Abstract
Ischemic stroke refers to the sudden loss of blood flow in a specific area of the brain. It is the fifth leading cause of mortality and the leading cause of permanent disability. The transcription factor nuclear factor erythroid 2‐related factor 2 (Nrf2) controls the production of several antioxidants and protective proteins and it has been investigated as a possible pharmaceutical target for reducing harmful oxidative events in brain ischemia. Each cell type exhibits different roles and behaviors in different phases post‐stroke, which is comprehensive yet important to understand to optimize management strategies and goals for care for stroke patients. In this review, we comprehensively summarize the protective effects of Nrf2 in experimental ischemic stroke, emphasizing the role of Nrf2 in different cell types including neurons, astrocytes, oligodendrocytes, microglia, and endothelial cells during acute and chronic phases of stroke and providing insights on the neuroprotective role of Nrf2 on each cell type throughout the long term of stroke care. We also highlight the importance of targeting Nrf2 in clinical settings while considering a variety of important factors such as age, drug dosage, delivery route, and time of administration.
Keywords: astrocytes, endothelial cells, ischemic preconditioning, MCAO, microglia, neurons, neuroprotection, oligodendrocytes, oxidative stress
1. INTRODUCTION
Ischemic stroke is the main cause of disability in the United States and a significant cause of death globally. 1 Every year, there are about 800,000 ischemic strokes in the United States, 600,000 of which are recurrent events. 2 Globally, one of six people has had an ischemic stroke in their lifetime, and 14 million people experience a stroke each year. 3 However, aside from reperfusion therapy, very few approaches are available clinically to protect the brain against ischemic injury or boost brain recovery. 4 Despite many attempts to design drugs that can lessen neural damage after ischemic stroke, practically none have translated to successful phase III trials. Recently, major flaws in preclinical studies have been identified, 5 reviving the opportunity to re‐test several failed approaches, such as neuronal protection against oxidative stress.
One of the most promising targets for ischemic stroke therapy is the nuclear factor erythroid 2‐related factor 2 (Nrf2). Nrf2 is a master transcription factor that regulates and maintains redox homeostasis. Ischemic injury with or without reperfusion is associated with excessive oxidative stress leading to DNA damage and cell death. Therefore, regulating redox levels might be a promising direction in the management of ischemic stroke. In addition, Nrf2 is involved in complex interactions with nuclear factor‐κB (NF‐κB), a major transcription factor that triggers a panel of inflammatory pathways, antagonizing NF‐κB at multiple levels to regulate neuroinflammation and prevent additional injury after stroke. 6 , 7
Cellular responses to ischemic stroke vary dramatically among different types of brain cells and between different phases of stroke. In this review, we provide an overview of the Nrf2 pathway in physiological and ischemic conditions and discuss the cell‐specific roles of Nrf2 in both acute and chronic phases of ischemic stroke (Figure 1).
FIGURE 1.
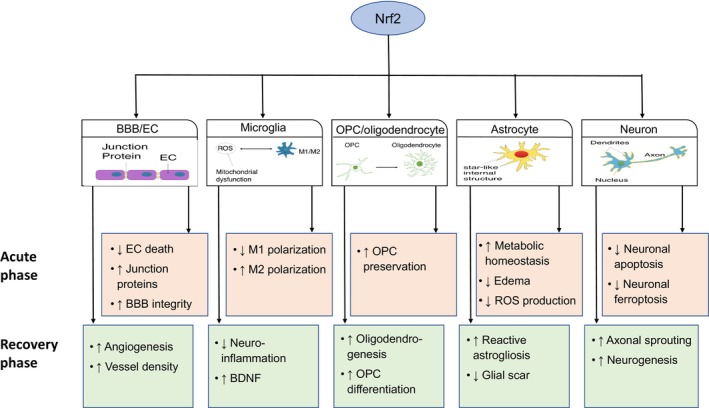
Cell‐specific effects of Nrf2 on ischemic stroke pathologies. Schematic image showing potential Nrf2 targets in acute and chronic recovery phases of ischemic stroke. BBB, blood–brain barrier; EC, endothelial cell; OPC, oligodendrocyte precursor cell; ROS, reactive oxygen species.
2. OVERVIEW OF THE NRF2 PATHWAY AND ITS IMPLICATIONS IN ISCHEMIC STROKE
Human Nrf2 consists of 605 amino acids and 7 Nrf2‐ECH domains (Neh1‐7) and is a member of the cap'n’collar family of transcription factors. 3 A nuclear localization signal is in charge of directing Nrf2 into the cell nucleus. 8 Neh1 mediates the formation of Nrf2‐musculoaponeurotic fibrosarcoma (Maf) heterodimer and binds to the antioxidant response element (ARE) of the DNA. 9 , 10 Neh4 and Neh5 are mediating domains for Nrf2 interaction with coactivators such as cAMP response element‐binding protein (CREB)‐binding protein which enhances its ARE‐binding capacity. 11 Neh7 domain binds to retinoic X receptor α, responsible for the competitive inhibition of Nrf2‐ARE binding. 12 Neh2 and Neh6, which interact with Kelch‐like‐ECH‐associated protein 1 (Keap1) and β‐transducin repeat‐containing protein, respectively, mediate proteasomal degradation of Nrf2. 3 , 13
The expression level of Nrf2 is finely tuned by complex cellular mechanisms. Under normal conditions, Nrf2 is constantly produced and degraded via either the canonical, Keap1‐dependent pathway, or the alternative, Keap1‐independent pathway, forming a dynamic balance. Nrf2 is activated upon electrophile reaction and oxidative stress, which withholds Keap1 from sequestering Nrf2 and prevents its degradation. 14 It has been shown that the Cys288 residue of Keap1 is critical for Keap1‐Nrf2 binding and dissociation. 15 The alternative, Keap1‐independent pathway for Nrf2 degradation involves Nrf2 phosphorylation, by glycogen synthase kinase 3β (GSK3β). GSK3β can be directly adducted by electrophiles via its Cys299 residue, 15 or indirectly regulated by the phosphatase and tensin homolog‐phosphoinositide 3‐kinase (PI3K)‐Akt axis. 13 The inactivation of Nrf2 is regulated through its negative feedback loop. For example, the presence of ARE in the Keap1 gene promotor region results in Keap1 upregulation and Nrf2 degradation. 16 Other factors such as the Keap1‐binding protein prothymosin and GSK3β‐mediated p‐Fyn also play a role in this negative feedback loop. 17 , 18 Additional details on Nrf2 structure, activation, inactivation, and degradation have been reviewed elsewhere. 19
Over 200 Nrf2 downstream genes have been identified based on the presence of ARE. They are associated with a wide array of functions, such as redox balance, metabolism, inflammation, and proteostasis. 20 , 21 Nrf2 activation after ischemic stroke in animal models has been reported consistently by multiple groups. The timeline varies, possibly due to different experimental stroke models in different animal species. Details have been thoroughly reviewed elsewhere. 22 In general, Nrf2 activation occurs hours after ischemic insult, and it lasts days to weeks before returning to baseline level. 23 , 24
The protective effects of Nrf2 depend on its temporal and spatial distribution after ischemic stroke. In this regard, Nrf2 activity has been evaluated using the ratio of nuclear to cytoplasmic Nrf2 levels after transient middle cerebral artery occlusion (MCAO) in rats. 25 At 4 h after reperfusion, Nrf2 activity was significantly greater in the ipsi‐lesional hemispheres when compared to the contra‐lesional hemispheres. Interestingly, at 72 h after perfusion, Nrf2 activity at both hemisphere dropped below the baseline, with no difference observed between them. Levels of Nrf2 in the peri‐infarct region were significantly greater than in the core region at 24 and 72 h after reperfusion, most abundantly in glial fibrillary acidic protein (GFAP)‐positive reactive astrocytes, 25 which is consistent with observation from our group. 26 , 27 Additionally, beneficial roles of Nrf2 in ischemic stroke have been demonstrated using Nrf2 activators or Nrf2 knockdown mice. Importantly, Nrf2 has critical neuroprotective roles in both acute and chronic phases after stroke, as reported previously by our group and others. 28 , 29 , 30
3. CELL‐SPECIFIC ROLE OF NRF2 IN THE ACUTE PHASE
3.1. Neurons
Ischemic insults result in several forms of neuronal cell death. Necrosis owing to excitotoxicity is the predominant form in the ischemic core, while apoptosis mainly occurs in the penumbra. 31 In the ischemic core, an abrupt cut‐off of ATP leads to a disruption in the Na+/K+ pump, and rapid K+ efflux results in transient hyperpolarization within seconds. Minutes later, the Na+/K+ pump fails and subsequently the disruption of the Ca2+/ATP pump and Na+/Ca2+ exchanger causes a massive efflux of K+ and an influx of Na+ and Ca2+, resulting in marked membrane depolarization. Excessive release of neurotransmitters such as glutamate ensues and further exacerbates neuronal membrane depolarization and energy consumption, resulting in an irreversible injury cascade, a process termed excitotoxicity. 32 Excitotoxicity is a common way of neuronal death not only seen in acute brain ischemia but also present in other diseases such as chronic ischemia, trauma, and neurodegenerative disorders. Over the past several decades, multiple studies have reported the role of Nrf2 in the mitigation of neuronal excitotoxicity. 33 , 34 , 35 , 36 Nevertheless, excitotoxicity owing to acute neurovascular insult typically results in irreversible necrotic cell death within minutes, which is hardly preventable or rescuable. Although Nrf2 has been studied extensively, the mechanism(s) of its neuroprotective effects is not fully understood and requires further research. Recent research advances supported that Nrf2 serves as a key mechanism in many preconditioning approaches. For example, our studies demonstrated the essential role of Nrf2 in ischemic preconditioning (IPC) in neuronal cultures from both WT and Nrf2 knockout (KO) animals, where IPC‐afforded protection against oxygen–glucose deprivation (OGD) is abolished in Nrf2 KO neurons. 37 This supports that Nrf2 plays a key role in protecting neurons against OGD in vitro. How exactly this may contribute to neuroprotection in vivo is not well understood.
3.1.1. Nrf2 and apoptosis
A more desirable goal for neuroprotection in ischemic stroke is to mitigate neuronal apoptosis in the penumbra. Studies by our group and others consistently demonstrated the pivotal role of Nrf2 in reducing infarct volume and rescuing neuronal loss after ischemic stroke. 37 , 38 , 39 , 40 Mechanistically, sublethal ischemia in the penumbra leads to sublethal intracellular calcium accumulation, resulting in hyperpermeability of the mitochondria membrane. Apoptosis is mainly triggered by the leakage of mitochondrial content. For example, cytochrome C directly activates caspases, and reactive oxygen species (ROS) lead to DNA fragmentation and subsequent apoptotic cell death. 41 Moreover, lipid peroxidation products such as 4‐hydroxynonenal could lead to DNA damage and subsequent apoptosis, and their scavenging with carbonyl reductase 1 or aldo‐keto reductase 1C15 could rescue neurons from DNA damage and ischemic injury in vivo and in vitro. 42 , 43 , 44
Several studies defined the role of Nrf2 in mitochondrial function. For example, 3‐n‐butylphthalide protected against OGD‐induced PC12 (rat pheochromocytoma) neuronal cell death, and this was associated with Nrf2 activation, enhanced mitochondrial membrane potential, and increased activity of the mitochondrial respiratory chain complex I‐IV and ATPase. 45 In a rat subarachnoid hemorrhage model, mitoquinone, a mitochondria‐target antioxidant, provided neuroprotection by reducing the release of Bax and cleaved Caspase‐3 from the mitochondria, which was completely abolished by ML385, an Nrf2 inhibitor. 39 Mitochondrial protection and the necessity of Nrf2 activation in ischemic stroke need further validation. A more direct role of Nrf2 in neuroprotection is through mitigating oxidative stress by clearance of ROS, and alleviating excitotoxic injury which has been thoroughly reviewed elsewhere. 46 Sublethal excitotoxicity in the penumbra predominantly leads to apoptosis rather than necrosis. It is worth noting that many studies only proved the association without further elucidating the causality. For example, in rats injected with monosodium glutamate, cyclooxygenase‐2 inhibitor flurbiprofen reduced hippocampal neuronal apoptosis, and this beneficial effect was associated with Nrf2 activation. 47 A chalcone analog, 1‐(2,3,4‐trimethoxyphenyl)‐2‐(3,4,5‐trimethoxyphenyl)‐acrylketone, also inhibited glutamate‐induced cell apoptosis and along with Nrf2 activation it improved scopolamine‐induced cognitive impairment. 48 In rats subjected to transient MCAO, carveol protected against oxidative stress (high lipid peroxidase) & cortical and striatal neuronal apoptosis via Nrf2 validated by injection of all‐trans retinoic acid (ATRA), an Nrf2 antagonist. 49 This supported the critical role of Nrf2, although the off‐target effect of ATRA should be considered. In addition, tert‐butylhydroquinone (tBHQ) treatment reduced glutamate‐induced death of retinal ganglion cells in vitro, and retina thinning in vivo; these effects were associated with increased Nrf2 activation and reduced NF‐κB, and were abolished by Nrf2‐shRNA transfection 33 , 50 Studies by our and other groups also demonstrated that Nrf2 activators protect against OGD‐induced neuronal death in vitro. 26 , 29 Collectively, these studies support the direct role of Nrf2 in mitigating excitotoxicity in ischemic neurons. Mechanistically, this seems to be associated with the PI3K/Akt pathway, since rosmarinic acid and bone morphogenetic proteins alleviated stroke‐related neuronal apoptosis, which was associated with Akt activation, and was abolished when Akt phosphorylation was inhibited. 51 , 52 These observations support that Nrf2 plays a critical role in mitigating neuronal apoptosis, mainly through preserving mitochondrial function.
3.1.2. Nrf2 and ferroptosis
Ferroptosis is a type of regulated cell death that is mechanistically and phenotypically unique from other forms of cell death. It is iron‐dependent, and lipid peroxide‐driven, 53 which can be suppressed by iron chelators and lipophilic antioxidants. Ferroptosis is implicated in many neurological disorders such as traumatic brain injury (TBI) and in chronic neurodegenerative diseases including Alzheimer's disease and Parkinson's disease. 54 , 55 In the field of ischemic stroke, ferroptosis is more extensively studied in hemorrhagic stroke 56 , 57 , 58 , 59 as heme and iron are blood components. Indeed, ferroptosis was observed in neonatal rat hypoxic–ischemic brain injury models. 60 A most recent study 61 pooled several RNAseq databases from ischemic stroke patients and matched healthy controls and revealed significantly enriched ferroptosis‐related differentially expressed genes and the potential role of ferroptosis‐related biomarkers in ischemic stroke patients, indicating the critical role of ferroptosis in ischemic stroke.
Ferroptosis can be initiated by direct inhibition of glutathione peroxidase 4 (GPX4) and glutathione (GSH) depletion, and similarly, can be mitigated by GPX4 and GSH. Nrf2 controls a variety of essential enzymes involved in glutathione production and metabolism, such as glutathione synthetase (GSS), and a subunit of the cystine/glutamate transporter (xCT), which are crucial for GSH synthesis. 62 , 63 Additionally, GPX4 is a transcriptional target of Nrf2. 64 , 65 Therefore, Nrf2 could be a major regulator of lipid peroxidation and ferroptosis. 66 Indeed, Nrf2 is critical for the anti‐ferroptotic effects of neuroprotective compounds such as vitexin, 67 elabela, 68 and inhaled propofol, 46 and miRNA‐27a directly binds to the Nrf2 gene, inhibiting its expression which exacerbates ferroptosis. 68 A limitation of ferroptosis research is that GSS and GPX4 are typically applied as markers for ferroptosis, but the potential involvement of other types of cell death has not been evaluated. Thus, such studies need to be interpreted with caution. Whether Nrf2 is essential for anti‐ferroptotis effects remains unsure, mainly due to a lack of specific ferroptosis markers.
3.2. Astrocytes
Astrocytes are the most abundant cell type in the central nervous system (CNS), presenting multiple supporting roles such as nutrient transportation, forming the blood–brain barrier (BBB), maintenance of ion balance, and participating in tissue repair. 69 In primary cell cultures, astrocytes harbor more robust Nrf2 than neurons under physiological conditions. 70 Nrf2 expression in astrocytes is detected in the acute phase of ischemic stroke. Studies from our group reported heme oxygenase‐1 (HO‐1) expression in astrocytes at 72 h post‐stroke in both the hippocampus and the cortex after transient MCAO in mice. 26 , 27 Another group used a rat ischemic stroke model and reported Nrf2 upregulation at 1d after stroke. 24 Collectively, these studies suggest a positive effect of astrocytic Nrf2 in the acute phase after stroke. Indeed, astrocyte Nrf2 has been shown to be involved in several neuroprotective approaches, such as 11‐keto‐β‐boswellic acid or resveratrol. 71 , 72
Astrocyte Nrf2 exerts neuroprotection via a variety of mechanisms. Firstly, and most importantly, it helps to boost more effective metabolic activity. Astrocyte‐specific Nrf2 overexpression could help maintain neuronal metabolic homeostasis after mitochondrial complex II inhibition. 73 In a resveratrol preconditioning model, Nrf2 was colocalized with the outer membrane of non‐synaptic, astrocytic mitochondria. Although no significant alterations were appreciated in the ratio of nuclear/mitochondrial subunits of oxidation phosphorylation (OXPHOS) or any of the complexes, Nrf2 KO mice had decreased formation of electron transport chain super complexes, 70 which were defined as an oligomer of electron transport chain complexes harboring more efficient bioenergetics and producing less ROS. 74 Secondly, astrocyte Nrf2 helps mitigate oxidative stress and subsequent cell death. For example, WD‐40 repeat protein 26 reduced H2O2‐mediated injury in primary human astrocyte cultures (U251‐MG, a glioblastoma cell line) associated with Nrf2/HO‐1 activation. 68 In another study, icariside II protected primary astrocytes against OGD; this effect was associated with activation of the Nrf2 pathway and OXPHOS/NF‐κB/ferroptosis axis, which is dependent on Nrf2 as proved using Nrf2‐siRNA. 69 Thirdly, astrocyte Nrf2 helps maintain the water and glutamate balance, as Nrf2 KO exhibited worsened edema and excitotoxicity. 70 Last but not least, vascular endothelial growth factor (VEGF) production in astrocytes is dependent on Nrf2, as demonstrated in a study using Nrf2 KO mice. 71
Astrocyte Nrf2 deserves to gain more attention in future studies. Mice overexpressing astrocyte‐specific Nrf2 have been used in studies of Alzheimer's disease, 75 Parkinson's Disease, 76 chronic hypoperfusion, 77 and amyotrophic lateral sclerosis, 78 and this could be a powerful tool for stroke research as well.
3.3. Oligodendrocytes
Studies related to Nrf2 and oligodendrocytes in the context of acute ischemic stroke are quite limited. In rat primary oligodendrocyte precursor cell (OPC) cultures subjected to OGD, miRNA‐146b‐5p overexpression prevented cell apoptosis in association with activation of Nrf2, supporting that Nrf2 might help maintain oligodendrocyte lineage homeostasis and prevent cell death. 79 Nrf2 was also found to be critical for cell survival and metabolic activity in OPC cultures upon exposure to complex IV inhibitors. 80 In experimental models of multiple sclerosis, Nrf2 was found to preserve oligodendrocytes and myelin. 81 , 82 These studies support that activation of Nrf2 in oligodendrocytes can enhance cell preservation and prevent cell apoptosis. Whether similar effects would be reproduced in ischemic stroke remains to be explored.
3.4. Microglia
Microglia are the main producers of proinflammatory cytokines which further exacerbate acute inflammation after ischemic stroke. 83 , 84 , 85 Although acute inflammation is critical to clear up the dead tissue for subsequent brain repair, excessive neuroinflammation leads to secondary brain injury and tissue disruption, which hinders long‐term regeneration and reestablishment of function. 86 , 87 As a result, proper control of the extent and duration of neuroinflammation is required. As CNS resident myeloid cells, microglia are subclassified as resting, pro‐inflammatory (formerly known as M1), and anti‐inflammatory (formerly known as M2) phenotypes. 88 , 89 After stroke, M1 phenotype started to increase from day 3 onward and remained high until 7–14 days while the M2 phenotype peaked at day 3–5 and then subsided. 90 It is important to note that resident microglia and infiltration macrophages are hard to differentiate after stroke, which is circumvented by most studies in the field and is beyond the scope of the present review. We, therefore, apply “microglia/macrophage” in the present review when these two cell types are undistinguishable in the referenced studies.
Studies have consistently reported that Nrf2 is associated with the inhibition of M1 polarization and enhancing M2 polarization in microglia/macrophages after stroke. For example, in a mouse permanent MCAO model, dexmedetomidine significantly improved stroke outcomes associated with enhanced M2 polarization in microglia/macrophages, which was abolished by Nrf2 inhibitor ML385, suggesting a critical role of Nrf2 in this process. 91 In the transient MCAO model, Nrf2 proved to be essential in sevoflurane preconditioning as adeno‐associated virus (AAV)‐shNrf2‐abolished this protection. 92 A similar association was also reported in the protection afforded by tanshinol borneol ester, 23 non‐mitogenic fibroblast growth factor 1, 40 and HP‐1c, a dual Nrf2/AMP‐activated protein kinase (AMPK) activator. 93 In primary microglial cultures or BV2 cultures, the causal relationship between Nrf2 and M2 has been proven consistently, upon either OGD or lipopolysaccharide stimuli. 40 , 91 , 92 , 93 Thus, the effects of Nrf2 activation on microglia polarization may contribute to reducing the pro‐inflammatory cytokines and increasing anti‐inflammatory markers after brain insults including ischemic stroke.
It is worth mentioning that the M1/M2 classification is an oversimplification that fails to capture the full spectrum of microglia behavior in stroke 94 and other neurodegenerative conditions. This classification has been derived primarily from in vitro cultures upon simple cytokine stimulus and it represents two rather extreme types of microglial activation. However, in vivo microglia display a complex spectrum with overlapping markers of both phenotypes, suggesting that microglia are highly flexible and highly dynamic cells with a complex role during stroke. 95 , 96 , 97 , 98
3.5. Endothelial cell (EC)/blood–brain barrier
Post‐stroke BBB disruption is a result of both direct ischemic injury to the ECs and indirect damage from oxidative stress and inflammation. Inflamed ECs allow leukocytes to travel across the BBB by interacting with the adhesion molecules such as E‐selectin and P‐selectin. 99 Details on how BBB disruption happens and its consequences after stroke have been thoroughly reviewed elsewhere. 100
Studies from our group and others consistently reported robust HO‐1 expression in the microvessels within the peri‐infarct region within 3 days after stroke, substantially more pronounced than in neurons or microglia. 15 , 25 Specifically, in rats subjected to hypoxia, Nrf2 binding to the ARE has been observed in the nuclear protein extracts from brain microvessel preparations as early as 1 h post hypoxic insult, 101 suggesting a critical role of Nrf2 in microvessels or, more directly, the BBB. Indeed, many studies including ours consistently reported BBB protection by Nrf2. For example, EC and BBB protection was observed through direct Nrf2 modulators such as sulforaphane (Sfn), dimethyl fumarate (DMF), and others. 25 , 102 , 103 , 104 Similar findings have also been reported in experimental models of TBI. 105
Mechanistically, Nrf2 protects against ischemic‐related EC death. For example, in primary mouse brain microvascular ECs, it was shown that Sfn reduced the cell death induced by OGD. 38 PC and dichloroacetic acid‐afforded protection against OGD‐induced EC death and in vitro BBB disruption is dependent on Nrf2 as well. 38 , 104 Another important mechanism of BBB protection is that Nrf2 preserves the junction proteins, which are critical for BBB integrity. For example, DMF protects against OGD‐mediated zonula occludens‐1 (ZO‐1) dysfunction by preventing gap formation on the ZO‐1 membranous lining, and this effect is dependent on Nrf2 activation. 103 In addition, studies from our group reported that Nrf2 could directly upregulate the expression of claudin 5 and cadherin 5 by enhancing their respective promoter activities. 15 , 38 It can be concluded that Nrf2 contributes to BBB protection after stroke, through lowering cell death and increasing cell junction proteins in ECs.
4. CELL‐SPECIFIC ROLE OF NRF2 IN THE CHRONIC RECOVERY PHASE
4.1. Neurons
The brain has the ability to change its network structure through growth, reorganization, and regeneration, termed neuroplasticity, as an intrinsic mechanism to facilitate recovery after stroke. 106 Our most recent study using a mouse distal MCAO model reported that Sfn treatment alleviated long‐term axonal disintegration at 35‐day post‐stroke and this effect was dependent on Nrf2. 29 A similar finding was also reported in a TBI model, 107 further supporting the role of Nrf2 in neuronal protection and neuroplasticity.
Mechanistically, Nrf2 is critical for axonal sprouting and neurite outgrowth after stroke. In the distal MCAO model in vivo, we showed that Sfn administration after stroke resulted in Nrf2‐enhanced axonal sprouting which was abolished in the Nrf2 KO mice. 29 Similar findings were noted in a spinal cord injury (SCI) model, where metformin promoted axonal growth after SCI in an Nrf2‐dependent manner, associated with reduced oxidative stress and improved mitochondrial function. 108 Several in vitro studies also demonstrated the critical role of Nrf2 in neurite outgrowth. For example, in PC12 cell cultures, Akt overexpression activated Nrf2/ARE and promoted axonal growth, 109 and in neuronal cultures subjected to hypoxic injury extracellular vesicles derived from human neural stem cells promoted elongation of neuronal axons along with Nrf2 activation. 110 More direct evidence is needed to tell whether Nrf2 is also critical for neuronal axonal sprouting in stroke in vivo.
Another important mechanism of neural recovery after stroke is neurogenesis. In the adult brain, the two best‐described neurogenic niches are the adult neural stem and progenitor cells (NSPCs) which reside in the ventricular–subventricular zone (SZV) and the dentate gyrus. 111 , 112 Interestingly, reduced neural stem cell number and self‐renewal capacity were observed with increased age. 113 , 114 Cortical neurogenesis has also been reported, though at variable degrees which was closely related to the severity of ischemic injury. 115 , 116 Nrf2 has been reported to boost neurogenesis both in vivo and in vitro. Inducing Nrf2 overexpression with AAV in middle‐aged mice (11 months) improved cognitive and motor function, along with increased NSPC proliferation, self‐renewal, neurogenesis, and migration. 117 Consistently, SVZ NSPC cultures show reduced Nrf2 expression with age. Nrf2 knockdown with siRNA reduced NSPC proliferation, and NSPCs from Nrf2 KO mice had lower survival and proliferation. 118 Collectively, these findings suggest a critical role of Nrf2 in neurogenesis and support the idea that targeting Nrf2 is a promising therapy strategy to promote neurogenesis during chronic recovery from stroke.
4.2. Astrocytes
Astrocyte function in the chronic phase after stroke is complex. On one hand, reactive astrogliosis in the penumbra is beneficial by preventing the expansion of the ischemic core. 119 , 120 Inhibition of reactive astrogliosis by fluorocitrate 5 days after stroke exacerbated neurobehavioral deficits, suppressed neurovascular remodeling, and worsened stroke outcomes. 121 On the other hand, severe gliosis led to glial scar formation, a maladaptive tissue reorganization pattern that prevents axonal regeneration and contributes to neurotoxicity and inflammation. 122 For example, gliosis surrounding the ischemic core is not completely impermeable, and leakage from the core results in sustained liquefactive necrosis and neurodegeneration after stroke. 123 Glial scar secretes inhibitory factors such as chondroitin sulfate proteoglycans which result in an environment inhibitory to axonal regeneration. 124 , 125
Several studies supported that Nrf2 is associated with reactive astrogliosis after stroke. tBHQ was able to promote reactive GFAP+ astrocytes proliferation in the peri‐infarct area from 7–28 days after MCAO, which was absent in the Nrf2 KO group, supporting that this upregulation of astrocytes proliferation is dependent on Nrf2. 39 Similar findings were also reported in DMF and Korean red ginseng mediated Nrf2 activation, where reactive gliosis correlated well with levels of glutamine synthetase and aquaporin 4, a possible link to excitotoxicity and brain edema. 56 , 126 It has also been suggested that reactive astrogliosis relates to decreased GSK3β. 37 Few studies have examined the effect of Nrf2 on glial scar formation. Nrf2 attenuates neuroinflammation which is a key mechanism contributing to a detrimental role of the glial scar. In addition, our recent study suggested that neurite sprouting after stroke is dependent on Nrf2, which may or may not be related to glial scar mitigation. 29
In summary, evidence exists that Nrf2 promotes reactive astrogliosis. Given the complicated role of astrocytes in the chronic phase after stroke, whether Nrf2 mitigates glial scar formation is inconclusive.
4.3. Oligodendrocytes
Remyelination is part of white matter plasticity and repair after stroke, due to major white matter loss. Oligodendrogenesis and OPC differentiation into mature oligodendrocytes are important elements of remyelination. By far, few studies examined this aspect in the chronic phase after stroke. Our group recently reported the indispensable role of Nrf2 in Sfn‐mediated myelin preservation and oligodendrogenesis 35 days after ischemic stroke. 29 The critical role of Nrf2 in oligodendrogenesis has also been reported after IPC 127 and cuprizone‐mediated demyelination, 128 and Nrf2 activator DMF‐induced differentiation in OPC cultures. 129 It is very likely that Nrf2 enhances oligodendrogenesis and OPC differentiation, yet more work is needed to further elucidate OPC‐ or oligodendrocyte‐specific Nrf2 after ischemic stroke.
4.4. Microglia
Microglia activation can be sustained into the chronic phase, which is related to progressive neurodegeneration. For example, a unique CD11c+ microglia subtype was observed in relation to degenerative changes in the thalamus 28 days after ischemic stroke in mice. Knocking down of resident microglia, using an intracerebroventricular injection of lentiviral particles carrying shRNA targeting colony‐stimulating factor 1 receptor, prolonged survival, relieved neuroinflammation, and improved white matter remyelination at 3 weeks after MCAO in diabetic mice. 130 Given the robust anti‐inflammatory property of Nrf2, it is expected that Nrf2 activators would attenuate neuroinflammation in the chronic phase of ischemic stroke which would lead to better outcomes. Indeed, this was reported using a dual AMPK/Nrf2 activator, and tanshinol borneol ester as well. 23 , 93 Cell type specificity of these responses needs to be elucidated in future studies.
In addition to their role in chronic neuroinflammation, microglia are important neurotrophic cells that could facilitate tissue repair, neuroplasticity, and recovery. During the recovery phase, microglia could produce local trophic gradients such as brain‐derived neurotrophic factor (BDNF), which promotes axonal regeneration 131 and enhances synaptic efficacy. 132 Indeed, knocking out of the BDNF specifically in microglia led to learning deficits and reduced synapse formation. 133 Importantly, Nrf2 is a direct transcriptional activator of BDNF in cultured BV2 cells, through binding with the BDNF exon I promoter. 134 After ischemic stroke in mice, Nrf2 activator bergenin enhanced BDNF expression in brain tissue. 135
In summary, Nrf2 inhibits prolonged microglial activation and boosts BDNF expression in microglia in the chronic phase after stroke.
4.5. Endothelial cells
Angiogenesis is an important aspect of long‐term recovery post‐ischemia as it leads to the formation of new blood vessels. It occurs 3–4 days after stroke, predominantly at the penumbra, and its extent seems to correlate with the length of survival after stroke. 136 , 137 , 138
tBHQ increased vessel density at d7, d14, and d28 post‐stroke in the peri‐infarct zone, and this effect was associated with higher VEGF levels in brain tissue at d7 and d14, and increased numbers of BrdU/CD31 double‐positive cells, a marker of newly generated ECs. Importantly, this increase was observed to a much lesser extent in the Nrf2 KO group, indicating a critical role of Nrf2 in angiogenesis. 39 Similarly, in a rat MCAO model, corilagin increased CD34/dextran double‐positive ECs 7d after MCAO, which was partially abolished by Nrf2‐siRNA intrathecal administration. 100 Such a causal relationship was also proved by the gain and loss of function of Nrf2 conducted through AAV and lentiviral interference in photothrombotic stroke. 139 Nrf2 pathway activation is also observed to accompany epigallocatechin‐3‐gallate‐mediated angiogenesis 35 days post‐MCAO in mice. 140 In cultured human brain microvascular ECs, quercetin promotes cell viability, migration and angiogenesis upon hypoxic injury, associated with Nrf2 activation. 141 And in cultured mouse cerebral microvascular ECs (bEnd.3), knockdown of Nrf2 inhibited proliferation, migration, and tube formation after hypoxia, associated with downregulation of PI3K/Akt pathway. 142 These findings consistently suggest a critical role of Nrf2 in angiogenesis after stroke.
An important point to consider is that angiogenesis after ischemic stroke may not always be beneficial. Indeed, pathological angiogenesis may further impede organ function. Interestingly, Nrf2 activation was observed in a mouse postnatal (P12‐17) oxygen‐induced retinopathy model, where Nrf2 KO mice showed impeded vascular regeneration and increased pathological neovascularization. Application of conditional KO mice where Nrf2 was knocked out in neurons, astrocytes, or ECs, respectively, revealed a critical role of neuronal and endothelial Nrf2 in regeneration. 143 Unfortunately, stroke research mainly focuses on newly generated ECs without further examining their functioning. Future studies should focus more on vascular functioning such as the BBB and local perfusion, aside from simply describing EC regeneration and angiogenesis.
5. CLINICAL CONSIDERATIONS AND FUTURE PERSPECTIVES
Preclinical investigations supported the neuroprotective effects of Nrf2 in ischemic stroke. For clinical use, however, it will be important to optimize drug delivery, drug dose, and timing of administration. In addition, previously tested Nrf2 modulators need to be evaluated to determine optimal treatment protocol and to avoid side effects. 144
It should be also emphasized that many other factors such as age could influence Nrf2 activation and its protective effects in vivo. 145 Aging has been associated with Nrf2 dysfunction across multiple species and in different organs. 6 The mechanisms responsible for this dysfunction involve miRNA, post‐translational protein modification, and impaired Nrf2‐Maf binding. 146 , 147 Indeed, (−)‐epicatechin reduced the activation and recruitment of microglia/macrophages at 7 days post‐stroke in young (2 months old) WT mice, but not in aged (12 months old) WT mice or aged Nrf2 KO mice, 148 suggesting that sensitivity to Nrf2 activators decreases with age.
The potential benefits of using Nrf2 activators in the context of ischemic stroke are threefold. First, in high‐risk patients, pretreatment with Nrf2 activators could mimic IPC and establish tolerance to protect against a subsequent, major stroke. Second, activation of the Nrf2 pathway in the acute‐phase post‐stroke may reduce neuronal loss due to less inflammation and better protection of BBB and white matter. Third, activation of the Nrf2 pathway in the chronic phase post‐stroke could contribute to tissue repair and regeneration. Several Nrf2 pharmaceutical activators have been reviewed elsewhere, 149 , 150 and the most promising ones such as Sfn, sulforadex, curcumin, and resveratrol have undergone, or are undergoing, clinical trial testing for the treatment of various disorders. 151 Notably, the majority of the Nrf2 activators have some off‐target effects. One possible solution to limit off‐target effects is to inject the drug in its inactive form, and then activate it once it crosses the BBB or when it is exposed to oxidative stress. This would mean that the bioactive component is released at the right time and in the right location. 152
In conclusion, targeting Nrf2 is a promising strategy for the management of ischemic stroke. More in‐depth research is needed to further evaluate the benefits of cell‐specific and phase‐specific Nrf2 activation in providing optimal neuroprotection and better functional outcome after stroke.
CONFLICT OF INTEREST STATEMENT
None.
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health/NINDS R01NS103810 and by start‐up funds from the Pittsburgh Institute of Brain Disorders and Recovery and the Department of Neurology at the University of Pittsburgh. We thank Pat Strickler for her administrative support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the United States Government or the Department of Veterans Affairs.
Fadoul G, Ikonomovic M, Zhang F, Yang T. The cell‐specific roles of Nrf2 in acute and chronic phases of ischemic stroke. CNS Neurosci Ther. 2024;30:e14462. doi: 10.1111/cns.14462
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Liu R, Zhao J, Li X, Messe S, Fisher M, Rudd A. To use stroke 911 to improve stroke awareness for countries where 911 is used as an emergency phone number. CNS Neurosci Ther. 2022;28(10):1473‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Esenwa C, Gutierrez J. Secondary stroke prevention: challenges and solutions. Vasc Health Risk Manag. 2015;11:437‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farina M, Vieira LE, Buttari B, Profumo E, Saso L. The Nrf2 pathway in ischemic stroke: a review. Molecules. 2021;26(16):5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiong Y, Wakhloo AK, Fisher M. Advances in acute ischemic stroke therapy. Circ Res. 2022;130(8):1230‐1251. [DOI] [PubMed] [Google Scholar]
- 5. Shi L, Rocha M, Leak RK, et al. A new era for stroke therapy: integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab. 2018;38(12):2073‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang T, Zhang F. Targeting transcription factor Nrf2 (nuclear factor erythroid 2‐related factor 2) for the intervention of vascular cognitive impairment and dementia. Arterioscler Thromb Vasc Biol. 2021;41(1):97‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ge JW, Deng SJ, Xue ZW, et al. Imperatorin inhibits mitogen‐activated protein kinase and nuclear factor kappa‐B signaling pathways and alleviates neuroinflammation in ischemic stroke. CNS Neurosci Ther. 2022;28(1):116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Theodore M, Kawai Y, Yang J, et al. Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J Biol Chem. 2008;283(14):8984‐8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313‐322. [DOI] [PubMed] [Google Scholar]
- 10. Hayes JD, McMahon M, Chowdhry S, Dinkova‐Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1‐Nrf2 pathway. Antioxid Redox Signal. 2010;13(11):1713‐1748. [DOI] [PubMed] [Google Scholar]
- 11. Krajka‐Kuźniak V, Paluszczak J, Baer‐Dubowska W. The Nrf2‐ARE signaling pathway: an update on its regulation and possible role in cancer prevention and treatment. Pharmacol Rep. 2017;69(3):393‐402. [DOI] [PubMed] [Google Scholar]
- 12. Wang H, Liu K, Geng M, et al. RXRα inhibits the NRF2‐ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73(10):3097‐3108. [DOI] [PubMed] [Google Scholar]
- 13. Perez DI, Palomo V, Pérez C, et al. Switching reversibility to irreversibility in glycogen synthase kinase 3 inhibitors: clues for specific design of new compounds. J Med Chem. 2011;54(12):4042‐4056. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi A, Kang MI, Okawa H, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3‐based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130‐7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang T, Sun Y, Mao L, et al. Brain ischemic preconditioning protects against ischemic injury and preserves the blood‐brain barrier via oxidative signaling and Nrf2 activation. Redox Biol. 2018;17:323‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaspar JW, Jaiswal AK. An autoregulatory loop between Nrf2 and Cul3‐Rbx1 controls their cellular abundance. J Biol Chem. 2010;285(28):21349‐21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jain AK, Jaiswal AK. GSK‐3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF‐E2 related factor 2. J Biol Chem. 2007;282(22):16502‐16510. [DOI] [PubMed] [Google Scholar]
- 18. Niture SK, Jaiswal AK. Prothymosin‐alpha mediates nuclear import of the INrf2/Cul3 Rbx1 complex to degrade nuclear Nrf2. J Biol Chem. 2009;284(20):13856‐13868. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2‐Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865(5):721‐733. [DOI] [PubMed] [Google Scholar]
- 21. Cuadrado A, Manda G, Hassan A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70(2):348‐383. [DOI] [PubMed] [Google Scholar]
- 22. Liu L, Locascio LM, Doré S. Critical role of Nrf2 in experimental ischemic stroke. Front Pharmacol. 2019;10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao S, Wu J, Liu R, et al. A novel compound DBZ ameliorates neuroinflammation in LPS‐stimulated microglia and ischemic stroke rats: role of Akt(Ser473)/GSK3β(Ser9)‐mediated Nrf2 activation. Redox Biol. 2020;36:101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dang J, Brandenburg LO, Rosen C, et al. Nrf2 expression by neurons, astroglia, and microglia in the cerebral cortical penumbra of ischemic rats. J Mol Neurosci. 2012;46(3):578‐584. [DOI] [PubMed] [Google Scholar]
- 25. Alfieri A, Srivastava S, Siow RCM, et al. Sulforaphane preconditioning of the Nrf2/HO‐1 defense pathway protects the cerebral vasculature against blood‐brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012‐1022. [DOI] [PubMed] [Google Scholar]
- 26. Zhang F, Wang S, Zhang M, et al. Pharmacological induction of heme oxygenase‐1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43(5):1390‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang M, Wang S, Mao L, et al. Omega‐3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J Neurosci. 2014;34(5):1903‐1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang B, Zhu X, Kim YT, et al. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med. 2012;52(5):928‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Q, Fadoul G, Ikonomovic M, Yang T, Zhang F. Sulforaphane promotes white matter plasticity and improves long‐term neurological outcomes after ischemic stroke via the Nrf2 pathway. Free Radic Biol Med. 2022;193(Pt 1):292‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang T, Sun Y, Li Q, et al. Ischemic preconditioning provides long‐lasting neuroprotection against ischemic stroke: the role of Nrf2. Exp Neurol. 2020;325:113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicotera P, Leist M, Ferrando‐May E. Apoptosis and necrosis: different execution of the same death. Biochem Soc Symp. 1999;66:69‐73. [DOI] [PubMed] [Google Scholar]
- 32. Lee MK, Kim SR, Sung SH, et al. Asiatic acid derivatives protect cultured cortical neurons from glutamate‐induced excitotoxicity. Res Commun Mol Pathol Pharmacol. 2000;108(1–2):75‐86. [PubMed] [Google Scholar]
- 33. An Y, Li H, Wang M, Xia Z, Ding L, Xia X. Nuclear factor erythroid 2‐related factor 2 agonist protects retinal ganglion cells in glutamate excitotoxicity retinas. Biomed Pharmacother. 2022;153:113378. [DOI] [PubMed] [Google Scholar]
- 34. Azar YO, Badawi GA, Zaki HF, Ibrahim SM. Agmatine‐mediated inhibition of NMDA receptor expression and amelioration of dyskinesia via activation of Nrf2 and suppression of HMGB1/RAGE/TLR4/MYD88/NF‐kappaB signaling cascade in rotenone lesioned rats. Life Sci. 2022;311(Pt A):121049. [DOI] [PubMed] [Google Scholar]
- 35. Jeong YH, Oh YC, Kim TI, Bae JS, Ma JY. The neuroprotective effects of Arecae Pericarpium against glutamate‐induced HT22 cell cytotoxicity. Curr Issues Mol Biol. 2022;44(12):5902‐5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zaki OS, Nassar NN, Abdallah DM, Safar MM, Mohammed RA. Cilostazol alleviates NLRP3 Inflammasome‐induced allodynia/hyperalgesia in murine cerebral cortex following transient ischemia: focus on TRPA1/glutamate and Akt/dopamine/BDNF/Nrf2 trajectories. Mol Neurobiol. 2022;59(12):7194‐7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Y, Chu L, Liu C, Zha Z, Shu Y. Protective effect of GSK‐3beta/Nrf2 mediated by dimethyl fumarate in middle cerebral artery embolization reperfusion rat model. Curr Neurovasc Res. 2021;18(4):456‐464. [DOI] [PubMed] [Google Scholar]
- 38. Mao L, Yang T, Li X, et al. Protective effects of sulforaphane in experimental vascular cognitive impairment: contribution of the Nrf2 pathway. J Cereb Blood Flow Metab. 2019;39(2):352‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Y, Zhang X, Yang Y, et al. Tert‐butylhydroquinone enhanced angiogenesis and astrocyte activation by activating nuclear factor‐E2‐related factor 2/heme oxygenase‐1 after focal cerebral ischemia in mice. Microvasc Res. 2019;126:103891. [DOI] [PubMed] [Google Scholar]
- 40. Dordoe C, Wang X, Lin P, et al. Non‐mitogenic fibroblast growth factor 1 protects against ischemic stroke by regulating microglia/macrophage polarization through Nrf2 and NF‐κB pathways. Neuropharmacology. 2022;212:109064. [DOI] [PubMed] [Google Scholar]
- 41. Wu H, Che X, Zheng Q, et al. Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. Int J Biol Sci. 2014;10(9):1072‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kwon JH, Lee J, Kim J, et al. Upregulation of carbonyl reductase 1 by Nrf2 as a potential therapeutic intervention for ischemia/reperfusion injury during liver transplantation. Mol Cells. 2019;42(9):672‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang C, Zhao M, Wang B, et al. The Nrf2‐NLRP3‐caspase‐1 axis mediates the neuroprotective effects of Celastrol in Parkinson's disease. Redox Biol. 2021;47:102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang T, Li Q, Fadoul G, Alraqmany N, Ikonomovic M, Zhang F. Aldo‐Keto reductase 1C15 characterization and protection in ischemic brain injury. Antioxidants (Basel). 2023;12(4):909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chu SF, Zhang Z, Zhou X, et al. Ginsenoside Rg1 protects against ischemic/reperfusion‐induced neuronal injury through miR‐144/Nrf2/ARE pathway. Acta Pharmacol Sin. 2019;40(1):13‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fan GB, Li Y, Xu GS, et al. Propofol inhibits ferroptotic cell death through the Nrf2/Gpx4 signaling pathway in the mouse model of cerebral ischemia‐reperfusion injury. Neurochem Res. 2022;48:956‐966. [DOI] [PubMed] [Google Scholar]
- 47. Farhat F, Nofal S, Raafat EM, Eissa Ahmed AA. Akt / GSK3beta / Nrf2 / HO‐1 pathway activation by flurbiprofen protects the hippocampal neurons in a rat model of glutamate excitotoxicity. Neuropharmacology. 2021;196:108654. [DOI] [PubMed] [Google Scholar]
- 48. Cui Y, Xiong Y, Li H, et al. Chalcone‐derived Nrf2 activator protects cognitive function via maintaining neuronal redox status. Antioxidants (Basel). 2021;10(11):1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malik I, Shah FA, Ali T, et al. Potent natural antioxidant Carveol attenuates MCAO‐stress induced oxidative, neurodegeneration by regulating the Nrf‐2 pathway. Front Neurosci. 2020;14:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shih AY, Li P, Murphy TH. A small‐molecule‐inducible Nrf2‐mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25(44):10321‐10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cui HY, Zhang XJ, Yang Y, et al. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regen Res. 2018;13(12):2119‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ding P, Chen W, Yan X, et al. BMPER alleviates ischemic brain injury by protecting neurons and inhibiting neuroinflammation via Smad3‐Akt‐Nrf2 pathway. CNS Neurosci Ther. 2022;28(4):593‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morris G, Walker AJ, Berk M, Maes M, Puri BK. Cell death pathways: a novel therapeutic approach for neuroscientists. Mol Neurobiol. 2018;55(7):5767‐5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S. Ferroptosis and cell death mechanisms in Parkinson's disease. Neurochem Int. 2017;104:34‐48. [DOI] [PubMed] [Google Scholar]
- 56. Chen J, Wang Y, Li M, et al. Netrin‐1 alleviates early brain injury by regulating Ferroptosis via the PPARgamma/Nrf2/GPX4 signaling pathway following subarachnoid hemorrhage. Transl Stroke Res. 2023. doi: 10.1007/s12975-022-01122-4 [DOI] [PubMed] [Google Scholar]
- 57. Liu Z, Zhou Z, Ai P, Zhang C, Chen J, Wang Y. Astragaloside IV attenuates ferroptosis after subarachnoid hemorrhage via Nrf2/HO‐1 signaling pathway. Front Pharmacol. 2022;13:924826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou ZX, Cui Q, Zhang YM, et al. Withaferin A inhibits ferroptosis and protects against intracerebral hemorrhage. Neural Regen Res. 2023;18(6):1308‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xiao L, Ji X, Zhao H, et al. A novel severe cerebral venous thrombosis rat model based on semi‐ligation combined with ferric chloride and thrombin. CNS Neurosci Ther. 2022;28(12):2129‐2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li C, Wu Z, Xue H, et al. Ferroptosis contributes to hypoxic‐ischemic brain injury in neonatal rats: role of the SIRT1/Nrf2/GPx4 signaling pathway. CNS Neurosci Ther. 2022;28(12):2268‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen G, Li L, Tao H. Bioinformatics identification of Ferroptosis‐related biomarkers and therapeutic compounds in ischemic stroke. Front Neurol. 2021;12:745240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chan JY, Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic‐leucine zipper protein. Biochim Biophys Acta. 2000;1517(1):19‐26. [DOI] [PubMed] [Google Scholar]
- 63. Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress‐inducible genes in macrophages. J Biol Chem. 2000;275(21):16023‐16029. [DOI] [PubMed] [Google Scholar]
- 64. Osburn WO, Wakabayashi N, Misra V, et al. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat‐mediated formation of superoxide anion. Arch Biochem Biophys. 2006;454(1):7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase‐3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem. 2006;281(21):14841‐14851. [DOI] [PubMed] [Google Scholar]
- 66. Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73(11–12):2195‐2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guo L, Shi L. Vitexin improves cerebral ischemia‐reperfusion injury by attenuating oxidative injury and ferroptosis via Keap1/Nrf2/HO‐1signaling. Neurochem Res. 2022;48:980‐995. [DOI] [PubMed] [Google Scholar]
- 68. Zhang J, Sun H, Zhu L, et al. Micro ribonucleic acid 27a aggravates Ferroptosis during early ischemic stroke of rats through nuclear factor Erythroid‐2‐related factor 2. Neuroscience. 2022;504:10‐20. [DOI] [PubMed] [Google Scholar]
- 69. Barros LF. How expensive is the astrocyte? J Cereb Blood Flow Metab. 2022;42(5):738‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Narayanan SV, Dave KR, Perez‐Pinzon MA. Ischemic preconditioning protects astrocytes against oxygen glucose deprivation via the nuclear erythroid 2‐related factor 2 pathway. Transl Stroke Res. 2018;9(2):99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ding Y, Chen MC, Wang MM, Li YW, Wen AD. Posttreatment with 11‐Keto‐beta‐Boswellic acid ameliorates cerebral ischemia‐reperfusion injury: Nrf2/HO‐1 pathway as a potential mechanism. Mol Neurobiol. 2015;52(3):1430‐1439. [DOI] [PubMed] [Google Scholar]
- 72. Narayanan SV, Dave KR, Saul I, Perez‐Pinzon MA. Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2‐related factor 2. Stroke. 2015;46(6):1626‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Calkins MJ, Vargas MR, Johnson DA, Johnson JA. Astrocyte‐specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci. 2010;115(2):557‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Genova ML, Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim Biophys Acta. 2014;1837(4):427‐443. [DOI] [PubMed] [Google Scholar]
- 75. Jiwaji Z, Tiwari SS, Avilés‐Reyes RX, et al. Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to tau and ass pathology. Nat Commun. 2022;13(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gan L, Vargas MR, Johnson DA, Johnson JA. Astrocyte‐specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha‐synuclein mutant (A53T) mouse model. J Neurosci. 2012;32(49):17775‐17787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sigfridsson E, Marangoni M, Johnson JA, Hardingham GE, Fowler JH, Horsburgh K. Astrocyte‐specific overexpression of Nrf2 protects against optic tract damage and behavioural alterations in a mouse model of cerebral hypoperfusion. Sci Rep. 2018;8(1):12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28(50):13574‐13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li X, Zhang W, Xiao M, et al. MicroRNA‐146b‐5p protects oligodendrocyte precursor cells from oxygen/glucose deprivation‐induced injury through regulating Keap1/Nrf2 signaling via targeting bromodomain‐containing protein 4. Biochem Biophys Res Commun. 2019;513(4):875‐882. [DOI] [PubMed] [Google Scholar]
- 80. Liessem‐Schmitz A, Teske N, Scheld M, et al. Nrf2 signaling in sodium azide‐treated oligodendrocytes restores mitochondrial functions. J Mol Neurosci. 2018;66(2):229‐237. [DOI] [PubMed] [Google Scholar]
- 81. Nellessen A, Nyamoya S, Zendedel A, et al. Nrf2 deficiency increases oligodendrocyte loss, demyelination, neuroinflammation and axonal damage in an MS animal model. Metab Brain Dis. 2020;35(2):353‐362. [DOI] [PubMed] [Google Scholar]
- 82. Draheim T, Liessem A, Scheld M, et al. Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model. Glia. 2016;64(12):2219‐2230. [DOI] [PubMed] [Google Scholar]
- 83. Dufort C, Wang Y, Hu X. Microglia: active participants in brain capillary function. J Cereb Blood Flow Metab. 2022;42(11):2161‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen W, Zhang Y, Zhai X, et al. Microglial phagocytosis and regulatory mechanisms after stroke. J Cereb Blood Flow Metab. 2022;42(9):1579‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zheng K, Lin L, Jiang W, et al. Single‐cell RNA‐seq reveals the transcriptional landscape in ischemic stroke. J Cereb Blood Flow Metab. 2022;42(1):56‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100(23):13632‐13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387‐1394. [DOI] [PubMed] [Google Scholar]
- 88. Hu X, Leak RK, Shi Y, et al. Microglial and macrophage polarization‐new prospects for brain repair. Nat Rev Neurol. 2015;11(1):56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu R, Liao XY, Pan MX, et al. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF‐kappaB p65/Hif‐1alpha signaling pathway. J Immunol. 2019;202(6):1704‐1714. [DOI] [PubMed] [Google Scholar]
- 90. Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063‐3070. [DOI] [PubMed] [Google Scholar]
- 91. Wang N, Nie H, Zhang Y, et al. Dexmedetomidine exerts cerebral protective effects against cerebral ischemic injury by promoting the polarization of M2 microglia via the Nrf2/HO‐1/NLRP3 pathway. Inflamm Res. 2022;71(1):93‐106. [DOI] [PubMed] [Google Scholar]
- 92. Cai M, Sun S, Wang J, et al. Sevoflurane preconditioning protects experimental ischemic stroke by enhancing anti‐inflammatory microglia/macrophages phenotype polarization through GSK‐3β/Nrf2 pathway. CNS Neurosci Ther. 2021;27(11):1348‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang Y, Huang Y, Xu Y, et al. A dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid Redox Signal. 2018;28(2):141‐163. [DOI] [PubMed] [Google Scholar]
- 94. Lyu J, Jiang X, Leak RK, Shi Y, Hu X, Chen J. Microglial responses to brain injury and disease: functional diversity and new opportunities. Transl Stroke Res. 2021;12(3):474‐495. [DOI] [PubMed] [Google Scholar]
- 95. Jassam YN, Izzy S, Whalen M, McGavern D, el Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95(6):1246‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19(8):987‐991. [DOI] [PubMed] [Google Scholar]
- 97. Zhang S. Microglial activation after ischaemic stroke. Stroke Vasc Neurol. 2019;4(2):71‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yu F, Wang Y, Stetler AR, Leak RK, Hu X, Chen J. Phagocytic microglia and macrophages in brain injury and repair. CNS Neurosci Ther. 2022;28(9):1279‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang R, Chopp M, Zhang Z, Jiang N, Powers C. The expression of P‐ and E‐selectins in three models of middle cerebral artery occlusion. Brain Res. 1998;785(2):207‐214. [DOI] [PubMed] [Google Scholar]
- 100. Ding Y, Ren D, Xu H, et al. Antioxidant and pro‐angiogenic effects of corilagin in rat cerebral ischemia via Nrf2 activation. Oncotarget. 2017;8(70):114816‐114828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ibbotson K, Yell J, Ronaldson PT. Nrf2 signaling increases expression of ATP‐binding cassette subfamily C mRNA transcripts at the blood‐brain barrier following hypoxia‐reoxygenation stress. Fluids Barriers CNS. 2017;14(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li W, Suwanwela NC, Patumraj S. Curcumin by down‐regulating NF‐kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res. 2016;106:117‐127. [DOI] [PubMed] [Google Scholar]
- 103. Kunze R, Urrutia A, Hoffmann A, et al. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood‐brain barrier integrity. Exp Neurol. 2015;266:99‐111. [DOI] [PubMed] [Google Scholar]
- 104. Zhao X, Li S, Mo Y, et al. DCA protects against oxidation injury attributed to cerebral ischemia‐reperfusion by regulating glycolysis through PDK2‐PDH‐Nrf2 axis. Oxid Med Cell Longev. 2021;2021:5173035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2‐driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27(38):10240‐10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fuchs E, Flugge G. Adult neuroplasticity: more than 40 years of research. Neural Plast. 2014;2014:541870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cheng T, Wang W, Li Q, et al. Cerebroprotection of flavanol (−)‐epicatechin after traumatic brain injury via Nrf2‐dependent and ‐independent pathways. Free Radic Biol Med. 2016;92:15‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang H, Zheng Z, Han W, et al. Metformin promotes axon regeneration after spinal cord injury through inhibiting oxidative stress and stabilizing microtubule. Oxid Med Cell Longev. 2020;2020:9741369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xia B, Liu H, Xie J, Wu R, Li Y. Akt enhances nerve growth factor‐induced axon growth via activating the Nrf2/ARE pathway. Int J Mol Med. 2015;36(5):1426‐1432. [DOI] [PubMed] [Google Scholar]
- 110. Liu Q, Tan Y, Qu T, et al. Therapeutic mechanism of human neural stem cell‐derived extracellular vesicles against hypoxia‐reperfusion injury in vitro. Life Sci. 2020;254:117772. [DOI] [PubMed] [Google Scholar]
- 111. Altman J. The discovery of adult mammalian neurogenesis. In: Seki T, Sawamoto K, Parent JM, et al., eds. Neurogenesis in the Adult Brain. Springer; 2011:3‐46. [Google Scholar]
- 112. Passarelli JP, Nimjee SM, Townsend KL. Stroke and neurogenesis: bridging clinical observations to new mechanistic insights from animal models. Transl Stroke Res. 2022. doi: 10.1007/s12975-022-01109-1 [DOI] [PubMed] [Google Scholar]
- 113. Conover JC, Shook BA. Aging of the subventricular zone neural stem cell niche. Aging Dis. 2011;2(1):49‐63. [PMC free article] [PubMed] [Google Scholar]
- 114. Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5(2):139‐152. [DOI] [PubMed] [Google Scholar]
- 115. Kreuzberg M, Kanov E, Timofeev O, Schwaninger M, Monyer H, Khodosevich K. Increased subventricular zone‐derived cortical neurogenesis after ischemic lesion. Exp Neurol. 2010;226(1):90‐99. [DOI] [PubMed] [Google Scholar]
- 116. Nakayama D, Matsuyama T, Ishibashi‐Ueda H, et al. Injury‐induced neural stem/progenitor cells in post‐stroke human cerebral cortex. Eur J Neurosci. 2010;31(1):90‐98. [DOI] [PubMed] [Google Scholar]
- 117. Xiong W, MacColl Garfinkel A, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125(4):1433‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Corenblum MJ, Ray S, Remley QW, et al. Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle‐age period. Aging Cell. 2016;15(4):725‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Choudhury GR, Ding S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol Dis. 2016;85:234‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Li H, Zhang N, Lin HY, et al. Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis‐induced ischemia in adult mice. BMC Neurosci. 2014;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hayakawa K, Nakano T, Irie K, et al. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2010;30(4):871‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Becerra‐Calixto A, Cardona‐Gomez GP. The role of astrocytes in neuroprotection after brain stroke: potential in cell therapy. Front Mol Neurosci. 2017;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zbesko JC, Nguyen TV, Yang T, et al. Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts. Neurobiol Dis. 2018;112:63‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17(1):120‐127. [DOI] [PubMed] [Google Scholar]
- 125. Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182(2):399‐411. [DOI] [PubMed] [Google Scholar]
- 126. Liu L, Kelly MG, Wierzbicki EL, et al. Nrf2 plays an essential role in Long‐term brain damage and neuroprotection of Korean red ginseng in a permanent cerebral ischemia model. Antioxidants (Basel). 2019;8(8):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Li Q, Lou J, Yang T, Wei Z, Li S, Zhang F. Ischemic preconditioning induces oligodendrogenesis in mouse brain: effects of Nrf2 deficiency. Cell Mol Neurobiol. 2022;42(6):1859‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sanadgol N, Barati M, Houshmand F, et al. Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self‐repairing period. Pharmacol Rep. 2020;72(3):641‐658. [DOI] [PubMed] [Google Scholar]
- 129. De Nuccio C, Bernardo A, Troiano C, et al. NRF2 and PPAR‐gamma pathways in oligodendrocyte progenitors: focus on ROS protection, mitochondrial biogenesis and promotion of cell differentiation. Int J Mol Sci. 2020;21(19):7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jackson L, Dumanli S, Johnson MH, Fagan SC, Ergul A. Microglia knockdown reduces inflammation and preserves cognition in diabetic animals after experimental stroke. J Neuroinflammation. 2020;17(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Batchelor PE, Porritt MJ, Martinello P, et al. Macrophages and microglia produce local trophic gradients that stimulate axonal sprouting toward but not beyond the wound edge. Mol Cell Neurosci. 2002;21(3):436‐453. [DOI] [PubMed] [Google Scholar]
- 132. Madinier A, Bertrand N, Mossiat C, et al. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS One. 2009;4(12):e8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning‐dependent synapse formation through brain‐derived neurotrophic factor. Cell. 2013;155(7):1596‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tang R, Cao QQ, Hu SW, et al. Sulforaphane activates anti‐inflammatory microglia, modulating stress resilience associated with BDNF transcription. Acta Pharmacol Sin. 2022;43(4):829‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhang X, Zhang F, Yao F, Wang P, Xiong Q, Neng P. Bergenin has neuroprotective effects in mice with ischemic stroke through antioxidative stress and anti‐inflammation via regulating Sirt1/FOXO3a/NF‐kappaB signaling. Neuroreport. 2022;33(13):549‐560. [DOI] [PubMed] [Google Scholar]
- 136. Krupinski J, Kałuza J, Kumar P, Kumar S, Wang JM. Some remarks on the growth‐rate and angiogenesis of microvessels in ischemic stroke. Morphometric and immunocytochemical studies. Patol Pol. 1993;44(4):203‐209. [PubMed] [Google Scholar]
- 137. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25(9):1794‐1798. [DOI] [PubMed] [Google Scholar]
- 138. Szpak GM, Lechowicz W, Lewandowska E, Bertrand E, Wierzba‐Bobrowicz T, Dymecki J. Border zone neovascularization in cerebral ischemic infarct. Folia Neuropathol. 1999;37(4):264‐268. [PubMed] [Google Scholar]
- 139. Wang P, Zhao Y, Li Y, et al. Sestrin2 overexpression attenuates focal cerebral ischemic injury in rat by increasing Nrf2/HO‐1 pathway‐mediated angiogenesis. Neuroscience. 2019;410:140‐149. [DOI] [PubMed] [Google Scholar]
- 140. Bai Q, Lyu Z, Yang X, Pan Z, Lou J, Dong T. Epigallocatechin‐3‐gallate promotes angiogenesis via up‐regulation of Nfr2 signaling pathway in a mouse model of ischemic stroke. Behav Brain Res. 2017;321:79‐86. [DOI] [PubMed] [Google Scholar]
- 141. Li MT, Ke J, Guo SF, et al. The protective effect of quercetin on endothelial cells injured by hypoxia and Reoxygenation. Front Pharmacol. 2021;12:732874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Huang Y, Mao Y, Li H, Shen G, Nan G. Knockdown of Nrf2 inhibits angiogenesis by downregulating VEGF expression through PI3K/Akt signaling pathway in cerebral microvascular endothelial cells under hypoxic conditions. Biochem Cell Biol. 2018;96(4):475‐482. [DOI] [PubMed] [Google Scholar]
- 143. Wei Y, Gong J, Xu Z, et al. Nrf2 in ischemic neurons promotes retinal vascular regeneration through regulation of semaphorin 6A. Proc Natl Acad Sci U S A. 2015;112(50):E6927‐E6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gazaryan IG, Thomas B. The status of Nrf2‐based therapeutics: current perspectives and future prospects. Neural Regen Res. 2016;11(11):1708‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sakamuri SS, Sure VN, Kolli L, et al. Aging related impairment of brain microvascular bioenergetics involves oxidative phosphorylation and glycolytic pathways. J Cereb Blood Flow Metab. 2022;42(8):1410‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cheng X, Ku CH, Siow RC. Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Free Radic Biol Med. 2013;64:4‐11. [DOI] [PubMed] [Google Scholar]
- 147. Kubben N, Zhang W, Wang L, et al. Repression of the antioxidant NRF2 pathway in premature aging. Cell. 2016;165(6):1361‐1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Leonardo CC, Mendes M, Ahmad AS, Doré S. Efficacy of prophylactic flavan‐3‐ol in permanent focal ischemia in 12‐mo‐old mice. Am J Physiol Heart Circ Physiol. 2015;308(6):H583‐H591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Robledinos‐Anton N, Fernández‐Ginés R, Manda G, Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid Med Cell Longev. 2019;2019:9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zolnourian AH, Franklin S, Galea I, Bulters DO. Study protocol for SFX‐01 after subarachnoid haemorrhage (SAS): a multicentre randomised double‐blinded, placebo controlled trial. BMJ Open. 2020;10(3):e028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mazur A, Fangman M, Ashouri R, Arcenas A, Doré S. Nrf2 as a therapeutic target in ischemic stroke. Expert Opin Ther Targets. 2021;25(3):163‐166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


