Abstract
Elongation factor SII interacts with RNA polymerase II and enables it to transcribe through arrest sites in vitro. The set of genes dependent upon SII function in vivo and the effects on RNA levels of mutations in different components of the elongation machinery are poorly understood. Using yeast lacking SII and bearing a conditional allele of RPB2, the gene encoding the second largest subunit of RNA polymerase II, we describe a genetic interaction between SII and RPB2. An SII gene disruption or the rpb2-10 mutation, which yields an arrest-prone enzyme in vitro, confers sensitivity to 6-azauracil (6AU), a drug that depresses cellular nucleoside triphosphates. Cells with both mutations had reduced levels of total poly(A)+ RNA and specific mRNAs and displayed a synergistic level of drug hypersensitivity. In cells in which the SII gene was inactivated, rpb2-10 became dominant, as if template-associated mutant RNA polymerase II hindered the ability of wild-type polymerase to transcribe. Interestingly, while 6AU depressed RNA levels in both wild-type and mutant cells, wild-type cells reestablished normal RNA levels, whereas double-mutant cells could not. This work shows the importance of an optimally functioning elongation machinery for in vivo RNA synthesis and identifies an initial set of candidate genes with which SII-dependent transcription can be studied.
The elongation phase of transcription is an important control point for the regulation of gene expression (reviewed in references 4, 29, and 41). Several general elongation factors, including TFIIF, ELL, and elongin (SIII), are able to increase the overall rate of transcription elongation of RNA polymerase II (PolII) in vitro (7, 13, 26, 37). SII enables PolII to transcribe through a variety of transcriptional blockages, including intrinsic arrest sites and nucleoprotein complexes (reviewed in references 29 and 41). SII reactivates arrested PolII complexes by binding to the enzyme and activating a nascent RNA cleavage activity, which eventually results in polymerase escape (reviewed in reference 28). It has been hypothesized that elongation factors such as TFIIF reduce the frequency of arrest by reducing the dwell time of PolII at arrest sites (6, 15).
Little is known about gene sequences that block transcription and the interaction of general elongation factors with PolII complexes in vivo. In yeast, mutation or disruption of the gene encoding SII, DST1 (gene names in this study are those designated in the Saccharomyces Genome Database; DST1 is also known as PPR2), renders cells sensitive to the base analog 6-azauracil (6AU) (19, 23, 24). 6AU depletes cellular levels of the RNA precursors UTP and GTP (12). Supplementing the drug-containing medium with uracil or guanine reverses this phenotype, suggesting that the drug inhibits growth because of the reduction in nucleoside triphosphate levels and thereby impairs transcription elongation (3, 12). The sensitivity of yeast to 6AU after disruption of DST1 may indicate an increased requirement by RNA polymerase for elongation factor assistance under conditions of substrate depletion (3). Hence, 6AU sensitivity has been exploited as a bioassay for transcription elongation. Indeed, mutations in two of the subunits of RNA PolII yield 6AU-sensitive yeast (3, 25, 34, 35). Some of these mutations generate enzymes that are defective in elongation in vitro, either by reducing the affinity of SII for polymerase (45) or by yielding an arrest-prone enzyme with a low elongation rate (25). Certain mutations in SPT4, SPT5, and SPT6 also confer sensitivity to 6AU and synthetic lethality when combined with an inactivated SII gene, suggesting that these genes are also involved in transcription elongation (17). Nevertheless, direct evidence that SII acts as an elongation factor in vivo is lacking, and the effect of mutations in DST1 or 6AU treatment on mRNA levels has not been explored. Little is known about target genes that are particularly reliant upon SII for their transcription or whose transcription rate is sensitive to a compromised elongation machinery.
rpb2-4 (A1016T) and rpb2-10 (P1018S) are two point mutations in the gene encoding the second largest subunit of PolII (RPB2) that confer 6AU sensitivity upon yeast (25, 34, 35). The rpb2-10 mutation yields polymerases which possess an abnormally low rate of elongation in vitro and a higher propensity to become arrested (25). Here, we show that cells bearing a combination of the rpb2-10 mutation and an inactivated DST1 gene have reduced levels of poly(A)+ RNA and specific mRNAs. 6AU treatment is accompanied by an additional reduction in RNA levels and a hypersensitive growth phenotype. These findings define for the first time a genetic interaction between RPB2 and DST1 and demonstrate a 6AU-induced effect on transcription. They also show that in vivo, efficient mRNA synthesis relies upon SII function and an efficiently elongating RNA PolII.
MATERIALS AND METHODS
Yeast strains and growth medium.
The genotypes of the Saccharomyces cerevisiae strains used in this study are listed in Table 1. The dst1::hisG allele consists of a disruption of the DST1 coding region at a unique PstI site located 61 nucleotides downstream from the initiating ATG of DST1. Yeast cells were transformed with KpnI- and BamHI-digested plasmid pC1002, described below, and a strain with the hisG disrupted allele was isolated as described previously (1). The hisG insertion was verified by yeast colony PCR (42) with primers 5′-GGCACTGGACTCTAAATCTC-3′ and 5′-TTTCTTTAGTTCTGACCGAGC-3′, which flank the region of insertion. The rpo21-18 allele was introduced into the chromosome by the pop-in/pop-out allele replacement method (31) with KpnI-digested plasmid pC1005, described below. This allele contains an XhoI linker insertion used to confirm integration by XhoI digestion of a PCR product generated from transformant genomic DNA with primers 5′-GATCCTGATCCACGTTCCAC-3′ and 5′-ATGAACATCTCATTAAGGCACC-3′.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype |
|---|---|
| Z96a | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP214 (LEU2 RPB2)] |
| Z100a | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP2-4L (LEU2 rpb2-4)] |
| Z106a | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP2-10L (LEU2 rpb2-10)] |
| SJR105b | MATα ura3-52 lys2-801am ade2-101oc trp1Δ1 his3Δ200 |
| DY95 | MATα ura3-52 lys2-801am ade2-101oc trp1Δ1 his3Δ200 rpb2-10 dst1::hisG |
| DY103 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP214 (LEU2 RPB2)] [pRS316 (URA3)] |
| DY104 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP2-4L (LEU2 rpb2-4)] [pRS316 (URA3)] |
| DY105 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP2-10L (LEU2 rpb2-10)] [pRS316 (URA3)] |
| DY106 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 dst1::hisG [pRP214 (LEU2 RPB2)] [pRS316 (URA3)] |
| DY107 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 dst1::hisG [pRP2-4L (LEU2 rpb2-4)] [pRS316 (URA3)] |
| DY108 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 dst1::hisG [pRP2-10L (LEU2 rpb2-10)] [pRS316 (URA3)] |
| DY109 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP214 (LEU2 RPB2)] [YCp50 (URA3)] |
| DY110 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP2-4L (LEU2 rpb2-4)] [YCp50 (URA3)] |
| DY111 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP2-10L (LEU2 rpb2-10)] [YCp50 (URA3)] |
| DY112 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 dst1::hisG [pRP214 (LEU2 RPB2)] [YCp50 (URA3)] |
| DY113 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 dst1::hisG [pRP2-4L (LEU2 rpb2-4)] [YCp50 (URA3)] |
| DY114 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 dst1::hisG [pRP2-10L (LEU2 rpb2-10)] [YCp50 (URA3)] |
| DY115 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP214 (LEU2 RPB2)] [pRP212 (URA3 RPB2)] |
| DY116 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP2-4L (LEU2 rpb2-4)] [pRP212 (URA3 RPB2)] |
| DY117 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 [pRP2-10L (LEU2 rpb2-10)] [pRP212 (URA3 RPB2)] |
| DY118 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 dst1::hisG [pRP214 (LEU2 RPB2)] [pRP212 (URA3 RPB2)] |
| DY119 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 dst1::hisG [pRP2-4L (LEU2 rpb2-4)] [pRP212 (URA3 RPB2)] |
| DY120 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 dst1::hisG [pRP2-10L (LEU2 rpb2-10)] [pRP212 (URA3 RPB2)] |
| DY123 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 rpo21-18 dst1::hisG [pRP214 (LEU2 RPB2)] [pRS316 (URA3)] |
| DY124 | MATα ura3-52 leu2-3,112 his3Δ200 rpb2Δ297::HIS3 rpo21-18 [pRP2-10L (LEU2 rpb2-10)] [pRS316 (URA3)] |
Obtained from R. Young (Massachusetts Institute of Technology).
Obtained from S. Jinks-Robertson (Emory University).
Yeast transformation was performed as previously described (14). Synthetic minimal growth medium (SD), synthetic complete medium lacking uracil (SC-Ura), and synthetic complete medium lacking leucine and uracil (SC-Leu-Ura) were prepared as described previously (36). 6AU, cycloheximide, or mycophenolic acid (Sigma, St. Louis, Mo.) dissolved in water, ethanol, or methanol, respectively, and filter sterilized was added to sterile medium as needed.
Plasmids.
Plasmid pC1001 consists of a 1.8-kb PCR product containing the DST1 gene generated from primers 5′-GGCACTGGACTCTAATCTC-3′ and 5′-AAAGATTTTACGTGAGACAGAC-3′ and cloned into the SmaI site of pGEM-7Zf+. Plasmid pC1002 consists of a blunt-ended 5.1-kb BamHI fragment from pHUHKH3 (11) (a gift from Gray Crouse, Emory University) carrying a hisG-URA3-hisG gene disruption cassette inserted into the nuclease-blunted PstI site of plasmid pC1001. pC1005 consists of a 5.7-kb EcoRI-HindIII fragment from plasmid pYF1540 (a gift from James Friesen, University of Toronto) carrying the rpo21-18 allele inserted into EcoRI- and HindIII-digested pRS306 (38).
Growth rate assays.
For solid medium growth assays, 5 ml of single-colony cultures grown for 48 h to saturation in SD were diluted to an optical density at 600 nm (OD600) of 1.0, and 3 μl was streaked onto plates of SC-Leu-Ura or SC-Leu-Ura with 75 μg of 6AU per ml. Plates were incubated at 30°C for 4 days. For liquid medium growth assays, 5 ml of single-colony cultures grown for 48 h to saturation in SD were diluted into fresh medium and grown to saturation. To test log-phase cells for 6AU sensitivity, 5 ml of single-colony cultures grown for 48 h to saturation in SD were diluted into fresh medium and grown to an OD600 of 0.4 to 0.6 before being rediluted into medium containing drug. For all liquid assays, growth was monitored by measuring the OD600. Doubling time was defined as the length of time it took to double the OD600 in the logarithmic part of the growth curve and was calculated by dividing the log(2) value by the slope of the curve.
RNA analysis.
Total RNA was isolated from cells by the hot phenol extraction method (5). RNA was quantitated by measuring the absorbance at 260 nm. For all experiments, similar amounts of total RNA were obtained from equal numbers of cells for all strains. Dot blot analysis was performed as described previously (8). For Northern analysis, 10 μg of total RNA was resolved on a 1% agarose–formaldehyde gel and blotted onto a Zeta-probe GT nylon membrane (Bio-Rad, Hercules, Calif.). The filter was air dried, baked at 80°C under vacuum for 30 min, and UV cross-linked in a Stratalinker 1800 (Stratagene, La Jolla, Calif.). Filters were prehybridized for 3 h at 42°C in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–5× Denhardt’s solution (5)–50% formamide–1% sodium dodecyl sulfate (SDS)–100 μg of salmon sperm DNA per ml and then hybridized at 42°C with 10 ng of 32P-labeled probes per ml overnight. Filters were washed twice at room temperature for 5 min each time in 2× SSC–0.1% SDS and then twice at room temperature for 5 min each time in 0.2× SSC–0.1% SDS. Probes were obtained from sequences PCR amplified with the respective yeast Genepairs primers (Research Genetics, Huntsville, Ala.) and labeled with 32P to a specific activity of 108 cpm/μg by the random priming method (5). Quantitation was performed with a Fujifilm BAS1000 imaging system.
RESULTS
To gain insight into the dependence of elongation-defective polymerases on transcription elongation factor SII in vivo, we disrupted the SII gene (we refer to the dst1::hisG allele as dst1) in an isogenic set of haploid cells harboring two previously isolated alleles of RPB2 which render cells sensitive to 6AU (rpb2-4 and rpb2-10) (25). We reasoned that two mutations conferring 6AU sensitivity, one that yields an elongation-defective polymerase and another that yields a DST1 disruption, might generate a synthetic phenotype. Polymerases with the rpb2-10 mutation have a two- to fourfold-lower average elongation rate than that of wild type and are arrest prone in vitro compared to wild-type enzymes, while rpb2-4-containing polymerases display nearly wild-type elongation characteristics (25). PolII with the rpb2-10 mutation is capable of responding to added SII for read-through in vitro (25).
The rpb2-10–dst1 double mutant displays synergistic sensitivity to nucleotide-depleting drugs.
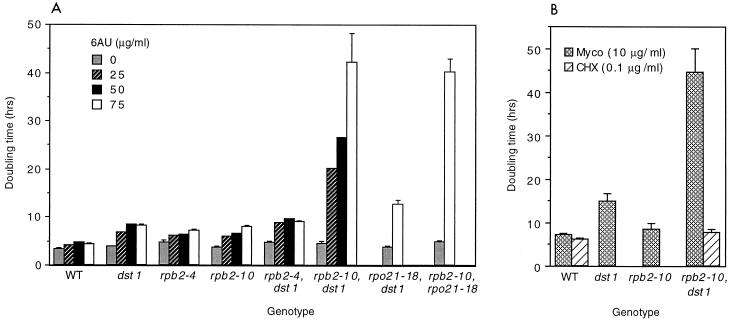
For initial experiments, cells from saturated cultures were diluted into fresh medium with and without 6AU, and the doubling times were calculated following the resumption of growth. This procedure yielded a linear growth response for all strains from which a constant doubling time could be readily defined. In the presence of 6AU, the double mutant was severely growth impaired, with a doubling time of 42 ± 5.9 h (n = 3) in SD containing 75 μg of 6AU per ml (Fig. 1A). This result was far greater than the drug sensitivity displayed by either mutant alone or the sum of both together (8.3 ± 0.2 h [n = 3] for dst1 and 8.0 ± 0.2 h [n = 3] for rpb2-10) (Fig. 1A). The sensitivity of the rpb2-10–dst1 strain, expressed as a ratio of the difference in doubling times in medium with and without 75 μg of 6AU per ml, compared to that of the wild type, was 34-fold. In addition, this effect was allele specific, since rpb2-4, when combined with a nonfunctional DST1 gene, did not display this synergistic effect (7.1 ± 0.4 h [n = 3] for rpb2-4 versus 9.0 ± 0.1 h [n = 3] for rpb2-4–dst1) (Fig. 1A). Hence, there is a synthetic effect of combining two mutations expected to compromise elongation.
FIG. 1.
Doubling times of strains at 30°C. (A) Liquid cultures of strains DY103 (wild type [WT]), DY106 (dst1), DY104 (rpb2-4), DY105 (rpb2-10), DY107 (rpb2-4–dst1), DY108 (rpb2-10–dst1), DY123 (rpo21-18–dst1), and DY124 (rpb2-10–rpo21-18) were grown for 48 h to saturation, diluted, and incubated in SD in the absence or presence of 6AU at 25, 50, and 75 μg/ml, and doubling times were calculated. Error bars indicate the standard deviation of the mean (n = 3) for all strains treated with 0 or 75 μg of 6AU per ml. Values for which no error bars are visible had standard deviations which were too small to permit error bars to be drawn. (B) Cells were grown as described above in SD in the presence of mycophenolic acid (Myco; 10 μg/ml; strains DY103, DY106, DY105, and DY108) or cycloheximide (CHX; 0.1 μg/ml; strains DY103 and DY108), and doubling times were calculated. Error bars indicate the standard deviations of the means (n = 3) for all strains. All strains carry plasmid pRS316 (38) and are Ura+.
The maximal inhibitory effect of 6AU was achieved at drug concentrations of 25 to 50 μg/ml for all strains except for the double mutant, for which increasing 6AU concentrations up to 75 μg/ml yielded a graded increase in cell doubling time (Fig. 1A). The 6AU-sensitive phenotype of the rpb2-4, rpb2-10, and dst1 mutants was initially described by monitoring colony growth on plates (12, 25). The sensitivity of these plating assays readily detected relatively small (1.4- to 1.9-fold) increases in doubling time. The synergistic 6AU sensitivity reported here had a surprisingly large dynamic range that has not been previously described, extending from a 1.4-fold to an almost 10-fold decrease in the growth rate.
Since rpb2-10–dst1 cells exhibited an extremely slow growth phenotype, we tested whether 6AU treatment resulted in lethality. Cells treated for 24 h with 75 μg of 6AU per ml returned to normal growth after removal of the drug, with a doubling time equivalent to that of untreated cells. In addition, the drug-treated cultures had plating efficiencies on solid medium very similar to those of mock-treated cultures (data not shown). Thus, the slow growth phenotype was fully reversible and was not lethal.
Like 6AU, mycophenolic acid also reduces intracellular levels of GTP, and cells lacking SII are sensitive to this drug (12). If the synergistic sensitivity to 6AU observed for the double mutant is representative of a severe transcription elongation defect, then mycophenolic acid should have an effect similar to that of 6AU on the doubling time of the double mutant. Indeed, rpb2-10–dst1 cells displayed a synergistic increase in doubling time compared to that of rpb2-10 or dst1 cells (Fig. 1B).
The synergistic sensitivity displayed by rpb2-10–dst1 cells could be the result of a general hypersensitivity to drugs and not directly related to effects on transcription. To address this possibility, wild-type and mutant cells were treated with the translation inhibitor cycloheximide. The doubling times of wild-type and rpb2-10–dst1 cells treated with 0.1 μg of cycloheximide per ml were not differentially affected (6.1 ± 0.3 h [n = 3] for RPB2 cells versus 7.8 ± 0.7 h [n = 3] for rpb2-10–dst1 cells) (Fig. 1B). Hence, the exquisite growth sensitivity of rpb2-10–dst1 cells does not represent general drug sensitivity. Hypersensitivity to 6AU was verified for the rpb2-10–dst1 double mutant when these mutations were moved into another strain of S. cerevisiae (SJR105) (Table 1). Since rpb2-10 PolII is hypersensitive to arrest but can be assisted by SII in vitro, the extreme 6AU sensitivity of the double mutant likely results from a severe transcription elongation defect in vivo.
The rpb2-10–rpo21-18 double mutant displays severe growth sensitivity to 6AU.
The rpo21-18 mutation is an in-frame linker insertion in RPO21, the gene encoding the largest subunit of PolII, which renders cells sensitive to 6AU and reduces by 50-fold the binding affinity of PolII for SII (2, 3, 45). Our results obtained with rpb2-10–dst1 suggested that interfering with SII function by use of the rpo21-18 mutation in combination with rpb2-10 might also yield a synergistic 6AU-sensitive phenotype. The rpo21-18 mutation was introduced into the RPO21 chromosomal locus in cells with the rpb2-10 mutation, and drug sensitivity was measured (Fig. 1A). The rpb2-10–rpo21-18 double mutant displayed profound 6AU sensitivity (doubling time, 40.1 ± 2.4 h [n = 3]), comparable to that seen with rpb2-10–dst1 cells (Fig. 1A). In contrast, inactivation of DST1 in an rpo21-18 mutant did not yield synergistic sensitivity (Fig. 1A). As expected, the loss of SII had little impact on sensitivity, since the mutant polymerase was already defective in binding SII. These data, coupled with the finding that polymerases with rpb2-10 are slowly elongating, arrest-prone enzymes in vitro (25), strongly suggest that the synthetic drug sensitivity reflects a severe transcription elongation defect.
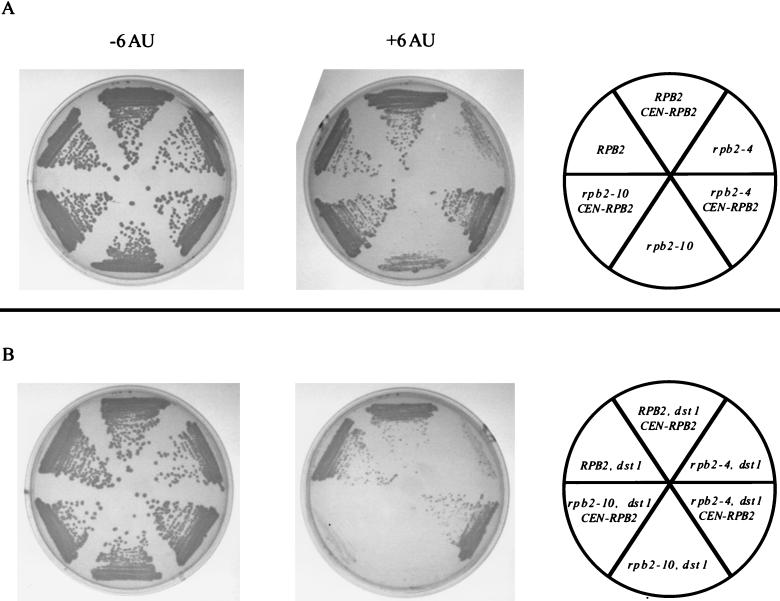
The rpb2-10 mutation becomes dominant upon inactivation of DST1.
The rpb2-4 and rpb2-10 mutations were originally isolated as recessive conditional alleles (34). Here we show that the drug-sensitive phenotype was also recessive, since supplying a wild-type copy of RPB2 rescued the 6AU sensitivity conferred by either mutation (Fig. 2A). However, upon disruption of DST1, the rpb2-10 mutation became dominant, i.e., was not complemented by wild-type RPB2 (Fig. 2B). The dominance was allele specific, since rpb2-4, when combined with a disrupted DST1 gene, remained recessive, as the growth of the cells was restored to a level similar to that with the dst1 mutation alone after a copy of wild-type RPB2 was provided (Fig. 2B).
FIG. 2.
Dominance test of 6AU-sensitive rpb2 alleles. Each strain (DY109 through DY120; Table 1) was transformed with control plasmid YCp50 (30) or plasmid pRP212 (CEN-RPB2) (35) containing the wild-type RPB2 gene followed by selection on SC-Leu-Ura. SD cultures were grown for 48 h to saturation and diluted to an OD600 of 1.0. Three microliters was streaked onto SC-Leu-Ura and grown for 4 days at 30°C in the absence (−6AU) or presence (+6AU) of 6AU (75 μg/ml). (A) Individual rpb2 alleles with or without plasmid-borne RPB2. (B) Individual rpb2 alleles in combination with the disrupted DST1 gene (dst1) and with or without plasmid-borne RPB2.
mRNA levels are severely depressed in 6AU-treated rpb2-10–dst1 cells.
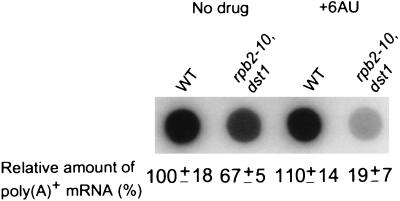
By genetic criteria, rpb2-10–dst1 cells appeared to possess severe transcription elongation defects. We therefore examined levels of poly(A)+ RNA in control and 6AU-treated wild-type and double-mutant cells. Since the average mRNA half-life is 15 min, the hybridization of labeled poly(dT) to equivalent total RNA was used to assess mRNA synthesis rates (8, 9, 18, 39). Poly(A)+ RNA levels in rpb2-10–dst1 cells were reduced to 67% ± 5% (n = 3) of the levels in wild-type cells (Fig. 3). Following treatment with 75 μg of 6AU per ml, RNA levels in mutant cells were reduced further, to 19% ± 7% (n = 3) of the levels in untreated wild-type cells (Fig. 3). In contrast, poly(A)+ RNA levels remained unchanged following 8 h of 6AU treatment in wild-type RPB2 cells (Fig. 3). Levels of poly(A)+ RNA in strains carrying either the dst1 or the rpb2-10 mutation alone were equivalent to wild-type levels in the absence of drug and were reduced to approximately half of untreated wild-type levels following treatment with 6AU (data not shown). Similar amounts of total RNA, as measured by the absorbance at 260 nm, were obtained from equal numbers of cells for all samples analyzed. Indeed, the yields of rRNA from wild-type and mutant cells were comparable (Fig. 4 and data not shown).
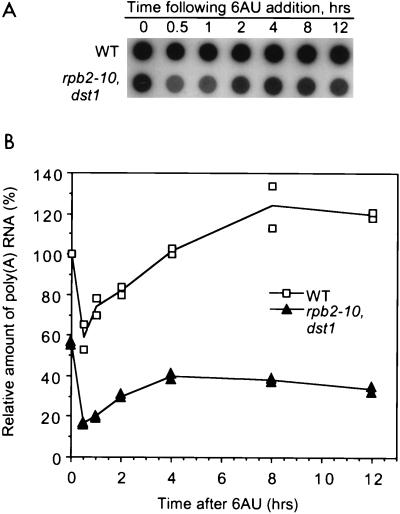
FIG. 3.
Poly(A)+ RNA levels in 6AU-treated wild-type and rpb2-10–dst1 cells. Strains DY103 (wild type [WT]) and DY108 (rpb2-10–dst1) were grown as described in the legend to Fig. 1A in the presence of 6AU (75 μg/ml) for 8 h. Equivalent numbers of cells were harvested, and total RNA was prepared. Equal amounts of RNA (2 μg) were applied to a dot blot in triplicate for each sample, and the filter was probed with [32P]poly(T). A representative image of one experiment is shown. The average of triplicate values was normalized to a value of 100% for untreated wild-type cells. The standard deviation as a percentage of the mean is shown for three independent experiments.
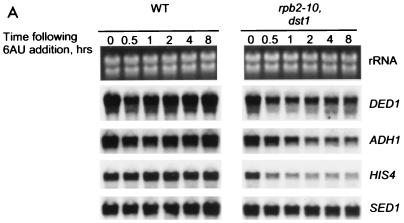
FIG. 4.
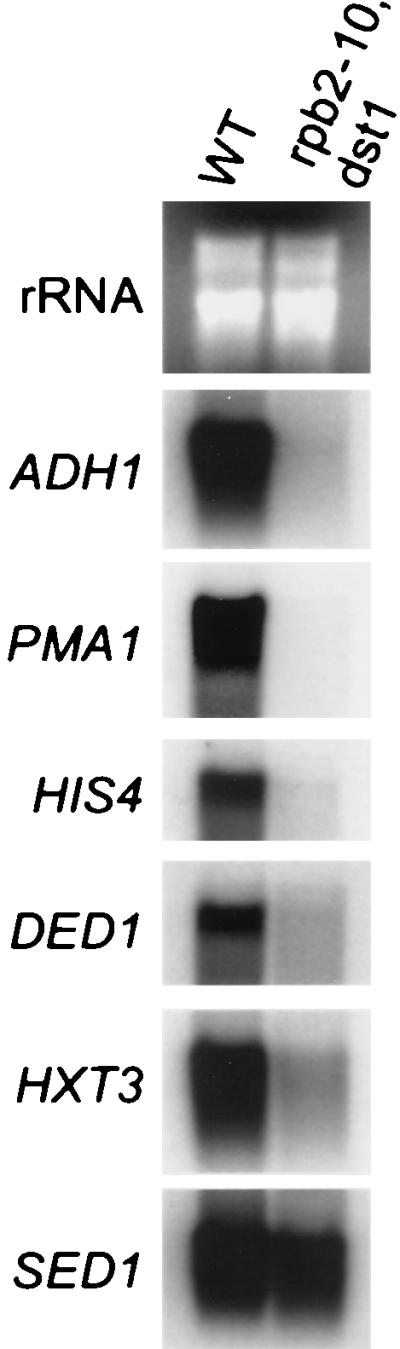
Northern blot analysis of wild-type and rpb2-10–dst1 cells. Strains DY103 (wild type [WT]) and DY108 (rpb2-10–dst1) were grown in the presence of 6AU (75 μg/ml), and total RNA was prepared. Blots were probed with the indicated sequences. rRNA was visualized by ethidium bromide staining.
The steady-state levels of specific mRNAs were examined by Northern blot analysis for ADH1, PMA1, HIS4, DED1, HXT3, and SED1. Following 6AU treatment, mRNA levels were significantly reduced in rpb2-10–dst1 cells for all genes examined except SED1 (Fig. 4). We conclude that the ability of these cells to reenter active growth and synthesize a number of mRNAs is impaired under these conditions.
rpb2-10–dst1 cells are unable to compensate for a rapid 6AU-induced reduction in mRNA levels.
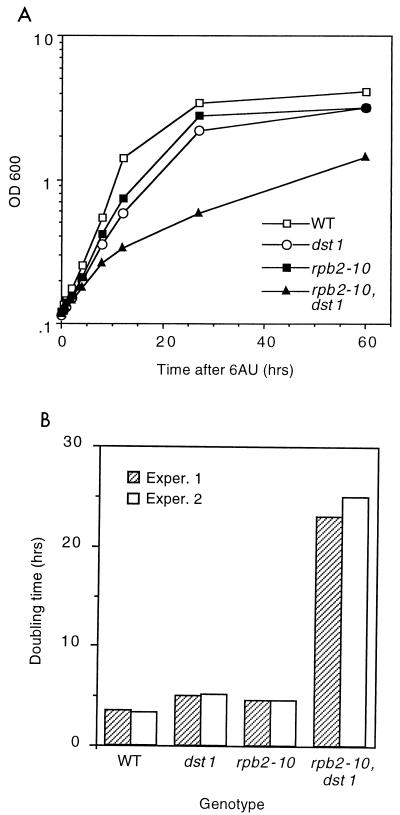
We tested the response of actively growing cells to 6AU by inoculating drug-containing medium with logarithmically growing cells (Fig. 5A). The response of the rpb2-10–dst1 mutant was complex and had two kinetic components, an initial doubling time (0 to 12 h) (Fig. 5A) of 7 h followed by a constant period of doubling every 23 h. While the other strains had reached saturation 27 h after inoculation, the double mutant did not reach a comparable cell density until 90 h after inoculation (Fig. 5A and data not shown). To facilitate a comparison of the effect of the drug across genotypes, we plotted the growth rates of the wild type and the single mutants and the lowest (12 to 60 h) (Fig. 5A) growth rate of the double mutant (Fig. 5B). The growth rate of double-mutant rpb2-10–dst1 cells was 7-fold lower than that of wild-type cells, while the growth rates of single-mutant rpb2-10 and dst1 cells were 1.4- and 1.5-fold lower, respectively, than that of wild-type cells. Hence, rpb2-10–dst1 cells in log growth also display synergistic sensitivity to 6AU. We used this graded response to monitor the time course of the loss of mRNAs.
FIG. 5.
(A) Response of logarithmically growing cells to 6AU. Mid-log-phase cells (OD600, 0.4 to 0.6) of strains DY103 (wild type [WT]), DY105 (rpb2-10), DY106 (dst1), and DY108 (rpb2-10–dst1) were diluted into SD containing 75 μg of 6AU per ml and grown at 30°C. Growth was monitored at OD600 and plotted versus time. (B) Doubling times calculated from the 0- to 12-h (DY103, DY105, and DY106) and 12- to 60-h (DY108) portions of the curves in panel A. Results from two independent experiments (Exper.) are shown.
RNA levels were analyzed for cells in log growth as a function of time after 6AU addition (Fig. 6A). Note that in the absence of drug treatment, rpb2-10–dst1 cells contained only 56% of the poly(A)+ RNA levels of wild-type cells (0 h in Fig. 6B). Surprisingly, 6AU rapidly reduced RNA levels in both wild-type cells and mutant cells 0.5 h after its addition. The reduction was more pronounced in mutant cells (3.3-fold) (Fig. 6B) than in wild-type cells (1.7-fold) (Fig. 6B). After 4 h of treatment, the amount of poly(A)+ RNA in wild-type cells returned to 100% or more of that prior to 6AU addition (Fig. 6B). Mutant cells were able to recover only to a maximum of 70% of their initial RNA levels, even after 12 h (Fig. 6B). We did not observe the rapid reduction in poly(A)+ RNA levels when mock-treated cells were diluted into fresh medium (data not shown). Regardless of genotype, 6AU has a recognizable effect on mRNA levels, most likely at the level of synthesis. Compared to the wild type, the double mutant was defective in its response to the drug.
FIG. 6.
Poly(A)+ RNA levels in logarithmically growing wild-type and rpb2-10–dst1 cells treated with 6AU. (A) Strains DY103 (wild type [WT]) and DY108 (rpb2-10–dst1) were grown as described in the legend to Fig. 5. At the indicated times, equivalent numbers of cells were harvested, and total RNA was prepared. Equal amounts of RNA (2 μg) were applied to a dot blot in duplicate for each sample, and the filter was probed with [32P]poly(T). A representative image of one experiment is shown. (B) Results were quantified and plotted. Each point represents the average of duplicate values and was normalized to a value of 100% for wild-type cells at time zero. Values from two independent growth experiments are shown. Lines connect averages of duplicate values for each time.
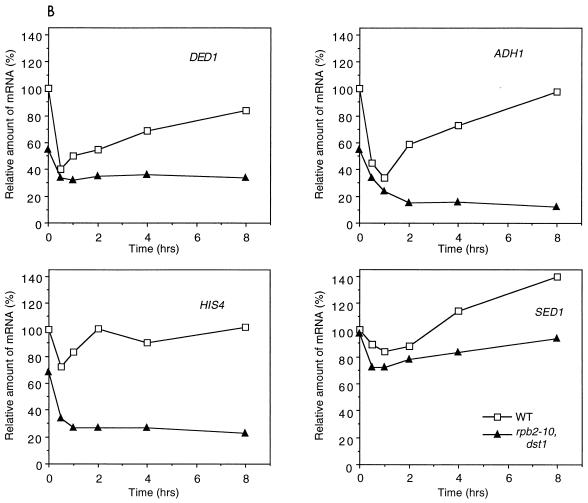
We used Northern blot analysis to investigate the effect of 6AU on the steady-state levels of specific mRNAs (Fig. 7A). Again, the mutations in rpb2-10–dst1 cells reduced the abundance of DED1, ADH1, and HIS4 mRNAs relative to the levels in wild-type cells. Following the addition of 6AU, the levels of DED1 and ADH1 mRNAs declined rapidly in both types of cells, while the reduction in HIS4 mRNA levels was less pronounced in wild-type cells (Fig. 7B). The levels of DED1, ADH1, and HIS4 transcripts in rpb2-10–dst1 cells remained severely depressed relative to those in wild-type cells, which recovered to starting levels (Fig. 7B). The starting levels of SED1 mRNA were comparable in wild-type and mutant cells and were only slightly reduced by 6AU after 1 h (Fig. 7B). rRNA levels in both types of cells did not significantly change over this interval following treatment with 6AU (Fig. 7A).
FIG. 7.
Northern blot analysis of logarithmically growing wild-type and rpb2-10–dst1 cells treated with 6AU. (A) Strains DY103 (wild type [WT]) and DY108 (rpb2-10–dst1) were grown as described in the legend to Fig. 5, and total RNA was prepared. Blots were probed with the indicated sequences. rRNA was visualized by ethidium bromide staining. (B) Results were quantified and plotted. Each point represents the value for each band normalized to a value of 100% for wild-type cells at time zero.
The dominance of rpb2-10 in the absence of DST1 is reflected in reduced RNA levels.
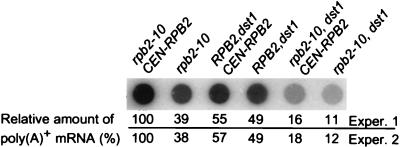
To further explore the association of growth rate with RNA content, we examined levels of poly(A)+ RNA in RPB2 merodiploids treated with 6AU (Fig. 2). Consistent with the ability of RPB2 to restore growth, the 60% reduction in poly(A)+ RNA levels in the rpb2-10 mutant could be reversed by providing a wild-type copy of RPB2 (Fig. 8). Predictably, RNA levels were relatively unchanged in RPB2–dst1 cells after an additional copy of RPB2 was supplied (Fig. 8). Providing 6AU-sensitive rpb2-10–dst1 cells with a wild-type RPB2 allele resulted in only a small increase in RNA levels (≈11 to ≈16% of wild-type levels), which was significantly smaller than that in RPB2–dst1 cells (≈50% of wild-type levels) (Fig. 8). Thus, RNA levels correlate with the apparent dominance of the rpb2-10 allele in the presence of wild-type RPB2 and the increased 6AU sensitivity of rpb2-10–dst1 cells.
FIG. 8.
Poly(A)+ RNA levels in rpb2-10, RPB2–dst1, and rpb2-10–dst1 cells carrying a wild-type copy of RPB2. Strains DY117 (rpb2-10–CEN-RPB2), DY111 (rpb2-10), DY118 (RPB2–dst1–CEN-RPB2), DY112 (RPB2-dst1), DY120 (rpb2-10–dst1–CEN-RPB2), and DY114 (rpb2-10–dst1) were grown as described in the legend to Fig. 1 in the presence of 6AU (75 μg/ml) for 8 h. Equivalent numbers of cells were harvested, and total RNA was prepared. Equal amounts of RNA (2 μg) were applied to a dot blot in duplicate for each sample, and the filter was probed with [32P]poly(T). A representative image of one experiment is shown. The average of duplicate values normalized to a value of 100% for strain DY117 (rpb2-10–CEN-RPB2) is presented for two independent growth experiments (Exper.).
DISCUSSION
The extent to which PolII gene expression depends upon transcription elongation factors in vivo is unknown. In this study, we investigated the sensitivity to nucleotide-depleting drugs of a collection of strains carrying mutations in different components of the transcription elongation machinery. We found that, depending on the combination of mutations, there was a wide range of relative sensitivity to these drugs. The most severe case was seen when a mutation which yields a slowly elongating and arrest-prone RNA PolII enzyme (rpb2-10) was introduced into cells lacking elongation factor SII. The result was a dramatic reduction in the levels of both total poly(A)+ RNA and individual transcripts. This is, to our knowledge, the first report that 6AU treatment reduces mRNA levels in living cells and the first indication that a mutation in DST1 can affect mRNA levels.
In contrast to the levels of total RNA and other individual transcripts, we found that the levels of SED1 transcripts in the rpb2-10–dst1 mutant did not decline following treatment with 6AU. SED1 mRNA is abundant and encodes a major cell surface glycoprotein expressed during postdiauxic growth (10a, 16a). The SED1 message may have an unusually long half-life, or its transcription may be resistant to these mutations and effects of nucleotide depletion. This gene appears to be in a unique category in this regard.
Our results also provide evidence for a genetic interaction between the second largest subunit of RNA PolII and elongation factor SII. This evidence complements the prior finding of a genetic interaction between SII and the RPO21 product, the largest PolII subunit (3). Mutations in RPO21 were shown to impair the ability of SII to bind RNA PolII (45). It is unlikely that the rpb2-10 mutation affects SII binding, since it is found near the catalytic pocket of the enzyme and influences the elongation properties of PolII in the absence of SII (25). Instead, we interpret this genetic interaction with the second largest subunit to reflect the ability of elongation-compromised PolII to use SII to facilitate elongation in vivo.
Sensitivity to 6AU is widely used in the transcription field as an assay for elongation defects. We propose that the phenotypes displayed by the mutants in this study are entirely consistent with data from in vitro biochemistry and represent various degrees of transcription elongation impairment in vivo. A slowly elongating polymerase with the rpb2-10 mutation would be expected to be hyperarresting. Combining the rpb2-10 and dst1 alleles results in an arrested polymerase which cannot benefit from the elongation-stimulating activity of SII. The result is severely compromised elongation, which yields synergistic sensitivity to 6AU. This finding is mirrored in cells containing PolII with both the rpb2-10 and the rpo21-18 mutations. This polymerase is compromised for elongation and, as the result of a binding defect, cannot use SII. Since the dst1 and rpo21-18 mutations both render polymerase unable to use SII and are functionally redundant, the rpo21-18–dst1 double mutant does not display synergistic sensitivity to 6AU. Perhaps 6AU-sensitive mutations in RPB2 which prevent the binding of SII could be isolated. We predict that these mutations, combined with the dst1 allele, would also not result in synergistic sensitivity. Whereas the overexpression of SII in vivo rescues the 6AU sensitivity conferred by RPO21 mutations that reduce SII binding (3), SII overexpression does not rescue the 6AU sensitivity conferred by the rpb2-4 or rpb2-10 mutation (data not shown). This result also suggests that the rpo21-18 and rpb2-10 mutations tested here are functionally distinct.
The fact that the otherwise recessive rpb2-10 allele became dominant upon inactivation of DST1 suggests that crippled rpb2-10 enzymes accumulate on genes to a level that can impede the ability of the wild-type enzyme to carry out gene expression. This phenotype is reminiscent of rpoB mutations in Escherichia coli which render the holoenzyme unable to clear a promoter and are dominant lethal when expressed in vivo (20–22, 32, 46). It is thought that this phenotype results from mutant polymerases occupying and occluding genes, thereby preventing their efficient transcription by wild-type polymerase (20, 32, 46).
Experiments measuring the effect of 6AU on cells in the logarithmic phase of growth revealed a complex growth response for the double mutant. We therefore chose to examine the 6AU sensitivity of the collection of mutants by diluting saturated cultures and monitoring their return to active growth. This approach, which measures the ability of cells to recover from a postdiauxic rate of growth, provided a clear and quantifiable means of measuring doubling times and was a robust assay for defining the genetic interaction between RPB2 and DST1. The synergistic sensitivity and mRNA synthesis defect of the rpb2-10–dst1 mutant were readily apparent, regardless of the initial phase of growth of the test cultures (Fig. 1 and 5). The slowing of growth displayed by cells at the end of the logarithmic phase is accompanied by a decrease in overall transcription (8, 43, 44). Our results are consistent with the apparent need for postexponential cells to turn on a large number of genes and that this process is particularly difficult for rpb2-10–dst1 cells in the presence of 6AU. Although these experiments do not reveal where in the pathway of gene expression 6AU exerts its effect in the double mutant, the simplest interpretation is that the inability of these cells to exit slow growth results from a defect in transcript elongation.
Following treatment with 6AU, the unexpected early phase of reduction of total RNA levels regardless of genotype is direct evidence that NTP depletion alone has an impact upon transcription in vivo. We suggest that the basis for the failure of the rpb2-10–dst1 double mutant to compensate for this reduction is its failure to mount a transcriptional response. The rapid and global effects on RNA levels of 6AU and the elongation-perturbing mutations described here are very similar to the effects of conditional mutations in the RNA PolII, TFIIB, TFIID, TFIIH, and SRB genes, suggesting that SII may act as a general elongation factor (10, 16, 18, 27, 33, 39, 40).
ACKNOWLEDGMENTS
We thank R. A. Young, J. D. Friesen, G. Crouse, S. Jinks-Robertson, and S. T. Warren for materials. We thank R. A. Young, J. D. Friesen, and colleagues at Emory University for helpful discussions.
This work was funded by NIH grant GM46331 to D.R. J.C.L. was supported by NIH training grant GM08490.
REFERENCES
- 1.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archambault J, Drebot M A, Stone J C, Friesen J D. Isolation and phenotypic analysis of conditional-lethal, linker-insertion mutations in the gene encoding the largest subunit of RNA polymerase II in Saccharomyces cerevisiae. Mol Gen Genet. 1992;232:408–414. doi: 10.1007/BF00266244. [DOI] [PubMed] [Google Scholar]
- 3.Archambault J, Lacroute F, Ruet A, Friesen J D. Genetic interaction between transcription elongation factor TFIIS and RNA polymerase II. Mol Cell Biol. 1992;12:4142–4152. doi: 10.1128/mcb.12.9.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aso T, Conaway J W, Conaway R C. The RNA polymerase II elongation complex. FASEB J. 1995;9:1419–1428. doi: 10.1096/fasebj.9.14.7589983. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates/Wiley-Interscience; 1988. [Google Scholar]
- 6.Bengal E, Flores O, Krauskopf A, Reinberg D, Aloni Y. Role of the mammalian transcription factors IIF, IIS, and IIX during elongation by RNA polymerase II. Mol Cell Biol. 1991;11:1195–1206. doi: 10.1128/mcb.11.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradsher J N, Tan S, McLaury H-J, Conaway J W, Conaway R C. RNA polymerase II transcription factor SIII. I. Identification, purification, and properties. J Biol Chem. 1993;268:25587–25593. [PubMed] [Google Scholar]
- 8.Choder M. A general topoisomerase I-dependent transcriptional repression in the stationary phase in yeast. Genes Dev. 1991;5:2315–2326. doi: 10.1101/gad.5.12a.2315. [DOI] [PubMed] [Google Scholar]
- 9.Choder M, Young R A. A portion of RNA polymerase II molecules has a component essential for stress responses and stress survival. Mol Cell Biol. 1993;13:6984–6991. doi: 10.1128/mcb.13.11.6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cormack B P, Struhl K. The TATA-binding protein is required for transcription by all three nuclear RNA polymerases in yeast cells. Cell. 1992;69:685–696. doi: 10.1016/0092-8674(92)90232-2. [DOI] [PubMed] [Google Scholar]
- 10a.DeRisi J L, Iyer V R, Brown P O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 11.Early M C, Crouse G F. Selectable cassettes for simplified construction of yeast gene disruption vectors. Gene. 1996;169:111–113. doi: 10.1016/0378-1119(95)00805-5. [DOI] [PubMed] [Google Scholar]
- 12.Exinger G, Lacroute F. 6-Azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr Genet. 1992;22:9–11. doi: 10.1007/BF00351735. [DOI] [PubMed] [Google Scholar]
- 13.Flores O, Ha I, Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II. Purification and subunit composition of transcription factor IIF. J Biol Chem. 1990;265:5629–5634. [PubMed] [Google Scholar]
- 14.Gietz D, St. Jean A, Woods R A, Schiestl R H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu W, Reines D. Identification of a decay in transcription potential that results in elongation factor-dependence of RNA polymerase II. J Biol Chem. 1995;270:11238–11244. doi: 10.1074/jbc.270.19.11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzder S N, Qiu H, Sommers C H, Sung P, Prakash L, Prakash S. DNA repair gene RAD3 of S. cerevisiae is essential for transcription by RNA polymerase II. Nature. 1994;367:91–94. doi: 10.1038/367091a0. [DOI] [PubMed] [Google Scholar]
- 16a.Hardwick K G, Boothroyd J C, Rudner A D, Pelham H R B. Genes that allow yeast cells to grow in the absence of the HDEL receptor. EMBO J. 1992;11:4187–4195. doi: 10.1002/j.1460-2075.1992.tb05512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartzog G A, Wada T, Handa H, Winston F. Evidence that SPT4, SPT5, and SPT6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev. 1998;12:357–369. doi: 10.1101/gad.12.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrick D, Parker R, Jacobson A. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:2269–2284. doi: 10.1128/mcb.10.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubert J-C, Guyonvarch A, Kammerer B, Exinger F, Liljelund P, Lacroute F. Complete sequence of a eukaryotic regulatory gene. EMBO J. 1983;2:2071–2073. doi: 10.1002/j.1460-2075.1983.tb01702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashlev M, Lee J, Zalenskaya K, Nikiforov V, Goldfarb A. Blocking of the initiation-to-elongation transition by a transdominant RNA polymerase mutation. Science. 1990;248:1006–1009. doi: 10.1126/science.1693014. [DOI] [PubMed] [Google Scholar]
- 21.Landick R, Stewart J, Lee D N. Amino acid changes in conserved regions of the β-subunit of Escherichia coli RNA polymerase alter transcription pausing and termination. Genes Dev. 1990;4:1623–1636. doi: 10.1101/gad.4.9.1623. [DOI] [PubMed] [Google Scholar]
- 22.Lee J Y, Zalenskaya K, Shin Y K, McKinney J D, Park J H, Goldfarb A. Expression of cloned rpoB gene of Escherichia coli: a genetic system for the isolation of dominant negative mutations and overproduction of defective beta subunit of RNA polymerase. J Bacteriol. 1989;171:3002–3007. doi: 10.1128/jb.171.6.3002-3007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakanishi T, Nakuno A, Nomura K, Sekimizu K, Natori S. Purification, gene cloning, and gene disruption of the transcription factor SII in Saccharomyces cerevisiae. J Biol Chem. 1992;267:13200–13204. [PubMed] [Google Scholar]
- 24.Nakanishi T, Shimoaraiso M, Natori S. Structure-function relationship of yeast SII in terms of stimulation of RNA polymerase II, arrest relief, and suppression of 6-azauracil sensitivity. J Biol Chem. 1995;270:8991. doi: 10.1074/jbc.270.15.8991. [DOI] [PubMed] [Google Scholar]
- 25.Powell W, Reines D. Mutations in the second largest subunit of RNA polymerase II cause 6-azauracil sensitivity in yeast and increased transcriptional arrest in vitro. J Biol Chem. 1996;271:6866–6873. doi: 10.1074/jbc.271.12.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price D H, Sluder A E, Greenleaf A L. Dynamic interaction between a Drosophila transcription factor and RNA polymerase II. Mol Cell Biol. 1989;9:1465–1475.8995. doi: 10.1128/mcb.9.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu H, Park E, Prakash L, Prakash S. The Saccharomyces cerevisiae DNA repair gene RAD25 is required for transcription by RNA polymerase II. Genes Dev. 1993;7:2161–2171. doi: 10.1101/gad.7.11.2161. [DOI] [PubMed] [Google Scholar]
- 28.Reines D. Nascent RNA cleavage by transcription elongation complexes. In: Conaway R C, Conaway J W, editors. Transcription mechanisms and regulation. New York, N.Y: Raven Press; 1994. pp. 263–278. [Google Scholar]
- 29.Reines D, Conaway J W, Conaway R C. The RNA polymerase II general elongation factors. Trends Biochem Sci. 1996;21:351–355. [PMC free article] [PubMed] [Google Scholar]
- 30.Rose M D, Novick P, Thomas J H, Bostein D, Fink G R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- 31.Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–309. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- 32.Sagitov V, Nikiforov V, Goldfarb A. Dominant lethal mutations near the 5′ substrate binding site affect RNA polymerase propagation. J Biol Chem. 1993;268:2195–2202. [PubMed] [Google Scholar]
- 33.Sakurai H, Ohishi T, Fukasawa T. Promoter structure-dependent functioning of the general transcription factor IIE in Saccharomyces cerevisiae. J Biol Chem. 1997;272:15936–15942. doi: 10.1074/jbc.272.25.15936. [DOI] [PubMed] [Google Scholar]
- 34.Scafe C, Nonet M, Young R A. RNA polymerase II mutants defective in transcription of a subset of genes. Mol Cell Biol. 1990;10:1010–1016. doi: 10.1128/mcb.10.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scafe C, Martin C, Nonet M, Podos S, Okamura S, Young R A. Conditional mutations occur predominantly in highly conserved residues of RNA polymerase II subunits. Mol Cell Biol. 1990;10:1271–1275. doi: 10.1128/mcb.10.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–20. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 37.Shilatifard A, Lane W S, Jackson K W, Conaway R C, Conaway J W. An RNA polymerase II elongation factor encoded by the human ELL gene. Science. 1996;271:1873–1876. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 38.Sikorski R, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson C M, Young R A. General requirement for RNA polymerase II holoenzymes in vivo. Proc Natl Acad Sci USA. 1995;92:4587–4590. doi: 10.1073/pnas.92.10.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tijerina P, Sayre M H. A debilitating mutation in transcription factor IIE with differential effects on gene expression in yeast. J Biol Chem. 1998;273:1107–1113. doi: 10.1074/jbc.273.2.1107. [DOI] [PubMed] [Google Scholar]
- 41.Uptain S M, Kane C M, Chamberlin M J. Basic mechanisms of transcript elongation and its regulation. Annu Rev Biochem. 1997;66:117–172. doi: 10.1146/annurev.biochem.66.1.117. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Kohlami S E, Cutler A J. An improved method for polymerase chain reaction using whole yeast cells. Anal Biochem. 1996;237:145–146. doi: 10.1006/abio.1996.0213. [DOI] [PubMed] [Google Scholar]
- 43.Werner-Washburne M, Becker J, Kosic-Smithers J, Craig E A. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989;171:2680–2688. doi: 10.1128/jb.171.5.2680-2688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werner-Washburne M, Braun E, Johnston G C, Singer R A. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993;57:383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Awrey D E, Edwards A M, Archambault J, Friesen J D. In vitro characterization of mutant yeast RNA polymerase II with reduced binding for elongation factor TFIIS. Proc Natl Acad Sci USA. 1996;93:1152–1157. doi: 10.1073/pnas.93.21.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaychikov E, Martin E, Denissova L, Kozlov M, Markovtsov V, Kashlev M, Heumann H, Nikiforov V, Goldfarb A, Mustaev A. Mapping of catalytic residues in the RNA polymerase active center. Science. 1996;273:107–109. doi: 10.1126/science.273.5271.107. [DOI] [PubMed] [Google Scholar]