Abstract
Nuclear hormone receptors are ligand-regulated transcription factors that play critical roles in metazoan homeostasis, development, and reproduction. Many nuclear hormone receptors exhibit bimodal transcriptional properties and can either repress or activate the expression of a given target gene. Repression appears to require a physical interaction between a receptor and a corepressor complex containing the SMRT/TRAC or N-CoR/RIP13 polypeptides. We wished to better elucidate the rules governing the association of receptors with corepressors. We report here that different receptors interact with different domains in the SMRT and N-CoR corepressors and that these divergent interactions may therefore contribute to distinct repression phenotypes. Intriguingly, different isoforms of a single nuclear hormone receptor class also differ markedly in their interactions with corepressors, indicative of their nonidentical actions in cellular regulation. Finally, we present evidence that combinatorial interactions between different receptors can, through the formation of heterodimeric receptors, result in novel receptor-corepressor interactions not observed for homomeric receptors.
Small lipophilic hormones regulate many diverse aspects of metazoan physiology by providing crucial signals that govern homeostasis, reproduction, and differentiation. These lipophilic hormones are sensed, in turn, by a family of nuclear hormone receptors that operate as hormone-regulated transcription factors (reviewed in references 3, 6, 26, 31, 36, 37, 39, and 49). Nuclear receptors include the thyroid hormone receptors (T3Rs), retinoic acid receptors (RARs), retinoid X receptors (RXRs), vitamin D3 receptors (VDRs), and peroxisome proliferator-activated receptors (PPARs) (37). Each nuclear hormone receptor binds both to its cognate hormone and to specific DNA sequences (denoted hormone-response elements) and either enhances or inhibits the transcription of adjacent target genes (3, 6, 14, 26, 31, 36, 37, 39, 40). In this fashion, a hormonal signal of extracellular origin is converted into a specific alteration in the pattern of gene expression in the target cell. More than one gene may encode a particular receptor class; for example, vertebrate cells possess two genes that encode T3R isoforms (denoted α and β) and three genes that encode RAR isoforms (denoted α, β, and γ) (3, 6, 26, 31, 36, 37, 39, 40).
Nuclear hormone receptors possess a modular structure comprised of a centrally located DNA-binding domain linked to a more C-terminal hormone-binding domain. Additional receptor domains that serve as sites of interaction with regulatory polypeptides and/or with downstream effectors that help mediate the transcriptional response have been identified (reviewed in reference 24). Nuclear hormone receptors are generally believed to function in cells as protein dimers, although monomers and oligomers may also operate in some contexts (7, 17, 20, 29, 30, 34). Notably, different nuclear receptors can associate to form heterodimers that exhibit nonadditive DNA-binding and transcriptional properties. RXRs appear to be particularly accommodating partners in these interactions and readily form heterodimers with RARs, T3Rs, PPARs, and VDRs (e.g., 20, 26, 29, 36, 37, 39, 49); heterodimer formation between T3Rs and VDRs, T3Rs and RARs, and T3Rs and PPARs may also be of physiological significance (4, 19, 42).
Many nuclear hormone receptors possess bimodal transcriptional properties and are capable of either repressing or activating target gene transcription, depending on the hormone status, the promoter, and the nature of the host cell (1, 2, 5, 13, 41). These alternative outcomes are manifested through the ability of these receptors to physically associate with auxiliary factors, denoted corepressors and coactivators, that help mediate the ultimate transcriptional response (24). We and others have isolated a clade of corepressor proteins (variously denoted SMRT/TRAC and N-CoR/RIP13) (Fig. 1) that appear to be required for transcriptional repression by nuclear hormone receptors and by a variety of nonreceptor transcription factors (10, 11, 15, 16, 22, 23, 25, 28, 33, 35, 38, 40, 45–48, 50, 52, 53). We report here that different receptors interact in markedly distinct ways with the SMRT corepressor. Intriguingly, even the different isoforms of a single receptor class can display dramatically different abilities to recruit corepressors. Furthermore, we demonstrate that receptor heterodimerization can produce novel corepressor interactions not observed in the homomeric context. Our results suggest that transcriptional silencing is a nonadditive combinatorial outcome that appears to be determined by the receptor class, isoform, and dimer partners that bind to a given target promoter.
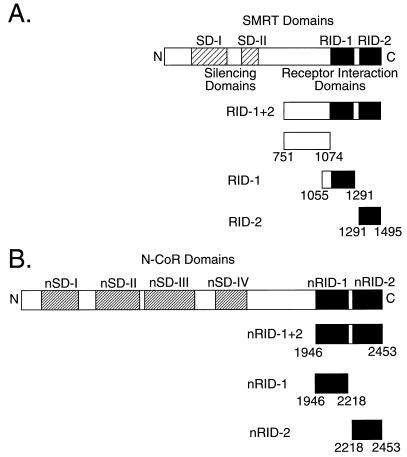
FIG. 1.
Schematic of the SMRT (A) and N-CoR (B) proteins and the fusions used in our studies. The SMRT and N-CoR corepressor proteins are depicted schematically from the N terminus to the C terminus. Indicated are the two SMRT silencing domains (SD-I and SD-II) and the four N-CoR silencing domains (nSD-I to nSD-IV) thought to be involved in transcriptional repression and the two RIDs in each corepressor protein (RID-1 and -2 and nRID-1 and -2) elucidated here. The different SMRT and N-CoR subdomains used in our assays are illustrated below each schematic.
MATERIALS AND METHODS
Molecular clones.
For expression in Escherichia coli, different regions of SMRT were subcloned into a pGEX-KG vector background (21) with appropriate restriction sites to create the glutathione S-transferase (GST) fusions described here. For expression in transiently transfected mammalian cells, different regions of SMRT, N-CoR, or the receptor C-terminal domains were fused with either the Saccharomyces cerevisiae GAL4 DNA-binding domain (GAL4DBD) or the GAL4 activation domain (GAL4AD) and then inserted into a pSG5 expression vector (22, 40). Human RAR (DraIII) chimeras were generated by appropriate cleavage and religation of the α and β isoforms at a shared, unique DraIII site. Human RAR (ClaI/DraIII) chimeras were generated by use of synthetic oligonucleotides to introduce a ClaI site at codon 138 in the RARα reading frame; this site was used in combination with a corresponding ClaI site preexisting in the RARβ sequence to create the appropriate chimeric DNA constructs.
GST fusion proteins and in vitro binding assays.
GST fusion proteins were expressed from the appropriate pGEX-KG recombinant vectors in transformed E. coli DH5α and were purified by immobilization on a glutathione-agarose matrix as previously described (21, 22, 35, 40, 51). Radiolabeled receptors were synthesized by a coupled in vitro transcription-translation protocol (TnT; Promega) and incubated with the immobilized GST fusion proteins (22, 40, 51). After extensive washing, the proteins remaining bound to the GST fusion protein matrix were eluted, resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and visualized and quantified by PhosphorImager analysis (Molecular Dynamics Storm System) (22, 40, 51). Unlabeled T3Rs and RARs were isolated from nuclear extracts of Sf9 cells infected with a corresponding recombinant baculovirus (9).
Transient transfections.
Approximately 2 × 105 CV-1 cells (maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum) were transfected by use of a calcium phosphate coprecipitation technique (8). Mammalian two-hybrid assays typically were done with 500 ng of the pSV-40-Luc reporter plasmid (composed of a simian virus 40 late promoter linked to five GAL4 17-mer DNA-binding sites and driving the expression of luciferase), 125 ng of the pSG5 GAL4DBD construct, 500 ng of the pSG5 GAL4AD construct, and 500 ng of a pCH110 vector (Pharmacia) used as an internal standard (22). Carrier DNA (generally pUC18) was added to bring the total DNA concentration per transfection to 5 μg. Eight hours after transfection, the cells were washed twice and fresh medium containing or lacking a suitable hormone ligand was added. The cells were harvested 40 h later and lysed in 1× lysis buffer (Promega), and the luciferase and β-galactosidase activities were determined (22).
RESULTS
Different nuclear hormone receptors interact with different domains of SMRT.
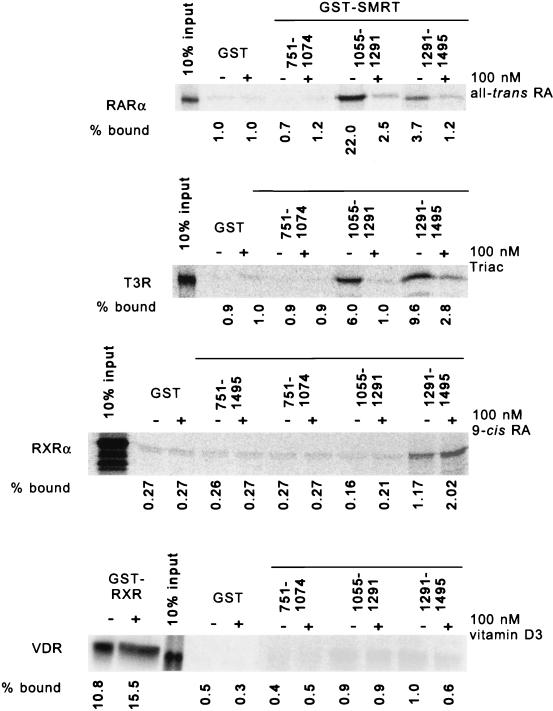
We previously demonstrated that both T3Rs and RARs bind to the C-terminal portion of SMRT; dissection of this phenomenon by yeast two-hybrid analysis (40) suggested that more than one receptor interaction domain (RID) may exist within this SMRT C terminus. To confirm and extend this observation, we used an in vitro binding assay. Defined portions of SMRT were expressed as GST fusions in E. coli (Fig. 1) and were tested for the ability to bind to radiolabeled receptors synthesized by transcription-translation in vitro. Receptor molecules bound to the immobilized GST-SMRT constructs were subsequently eluted and analyzed by SDS-PAGE (Fig. 2).
FIG. 2.
Different receptors preferentially interact with different domains of SMRT in an in vitro assay. Different nuclear hormone receptors were synthesized as radiolabeled proteins by transcription and translation in vitro and were tested for the ability to bind to nonrecombinant GST or to different GST-SMRT fusions immobilized on a glutathione-agarose matrix (as indicated above the panels). The assays were performed in the presence (+) or absence (−) of 100 nM cognate hormone. After the matrix was washed, the receptors remaining bound to the immobilized GST fusion proteins were eluted, resolved by SDS-PAGE, and visualized by PhosphorImager analysis. The amount of a radiolabeled receptor bound to a GST fusion polypeptide was also quantified and is presented numerically below each panel as a percentage of the total radiolabeled receptor (input) used in each binding reaction. The receptors tested were T3Rα, RARα, RXRα, and VDR. The ability of VDR to bind to an immobilized GST-RXR construct was also tested as a positive control (first two lanes of the bottom panel) to confirm the functionality of the in vitro-synthesized receptor. RA, retinoic acid.
Two distinct domains within the SMRT C terminus were able to bind to T3R in vitro; these were denoted RID-1 (SMRT codons 1055 to 1291) and RID-2 (SMRT codons 1291 to 1495) (Fig. 1 and 2). In contrast, little or no binding of T3R was detected with a nonrecombinant GST construct or with GST fusion proteins representing more N-terminal SMRT domains (Fig. 2 and data not shown). Intriguingly, T3R exhibited nearly equal interactions with both RID-1 and RID-2 of SMRT (with a slight preference for RID-2), whereas RAR preferentially interacted with RID-1 (Fig. 2). T3Rs (and RARs) repress transcription primarily in the absence of hormone, whereas the addition of hormone causes release of the corepressor and conversion of the receptor into a transcriptional activator (1, 2, 5, 10, 23, 35, 40, 41, 43). Notably, this hormone-mediated release of the corepressor was observed with either the RID-1 or the RID-2 construct, indicating that the binding of hormone concurrently destabilizes receptor interactions with both RIDs in SMRT (Fig. 2).
Although T3Rs and RARs exemplify the receptors known to function as transcriptional repressors, we wished to determine if SMRT might also participate in the transcriptional functions of other members of the nuclear hormone receptor family. We determined that SMRT also interacted with RXRs in vitro (Fig. 2), consistent with previous observations of an RXR-SMRT interaction by two-hybrid analysis in yeast (40, 45, 46). Although this RXR-SMRT interaction was significantly weaker than that between SMRT and T3R or RAR, it was highly reproducible and clearly above the background observed with nonrecombinant GST or with GST fusions containing the SMRT N terminus (Fig. 2). Unlike RAR or T3R, RXR interacted exclusively with RID-2 of SMRT, and an RXR ligand (9-cis retinoic acid) actually slightly stimulated rather than inhibited the RXR-SMRT interaction (45) (Fig. 2 and data not shown). Extending these experiments to other receptors revealed moderate to strong interactions between PPARγ and the RID-2 region of SMRT, whereas no interaction above the background could be detected between any of our SMRT constructs and VDR or the glucocorticoid receptor in either the presence or the absence of the cognate hormone (Fig. 2 and data not shown).
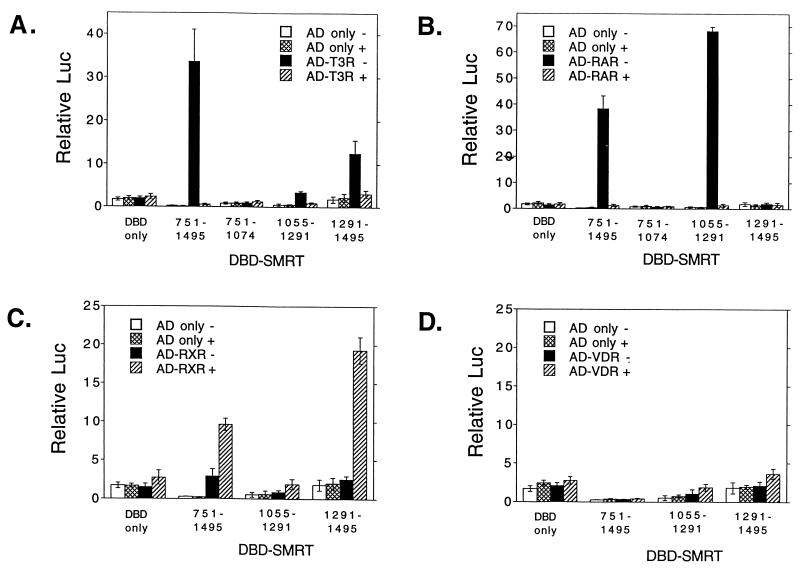
We next used a mammalian two-hybrid assay to determine if the SMRT-nuclear hormone receptor interactions observed in vitro extended to a more physiological context in vivo. For this assay, different portions of SMRT were fused to GAL4DBD and inserted into a mammalian expression vector, pSG5. In parallel, relevant portions of the nuclear hormone receptors were fused to GAL4AD and placed into the same pSG5 vector. In this fashion, interactions between SMRT and the receptors should lead to a functional reconstitution of the GAL4 transcriptional activator, assayed as the stimulation of a GAL4 (17-mer)-luciferase reporter, when all three constructs are cointroduced into mammalian CV-1 cells.
Consistent with our in vitro data, both RARα and T3Rα strongly interacted with SMRT in our mammalian two-hybrid assay, whereas no stimulation of the reporter was observed if either the GAL4DBD-SMRT or the GAL4AD-receptor construct was replaced by an equivalent nonrecombinant GAL4 vector (Fig. 3A and B). RXRα also demonstrated a reproducible interaction with SMRT in the two-hybrid assay, although, again, at a much lower level than T3R or RAR (Fig. 3C; note the change in scale), whereas VDR exhibited no detectable interaction with SMRT in this assay (Fig. 3D). In vivo, as in vitro, T3R interacted with both RID-1 and RID-2 (although it exhibited a preference for RID-2), whereas RAR interacted almost exclusively with RID-1 and RXR interacted almost exclusively with RID-2 (Fig. 3). Also paralleling our in vitro experiments, the two-hybrid interaction between SMRT and RAR or T3R was virtually abolished by the addition of the cognate hormone, whereas the interaction between SMRT and RXR was actually enhanced by 9-cis retinoic acid (Fig. 3). We conclude that the two RIDs in SMRT are nonequivalent in their interactions with different members of the nuclear hormone receptor family and that this nonequivalence can be observed in both in vivo and in vitro assays.
FIG. 3.
Different receptors preferentially interact with different domains of SMRT in a two-hybrid assay in vivo. pSG5 vectors expressing GAL4DBD (DBD) only or GAL4DBD fused with different domains of SMRT (as indicated below each panel) were introduced into CV-1 cells together with a GAL4 (17-mer)-luciferase reporter and a series of pSG5 GAL4AD constructs. The GAL4AD constructs contained GAL4AD only (open or cross-hatched bars) or a GAL4AD-receptor fusion (closed or hatched bars). The cells were incubated in the absence (open or closed bars) or presence (cross-hatched or hatched bars) of cognate hormone; after 48 h, the cells were harvested and luciferase activity was determined relative to that of pCH110, used as an internal control (Relative Luc). The results represent the averages and standard deviations from at least two duplicate experiments. (A) GAL4AD-T3Rα fusion. (B) GAL4AD-RARα fusion. (C) GAL4AD-RXRα fusion. (D) GAL4AD-VDR fusion.
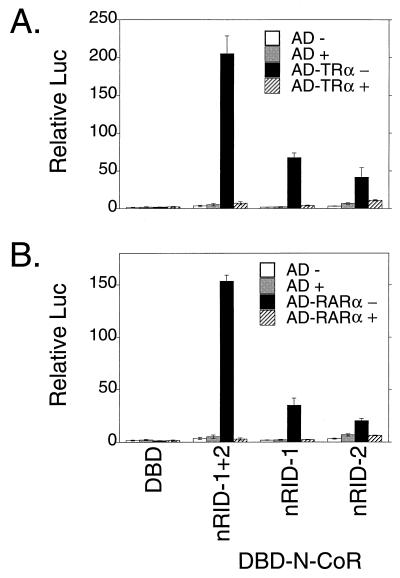
We wished to extend these interaction studies to N-CoR, a second member of the SMRT corepressor family that exhibits approximately 50% amino acid relatedness to SMRT over regions of overlap. T3Rα displayed a pattern of interactions with N-CoR similar to that observed with SMRT, interacting independently with two distinct C-terminal domains of N-CoR, denoted here as nRID-1 and nRID-2, that correspond in general location to RID-1 and RID-2 of SMRT, respectively (compare Fig. 4A and 3A). Intriguingly, RARα also interacted with both nRID-1 and nRID-2 of N-CoR (Fig. 4B). This result is in marked contrast to the strong specificity that RARα exhibited for RID-1 of SMRT (compare Fig. 4B and 3B). It should be noted that the nRID-2 construct used here is 235 amino acids long; the RID-2 SMRT construct is 204 amino acids long. Thus, the precise limits of the N-CoR and SMRT constructs used in these experiments were similar but not identical. Nonetheless, within the limits of our experimental methodology, we conclude that the RIDs of SMRT and N-CoR possess distinguishable, although overlapping, specificities for the different nuclear hormone receptors.
FIG. 4.
Different receptors interact with different domains of N-CoR in a two-hybrid assay in vivo. pSG5 vectors expressing GAL4DBD only (DBD) or GAL4DBD fused with the domains of N-CoR indicated below the panels (corresponding to the schematic in Fig. 1B) were introduced into CV-1 cells together with a GAL4 (17-mer)-luciferase reporter and a series of pSG5 GAL4AD constructs. The GAL4AD constructs contained GAL4AD only (open or grey bars) or a GAL4AD-receptor fusion (black or hatched bars). The cells were incubated in the absence (open or closed bars) or presence (grey or hatched bars) of cognate hormone; after 48 h, the cells were harvested and luciferase activity was determined relative to that of pCH110, used as an internal control (Relative Luc). The results represent the averages and standard deviations from at least two duplicate experiments. (A) GAL4AD-T3Rα fusion. (B) GAL4AD-RARα fusion.
The three different RAR isoforms diverge in their ability to interact with SMRT.
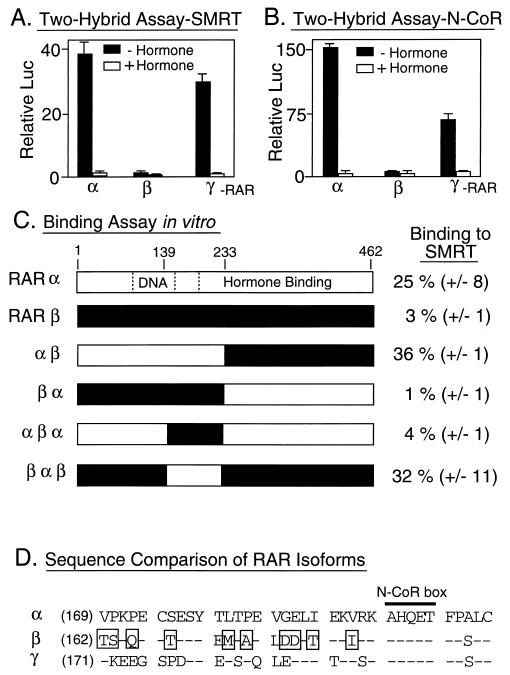
RARs are encoded by three different loci in the mammalian genome, resulting in the synthesis of three major subclasses, or isoforms, of RARs (denoted RARα, RARβ, and RARγ; reviewed in references 6 and 26). Although believed to serve distinct, if partially redundant, functions in vivo, these three different RAR isoforms are virtually indistinguishable in most of their biochemical properties in vitro. Unexpectedly, RARβ exhibited a severely limited ability to interact with SMRT in the mammalian two-hybrid assay compared to either the RARα or the RARγ isoforms (Fig. 5A); notably, all three GAL4AD-RAR isoform fusions were nonetheless expressed and exhibited nearly equal abilities to interact with a GAL4DBD-RXR fusion used in the same assay as a positive control (data not shown). This relative inability of the RARβ isoform to associate with the SMRT corepressor could also be observed in analogous two-hybrid experiments with N-CoR (Fig. 5B) and was also evident in our in vitro binding assay (Fig. 5C). Analysis of chimeras of RARα and RARβ localized the sequences responsible for this isoform-specific SMRT interaction to a small region within the central domain of the receptor, in between the DNA-binding and hormone-binding domains (Fig. 5C). RAR derivatives that possess the α sequence in this region bound to SMRT very strongly in vitro and in vivo, whereas receptor derivatives that contain the equivalent β sequences exhibited a greatly reduced ability to interact with SMRT (Fig. 5C). This region contains a cluster of amino acids that are present in RARα or RARγ but divergent in RARβ and that are likely to account for the different SMRT interaction phenotypes (Fig. 5D). Notably, this isoform-specific amino acid cluster is adjacent to an N-CoR box previously proposed to be necessary for the interaction of the receptor with the corepressor (23).
FIG. 5.
Different RAR isoforms differ in their abilities to interact with SMRT. (A) Interactions of different RAR isoforms with SMRT, as determined with a mammalian two-hybrid assay in vivo. RARα, RARβ, and RARγ were expressed as GAL4AD fusions in CV-1 cells and tested for the ability to interact with GAL4DBD-SMRT (amino acids 751 to 1495) and induce the expression of the GAL4 (17-mer)-luciferase reporter. The cells were incubated in the absence or presence of cognate hormone; after 48 h, the cells were harvested and luciferase activity was determined relative to that of pCH110, used as an internal control (Relative Luc). The results represent the averages and standard deviations from at least two duplicate experiments. (B) Interactions of different RAR isoforms with N-CoR, as determined with a mammalian two-hybrid assay in vivo. The same assay as that in panel A was performed, but with a GAL4DNA–N-CoR construct in place of the GAL4DBD-SMRT construct. (C) Abilities of RARα, RARβ, or RARα-RARβ chimeras to bind to GST-SMRT in vitro. The different receptors, depicted schematically, were synthesized in vitro and tested for their abilities to bind to GST-SMRT (amino acids 751 to 1495) as described in the legend to Fig. 2. The locations of the DNA-binding and hormone-binding domains are indicated within the RARα schematic. The amount of receptor bound to the immobilized GST-SMRT polypeptide, relative to the input amount of receptor, is indicated numerically to the right of each protein schematic. The averages and standard deviations of two or more determinations are presented. (D) Amino acid sequence comparison of the central domains of RARα, RARβ, and RARγ. The amino acid sequences of the central domains of the human RAR isoforms are presented beginning with the amino acid indicated parenthetically to the left of each sequence. Amino acids in RARβ or RARγ that are identical to those in equivalent positions in RARα are depicted by dashes, whereas amino acids in RARβ that are not conserved in either RARα or RARγ are boxed. The location of an N-CoR box is also shown (see the text).
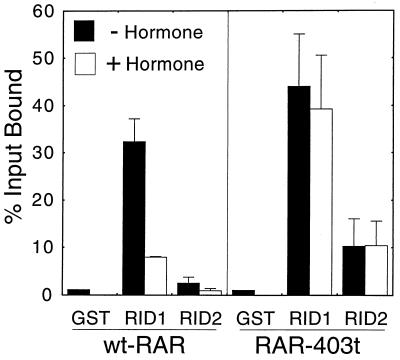
When mapping the determinants of RAR involved in these isoform-specific SMRT interactions, we observed that deletions of the AF-2 domain at the C terminus of either RARα (denoted RARα 403-t) or RARβ resulted in a modestly enhanced interaction of these receptor derivatives with SMRT (Fig. 6 and data not shown). This enhanced interaction appeared to be mediated, at least in part, by an increased ability of the receptor to interact with RID-2 of SMRT; notably, however, the vast majority of the RAR-SMRT interaction remained mediated through RID-1 (Fig. 6). We suggest that there are cryptic interaction sites for SMRT that, within native RARs, are obscured by the extreme C terminus of the receptor. Also, as reported previously, removal of the RAR C-terminal domain renders the receptor-SMRT interaction refractory to hormone (Fig. 6). These results are consistent with proposals that changes in the conformation of the receptor C terminus can regulate the association of the receptor with SMRT (2, 33, 35, 40, 43).
FIG. 6.
Deletion of the RAR C terminus enhances binding to SMRT. Full-length wild-type RARα (wt-RAR) or a C-terminal truncation (RAR-403t) were synthesized as radiolabeled proteins by transcription and translation in vitro and were tested for the ability to bind to nonrecombinant GST, to GST-SMRT RID-1 (SMRT amino acids 1055 to 1291), or to GST-SMRT RID-2 (SMRT amino acids 1291 to 1495) as described in the legend to Fig. 2. The assays were performed in the presence (+) or absence (−) of 1 μM all-trans retinoic acid. After washing was done, the receptors remaining bound to the immobilized GST fusion proteins were eluted, resolved by SDS-PAGE, and visualized and quantified by PhosphorImager analysis. The amount of a radiolabeled receptor bound to a GST fusion polypeptide is presented as a percentage of the total radiolabeled receptor (input) used in each binding reaction. The averages and standard deviations of at least two experiments are presented.
Heterodimer formation by different nuclear hormone receptors can result in novel modes of SMRT interaction.
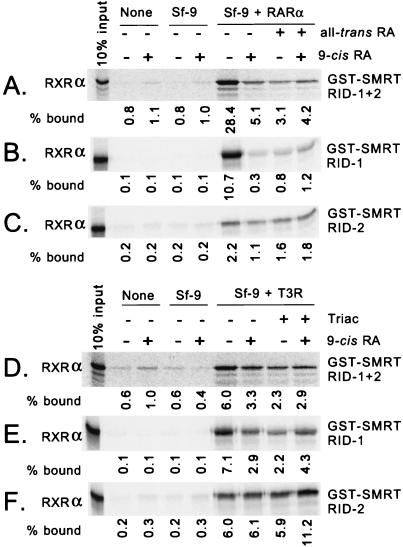
Many nuclear hormone receptors can form heterodimers with other receptors, resulting in novel DNA and hormone recognition properties (6, 12, 18, 26, 27, 31, 32, 36, 37, 39, 42, 44, 49). We examined whether receptor heterodimerization could also be manifested as an altered interaction with SMRT. We first tested RXR heterodimers, given the proposed preeminent role of RXRs as a partner for RARs and T3Rs. Although radiolabeled RXR interacted only weakly with immobilized GST-SMRT, the addition of unlabeled RAR (obtained from recombinant baculovirus-infected Sf9 cell extracts) greatly enhanced the binding of RXR to SMRT: 0.8% of the input RXR bound to SMRT in the absence of RAR, but 28.4% bound in the presence of RAR (Fig. 7A; Sf9 + RARα). In contrast, extracts of Sf9 cells infected with nonrecombinant baculovirus had no effect (Fig. 7A; Sf9). The addition of all-trans retinoic acid greatly reduced the amount of RXR retained by the GST-SMRT matrix, consistent with the participation of the RAR partner in tethering radiolabeled RXR to SMRT. The hormone 9-cis retinoic acid is a high-affinity ligand for RARs as well as for RXRs (6, 36); similar to the effects of all-trans retinoic acid, the addition of 9-cis retinoic acid led to the dissociation of the presumptive RXR-RAR heterodimer from the GST-SMRT matrix.
FIG. 7.
Enhancement of the RXR-SMRT interaction by the addition of RAR or T3R in vitro. Radiolabeled RXRα was synthesized by transcription and translation in vitro and tested for the ability to bind to a GST-SMRT (RID-1 plus RID-2) polypeptide (A and D), a GST-SMRT (RID-1) polypeptide (B and E), or a GST-SMRT (RID-2) polypeptide (C and F), each immobilized on glutathione-agarose. The incubations were performed in the presence (+) or absence (−) of various combinations of hormone, as indicated above each panel, and without further additions (None), in the presence of lysates of Sf9 cells infected with a nonrecombinant baculovirus (Sf-9), or in the presence of lysates of Sf9 cells infected with a baculovirus expressing high levels of either RARα (Sf-9 + RARα) (A to C) or T3Rα (Sf-9 + T3R) (D to F). The amount of radiolabeled RXR bound to a GST fusion polypeptide was visualized and quantified by PhosphorImager analysis and is presented numerically below each panel as a percentage of the total radiolabeled RXR (input) used in each binding reaction.
We also repeated these experiments with constructs of SMRT limited to the individual RID-1 or RID-2 regions (Fig. 7B and C). The ability of RARα to enhance the binding of RXRα to SMRT was also clearly observed with these individual RID constructs, if at a somewhat reduced level compared to the effects seen when SMRT constructs containing both RIDs were used (Fig. 7A). Given the specificity, when tested alone, of RXRα for SMRT RID-2 and of RARα for SMRT RID-1 (Fig. 2), these results suggest that the presumptive RAR-RXR heterodimer can be tethered to SMRT by either receptor moiety in the dimer.
The addition of unlabeled T3Rα was also able to enhance the binding of RXRα to SMRT; approximately 0.6% of input RXR bound to SMRT (RID-1 plus RID-2) in the absence of T3R (Fig. 7D; Sf9), compared to 6.0% in the presence of T3R (Fig. 7D; Sf9 + T3Rα). A similar enhancement was observed when the individual SMRT RIDs were tested separately (Fig. 7E and F). The addition of a T3R ligand, Triac, interfered with this enhancement when either type of SMRT construct (RID-1 plus RID-2 or RID-1 alone) was used, consistent with the participation of T3R in the tethering of radiolabeled RXR to SMRT under these conditions (Fig. 7D and E). In contrast, Triac did not inhibit the binding of the presumptive RXR-T3R heterodimer to the SMRT (RID-2) construct (Fig. 7F), perhaps suggesting that the abstracted SMRT RID-2 interacts primarily with the RXR moiety under these conditions. Intriguingly, 9-cis retinoic acid, which stabilized the interactions of RXR with the corepressor in the absence of T3R (Fig. 3C), inhibited the binding of the RXR-T3R heterodimer to the SMRT (RID-1 plus RID-2) and SMRT (RID-1) constructs. A similar paradoxical effect, suggestive of differences in the effects of hormone ligands on heterodimers versus homodimers, was also observed in our two-hybrid studies (see below).
These heterodimeric interactions could be mimicked in the mammalian two-hybrid assay. As previously noted, the GAL4AD-RXR fusion by itself exhibited only a weak interaction with GAL4DBD-SMRT (RID-1 plus RID-2) in the two-hybrid assay, whereas GAL4AD-RAR exhibited a moderate interaction with GAL4DBD-SMRT (Fig. 8A). The simultaneous introduction of both GAL4AD-RAR and GAL4AD-RXR, however, resulted in a stronger interaction with GAL4DBD-SMRT that was much greater than the sum of the interactions of the two receptor constructs introduced separately (Fig. 8A). An analogous synergistic interaction of GAL4AD-T3R and GAL4AD-RXR with GAL4DBD-SMRT was also observed (Fig. 8B). In vivo as in vitro, the combined interaction of RXR and RAR with SMRT was abolished by RAR ligands (all-trans or 9-cis retinoic acid), whereas the combined interaction of RXR and T3R with SMRT was slightly reduced by the addition of 9-cis retinoic acid and strongly inhibited by the addition of Triac.
FIG. 8.
Combinatorial interactions of receptors with SMRT in a two-hybrid analysis in vivo. A mammalian two-hybrid protocol similar to that in Fig. 3 was used, but with a pSG5 GAL4DBD-SMRT (amino acids 751 to 1495) construct in all cases and with one or more nuclear hormone receptors being introduced simultaneously as GAL4AD fusions. The cells were incubated in the absence or presence of cognate hormones, as indicated below each panel; the cells were harvested after 48 h, and luciferase activity was determined relative to that of pCH110, used as an internal control (Relative Luc). The results represent the averages and standard deviations from at least two duplicate experiments. (A) Introduction of GAL4AD fusions of RXRα, RARα, or both. (B) Introduction of GAL4AD fusions of RXRα, T3Rα, or both. (C) Introduction of GAL4AD fusions of VDR, RXRα, or both. (D) Introduction of GAL4AD fusions of VDR, T3Rα, or both.
Although VDR did not exhibit an autonomous ability to associate with SMRT, vitamin D3 signal transduction in vivo is thought to be primarily mediated by RXR-VDR heterodimers. We therefore tested if RXR-VDR heterodimers displayed novel interactions with SMRT not observed when these receptors were tested individually. Indeed, RXRs and VDRs cointroduced as GAL4AD fusions exhibited a clear and robust interaction with GAL4DBD-SMRT, in contrast to the much weaker, or undetectable, SMRT interaction observed when either of these receptors was introduced individually (Fig. 8C). An analogous enhancement of the abilities of VDR and RXR to interact with SMRT was also observed when these receptors were tested in combination in vitro (data not shown). Intriguingly, the addition of either vitamin D3 or 9-cis retinoic acid destabilized the RXR-VDR two-hybrid interaction with SMRT (Fig. 8C). Thus, it appears that heterodimerization with RXR can enhance an otherwise cryptic ability of VDR to interact with SMRT and that attachment of a hormone ligand to either receptor partner can, at least in vivo, partially disrupt this interaction. In contrast to these RXR heterodimers, the cointroduction of T3R-RAR, T3R-VDR, or T3R-PPAR into the mammalian two-hybrid system yielded largely additive interactions with SMRT, without any indication of a synergistic or combinatorial outcome (e.g., Fig. 8D and data not shown). We conclude that certain receptor heterodimers are capable of conferring a variety of interactions with corepressors that are not observed with the parental receptors tested individually and that the effects of hormones on these interactions differ in the homodimeric and heterodimeric contexts.
DISCUSSION
Nuclear hormone receptors differ in their abilities to interact with the SMRT corepressor.
Nuclear hormone receptors are key signal transducers through which extracellular hormones invoke changes in target cell gene expression. The ability of many of these receptors to not only activate but also repress gene transcription is a crucial component of the repertoire by which the nuclear hormone receptors regulate physiology and development (6, 26, 31, 36, 37, 39, 49). Transcriptional repression by T3Rs and RARs appears to depend on the ability of these receptors to recruit a corepressor complex composed, in part, of the SMRT/TRAC and/or N-CoR/RIP13 corepressor proteins (10, 11, 23, 28, 33, 35, 40, 46, 51, 52, 53). In this study, we have sought to better elucidate the rules governing receptor-SMRT interactions.
Our results indicate that SMRT contains within its C-terminal region at least two subdomains, denoted RID-1 and RID-2, that are independently able to confer physical and functional interactions with a defined subset of the nuclear hormone receptor family. Intriguingly, there is no extensive amino acid relatedness between RID-1 and RID-2, and different receptors display different abilities to interact with these two SMRT subdomains. T3Rα interacts with both SMRT RID-1 and RID-2 in vitro and in two-hybrid assays in vivo in both yeast (40) and mammalian cells. T3Rα also interacts with both of the analogous RIDs of N-CoR; these interaction domains of N-CoR are related but are not identical in sequence to the corresponding interaction domains of SMRT. Perhaps reflecting this nonidentity of the SMRT and N-CoR RIDs, RARα interacts almost exclusively with RID-1 of SMRT but interacts moderately well with both RID-1 and RID-2 of N-CoR. Thus, different receptors make different patterns of contact with the SMRT and N-CoR corepressors, and these distinct patterns of contact may potentially be manifested as differences in transcriptional regulation.
Unexpectedly, not all isoforms within a given receptor family interact equally well with a corepressor; specifically, RARβ interacts very poorly with SMRT and N-CoR, whereas RARα and RARγ interact quite well with both corepressors. These different RAR isoforms are thought to perform distinct functions in development and differentiation (reviewed in reference 6); our determination that they possess distinct corepressor interaction properties suggests at least one biochemical basis for their nonidentical physiological roles. The divergent corepressor association properties of the RARβ isoform map to a small cluster of amino acids within the D domain of the receptor that differ from the equivalent sequences in RARα and RARγ. Our preliminary analysis suggests that changing individual nonconserved amino acids from the RARβ sequence to that of RARα (such as an A175P or a T181I substitution) fails to confer strong corepressor association (50a); apparently more subtle, or multiple, amino acid divergences within this small cluster contribute to the isoform specificity. Notably, this amino acid cluster is proximal to the N-CoR box, a domain previously implicated in corepressor binding by RARs and T3Rs (10, 23, 40). Recently, it was proposed that the N-CoR box may itself play only an indirect role in the receptor-corepressor interaction, perhaps by stabilizing the conformation of the receptor rather than by providing the actual amino acid contacts involved in the binding of the corepressor. Consistent with this view, conservation of the N-CoR box itself is not necessary for corepressor binding; COUP-TF, RXRs, and PPARs, for example, all lack a detectable N-CoR box but, nonetheless, tether SMRT and N-CoR (40, 45–47). However, our own results indicate that, whether by direct or indirect means, the amino acids within and immediately flanking the N-CoR box play a critical role in defining the ability of RARs and T3Rs to associate with corepressors.
Whatever the precise sites of corepressor interaction, it is intriguing that the addition of a cognate hormone destabilizes the association between the corepressor and T3Rs or RARs but not between the corepressor and RXRs. We recently implicated a conformational change in the C termini of these receptors as participating in this hormone-mediated release of the corepressor, and we attributed a divergence between the C termini of RXRs and T3Rs or RARs as being potentially responsible for the different responses of these different receptor classes (35). The results presented here extend this work by demonstrating that excision of the C terminus of RARs can, in fact, further enhance the interaction of these receptors with SMRT, even in the absence of hormone. Thus, the C terminus of these receptors may play a general role in defining the access of the receptor to the corepressor, and changes in the nature and folding of the receptor C terminus, perhaps influenced by different agonists and antagonists, may be important in modulating this phenomenon.
Heterodimer formation can lead to combinatorial effects on corepressor recruitment.
T3Rs, RARs, and VDRs are believed to exist in most cells primarily in the form of heterodimers with RXRs, although homodimers and alternative heterodimeric interactions between these receptors have also been observed and may be of physiological significance (e.g., 4, 19, 36, 42). We therefore examined if heterodimer formation could influence the ability of these different receptor classes to interact with the SMRT corepressor. T3R-RXR and RAR-RXR heterodimers were indeed able to strongly interact with SMRT both in vivo and in vitro, indicating that heterodimer formation with RXRs did not inhibit and in fact may well have enhanced the ability of T3Rs and RARs to recruit the corepressor. This work is consistent with the results of Zamir et al. (52), Zhang et al. (53), and Li et al. (33), demonstrating that N-CoR and SMRT may preferentially bind to receptor dimers rather than receptor monomers. In our experiments, this ability of heterodimer formation to influence corepressor recruitment was particularly evident for the VDRs, which failed to interact detectably with the corepressor when expressed independently but which exhibited a robust interaction with SMRT when coexpressed with RXRs. In contrast, coexpression of T3Rs and RARs or of T3Rs and VDRs yielded no greater interaction with SMRT than did a simple sum of the interactions of individual receptors analyzed independently. We suggest, therefore, that the combinatorial interactions of different receptors may play an important role in determining the transcriptional repression properties of the resulting heterodimer. These combinatorial effects may also help reconcile the apparent contradiction between the observations that RXRs, PPARs, and VDRs can associate with corepressors yet have not been reported to function as transcriptional repressors; it appears likely that these receptors may well be able to confer repression, but only in the correct heterodimer and promoter context.
ACKNOWLEDGMENTS
We sincerely thank P. Chambon, R. Evans, L. Freedman, A. Horlein, M. G. Rosenfeld, and B. Speigelmann for their generosity in providing molecular clones and H.-W. Chen and S. Sande for their help in creating many of the constructs used in these studies.
This work was supported by Public Health Service grant CA53394 from the National Cancer Institute.
REFERENCES
- 1.Baniahmad A, Kohne A C, Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-Erb A oncogene product, and in the retinoic acid receptor. EMBO J. 1992;11:1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baniahmad A, Leng X, Burris T P, Tsai S Y, Tsai M J, O’Malley B W. The τ4 activation domain of the thyroid hormone receptor is required for release of a putative corepressor(s) necessary for transcriptional silencing. Mol Cell Biol. 1995;15:76–86. doi: 10.1128/mcb.15.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–858. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 4.Bogazzi F, Hudson L D, Nikodem V M. A novel heterodimerization partner for thyroid hormone receptor. J Biol Chem. 1994;269:11683–11686. [PubMed] [Google Scholar]
- 5.Casanova J, Helmer E, Selmi-Ruby S, Qi J S, Au-Flieger M, Desai-Yajnik V, Koudinova N, Yarm F, Raaka B M, Samuels H H. Functional evidence for ligand-dependent dissociation of thyroid hormone and retinoid acid receptors from an inhibitory cellular factor. Mol Cell Biol. 1994;14:5756–5765. doi: 10.1128/mcb.14.9.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambon P. The retinoid signaling pathway. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Privalsky M L. Cooperative formation of high order oligomers by retinoid X receptors: an unanticipated mode of DNA recognition. Proc Natl Acad Sci USA. 1995;92:422–426. doi: 10.1073/pnas.92.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H-W, Privalsky M L. The erbA oncogene represses the actions of both retinoid X and retinoid A receptors, but does so by distinct mechanisms. Mol Cell Biol. 1993;13:5970–5980. doi: 10.1128/mcb.13.10.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H W, Smit-McBride Z, Sharif M, Lewis S, Privalsky M L. Nuclear hormone receptors involved in neoplasia: erbA exhibits a novel DNA sequence specificity determined by amino acids outside of the zinc-finger domain. Mol Cell Biol. 1993;13:2366–2376. doi: 10.1128/mcb.13.4.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J D, Evans R M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 11.Chen J D, Umesono K, Evans R M. SMRT isoforms mediate repression and anti-repression of nuclear receptor heterodimers. Proc Natl Acad Sci USA. 1996;93:7567–7571. doi: 10.1073/pnas.93.15.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claret F-X, Antakly T, Karin M, Saatcioglu F. A shift in the ligand responsiveness of thyroid hormone receptor α induced by heterodimerization with retinoid X receptor α. Mol Cell Biol. 1996;16:219–227. doi: 10.1128/mcb.16.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damm K, Thompson C C, Evans R M. Protein encoded by v-Erb A functions as a thyroid hormone receptor antagonist. Nature. 1989;339:593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- 14.Damm K, Heyman R A, Umesono K, Evans R M. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc Natl Acad Sci USA. 1993;90:2989–2993. doi: 10.1073/pnas.90.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhordain P, Albagli O, Lin R J, Ansieau S, Quief S, Leutz A, Kerckaert J P, Evans R M, Leprince D. Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL-6 oncoprotein. Proc Natl Acad Sci USA. 1997;94:10762–10767. doi: 10.1073/pnas.94.20.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downes M, Burke L J, Bailey P J, Muscat G E. Two receptor interaction domains in the corepressor, N-CoR/RIP13, are required for an efficient interaction with Rev-erbA alpha and RVR: physical association is dependent on the E region of the orphan receptors. Nucleic Acids Res. 1996;2:4379–4386. doi: 10.1093/nar/24.22.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman B M, Samuels H H. Dimerization among nuclear hormone receptors. New Biol. 1990;2:587–594. [PubMed] [Google Scholar]
- 18.Forman B M, Umesono K, Chen J, Evans R M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 19.Glass C K, Lipkin S M, Devary O V, Rosenfeld M G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989;59:697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- 20.Glass C K. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocrine Rev. 1994;15:391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- 21.Guan K L, Dixon J E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 22.Hong S-H, David G, Wong C W, Dejean A, Privalsky M L. SMRT corepressor interacts with PLZF, and with the PML-RARα and PLZF-RARα oncoproteins associated with acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:9028–9033. doi: 10.1073/pnas.94.17.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horlein A J, Naar A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamel Y, Soderstrom M, Glass C K, Rosenfeld M G. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz K B, Jackson T A, Bain D L, Richer J K, Takimoto G S, Tung L. Nuclear hormone receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 25.Jackson T A, Richer J K, Bain D L, Takimoto G S, Tung L, Horwitz K B. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Mol Endocrinol. 1997;11:693–705. doi: 10.1210/mend.11.6.0004. [DOI] [PubMed] [Google Scholar]
- 26.Kastner P, Mark M, Chambon P. Nonsteroidal nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–870. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 27.Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld M G, Heyman R A, Glass C K. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994;371:528–531. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- 28.Kurokawa R, Soderstrom M, Horlein A, Halachmi S, Brown M, Rosenfeld M G, Glass C K. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 29.Laudet V, Stehelin D. Flexible friends. Curr Biol. 1992;2:293–295. [Google Scholar]
- 30.Laudet V, Adelmant G. Nuclear receptors: lonesome orphans. Curr Biol. 1995;5:124–127. doi: 10.1016/s0960-9822(95)00031-5. [DOI] [PubMed] [Google Scholar]
- 31.Lazar M A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocrinol Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 32.Leng X, Tsai S Y, O’Malley B W, Tsai M J. Ligand-dependent conformational changes in thyroid hormone and retinoic acid receptors are potentially enhanced by heterodimerization with retinoic X receptor. J Steroid Biochem Mol Biol. 1993;46:643–661. doi: 10.1016/0960-0760(93)90306-h. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Leo C, Schroen D J, Chen J D. Characterization of receptor interaction and transcriptional repression by the corepressor SMRT. Mol Endocrinol. 1997;11:2025–2037. doi: 10.1210/mend.11.13.0028. [DOI] [PubMed] [Google Scholar]
- 34.Lin B C, Wong C-W, Chen H-W, Privalsky M L. Plasticity of tetramer formation by retinoid X receptors: an alternative paradigm for DNA recognition. J Biol Chem. 1997;272:9860–9867. doi: 10.1074/jbc.272.15.9860. [DOI] [PubMed] [Google Scholar]
- 35.Lin B C, Hong S-H, Krig S, Yoh S M, Privalsky M L. A conformational switch in nuclear hormone receptors is involved in coupling hormone binding to corepressor release. Mol Cell Biol. 1997;17:6131–6138. doi: 10.1128/mcb.17.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangelsdorf D J, Evans R M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 37.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Overview: the nuclear receptor superfamily: the second decade. Cell. 1995;83:835–840. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pazin M J, Kadonaga J T. What’s up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro R C, Apriletti J W, West B L, Wagner R L, Fletterick R J, Schaufele F, Baxter J D. The molecular biology of thyroid hormone action. Ann N Y Acad Sci. 1993;758:366–389. doi: 10.1111/j.1749-6632.1995.tb24843.x. [DOI] [PubMed] [Google Scholar]
- 40.Sande S, Privalsky M L. Identification of TRACs (T3 receptor-associating cofactors), a family of cofactors that associate with, and modulate the activity of, nuclear hormone receptors. Mol Endocrinol. 1996;10:813–825. doi: 10.1210/mend.10.7.8813722. [DOI] [PubMed] [Google Scholar]
- 41.Sap J, Munoz A, Schmitt H, Stunnenberg H, Vennstrom B. Repression of transcription mediated by a thyroid hormone response element by the v-Erb A oncogene product. Nature. 1989;340:242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- 42.Schrader M, Muller K M, Nayeri S, Kahlen J-P, Carlberg C. Vitamin D3-thyroid hormone receptor heterodimer polarity directs ligand sensitivity of transactivation. Nature. 1994;370:382–386. doi: 10.1038/370382a0. [DOI] [PubMed] [Google Scholar]
- 43.Schulman I G, Juguilon H, Evans R M. Activation and repression by nuclear hormone receptors: hormone modulates an equilibrium between active and repressive states. Mol Cell Biol. 1996;16:3807–3813. doi: 10.1128/mcb.16.7.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulman I G, Li C, Schwabe J W R, Evans R M. The phantom ligand effect: allosteric control of transcription by the retinoid X receptor. Genes Dev. 1997;11:299–308. doi: 10.1101/gad.11.3.299. [DOI] [PubMed] [Google Scholar]
- 45.Seol W, Choi H S, Moore D D. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 46.Seol W, Mahon M J, Lee Y K, Moore D D. Two receptor interacting domains in the nuclear hormone receptor corepressor RIP13/N-CoR. Mol Endocrinol. 1996;10:1646–1655. doi: 10.1210/mend.10.12.8961273. [DOI] [PubMed] [Google Scholar]
- 47.Shibata H, Nawaz Z, Tsai S Y, O’Malley B W. Gene silencing by COUP-TF is mediated by transcriptional corepressors, N-CoR and SMRT. Mol Endocrinol. 1997;11:714–724. doi: 10.1210/mend.11.6.0002. [DOI] [PubMed] [Google Scholar]
- 48.Smith C L, Nawaz Z, O’Malley B W. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 49.Tsai M J, O’Malley B W. Molecular mechanisms of action of steroid/thyroid hormone receptor superfamily members. Annu Rev Biochem. 1994;63:451–483. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 50.Wolffe A P. Sinful repression. Nature. 1997;387:16–17. doi: 10.1038/387016a0. [DOI] [PubMed] [Google Scholar]
- 50a.Wong, C.-W., and M. L. Privalsky. Unpublished observations.
- 51.Yoh S M, Chatterjee V K K, Privalsky M L. Thyroid hormone resistance syndrome manifests as an aberrant interaction between mutant T3 receptors and transcriptional corepressors. Mol Endocrinol. 1997;11:470–480. doi: 10.1210/mend.11.4.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zamir I, Zhang J, Lazar M A. Stoichiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 1997;11:835–846. doi: 10.1101/gad.11.7.835. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Zamir I, Lazar M A. Differential recognition of liganded and unliganded thyroid hormone receptor by retinoid X receptor-regulated transcriptional repression. Mol Cell Biol. 1997;17:6887–6897. doi: 10.1128/mcb.17.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]