ABSTRACT
Antimicrobial resistance is emerging in clinical strains of Clostridioides difficile. Ibezapolstat (IBZ) is a DNA polymerase IIIC inhibitor that has completed phase II clinical trials. IBZ has potent in vitro activity against wild-type, susceptible strains but its effect on C. difficile strains with reduced susceptibility to metronidazole (MTZ), vancomycin (VAN), or fidaxomicin (FDX) has not been tested. The primary objective of this study was to test the antibacterial properties of IBZ against multidrug-resistant C. difficile strains. The in vitro activity, bactericidal, and time-kill activity of IBZ versus comparators were evaluated against 100 clinical strains of which 59 had reduced susceptibility to other C. difficile antibiotics. Morphologic changes against a multidrug resistance strain were visualized by light and scanning electron microscopy. The overall IBZ MIC50/90 values (µg/mL) for evaluated C. difficile strains were 4/8, compared with 2/4 for VAN, 0.5/1 for FDX, and 0.25/4 for MTZ. IBZ MIC50/90 values did not differ based on non-susceptibility to antibiotic class or number of classes to which strains were non-susceptible. IBZ bactericidal activity was similar to the minimum inhibitory concentration (MIC) and maintained in wild-type and non-susceptible strains. Time-kill assays against two laboratory wild-type and two clinical non-susceptible strains demonstrated sustained IBZ activity despite reduced killing by comparator antibiotics for IBZ and VAN non-susceptible strains. Microscopy visualized increased cell lengthening and cellular damage in multidrug-resistant strains exposed to IBZ sub-MIC concentrations. This study demonstrated the potent antibacterial activity of IBZ against a large collection of C. difficile strains including multidrug-resistant strains. This study highlights the therapeutic potential of IBZ against multidrug-resistant strains of C. difficile.
KEYWORDS: Clostridioides difficile infection, time-kill studies, bactericidal effects, multidrug-resistant organisms
INTRODUCTION
Clostridioides difficile is a Gram-positive, toxin- and spore-forming obligate anaerobe and the most common cause of infectious diarrhea in hospitalized patients (1). Only two antibiotics, vancomycin (VAN) and fidaxomicin (FDX), are guidelines recommended for C. difficile infection (CDI) (2), and antimicrobial resistance has been reported to both (3, 4). A third antibiotic, metronidazole (MTZ), is no longer a guideline recommended due to decreasing clinical response rates most likely due to antimicrobial resistance (5, 6). Although novel therapeutics are in development for CDI, including the recent approval of live biotherapeutic products, only three antibiotics have made it to phase III trials since the approval of FDX in 2011, and none have filed for Food and Drug Administration approval (7–12). Thus, new antibiotics and particularly those with activity against multidrug-resistant (MDR) strains are urgently needed.
Ibezapolstat (IBZ) is a DNA polymerase IIIC (polIIIC) inhibitor that has recently completed phase II clinical trials (13, 14). PolIIIC is a distinct site of action compared to those targeted by currently available antimicrobials. The DNA polIIIC enzyme is essential for the replication of low G + C content of Gram-positive bacteria and is selective for species within the Bacillota phylum (15). As a unique target of the replisome, IBZ may have activity against C. difficile strains with resistance to other antimicrobial classes. However, the activity of IBZ on MDR C. difficile strains has not been studied. Therefore, this project’s purpose was to determine the efficacy of IBZ against C. difficile strains with various patterns of antimicrobial resistance.
MATERIALS AND METHODS
Strains and ribotyping
Clinical C. difficile strains (n = 100) comprising the most common PCR ribotypes (RT) in Texas were selected from our ongoing Texas Surveillance System (16) and included along with laboratory strains R20291 (RT027) and CD630 (RT012) for minimum inhibitory concentration (MIC), bactericidal testing, and microscopy. MDR strains were selected from our collection to fulfill the study objectives. Fluorescent PCR ribotyping was done as previously described (17). The surveillance study from which clinical strains were selected is approved by the University of Houston Committee for the Protection of Human Subjects (CPHS00128).
Antibiotics
IBZ powder was provided by the study sponsor (Acurx Pharmaceuticals, Inc., Staten Island, NY, USA). FDX and VAN were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). For all assays, antibiotics were diluted in dimethyl sulfoxide (IBZ and FDX) or diluted water (VAN, MTZ) and further diluted with distilled water to reach the final desired concentration. Non-susceptibility to each antibiotic agent was assessed using agar dilution MIC values. Determination of VAN (MIC > 2 mg/L) and FDX (MIC > 1 mg/L) non-susceptibility was based on epidemiologic cutoff values (18–20).
Agar dilution MIC testing
Isolates were streaked onto a blood agar plate and incubated overnight. A single isolated colony from the blood agar plate was then suspended in brain heart infusion (BHI) broth to achieve turbidity equal to the 0.5 McFarland standard. Agar dilution MIC testing was performed in compliance with Clinical and Laboratory Standards Institute (CLSI) guidance for anaerobic bacteria and as previously described (6, 19). Briefly, the C. difficile suspension at a final concentration of ~105-6 colony-forming units (CFU)/m was spotted on Brucella agar (Criterion Media) plates supplemented with hemin (5 µg/mL) (Sigma), vitamin K (10 µg/mL) (Sigma), defibrinated sheep blood (5% vol/vol) (Northeast Lab Services), and doubling the dilution of antibiotics (IBZ, VAN, FDX, and MTZ) from 0.25 mg/L to 16 mg/L. All assays were performed at least in duplicate. MIC assays were repeated for any results with discordant results (>1 dilution difference) within duplicate runs. Isolates out of range were repeated with dilutions up to 64 µg/mL. Reference strain C. difficile R20291 was included as a control strain within each experimental run as opposed to the CLSI control strain ATCC 70057.
ATP-bioluminescence assay
The BacTiter-Glo assay (Promega Corp., Madison, WI, USA) was used to assess IBZ bactericidal activity on the 100 clinical strains stratified by antibiotic susceptibility. Methods were adapted from the manufacturer instructions based on the publication by Jarrad et al. (21). Total unfiltered relative luminescence units were measured with a microplate reader and normalized to the positive growth control (no antibiotic) to account for signal decays according to the formula: % Relative luminescence = (well luminescence − mean of media background)/(mean of growth control − mean of media background) × 100%.
Time-kill kinetic studies
Two laboratory strains (R20291 and CD630) and two clinical strains [MT5094, a VAN non-susceptible (MIC = 16) strain and FDXR28, an FDX non-susceptible (MIC > 16) strain] were used to test bactericidal effect of wild-type versus non-susceptible strains for IBZ and comparators. C. difficile suspension was added to microtiter plates along with fixed concentrations (4–64 µg/mL) of IBZ or comparators (VAN, FDX). Total viable counts were determined immediately (T0; control) and at 24-h post-inoculation. Samples were withdrawn at each time point, centrifuged (1 min at 16 ,000 × g), and washed twice in sterile pre-reduced phosphate buffered saline (Oxoid Ltd, Waltham, MA, USA) to reduce residual drug carryover, before 10-fold serial dilutions were performed prior to plating on BHIS agar supplemented with yeast extract and L-cysteine. Agar plates were incubated for 24 h, following which the number of viable C. difficile (CFU/mL) was determined. The limit of detection for killing kinetic assays was 200 CFU/mL. Bactericidal activity was defined as a reduction of ≥3log10 in viability relative to the starting inoculum after 24-h exposure to antibiotics. Bacteriostatic was defined as <3log10 killing compared to the starting inoculum.
Light microscopy
Light microscopy was done to visualize morphologic differences upon IBZ exposure to a reference strain (R20291: RT027) and clinical strains with reduced susceptibility to VAN (MT4883: RT027) and FDX (FDXR28; RT255). Samples (5 mL) from time-kill studies performed with IBZ 0.5 MIC and 24 h of incubation were centrifuged for 1 min at 10,000 rpm. The pellets were resuspended in 200 µL of 4% paraformaldehyde and incubated for 1 h at room temperature. The samples were centrifuged again for 1 min at 10,000 rpm, resuspended in 1 mL H2O, and stored at 4°C prior to microscopy experiments. Light microscopy was performed using an inverted light microscope (Evos Cell Imaging System; Thermo Fisher).
Experimental plan and analysis
Summary values were calculated and tabulated for all MIC (MIC50 and MIC90) and ATP (ATP50 and ATP90) assays. MIC differences between antibiotics were assessed using analysis of variance (ANOVA). Log10 CFU/mL reduction from time-kill curves were graphed, tabulated, and compared between antibiotics using the Kruskal Wallis ANOVA. Data visualization and analysis were done with SAS version 9.1 (SAS Institute, Cary, NC, USA) and RStudio (RStudio package version 2023.09.1+494).
RESULTS
Activity of ibezapolstat against antibiotic non-susceptible C. difficile strains
One hundred clinical isolates were tested to assess the antibacterial of IBZ against multidrug-resistant strains. Isolates were collected between 2017 and 2018 in Texas, USA. The most common ribotypes included were F027, F014-020, F002, and F106. The strains included 41 antibiotic-susceptible strains, 39 VAN non-susceptible strains, 39 FDX non-susceptible strains, and 34 MTZ non-susceptible strains. Many (n = 33) non-susceptible strains displayed reduced susceptibility to more than one antibiotic. The activity of IBZ and comparator antibiotics is shown for duplicate independent inocula in Table 1. The overall IBZ MIC50/90 values (µg/mL) for IBZ for evaluated C. difficile strains were 4/8, compared with 2/4 for VAN, 0.5/1 for FDX, and 0.25/4 for MTZ. Results from the ANOVA demonstrated that all MIC determinations were statistically different from each other (P < 0.01 for each comparison) except for FDX and MTZ which had similar MIC distribution. IBZ MIC50/90 values did not differ based on non-susceptible antibiotic class or number of classes to which strains were non-susceptible. IBZ bactericidal activity was like the MIC and maintained in wild-type and non-susceptible strains. The overall IBZ ATP50/90 values for IBZ for evaluated C. difficile strains were 4/16 with no differences observed between ribotype 027 (4/8) compared to other ribotype strains.
TABLE 1.
Ibezapolstat agar dilution and ATP bactericidal activity against multidrug-resistant Clostridioides difficile strains
| Ibezapolstat | |||||
|---|---|---|---|---|---|
| N | MIC50 | MIC90 | ATP50 | ATP90 | |
| Ribotype | |||||
| All | 100 | 4 | 8 | 4 | 16 |
| F027 | 27 | 4 | 8 | 4 | 8 |
| F106 | 13 | 8 | 16 | 4 | 32 |
| F014-020 | 12 | 4 | 8 | 4 | 8 |
| F002 | 7 | 8 | 8 | 4 | 16 |
| Other | 41 | 4 | 8 | 4 | 32 |
| Susceptibility | |||||
| VAN susceptible | 61 | 8 | 8 | 4 | 32 |
| VAN non-susceptible | 39 | 4 | 8 | 4 | 8 |
| FDX susceptible | 61 | 4 | 8 | 4 | 16 |
| FDX non-susceptible | 39 | 4 | 8 | 4 | 32 |
| MTZ susceptible | 66 | 8 | 8 | 4 | 32 |
| MTZ non-susceptible | 34 | 4 | 8 | 4 | 8 |
| No. of antibioticsa non-susceptible | |||||
| 0 | 41 | 4 | 8 | 4 | 32 |
| 1 | 26 | 8 | 8 | 4 | 32 |
| 2 | 13 | 4 | 8 | 4 | 8 |
| 3 | 20 | 4 | 4 | 2 | 8 |
Antibiotics: VAN, FDX, and MTZ.
Time-kill studies
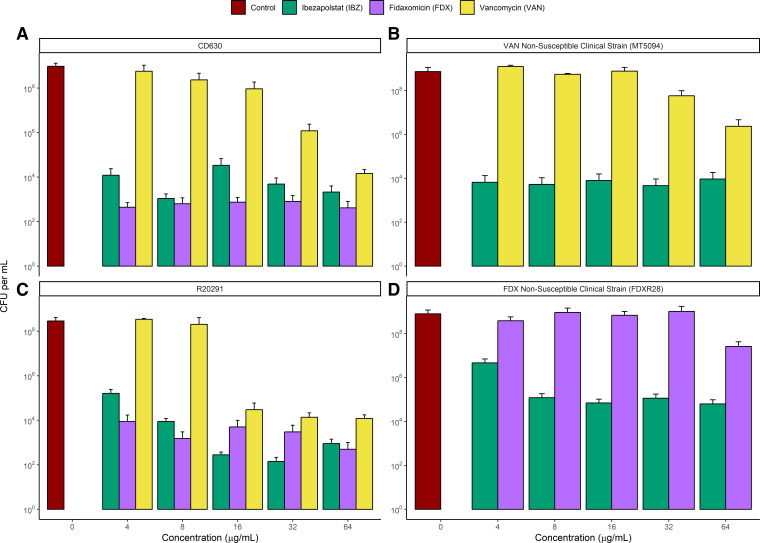
The results of time-kill studies at 24-h evaluation for IBZ, FDX, and VAN against CD630, R20291, MT5094, a VAN non-susceptible isolate, and FDXR28, an FDX non-susceptible isolate are shown in Fig. 1. The corresponding changes in log10 CFU/mL relative to baseline are shown in Table 2. All antibiotics exhibited bacteriostatic activity against wild-type, laboratory strains. For laboratory strains (R20291 and CD630), FDX bactericidal activity for both strains (>3log10 change) at all concentrations except for higher concentrations (32–64 µg/mL) for R20291, an RT027 strain. For IBZ, >2log10 change was observed for both strains except for lower concentrations (4–8 µg/mL) for RT027. VAN achieved >2log10 killing for CD630 only at the highest concentration tested (64 µg/mL) but consistently achieved >2log10 killing for R20291 except for one concentration (8 µg/mL). Results from the ANOVA demonstrated that all antibiotics were superior to control for wild-type, laboratory strains (P < 0.0001). For CD630, the killing activity of FDX and IBZ was significantly better than VAN while for R20291, the killing activity of FDX was significantly better than IBZ which was significantly better than VAN. IBZ maintained at least bacteriostatic concentrations for FDX and VAN non-susceptible strains which was not maintained by FDX and VAN. IBZ log10 killing ranged from 0.68 to 1.9 relative to baseline for the FDXR28 while FDX log10 killing showed increased growth (1.26–2.64log10 increase). IBZ log10 killing was bactericidal (2.92–3.73log10 decrease) for most concentrations against MT5094 while VAN log10 killing showed increased growth (1.52–3.09) except for the highest concentration tested (64 µg/mL, 1.44log10 killing). For FDXR28, IBZ was statistically better than FDX (P < 0.0001) and for MT5094, IBZ was statistically better than VAN (P < 0.0001).
Fig 1.
Time-kill results for IBZ, FDX, and VAN against C. difficile laboratory strains CD630 (panel A), R20291 (panel C), MT5034, a VAN non-susceptible strain (panel B), and FDXR28, a FDX non-susceptible strain (panel D). Limit of detection, 50 CFU/mL. Error bars represent standard error.
TABLE 2.
Time-kill kinetic data for IBZ, FDX, and VAN against C. difficile isolatesa
| Drug concentration (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Test strain | Drug | 0 | 4 | 8 | 16 | 32 | 64 |
| CD630 | IBZ | . | −2.22 | −2.82 | −2.22 | −2.4 | −2.75 |
| FDX | . | −3.52 | −3.57 | −3.57 | −3.14 | −4.01 | |
| VAN | . | 1.45 | −0.08 | −0.68 | −1.72 | −2.52 | |
| Control | 2.92 | . | . | . | . | . | |
| R20291 | IBZ | . | −1.61 | −1.94 | −3.43 | −3.94 | −2.74 |
| FDX | . | −3.53 | −4.25 | −4.45 | −1.92 | −2.7 | |
| VAN | . | 2.53 | −1.18 | −2.61 | −2.13 | −2.1 | |
| Control | 2.27 | . | . | . | . | . | |
| FDXR28 | IBZ | . | −0.68 | −1.9 | −0.98 | −0.77 | −1.04 |
| FDX | . | 2.29 | 2.56 | 2.56 | 2.64 | 1.26 | |
| Control | 2.55 | . | . | . | . | . | |
| MT5094 | IBZ | . | −3.6 | −3.73 | −2.92 | −3.13 | −3.55 |
| VAN | . | 3.09 | 2.74 | 2.77 | 1.52 | −1.44 | |
| Control | 2.72 | . | . | . | . | . | |
Results represent the change in log10 CFU/mL relative to 0 h (initial concentration). CD630 and R20291 are laboratory C. difficile strains. FDXR28 is a fidaxomicin non-susceptible isolate, and MT5094 is a vancomycin non-susceptible isolate.
Light microscopy
At IBZ 0.5× MIC, a similar increased cell length and filamentous phenotype were observed in isolates exposed to sub-MIC IBZ in reference strains and VAN/FDX reduced susceptibility isolates (Fig. 2).
Fig 2.
Representative light microscopy images demonstrating IBZ pharmacologic activity against C. difficile clinical strain MT4883, a multidrug-resistant strain with increased VAN (8 µg/mL), MTZ (2 µg/mL), and FDX (2 µg/mL) MICs and FDXR28 with reduced susceptibility to FDX (>16 µg/mL).
DISCUSSION
Antimicrobial resistance is important in the pathogenic spread of CDI including the most recent ribotype 027 epidemic. This epidemic was characterized by the emergence of two novel fluoroquinolone resistance genes at a time when fluoroquinolone antibiotics were commonly used (22). Recently, antimicrobial resistance has emerged to antibiotics that are commonly used to treat CDI, namely MET, VAN, and FDX (23). MET resistance emerged co-incident with the ribotype 027 epidemic possibly contributing to the increased virulence observed (5). Our group also demonstrated reduced clinical response rates in patients with MTZ non-susceptible strains (6). Although not as common or as well elucidated, antimicrobial resistance to C. difficile antibiotics is also emerging with VAN and FDX non-susceptible strains reported (3, 4). These results show that new antibiotic development in CDI must consider the emergence of antimicrobial resistance to antibiotics commonly used to treat CDI.
IBZ is a DNA polIIIC inhibitor with selective activity against C. difficile. In a phase IIa study, IBZ demonstrated a 100% clinical success rate in treating CDI with no recurrence (14). Phase III studies are being planned and a better knowledge of IBZ activity against MDR strains would allow for a better understanding of IBZ potential. Pharmacologic laboratory investigations allowed us to test the antibacterial activity of IBZ against comparator antibiotics (MET, VAN, and FDX) in our clinical strains with reduced susceptibility. Major findings include continued IBZ activity against multidrug non-susceptible strains using traditional MIC testing, an ATP-bioluminescence bactericidal assay, and time-kill assays. IBZ bacteriostatic or bactericidal activity was maintained for clinical strains with reduced susceptibility to VAN or FDX. These results expand and confirm the previous findings from Murray et al. that demonstrated similar MIC50/90 values (2/4 µg/mL) from 104 susceptible clinical strains and bactericidal activity against three clinical isolates (24). Like Murray et al., the activity of IBZ was significantly different than comparator antibiotics with MIC values for FDX being significantly lower than IBZ, VAN, or MET. It is worth noting that there was a wide spectrum of FDX MIC values potentially showing MIC creep with increased usage. Also, for the non-absorbable antibiotics such as IBZ, FDX, or VAN, further studies are needed to better understand how these MIC values relate to the concentrations in the gut. Also like Murray et al., IBZ exhibited bacteriostatic reduction from initial concentrations. With only two guideline-recommended antibiotics available for a common infection, the likelihood of resistance development is high. New antibiotic with a novel mechanism of action is needed. Taken together, these experiments provide strong in vitro evidence for the further development of IBZ as a treatment option for CDI including multidrug-resistant strains. The effect of IBZ against multidrug-resistant strains should be further explored in animal models to take full advantage of this unique mechanism of action.
In conclusion, this study demonstrated the potent bactericidal activity of IBZ against a large collection of C. difficile strains including multidrug-resistant strains. This study highlights the therapeutic potential of IBZ against multidrug-resistant strains of C. difficile.
ACKNOWLEDGMENTS
This work was supported by an investigator-initiated grant from Acurx Pharmaceuticals paid directly to the University of Houston.
E.B.: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—review and editing. K.B.: Data curation, Formal Analysis, Methodology, Supervision, Validation, Visualization, Writing—review and editing. M.J.A.: Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing—review and editing. T.A.E.: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing—review and editing. J.J.: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing—original draft. T.M.L.: Data curation, Methodology, Project administration, Resources, Validation, Visualization, Writing—review and editing. C.K.L.: Data curation, Methodology, Project administration, Resources, Software, Supervision, Writing—review and editing. A.J.G.L.: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing—original draft, Writing—review and editing. K.W.G.: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review and editing.
Contributor Information
Kevin W. Garey, Email: kgarey@uh.edu.
Ryan K. Shields, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
REFERENCES
- 1. Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, et al. 2018. Changes in prevalence of health care–associated infections in U.S. hospitals. N Engl J Med 379:1732–1744. doi: 10.1056/NEJMoa1801550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, Kelly CP, Wilcox MH. 2021. Clinical practice guideline by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 73:e1029–e1044. doi: 10.1093/cid/ciab549 [DOI] [PubMed] [Google Scholar]
- 3. Shen WJ, Deshpande A, Hevener KE, Endres BT, Garey KW, Palmer KL, Hurdle JG. 2020. Constitutive expression of the cryptic vanGCd operon promotes vancomycin resistance in Clostridioides difficile clinical isolates. J Antimicrob Chemother 75:859–867. doi: 10.1093/jac/dkz513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marchandin H, Anjou C, Poulen G, Freeman J, Wilcox M, Jean-Pierre H, Barbut F. 2023. In vivo emergence of a still uncommon resistance to fidaxomicin in the urgent antimicrobial resistance threat Clostridioides difficile. J Antimicrob Chemother 78:1992–1999. doi: 10.1093/jac/dkad194 [DOI] [PubMed] [Google Scholar]
- 5. Olaitan AO, Dureja C, Youngblom MA, Topf MA, Shen WJ, Gonzales-Luna AJ, Deshpande A, Hevener KE, Freeman J, Wilcox MH, Palmer KL, Garey KW, Pepperell CS, Hurdle JG. 2023. Decoding a cryptic mechanism of metronidazole resistance among globally disseminated fluoroquinolone-resistant Clostridioides difficile. Nat Commun 14:4130. doi: 10.1038/s41467-023-39429-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonzales-Luna AJ, Olaitan AO, Shen WJ, Deshpande A, Carlson TJ, Dotson KM, Lancaster C, Begum K, Alam MJ, Hurdle JG, Garey KW. 2021. Reduced susceptibility to metronidazole is associated with initial clinical failure in Clostridioides difficile infection. Open Forum Infect Dis 8:ofab365. doi: 10.1093/ofid/ofab365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khanna S, Assi M, Lee C, Yoho D, Louie T, Knapple W, Aguilar H, Garcia-Diaz J, Wang GP, Berry SM, Marion J, Su X, Braun T, Bancke L, Feuerstadt P. 2022. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs 82:1527–1538. doi: 10.1007/s40265-022-01797-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, Sims M, Kraft CS, Cohen SH, Berenson CS, Korman LY, Ford CB, Litcofsky KD, Lombardo M-J, Wortman JR, Wu H, Auniņš JG, McChalicher CWJ, Winkler JA, McGovern BH, Trucksis M, Henn MR, von Moltke L. 2022. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med 386:220–229. doi: 10.1056/NEJMoa2106516 [DOI] [PubMed] [Google Scholar]
- 9. Gerding DN, Cornely OA, Grill S, Kracker H, Marrast AC, Nord CE, Talbot GH, Buitrago M, Gheorghe Diaconescu I, Murta de Oliveira C, Preotescu L, Pullman J, Louie TJ, Wilcox MH. 2019. Cadazolid for the treatment of Clostridium difficile infection: results of two double-blind, placebo-controlled, non-inferiority, randomised phase 3 trials. Lancet Infect Dis 19:265–274. doi: 10.1016/S1473-3099(18)30614-5 [DOI] [PubMed] [Google Scholar]
- 10. Boix V, Fedorak RN, Mullane KM, Pesant Y, Stoutenburgh U, Jin M, Adedoyin A, Chesnel L, Guris D, Larson KB, Murata Y. 2017. Primary outcomes from a phase 3, randomized, double-blind, active-controlled trial of surotomycin in subjects with Clostridium difficile infection. Open Forum Infect Dis 4:ofw275. doi: 10.1093/ofid/ofw275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daley P, Louie T, Lutz JE, Khanna S, Stoutenburgh U, Jin M, Adedoyin A, Chesnel L, Guris D, Larson KB, Murata Y. 2017. Surotomycin versus vancomycin in adults with Clostridium difficile infection: primary clinical outcomes from the second pivotal. J Antimicrob Chemother 72:3462–3470. doi: 10.1093/jac/dkx299 [DOI] [PubMed] [Google Scholar]
- 12. Okhuysen PC, Ramesh M, Garey KW, Louie TJ, Cisneros JT, Stychneuskaya A, Kiknadze N, Li J, Duperchy E, Wilcox PMH, Montoya JG, Styles L, Clow F, James D, Dubberke ER, De Oliveira CM, Van Steenkiste C. 2022. A phase 3, randomized, double-blind study to evaluate the efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridioides difficile infection. Open Forum Infect Dis 9:ofac492.021. doi: 10.1093/ofid/ofac492.021 [DOI] [Google Scholar]
- 13. Xu WC, Silverman MH, Yu XY, Wright G, Brown N. 2019. Discovery and development of DNA polymerase IIIC inhibitors to treat Gram-positive infections. Bioorg Med Chem 27:3209–3217. doi: 10.1016/j.bmc.2019.06.017 [DOI] [PubMed] [Google Scholar]
- 14. Garey KW, McPherson J, Dinh AQ, Hu C, Jo J, Wang W, Lancaster CK, Gonzales-Luna AJ, Loveall C, Begum K, Jahangir Alam M, Silverman MH, Hanson BM. 2022. Efficacy, safety, pharmacokinetics, and microbiome changes of ibezapolstat in adults with Clostridioides difficile infection: a phase 2A multicenter clinical trial. Clin Infect Dis 75:1164–1170. doi: 10.1093/cid/ciac096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Eijk E, Wittekoek B, Kuijper EJ, Smits WK. 2017. DNA replication proteins as potential targets for antimicrobials in drug-resistant bacterial pathogens. J Antimicrob Chemother 72:1275–1284. doi: 10.1093/jac/dkw548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzales-Luna AJ, Carlson TJ, Dotson KM, Poblete K, Costa G, Miranda J, Lancaster C, Walk ST, Tupy S, Begum K, Alam MJ, Garey KW. 2020. PCR ribotypes of Clostridioides difficile across Texas from 2011 to 2018 including emergence of ribotype 255. Emerg Microbes Infect 9:341–347. doi: 10.1080/22221751.2020.1721335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinson JNV, Broadaway S, Lohman E, Johnson C, Alam MJ, Khaleduzzaman M, Garey KW, Schlackman J, Young VB, Santhosh K, Rao K, Lyons RH Jr, Walk ST. 2015. Evaluation of portability and cost of a fluorescent PCR ribotyping protocol for Clostridium difficile epidemiology. J Clin Microbiol 53:1192–1197. doi: 10.1128/JCM.03591-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyanova L, Dimitrov G, Gergova R, Hadzhiyski P, Markovska R. 2023. Clostridioides difficile resistance to antibiotics, including post-COVID-19 data. Expert Rev Clin Pharmacol 16:925–938. doi: 10.1080/17512433.2023.2252331 [DOI] [PubMed] [Google Scholar]
- 19. Clinical Laboratory and Standards Institute. 2023. Performance standards for antimicrobial susceptibility testing. 33rd ed. CLSI Supplement M100. Wayne, Pennsylvania. [Google Scholar]
- 20.Data from the EUCAST MIC distribution website. Accessed 30 March 2023
- 21. Jarrad AM, Blaskovich MAT, Prasetyoputri A, Karoli T, Hansford KA, Cooper MA. 2018. Detection and investigation of eagle effect resistance to vancomycin in Clostridium difficile with an ATP-bioluminescence assay. Front Microbiol 9:1420. doi: 10.3389/fmicb.2018.01420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He M, Miyajima F, Roberts P, Ellison L, Pickard DJ, Martin MJ, Connor TR, Harris SR, Fairley D, Bamford KB, et al. 2013. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet 45:109–113. doi: 10.1038/ng.2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dureja C, Olaitan AO, Hurdle JG. 2022. Mechanisms and impact of antimicrobial resistance in Clostridioides difficile. Curr Opin Microbiol 66:63–72. doi: 10.1016/j.mib.2022.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murray B, Wolfe C, Marra A, Pillar C, Shinabarger D. 2020. In vitro activity of the novel antibacterial agent ibezapolstat (ACX-362E) against Clostridioides difficile. J Antimicrob Chemother 75:2149–2155. doi: 10.1093/jac/dkaa134 [DOI] [PubMed] [Google Scholar]