Summary
Background
Adjunctive newer antiseizure medications (ASMs) are being used in patients with treatment-resistant focal-onset seizures (FOS). An updated network meta-analysis (NMA) was necessary to compile evidence in this critical area.
Methods
We systematically searched PubMed, Embase, Cochrane Library, Web of Science, and Scopus from their inception until 17 January 2024, evaluating the efficacy, tolerability, and safety of rufinamide (RUF), brivaracetam (BRV), cenobamate (CNB), eslicarbazepine (ESL), lacosamide (LCM), retigabine (RTG), and perampanel (PER) as adjunctive treatments for FOS. Efficacy outcomes included seizure response and seizure freedom. Tolerability was assessed by discontinuation due to adverse events (AEs). Safety outcomes were evaluated based on the number of patients experiencing at least one AE and serious adverse events (SAEs). This review is registered with PROSPERO (CRD42023485130).
Findings
A total of 29 studies involving 11,750 participants were included. For seizure response, all ASMs were significantly superior to placebo, with RTG ranking highest, followed by CNB. Considering dosage, CNB 400 mg/d was top-ranked, followed by RTG 1200 mg/d. For seizure freedom, BRV was highest-ranked, followed by CNB, with BRV 100 mg/d leading, followed by CNB 400 mg/d. Regarding tolerability, LCM 600 mg/d had the lowest ranking, followed by CNB 400 mg/d. For the safety outcome of AEs, ESL 1200 mg/d was ranked lowest, followed by CNB 400 mg/d. Regarding SAEs, LCM 400 mg/d was ranked lowest, followed by RTG 1200 mg/d.
Interpretation
ASMs at different dosages have varying efficacy and tolerability profiles. We have provided hierarchical rankings of ASMs for efficacy and safety outcomes. Our findings offer the most comprehensive evidence available to inform patients, families, physicians, guideline developers, and policymakers about the choice of ASMs in patients with treatment-resistant FOS.
Funding
None.
Keywords: Focal seizures, Seizure, Epilepsy, Antiseizure medications, Network meta-analysis
Research in context.
Evidence before this study
Newer antiseizure medications (ASMs) are being used to improve seizure control in patients with refractory epilepsy. There has been a rise in randomised controlled trials (RCTs) investigating new ASMs as an additional treatment for focal-onset seizures (FOS). Given these developments, an updated and comprehensive network meta-analysis (NMA) was necessary to compile evidence in this critical area of clinical research. We conducted a search on PubMed, Embase, Cochrane Library, Web of Science and Scopus from inception to 17 January 2024 using a combination of the search terms (retigabine OR brivaracetam OR eslicarbazepine OR lacosamide OR perampanel OR cenobamate OR rufinamide) AND (epilepsy OR seizure), with no restrictions on publication type or language.
Added value of this study
A total of 29 studies involving 11,750 participants were included in this study. This systematic review and Bayesian NMA provided an overview of the relative efficacy, tolerability and safety of the most common ASMs used in patients with drug-resistant partial epilepsy. Additionally, we also compared ASMs across different daily dosage. Thus, we provide the best currently available evidence base to guide the choice about pharmacological treatment in this important clinical area.
Implications of all the available evidence
We aim to give developers and prescribers practical advice for making informed choices among various treatment options, including different ASMs or dosage levels. Our findings should inform clinical guidelines and support the decision-making process between patients and clinicians, helping to choose the most suitable treatment strategies for drug-resistant partial epilepsy. Furthermore, future research should include individual patient data in NMA to achieve more accurate estimations in this critical area.
Introduction
Epilepsy is a chronic neurological disorder characterised by a tendency to experience seizures. It is among the most prevalent neurological disorders.1 The estimated incidence is about 80 per 100,000, with a prevalence of 5–10 per 1,000, affecting approximately 70 million people worldwide.2,3 This represents about 0.5–1% of the global population,4 with around 80% of cases occurring in developing countries.5
Focal-onset seizures (FOS) are the most common type of seizure, accounting for 61% of all epilepsy cases.6 The primary therapy for treating focal seizures is medicinal intervention. In the treatment of epilepsy, antiepileptic medications (ASMs) are typically employed for seizure management. Clinical guidelines have been established to direct the care of epilepsy.7,8 The first line of treatment for epilepsy is ASM, and the use of a single ASM is considered to be the best approach for patients with newly diagnosed epilepsy.9 About 50–60% of patients achieve lasting seizure freedom with their first ASM.10,11 However, for some patients, additional treatment may be required, as only 15–25% of patients will experience satisfactory seizure remission after switching or adding other treatment options, and the remaining 20–30% patients will not achieve satisfactory seizure remission.12,13 If the first ASM is not effective in controlling seizures, there are a few options to consider. Increasing the dosage of the same ASM, trying a different ASM, or using a combination of at least two ASMs with different actions can be attempted.14, 15, 16 Some patients, who are unable to achieve sustained seizure freedom after trying 2–3 ASMs,17 will require treatment with a combination of drugs.15,18,19
Epilepsy impacts people of all ages, races, social classes, and geographical locations. As a chronic condition, it poses additional physical and psychological challenges for patients compared to the general population. It significantly burdens individuals and society. Patients with epilepsy commonly experience psychiatric illnesses and cognitive abnormalities alongside their condition.20,21 Notably, many ASMs can cause psychiatric side effects, such as hyperactivity and depression, and some may increase suicide risk.22, 23, 24, 25 Moreover, ASMs can adversely affect cognitive functions, leading to difficulties in learning, driving, and memory retention.25,26 It is essential to consider these potential side effects when treating individuals with epilepsy. Various factors, including the type of seizures and the response to ASMs, impact the burden of epilepsy.27 According to the Global Burden of Epilepsy study in 2019,28 both idiopathic epilepsy and epilepsy due to other causes resulted in a global loss of 18.3 million years due to disability (YLDs), accounting for 2.1% of total global YLDs.29 Uncontrolled epilepsy can be disabling, leading to significant psychological and social dysfunction, reduced educational and employment opportunities, impaired quality of life, and a 2–3 times higher risk of premature death compared to the general population.30, 31, 32, 33, 34 Despite the increased availability of ASMs over the past 25 years, the challenge of treatment-resistant epilepsy has remained constant, highlighting the need for new and more effective treatment options.35
Newer ASMs are being used to improve seizure control in patients with refractory epilepsy, defined as those who have not achieved satisfactory seizure control with two or more different ASMs.12,13 Traditional ASMs have limitations in achieving seizure freedom, which is why newer ASMs with distinct mechanisms of action are being explored.36 These newer ASMs are designed to target various processes involved in seizure development, such as blocking sodium or calcium channels, activating potassium channels, enhancing gamma-aminobutyric acid (GABA) activity, and inhibiting excitatory amino acids.37 However, studies have shown that using two traditional ASMs together does not benefit patients with refractory epilepsy.10,35 As a result, adding newer ASMs to the treatment regimen has become standard practice for these patients.8 Recently, several newer ASMs with better safety profiles have been introduced. These aim to improve seizure control, particularly for patients with refractory epilepsy. Examples include rufinamide (RUF), brivaracetam (BRV), cenobamate (CNB), eslicarbazepine (ESL), lacosamide (LCM), retigabine (RTG), and perampanel (PER).
The increasing number of drugs available poses a challenge for doctors in choosing the treatment due to limited comparative data on their effectiveness. To address this issue, several randomised controlled trials (RCTs) have compared newer ASMs with a placebo (PBO) as additional therapy for patients with FOS. As expected, most of these trials have found that newer ASMs provide superior seizure control and are generally safe and well-tolerated by this patient group. Although direct comparisons between different treatments are scarce, network meta-analysis (NMA) can offer an objective comparison between alternative therapies. Despite some NMAs being published previously,38, 39, 40, 41, 42, 43 there is still some controversy surrounding this topic. Therefore, a comprehensive and up-to-date network meta-analysis is needed to compile evidence on the efficacy, safety, and tolerability of newer ASMs as add-on treatments for treatment-resistant FOS.
Methods
Search strategy
This review was conducted in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Network Meta-Analyses (PRISMA) extension statement for network meta-analyses,44 and registered in PROSPERO (registration number: CRD42023485130). We conducted a search on PubMed, Embase, Cochrane Library, Web of Science and Scopus from inception to 17 January 2024 using a combination of the search terms (retigabine OR brivaracetam OR eslicarbazepine OR lacosamide OR perampanel OR cenobamate OR rufinamide) AND (epilepsy OR seizure), with no restrictions on publication type or language.
Eligibility criteria
Initially, articles were considered eligible if they met certain criteria. These criteria included: 1) being randomised, double-blinded, controlled, parallel-group add-on study that compared various medications (RUF, BRV, CNB, ESL, LCM, RTG, and PER) with any other comparator; 2) having maintenance periods (or stable dosing periods for dose-escalation trials); 3) including patients with focal epilepsy and seizures that were not controlled by one or more concomitant ASMs; and 4) having no restrictions based on date, gender, or ethnicity.
Outcome measures
The primary outcomes for measuring efficacy were the proportion of patients with ≥50% responder rate and the seizure freedom rates (100% reduction) during the maintenance treatment period. Tolerability was assessed by the withdrawal rate due to adverse events (AES). The safety outcomes were at least one AE rate and serious adverse events (SAEs) in the treatment period.
Study selection, data extraction, assessment of the risk of bias and confidence in the evidence
Two reviewers (Yankun Chen and Wenze Li) independently searched and selected potentially relevant publications identified by the search strategy. The following information was extracted for each included study: first author and year of publication, country in which patients were recruited, characteristics (including gender, age, and seizure type), sample size, duration of the maintenance period of treatment, ASMs used in each group, and prior therapy. Any disagreement was resolved by discussion with a third review author (Yangmei Chen). The risk of bias of the identified studies was assessed following the recommendations of the Cochrane Collaboration.45 We used a web application, CINeMA (Confidence in Network Meta-Analysis), to evaluate the confidence in evidence.46,47
Statistical analysis
Both different drugs and their dosages were compared in our network meta-analysis. For the first case, when RCTs included multiple treatment arms with different doses of an ASM, the numbers of events and patients receiving different doses of ASMs were pooled into one arm for each ASM as a node in the network meta-analysis. For the second case, the same ASM at a different dosage was treated as a separate node. The intention-to-treat (ITT) population was used if available. Bayesian network meta-analysis was performed using the “GeMTC” package in R software (version 4.2.3) and JAGS (version 4.3.1). We employed the Markov Chain Monte Carlo (MCMC) approach to obtain the posterior distribution of differences between ASMs. Three chains were used, with 50,000 iterations after a 20,000 burn-in. Convergence assessment was performed using the potential scale reduction factor (PSRF). Risk ratios (RRs) and their corresponding 95% credible intervals (95% CIs) were reported as effect sizes. Global inconsistency was evaluated by comparing the values of the Deviance Information Criterion (DIC) between the consistency model and the inconsistency model. Local inconsistency was assessed using the node-splitting approach. Heterogeneity was assessed by calculating the between-trial variance (τ2). Transitivity was assessed by considering several potential covariates (sample size, publication year, mean age, male percentage, maintenance period, and epilepsy duration) across treatment arms. Univariate meta-regression was also performed using each covariate for all outcomes. The surface under the cumulative ranking curve (SUCRA) was used to rank the ASMs.
Ethics
Institutional ethical review board approval was not required, and participant informed consent was not needed for this review as all study data had been previously published. The data supporting the results of this review are available upon request from the corresponding author.
Role of the funding source
There was no funding source for this study. All authors have full access to all the data in the study and accept responsibility for the decision to submit for publication.
Results
Study selection and characteristics of included studies
Of the 4253 studies screened (496 from PubMed, 875 from Embase, 845 from Cochrane, 918 from Web of Science and 1119 from Scopus), 29 trials (RUF, n = 3; BRV, n = 5; CNB, n = 2; ESL, n = 7; LCM, n = 5; RTG, n = 3; PER, n = 4)48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 with approximately 11,750 patients were included in the data analysis. The selection process is descried in Fig. 1. Network plots are presented in Fig. 2 for all outcomes. The key characteristics of the studies and participants included are summarised in Tables 1 and 2.
Fig. 1.
Search process.
Fig. 2.
Network map for all outcomes. Considering each ASM as a separate node: a, network map for seizure response; b, network map for seizure freedom; c, network map for tolerability; d, network map for AEs; e, network map for SAEs; Considering different dosage as separate nodes: f, network map for seizure response; g, network map for seizure freedom; h, network map for tolerability; i, network map for AEs; j, network map for SAEs. Each node represents an intervention, and the size of the node is proportional to the number of patients assigned to the intervention. The lines indicate direct comparisons between nodes, and the size of the line is proportional to the number of trials comparing each pair of nodes. PBO, Placebo; RUF, Rufinamide; BRV, Brivaracetam; CNB, Cenobamate; ESL, Eslicarbazepine; LCM, Lacosamide; RTG, Retigabine; PER, Perampanel; AEs, adverse effects; SAEs, serious adverse events.
Table 1.
Characteristics of the included studies.
| Study | Study design | Main inclusion criteria | Treatment arms |
|---|---|---|---|
| Brodie MJ et al. (2010)48 | Multicenter, multinational (71 centers in Australia, Belgium, France, Germany, Hungary, Israel, Poland, Russia, South Africa, Spain, the United Kingdom, Ukraine, and the United States) Randomised, double-blind, parallel-group study,placebo-controlled trial: 8-week observational baseline period 4-week titration phase 12-week maintenance phase 4-week transition phase |
Aged 18–75 years Localization-related epilepsy refractory to stable doses of 1–3 AEDs 4 qualifying seizures per 28 d without a seizure-free period of >21 d during the baseline period |
PBO (n = 179) RTG 600 mg/d (n = 181) RTG 900 mg/d (n = 178) |
| French JA et al. (2011)49 | Multicenter, multinational (the United States, Canada,Mwxico, Argentina) Randomised,double-blind, parallel-group, placebo-controlled trial: 8-week observational baseline period 6-week titration phase 12-week maintenance phase |
Aged 18–75 years With drug-resistant partial epilepsy characterized by simple or complex partial-onset seizures, with or without secondary generalization, a 28-day partial seizure frequency of ≥4 seizures over 8 weeks Current treatment on a stable dose of 1–3 ASMs with or without vagus nerve stimulator |
PBO (n = 152) EZG [RTG] 1200 mg/d (n = 153) |
| Porter RJ et al. (2007)50 | Multicenter, nultinational (Europe, Australia and the United States) Parallel-group, double-blind, placebo-controlled, randomised clinical trial: 8-week observational baseline period 8-week titration phase 8-week maintenance phase |
Aged 16–70 years (inclusive) Had inadequately controlled partialonset seizures Patients had to experience a minimum of four partial-onset seizures per month during the 8-week baseline Phase with no 30-day seizure-free period, while maintained on stable doses of one or two AEDs (valproate, carbamazepine, phenytoin, topiramate, lamotrigine, gabapentin, oxcarbazepine, benzodiazepines, or barbiturates) Patients receiving treatment with vagal nerve stimulator were eligible as long as the stimulation parameters were kept constant throughout the study (considered as one AED) Women of childbearing potential were not to be pregnant or lactating and were to be using a reliable method of contraception |
PBO (n = 96) RTG 600 mg/d (n = 99) RTG 900 mg/d (n = 95) RTG 1200 mg/d (n = 106) |
| Biton V et al. (2014)51 | Multicentre, multinational (Australia, Brazil, Canada, Mexico, USA) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 12-week maintenance phas 1-week downtitration period |
Aged 16–70 years Focal epilepsy uncontrolled despite treatment with one or two ASMs At least 2 focal seizures/month during the 3 months before screening and at least 8 focal seizures during the 8-week baseline period Current treatment on a stable dose of 1–2 ASMs for at least 4 weeks before screening |
PBO (n = 98) BRV 5 mg/d (n = 97) BRV 20 mg/d (n = 100) BRV 50 mg/d (n = 101) |
| Ryvlin P et al. (2014)52 | Multicentre, multinational (Belgium, Finland, France, Germany, Hungary, India, Italy, Poland, Spain, Swit- zerland, The Netherlands, UK) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 12-week maintenance phase |
Aged 16–70 years Focal epilepsy uncontrolled despite treatment with one or two ASMs At least 2 focal seizures/month during the 3 months before screening and at least 8 focal seizures during the 8-week baseline period Current treatment on a stable dose of 1–2 ASMs for at least 4 weeks before screening |
PBO (n = 100) BRV 20 mg/d (n = 99) BRV 50 mg/d (n = 99) BRV 100 mg/d (n = 100) |
| Klein P et al. (2015)53 | Multicentre, multinational (Austria, Belgium, Brazil, Bulgaria, Canada, Czech Republic, Estonia, Finland, France, Germany, Hong Kong, Hungary, India, Italy, Japan, Latvia, Lithuania, Mexico, Poland, Puerto Rico, Republic of Korea, Russia, The Netherlands, Spain, Sweden, Taiwan, UK, USA) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 12-week maintenance phase 4-week down-titeation period 2-week drug-free period |
Aged 16–80 years Focal epilepsy uncontrolled despite treatment with one or two ASMs At least 2 focal seizures/month during the 3 months before screening and at least 8 focal seizures during the 8-week baseline period Current treatment on a stable dose of 1–2 ASMs for at least 4 weeks before screening |
PBO (n = 261) BRV 100 mg/d (n = 253) BRV 200 mg/d (n = 250) |
| Van Paesschen W et al. (2013)54 | Multicenter, nultinational (42 centers in nine European countries:Belgium, Czech Republic, Finland, France, Germany, The Netherlands, Poland, Spain and the United Kingdom) Randomised, double-blind, placebo-controlled, parallel group, dose-ranging study: 4- week prospective baseline 3-week up-titration period 7-week maintenance period 2-week double-blinded down-titration period 2-week drug-free period |
Aged 16–65 years With partial-onset seizures, with or without secondary generalization required to have wellcharacterized focal epilepsy or epileptic syndrome Two or more partial-onset seizures per month during the 3 months prior to study entry, four or more partial-onset seizures during the 4-week prospective baseline period, and a stable regimen of one to two concomitant AEDs The same doses and AEDs were required to have been maintained for 1 month before screening and throughout study participation |
PBO (n = 52) BRV 50 mg/d (n = 53) BRV 150 mg/d (n = 52) |
| French JA et al. (2010)55 | Multicenter, nultinational (41 epilepsy centers in Brazil, India, Mexico, the United States) an exploratory, phase IIb, double-blind, randomised, Parallel-group, placebocontrolled, dose-ranging study: 4-week observational baseline period 7-week treatment period 2-week follow-up drug free period |
Aged 16–65 years With refractory POS, whether or not secondarily generalized and well-characterized focal epilepsy/epileptic syndrome Experiencing at least 4 POS during a 4-week prospective baseline period and taking 1 or 2 concomitant AEDs maintained at stable dose from at least 1 month before screening and throughout the study Use of felbamate in the past year or current use of vigabatrin were forbidden for safety reasons; patients previously treated with vigabatrin were required to have stable visual fields; phenobarbital usage in the past 6 months was not permitted due to a possible pharmacokinetic interaction with BRV Taking any concomitant medications that could affect the metabolism of BRV, or medications with possible CNS effects determined at the investigator's discretion, must have been on a stable dose for at least 1 month before the screening visit, and remain on this dose during the study |
PBO (n = 54) BRV 5 mg/d (n = 50) BRV 20 mg/d (n = 52) BRV 50 mg/d (n = 52) |
| Elger C et al. (2009)56 | Multicentre, multinational (Austria, Croatia, Czech Republic, Germany, Hungary, Lithuania, Poland, Romania, Russia, Switzerland) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 2-week titration phase 12-week maintenance phase |
Aged 18 years or older Focal onset seizures for a minimum of 12 months before screening At least 4 focal seizures in the two 4-week periods of the 8-week baseline period with no seizure-free interval >21 consecutive days Current treatment on a stable dose of 1–2 ASMs for at least 2 months before screening |
PBO (n = 102) ESL 400 mg/d (n = 100) ESL 800 mg/d (n = 98) ESL 1200 mg/d (n = 102) |
| Gil-Nagel A et al. (2009)57 | Multicentre, multinational (Mexico, Portugal, Spain) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 2-week titration phase 12-week maintenance phase 4-week tapering-off period |
Aged 18 years or older Focal onset seizures for a minimum of 12 months before screening At least 4 focal seizures in the two 4-week periods before screening as well as during each of the 4-weeks periods of the 8-week baseline period Current treatment on a stable dose of 1–2 ASMs for at least 2 months before screening |
PBO (n = 87) ESL 800 mg/d (n = 85) ESL 1200 mg/d (n = 80) |
| Ben-Menachem E et al. (2010)58 | Multicentre, multinational (Argentina, Australia, Bel- gium, Brazil, Denmark, Germany, The Netherlands, Portugal, Romania, South Africa, Spain, Sweden, UK) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 14-week treatment period |
Aged 18 years or older Focal onset seizures for a minimum of 12 months before screening At least 4 focal seizures in the two 4-week periods before screening as well as during each of the two 4-week periods of the 8-week baseline period Current treatment on a stable dose of 1–3 ASMs for at least 2 months before screening |
PBO (n = 100) ESL 400 mg/d (n = 96) ESL 800 mg/d (n = 101) ESL 1200 mg/d (n = 98) |
| Sperling MR et al. (2015)59 | Multicentre, multinational (Argentina, Australia, Belgium, Brazil, Canada, Cyprus, France, Germany, Greece, Hungary, India, Italy, Poland, Turkey, South Korea, Romania, South Africa, Ukraine, US) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 2-week titration phase 12-week maintenance phase 2-week tapering off period |
Aged 16 years or older Focal onset seizures for a minimum of 12 months before screening At least 4 focal seizures within the 4-week period before screening and at least 8 focal seizures during the baseline period (with ≥3 seizures during each 4-week period and no seizure-free interval exceeding 28 con- secutive days) Current treatment on a stable dose of 1–2 ASMs for at least 1 month before screening |
PBO (n = 224) ESL 800 mg/d (n = 216) ESL 1200 mg/d (n = 210) |
| Jóźwiak S et al. (2018)60 | Multicenter, nultinational (Italy, Poland, Russia, and Ukraine) Phase-II, randomised, double-blind, placebo-controlled study: 4-week baseline period 4-week up-titration period 8-week maintenance phase 4-week tapering-off period 4-week observational follow-up period |
Aged 6–16 years Diagnosed with epilepsy for ≥12 months prior to enrolment, with at least 2 epileptic FOS (≥4 in the month before enrolment), receiving 1–2 AEDs (except oxcarbazepine), and intelligence quotient (IQ) ≥70, were randomised (2:1) to ESL or placebo |
PBO (n = 40) ESL 30 mg/kg/d, maximum of 1200 mg/d (n = 83) |
| Kirkham F et al. (2020)61 | Multicenter, nultinational (Czech Republic, Hungary, Poland, Romania, Russia, Slovakia, Ukraine, Austria, France, Germany, United Kingdom, Bosnia and Herzegovina, Croatia, Italy, Portugal, Serbia, Spain, Malaysia, Philippines, and Taiwan) Phase-III, randomised, double-blind, placebo-controlled study: 8-week baseline period 6-week titration period 12-week maintenance phase 4-week tapering-off period 4-week observational follow-up period |
Aged 2–18 years Diagnosed as having epilepsy for ≥6months prior to enrollment, with FOS (≥4 seizures in the month before enrollment), receiving 1–2 AEDs (except oxcarbazepine), were randomised (1:1) to ESL or placebo |
PBO (n = 129) ESL 20–30 mg/kg/d, maximum of 1200 mg/d (n = 134) |
| Elger C et al. (2007)62 | Multicenter, nultinational (Croatia, Czech Republic, Germany, Lithuania, and Poland) Randomised, placebo-controlled, parallel-group, therapeutic exploratory (phase II) study: 2-month baseline period 12-week treatment phase 1-week tapering-off phase |
Aged 18–65 years With at least four partial-onset seizures per month in spite of treatment with one or two of the following AEDs: phenytoin, valproic acid, primidone, phenobarbital, lamotrigine, gabapentin, topiramate, and clonazepam in stable doses during at least 2 months prior to randomization |
PBO (n = 47) ESL 1200 mg/d QD (n = 50) ESL 1200 mg/d BID (n = 46) |
| Ben-Menachem E et al. (2007)63 | Multicentre, multinational (Germany, Hungary, Lithu- ania, Poland, Switzerland, UK, USA) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 6-week titration phase 12-week maintenance phase |
Aged 18–65 years Focal onset seizures for at least the preceding 2 years despite treatment with at least two ASMs At least 4 focal seizures per 28 days on average, with no seizure-free period longer than 21 days during the 8-week baseline period Current treatment on a stable dose of 1–2 ASMs in the 4 weeks before enrolment and during the baseline period |
PBO (n = 97) LCM 200 mg/d (n = 107) LCM 400 mg/d (n = 108) LCM 600 mg/d (n = 106) |
| Halász P et al. (2009)64 | Multicentre, multinational (Australia, Croatia, Czech Republic, Finland, France, Germany, Hungary, Lithu- ania, Poland, Russia, Spain, Sweden, UK) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 4-week titration phase 12-week maintenance phase 2-week transition or taper period |
Aged 16–70 years Focal onset seizures for at least the preceding 2 years despite treatment with at least two ASMs At least 4 focal seizures per 28 days on average, with no seizure-free period longer than 21 days during the 8-week period before enrolment as well as during the 8-week baseline period Current treatment on a stable dose of 1–3 ASMs in the 4 weeks before enrolment and during the baseline period |
PBO (n = 163) LCM 200 mg/d (n = 163) LCM 400 mg/d (n = 159) |
| Chung S et al. (2010)65 | Multicentre, national (USA) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 6-week titration phase 12-week maintenance phase 2-week transition or 3-week taper period |
Aged 16–70 years Focal onset seizures for at least the preceding 2 years despite treatment with at least two ASMs At least 4 focal seizures per 28 days, with no seizure-free period longer than 21 days during the 8-week baseline period Current treatment on a stable dose of 1–3 ASMs in the 4 weeks before enrolment and during the baseline period |
PBO (n = 104) LCM 400 mg/d (n = 204) LCM 600 mg/d (n = 97) |
| Hong Z et al. (2016)66 | Multicentre, multinational (China, Japan) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 4-week titration phase 12-week maintenance phase 2-week transition or 3-week taper period |
Aged 16–70 years Focal onset seizures for at least the preceding 2 years despite treatment with at least two ASMs At least 4 focal seizures with motor sign per 28d and no seizurefree period or longer than 21d during the 8 weeks before baseline Current treatment on a stable dose of 1–3 ASMs in the 4 weeks before entry into the baseline period |
PBO (n = 184) LCM 200 mg/d (n = 183) LCM 400 mg/d (n = 180) |
| Farkas V et al. (2019)67 | Multicentre, multinational (at 114 sites in Europe, North America, Latin America, and the Asia Pacific region) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 6-week titration phase 10-week maintenance phase 4-week taper/transition period 30-day safety follow-up period |
Children and adolescents (≥4–<17 years of age) Focal (partial-onset) seizures, with ≥1 prior EEG and MRI/CT scans consistent with this diagnosis Uncontrolled focal seizures after an adequate course of treatment (in the opinion of the investigator) with ≥2 AEDs (concurrently or sequentially) An average of ≥2 focal seizures per 28 days, with no more than 21 days without seizures in the 8-week period before entering the baseline period, and at least 2 focal seizures during the 8-week prospective baseline A stable dose regimen of 1–3 AEDs for ≥4 weeks before the baseline period and throughout the trial |
PBO (n = 172) <30 kg (n = 52); ≥30 kg–<50 kg (n = 60); ≥50 kg (n = 60) LCM (n = 171) <30 kg: 8–12 mg/kg/d (n = 61); ≥30 kg–<50 kg: 6–8 mg/kg/d (n = 46); ≥50 kg: 300–400 mg/d (n = 64) |
| Krauss GL et al. (2012)68 | Multicentre, multinational (Australia, Bulgaria, Czech Republic, Estonia, Germany, Hong Kong, Hungary, India, Italy, Latvia, Lithuania, Malaysia, Philippines, Poland, Portugal, Republic of Korea, Romania, Russia, Serbia, Spain, Taiwan, Thailand, Ukraine) Parallel-group, randomised, placebo-controlled trial: 6-week observational baseline period 6-week titration phase 13-week maintenance phase 4-week after stopping treatment period |
Aged 12 years or older Focal onset seizures despite treatment with at least two ASMs within the past 2 years At least 5 focal seizures in the 6-week baseline period, with ≥2 focal seizures per each of 3-week period and no seizure-free interval exceeding 25 days Current treatment on a stable dose of 1–3 ASMs for at least 3 weeks before randomisation |
PBO (n = 185) PER 2 mg/d (n = 180) PER 4 mg/d (n = 172) PER 8 mg/d (n = 169) |
| French JA et al. (2012)69 | Multicentre, multinational (Argentina, Canada, Chile, Mexico, USA) Parallel-group, randomised, placebo-controlled trial: 6-week observational baseline period 6-week titration phase 13-week maintenance phase 4-week follow-up phase |
Aged 12 years or older Focal onset seizures despite treatment with at least two ASMs within the past 2 years At least 5 focal seizures in the 6-week baseline period, with ≥2 focal seizures per each of 3-week period and no seizure-free interval exceeding 25 days Current treatment on a stable dose of 1–3 ASMs for at least 3 weeks before randomisation |
PBO (n = 121) PER 8 mg/d (n = 133) PER 12 mg/d (n = 134) |
| French JA et al. (2013)70 | Multicentre, multinational (Australia, Austria, Belgium, Germany, Finland, France, UK, Greece, India, Israel, Italy, The Netherlands, Russia, South Africa, Sweden, USA) Parallel-group, randomised, placebo-controlled trial: 6-week observational baseline period 6-week titration phase 13-week maintenance phase |
Aged 12 years or older Focal onset seizures despite treatment with at least two ASMs within the past 2 years At least 5 focal seizures in the 6-week baseline period, with ≥2 focal seizures per each of 3-week period and no seizure-free interval exceeding 25 days Current treatment on a stable dose of 1–3 ASMs for at least 3 weeks before randomisation |
PBO (n = 136) PER 8 mg/d (n = 129) PER 12 mg/d (n = 121) |
| Nishida T et al. (2018)71 | Multicentre, multinational (Australia, China, Japan, Malaysia, Republic of Korea, Taiwan, Thailand) Parallel-group, randomised, placebo-controlled trial: 6-week observational baseline period 6-week titration phase 13-week maintenance phase |
Aged 12 years or older Focal onset seizures despite treatment with at least two ASMs within the past 2 years At least 5 focal seizures in the 6-week baseline period, with ≥2 focal seizures per each of 3-week period and no seizure-free interval exceeding 25 days Current treatment on a stable dose of 1–3 ASMs |
PBO (n = 175) PER 4 mg/d (n = 174) PER 8 mg/d (n = 175) PER 12 mg/d (n = 180) |
| Krauss GL et al. (2020)72 | Multicentre, multinational (Australia, Bulgaria, Czech Republic, France, Germany, Hungary, Israel, Poland, Romania, Serbia, South Korea, Spain, Thailand, Ukraine, UK, USA) Parallel-group, randomised, placebo-controlled trial: 8-week observational baseline period 6-week titration phase 12-week maintenance phase |
Aged 18–70 years Focal epilepsy uncontrolled despite treatment with at least one ASM within the past 2 years At least 8 focal seizures with a seizure-free interval fewer than 25 days during the 8-week baseline assessment with at least 3 of these seizures occurring during each of the two consecutive 4-week segments of the baseline period Current treatment on a stable dose of 1–3 ASMs for at least 4 weeks before screening |
PBO (n = 108) CNB 100 mg/d (n = 108) CNB 200 mg/d (n = 110) CNB 400 mg/d (n = 111) |
| Chung SS et al. (2020)73 | This phase 2, multicenter, multinational (the United States, India, Republic of Korea, and Poland) Randomised, double-blind, placebocontrolled, parallel-group study: 8-week baseline period 6-week titration phase 6-week maintenance phase |
Aged 18–65 years With a diagnosis of treatment-resistant focal (partial-onset) epilepsy had an EEG consistent with the diagnosis of focal epilepsy and a CT or MRI scan performed within the last 5 years |
PBO (n = 109) CNB 200 mg/d (n = 113) |
| Elger CE et al. (2010)74 | Multicenter, randomised, double-blind, double-dummy, Placebo-controlled, parallel-group study: 12-week baseline phase 12-week double-blind phase |
Aged 15–65 years Had a diagnosis of partial seizures, simple and/or complex, with or without secondary generalization, who were receiving stable dosages of one to three AEDs for at least 4 weeks prior to starting the baseline phase and were experiencing at least four seizures per month during the 6 months prior to the baseline phase |
PBO (n = 133) RUF 200 mg/d (n = 127) RUF 400 mg/d (n = 125) RUF 800 mg/d (n = 129) RUF 1600 mg/d (n = 133) |
| Biton V et al. (2011)75 | Randomised, double-blind, placebo-controlled, parallel-group study: 56-day baseline phase 12-day titration phase 84-day maintenance phase |
Aged 12–80 years With partial-onset seizures with or without secondarily generalized seizures Vagus nerve stimulators had to have been implanted for at least 6 months before randomization and stimulator parameters had to have been unchanged for at least 1 month prior to screening and for the duration of the study |
PBO (n = 180) RUF 3200 mg/d (n = 176) |
| Brodie MJ et al. (2009)76 | Multicenter, nultinational (the United States, Argentina, Chile, France, Germany, Great Britain, Italy, Russia, Slovakia, South Africa, Spain, Switzerland, and Uruguay) Double-blind, placebo-controlled, randomised, parallel-group: 8- week baseline phase 2-week titration period 11-week maintenance phase |
Aged 16 years or older and weighed at least 18 kg Had a diagnosis of partial seizures, had been on a stable dose of one or two AEDs during the 8-week baseline phase (any additional AEDs or nonallowed medication must have been discontinued 30 days prior to the 8-week baseline phase) Had at least six documented partial seizures during the 8-week baseline phase (with at least one partial seizure occurring in each 4-week period) Eligible women capable of bearing children were required to use acceptable methods of contraception (an intrauterine device, spermicide and barrier, or abstinence) for at least 1 month prior to study enrollment and throughout the study and have a negative pregnancy test at the time of randomization |
PBO (n = 157) RUF 3200 mg/d (n = 156) |
PBO: Placebo; RUF: Rufinamide, BRV: Brivaracetam, CNB: Cenobamate, ESL: Eslicarbazepine, LCM: Lacosamide, RTG: Retigabine, PER: Perampanel.
Table 2.
Characteristics of the study participants.
| Treatment arm | Number of participants | Age, years, mean (SD) | Male (percentage)/female (percentage) | Epilepsy duration, years, mean (SD) | Number of concomitant ASMs, (%) |
Baseline seizure frequency per 28 days, median | |||
|---|---|---|---|---|---|---|---|---|---|
| One | Two | Three or more | |||||||
| Brodie MJ et al. (2010)48 | PBO | 179 | 37.7 (11.8) | 89 (50.0)/90 (50.0) | 22.8 (11.8) | 40 (22.3) | 87 (48.6) | 52 (29.1) | 9.3 |
| EZG (RTG) 600 mg/d | 181 | 37.5 (12.0) | 76 (42.0)/105 (58.0) | 22.5 (13.0) | 49 (27.1) | 76 (42.0) | 56 (30.9) | 9.5 | |
| EZG (RTG) 900 mg/d | 178 | 37.7 (12.8) | 93 (52.0)/85 (48.0) | 22.5 (12.7) | 35 (19.7) | 99 (55.6) | 44 (24.7) | 10.3 | |
| French JA et al. (2011)49 | PBO | 152 | 36.7 (11.6) | 72 (47.4)/80 (52.6) | 23.1 (12.8) | 21 (13.8) | 70 (46.1) | 61 (40.1) | 11.3 |
| EZG (RTG) 1200 mg/d | 153 | 37.7 (12.6) | 68(44.4)/85(55.6) | 23.7 (13.0) | 32 (20.9) | 79 (51.6) | 42 (27.5) | 12.1 | |
| Porter RJ et al. (2007)50 | PBO | 96 | 34.5 (10.3) | 48 (50.0)/48 (50.0) | 20.8 (11.2) | 33 (34.3) | 62 (64.6) | 1 (1.1) | 8.5 |
| RTG 600 mg/d | 99 | 36.8 (10.9) | 53 (53.5)/46 (46.5) | 21.2 (12.0) | 26 (26.0) | 72 (72.0) | 2 (2.0) | 8.5 | |
| RTG 900 mg/d | 95 | 37.0 (10.2) | 48 (50.5)/47 (49.5) | 19.7 (12.0) | 26 (27.4) | 69 (72.6) | 0 | 7.9 | |
| RTG 1200 mg/d | 106 | 38.3 (11.9) | 55 (51.9)/51 (48.1) | 20.1 (11.4) | 31 (29.2) | 74 (69.8) | 1 (0.9) | 10.4 | |
| Biton V et al. (2014)51 | PBO | 98 | 37.5 (12.6) | 43 (43.9)/55 (56.1) | 24.3 (12.2) | 13 (13.3) | 80 (81.6) | 4 (4.1) | 2.6b |
| BRV 5 mg/d | 97 | 38.9 (11.6) | 49 (50.5)/48 (49.5) | 22.2 (12.1) | 14 (14.4) | 76 (78.4) | 7 (7.2) | 2.4b | |
| BRV 20 mg/d | 100 | 37.3 (13.3) | 52 (52.0)/48 (48.0) | 22.9 (14.0) | 16 (16.0) | 72 (72.0) | 12 (12.0) | 2.2b | |
| BRV 50 mg/d | 101 | 38.9 (12.3) | 51 (50.5)/50 (49.5) | 26.2 (12.0) | 13 (12.9) | 82 (81.2) | 6 (5.9) | 2.9b | |
| Ryvlin P et al. (2014)52 | PBO | 100 | 36.4 (13.0) | 54 (54.0)/46 (46.0) | 20.4 (12.3) | 14 (14.0) | 83 (83.0) | 3 (3.0) | 2.07b |
| BRV 20 mg/d | 99 | 35.7 (12.5) | 61 (61.6)/38 (38.4) | 22.1 (13.6) | 18 (18.2) | 77 (77.8) | 4 (4.0) | 1.93b | |
| BRV 50 mg/d | 99 | 38.9 (13.6) | 54 (54.5)/45 (45.5) | 22.3 (13.0) | 20 (20.2) | 77 (77.8) | 2 (2.0) | 1.80b | |
| BRV 100 mg/d | 100 | 38.0 (13.1) | 58 (58.0)/42 (42.0) | 22.1 (12.8) | 16 (16.0) | 77 (77.0) | 7 (7.0) | 2.02b | |
| Klein P et al. (2015)53 | PBO | 261 | 39.8 (12.5) | 133 (51.0)/128 (49.0) | 22.7 (13.3) | 75 (29.0) | 181 (69.9) | 3 (1.2) | 10.0 |
| BRV 100 mg/d | 253 | 39.1 (13.4) | 102 (40.3)/151 (59.7) | 22.2 (13.3) | 70 (27.8) | 182 (72.2) | 0 | 9.5 | |
| BRV 200 mg/d | 250 | 39.8 (12.8) | 133 (53.2)/117 (46.8) | 23.4 (14.6) | 69 (27.7) | 179 (71.9) | 1 (0.4) | 9.3 | |
| Van Paesschen W et al. (2013)54 | PBO | 52 | 40.0 (11.7) | 25 (48.1)/27 (51.9) | 21.0 (12.9) | 7 (13.5) | 43 (82.7) | 1 (1.9) | 2.27b |
| BRV 50 mg/d | 53 | 38.2 (12.1) | 24 (45.3)/29 (54.7) | 25.1 (14.8) | 13 (24.5) | 35 (66.0) | 5 (9.4) | 1.75b | |
| BRV 150 mg/d | 52 | 34.4 (10.1) | 21 (40.4)/31 (59.6) | 19.8 (11.6) | 9 (17.3) | 39 (75.0) | 4 (7.7) | 2.94b | |
| French JA et al. (2010)55 | PBO | 54 | 33.6 (11.3) | 24 (44.4)/30 (55.6) | 21.7 (13.0) | 20 (37.0) | 31 (57.4) | 3 (5.6) | 2.23b |
| BRV 5 mg/d | 50 | 32.7 (12.2) | 30 (60.0)/20 (40.0) | 16.0 (11.5) | 15 (30.0) | 29 (58.0) | 6 (12.0) | 2.21b | |
| BRV 20 mg/d | 52 | 35.3 (13.7) | 28 (53.8)/24 (46.2) | 22.9 (13.5) | 22 (42.3) | 28 (53.8) | 2 (3.8) | 2.34b | |
| BRV 50 mg/d | 52 | 30.9 (11.6) | 28 (53.8)/24(46.2) | 19.1 (10.8) | 16 (30.8) | 34 (65.4) | 2 (3.8) | 1.95b | |
| Elger C et al. (2009)56 | PBO | 102 | 37.0 (11.9) | 48 (47.1)/54 (52.9) | 19.4 (12.6) | 34 (33.3) | 67 (65.7) | 1 (1.0) | 6.7 |
| ESL 400 mg/d | 100 | 37.8 (11.4) | 50 (50.0)/50 (50.0) | 21.0 (11.7) | 39 (39.0) | 60 (60.0) | 1 (1.0) | 7.5 | |
| ESL 800 mg/d | 98 | 41.3 (12.0) | 54 (55.1)/44 (44.9) | 23.1 (13.5) | 31 (31.6) | 67 (68.4) | 0 | 7.0 | |
| ESL 1200 mg/d | 102 | 38.4 (11.7) | 44 (43.1)/58 (56.9) | 20.4 (11.9) | 39 (38.2) | 63 (61.8) | 0 | 7.4 | |
| Gil-Nagel A et al. (2009)57 | PBO | 87 | 37.7 (12.1) | 43 (49.4)/44 (50.6) | 23.8 (13.0) | 16 (18.4) | 66 (75.9) | 5 (5.7) | 11.3 (18.5)c |
| ESL 800 mg/d | 85 | 36.8 (10.7) | 35 (41.2)/50 (58.8) | 22.5 (11.8) | 22 (25.9) | 58 (68.2) | 5 (5.9) | 11.6 (22.1)c | |
| ESL 1200 mg/d | 80 | 36.0 (11.4) | 35 (43.8)/45 (56.3) | 23.0 (13.0) | 12 (15.0) | 63 (78.8) | 5 (6.3) | 11.3 (10.3)c | |
| Ben-Menachem E et al. (2010)58 | PBO | 100 | 36.7 (12.2) | 52 (52.0)/48 (48.0) | 25.4 (13.1) | 15 (15.0) | 76 (76.0) | 9 (9.0) | 8.0 |
| ESL 400 mg/d | 96 | 37.6 (11.2) | 39 (40.6)/57 (59.4) | 24.7 (11.5) | 22 (22.9) | 68 (70.8) | 6 (6.3) | 8.0 | |
| ESL 800 mg/d | 101 | 36.4 (12.6) | 51 (50.5)/50 (49.5) | 22.4 (11.6) | 17 (16.8) | 73 (72.3) | 11 (10.9) | 9.0 | |
| ESL 1200 mg/d | 98 | 36.9 (11.6) | 52 (53.1)/46 (46.9) | 23.0 (12.9) | 20 (20.4) | 68 (69.4) | 10 (10.2) | 9.0 | |
| Sperling MR et al. (2015)59 | PBO | 224 | 39.0 (16, 67)a | 112 (50.0)/112 (50.0) | 21.3 (14.6) | 64 (28.6) | 158 (70.5) | 1 (0.4) | 8.0 |
| ESL 800 mg/d | 216 | 38.5 (16, 71)a | 109 (50.5)/107 (49.5) | 21.6 (13.0) | 60 (27.8) | 153 (70.8) | 0 | 8.0 | |
| ESL 1200 mg/d | 210 | 38.0 (16, 69)a | 105 (50.0)/105 (50.0) | 21.2 (13.0) | 59 (28.1) | 151 (71.9) | 0 | 9.0 | |
| Jóźwiak S et al. (2018)60 | PBO | 40 | 11.6 (2.8) | 26 (65.0)/14 (35.0) | NA | 19 (47.5) | 18 (45.0) | NA | 5.2 |
| ESL 30 mg/kg/d, maximum of 1200 mg/d | 83 | 11.8 (3.1) | 47 (56.6)/36 (43.4) | NA | 44 (53.0) | 37 (44.6) | NA | 5.0 | |
| Kirkham F et al. (2020)61 | PBO | 129 | 9.5 (3.9) | 62 (48.1)/67 (51.9) | 2–6 years 3.6 (1.4)d 7–11 years 6.3 (2.7)d 12–18 years 8.8 (4.1)d |
25 (19.4) | 94 (72.9) | 10 (7.8) | 17.0 |
| ESL 20–30 mg/kg/d, maximum of 1200 mg/d | 134 | 9.9 (4.2) | 64 (47.8)/70 (52.2) | 2–6 years 3.1 (1.4)d 7–11 years 6.6 (3.0)d 12–18 years 8.9 (4.3)d |
21 (15.7) | 98 (73.1) | 15 (11.2) | 11.5 | |
| Elger C et al. (2007)62 | PBO | 47 | 40.4 (10.8) | (42.6)/(57.4) | 20.0 (13.6) | (32.0) | (68.0) | 0 | 11.8 (4, 120)c |
| ESL 1200 mg/d QD | 50 | 39.3 (11.4) | (44.0)/(56.0) | 16.7 (11.7) | (30.0) | (70.0) | 0 | 14.1 (4, 106)c | |
| ESL 1200 mg/d BID | 46 | 39.8 (11.9) | (34.8)/(65.2) | 19.5 (12.6) | (39.0) | (61.0) | 0 | 13.6 (4, 128)c | |
| Ben-Menachem E et al. (2007)63 | PBO | 97 | 38.9 (11.1) | 47 (48.0)/50 (52.0) | 24.6 (11.8) | 16% and 84% of the patients were taking 1 and 2 ASMs | Median seizure frequency ranged from 11 to 13 across all arms | ||
| LCM 200 mg/d | 107 | 39.9 (11.7) | 46 (43.0)/61 (57.0) | 25.1 (12.9) | |||||
| LCM 400 mg/d | 108 | 41.2 (11.6) | 53 (49.0)/55 (51.0) | 24.7 (13.1) | |||||
| LCM 600 mg/d | 106 | 39.4 (10.5) | 45 (42.0)/61 (58.0) | 23.6 (12.7) | |||||
| Halász P et al. (2009)64 | PBO | 163 | 38.5 (10.9) | 91 (55.8)/72 (44.2) | 21.1 (12.2) | 21 (13.2) | 82 (51.6) | 56 (35.2) | 9.9 |
| LCM 200 mg/d | 163 | 36.9 (11.7) | 90 (55.2)/73 (44.8) | 22.9 (12.3) | 17 (10.6) | 77 (48.1) | 66 (41.3) | 11.5 | |
| LCM 400 mg/d | 159 | 37.9 (13.0) | 69 (43.4)/90 (56.6) | 22.8 (13.2) | 25 (15.8) | 79 (50.0) | 54 (34.2) | 10.3 | |
| Chung S et al. (2010)65 | PBO | 104 | 38.1 (12.0) | 49 (47.1)/55 (52.9) | 25.4 (13.3) | 18 (17.3) | 54 (51.9) | 32 (30.8) | 15.0 |
| LCM 400 mg/d | 204 | 39.1 (12.4) | 104 (51.0)/100 (49.0) | 24.5 (13.2) | 36 (17.9) | 110 (54.7) | 55 (27.4) | 11.5 | |
| LCM 600 mg/d | 97 | 36.8 (11.8) | 47 (48.5)/50 (51.5) | 23.4 (13.3) | 18 (18.6) | 57 (58.8) | 22 (22.7) | 16.5 | |
| Hong Z et al. (2016)66 | PBO | 184 | 31.8 (12.0) | 102 (55.4)/82 (44.6) | 16.8 (11.5) | 41 (22.4) | 71 (38.8) | 71 (38.8) | 10.5 |
| LCM 200 mg/d | 183 | 33.2 (12.2) | 94 (51.4)/89 (48.6) | 18.3 (10.9) | 45 (24.7) | 79 (43.4) | 58 (31.9) | 11.0 | |
| LCM 400 mg/d | 180 | 32.3 (11.9) | 104 (57.8)/76 (42.2) | 17.9 (11.7) | 35 (19.6) | 81 (45.3) | 63 (35.2) | 10.0 | |
| Farkas V et al. (2019)67 | PBO <30 kg | 52 | 10.9 (3.5) | 99 (57.6)/73 (42.4) | 6.04 (0.4, 16.2)a | 29 (16.9) | 82 (47.7) | 61 (35.5) | 8.7 |
| PBO ≥30 kg ≤50 kg | 60 | ||||||||
| PBO ≥50 kg | 60 | ||||||||
| LCM <30 kg: 8–12 mg/kg/d | 61 | 10.5 (3.6) | 91 (53.2)/80 (46.8) | 6.00 (0.4, 15.7)a | 30 (17.5) | 78 (45.6) | 63 (36.8) | 10.4 | |
| LCM ≥30 kg ≤50 kg: 6–8 mg/kg/d | 46 | ||||||||
| LCM ≥50 kg: 300–400 mg/d | 64 | ||||||||
| Krauss GL et al. (2012)68 | PBO | 185 | 33.4 (12.6) | 95 (51.4)/90 (48.6) | 17.5 (10.7)d | 28 (15.1) | 90 (48.6) | 67 (36.2) | 9.3 |
| PER 2 mg/d | 180 | 33.8 (13.6) | 85 (47.2)/95 (52.8) | 19.4 (12.1)d | 30 (16.7) | 80 (44.4) | 70 (38.9) | 10.1 | |
| PER 4 mg/d | 172 | 33.6 (12.2) | 88 (51.2)/84 (48.8) | 19.7 (12.1)d | 19 (11.0) | 88 (51.2) | 65 (37.8) | 10.0 | |
| PER 8 mg/d | 169 | 34.6 (12.8) | 77 (45.6)/92 (54.4) | 20.0 (11.9)d | 27 (16.0) | 82 (48.5) | 60 (35.5) | 10.9 | |
| French JA et al. (2012)69 | PBO | 121 | 35.6 (14.7) | 54 (44.6)/67 (55.4) | 24.1 (12.9)d | 15 (12.4) | 64 (52.9) | 42 (34.7) | 13.7 |
| PER 8 mg/d | 133 | 35.8 (14.2) | 65 (48.9)/68 (51.1) | 23.6 (13.5)d | 26 (19.5) | 70 (52.9) | 37 (27.8) | 14.3 | |
| PER 12 mg/d | 134 | 36.7 (14.6) | 69 (51.5)/65 (48.5) | 23.3 (14.4)d | 19 (14.2) | 82 (61.2) | 33 (24.6) | 12.0 | |
| French JA et al. (2013)70 | PBO | 136 | 34.4 (13.6) | 71 (52.2)/65 (47.8) | 22.0 (12.9)d | 17 (12.5) | 64 (47.1) | 55 (40.4) | 11.8 |
| PER 8 mg/d | 129 | 36.7 (14.4) | 65 (50.4)/64 (49.6) | 22.5 (13.6)d | 16 (12.4) | 68 (52.7) | 45 (34.9) | 13.0 | |
| PER 12 mg/d | 121 | 35.5 (14.1) | 50 (41.3)/71 (58.7) | 21.3 (13.2)d | 9 (7.4) | 63 (52.1) | 49 (40.5) | 13.7 | |
| Nishida T et al. (2018)71 | PBO | 175 | 34.5 (13.2) | 86 (49.1)/89 (50.9) | 17.5 (10.9) | 11 (6.3) | 67 (38.3) | 97 (55.4) | Median seizure frequency ranged from 9.1 to 10.0 across all arms |
| PER 4 mg/d | 174 | 33.1 (13.2) | 80 (46.0)/94 (54.0) | 17.4 (11.1) | 9 (5.2) | 70 (40.2) | 95 (54.5) | ||
| PER 8 mg/d | 175 | 33.6 (14.1) | 91 (52.0)/84 (48.0) | 16.9 (11.5) | 15 (8.6) | 60 (34.3) | 100 (57.1) | ||
| PER 12 mg/d | 180 | 32.3 (12.3) | 87 (48.3)/93 (51.7) | 17.4 (11.2) | 13 (7.2) | 75 (41.7) | 92 (51.1) | ||
| Krauss GL et al. (2020)72 | PBO | 108 | 39.6 (12.4) | 58 (54.0)/50 (46.0) | 23.0 (14.2) | 27 (25.0) | 54 (50.0) | 27 (25.0) | 8.4 |
| CNB 100 mg/d | 108 | 39.0 (12.1) | 57 (53.0)/51 (47.0) | 25.5 (13.4) | 25 (23.0) | 48 (44.0) | 35 (33.0) | 9.5 | |
| CNB 200 mg/d | 110 | 40.9 (12.4) | 54 (49.0)/56 (51.0) | 22.8 (13.2) | 39 (36.0) | 47 (43.0) | 24 (22.0) | 11.0 | |
| CNB 400 mg/d | 111 | 39.6 (10.3) | 52 (47.0)/59 (53.0) | 24.4 (14.2) | 24 (22.0) | 62 (56.0) | 25 (22.0) | 9.0 | |
| Chung SS et al. (2020)73 | PBO | 109 | 38.0 (18, 59)a | 58 (53.2)/51 (46.8) | 21.1 (2.4, 60.8)d | 12 (11.0) | 52 (47.7) | 45 (41.3) | 5.5 |
| CNB 200 mg/d | 113 | 36.0 (18, 61)a | 55 (48.7)/58 (51.3) | 20.0 (2.3, 52.5)d | 19 (16.8) | 53 (46.9) | 41 (36.3) | 7.5 | |
| Elger CE et al. (2010)74 | PBO | 133 | 37.3 (17, 68) | 80 (60.0)/53 (40.0) | NA | 26 (19.6) | 70 (52.6) | 37 (27.9) | 11.7 |
| RUF 200 mg/d | 127 | 35.9 (15, 63) | 64 (50.0)/63 (50.0) | NA | 38 (29.9) | 57 (44.9) | 32 (25.2) | 11.1 | |
| RUF 400 mg/d | 125 | 34.3 (14, 62) | 74 (59.0)/51 (41.0) | NA | 29 (23.2) | 68 (54.4) | 28 (22.4) | 11.8 | |
| RUF 800 mg/d | 129 | 37.1 (16, 64) | 68 (53.0)/61 (47.0) | NA | 39 (30.2) | 57 (44.2) | 33 (25.6) | 12.7 | |
| RUF 1600 mg/d | 133 | 36.0 (16, 62) | 61 (46.0)/72 (54.0) | NA | 31 (23.3) | 73 (54.9) | 29 (21.8) | 11.3 | |
| Biton V et al. (2011)75 | PBO | 180 | 38.1 (14.8) | 83 (46.1)/97 (53.9) | NA | 49 (13.8) | 157 (44.1) | 150 (42.1) | 13.8 |
| RUF 3200 mg/d | 176 | 36.4 (14.8) | 84 (47.7)/92 (52.3) | NA | 13.0 | ||||
| Brodie MJ et al. (2009)76 | PBO | 157 | 37.9 (17, 68) | 76 (48.4)/81 (51.6) | NA | 46 (29.2) | 111 (70.8) | NA | 8.0 |
| RUF 3200 mg/d | 156 | 35.8 (16, 72) | 63 (40.4)/93 (59.6) | NA | 46 (29.5) | 110 (70.5) | NA | 8.5 | |
Data are mean (SD) or median (min, max) unless otherwise specified.
Median.
Baseline partial-onset seizure frequency per week.
Mean (SD) or Mean (min, max).
Converted to years by dividing the number of months by 12. NA, Not Available; PBO, Placebo; RUF, Rufinamide; BRV, Brivaracetam; CNB, Cenobamate; ESL, Eslicarbazepine; LCM, Lacosamide; RTG, Retigabine; PER, Perampanel; ASM, antiseizure medication; SD, standard deviation.
Risk of bias assessment
Among the 29 included RCTs, all studies were double-blinded and exhibited a relatively low overall risk of bias (Appendix 1).
Seizure response
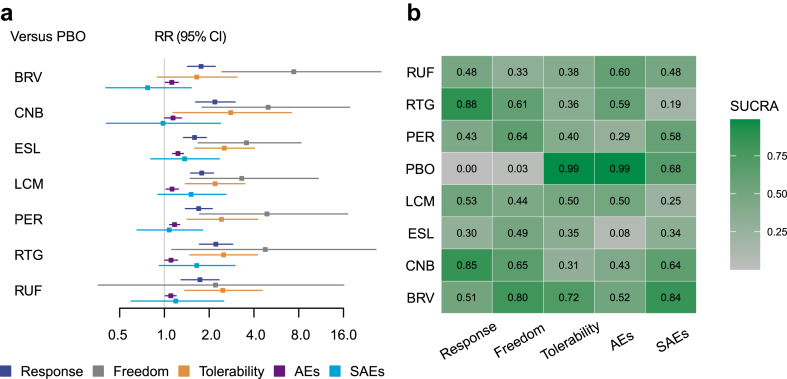
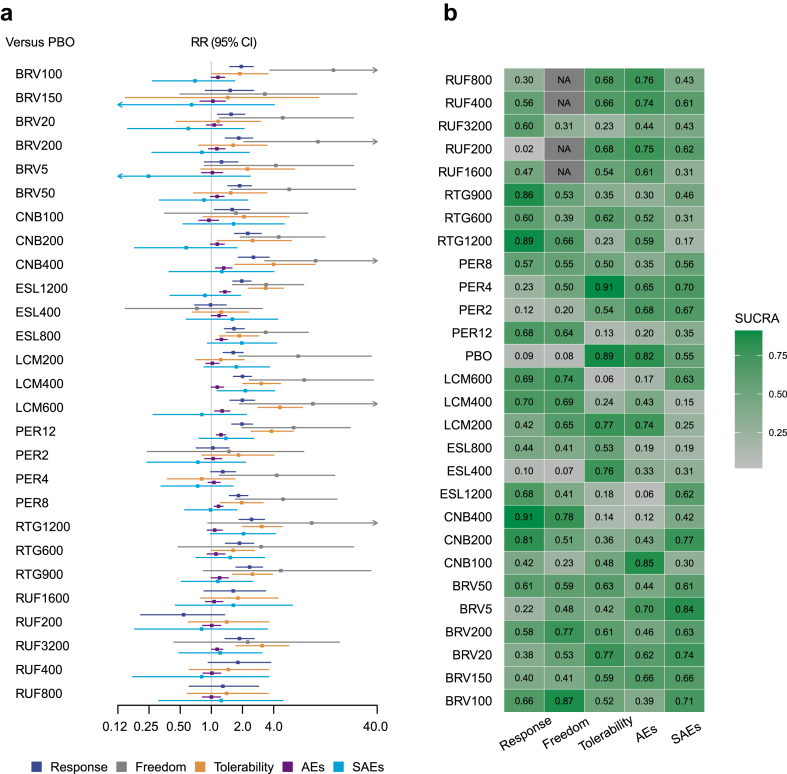
The 50% responder rates were reported in all 29 trials.48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 An assessment of transitivity with different drugs treated as separate nodes is detailed in Appendix 2. All seven ASMs significantly outperformed placebo (Fig. 3a), with detailed results for seizure response outcomes available in Appendix 3. RTG achieved the highest SUCRA value, followed by CNB (Fig. 3b). When analysing different dosages as separate nodes, three studies were excluded due to dosage adjustments based on participant weights, lacking a fixed target dosage.60,61,67 The assessment of transitivity for this analysis is in Appendix 8, with detailed results in Appendix 9. Most ASMs at varying dosages were superior to placebo (Fig. 4a), with CNB 400 mg/d achieving the highest SUCRA value, followed by RTG 1200 mg/d (Fig. 4b).
Fig. 3.
Results of network meta-analysis considering each ASM as a separate node. a, Forest plot between ASMs and placebo for the efficacy, tolerability and safety outcomes; b. the SUCRA values of all ASMs for all outcomes. PBO, Placebo; RUF, Rufinamide; BRV, Brivaracetam; CNB, Cenobamate; ESL, Eslicarbazepine; LCM, Lacosamide; RTG, Retigabine; PER, Perampanel; AEs, adverse effects; SAEs, serious adverse events; RR, risk ratio; CI, credible interval; SUCRA, the surface under the cumulative ranking curve.
Fig. 4.
Results of network meta-analysis considering each dosage as a separate node. a, Forest plot between ASMs with different dosages and placebo for the efficacy, tolerability and safety outcomes; b. the SUCRA values of all ASMs with different dosages for all outcomes. PBO, Placebo; RUF, Rufinamide; BRV, Brivaracetam; CNB, Cenobamate; ESL, Eslicarbazepine; LCM, Lacosamide; RTG, Retigabine; PER, Perampanel; AEs, adverse effects; SAEs, serious adverse events; RR, risk ratio; CI, credible interval; SUCRA, the surface under the cumulative ranking curve.
Seizure freedom
Seizure freedom data were absent in four studies,50,61,74,75 resulting in 25 studies being analysed further. When treating different drugs as separate nodes, all ASMs except RUF were significantly better than placebo (Fig. 3a), with BRV leading according to SUCRA values, followed by CNB (Fig. 3b). Detailed results are in Appendix 4. With different dosages treated as separate nodes, BRV 100 mg/d ranked highest, followed by CNB 400 mg/d, with details in Appendix 10.
Tolerability
Withdrawals due to AEs were reported in all studies. With different drugs as separate nodes, all ASMs except BRV had significantly more withdrawals than placebo (Fig. 3a), with placebo having the highest SUCRA value, followed by BRV (Fig. 3b). CNB had the lowest ranking. Details are in Appendix 7. When considering different dosages as separate nodes, PER 4 mg/d led, followed by placebo, with LCM 600 mg/d ranking lowest. Details are in Appendix 13.
Safety outcomes
At least one AE was not reported in three studies,50,64,65 allowing 26 studies for subsequent analysis. Treating different drugs as separate nodes showed all ASMs had more patients with at least one AE than placebo, though the effect sizes were relatively small (Fig. 3a). Placebo ranked highest according to SUCRA values, followed by RUF, with ESL ranking lowest. Detailed outcomes are in Appendix 5. For different dosages as separate nodes, CNB 100 mg/d led, followed by placebo, with ESL 1200 mg/d ranking lowest. Details are in Appendix 11.
For SAEs, all studies reported data for each arm. No significant differences were found between ASMs and placebo when treating different drugs as separate nodes (Fig. 3a), with BRV ranking highest according to SUCRA values, followed by placebo, and RTG ranking lowest. Details are in Appendix 6. With different dosages as separate nodes, BRV 5 mg/d led, followed by CNB 200 mg/d, with LCM 400 mg/d ranking lowest. Details are in Appendix 12.
Discussion
To our knowledge, this study is the most comprehensive quantitative synthesis to date, examining the use of third-generation ASMs as an add-on strategy in patients with treatment-resistant FOS, boasting the largest sample size. Compared to previous studies addressing the same issue, we provide an updated and detailed assessment of efficacy, tolerability, and safety outcomes for both ASMs and their different dosages. Our findings aim to inform clinical guidelines and support decision-making for patients and clinicians, helping to select the most appropriate treatment strategies in this critical clinical area.
Achieving seizure freedom is a primary objective in the treatment of epilepsy.77 However, only 50% of patients who receive their first antiepileptic drug treatment become seizure-free.35 This means that many patients require additional treatment in order to reach this goal. Unfortunately, more than a third of patients with epilepsy continue to experience uncontrolled seizures even after being treated with an antiepileptic drug. Additionally, very few patients with treatment-resistant epilepsy are able to achieve seizure freedom, even with the use of newer drugs.35,78,79 Uncontrolled epilepsy can have a profound impact on patients' lives, leading to psychological and social dysfunction, limited educational and employment opportunities, and a diminished quality of life. It also poses a risk of premature death,32,33,80 with persistent generalised tonic-clonic seizures being a particular risk factor for sudden unexpected death in epilepsy.81,82 Given these challenges, there is a critical need for new and more effective antiepileptic drugs that can reduce seizures in patients with uncontrolled epilepsy and help a greater number of individuals attain seizure freedom.
Our findings revealed that CNB secured the second position in terms of both seizure response and seizure freedom. Upon analyzing based on dosages, CNB at a daily dose of 400 mg emerged as the top performer for seizure response, while it maintained its second-place ranking for seizure freedom. However, CNB is not covered by current guidelines.83,84 CNB is a new antiseizure medication which has been approved for the treatment of FOS in adults.85 In November 2019, Xcopri®, marketed by SK Life Science Inc (Paramus, NJ, USA), was approved by the Food and Drug Administration for adult focal seizures.86 The European Medicines Agency (EMA) approved Ontozry® by Arvelle Therapeutics Netherlands B.V. (Amsterdam, The Netherlands) for the adjunctive treatment of FOS in treatment-resistant adult patients in March 2021.87 Laskier et al. (2023) estimated the cost per quality-adjusted life-year (QALY) for CNB compared to BRV, ESL, LCM, and PER over a lifetime horizon.88 CNB improved QALYs and was less costly than BRV, ESL, LCM, and PER. Villanueva et al. (2023) found that CNB could represent the most effective ASM in all doses studied compared to the third-generation ASMs and the most efficient option at DDD for both ≥50% responder rate and seizure freedom.89 This study could represent an important contribution towards informed decision-making regarding the selection of the most appropriate therapy for FOS in adult patients with DRE from a clinical and economic perspective in Spain. CNB is a medication that could potentially change the perspectives regarding the management and prognosis of refractory epilepsy.90 CNB has a dual complementary mechanism of action. It works by both inhibiting the persistent component of the sodium current, thus decreasing excitatory currents, and enhancing the inactivated state of voltage-gated sodium channels.91 This dual activity contributes to its antiseizure properties. Additionally, it has been found that CNB enhances inhibitory currents through allosteric modulation of the GABAA receptor, leading to a decrease in neuronal excitability.91,92 Experimental evidence suggests that CNB may target both synaptic and extrasynaptic GABAA receptors. CNB has the potential to significantly impact the management and prognosis of refractory epilepsy,93 especially if patients can tolerate higher dosages. Therefore, CNB may be considered as a cost-effective adjunctive medication for patients with treatment-resistant FOS.
BRV is also a new type of medication derived from pyrrolidine. Our findings indicate that BRV secured the top position for both seizure freedom and SAEs. When considering dosages, BRV at 100 mg/d emerged as the leading option for seizure freedom. It acts as a potent inhibitor for synaptic vesicle protein 2A (SV2A), showing greater affinity compared to levetiracetam (LEV) in the human cerebral cortex.94 Additionally, BRV also exhibits inhibitory effects on neuronal voltage-dependent sodium channels.94, 95, 96 These properties make BRV a promising candidate for the treatment of both focal and generalised seizures, as observed in animal models.97 In terms of pharmacokinetics, BRV displays linear characteristics across a wide range of doses. It is rapidly and almost completely absorbed by the body, with an elimination half-life of approximately 8 h. The medication also has a plasma protein binding rate of 20%.98, 99, 100 The majority of the BRV dose, including 8.6% of the drug unchanged, is excreted in the urine.100 Metabolism mainly occurs through hydrolysis and secondarily through hydroxylation, which is mediated by cytochrome (CYP) 2C19. The primary metabolites (acid, hydroxyl, and hydroxyacid) do not have any pharmacological activity. In vitro and in vivo studies have shown that BRV may inhibit epoxide hydrolase, resulting in an increase in plasma carbamazepine (CBZ)—epoxide levels when taken concurrently with CBZ treatment.101 In a study conducted on patients with photosensitive epilepsy, it was observed that a single dose of BRV was effective at reducing or eliminating EEG discharges caused by photic stimulation at all tested doses (ranging from 10 to 80 mg).102
RUF is an oral antiepileptic drug, and it is a triazole derivative that is a unique chemical structure different from other current antiepileptic drugs on the market.103 RUF can be used to treat seizures associated with Lennox-Gastaut syndrome (LGS) in patients aged four and older.104,105 RUF has been approved by regulatory authorities in the USA and EU for adjunctive therapy in LGS. One of the advantages of RUF is its simple pharmacokinetic profile. It has low plasma protein binding and is metabolised through hydrolysis. Unlike other drugs, it does not significantly inhibit or induce the cytochrome P450 system, which is involved in the metabolism of many drugs. As a result, it has minimal risks of drug–drug interactions. Additionally, RUF has been found to have no significant impact on cognitive function.106, 107, 108, 109, 110 Our results showed RUF displayed average performance across all outcomes, except for the AE safety outcome, where it ranked second, trailing behind placebo. However, the efficacy evaluation of RUF for seizure freedom was constrained, as only one study provided relevant data.76
ESL has been approved for use as adjunctive therapy in adults with FOS with or without secondary generalisation by the EMA, FDA, and Health Canada. Subsequently, both EMA and FDA have also approved ESL for use as monotherapy in the same group of patients.111 ESL is a novel voltage-gated sodium channel (VGSC) blocker. It is chemically related to CBZ and oxcarbazepine (OXC), as they both share a similar basic chemical structure of a dibenzazepine nucleus with a 5-carboxamide substituent. However, ESL differs structurally from CBZ and OXC at the 10, 11-position.112,113 One notable difference between ESL and CBZ is that ESL does not undergo metabolism to CBZ-10, 11-epoxide. This means that ESL is not susceptible to enzyme induction or autoinduction. On the other hand, OXC is a prodrug that is converted to both enantiomers of the OXC monohydroxy derivative (MHD). In contrast, ESL is a prodrug that is converted only to S-MHD, which is also known as S-licarbazepine or ESL.113,114 The pharmacokinetics of ESL are not affected by factors such as food consumption,115,116 age,117 or gender.118 Our results showed ESL 1200 mg/d was ranked seventh in terms of seizure response. However, dosages of 800 mg/d and 400 mg/d were ranked 16th and 26th, respectively, indicating that a higher dose of ESL might be necessary to achieve a satisfactory response. In the context of seizure freedom, ESL at dosages of 400 mg/d, 800 mg/d, and 1200 mg/d ranked first, seventh, and eighth from the bottom, respectively. This pattern suggests that ESL may offer limited advantages for achieving seizure freedom compared to other ASMs.
Our results showed LCM was ranked third for seizure response. Upon evaluating dosages, LCM at 400 mg/d and 600 mg/d were ranked fifth and eighth for seizure response, respectively. Regarding seizure freedom, LCM at 600 mg/d and 400 mg/d were ranked fourth and fifth, respectively. LCM is a recently introduced ASM that has been approved by both the US FDA and EMA for the treatment of FOS in adults and children aged four and older.119,120 LCM is a modified amino acid, specifically the R-enantiomer of 2-acetamido-N-benzyl-3-methoxypropionamide, and belongs to the third generation of ASMs, which work through different mechanisms compared to previous generations.121 Unlike traditional ASMs that block sodium channels and mainly target fast inactivation by altering the voltage dependence of inactivation to more hyperpolarised potentials, LCM specifically enhances slow inactivation of voltage-gated sodium channels by binding to the collapsin response mediator protein 2.122,123 The collapsin response mediator protein 2 is involved in neuronal differentiation, growth, and polarisation processes.124 These mechanisms result in significant anti-epileptic effects whilst also maintaining normal brain function, including cognition.125,126 LCM has a pharmacokinetic profile that is advantageous, with linear kinetics, high bioavailability, low potential for drug interactions, and good tolerability.127 The body rapidly absorbs LCM, with peak blood concentration achieved within 0.5–4 h after ingestion and taking 800 mg orally can result in complete absorption and the drug maintains a stable blood concentration.121 As a result, it can be taken twice daily for antiepileptic treatment.122
RTG emerged as the leading medication for seizure response and secured the 4th position for seizure freedom. When examining dosages, RTG at 1200 mg/d and 900 mg/d were ranked second and third for seizure response, respectively. Despite its promising efficacy, it is important to highlight that RTG was ranked lowest for SAE safety outcomes. RTG was approved by the EMA in March 2011 under the name RTG (Trobalt®) and by the US FDA in June 2011 under the name ezogabine (EZG, Potiga®) for use as an additional therapy for FOS in adult patients.67, 68, 69 RTG/EZG facilitates the opening of specific neuronal voltage-gated potassium channels, resulting in an increase in potassium current. This shift in current leads to a hyperpolarising effect and reduces the excitability of the brain cells.128 RTG/EZG also enhances gamma-aminobutyric acid (GABA)-evoked currents in cortical neurons and inhibits the production of neuroactive amino acids induced by 4-aminopyridine and promotes the synthesis of GABA in hippocampal slices.128,129 These effects may contribute to the stabilisation of neuronal excitability.129 Research suggests that activating M-channels through the opening of Kv 7.2 and Kv 7.3 channels could be a powerful method for treating epilepsy and other neuropsychiatric disorders.130, 131, 132, 133, 134 However, concerns have been raised about the potential risk of retinal pigmentation and visual impairment associated with RTG/EZG treatment. As a result, in May 2013, the patient population eligible for RTG/EZG treatment was revised to allow its use only after other drug combinations have proved ineffective or intolerable, and the benefits of treatment outweigh the risk of retinal abnormalities.135 The pharmacokinetic profile of RTG is dose-proportional up to a maximum daily dose of 1200 mg.136 It is rapidly absorbed after oral administration, with peak plasma concentrations reached within 1.5 h. Food does not affect the overall absorption and exposure of RTG, although it may slightly delay the time to reach maximal plasma drug concentrations to approximately 2 h.136 RTG undergoes two processes in the body to form metabolites. One process is acetylation, which leads to the formation of the primary metabolite. The second process is glucuronidation, resulting in the creation of an N-glucuronide structure.137 After administration, the maximum plasma concentration of the primary active metabolite is reached about 2 h later than the parent compound. Both RTG and its primary metabolite have a plasma half-life of approximately 8 h. Furthermore, there are no significant pharmacokinetic interactions between RTG and other antiepileptic drugs such as phenobarbitone, valproate, topiramate, phenytoin, lamotrigine, or carbamazepine.138 However, it should be noted that phenytoin and carbamazepine do moderately increase the clearance of RTG.138
Our findings also indicated that PER was ranked third for seizure freedom. At a dosage of 12 mg/d, PER was positioned 6th for seizure response and 8th for seizure freedom. PER is a highly selective and non-competitive antagonist of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor.139,140 PER is approved as an additional treatment for focal and primary generalised tonic-clonic seizures in patients aged 12 and above.141,142 It can be used as monotherapy or as an adjunct therapy for FOS with or without generalised seizures in individuals aged 4 years or older.143,144 It can also be used as an add-on therapy for primary generalised tonic-clonic seizures in those aged 7 years and older.145 In 2014, the European Union and the United States approved the use of PER for patients who are 18 years old or older. Since then, over 40 countries worldwide have also approved the use of this drug.143,146 AMPA receptors are responsible for mediating fast excitatory synaptic transmission and play a crucial role in triggering and spreading epileptic seizures.139,140,147 PER works by inhibiting excitatory neurotransmission through the modulation of post-synaptic glutamate activity. Additionally, it has the ability to block excessive levels of glutamate due to its non-competitive nature, meaning it cannot be displaced even under high concentrations of AMPA receptor agonists. This unique mechanism of action contributes to the drug's strong anti-seizure properties.147 In laboratory studies using liver cells, it has been found that PER is mainly broken down by a specific enzyme called cytochrome P450 (CYP) isotype CYP3A4/5.148 This means that the clearance of PER may be affected by other drugs.141,142,149,150 When researchers investigated the effects of PER on other drugs, they found that it only weakly inhibits certain enzymes (CYP2C8, CYP3A4) and uridine diphosphate glucuronosyltransferase (UGT) enzymes (UGT1A9, UGT2B7) in laboratory studies. It also weakly induces other enzymes (CYP2B6, CYP3A4/5, UGT1A1, UGT1A4). However, PER is not a substance that is affected by or affects drug transporters in laboratory studies.140,142,148 Therefore, it is not expected to have a significant impact on the way other drugs are processed in the body. PER has not been found to interact with other types of glutamate receptors, such as N-methyl-d-aspartate (NMDA) and kainate receptors.151,152 Unlike some medications that block NMDA receptors, PER does not cause behavioural adverse events similar to those associated with phencyclidine.152
However, there are several limitations to consider. Firstly, the majority of RCTs compare ASMs with placebos, with few RCTs directly comparing different ASMs. Consequently, all effect sizes between different ASMs were derived from indirect comparisons. While we have ranked all ASMs across all outcomes, these rankings should be treated with caution, underscoring the need for future head-to-head trials. Secondly, achieving seizure freedom in patients with treatment-resistant FOS is challenging, leading to a scarcity of adequate case numbers for seizure freedom in most RCTs. This scarcity may introduce bias in estimating seizure freedom outcomes.153 Lastly, our analysis was based on traditional NMA; future studies should incorporate individual patient data into NMA for more precise estimates in this field.
In summary, this systematic review and Bayesian NMA offer insights into the relative efficacy, tolerability, and safety of third-generation ASMs in treating patients with treatment-resistant FOS. By providing the most comprehensive current evidence base, our analysis aims to inform pharmacological treatment choices in this critical clinical area. The insights gained should assist in shaping clinical guidelines and supporting the decision-making process for patients and clinicians, facilitating the identification of the most suitable treatment strategies for treatment-resistant FOS.
Contributors
Yangmei Chen and Yankun Chen designed the study. Yankun Chen, Wenze Li, Xinxia Gao and Chenfei Lu conducted the literature search and searched the articles. Wenze Li, Yankun Chen, Xinxia Gao, Huizhen Song, Yanli Zhang, Sihao Zhao, Gaoang Cai, Qing Guo and Chenfei Lu contributed to the data extraction process. Yankun Chen, Dongdong Zhou and Chenfei Lu analysed or interpreted the data. Wenze Li, Yankun Chen, Xinxia Gao and Dongdong Zhou verified the underlying data. Yankun Chen, Wenze Li, Chenfei Lu and Xinxia Gao drafted the manuscript. Dongdong Zhou and Yangmei Chen conducted the study supervision and critical revision. All the authors revised the article and approved the final version. All authors have full access to all the data in the study and accept responsibility for decision to submit for publication.
Data sharing statement
Data are presented in the current manuscript, or within the manuscripts or appendices of the included studies.
Declaration of interests
The authors report no conflicts of interest in this work.
Acknowledgements
The authors would like to thank all the staff at the Library in the Chongqing Medical University for their great support and conscientious work during the literature search of this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102513.
Contributor Information
Dongdong Zhou, Email: zhoudongdong@cqmu.edu.cn.
Yangmei Chen, Email: chenym1997@cqmu.edu.cn.
Appendix A. Supplementary data
References
- 1.Chang B.S., Lowenstein D.H. Epilepsy. N Engl J Med. 2003;349(13):1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 2.Hirtz D., Thurman D.J., Gwinn-Hardy K., Mohamed M., Chaudhuri A.R., Zalutsky R. How common are the "common" neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 3.Cagnetti C., Lattanzi S., Foschi N., Provinciali L., Silvestrini M. Seizure course during pregnancy in catamenial epilepsy. Neurology. 2014;83(4):339–344. doi: 10.1212/WNL.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 4.Forsgren L., Beghi E., Oun A., Sillanpää M. The epidemiology of epilepsy in Europe - a systematic review. Eur J Neurol. 2005;12(4):245–253. doi: 10.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . 2012. Fact sheets-epilepsy.http://www.who.int/mediacentre/factsheets/fs999/en/index.html Accessed 27 Feb 2013. [Google Scholar]
- 6.Gupta S., Ryvlin P., Faught E., Tsong W., Kwan P. Understanding the burden of focal epilepsy as a function of seizure frequency in the United States, Europe, and Brazil. Epilepsia Open. 2017;2(2):199–213. doi: 10.1002/epi4.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and Clinical Excellence . 2012. CG137 epilepsy: NICE guidance.http://guidance.nice.org.uk/CG137/NICEGuidance/pdf/English Accessed 27 Feb 2013. [Google Scholar]
- 8.French J.A., Kanner A.M., Bautista J., et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the therapeutics and technology assessment subcommittee and quality standards subcommittee of the American academy of neurology and the American epilepsy society. Neurology. 2004;62(8):1261–1273. doi: 10.1212/01.wnl.0000123695.22623.32. [DOI] [PubMed] [Google Scholar]
- 9.Tsai J.J., Wu T., Leung H., et al. Perampanel, an AMPA receptor antagonist: from clinical research to practice in clinical settings. Acta Neurol Scand. 2018;137(4):378–391. doi: 10.1111/ane.12879. [DOI] [PubMed] [Google Scholar]
- 10.Kwan P., Brodie M.J. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 11.Brodie M.J., Barry S.J., Bamagous G.A., Norrie J.D., Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begley C.E., Annegers J.F., Lairson D.R., Reynolds T.F., Hauser W.A. Cost of epilepsy in the United States: a model based on incidence and prognosis. Epilepsia. 1994;35(6):1230–1243. doi: 10.1111/j.1528-1157.1994.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 13.Preux P.M., Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4(1):21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- 14.Perucca E., Tomson T. The pharmacological treatment of epilepsy in adults. Lancet Neurol. 2011;10(5):446–456. doi: 10.1016/S1474-4422(11)70047-3. [DOI] [PubMed] [Google Scholar]
- 15.Shih J.J., Whitlock J.B., Chimato N., Vargas E., Karceski S.C., Frank R.D. Epilepsy treatment in adults and adolescents: expert opinion, 2016. Epilepsy Behav. 2017;69:186–222. doi: 10.1016/j.yebeh.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Chi X., Li R., Hao X., et al. Response to treatment schedules after the first antiepileptic drug failed. Epilepsia. 2018;59(11):2118–2124. doi: 10.1111/epi.14565. [DOI] [PubMed] [Google Scholar]
- 17.Kwan P., Arzimanoglou A., Berg A.T., et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 18.Cockerell O.C., Johnson A.L., Sander J.W., Hart Y.M., Shorvon S.D. Remission of epilepsy: results from the national general practice study of epilepsy. Lancet. 1995;346(8968):140–144. doi: 10.1016/s0140-6736(95)91208-8. [DOI] [PubMed] [Google Scholar]
- 19.Devinsky O. Patients with refractory seizures. N Engl J Med. 1999;340(20):1565–1570. doi: 10.1056/NEJM199905203402008. [DOI] [PubMed] [Google Scholar]
- 20.Leeman B., Schachter S.C. In: Wyllie's treatment of epilepsy: principles and practice. 5th ed. Wyllie E., editor. Lippincott Williams and Wilkins; Philadelphia, PA: 2011. Psychiatric comorbidity of epilepsy; pp. 1037–1050. [Google Scholar]
- 21.Kleen K., Scott R.C., Lenck-Santini P.P., Holmes G.L. In: Jasper's basic mechanisms of the epilepsies. 4th ed. Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., DelgadoEscueta A.V., editors. National Center for Biotechnology Information (US); Bethesda, MD: 2012. Cognitive and behavioral comorbidities of epilepsy. [Google Scholar]
- 22.Kaufman K.R. Antiepileptic drugs in the treatment of psychiatric disorders. Epilepsy Behav. 2011;21(1):1–11. doi: 10.1016/j.yebeh.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger A.B. Psychotropic effects of antiepileptic drugs. Neurology. 2006;67(11):1916–1925. doi: 10.1212/01.wnl.0000247045.85646.c0. [DOI] [PubMed] [Google Scholar]
- 24.Mula M., Kanner A.M., Schmitz B., Schachter S. Antiepileptic drugs and suicidality: an expert consensus statement from the task force on therapeutic strategies of the ILAE commission on neuropsychobiology. Epilepsia. 2013;54(1):199–203. doi: 10.1111/j.1528-1167.2012.03688.x. [DOI] [PubMed] [Google Scholar]
- 25.Perucca P., Gilliam F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):792–802. doi: 10.1016/S1474-4422(12)70153-9. [DOI] [PubMed] [Google Scholar]
- 26.Aldenkamp A.P., De Krom M., Reijs R. Newer antiepileptic drugs and cognitive issues. Epilepsia. 2003;44(Suppl 4):21–29. doi: 10.1046/j.1528-1157.44.s4.3.x. [DOI] [PubMed] [Google Scholar]
- 27.Strzelczyk A., Reese J.P., Dodel R., Hamer H.M. Cost of epilepsy: a systematic review. Pharmacoeconomics. 2008;26(6):463–476. doi: 10.2165/00019053-200826060-00002. [DOI] [PubMed] [Google Scholar]
- 28.GHDx . 2019. GBD results: idiopathic epilepsy.https://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/18c71f2e1f39764dd16f7a20a625aa93 [Google Scholar]
- 29.Epilepsy—level 1 impairment. http://www.healthdata.org/results/gbd_summaries/2019/epilepsy-level-1-impairment
- 30.World Health Organization . 2023. Epilepsy. Fact sheet.https://www.who.int/en/news-room/fact-sheets/detail/epilepsy Available at: Accessed 12 March 2023. [Google Scholar]
- 31.Zhang D., Li X., Ding J., et al. Value of perampanel as adjunctive treatment for partial-onset seizures in epilepsy: cost-effectiveness and budget impact analysis. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.670108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laxer K.D., Trinka E., Hirsch L.J., et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59–70. doi: 10.1016/j.yebeh.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Josephson C.B., Patten S.B., Bulloch A., et al. The impact of seizures on epilepsy outcomes: a national, community-based survey. Epilepsia. 2017;58(5):764–771. doi: 10.1111/epi.13723. [DOI] [PubMed] [Google Scholar]
- 34.Strzelczyk A., Griebel C., Lux W., Rosenow F., Reese J.P. The burden of severely drug-refractory epilepsy: a comparative longitudinal evaluation of mortality, morbidity, resource use, and cost using German health insurance data. Front Neurol. 2017;8:712. doi: 10.3389/fneur.2017.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z., Brodie M.J., Liew D., Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–286. doi: 10.1001/jamaneurol.2017.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mäkinen J., Rainesalo S., Raitanen J., Peltola J. The effect of newer antiepileptic drugs in combination therapy. Epilepsy Res. 2017;132:15–20. doi: 10.1016/j.eplepsyres.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Lasoń W., Dudra-Jastrzębska M., Rejdak K., Czuczwar S.J. Basic mechanisms of antiepileptic drugs and their pharmacokinetic/pharmacodynamic interactions: an update. Pharm Rep. 2011;63(2):271–292. doi: 10.1016/s1734-1140(11)70497-2. [DOI] [PubMed] [Google Scholar]
- 38.Beyenburg S., Stavem K., Schmidt D. Placebo-corrected efficacy of modern antiepileptic drugs for refractory epilepsy: systematic review and meta-analysis. Epilepsia. 2010;51(1):7–26. doi: 10.1111/j.1528-1167.2009.02299.x. [DOI] [PubMed] [Google Scholar]
- 39.Bodalia P.N., Grosso A.M., Sofat R., et al. Comparative efficacy and tolerability of anti-epileptic drugs for refractory focal epilepsy: systematic review and network meta-analysis reveals the need for long term comparator trials. Br J Clin Pharmacol. 2013;76(5):649–667. doi: 10.1111/bcp.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campos M.S., Ayres L.R., Morelo M.R., Marques F.A., Pereira L.R. Efficacy and tolerability of antiepileptic drugs in patients with focal epilepsy: systematic review and network meta-analyses. Pharmacotherapy. 2016;36(12):1255–1271. doi: 10.1002/phar.1855. [DOI] [PubMed] [Google Scholar]
- 41.Costa J., Fareleira F., Ascenção R., Borges M., Sampaio C., Vaz-Carneiro A. Clinical comparability of the new antiepileptic drugs in refractory partial epilepsy: a systematic review and meta-analysis. Epilepsia. 2011;52(7):1280–1291. doi: 10.1111/j.1528-1167.2011.03047.x. [DOI] [PubMed] [Google Scholar]
- 42.Khan N., Shah D., Tongbram V., Verdian L., Hawkins N. The efficacy and tolerability of perampanel and other recently approved anti-epileptic drugs for the treatment of refractory partial onset seizure: a systematic review and Bayesian network meta-analysis. Curr Med Res Opin. 2013;29(8):1001–1013. doi: 10.1185/03007995.2013.803461. [DOI] [PubMed] [Google Scholar]
- 43.Martyn-St James M., Glanville J., McCool R., et al. The efficacy and safety of retigabine and other adjunctive treatments for refractory partial epilepsy: a systematic review and indirect comparison. Seizure. 2012;21(9):665–678. doi: 10.1016/j.seizure.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Hutton B., Salanti G., Caldwell D.M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 45.Higgins J.P.T., Green S., editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011. http://handbook-5-1.cochrane.org/ (The cochrane collaboration). Accessed June 2021. [Google Scholar]
- 46.Nikolakopoulou A., Higgins J.P.T., Papakonstantinou T., et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4) doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papakonstantinou T., Nikolakopoulou A., Higgins J.P.T., Egger M., Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16(1) doi: 10.1002/cl2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brodie M.J., Lerche H., Gil-Nagel A., et al. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 2010;75(20):1817–1824. doi: 10.1212/WNL.0b013e3181fd6170. [DOI] [PubMed] [Google Scholar]
- 49.French J.A., Abou-Khalil B.W., Leroy R.F., et al. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 2011;76(18):1555–1563. doi: 10.1212/WNL.0b013e3182194bd3. [DOI] [PubMed] [Google Scholar]
- 50.Porter R.J., Partiot A., Sachdeo R., Nohria V., Alves W.M., 205 Study Group Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68(15):1197–1204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- 51.Biton V., Berkovic S.F., Abou-Khalil B., Sperling M.R., Johnson M.E., Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. 2014;55(1):57–66. doi: 10.1111/epi.12433. [DOI] [PubMed] [Google Scholar]
- 52.Ryvlin P., Werhahn K.J., Blaszczyk B., Johnson M.E., Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomized, placebo-controlled trial. Epilepsia. 2014;55(1):47–56. doi: 10.1111/epi.12432. [DOI] [PubMed] [Google Scholar]
- 53.Klein P., Schiemann J., Sperling M.R., et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. 2015;56(12):1890–1898. doi: 10.1111/epi.13212. [DOI] [PubMed] [Google Scholar]
- 54.Van Paesschen W., Hirsch E., Johnson M., Falter U., von Rosenstiel P. Efficacy and tolerability of adjunctive brivaracetam in adults with uncontrolled partial-onset seizures: a phase IIb, randomized, controlled trial. Epilepsia. 2013;54(1):89–97. doi: 10.1111/j.1528-1167.2012.03598.x. [DOI] [PubMed] [Google Scholar]
- 55.French J.A., Costantini C., Brodsky A., von Rosenstiel P., N01193 Study Group Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology. 2010;75(6):519–525. doi: 10.1212/WNL.0b013e3181ec7f7f. [DOI] [PubMed] [Google Scholar]
- 56.Elger C., Halász P., Maia J., Almeida L., Soares-da-Silva P., BIA-2093-301 Investigators Study Group Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures: a randomized, double-blind, placebo-controlled, parallel-group phase III study. Epilepsia. 2009;50(3):454–463. doi: 10.1111/j.1528-1167.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- 57.Gil-Nagel A., Lopes-Lima J., Almeida L., Maia J., Soares-da-Silva P., BIA-2093-303 Investigators Study Group Efficacy and safety of 800 and 1200 mg eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures. Acta Neurol Scand. 2009;120(5):281–287. doi: 10.1111/j.1600-0404.2009.01218.x. [DOI] [PubMed] [Google Scholar]
- 58.Ben-Menachem E., Gabbai A.A., Hufnagel A., Maia J., Almeida L., Soares-da-Silva P. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res. 2010;89(2-3):278–285. doi: 10.1016/j.eplepsyres.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Sperling M.R., Abou-Khalil B., Harvey J., et al. Eslicarbazepine acetate as adjunctive therapy in patients with uncontrolled partial-onset seizures: results of a phase III, double-blind, randomized, placebo-controlled trial. Epilepsia. 2015;56(2):244–253. doi: 10.1111/epi.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jóźwiak S., Veggiotti P., Moreira J., Gama H., Rocha F., Soares-da-Silva P. Effects of adjunctive eslicarbazepine acetate on neurocognitive functioning in children with refractory focal-onset seizures. Epilepsy Behav. 2018;81:1–11. doi: 10.1016/j.yebeh.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 61.Kirkham F., Auvin S., Moreira J., et al. Efficacy and safety of eslicarbazepine acetate as adjunctive therapy for refractory focal-onset seizures in children: a double-blind, randomized, placebo-controlled, parallel-group, multicenter, phase-III clinical trial. Epilepsy Behav. 2020;105 doi: 10.1016/j.yebeh.2020.106962. [DOI] [PubMed] [Google Scholar]
- 62.Elger C., Bialer M., Cramer J.A., Maia J., Almeida L., Soares-da-Silva P. Eslicarbazepine acetate: a double-blind, add-on, placebo-controlled exploratory trial in adult patients with partial-onset seizures. Epilepsia. 2007;48(3):497–504. doi: 10.1111/j.1528-1167.2007.00984.x. [DOI] [PubMed] [Google Scholar]
- 63.Ben-Menachem E., Biton V., Jatuzis D., Abou-Khalil B., Doty P., Rudd G.D. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48(7):1308–1317. doi: 10.1111/j.1528-1167.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 64.Halász P., Kälviäinen R., Mazurkiewicz-Beldzińska M., et al. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50(3):443–453. doi: 10.1111/j.1528-1167.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 65.Chung S., Sperling M.R., Biton V., et al. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia. 2010;51(6):958–967. doi: 10.1111/j.1528-1167.2009.02496.x. [DOI] [PubMed] [Google Scholar]
- 66.Hong Z., Inoue Y., Liao W., et al. Efficacy and safety of adjunctive lacosamide for the treatment of partial-onset seizures in Chinese and Japanese adults: a randomized, double-blind, placebo-controlled study. Epilepsy Res. 2016;127:267–275. doi: 10.1016/j.eplepsyres.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 67.Farkas V., Steinborn B., Flamini J.R., et al. Efficacy and tolerability of adjunctive lacosamide in pediatric patients with focal seizures. Neurology. 2019;93(12):e1212–e1226. doi: 10.1212/WNL.0000000000008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krauss G.L., Serratosa J.M., Villanueva V., et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78(18):1408–1415. doi: 10.1212/WNL.0b013e318254473a. [DOI] [PubMed] [Google Scholar]
- 69.French J.A., Krauss G.L., Biton V., et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79(6):589–596. doi: 10.1212/WNL.0b013e3182635735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.French J.A., Krauss G.L., Steinhoff B.J., et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54(1):117–125. doi: 10.1111/j.1528-1167.2012.03638.x. [DOI] [PubMed] [Google Scholar]
- 71.Nishida T., Lee S.K., Inoue Y., Saeki K., Ishikawa K., Kaneko S. Adjunctive perampanel in partial-onset seizures: Asia-Pacific, randomized phase III study. Acta Neurol Scand. 2018;137(4):392–399. doi: 10.1111/ane.12883. [DOI] [PubMed] [Google Scholar]
- 72.Krauss G.L., Klein P., Brandt C., et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38–48. doi: 10.1016/S1474-4422(19)30399-0. [DOI] [PubMed] [Google Scholar]
- 73.Chung S.S., French J.A., Kowalski J., et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311–e2322. doi: 10.1212/WNL.0000000000009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elger C.E., Stefan H., Mann A., Narurkar M., Sun Y., Perdomo C. A 24-week multicenter, randomized, double-blind, parallel-group, dose-ranging study of rufinamide in adults and adolescents with inadequately controlled partial seizures. Epilepsy Res. 2010;88(2-3):255–263. doi: 10.1016/j.eplepsyres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Biton V., Krauss G., Vasquez-Santana B., et al. A randomized, double-blind, placebo-controlled, parallel-group study of rufinamide as adjunctive therapy for refractory partial-onset seizures. Epilepsia. 2011;52(2):234–242. doi: 10.1111/j.1528-1167.2010.02729.x. [DOI] [PubMed] [Google Scholar]
- 76.Brodie M.J., Rosenfeld W.E., Vazquez B., et al. Rufinamide for the adjunctive treatment of partial seizures in adults and adolescents: a randomized placebo-controlled trial. Epilepsia. 2009;50(8):1899–1909. doi: 10.1111/j.1528-1167.2009.02160.x. [DOI] [PubMed] [Google Scholar]
- 77.Fountain N.B., Van Ness P.C., Bennett A., et al. Quality improvement in neurology: epilepsy update quality measurement set. Neurology. 2015;84(14):1483–1487. doi: 10.1212/WNL.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian N., Boring M., Kobau R., Zack M.M., Croft J.B. Active epilepsy and seizure control in adults - United States, 2013 and 2015. MMWR Morb Mortal Wkly Rep. 2018;67(15):437–442. doi: 10.15585/mmwr.mm6715a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leppik I., De Rue K., Edrich P., Perucca E. Measurement of seizure freedom in adjunctive therapy studies in refractory partial epilepsy: the levetiracetam experience. Epileptic Disord. 2006;8(2):118–130. [PubMed] [Google Scholar]
- 80.Thurman D.J., Logroscino G., Beghi E., et al. The burden of premature mortality of epilepsy in high-income countries: a systematic review from the mortality task force of the international league against epilepsy. Epilepsia. 2017;58(1):17–26. doi: 10.1111/epi.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hesdorffer D.C., Tomson T., Benn E., et al. Combined analysis of risk factors for SUDEP. Epilepsia. 2011;52(6):1150–1159. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]
- 82.Bialer M., Johannessen S.I., Levy R.H., Perucca E., Tomson T., White H.S. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI) Epilepsy Res. 2013;103(1):2–30. doi: 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Fisher R.S. The new classification of seizures by the international league against epilepsy 2017. Curr Neurol Neurosci Rep. 2017;17(6):48. doi: 10.1007/s11910-017-0758-6. [DOI] [PubMed] [Google Scholar]
- 84.Neurology Branch of Chinese Medical Association, Group of EEG and Epilepsy, Neurology Branch of Chinese Medical Association Guidelines for standardized diagnosis and treatment of focal seizures in Chinese adults. Chin J Neurol. 2022;555(12):1341–1352. doi: 10.3760/cma.j.cn113694-20220411-00289. [DOI] [Google Scholar]
- 85.Singh A. Cenobamate for treatment-resistant focal seizures: current evidence and place in therapy. J Cent Nerv Syst Dis. 2022;14 doi: 10.1177/11795735211070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Food and Drug Administration . 2019. XCOPRI prescribing information.https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212839s000lbl.pdf Accessed July 29, 2023. [Google Scholar]
- 87.European Medicines Agency . 2021. ONTOZRY. Summary of product characteristics.https://www.ema.europa.eu/en/documents/product-information/ontozry-epar-productinformation_en.pdf Accessed July 17, 2023. [Google Scholar]
- 88.Laskier V., Agyei-Kyeremateng K.K., Eddy A.E., et al. Cost-effectiveness of cenobamate for focal seizures in people with treatment-resistant epilepsy. Epilepsia. 2023;64(4):843–856. doi: 10.1111/epi.17506. [DOI] [PubMed] [Google Scholar]
- 89.Villanueva V., Serratosa J.M., Toledo M., et al. Number needed to treat and associated cost analysis of cenobamate versus third-generation anti-seizure medications for the treatment of focal-onset seizures in patients with treatment-resistant epilepsy in Spain. Epilepsy Behav. 2023;139 doi: 10.1016/j.yebeh.2022.109054. [DOI] [PubMed] [Google Scholar]
- 90.Rissardo J.P., Fornari Caprara A.L. Cenobamate (YKP3089) and treatment-resistant epilepsy: a review of the literature. Medicina (Kaunas) 2023;59(8):1389. doi: 10.3390/medicina59081389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakamura M., Cho J.H., Shin H., Jang I.S. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur J Pharmacol. 2019;855:175–182. doi: 10.1016/j.ejphar.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 92.Sharma R., Nakamura M., Neupane C., et al. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur J Pharmacol. 2020;879 doi: 10.1016/j.ejphar.2020.173117. [DOI] [PubMed] [Google Scholar]
- 93.Janson M.T., Bainbridge J.L. Continuing burden of refractory epilepsy. Ann Pharmacother. 2021;55(3):406–408. doi: 10.1177/1060028020948056. [DOI] [PubMed] [Google Scholar]
- 94.Gillard M., Fuks B., Leclercq K., Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti-convulsant properties. Eur J Pharmacol. 2011;664(1-3):36–44. doi: 10.1016/j.ejphar.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 95.Kenda B.M., Matagne A.C., Talaga P.E., et al. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem. 2004;47(3):530–549. doi: 10.1021/jm030913e. [DOI] [PubMed] [Google Scholar]
- 96.Zona C., Pieri M., Carunchio I., Curcio L., Klitgaard H., Margineanu D.G. Brivaracetam (ucb 34714) inhibits Na(+) current in rat cortical neurons in culture. Epilepsy Res. 2010;88(1):46–54. doi: 10.1016/j.eplepsyres.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 97.Matagne A., Margineanu D.G., Kenda B., Michel P., Klitgaard H. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154(8):1662–1671. doi: 10.1038/bjp.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rolan P., Sargentini-Maier M.L., Pigeolet E., Stockis A. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after multiple increasing oral doses in healthy men. Br J Clin Pharmacol. 2008;66(1):71–75. doi: 10.1111/j.1365-2125.2008.03158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sargentini-Maier M.L., Rolan P., Connell J., et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males. Br J Clin Pharmacol. 2007;63(6):680–688. doi: 10.1111/j.1365-2125.2006.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sargentini-Maier M.L., Espié P., Coquette A., Stockis A. Pharmacokinetics and metabolism of 14C-brivaracetam, a novel SV2A ligand, in healthy subjects. Drug Metab Dispos. 2008;36(1):36–45. doi: 10.1124/dmd.107.017129. [DOI] [PubMed] [Google Scholar]
- 101.von Rosenstiel P., Perucca E. In: The treatment of epilepsy. 3rd ed. Shorvon S.D., Perucca E., Engel E. Jr., editors. Wiley-Blackwell; Chichester: 2009. Brivaracetam; pp. 447–457. [Google Scholar]
- 102.Kasteleijn-Nolst Trenité D.G., Genton P., Parain D., et al. Evaluation of brivaracetam, a novel SV2A ligand, in the photosensitivity model. Neurology. 2007;69(10):1027–1034. doi: 10.1212/01.wnl.0000271385.85302.55. [DOI] [PubMed] [Google Scholar]
- 103.Wheless J.W., Vazquez B. Rufinamide: a novel broad-spectrum antiepileptic drug. Epilepsy Curr. 2010;10(1):1–6. doi: 10.1111/j.1535-7511.2009.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strom L.A., Koh S., Frey L. New antiepileptic drugs: lacosamide, rufinamide, and vigabatrin. Curr Treat Options Neurol. 2010;12(4):287–299. doi: 10.1007/s11940-010-0079-4. [DOI] [PubMed] [Google Scholar]
- 105.Verrotti A., Loiacono G., Ballone E., Mattei P.A., Chiarelli F., Curatolo P. Efficacy of rufinamide in treatment-resistant epilepsy: a meta-analysis. Pediatr Neurol. 2011;44(5):347–349. doi: 10.1016/j.pediatrneurol.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Perucca E., Cloyd J., Critchley D., Fuseau E. Rufinamide: clinical pharmacokinetics and concentration-response relationships in patients with epilepsy. Epilepsia. 2008;49(7):1123–1141. doi: 10.1111/j.1528-1167.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- 107.Hakimian S., Cheng-Hakimian A., Anderson G.D., Miller J.W. Rufinamide: a new anti-epileptic medication. Expert Opin Pharmacother. 2007;8(12):1931–1940. doi: 10.1517/14656566.8.12.1931. [DOI] [PubMed] [Google Scholar]
- 108.Aldenkamp A.P., Alpherts W.C. The effect of the new antiepileptic drug rufinamide on cognitive functions. Epilepsia. 2006;47(7):1153–1159. doi: 10.1111/j.1528-1167.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 109.Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3(11):663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- 110.Satoh T., Hosokawa M. Structure, function and regulation of carboxylesterases. Chem Biol Interact. 2006;162(3):195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 111.Trinka E., Ben-Menachem E., Kowacs P.A., et al. Efficacy and safety of eslicarbazepine acetate versus controlled-release carbamazepine monotherapy in newly diagnosed epilepsy: a phase III double-blind, randomized, parallel-group, multicenter study. Epilepsia. 2018;59(2):479–491. doi: 10.1111/epi.13993. [DOI] [PubMed] [Google Scholar]
- 112.Ambrósio A.F., Soares-Da-Silva P., Carvalho C.M., Carvalho A.P. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem Res. 2002;27(1-2):121–130. doi: 10.1023/a:1014814924965. [DOI] [PubMed] [Google Scholar]
- 113.Benes J., Parada A., Figueiredo A.A., et al. Anticonvulsant and sodium channel-blocking properties of novel 10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide derivatives. J Med Chem. 1999;42(14):2582–2587. doi: 10.1021/jm980627g. [DOI] [PubMed] [Google Scholar]
- 114.Hainzl D., Parada A., Soares-da-Silva P. Metabolism of two new antiepileptic drugs and their principal metabolites S(+)- and R(-)-10,11-dihydro-10-hydroxy carbamazepine. Epilepsy Res. 2001;44(2-3):197–206. doi: 10.1016/s0920-1211(01)00231-5. [DOI] [PubMed] [Google Scholar]
- 115.Fontes-Ribeiro C., Macedo T., Nunes T., et al. Dosage form proportionality and food effect of the final tablet formulation of eslicarbazepine acetate: randomized, open-label, crossover, single-centre study in healthy volunteers. Drugs R D. 2008;9(6):447–454. doi: 10.2165/0126839-200809060-00007. [DOI] [PubMed] [Google Scholar]
- 116.Maia J., Vaz-da-Silva M., Almeida L., et al. Effect of food on the pharmacokinetic profile of eslicarbazepine acetate (BIA 2-093) Drugs R D. 2005;6(4):201–206. doi: 10.2165/00126839-200506040-00002. [DOI] [PubMed] [Google Scholar]
- 117.Almeida L., Potgieter J.H., Maia J., Potgieter M.A., Mota F., Soares-da-Silva P. Pharmacokinetics of eslicarbazepine acetate in patients with moderate hepatic impairment. Eur J Clin Pharmacol. 2008;64(3):267–273. doi: 10.1007/s00228-007-0414-1. [DOI] [PubMed] [Google Scholar]
- 118.Falcão A., Maia J., Almeida L., Mazur D., Gellert M., Soares-da-Silva P. Effect of gender on the pharmacokinetics of eslicarbazepine acetate (BIA 2-093), a new voltage-gated sodium channel blocker. Biopharm Drug Dispos. 2007;28(5):249–256. doi: 10.1002/bdd.549. [DOI] [PubMed] [Google Scholar]
- 119.Hoy S.M. Lacosamide: a review in focal-onset seizures in patients with epilepsy. CNS Drugs. 2018;32(5):473–484. doi: 10.1007/s40263-018-0523-7. [DOI] [PubMed] [Google Scholar]
- 120.Perucca E., Yasothan U., Clincke G., Kirkpatrick P. Lacosamide. Nat Rev Drug Discov. 2008;7(12):973–974. doi: 10.1038/nrd2764. [DOI] [PubMed] [Google Scholar]
- 121.Cawello W. Clinical pharmacokinetic and pharmacodynamic profile of lacosamide. Clin Pharmacokinet. 2015;54(9):901–914. doi: 10.1007/s40262-015-0276-0. [DOI] [PubMed] [Google Scholar]
- 122.Rogawski M.A., Tofighy A., White H.S., Matagne A., Wolff C. Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res. 2015;110:189–205. doi: 10.1016/j.eplepsyres.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 123.Wilson S.M., Khanna R. Specific binding of lacosamide to collapsin response mediator protein 2 (CRMP2) and direct impairment of its canonical function: implications for the therapeutic potential of lacosamide. Mol Neurobiol. 2015;51(2):599–609. doi: 10.1007/s12035-014-8775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung S.S. Lacosamide: new adjunctive treatment option for partial-onset seizures. Expert Opin Pharmacother. 2010;11(9):1595–1602. doi: 10.1517/14656566.2010.488639. [DOI] [PubMed] [Google Scholar]
- 125.IJff D.M., van Veenendaal T.M., Majoie H.J., de Louw A.J., Jansen J.F., Aldenkamp A.P. Cognitive effects of lacosamide as adjunctive therapy in refractory epilepsy. Acta Neurol Scand. 2015;131(6):347–354. doi: 10.1111/ane.12372. [DOI] [PubMed] [Google Scholar]
- 126.Bang S.R., Ambavade S.D., Jagdale P.G., Adkar P.P., Waghmare A.B., Ambavade P.D. Lacosamide reduces HDAC levels in the brain and improves memory: potential for treatment of Alzheimer's disease. Pharmacol Biochem Behav. 2015;134:65–69. doi: 10.1016/j.pbb.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 127.Beydoun A., D'Souza J., Hebert D., Doty P. Lacosamide: pharmacology, mechanisms of action and pooled efficacy and safety data in partial-onset seizures. Expert Rev Neurother. 2009;9(1):33–42. doi: 10.1586/14737175.9.1.33. [DOI] [PubMed] [Google Scholar]
- 128.Rundfeldt C. The new anticonvulsant retigabine (D-23129) acts as an opener of K+ channels in neuronal cells. Eur J Pharmacol. 1997;336(2-3):243–249. doi: 10.1016/s0014-2999(97)01249-1. [DOI] [PubMed] [Google Scholar]
- 129.Sills G.J., Rundfeldt C., Butler E., Forrest G., Thompson G.G., Brodie M.J. A neurochemical study of the novel antiepileptic drug retigabine in mouse brain. Pharm Res. 2000;42(6):553–557. doi: 10.1006/phrs.2000.0738. [DOI] [PubMed] [Google Scholar]
- 130.Meldrum B.S., Rogawski M.A. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4(1):18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Benarroch E.E. Potassium channels: brief overview and implications in epilepsy. Neurology. 2009;72(7):664–669. doi: 10.1212/01.wnl.0000343739.72081.4e. [DOI] [PubMed] [Google Scholar]
- 132.Blackburn-Munro G., Jensen B.S. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur J Pharmacol. 2003;460(2-3):109–116. doi: 10.1016/s0014-2999(02)02924-2. [DOI] [PubMed] [Google Scholar]
- 133.Korsgaard M.P., Hartz B.P., Brown W.D., Ahring P.K., Strøbaek D., Mirza N.R. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther. 2005;314(1):282–292. doi: 10.1124/jpet.105.083923. [DOI] [PubMed] [Google Scholar]
- 134.Dencker D., Dias R., Pedersen M.L., Husum H. Effect of the new antiepileptic drug retigabine in a rodent model of mania. Epilepsy Behav. 2008;12(1):49–53. doi: 10.1016/j.yebeh.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 135.GlaxoSmithKline POTIGA™ prescribing information. https://www.gsksource.com/gskprm/htdocs/documents/POTIGA-PI-MG.PDF Available at: [revised 2015 [accessed March 4, 2016]]
- 136.Ferron G.M., Paul J., Fruncillo R., et al. Multiple-dose, linear, dose-proportional pharmacokinetics of retigabine in healthy volunteers. J Clin Pharmacol. 2002;42(2):175–182. doi: 10.1177/00912700222011210. [DOI] [PubMed] [Google Scholar]
- 137.Hempel R., Schupke H., McNeilly P.J., et al. Metabolism of retigabine (D-23129), a novel anticonvulsant. Drug Metab Dispos. 1999;27(5):613–622. [PubMed] [Google Scholar]
- 138.Hermann R., Knebel N.G., Niebch G., Richards L., Borlak J., Locher M. Pharmacokinetic interaction between retigabine and lamotrigine in healthy subjects. Eur J Clin Pharmacol. 2003;58(12):795–802. doi: 10.1007/s00228-003-0558-6. [DOI] [PubMed] [Google Scholar]
- 139.Chinvarun Y., Huang C.W., Wu Y., et al. Optimal use of perampanel in asian patients with epilepsy: expert opinion. Ther Clin Risk Manag. 2021;17:739–746. doi: 10.2147/TCRM.S316476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang R., Qiao S., Fang X., et al. Efficacy and tolerability of perampanel as adjunctive therapy in Chinese patients with focal-onset seizures: an observational, prospective study. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.731566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eisai Group . Eisai Europe Ltd; Hatfield: 2015. Fycompa summary of product characteristics. [Google Scholar]
- 142.Eisai Inc . Eisai Inc.; Woodcliff Lake, NJ: 2014. Fycompa US prescribing information. [Google Scholar]
- 143.Tyrlikova I., Brazdil M., Rektor I., Tyrlik M. Perampanel as monotherapy and adjunctive therapy for focal onset seizures, focal to bilateral tonic-clonic seizures and as adjunctive therapy of generalized onset tonic-clonic seizures. Expert Rev Neurother. 2019;19(1):5–16. doi: 10.1080/14737175.2019.1555474. [DOI] [PubMed] [Google Scholar]
- 144.Ledingham D.R., Patsalos P.N. Perampanel: what is its place in the management of partial onset epilepsy? Neurol Ther. 2013;2(1-2):13–24. doi: 10.1007/s40120-013-0012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Trinka E., Lattanzi S., Carpenter K., et al. Exploring the evidence for broad-spectrum effectiveness of perampanel: a systematic review of clinical data in generalised seizures. CNS Drugs. 2021;35(8):821–837. doi: 10.1007/s40263-021-00831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schmidt L.A., Myers J.L., McHugh J.B. Napsin a is differentially expressed in sclerosing hemangiomas of the lung. Arch Pathol Lab Med. 2012;136(12):1580–1584. doi: 10.5858/arpa.2011-0486-OA. [DOI] [PubMed] [Google Scholar]
- 147.Potschka H., Trinka E. Perampanel: does it have broad-spectrum potential? Epilepsia. 2019;60(Suppl 1):22–36. doi: 10.1111/epi.14456. [DOI] [PubMed] [Google Scholar]
- 148.Patsalos P.N. The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel noncompetitive AMPA receptor antagonist. Epilepsia. 2015;56(1):12–27. doi: 10.1111/epi.12865. [DOI] [PubMed] [Google Scholar]
- 149.Gidal B.E., Laurenza A., Hussein Z., et al. Perampanel efficacy and tolerability with enzyme-inducing AEDs in patients with epilepsy. Neurology. 2015;84(19):1972–1980. doi: 10.1212/WNL.0000000000001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gidal B.E., Ferry J., Majid O., Hussein Z. Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia. 2013;54(8):1490–1497. doi: 10.1111/epi.12240. [DOI] [PubMed] [Google Scholar]
- 151.Hanada T., Hashizume Y., Tokuhara N., et al. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 2011;52(7):1331–1340. doi: 10.1111/j.1528-1167.2011.03109.x. [DOI] [PubMed] [Google Scholar]
- 152.Rogawski M.A. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011;11(2):56–63. doi: 10.5698/1535-7511-11.2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Greenland S., Mansournia M.A., Altman D.G. Sparse data bias: a problem hiding in plain sight. BMJ. 2016;352:i1981. doi: 10.1136/bmj.i1981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.