Abstract
Terminal differentiation of skeletal muscle cells in culture is inhibited by a number of different growth factors whose subsequent intracellular signaling events are poorly understood. In this study, we have investigated the role of heterotrimeric G proteins in mediating fibroblast growth factor (FGF)-dependent signals that regulate myogenic differentiation. Pertussis toxin, which ADP-ribosylates and inactivates susceptible G proteins, promotes terminal differentiation in the presence of FGF-2, suggesting that Gα or Gβγ subunits or both are involved in transducing the FGF-dependent signal(s) that inhibits myogenesis. We found that Gβγ subunits are likely to be involved since the expression of the C terminus of β-adrenergic receptor kinase 1, a Gβγ subunit-sequestering agent, promotes differentiation in the presence of FGF-2, and expression of the free Gβγ dimer can replace FGF-2, rescuing cells from pertussis toxin-induced differentiation. Addition of pertussis toxin also blocked FGF-2-mediated activation of mitogen-activated protein kinases (MAPKs). Ectopic expression of dominant active mutants in the Ras/MAPK pathway rescued cells from pertussis toxin-induced terminal differentiation, suggesting that the Gβγ subunits act upstream of the Ras/MAPK pathway. It is unlikely that the pertussis toxin-sensitive pathway is activated by other, as yet unidentified FGF receptors since PDGF (platelet-derived growth factor)-stimulated MM14 cells expressing a chimeric receptor containing the FGF receptor-1 intracellular domain and the PDGF receptor extracellular domain were sensitive to pertussis toxin. Our data suggest that FGF-mediated signals involved in repression of myogenic differentiation are transduced by a pertussis toxin-sensitive G-protein-coupled mechanism. This signaling pathway requires the action of Gβγ subunits and activation of MAPKs to repress skeletal muscle differentiation.
Of the soluble growth factors thought to play critical roles in the development of skeletal muscle, fibroblast growth factors (FGFs), Sonic hedgehog, scatter factor/hepatocyte growth factor, and transforming growth factor β are thought to be required for skeletal muscle development in vivo (2, 5, 16, 23, 48). We are attempting to delineate the signaling pathways utilized by FGFs that regulate the proliferation and differentiation of skeletal muscle cells. Previous studies performed by other groups as well as data obtained in our laboratory have demonstrated that (i) distinct FGF pathways are involved in regulating MM14 myoblast growth and differentiation (37), (ii) FGF signaling pathways cannot be replaced by stimulation of other growth factor receptors (36, 38, 39), and (iii) FGFs stimulate activation of mitogen-activated protein kinase (MAPK) pathways (8, 36, 45, 47). Of the four identified FGF receptor tyrosine kinases, only one, FGF receptor-1, is detectably expressed in MM14 cells (37, 63); it is required for FGF-mediated repression of terminal differentiation (22). Additionally, high-affinity binding and subsequent signaling events require that FGFs bind to both the tyrosine kinase and a heparan sulfate proteoglycan (49, 53, 54).
Pertussis toxin-sensitive, G-protein-coupled mechanisms have been reported to affect myoblast differentiation and proliferation, although the mechanisms involved have not been investigated (30, 67). Pertussis toxin (PT), a protein virulence factor produced by Bordetella pertussis, is composed of an A protomer and a B oligomer. The A protomer consists of a single peptide that ADP-ribosylates specific eucaryotic G proteins (Gi/o), locking the G protein in the GDP-bound state and preventing dissociation of Gα and Gβγ subunits, thus leading to inactivation of the G-protein signal. The B oligomer binds to cell surface receptor proteoglycans and transfers the A protomer to the interior of the cell (29).
The heterotrimeric G proteins are composed of distinct α, β, and γ subunits, and all three can participate in signal transduction. Following receptor activation by agonist, Gα subunits of PT-sensitive proteins transmit signals to adenylyl cyclase and other effector molecules (66). The Gβγ heterodimer, released upon activation of PT-sensitive G proteins, activates K+ channels (35), mediates the translocation of the β-adrenergic receptor kinase 1 (βARK1) (64), regulates specific isoforms of adenylyl cyclase (62) and phospholipase C (PLC) (9), and stimulates the MAPKs (12, 15, 41). Stimulation of MAPK activity by the insulin-like growth factor 1 (IGF-1) receptor tyrosine kinase depends on participation of Gβγ subunits derived from PT-sensitive G proteins (41). As for the G-protein-coupled receptor-mediated pathways, IGF-1 signaling can be inhibited by PT treatment or by a Gβγ subunit inhibitor (41).
A large number of polypeptide growth factor receptors stimulate activation of MAPKs (4, 46, 55). A few reports have demonstrated that MAPK stimulation is PT sensitive. Among the receptor tyrosine kinases, PT interferes with the activation of MAPK by epidermal growth factor in hepatocytes (20) and by IGF-1 in Rat-1 fibroblasts (66). Activation of MAPKs is known to occur via the Ras/Raf/MKK1/2 pathway (27, 60). Recently, MAPKs were reported to be activated by Ras-independent mechanisms that include c-Src protein tyrosine kinase (18) and protein kinase C (7, 44, 46, 65) pathways. Additional complexity in these signaling pathways is suggested by the existence of MAPK kinase kinases other than Raf (3). G-protein-dependent signaling can be coupled to the MAPK cascade through release of free βγ subunits, which is linked to activation of a Ras-dependent pathway (32), or through activation of MAPK by PT-sensitive Gα subunits (31, 64).
Here we report that PT stimulates myogenic differentiation in the presence of FGF-2, inhibits FGF-induced proliferation of MM14 cells, and blocks FGF-2-stimulated MAPK activity. In addition, FGF-2 signaling can be blocked by inhibitors of Gβγ subunits. Expression of the free Gβγ dimer suppresses PT-stimulated differentiation and mimics the effect of FGF-2 on MM14 cells. Thus, we demonstrate for the first time that signaling pathways regulated by binding of FGF-2 to FGF receptor-1 can be mediated by Gβγ subunits of PT-sensitive heterotrimeric G proteins.
MATERIALS AND METHODS
Cell culture.
Mouse MM14 cells (39) were cultured on gelatin-coated plates in growth medium consisting of Ham’s F10 (Life Technologies, Gaithersburg, Md.) supplemented with 0.8 mM CaCl2, 100 U of penicillin G per ml, 5 μg of streptomycin sulfate per ml, and 15% horse serum. The concentration of FGF-2 was increased from 0.3 to 2.5 nM with increasing cell density. Human recombinant FGF-2 was purified from a yeast strain expressing this growth factor (53). PT and cholera toxin (CT) were purchased from Life Technologies), B oligomer of PT was purchased from Calbiochem (San Diego, Calif.), and forskolin was purchased from Sigma (St. Louis, Mo.).
Clonal growth assay.
Cells were plated onto six-well plates at a density of 50 cells per well in growth medium containing 0.3 nM FGF-2, cultured for 48 h, then fixed with AFA (70% ethanol–37% formaldehyde–glacial acetic acid, 20:2:1) at 4°C, and immunostained for myosin heavy chain (MHC) as previously described (36). Colonies were analyzed by phase-contrast microscopy. The number of nuclei per colony was determined, and percent MHC-positive cells per well was quantified. PT at a concentration of 50 ng/ml was used in a first series of experiments. In subsequent experiments, we determined that PT at 20 ng/ml produced an equivalent effect, and this concentration was used.
DNA transfection.
DNA was transiently transfected into MM14 cells by a calcium phosphate-DNA precipitate method as described previously (36). The expression vector pBJ5 PDGFRβ/FGFR1, encoding a chimeric platelet-derived growth factor (PDGF) β receptor/FGF receptor 1 construct (PDGF/FGF receptor chimera), is composed of the PDGF β-receptor extracellular domain and the FGF receptor-1 transmembrane and intracellular domains. This vector was previously constructed in our laboratory (37). Eucaryotic expression vectors pCDM8.1Gβ1 and pCDM8.1Gγ2, encoding Gβ1 (17) and Gγ2 (19), respectively, were a gift from M. Simon (California Institute of Technology). pCEV CD8 βARK, an expression vector that encodes a membrane-targeted C-terminal fragment of βARK1 (11), and a control vector (pCEV CD8) were provided by S. Gutkind (National Institute of Dental Research, National Institutes of Health). MMTV-LTR Ras Ej6, carrying the Ha-ras oncogene (51), RSV-Raf-BXB, carrying a constitutively active form of the raf-1 proto-oncogene, Raf-BXB (6) (referred to as BXB-Raf in this report), and CMV-MKK1(R4F), a cytomegalovirus (CMV)-based vector encoding a constitutively active mutant of MAPK kinase 1 (R4F-MKK1) (43), were provided by R. Palmiter (Howard Hughes Medical Institute, University of Washington), U. Rapp (National Cancer Institute, Frederick Cancer Research and Development Center), and N. Ahn (Howard Hughes Medical Institute, University of Colorado), respectively.
Muscle-specific promoter assay.
A differentiation-sensitive muscle-specific reporter activity assay was used to determine the extent of MM14 differentiation following transient transfection. The reporter contained the firefly luciferase gene driven by a muscle-specific promoter (MSP; human α-cardiac actin promoter) (36). MM14 cells were plated on six-well plates at a density of 10,000 cells/well and cotransfected with 1 μg of MSP reporter vector, 1 μg of CMV-LacZ, and different amounts of expression vector or control vector as indicated. Equivalent DNA concentrations were maintained by the addition of pcDNA3 vector (Invitrogen, San Diego, Calif.). Cells were harvested and luciferase activity was determined 36 h following transfection. Luciferase activity was determined by using a Tropix (Bedford, Mass.) Dual Light assay kit and quantitated with a luminometer (Optocomp I; MGM Instruments, Inc., Hamden, Conn.). Luciferase activity values (relative light units) were normalized to β-galactosidase activity values (relative light units) to correct for transfection efficiency. The CMV promoter was chosen to drive the lacZ gene since this promoter exhibits the lowest level of change of all promoters tested (<1.5-fold) between proliferating and differentiated MM14 cell populations.
MAPK activity assay.
MAPK activity was determined by using the PathDetect Elk1 reporting system (Stratagene, San Diego, Calif.). (68). MM14 cells were plated on six-well plates at a density of 40,000 cells/well or on 24-well plates at a density of 8,000 cells/well and cotransfected with 1 μg (250 ng for the 24-well plate) of pFR-Luc reporter vector per well, 250 ng (50 ng for the 24-well plate) of pFA-Elk1 vector per well, and 1 μg (200 ng for the 24-well plate) of CMV-LacZ vector per well; 12 to 16 h after the transfection, cells were washed twice with phosphate-buffered saline (pH 7.2) and incubated in 2.5% serum without FGF for 6 h. The cells were then kept in the same medium or stimulated with 0.1 nM FGF-2. Cells were harvested and luciferase activity was determined 6 h following FGF stimulation or treatment. Luciferase activity was determined, quantitated, and normalized as described for the MSP assay.
RESULTS
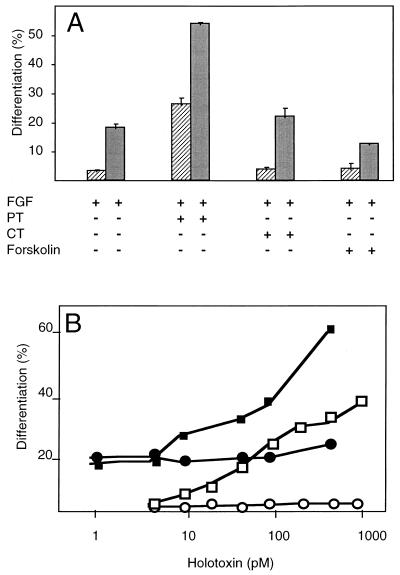
Proper development and regeneration of skeletal muscle in vivo is likely to be dependent on FGFs (16, 24). MM14, a skeletal muscle satellite cell line, like skeletal muscle primary cultures, is dependent on FGFs (10, 52, 59). MM14 cells thus serve as a model for investigating signaling in primary cells. We have previously demonstrated that ERK1/2 (extracellular-regulated kinases 1 and 2) can be activated by FGF-2 in MM14 cells (36). We wanted to investigate further the signaling mechanisms activated by FGF in MM14 cells and to identify pathways involved in the regulation of myogenesis, specifically G-protein signaling. We therefore treated MM14 cells with PT, CT, and forskolin to examine whether cyclic AMP-dependent signaling plays a role in the FGF response. While PT blocked FGF activity and promoted terminal differentiation in a dose-dependent fashion (Fig. 1), neither CT, which ADP-ribosylates G proteins involved in adenylate cyclase activation, nor forskolin, a direct activator of adenylate cyclase (25), affected the proliferation or differentiation of MM14 cells (Fig. 1A). These data suggest that the action of PT is distinct from its potential effects on adenylate cyclase and protein kinase A. The B oligomer of PT is known to bind membrane proteoglycans (29). To rule out a possible effect of the B oligomer on FGF binding to its receptor complex, we examined whether the B oligomer of the holotoxin was sufficient to induce skeletal muscle differentiation. Treatment of MM14 cells with the B oligomer over a wide range of concentrations elicited no detectable effect on MM14 cell differentiation (Fig. 1B). In contrast to the B oligomer, treatment with the PT holotoxin promoted myogenesis under identical culture conditions, demonstrating that the effect of the toxin is likely to be mediated via the ADP-ribosylation of a Gi/o protein(s) (Fig. 1B). Consistent with the ability of PT to block FGF signaling events that repress myogenesis, PT treatment also prevented proliferation in the presence of FGF-2 and 15% horse serum (Fig. 2). Neither forskolin, CT, nor the B oligomer of PT had any detectable effect on MM14 cell proliferation (Fig. 2).
FIG. 1.
PT stimulates skeletal muscle differentiation in the presence of FGF-2. (A) MM14 cells were incubated in the presence of 15% (▨) or 2.5% ( ) serum in medium containing 0.3 nM FGF-2. PT (50 ng/ml; 480 pM), CT (1,000 ng/ml; 11.9 nM), or forskolin (10 μM) was added 1 h after plating. Cells were fixed and stained 48 h after plating. Differentiation of MM14 cells was assayed by clonal analysis as described in Materials and Methods and determined as the number of nuclei in MHC-positive cells. Mean values and standard deviations represent three independent experiments performed in triplicate. No fewer than 75 colonies/100 cells were counted per point per experiment. (B) The B oligomer of pertussis toxin does not affect myogenic differentiation. MM14 cells were incubated in the presence of 15% (□ and ○) or 2.5% (■ and •) serum in medium containing 0.3 nM FGF-2. Cells received the indicated concentrations of holotoxin (■ and □) or B oligomer (• and ○), added at equivalent molar concentrations. Mean values represent the averages of three independent experiments performed in triplicate. Standard deviations were no more than 5% for PT in 2.5% serum, 2.4% for PT in 15% serum, 3.3% for B oligomer in 2.5% serum, and 0.5% for B oligomer in 15% serum.
) serum in medium containing 0.3 nM FGF-2. PT (50 ng/ml; 480 pM), CT (1,000 ng/ml; 11.9 nM), or forskolin (10 μM) was added 1 h after plating. Cells were fixed and stained 48 h after plating. Differentiation of MM14 cells was assayed by clonal analysis as described in Materials and Methods and determined as the number of nuclei in MHC-positive cells. Mean values and standard deviations represent three independent experiments performed in triplicate. No fewer than 75 colonies/100 cells were counted per point per experiment. (B) The B oligomer of pertussis toxin does not affect myogenic differentiation. MM14 cells were incubated in the presence of 15% (□ and ○) or 2.5% (■ and •) serum in medium containing 0.3 nM FGF-2. Cells received the indicated concentrations of holotoxin (■ and □) or B oligomer (• and ○), added at equivalent molar concentrations. Mean values represent the averages of three independent experiments performed in triplicate. Standard deviations were no more than 5% for PT in 2.5% serum, 2.4% for PT in 15% serum, 3.3% for B oligomer in 2.5% serum, and 0.5% for B oligomer in 15% serum.
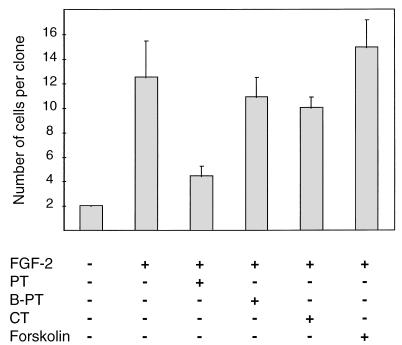
FIG. 2.
PT inhibits proliferation of MM14 cells. MM14 cells were plated onto six-well plates at a density of 50 cells per well and incubated in media containing 15% serum in the absence or presence of 0.3 nM FGF-2. PT (480 pM), B oligomer of PT (B-PT; 480 pM), CT (11.9 nM), and forskolin (10 mM) were added 1 h after plating. Cells were fixed and stained, and the numbers of cells per clone were determined 48 h after plating. Mean values and standard deviations represent three independent experiments performed in triplicate. No fewer than 75 colonies were counted per point per experiment.
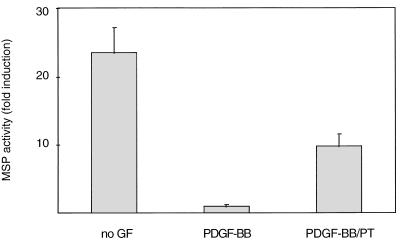
To determine whether PT directly interfered with signaling from FGF receptor-1, we studied the PT sensitivity of MM14 cells transiently transfected with a construct encoding a PDGF/FGF receptor chimera (37). MM14 cells do not express endogenous PDGF receptors (36), and expression of the chimeric receptor confers PDGF-BB-dependent inhibition of myogenic differentiation in MM14 cells (37). In the presence of PDGF-BB, PT induces differentiation of MM14 cells transiently transfected with the chimeric receptor (Fig. 3). These data suggest that PT inhibits signals transduced directly from activation of the FGF receptor-1 tyrosine kinase.
FIG. 3.
PT stimulates differentiation in MM14 cells transiently transfected with a PDGF/FGF receptor chimera expression vector. MM14 cells were cotransfected with the MSP reporter, a CMV-LacZ expression vector, and a vector encoding the PDGF/FGF receptor chimera. Cells were incubated in the presence of 2.5% serum, and PT (20 ng/ml; 192 pM) was added 6 h after transfection. Luciferase activity was determined 36 h after transfection and normalized for transfection efficiency. Data are expressed as luciferase activity relative to activity in cells cultured in the presence of 0.2 nM PDGF-BB. Mean values and standard deviations represent three independent experiments performed in triplicate.
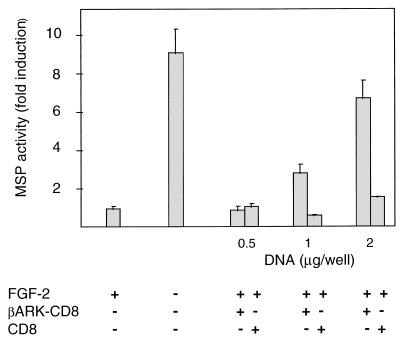
Recent data have shown that G-protein-coupled mechanisms of signal transduction often require the Gβγ subunits (14). Expression of a specific Gβγ subunit binding peptide derived from the carboxyl terminus of βARK1 (βARK1-CT) can block Gβγ subunit-mediated signal transduction in stably and transiently transfected cell lines (11, 33). The βARK1-CT fragment is localized to the cell membrane by the fusing of βARK1-CT to the transmembrane domain from the CD8 receptor (βARK-CD8), thus effectively excluding Gβγ subunits from participating in intracellular signaling. To determine whether the inhibition of myogenic differentiation was mediated by Gβγ or Gα subunits, we examined the effects of transient expression of βARK-CT on MM14 cell differentiation. Transient expression of this Gβγ-sequestering agent stimulated differentiation in the presence of added FGF-2, as assayed by induction of a muscle-specific promoter (Fig. 4). Transfection with a control vector containing the coding sequences for the CD8 transmembrane domain but lacking the βARK-CT sequences elicited no detectable effect, indicating that the induction of differentiation was likely to be due to Gβγ subunit sequestration (Fig. 4).
FIG. 4.
Sequestration of free Gβγ subunits by the C-terminal fragment of βARK1 stimulates differentiation in the presence of FGF. MM14 cells were cotransfected with the MSP reporter, CMV-LacZ expression vector, and increasing amounts of either the vector encoding the membrane-targeted C-terminal fragment of βARK1 (βARK-CD8) or the control vector containing only the membrane-targeting sequence (CD8). The total amount of transfected DNA in each well was equalized to 4 μg with pcDNA3 (Invitrogen). Cells were incubated in the presence of FGF (0.3 nM) in medium supplemented with 15% serum. Luciferase activity was determined 36 h after transfection and normalized for transfection efficiency. Luciferase activity relative to activity in cells cultured in the presence of 0.3 nM FGF-2 is shown. Data shown are the means and standard deviations of triplicate measurements from one representative transfection. The experiment was repeated three times with comparable results.
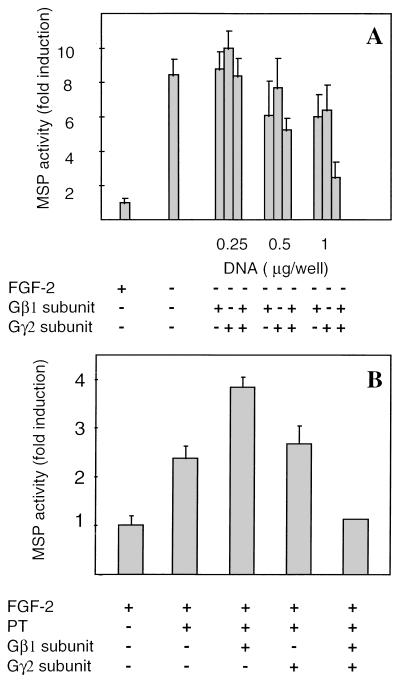
If Gβγ subunits are critical for transducing FGF signals in skeletal muscle cells, then expression of the appropriate Gβγ subunits would be expected to substitute for FGF. Transfection of MM14 cells with increasing amounts of either a Gβ1 or a Gγ2 expression vector inhibited MSP activity less than twofold (Fig. 5A). However, transfection of cells with both Gβ1 and Gγ2 expression vectors was synergistic and completely inhibited terminal differentiation, similar to what was observed for control cells given FGF-2 (Fig. 5A). A similar experiment was performed in the presence of FGF-2 and PT. As expected, transient transfection of Gβγ subunits rescued MM14 cells from PT-stimulated differentiation (Fig. 5B). However, transfection with Gγ2 alone elicited no detectable effect, while transfection with only Gβ1 consistently increased MSP activity to levels greater than those for cells treated with PT alone (Fig. 5B). Taken together, these data suggest that the Gβγ subunits play a central role in FGF-dependent regulation of myogenesis.
FIG. 5.
Repression of MM14 differentiation by Gβγ subunits. Transient transfection of MM14 cells with vectors encoding Gβγ subunits represses myogenic differentiation induced by removal of FGF-2 (A) or by addition of PT (B). MM14 cells were cotransfected with the MSP reporter, a CMV-LacZ expression vector, and either a vector encoding Gβ1 or one encoding Gγ2. The total amount of transfected DNA in each well was equalized to 3 μg with pcDNA3 (Invitrogen). Cells were incubated in the absence or presence of FGF-2 (0.3 nM) in medium supplemented with 15% serum. (B) MM14 cells were cotransfected with the MSP reporter, the CMV-LacZ expression vector, and either 1 μg of Gβ1, 1 μg of Gγ2, or 1 μg of each for both Gβ1 and Gγ2 expression vectors. PT (192 pM) was added 6 h after transfection. For both panels, luciferase activity was determined 36 h after transfection and normalized for transfection efficiency. Luciferase activity relative to activity in cells cultured in the presence of 0.3 nM FGF-2 is shown. Mean values and standard deviations represent three (A) and two (B) independent experiments performed in triplicate.
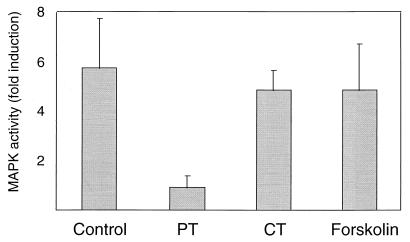
As a further measure of the dependence of FGF signaling on a Gi/o-dependent mechanism, we examined the ability of FGF to activate MAPKs in cells pretreated with PT. The PathDetect Elk1 system detects MAPK activation by phosphorylation of an Elk transcriptional activator fragment (amino acids 307 to 428) fused to the GAL4 DNA binding domain (68). Phosphorylation of this fusion protein by MAPKs then activates a reporter (pFR-Luc) consisting of the firefly luciferase gene placed downstream of a basic promoter element and located 3′ to five tandem repeats of the 17-bp GAL4 binding element. Control experiments with cells transiently cotransfected with either pFA-Elk1 or pFR-Luc alone, or with the combination of pFR-Luc with the transactivating vector lacking the Elk1 domain, displayed no luciferase activity (data not shown). MM14 cells transiently cotransfected with pFR-Luc and pFA-Elk1 were stimulated with FGF-2. Upon FGF-2 stimulation, a 4.0- to 7.5-fold increase in MAPK activity was observed, consistent with our previous observations (36). Pretreatment of MM14 cells with PT completely abolished MAPK activation, while pretreatment with forskolin and CT had minimal effects on MAPK activity (Fig. 6).
FIG. 6.
Pretreatment with PT blocks FGF-stimulated MAPK activity. MM14 cells cotransfected with pFR-Luc, pFA-Elk1, and CMV-LacZ expression vectors were starved for 6 h in medium supplemented with 2.5% serum and no FGF-2. The cells then were stimulated with 0.1 nM FGF-2 for 6 h. Pretreatment included incubation with PT (192 pM) and CT (11.9 nM) 1 h prior to addition of FGF-2. Forskolin was added 15 min prior to FGF-2 addition. Control cells (with or without FGF-2) were incubated in the presence or absence of 0.1% dimethyl sulfoxide (not shown). Cells were harvested and normalized values of luciferase activities were determined 6 h following FGF-2 addition. Luciferase activity relative to activity in unstimulated cells is shown. Mean values and standard deviations represent three independent experiments performed in triplicate.
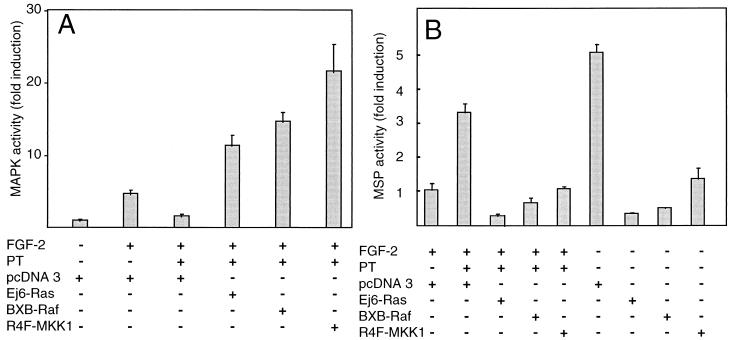
The effects of PT treatment on MAPK activity and differentiation suggest that a Gi/o protein-dependent pathway may be involved in activation of MAPKs following FGF stimulation. To test this hypothesis, we examined whether the PT-induced block in FGF signaling could be overcome by known activators of the Ras/MAPK pathway. Activators of the MAPK pathway including Ha-Ras (Ej6-Ras), Raf (BXB-Raf), and MKK1 (R4F-MKK1) all activate the Elk1 reporter system in MM14 cells in the presence of PT (Fig. 7A). Moreover, these MAPK pathway activators repress differentiation in the presence or absence of FGF (Fig. 7B), suggesting that they act on signaling pathways directly involved in regulating terminal differentiation. The observation that constitutively active mutants of Ras (Ej6-Ras), Raf (BXB-Raf), and MKK1 (R4F-MKK1) all overcome PT-induced differentiation as well as MAPK activity suggests that the Gi/o proteins inhibited by PT act in a pathway parallel to a MAPK cascade or more likely at an early step in an FGF signaling cascade.
FIG. 7.
Constitutively active Ras, Raf, and MKK1 prevent PT-mediated inhibition of MAPK activity and stimulation of differentiation in MM14 cells. MM14 cells were cotransfected with 1 μg of pFR-Luc, 0.25 μg of pFA-Elk1, and 1 μg of CMV-LacZ expression vectors (A) or with 1 μg of MSP reporter and 1 μg of CMV-LacZ vector together with 1.5 μg of either pcDNA3 or vectors encoding constitutively active mutants of Ras (Ej6-Ras), Raf (BXB-Raf), and MKK1 (R4F-MKK1) (B). Cells were either left untreated or treated with PT in the presence and absence of FGF-2 (0.3 nM) in medium supplemented with 15% serum. PT (192 pM) was added 6 h after transfection. Luciferase activity was determined 36 h after transfection and normalized for transfection efficiency. Luciferase activity relative to activity in cells cultured without the growth factor (A) or to activity in cells cultured in the presence of 0.3 nM FGF-2 (B) is shown. Mean values and standard deviations represent three (A) and two (B) independent experiments performed in triplicate.
DISCUSSION
The molecular mechanisms involved in the regulation of skeletal muscle differentiation by members of the FGF family are poorly understood. We and others have previously demonstrated that skeletal muscle cells, which are dependent on FGFs, stimulate ERK1/2 activity (1, 8, 36, 45, 47). To better understand the events leading to activation of MAPKs, we have examined the role of Gi/o proteins in FGF receptor-1 signaling. In this study, we found that repression of myogenesis by FGFs involves a Gi/o protein-mediated event that is required for MAPK activation.
MM14 cells exhibit an absolute requirement for FGFs that cannot be replaced by serum or other growth factors (10, 36). In the presence of FGF, removal or reduction of serum from MM14 cells causes the cells to enter into a reversible G0 phase without initiating terminal differentiation (10). Thus, inhibitors or activators of FGF signaling in skeletal muscle myoblasts can be readily identified. An inhibitor will promote differentiation in the presence of FGF, while an activator will prevent differentiation in the presence or absence of FGFs. In low serum, we observed that PT induced differentiation, clearly blocking the effects of FGF-2. The induction of differentiation was specific and dose dependent. Neither CT, which ADP-ribosylates PT-insensitive G proteins involved in adenylate cyclase activation, nor forskolin, a direct activator of adenylate cyclase (25), affected the growth or differentiation of MM14 myoblasts.
The biological activity of PT is usually due to the S1 subunit, which ADP-ribosylates Gi/o proteins (29). However, the binding of the B oligomer to cell surface proteoglycans can increase inositol triphosphate production and intracellular calcium levels in Jurkat cells (57), stimulate proliferation in human T lymphocytes (21), and enhance glucose oxidation in adipocytes (61). The B oligomer does not detectably affect myogenic differentiation or proliferation in MM14 skeletal muscle myoblasts, demonstrating that the effect of PT is likely to be mediated by the activity of the S1 subunit, which ADP-ribosylates Gi/o protein(s). Thus, in MM14 cells, as in other cells (40, 56, 58), FGF-dependent signals appear to require the action of a PT-sensitive Gi/o protein(s). We have previously demonstrated that FGF-dependent repression of differentiation in MM14 cells requires a functional FGF receptor-1 (22), the only detectable FGF receptor isoform expressed in MM14 cells (37, 63). The capacity of a truncated dominant negative FGF receptor-1 mutant to block FGF signaling and promote differentiation in these cells demonstrates that repression of myogenic differentiation by FGF requires FGF receptor-1 (22). Furthermore, the PDGF/FGF receptor chimera is capable of repressing differentiation in the absence of FGFs and in the presence of a dominant negative FGF receptor mutant (37). With few exceptions, PT-sensitive Gβγ-mediated signal transduction events are usually initiated by binding of a specific ligand to a membrane-spanning G-protein-coupled receptor. In this report, we demonstrated that repression of myogenic differentiation upon addition of PDGF-BB to cells expressing the PDGF/FGF receptor chimera is PT sensitive. Thus, the FGF receptor-1 tyrosine kinase appears to mediate signals via a PT-sensitive Gi/o protein(s). Furthermore, it is unlikely that an FGF receptor other than FGF receptor-1 is involved.
Upon ligand-dependent receptor activation and binding of GTP to the α subunit of G proteins, Gα and Gβγ subunits dissociate. In this active state, both α and βγ subunits can activate or inhibit their effectors and thus participate in intracellular signaling. We demonstrated that expression of a specific Gβγ subunit binding peptide derived from βARK1-CT induced myogenic differentiation in the presence of FGF-2. Moreover, transient transfection of Gβγ subunits rescued MM14 cells from PT-stimulated differentiation and prevented differentiation in the absence of added FGF-2. Expression of Gβ1 or Gγ2 inhibited MSP activity at the highest concentrations tested; alone, each was capable of reducing MSP activity by only 25%. However, coexpression of both subunits elicited a synergistic effect and reduced MSP activity by 78% (∼3-fold) in the absence of added FGF-2. These data suggest that the levels of Gβγ subunits involved in FGF signaling may be limiting since neither subunit alone was effective at reducing MSP activity.
In the presence of PT, overexpression of Gγ2 had no effect but overexpression of Gβ1 enhanced MSP activity 1.8-fold. In contrast to the effects of either subunit transfected individually, overexpression of both Gβ1 and Gγ2 rescued the PT-induced block of FGF signaling and reduced MSP activity to control levels in the absence of PT. We propose that specific combinations of Gβγ subunits may be required for FGF signaling in skeletal muscle myoblasts. Thus, overexpression of an individual Gβ or Gγ subunit could negatively or positively affect FGF signaling, depending on the concentration and distribution of Gβ and Gγ subunits within the cell.
The molecular mechanisms involved in activation of Gβγ by FGF are unclear, as are the downstream targets of Gβγ in skeletal muscle cells. In other cell types, the participation of Gβγ signaling in tyrosine kinase-mediated activation of ERKs requires calcium and/or PLCs (13). Preliminary data from our laboratory also suggest that PLCs are required for FGF signaling and that PLC activation follows stimulation of Gβγ by FGFs (unpublished data). A potential mechanism for coupling Gβγ with the Ras/MAPK cascade in skeletal muscle cells may also involve regulation of Ras. The pleckstrin homology (PH) domain shared by several proteins that regulate the activity of p21ras, including Ras–GDP-releasing factor, Ras–GTPase-activating protein, and IRS-1, binds Gβγ subunits (64, 69). Interactions between Gβγ subunits and the PH domains of one or more p21ras-regulatory proteins may provide the coupling of Gβγ subunit-mediated signaling to activation of MAPKs, thereby inhibiting myogenic differentiation. Recently, FRS2, a potential substrate for FGF receptor-1, was identified in fibroblasts (34). It is not yet known if FRS2 is present in skeletal muscle cells or if phosphorylation of FRS2 is dependent on G-protein activation. However, it is interesting that Gβγ subunits bind to a similar substrate for the insulin receptor through the PH domains (64). Alternatively, Gβγ subunits may directly or indirectly affect Ca2+ channels and activate Ras- and/or MAPK-dependent pathways through modulation of intracellular Ca2+ (42, 50). Recently, a second mechanism involving Ras-independent stimulation of MAPKs via Gαo was described (66).
Activation of the MAPK cascade(s) is widely considered to be essential for growth factor-induced proliferation responses. To obtain further data in support of PT-sensitive G-protein involvement in MAPK activation, we examined induction of an Elk1-dependent reporter gene in MM14 cells in the presence and absence of PT. Pretreatment of MM14 cells with PT abolished increases in FGF-2-mediated MAPK activity, demonstrating that activation of MAPKs in MM14 cells requires the action of PT-sensitive G proteins. This assay does not distinguish between different MAPKs because Elk1 is known to be phosphorylated by several MAPKs (ERKs = JNK > p38 [26]). Typically, activation of ERK1/2 is thought to occur via a pathway beginning with a growth factor receptor and proceeding through Ras, Raf, and MKK1/2, which phosphorylate ERK. To determine if the Gβγ subunit signaling event occurs upstream of downstream of, or parallel to the Ras/ERK pathway, we examined the effects of overexpression of constitutive active mutants of Ras, Raf, or MKK1/2 on differentiation in the presence and absence of PT. The capability of all mutants to rescue PT-induced differentiation suggests that signal transducers in the Ras/ERK1/2 pathway may act downstream of PT-sensitive G proteins in MM14 cells. Alternatively, it is possible that the signaling mutants in the Ras/ERK pathway independently overcome PT-mediated inhibition of FGF signaling through parallel signaling pathways. We favor the former hypothesis since (i) a constitutively active MKK1 mutant represses differentiation and activates the Elk1 reporter (28); (ii) PT inhibits FGF signals that both repress myogenesis and activate the Elk1 reporter; and (iii) constitutively active mutants of Ras, Raf, and MKK1 can replace FGF and can overcome the PT-induced block to FGF signaling. The molecular mechanisms leading to stimulation of MAPKs by a PT-sensitive Gi/o protein(s) initiated by activation of the FGF receptor-1 tyrosine kinase are unusual and not yet understood.
Our data suggest that a unique mechanism may be involved in FGF-mediated repression of myogenesis and support a role for Gβγ in activation of FGF signaling pathways regulating myogenesis. We do not know if the PT-sensitive event is directly involved in activation of MAPKs by FGFs, or whether it is necessary for intracellular FGF signaling but not directly involved in MAPK activation. Although Gβγ signaling is the earliest event that we have detected following activation of FGF receptor-1, further experimentation will be required to elucidate the molecular mechanisms involved in regulation of skeletal muscle differentiation by FGFs.
ACKNOWLEDGMENTS
This work was supported by grants from the Walther Cancer Institute and the National Institutes Health to B.B.O.
We thank J. Martin and N. Ahn for their thoughtful comments on the manuscript.
REFERENCES
- 1.Bennett A M, Tonks N K. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- 2.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 3.Blank J L, Gerwins P, Elliott E M, Sather S, Johnson G L. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J Biol Chem. 1996;271:5361–5368. doi: 10.1074/jbc.271.10.5361. [DOI] [PubMed] [Google Scholar]
- 4.Bogoyevitch M A, Glennon P E, Andersson M B, Clerk A, Lazou A, Marshall C J, Parker P J, Sugden P H. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes. The potential role of the cascade in the integration of two signaling pathways leading to myocyte hypertrophy. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 5.Brand-Saberi B, Muller T S, Wilting J, Christ B, Birchmeier C. Scatter factor/hepatocyte growth factor (SF/HGF) induces emigration of myogenic cells at interlimb level in vivo. Dev Biol. 1996;179:303–308. doi: 10.1006/dbio.1996.0260. [DOI] [PubMed] [Google Scholar]
- 6.Bruder J T, Heidecker G, Rapp U R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992;6:545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco M T, Moscat J, Rapp U, Cooper G M. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell J S, Wenderoth M P, Hauschka S D, Krebs E G. Differential activation of mitogen-activated protein kinase in response to basic fibroblast growth factor in skeletal muscle cells. Proc Natl Acad Sci USA. 1995;92:870–874. doi: 10.1073/pnas.92.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camps M, Carozzi A, Schnabel P, Scheer A, Parker P J, Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992;360:684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- 10.Clegg C H, Linkhart T A, Olwin B B, Hauschka S D. Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 1987;105:949–956. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crespo P, Cachero T G, Xu N, Gutkind J S. Dual effect of beta-adrenergic receptors on mitogen-activated protein kinase. Evidence for a beta gamma-dependent activation and a G alpha s-cAMP-mediated inhibition. J Biol Chem. 1995;270:25259–25265. doi: 10.1074/jbc.270.42.25259. [DOI] [PubMed] [Google Scholar]
- 12.Crespo P, Xu N, Simonds W F, Gutkind J S. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 13.Della Rocca G J, van Biesen T, Daaka Y, Luttrell D K, Luttrell L M, Lefkowitz R J. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 14.Exton J H. Cell signalling through guanine-nucleotide-binding regulatory proteins (G proteins) and phospholipases. Eur J Biochem. 1997;243:10–20. doi: 10.1111/j.1432-1033.1997.t01-1-00010.x. [DOI] [PubMed] [Google Scholar]
- 15.Faure M, Voyno-Yasenetskaya T A, Bourne H R. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- 16.Floss T, Arnold H H, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997;11:2040–2051. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong H K, Hurley J B, Hopkins R S, Miake-Lye R, Johnson M S, Doolittle R F, Simon M I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci USA. 1986;83:2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner A M, Vaillancourt R R, Johnson G L. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase by G protein and tyrosine kinase oncoproteins. J Biol Chem. 1993;268:17896–17901. [PubMed] [Google Scholar]
- 19.Gautam N, Baetscher M, Aebersold R, Simon M I. A G protein gamma subunit shares homology with ras proteins. Science. 1989;244:971–974. doi: 10.1126/science.2499046. [DOI] [PubMed] [Google Scholar]
- 20.Gines P, Li X, Brown S E, Nakamura T, Guzelian P S, Heasley L E, Schrier R W, Nemenoff R A. Inhibitory actions of cyclic adenosine monophosphate and pertussis toxin define two distinct epidermal growth factor-regulated pathways leading to activation of mitogen-activated protein kinase in rat hepatocytes. Hepatology. 1996;23:1167–1173. doi: 10.1002/hep.510230535. [DOI] [PubMed] [Google Scholar]
- 21.Gray L S, Huber K S, Gray M C, Hewlett E L, Engelhard V H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989;142:1631–1638. [PubMed] [Google Scholar]
- 22.Hannon K, Kudla A J, McAvoy M J, Clase K L, Olwin B B. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J Cell Biol. 1996;132:1151–1159. doi: 10.1083/jcb.132.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannon, K., M. M. McAvoy, and B. B. Olwin. 1998. Unpublished observation.
- 24.Itoh N, Mima T, Mikawa T. Loss of fibroblast growth factor receptors is necessary for terminal differentiation of embryonic limb muscle. Development. 1996;122:291–300. doi: 10.1242/dev.122.1.291. [DOI] [PubMed] [Google Scholar]
- 25.Iyengar R. Molecular and functional diversity of mammalian Gs-stimulated adenylyl cyclases. FASEB J. 1993;7:768–775. doi: 10.1096/fasebj.7.9.8330684. [DOI] [PubMed] [Google Scholar]
- 26.Janknecht R, Hunter T. Convergence of MAP kinase pathways on the ternary complex factor Sap-1a. EMBO J. 1997;16:1620–1627. doi: 10.1093/emboj/16.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson G L, Vaillancourt R R. Sequential protein kinase reactions controlling cell growth and differentiation. Curr Opin Cell Biol. 1994;6:230–238. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 28.Jones, N. C., Y. V. Fedorov, and B. B. Olwin. Submitted for publication.
- 29.Kaslow H R, Burns D L. Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J. 1992;6:2684–2690. doi: 10.1096/fasebj.6.9.1612292. [DOI] [PubMed] [Google Scholar]
- 30.Kelvin D J, Simard G, Tai H H, Yamaguchi T P, Connolly J A. Growth factors, signaling pathways, and the regulation of proliferation and differentiation in BC3H1 muscle cells. I. A pertussis toxin-sensitive pathway is involved. J Cell Biol. 1989;108:159–167. doi: 10.1083/jcb.108.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinane T B, Kang I, Chu A, Chin S H, Ercolani L. G alpha(i-2) mediates renal LLC-PK1 growth by a Raf-independent activation of p42/p44 MAP kinase. Am J Physiol. 1997;272:F273–F282. doi: 10.1152/ajprenal.1997.272.2.F273. [DOI] [PubMed] [Google Scholar]
- 32.Koch W J, Hawes B E, Allen L F, Lefkowitz R J. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci USA. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch W J, Hawes B E, Inglese J, Luttrell L M, Lefkowitz R J. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 34.Kouhara H, Hadari Y R, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 35.Krapivinsky G, Krapivinsky L, Wickman K, Clapham D E. G beta gamma binds directly to the G protein-gated K+ channel, IKACh. J Biol Chem. 1995;270:29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- 36.Kudla A J, John M L, Bowen-Pope D F, Rainish B, Olwin B B. A requirement for fibroblast growth factor in regulation of skeletal muscle growth and differentiation cannot be replaced by activation of platelet-derived growth factor signaling pathways. Mol Cell Biol. 1995;15:3238–3246. doi: 10.1128/mcb.15.6.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudla A J, Jones N C, Rosenthal R S, Arthur K, Clase K L, Olwin B B. The FGF receptor-1 tyrosine kinase domain regulates myogenesis but is not sufficient to stimulate proliferation. J Cell Biol. 1998;142:241–250. doi: 10.1083/jcb.142.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim R W, Hauschka S D. EGF responsiveness and receptor regulation in normal and differentiation-defective mouse myoblasts. Dev Biol. 1984;105:48–58. doi: 10.1016/0012-1606(84)90260-4. [DOI] [PubMed] [Google Scholar]
- 39.Linkhart T A, Clegg C H, Hauschka S D. Control of mouse myoblast commitment to terminal differentiation by mitogens. J Supramol Struct. 1980;14:483–498. doi: 10.1002/jss.400140407. [DOI] [PubMed] [Google Scholar]
- 40.Logan A, Logan S D. Studies on the mechanisms of signalling and inhibition by pertussis toxin of fibroblast growth factor-stimulated mitogenesis in Balb/c 3T3 cells. Cell Signalling. 1991;3:215–223. doi: 10.1016/0898-6568(91)90047-x. [DOI] [PubMed] [Google Scholar]
- 41.Luttrell L M, van Biesen T, Hawes B E, Koch W J, Touhara K, Lefkowitz R J. G beta gamma subunits mediate mitogen-activated protein kinase activation by the tyrosine kinase insulin-like growth factor 1 receptor. J Biol Chem. 1995;270:16495–16498. doi: 10.1074/jbc.270.28.16495. [DOI] [PubMed] [Google Scholar]
- 42.Macrez N, Morel J L, Kalkbrenner F, Viard P, Schultz G, Mironneau J. A betagamma dimer derived from G13 transduces the angiotensin AT1 receptor signal to stimulation of Ca2+ channels in rat portal vein myocytes. J Biol Chem. 1997;272:23180–23185. doi: 10.1074/jbc.272.37.23180. [DOI] [PubMed] [Google Scholar]
- 43.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 44.Marquardt B, Frith D, Stabel S. Signalling from TPA to MAP kinase requires protein kinase C, raf and MEK: reconstitution of the signalling pathway in vitro. Oncogene. 1994;9:3213–3218. [PubMed] [Google Scholar]
- 45.Milasincic D J, Calera M R, Farmer S R, Pilch P F. Stimulation of C2C12 myoblast growth by basic fibroblast growth factor and insulin-like growth factor 1 can occur via mitogen-activated protein kinase-dependent and -independent pathways. Mol Cell Biol. 1996;16:5964–5973. doi: 10.1128/mcb.16.11.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitev V, Le Panse R, Coulomb B, Miteva L, Houdebine L M. Epidermal growth factor stimulates mitogen-activated protein kinase by a PKC-dependent pathway in human keratinocytes. Biochem Biophys Res Commun. 1995;208:245–252. doi: 10.1006/bbrc.1995.1330. [DOI] [PubMed] [Google Scholar]
- 47.Mourey R J, Vega Q C, Campbell J S, Wenderoth M P, Hauschka S D, Krebs E G, Dixon J E. A novel cytoplasmic dual specificity protein tyrosine phosphatase implicated in muscle and neuronal differentiation. J Biol Chem. 1996;271:3795–3802. doi: 10.1074/jbc.271.7.3795. [DOI] [PubMed] [Google Scholar]
- 48.Munsterberg A E, Kitajewski J, Bumcrot D A, McMahon A P, Lassar A B. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- 49.Olwin B B, Rapraeger A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J Cell Biol. 1992;118:631–639. doi: 10.1083/jcb.118.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poiraudeau S, Lieberherr M, Kergosie N, Corvol M T. Different mechanisms are involved in intracellular calcium increase by insulin-like growth factors 1 and 2 in articular chondrocytes: voltage-gated calcium channels, and/or phospholipase C coupled to a pertussis-sensitive G-protein. J Cell Biochem. 1997;64:414–422. [PubMed] [Google Scholar]
- 51.Quaife C J, Pinkert C A, Ornitz D M, Palmiter R D, Brinster R L. Pancreatic neoplasia induced by ras expression in acinar cells of transgenic mice. Cell. 1987;48:1023–1034. doi: 10.1016/0092-8674(87)90710-0. [DOI] [PubMed] [Google Scholar]
- 52.Rando T A, Blau H M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rapraeger A C, Guimond S, Krufka A, Olwin B B. Regulation by heparan sulfate in fibroblast growth factor signaling. Methods Enzymol. 1994;245:219–240. doi: 10.1016/0076-6879(94)45013-7. [DOI] [PubMed] [Google Scholar]
- 54.Rapraeger A C, Krufka A, Olwin B B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 55.Reimann T, Hempel U, Krautwald S, Axmann A, Scheibe R, Seidel D, Wenzel K W. Transforming growth factor-beta1 induces activation of Ras, Raf-1, MEK and MAPK in rat hepatic stellate cells. FEBS Lett. 1997;403:57–60. doi: 10.1016/s0014-5793(97)00024-0. [DOI] [PubMed] [Google Scholar]
- 56.Rodan S B, Wesolowski G, Thomas K A, Yoon K, Rodan G A. Effects of acidic and basic fibroblast growth factors on osteoblastic cells. Connect Tissue Res. 1989;20:283–288. doi: 10.3109/03008208909023898. [DOI] [PubMed] [Google Scholar]
- 57.Rosoff P M, Walker R, Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987;139:2419–2423. [PubMed] [Google Scholar]
- 58.Sa G, Fox P L. Basic fibroblast growth factor-stimulated endothelial cell movement is mediated by a pertussis toxin-sensitive pathway regulating phospholipase A2 activity. J Biol Chem. 1994;269:3219–3225. [PubMed] [Google Scholar]
- 59.Seed J, Hauschka S D. Clonal analysis of vertebrate myogenesis. VIII. Fibroblasts growth factor (FGF)-dependent and FGF-independent muscle colony types during chick wing development. Dev Biol. 1988;128:40–49. doi: 10.1016/0012-1606(88)90264-3. [DOI] [PubMed] [Google Scholar]
- 60.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 61.Tamura M, Nogimori K, Yajima M, Ase K, Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983;258:6756–6761. [PubMed] [Google Scholar]
- 62.Tang W J, Gilman A G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991;254:1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- 63.Templeton T J, Hauschka S D. FGF-mediated aspects of skeletal muscle growth and differentiation are controlled by a high affinity receptor, FGFR1. Dev Biol. 1992;154:169–181. doi: 10.1016/0012-1606(92)90057-n. [DOI] [PubMed] [Google Scholar]
- 64.Touhara K, Inglese J, Pitcher J A, Shaw G, Lefkowitz R J. Binding of G protein beta gamma-subunits to pleckstrin homology domains. J Biol Chem. 1994;269:10217–10220. [PubMed] [Google Scholar]
- 65.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 66.van Biesen T, Hawes B E, Raymond J R, Luttrell L M, Koch W J, Lefkowitz R J. G(o)-protein alpha-subunits activate mitogen-activated protein kinase via a novel protein kinase C-dependent mechanism. J Biol Chem. 1996;271:1266–1269. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- 67.Vandenburgh H H, Shansky J, Solerssi R, Chromiak J. Mechanical stimulation of skeletal muscle increases prostaglandin F2 alpha production, cyclooxygenase activity, and cell growth by a pertussis toxin sensitive mechanism. J Cell Physiol. 1995;163:285–294. doi: 10.1002/jcp.1041630209. [DOI] [PubMed] [Google Scholar]
- 68.Xu L, Sanchez T, Zheng C-F. In vivo signal transduction pathway reporting systems. Strategies. 1997;10:1–3. [Google Scholar]
- 69.Xu N, Coso O, Mahadevan D, De Blasi A, Goldsmith P K, Simonds W F, Gutkind J S. The PH domain of Ras-GAP is sufficient for in vitro binding to beta gamma subunits of heterotrimeric G proteins. Cell Mol Neurobiol. 1996;16:51–59. doi: 10.1007/BF02578386. [DOI] [PMC free article] [PubMed] [Google Scholar]