendorfer, et al., 2020; Korb, Massaccesi, et al., 2020), primacy (both can be classified as primary rewards, in contrast to money or social feedback), temporal proximity, tangibility, and familiarity (Matyjek et al., 2020).
FIGURE 1.

Trial sequence of the reward task. Participants could obtain social and nonsocial rewards in three levels (high, low, very low). Social rewards consisted of skin‐to‐skin caresses delivered to the forearm from a trained same‐gender confederate at three speeds: 6, 21, and 27 cm/s. Nonsocial rewards consisted of milk with three different concentrations of cocoa: chocolate milk, a 4:1 mix of milk and chocolate milk, and milk. At the beginning of each trial, a cue announced the attainable reward (high or low), and participants were asked to rate their wanting of the announced reward. Then, participants exerted effort by squeezing a hand dynamometer to obtain the announced reward. The applied force, displayed via online visual feedback, was expressed as percentage of the participants' maximum voluntary contraction (MVC, measured immediately before the task) and translated into the probability of obtaining the announced reward (0%–100%). The obtained reward was then announced (high, low, or very low in case of low effort) and delivered. Following a relaxation phase, participants rated their liking of the stimulus. At the end of nonsocial trials, participants received water to rinse their mouth.
We formulated our hypothesis based on evidence from existing animal and human research, which indicates that the hedonic effect associated with reward consumption solely depends on opioids, while the effect of incentive salience during anticipation also relies on dopamine. Accordingly, during reward anticipation, we expected dopamine and opioid antagonism to reduce ratings of wanting, effort, and activity in brain regions associated with incentive salience (e.g., ventral tegmental area [VTA], NAc, and vmPFC). During reward consumption, we expected opioid antagonism to reduce ratings of liking and activity in brain areas associated with hedonic pleasure (e.g., NAc, OFC). Furthermore, if social and nonsocial rewards are subtended by different substrates, dopamine and opioid antagonism should act differently on reward‐related brain regions. In particular, based on our previous findings (Korb, Götzendorfer, et al., 2020), we expected stronger pharmacological modulation of food compared to touch rewards, possibly due to the involvement of other neurochemical systems specific to social rewards, such as oxytocin and serotonin (Fischer & Ullsperger, 2017; Tang et al., 2020).
2. MATERIALS AND METHODS
2.1. Sample
The study included 94 healthy volunteers (57 females; Table 1). Five participants did not undergo magnetic resonance imaging (MRI) scanning because of sickness (3), claustrophobia (1), or impossibility of providing a urine sample for the drug/pregnancy test (1) and were therefore excluded, leading to a final sample size of 89 (age M = 23.7, SD = 3.7; but N = 85 for functional MRI data analyses, see Section 2.4). All participants reported being right‐handed, smoking less than five cigarettes daily, having no history of current or former drug abuse, liking milk and chocolate, not suffering from diabetes, lactose intolerance, lesions, or skin diseases on the right forearm, and being free of psychiatric or neurological disorders. Other exclusion criteria included contraindications to MRI and having taken part in a pharmacological study in the 2 months preceding the experiment. To avoid sexual connotations, social touch was always administered by a same‐gender experimenter, and only participants who reported to be heterosexual were included. The study was approved by the Ethical Committee of the Medical University of Vienna (EK N. 1393/2017) and was performed in line with the Declaration of Helsinki (World Medical Association, 2013). Participants signed the informed consent and received a monetary compensation of 90€.
TABLE 1.
Sample characteristics.
| Amisulpride | Naltrexone | Placebo | Group differences | ||
|---|---|---|---|---|---|
| F | p | ||||
| N (male, female) | 32 (14, 18) | 29 (10, 19) | 28 (11, 17) | ||
| Age | 24.0 ± 4.0 | 23.9 ± 3.6 | 23.3 ± 3.6 | .36 | .70 |
| BMI | 23.0 ± 2.6 | 22.3 ± 2.2 | 22.8 ± 2.2 | .83 | .44 |
| MVC pre | 153.5 ± 56.8 | 153.6 ± 62.8 | 139.9 ± 54.8 | .50 | .61 |
| MVC post | 169.2 ± 54.2 | 160.5 ± 58.0 | 160.8 ± 53.5 | .23 | .80 |
| Hunger | 3.2 ± 2.0 | 3.6 ± 2.0 | 3.5 ± 4.8 | .36 | .70 |
| Sociability | 5.0 ± 1.2 | 4.8 ± 1.7 | 4.8 ± 1.3 | .13 | .88 |
| AQ‐k | 7.2 ± 4.7 | 5.4 ± 3.1 | 5.5 ± 3.1 | 2.47 | .09 |
| HTAS | 66.3 ± 14.7 | 72.4 ± 13.7 | 67.4 ± 13.7 | 1.82 | .17 |
| STQ | 22.7 ± 8.5 | 21.6 ± 8.1 | 23.9 ± 8.1 | .57 | .57 |
| Mood | |||||
| PANAS‐positive T1 | 32.0 ± 6.1 | 32.0 ± 5.1 | 32.7 ± 5.5 | .16 | .85 |
| PANAS‐positive T2 | 26.4 ± 7.7 | 23.9 ± 6.7 | 29.4 ± 8.5 | 3.66 | .03* |
| PANAS‐negative T1 | 13.2 ± 4.0 | 12.0 ± 2.1 | 12.1 ± 2.2 | 1.54 | .22 |
| PANAS‐negative T2 | 12.3 ± 3.4 | 11.2 ± 1.8 | 11.1 ± 1.6 | 2.26 | .11 |
| Side effects | |||||
| Weakness T2 | 1.48 ± 0.63 | 1.45 ± 0.57 | 1.14 ± 0.36 | 3.53 | .03* |
| Dizziness T2 | 1.19 ± 0.40 | 1.52 ± 0.57 | 1.11 ± 0.31 | 6.84 | <.01* |
| Dry mouth T2 | 1.03 ± 0.18 | 1.21 ± 0.41 | 1.04 ± 0.19 | 3.70 | .03* |
| % of rewards obtained | |||||
| High | 35.1% | 36.2% | 37.4% | ||
| Low | 34.8% | 31.3% | 31.2% | ||
| Very low | 28.6% | 31.9% | 29.3% | ||
Abbreviations: AQ‐k, short version of the Autism Spectrum Quotient (Freitag et al., 2007); BMI, body mass index; HTAS, Health and Taste Attitudes Scale (Roininen & Tuorila, 1999); M, mean; MVC, maximum voluntary contraction; PANAS, Positive and Negative Affect Scale (Watson et al., 1988); STQ, Social Touch Questionnaire (Wilhelm et al., 2001); T1, before drug administration; T2, ~4.5 h after drug administration.
p < .05.
2.2. Procedure
Before participating in the study, participants underwent a health screening, including electrocardiogram and blood examination, and a neuropsychiatric interview. To enhance and equate drugs' absorption time, participants were instructed not to eat in the preceding 6 hours before coming to the lab. At arrival, they filled out the Positive and Negative Affect Scale (PANAS) (Watson et al., 1988) and a questionnaire about physical symptoms (Figure S1), performed a urine drug test and, if females, a pregnancy test, and received a capsule filled with either 400 mg of amisulpride, 50 mg of naltrexone, or 650 mg of mannitol from the study doctor. Then participants received a snack and, after a waiting time of 3 h, they were transferred to the MRI center where they performed the reward task during functional MRI (fMRI) scanning.
The reward task (Chiappini et al., 2022) involved the anticipation and consumption of nonsocial and social rewards (Figure 1). Chocolate milk, a 4:1 mix of milk and chocolate milk, and milk were used as high, low, and very low nonsocial rewards. Skin‐to‐skin caresses delivered by a same‐gender experimenter to the participants' right forearm at 6, 21, and 27 cm/s were used as high, low, and very low social rewards (see Section 2 in Supplementary Material for details). Participants first experienced each social and nonsocial reward once outside the MRI scanner. Once in the MRI scanner, participants' MVC was established (peak force exerted by squeezing a hand dynamometer for 3 s across the three trials) to be used as threshold in the reward task. Following four practice trials, participants performed four blocks of the reward task (acquired in four fMRI runs), two in the social and two in the nonsocial condition. The order of the blocks (ABAB or BABA) was randomized across participants. Each block consisted of 16 trials, including the following main components (Figure 1): (i) announcement of reward (high or low; 1 s); (ii) rating of wanting on a visual analog scale (VAS) from −10 (not at all) to +10 (very much) (4 s); (iii) effort task: to obtain the announced reward, participants had to squeeze the hand dynamometer with their left hand (the applied force was translated into a probability of obtaining the reward; 4 s); (iv) announcement of reward gained (high, low, or, in case of low effort, very low; 1 s); (v) prepare for reward delivery (6 s); (vi) reward delivery (7 s); (vii) relaxation phase (8 s); (viii) rating of liking on VAS from −10 (not at all) to +10 (very much) (4 s); (ix) only in the nonsocial condition, water delivery for rinsing (7 s).
After completing the task, the participants' MVC was assessed again. Then, participants completed the PANAS and physical symptoms questionnaire (~4.5 h after drug intake). At the end of the session, participants were asked to guess the identity of the drug they received and were debriefed about the aim of the study.
2.3. Drug administration
Naltrexone is a nonselective opioid antagonist with high affinity to the μ‐ and κ‐opioid receptors, while amisulpride is a selective dopamine D2/D3 receptor antagonist. We used 50 mg per‐oral naltrexone (Dependex®), which blocks more than 90% of μ‐opioid receptors (Lee et al., 1988), and 400 mg per‐oral amisulpride (Solian®), which results in 50%–80% D2/ receptor blockade (la Fougère et al., 2005; Meisenzahl et al., 2008). Higher doses of amisulpride are not recommended in research on healthy subjects due to the increased risk of extrapyramidal side effects. The length of the time interval between drug intake and task onset (3 h) was modeled on previous studies using the same compounds and doses (Korb, Götzendorfer, et al., 2020; Weber et al., 2016) and on drugs' pharmacodynamics (Meyer et al., 1984; Rosenzweig et al., 2002).
2.4. MRI acquisition and data preprocessing
MRI data were acquired using a 3T Siemens Prisma fit MRI scanner (Siemens Healthineers, Erlangen, Germany) with a 64‐channel head coil. Functional whole‐brain scans were collected using a multiband‐accelerated T2*‐weighted 2D echoplanar imaging sequence (40 slices, TE/TR = 35/1000 ms, flip angle = 62°, voxel size = 2.3 × 2.3 × 3.0 mm, FOV = 220 × 220 mm). Structural images were acquired with a magnetization‐prepared two inversion time rapid gradient‐echo (MP2RAGE) sequence (TE/TR = 2.98/4000 ms, flip angle = 4°, voxel size = 1 × 1 × 1 mm, FOV = 256 × 216 x 160 mm). Imaging data were preprocessed with Statistical Parametric Mapping (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK) and FMRIB Software Library (FSL; Analysis Group, FMRIB, Oxford, UK). Preprocessing included: realignment to the first image of each run, magnetic field inhomogeneity distortion correction (topup; Andersson et al., 2003), co‐registration to T1 image, segmentation, normalization to Montreal Neurological Institute (MNI) template space, smoothing with an 8‐mm full width at half‐maximum Gaussian kernel. Runs containing framewise displacement greater than 0.5 mm on more than 40% of the total frames were excluded from additional analyses (one food run in six participants). Following smoothing, the ICA‐AROMA algorithm was applied to reduce motion artifacts (Pruim et al., 2015). Due to technical issues, fMRI data of one participant is not available and data from three participants could not be analyzed as they completed only the social touch blocks. fMRI analyses thus included 85 participants in total (30 AMI, 27 NAL, 27 PLA).
2.5. Statistical analyses
The statistical analyses were preregistered on OSF prior to execution (but after data collection) (https://osf.io/6xkph). Differently from Korb, Götzendorfer, et al. (2020), high, low, and very low reward levels were defined a priori in this study. However, for some participants, the actual wanting and liking of the stimuli did not match those a priori levels (27.4% for food and 15.7% for touch). Therefore, differently from our preregistration, reward levels were re‐defined based on the average actual ratings of wanting and liking of each participant.
2.6. Behavioral data
Statistical analyses of behavioral data were conducted in R (R Core Team, 2021). Drug effects on wanting, liking, and effort were investigated using three linear mixed models (LMMs). Each model included Drug (amisulpride, naltrexone, placebo), Reward Type (food, touch), and Reward Level (high, low, very low) as fixed effects, and by‐subjects random intercepts and slopes for Reward Type, Reward Level, and their interaction. Group comparis age, scores of the trait questionnaires, body mass index (BMI), and MVC were assessed with analysis of variances (ANOVAs). Planned comparisons were corrected using the Tukey method. Null findings in behavioral analyses were followed up with post hoc Bayesian analyses in JASP 0.15 (JASP Team, 2021). We implemented repeated measures ANOVAs with a default multivariate Cauchy prior (r scale prior width for fixed effects = .5). Then, robustness checks were conducted using a narrower (r = .2) and a wider prior (r = 1), as recommended by Van Doorn et al. (2021). We computed Bayes factors (BF01) to estimate the evidence in favor of both the null (H0) and the alternative hypotheses (H1, drug model), using the following thresholds: moderate support for H0 with a BF01 between 3 and 10, strong support for H0 with a BF01 larger than 10, moderate support for H1 with BF01 between 0.3 and 0.1 and strong support for H1 with a BF01 smaller than 0.1 (Van Doorn et al., 2021).
2.7. fMRI data
Neural data were analyzed using SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). Following our preregistered analysis plan (https://osf.io/6xkph), we conducted whole‐brain and region of interest (ROI) analyses, using either the trial‐by‐trial subjective ratings of wanting, rating of liking, and effort as parametric modulators (GLM.1 and GLM.2), or the categorical predictor Reward Level (high > low, GLM.3).
2.7.1. Whole brain analysis with parametric modulation
Preprocessed data were analyzed as an event‐related design in the context of the GLM approach in a two‐level procedure. At the first level, two design matrices, one for food runs and one for touch runs, were fitted for each subject. Each design matrix included regressors for each distinct phase of the trials (fix cross, anticipation preeffort, rate wanting, effort, anticipation post‐effort, prepare delivery, delivery, relax, rate liking, prepare rinsing [only for food trials], rinsing [only for food trials]). To explore the brain activation in response to wanting during reward anticipation (anticipation preeffort) and to liking during and after reward consumption (delivery), parametric modulation was implemented at the first level. Two models were fitted with each of the two parametric modulators for the anticipation phase (ratings of wanting and effort exerted). In the first model (GLM.1), the ratings of wanting were orthogonalized with respect to effort and therefore included as first and second parametric modulators of the anticipation preeffort phase, while the ratings of liking were included as parametric modulator of the delivery phase. The second model (GLM.2) was identical to the first one, but for the phases of anticipation preeffort, effort was orthogonalized with respect to wanting. We further analyzed only the first parametric modulator for each model, ignoring the second one. For each design matrix (food runs and touch runs), the following contrast images were calculated and taken to the second‐level analysis: (i) first‐order parametric modulation of ratings of wanting (GLM.1) or effort (GLM.2) in anticipation preeffort, and (ii) first‐order parametric modulation of ratings of liking in delivery.
Second‐level analysis included the factors Drug (amisulpride, naltrexone, placebo) and Reward Type (food, touch). A mixed‐model ANOVA (flexible factorial design) was fitted for each first‐level contrast to explore the main effects of Drug and Reward Type, as well as their interaction.
2.7.2. Whole brain analysis with categorical predictor Reward Level (high > low)
In order to reproduce the analysis performed in the majority of the previous papers (e.g., Buchel et al., 2018; Rademacher et al., 2010; Spreckelmeyer et al., 2009), another first‐level analysis (GLM.3), including two design matrices for food runs and touch runs separately, were fitted for each participant, using the categorical variable Reward Level (high > low reward) instead of the continuous parametric modulators (trial by trial ratings of wanting and liking, and effort exerted). The category “low reward” included both low and very low reward types. The simple contrasts of high reward versus low reward in the main phases of (i) reward anticipation (anticipation preeffort) and (ii) reward consumption (delivery) were taken to the second‐level for group comparison. Similar to what was explained above, a mixed‐model ANOVA (flexible factorial design) was fitted for each phase (anticipation preeffort, delivery) to explore the main effects of Drug and Reward Type, as well as their interactions. All reported results are based on family‐wise error (FWE) correction for voxel intensity tests (FWE, p < .05) unless differently specified.
2.7.3. ROI analyses
In addition to the whole‐brain analyses, we performed a ROI analysis with a priori defined masks. ROIs were chosen based on findings from previous metanalyses on monetary, erotic, food, and social rewards (Gu et al., 2019; Sescousse et al., 2013) and relevant neuroimaging research (e.g., Izuma et al., 2008; O'Doherty et al., 2002; Rademacher et al., 2010) and include four ROIs generated based on anatomical masks: NAc (AAL3 atlas), VTA (Trutti et al., 2021), medial OFC (Jülich Brain MPM atlas) and vmPFC (AAL3 atlas). For each subject, brain activity in each ROI was extracted for the following contrast images: (i) first‐order parametric modulation of rating of wanting in reward anticipation and (ii) first‐order parametric modulation of rating of liking in reward consumption. To investigate the effect of the administered drugs on social and nonsocial reward anticipation and consumption, ANOVAs (one for each ROI) were performed, including the between‐subject factor Drug (amisulpride, naltrexone, placebo) and the within‐subject factors Reward Type (food, touch) and Phase (anticipation, consumption).
The same ROI analysis was conducted also on the contrasts calculated from GLM.3: high > low reward in (i) anticipation and (ii) consumption. We adjusted the threshold of statistical significance for both ROI analyses based on the number of ROIs (p < .0125).
3. RESULTS
3.1. No changes in behavioral measures of wanting and liking following opioid and dopamine antagonism
The LMM conducted on the ratings of wanting (Figure 2a,c) revealed only a significant main effect of Reward Level (F[1,86.8] = 142.42, p < .001). No significant Drug main or interaction effects were observed (all p > .14, Figure 2a,c). Bayesian analyses provided moderate support against the Drug effect (BF01 = 6.1) and strong support against the Drug interaction effect (BF01 > 11.7) (Table S1).
FIGURE 2.

No significant drug effects on the ratings of (a) wanting and (b) liking. (c) Ratings of wanting did not differ for food and touch, but (d) ratings of liking were higher for low food rewards compared to low touch rewards (p = .04), regardless of the drug group. *p < .05. The violin plots depicted here consist of box plots, representing the median (thick horizontal line), the interquartile range (box), and the lower/upper adjacent values (whiskers) and kernel density plots, representing kernel probability density of the data at different values. AMI, amisulpride; NAL, naltrexone; PLA, placebo.
The LMM conducted on the ratings of liking (Figure 2b,d) revealed a significant main effect of Reward Level (F[2,85.5] = 150.00, p < .001) and Reward Level by Reward Type interaction (F[2,83.2] = 6.11, p < .01). Ratings of liking for low nonsocial rewards were higher than for low social rewards (p = .041; Figure 2d). No significant Drug main or interaction effects were observed (all p > .34; Figure 2b). Bayesian analyses provided moderate support against the Drug effect (BF01 = 8.4) and strong support against the Drug interaction effects (BF01 > 71.4) (Table S2).
The LMM conducted on the effort exerted to obtain the announced reward revealed a significant main effect of Reward Level (F[1,86.9] = 68.8, p < .001), a Drug by Reward Type interaction (F[2,83.9] = 3.67, p = .030), and a Reward Level by Reward Type interaction (F[1,86.1] = 5.92, p = .017) (Figure 3). Regarding the Drug by Reward Type interaction (Figure 3b), participants who received amisulpride exerted greater effort for nonsocial compared to social rewards, but the comparison was not statistically significant (p = .07, all other comparisons p > .35). Regarding the Reward Level by Reward Type interaction (Figure 3c), effort exerted was slightly higher for high nonsocial compared to high social rewards, but the comparison was not statistically significant (p = .15), nor there was a statistically significant difference between the effort exerted for low social and low nonsocial rewards (p = .95). To further examine the Drug by Reward Type interaction, we fitted the same LMM separating the Drug predictor in two dummy‐coded predictors (amisulpride‐dummy: 1 = amisulpride, 0 = placebo; naltrexone‐dummy: 1 = naltrexone, 0 = placebo). This LMM showed a significant Naltrexone‐dummy by Reward Type interaction (F[1,83.8] = 4.68, p = .034). To test whether this interaction was driven by differences between naltrexone and placebo groups in the effort exerted to obtain food or touch rewards, we calculated the simple contrasts, which (in keeping with the results from the first model) did not reveal any significant difference (all p > .17).
FIGURE 3.
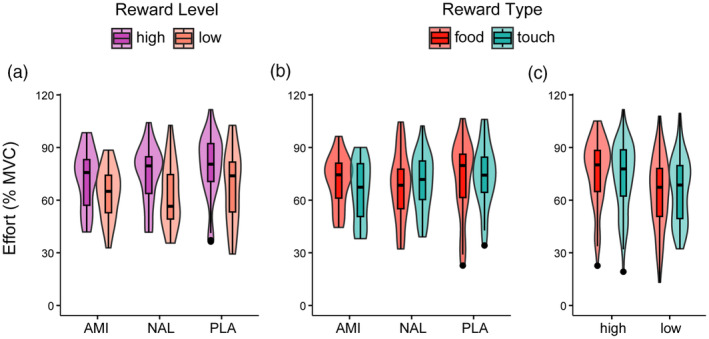
No significant drug effects on the effort exerted (% of maximum voluntary contraction, MVC) to obtain rewards of different (a) levels (high, low) and (b) types (food, touch). (c) Effort exerted also did not differ for food and touch, regardless of the drug group. The violin plots depicted here consist of box plots, representing the median (thick horizontal line), the interquartile range (box), and the lower/upper adjacent values (whiskers), and kernel density plots, representing kernel probability density of the data at different values. AMI, amisulpride; NAL, naltrexone; PLA, placebo.
Bayesian analyses provided moderate support against the Drug main effect (BF01 = 4.5, anecdotal BF01 = 1.6 when using a narrower prior), but anecdotal evidence for the Drug by Reward Type interaction effect (BF01 = 1.8) (Table S3).
3.2. Opioid antagonism reduces liking‐related activity in the medial orbitofrontal cortex during consumption of social and nonsocial rewards
As a manipulation check, we first confirmed that reward anticipation and consumption in our task elicited the expected neural activity in regions associated with reward processing in the placebo group only. We found greater activity during anticipation of high compared to low rewards (GLM.3) in the right lingual gyrus, the medial part of the superior frontal gyrus, including vmPFC and anterior and posterior portions of OFC, left supramarginal gyrus, right middle temporal gyrus, right superior temporal gyrus, cingulate cortex, precuneus, and right putamen (Figure 4a and Table S4 for a complete list). The analysis including trial‐by‐trial ratings of wanting (GLM‐1) and effort (GLM‐2) as parametric modulators showed similar results, but only at a lenient statistical threshold (p < .001 uncorrected, k > 20, see Table S5). During consumption (GLM.3), we observed greater activity for high compared to low rewards in the subgenual portion of the anterior cingulate cortex (ACC), medial OFC, VTA, and thalamus (all FWE p < .05), as well as in the left insula, parietal and frontal regions at a more lenient threshold (p < .001 uncorrected) (Figure 4b and Table S4). Trial‐by‐trial ratings of liking did not significantly correlate with activity in any brain region.
FIGURE 4.

Brain regions showing greater activity during high compared to low reward (a) anticipation and (b) consumption (GLM.3) in the placebo group. (c) Reduced activity in the medial orbitofrontal cortex following naltrexone (Nal) administration compared to placebo (Pla) during reward consumption (GLM.3). (d) Reduced activity in middle frontal and precentral gyri, thalamus, and caudate following amisulpride (Ami) administration compared to placebo (Pla) during reward consumption (GLM.3). Images are thresholded at p < .001 uncorrected.
We then investigated the effect of naltrexone and amisulpride administration, compared to placebo, on the neural representations of reward anticipation (wanting) and consumption (liking). No drug effects were observed during anticipation of high compared to low rewards (GLM.1). During reward consumption (GLM.3, high > low), administration of naltrexone was associated with reduced activity compared to placebo in the right medial OFC, left medial superior frontal gyrus (including dorsomedial prefrontal cortex), left superior and middle frontal gyrus (including dorsolateral prefrontal cortex) and right lateral OFC (Figure 4c and Table S6). Furthermore, during the same phase, administration of amisulpride compared to placebo was associated with reduced activity in the right middle frontal gyrus (premotor cortex), right thalamus (ventral lateral nucleus), left precentral gyrus (primary motor cortex), right caudate, Rolandic operculum, and inferior frontal gyrus (Figure 4d and Table S6). The analyses including the ratings of wanting and liking and effort exerted as parametric modulators (GLM.1–2) did not reveal any significant drug effects during reward anticipation or consumption. We also did not observe significant differences between reward types (food and touch) in any of the categorical and parametric analyses conducted.
ROI analyses conducted on the parametrically modulated contrasts in anticipation (ratings of wanting) and consumption (ratings of liking) (GLM.1) revealed a significant Reward Type × Phase interaction effect in the NAc (F[1,82] = 9.69, p = .003) and medial OFC (F[1,82] = 8.61, p = .004). Specifically, NAc and medial OFC wanting‐related activity were higher during the anticipation of food rewards than touch rewards (p < .001). Furthermore, for food rewards, NAc and medial OFC wanting‐related activity in anticipation was greater than liking‐related activity in consumption (p < .01). We also observed a significant main effect of Phase in vmPFC (F[1,82] = 15.95, p < .001), reflecting greater wanting‐related activity during reward anticipation compared to liking‐related activity during reward consumption. No significant drug effects were observed. Analyses conducted on the categorical contrasts (high > low reward) in anticipation and consumption (GLM.3) revealed only a significant main effect of Phase in the NAc, VTA, medial OFC, and vmPFC, reflecting a greater activity for high compared to low rewards during anticipation compared to consumption (all p < .001).
3.3. Matching of drug groups
Last, we conducted a series of statistical tests to exclude potential group differences in secondary measures, which could have influenced the results.
First, the three drug groups did not significantly differ in terms of age, BMI, MVC before and after the reward task, autistic traits (short version of the Autism Spectrum Quotient [AQ‐k]), social touch appreciation (Social Touch Questionnaire [STQ]), attitudes to hedonic characteristics of food (Health and Taste Attitudes Questionnaire [HTAS]), as well as in their reported mood (PANAS), hunger, and sociability at the beginning of the session (see Table 1). Regarding mood, the naltrexone group reported significantly lower positive mood 4.5 h after pill intake compared to the placebo group (p = .03; Table 1).
Regarding side effects, no significant group differences before drug administration were found. At 4.5 h following drug administration, we observed a significant main effect of Drug for weakness (F[2, 85] = 3.53, p = .034), dizziness (F[2, 85] = 6.84, p = .002), and dry mouth (F[2, 85] = 3.70, p = .029) (Table 1). Participants administered with amisulpride reported greater weakness than participants administered with placebo (p = .04). Naltrexone was associated with greater reported dizziness compared to placebo (p < .01) and amisulpride (p = .02) and greater reported dry mouth compared to amisulpride (p = .03). Nevertheless, the average values of all reported side effects were between 1 (“not all”) and 2 (“slightly”), indicating the absence of severe side effects in all drug groups (Figure S1). Drug blinding was successful as participants' guesses were not significantly related to their group allocation (Χ 2[4] = 5.79, p = .22). Overall, 42% of participants correctly guessed the content of the pill they received.
Finally, no significant differences between drug groups in the average number of high, low, and very low rewards obtained during the task were observed (all p > .31, Table 1).
4. DISCUSSION
Prior animal and human findings suggest a partial neurobiological dissociation in the processing of the motivational (wanting) and hedonic (liking) components of reward, with dopaminergic neurotransmission involved in wanting and opioidergic neurotransmission involved in both wanting and liking (e.g., Berridge & Kringelbach, 2015; Korb, Götzendorfer, et al., 2020). However, previous studies mainly investigated the neuroanatomical and neurochemical dissociation of reward dimensions within the domain of nonsocial rewards. Whether a similar neurocircuitry is involved in wanting and liking of rewards of social nature remains unclear. Here, we examined how blocking of the dopamine and opioid systems modulates the neural processing of wanting and liking of social (touch) and nonsocial (food) rewards. We found that opioid antagonism reduced the activity of the medial orbitofrontal and prefrontal cortices in response to receiving food and touch. Dopaminergic antagonism did not significantly modulate brain activity during reward anticipation but reduced brain activity in sensory and motor processing regions during reward consumption. No effects of the drugs on the behavioral measures of wanting and liking were observed.
Naltrexone and amisulpride administration did not significantly change subjective wanting and liking of food and touch rewards. Previous research has been inconsistent in regard to the effects of opioid and dopamine antagonism on self‐report ratings of wanting and liking, with some studies reporting significant reductions (e.g., Buchel et al., 2018; Soutschek et al., 2021) and others reporting no effects (Korb, Götzendorfer, et al., 2020; Løseth et al., 2019). Especially concerning the effects of dopaminergic manipulations on wanting, most were observed in studies involving objective measures, like effort, rather than subjective ratings. Specifically, prior studies consistently showed that increasing dopaminergic function enhances the willingness to exert effort (i.e., choice of the effortful task) during choice phase (Soutschek et al., 2020; Wardle et al., 2011; Westbrook et al., 2020), while the opposite effect was observed for dopamine blockade (Cawley et al., 2013; Venugopalan et al., 2011). Fewer studies investigated the effects of dopamine on effort exertion (measured as the amount of grip force to gain a reward or button presses in Pavlovian‐to‐instrumental transfer). Two studies reported increased effort allocation for monetary rewards following l‐DOPA administration (Michely et al., 2020; Zenon et al., 2016). Dopamine antagonism did not affect grip force to gain monetary rewards (Michely et al., 2020), but reduced button presses associated with chocolate (Weber et al., 2016). Regarding opioids, studies showed effects of opioid agonism and antagonism on ratings of liking of money, attractive faces, and sugar (Buchel et al., 2018; Chelnokova et al., 2014; Eikemo et al., 2016, 2017; Yeomans & Gray, 1996; Ziauddeen et al., 2013; but see Korb, Götzendorfer, et al., 2020; Løseth et al., 2019). Some studies also reported reduced effort for different kinds of reward following opioid antagonism (Chelnokova et al., 2014; Eikemo et al., 2017; Korb, Massaccesi, et al., 2020; Weber et al., 2016), while Nunez et al. (2022) observed null effects of naltrexone on the willingness to exert effort for money. Using the same reward task employed in the present study, we recently showed reduced effort exerted to gain food and, to a lesser extent, social touch following administration of the dopamine antagonist amisulpride and opioid antagonist naltrexone (Korb, Götzendorfer, et al., 2020). However, in contrast to our previous findings, here we did not observe significant drug effects on effort. This may be due to lower statistical power, as the sample size of this study was tailored mainly to investigate effects on brain activity rather than on behavior. The Bayesian analysis provided moderate support for this null finding, but results partly depended on prior selection. We, therefore, refrain from making any claim concerning whether this null finding represents an actual null effect of the pharmacological challenge.
At the neural level, opioid receptor blockade via naltrexone resulted in reduced activity in the medial OFC during reward consumption. In humans, opioid signaling has been previously linked to hedonic processing of different kinds of rewarding stimuli, such as palatable food (e.g., Drewnowski et al., 1992; Eikemo et al., 2016; Korb, Götzendorfer, et al., 2020; Nummenmaa et al., 2018; Weber et al., 2016; Yeomans & Gray, 1996; Ziauddeen et al., 2013), money (e.g., Eikemo et al., 2017; Petrovic et al., 2008; Weber et al., 2016), social and erotic stimuli (e.g., Buchel et al., 2018; Chelnokova et al., 2014; Koepp et al., 2009; Korb, Götzendorfer, et al., 2020). The OFC is a major reward processing hub and its medial portion, in particular, is involved in encoding reward magnitude during the receipt of different types of reward (Diekhof et al., 2012; Rolls et al., 2020), including food pleasantness (Kringelbach et al., 2003; Kringelbach & Rolls, 2004; Small et al., 2001). Importantly, in rodents, opioid stimulation in the OFC causally enhances hedonic reactions to sweet food rewards (Castro & Berridge, 2017). In humans, reduced OFC activation to erotic stimuli was reported following naloxone infusion (Buchel et al., 2018), and opioid availability in the thalamus (measured by positron emission tomography) was negatively associated with food reward BOLD responses in the OFC (Nummenmaa et al., 2018). While we also observed reduced activity in small clusters within the middle frontal gyrus, we did not observe the effects of opioid antagonism in the ACC, as previously shown by two studies examining monetary and food rewards (Murray et al., 2014; Petrovic et al., 2008). Overall, the present and previous findings point to an involvement of opioid signaling in the medial OFC related to the consumption of both social and nonsocial primary rewards, such as food, sex, and touch. However, it is fundamental to remember that our analyses do not allow to distinguish among different neuronal populations in the same brain region. Indeed, distinct but interacting neuronal populations responding to caloric consumption and social interaction have been observed in animal models (Jennings et al., 2019).
Contrary to our initial hypotheses, dopamine antagonism was associated with neural modulation during reward consumption. Specifically, amisulpride reduced activity in sensory and motor processing regions, including the ventrolateral thalamus, premotor and primary motor cortices, and the caudate, compared to placebo. These regions are part of the basal ganglia‐thalamocortical pathway involved in motor control, also through dopaminergic signaling (Bosch‐Bouju et al., 2013). We speculate that here dopamine antagonism may have affected the processing of the sensorimotor properties of the stimuli (e.g., speed of touch or food taste) rather than reward‐related features (Macedo‐Lima & Remage‐Healey, 2021). However, it should be noted that the clusters of reduced activity following amisulpride administration surviving FWE correction were rather small. Thus, evidence of such modulation will need further investigation. While studies examining self‐report liking indicate little to no effects of dopamine antagonism (see Webber et al., 2020 for a recent review), previous research examining brain responses during reward consumption yielded mixed results. Some studies reported no effects (Bjork et al., 2014; Graf et al., 2014; Sescousse et al., 2016; Soutschek et al., 2021), whereas others indicated reduced activity in striatal and prefrontal regions in food, monetary, and erotic stimuli (Frank et al., 2016; McCabe et al., 2011, 2013; Oei et al., 2012; Riba et al., 2008), indicating the need for further research.
In addition, we did not observe dopaminergic modulation of regions within the mesocorticolimbic pathway usually associated with reward anticipation, such as the VTA or the NAc. Despite not initially hypothesized, the finding is consistent with a recent study that showed no modulation of wanting‐related brain activity for nonconsumable goods following the administration of 400 mg of amisulpride (Soutschek et al., 2021). Most of the prior research examining the role of dopamine in reward anticipation used the Monetary Incentive Delay (MID) task (Knutson et al., 2000). A number of studies supported the hypothesis that increasing dopamine enhances, while blocking dopamine reduces, striatal activity during reward anticipation. On the other hand, other studies reported no effects of dopamine agonism and antagonism on the anticipation of monetary rewards (see Webber et al., 2020 for a recent review). For example, Grimm et al. (2021) recently reported no effects of dopamine antagonism (200 mg amisulpride) and agonism (125 mg l‐DOPA) on behavioral and neural responses to monetary reward anticipation using the MID task. Together, the findings highlight the need for a more systematic investigation of dopaminergic modulation of reward processing in humans, which should take into account different types of reward, individual differences (e.g., dopamine baseline levels), and other sources of variation (Martins et al., 2017; Webber et al., 2020). We also did not observe any significant effect of blocking opioid receptors on neural responses during anticipation of food and touch stimuli. This is in line with a previous study by Buchel et al. (2018) showing that naltrexone reduced neural activity in the mesocorticolimbic system during exclusively during consumption. Similarly, Soutschek et al. (2021) observed a modulation of fronto‐striatal connectivity by naltrexone but also reported no significant effects of opioid antagonism on the neural representation of wanting.
Finally, regardless of the drug group, in the ROI analysis we observed that wanting‐related activity in the NAc and medial OFC was mainly elicited by the anticipation of food rewards, but not touch rewards, suggesting possible differences in neuroanatomical circuitry underlying social and nonsocial reward processing. Social touch is considered a reward as it triggers approach behavior (wanting)—for example, individuals are willing to work to obtain it—and its experience is commonly associated with pleasure (liking), expressed as subjective ratings or positive facial expressions (e.g., Korb, Götzendorfer, et al., 2020; Korb, Massaccesi, et al., 2020; Løseth et al., 2019; Massaccesi et al., 2021, 2022; Mayo et al., 2018; Perini et al., 2015). Nevertheless, prior neuroimaging research showed that social touch is commonly associated with activity in the insula, secondary somatosensory cortex, and temporal regions, while activity of reward‐related brain regions, like the OFC and the NAc, has been less consistently reported (e.g., Bjornsdotter et al., 2014; Gordon et al., 2013; Sailer et al., 2016; Sander & Nummenmaa, 2021). It also has to be noted that, despite our efforts to enhance the social properties of the administered touch (e.g., skin‐to‐skin caresses administered by another person to whom the participants were personally introduced prior to entering the scanner), the absence of a social relationship between the toucher and the participant, as well as methodological aspects (e.g., lying in the scanner without the possibility to see the person administering the touch), might have affected our results.
Some limitations of the present study should be considered. First, drug effects were only observed when operationalizing reward‐related activity as BOLD responses for high compared to low rewards and not when using ratings of wanting/liking and effort as parametric modulators. This may be due to a lack of power for such parametric analysis and individual variability in these measures. Null drug findings were also observed for the ROI analyses. Despite the observed effect of naltrexone on the activity of medial OFC during reward consumption, our ROI analysis using an anatomical mask of medial OFC did not reveal any significant drug effect. This may be due to the relatively large size of the mask employed and/or to a lack of power to detect the effect in the ROI analysis. In light of this, results should be interpreted with caution. Future studies should aim to replicate the present evidence in larger samples. Second, due to feasibility reasons, we employed a between‐subject design. Therefore, although participants were randomly assigned to the three treatments and the groups did not differ in several relevant characteristics (e.g., demographics; mood, hunger, and sociability at the beginning of the study; general attitudes toward social touch and hedonic aspects of food; see Table 1), we cannot rule out entirely the possibility of group differences in reward processing unrelated to the drug administration. Third, despite the long interval between the anticipation and consumption phases in our design (15 s), possibly due to the absence of random jitters between the two task phases, BOLD signal responses related to liking during consumption may have been masked by those pertaining wanting during anticipation, as wanting and liking are highly correlated measures. Future studies should aim to better disentangle these two components by adding jittered intervals among the task phases. Our study also lacked an implicit measure of liking, like facial electromyography (Chiappini et al., 2023; Korb, Massaccesi, et al., 2020), which should be included in future work. Last, amisulpride can have both presynaptic and postsynaptic effects depending on the dosage. The dose of 400 mg employed here is considered the lowest dose to induce postsynaptic effects (Racagni et al., 2004; Schoemaker et al., 1997) and was chosen to ensure participants' safety and minimal side effects (see, e.g., Korb, Götzendorfer, et al., 2020; Soutschek et al., 2021; Weber et al., 2016). Results should be interpreted in the light of the dose employed and possible interindividual differences in drug absorption.
In conclusion, by combining pharmacological challenges, neuroimaging, and a behavioral paradigm inspired by animal research, we showed that blocking the opioid system reduced liking‐related activity in OFC during the receipt of primary social and nonsocial stimuli, in line with the common currency schema of reward. The findings represent a significant step toward deepening our understanding of the neurochemical and neuroanatomical foundations of wanting and liking of social and nonsocial rewards. This research also holds promise for a better comprehension of clinical conditions characterized by general disturbances in reward processing, such as anorexia nervosa and depression.
AUTHOR CONTRIBUTIONS
Conceptualization: GS, CE, SK, MW. Methodology: GS, SK, JL, CW. Software: SK. Formal analysis: CM. Visualization: CM. Investigation: SG, EC. Data curation: CM, SK, EC, SG. Supervision: GS, SK. Project administration: SK, MW, EC, SG. Funding acquisition: GS, CE. Writing—original draft: CM. Writing – review and editing: CM, GS, SK, JL, EC, CW.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGMENTS
This work was supported by the Vienna Science and Technology Fund (WWTF) with a grant (10.47379/CS15003) awarded to GS and CE. Open‐access funding was provided by the University of Vienna. The funding sources had no role in the elaboration of the study design, the data collection, analysis, and interpretation, the writing of this report, and the decision to submit this manuscript for publication. We thank Eva Pool for valuable input regarding the fMRI analyses, Gheorghe L. Preda for his contribution in carrying out the medical procedures, and the students involved in data collection: Raimund Buehler, Merit Pruin, Björn Bartuska, Luca Wiltgen, Ariane Hohl, Berit Hansen.
Massaccesi, C. , Korb, S. , Götzendorfer, S. , Chiappini, E. , Willeit, M. , Lundström, J. N. , Windischberger, C. , Eisenegger, C. , & Silani, G. (2024). Effects of dopamine and opioid receptor antagonism on the neural processing of social and nonsocial rewards. Human Brain Mapping, 45(4), e26645. 10.1002/hbm.26645
Contributor Information
Claudia Massaccesi, Email: claudia.massaccesi@univie.ac.at.
Giorgia Silani, Email: giorgia.silani@univie.ac.at.
DATA AVAILABILITY STATEMENT
The behavioral data and analysis scripts that support the findings are available on Open Science Framework https://osf.io/kw623/.
REFERENCES
- Andersson, J. L. R. , Skare, S. , & Ashburner, J. (2003). How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. NeuroImage, 20(2), 870–888. 10.1016/S1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- Berridge, K. C. , & Kringelbach, M. L. (2015). Pleasure systems in the brain. Neuron, 86(3), 646–664. 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, K. C. , & Robinson, T. E. (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. 10.1016/S0166-2236(03)00233-9 [DOI] [PubMed] [Google Scholar]
- Berridge, K. C. , & Valenstein, E. S. (1991). What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behavioral Neuroscience, 105(1), 3–14. 10.1037/0735-7044.105.1.3 [DOI] [PubMed] [Google Scholar]
- Bjork, J. M. , Grant, S. J. , Chen, G. , & Hommer, D. W. (2014). Dietary tyrosine/phenylalanine depletion effects on behavioral and brain signatures of human motivational processing. Neuropsychopharmacology, 39(3), 595–604. 10.1038/npp.2013.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdotter, M. , Gordon, I. , Pelphrey, K. , Olausson, H. , & Kaiser, M. (2014). Development of brain mechanisms for processing affective touch. Frontiers in Behavioral Neuroscience, 8, 24. 10.3389/fnbeh.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch‐Bouju, C. , Hyland, B. , & Parr‐Brownlie, L. (2013). Motor thalamus integration of cortical, cerebellar and basal ganglia information: Implications for normal and parkinsonian conditions. Frontiers in Computational Neuroscience, 7, 163. 10.3389/fncom.2013.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel, C. , Miedl, S. , & Sprenger, C. (2018). Hedonic processing in humans is mediated by an opioidergic mechanism in a mesocorticolimbic system. eLife, 7, e39648. 10.7554/eLife.39648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, D. C. , & Berridge, K. C. (2017). Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proceedings of the National Academy of Sciences, 114(43), E9125–E9134. 10.1073/pnas.1705753114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley, E. I. , Park, S. , aan het Rot, M. , Sancton, K. , Benkelfat, C. , Young, S. N. , Boivin, D. B. , & Leyton, M. (2013). Dopamine and light: Dissecting effects on mood and motivational states in women with subsyndromal seasonal affective disorder. Journal of Psychiatry and Neuroscience, 38(6), 388–397. 10.1503/jpn.120181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelnokova, O. , Laeng, B. , Eikemo, M. , Riegels, J. , Løseth, G. , Maurud, H. , Willoch, F. , & Leknes, S. (2014). Rewards of beauty: The opioid system mediates social motivation in humans. Molecular Psychiatry, 19(7), 746–747. 10.1038/mp.2014.1 [DOI] [PubMed] [Google Scholar]
- Chevallier, C. , Kohls, G. , Troiani, V. , Brodkin, E. S. , & Schultz, R. T. (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16(4), 231–239. 10.1016/j.tics.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini, E. , Silani, G. , & Korb, S. (2022). Anticipatory and consummatory responses to touch and food rewards: A protocol for human research. Bio‐Protocol, 12(4), e4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini, E. , Silani, G. , Lundström, J. N. , & Korb, S. (2023). Facial electromyography in food research in a behavioral and MR setting. In Bensafi M. (Ed.), Basic protocols on emotions, senses, and foods (pp. 185–201). Springer US. 10.1007/978-1-0716-2934-5_15 [DOI] [Google Scholar]
- Diekhof, E. K. , Kaps, L. , Falkai, P. , & Gruber, O. (2012). The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude – An activation likelihood estimation meta‐analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia, 50(7), 1252–1266. 10.1016/j.neuropsychologia.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Drewnowski, A. , Krahn, D. D. , Demitrack, M. A. , Nairn, K. , & Gosnell, B. A. (1992). Taste responses and preferences for sweet high‐fat foods: Evidence for opioid involvement. Physiology & Behavior, 51(2), 371–379. 10.1016/0031-9384(92)90155-U [DOI] [PubMed] [Google Scholar]
- Eikemo, M. , Biele, G. , Willoch, F. , Thomsen, L. , & Leknes, S. (2017). Opioid modulation of value‐based decision‐making in healthy humans. Neuropsychopharmacology, 42(9), 1833–1840. 10.1038/npp.2017.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikemo, M. , Løseth, G. E. , Johnstone, T. , Gjerstad, J. , Willoch, F. , & Leknes, S. (2016). Sweet taste pleasantness is modulated by morphine and naltrexone. Psychopharmacology, 233(21–22), 3711–3723. 10.1007/s00213-016-4403-x [DOI] [PubMed] [Google Scholar]
- Fischer, A. G. , & Ullsperger, M. (2017). An update on the role of serotonin and its interplay with dopamine for reward. Frontiers in Human Neuroscience, 11, 484. 10.3389/fnhum.2017.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, S. , Veit, R. , Sauer, H. , Enck, P. , Friederich, H.‐C. , Unholzer, T. , Bauer, U.‐M. , Linder, K. , Heni, M. , Fritsche, A. , & Preissl, H. (2016). Dopamine depletion reduces food‐related reward activity independent of BMI. Neuropsychopharmacology, 41(6), 1551–1559. 10.1038/npp.2015.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag, C. M. , Retz‐Junginger, P. , Retz, W. , Seitz, C. , Palmason, H. , Meyer, J. , Rösler, M. , & von Gontard, A. (2007). Evaluation der deutschen Version des Autismus‐Spektrum‐Quotienten (AQ)—Die Kurzversion AQ‐k. Zeitschrift für Klinische Psychologie und Psychotherapie, 36(4), 280–289. 10.1026/1616-3443.36.4.280 [DOI] [Google Scholar]
- Gordon, I. , Voos, A. C. , Bennett, R. H. , Bolling, D. Z. , Pelphrey, K. A. , & Kaiser, M. D. (2013). Brain mechanisms for processing affective touch. Human Brain Mapping, 34(4), 914–922. 10.1002/hbm.21480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf, H. , Wiegers, M. , Metzger, C. D. , Walter, M. , Grön, G. , & Abler, B. (2014). Erotic stimulus processing under amisulpride and reboxetine: A placebo‐controlled fMRI study in healthy subjects. The International Journal of Neuropsychopharmacology, 18(2), pyu004. 10.1093/ijnp/pyu004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, O. , Nägele, M. , Küpper‐Tetzel, L. , de Greck, M. , Plichta, M. , & Reif, A. (2021). No effect of a dopaminergic modulation fMRI task by amisulpride and L‐DOPA on reward anticipation in healthy volunteers. Psychopharmacology, 238(5), 1333–1342. 10.1007/s00213-020-05693-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, R. , Huang, W. , Camilleri, J. , Xu, P. , Wei, P. , Eickhoff, S. B. , & Feng, C. (2019). Love is analogous to money in human brain: Coordinate‐based and functional connectivity meta‐analyses of social and monetary reward anticipation. Neuroscience & Biobehavioral Reviews, 100, 108–128. 10.1016/j.neubiorev.2019.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma, K. , Saito, D. N. , & Sadato, N. (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58(2), 284–294. 10.1016/j.neuron.2008.03.020 [DOI] [PubMed] [Google Scholar]
- JASP Team (2021). JASP (Version 0.15)[Computer software]. https://jasp‐stats.org/.
- Jennings, J. H. , Kim, C. K. , Marshel, J. H. , Raffiee, M. , Ye, L. , Quirin, S. , Pak, S. , Ramakrishnan, C. , & Deisseroth, K. (2019). Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature, 565(7741), 645–649. 10.1038/s41586-018-0866-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, B. , Westdorp, A. , Kaiser, E. , & Hommer, D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–27. 10.1006/nimg.2000.0593 [DOI] [PubMed] [Google Scholar]
- Koepp, M. J. , Hammers, A. , Lawrence, A. D. , Asselin, M. C. , Grasby, P. M. , & Bench, C. J. (2009). Evidence for endogenous opioid release in the amygdala during positive emotion. NeuroImage, 44(1), 252–256. 10.1016/j.neuroimage.2008.08.032 [DOI] [PubMed] [Google Scholar]
- Korb, S. , Götzendorfer, S. J. , Massaccesi, C. , Sezen, P. , Graf, I. , Willeit, M. , Eisenegger, C. , & Silani, G. (2020). Dopaminergic and opioidergic regulation during anticipation and consumption of social and nonsocial rewards. eLife, 9, e55797. 10.7554/eLife.55797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb, S. , Massaccesi, C. , Gartus, A. , Lundström, J. N. , Rumiati, R. , Eisenegger, C. , & Silani, G. (2020). Facial responses of adult humans during the anticipation and consumption of touch and food rewards. Cognition, 194, 104044. 10.1016/j.cognition.2019.104044 [DOI] [PubMed] [Google Scholar]
- Kringelbach, M. L. , & Rolls, E. T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72(5), 341–372. 10.1016/j.pneurobio.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Kringelbach, M. L. , O'Doherty, J. , Rolls, E. T. , & Andrews, C. (2003). Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex, 13(10), 1064–1071. 10.1093/cercor/13.10.1064 [DOI] [PubMed] [Google Scholar]
- la Fougère, C. , Meisenzahl, E. , Schmitt, G. , Stauss, J. , Frodl, T. , Tatsch, K. , Hahn, K. , Möller, H.‐J. , & Dresel, S. (2005). D2 receptor occupancy during high‐ and low‐dose therapy with the atypical antipsychotic amisulpride: A 123I‐iodobenzamide SPECT study. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine, 46(6), 1028–1033. [PubMed] [Google Scholar]
- Lee, M. C. , Wagner, H. N. , Tanada, S. , Frost, J. J. , Bice, A. N. , & Dannals, R. F. (1988). Duration of occupancy of opiate receptors by naltrexone. Journal of Nuclear Medicine, 29(7), 1207–1211. [PubMed] [Google Scholar]
- Lin, A. , Adolphs, R. , & Rangel, A. (2012). Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience, 7(3), 274–281. 10.1093/scan/nsr006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Hairston, J. , Schrier, M. , & Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35(5), 1219–1236. 10.1016/j.neubiorev.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løseth, G. E. , Eikemo, M. , & Leknes, S. (2019). Effects of opioid receptor stimulation and blockade on touch pleasantness: A double‐blind randomised trial. Social Cognitive and Affective Neuroscience, 14(4), 411–422. 10.1093/scan/nsz022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo‐Lima, M. , & Remage‐Healey, L. (2021). Dopamine modulation of motor and sensory cortical plasticity among vertebrates. Integrative and Comparative Biology, 61(1), 316–336. 10.1093/icb/icab019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, D. , Mehta, M. A. , & Prata, D. (2017). The “highs and lows” of the human brain on dopaminergics: Evidence from neuropharmacology. Neuroscience & Biobehavioral Reviews, 80, 351–371. 10.1016/j.neubiorev.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Massaccesi, C. , Korb, S. , Skoluda, N. , Nater, U. M. , & Silani, G. (2021). Effects of appetitive and aversive motivational states on wanting and liking of interpersonal touch. Neuroscience, 464, 12–25. 10.1016/j.neuroscience.2020.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaccesi, C. , Willeit, M. , Quednow, B. B. , Nater, U. M. , Lamm, C. , Müller, D. , & Silani, G. (2022). Opioid‐blunted cortisol response to stress is associated with increased negative mood and wanting of social reward. Neuropsychopharmacology, 1–10, 1798–1807. 10.1038/s41386-022-01283-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyjek, M. , Meliss, S. , Dziobek, I. , & Murayama, K. (2020). A multidimensional view on social and non‐social rewards. Frontiers in Psychiatry, 11, 818. 10.3389/fpsyt.2020.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo, L. M. , Lindé, J. , Olausson, H. , Heilig, M. , & Morrison, I. (2018). Putting a good face on touch: Facial expression reflects the affective valence of caress‐like touch across modalities. Biological Psychology, 137, 83–90. 10.1016/j.biopsycho.2018.07.001 [DOI] [PubMed] [Google Scholar]
- McCabe, C. , Harwood, J. , Brouwer, S. , Harmer, C. J. , & Cowen, P. J. (2013). Effects of pramipexole on the processing of rewarding and aversive taste stimuli. Psychopharmacology, 228(2), 283–290. 10.1007/s00213-013-3033-9 [DOI] [PubMed] [Google Scholar]
- McCabe, C. , Huber, A. , Harmer, C. J. , & Cowen, P. J. (2011). The D2 antagonist sulpiride modulates the neural processing of both rewarding and aversive stimuli in healthy volunteers. Psychopharmacology, 217(2), 271–278. 10.1007/s00213-011-2278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, I. M. , Eikemo, M. , & Leknes, S. (2021). The role of mu‐opioids for reward and threat processing in humans: Bridging the gap from preclinical to clinical opioid drug studies. Current Addiction Reports, 8, 306–318. 10.1007/s40429-021-00366-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenzahl, E. M. , Schmitt, G. , Gründer, G. , Dresel, S. , Frodl, T. , la Fougère, C. , Scheuerecker, J. , Schwarz, M. , Boerner, R. , Stauss, J. , Hahn, K. , & Möller, H.‐J. (2008). Striatal D2/D3 receptor occupancy, clinical response and side effects with amisulpride: An iodine‐123‐iodobenzamide SPET study. Pharmacopsychiatry, 41(5), 169–175. 10.1055/s-2008-1076727 [DOI] [PubMed] [Google Scholar]
- Meyer, M. C. , Straughn, A. B. , Lo, M. W. , Schary, W. L. , & Whitney, C. C. (1984). Bioequivalence, dose‐proportionality, and pharmacokinetics of naltrexone after oral administration. The Journal of Clinical Psychiatry, 45(9 Pt 2), 15–19. [PubMed] [Google Scholar]
- Michely, J. , Viswanathan, S. , Hauser, T. U. , Delker, L. , Dolan, R. J. , & Grefkes, C. (2020). The role of dopamine in dynamic effort‐reward integration. Neuropsychopharmacology, 45(9), 1448–1453. 10.1038/s41386-020-0669-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, E. , Brouwer, S. , McCutcheon, R. , Harmer, C. J. , Cowen, P. J. , & McCabe, C. (2014). Opposing neural effects of naltrexone on food reward and aversion: Implications for the treatment of obesity. Psychopharmacology, 231(22), 4323–4335. 10.1007/s00213-014-3573-7 [DOI] [PubMed] [Google Scholar]
- Nummenmaa, L. , Saanijoki, T. , Tuominen, L. , Hirvonen, J. , Tuulari, J. J. , Nuutila, P. , & Kalliokoski, K. (2018). μ‐Opioid receptor system mediates reward processing in humans. Nature Communications, 9(1), 1500. 10.1038/s41467-018-03848-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez, C. , Hoots, J. K. , Schepers, S. T. , Bower, M. , de Wit, H. , & Wardle, M. C. (2022). Pharmacological investigations of effort‐based decision‐making in humans: Naltrexone and nicotine. PLoS One, 17(10), e0275027. 10.1371/journal.pone.0275027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty, J. P. , Deichmann, R. , Critchley, H. D. , & Dolan, R. J. (2002). Neural responses during anticipation of a primary taste reward. Neuron, 33(5), 815–826. 10.1016/S0896-6273(02)00603-7 [DOI] [PubMed] [Google Scholar]
- Oei, N. Y. L. , Rombouts, S. A. , Soeter, R. P. , van Gerven, J. M. , & Both, S. (2012). Dopamine modulates reward system activity during subconscious processing of sexual stimuli. Neuropsychopharmacology, 37(7), 1737. 10.1038/npp.2012.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña, S. , & Smith, K. S. (2010). Hedonic and motivational roles of opioids in food reward: Implications for overeating disorders. Pharmacology Biochemistry and Behavior, 97(1), 34–46. 10.1016/j.pbb.2010.05.016 [DOI] [PubMed] [Google Scholar]
- Peciña, S. , Smith, K. S. , & Berridge, K. C. (2006). Hedonic hot spots in the brain. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 12(6), 500–511. 10.1177/1073858406293154 [DOI] [PubMed] [Google Scholar]
- Perini, I. , Olausson, H. , & Morrison, I. (2015). Seeking pleasant touch: Neural correlates of behavioral preferences for skin stroking. Frontiers in Behavioral Neuroscience, 9, 8. 10.3389/fnbeh.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic, P. , Pleger, B. , Seymour, B. , Klöppel, S. , De Martino, B. , Critchley, H. , & Dolan, R. J. (2008). Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. The Journal of Neuroscience, 28(42), 10509–10516. 10.1523/JNEUROSCI.2807-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim, R. H. R. , Mennes, M. , van Rooij, D. , Llera, A. , Buitelaar, J. K. , & Beckmann, C. F. (2015). ICA‐AROMA: A robust ICA‐based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Racagni, G. , Canonico, P. L. , Ravizza, L. , Pani, L. , & Amore, M. (2004). Consensus on the use of substituted benzamides in psychiatric patients. Neuropsychobiology, 50(2), 134–143. 10.1159/000079104 [DOI] [PubMed] [Google Scholar]
- Rademacher, L. , Krach, S. , Kohls, G. , Irmak, A. , Gründer, G. , & Spreckelmeyer, K. N. (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage, 49(4), 3276–3285. 10.1016/j.neuroimage.2009.10.089 [DOI] [PubMed] [Google Scholar]
- Riba, J. , Krämer, U. M. , Heldmann, M. , Richter, S. , & Münte, T. F. (2008). Dopamine agonist increases risk taking but blunts reward‐related brain activity. PLoS One, 3(6), e2479. 10.1371/journal.pone.0002479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roininen, K. , & Tuorila, H. (1999). Health and taste attitudes in the prediction of use frequency and choice between less healthy and more healthy snacks. Food Quality and Preference, 10(4), 357–365. 10.1016/S0950-3293(98)00057-3 [DOI] [Google Scholar]
- Rolls, E. T. , Cheng, W. , & Feng, J. (2020). The orbitofrontal cortex: Reward, emotion and depression. Brain Communications, 2(2), fcaa196. 10.1093/braincomms/fcaa196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig, P. , Canal, M. , Patat, A. , Bergougnan, L. , Zieleniuk, I. , & Bianchetti, G. (2002). A review of the pharmacokinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers. Human Psychopharmacology, 17(1), 1–13. 10.1002/hup.320 [DOI] [PubMed] [Google Scholar]
- Ruff, C. C. , & Fehr, E. (2014). The neurobiology of rewards and values in social decision making. Nature Reviews Neuroscience, 15(8), 549–562. 10.1038/nrn3776 [DOI] [PubMed] [Google Scholar]
- Sailer, U. , Triscoli, C. , Häggblad, G. , Hamilton, P. , Olausson, H. , & Croy, I. (2016). Temporal dynamics of brain activation during 40 minutes of pleasant touch. NeuroImage, 139, 360–367. 10.1016/j.neuroimage.2016.06.031 [DOI] [PubMed] [Google Scholar]
- Sander, D. , & Nummenmaa, L. (2021). Reward and emotion: An affective neuroscience approach. Current Opinion in Behavioral Sciences, 39, 161–167. 10.1016/j.cobeha.2021.03.016 [DOI] [Google Scholar]
- Schoemaker, H. , Claustre, Y. , Fage, D. , Rouquier, L. , Chergui, K. , Curet, O. , Oblin, A. , Gonon, F. , Carter, C. , Benavides, J. , & Scatton, B. (1997). Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynaptic and limbic selectivity. Journal of Pharmacology and Experimental Therapeutics, 280(1), 83–97. [PubMed] [Google Scholar]
- Sescousse, G. , Caldú, X. , Segura, B. , & Dreher, J.‐C. (2013). Processing of primary and secondary rewards: A quantitative meta‐analysis and review of human functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37(4), 681–696. 10.1016/j.neubiorev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Sescousse, G. , Janssen, L. K. , Hashemi, M. M. , Timmer, M. H. M. , Geurts, D. E. M. , ter Huurne, N. P. , Clark, L. , & Cools, R. (2016). Amplified striatal responses to near‐miss outcomes in pathological gamblers. Neuropsychopharmacology, 41(10), 2614–2623. 10.1038/npp.2016.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse, G. , Redouté, J. , & Dreher, J.‐C. (2010). The architecture of reward value coding in the human orbitofrontal cortex. Journal of Neuroscience, 30(39), 13095–13104. 10.1523/JNEUROSCI.3501-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, D. M. , Zatorre, R. J. , Dagher, A. , Evans, A. C. , & Jones‐Gotman, M. (2001). Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain, 124(9), 1720–1733. 10.1093/brain/124.9.1720 [DOI] [PubMed] [Google Scholar]
- Soutschek, A. , Gvozdanovic, G. , Kozak, R. , Duvvuri, S. , de Martinis, N. , Harel, B. , Gray, D. L. , Fehr, E. , Jetter, A. , & Tobler, P. N. (2020). Dopaminergic D1 receptor stimulation affects effort and risk preferences. Biological Psychiatry, 87(7), 678–685. 10.1016/j.biopsych.2019.09.002 [DOI] [PubMed] [Google Scholar]
- Soutschek, A. , Weber, S. C. , Kahnt, T. , Quednow, B. B. , & Tobler, P. N. (2021). Opioid antagonism modulates wanting‐related frontostriatal connectivity. eLife, 10, e71077. 10.7554/eLife.71077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer, K. N. , Krach, S. , Kohls, G. , Rademacher, L. , Irmak, A. , Konrad, K. , Kircher, T. , & Gründer, G. (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4(2), 158–165. 10.1093/scan/nsn051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y. , Benusiglio, D. , Lefevre, A. , Hilfiger, L. , Althammer, F. , Bludau, A. , Hagiwara, D. , Baudon, A. , Darbon, P. , Schimmer, J. , Kirchner, M. K. , Roy, R. K. , Wang, S. , Eliava, M. , Wagner, S. , Oberhuber, M. , Conzelmann, K. K. , Schwarz, M. , Stern, J. E. , … Grinevich, V. (2020). Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nature Neuroscience, 23(9), 1125–1137. 10.1038/s41593-020-0674-y [DOI] [PubMed] [Google Scholar]
- Trutti, A. C. , Fontanesi, L. , Mulder, M. J. , Bazin, P.‐L. , Hommel, B. , & Forstmann, B. U. (2021). A probabilistic atlas of the human ventral tegmental area (VTA) based on 7 Tesla MRI data. Brain Structure and Function, 226(4), 1155–1167. 10.1007/s00429-021-02231-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn, J., van den Bergh, D., Böhm, U., Dablander, F., Derks, K., Draws, T., Etz, A., Evans, N. J., Gronau, Q. F., Haaf, J. M., Hinne, M., Kucharský, Š., Ly, A., Marsman, M., Matzke, D., Gupta, A. R. K. N., Sarafoglou, A., Stefan, A., Voelkel, J. G., & Wagenmakers, E.‐J. (2021). The JASP guidelines for conducting and reporting a Bayesian analysis. Psychonomic Bulletin & Review, 28(3), 813–826. https://doi.org/10.3758/s13423‐020‐01798‐5 [DOI] [PMC free article] [PubMed]
- Venugopalan, V. V. , Casey, K. F. , O'Hara, C. , O'Loughlin, J. , Benkelfat, C. , Fellows, L. K. , & Leyton, M. (2011). Acute phenylalanine/tyrosine depletion reduces motivation to smoke cigarettes across stages of addiction. Neuropsychopharmacology, 36(12), 2469–2476. 10.1038/npp.2011.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake, S. J. , & Izuma, K. (2017). A common neural code for social and monetary rewards in the human striatum. Social Cognitive and Affective Neuroscience, 12(10), 1558–1564. 10.1093/scan/nsx092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle, M. C. , Treadway, M. T. , Mayo, L. M. , Zald, D. H. , & de Wit, H. (2011). Amping up effort: Effects of d‐amphetamine on human effort‐based decision‐making. Journal of Neuroscience, 31(46), 16597–16602. 10.1523/JNEUROSCI.4387-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, D. , Clark, L. A. , & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Webber, H. E. , Lopez‐Gamundi, P. , Stamatovich, S. N. , de Wit, H. , & Wardle, M. C. (2020). Using pharmacological manipulations to study the role of dopamine in human reward functioning: A review of studies in healthy adults. Neuroscience & Biobehavioral Reviews, 120, 123–158. 10.1016/j.neubiorev.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, S. C. , Beck‐Schimmer, B. , Kajdi, M.‐E. , Müller, D. , Tobler, P. N. , & Quednow, B. B. (2016). Dopamine D2/3‐ and μ‐opioid receptor antagonists reduce cue‐induced responding and reward impulsivity in humans. Translational Psychiatry, 6(7), e850. 10.1038/tp.2016.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook, A. , van den Bosch, R. , Määttä, J. I. , Hofmans, L. , Papadopetraki, D. , Cools, R. , & Frank, M. J. (2020). Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science, 367(6484), 1362–1366. 10.1126/science.aaz5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm, F. H. , Kochar, A. S. , Roth, W. T. , & Gross, J. J. (2001). Social anxiety and response to touch: Incongruence between self‐evaluative and physiological reactions. Biological Psychology, 58(3), 181–202. 10.1016/S0301-0511(01)00113-2 [DOI] [PubMed] [Google Scholar]
- World Medical Association . (2013). World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. Journal of the American Medical Association, 310(20), 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- Yeomans, M. R. , & Gray, R. W. (1996). Selective effects of naltrexone on food pleasantness and intake. Physiology & Behavior, 60(2), 439–446. 10.1016/S0031-9384(96)80017-5 [DOI] [PubMed] [Google Scholar]
- Zenon, A. , Devesse, S. , & Olivier, E. (2016). Dopamine manipulation affects response vigor independently of opportunity cost. Journal of Neuroscience, 36(37), 9516–9525. 10.1523/JNEUROSCI.4467-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen, H. , Chamberlain, S. R. , Nathan, P. J. , Koch, A. , Maltby, K. , Bush, M. , Tao, W. X. , Napolitano, A. , Skeggs, A. L. , Brooke, A. C. , Cheke, L. , Clayton, N. S. , Sadaf Farooqi, I. , O'Rahilly, S. , Waterworth, D. , Song, K. , Hosking, L. , Richards, D. B. , Fletcher, P. C. , & Bullmore, E. T. (2013). Effects of the mu‐opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: A proof of mechanism study in binge‐eating obese subjects. Molecular Psychiatry, 18(12), 1287–1293. 10.1038/mp.2012.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The behavioral data and analysis scripts that support the findings are available on Open Science Framework https://osf.io/kw623/.


