Abstract
Patients with advanced pancreatic cancer (PC) need a cost-effective treatment regimen. The present study was designed to compare the efficacy and safety of nab-paclitaxel plus S-1 (AS) and gemcitabine plus S-1 (GS) regimens in patients with chemotherapy-naïve advanced PC. In this open-label, multicenter, randomized study named AvGmPC, eligible patients with chemotherapy-naïve advanced PC were randomly assigned (1:1) to receive AS (125 mg/m2 nab-paclitaxel, days 1 and 8; 80–120 mg S-1, days 1–14) or GS (1,000 mg/m2 gemcitabine, days 1 and 8; 80–120 mg S-1, days 1–14). The treatment was administered every 3 weeks until intolerable toxicity or disease progression occurred. The primary endpoint was progression-free survival (PFS). Between December 2018 and March 2022, 101 of 106 randomized patients were treated and evaluated for analysis (AS, n=49; GS, n=52). As of the data cutoff, the median follow-up time was 11.37 months [95% confidence interval (CI), 9.31–13.24]. The median PFS was 7.16 months (95% CI, 5.19–12.32) for patients treated with AS and 6.41 months (95% CI, 3.72–8.84) for patients treated with GS (HR=0.78; 95% CI, 0.51–1.21; P=0.264). The AS regimen showed a slightly improved overall survival (OS; 13.27 vs. 10.64 months) and a significantly improved ORR (44.90 vs. 15.38%; P=0.001) compared with the GS regimen. In the subgroup analyses, PFS and OS benefits were observed in patients treated with the AS regimen who had KRAS gene mutations and high C-reactive protein (CRP) levels (≥5 mg/l). The most common grade ≥3 adverse events were neutropenia, anemia and alopecia in the two groups. Thrombocytopenia occurred more frequently in the GS group than in the AS group. While the study did not meet the primary endpoint, the response benefit observed for AS may be suggestive of meaningful clinical activity in this population. In particular, promising survival benefits were observed in the subsets of patients with KRAS gene mutations and high CRP levels, which is encouraging and warrants further investigation. This trial was retrospectively registered as ChiCTR1900024588 on July 18, 2019.
Keywords: nab-paclitaxel, gemcitabine, S-1, pancreatic cancer, first-line chemotherapy
Introduction
Pancreatic cancer (PC) is among the most lethal malignancies and its global mortality and incidence rates have been rising continuously, with the lowest 5-year survival rate among all cancers (9%) (1–3). In particular, recent cancer statistics indicate that China is faced with a rising burden of cases of PC (4), which merits attention. Surgery with adjuvant chemotherapy is the only method of curing this disease, but ~80% of patients are diagnosed with unresectable or metastatic PC (5). Therefore, chemotherapy remains the mainstay for advanced PC (6).
According to current guidelines of the National Comprehensive Cancer Network, first-line regimens for advanced PC include a combination of fluorouracil + leucovorin + irinotecan + oxaliplatin (FOLFIRINOX), and nanoparticle albumin-bound paclitaxel (nab-paclitaxel) plus gemcitabine (AG) (7,8). Although FOLFIRINOX and AG regimens demonstrated significant survival advantages in clinical trials, the objective response rate (ORR) was limited to 23–31.6% (9,10). In addition, a high level of toxicity was reported among patients receiving FOLFIRINOX, resulting in it being necessary to use rigorous patient selection criteria and a low dose intensity of each agent. In China, AG was considered to be a more practical and convenient regimen than FOLFIRINOX (11). However, nab-paclitaxel was not covered for use in PC by health insurance policies in China when the present study was designed in 2018; therefore, the use of the AG regimen was limited in Chinese clinical practice at that time.
Numerous attempts have been made to develop high-efficacy chemotherapeutic regimens to improve the prognosis of patients with advanced PC (12–14). S-1 is a fourth-generation oral fluoropyrimidine, consisting of tegafur, gimeracil, and oteracil potassium. Due to S-1 being convenient to administer and highly effective, S-1 has been studied as a monotherapy in the MPACA-3 and ASPAC-01 trials, and as a combination therapy in the GEST trial (14–16). Two phase II trials of S-1 monotherapy reported promising results with an ORR of 21.1–37.5% and overall survival (OS) of 5.6–9.2 months (14,17). Subsequently, large-scale clinical trials showed that the ORR of gemcitabine plus S-1 (GS) was 44–48%, with a median OS of 10–12 months in patients with metastatic PC (18,19). Notably, a combination of gemcitabine with a fluoropyrimidine, such as S-1 or capecitabine, was affordable and more widely used than the AG regimen in China in 2018, particularly for patients with a poor economic status. This regimen was also recommended as a first-line treatment for advanced PC in Chinese guidelines since 2018 (20). Although it exhibited a good safety profile, patient survival was still not adequate (21). Accordingly, alternative agents were sought to improve patient survival.
Nab-paclitaxel was developed as a solvent-free paclitaxel formulation that eliminates the risk of hypersensitivity reactions (22). Owing to its promising activity, nab-paclitaxel has been approved as a therapeutic option for various cancers, including breast (23), non-small cell lung cancer (24) and PC (10). Given that nab-paclitaxel and S-1 in combination with gemcitabine were found to significantly improve outcomes (25), efforts were made to develop a novel combination of nab-paclitaxel and S-1 (AS). This combination was reported to exhibit a synergetic effect with good tolerability in preclinical models of PC (26,27). Subsequently, two single-arm phase II trials of AS were performed in China, which reported noteworthy response rates of 50.0–53.1% and an OS of 9.4–13.6 months in patients with advanced PC (28,29). Additionally, the efficacy and safety of this treatment combination were established in advanced breast cancer and gastric cancer (30,31). However, the use of nab-paclitaxel increases the medical expenses of patients with PC. At present, there is no direct evidence regarding the comparable efficacy of AS and GS in advanced PC. In view of the potential favorable survival benefits and controllable safety profiles of the AS regimen, the present prospective study was designed to compare the efficacy and safety of AS with that of GS as first-line chemotherapy in patients with advanced PC. If the more expensive treatment provides no increase in survival, this treatment may be considered to be limited in value.
Materials and methods
Study design and participants
The present study (AvGmPC) was an open-label, multicenter, prospective, randomized clinical study conducted across three centers (Zhongshan Hospital affiliated to Fudan University, Huashan Hospital affiliated to Fudan University, and Ruijin Hospital affiliated to Shanghai Jiaotong University; all Shanghai, China). Eligible patients were aged between 18 and 75 years with histologically or cytologically confirmed unresectable locally advanced or metastatic PC; tumor staging was reported using the eighth edition of the American Joint Committee on Cancer staging system for PC (32). Unresectable tumors were initially identified using imaging techniques and subsequently confirmed by discussion among the multidisciplinary team responsible for pancreatic care. Additional eligibility criteria included: No prior history of antitumor therapy including radiotherapy and chemotherapy; at least one measurable lesion according to Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST 1.1) (33); Eastern Cooperative Oncology Group (ECOG) performance status 0–1; adequate hematologic function, as indicated by an absolute neutrophil count ≥1.5×109/l, a platelet count ≥100×109/l and a hemoglobin level ≥100 g/l); adequate hepatic function, as indicated by bilirubin ≤1.5 upper limit of the normal (ULN), alanine aminotransferase and aspartate aminotransferase ≤2.5 ULN; and adequate renal function (serum creatinine ≤1 ULN). Patients with recurrent diseases after surgery were eligible for enrolment. However, patients who had received other investigational drugs within 4 weeks prior to study initiation were ineligible for inclusion in the present trial. Exclusion criteria also comprised: Other malignancies within 5 years, with the exception of cured cervical carcinoma or skin basal cell carcinoma; uncontrolled brain metastases; congestive heart failure (New York Heart Association class ≥II); peripheral nerve injury (Sunderland grade ≥II); allergy or intolerance to study drugs; severe systemic infection or concomitant diseases; and pregnant or lactating women.
The study protocol was approved by the Ethics Committees of Zhongshan Hospital affiliated to Fudan University (approval no. B2018-260), Huashan Hospital affiliated to Fudan University [approval no. 2019 (001)] and Ruijin Hospital affiliated to Shanghai Jiaotong University [approval no. 2019 (143)]. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient before enrolment. The study was retrospectively registered with the Chinese Clinical Trial Registry as ChiCTR1900024588 on July 18, 2019.
Patients were enrolled between December 19, 2018 and March 8, 2022. The database was closed for final analysis on August 15, 2022.
Randomization and masking
Patients were enrolled by the investigators and randomly assigned in a 1:1 ratio to receive either AS or GS, using a central computerized dynamic hierarchical randomization system. Randomization was stratified according to PC status (locally advanced vs. metastatic) and baseline CA 19-9 levels (<500 vs. ≥500 U/ml). Patients and investigators were not masked to study treatment in this open-label trial.
Treatment
Patients randomized to the AS group received nab-paclitaxel intravenously (125 mg/m2 on days 1 and 8) and oral S-1 twice daily on days 1-14 at a dose calculated according to the body surface area (BSA) of the patient (80 mg for BSA <1.25 m2, 100 mg for BSA 1.25–1.5 m2, 120 mg for BSA ≥1.5 m2) every 3 weeks. Patients assigned to the GS group received gemcitabine intravenously (1,000 mg/m2 on days 1 and 8) and S-1 at the same doses as those in the AS group every 3 weeks. Treatment continued until the occurrence of progressive disease (PD) as evaluated by the investigators according to RECIST 1.1, any intolerable adverse events (AEs), or at the discretion of the investigators or patients. Crossover within the two groups was permitted in the event of disease progression.
Toxicity was managed with dosing interruption, dose reduction or supportive care. Dosing interruption was performed according to protocol guidelines; specifically, the treatment cycle was delayed until non-hematological (≤ grade 1) and hematological toxicities were resolved, and an absolute neutrophil count (ANC) ≥1.5×109/l and platelet count (PLT) ≥7×109/l were achieved. Dose reduction was considered if one of the following events occurred: i) ANC <0.5×109/l; ii) three consecutive occurrences of grade 2 ANC reductions (1.0×109/l < ANC <1.5×109/l); iii) febrile neutropenia; iv) 0.5×109/l < ANC <1.0×109/l and 25×109/l< PLT <50×109/l; v) PLT <25×109/l; vi) ≥ grade 2 peripheral neuropathy or gastrointestinal toxicity. Dose re-escalation was not allowed after dose reduction.
Assessments
Tumor response was assessed by two independent oncologists according to RECIST 1.1 guidelines with computed tomography (CT) or magnetic resonance imaging at baseline, then at every 6 weeks from the start of the first 3-week cycle, until disease progression or discontinuation of the treatment protocol. Patients were followed up for survival until death or study closure. Safety assessments were performed by investigators at every clinical visit in accordance with the study protocol. AEs were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0) (34). Physical examinations and routine laboratory tests, including hematology, liver and kidney function, CA199 and CA125 tests, were performed prior to each cycle of chemotherapy.
Outcomes
The primary endpoint was progression-free survival (PFS), defined as the time between the initiation of treatment and the observation of disease progression or death due to any cause. Secondary endpoints included: OS, defined as the time between the initiation of treatment and the occurrence of death from any cause; ORR, defined as the proportion of patients achieving a complete response (CR) or partial response (PR); disease control rate (DCR); 12- and 24-week PFS rates, defined as the percentage of patients who did not have a PD or had died by week 12 or 24, respectively; 12- and 24-week OS rates, defined as the percentage of patients who had died by week 12 or 24, respectively; and safety.
Statistical analysis
Based on the results of a previous study (35), it was presumed that the median PFS of AS and GS groups would be 7.1 and 3.6 months, respectively. Assuming an enrolment period of 24 months and a follow-up period of 12 months, the PFS was tested by a log-rank test with a significance level of 5% (two-sided) and 80% power; after adjustment for a dropout rate of 15%, 106 patients were enrolled in the study.
Efficacy was assessed in the modified intent-to-treat (mITT) population, which included all participants randomly assigned to treatment who received at least one dose of the assigned trial treatment. Safety was evaluated in the safety analysis set, which consisted of all patients who had at least one dose of assigned trial treatment. The Kaplan-Meier method was used to analyze the PFS and OS, with medians and corresponding 95% confidence intervals (CIs). Data for patients who were alive and without disease progression or who were lost to follow-up were censored for the analysis of PFS at the time of the last imaging assessment. Patients who received other anticancer therapies, such as radiotherapy, without disease progression were recorded as censored. Data for patients who were alive or lost to follow-up were censored for OS at the time they were last known to be alive. For post hoc analyses of the interactions between the treatment and subgroup, the hazard ratio (HR) with two-sided 95% CIs was estimated using the Cox proportional hazards regression model in the pre-planned subgroups. ORR, DCR and safety were compared between the two treatment groups using the Chi-square or Fisher's exact test. The same stratification factors used in randomization were used for all stratified analyses. Statistical analyses were conducted using IBM SPSS software (version 25; IBM Corp.). P≤0.05 was considered to indicate a statistically significant result.
Results
Baseline characteristics
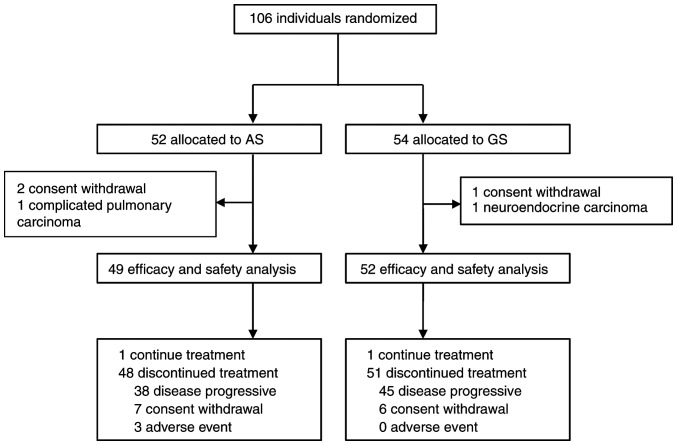
A total of 106 patients from 3 medical centers were enrolled in the study, of which 52 patients were assigned to the AS group and 54 patients were assigned to the GS group. However, 5 of these patients were excluded before treatment initiation due to neuroendocrine carcinoma (n=1), complicated pulmonary carcinoma (n=1) and withdrawal before treatment (n=3). Thus, 49 (94.23%) of 52 patients assigned to the AS group and 52 (96.29%) of 54 patients in the GS group received at least one dose of the study treatment, and were included in the mITT population. A flow chart of patient enrolment and the study design is presented in Fig. 1. An ECOG PS score of 1 was reported in 97.96% of the patients and 65.30% had one or more metastasis sites in the AS group. Baseline demographics and clinical characteristics were well-balanced between the groups (Table I). The median age was 63 (interquartile range (IQR) 36–75) years in the AS group and 62 (IQR 39–73) years in the GS group. Most patients were male in the AS (61.22%) and GS (75.00%) groups.
Figure 1.
Profile of the trial. AS, nab-paclitaxel plus S-1; GS, gemcitabine plus S-1.
Table I.
Patient demographic characteristics at baseline.
| Characteristic | AS group (n=49) | GS group (n=52) |
|---|---|---|
| Age, years | ||
| Median (IQR) | 63 (36–75) | 62 (39–73) |
| <65, n (%) | 30 (61.22) | 36 (69.23) |
| ≥65, n (%) | 19 (38.78) | 16 (30.77) |
| Sex, n (%) | ||
| Male | 30 (61.22) | 39 (75.00) |
| Female | 19 (38.78) | 13 (25.00) |
| ECOG PS, n (%) | ||
| 0 | 1 (2.04) | 1 (1.92) |
| 1 | 48 (97.96) | 51(98.08) |
| CA199a, U/ml, median (range) | 345.00 (2.00–10,000.00) | 276.00 (2.00–10,000.00) |
| CA125b, U/ml, median (range) | 35.50 (7.20–3,505.00) | 37.45 (9.00–852.00) |
| Location, n (%) | ||
| Pancreatic head | 26 (53.06) | 22 (42.31) |
| Pancreatic body | 6 (12.24) | 12 (23.08) |
| Pancreatic tail | 8 (16.33) | 9 (17.31) |
| Accumulated multiple sites | 9 (18.37) | 9 (17.31) |
| Stages, n (%) | ||
| III | 17 (34.69) | 20 (38.46) |
| IV | 32 (65.31) | 32 (61.54) |
| Metastasis sites, n (%) | ||
| 0 | 17 (34.69) | 20 (38.46) |
| 1 | 20 (40.81) | 22 (42.31) |
| 2 | 7 (14.29) | 7 (13.46) |
| 3 | 4 (8.16) | 2 (3.85) |
| 4 | 1 (2.04) | 1 (1.92) |
Normal range, 0–40 U/ml;
normal range, 0–35 U/ml. ECOG PS scores: 0, normal activity; 1, symptoms but the patient is nearly fully ambulatory. Stages: III, regional lymph node metastasis without distant metastasis; IV, distant metastasis. AS, nab-paclitaxel plus S-1; GS, gemcitabine plus nab-paclitaxel; IQR, interquartile range; ECOG PS, Eastern Cooperative Oncology Group performance status.
Treatment and subsequent therapy
Of the 106 enrolled patients, 49 in the AS group and 52 in the GS group received at least one dose of the assigned combination therapy. At the time of the data cutoff in the mITT population (August 15, 2022), one patient in each group was still receiving the assigned treatment. The median number of treatment cycles was 7.00 (IQR 2.50–11.00) for patients in the AS group and 6.50 (IQR 3.25–12.75) for those in the GS group (Table SI). There were 20 patients (14 in AS group vs. 6 in the GS group) who had not progressed after >6 cycles of combination therapy and were unable to tolerate intensive regimens who chose to undergo maintenance with S-1, nab-paclitaxel or gemcitabine monotherapy as decided by the investigators. As the data cut-off, 99 (98.02%) patients had discontinued the protocol therapy early (AS, n=48 vs. GS, n=51) due to AEs (AS, n=3 vs. GS, n=0), disease progression (AS, n=38 vs. GS, n=45) and patient choice (AS, n=7 vs. GS, n=6; Fig. 1). The relative dose intensities for nab-paclitaxel and S-1 in the AS regimen and for gemcitabine and S-1 in the GS regimen were 92.30, 93.10, 95.90 and 97.20%, respectively.
Second-line and third-line treatments were respectively administered to 29 (59.18%) and 8 (16.33%) patients assigned to the AS group, and 34 (65.38%) and 7 (13.46%) patients assigned to the GS group (Table SII). After the discontinuation of their assigned treatment, the 29 patients in the AS group who received subsequent therapies with second-line regimens were mostly treated with gemcitabine-based and radiotherapy-based regimens. By contrast, the 34 patients in the GS group selected mostly nab-paclitaxel-based regimens and irinotecan/oxaliplatin-based regimens as subsequent therapies.
Efficacy
At the data cut-off, the median follow-up period was 11.37 months (95% CI, 9.31–13.24), with 83 PFS events observed [AS, n=38 (77.55%); GS, n=45 (86.54%)]. At the final analysis, the median PFS was 7.16 months (95% CI, 5.19–12.32) in the AS group as compared with 6.41 months (95% CI, 3.72–8.84) in the GS group, with an HR of 0.78 (95% CI, 0.51–1.21, P=0.264; Fig. 2A). The 12-week PFS rates were 87.29 and 78.85% in the AS and GS groups (P=0.233), respectively. With regard to 24-week PFS rates, similar results were also observed for the AS and GS groups (59.65 vs. 57.41%, respectively; P=0.638). A post hoc subgroup analysis of PFS based on patient characteristics revealed that patients with KRAS gene mutations (HR 0.42; 95% CI, 0.21–0.85) and baseline C-reactive protein (CRP) ≥5 mg/l (HR 0.46; 95% CI, 0.23–0.95) were more likely to benefit from the AS regimen (Fig. 2B).
Figure 2.
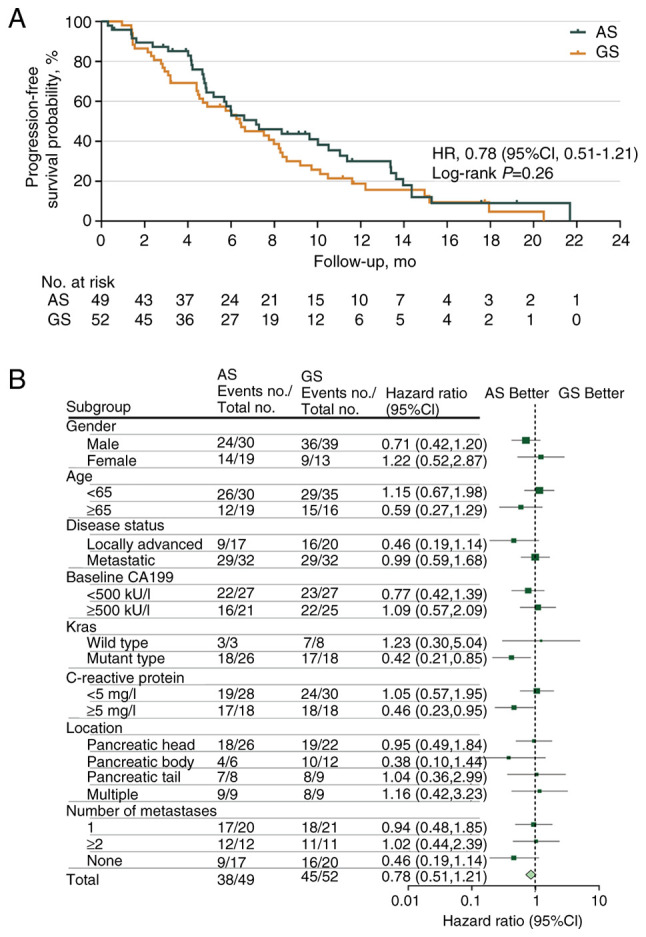
Analyses of progression-free survival. (A) Kaplan-Meier estimates of progression-free survival and (B) Forest plot of progression-free survival according to various patient subgroups. HR, hazard ratio; CI, confidence interval; AS, nab-paclitaxel plus S-1; GS, gemcitabine plus S-1.
At the time of final analysis, when 71 death events had been recorded [AS, n=34 (69.39%); GS, n=37 (71.15%)], the median OS durations were 13.27 months (95% CI, 10.39–18.52) and 10.64 months (95% CI, 5.36–13.59) in the AS and GS groups, respectively, with an HR of 0.92 (95% CI, 0.58–1.47; P=0.551; Fig. 3A). The 24-week OS rate was 85.48% in the AS group compared with 78.71% in the GS group. Marked OS benefits were observed in patients with KRAS gene mutations [HR 0.44 (95% CI, 0.21–0.93) and high CRP levels (≥5 mg/l) (HR 0.46 (95% CI, 0.22–0.95)] from the AS regimen (Fig. 3B).
Figure 3.
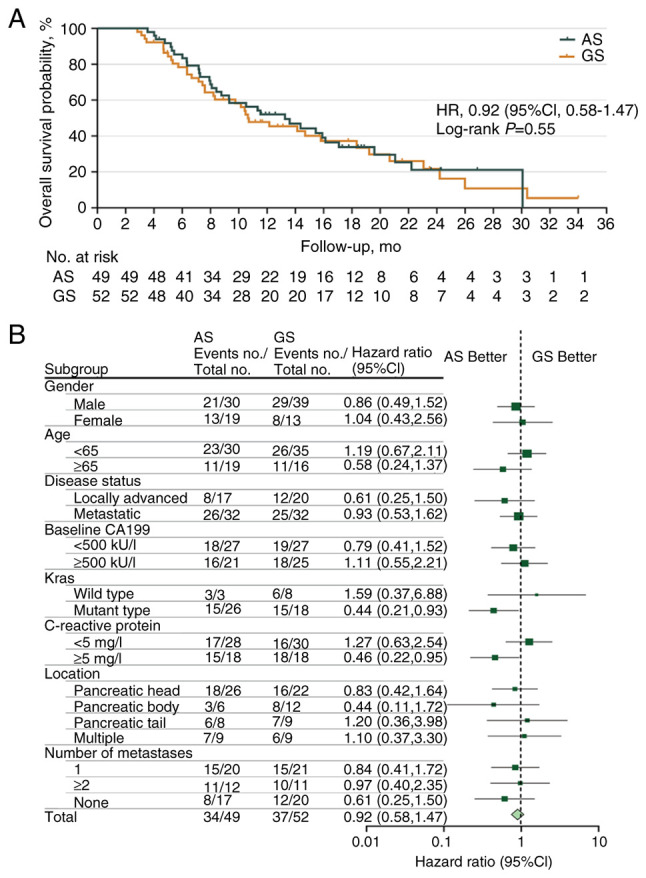
Analyses of overall survival. (A) Kaplan-Meier estimates of overall survival and (B) Forest plot of overall survival according to various patient subgroups. HR, hazard ratio; CI, confidence interval; AS, nab-paclitaxel plus S-1; GS, gemcitabine plus S-1.
No patient in either group achieved a CR as the best response according to RECIST 1.1 (Table II). However, 22 patients (44.90%) in the AS group and 8 patients (15.38%) in the GS group had a PR. Among the evaluated patients, the ORR was significantly higher in the AS group than in the GS group [44.90% (95% CI, 30.67–59.77%) vs. 15.38% (95% CI, 6.88–28.08%), respectively; P=0.001]. There were 20 patients (40.82%) in the AS group and 35 patients (67.31%) in the GS group who achieved a stable disease; thus, the DCRs in the AS and GS groups were 85.71% (95% CI, 72.76–94.06%) and 82.69% (95% CI, 69.67–91.77%), respectively (P=0.678). The maximal percentage change from baseline in the sum of the longest target lesion diameters during treatment is presented as a waterfall plot (Fig. S1).
Table II.
Tumor response according to RECIST 1.1.
| Variable | AS group (n=49) | GS group (n=52) | P-value |
|---|---|---|---|
| Objective response | 0.001 | ||
| No. of patients | 22 | 8 | |
| % of patients (95% CI)a | 44.90 (30.67–59.77) | 15.38 (6.88–28.08) | |
| Best overall response, n (%) | 0.005 | ||
| Complete response | 0 (0.00) | 0 (0.00) | |
| Partial response | 22 (44.90) | 8 (15.38) | |
| Stable disease | 20 (40.82) | 35 (67.31) | |
| Progressive disease | 5 (10.20) | 8 (15.38) | |
| Not evaluated | 2 (4.08) | 1 (1.92) |
Difference in % vs. GS (95% CI) is 29.52 (12.48–46.55). RECIST, response evaluation criteria in solid tumors version; AS, nab-paclitaxel plus S-1; GS, gemcitabine plus S-1; CI, confidence interval.
AEs
The main AEs are summarized in Table III. The number of AEs of any grade was 46 (93.88%) in the AS group vs. 44 (84.62%) in the GS group. AEs of grade ≥3 occurred in 34 patients (69.39%) treated with AS and in 22 patients (42.31%) treated with GS (P=0.009). AEs of any grade that led to treatment discontinuation were only observed in 3 patients (6.12%) with the AS therapy, for which the events were myelosuppression (n=3) and peripheral neurotoxicity (n=1). The frequency of the most common AEs of any grade in the two groups (AS vs. GS) was generally similar, including neutropenia (55.10 vs. 44.23%), anemia (32.65 vs. 23.08%) and alopecia (42.86 vs. 32.69%). Most AEs of both groups were grade 1 or 2 and manageable. Dose reductions occurred in 21 participants (42.85%) with AS and 13 (25.00%) with GS, and all were due to AEs. The most common grade ≥3 hematological toxicity was neutropenia (AS, 44.89% vs. GS, 23.08%; P=0.020). The non-hematological toxicities with high incidence in the AS group were peripheral neurotoxicity (46.94%), alopecia (42.86%) and fatigue (26.53%), and in the GS group were alopecia (32.69%) and rash (13.46%). The proportion of patients with peripheral neurotoxicities was higher in the AS group than in the GS group (46.94 vs. 1.92%, respectively; P<0.001). No treatment-associated mortalities occurred in either of the groups.
Table III.
Major adverse events.
| All grades | Grades 3–4 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Events | AS group (n=49) | GS group (n=52) | P-value | AS group (n=49) | GS group (n=52) | P-value |
| All events, n (%) | 46 (93.88) | 44 (84.62) | 0.202 | 34 (69.39) | 22 (42.31) | 0.009 |
| Hematology toxicity, n (%) | ||||||
| Neutropenia | 27 (55.10) | 23 (44.23) | 0.275 | 22 (44.89) | 12 (23.08) | 0.020 |
| Anemia | 16 (32.65) | 12 (23.08) | 0.283 | 2 (4.08) | 2 (3.85) | 1.000 |
| Thrombocytopenia | 4 (8.16) | 12 (23.08) | 0.040 | 0 (0) | 5 (9.62) | 0.077 |
| Non-hematological toxicity, n (%) | ||||||
| Peripheral neurotoxicity | 23 (46.94) | 1 (1.92) | <0.001 | 4 (8.16) | 0 (0) | 0.052 |
| Alopecia | 21 (42.86) | 17 (32.69) | 0.292 | 0 (0) | 0 (0) | - |
| Fatigue | 13 (26.53) | 5 (9.62) | 0.026 | 4 (8.16) | 3 (5.77) | 0.710 |
| Diarrhea | 9 (18.37) | 5 (9.62) | 0.203 | 5 (10.20) | 1 (1.92) | 0.105 |
| Vomiting | 9 (18.37) | 2 (3.85) | 0.019 | 3 (6.12) | 0 (0) | 0.111 |
| Oral mucositis | 7 (14.28) | 1 (1.92) | 0.028 | 2 (4.08) | 0 (0) | 0.233 |
| Rash | 1 (2.04) | 7 (13.46) | 0.061 | 0 (0.00) | 1 (1.92) | 1.000 |
| Hand-foot syndrome | 7 (14.29) | 1 (1.92) | 0.028 | 0 (0.00) | 0 (0.00) | - |
AS, nab-paclitaxel plus S-1; GS, gemcitabine plus S-1.
Discussions
To the best of our knowledge, the present study is the first multicenter, open-label phase II trial to compare AS with GS as first-line chemotherapies in patients with advanced PC. Despite the study failing to meet the primary endpoint of PFS, the AS regimen showed a numerical improvement of 2.63 months in OS compared with the GS regimen. More encouragingly, the ORR of patients treated with AS was significantly higher compared with that of patients treated with GS. Further analysis revealed that several subpopulations of patients, including patients who had KRAS gene mutations and CRP levels ≥5 mg/l, benefited more from the AS regimen. Additionally, the present study found a favorable and acceptable safety profile for AS in Chinese patients with advanced PC. Overall, the data from the present trial provide an important benchmark for investigation of the AS regimen in a subset of patients.
Although the results obtained in the present study did not demonstrate an advantage of AS over GS in terms of PFS (7.16 vs. 6.41 months), the results were generally comparable with those of other studies of AS, such as the NPSPAC trial (5.6 months) (29) and another trial performed in China (6.3 months) (36). It should also be noted that the relatively high PFS obtained with the GS regimen in the present study may be the main reason for the primary endpoint of PFS not being met. When compared with a previous retrospective study of GS, with its inherent selection bias and enrolment of patients only with stage IV disease (35), the improved baseline characteristics of the patients in the current study, with 38.46% in stage III and 61.54% in stage IV, may have contributed to the longer PFS (6.41 vs. 3.6 months) of the GS regimen. Furthermore, the patients who received the AS regimen had a numerically higher OS compared with those who received the GS regimen (13.27 vs. 10.64 months, respectively), suggesting a possible survival advantage for patients who were able to adhere to this regimen. Compared with the OS found in other studies (29,36), an improved survival benefit was observed in the present study, which may be due to the use of subsequent therapies in the two groups. Despite response activity not being a primary endpoint of the present study, a markedly increased ORR (44.90 vs. 15.38%) was achieved in patients treated with the AS regimen. Zhang et al (28) reported that the ORR was as high as 53.1% among 32 patients with advanced pancreatic ductal adenocarcinoma receiving the same triweekly regimen, which was similar to the present results. On the basis of this finding, the response benefit of AS is suggestive of meaningful clinical activity in this population. Despite an increased ORR of the AS regimen compared with the GS regimen being observed in the present AvGmPC study, the response did not translate into improved survival benefits, which may be partly explained by more patients in the AS group discontinuing treatment due to intolerable toxicity. Another explanation is that crossover within two groups was permitted in the event of disease progression; thus, the proportion of patients with second-line use of nab-paclitaxel and gemcitabine was similar, potentially leading to the OS of the two groups being comparable. Although these are interesting findings, considering economic limitations, further study with AS in a larger cohort of advanced PC requires careful assessment.
Distinct advantages of AS with regard to PFS/OS were not obtained in the present study population; however, the subgroup analysis showed that patients with KRAS gene mutations and elevated CRP levels were more likely to benefit from the AS regimen. A number of clinical trials have shown significant OS advantages in KRAS wild-type patients (13.4 vs. 9.1 months; 479 vs. 255 days; and 352 vs. 333 days) (37–39). The findings of the present study suggest that the AS regimen may be able to reverse the adverse effects of oncogenic KRAS mutations. Considering these results, they may be partially explained by the finding that KRAS mutations increase nab-paclitaxel uptake by 5–25-fold compared with that in KRAS wild-type PC cells, which may be associated with extracellular signal-regulated kinase activation (40). In addition, the subgroup analysis in the present study showed improvements in PFS and OS among patients with high CRP levels. This suggests the predictive potential of CRP as a biomarker for the efficacy of AS. Considering the maximization of benefits, further study should focus on subpopulations with KRAS gene mutations and/or elevated CRP levels to explore the survival benefits of the AS regimen.
In order to identify the less toxic regimen, the safety of the AS and GS regimens was compared. The safety profiles of AS in the present study were generally consistent with known profiles, as previously reported in other studies with similar populations (29,36). In addition, AS has been shown to be less safe than nab-paclitaxel or S-1 monotherapy in previous studies, indicating that this combination might increase the risk of various AEs including neutropenia, oral mucositis, diarrhea and vomiting (15,29,41). Seven patients in the AS group experienced oral mucositis accompanied by gastrointestinal symptoms, which resulted in reductions in daily food intake and the recovery of white blood cell numbers. This may be associated with the status of dihydropyrimidine dehydrogenase (DPD) and the enzymes that metabolize paclitaxel; however, the analysis of this data has not yet been completed. No treatment-associated deaths occurred in either of the groups in the present study, and no unexpected safety signals were identified in the study population. Notably, the GS regimen was associated with a high incidence of thrombocytopenia and rash. We hypothesize that this result may be partly due to a generic gemcitabine drug being used. In addition, although a greater proportion of patients experienced peripheral neuropathy in the AS group, in most patients, these events could be mitigated with dose reduction or the suspension of nab-paclitaxel treatment. In general, the AS regimen was well-tolerated and was delivered safely in patients with advanced PC.
Overall support for the AS regimen is based on several considerations: i) A significantly higher ORR and numerical improvement of 2.6 months in OS was observed in the AS group compared with the GS group; and subpopulations with KRAS gene mutations and CRP levels ≥5 mg/l gained more benefit from the AS regimen. ii) The study being designed on the basis of retrospective data led to an underestimation of the potential improvement in PFS for the GS group, and several cases did not adhere to the protocol due to mucosal reactions, leading to no significant improvement in PFS being observed between the AS and GS regimens. iii) Despite there being a greater number of AEs in the AS group than in the GS group, most AEs were grade 1 or 2 and manageable. and no treatment-associated deaths occurred. Together, considering the overall risk-benefit, the AS regimen may be deemed to be favorable in patients with PC. Importantly, based on these findings, future studies should focus on exploration of the efficacy of the AS regimen in selected populations, such as those with RAS mutations or high CRP levels. Dose adjustments should also be considered, as it may be necessary to reduce the initial dose of nab-paclitaxel. Also, the DPD enzyme or the DPYD gene that encodes it should be investigated to assist in the dosage selection for S-1, and the impact of taxane metabolism-associated genetic phenotypes on adverse reactions require exploration.
Although the present study did not reach the primary endpoint, it is not possible to make an absolute determination of whether this is a negative study or a failed study. Several important limitations of the study should be recognized. Firstly, the study was conducted in China and included only Asian participants; it is unclear whether the results can be simply extrapolated to Western patients because the pharmacokinetics and pharmacodynamics of S-1 between Western and East Asian patients may differ (42,43). The prominent factors for inter-ethnic differences in drug effects between Asian and Caucasians populations among the three S-1 components are differences in genetic polymorphisms, cultural differences and dietary habits. Tegafur (FT) is a prodrug of 5-fluorouracil (5FU), which is converted to 5FU in vivo mainly in the liver through hydroxylation by cytochrome P-450 2A6 (CYP2A6), a highly polymorphic enzyme with a higher frequency of common allelic variants CYP2A6*4, *7 and *9 in East Asians than Caucasians. As these variants are associated with reduced enzymatic activity, reduced activation of FT may explain the lower 5FU exposure in Asian patients (43). Also, DPD catabolizes 85% of 5FU to fluoro-β-alanine and has a major influence on 5FU levels (44); therefore, the variable inhibition of DPD by gimeracil is likely to impact 5FU exposure. Secondly, the study had an open-label design due to the different administration, which may have lead to a subconscious bias in favor of the experimental group. Nevertheless, radiological results were required to be assessed by at least two independent oncologists, which eliminated this limitation and ensured the quality of the study findings to a large extent. Thirdly, the high dose in the AS regimen without a dose-escalation design may have lead to discontinuation due to AEs. However, patients were allowed to receive supportive care and further second- or third-line treatment, which made the two groups of patients more comparable. Finally, due to COVID-19, changes in the treatment plan and survival data of patients were inevitably delayed.
Although the study failed to reach the primary endpoint, the improved response observed in patients with AS may indicate meaningful clinical benefits in this population. The promising PFS and OS benefits in certain predefined subsets and manageable toxicity indicate that the AS regimen is comparable with GS and a convenient alternative first-line chemotherapy for advanced PC. However, a larger-scale randomized trial is required for further evaluation of the AS regimen in the near future.
Supplementary Material
Acknowledgements
The authors would like to thank CSPC Ouyi Pharmaceutical Co., Ltd. (Shijiazhuang, Hebei, China) for supplying nab-paclitaxel.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
WL, YZ and TL had full access to all the data and are responsible for the integrity of the data and the accuracy of the data analysis. WL, XG, RZ and YZ conceived and designed the study. XG, YX, TL, LY, JW, DF, JZ, JL, XL, WL, XY, TK, LL, YR, DJ, WWu and YZ acquired, analyzed and interpretated the data. XG, YX and XL drafted the manuscript. YX, YR, DJ, WWu, WL and YZ critically revised the manuscript for important intellectual content. XX, YJ, LW, DW, WWa, XL and ML performed the statistical analysis. WL and YZ obtained funding. XG, RZ, LY, JW, DF, JZ, TL, JL, YR, DJ, WWu, XX, YJ, LW, XY, DW, TK, LL and WWa provided administrative, technical or material support. XL, WL and YZ supervised the study. RZ and TL confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the ethics committees of Zhongshan Hospital affiliated to Fudan University (approval no. B2018-260), Huashan Hospital affiliated to Fudan University [approval no. 2019 (001)] and Ruijin Hospital affiliated to Shanghai Jiaotong University (approval no. 2019 (143). The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before enrolment. The study is registered with Chinese Clinical Trial Registry: ChiCTR1900024588.
Patient consent for publication
The subjects gave written informed consent for the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Viale PH. The American Cancer Society's facts & figures: 2020 Edition. J Adv Pract Oncol. 2020;11:135–136. doi: 10.6004/jadpro.2020.11.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Maréchal R, Van Laethem JL, Ducreux M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer. 2016;57:10–22. doi: 10.1016/j.ejca.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN), corp-author Pancreatic Adenocarcinoma. NCCN; Plymouth Meeting, PA: 2022. Clinical Practice Guidelines in Oncology. (v. 1 2022) [Google Scholar]
- 8.CSCO, corp-author. Guidelines of The Chinese Society of Clinical Oncology (CSCO) pancreatic cancer 2020. People's Medical Publishing House; Beijing: 2020. [Google Scholar]
- 9.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui J, Jiao F, Li Q, Wang Z, Fu D, Liang J, Liang H, Xia T, Zhang T, Zhang Y, et al. Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of pancreatic cancer. J National Cancer Center. 2022;2:205–215. doi: 10.1016/j.jncc.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Gorner M, Molle M, Greten TF, Lakner V, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: Outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423–2429. doi: 10.1200/JCO.2013.53.6995. [DOI] [PubMed] [Google Scholar]
- 13.Ohba A, Ueno H, Shiba S, Okano N, Kobayashi T, Nagashima F, Sasahira N, Sasaki M, Imaoka H, Sakamoto Y, et al. Safety and efficacy of S-IROX (S-1, irinotecan and oxaliplatin combination therapy) in patients with advanced pancreatic cancer: A multicenter phase 1b dose-escalation and dose-expansion clinical trial. Eur J Cancer. 2022;174:40–47. doi: 10.1016/j.ejca.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Go SI, Lee SC, Bae WK, Zang DY, Lee HW, Jang JS, Ji JH, Kim JH, Park S, Sym SJ, et al. Modified FOLFIRINOX versus S-1 as second-line chemotherapy in gemcitabine-failed metastatic pancreatic cancer patients: A randomised controlled trial (MPACA-3) Eur J Cancer. 2021;157:21–30. doi: 10.1016/j.ejca.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/jco.2013.31.15_suppl.e15109. [DOI] [PubMed] [Google Scholar]
- 16.Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01) Lancet. 2016;388:248–257. doi: 10.1016/S0140-6736(16)30583-9. [DOI] [PubMed] [Google Scholar]
- 17.Okusaka T, Funakoshi A, Furuse J, Boku N, Yamao K, Ohkawa S, Saito H. A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2008;61:615–621. doi: 10.1007/s00280-007-0514-8. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Yamaguchi T, Ishihara T, Sudo K, Kato H, Saisho H. Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer. 2006;94:1575–1579. doi: 10.1038/sj.bjc.6603168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueno H, Okusaka T, Furuse J, Yamao K, Funakoshi A, Boku N, Ohkawa S, Yokosuka O, Tanaka K, Moriyasu F, et al. Multicenter phase II study of gemcitabine and S-1 combination therapy (GS Therapy) in patients with metastatic pancreatic cancer. Jpn J Clin Oncol. 2011;41:953–958. doi: 10.1093/jjco/hyr090. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Yu X. Comprehensive guidelines for the diagnosis and treatment of pancreatic cancer (2018 edition) J Clin Hepatol. 2018;34:2109–2120. [Google Scholar]
- 21.Song H, Han B, Park CK, Kim JH, Jeon JY, Kim IG, Kim HJ, Jung JY, Kim JH, Kwon JH, et al. Phase II trial of gemcitabine and S-1 for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2013;72:845–852. doi: 10.1007/s00280-013-2265-z. [DOI] [PubMed] [Google Scholar]
- 22.Roy V, LaPlant BR, Gross GG, Bane CL, Palmieri FM, North Central Cancer Treatment Group Phase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531) Ann Oncol. 2009;20:449–453. doi: 10.1093/annonc/mdn661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 24.Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, Soo R, Conter HJ, Kozuki T, Huang KC, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. J Thorac Oncol. 2020;15:1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Tahara J, Shimizu K, Otsuka N, Akao J, Takayama Y, Tokushige K. Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2018;82:245–250. doi: 10.1007/s00280-018-3611-y. [DOI] [PubMed] [Google Scholar]
- 26.Suenaga M, Yamada S, Fujii T, Tanaka C, Kanda M, Nakayama G, Sugimoto H, Koike M, Fujiwara M, Kodera Y. S-1 plus nab-paclitaxel is a promising regimen for pancreatic cancer in a preclinical model. J Surg Oncol. 2016;113:413–419. doi: 10.1002/jso.24147. [DOI] [PubMed] [Google Scholar]
- 27.Li JA, Xu XF, Han X, Fang Y, Shi CY, Jin DY, Lou WH. Nab-Paclitaxel plus S-1 shows increased antitumor activity in patient-derived pancreatic cancer xenograft mouse models. Pancreas. 2016;45:425–433. doi: 10.1097/MPA.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Du C, Sun Y, Yang L, Cui C, Jiang Z, Wang C, Wang J, Zhou A. Nab-paclitaxel plus S-1 as first-line followed by S-1 maintenance for advanced pancreatic adenocarcinoma: A single-arm phase II trial. Cancer Chemother Pharmacol. 2018;82:655–660. doi: 10.1007/s00280-018-3650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y, Zhang S, Han Q, Li J, Yan H, Lv Y, Shi H, Liu R, Dai G. Nab-paclitaxel plus S-1 in advanced pancreatic adenocarcinoma (NPSPAC): A single arm, single center, phase II trial. Oncotarget. 2017;8:92401–92410. doi: 10.18632/oncotarget.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai YH, Yu XJ, Xu HT, Zhuang L, Zhang MS, Zou YM, Fu Q, Qiu H, Yuan XL. Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: Results of a multicenter, randomized, phase III trial (GAPSO study) Ther Adv Med Oncol. 2022;14:17588359221118020. doi: 10.1177/17588359221118020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto M, Toba H, Aoyama M, Nakagawa M, Takechi H, Yoshida T, Tangoku A. Phase 1 Dose-Escalation study of triweekly Nab-Paclitaxel combined with S-1 for HER2-Negative metastatic breast cancer. Clin Breast Cancer. 2020;20:448–453. doi: 10.1016/j.clbc.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE-Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90–92. doi: 10.1016/j.ad.2019.05.009. (English, Spanish) [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Guo X, Fan Y, Wang D, Wu W, Wu L, Liu T, Xu B, Feng Y, Wang Y, et al. Efficacy and safety comparison of nabpaclitaxel plus S-1 and gemcitabine plus S-1 as first-line chemotherapy for metastatic pancreatic cancer. Jpn J Clin Oncol. 2018;48:535–541. doi: 10.1093/jjco/hyy063. [DOI] [PubMed] [Google Scholar]
- 36.Zong Y, Yuan J, Peng Z, Lu M, Wang X, Shen L, Zhou J. Nab-paclitaxel plus S-1 versus nab-paclitaxel plus gemcitabine as first-line chemotherapy in patients with advanced pancreatic ductal adenocarcinoma: A randomized study. J Cancer Res Clin Oncol. 2021;147:1529–1536. doi: 10.1007/s00432-020-03442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Jang KT, Ki CS, Lim T, Park YS, Lim HY, Choi DW, Kang WK, Park K, Park JO. Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications, and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer. 2007;109:1561–1569. doi: 10.1002/cncr.22559. [DOI] [PubMed] [Google Scholar]
- 38.Ogura T, Yamao K, Hara K, Mizuno N, Hijioka S, Imaoka H, Sawaki A, Niwa Y, Tajika M, Kondo S, et al. Prognostic value of K-ras mutation status and subtypes in endoscopic ultrasound-guided fine-needle aspiration specimens from patients with unresectable pancreatic cancer. J Gastroenterol. 2013;48:640–646. doi: 10.1007/s00535-012-0664-2. [DOI] [PubMed] [Google Scholar]
- 39.Philip PA, Azar I, Xiu J, Hall MJ, Hendifar AE, Lou E, Hwang JJ, Gong J, Feldman R, Ellis M, et al. Molecular characterization of KRAS Wild-type tumors in patients with pancreatic adenocarcinoma. Clin Cancer Res. 2022;28:2704–2714. doi: 10.1158/1078-0432.CCR-21-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li R, Ng TSC, Wang SJ, Prytyskach M, Rodell CB, Mikula H, Kohler RH, Garlin MA, Lauffenburger DA, Parangi S, et al. Therapeutically reprogrammed nutrient signalling enhances nanoparticulate albumin bound drug uptake and efficacy in KRAS-mutant cancer. Nat Nanotechnol. 2021;16:830–839. doi: 10.1038/s41565-021-00897-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C. An early phase II study of S-1 in patients with metastatic pancreatic cancer. Oncology. 2005;68:171–178. doi: 10.1159/000086771. [DOI] [PubMed] [Google Scholar]
- 42.Haller DG, Cassidy J, Clarke SJ, Cunningham D, Van Cutsem E, Hoff PM, Rothenberg ML, Saltz LB, Schmoll HJ, Allegra C, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol. 2008;26:2118–2123. doi: 10.1200/JCO.2007.15.2090. [DOI] [PubMed] [Google Scholar]
- 43.Chuah B, Goh BC, Lee SC, Soong R, Lau F, Mulay M, Dinolfo M, Lim SE, Soo R, Furuie T, et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci. 2011;102:478–483. doi: 10.1111/j.1349-7006.2010.01793.x. [DOI] [PubMed] [Google Scholar]
- 44.Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;47:2203–2206. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.