Abstract
Objectives:
Left-sided portal hypertension (LSPH) leads to life-threatening gastrointestinal (GI) bleeding. There are no recommendations or consensus about the management of GI bleeding caused by LSPH. This systematic review and meta-analysis were conducted to evaluate the incidence of GI bleeding and the mortality of patients with LSPH receiving different therapeutic strategies.
Design:
A systematic review and meta-analysis were performed to determine the efficacy of different therapeutic strategies for GI bleeding caused by LSPH.
Data sources and methods:
All relevant studies were searched from PubMed, Embase, Web of Science, Cochrane Library, Scopus, ScienceDirect, MEDLINE, Google Scholar, CNKI, and Wanfang Data without language restriction through 15 November 2023. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated through RevMan5.3 software. (The Cochrane Collaboration, Copenhagen, Denmark).
Results:
Seventeen retrospective studies and one prospective study involving 624 patients were included. This systematic review and meta-analysis found that: (1) splenectomy was more effective than non-splenectomy therapeutic strategies in reducing the incidence of GI bleeding caused by LSPH (OR: 0.12; 95% CI: 0.06–0.27); (2) splenectomy was superior to partial splenic artery embolism (PSAE) (OR: 0.06; 95% CI: 0.01–0.62) or endoscopic interventions (OR: 0.04; 95% CI: 0.01–0.19) in the prevention of GI bleeding, respectively; (3) no significant difference in the mortality was observed between splenectomy and non-splenectomy therapeutic strategies (OR: 0.46; 95% CI: 0.20–1.08); and (4) patients receiving preoperative PSAE followed by splenectomy had less intraoperative bleeding and shorter operative time than those receiving splenectomy.
Conclusion:
This meta-analysis demonstrated that splenectomy is superior to non-splenectomy therapeutic strategies in reducing the incidence of GI bleeding from LSPH, which revealed that splenectomy should be recommended in the management of these patients.
Trial registration:
This study has been registered on the PROSPERO database with the registration number CRD42023483764.
Keywords: endoscopic interventions, gastrointestinal bleeding, left-sided portal hypertension, partial splenic artery embolization, splenectomy
Introduction
Left-sided portal hypertension (LSPH), a rare extrahepatic portal hypertension, is also known as localized, regional, or sinistral portal hypertension.1,2 LSPH is characterized by increased pressure on the left portal system secondary to the compression or the obstruction of splenic vein. Normal liver function and main portal vein patency are observed in patients with LSPH. 3 LSPH is caused mainly by pancreatic diseases, including chronic pancreatitis, pancreatic pseudocysts, pancreatic carcinoma, etc.4,5 Isolated gastric varices are a typical manifestation of LSPH, which results in severe or persistent gastrointestinal (GI) bleeding.5–7
The main therapeutic goal for GI bleeding from gastric varices is to manage the occurrence and recurrence of the bleeding. Generally, therapeutic strategies for cirrhosis-derived gastric varices contain endoscopic interventions, balloon-occluded retrograde transvenous obliteration, transjugular intrahepatic portosystemic shunt, etc.8,9 However, gastric varices derived from liver cirrhosis or LSPH have different vascular anatomy, which results in the difference in the management of GI bleeding. Therapeutic strategies for bleeding from LSPH include endoscopic interventions, partial splenic arterial embolization (PSAE), and splenectomy.10–13 Endoscopic interventions are widely used for gastroesophageal varices in patients with liver cirrhosis but have a high failure rate in the management of GI bleeding caused by LSPH. 12 PSAE is a major therapeutic strategy for hypersplenism. Some studies demonstrated that PSAE was an effective approach for GI bleeding caused by LSPH.12,14,15 However, the efficacy of PSAE needs to be investigated. Splenectomy is used for GI bleeding caused by LSPH by removing the spleen. Limited data demonstrated splenectomy were effective in reducing GI bleeding caused by LSPH. 16 In summary, there is no consensus guideline for the management of GI bleeding from LSPH. Additionally, meta-analyses evaluating the efficacy of different therapeutic strategies for GI bleeding from LSPH have not been reported until now. 6 Therefore, this systematic review and meta-analysis aimed to determine the incidence of GI bleeding and the mortality of patients with LSPH after the patients had received different therapeutic strategies.
Methods
Search strategy
This systematic review and meta-analysis were registered in PROSPERO (registration number: CRD42023483764) and performed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement 17 (Supplemental Table S1). The two authors independently searched English databases (PubMed, Embase, Web of Science, Cochrane Library, Scopus, ScienceDirect, MEDLINE, and Google Scholar) and Chinese databases (CNKI, Wanfang Data) from inception to 15 November 2023. Search keywords included LSPH, sinistral portal hypertension, bleeding, splenectomy, partial splenic artery embolization, and endoscopic interventions. The detailed search strategy was shown in Supplemental Table S2.
Selection criteria
Studies were included if they met the following criteria: (i) patients with LSPH; (ii) patients receiving different therapeutic strategies for LSPH; (iii) reported incidence of GI bleeding in patients after treatment; (iv) reported mortality in patients after treatment; (v) the patients with a minimum follow-up period of 6 months; (vi) odds ratios (ORs) or other data for the calculation of ORs and 95% confidence intervals (CIs) were reported. Studies presented as case series, reports, reviews, or comments were excluded. For the studies with overlapping cohorts, recent studies with comprehensive data were enrolled.
Data abstraction
We collected the first author’s name, the publication year, and the type of research and extracted the number, age, sex, and etiology of the patients with LSPH from the cohorts. The number of patients, the surgical procedures for splenectomy, the rate of GI bleeding, and the mortality of patients in different treatment groups were collected for this meta-analysis.
Quality assessment
The Newcastle-Ottawa Scale (NOS) was utilized to evaluate the quality of included articles by two researchers. 18 If there were a disagreement between the two researchers, all the researchers would reach an agreement after careful discussion. The literature with an NOS score of <5 was considered to be low quality. 19
Statistical analysis
Heterogeneity was assessed using the Chi-square-based Q-tests and I2 statistic. Studies with an I2 statistics of 0%, 25%, 50%, and 75% corresponded to no, low, moderate, and high heterogeneity. p Value >0.10 or I2 < 50% indicates low heterogeneity and a fixed-effect model would be performed. 20 Publication bias was analyzed and represented by funnel plots and Egger’s test. 21 State MP15 (Stata Corporation, College Station, TX, USA) was used to run Egger’s test, and RevMan5.3 software (The Cochrane Collaboration, Copenhagen, Denmark) was used to produce all calculations and graphics.
Results
Search results and quality of included studies
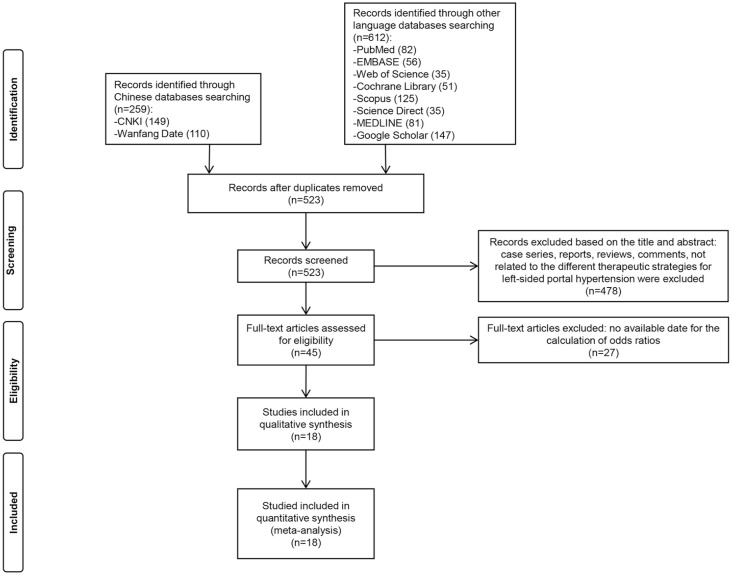
Figure 1 illustrates the selection process of literature. After a comprehensive search, 523 papers were chosen, and 478 papers were excluded because the titles and the abstracts were not associated with the efficacy of therapeutic strategies for GI bleeding from LSPH, or the studies were presented as case series, reports, reviews, or comments.
Figure 1.
Flow diagram of the selection.
Twenty-seven articles were removed due to no available data for the calculation of ORs and 95% CIs. Finally, a total of 18 studies were included in our meta-analysis. The results of the quality assessment were shown in Supplemental Table S3 and the quality scores of all included studies were ⩾5 in this meta-analysis. Therefore, all included studies were considered to be high quality.
Characteristics of included studies
A summary of characteristics of the included studies were showed in Table 1. These studies were published between 1992 and 2022. Included studies contained 1 prospective study 22 and 17 retrospective studies.16,23–38 Two studies were performed in the United States,39,26 one study was from Spain, 23 one study was from Japan, 38 and the rest of the studies were from China. A total of 624 patients were included in this meta-analysis. 71.72% of patients were male because LSPH is more common in men than in women, with a male-to-female ratio of nearly 2:1.27,40,41 The etiologies of enrolled patients with LSPH included acute pancreatitis, chronic pancreatitis, pancreatic pseudocyst, pancreatic carcinoma, etc.4,42 The patients with chronic pancreatitis were recruited in two studies,25,39 while the other studies included patients with different pancreatic diseases. The follow-up times were variable among different studies, which ranged from 6 to 128 months. There are two types of splenectomy: splenectomy and splenectomy combined with pancreatic surgery. Major non-splenectomy therapeutic strategies include endoscopic interventions and PSAE. Endoscopic interventions include endoscopic sclerotherapy using N-butyl-2-cyanoacrylate and endoscopic variceal ligation. In addition, four studies determined the efficacy of preoperative PSAE followed by splenectomy and splenectomy.22,23,27,33
Table 1.
Summary of characteristics of included studies.
| First author, year | Country | Number of patients | Characteristics of enrolled patients | Surgical procedures of splenectomy | Therapeutic strategies | Duration of follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|
| Age (years) | Gender (M/F) | Etiology of LSPH | ||||||
| Alexandra Fernandes, 2015 23 | Spain | 22 | 59.6 ± 10.6 | 17/5 | Chronic pancreatitis (7), acute pancreatitis (7), pancreatic carcinoma (4), pancreatic surgery (3), pancreatic arteriovenous malformations (1) | Splenectomy (alone), splenectomy combined with other surgery (pancreatic resection or pseudocyst drainage) | Endoscopic interventions, PSAE, medications | 24 |
| George Sakorafas, 1999 24 | America | 34 | 46 (19–74) | 23/11 | Chronic pancreatitis (34) | Splenectomy (alone), splenectomy combined with other surgery (pancreatic surgery) | – | 58 |
| Jingjing Liu, 2022 25 | China | 33 | 44.74 | 29/4 | Chronic pancreatitis (33) | Splenectomy (alone) | Splenic vein stent implantation, medications | 6 |
| John Loftus, 1992 26 | America | 37 | 50.3 | – | Chronic pancreatitis (10), islet cell carcinoma (6), adenocarcinoma (4), acute pancreatitis (1), chronic pancreatitis and acute pancreatitis (1), unknown (15) | – | – | 54 |
| Quanda Liu, 2014 16 | China | 21 | 47 ± 8 | 14/7 | Acute pancreatitis (1), chronic pancreatitis (7), primary pancreatic carcinoma (11), benign pancreatic carcinoma (1), metastatic pancreatic carcinoma (1) | Splenectomy combined with other surgery (pancreatic duct-jejunum Roux-en-Y anastomosis, external drainage of the pancreatic cyst, or distal pancreatectomy) | PSAE, endoscopic interventions | 72 |
| Zihe Wang, 2021 27 | China | 59 | 44.7 | 43/16 | Pancreatic pseudocyst (33), pancreatic abscess (9), acute pancreatitis (2), chronic pancreatitis (14), gastric ulcer (1) | Splenectomy (alone), preoperative PSAE followed by splenectomy | Preoperative PSAE followed by splenectomy | 20 |
| Guijin Chen, 2013 28 | China | 45 | M: 45.94 ± 9.42, F: 49.64 ± 6.92 | 31/14 | Pancreatic pseudocyst (15), acute pancreatitis (1), chronic pancreatitis (22), pancreatic carcinoma (1), Hodgkin lymphoma (2), translocation of spleen (1), splenic aneurysm (1), arteriovenous malformations (1), abdominal trauma (1) | Splenectomy (alone), splenectomy combined with other surgery (pericardial vascular dissection) |
Endoscopic interventions | 54 |
| Liushun Feng, 1995 29 | China | 14 | 28.5 (3–65) | 9/5 | Chronic pancreatitis (6), pancreatic carcinoma (3), gastric ulcer (1), pancreatic aneurysm (1), splenic lymph node tuberculosis (1), unknown (2) | Splenectomy (alone), splenectomy combined with other surgery (subtotal gastrectomy) | Ligation of the splenic artery | 18 |
| Angzhi Li*, 2021 22 | China | 60 | 44.8 | 44/16 | – | Splenectomy (alone), preoperative PSAE followed by splenectomy | Preoperative PSAE followed by splenectomy | – |
| Liting Liao, 2019 30 | China | 47 | 41.4 (21–77) | 41/6 | Pancreatic carcinoma (2), chronic pancreatitis (7), acute pancreatitis (33), autoimmune pancreatitis (5) | Splenectomy combined with other surgery (cardiac vascular dissection) | PSAE, conservative treatment | – |
| Bei Sun, 2006 31 | China | 67 | 43.2 (15–79) | 43/24 | Pancreatic pseudocyst (19), pancreatic abscess (5), chronic pancreatitis (23), pancreatic carcinoma (14), pancreatic trauma (2), unknown (4) | Splenectomy (alone), splenectomy combined with other surgery (pericardial vascular dissection, Roux-en-Y anastomosis of cyst and jejunum, or pancreatectomy) | PSAE, endoscopic interventions | 33.1 |
| Yu Tang, 2008 32 | China | 67 | 48 (16–78) | 47/20 | Pancreatic pseudocyst (10), pancreatic carcinoma (23), unknown (34) | Splenectomy (alone), splenectomy combined with other surgery (portal disconnection, pancreatectomy, or pseudocyst drainage) | Medications | – |
| Guangping Tu, 2019 33 | China | 32 | 49 ± 21 | 27/5 | Pancreatic pseudocyst (1), acute pancreatitis (8), chronic pancreatitis (23) | splenectomy (alone), preoperative PSAE followed by splenectomy | Preoperative PSAE followed by splenectomy | 25 |
| Kai Wang, 2008 34 | China | 21 | 50.5 (34–72) | 14/7 | Pancreatic pseudocyst (4), pancreatic carcinoma (6), chronic pancreatitis (11) | Splenectomy (alone), splenectomy combined with other surgery (pericardial vascular dissection or internal drainage) | Ligation of the splenic artery | 60 |
| Qingsong Xie, 2020 35 | China | 24 | 47.6 (27–70) | 14/10 | Pancreatic pseudocyst (3), pancreatic carcinoma (4), acute or chronic pancreatitis (15), pancreatic duct stones (1), pancreatic injury (1) | Splenectomy (alone), splenectomy combined with other surgery (tail pancreatectomy, intestinal drainage, fenestration and drainage of pancreatic pseudocysts, residual cholecystectomy and choledocholithotomy and T-tube drainage, portal azygos vein disconnection or pancreatectomy) | PSAE, conservative treatment | 128 |
| Xiangyu Zhong, 2015 36 | China | 23 | 46 (24–73) | 14/9 | Pancreatic pseudocyst (6), pancreatic carcinoma (5), chronic pancreatitis (10), acute pancreatitis (1), pancreatic injury (1) | Splenectomy (alone), splenectomy combined with other surgery (disconnection of gastric fundus blood vessels, disconnection of gastric fundus and cardiac blood vessels, cyst jejunal drainage, pancreaticoduodenectomy or pancreatectomy) | PSAE, conservative treatment | 33.1 |
| Yang Song, 2005 37 | China | 9 | 41 ± 10 | 6/3 | Pancreatic carcinoma (3), acute pancreatitis (1), chronic pancreatitis (5) | Splenectomy combined with other surgery (pancreatectomy) | Endoscopic interventions | 48 |
| Shugo Mizuno, 2019 38 | Japan | 9 | 61.3 | 5/4 | Pancreatic carcinoma (9) | Splenectomy (alone) | PSAE, medications, endoscopic interventions | 89 |
Except for one prospective study, all the remaining articles were retrospective observational studies.
PSAE, partial splenic artery embolization.
Incidence of GI bleeding after treatment
Meta-analysis
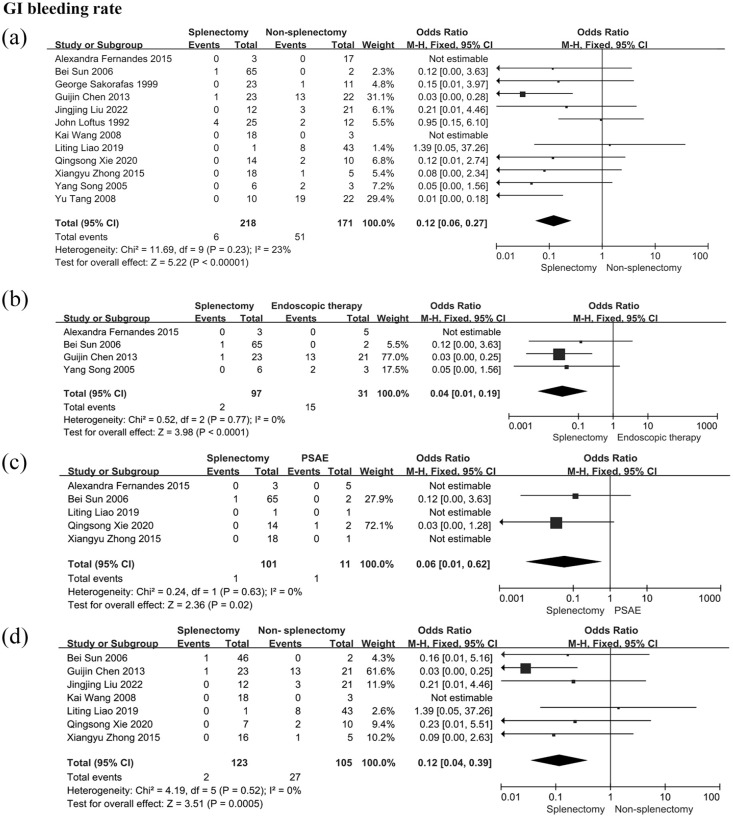
A total of 12 studies provided evaluable data, involving 218 patients in the splenectomy group and 170 patients in the non-splenectomy group. Forest plots showed that splenectomy was more effective than non-splenectomy therapeutic strategies in reducing the incidence of GI bleeding caused by LSPH (OR: 0.12; 95% CI: 0.06–0.27; p = 0.23; I2 = 23%, Figure 2). The difference was statistically significant and the heterogeneity among the 12 studies was low.
Figure 2.
GI bleeding rate in patients with LSPH. (a) GI bleeding rate in patients receiving splenectomy or non-splenectomy; (b) GI bleeding rate in patients receiving splenectomy or endoscopic interventions; (c) GI bleeding rate in patients receiving splenectomy or PSAE; (d) GI bleeding rate in patients receiving splenectomy or non-splenectomy after excluding patients who underwent pancreatic surgery.
Events, the number of bleeding patients; GI, gastrointestinal; LSPH, left-sided portal hypertension; PSAE, partial splenic artery embolization; total, the number of patients enrolled in this group.
Subgroup analysis
Subgroup analyses were performed to determine the efficacy of splenectomy and non-splenectomy strategies containing endoscopic interventions and PSAE (Figure 2). Firstly, the patients undergoing splenectomy had a lower risk of GI bleeding than those undergoing endoscopic interventions (OR: 0.04; 95%CI: 0.01–0.19; p = 0.77; I2 = 0%). Secondly, splenectomy was superior to PSAE in the prevention of GI bleeding (OR: 0.06; 95% CI: 0.01–0.62; p = 0.63; I2 = 0%). Additionally, pancreatic surgery leads to LSPH, and the mechanism underlying LSPH from pancreatic surgery is different from that of LSPH caused by pancreatitis.43,44 Thus, the patients who underwent pancreatic surgery were excluded when a subgroup analysis was performed. The patients in the splenectomy group had a lower rate of GI bleeding than those in the non-splenectomy group (OR: 0.12; 95% CI: 0.04–0.39; p = 0.52; I2 = 0%, Figure 2).
All-cause mortality after treatment
Meta-analysis
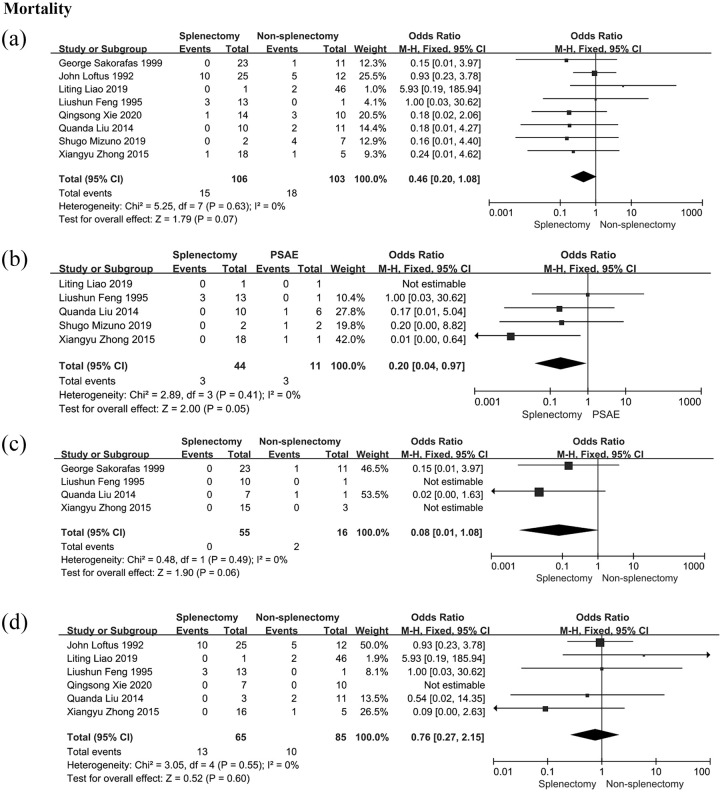
There were 106 patients in the splenectomy group and 103 patients in the non-splenectomy group from eight studies. However, the result revealed no statistical difference in all-cause mortality between the splenectomy group and the non-splenectomy group (OR: 0.46; 95% CI: 0.20–1.08; p = 0.63; I2 = 0%, Figure 3).
Figure 3.
All-cause mortality in patients with LSPH. (a) All-cause mortality in patients receiving splenectomy or non-splenectomy; (b) all-cause mortality in patients receiving splenectomy or PSAE; (c) all-cause mortality in patients receiving splenectomy or non-splenectomy after excluding patients with pancreatic carcinoma; (d) all-cause mortality in patients receiving splenectomy or non-splenectomy after excluding patients who underwent pancreatic surgery.
Events, the number of dead patients; GI bleeding, gastrointestinal bleeding; LSPH, left-sided portal hypertension; PSAE, partial splenic artery embolization; total, the number of patients enrolled in this group.
Subgroup analysis
Subgroup analyses were performed according to the etiology, operative approach, or therapeutic strategy. Firstly, patients with pancreatic carcinoma were excluded due to poor prognosis; then, the mortality was determined in patients who received splenectomy or non-splenectomy. There was no significant difference in mortality between the splenectomy group and the non-splenectomy group (OR: 0.08; 95% CI: 0.01–1.08; p = 0.49; I2 = 0%, Figure 3). Secondly, there was no statistically significant difference in all-cause mortality between the splenectomy group and the non-splenectomy group after removing patients who had received pancreatic surgery (OR: 0.76; 95% CI: 0.27–2.15; p = 0.55; I2 = 0%, Figure 3). Finally, all-cause mortality of patients receiving the splenectomy was lower than that of those receiving PSAE (OR: 0.20; 95% CI: 0.04–0.97; p = 0.41; I2 = 0%, Figure 3).
The efficacy and the safety of preoperative PSAE followed by splenectomy
Incidence of GI bleeding after treatment
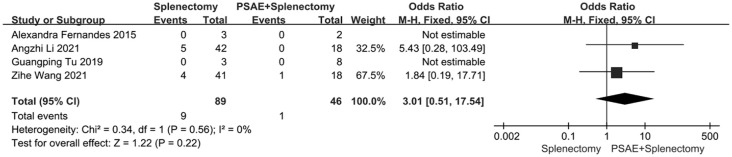
A total of four studies provided evaluable data, with 89 patients who received splenectomy (splenectomy group) and 46 patients who received preoperative PSAE followed by splenectomy (preoperative PSAE group). The findings demonstrated no statistical difference in the incidence of bleeding between the splenectomy group and the preoperative PSAE group (OR: 3.01; 95% CI: 0.51–17.54; p = 0.56; I2 = 0%, Figure 4).
Figure 4.
GI bleeding rate in patients receiving splenectomy or preoperative PSAE followed by splenectomy.
GI bleeding, gastrointestinal bleeding; PSAE, partial splenic artery embolization.
Intraoperative and postoperative outcomes
The intraoperative and postoperative outcomes of three studies were summarized in Table 2.22,27,33 Less intraoperative bleeding was observed in the patients receiving preoperative PSAE followed by splenectomy compared with those receiving splenectomy only. Tu et al. demonstrated the difference in operative time between the two groups was not significant, 33 but other studies showed shorter operative time in the preoperative PSAE group compared with the splenectomy group. Postoperative pancreatic fistula (POPF) was the most common complication of splenectomy, and no statistical difference in the occurrence of POPF was observed between the preoperative PSAE group and the splenectomy group. In Table 3, we provided a summary of the results of meta-analysis.
Table 2.
Intraoperative and postoperative outcomes of patients who received splenectomy or preoperative PSAE followed by splenectomy.
| First author, year | Splenectomy | Preoperative PSAE followed by splenectomy | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Intraoperative blood loss (ml) | Operation time (min) | POPF | Number of patients | Intraoperative blood loss (ml) | Operation time (min) | POPF | Intraoperative blood loss | Operation time | |
| Zhihe Wang, 2021 27 | 41 | 637.0 (416.5–1109.9) | 174.0 (145–212) | 7 | 18 | 420.3 (278.1–620.1) | 141.5 (120–166.25) | 6 | 0.041 | 0.012 |
| Angzhi Li, 2021 22 | 42 | 858.3 | 174.9 | 1 | 18 | 559.2 | 146.8 | 1 | 0.035 | 0.027 |
| Guangping Tu, 2019 33 | 3 | 728.0 | 214.3 | – | 8 | 541.6 | 201.2 | – | – | – |
POPF, Postoperative pancreatic fistula; PSAE, partial splenic artery embolization.
Table 3.
Summary of meta-analysis results.
| Group | No. studies | Pooled OR (95% CI) |
|---|---|---|
| Incidence of GI bleeding after treatment | ||
| Splenectomy versus non-splenectomy | 12 | 0.12 (0.06–0.27) |
| Subgroup | ||
| Splenectomy versus endoscopic interventions | 4 | 0.04 (0.01–0.19) |
| Splenectomy versus PSAE | 5 | 0.06 (0.01–0.62) |
| Splenectomy versus non-splenectomy (pancreatic surgery excluded) | 7 | 0.12 (0.04–0.39) |
| All-cause mortality after treatment | ||
| Splenectomy versus non-splenectomy | 8 | 0.46 (0.20–1.08) |
| Subgroup | ||
| Splenectomy versus PSAE | 5 | 0.20 (0.04–0.97) |
| Splenectomy versus non-splenectomy (pancreatic carcinoma excluded) | 4 | 0.08 (0.01–1.08) |
| Splenectomy versus non-splenectomy (pancreatic surgery excluded) | 6 | 0.76 (0.27–2.15) |
| Splenectomy versus preoperative PSAE followed by splenectomy | ||
| Incidence of GI bleeding after treatment | 4 | 3.01 (0.51–17.54) |
GI bleeding, gastrointestinal bleeding; PSAE, partial splenic artery embolization.
Sensitivity analysis and publication bias
According to the results of Chi-square-based Q-tests and I2 statistics, all meta-analyses showed low heterogeneity and the fixed-effect model was performed to pool ORs and 95% CIs. For the risk of potential heterogeneity, we performed subgroup analyses to eliminate this risk. p Value >0.10 and I2 < 50% were observed in all subgroup analyses, which revealed low heterogeneity. Funnel plots for meta-analysis were roughly symmetrical, showing that there was no obvious publication bias among the studies (Supplemental Figures S1–S3). p > 0.05 of Egger’s test in all meta-analyses and no publication bias was observed (Supplemental Table S4).
Discussion
Our systematic review and meta-analysis evaluated the efficacy of different therapeutic strategies for GI bleeding caused by LSPH. Our meta-analysis demonstrated that splenectomy reduced the incidence of GI bleeding more effectively than other therapeutic strategies containing PSAE and endoscopic interventions. These indicated that splenectomy should be recommended in the management of GI bleeding caused by LSPH. Additionally, the mortality in the splenectomy group was lower than that in the PSAE group. LSPH is caused by the obstruction or embolization of the splenic vein. Then, the blood in the splenic vein reverses into the fundal venous plexus through the short gastric veins, producing isolated fundal varices. 3 Gastric varices is a life-threatening cause of bleeding in the upper GI tract. The management of bleeding gastric varices presents a challenge for patients with LSPH. 45 In theory, the blockage of afferent veins is an effective strategy for gastric varices. Splenectomy removes the entire spleen and cuts off the blood supply from the spleen artery, which reduces blood flow in the splenic vein and the short gastric veins effectively. Endoscopic interventions is recommended in the management of bleeding gastric varices. 46 Although endoscopic interventions are effective in controlling bleeding gastric varices from LSPH, our meta-analysis revealed the incidence of bleeding in patients receiving endoscopic interventions was higher than that in those who received splenectomy. PSAE occludes the artery supply of the spleen peripherally, which results in ischemic necrosis of splenic tissue followed by a decrease in spleen size.11,14 Thus, splenectomy and PSAE prevent bleeding by reducing the blood flow of the afferent vein (the short gastric veins). Because splenectomy reduces blood flow of the short gastric veins more efficiently than PSAE, our meta-analysis showed splenectomy was superior to PSAE in GI bleeding control and mortality improvement.
There was no significant difference in all-cause mortality between the splenectomy group and the non-splenectomy group. Since the patients with pancreatic carcinoma had a poor prognosis, the mortality of enrolled patients was determined after excluding the patients with pancreatic carcinoma. Similarly, statistical results showed no significance in mortality between the splenectomy group and the non-splenectomy group. However, the forest plot showed lower mortality in the splenectomy group compared with the non-splenectomy group. No statistical significance between the two groups was probably attributed to sample size and the quality of the study.
The resection and reconstruction of the portal vein and/or superior mesenteric vein has become a standard procedure when patients with pancreatic head carcinoma and venous invasion receive pancreatoduodenectomy.47,48 The splenic vein has been ligated traditionally during mesenteric-portal venous reconstruction because it simplifies surgical operation and facilitates the removal of tissue. 49 However, this leads to LSPH. 38 Thus, the patients who underwent pancreatic surgery were excluded in our meta-analysis. Splenectomy was effective in preventing GI bleeding in the remaining patients.
PSAE followed by splenectomy was an alternative strategy for GI bleeding caused by LSPH.50–52 Our meta-analysis found no significant difference in reducing the GI bleeding rate between the splenectomy group and the preoperative PSAE group. Additionally, the preoperative PSAE group exhibited less blood loss and shorter operation time; thus, PSAE followed by splenectomy could be performed in high-risk patients for splenectomy. 53
The complications of splenectomy, including hemorrhage, infection, pancreatic injury, portal, and splenic vein thrombosis, are less common in patients with LSPH compared with patients with liver cirrhosis. Meanwhile, some new therapeutic strategies, such as splenic vein stenting and endoscopic ultrasound-guided coil and glue injection for obliteration of splenic artery, have emerged. More studies should be performed to assess their efficacy and safety in future.54,55
Study limitations
There were several limitations existed in our meta-analysis. Firstly, it is difficult to evaluate the efficacy and the safety of therapeutic interventions through randomized controlled trials due to the low incidence of LSPH. Thus, there were 18 studies included in our meta-analysis and only one of them was a prospective study. Secondly, Chinese-language studies were included in this meta-analysis, which revealed further studies should be performed in future. Thirdly, only 624 patients were involved in this meta-study. Fourthly, the follow-up times were variable among different studies, which ranged from 6 to 128 months.
Conclusion
In conclusion, splenectomy is effective in reducing the incidence of GI bleeding caused by LSPH, which revealed that splenectomy should be recommended in the management of these patients.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848241234501 for Splenectomy versus non-splenectomy for gastrointestinal bleeding from left-sided portal hypertension: a systematic review and meta-analysis by Minghui Liu, Ning Wei and Yuhu Song in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iDs: Minghui Liu  https://orcid.org/0000-0002-3220-7925
https://orcid.org/0000-0002-3220-7925
Yuhu Song  https://orcid.org/0000-0003-1952-654X
https://orcid.org/0000-0003-1952-654X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Minghui Liu, Department of Gastroenterology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Ning Wei, Department of Gastroenterology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Yuhu Song, Department of Gastroenterology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Minghui Liu: Data curation; Formal analysis; Resources; Software; Writing – original draft.
Ning Wei: Methodology; Supervision; Validation; Writing – review & editing.
Yuhu Song: Conceptualization; Funding acquisition; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 82070631).
The authors declare that there is no conflict of interest.
Availability of data and materials: All data relevant to the study are either included in the article or uploaded as supporting information.
References
- 1. Pereira P, Peixoto A. Left-sided portal hypertension: a clinical challenge. GE Port J Gastroenterol 2015; 22: 231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans GR, Yellin AE, Weaver FA, et al. Sinistral (left-sided) portal hypertension. Am Surg 1990; 56: 758–763. [PubMed] [Google Scholar]
- 3. Song YH, Xiang HY, Si KK, et al. Difference between type 2 gastroesophageal varices and isolated fundic varices in clinical profiles and portosystemic collaterals. World J Clin Cases 2022; 10: 5620–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koklu S, Coban S, Yuksel O, et al. Left-sided portal hypertension. Dig Dis Sci 2007; 52: 1141–1149. [DOI] [PubMed] [Google Scholar]
- 5. Hakim S, Bortman J, Orosey M, et al. Case report and systematic literature review of a novel etiology of sinistral portal hypertension presenting with UGI bleeding: left gastric artery pseudoaneurysm compressing the splenic vein treated by embolization of the pseudoaneurysm. Medicine 2017; 96: e6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng K, Guo X, Feng J, et al. Gastrointestinal bleeding due to pancreatic disease-related portal hypertension. Gastroenterol Res Pract 2020; 2020: 3825186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ono Y, Inoue Y, Kato T, et al. Sinistral portal hypertension after pancreaticoduodenectomy with splenic vein resection: pathogenesis and its prevention. Cancers 2021; 13: 5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo X, Hernandez-Gea V. Update on the management of gastric varices. Liver Int 2022; 42: 1250–1258. [DOI] [PubMed] [Google Scholar]
- 9. de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII – renewing consensus in portal hypertension. J Hepatol 2022; 76: 959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caletti G, Brocchi E, Baraldini M, et al. Assessment of portal hypertension by endoscopic ultrasonography. Gastrointest Endosc 1990; 36: S21–S27. [DOI] [PubMed] [Google Scholar]
- 11. Grout-Smith H, Dumenci O, Tait P, et al. Splenic artery embolisation for the emergency treatment of sinistral portal hypertension: a systematic review. J Clin Interv Radiol 2021; 5: 79–85. [Google Scholar]
- 12. Patel U, Mingbunjerdsuk T, Gabr M, et al. Advances in management of pancreatitis related portal hypertension. Dig Dis Interv 2022; 6: 108–112. [Google Scholar]
- 13. Brooks DH. Surgery of the spleen. Surg Clin North Am 1975; 55: 287–301. [DOI] [PubMed] [Google Scholar]
- 14. Ou Y, Huang L, Chen Y, et al. Emergency splenic arterial embolization for massive variceal bleeding in liver recipient with left-sided portal hypertension. Liver Transpl 2005; 11: 1136–1139. [DOI] [PubMed] [Google Scholar]
- 15. Ai M, Lu G, Xu J. Application of splenic artery embolization in pancreatic sinistral portal hypertension; preliminary experience in one case. J Interv Radiol 2019; 28: 366–368. [Google Scholar]
- 16. Liu Q, Song Y, Xu X, et al. Management of bleeding gastric varices in patients with sinistral portal hypertension. Dig Dis Sci 2014; 59: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 17. Vogt Y. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011; 39: 91–92. [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Hu P, Chen X, et al. Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized trials. PLoS One Dataset, 2015. [Google Scholar]
- 19. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, https://api.semanticscholar.org/CorpusID:79550924
- 20. Sun J, Freeman B, Natanson C. Meta-analysis of clinical trials. In: Gallin JI, Ognibene FP, Johnson LL. (eds.) Principles and practice of clinical research (4th ed), 2018; 22: 317–327. [Google Scholar]
- 21. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2018; 74: 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li A, Li H, Xiong J, et al. Splenic artery embolization in the treatment of pancreatic sinistral portal hypertension with high risk of bleeding during surgery: a prospective non-randomized study. J Clin Emerg 2021; 22: 169–171. [Google Scholar]
- 23. Fernandes A, Almeida N, Ferreira AM, et al. Left-sided portal hypertension: a sinister entity. GE Port J Gastroenterol 2015; 22: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakorafas GH, Sarr MG, Farley DR, et al. The significance of sinistral portal hypertension complicating chronic pancreatitis. Am J Surg 2000; 179: 129–133. [DOI] [PubMed] [Google Scholar]
- 25. Liu J, Wang Q, Ding X, et al. The clinical applicability of percutaneous splenic vein stent implantation for pancreatic portal hypertension. BMC Gastroenterol 2022; 22: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loftus JP, Nagorney M, Ilstrup D, et al. Sinistral portal hypertension: splenectomy or expectant management. Ann Surg 1993; 217: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Z, Li M, Huang X, et al. Preoperative splenic artery embolism followed by splenectomy is safe and effective in patients with sinistral portal hypertension. Langenbecks Arch Surg 2022; 407: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen G, Wang C, Li S. Diagnosis and treatment of regional portal hypertension: an analysis of 45 cases. Acad J Chin PLA Med Sch 2013; 34: 2095–5227. [Google Scholar]
- 29. Feng L, Zhao L, Liu Q. Segmental portal hypertension caused by splenic vein thrombosis-14 cases report. Chin J Gastroenterol Hepatol 1995; 4: 142–159. [Google Scholar]
- 30. Liao L. Clinical analysis of 47 cases of pancreatic portal hypertension. MS thesis, China Medical University, Liaoning, China, 2019. [Google Scholar]
- 31. Sun B, Kong R, Jiang H. Diagnosis and treatment of pancreatic sinistral portal hypertension:review of literature and a report of 67 cases. Chin J Pract Surg 2006; 26: 684–686. [Google Scholar]
- 32. Tang Y, Ou Z, Ma H. Analysis of 67 cases of segmental portal hypertension. Clin Focus 2008; 23: 193–194. [Google Scholar]
- 33. Tu G, Sun J, Liu Y, et al. Clinical experience of pancreatitis causing regional portal hypertension of pancreatic origin. J Hepatobiliary Surg 2019; 27: 418–422. [Google Scholar]
- 34. Wang K, Yu B, Guan H. The study of the clinical diagnosis and treatment of pancreatic segmental portal hypertension (PSPH). Mod Med J China 2008; 10: 18–19. [Google Scholar]
- 35. Xie Q, Chen Z, Gu H. Diagnosis and treatment of left portal hypertension. J Hepatobiliary Surg 2022; 28: 179–185. [Google Scholar]
- 36. Zhong X, Zhao X, Wen T. Diagnosis and treatment of pancreatic segmental portal hypertension: an analysis of 23 cases. Fu Bu Wai Ke 2015; 28: 381–385. [Google Scholar]
- 37. Song Y, Shen H, Liu Q. The diagnosis and management of upper gastrointestinal bleeding due to pancreat ogenic portal hypertension Chin J Pract 2005; 9: 791–795. [Google Scholar]
- 38. Mizuno S, Kato H, Yamaue H, et al. Left-sided portal hypertension after pancreaticoduodenectomy with resection of the portal vein/superior mesenteric vein confluence in patients with pancreatic cancer: a project study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. Ann Surg 2021; 274: e36–e44. [DOI] [PubMed] [Google Scholar]
- 39. Sakorafas GH, Sarr MG, Farley DR, et al. The significance of sinistral portal. Am J Surg 2000; 179: 129–133. [DOI] [PubMed] [Google Scholar]
- 40. Shi Y, Pan M, Nie S, et al. 238 cases of pancreatogenic portal hypertension. Chin J Gerontol 2015; 35: 5000–5001. [Google Scholar]
- 41. Huang W, Wang L, Liu T, et al. Analysis of 44 cases pancreatis regional portal hypertension. J Clin Intern Med 2006; 23: 1994–2023. [Google Scholar]
- 42. Li N, Zhang Y, Yan Q, et al. Upper gastrointestinal bleeding caused by pancreatic diseases: clinical analysis of 22 cases. Chin J Gastroenterol 2017; 22: 474–477. [Google Scholar]
- 43. Addeo P, Nappo G, Felli E, et al. Management of the splenic vein during a pancreaticoduodenectomy with venous resection for malignancy. Updates Surg 2016; 68: 241–246. [DOI] [PubMed] [Google Scholar]
- 44. Al-Saeedi M, Frank-Moldzio L, Contin P, et al. Splenorenal shunt for reconstruction of the gastric and splenic venous drainage during pancreatoduodenectomy with resection of the portal venous confluence. Langenbecks Arch Surg 2021; 406: 2535–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weber S, Rikkers L. Splenic vein thrombosis and gastrointestinal bleeding in chronic pancreatitis. World J Surg 2003; 27: 1271–1274. [DOI] [PubMed] [Google Scholar]
- 46. Chirapongsathorn S, Manatsathit W, Farrell A, et al. Safety and efficacy of endoscopic cyanoacrylate injection in the management of gastric varices: a systematic review and meta-analysis. JGH Open 2021; 5: 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Javed AA, Bleich K, Bagante F, et al. Pancreaticoduodenectomy with venous resection and reconstruction: current surgical techniques and associated postoperative imaging findings. Abdom Radiol 2018; 43: 1193–1203. [DOI] [PubMed] [Google Scholar]
- 48. Wang WL, Ye S, Yan S, et al. Pancreaticoduodenectomy with portal vein/superior mesenteric vein resection for patients with pancreatic cancer with venous invasion. Hepatobiliary Pancreat Dis Int 2015; 14: 429–435. [DOI] [PubMed] [Google Scholar]
- 49. Petrucciani N, Debs T, Rosso E, et al. Left-sided portal hypertension after pancreatoduodenectomy with resection of the portal/superior mesenteric vein confluence. Results of a systematic review. Surgery 2020; 168: 434–439. [DOI] [PubMed] [Google Scholar]
- 50. Adams DB, Mauterer DJ, Vujic IJ, et al. Preoperative control of splenic artery inflow in patients with splenic venous occlusion. South Med J 1990; 83: 1021–1024. [DOI] [PubMed] [Google Scholar]
- 51. Wu Z, Zhou J, Pankaj P. Comparative treatment and literature review for laparoscopic splenectomy alone versus preoperative splenic artery embolization splenectomy. Surg Endosc 2012; 26: 2758–2766. [DOI] [PubMed] [Google Scholar]
- 52. Patrono D, Benvenga R, Moro F, et al. Left-sided portal hypertension: Successful management by laparoscopic splenectomy following splenic artery embolization. Int J Surg Case Rep 2014; 5: 652–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fujitani RM, Johs SM, Cobb SR, et al. Preoperative splenic artery occlusion as an adjunct for high risk splenectomy. Am Surg 1988; 54: 602–608. [PubMed] [Google Scholar]
- 54. Braden B, Gupta V, Dietrich C. Therapeutic EUS: new tools, new devices, new applications. Endosc Ultrasound 2019; 8: 370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei B, Zhang L, Tong H, et al. Retrospective comparison of clinical outcomes following splenic vein stenting and splenic arterial embolization in sinistral portal hypertension-related gastrointestinal bleeding. Am J Roentgenol 2021; 216: 1579–1587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848241234501 for Splenectomy versus non-splenectomy for gastrointestinal bleeding from left-sided portal hypertension: a systematic review and meta-analysis by Minghui Liu, Ning Wei and Yuhu Song in Therapeutic Advances in Gastroenterology