ABSTRACT
Mycobacterium avium complex pulmonary disease is treated with an azithromycin, ethambutol, and rifampicin regimen, with limited efficacy. The role of rifampicin is controversial due to inactivity, adverse effects, and drug interactions. Here, we evaluated the efficacy of clofazimine as a substitute for rifampicin in an intracellular hollow-fiber infection model. THP-1 cells, which are monocytes isolated from peripheral blood from an acute monocytic leukemia patient, were infected with M. avium ATCC 700898 and exposed to a regimen of azithromycin and ethambutol with either rifampicin or clofazimine. Intrapulmonary pharmacokinetic profiles of azithromycin, ethambutol, and rifampicin were simulated. For clofazimine, a steady-state average concentration was targeted. Drug concentrations and bacterial densities were monitored over 21 days. Exposures to azithromycin and ethambutol were 20%–40% lower than targeted but within clinically observed ranges. Clofazimine exposures were 1.7 times higher than targeted. Until day 7, both regimens were able to maintain stasis. Thereafter, regrowth was observed for the rifampicin-containing regimen, while the clofazimine-containing regimen yielded a 2 Log10 colony forming unit (CFU) per mL decrease in bacterial load. The clofazimine regimen also successfully suppressed the emergence of macrolide tolerance. In summary, substitution of rifampicin with clofazimine in the hollow-fiber model improved the antimycobacterial activity of the regimen. Clofazimine-containing regimens merit investigation in clinical trials.
KEYWORDS: nontuberculous mycobacteria, clofazimine, hollow-fiber infection model, pharmacokinetics, pharmacodynamics, PK/PD
INTRODUCTION
Mycobacterium avium complex pulmonary disease (MAC-PD) is emerging as an important opportunistic infection. The recommended treatment for MAC-PD consists of long-term (18 months) multi-drug therapy combining azithromycin (AZT), ethambutol (EMB), and rifampicin (RIF) with or without (inhaled) amikacin (1). Despite this prolonged multi-drug therapy, outcomes are poor, with only 60%–70% of patients achieving sustained culture conversion (2).
The role of RIF in these treatment regimen is subject of debate since it shows limited activity in vitro against MAC bacteria (3), causes frequent and potentially severe adverse events (4), and has pharmacokinetic (PK) interactions with macrolides such as AZT through induction of the CYP3A4 enzyme, resulting in reduced exposure and thus likely reduced efficacy (5, 6).
Replacing RIF with a more effective drug with fewer adverse effects and pharmacokinetic interactions is an important strategy to improve the antibiotic treatment outcome of MAC-PD. Retrospective studies have suggested that clofazimine (CFZ) may be a good alternative to RIF (7, 8) since it is active alone, synergistic with macrolides and amikacin in vitro (9), and does not impact AZT exposures in vivo (5, 10).
We employed the hollow-fiber infection model (HFIM) to investigate whether CFZ can replace RIF in the recommended treatment of MAC-PD, both in terms of antimycobacterial effect and suppression of the emergence of macrolide tolerance and resistance.
MATERIALS AND METHODS
Bacteria, cells, and antibiotics
The Mycobacterium avium subsp. hominissuis ATCC 700898 reference strain was obtained from the American Type Culture Collection (ATCC; Manassas, VA) and cultured in Middlebrook 7H9 supplemented with 10% oleic albumin dextrose catalase (OADC; Beckton-Dickinson, Vianen, the Netherlands) for 5 days prior to the experiment. THP-1 cells, which are monocytes isolated from peripheral blood from an acute monocytic leukemia patient, were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany; ACC 16 Lot 32) and cultured in RPMI (Roswell Park Memorial Institute medium) 1640 with 20% heat-inactivated fetal bovine serum (FBS; Life Technologies Limited, Paisley, UK) for the first three passages and with 10% FBS afterward at 36°C and 5% CO2.
AZT, EMB, RIF, and CFZ were purchased from Sigma Aldrich (Zwijndrecht, the Netherlands). Stock solutions of the compounds were prepared in ethanol, Milli-Q water, dimethyl sulfoxide (DMSO) and DMSO, respectively, and stored at −20°C until use. Syringe solutions of AZT (40%/60% (vol/vol) ethanol/Milli-Q water) and EMB (Milli-Q water) were prepared every 3 days. RIF (in 0.08%/99.2% (vol/vol) DMSO/Milli-Q water) was replaced every 2 days due to drug instability. CFZ bolus solutions at 0.1 mg/mL were prepared daily in 10% DMSO, 0.5% Tween 80 in RPMI-2% FBS.
Minimum inhibitory concentration (MIC) determinations
To assess the emergence of resistance, the MICs of AZT, RIF, and CFZ against M. avium ATCC 700898 were determined prior and post experiment by broth microdilution in cation-adjusted Mueller Hinton broth according to Clinical and Laboratory Standards Institute guidelines (11). For AZT and RIF, microdilution plate concentrations ranged from 256 to 0.125 and from 64 to 0.03 mg/L, respectively. The MIC of CFZ was measured using Sensititre SLOMYCO2 plates (ThermoFisher, Breda, the Netherlands) according to manufacturer’s recommendations.
Hollow-fiber setup
The intracellular HFIM experiment was performed as previously described (12), but with minor modifications, i.e., a 2·106 cells/mL suspension of THP-1 cells, representing human macrophages, in RPMI 1640 broth containing 2% heat-inactivated FBS was infected with a 0.5 McFarland suspension of M. avium and incubated at 36°C in 5% CO2 atmosphere for 24 h prior to starting the experiment. Infected THP-1 cells (30 mL) were inoculated into each hollow-fiber cartridge (C8008; FiberCell Systems, New Market, MD, USA) for 4 h before the start of the experiment. All hollow-fiber cartridges were rinsed extensively before use (see Supplement).
Drug penetration into the extracapillary space of the C8008 cartridges was confirmed for AZT, EMB, and RIF (Fig. S1 to S3). CFZ does not penetrate the extracapillary space (Fig. S4) and was administered daily into the extracapillary space via the sampling ports. The cartridges were primed with CFZ before simulating the pharmacokinetic profile to mitigate CFZ’s binding to plastics (Fig. S4).
Hollow-fiber study design and simulated pharmacokinetic profiles
Three experimental arms were included in triplicate: the rifampicin regimen (AZT, EMB, and RIF), the clofazimine regimen (AZT, EMB, and CFZ), and a growth control.
AZT, EMB, and RIF protein-unbound epithelial lining fluid (ELF) drug concentrations were simulated corresponding to once-daily doses of 250 mg, 900 mg (15 mg/kg), and 600 mg (10 mg/kg), respectively (5, 13–16). We accounted for a 25% increase in AZT exposure when not administered concomitantly with RIF (5). In addition, we considered a [RIF]ELF/[RIF]plasma ratio of 2.6, based on available data (15), a free fraction of 10% in plasma and an assumed 100% free fraction in ELF. ELF pharmacokinetic parameters are listed below in Table 1. The diluent inflow was set to simulate the half-life of RIF, and the difference between the half-lives of EMB and AZT was corrected by zero-order top-up.
TABLE 1.
Pharmacokinetic parameters realized in the HFIM experimenta
| Parameter | Drug | Rifampicin therapy | Clofazimine therapy | ||
|---|---|---|---|---|---|
| Target (5, 13–16) | Actual | Target (5, 13–20) | Actual | ||
| T1/2 (h) | AZT | 20 | 19.2 ± 7.3 | 20 | 17.6 ± 1.4 |
| EMB | 10 | 14.4 ± 5.0 | 10 | 10.3 ± 1.5 | |
| RIF | 2 | 3.5 ± 0.7 | – | – | |
| Tmax (h) | AZT | 10 | 15.2 ± 6.0 | 10 | 10 ± 0 |
| EMB | 3 | 3 ± 0 | 3 | 2.6 ± 0.6 | |
| RIF | 2 | 2 ± 0 | – | – | |
| Css (mg/L) | AZT | 3 | 4.3 ± 1.2 | 3.75 | 4.1 ± 0.2 |
| EMB | 3 | 2.4 ± 0.1 | 3 | 2.6 ± 0.4 | |
| RIF | 3.9 | 2.9 ± 0.3 | – | – | |
| AUC0-24 (mg·h/L) |
AZT | 108 | 75.7 ± 17.2 | 135 | 79.1 ± 6.7 |
| EMB | 48.5 | 39.2 ± 6.1 | 48.5 | 32.5 ± 3.3 | |
| RIF | 15.2 | 16.0 ± 2.7 | – | – | |
| Cavg (mg/L) | CFZ | – | – | 2.2 | 3.7 ± 0.5 |
Values represent geometric mean ± standard deviation. T1/2, elimination half-life; Tmax, time at which Css is reached; Css, peak concentration at steady state; AUC0-24, area under the 24-h concentration-time curve at steady state; Cavg, average concentration at steady state. Dashes represent PK parameters that were not simulated in each regimen (e.g. RIF PK parameters in the CFZ regimen and vice versa).
An average CFZ concentration at clinical steady state (Cavg) was targeted at a daily dose of 100 mg. Plasma CFZ concentrations were extrapolated to lung concentrations. A plasma Cavg of 0.86 mg/L (17), a [CFZ]lung/[CFZ]plasma ratio of 250 (18, 19) and binding to lung tissue similar to that to plasma proteins (99%) (20) were considered, resulting in a Cavg of 2.2 mg/L. A CFZ bolus was administered every 24 h. Preparative experiments showed CFZ instability in the HFIM following first-order kinetics with a T1/2 degradation time of 14 h (Fig. S4; Table S1). Considering its instability, a C0 of 3.7 mg/L was targeted to achieve the desired Cavg during the dosing interval.
Bacterial and THP-1 cell enumerations
Bacterial and THP-1 cell enumeration was performed as previously described (12). Bacterial and THP-1 cell samples were collected on days 0, 3, 7, 14, and 21, and cell densities were determined. Samples were taken directly from the extracapillary space after homogenizing the contents using two 20 mL syringes. A brief overview can also be found in the supplementary materials.
To investigate the emergence of macrolide tolerance, Middlebrook 7H10 agar (M7H10; Beckton-Dickinson, Vianen, the Netherlands) plates were prepared following the manufacturer’s recommendation with the addition of AZT at a final concentration of eight times the pre-treatment MIC. When samples were drawn to determine bacterial density, the samples were also plated on the drug-containing agar plates to detect emergence of macrolide tolerance.
Pharmacokinetic measurements
Pharmacokinetic evaluation was performed at day 0 of the experiment and at steady state (day 16). For AZT, EMB, and RIF, pharmacokinetic samples were drawn from the central reservoir at timepoints 0, 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, 22, and after 24 h. CFZ samples were drawn 15 minutes after bolus injection, at 12 h and at 24 h from the extracapillary space. Additional CFZ samples were taken simultaneously when samples for bacterial enumeration (pharmacodynamic samples) were drawn from the hollow-fiber cartridge and frozen until further evaluation. Additional samples of CFZ at 1 mg/L were simultaneously frozen as quality controls.
Briefly, all samples were processed by precipitating proteins in the growth medium, followed by a centrifugation step before antibiotic concentrations were analyzed using an ultra-high performance liquid chromatography-mass spectrometer (XEVO TQ-S micro triple quadrupole mass spectrometer, Waters, Etten-Leur, The Netherlands) (see Supplement).
Calculations and statistics
Pharmacodynamic, THP-1 cell density and pharmacokinetic plots were generated using GraphPad Prism version 7.0.0 (GraphPad Software Inc., LA Jolla, CA, USA). Pharmacokinetic analyses were performed using Phoenix 64 WinNonlin (Build 8.3.1.5.014). The area under the curve (AUC) was calculated using the linear-up log-down trapezoidal rule. The log-linear period (log concentrations versus time) was based on the last data points. The absolute value of the slope (λz /2.303, where λz is the first-order elimination rate constant) was calculated using linear regression analysis. λz allowed the calculation of the antibiotic half-lives (half-life = 0.693/λz). CFZ Cavg was calculated by dividing its AUC by 24 h using GraphPad Prism version 7.0.0 (GraphPad Software Inc., LA Jolla, CA, USA). All pharmacokinetic parameters are depicted as the geometric mean of the triplicate values, together with a standard error of the mean.
RESULTS
Minimum inhibitory concentrations
The MICs of AZT, EMB, RIF, and CFZ were 64 mg/L, 8 mg/L, 4 mg/L, and 0.12 mg/L, respectively, before the hollow-fiber experiment and remained stable throughout.
Pharmacodynamic effect and the emergence of macrolide tolerance
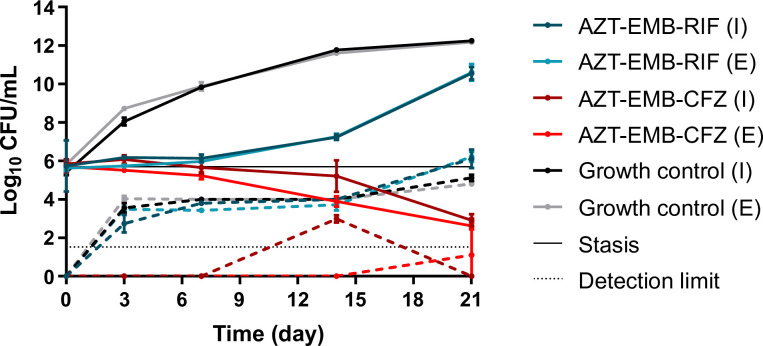
Both treatment arms were able to suppress growth of the mycobacterial population until day 7, after which regrowth was observed in the RIF-containing treatment arm (Fig. 1). From day 7, the CFZ-containing regimen was able to reduce the bacterial load by 2 Log10 colony forming unit (CFU) per mL at day 21. Specifically, the area under the bacterial kill curve (AUBKC) reduction of the CFZ regimen in comparison to the RIF arm was 35.6%–39.2 % and 22.6%–35.1 % for the extracellular and intracellular fractions, respectively. As seen, the effects on both fractions were similar throughout the experiment. In addition, the CFZ regimen proved to be better at suppressing the emergence of macrolide tolerance (there was an AUBKC reduction between the RIF and CFZ arms of 85%–100% and 72.3%–75.7% for the extracellular and intracellular fractions, respectively), as shown in Fig. 1. On day 7, one growth control system was contaminated and excluded from further analysis.
Fig 1.
Hollow-fiber pharmacodynamic effects (solid lines) and emergence of macrolide tolerance (dashed lines) of the growth control, rifampicin therapy, and clofazimine therapy in the hollow-fiber experiment. I, intracellular fraction; E, extracellular fraction. Detection limit and stasis correspond to 1.5 Log10 CFU/mL and 5.7 Log10 CFU/mL, respectively.
Pharmacokinetic evaluation
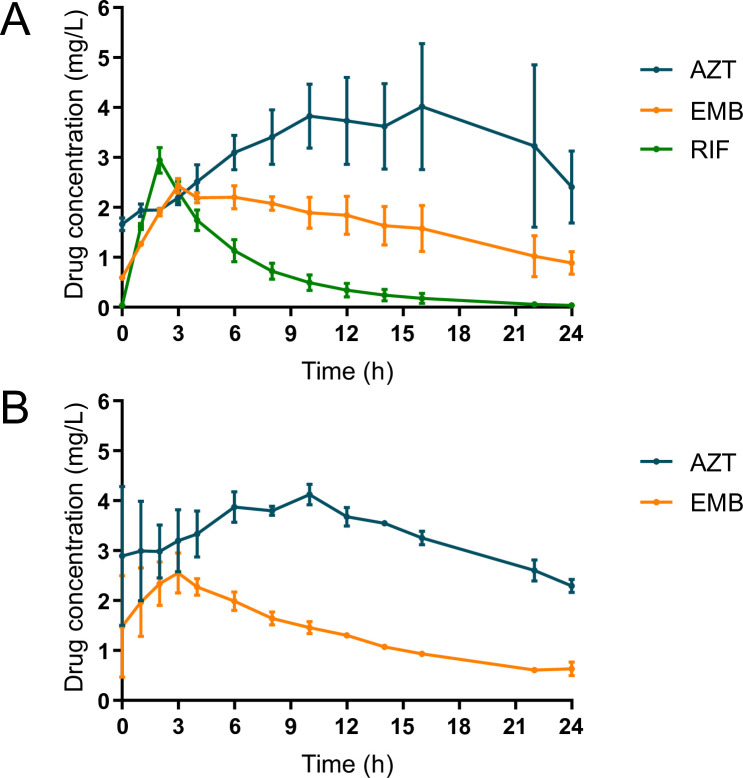
Pharmacokinetic parameters and corresponding pharmacokinetic profiles at steady state (at day 16) are shown in Table 1 and Fig. 2. One data point in two systems was not included in the calculation of λz of AZT because of a technical error. The observed AZT exposures were lower than targeted, rendering a 5% increased AZT exposure when it was not administered concomitantly with RIF instead of the 25% targeted. Lower (19%–33%) EMB exposures were also reached, as a result of a low Cmax. RIF exposure followed the targeted profile. The average CFZ concentration was higher than the target (3.8 mg/L instead of 2.2 mg/L).
Fig 2.
Steady-state pharmacokinetic graph of the rifampicin-containing therapy (A) and the clofazimine-containing therapy (B) on day 16. Dots represent mean drug concentrations values, which are connected by lines, whereas bars correspond to standard deviations.
On day 16, the CultureGuard filter connecting the diluent to one of the CFZ-containing systems was clogged and its PK calculations were excluded from the overall PK analysis.
THP-1 cells
THP-1 cell density was stable throughout the experiment and between systems (Fig. S5). Due to the vigorous mixing required in the CFZ-containing systems during drug administration, a reduction of THP-1 cells was registered at day 7. Thus, to maintain THP-1 cell counts identical to the other systems, we added 1.7·108 THP-1 cells. The multiplicity of infection started at 1 (bacterium:cells) and increased slightly over time in the growth control and RIF regimens, while the CFZ therapy showed a decrease from day 7 (Fig. S6). It coincided with the decrease in bacterial cells in this arm (Fig. 1). This demonstrates that we were able to maintain the intracellular infection model during the experiment.
DISCUSSION
Replacing RIF with CFZ improved the antimycobacterial activity of the three-drug macrolide-based regimen in the hollow-fiber model. After an initial phase on par with the RIF-containing regimen, the CFZ-containing regimen showed sustained antimycobacterial activity and suppression of macrolide tolerance and resistance, outperforming the RIF regimen. Given the small differences in AZT exposure between the two regimens (Fig. 2; Table 1), CFZ is the most likely driver of the increased efficacy of the studied regimen. These in vitro findings suggest a role for CFZ in MAC-PD treatment regimens and support the inclusion of such a regimen in clinical trials as well as the use of CFZ in patients in whom RIF is contraindicated or intolerable (4, 8).
Biphasic activity, with stasis similar to rifampicin regimen first and bactericidal activity in a later phase, has already been documented for clofazimine. Fourteen days of CFZ monotherapy showed no significant early bactericidal activity in tuberculosis patients (21). Early assessments of CFZ efficacy have not been conducted at MAC-PD, where culture conversion at 6 months of treatment is the first read-out. CFZ-based regimens have shown mixed results there. The recently completed PERC (Pulmonary NTM disease: A regimen of ethambutol and azithromycin with as adjunctive rifampicin vs clofazimine) randomized controlled clinical trial compared CFZ with RIF in the treatment of MAC-PD and recorded equal culture conversion rates (62% vs 58%) after 6 months of treatment (22, 23), similar to the first week in our experiment, while an earlier cohort study suggested higher culture conversion rates for CFZ-containing regimens (8). The late onset of the bactericidal effect of the CFZ regimen is in line with previous observations in mouse models of tuberculosis, where an exposure-dependent bactericidal effect and treatment-shortening potential were observed with prolonged treatment (24). However, recent mouse models of MAC disease showed superior efficacy of CFZ- versus RIF-containing regimens, with early onset of action in both regimens (25).
The effect of CFZ increased equally with time against intracellular and extracellular M. avium (Fig. 1), suggesting that intracellular accumulation is not a rate-limiting step. In clinical studies, the late effect is partly due to the very long elimination half-life of CFZ, which consequently takes up to 4 months to reach steady state (26). We simulated steady-state pharmacokinetics yet the full effect was still delayed; in fact, the CFZ exposures in our experiment were higher than targeted, probably due to the mixing process we had to perform after loading CFZ directly into the extracapillary space of the hollow-fiber cartridges, which may better reflect a daily dose of 200 mg. Loading doses, higher doses, or alternative routes of administration such as inhalation (27) may increase and accelerate the effect of CFZ in MAC-PD treatment, but the dose-response relationship of CFZ required to achieve antimicrobial activity remains unknown (24, 26); higher doses up to 600 mg per day and 300 mg per day for 3 months have been used in the treatment of leprosy and leprosy reactions (28, 29). On the other hand, CFZ use has been related to higher mortality rates in the treatment of disseminated MAC infection in patients with AIDS in a single study (30). However, other aspects such as the higher bacterial loads in the CFZ arm may also explain this higher mortality. A study evaluating the safety and pharmacokinetics of a 4-week 300 mg once-daily loading phase of CFZ has recently completed enrolment (clinicaltrials.gov; registration number: NCT05294146). To show the true added benefit of CFZ in the context of MAC-PD, regimens might thus require dose and delivery optimization and benefits might be best shown in longer-term follow-up, e.g., in rates of prolonged culture conversion and relapse rates. Currently, there is very little evidence to support treatment duration in MAC-PD (1) and a potential treatment-shortening effect of CFZ is also an important subject for clinical trials.
RIF lacks in vitro activity (3), but it is part of currently recommended treatment regimens for MAC-PD for its presumed potential, when combined with EMB, to prevent the emergence of macrolide resistance (31). Yet, the studies that support this recommendation suggest that EMB is more important than RIF in suppression of macrolide resistance (1, 31), and two recent clinical cohort studies have shown that in low bacterial burden MAC-PD, two-drug regimens of EMB and a macrolide perform as well as the recommended three-drug RIF-containing regimens (32, 33). Our results demonstrate that in the hollow-fiber model, the CFZ regimen is more effective in suppressing macrolide tolerance and resistance. Whether this effect is driven by CFZ or by the higher exposure to AZT cannot be inferred from the current experiment, but the moderate differences in macrolide exposure between the two arms of the experiment and prior studies of CFZ-macrolide synergy (9) suggest that CFZ itself prevents macrolide tolerance.
All limitations inherent to preclinical models also apply to this study. The success of preclinical models in predicting clinical trial outcomes is limited by the assumptions inherent to models. Here, the pharmacokinetics of CFZ in the lung is an important potential limitation. We simulated steady-state pharmacokinetics in the epithelial lining fluid for AZT, EMB, and RIF, but literature on lung concentrations of CFZ is limited and a wide range of drug concentrations in lung tissue and plasma:lung ratios has been reported (18, 19, 34). CFZ pharmacokinetics in ELF have never been reported and our Cavg target (0.86 mg/L) was extrapolated from lung tissue concentrations (17). Of note, other authors measured lower concentrations of clofazimine in the lung (about 0.4 mg/L) (5, 26). Moreover, in our experiment, a higher CFZ Cavg than targeted was achieved, most likely corresponding to a daily dose of 200 mg instead of 100 mg and this may also be reflected in the positive outcome of the CFZ-containing regimen compared to the RIF regimen. We attained lower AZT exposures than targeted in the CFZ regimen, where targets were higher due to the absence of RIF and its induction of cytochrome P450 enzymes (5). Higher exposures have been shown to improve treatment outcomes in patients (6), so the effect of the CFZ regimen may be underestimated. Finally, our work mainly reflects nodular/bronchiectatic MAC-PD rather than fibro-cavitary disease as we simulate ELF pharmacokinetics and this does not reflect the lower exposures observed in cavity walls and contents (35).
In conclusion, replacing RIF with CFZ improved the efficacy of MAC-PD treatment in the hollow-fiber model. The CFZ concentrations we achieved are steady-state concentrations and may better correspond to a daily dose of 200 mg instead of 100 mg. Therefore, higher doses, the safety of higher loading doses, and targeted delivery of high doses should be investigated in human subjects. Findings from this study bring hope for an improved regimen but should be confirmed in clinical trials.
ACKNOWLEDGMENTS
We acknowledge José Antonio Aínsa, Ainhoa Lucía, and Santiago Ramón-García for funding acquisition, as well as participation and critical comments on the project.
S.S. is recipient of a personal grant from the Programme Margarita Salas which was launched by the Ministry of Universities of Spain, summoned and resolved by the University of Zaragoza, and financed by the European Union-Next Generation EU. J.R. is supported by a grant from the Radboud Institute for Health Sciences (Junior researcher grant).
The funders had no role in the study design, data collection, and interpretation or the decision to submit the work for publication.
Contributor Information
Sandra Salillas, Email: sandrasb@unizar.es.
Jelmer Raaijmakers, Email: jelmer.raaijmakers@radboudumc.nl.
Sean Wasserman, St George's, University of London, London, United Kingdom.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.01157-23.
Supplemental text, figures, and tables.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. 2020. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 56:2000535. doi: 10.1183/13993003.00535-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diel R, Nienhaus A, Ringshausen FC, Richter E, Welte T, Rabe KF, Loddenkemper R. 2018. Microbiologic outcome of interventions against Mycobacterium avium complex pulmonary disease: a systematic review. Chest 153:888–921. doi: 10.1016/j.chest.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 3. Fröberg G, Maurer FP, Chryssanthou E, Fernström L, Benmansour H, Boarbi S, Mengshoel AT, Keller PM, Viveiros M, Machado D, et al. 2023. Towards clinical breakpoints for non-tuberculous mycobacteria – determination of epidemiological cut off values for the Mycobacterium avium complex and Mycobacterium abscessus using broth microdilution. Clin Microbiol Infect 29:758–764. doi: 10.1016/j.cmi.2023.02.007 [DOI] [PubMed] [Google Scholar]
- 4. van Ingen J, Aliberti S, Andrejak C, Chalmers JD, Codecasa LR, Daley CL, Hasegawa N, Griffith DE, Hoefsloot W, Huitt G, Jarand J, Jhun BW, Loebinger MR, Marras TK, Morimoto K, Polverino E, Ringshausen FC, Santin M, Thomson R, Wagner D, Wallace RJ, Winthrop KL, Yim J-J. 2021. Management of drug toxicity in Mycobacterium avium complex pulmonary disease: an expert panel survey. Clin Infect Dis 73:e256–e259. doi: 10.1093/cid/ciaa1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, Aarnoutse RE, Heifets LB, Peloquin CA, Daley CL. 2012. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med 186:559–565. doi: 10.1164/rccm.201204-0682OC [DOI] [PubMed] [Google Scholar]
- 6. Jeong B-H, Jeon K, Park HY, Moon SM, Kim S-Y, Lee S-Y, Shin SJ, Daley CL, Koh W-J. 2016. Peak plasma concentration of azithromycin and treatment responses in Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 60:6076–6083. doi: 10.1128/AAC.00770-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roussel G, Igual J. 1998. Clarithromycin with minocycline and clofazimine for Mycobacterium avium intracellulare complex lung disease in patients without the acquired immune deficiency syndrome. Int J Tuberc Lung Dis 2:462–470. [PubMed] [Google Scholar]
- 8. Jarand J, Davis JP, Cowie RL, Field SK, Fisher DA. 2016. Long-term follow-up of Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest 149:1285–1293. doi: 10.1378/chest.15-0543 [DOI] [PubMed] [Google Scholar]
- 9. Ferro BE, Meletiadis J, Wattenberg M, de Jong A, van Soolingen D, Mouton JW, van Ingen J. 2016. Clofazimine prevents the regrowth of Mycobacterium abscessus and Mycobacterium avium type strains exposed to amikacin and clarithromycin. Antimicrob Agents Chemother 60:1097–1105. doi: 10.1128/AAC.02615-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horita Y, Doi N. 2014. Comparative study of the effects of antituberculosis drugs and antiretroviral drugs on cytochrome P450 3A4 and P-glycoprotein. Antimicrob Agents Chemother 58:3168–3176. doi: 10.1128/AAC.02278-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wayne P. 2018. Susceptibility testing of mycobacteria, Nocardia spp. and other aerobic actinomycetes. 3rd ed. Clinical and Laboratory Standards Institute. [PubMed] [Google Scholar]
- 12. Ruth MM, Raaijmakers J, van den Hombergh E, Aarnoutse R, Svensson EM, Susanto BO, Simonsson USH, Wertheim H, Hoefsloot W, van Ingen J. 2022. Standard therapy of Mycobacterium avium complex pulmonary disease shows limited efficacy in an open source hollow fibre system that simulates human plasma and epithelial lining fluid pharmacokinetics. Clin Microbiol Infect 28:448. doi: 10.1016/j.cmi.2021.07.015 [DOI] [PubMed] [Google Scholar]
- 13. Magis-Escurra C, Alffenaar JW, Hoefnagels I, Dekhuijzen PNR, Boeree MJ, van Ingen J, Aarnoutse RE. 2013. Pharmacokinetic studies in patients with nontuberculous mycobacterial lung infections. Int J Antimicrob Agents 42:256–261. doi: 10.1016/j.ijantimicag.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 14. Olsen KM, San Pedro GS, Gann LP, Gubbins PO, Halinski DM, Campbell GD. 1996. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob Agents Chemother 40:2582–2585. doi: 10.1128/AAC.40.11.2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clewe O, Goutelle S, Conte JE, Simonsson USH. 2015. A pharmacometric pulmonary model predicting the extent and rate of distribution from plasma to epithelial lining fluid and alveolar cells - using rifampicin as an example. Eur J Clin Pharmacol 71:313–319. doi: 10.1007/s00228-014-1798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conte JE, Golden JA, Kipps JE, Lin ET, Zurlinden E. 2004. Effect of sex and AIDS status on the plasma and intrapulmonary pharmacokinetics of rifampicin. Clin Pharmacokinet 43:395–404. doi: 10.2165/00003088-200443060-00003 [DOI] [PubMed] [Google Scholar]
- 17. Watanabe F, Furuuchi K, Hanada K, Fujiwara K, Uesugi F, Hiramatsu M, Yoshiyama T, Shiraishi Y, Kurashima A, Ohta K, Morimoto K. 2022. Pharmacokinetics and adverse effects of clofazimine in the treatment of pulmonary non-tuberculous mycobacterial infection. Antimicrob Agents Chemother 66:e0044122. doi: 10.1128/aac.00441-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barry VC, Buggle K, Byrne J, Conalty ML, Winder F. 1960. Absoption, distribution and retention of the rimino-compounds in the experimental animal. Ir J Med Sci 416:345–352. doi: 10.1007/BF02945619 [DOI] [PubMed] [Google Scholar]
- 19. Venkatesan K, Deo N, Gupta UD. 2007. Tissue distribution and deposition of clofazimine in mice following oral administration of isoniazid. Arzneimittelforschung 57:472–474. doi: 10.1055/s-0031-1296634 [DOI] [PubMed] [Google Scholar]
- 20. Irwin SM, Gruppo V, Brooks E, Gilliland J, Scherman M, Reichlen MJ, Leistikow R, Kramnik I, Nuermberger EL, Voskuil MI, Lenaerts AJ. 2014. Limited activity of clofazimine as a single drug in a mouse model of tuberculosis exhibiting caseous necrotic granulomas. Antimicrob Agents Chemother 58:4026–4034. doi: 10.1128/AAC.02565-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Hutchings J, Burger DA, Schall R, Mendel CM. 2015. Bactericidal activity of pyrazinamide and clofazimine alone and in combinations with pretomanid and bedaquiline. Am J Respir Crit Care Med 191:943–953. doi: 10.1164/rccm.201410-1801OC [DOI] [PubMed] [Google Scholar]
- 22. Zweijpfenning S. 2022. Clofazimine is a safe and effective replacement for rifampicin in Mycobacterium avium complex pulmonary disease treatment – primary outcomes of the PERC trial. Bronchiectasis conference. Available from: https://www.world-bronchiectasis-conference.org/2022/index21a8.html?page_id=1318World [Google Scholar]
- 23. Zweijpfenning SMH, Aarnoutse R, Boeree MJ, Magis-Escurra C, Stemkens R, Geurts B, van Ingen J, Hoefsloot W. 2023. Clofazimine is a safe and effective alternative for rifampicin in Mycobacterium avium complex pulmonary disease treatment - outcomes of a randomized trial. Chest:S0012-3692(23)05830-0. doi: 10.1016/j.chest.2023.11.038 [DOI] [PubMed] [Google Scholar]
- 24. Ammerman NC, Swanson RV, Bautista EM, Almeida DV, Saini V, Omansen TF, Guo H, Chang YS, Li S-Y, Tapley A, Tasneen R, Tyagi S, Betoudji F, Moodley C, Ngcobo B, Pillay L, Bester LA, Singh SD, Chaisson RE, Nuermberger E, Grosset JH. 2018. Impact of clofazimine dosing on treatment shortening of the first-line regimen in a mouse model of tuberculosis. Antimicrob Agents Chemother 62:e00636-18. doi: 10.1128/AAC.00636-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JM, Park J, Choi S, Jhun BW, Kim SY, Jo KW, Hong JJ, Kim LH, Shin SJ. 2020. A clofazimine-containing regimen confers improved treatment outcomes in macrophages and in a murine model of chronic progressive pulmonary infection caused by the Mycobacterium avium complex. Front Microbiol 11:626216. doi: 10.3389/fmicb.2020.626216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abdelwahab MT, Wasserman S, Brust JCM, Gandhi NR, Meintjes G, Everitt D, Diacon A, Dawson R, Wiesner L, Svensson EM, Maartens G, Denti P. 2020. Clofazimine pharmacokinetics in patients with TB: dosing implications. J Antimicrob Chemother 75:3269–3277. doi: 10.1093/jac/dkaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banaschewski B, Verma D, Pennings LJ, Zimmerman M, Ye Q, Gadawa J, Dartois V, Ordway D, van Ingen J, Ufer S, Stapleton K, Hofmann T. 2019. Clofazimine inhalation suspension for the aerosol treatment of pulmonary nontuberculous mycobacterial infections. J Cyst Fibros 18:714–720. doi: 10.1016/j.jcf.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 28. Waters MF. 1969. G 30 320 or B 663 - lampren (Geigy). Lepr Rev 40:21–47. doi: 10.5935/0305-7518.19690006 [DOI] [PubMed] [Google Scholar]
- 29. Karat AB, Jeevaratnam A, Karat S, Rao PS. 1970. Double-blind controlled clinical trial of clofazimine in reactive phases of lepromatous leprosy. Br Med J 1:198–200. doi: 10.1136/bmj.1.5690.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chaisson RE, Keiser P, Pierce M, Fessel WJ, Ruskin J, Lahart C, Benson CA, Meek K, Siepman N, Craft JC. 1997. Clarithromycin and ethambutol with or without clofazimine for the treatment of bacteremic Mycobacterium avium complex disease in patients with HIV infection. AIDS 11:311–317. doi: 10.1097/00002030-199703110-00008 [DOI] [PubMed] [Google Scholar]
- 31. Griffith DE, Brown-Elliott BA, Langsjoen B, Zhang Y, Pan X, Girard W, Nelson K, Caccitolo J, Alvarez J, Shepherd S, Wilson R, Graviss EA, Wallace RJ Jr. 2006. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 174:928–934. doi: 10.1164/rccm.200603-450OC [DOI] [PubMed] [Google Scholar]
- 32. Miwa S, Shirai M, Toyoshima M, Shirai T, Yasuda K, Yokomura K, Yamada T, Masuda M, Inui N, Chida K, Suda T, Hayakawa H. 2014. Efficacy of clarithromycin and ethambutol for Mycobacterium avium complex pulmonary disease: a preliminary study. Ann Am Thorac Soc 11:23–29. doi: 10.1513/AnnalsATS.201308-266OC [DOI] [PubMed] [Google Scholar]
- 33. Moon SM, Yoo IY, Huh HJ, Lee NY, Jhun BW. 2019. Intermittent treatment with azithromycin and ethambutol for noncavitary Mycobacterium avium complex pulmonary disease. Antimicrob Agents Chemother 64:e01787-19. doi: 10.1128/AAC.01787-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Swanson RV, Adamson J, Moodley C, Ngcobo B, Ammerman NC, Dorasamy A, Moodley S, Mgaga Z, Tapley A, Bester LA, Singh S, Grosset JH, Almeida DV. 2015. Pharmacokinetics and pharmacodynamics of clofazimine in a mouse model of tuberculosis. Antimicrob Agents Chemother 59:3042–3051. doi: 10.1128/AAC.00260-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strydom N, Gupta SV, Fox WS, Via LE, Bang H, Lee M, Eum S, Shim TS, Barry CE, Zimmerman M, Dartois V, Savic RM. 2019. Tuberculosis drugs’ distribution and emergence of resistance in patient’s lung lesions: a mechanistic model and tool for regimen and dose optimization. PLoS Med 16:e1002773. doi: 10.1371/journal.pmed.1002773 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental text, figures, and tables.