Abstract
CRISPR-Cas9 has emerged as a revolutionary tool that enables precise and efficient modifications of the genetic material. This review provides a comprehensive overview of CRISPR-Cas9 technology and its applications in genome editing. We begin by describing the fundamental principles of CRISPR-Cas9 technology, explaining how the system utilizes a single guide RNA (sgRNA) to direct the Cas9 nuclease to specific DNA sequences in the genome, resulting in targeted double-stranded breaks. In this review, we provide in-depth explorations of CRISPR-Cas9 technology and its applications in agriculture, medicine, environmental sciences, fisheries, nanotechnology, bioinformatics, and biotechnology. We also highlight its potential, ongoing research, and the ethical considerations and controversies surrounding its use. This review might contribute to the understanding of CRISPR-Cas9 technology and its implications in various fields, paving the way for future developments and responsible applications of this transformative technology.
Keywords: CRISPR-Cas9, genome editing, medicine, molecular biology, molecular genetics
Introduction
CRISPR-Cas9 technology has revolutionized the field of genome editing, offering precise and efficient methods to modify genetic material [1,2,3]. CRISPR-Cas9 is a powerful gene-editing tool that harnesses the bacterial immune system’s ability to target and cleave specific DNA sequences. The system utilizes a short RNA molecule called single guide RNA (sgRNA) to guide the Cas9 nuclease to the target DNA sequence, resulting in a double-stranded break. This break can then be repaired through cellular repair mechanisms, allowing for the introduction of desired genetic modifications. The simplicity, cost-effectiveness, and high efficiency of CRISPR-Cas9 have made it a game-changer in the field of genetic engineering [4,5,6].
Numerous studies have showcased the versatility and potential of CRISPR-Cas9 technology in various applications. In biomedical research, CRISPR-Cas9 has facilitated the study of gene functions and disease mechanisms. It has enabled researchers to create targeted gene knockouts, generate disease models, and explore potential therapeutic strategies. For instance, in a study by Hsu et al., CRISPR-Cas9 was used to successfully edit multiple genes simultaneously, providing a powerful tool for functional genomics research [7]. CRISPR-Cas9 has also found applications in agriculture, bioinformatics, and biotechnology. In agriculture, it offers a promising approach for crop improvement by modifying genes related to disease resistance, yield, and nutritional content. This technology provides a more precise and rapid alternative to traditional breeding methods. An example of its application in crop improvement can be seen in the work of Li et al., where CRISPR-Cas9 was used to enhance rice grain yield by targeting a gene involved in grain size regulation [8].
Overall, CRISPR-Cas9 technology has revolutionized genome editing, providing researchers with a versatile and efficient tool for genetic modification. Its applications in biomedical research, agriculture, bioinformatics, and biotechnology hold immense promise for addressing various challenges and improving human lives. The aim of this review was to comprehensively review the mechanism of CRISPR-Cas9 and explore its applications and future prospects, as well as the ethical and safety considerations associated with its use.
CRISPR-Cas9 technology
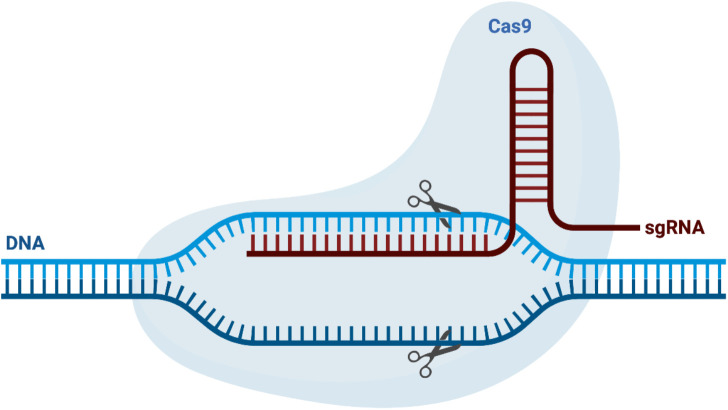
CRISPR-Cas9 technology is a powerful gene-editing tool that has gained significant attention and transformed the field of genome editing [9]. The system is derived from a bacterial immune system and utilizes a combination of two main components: the clustered regularly interspaced short palindromic repeats (CRISPR) and the CRISPR-associated protein 9 (Cas9) nuclease (Figure 1).
Figure 1. The components of the CRISPR-Cas9 system. CRISPR/Cas9 technology comprises several essential components that enable precise genome editing. Firstly, the Cas9 protein acts as a molecular scissor and nuclease, capable of cleaving the DNA strands at specific locations. Secondly, the single guide RNA (sgRNA) serves as a molecular guide, providing Cas9 with the sequence information necessary to target and bind to the desired DNA site. The sgRNA consists of a CRISPR RNA, which recognizes the target sequence, and a trans-activating CRISPR RNA, which aids in Cas9 binding.
The CRISPR component consists of short repeated DNA sequences interspersed with unique spacer sequences, which serve as a memory of past viral infections. The Cas9 nuclease, on the other hand, is an enzyme that acts as a molecular scissor that is capable of cleaving DNA strands. Together, the CRISPR and Cas9 components work in a coordinated manner to precisely target and modify specific DNA sequences [10].
The key to the specificity of CRISPR-Cas9 technology lies in the sgRNA, which is designed to be complementary to the target DNA sequence. The sgRNA serves as a molecular guide, directing the Cas9 nuclease to the precise location in the genome that needs to be modified. Once the Cas9 nuclease reaches the target site, it introduces a double-stranded break in the DNA [11].
CRISPR-Cas9 discovery and development
CRISPR-Cas9 technology has revolutionized genome editing due to its simplicity, efficiency, and versatility [12]. The CRISPR-Cas9 system was first harnessed for genome editing by Jennifer Doudna and Emmanuelle Charpentier, who demonstrated its potential in a seminal study published in Science in 2014 [13]. They showed that the Cas9 nuclease could be guided by a synthetic single-guide RNA (sgRNA) to introduce targeted DNA cleavage in vitro. This groundbreaking work laid the foundation for the rapid advancement of CRISPR-Cas9 technology. [13].
Since its initial discovery, CRISPR-Cas9 has been widely adopted and extensively optimized for various organisms and cell types. Numerous studies have further refined the system and expanded its applications. For example, Mali et al. demonstrated the successful use of CRISPR-Cas9 to edit the genomes of human cells, highlighting its potential for biomedical research [14].
The specificity of CRISPR-Cas9 technology has been a topic of great interest and improvement. Studies have explored strategies to reduce off-target effects and enhance precision. Fu et al. developed a modified Cas9 variant, termed SpCas9-HF1, which exhibited reduced off-target activity while maintaining high on-target efficiency. This study demonstrated the feasibility of engineering the Cas9 nuclease to improve its specificity [15].
The versatility of CRISPR-Cas9 extends beyond gene knockout. Researchers have harnessed the technology for a range of applications, including gene activation and repression. In a study by Gilbert et al., the dCas9 protein (a catalytically inactive form of Cas9) was fused to transcriptional activators or repressors, enabling precise control of gene expression [16]. This approach has facilitated the investigation of gene function and regulatory networks.
CRISPR-Cas9 technology has transformed the field of genome editing. Key studies by Doudna and Charpentier have paved the way for its widespread adoption and optimization. Advances in specificity, versatility, and applications continue to propel the field forward, opening up new possibilities for scientific research, therapeutic interventions, and agricultural advancements [13].
CRISPR-Cas9 technology application in agriculture
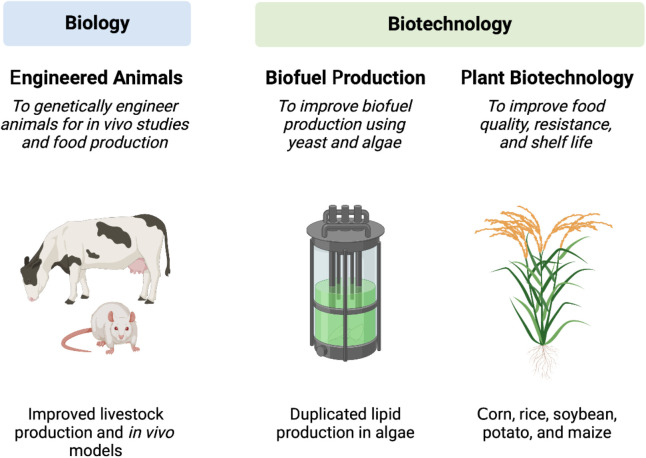
CRISPR-Cas9 technology holds significant promise for revolutionizing agriculture by enabling precise and efficient genetic modifications in crops and livestock (Figure 2) [13]. This sub-section highlights the applications and advancements of CRISPR-Cas9 in agricultural settings.
Figure 2. Applications of CRISPR-Cas9 technology for genome editing in the field of agriculture.
Crop improvement is a major focus of CRISPR-Cas9 technology in agriculture. The ability to precisely edit plant genomes allows for the enhancement of desirable traits, such as disease resistance, yield, nutritional content, and stress tolerance. For example, in a study by Shan et al., CRISPR-Cas9 was used to successfully target the susceptibility gene in rice (Figure 2), leading to enhanced resistance against bacterial blight [17].
CRISPR-Cas9 has also shown promise in accelerating the breeding process. Traditional breeding methods often require multiple generations to achieve desired traits. With CRISPR-Cas9, targeted genetic modifications can be introduced directly into the germline of plants, significantly reducing the breeding timeline. In a study by Li et al., CRISPR-Cas9 was utilized to enhance rice grain yield by precisely editing genes associated with grain size regulation [18].
Furthermore, CRISPR-Cas9 technology has the potential to address global food security challenges. By modifying crop genomes, it is possible to develop crops that are more resistant to pests, diseases, and environmental stresses, thereby increasing productivity and reducing yield losses. This technology also offers opportunities for the production of nutritionally fortified crops, which can help combat malnutrition and improve human health [17,18].
The use of CRISPR-Cas9 in livestock breeding is another emerging area in agricultural applications. Researchers are exploring the potential to introduce specific genetic modifications in livestock to improve traits such as meat quality, disease resistance, and animal welfare. While progress in this field is still in its early stages, CRISPR-Cas9 holds promise for revolutionizing livestock production and addressing challenges faced by the livestock industry [19,20].
Although the application of CRISPR-Cas9 in agriculture offers immense potential, it is not without challenges. Regulatory frameworks and public acceptance remain important considerations, particularly regarding genetically modified organisms (GMOs). Efforts are underway to address these concerns and establish guidelines for the responsible use of CRISPR-Cas9 in agriculture [21,22].
Furthermore, CRISPR-Cas9 technology presents significant opportunities for agricultural advancements. Its precise genome editing capabilities enable targeted modifications in crops and livestock, leading to improved traits, increased productivity, and enhanced food security [21,22]. As research progresses and regulatory frameworks develop, CRISPR-Cas9 holds the potential to revolutionize agricultural practices and contribute to a sustainable and resilient global food system.
CRISPR-Cas9 technology in medicine
CRISPR-Cas9 technology has emerged as a powerful tool with transformative implications in the field of medicine [23,24] (Figure 3). This sub-section provides an overview of the applications and advancements of CRISPR-Cas9 in medical research and therapy.
Figure 3. Applications of CRISPR-Cas9 technology for genome editing in the field of medicine.
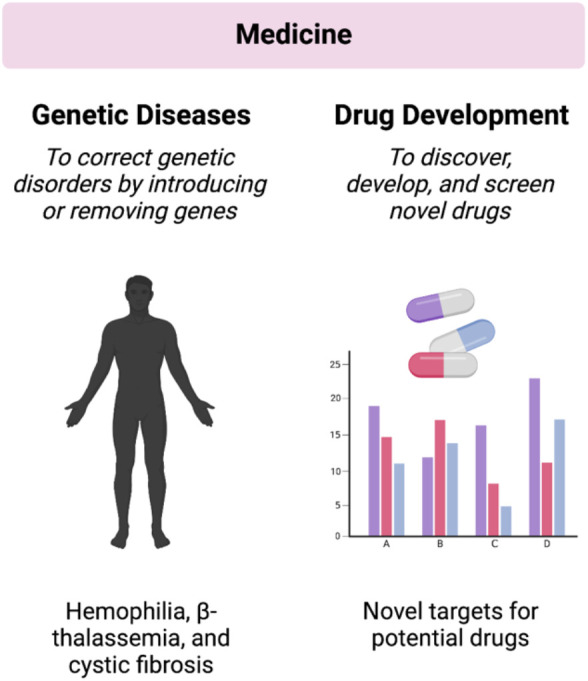
One of the key applications of CRISPR-Cas9 in medicine is in the study of gene function and disease mechanisms. By selectively modifying genes in human cells or model organisms, researchers can gain insights into the underlying causes of genetic diseases. For example, in a study by Shalem et al., CRISPR-Cas9 was employed to systematically knock out each gene in the human genome, leading to the identification of genes essential for cancer cell survival [25].
CRISPR-Cas9 also holds promise for the development of gene therapies. It offers the potential to correct disease-causing mutations directly in the genomes of patient cells. In addition, CRISPR-Cas9 technology is possible to correct a mutation associated with a genetic blood disorder, β-thalassemia, in patient-derived stem cells [13].
Furthermore, CRISPR-Cas9 technology has opened up new avenues for cancer research and therapy. It allows for the targeted disruption of genes involved in cancer progression or the introduction of specific modifications to sensitize cancer cells to existing treatments. In a study, CRISPR-Cas9 was used to knock out a gene involved in chemotherapy resistance, enhancing the effectiveness of the treatment in cancer cells [26].
The potential of CRISPR-Cas9 extends beyond gene editing. It has been harnessed for diagnostic purposes, such as the detection of specific DNA sequences associated with diseases. CRISPR-based diagnostic tools, such as specific high-sensitivity enzymatic reporter UnLOCKing (SHERLOCK) and DNA endonuclease-targeted CRISPR trans reporter (DETECTR) have been developed to enable rapid and accurate detection of viral infections and genetic mutations [26].
Moreover, the combination use of CRISPR-innovative technology and stem cell research could enable the creation of disease models for the investigation of therapy. The gene editing by using CRISPR-Cas9 was applied to clarify the mechanism autosomal dominant polycystic kidney disease (ADPKD) by deleting the PKD2 gene [27]. Furthermore, CRISPR also helped to explain the hypoglycemia by insertion of MEN1 gene with artificial specific point mutation into iPSCs [28]. The CRISPR-Cas9 is also subjected for drug screening by inducing deoxyguanosine kinase (DGUOK) knockout to the stem cells which developed as hepatocytes. It generated the mitochondrial dysfunction in hepatocytes as a board for compound screening [29].
While the potential of CRISPR-Cas9 in medicine is immense, there are challenges that need to be addressed. These include off-target effects, delivery methods, and ethical considerations, particularly when it comes to germline editing. Ongoing research aims to improve the specificity and safety of CRISPR-Cas9, and discussions around ethical and regulatory frameworks continue to evolve [13].
Nonetheless, CRISPR-Cas9 technology holds significant promise in medicine. Its applications range from understanding gene function and disease mechanisms to developing precise gene therapies and diagnostic tools. As research progresses and challenges are overcome, CRISPR-Cas9 is poised to revolutionize the field of medicine, offering new possibilities for treating genetic diseases and advancing personalized medicine [30].
CRISPR-Cas9 technology in clinical sciences
The application of CRISPR-Cas9 technology in clinical sciences holds great promise for advancing therapeutic interventions and precision medicine [13]. This sub-section provides an overview of the use of CRISPR-Cas9 in clinical research and potential clinical applications.
Clinical research utilizing CRISPR-Cas9 has contributed to a better understanding of disease mechanisms and the development of potential treatments [25]. For instance, CRISPR-Cas9 was used to target the PCSK9 gene, known to be involved in cholesterol regulation. The study demonstrated the potential of CRISPR-Cas9 as a therapeutic approach for lowering cholesterol levels in individuals with familial hypercholesterolemia [31].
The potential of CRISPR-Cas9 for treating genetic diseases is a major focus of clinical applications. A study explored the use of CRISPR-Cas9 to correct disease-causing mutations in patient cells or tissues [32]. In a landmark study, CRISPR-Cas9 was employed to edit the CCR5 gene in hematopoietic stem cells, aiming to confer resistance to HIV infection [33].
Another area of interest is the development of CRISPR-based cancer therapies. Researchers are investigating the potential of using CRISPR-Cas9 to target and eliminate cancer cells with precision. A study demonstrated the feasibility of using CRISPR-Cas9 to selectively kill cancer cells by targeting specific fusion genes involved in leukemia [32].
Furthermore, CRISPR-Cas9 technology has been explored for the development of ex vivo gene therapies. In this approach, patient cells are isolated, edited using CRISPR-Cas9, and then reinfused back into the patient. A notable example is a clinical trial conducted at the University of Pennsylvania, where CRISPR-Cas9 was used to modify T cells from patients with advanced cancer to enhance their ability to recognize and kill cancer cells [32].
It is important to note that while CRISPR-Cas9 technology shows tremendous potential, there are still challenges and ethical considerations that need to be addressed before widespread clinical implementation. Issues such as off-target effects, delivery methods, long-term safety, and ethical guidelines surrounding germline editing require careful evaluation and regulation [31,32]. The application of CRISPR-Cas9 in clinical sciences has the potential to revolutionize therapeutic interventions and precision medicine [13]. Ongoing research and clinical trials are exploring the feasibility and safety of CRISPR-based treatments for genetic diseases, cancer, and other conditions. With further advancements, CRISPR-Cas9 holds promise for addressing unmet medical needs and improving patient outcomes [33].
CRISPR-Cas9 technology in environmental sciences
The application of CRISPR-Cas9 technology in environmental sciences presents exciting possibilities for addressing environmental challenges, conservation efforts, and sustainable practices [34]. This sub-section provides an overview of the use of CRISPR-Cas9 in environmental research and potential applications.
One area of interest in environmental sciences is the use of CRISPR-Cas9 for conservation purposes. Researchers are exploring the potential of CRISPR-Cas9 to mitigate threats to endangered species by editing their genomes to enhance their resilience or adaptability to changing environments. The successful use of CRISPR-Cas9 to introduce genetic resistance to white-nose syndrome in bats, a devastating fungal disease that has caused significant declines in bat populations [35,36].
CRISPR-Cas9 technology is also being investigated for controlling or eradicating invasive species. Invasive species pose significant threats to ecosystems and native biodiversity. Researchers are exploring the possibility of using CRISPR-Cas9 to genetically modify invasive species to suppress their populations or make them less harmful to native species. While still in the early stages of research, this approach has the potential to provide effective and environmentally friendly solutions for invasive species management [36].
Additionally, CRISPR-Cas9 has the potential to enhance agricultural practices and promote sustainable farming methods. The use of CRISPR-Cas9 to develop crops with improved nutrient uptake, drought resistance, and reduced pesticide reliance [34]. These genetic modifications can contribute to increased crop productivity, reduced environmental impact, and more sustainable food production [34].
Furthermore, CRISPR-Cas9 technology offers opportunities for environmental monitoring and assessment. A study stated that the detection and identification of specific organisms, pathogens, or contaminants in environmental samples [35]. This can aid in the early detection of harmful agents, helping to prevent or mitigate environmental pollution or disease outbreaks. CRISPR-based diagnostic tools have shown promise in detecting waterborne pathogens and monitoring environmental health [35].
While the application of CRISPR-Cas9 in environmental sciences is still in its early stages, it holds tremendous potential for addressing environmental challenges and promoting sustainable practices. However, it is crucial to consider potential ecological and ethical implications, and careful risk assessments and regulatory frameworks should accompany the development and deployment of CRISPR-based environmental interventions [35].
CRISPR-Cas9 technology presents exciting opportunities for environmental sciences. Its applications range from conservation efforts and invasive species management to sustainable agriculture and environmental monitoring. Continued research and responsible implementation of CRISPR-Cas9 in environmental contexts can contribute to the preservation and restoration of ecosystems and support sustainable practices [35,36].
CRISPR-Cas9 technology in biotechnology
The innovative CRISPR-Cas9 technology has revolutionized the field of biotechnology, offering powerful tools for genome editing, gene regulation, and synthetic biology [36,37]. This sub-section provides an overview of the applications and advancements of CRISPR-Cas9 in biotechnology [37].
Genome editing is one of the primary applications of CRISPR-Cas9 in biotechnology. It enables precise modifications in the DNA sequences of various organisms, including bacteria, yeast, plants, and animals [10,11]. This capability has accelerated the development of genetically modified organisms (GMOs) with improved traits or novel functionalities. For instance, CRISPR-Cas9 was utilized to engineer a strain of yeast, demonstrating the potential of CRISPR-Cas9 in the production of valuable compounds [37].
CRISPR-Cas9 technology also plays a crucial role in gene regulation and functional genomics. It allows for the precise manipulation of gene expression, enabling researchers to investigate gene function and unravel the complexities of biological systems [5]. By using CRISPR-Cas9 to target and control gene regulatory elements, such as promoters or enhancers, researchers can gain insights into the roles of specific genes and their interactions [7]. A study demonstrated the application of CRISPR-Cas9 in activating or repressing specific genes, providing a valuable tool for functional genomics studies [38].
Synthetic biology, a field focused on engineering biological systems with novel functions, has been greatly empowered by CRISPR-Cas9 technology [9,12]. CRISPR-based tools, such as CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa), allow for precise control of gene expression and modulation of cellular pathways. These tools have been applied in the design of biosensors, metabolic engineering, and the development of new biotechnological applications [37,38].
Moreover, the use of CRISPR-Cas9 also could improve the quality of crops. The mutation induction of OsProDH gene in rice could increase the accumulation of proline [39]. It played an essential role for protection from abiotic and biotic stress by reactive oxygen species (ROS). Therefore, the manipulation of OsProDH gene could escalate the thermotolerance of rice plant [39].
Furthermore, CRISPR-Cas9 technology has enabled the rapid and cost-effective generation of animal models for studying human diseases. It allows for the introduction of specific mutations associated with human genetic disorders, providing valuable tools for understanding disease mechanisms and developing therapeutic interventions. In a study, CRISPR-Cas9 was used to create a pig model of cardiovascular disease, demonstrating the potential of CRISPR-Cas9 in modeling complex human diseases in animals [39].
As with any powerful technology, responsible use and ethical considerations are important in the field of biotechnology. Ongoing research and discussions continue to address safety, regulatory, and ethical concerns surrounding the use of CRISPR-Cas9 in biotechnological applications [38,39].
CRISPR-Cas9 technology has revolutionized biotechnology, enabling precise genome editing, gene regulation, and synthetic biology. Its applications range from the development of GMOs with improved traits to the generation of animal models for disease research. As research and advancements continue, CRISPR-Cas9 holds great promise for shaping the future of biotechnology and driving innovation in various sectors [39].
CRISPR-Cas9 technology in fisheries
The application of CRISPR-Cas9 technology in fisheries holds potential for improving aquaculture practices, enhancing fish health, and addressing challenges in fisheries management [40,41]. This sub-section provides an overview of the use of CRISPR-Cas9 in fisheries research and potential applications.
CRISPR-Cas9 has been explored as a tool for genetic improvement in aquaculture species. Liu et al. demonstrating the use of CRISPR-Cas9 to introduce beneficial traits, such as disease resistance, improved growth rates, and enhanced stress tolerance, into commercially important fish species [40]. Li et al. mentioned that the successful application of CRISPR-Cas9 in zebrafish to produce offspring with increased disease resistance [41].
The technology also offers the potential for controlling invasive species and preventing their negative impacts on native fish populations and ecosystems [42]. Invasive species can disrupt natural habitats and compete with native species for resources. CRISPR-Cas9 can be employed to modify the genomes of invasive fish species, aiming to reduce their reproductive success or increase their susceptibility to environmental factors. Although still in its early stages, CRISPR-based approaches hold promise for managing and mitigating the impacts of invasive species [43].
Furthermore, CRISPR-Cas9 has the potential to contribute to fish health management. It can be used to develop disease-resistant strains of fish by targeting and modifying genes associated with susceptibility to viral, bacterial, or parasitic infections. By enhancing the innate immune responses of fish, CRISPR-Cas9 technology may offer new possibilities for controlling disease outbreaks in aquaculture settings [40]. A study highlighted the potential of CRISPR-Cas9 for enhancing fish immunity against viral pathogens [41].
It is worth noting that the application of CRISPR-Cas9 technology in fisheries is still in the early stages, and further research and evaluations are needed to assess its safety, efficacy, and potential ecological impacts. Okoli et al. suggested the regulatory frameworks and guidelines must be established to ensure responsible and ethical use of CRISPR-Cas9 in fisheries [41].
CRISPR-Cas9 technology offers exciting prospects for fisheries research and applications. Its potential includes genetic improvement of aquaculture species, management of invasive species, and enhancement of fish health and disease resistance [40,43]. Continued research and responsible implementation of CRISPR-Cas9 in fisheries can contribute to sustainable aquaculture practices and the conservation of fish populations [42].
CRISPR-Cas9 technology in nanotechnology
CRISPR-Cas9 technology has the potential to synergize with nanotechnology in various ways. Nanotechnology offers precise control at the nanoscale, and when combined with the precision of CRISPR-Cas9, it opens up new possibilities for targeted delivery, imaging, and sensing applications. Here are a few potential areas where the integration of CRISPR-Cas9 and nanotechnology could be explored: (a) Targeted delivery systems. Nanoparticles can be engineered to carry CRISPR-Cas9 components, such as guide RNA and Cas9 protein, to specific cells or tissues. This targeted delivery approach could enhance the efficiency and specificity of genome editing while minimizing off-target effects [44]. (b) Imaging and tracking. Nanoparticles with imaging agents can be used to monitor the delivery and activity of CRISPR-Cas9 components in real-time. This allows researchers to visualize and track the editing process, providing valuable insights into its efficacy and potential optimization [45]. (c) Gene regulation and epigenetic modifications. Nanotechnology-based platforms can be employed to modulate gene expression and epigenetic modifications, either by directly targeting DNA or influencing regulatory factors. Integration of CRISPR-Cas9 with nanotechnology can enable precise control over gene expression and epigenetic regulation, offering new avenues for disease treatment and fundamental biological research [46].
While the specific references on CRISPR-Cas9 technology in nanotechnology are limited, some researchers published articles and reviews on CRISPR-based delivery systems, nanocarriers for gene editing, or nanotechnology-enabled gene regulation for further insights into the potential synergies between CRISPR-Cas9 technology and nanotechnology [47].
CRISPR-Cas9 technology in bioinformatics and machine learning
The integration of CRISPR-Cas9 technology with bioinformatics and machine learning has yielded significant advancements in genomic research and data analysis. CRISPR-Cas9, a powerful gene editing tool, combined with computational methods has enabled researchers to gain deeper insights into the structure, function, and regulation of genomes. The application of machine learning algorithms to CRISPR-Cas9 data has further enhanced the analysis of large-scale genomic datasets, enabling the discovery of novel patterns and relationships [48,49].
One area where CRISPR-Cas9 technology has merged with bioinformatics and machine learning is in the prediction of off-target effects. By leveraging machine learning algorithms and genomic sequence data, researchers have developed computational models that can predict potential off-target sites of CRISPR-Cas9 gene editing. These models help guide experimental design and increase the specificity and accuracy of CRISPR-Cas9-mediated gene modifications [50,51].
Furthermore, machine learning techniques have been employed to analyze CRISPR-Cas9 screening data. CRISPR screening involves perturbing genes and observing the resulting phenotypic changes, generating large-scale datasets [52]. Machine learning algorithms, such as neural networks and support vector machines, have been used to extract meaningful information from these datasets, identify gene functions, and uncover genetic interactions [53,54].
In addition, the integration of CRISPR-Cas9 technology with machine learning has facilitated the development of computational tools for predicting the functional consequences of genetic variants. Machine learning algorithms can be trained on CRISPR-Cas9 experimental data to predict the impact of specific genetic variants on gene function or disease risk [48,49]. These tools assist in the interpretation of genomic variations and aid in prioritizing potential disease-associated variants [50].
Moreover, machine learning has been utilized to improve the efficiency and accuracy of CRISPR-Cas9 experimental designs. By leveraging large genomic datasets and computational models, machine learning algorithms can guide the selection of optimal target sites for CRISPR-Cas9 gene editing, taking into account factors such as on-target activity and off-target effects. This approach streamlines the experimental process and increases the success rate of CRISPR-Cas9 experiments [50,51].
The integration of CRISPR-Cas9 technology with bioinformatics and machine learning holds great promise for advancing our understanding of genomic biology and accelerating discoveries in personalized medicine. By combining experimental data, computational algorithms, and machine learning, researchers can unlock the potential of CRISPR-Cas9 for precision genomics and pave the way for targeted therapies [48,49].
Future directions of CRISPR-Cas9 technology
CRISPR-Cas9 technology has made significant contributions to various fields, from agriculture and medicine to environmental sciences and biotechnology. Looking ahead, ongoing research and advancements are paving the way for exciting future directions in the development and application of CRISPR-Cas9 [55,56]. This sub-section highlights some of the potential future directions and areas of exploration for CRISPR-Cas9 technology.
One of the key focuses of future research is to improve the precision and specificity of CRISPR-Cas9 editing. Efforts are underway to reduce off-target effects, increase the efficiency of homology-directed repair (HDR), and minimize unintended modifications to the genome. Various strategies, including the use of high-fidelity Cas9 variants, modified guide RNA designs, and novel delivery methods, are being explored. These advancements will enhance the accuracy and safety of CRISPR-Cas9 technology in clinical applications. A study described the development of an improved Cas9 variant, SpCas9-HF1, with enhanced specificity and reduced off-target activity [55].
Expanding Editing Capabilities: Researchers are actively working on expanding the editing capabilities of CRISPR-Cas9 beyond DNA modifications. Efforts are being made to develop RNA-targeting CRISPR systems for precise RNA editing and manipulation. This includes the use of Cas13 enzymes for programmable RNA targeting and the development of base editors that allow for precise single-nucleotide changes. These advancements will enable researchers to edit RNA molecules directly, opening up new possibilities for treating diseases at the RNA level. A study demonstrated the use of CRISPR/Cas13 for programmable RNA editing in human cells [56].
Therapeutic Applications: CRISPR-Cas9 holds great promise for therapeutic applications, including the treatment of genetic disorders and cancers. Future research will focus on advancing the development of CRISPR-based gene therapies, improving delivery methods, and addressing immune responses to CRISPR components. Additionally, the potential application of CRISPR-Cas9 in precision oncology, such as targeting specific cancer driver mutations or immune checkpoint genes, is an area of active investigation. A study reported the successful use of CRISPR-Cas9 to treat a genetic form of blindness in a clinical trial [57].
Multiplex Genome Editing: Multiplex genome editing, the ability to target and modify multiple genomic loci simultaneously, is an area of ongoing research. Enhancing the efficiency and scalability of multiplex editing will enable researchers to study complex gene networks and pathways more comprehensively. This will have implications for understanding disease mechanisms, developing synthetic biology applications, and advancing agricultural biotechnology [57,58].
Ethical and Regulatory Considerations: As CRISPR-Cas9 technology progresses, it is crucial to address ethical, legal, and societal considerations. Discussions on responsible use, equitable access, and potential risks associated with CRISPR-Cas9 will continue to shape the future development and deployment of this technology. Regulatory frameworks and guidelines are being established to ensure the safe and ethical use of CRISPR-Cas9 in various applications [57,58].
The future of CRISPR-Cas9 technology holds tremendous potential. Advancements in precision, expanding editing capabilities, therapeutic applications, multiplex editing, and addressing ethical considerations will shape the future directions of CRISPR-Cas9 research and applications.
CRISPR-Cas9 technology controversy
Despite the enormous potential of CRISPR-Cas9 technology, it has also sparked various ethical, legal, and societal concerns. This sub-section explores some of the controversies surrounding CRISPR-Cas9 and the discussions they have ignited. One major controversy revolves around the ethical implications of germline editing, which involves making heritable changes to the DNA of embryos or reproductive cells [59,60]. The ability to modify the germline raises questions about the potential long-term effects on future generations, the unintended consequences of genetic manipulation, and the potential for creating “designer babies”. In 2018, the birth of twin girls in China, whose genomes were edited using CRISPR-Cas9, sparked international outcry and renewed calls for strict regulations and oversight in germline editing [59].
Another area of controversy is the potential for off-target effects. Although efforts have been made to improve the specificity of CRISPR-Cas9, concerns remain about unintended modifications to the genome, which could have unintended consequences for human health and the environment. Researchers are actively working to reduce off-target effects and improve the accuracy of CRISPR-Cas9 editing. Ongoing discussions involve establishing rigorous safety protocols, ensuring proper risk assessment, and promoting transparency in reporting off-target effects [61,62].
Intellectual property rights and patent disputes have also been a source of controversy in the CRISPR-Cas9 field. Various institutions and researchers have filed patent claims over the technology, leading to legal battles and debates over who should have control and ownership of this revolutionary technology. These disputes have implications for the accessibility and affordability of CRISPR-Cas9 applications, particularly in the context of developing countries and global health initiatives [61,62].
The controversy surrounding CRISPR-Cas9 has spurred broader discussions on the ethical, legal, and social implications (ELSI) of gene editing technologies [63]. These discussions encompass topics such as equitable access to CRISPR-based therapies, potential uses for enhancement rather than therapeutic purposes, and the importance of public engagement and involvement in decision-making processes [64,65]. International conferences and expert panels have been convened to address these ELSI concerns and provide guidelines for responsible and ethical use of CRISPR-Cas9 technology. It is essential to continue these discussions and debates surrounding CRISPR-Cas9 technology to ensure responsible and ethical practices, consider the potential risks and benefits, and promote transparency and public trust in the field of genome editing [62].
Conclusion
We discussed the topic of CRISPR-Cas9 technology for editing the genome. The manuscript was divided into several sub-sections, each exploring different aspects of this technology and its applications. In summary, this comprehensive conversation provided an in-depth exploration of CRISPR-Cas9 technology, its applications in agriculture, medicine, environmental sciences, fisheries, nanotechnology, bioinformatics, and biotechnology. It highlighted its potential, ongoing research, and the ethical considerations and controversies surrounding its use. Furthermore, this manuscript contributes to the understanding of CRISPR-Cas9 and its implications in various fields, paving the way for future developments and responsible applications of this transformative technology.
Acknowledgments
None.
Ethics approval
Not required.
Competing interests
All the authors declare that there are no conflicts of interest.
Funding
This study received no external funding.
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
How to cite
Ansori ANM, Yulanda A, Susilo RJK, et al. Application of CRISPR-Cas9 genome editing technology in various fields: A review. Narra J 2023; 3 (2): e184 - http://doi.org/10.52225/narra.v3i2.184.
References
- 1.Bhatia S, Pooja Yadav SK. CRISPR-Cas for genome editing: Classification, mechanism, designing and applications. Int J Biol Macromol 2023; 238:124054. [DOI] [PubMed] [Google Scholar]
- 2.Bhokisham N, Laudermilch E, Traeger LL, et al. CRISPR-Cas system: The current and emerging translational landscape. Cells 2023; 12(8):1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng H, Nan M, Li Y, et al. Application of CRISPR-Cas9 gene editing technology in basic research, diagnosis and treatment of colon cancer. Front Endocrinol 2023; 14:1148412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilahibaks NF, Hulsbos MJ, Lei Z, et al. Enabling precision medicine with CRISPR-Cas genome editing technology: A translational perspective. Adv Exp Med Biol 2023; 1396:315–339. [DOI] [PubMed] [Google Scholar]
- 5.Wang JY, Doudna JA. CRISPR technology: A decade of genome editing is only the beginning. Science 2023; 379(6629):eadd8643. [DOI] [PubMed] [Google Scholar]
- 6.Verma V, Kumar A, Partap M, et al. CRISPR-Cas: A robust technology for enhancing consumer-preferred commercial traits in crops. Front Plant Sci 2023; 14:1122940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu PD, Scott DA, Weinstein JA. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 2013; 31(9):827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JF, Norville JE, Aach J.. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol. 2013; 31(8):688–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T, Yang Y, Qi H, et al. CRISPR-Cas9 therapeutics: Progress and prospects. Signal Transduct Target Ther 2023; 8(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson C, Kelsh RN JRichardson R.. New advances in CRISPR/Cas-mediated precise gene-editing techniques. Dis Model Mech 2023; 16(2):dmm049874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mingarro G, Del Olmo ML. Improvements in the genetic editing technologies: CRISPR-Cas and beyond. Gene 2023; 852:147064. [DOI] [PubMed] [Google Scholar]
- 12.Naeem M, Alkhnbashi OS. Current bioinformatics tools to optimize CRISPR-Cas9 experiments to reduce off-target effects. Int J Mol Sci 2023; 24(7):6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doudna JA, Charpentier E.. The new frontier of genome engineering with CRISPR-Cas9. Science 2014; 346(6213):1258096. [DOI] [PubMed] [Google Scholar]
- 14.Mali P, Yang L, Esvelt KM. RNA-guided human genome engineering via Cas9. Science 2013; 339(6121):823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Sander JD, Reyon D, et al. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 2014; 32(3):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert LA, Horlbeck MA, Adamson B.. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 2014; 159(3):647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan Q, Wang Y, Li J, et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol 2013; 31(8):686–688. [DOI] [PubMed] [Google Scholar]
- 18.Li T, Liu B, Spalding MH, et al. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol 2012; 30(5):390–392. [DOI] [PubMed] [Google Scholar]
- 19.Dawood AA, Jasim BI. The CRISPR genome editing process is an effective advancement of short-term cancer treatment. Res J Pharm Dosage Forms Tech 2021; 13(1):54–56. [Google Scholar]
- 20.González MN, Massa GA, Andersson M, et al. CRISPR-Cas9 technology for potato functional genomics and breeding. Methods Mol Biol 2023; 2653:333–361. [DOI] [PubMed] [Google Scholar]
- 21.Yin W, Chen Z, Huang J, et al. Application of CRISPR-Cas9 gene editing technology in crop breeding. Chinese J Biiotechnol 2023; 39(2):399–424. [DOI] [PubMed] [Google Scholar]
- 22.Tiwari JK, Singh AK, Behera TK. CRISPR/Cas genome editing in tomato improvement: Advances and applications. Front Plant Sci 2023; 14:1121209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mark JKK, Lim CSY, Nordin F, et al. Expression of mammalian proteins for diagnostics and therapeutics: A review. Mol Biol Rep 2022; 49(11):10593–10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang P, Xu Y, Zhang X, et al. CRISPR-Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 2015; 6(5):363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shalem O, Sanjana NE, Zhang F, et al. High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet 2015; 16(5):299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhen S, Hua L, Liu YH, et al. Mutant p53 gain of function in cancer. Cancer Lett 2017; 376(1):144–153. [Google Scholar]
- 27.Trionfini P, Ciampi O, Romano E, et al. Generation of two isogenic knockout PKD2 iPS cell lines, IRFMNi003-A-1 and IRFMNi003-A-2 using CRISPR-Cas9 technology. Stem Cell Res 2020; 42:101667. [DOI] [PubMed] [Google Scholar]
- 28.Guo D, Liu H, Gao G, et al. Creating a patient carried Men1 gene point mutation on wild type iPSCs locus mediated by CRISPR-Cas9 and ssODN. Stem Cell Res 2017; 18:67–69. [DOI] [PubMed] [Google Scholar]
- 29.Jing R, Corbett JL, Cai J, et al. A screening using iPSCs-derived hepatocytes reveals NAD+ as a potential treatment for mtDNA depletion syndrome. Cell Rep 2018; 25(6):1469–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci 2017; 372(1732):20160270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chadwick AC, Musunuru K.. Treatment of dyslipidemia using CRISPR/Cas9 genome editing. Curr Atheroscler Rep 2017; 19(7):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stadtmauer EA, Fraietta JA, Davis MM. CRISPR-engineered T cells in patients with refractory cancer. Science 2020; 367(6481):eaba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Wang J, Liu Y.. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med 2019; 381(13):1240–1247. [DOI] [PubMed] [Google Scholar]
- 34.Gajardo HA, Gómez-Espinoza O, Boscariol Ferreira P, et al. The potential of CRISPR/Cas technology to enhance crop performance on adverse soil conditions. Plants 2023; 12(9):1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S, Rahangdale S, Pandita S, et al. CRISPR/Cas9 for insect pests management: A comprehensive review of advances and applications. Agriculture 2022; 12(11):1896. [Google Scholar]
- 36.Lander N, Chiurillo MA. State-of-the-art CRISPR/Cas9 technology for genome editing in trypanosomatids. J Eukaryot Microbiol 2019; 66(6):981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen J. Synthetic biology for engineering acetyl coenzyme A metabolism in yeast. Biotechnol Bioeng 2019; 116(4):723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert LA, Larson MH, Morsut L, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013; 154(2):442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo M, Zhang X, Liu J, et al. OsProDH negatively regulates thermotolerance in rice by modulating proline metabolism and reactive oxygen species scavenging. Rice 2020; 13(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Zhou Y, Qi X, et al. CRISPR/Cas9 in zebrafish: An efficient combination for human genetic diseases modeling. Hum Genet 2017; 136(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Jia Z, Zhang S, et al. Progress in gene-editing technology of zebrafish. Biomolecules 2021; 11(9):1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okoli AS, Blix T, Myhr AI, et al. Sustainable use of CRISPR/Cas in fish aquaculture: The biosafety perspective. Transgenic Res 2022; 31(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harvey-Samuel T, Ant T, Alphey L.. Towards the genetic control of invasive species. Biol Invasions 2017; 19(6):1683–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dilnawaz F, Acharya S.. Nanoparticle-based CRISPR/Cas delivery: An emerging tactic for cancer therapy. Curr Med Chem 2023; 30(31):3562–3581. [DOI] [PubMed] [Google Scholar]
- 45.Ma L, Dong C, Yu M, et al. Nanomedicine enables efficient CRISPR-Cas9 genome editing for disease treatment. Sci Bull. 2022; 67(6):572–576. [DOI] [PubMed] [Google Scholar]
- 46.Xu X, Liu C, Wang Y, et al. Nanotechnology-based delivery of CRISPR-Cas9 for cancer treatment. Adv Drug Deliv Rev 2021; 176:113891. [DOI] [PubMed] [Google Scholar]
- 47.Demirer GS, Silva TN, Jackson CT, et al. Nanotechnology to advance CRISPR-Cas genetic engineering of plants. Nat Nanotechnol 2021; 16(3):243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muhammad RAH, Toufikuzzaman M, Rahman MS, et al. CRISPRpred(SEQ): A sequence-based method for sgRNA on target activity prediction using traditional machine learning. BMC Bioinformatics 2020; 21(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konstantakos V, Nentidis A, Krithara A, et al. CRISPR-Cas9 gRNA efficiency prediction: An overview of predictive tools and the role of deep learning. Nucleic Acids Res 2022; 50(7):3616–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alkhnbashi OS, Meier T, Mitrofanov A, et al. CRISPR-Cas bioinformatics. Methods 2020; 172:3–11. [DOI] [PubMed] [Google Scholar]
- 51.Makarova KS, Koonin EV. Annotation and classification of CRISPR-Cas systems. Methods Mol Biol 2015; 1311:47–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaul T, Raman NM, Eswaran M, et al. Data mining by pluralistic approach on CRISPR gene editing in plants. Front Plant Sci 2019; 10:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alipanahi R, Safari L, Khanteymoori A.. CRISPR genome editing using computational approaches: A survey. Front Bioinform 2023; 2:1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palermo G, Miao Y, Walker RC, et al. CRISPR-Cas9 conformational activation as elucidated from enhanced molecular simulations. Proc Natl Acad Sci USA 2017; 114(28):7260–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleinstiver BP, Prew MS, Tsai SQ, et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015; 523(7561):481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abudayyeh OO, Gootenberg JS, Essletzbichler P, et al. RNA targeting with CRISPR-Cas13. Nature 2017; 550(7675):280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maeder ML, Stefanidakis M, Wilson CJ, et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat Med 2019; 25(2):229–233. [DOI] [PubMed] [Google Scholar]
- 58.Shalem O, Sanjana NE, Hartenian E, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014; 343(6166):84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greely HT. CRISPR’d babies: Human germline genome editing in the ‘He Jiankui affair’. J Law Biosci 2019; 6(1):111–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haeussler M, Concordet JP. CRISPR-Cas9 in the era of big data biology. J Mol Biol 2016; 428(5 Pt B):928–931.26546279 [Google Scholar]
- 61.Ayanoğlu FB, Elçin AE, Elçin YM. Bioethical issues in genome editing by CRISPR-Cas9 technology. Turk J Biol 2020; 44(2):110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson JP, Selin CL, Scott CT. Toward anticipatory governance of human genome editing: A critical review of scholarly governance discourse. J Responsible Innov 2021; 8(3):382–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mark JKK, Lim CSY, Nordin F, et al. Expression of mammalian proteins for diagnostics and therapeutics: a review. Mol Biol Rep 2022; 49(11):10593–10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chin DS, Lim CSY, Nordin F, et al. Antibody-dependent cell-mediated cytotoxicity through natural killer (NK) cells: Unlocking NK Cells for Future Immunotherapy. Curr Pharm Biotechnol 2022; 23(4):552–578. [DOI] [PubMed] [Google Scholar]
- 65.Suwito BE, Adji AS, Wardani VAK, et al. A review of CRISPR Cas9 for ASCVD: treatment strategies and could target PSCK9 gene using CRISPR cas9 prevent the patient from atherosclerotic vascular disease? Bali Med J 2022; 11(2):985–993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.