Abstract
Background:
Standardized practices are needed in the analysis of inflammation biomarker values outside limits of detection (LOD) when used for inflammation correction of nutritional biomarkers.
Objective:
We assessed the direction and extent to which serum C-reactive protein (CRP) and alpha-1-acid-glycoprotein (AGP) values outside LODs (<0.05 mg/L and >4.0 g/L, respectively) affect inflammation regression correction of serum ferritin and compared approaches to addressing such values when estimating inflammation-adjusted ferritin and iron deficiency (ID).
Methods:
Examined 29 cross-sectional datasets from 7 countries with reproductive-age women (15–49y) (n=12,944), preschool-age children (6–59m) (n=18,208) and school-age children (6–14y) (n=4,625). For each dataset, we compared 6 analytic approaches for addressing CRP <LOD: listwise deletion, single imputation (lower, middle, or upper bound; LOD/√2; random number), with multiple imputation (MI). For each approach, inflammation-adjusted ferritin and ID using BRINDA regression correction were estimated. We calculated deviance of each estimate from that given by MI within each dataset and performed fixed effects multivariate meta-regression with analytic approach as moderator to compare the reliability of each approach to MI.
Results:
Across datasets, observations outside LOD ranged from 0.0 to 35.0% of CRP values and 0.0 to 2.5% of AGP values. Pooled deviance estimates for mean ferritin (μg/L) and ID (percentage points) were: listwise deletion −0.46 (95%CI: −0.76, −0.16) and 0.14 (−0.43, 0.72), lower bound 0.45 (0.14, 0.76) and −0.36 (−0.91, 0.20), middle bound −0.21 (−0.51, 0.09) and 0.22 (−0.34, 0.79), LOD/√(2) −0.26 (−0.57, 0.04) and 0.25 (−0.31, 0.81), upper bound −0.31 (−0.61, −0.01) and 0.30 (−0.27, 0.86), and random number −0.08 (−0.38, 0.22) and 0.11 (−0.46, 0.67). There was moderation by approach in the ferritin model (p<0.001).
Conclusions:
Findings demonstrate the need for standardized analyses of inflammation biomarker values outside LODs and suggest that random number single imputation may be a reliable and feasible alternative to MI for CRP <LOD.
Keywords: limit of detection, inflammation, iron deficiency, regression correction, imputation, left censored, acute phase proteins
Background
Iron deficiency is a cause of an estimated 30 million disability-adjusted life-years (1), and accurate quantification of the condition is needed to design and evaluate population interventions to address it. Biomarkers of iron status including serum ferritin and soluble transferrin receptor (sTfR) are influenced by systemic inflammation (2), which can lead to inaccurate identification of iron deficiency. For example, Ferritin concentration increases in the presence of inflammation resulting in an underestimation of iron deficiency in both individuals and populations. Several methods have been used to account for the influence of inflammation on ferritin for defining iron deficiency. One approach is to raise ferritin cut-points when infection or inflammation are present in individuals (3). In settings with suspected or known infection or inflammation, concurrent measurement of ferritin with C-reactive protein (CRP) and alpha-1-acid glycoprotein (AGP) is recommended to adjust for inflammation using one of a variety of methods including raising fixed ferritin cut-points, excluding persons with systemic inflammation, or applying arithmetic or regression correction factors based on CRP and AGP to ferritin values (3).
The regression correction approach developed by the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project has become a commonly used method of adjusting ferritin for inflammation in population-based surveys. The BRINDA approach can simultaneously account for CRP, a marker of acute infection, and AGP, a marker of chronic infection or convalescence, and accounts for the effects of inflammation at the full range of measured CRP and AGP concentrations (2, 4). The simplified BRINDA regression equation follows:
where CRPreference is 0.10 mg/L for preschool-age children (PSC) or 0.16 mg/L for nonpregnant women of reproductive age (WRA) and AGPreference is 0.59 g/L for PSC or 0.54 g/L for WRA or the maximum values of the lowest decile of CRP and AGP, respectively, within a population (5). For school-age children (SAC) there is no external reference, due to limited numbers of surveys compiled by the BRINDA working group, and thus the lowest decile within a survey is used. The references values for CRP and AGP represent an apparently healthy population with no inflammation, and ferritin levels at or below these values within a sample are not adjusted for inflammation.
A unique requirement for the BRINDA approach is continuous measures of inflammation. However, biological assays are rarely without limits of detection (LOD), the minimum or maximum concentration distinguishable from “analytical noise”, which censor values outside these limits (6). For example, a commonly used and cost-effective sandwich enzyme-linked immunosorbent assay (ELISA) from the VitMin Lab (Willstaett, Germany) (7) reports a lower LOD for CRP of 0.05 mg/L and an upper LOD for AGP of 4.0 g/L at the time of this analysis [Personal communication, Juergen Georg Erhardt, VitMin Lab (Willstaett, Germany)] Until 2018, the practice of this lab was to report measured values down to and including zero. Analysts, therefore, decide how to account for imprecision at the lowest concentrations of CRP.
O’Callaghan and Roth recently called for standardization of nutrition biomarker data, highlighted inconsistencies in laboratory practices and reporting in nutrition studies and pointed to the potential for non-negligible effects of variable LODs (8). Even when using identical laboratory practices and LODs, researchers must decide how to treat values outside limits of detection, especially when excluding such values from analysis has the potential to bias results (9). In the case of CRP, for example, excluding values below the lower LOD means excluding the “least inflamed” individuals from estimates of inflammation-adjusted ferritin and the prevalence of iron deficiency. Thus, excluding values outside limits of detection (listwise deletion) may be problematic and imputation methods may be more useful.
Some common single imputation methods for values less than the LOD are using the lower bound (zero), middle bound (half LOD), the LOD divided by the square root of 2, upper bound (LOD), and a constrained random number. Imputing values exceeding upper LOD is less common (10). Multiple imputation is considered the standard for addressing missing data (9), but it relies on various assumptions about missingness and requires a high level of technical capacity.
Our study was guided by two objectives: 1) describe how and to what extent CRP and AGP values outside LODs affect regression correction of ferritin for inflammation and 2) compare analytic methods of addressing LOD values in estimating inflammation-adjusted ferritin and iron deficiency.
Methods
Data Sources
We focused on household nutrition surveys conducted in low- and middle-income countries with technical assistance from the Centers for Disease Control and Prevention’s (CDC) International Micronutrient Malnutrition Prevention and Control team during the previous 10 years. To limit variation in laboratory methods and because it is the preferred assay in low-resource settings which often have a high burden of inflammation, we focused on 16 surveys with data from the VitMin Lab (Willstaett, Germany): 9 surveys had data from WRA (n=12,944), 15 had PSC (n=18,208), and 5 had SAC (n=4,625) (Table 1). Eight of the surveys were nationally representative, and eight were part of population-based program evaluations. Details of each survey are reported elsewhere (11–25). All surveys received ethical approval in their respective countries and were conducted in line with their ethical standards. The present secondary analysis used deidentified datasets with permission from partner institutions within each included country and CDC considered it public health practice and nonhuman subjects research and exempt from IRB review.
Table 1:
Summary of Data Sources and Basic Characteristics of Participants of 29 Surveys Across 7 Countries Examining Analytic Approaches to Address Limits of Detection in Inflammatory Biomarkers 1
| Country (reference) | Year | Population 2 | n | Age, y or mo 3 | Female, % | Malaria, % 4 | CRP>5 mg/L, % 5 | CRP<0.05 mg/L, % 6 | AGP>1 g/L, % 7 | AGP>4 g/L, % 8 |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Democratic Republic of Congo (19) | 2011 | PSC | 1,281 | 11 (6, 17) | 47.2 | 21.6 | 42.2 | 2.3 | 57.4 | 0.0 |
| Democratic Republic of Congo (19) | 2015 | PSC | 1,300 | 11 (6, 17) | 50.9 | 41.2 | 43.1 | 0.2 | 60.5 | 0.0 |
| Ghana (25) | 2019 | WRA | 949 | 17 (15, 22) | 100.0 | 30.5 | 5.1 | 35.0 | 7.3 | 0.0 |
| SAC | 621 | 13 (10, 14) | 70.6 | 40.3 | 7.8 | 32.4 | 8.1 | 0.0 | ||
| Guatemala (22) | 2013 | WRA | 1,639 | 28 (15, 49) | 100.0 | - | 16.9 | 0.6 | 16.8 | 0.0 |
| PSC | 862 | 33 (6, 59) | 50.5 | - | 12.5 | 1.5 | 21.0 | 0.0 | ||
| Guatemala (21) | 2015 | WRA | 1,514 | 29 (15, 49) | 100.0 | - | 15.7 | 0.1 | 8.5 | 0.0 |
| PSC | 682 | 34 (6, 59) | 50.5 | - | 11.9 | 4.1 | 15.3 | 0.0 | ||
| Guatemala (20) | 2016 | WRA | 1,490 | 29 (15, 49) | 100.0 | - | 16.1 | 0.5 | 9.5 | 0.1 |
| PSC | 581 | 35 (6, 59) | 51.8 | - | 11.5 | 1.9 | 16.4 | 0.1 | ||
| Guatemala (24) | 2017/18 | WRA | 1,494 | 30 (15, 49) | 100.0 | - | 22.0 | 0.0 | 14.7 | 0.0 |
| PSC | 570 | 35 (6, 59) | 49.0 | - | 12.3 | 2.3 | 17.5 | 0.0 | ||
| SAC | 1,011 | 10 (6, 14) | 54.4 | - | 5.0 | 1.8 | 13.3 | 0.0 | ||
| Guatemala (23) | 2018/19 | WRA | 1,507 | 30 (15, 49) | 100.0 | - | 17.5 | 11.6 | 12.6 | 0.3 |
| PSC | 599 | 34 (6, 59) | 48.9 | - | 11.4 | 31.4 | 14.0 | 0.0 | ||
| Malawi (18) | 2009 | WRA | 591 | 27 (15, 49) | 100.0 | 5.0 | 14.6 | 1.2 | 9.3 | 0.0 |
| PSC | 1,016 | 30 (6, 59) | 52.4 | 20.3 | 39.3 | 1.6 | 44.9 | 0.0 | ||
| SAC | 646 | 9 (5, 17) | 50.8 | 16.6 | 18.0 | 4.5 | 19.0 | 0.0 | ||
| Malawi (17) | 2015/16 | WRA | 776 | 27 (15, 49) | 100.0 | 14.9 | 8.9 | 3.1 | 12.6 | 0.3 |
| PSC | 1,102 | 33 (6, 59) | 49.3 | 26.2 | 25.3 | 2.5 | 57.8 | 1.6 | ||
| SAC | 758 | 9 (5, 14) | 51.6 | 37.9 | 16.4 | 5.0 | 33.1 | 0.3 | ||
| Mozambique (16) | 2015 | PSC | 1,230 | 18 (6, 59) | 52.5 | 29.6 | 39.8 | 0.1 | 61.1 | 0.4 |
| Nepal (11) | 2012/13 | PSC | 2,347 | 14 (6, 23) | 52.9 | 0.0 | 18.2 | 1.4 | 39.0 | 0.0 |
| Nepal (15) | 2016 | PSC | 2,203 | 14 (6, 23) | 45.2 | 0.1 | 16.8 | 0.3 | 31.6 | 0.2 |
| Nepal (14) | 2016 | WRA | 2,984 | 24 (15, 49) | 100.0 | 0.0 | 4.4 | 4.4 | 5.7 | 0.0 |
| PSC | 1,651 | 34 (6, 59) | 49.3 | 0.0 | 10.1 | 3.4 | 26.5 | 0.1 | ||
| SAC | 1,589 | 12 (10, 14) | 62.4 | 0.0 | 2.3 | 10.4 | 5.2 | 0.0 | ||
| Uganda (13) | 2015 | PSC | 1,249 | 18 (12, 23) | 47.5 | 48.9 | 34.5 | 0.7 | 63.5 | 2.5 |
| Uganda (12) | 2016 | PSC | 1,535 | 17 (12, 23) | 47.2 | 48.8 | 33.9 | 0.5 | 55.4 | 2.3 |
Unweighted estimates.
PSC, Preschool-Age Children; SAC, School-Age Children; WRA, Women of Reproductive Age
Age in years for WRA and SAC. Age in months for PSC. Values median (range).
Malaria rapid diagnostic test.
CRP, Serum C-reactive protein; Proportion of CRP >5 mg/L, commonly used cut-point indicating the presence of systemic inflammation (34, 35).
Proportion outside limit of detection for CRP.
AGP, Serum alpha-1-acid glycoprotein; Proportion of AGP >1 g/L, commonly used cut-point indicating the presence of systemic inflammation (34).
Proportion outside limit of detection for AGP.
Laboratory Measures
VitMin Lab’s combination sandwich ELISA technique concurrently measures ferritin, retinol-binding protein, sTfR, CRP, and AGP from serum or plasma (7). This assay produces results for CRP with a lower LOD of <0.05 mg/L (no upper LOD) and results for AGP with an upper LOD of >4.0 g/L (no lower LOD). We focused the present analysis on ferritin because it is the recommended biomarker for assessing population iron deficiency and is a sensitive measure of iron stores.
Statistical Analyses
For each survey and by population group, basic characteristics including age, sex, and malaria status were presented as median (minimum, maximum) or percent. Within each survey and population group, we also examined the proportion (%) of CRP and AGP values falling outside LODs and the proportion exceeding commonly used cut-points for inflammation (CRP> 5 mg/L and AGP >1 g/L). Because of the very low proportion of AGP observations greater than the LOD (Table 1), we imputed the LOD value (4.0 g/L) for these cases and focused primarily on CRP. We examined seven candidate analytic approaches for addressing the left censored CRP values. The first analytic approach was listwise deletion defined as using only a sub-sample of observations with CRP ≥ 0.05 mg/L in formulaic analysis. The following five approaches used single imputation: lower bound (0.0 imputed), middle bound (0.025 imputed), LOD/√2 (0.354 imputed), upper bound (0.05 imputed), and a uniform random number between 0.0 and 0.05 imputed once where values were <0.05 mg/L. For the final approach, we used multiple imputation by monotone method modeled on AGP and constrained by 0.0 and 0.05 with 100 iterations (26–28). Multiple imputation was considered the gold standard approach.
We calculated inflammation-adjusted ferritin and iron deficiency (WRA and SAC: ferritin <15 μg/L, PSC: ferritin <12 μg/L) using the BRINDA method for each of the seven analytic approaches (3). For the multiple imputation approach, we combined results from each imputation using PROC MIANALYZE (26). To inform how values of CRP less than the lower LOD (CRP <LOD) affect regression correction for inflammation, we examined differences between approaches in three components of the BRINDA regression equation: 1) the lowest decile of CRP for populations without an external reference decile (school aged children), 2) ß-value of CRP, 3) and ß-value of AGP. Since the BRINDA inflammation correction method is based on a log e scale (of ferritin, CRP and AGP), ß-values were exponentiated and interpreted as additive percent change in expected geometric mean ferritin per 1 percent increase in CRP and AGP concentration. For surveys employing a multi-stage sampling design, complex survey procedures were used to appropriately adjust standard errors (via Taylor series) and sampling weights were applied.
To understand the extent to which CRP <LOD biases estimates of inflammation-adjusted ferritin and iron deficiency (ID), we calculated the deviance of each approach and conducted a multivariate meta-analysis. Deviance was calculated by subtracting the estimate of ferritin or ID from the estimate given by multiple imputation within each survey and population group. Each analytic method (listwise, lower bound, etc.) chosen is a qualitative fixed effect with respect to the calculated point estimates (for ID, or mean ferritin concentration) within a survey and population group. Unlike univariate meta-analysis of only effect sizes, in our multi-survey effect size and multi-LOD method analyses, each candidate analytic approach was modeled as a moderator. This ensures that the pooled estimates (grand intercept of the meta-regression) are method-specific and based on independent constituent surveys estimates (29, 30). We also created forest plots to compare the pooled estimates and reliability of each approach relative to a multiple imputation referent point estimate within each method. We tested for differences in the pooled estimates of the subgroups using a Q-test for moderators (29). In this analysis, consistency is defined as an approach-specific pooled estimate having a low deviance from the from the multiple imputation approach and reliability is defined by how similar individual surveys’ (with their inherent proportion CRP <LOD) deviance estimates are within an analytic approach. The meta-analysis was conducted in R 4.0.2 (The R Foundation for Statistical Computing) and all other analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC).
Results
Table 1 shows the age, sex, malaria status, proportion exceeding cut-points for inflammation, and proportion outside LODs for each marker of systemic inflammation within each survey and population group. Among the 9 surveys of WRA, the proportion of CRP <LOD was <1% for 4, 1–5% for 3, and >10% for 2 surveys, and the proportion of CRP >5 mg/L ranged from 4.4 to 22.0%. Among the 15 surveys of PSC, the proportion of CRP <LOD was <1% for 5, 1–5% for 9, and >10% for 1 survey, and the proportion of CRP>5 mg/L ranged from 11.4 to 43.1%. Among the 5 surveys of SAC, the proportion of CRP <LOD was 1–5% for 3, and >10% for 2 surveys, and the proportion of CRP>5 mg/L ranged from 2.3 to 18.0%. Of the 29 survey populations, 19 had no values of AGP greater than the upper LOD, 7 had a proportion <0.5%, and 3 had 1.6–2.5% of AGP values less than the upper LOD. The proportion of AGP >1 g/L ranged from 5.2 to 63.5% within the 29 datasets.
Supplementary Table 1 shows three components of interest in the BRINDA regression equation. For WRA and PSC, the reference deciles are unchanged under differing approaches for CRP <LOD because external deciles were used as recommended by the BRINDA project. However, when no external decile is used, as is the case for SAC, the reference decile can be affected by the method of choice for dealing with values of CRP <LOD. In all surveys with SAC, listwise deletion changed the reference decile since observations at the lower end of the distribution of CRP were deleted. In the two surveys with >10% of CRP <LOD, Ghana and Nepal, the reference decile depended directly on the <LOD values imputed.
For all population groups, the regression slope of the relationship of CRP to ferritin, adjusted for AGP, was increased for listwise deletion, and all single imputation approaches except for lower bound imputation as compared to multiple imputation. The differences in the slope of the relationship of AGP to ferritin were less consistent; however, it is notable that changing even a small proportion of the values at the lowest end of the distribution of CRP also changed the adjusted relationship between AGP and ferritin. For example, in the survey of Guatemalan WRA in 2018/19, the weakly positive relationship between AGP and ferritin when using lower bound imputation (ß=1.11, increase of 11% from expected mean ferritin) effectively disappears when 11.6% of CRP <LOD are removed from the analysis using listwise deletion (ß=0.99, decrease of 1%). Another example of the effect of CRP values on AGP is more pronounced among the PSC from the Democratic Republic of the Congo, ranging from ß=4.34 (an excess additive increase of 334%) for lower bound imputation to ß=3.69 (additive increase of 269%) for listwise deletion despite there being only 2.3% of CRP <LOD. Percent change in the slope of CRP and AGP tended to be higher in datasets with a higher burden of inflammation, a higher proportion of CRP <LOD, and populations without an external reference decile (SAC).
Figure 1 summarizes the pooled deviance (represented by polygons) and reliability of each approach in estimating inflammation-adjusted serum ferritin concentration. Along the left y-axis are each survey labeled by the proportion of CRP <LOD, and the x-axis is the deviance of the estimate of inflammation-adjusted ferritin from the estimate given by multiple imputation (0 reference line) within each survey and population group in μg/L. The right y-axis provides information about the population group of each survey. The pooled estimate for listwise deletion had a significantly lower mean ferritin by an average of −0.46 μg/L (95% CI: −0.76, −0.16) as compared to multiple imputation. The reliability of listwise deletion was low as evidenced by how point estimates are dotted inconsistently around the vertical reference line. The pooled estimate of mean ferritin was significantly higher for lower bound imputation at a deviance of +0.45 μg/L (0.14, 0.76). The reliability of lower bound imputation was low with individual survey estimates significantly higher and lower than multiple imputation. Middle bound and LOD/√2 imputation resulted in pooled estimates somewhat lower than multiple imputation with a deviance of −0.21 (−0.51, 0.09) and −0.26 (−0.57, 0.04), respectively, and their estimates were reliable for WRA and PSC but not SAC. Upper bound imputation yielded estimates of inflammation-adjusted ferritin that were significantly lower than multiple imputation on average. Random number imputation yielded a pooled estimate with a deviance of −0.08 μg/L (−0.38, 0.22) from multiple imputation, making it the most consistent and reliable approach. There was significant moderation by approach (p<.001). Across all approaches, reliability of ferritin estimates tended to decrease as the proportion of CRP <LOD increased.
Figure 1: Deviance and Reliability of Inflammation-Adjusted Mean Serum Ferritin Concentration under Differing Analytic Approaches for C-reactive Protein (CRP) Less Than the Limit of Detection (LOD) Within 29 Surveys Across 7 Countries (n=33,053).
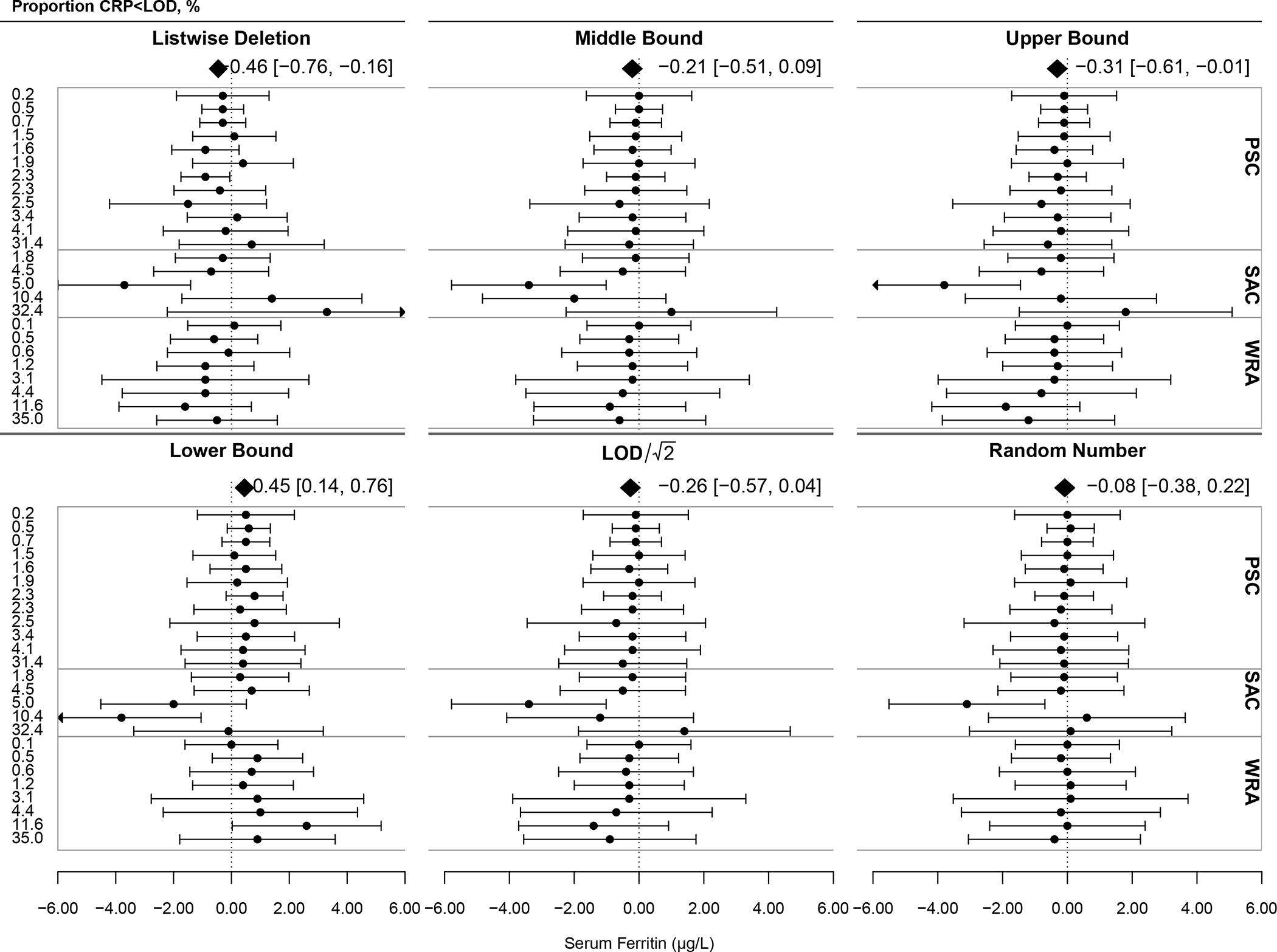
LOD for CRP=0.05 mg/L. The dotted reference line at 0 indicates the estimated mean serum ferritin adjusted for inflammation using multiple imputation within each survey and population group. Points and error bars represent the deviance of each approach from the multiple imputation estimate within each survey and population group with 95% confidence intervals. The polygon represents the mean deviance of all surveys using the labelled approach. Test for differences by analytic approach (subgroup differences): QM=20.19, df=5, p<.001.
Figure 2 shows complementary forest plots with estimates of inflammation-adjusted iron deficiency in percentage points (p.p.). The pooled estimate for listwise deletion did not deviate significantly from multiple imputation; however, this method yielded unreliable results, at its highest significantly overestimating iron deficiency by 4.7 p.p. and its lowest underestimating by 2.7 p.p. For example, among preschool-age children in the Democratic Republic of Congo, the prevalence of iron deficiency was 61.0% after adjusting for the high levels of inflammation in the population under multiple imputation of CRP<LOD. Though only 0.2% of CRP values are <LOD, the prevalence rises further to 65.7% when those left-censored observations are dropped under listwise deletion rather than imputed: a 7.7 percent difference (data not shown). In another example among school-age children in Nepal, the prevalence of iron deficiency was 8.5% after adjusting for relatively low levels of inflammation in the population under multiple imputation of CRP<LOD. In this dataset, 10.4% of CRP values are <LOD, and the prevalence falls to 5.8% when those left-censored observations are dropped under listwise deletion rather than imputed: a 31.8 percent difference (data not shown). The pooled estimate for lower bound imputation also did not differ significantly from multiple imputation, though this approach underestimated iron deficiency as compared to multiple imputation. The pooled estimate for middle bound imputation did not differ significantly from multiple imputation [0.22 (−0.34, 0.79)] and reliably yielded estimates of iron deficiency within 1 p.p. of multiple imputation. LOD/√2 was similar to middle bound though slightly less compared to multiple imputation and slightly less reliable [0.25 (−0.31, 0.81)]. Though not significantly different, upper bound imputation overestimated the prevalence of inflammation-adjusted iron deficiency compared to multiple imputation. Random number imputation yielded the pooled estimate nearest multiple imputation [0.11 (−0.46, 0.67)], and all estimates had a low deviance indicating high reliability. The test for differences by approach failed to reject the null hypothesis of homogeneity of subgroups (p=0.61). Reliability tended to decrease as the proportion of CRP <LOD increased for all approaches though less pronounced with middle bound, LOD/√2, and random number imputation.
Figure 2: Deviance and Reliability of Inflammation-Adjusted Prevalence of Iron Deficiency under Differing Imputation Analytic Approaches for C-reactive Protein (CRP) Less Than the Limit of Detection (LOD) Within 29 Surveys Across 7 Countries (n=33,053).
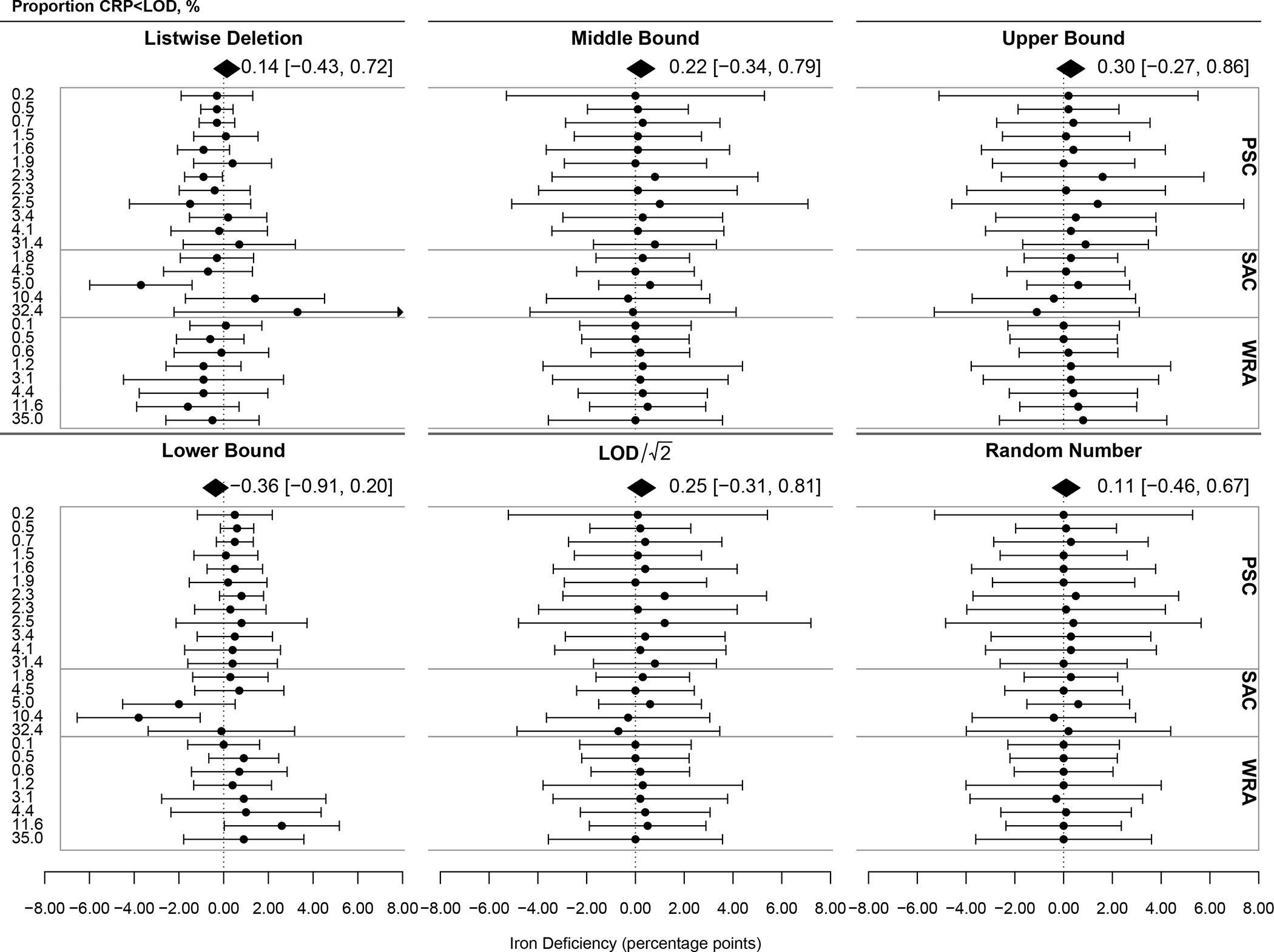
LOD for CRP=0.05 mg/L. The dotted reference line at 0 indicates the estimated prevalence of inflammation-adjusted iron deficiency using multiple imputation within each survey and population group. Points and error bars represent the deviance of each approach from the multiple imputation estimate within each survey and population group with 95% confidence intervals. The polygon represents the mean deviance of all surveys using the labelled approach. Test for differences by analytic approach (subgroup differences): QM=3.59, df=5, p=0.61.
Discussion
The results of this study provide additional evidence of the need to standardize practices in the treatment of biomarker values outside LODs. Our data showed the need was greater for CRP than AGP. Though the proportion of observations exceeding the upper LOD of AGP in the examined datasets was very low, further study may be necessary to determine best approaches for settings with higher AGP when sample dilution is not appropriate. Though adoption of more sensitive laboratory analyses may reduce the proportion of CRP <LOD, all analyses will have LOD with which to contend (31, 32). This laboratory limitation may cause misclassification of inflammation-adjusted iron deficiency when appropriate measures are not taken to account for this left-censoring. This may lead to an over- or underestimate of the prevalence of iron deficiency in populations with downstream effects on public health resources. Imputation may be a practical and reliable approach for addressing CRP <LOD.
In our data, overestimates ranged as high as 4.7 p.p. and underestimates as low at −2.7 p.p. when using listwise deletion of CRP <LOD. These differences can move estimates of the magnitude of the public health problem from one category to another, impacting the policies, interventions, and resources that are dedicated to improving iron status.
In the BRINDA equation, the slope of the relationship of CRP and AGP to ferritin were altered by listwise deletion or imputation of CRP <LOD. The changes were more pronounced among datasets with a high burden of inflammation or high proportion of CRP <LOD. Manipulation of the lowest decile where the proportion of CRP <LOD exceeds 10% raises the potential need for external reference deciles for all population groups.
Multiple imputation is considered the gold standard for addressing missing data and should be used where the technical capacity permits. Pooled results from 29 surveys showed that single imputation of a uniform random number bounded by 0 and the LOD may be a feasible alternative for addressing CRP <LOD in the BRINDA approach and yields reliable estimates that are consistent with multiple imputation. A random number approach is appropriate because the missingness of CRP <LOD is bounded and denotes a ‘healthy’ status with respect to the BRINDA inflammation correction. This approach would not address missingness in the distribution of CRP exceeding the LOD.
At least one other study has attempted to address the issue of left-censored inflammatory biomarkers in regression correction of iron deficiency (33). Though validated only on a small sample of SAC, the authors’ probability method appears robust but has several disadvantages including its complexity, lower precision relative to the BRINDA method, and inability to provide individual-level inflammation-adjusted ferritin for subsequent analyses, such as iron deficiency anemia or individual-level control for confounding (33).
The present study is strengthened by its use of large and diverse datasets drawn from a commonly used laboratory for population-based micronutrient status assessments using a single assay to reduce variability from laboratory methods. While this enabled isolation of the research question to the performance of imputation approaches under a single LOD, this limited our understanding of each approach’s performance if the LODs were increased or decreased, which may need further investigation. The analysis was conducted within populations with broadly differing burdens of inflammation, strengthening the applicability of findings. While primarily applicable to inflammation regression correction, these findings may be applicable to other inflammation correction approaches if only to caution against listwise deletion of values <LOD.
This study is limited by the unknowable truth of left-censored values which may differ from multiple imputation against which our approaches were tested. Additionally, it was not feasible to test every possible method of imputation forcing us to limit the number of approaches.
Population-level micronutrient surveys suffer from several potential errors that can produce bias from selection and non-response to measurement errors. While this analysis finds that several imputation approaches to CRP <LOD are relatively consistent, our results highlight that there is potential for bias in the analysis of inflammation-adjusted iron status if standard approaches are not taken. Multiple imputation may be used to address left-censored data, and random number single imputation may be a consistent, simple, and reliable solution for CRP <LOD within a diverse set of population parameters.
Supplementary Material
Acknowledgements:
We acknowledge the contributions of the BRINDA working group and steering committee members (https://brinda-nutrition.org).
Sources of Support:
The authors are supported by their respective institutions. Author L.G. is supported by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC. The Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project (authors P.S.S. and M.F.Y.) is supported by the Bill & Melinda Gates Foundation, Centers for Disease Control and Prevention, Eunice Kennedy Shriver National Institute of Child Health and Human Development, HarvestPlus, and the United States Agency for International Development.
Abbreviations:
- AGP
alpha-1-acid glycoprotein
- BRINDA
Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia
- CDC
Centers for Disease Control and Prevention
- CRP
C-reactive protein
- ELISA
enzyme-linked immunosorbent assay
- LOD
limit of detection
- PSC
preschool age children
- SAC
school age children
- sTfR
serum transferrin receptor
- WRA
women of reproductive age
Footnotes
Conflicts of Interest: Authors P.S.S., and M.F.Y. developed the BRINDA inflammation correction method. Authors L.G., A.J.S., O.Y.A and M.E.J. have no conflicts of interest to disclose
Contributor Information
Lucas Gosdin, Nutrition Branch, Division of Nutrition, Physical Activity, and Obesity, Centers for Disease Control and Prevention.
Andrea J. Sharma, Nutrition Branch, Division of Nutrition, Physical Activity, and Obesity, Centers for Disease Control and Prevention
Parminder S. Suchdev, Nutrition Branch, Division of Nutrition, Physical Activity, and Obesity, Centers for Disease Control and Prevention Hubert Department of Global Health, Rollins School of Public Heath, Emory University.
Maria Elena Jefferds, Nutrition Branch, Division of Nutrition, Physical Activity, and Obesity, Centers for Disease Control and Prevention.
Melissa F. Young, Hubert Department of Global Health, Rollins School of Public Heath, Emory University
O. Yaw Addo, Nutrition Branch, Division of Nutrition, Physical Activity, and Obesity, Centers for Disease Control and Prevention.
References
- 1.Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018;392(10159):1859–922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thurnham D, McCabe G. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. Edtion ed. World Health Organization Report: Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. Geneva: World Health Organization, 2012. [Google Scholar]
- 3.WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Geneva: World Health Organization, 2020. Available from https://www.who.int/publications/i/item/9789240000124 [PubMed] [Google Scholar]
- 4.Suchdev PS, Namaste SML, Aaron GJ, Raiten DJ, Brown KH, Flores-Ayala R, Group BW. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Advances in nutrition (Bethesda, Md) 2016;7(2):349–56. doi: 10.3945/an.115.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ, et al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl 1):359S–71S. doi: 10.3945/ajcn.116.141762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev 2008;29 Suppl 1(Suppl 1):S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 7.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr 2004;134(11):3127–32. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- 8.O’Callaghan KM, Roth DE. Standardization of laboratory practices and reporting of biomarker data in clinical nutrition research. The American Journal of Clinical Nutrition 2020;112(Supplement_1):453S–7S. doi: 10.1093/ajcn/nqaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006;59(10):1087–91. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Mikšová D, Filzmoser P, Middleton M. Imputation of values above an upper detection limit in compositional data. Computers & Geosciences 2020;136:104383. doi: 10.1016/j.cageo.2019.104383. [DOI] [Google Scholar]
- 11.Ministry of Health and Population, UNICEF, European Union, Centers for Disease Control and Prevention. Baseline survey in Kapilvastu and Accham districts for the Integrated Infant and Young Child Feeding and Baal Vita Micronutrient Powder intervention in Nepal, 2012–2013. Kathmandu, Nepal: UNICEF, 2015. [Google Scholar]
- 12.Ministry of Health, World Food Programme (WFP), Makerere University, Centers for Disease Control and Prevention. Micronutrient powder and infant and young child feeding intervention endline survey and impact evaluation, Amuria and Soroti districts, Uganda, 2015–16. Kampala, Uganda: WFP, 2018. [Google Scholar]
- 13.Ministry of Health, World Food Programme (WFP), Makerere University, Centers for Disease Control and Prevention. Baseline micronutrient survey report among children 6 to 23 months of age in Amuria and Soroti Districts, Uganda. Kampala, Uganda: WFP, 2017. [Google Scholar]
- 14.Ministry of Health and Population (MoH), New ERA, UNICEF, European Union, United States Agency for International Development (USAID), Centers for Disease Control and Prevention (CDC). Nepal National Micronutrient Status Survey, 2016. Kathmandu, Nepal: MoH, 2018. [Google Scholar]
- 15.Ministry of Health and Population (MoH), New ERA, UNICEF/Nepal, Centers for Disease Control and Prevention (CDC). Endline impact evaluation survey for the integrated infant and young child feeding and Baal Vita micronutrient powders intervention in Kapilvastu and Achham districts of Nepal. Kathmandu, Nepal: MoH, New ERA, UNICEF/Nepal, and CDC, 2016. [Google Scholar]
- 16.Ministry of Health (MISAU), Global Alliance for Improved Nutrition (GAIN), Centers for Disease Control and Prevention (CDC), COWI Mozambique. Baseline Survey Report of Micronutrient Powder Intervention among Young Children in Sofala Province, Mozambique. Maputo, Mozambique: GAIN, 2017. [Google Scholar]
- 17.National Statistical Office (NSO), Community Health Services Unit (CHSU) [Malawi], Centers for Disease Control and Prevention (CDC), Emory University. Malawi Micronutrient Survey 2015–16. Atlanta, GA, USA: NSO, CHSU, CDC, and Emory University, 2017. [Google Scholar]
- 18.UNICEF/Malawi, Irish Aid, Centers for Disease Control and Prevention. A Report for the National Micronutrient Survey. Malawi Government, 2009. [Google Scholar]
- 19.PRONANUT (National Nutrition Program), National Statistics Office, UNICEF/DRC, Centers for Disease Control and Prevention (CDC). Impact evaluation of an integrated infant and young child feeding and lipid based nutrition supplement program in Kasenga and Kipushi health zones, Katanga Province, Democratic Republic of Congo (DRC). PRONANUT (National Nutrition Program), National Statistics Office Lubumbashi, UNICEF/DRC, and Centers for Disease Control and Prevention (CDC; Atlanta: ), 2020. [Google Scholar]
- 20.Instituto de Nutrición de Centro América y Panamá (INCAP). Informe del Sistema de Vigilancia Epidemiológica de Salud y Nutrición - SIVESNU - 2016. Guatemala: INCAP, 2020. [Google Scholar]
- 21.Instituto de Nutrición de Centro América y Panamá (INCAP). Informe del Sistema de Vigilancia Epidemiológica de Salud y Nutrición - SIVESNU - 2015. Guatemala: INCAP, 2018. [Google Scholar]
- 22.Instituto de Nutrición de Centro América y Panamá (INCAP). Informe del Sistema de Vigilancia Epidemiológica de Salud y Nutrición - SIVESNU - 2013. Guatemala: INCAP, 2015. [Google Scholar]
- 23.Instituto de Nutrición de Centro América y Panamá (INCAP). Informe del Sistema de Vigilancia Epidemiológica de Salud y Nutrición - SIVESNU - agosto 2018 – mayo 2019 – Módulo 2: Salud y nutrición infantil. Guatemala: INCAP, 2020. [Google Scholar]
- 24.Instituto de Nutrición de Centro América y Panamá (INCAP). Informe del Sistema de Vigilancia Epidemiológica de Salud y Nutrición - SIVESNU - mayo 2017 – febrero 2018 – Módulo 2: Salud y nutrición infantil. Guatemala: INCAP, 2020. [Google Scholar]
- 25.Ghana Health Service, Ghana Education Service, UNICEF-Ghana, Centers for Disease Control and Prevention (CDC). Girls’ Iron-Folic acid Tablet Supplementation Program: Phase III baseline survey of adolescent boys and girls in Upper West, Western, and Western-North regions, Republic of Ghana. Cantonments, Accra, Ghana: UNICEF, 2020. [Google Scholar]
- 26.Berglund P, Heeringa S. Multiple Imputation of Missing Data Using SAS®. Cary, NC: SAS Institute Inc., 2014. [Google Scholar]
- 27.Allison P Internet: http://www.statisticalhorizons.com/more-imputations (accessed May 12, 2021.
- 28.Little RJA. Pattern-Mixture Models for Multivariate Incomplete Data. Journal of the American Statistical Association 1993;88(421):125–34. doi: 10.1080/01621459.1993.10594302. [DOI] [Google Scholar]
- 29.Viechtbauer W Conducting meta-analyses in R with the metafor package. Journal of statistical software 2010;36(3):1–48. [Google Scholar]
- 30.Jackson D, Riley R, White IR. Multivariate meta-analysis: potential and promise. Stat Med 2011;30(20):2481–98. doi: 10.1002/sim.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo L, Yang Z, Zhi S, Feng Z, Lei C, Zhou Y. A sensitive and innovative detection method for rapid C-reactive proteins analysis based on a micro-fluxgate sensor system. PLOS ONE 2018;13(3):e0194631. doi: 10.1371/journal.pone.0194631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv Y, Wu R, Feng K, Li J, Mao Q, Yuan H, Shen H, Chai X, Li LS. Highly sensitive and accurate detection of C-reactive protein by CdSe/ZnS quantum dot-based fluorescence-linked immunosorbent assay. J Nanobiotechnology 2017;15(1):35-. doi: 10.1186/s12951-017-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S, Kurpad AV, Sachdev HS, Thomas T. Inflammation correction in micronutrient deficiency with censored inflammatory biomarkers. The American Journal of Clinical Nutrition 2020;113(1):47–54. doi: 10.1093/ajcn/nqaa285. [DOI] [PubMed] [Google Scholar]
- 34.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. The American Journal of Clinical Nutrition 2010;92(3):546–55. doi: 10.3945/ajcn.2010.29284. [DOI] [PubMed] [Google Scholar]
- 35.World Health O. C-reactive protein concentrations as a marker of inflammation or infection for interpreting biomarkers of micronutrient status. Geneva: World Health Organization, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


