Abstract
Purpose:
Several sentinel phase III randomized trials have recently been published challenging traditional radiation therapy (RT) practices for small cell lung cancer (SCLC). This American Society for Radiation Oncology guideline reviews the evidence for thoracic RT and prophylactic cranial irradiation (PCI) for both limited-stage (LS) and extensive-stage (ES) SCLC.
Methods:
The American Society for Radiation Oncology convened a task force to address 4 key questions focused on indications, dose fractionation, techniques and timing of thoracic RT for LS-SCLC, the role of stereotactic body radiation therapy (SBRT) compared with conventional RT in stage I or II node negative SCLC, PCI for LS-SCLC and ES-SCLC, and thoracic consolidation for ES-SCLC. Recommendations were based on a systematic literature review and created using a consensus-building methodology and system for grading evidence quality and recommendation strength.
Results:
The task force strongly recommends definitive thoracic RT administered once or twice daily early in the course of treatment for LS-SCLC. Adjuvant RT is conditionally recommended in surgically resected patients with positive margins or nodal metastases. Involved field RT delivered using conformal advanced treatment modalities to postchemotherapy volumes is also strongly recommended. For patients with stage I or II node negative disease, SBRT or conventional fractionation is strongly recommended, and chemotherapy should be delivered before or after SBRT. In LS-SCLC, PCI is strongly recommended for stage II or III patients who responded to chemoradiation, conditionally not recommended for stage I patients, and should be a shared decision for patients at higher risk of neurocognitive toxicities. In ES-SCLC, radiation oncologist consultation for consideration of PCI versus magnetic resonance surveillance is strongly recommended. Lastly, the use of thoracic RT is strongly recommended in select patients with ES-SCLC after chemotherapy treatment, including a conditional recommendation in those responding to chemotherapy and immunotherapy.
Conclusions:
RT plays a vital role in both LS-SCLC and ES-SCLC. These guidelines inform best clinical practices for local therapy in SCLC.
Preamble
As the leading organization in radiation oncology, the American Society for Radiation Oncology (ASTRO) is dedicated to improving quality of care and patient outcomes. A cornerstone of this goal is the development and dissemination of clinical practice guidelines based on systematic methods to evaluate and classify evidence, combined with a focus on patient-centric care and shared decision-making. ASTRO develops and publishes guidelines without commercial support, and members volunteer their time.
Disclosure Policy —
ASTRO has detailed policies and procedures related to disclosure and management of industry relationships to avoid actual, potential, or perceived conflicts of interest. All task force members are required to disclose industry relationships and personal interests beginning 12 months before initiation of the writing effort. Disclosures go through a rigorous review process with final approval by ASTRO’s Conflict of Interest Review Committee. For the purposes of full transparency, task force members’ comprehensive disclosure information is included in this publication. The complete disclosure policy for Formal Papers is online.
Selection of Task Force Members —
The Guideline Subcommittee strives to avoid bias by selecting a multidisciplinary group of experts with variation in geographic region, gender, ethnicity, race, practice setting, and areas of expertise. Representatives from organizations and professional societies with related interests and expertise are also invited to serve on the task force.
Methodology —
The task force uses evidence-based methodologies to develop guideline recommendations in accordance with the National Academy of Medicine standards. The evidence identified from key questions (KQs) is assessed using the Population, Intervention, Comparator, Outcome, Timing, Setting (PICOTS) framework. A systematic review of the KQs is completed, which includes creation of evidence tables that summarize the evidence base task force members use to formulate recommendations. Table 1 describes ASTRO’s recommendation grading system.
Table 1.
ASTRO recommendation grading classification system
| ASTRO’s recommendations are based on evaluation of multiple factors including the QoE, individual study quality, and panel consensus, all of which inform the strength of recommendation. QoE is based on the body of evidence available for a particular key question and includes consideration of number of studies, study design, adequacy of sample sizes, consistency of findings across studies, and generalizability of samples, settings, and treatments. | ||||
|---|---|---|---|---|
|
| ||||
| Strength of Recommendation | Definition | Overall QoE Grade | Recommendation Wording | |
|
| ||||
| Strong | • | • Benefits clearly outweigh risks and burden, or risks and burden clearly outweigh benefits. |
Any (usually high, moderate, or expert opinion) | “Recommend/Should” |
| • | • All or almost all informed people would make the recommended choice. |
|||
| Conditional | • | • Benefits are finely balanced with risks and burden or appreciable uncertainty exists about the magnitude of benefits and risks. |
Any (usually moderate, low, or expert opinion) | “Conditionally Recommend” |
| • | • Most informed people would choose the recommended course of action, but a substantial number would not. |
|||
| • | • A shared decision-making approach regarding patient values and preferences is particularly important. |
|||
|
| ||||
| Overall QoE Grade | Type/Quality of Study | Evidence Interpretation | ||
|
| ||||
| High | • | • 2 or more well-conducted and highly generalizable RCTs or meta-analyses of such trials. |
The true effect is very likely to lie close to the estimate of the effect based on the body of evidence. | |
| Moderate | • | • 1 well-conducted and highly generalizable RCT or a meta-analysis of such trialsOR |
The true effect is likely to be close to the estimate of the effect based on the body of evidence, but it is possible that it is substantially different. | |
| • | • 2 or more RCTs with some weaknesses of procedure or generalizabilityOR |
|||
| • | •2 or more strong observational studies with consistent findings. | |||
| Low | • | • 1 RCT with some weaknesses of procedure or generalizabilityOR |
The true effect may be substantially different from the estimate of the effect. There is a risk that future research may significantly alter the estimate of the effect size or the interpretation of the results. | |
| • | • 1 or more RCTs with serious deficiencies of procedure or generalizability or extremely small sample sizesOR |
|||
| • | • 2 or more observational studies with inconsistent findings, small sample sizes, or other problems that potentially confound interpretation of data. |
|||
| Expert Opinion* | • | • Consensus of the panel based on clinical judgment and experience, due to absence of evidence or limitations in evidence. |
Strong consensus (≥90%) of the panel guides the recommendation despite insufficient evidence to discern the true magnitude and direction of the net effect. Further research may better inform the topic. | |
Abbreviations: ASTRO = American Society for Radiation Oncology; QoE = quality of evidence; RCT = randomized controlled trial.
A lower quality of evidence, including expert opinion, does not imply that the recommendation is conditional. Many important clinical questions addressed in guidelines do not lend themselves to clinical trials but there still may be consensus that the benefits of a treatment or diagnostic test clearly outweigh its risks and burden.
Consensus Development —
Consensus is evaluated using a modified Delphi approach. Task force members confidentially indicate their level of agreement on each recommendation based on a 5-point Likert scale, from “strongly agree” to “strongly disagree.” A prespecified threshold of ≥75% (≥90% for expert opinion recommendations) of raters that select “strongly agree” or “agree” indicates consensus is achieved. Recommendation(s) that do not meet this threshold are removed or revised. Recommendations edited in response to task force or reviewer comments are resurveyed before submission of the document for approval.
Annual Evaluation and Updates —
Guidelines are evaluated annually beginning 2 years after publication for new potentially practice-changing studies that could result in a guideline update. In addition, the Guideline Subcommittee will commission a replacement or reaffirmation within 5-years of publication.
1. Introduction
Small cell lung cancer (SCLC) is the second most common thoracic malignancy, representing approximately 13% of newly diagnosed lung cancers.1 SCLC is a particularly aggressive malignancy, with only about one-third of patients diagnosed with localized or locoregional disease (American Joint Committee on Cancer [AJCC] stage I-III disease, historically defined by the Veterans Affairs Lung Study Group as limited-stage [LS]) that is potentially amenable to curative local therapy. The remaining two-thirds present with distant metastatic disease (AJCC stage IV, historically defined as extensive-stage [ES]).2,3 The standard treatment for LS-SCLC has consisted of chemotherapy with early administration of concurrent twice-daily thoracic radiation therapy (RT) and prophylactic cranial irradiation (PCI).4–8 In ES disease, treatment typically involves chemotherapy alone9 with or without PCI.10 These approaches have been established over the past 30 years, with incremental progress in treatment options and outcomes until recently.
Multiple high-impact phase III clinical trials reported for both LS- and ES-SCLC have challenged traditional RT practices. In 2015, investigators from Chest Radiotherapy Extensive-Stage Trial (CREST) reported a long-term survival benefit with thoracic consolidative RT for ES-SCLC.11 In 2017, the Concurrent Once-Daily Versus Twice-Daily Radiotherapy (CONVERT) trial reported on survival and toxicity differences between the 2 schedules with modern RT doses, fields, and techniques in LS-SCLC.12 A 2017 Japanese PCI trial called into question the role of PCI when modern imaging surveillance is performed in ES-SCLC.13 Finally, in 2018, a Study of Carboplatin Plus Etoposide With or Without Atezolizumab in Participants With Untreated Extensive-Stage Small Cell Lung Cancer (IMpower133) study reported that the addition of immunotherapy to cytotoxic chemotherapy improved survival for ES-SCLC.14
With these seminal publications, along with the advent of new RT approaches to treat SCLC, including stereotactic body radiation therapy (SBRT), intensity modulated radiation therapy (IMRT), and proton therapy, ASTRO created this guideline on SCLC covering thoracic RT for LS-SCLC, SBRT for stage I and II node negative SCLC, PCI for LS- and ES-SCLC, and thoracic RT consolidation in ES-SCLC with the goal of best informing clinical care.
2. Methods
2.1. Task Force Composition
The task force consisted of a multidisciplinary team of radiation, medical, and surgical oncologists, a radiation oncology resident, and a patient representative. This guideline was developed in collaboration with the American Society of Clinical Oncology and the American College of Chest Physicians, who provided representatives and peer reviewers.
2.2. Document Review and Approval
The guideline was reviewed by 14 official peer reviewers (see Appendix E1 for the reviewers’ names and disclosure information) and revised accordingly. The modified guideline was posted on the ASTRO website for public comment in September and October 2019. The final guideline was approved by the ASTRO Board of Directors and endorsed by the American College of Chest Physicians (CHEST), European Society of Radiotherapy, International Association for the Study of Lung Cancer, and the Royal Australian and New Zealand College of Radiologists.
2.3. Evidence Review
A systematic literature review of human subject studies indexed in MEDLINE (through PubMed) was conducted. The inclusion criteria were literature about adults with a diagnosis of SCLC receiving RT and published in English from July 1998 through December 2018. Preclinical or nonhuman studies, dosimetric studies without clinical outcomes, studies available in abstract only, health economics or cost analysis studies, review articles, and comments or editorials were excluded. Inclusion of retrospective studies was restricted to those with at least 200 patients for KQ1 (unless addressing proton therapy or IMRT), 30 patients for KQ2, and 100 patients for KQ3 and KQ4. For KQ1, prospective studies were only included if they had 50 patients or more, unless they covered proton therapy or IMRT. There was no required number of patients for inclusion of prospective studies for the other KQs. Both medical subject headings (MeSH) terms and key search terms were used, and terms common to all searches included: small cell lung cancer, SCLC, small cell lung carcinoma, Small Cell Lung Carcinoma [Mesh], oat cell, radiation therapy, radiotherapy, Radiotherapy[Mesh], and irradiation. Additional terms specific to the KQs were also used and hand searches supplemented the electronic searches.
The data used by the task force to formulate recommendations are summarized in evidence tables (Appendix E2). References selected and published in this document are representative and not all-inclusive. The outcomes of interest were overall, progression-free, and metastasis-free survival; local and nodal control; toxicity; and quality of life. See Figure 1 for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) diagram showing the number of articles screened, excluded, and included in the evidence review. Lastly, see Appendix E3 for a list of abbreviations and Appendix E4 for the detailed search protocol.
Figure 1.
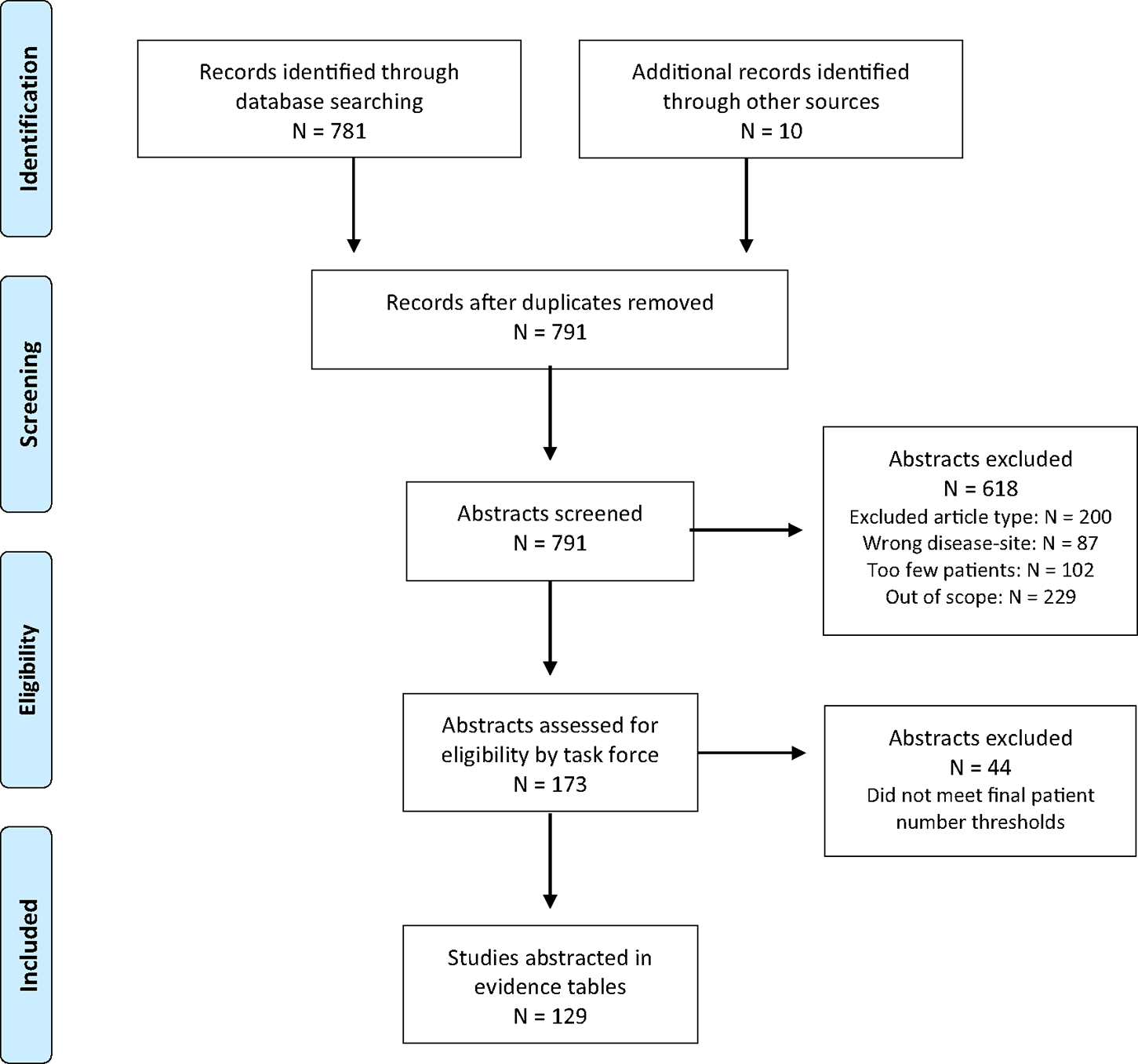
PRISMA diagram, based on Moher et al.111
Abbreviation: PRISMA = preferred reporting items for systematic reviews and meta-analyses.
2.4. Scope of the Guideline
This guideline covers only the subjects specified in the KQs (Table 2). The guideline refers to the AJCC staging, eighth edition.15 Outside the scope of this guideline are many other important questions that may be subjects of other guidelines, including SCLC treated with surgery or chemotherapy alone, whole brain RT for metastases, extrathoracic consolidation, and urgent or palliative unplanned RT due to active symptoms.
Table 2.
KQs in Population, Intervention, Comparator, Outcome (PICO) format
| KQ | Population | Intervention | Comparator | Outcomes | |||
|---|---|---|---|---|---|---|---|
| 1 | What are the indications, appropriate dose-fractionation schedules, techniques, and timing of thoracic RT for LS-SCLC? | ||||||
| Patients with pathologically confirmed LS-SCLC with no evidence of M1 disease | • | • Twice daily RT |
• | • Once daily RT to moderate dose (≤5000 cGy) |
• | • OS |
|
| • | • Once daily RT to higher dose (>5000 cGy) |
• | • Starting thoracic RT at beginning of cycle 1 or cycle 2 of chemo |
• | • Progression-free survival |
||
| • | • Starting thoracic RT after cycle 2 of chemo or in between chemo cycles |
• | • Elective nodal irradiation |
• | • Local control |
||
| • | • Involved field RT |
• | • 3-D CRT |
• | • Grade ≥3 esophagitis |
||
| • | • Adjuvant RT |
• | • Grade ≥3 pneumonitis |
||||
| • | • IMRT |
• | • Major cardiac events |
||||
| • | • Proton therapy |
• | • Hematologic toxicity |
||||
| • | • Hypofractionated RT |
• | • Quality of life |
||||
| 2 | What is the role of SBRT compared with conventional RT in stage I or II node negative SCLC? | ||||||
| Patients with pathologically confirmed AJCC stage IA, IB, or IIA LS-SCLC | • | • SBR |
• | • Conventionally fractionated RT |
• | • OS |
|
| • | • Use of chemo before or after SBRT |
• | • SBRT alone without chemo |
• | • Progression-free survival |
||
| • | • Local control |
||||||
| • | • Nodal control |
||||||
| • | • Distant metastasis-free survival |
||||||
| • | • Grade ≥2 toxicities to the lungs, mediastinal structures, chest wall/ribs, brachial plexus |
||||||
| 3 | What are the indications, appropriate dose-fractionation schedules, and timing of prophylactic cranial RT for LS- and ES-SCLC? | ||||||
| Patients with pathologically confirmed SCLC with no known brain metastases | • | • PCI |
• | • MRI brain surveillance/clinical observation followed by salvage whole brain RT |
• | • Brain metastasis-free survival |
|
| • | • Dose-fractionation schedules for PCI other than 2500 cGy in 10 fx |
• | • PCI in 2500 cGy in 10 fx |
• | • Time to development of brain metastasis |
||
| • | • Different timing of PCI and chemo |
• | • Progression-free survival |
||||
| • | • Different timing of thoracic RT and PCI |
• | • Quality of life |
||||
| • | • Neurocognitive function |
||||||
| • | • Neurotoxicities |
||||||
| 4 | What are the indications, appropriate dose-fractionation schedules, and timing of thoracic consolidation in patients with ES-SCLC? | ||||||
| Patients with pathologically confirmed ES-SCLC | • | • Thoracic RT consolidation |
• | • Chemo alone without thoracic RT consolidation |
• | • OS |
|
| • | • Different dose-fractionation schedules for consolidation |
• | • Elective (not palliative) treatment of distant metastatic disease |
• | • Local control |
||
| • | • Different timing of RT and chemo |
• | • Grade ≥3 acute and late toxicities |
||||
Abbreviations: 3-D CRT = 3-dimensional conformal radiation therapy; AJCC = American Joint Committee on Cancer; chemo = chemotherapy; ES = extensive-stage; fx = fractions; IMRT = intensity modulated radiation therapy; KQs = key questions; LS = limited-stage; MRI = magnetic resonance imaging; OS = overall survival; PCI = prophylactic cranial irradiation; RT = radiation therapy; SBRT = stereotactic body radiation therapy; SCLC = small cell lung cancer.
3. Key Questions and Recommendations
3.1. Key Question 1: Thoracic RT for LS-stage SCLC (Table 3)
Table 3.
Recommendations for thoracic RT for limited-stage SCLC
| KQ1 Recommendations | Strength of Recommendation | Quality of Evidence (Refs) |
|---|---|---|
|
| ||
| • 1. For patients with LS-SCLC who can tolerate definitive therapy, thoracic RT is recommended. |
Strong | High 16, 17, 18, 19, 20 |
| • 2. For patients with LS-SCLC receiving chemotherapy and RT, thoracic RT should begin with cycle 1 or 2 of chemotherapy. • Implementation Remark: It is important to maintain the dosage and timing of chemotherapy with RT based on trial data. Timing is more critical for accelerated dose-intensive RT. |
Strong | Moderate 21, 22, 23, 24, 25, 26, 27, 28 |
| • 3. For postoperative patients with LS-SCLC and R1 or R2 resection, postoperative RT is conditionally recommended. |
Conditional | Expert Opinion 29 |
| • 4. For postoperative patients with LS-SCLC that is clinically node negative and pathologically N2-positive, mediastinal RT is conditionally recommended. |
Conditional | Expert Opinion 29 |
| • 5. For patients with LS-SCLC, twice-daily RT in 150 cGy fractions to 4500 cGy is recommended. |
Strong | High 5,12,30, 31, 32, 33, 34 |
| • 6. For patients with LS-SCLC, daily RT in 200 cGy fractions to 6000–7000 cGy is conditionally recommended as an acceptable alternative to twice-daily RT. |
Conditional | Moderate 12,35, 36, 37 |
| • 7. For patients with LS-SCLC, involved field RT is recommended as the standard of care (defined as fluorodeoxyglucose avid on PET, enlarged on CT, and/or biopsy-positive). |
Strong | Moderate 38, 39, 40, 41, 42, 43 |
| • 8. For tumors that experience shrinkage with chemotherapy in patients with LS-SCLC, treating all involved nodal stations (at time of diagnosis) and postchemotherapy lung parenchymal tumor is recommended. |
Strong | Moderate 12,38 |
| • 9. For patients with LS-SCLC, highly conformal techniques are recommended to minimize normal tissue dose. |
Strong | Low 44, 45, 46 |
Abbreviations: cGy = centigray; CT = computed tomography; KQ = key question; LS = limited-stage; PET = positron emission tomography; RT = radiation therapy; SCLC = small cell lung cancer.
See Appendix E2 for the evidence supporting the recommendations for KQ1.
What are the indications, appropriate dose-fractionation schedules, techniques, and timing of thoracic RT for LS-SCLC?
RT is part of curative-intent treatment for patients with LS-SCLC with a benefit in survival evident in both recent and historical data.7,16–20 For patients in good health with adequate performance status, concurrent chemoradiation is the standard of care and can be used for patients with good performance, despite comorbidities, and is for fit elderly (>70 years) patients with careful selection.47–49
Earlier thoracic RT is superior to delayed thoracic RT when delivering concurrent chemoradiation for LS-SCLC and, ideally, RT should start with cycle 1 or 2 of chemotherapy.21,23,25,26,50 However, chemotherapy should not be delayed with the goal of starting RT concurrent with cycle 1 of chemotherapy. If tumor shrinkage might allow for a decrease in radiation toxicities, starting RT with cycle 3 of chemotherapy may be more optimal for a subset of patients and may provide comparable results to starting with cycle 1 or 2, although the data are more limited.51,52
Based on 2 randomized trials and other smaller studies, the optimal dose and fractionation for RT in LS-SCLC is 4500 cGy delivered in 30 twice daily fractions of 150 cGy, delivered with at least a 6-hour interfractional interval, over 3 weeks.5,12,30–34 For patients who are unwilling or unable to undergo twice-daily RT, or in clinics where it is not feasible to deliver twice-daily treatment, daily RT of 6000 to 7000 cGy is an acceptable alternative.12,35,53 Studies show that this dose range, and perhaps doses as low as 5000 cGy, are comparable to the twice daily regimen.27,28,36,37,54–58 Mild hypofractionation greater than 300 cGy per fraction59 is not routinely recommended owing to limited evidence for its equivalence.30
The recommended treatment field for LS-SCLC is involved field RT, as defined on imaging (positron emission tomography/computed tomography [CT] avidity or abnormal/enlarged nodes on CT), or pathology.38–43 Involved field RT is consistent with the overall trend in lung cancer treatment to minimize toxicity by limiting treatment fields. There is variation in the management of an uninvolved ipsilateral hilum, as some trials electively included it, and some have not. Additionally, if the subcarinal or lower paratracheal lymph node stations are involved, the hilum would receive a substantial incidental dose of radiation. Because SCLC is chemo-sensitive and RT may not start until cycle 2 or later, tumor shrinkage can occur from diagnosis to the beginning of RT. The recommendation of the task force is to treat all involved nodal stations at the time of diagnosis, but primary lung tumor contours can be matched to the postchemotherapy volume. This is the approach taken in most of the large randomized trials.12,60
Use of modulated techniques (eg, IMRT or volumetric modulated arc therapy) over 3-dimensional conformal treatment is recommended in an attempt to decrease normal tissue toxicities, as is the use of tumor motion management techniques and image guidance where applicable. However, unlike non-small cell lung cancer (NSCLC), there are limited data on advanced RT techniques in SCLC treatment.44 Proton therapy could potentially further decrease normal tissue toxicities, but there are limited prospective data on its role in SCLC treatment.45,46 Generation of evidence is encouraged through treatment of patients in prospective clinical trials or multi-institutional registries.
There are limited data on the role of postoperative RT for SCLC, so the recommendation on indications for RT in this setting is based on NSCLC. For positive margin(s) or incomplete resection, postoperative RT should be given.61 For patients who underwent negative nodal sampling preoperatively and are found at the time of surgery to be N2 positive, postoperative RT can be considered, as the disease control benefit may outweigh potential harm from RT.29 Because the benefit-to-harm ratio in SCLC is likely even less advantageous for N0–1 disease than N2 disease, postoperative RT for N0–1 disease is generally not recommended for SCLC. There is no current standard for treatment volumes in the postoperative setting for SCLC, and extrapolation from NSCLC literature can be considered.
3.2. Key Question 2: Role of SBRT in stage I or II node negative SCLC (Table 4)
Table 4.
Recommendations for SBRT in stage I or II node negative SCLC
| KQ2 Recommendations | Strength of Recommendation | Quality of Evidence (Refs) |
|---|---|---|
|
| ||
| • 1. For patients with stage I or II node negative LS-SCLC who are medically inoperable, either SBRT or conventional fractionation is recommended. •Implementation Remarks: ∘ • Ideally the node negative status should be confirmed by invasive nodal staging. ∘ • Ultracentral tumors may be more appropriately treated with conventional fractionation schema. |
Strong | Moderate 20,62, 63, 64 |
| • 2. For patients with stage I or II node negative LS-SCLC receiving SBRT, chemotherapy should be delivered to patients in whom it is medically tolerated. |
Strong | Moderate 18,62,63 |
Abbreviations: KQ = key question; LS = limited-stage; SBRT = stereotactic body radiation therapy; SCLC = small cell lung cancer.
See Appendix E2 for the evidence supporting the recommendations for KQ2.
What is the role of SBRT compared with conventional RT in stage I or II node negative SCLC?
SBRT, a strategy that employs very high ablative doses of radiation delivered to the cancer target over 1 to 5 fractions with highly conformal techniques,59 is an effective modality that is increasingly being used for stage I and II SCLC. Although data to date are limited and there are no completed randomized controlled trials of SBRT for SCLC, this modality is of particular utility for patients who are not operative candidates owing to medical comorbidities, functional status, poor baseline lung function, or preference to avoid surgery. In light of its favorable side effect profile, SBRT is most suitable in elderly patients or those with limited performance status who have histologically confirmed stage I to II, node negative, peripherally located SCLC.20,62–64 SBRT doses for SCLC should mirror prior ASTRO guideline dose recommendations.59 Whenever feasible, invasive mediastinal staging should be employed to confirm the status of the mediastinal lymph nodes. As with NSCLC,65,66 this mediastinal staging assures lymph node negativity and corroborates the findings of the requisite thoracic imaging with chest CT and positron emission tomography scan.
Although SBRT may be reasonable for SCLCs in the aforementioned populations, SBRT is less suitable for patients with “ultracentral” tumors, which includes tumors whose planning target volume directly contacts or overlaps the proximal bronchial tree, trachea, mainstem bronchus, esophagus, pulmonary vein, or pulmonary artery. The propensity for lymph node metastases may be even higher with central versus peripheral lesions, and ultracentral tumors also often encroach on mediastinal structures, which increases the risk of toxicity with SBRT.67 As a result, conventionally fractionated RT is more appropriate in these patients. More mild hypofractionation could be considered in very select patients, akin to treatment approaches in NSCLC,68 but data for such an approach in patients with ultracentral early-stage SCLC are lacking.
In evaluating the use of SBRT based on data from the National Cancer Database, a study found that the percentage of patients with stage I SCLC who were treated with SBRT increased from 2004 (0.4%) to 2013 (6.4%), as did the use of definitive surgical management during this period.69 The CONVERT trial included a significant number of patients with LS-SCLC and N1 disease who received chemotherapy and had good performance status (Eastern Cooperative Oncology Group 0–1). The local progression-free survival was 56% at 2 years and 47% at 4 years, and the median time to local failure was 38 months.64 In addition, a retrospective study of SBRT for LS-SCLC from multiple institutions evaluated 76 lesions in 74 patients. Only 59% of the patients received chemotherapy, and >30% of patients had poor performance statuses (Eastern Cooperative Oncology Group 2–3). Despite this, local progression-free survival was 96.1% at 3 years.63,70 Despite the seemingly small numbers of patients, these 2 cohorts are relatively sizable given the low incidence of stage I and II node negative SCLC.63,64,70 These results suggest SBRT may provide similar, if not possibly superior, local control outcomes to conventionally fractionated RT, with a mild toxicity profile, and that the utility of SBRT may extend to stage I and II node negative patients with LS-SCLC who qualify for concurrent chemoradiation.
In patients with stage I and II node negative LS-SCLC receiving SBRT, chemotherapy should be delivered to patients who can medically tolerate it, given the known relapse patterns with a proclivity for distant metastases. Chemotherapy is likely to improve overall survival (OS) when combined with local therapy for stage I and II SCLC.70,71 Given the short time frame over which SBRT can be delivered, it lends itself to rapid, sequential initiation of systemic therapy, with SBRT started and completed before initiation of chemotherapy or delivered between early cycles of chemotherapy. Although there is a lack of modality sequencing comparative data, these are the preferred treatment approaches over concurrent SBRT and chemotherapy delivered on the same days. In cases where SBRT is not delivered before initiating chemotherapy or in between cycles of chemotherapy, both SBRT64 and hypofractionated RT62 have been studied with concurrent chemotherapy beginning on day 1 of RT with good safety and efficacy in LS-SCLC. Because of the known responsiveness of SCLC to chemotherapy, it is advisable to incorporate SBRT early in the treatment course. After the initiation of even 1 to 2 cycles of chemotherapy, the lung tumor may decrease in size significantly owing to treatment response and may be more difficult to visualize on imaging. Adding RT to chemotherapy is likely to improve survival rates compared with chemotherapy alone for LS-SCLC.20 SBRT should also incorporate volumetric image guidance (ie, cone beam CT) given the need to allow for 3-dimensional targeting of the tumor and possible changes in tumor volume during the course of RT.72
3.3. Key Question 3: Prophylactic cranial RT (Table 5)
Table 5.
Recommendations for prophylactic cranial RT
| KQ3 Recommendations | Strength of Recommendation | Quality of Evidence (Refs) |
|---|---|---|
|
| ||
| • 1. For patients with SCLC who respond to initial therapy, restaging with brain MRI to guide decision-making regarding PCI is recommended. |
Strong | Low 73,74 |
| • 2. For patients with stage I SCLC, PCI is conditionally not recommended. • Implementation Remark: In lieu of PCI, surveillance using brain MRI with contrast can serve as an alternative. |
Conditional | Low 75, 76, 77, 78 |
| • 3. For patients with stage II-III LS-SCLC who are less than 70 years of age with good performance status (ECOG 0–2) and respond to thoracic chemoradiation, PCI is recommended. |
Strong | High 8,75,79, 80, 81, 82, 83, 84, 85, 86, 87 |
| • 4. For patients with LS-SCLC who have limited performance status, older age, and/or significant comorbidities, shared decision-making on PCI (considering patient- and disease-specific characteristics) is recommended. |
Strong | Low 80,85,88, 89, 90, 91, 92 |
| • 5. For patients with LS-SCLC receiving PCI, 2500 cGy in 10 fractions is recommended. |
Strong | Moderate 82,83,91, 92, 93 |
| • 6. For patients with ES-SCLC who respond to chemotherapy, consultation with a radiation oncologist to enhance shared decision-making on PCI versus MRI surveillance (considering patient- and disease-specific characteristics) is recommended. |
Strong | Moderate 10,13,81,94, 95, 96 |
| • 7. For patients with ES-SCLC who elect PCI, 2500 cGy in 10 fractions or 2000 cGy in 5 fractions is recommended. |
Strong | Moderate 10,13,92,93,97,98 |
Abbreviations: cGy = centigray; ECOG = Eastern Cooperative Oncology Group; ES = extensive-stage; KQ = key question; LS = limited-stage; MRI = magnetic resonance imaging; PCI = prophylactic cranial irradiation; RT = radiation therapy; SCLC = small cell lung cancer.
See Appendix E2 for the evidence supporting the recommendations for KQ3.
What are the indications, appropriate dose-fractionation schedules, and timing of prophylactic cranial RT for LS-SCLC and ES-SCLC?
Standard platinum-etoposide chemotherapy for SCLC has limited efficacy in the central nervous system owing to low penetrance of the blood–brain barrier. As a result, among patients with SCLC who undergo chemoradiation, approximately 59% to 69% ultimately develop brain metastases (BMs).13,80,99 This recognition has led to investigations of PCI to minimize rates of BMs and associated morbidity and mortality. A meta-analysis of clinical trials of mostly patients with LS-SCLC demonstrated an absolute 5.4% OS advantage at 3 years with the use of PCI, establishing it as an important consideration in SCLC management,8 with similar findings confirmed in a subsequent meta-analysis.81 Although the Aupérin meta-analysis only included patients with a complete remission to initial therapy (primarily assessed by chest radiograph),8 the support for PCI can be extrapolated to patients with complete and partial responses owing to differences in how responses are assessed using modern imaging.100
Given the importance of central nervous system as a site of failure in SCLC, and in the context of PCI consideration, magnetic resonance imaging (MRI) restaging after completion of chemoradiation is recommended. Studies examining the value of restaging MRI after upfront chemoradiation indicate interval development of BMs in 20% to32%ofpatients.73,74 This information imparts prognostic information and guides decision making on whole-brain RT intent and dosing. MRI with contrast is the recommended imaging modality given higher sensitivity for small lesions; however, contrast-enhanced CT maintains a role in staging and surveillance for patients with pacemakers or other MRI contraindications.101
For patients with LS-SCLC who respond to initial chemoradiation and remain without evidence of BMs upon MRI restaging, consultation regarding PCI is necessary. In a pooled analysis of 2 parallel randomized studies assessing over 500 patients, the 5-year cumulative rate of brain metastasis as the first site of relapse was 37% in the control group and 20% in the PCI group.74 Similar findings were seen in a second, smaller randomized trial in which the development decreased from 32% without PCI to 3.8% with PCI.79 Additionally, multiple meta-analyses and pooled analyses of older studies with LS-SCLC populations suggest an OS benefit to the use of PCI, with risk ratios for ultimate BM development consistently ranging from 0.45 to 0.50.8,75,81–83,98
Although the studies that provide the strongest evidence for a survival advantage largely predate the use of MRI, the risk for BMs remains high in the current era. Studies in LS-SCLC after negative restaging MRI following chemoradiation show BM development in 37% to 41% of patients managed without PCI.102,103 Further contemporary reports, albeit not randomized, continue to show significant advantages to the use of PCI in patients with LS-SCLC, including 3 large retrospective studies that suggest a survival benefit even among patients restaged with MRI following initial therapy.85–87 Taken together, the literature suggests that PCI remains the standard of care for most patients with LS-SCLC who respond to upfront chemoradiation.
However, numerous series have identified subgroups less likely to benefit. For example, multiple studies suggest that the risk for BM development in stage I disease is relatively low and an OS benefit associated with PCI has not been established.75–78 Given the negative effect on quality of life104 and neurotoxicity82,95 from PCI, this population should not be routinely treated with PCI, and the risk-benefit ratio of PCI should be discussed with the individual patient in the context of shared decision-making. Another consideration is that the SCLC population tends to be elderly, with heavy smoking histories and associated comorbidities. Although age is an imperfect measure, much of the randomized data demonstrating a benefit from PCI excluded patients >70 years old,80 whereas other series suggest that patients >70 years old are unlikely to derive an OS benefit.85 In addition, increasing age (as a continuous variable) is the most significant factor for developing chronic neurotoxicity in an analysis of a prospective PCI trial.92,104 Beyond age, 2 Canadian studies have suggested that patients with LS-SCLC and incomplete responses to chemoradiation may not benefit from PCI.90,91 Given the uncertain benefit in such subgroups, and given the toxicities that accompany PCI, shared decision making regarding PCI versus MRI surveillance should be facilitated for patients with advanced age, limited performance status, preexisting neurocognitive conditions, or significant comorbid conditions.
The recommended regimen of 2500 cGy in 10 fractions of 250 cGy for patients with LS-SCLC who undergo PCI is supported by randomized data.93 No significant reduction in the 2-year incidence of BMs was noted with higher doses. Given the increased mortality in the higher dose arm, 2500 cGy is recommended as the standard of care in LS-SCLC. Other studies similarly suggest that increasing dose in the PCI setting is not advantageous,82,83,91 and an analysis of the neurocognitive outcomes within the randomized Radiation Therapy Oncology Group (RTOG) 0212 study (2500 cGy vs 3600 cGy) demonstrated a significant increase in the occurrence of chronic neurotoxicity in the higher dose cohort.92 Strategies to reduce the risk of neurotoxicity, including medical interventions and hippocampal avoidance PCI, are currently being assessed in ongoing clinical trials (NRG Oncology trial CC003), and the rate of brain metastatic failure from this approach is yet to be defined in this population.
In ES-SCLC, the use of PCI was examined in a randomized trial of patients with any response to upfront chemotherapy.10 Patients had no known BMs, but brain imaging was not mandated as part of the standard staging or follow-up procedures. PCI reduced symptomatic BMs and resulted in improved OS. Similarly, meta-analyses8,81,94,95 and some pooled analyses82,83 demonstrated OS to be favorably associated with PCI usage in ES-SCLC. However, a different meta-analysis questioned the OS benefit of PCI in ES-SCLC.96 A more contemporary Japanese randomized study was designed to examine MRI surveillance compared with PCI.13 PCI resulted in a significantly decreased burden of BMs compared with MRI surveillance (48% vs 69%). However, OS was not improved.13 It should be noted that no quality of life and limited neurocognition data are available from this trial. Therefore, for patients who can adhere to the schedule, MRI surveillance can be considered an alternative to PCI. The task force recommends consultation with a radiation oncologist regarding the benefits and risks of PCI versus MRI surveillance. Similar to patients with LS-SCLC, patients with ES-SCLC who progress after initial therapy should not be recommended for PCI.
Among patients with ES-SCLC who elect to have PCI, the task force agreed that data supporting utilization of 2500 cGy in 10 fractions are likely applicable regarding disease control and neurotoxicity.92,93 This regimen has been extensively used in patients with ES-SCLC undergoing PCI.10,97,98 However, given that the strongest randomized data supporting PCI for ES-SCLC delivered 2000 cGy in 5 fractions of 400 cGy to 62% of the patients receiving PCI,10 that fractionation scheme also garnered support as an evidence-based regimen, although neurocognitive effects for this scheme have not been studied.
3.4. Key Question 4: Thoracic consolidation for ES-SCLC (Table 6)
Table 6.
Recommendations for thoracic consolidation for ES-SCLC
| KQ4 Recommendations | Strength of Recommendation | Quality of Evidence (Refs) |
|---|---|---|
|
| ||
| • 1. For patients with ES-SCLC with a response to chemotherapy alone but residual tumor in the thorax, thoracic RT is recommended. |
Strong | High 11,105, 106, 107 |
| • 2. For patients with ES-SCLC with a response to chemotherapy alone, thoracic RT to a dose of 3000 cGy in 10 fractions is conditionally recommended. • Implementation Remark: In patients expected to have a prolonged survival, higher doses may be appropriate. |
Conditional | Moderate 11,106,107 |
| • 3. For patients with ES-SCLC who will receive thoracic RT, the treatment should be given after completion of chemotherapy alone. |
Strong | High 11,106,107 |
| • 4. For patients with ES-SCLC with a response to chemotherapy and immunotherapy and residual disease in the thorax, thoracic RT to 3000 cGy in 10 fractions within 6–8 weeks is conditionally recommended. |
Conditional | Expert Opinion |
Abbreviations: cGy = centigray; ES = extensive-stage; KQ = key question; RT = radiation therapy; SCLC = small cell lung cancer.
See Appendix E2 for the evidence supporting the recommendations for KQ4.
What are the indications, appropriate dose-fractionation schedules, and timing of thoracic consolidation in patients with ES-SCLC?
The role of thoracic RT in patients with ES-SCLC is addressed in 3 randomized controlled trials11,105,106 and was confirmed in 1 meta-analysis.107 In 1 study, a significant benefit in OS was seen when high-dose thoracic RT was given in combination with chemotherapy after a response to 3 cycles of chemotherapy.105 These patients had a complete response outside the thorax and at least a partial response inside the thorax. This highly selective patient group and the high-dose chemoradiation scheme for patients with disseminated disease have probably contributed to the fact that this approach did not become a standard treatment approach. CREST, a randomized trial, demonstrated a significant improvement in OS at 2 years.11 Further analysis showed that in patients with residual intrathoracic disease, there was a statistically significant benefit in OS for thoracic RT, and the risk of disease progression inside the thorax was reduced by approximately 50%.108 The prolonged time to progression was confirmed in the RTOG 0937 trial, although it was closed prematurely and no significant differences in OS were observed between patients receiving RT to all disease sites and those receiving PCI only.106
If thoracic RT is given, it is advised to start after the completion of chemotherapy, and to eventually deliver thoracic RT simultaneously with PCI, if given. For a very select group of patients with ES-SCLC with minimal systemic disease, a more aggressive approach with a higher dose of thoracic RT can be considered.
The doses in CREST (3000 cGy in 10 fractions of 300 cGy)11 could be delivered without significant toxicities. That trial showed no difference in toxicity between patients who did and who did not receive thoracic RT. Patients with good performance statuses who have had an excellent response to chemotherapy might be expected to have a longer survival than the typical patient with ES-SCLC. As a result, higher doses of thoracic RT (eg, 4500 cGy in 15 fractions as was delivered in the RTOG study)106 might be considered as a way to improve local control in this population.105
There is also increasing interest in the use of immunotherapy for patients with ES-SCLC, especially after the publicationofIMpower-133ontheuseofatezolizumaband the Durvalumab ± Tremelimumab in Combination With Platinum Based Chemotherapy in Untreated Extensive-Stage Small Cell Lung Cancer (CASPIAN) trial on the use of durvalamab.14,109 Although thoracic RT was not routinely administered in this trial, a secondary analysis presented in abstract form reported that some patients did receive palliative thoracic RT in this study without additional toxicity. Because palliative doses are expected to have limited toxicity, the task force’s expert opinion suggests that 3000 cGy of thoracic RT can safely be given in these patients with residual thoracic disease after the completion of chemotherapy and immunotherapy.110 Additional research on thoracic RT and immunotherapy is needed to address indications, timing, and dose.
4. Conclusion/Future Directions
After many decades of minimal progress in the treatment of SCLC, we are now in an era of novel therapeutics that have demonstrated significant improvement in survival. Previously, it was impractical to plan or implement clinical trials in many of these patients, especially those with extensive disease because of poor prognosis, and rapid decline was frequently observed. Due to the increase in survival after a diagnosis of SCLC, radiation oncologists are seeing a greater number of patients and clinical scenarios in the course of standard clinical practice.
For LS-SCLC, the typical questions regarding dose, fractionation, and timing are addressed in the recommendations. Additionally, adjuvant RT is conditionally recommended in surgically resected patients, and conformal advanced treatment modalities are also strongly recommended. For patients with stage I and II node negative disease, SBRT is a technique that in the thorax is typically reserved for early-stage NSCLC or oligometastases in the lung and has emerged as an effective treatment option.
The decision to use PCI is very challenging for the radiation oncologist, and so the task force’s recommendations delve into which patients can benefit from treatment and which can be offered MRI surveillance. The use of thoracic RT in patients with ES-SCLC after chemotherapy treatment is recommended, including a conditional recommendation in patients with a response to chemotherapy and immunotherapy.
The future of RT for SCLC—particularly in LS disease—will continue to evolve with the expected publication of the phase III Cancer and Leukemia Group B 30610 trial assessing once or twice daily irradiation with concurrent chemotherapy for LS-SCLC, and with the initiation of the NRG LU005 phase II/III randomized trial assessing the role of immunotherapy with concurrent chemoradiation for LS-SCLC. Additionally, other immunotherapy agents and completely new therapeutic classes continue to be studied in ES-SCLC, leading to new opportunities to help our patients. ASTRO will evaluate the need to update these guidelines in the future as potentially practice-changing data, treatment approaches, or technologies emerge.
Supplementary Material
5. Acknowledgments
Thank you to Cristina Decesaris, MD, Elisha Fredman, MD, Blair Murphy, MD, Michael Stolten, MD, and Sherry Yan, MD, for literature review assistance; and ASTRO staff members, Caroline Patton and Lisa Bradfield, for guidance regarding guideline methodology and literature search support.
The task force also thanks the peer reviewers for their comments and time spent reviewing the guideline. See Appendix E1 for their names and disclosures.
Sources of support: This work was funded by the American Society for Radiation Oncology.
Footnotes
Task Force Members’ Disclosure Statements
All task force members’ disclosure statements were rigorously reviewed before being invited and were shared with other task force members throughout the guideline’s development. Those disclosures are published within this report. Where potential conflicts were detected, remedial measures to address them were taken.
Jeffrey Bogart: Alliance for Clinical Trials in Oncology (chair, Radiation Oncology Committee, travel expenses), Cardan Robotics (partnership), Mobius Imaging (stock); Megan Daly (ASCO representative): Boston Scientific (advisory board), Department of Defense, EMD Serono, and NIH (all research grants), International Journal of Radiation Oncology, Biology, Physics (associate editor), Practical Radiation Oncology (executive editor), Seminars in Oncology (editorial board member), Triptych Health Partners (consultant); Corinne Faivre-Finn: AstraZeneca, Elekta, and Merck (all research grants, travel expenses), Cancer Research UK (Clinical Expert Review Panel), Christie Hospital and EORTC headquarters (contact NOCI clinician), CRUK Lung Cancer Center of Excellent (RT research lead), EORTC Lung Group (early disease chair), EORTC General Assembly (voting member), IASLC Advanced Radiation Technology Committee (member), National Cancer Research Network (Lung Cancer Locoregional Disease Subgroup and Workstream 3 CTRAD Group member), Pfizer (travel expenses); Nancy Gatschet (patient representative): Abbvie (patient advocate [unpaid]); Elizabeth Gore: NRG Oncology (chair, Publication Committee); Salma Jabbour: Big Ten Cancer Research Consortium (member, Steering and GI Committees), International Journal of Radiation Oncology, Biology, Physics (senior editor), Merck (research grants, consultant), NCI Radiation Research Program (cochair, Upper GI Working Group), Nestle (research grants); Timothy Kruser: AstraZeneca (speaker’s bureau, advisory board initiated in July 2019); Bryan Schneider (ASCO representative): Bristol-Myers Squibb, Genentech, Medimmune, and OncoMed Pharmaceuticals (all research grants); Charles Simone: Annals of Palliative Medicine (editor-in-chief), Proton Collaborative Group (chair, Executive Council and Lung Committee); Ben Slotman: Varian (research grants, travel expenses), Viewray (research grants, honoraria); Abraham Wu: AlphaTau Medical (travel expenses), AstraZeneca (consultant), CivaTech Oncology (research grants), NIH (research grants); Jing Zeng: NIH (research grants); Alvin Cabrera, Nicholas DeNunzio, Frank Detterbeck (American College of Chest Physicians representative), Kenneth Rosenzweig, and Andrew Turrisi reported no disclosures.
Disclaimer and Adherence — American Society for Radiation Oncology (ASTRO) guidelines present scientific, health, and safety information and may reflect scientific or medical opinion. They are available to ASTRO members and the public for educational and informational purposes only. Commercial use of any content in this guideline without the prior written consent of ASTRO is strictly prohibited.
Adherence to this guideline does not ensure successful treatment in every situation. This guideline should not be deemed inclusive of all proper methods of care or exclusive of other methods reasonably directed to obtaining the same results. The physician must make the ultimate judgment regarding therapy considering all circumstances presented by the patient. ASTRO assumes no liability for the information, conclusions, and findings contained in its guidelines. This guideline cannot be assumed to apply to the use of these interventions performed in the context of clinical trials. This guideline is based on information available at the time the task force conducted its research and discussions on this topic. There may be new developments that are not reflected in this guideline and that may, over time, be a basis for ASTRO to revisit and update the guideline.
Supplementary data
Supplementary material for this article can be found at https://doi.org/10.1016/j.prro.2020.02.009.
References
- 1.Howlader N, Noone A, Krapcho M, et al. , eds. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2016. Available at: https://seer.cancer.gov/csr/1975_2016/. Accessed November 22, 2019. [Google Scholar]
- 2.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—What limits limited disease? Lung Cancer. 2002;37:271–276. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 4.Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336–344. [DOI] [PubMed] [Google Scholar]
- 5.Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. [DOI] [PubMed] [Google Scholar]
- 6.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890–895. [DOI] [PubMed] [Google Scholar]
- 7.Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618–1624. [DOI] [PubMed] [Google Scholar]
- 8.Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–484. [DOI] [PubMed] [Google Scholar]
- 9.Green RA, Humphrey E, Close H, Patno ME. Alkylating agents in bronchogenic carcinoma. Am J Med. 1969;46:516–525. [DOI] [PubMed] [Google Scholar]
- 10.Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. [DOI] [PubMed] [Google Scholar]
- 11.Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: A phase 3 randomised controlled trial. Lancet. 2015;385:36–42. [DOI] [PubMed] [Google Scholar]
- 12.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): An open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:663–671. [DOI] [PubMed] [Google Scholar]
- 14.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. [DOI] [PubMed] [Google Scholar]
- 15.Amin M, Edge S, Greene F, et al. , eds. American Joint Committee on Cancer Staging Manual. Eighth Edition. Philadelphia, PA: Springer International Publishing; 2017. [Google Scholar]
- 16.Perry MC, Herndon JE 3rd, Eaton WL, Green MR. Thoracic radiation therapy added to chemotherapy for small-cell lung cancer: An update of Cancer and Leukemia Group B Study 8083. J Clin Oncol. 1998;16:2466–2467. [DOI] [PubMed] [Google Scholar]
- 17.Corso CD, Rutter CE, Park HS, et al. Role of chemoradiotherapy in elderly patients with limited-stage small-cell lung cancer. J Clin Oncol. 2015;33:4240–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaspar LE, Gay EG, Crawford J, Putnam JB, Herbst RS, Bonner JA. Limited-stage small-cell lung cancer (stages I-III): Observations from the National Cancer Data Base. Clin Lung Cancer. 2005;6:355–360. [DOI] [PubMed] [Google Scholar]
- 19.Gaspar LE, McNamara EJ, Gay EG, et al. Small-cell lung cancer: Prognostic factors and changing treatment over 15 years. Clin Lung Cancer. 2012;13:115–122. [DOI] [PubMed] [Google Scholar]
- 20.Lally BE, Geiger AM, Urbanic JJ, et al. Trends in the outcomes for patients with limited stage small cell lung cancer: An analysis of the Surveillance, Epidemiology, and End Results database. Lung Cancer. 2009;64:226–231. [DOI] [PubMed] [Google Scholar]
- 21.Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–3060. [DOI] [PubMed] [Google Scholar]
- 22.De Ruysscher D, Lueza B, Le Pechoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: Usefulness of the individual patient data meta-analysis. Ann Oncol. 2016;27:1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22:4837–4845. [DOI] [PubMed] [Google Scholar]
- 24.Pijls-Johannesma M, De D, Vansteenkiste J, Kester A, Rutten I, Lambin P. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: A systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev. 2007;33:461–473. [DOI] [PubMed] [Google Scholar]
- 25.Damhuis R, Widder J, Senan S. Population-based results of chemoradiotherapy for limited stage small cell lung cancer in The Netherlands. PLoS One. 2018;30:17–22. [DOI] [PubMed] [Google Scholar]
- 26.Wong AT, Rineer J, Schwartz D, et al. Effect of thoracic radiotherapy timing and fractionation on survival in nonmetastatic small cell lung carcinoma. Clin Lung Cancer. 2017;18:207–212. [DOI] [PubMed] [Google Scholar]
- 27.Bonner JA, Sloan JA, Shanahan TG, et al. Phase III comparison of twice-daily split-course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. J Clin Oncol. 1999;17:2681–2691. [DOI] [PubMed] [Google Scholar]
- 28.McClay EF, Bogart J, Herndon JE 2nd, et al. A phase III trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited-stage small cell lung cancer: Cancer and Leukemia Group B Study (9235). Am J Clin Oncol. 2005;28:81–90. [DOI] [PubMed] [Google Scholar]
- 29.Wong AT, Rineer J, Schwartz D, Schreiber D. Assessing the impact of postoperative radiation therapy for completely resected limited-stage small cell lung cancer using the National Cancer Database. J Thorac Oncol. 2016;11:242–248. [DOI] [PubMed] [Google Scholar]
- 30.Gronberg BH, Halvorsen TO, Flotten O, et al. Randomized phase II trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncol. 2016;55:591–597. [DOI] [PubMed] [Google Scholar]
- 31.Chen GY, Jiang GL, Wang LJ, et al. Cisplatin/etoposide chemotherapy combined with twice daily thoracic radiotherapy for limited small-cell lung cancer: A clinical phase II trial. Int J Radiat Oncol Biol Phys. 2005;61:70–75. [DOI] [PubMed] [Google Scholar]
- 32.Ettinger DS, Berkey BA, Abrams RA, et al. Study of paclitaxel, etoposide, and cisplatin chemotherapy combined with twice-daily thoracic radiotherapy for patients with limited-stage small-cell lung cancer: A Radiation Therapy Oncology Group 9609 phase II study. J Clin Oncol. 2005;23:4991–4998. [DOI] [PubMed] [Google Scholar]
- 33.Glisson B, Scott C, Komaki R, Movsas B, Wagner H. Cisplatin, ifosfamide, oral etoposide, and concurrent accelerated hyperfractionated thoracic radiation for patients with limited small-cell lung carcinoma: Results of radiation therapy oncology group trial 93–12. J Clin Oncol. 2000;18:2990–2995. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber D, Wong AT, Schwartz D, Rineer J. Utilization of hyperfractionated radiation in small-cell lung cancer and its impact on survival. J Thorac Oncol. 2015;10:1770–1775. [DOI] [PubMed] [Google Scholar]
- 35.Horn L, Bernardo P, Sandler A, et al. A phase II study of paclitaxel + etoposide + cisplatin + concurrent radiation therapy for previously untreated limited stage small cell lung cancer (E2596): A trial of the Eastern Cooperative Oncology Group. J Thorac Oncol. 2009;4:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu L, Zhang S, Xu X, et al. Increased biological effective dose of radiation correlates with prolonged survival of patients with limited-stage small cell lung cancer: A systematic review. PLoS One. 2016;11:e0156494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogart JA, Herndon JE 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: Analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004;59:460–468. [DOI] [PubMed] [Google Scholar]
- 38.Hu X, Bao Y, Zhang L, et al. Omitting elective nodal irradiation and irradiating postinduction versus preinduction chemotherapy tumor extent for limited-stage small cell lung cancer: Interim analysis of a prospective randomized noninferiority trial. Cancer. 2012;118:278–287. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Liu W, Ji K, Wang P, Wang X, Zhao L. Simultaneous integrated dose reduction intensity-modulated radiotherapy applied to an elective nodal area of limited-stage small-cell lung cancer. Exp Ther Med. 2015;10:2083–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Wang J, Yuan Z, et al. Preliminary results about application of intensity-modulated radiotherapy to reduce prophylactic radiation dose in limited-stage small cell lung cancer. J Cancer. 2018;9:2625–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirvani SM, Komaki R, Heymach JV, Fossella FV, Chang JY. Positron emission tomography/computed tomography-guided intensity-modulated radiotherapy for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:e91–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Loon J, De Ruysscher D, Wanders R, et al. Selective nodal irradiation on basis of (18)FDG-PET scans in limited-disease small-cell lung cancer: A prospective study. Int J Radiat Oncol Biol Phys. 2010;77:329–336. [DOI] [PubMed] [Google Scholar]
- 43.Colaco R, Sheikh H, Lorigan P, et al. Omitting elective nodal irradiation during thoracic irradiation in limited-stage small cell lung cancer—evidence from a phase II trial. Lung Cancer. 2012;76:72–77. [DOI] [PubMed] [Google Scholar]
- 44.Shirvani SM, Juloori A, Allen PK, et al. Comparison of 2 common radiation therapy techniques for definitive treatment of small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87:139–147. [DOI] [PubMed] [Google Scholar]
- 45.Rwigema JM, Verma V, Lin L, et al. Prospective study of proton-beam radiation therapy for limited-stage small cell lung cancer. Cancer. 2017;123:4244–4251. [DOI] [PubMed] [Google Scholar]
- 46.Colaco RJ, Huh S, Nichols RC, et al. Dosimetric rationale and early experience at UFPTI of thoracic proton therapy and chemotherapy in limited-stage small cell lung cancer. Acta Oncol. 2013;52:506–513. [DOI] [PubMed] [Google Scholar]
- 47.Halvorsen TO, Sundstrom S, Flotten O, et al. Comorbidity and outcomes of concurrent chemo- and radiotherapy in limited disease small cell lung cancer. Acta Oncol. 2016;55:1349–1354. [DOI] [PubMed] [Google Scholar]
- 48.Christodoulou M, Blackhall F, Mistry H, et al. Compliance and outcome of elderly patients treated in the Concurrent Once-Daily Versus Twice-Daily Radiotherapy (CONVERT) Trial. J Thorac Oncol. 2019;14:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stinchcombe TE, Fan W, Schild SE, et al. A pooled analysis of individual patient data from National Clinical Trials Network clinical trials of concurrent chemoradiotherapy for limited-stage small cell lung cancer in elderly patients versus younger patients. Cancer. 2019;125:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, Kester A, Rutten I, Lambin P. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol. 2006;17:543–552. [DOI] [PubMed] [Google Scholar]
- 51.Skarlos DV, Samantas E, Briassoulis E, et al. Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrently with chemotherapy in limited disease small-cell lung cancer: A randomized phase II study of the Hellenic Cooperative Oncology Group (HeCOG). Ann Oncol. 2001;12:1231–1238. [DOI] [PubMed] [Google Scholar]
- 52.Sun JM, Ahn YC, Choi EK, et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol. 2013;24:2088–2092. [DOI] [PubMed] [Google Scholar]
- 53.Sloan JA, Bonner JA, Hillman SL, et al. A quality-adjusted reanalysis of a Phase III trial comparing once-daily thoracic radiation vs. twice-daily thoracic radiation in patients with limited-stage small-cell lung cancer(1). Int J Radiat Oncol Biol Phys. 2002;52:371–381. [DOI] [PubMed] [Google Scholar]
- 54.Blackstock AW, Bogart JA, Matthews C, et al. Split-course versus continuous thoracic radiation therapy for limited-stage small-cell lung cancer: Final report of a randomized phase III trial. Clin Lung Cancer. 2005;6:287–292. [DOI] [PubMed] [Google Scholar]
- 55.Bogart JA, Watson D, McClay EF, et al. Interruptions of once-daily thoracic radiotherapy do not correlate with outcomes in limited stage small cell lung cancer: Analysis of CALGB phase III trial 9235. Lung Cancer. 2008;62:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lebeau B, Urban T, Brechot JM, et al. A randomized clinical trial comparing concurrent and alternating thoracic irradiation for patients with limited small cell lung carcinoma. “Petites Cellules” Group. Cancer. 1999;86:1480–1487. [PubMed] [Google Scholar]
- 57.Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59:943–951. [DOI] [PubMed] [Google Scholar]
- 58.Schild SE, Stella PJ, Brooks BJ, et al. Results of combined-modality therapy for limited-stage small cell lung carcinoma in the elderly. Cancer. 2005;103:2349–2354. [DOI] [PubMed] [Google Scholar]
- 59.Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7:295–301. [DOI] [PubMed] [Google Scholar]
- 60.Radiation Therapy Regimens in Treating Patients With Limited-Stage Small Cell Lung Cancer Receiving Cisplatin and Etoposide. Available at: https://clinicaltrials.gov/ct2/show/NCT00632853. Accessed June 17, 2019.
- 61.Wang EH, Corso CD, Rutter CE, et al. Postoperative radiation therapy is associated with improved overall survival in incompletely resected stage II and III non-small-cell lung cancer. J Clin Oncol. 2015;33:2727–2734. [DOI] [PubMed] [Google Scholar]
- 62.Li C, Xiong Y, Zhou Z, et al. Stereotactic body radiotherapy with concurrent chemotherapy extends survival of patients with limited stage small cell lung cancer: A single-center prospective phase II study. Med Oncol. 2014;31:369. [DOI] [PubMed] [Google Scholar]
- 63.Verma V, Simone CB 2nd, Allen PK, et al. Multi-institutional experience of stereotactic ablative radiation therapy for stage I small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salem A, Mistry H, Hatton M, et al. Association of chemoradiotherapy with outcomes among patients with stage I to II vs stage III small cell lung cancer: Secondary analysis of a randomized clinical trial. JAMA Oncol. 2018:e185335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S–e250S. [DOI] [PubMed] [Google Scholar]
- 66.Putnam JB Jr. Disciplined practice and improving clinical and pathologic staging for non-small cell lung cancer. Ann Thorac Surg. 2014;97:744–746. [DOI] [PubMed] [Google Scholar]
- 67.Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. J Thorac Oncol. 2016;11:1081–1089. [DOI] [PubMed] [Google Scholar]
- 68.Chen H, Laba JM, Zayed S, Boldt RG, Palma DA, Louie AV. Safety and effectiveness of stereotactic ablative radiotherapy for ultra-central lung lesions: A systematic review. J Thorac Oncol. 2019;14:1332–1342. [DOI] [PubMed] [Google Scholar]
- 69.Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11–16. [DOI] [PubMed] [Google Scholar]
- 70.Verma V, Simone CB 2nd, Allen PK, Lin SH. Outcomes of stereotactic body radiotherapy for T1-T2N0 small cell carcinoma according to addition of chemotherapy and prophylactic cranial irradiation: A multicenter analysis. Clin Lung Cancer. 2017;18:675–681.e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paximadis P, Beebe-Dimmer JL, George J, Schwartz AG, Wozniak A, Gadgeel S. Comparing treatment strategies for stage I small-cell lung cancer. Clin Lung Cancer. 2018;19:e559–e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corradetti MN, Mitra N, Bonner Millar LP, et al. A moving target: Image guidance for stereotactic body radiation therapy for early-stage non-small cell lung cancer. Pract Radiat Oncol. 2013;3:307–315. [DOI] [PubMed] [Google Scholar]
- 73.Manapov F, Klautke G, Fietkau R. Prevalence of brain metastases immediately before prophylactic cranial irradiation in limited disease small cell lung cancer patients with complete remission to chemoradiotherapy: A single institution experience. J Thorac Oncol. 2008;3:652–655. [DOI] [PubMed] [Google Scholar]
- 74.Yilmaz U, Kirakli EK, Gurlek U, Ozdogan Y, Gumus B, Aksit S. Frequency of silent brain metastasis before prophylactic cranial irradiation in small cell lung cancer. Turk Thorac J. 2017;18:11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y, Zhang D, Zhou X, et al. Prophylactic cranial irradiation in resected small cell lung cancer: A systematic review with meta-analysis. J Cancer. 2018;9:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu J, Yang H, Fu X, et al. Prophylactic cranial irradiation for patients with surgically resected small cell lung cancer. J Thorac Oncol. 2017;12:347–353. [DOI] [PubMed] [Google Scholar]
- 77.Zhu H, Guo H, Shi F, et al. Prophylactic cranial irradiation improved the overall survival of patients with surgically resected small cell lung cancer, but not for stage I disease. Lung Cancer. 2014;86:334–338. [DOI] [PubMed] [Google Scholar]
- 78.Wu AJ, Gillis A, Foster A, et al. Patterns of failure in limited-stage small cell lung cancer: Implications of TNM stage for prophylactic cranial irradiation. Radiother Oncol. 2017;125:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao KJ, Huang HY, Tu MC, Pan GY. Long-term results of prophylactic cranial irradiation for limited-stage small-cell lung cancer in complete remission. Chin Med J (Engl). 2005;118:1258–1262. [PubMed] [Google Scholar]
- 80.Arriagada R, Le Chevalier T, Riviere A, et al. Patterns of failure after prophylactic cranial irradiation in small-cell lung cancer: Analysis of 505 randomized patients. Ann Oncol. 2002;13:748–754. [DOI] [PubMed] [Google Scholar]
- 81.Meert AP, Paesmans M, Berghmans T, et al. Prophylactic cranial irradiation in small cell lung cancer: A systematic review of the literature with meta-analysis. BMC Cancer. 2001;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schild SE, Foster NR, Meyers JP, et al. Prophylactic cranial irradiation in small-cell lung cancer: Findings from a North Central Cancer Treatment Group pooled analysis. Ann Oncol. 2012;23:2919–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Viani GA, Boin AC, Ikeda VY, Vianna BS, Silva RS, Santanella F. Thirty years of prophylactic cranial irradiation in patients with small cell lung cancer: A meta-analysis of randomized clinical trials. J Bras Pneumol. 2012;38:372–381. [DOI] [PubMed] [Google Scholar]
- 84.Zhang W, Jiang W, Luan L, Wang L, Zheng X, Wang G. Prophylactic cranial irradiation for patients with small-cell lung cancer: A systematic review of the literature with meta-analysis. BMC Cancer. 2014;14:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farooqi AS, Holliday EB, Allen PK, Wei X, Cox JD, Komaki R. Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: Do all patients benefit? Radiother Oncol. 2017;122:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giuliani M, Sun A, Bezjak A, et al. Utilization of prophylactic cranial irradiation in patients with limited stage small cell lung carcinoma. Cancer. 2010;116:5694–5699. [DOI] [PubMed] [Google Scholar]
- 87.Qiu G, Du X, Zhou X, et al. Prophylactic cranial irradiation in 399 patients with limited-stage small cell lung cancer. Oncol Lett. 2016;11:2654–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eaton BR, Kim S, Marcus DM, et al. Effect of prophylactic cranial irradiation on survival in elderly patients with limited-stage small cell lung cancer. Cancer. 2013;119:3753–3760. [DOI] [PubMed] [Google Scholar]
- 89.Patel S, Macdonald OK, Suntharalingam M. Evaluation of the use of prophylactic cranial irradiation in small cell lung cancer. Cancer. 2009;115:842–850. [DOI] [PubMed] [Google Scholar]
- 90.Tai P, Assouline A, Joseph K, Stitt L, Yu E. Prophylactic cranial irradiation for patients with limited-stage small-cell lung cancer with response to chemoradiation. Clin Lung Cancer. 2013;14:40–44. [DOI] [PubMed] [Google Scholar]
- 91.Tai P, Tonita J, Yu E, Skarsgard D. Twenty-year follow-up study of long-term survival of limited-stage small-cell lung cancer and overview of prognostic and treatment factors. Int J Radiat Oncol Biol Phys. 2003;56:626–633. [DOI] [PubMed] [Google Scholar]
- 92.Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le Pechoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99–01, EORTC 22003–08004, RTOG 0212, and IFCT 99–01): A randomised clinical trial. Lancet Oncol. 2009;10:467–474. [DOI] [PubMed] [Google Scholar]
- 94.Ge W, Xu H, Yan Y, Cao D. The effects of prophylactic cranial irradiation versus control on survival of patients with extensive-stage small-cell lung cancer: a meta-analysis of 14 trials. Radiat Oncol. 2018;13:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rule WG, Foster NR, Meyers JP, et al. Prophylactic cranial irradiation in elderly patients with small cell lung cancer: Findings from a North Central Cancer Treatment Group pooled analysis. J Geriatr Oncol. 2015;6:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maeng CH, Song JU, Shim SR, Lee J. The Role of prophylactic cranial irradiation in patients with extensive stage small cell lung cancer: A systematic review and meta-analysis. J Thorac Oncol. 2018;13:840–848. [DOI] [PubMed] [Google Scholar]
- 97.Bang A, Kendal WS, Laurie SA, Cook G, MacRae RM. Prophylactic cranial irradiation in extensive stage small cell lung cancer: Outcomes at a comprehensive cancer centre. Int J Radiat Oncol Biol Phys. 2018;101:1133–1140. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y, Li J, Hu Y, et al. Prophylactic cranial irradiation could improve overall survival in patients with extensive small cell lung cancer: A retrospective study. Strahlenther Onkol. 2016;192:905–912. [DOI] [PubMed] [Google Scholar]
- 99.Sas-Korczynska B, Korzeniowski S, Wojcik E. Comparison of the effectiveness of “late” and “early” prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. Strahlenther Onkol. 2010;186:315–319. [DOI] [PubMed] [Google Scholar]
- 100.Kesarwala AH, Lu DJ, Xanthopoulos E, et al. The role of advanced imaging in assessing response to definitive chemoradiation before prophylactic cranial irradiation in limited-stage small-cell lung cancer. Clin Lung Cancer. 2018;19:e205–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pope WB. Brain metastases: Neuroimaging. Handb Clin Neurol. 2018;149:89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eze C, Roengvoraphoj O, Niyazi M, et al. Treatment response and prophylactic cranial irradiation are prognostic factors in a real-life limited-disease small-cell lung cancer patient cohort comprehensively staged with cranial magnetic resonance imaging. Clin Lung Cancer. 2017;18:e243–e249. [DOI] [PubMed] [Google Scholar]
- 103.Zheng Y, Wang L, Zhao W, et al. Risk factors for brain metastasis in patients with small cell lung cancer without prophylactic cranial irradiation. Strahlenther Onkol. 2018;194:1152–1162. [DOI] [PubMed] [Google Scholar]
- 104.Le Pechoux C, Laplanche A, Faivre-Finn C, et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99–01, EORTC 22003–08004, RTOG 0212 and IFCT 99–01). Ann Oncol. 2011;22:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol. 1999;17:2092–2099. [DOI] [PubMed] [Google Scholar]
- 106.Gore EM, Hu C, Sun AY, et al. Randomized phase II study comparing prophylactic cranial irradiation alone to prophylactic cranial irradiation and consolidative extracranial irradiation for extensive-disease small cell lung cancer (ED SCLC): NRG Oncology RTOG 0937. J Thorac Oncol. 2017;12:1561–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palma DA, Warner A, Louie AV, Senan S, Slotman B, Rodrigues GB. Thoracic radiotherapy for extensive stage small-cell lung cancer: A meta-analysis. Clin Lung Cancer. 2016;17:239–244. [DOI] [PubMed] [Google Scholar]
- 108.Slotman BJ, van Tinteren H, Praag JO, et al. Radiotherapy for extensive stage small-cell lung cancer - Authors’ reply. Lancet. 2015;385:1292–1293. [DOI] [PubMed] [Google Scholar]
- 109.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet (London, England). 2019;394:1929–1939. [DOI] [PubMed] [Google Scholar]
- 110.Mok TSK, Reck M, Horn L, et al. IMpower133: Primary efficacy and safety + CNS-related adverse events in a phase I/III study of first-line (1L) atezolizumab + carboplatin + etoposide in extensive-stage SCLC (ES-SCLC). Ann Oncol. 2018;29:ix173–ix178. [Google Scholar]
- 111.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


