Abstract
The medicinal potential of Coelogyne suaveolens, a traditional medicinal plant, was investigated through in vivo and molecular docking studies. The ethyl acetate fraction of the plant's acetonic extract was subjected to various bioactivity tests to assess its analgesic, anxiolytic, and sedative effects on Swiss albino mice. Furthermore, we used GCMS to identify the bioactive chemicals in the extract's ethyl acetate fraction. The root and bulb extracts demonstrated significant analgesic activity in acetic acid‐induced writhing, hot plate, and tail immersion tests in a dose‐dependent manner when compared to the control. Again, the extract exhibited moderate anxiolytic activity in the elevated plus maze test at a dosage of 400 mg/kg body weight, while the root extract showed significant anxiolytic activity in the hole board test at the same dosage. Significant sedative activity was observed in the hole cross, open field, and rotarod tests at a dosage of 400 mg/kg. According to molecular docking studies, the extract has the potential to serve as an analgesic medication by reducing the enzymatic activity of cyclooxygenases 1 and 2. Overall, the findings suggest that C. suaveolens has substantial therapeutic potential for the development of novel treatments for pain, anxiety, and sleep disorders.
Keywords: analgesic, anxiolytic, Coelogyne suaveolens, computational work, GCMS, sedative activity
The study explored the medicinal properties of Coelogyne suaveolens through in vivo experiments and molecular docking. The plant extract exhibited analgesic, anxiolytic, and sedative effects in mice with identified bioactive compounds. Molecular docking suggested its potential as an analgesic targeting cyclooxygenases, highlighting its therapeutic potential for pain, anxiety, and sleep disorders.

1. INTRODUCTION
Natural medications have been used for centuries to heal complex ailments without side effects and at affordable prices. Different varieties of naturally occurring chemical compounds in medicinal plants contribute to their therapeutic qualities. With the development of scientific studies researchers have been able to successfully discover many phytochemical components in medicinal plants and accelerate these findings by using in silico studies. These chemicals have been shown to exhibit a variety of physiological actions and are utilized for preventative measures (Sofowora, 1996). Due to a lack of scientific facts, mainstream drugs have made experts distrust the safety of traditional medicine. In the nineteenth century, humans isolated medicinal plant active ingredients and invented quinine using Cinchona bark (Phillipson, 2001). The results of these studies convinced medical researchers to put their faith in alternative medicine and pursue it further. Additionally, it has begun to gain popularity among the public as an alternative to conventional medication because of its higher efficacy, lower risk of health complications, and lower cost. Many powerful conventional medicinal agents, such as vincristine, paclitaxel, and vinblastine (Cragg & Newman, 2005), cardioprotective drugs like digoxin (Morris et al., 2006), narcotic analgesics such as morphine (Rates, 2001), and anti‐malarial drugs such as artemisinin and quinine (Queiroz et al., 2009), were first discovered as a result of the influence of phytochemistry. Studies have been conducted recently on a variety of medicinal plants, including Hedychium spicatum, whose rhizomes are used to treat pain, diarrhea, nausea, liver issues, vomiting, inflammation, headache, stomachaches, and fever, as well as Erigeron bonariensis, which is said to have antioxidant, antibacterial, and anti‐inflammatory properties, and Paederia foetida, which has anti‐diabetic, anti‐hyperlipidemic, antioxidant, nephro‐protective, anti‐inflammatory, antinociceptive, antitussive, thrombolytic, anti‐diarrhoeal, sedative‐anxiolytic, anti‐ulcer, hepatoprotective, and anthelmintic activity (Mahanur et al., 2023; Sarma et al., 2023; Singh et al., 2023). On the other hand, in silico methods, as described in recent research (Hossain et al., 2023; Madden et al., 2020), create predictions about numerous characteristics of chemical compounds, particularly ADME traits, along with their biological function and toxicity, using the current data and molecular composition information. These models are based on the basic concept that a chemical's molecular structure encodes both its inherent characteristics and its potential interactions. As a result, QSAR (quantitative structure–activity relationship) models can be constructed, enabling chemical response predictions based purely on structural data.
Pain is a mental, physical, and unpleasant phenomenon that is acknowledged as a worldwide health issue (Kumar Paliwal et al., 2016). Most pain in the body begins with some sort of illness or injury (Mills et al., 2019), where analgesics, a class of drug, are used to treat pain by blocking different pathways (Van Rensburg & Reuter, 2019). Nonsteroidal anti‐inflammatory medications (NSAIDs) and opioids are the most commonly utilized analgesics nowadays (Rosenblum et al., 2008). NSAIDs typically work by inhibiting the formation of prostaglandins via the blocking of an enzyme called cyclooxygenase (Gunaydin & Bilge, 2018). NSAIDs have been especially helpful in the treatment of inflammation and pain; however, they are well known to be related to gastrointestinal injury. Selective Cyclooxygenase‐2 inhibitors have been shown to reduce the risk of digestive tract issues, but they have also been linked to adverse cardiovascular events (Zarghi & Arfaei, 2011). Serious NSAID‐related problems, including bleeding, perforation, and mortality, occur at a yearly estimated rate of around 2% for NSAID consumers (De Cosmo & Congedo, 2015). On the other hand, opioids are known to promote addiction, physical dependency, and tolerance. When people discontinue opioids after a lengthy period of use, they develop bodily (e.g., muscle cramps, diarrhea, and anxiety) and psychological symptoms of depression (Hijazi et al., 2017). In addition to physical pain, the world is affected by several other serious conditions, such as anxiety disorders, which rank as the most frequent mental illness in the United States (7.3%). Anxiety disorders (especially GAD and panic disorder) frequently overlap with depressive illness, which complicates treatments (Bandelow & Michaelis, 2015). Among different drug options, bupropion's ability to inhibit norepinephrine and dopamine reuptake makes it a potent antidepressant with fewer side effects (Friesner et al., 2004). Drugs like MAOIs and tricyclic antidepressants (TCAs) are commonly used in therapy because of their ability to inhibit the increase of monoamine metabolism and decrease reuptake capability. Many of the standard treatments for depression, however, come with serious drawbacks. Therefore, new antidepressant medicines are necessary to avoid these consequences (Nasrin et al., 2022).
Orchids are renowned for their aesthetic appeal, and they are frequently utilized as decorative items in households, offices, and public spaces. While most individuals appreciate their beauty, others have discovered practical applications for them. For a long time, people from various regions of the world have employed orchids for medicinal purposes. However, the use of orchids in medicine has decreased over time due to insufficient research to determine their efficacy and adverse effects. The Chinese were the first to discover the medicinal properties of orchids, and they continue to use them for medicinal purposes today, mainly in the form of medicinal tea. Dendrobium, in particular, is believed to have several medicinal properties, including antioxidant (Luo et al., 2016), hypoglycemic (Pan et al., 2014), immune modulatory (He et al., 2016), cardioprotective (Dou et al., 2016), hepatoprotective, and antitumor (Tang et al., 2017). To advocate for the traditional functions of orchids, Coelogyne suaveolens, a member of the family Orchidaceae native to central China, Assam, the eastern Himalayas, and Thailand, has been selected. The central question addressed by this research is whether C. suaveolens, a traditional medicinal plant, possesses therapeutic properties that can be scientifically validated. In comparison to previously published literature on medicinal orchids, this research offers a unique contribution by focusing on C. suaveolens, which has received limited attention in prior studies. While orchids, especially Dendrobium species, have been investigated for their medicinal properties, including antioxidant, hypoglycemic, immune modulatory, cardioprotective, hepatoprotective, and antitumor effects, this study distinguishes itself by delving into the potential therapeutic benefits of C. suaveolens.
As no prior research exists on the analgesic, anxiolytic, sedative activities of this orchid, the purpose of this research is to assess its bioactive phytochemicals and their pharmacological actions. This research addresses a specific research gap in the field of medicinal plant‐based therapies by providing empirical evidence for the therapeutic potential of C. suaveolens. It fills the gap between traditional medicinal knowledge and modern scientific validation, demonstrating its effectiveness in pain relief, anxiety reduction, and sleep disorders, paving the way for the development of novel treatments in these areas.
2. MATERIALS AND METHODS
2.1. Chemicals
Aceclofenac, diazepam, and morphine were provided by United Chemicals & Pharmaceuticals Limited, Chittagong, Bangladesh. The Department of Pharmacy, Faculty of Biological Science, University of Chittagong provided other analytical‐grade reagents.
2.2. Collection and identification of the plant
In 2021, with the help of a well‐known local traditional healer, the bulbs and roots of the matured plant were collected, which was newly identified in Bangladesh (Huda et al., 2021). Then it was approved by a noted taxonomist, Dr. KamrulHuda and Assistant horticulturist Mr. Md. Owahidul Alam from department of Botany, University of Chittagong under herbarium no. Dpcu/2021/011.
2.3. Crude extract preparation
The study materials (bulb and root) were washed, sliced into tiny pieces, and dried in the sunlight for seven days in a semi‐shed. The plant components were processed into a powder using a mechanical grinder after drying. The bulb and root of the plant were powdered and immersed in acetone. After 13 days of intermittent stirring, the filtrate was concentrated after the solution was filtered using a rotary evaporator by evaporation under reduced pressure and below 50°C (Stu‐art, UK).
2.4. Solvent–solvent partitioning
Using ethyl acetate solvent, crude acetone extracts of the bulb and root of C. suaveolens underwent solvent–solvent partitioning using the technique developed by VanWagenen et al. (1993).
2.5. GC MS analysis
A mass spectrometer from Agilent Technologies (Santa Clara, CA, USA) was used to analyze the chemical constituents of C. suaveolens extract using a 7890A capillary gas chromatography system. For the analysis, approximately 6 μL of crude extract (1% w/v) was diluted with methanol:chloroform:water (2.5:1:1) and injected into a fused silica capillary column (HP‐5MSI, 90 m × 0.25 mm) coated with a 0.25 μm film consisting of 95% dimethyl‐poly‐siloxane and 5% phenyl. Helium (99.999%) was used as the carrier gas, flowing at a rate of 1 mL/min. The mass chromatogram was analyzed with the MS quad at 150°C and the source temperatures at 250°C, respectively. The National Institute of Standards and Technology (NIST) mass spectrometry data center serves as a reference for identifying the chemical components of the extracts by comparing their MS spectra to the NIST database (Al‐Nuri et al., 2022).
2.6. Experimental animals
We acquired Swiss albino mice from the BCSIR laboratory in Chittagong for our experiment. These mice were of both sexes, aged 4–5 weeks, and had a weight ranging from 20 to 25 grams. These mice were housed in clean, dry polypropylene cages within the Animal House of the Department of Pharmacy at the University of Chittagong. They were subjected to a 12‐hour light–dark cycle at a temperature of 25 ± 2°C and a relative humidity level ranging from 45% to 55%. Throughout the duration of the investigation, the mice were provided with a nutritionally adequate diet and a free water supply. Twelve hours prior to and throughout the duration of the trial, food was not given. The Departmental Ethical Review Committee of the University of Chittagong's Department of Pharmacy granted approval for the clinical animal experiment under consent number Pharm/CUDP‐16, 2022:08. At the completion of each experiment, all mice were sacrificed under diethyl ether anesthesia.
2.7. Study design for in vivo testing
During each evaluation, six groups of mice, with five mice in each group, were used for each investigation. For analgesic activity, Group (I) served as the control (1% tween‐80 10 mL/kg), Group (II) served as the standard (Diclofenac sodium 50 mg/kg was used in the acetic acid writhing study, and morphine sulfate 10 mg/kg was used for tail immersion and the hot plate method), and Groups (III) and (IV) received bulb extract at 200 and 400 mg/kg, respectively, and Groups (V) and (VI) received root extract at 200 and 400 mg/kg, respectively. For anxiolytic and sedative activity, Group (I) served as the control (1% tween‐80 10 mL/kg), Group (II) served as the standard (Diazepam 1 mg/kg was used in both the anxiolytic and sedative tests), and Groups (III)–(VI) received the bulb and root extract at doses of 200 and 400 mg/kg, like in the previous manner.
2.8. Acute toxicity study
The previously outlined approach was used to conduct an acute toxicity investigation. Each group consists of five mice that overnight fasted before receiving the extract. Each animal group received oral dosages of 1000, 2000, 3000, and 4000 mg/kg of body weight for each extract. After receiving the plant extract, they were not given food for an additional 3–4 h. Each animal was observed for thirty minutes, twenty‐four hours, and then three days. At least once per day, the mice were examined for any changes in their epidermis, fur, mucous membrane, eyes, respiration rate, circulation rate, and central and autonomic nervous systems. One‐tenth of the median lethal dose would be the effective dose (LD50) (Afrin et al., 2021).
2.9. Investigation of analgesic activity
2.9.1. Acetic acid‐induced writhing test
The acetic acid‐induced writhing test was a behavioral observation and measurement approach that revealed painful stimulation in mice. This research was conducted using the approach developed by Koster, with some adjustments made by Dambisya and Lee (Dambisya et al., 1999; Koster et al., 1959). After giving the standard and extract to the mice, 0.7% glacial acetic acid (10 mL/kg) was administered intraperitoneally (IP) 15 min after giving the standard and 30 min after giving the extract. This caused pain that showed up as constrictions or writhing of the abdomen. 5 min later, each mouse in each group was carefully watched for 20 min to count how many times it had writhed. Diethyl ether was used to euthanize the treated mice after each observation. The percent inhibition of abdominal writhing was used to measure the level of analgesia. This was done with the following formula:
where Nc denotes the number of writhings in the control group and Nt denotes the number of writhing's in the treatment group.
2.9.2. Tail immersion method
The methodology created by Di Stasi et al. was used in this study. Using this thermal approach, the central analgesic activity of the extracts under investigation was assessed (di Stasi et al., 1988). Before the 30 min of treatment, about 2–3 cm of each mouse's tail was put into a water bath with warm water kept at 50 ± 1°C, and the time it needed for the mouse to pull its tail out of the warm water was marked down. The animals that flicked their tails within 3–5 s were chosen for this research. To keep the tail from getting hurt, a cut‐off time of 15 s was chosen. After the initial test, the mice that were given the drug were checked at 30, 60, 90, and 120 min (Malairajan et al., 2006). Wrapping the animals carefully immobilized them so that measurements could be taken. The following formula was used to determine what proportion of the maximum possible effect (% MPE) was achieved (Fan et al., 2014):
The percentage of time that the tail immersion elongated compared to the control time was measured. The central analgesic effect will be stronger if the percentage of elongation period is higher.
The following equation was used to get the percentage of time elongation (Kumawat et al., 2012):
2.9.3. Hot plate method
In this investigation, a commonly accessible Eddy's hot plate with an electrical heating surface maintained at 55–56°C was used. The methodology by Arunkumar et al was followed with a slight modification (Arunkumar et al., 2019). Animals in each group were subjected to the hot plate individually; reaction times were recorded as the number of seconds it took for the animals to lick their forepaws or jump in response; and the reaction strengths of individual mice were measured at 30, 60, and 120 min intervals before and after drug treatment. To prevent injury to the paws, a 15‐second cutoff time was instituted. The following formula was used to detect the % increase in reaction time:
2.10. Anxiolytic profiling
2.10.1. Elevated plus maze method
The elevated plus maze is a crucial tool for studying the neuroprotective and anxiolytic effects of test medications (Sunanda et al., 2014). Since rodent's fear heights and prefer confined areas, they spend a disproportionate amount of time there. When animals enter an open‐arms environment, they often get frightened and are unable to move (Pellow et al., 1985). The main benefits of this test approach are (a) its speed, simplicity, and reduced testing time; (b) the absence of the need for unpleasant stimuli or training; and (c) its predictability and reliability in assessing the anti‐anxiety and anti‐anxiety medication properties (Emamghoreishi et al., 2005; Vogel et al., 2010). The elevated plus maze is a plus‐shaped tool 40 cm above the ground, featuring two open arms (25 × 5 cm) and two closed arms (25 × 5 cm, 16 cm long) perpendicular to each other [Miyakawa et al., 1996]. Each test subject was positioned in the center of the maze, oriented toward an open arm, and the stopwatch was initiated. Over a 5‐minute period, several factors were observed. Initially, the mice exhibited a preference for either the open or closed arms. The count of entries into both open and closed arms was noted, with an entry defined as all four paws of the mouse being within an arm. Afterwards, the animals received various substances, including a control group receiving saline, a standard group administered with diazepam, and multiple test samples of C. suaveolens. After a 30‐minute interval from treatment, each animal was placed back in the maze's center. For a final 5‐minute period, the duration that each animal spent in both the open and closed arms was documented.
2.10.2. Hole‐board test
Hole‐board equipment is an installation with holes in the floor that an animal can insert its head into. This is called “head‐dipping.” (File & Wardill, 1975). The length and regularity of head dipping can be used to measure neophilia, which means “directed exploration.” This shows that the animal's ability to move around is independent (Ljungberg & Ungerstedt, 1976). An increased level of head dipping is usually a sign of neophilia, while low levels are often caused by a lack of neophilia or show that the animals get more anxious (Crawley, 1985). Hence, the anxiety level rises as head‐dipping is reduced and lowers when the opposite occurs (Kliethermes & Crabbe, 2006). Mice were placed individually on the hole board device for 30 min before being administered with control, standard, and test samples. We then used a tally counter to measure the number of times each mouse dipped its head into a hole at eye level over a 5‐minute trial session.
2.11. Sedative profiling
2.11.1. Open‐field method
The equipment used for this test had a floor space of roughly 0.5 m2 and was enclosed by a wall that was 50 cm in height (Gupta et al., 1971). The ground is covered in a pattern of tiny squares that alternate between being white and black in color. The count of squares explored by the mice was documented at intervals of 0, 30, 60, 90, and 120 min post‐oral treatment. This involved the control group (saline), the standard group (diazepam), and varying doses of test samples (200 and 400 mg/kg). A tally counter was used to track the mice's movements within a 3‐minute timeframe.
2.12. Hole cross method
This test was conducted in a wooden‐walled chamber (30 cm × 20 cm × 14 cm) without a ceiling. In the middle of the room, a fixed wooden frame divides it into two parts. The wooden barrier had a round hole that was 3.5 cm in diameter and 7.5 cm high. At 0, 30, 90, and 120 min, the number of times the mice passed through the opening between the two chambers was recorded using a tally counter for a period of 3 min (Hossen et al., 2022).
2.13. Rotarod method
A rotarod device was used to evaluate the impact on motor coordination; it featured a plant‐shaped base and a 3 cm diameter by 30 cm long iron rod that had a non‐slippery surface. The rod had four disks, dividing it into five equal parts. In a training lesson 24 h before the test, the animals were selected based on their ability to stay on the bar (at 12 rpm) for 2 min. Then, five mice were put on the rod and walked at 12 rpm at the same time, and this was watched for 30, 60, and 90 min. The performance time was automatically recorded as the time between when the animal was put on the spinning bar and when it fell off. The amount of time the animals spent in the machine was tracked for 5 min (300 s) (Doukkali et al., 2015).
2.14. In silico study
2.14.1. Ligand preparation
Seven major compounds were chosen from the GC–MS profiling Tables 2a and 2b for a molecular docking analysis to further assess analgesic efficacy. These included alpha‐linolenic acid, {1,6‐Octadien‐3‐ol, 3,7‐dimethyl‐}, isopulegol, geranyl acetate, {bicyclo[3.1.1]heptan‐3‐one, 2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐}, {3,6‐dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran}, and {9,12‐octadecadienoic acid (Z,Z)‐}. All of the compounds were obtained in SDF format from the PubChem database. Then, using Open Babel (version 2.3.1), the structures were exported to the pdb format. AutoDock Tools (version 1.5.6)'s ligand preparation module was used to convert these pdb files into pdbqt format.
TABLE 2a.
Compounds identified by GCMS analysis in the bulb of Coelogyne suaveolens.
| SL No | Compound name | Molecular formula | Molecular weight (g/mol) | RT (min) | Concentration (%) |
|---|---|---|---|---|---|
| 1 | Naphthalene, decahydro‐1‐pentadecyl‐ | C25H48 | 348.6 | 25.978 | 16.634 |
| 2 | (2,6,6‐Trimethylcyclohex‐1‐enylmethanesulfonyl)benzene | C16H22O2S | 278.4 | 25.186 | 35.540 |
| 3 | 4,4′‐Dimethoxy‐2,2′‐dimethylbiphenyl | C16H18O2 | 242.31 | 26.861 | 29.340 |
| 4 | 2‐Methyltetracosane | C25H52 | 352.7 | 27.555 | 9.793 |
| 5 | 7‐Hexadecenal, (Z)‐ | C16H30O | 238.41 | 29.381 | 4.847 |
| 6 | .alpha.‐Linolenic acid, TMS derivative | C21H38O2Si | 350.6 | 36.110 | 0.833 |
| 7 | Undec‐10‐ynoic acid, decyl ester | C21H38O2 | 322.5 | 14.017 | 0.169 |
| 8 | [1,1′‐Bicyclopropyl]‐2‐octanoic acid, 2′‐hexyl‐, methyl ester | C21H38O2 | 322.5 | 14.833 | 0.445 |
| 9 | 9,12‐Tetradecadien‐1‐ol, acetate, (Z,E)‐ | C16H28O2 | 252.39 | 15.246 | 0.207 |
| 10 | E,E,Z‐1,3,12‐Nonadecatriene‐5,14‐diol | C19H34O2 | 294.5 | 16.697 | 0.210 |
| 11 | (R)‐(−)‐14‐Methyl‐8‐hexadecyn‐1‐ol | C17H32O | 252.4 | 19.100 | 0.297 |
| 12 | 2‐Octylcyclopropene‐1‐heptanol | C18H34O | 266.5 | 20.520 | 0.096 |
| 13 | Ethanol, 2‐(9,12‐octadecadienyloxy)‐, (Z,Z)‐ | C20H38O2 | 310.5 | 20.941 | 0.259 |
| 14 | 2‐(4‐Hydroxybutyl)cyclohexanol | C10H20O2 | 172.26 | 22.132 | 0.138 |
| 15 | 7‐Hexadecyn‐1‐ol | C16H30O | 238.41 | 22.475 | 0.148 |
| 16 | (2R,3R,4aR,5S,8aS)‐2‐Hydroxy‐4a,5‐dimethyl‐3‐(prop‐1‐en‐2‐yl)octahydronaphthalen‐1(2H)‐one | C15H24O2 | 236.35 | 34.891 | 0.556 |
TABLE 2b.
Compounds identified by GCMS analysis in the root of Coelogyne suaveolens.
| SL No | Compound name | Molecular formula | Molecular weight (g/mol) | RT (min) | Concentration (%) |
|---|---|---|---|---|---|
| 1 | 1,6‐Octadien‐3‐ol, 3,7‐dimethyl‐ | C10H18O | 154.25 | 9.537 | 0.477 |
| 2 | Geranyl Acetate | C12H20O2 | 196.29 | 13.812 | 0.492 |
| 3 | 7‐Tetradecene | C14H28 | 196.37 | 14.027 | 0.204 |
| 4 | 1‐Eicosanol | C20H42O | 298.5 | 16.705 | 0.294 |
| 5 | Bicyclo[3.1.1]heptan‐3‐one, 2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐ | C10H16O | 152.23 | 17.702 | 0.882 |
| 6 | 7‐Hexadecenal, (Z)‐ | C16H30O | 238.41 | 19.103 | 0.349 |
| 7 | 9,12‐Octadecadienoic acid (Z,Z)‐ | C18H32O2 | 280.4 | 22.359 | 0.381 |
| 8 | Isopulegol | C10H18O | 154.25 | 22.988 | 0.180 |
| 9 | 3,6‐Dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran | C10H16O | 152.23 | 25.171 | 23.096 |
| 10 | Androstan‐17‐one, oxime, (5.alpha.)‐ | C19H31NO | 289.5 | 25.969 | 8.130 |
| 11 | 4‐Biphenyltrimethylsiloxane | C15H18OSi | 242.39 | 26.849 | 41.098 |
| 12 | 5.beta.‐Androstan‐17‐one, 3.alpha.‐(trimethylsiloxy)‐, O‐methyloxime | C23H41NO2Si | 391.7 | 27.493 | 7.633 |
| 13 | Cyclobarbital | C12H16N2O3 | 236.27 | 28.838 | 0.385 |
| 14 | Diethofencarb | C14H21NO4 | 267.32 | 30.278 | 7.903 |
| 15 | Tetraconazole | C13H11Cl2F4N3O | 372.14 | 29.309 | 0.046 |
| 16 | 3‐Chloropropionic acid, heptadecyl ester | C20H39ClO2 | 347 | 19.103 | 0.349 |
2.14.2. Protein preparation
Three‐dimensional structures of cyclooxygenase‐1 (PDB ID: 5wbe) (Cingolani et al., 2017) and human cyclooxygenase‐2 (PDB ID: 5ikq) (Orlando & Malkowski, 2016) were downloaded in pdb format from the protein data bank (Berman et al., 2000) for the assessment of analgesic activity. Discovery Studio 2020 was used to make both of the protein structures ready by eliminating water molecules and complex co‐structures. The proteins subsequently went through an energy minimization procedure utilizing the steepest descent and conjugate gradient methods. Energy calculations were done in vacuo using the GROMOS 96 43B1 parameters with the implementation of Swiss‐PDB Viewer (Version 4.1.0). The pdb format was transformed to the pdbqt format employing AutoDock Tools (version 1.5.6), and the final macromolecules have been conserved in this format.
2.14.3. Molecular docking simulation
Molecular docking analyses were carried out via AutodockVina (version 1.1.2) to determine how the compounds selected might work to prevent analgesia by blocking COX‐1 and COX‐2 enzymes. AutodockVina's grid box was maintained at 66.9216, 75.6914, and 64.8721 angstroms (X, Y, and Z) for the cyclooxygenase‐1 and 77.1175, 62.5380, and 57.5579 angstroms (X, Y, and Z) for the human cyclooxygenase‐2. The shell script given by the AutoDockVina developers was used to implement AutodockVina, and the binding affinity of the ligands was measured in terms of kcal/mol (Kumar et al., 2023 Trott & Olson, 2009).
3. STATISTICAL ANALYSIS
The data were presented in the form of a mean ± SEM (standard error of mean). “Statistical Package for the Social Sciences” (SPSS, Version 16.0, IBM Corporation, New York) was used for statistical analysis, and a one‐way ANOVA followed by a post hoc Dunnett's test for comparisons was performed. Significance levels were determined as follows: *p < .05, **p < .01, and ***p < .001, indicating statistical significance when compared to the control group.
4. RESULTS
4.1. Acute toxicity study
There was no indication of any negative consequences in the mice, including reduced motor function, agitation, seizures, diarrhea, coma, or lacrimation, at any of the doses tested. At the concentrations utilized in the tests, no mice died. As a result, the LD50 was confirmed to be greater than 4000 mg/kg.
4.2. Phytochemical investigation
4.2.1. Qualitative screening
This study was conducted to verify the presence or absence of preliminary phytochemicals. A variety of phytochemicals, including alkaloids, flavonoids, saponins, tannins, reducing sugars, phenolic compounds, proteins, amino acids, phytosterols, and terpenoids were detected in the C. suaveolens bulb and root extracts upon phytochemical screening, as demonstrated in Table 1.
TABLE 1.
Phytochemical analysis of the ethyl acetate fraction of C. suaveolens bulb and root extracts.
| Phytochemicals | Bulb | Root |
|---|---|---|
| Alkaloids | − | − |
| Flavonoids | + | + |
| Saponins | + | + |
| Tannins | + | + |
| Phenolic compounds | − | + |
| Glycosides | + | + |
| Carbohydrates | − | − |
| Reducing sugar | − | − |
| Protein and amino acid | − | + |
| Phytosterol | + | + |
| Terpenoids | + | − |
4.3. GC–MS profiling
The phytoconstituent‐rich ethyl acetate fraction of the acetonic extract of C. suaveolens bulb and root was subjected to GC–MS analysis (gas chromatography–mass spectrometry). A total of 16 compounds were identified from the bulb extract, and an additional 16 compounds were found in the root extract, each exhibiting diverse phytochemical activities. Figures 1 and 2 show the chromatogram, and Tables 2a and 2b list the chemical components and their molecular formula, molecular weight (MW), retention time (RT), and concentration (%).
FIGURE 1.

Coelogyne suaveolens ethyl acetate fraction's GC–MS chromatogram (Bulb).
FIGURE 2.

Coelogyne suaveolens ethyl acetate fraction's GC–MS chromatogram (Root).
4.4. In vivo analgesic activity
4.4.1. Acetic acid‐induced writhing method
In this procedure, the examined extracts administered at 200 and 400 mg per body weight exhibited a reduction in writhing occurrences among rodents when compared to the control group. Notably, the bulb and root extract at the 400 mg/kg dose displayed a marked reduction in writhing incidents (indicated by a significant p‐value of less than .001). This substantial decrease in writhing pointed to their enhanced efficacy as peripheral analgesic agents, as detailed in Table 3. Additionally, when comparing the effects of bulb and root extracts, the bulb extract displayed the highest percentage of writhing inhibition at both the 200 and 400 mg/kg doses, surpassing the performance of the root extract.
TABLE 3.
Evaluation of the analgesic effect of Coelogyne suaveolens bulb and root extracts by the acetic acid‐induced writhing method.
| Treatment | Writhing count | Mean ± SEM | % Inhibition | ||||
|---|---|---|---|---|---|---|---|
| M‐1 | M‐2 | M‐3 | M‐4 | M‐5 | |||
| Control | 57 | 32 | 45 | 34 | 56 | 45 ± 5.15 | 0 |
| Standard | 6 | 6 | 7 | 3 | 5 | 5.4 ± 0.68*** | 88 |
| Bulb 200 | 32 | 16 | 18 | 33 | 33 | 26.4 ± 3.85** | 41.33 |
| Bulb 400 | 10 | 13 | 10 | 14 | 8 | 11 ± 1.09*** | 75.56 |
| Root 200 | 21 | 33 | 40 | 28 | 44 | 33.2 ± 4.12 | 26.22 |
| Root 400 | 20 | 16 | 12 | 10 | 22 | 16 ± 2.28*** | 64.44 |
Note: All values are Mean ± SEM and statistically analyzed using One‐Way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison test, n = 5. *p < .05, **p < .01, ***p < .001 when compared with control.
4.4.2. Tail immersion method
The extracts administered at 200 and 400 mg/kg body weight demonstrated varying degrees of elevation in pain reaction time (PRT), percentage elongation of latency, and percentage of maximal possible effect (%MPE) in comparison to the control group, with the effect becoming more pronounced with increasing doses. Conversely, the standard substance, morphine sulfate (10 mg/kg), markedly intensified these measures. The anti‐nociceptive effect of the ethyl acetate fraction of the bulb of C. suaveolens at the 400 mg/kg dose level was found to be significant (p < .001) with 32.13% elongation of reaction time at 30 min and p < .01 with 23.03% elongation of reaction time at 60 min in the tail immersion model. Again, a significant result (p < .001) with 27.22% elongation of reaction time at 60 min is shown by the root of C. suaveolens at the 400 mg/kg dose level in the tail immersion model. This suggests that the extracts have central analgesic activity, which may be due to their effects on the spinal cord or brain. The delayed onset of action of the root extract compared to the bulb extract may be due to differences in the active compounds or their pharmacokinetics. Table 4a displays the findings for PRT and %MPE, while Table 4b exhibits the percentages of elongation of latency.
TABLE 4a.
Effect of Coelogyne suaveolens bulb and root extract on reaction time and %MPE.
| Test samples | Dose (mg/kg) | Pretreatment | Reaction times in seconds (mean ± SEM) and %MPE | |||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | |||
| Control | 10 mL/kg | 2.42 ± 0.07 | 2.45 ± 0.05 (0.24%) | 2.54 ± 0.07 (0.95%) | 2.48 ± 0.44 (0.48%) | 2.44 ± 0.03 (0.16%) |
| Standard | 50 | 2.55 ± 0.13 | 6.32 ± 0.07*** (30.28%) | 8.22 ± 0.11*** (45.54%) | 8.03 ± 0.07*** (44.01%) | 7.82 ± 0.16*** (42.33%) |
| Bulb | 200 | 2.46 ± 0.03 | 2.52 ± 0.04 (0.48%) | 2.59 ± 0.01 (1.04%) | 2.50 ± 0.07 (0.32%) | 2.46 ± 0.06 (0) |
| Bulb | 400 | 2.43 ± 0.01 | 3.61 ± 0.12*** (9.39%) | 3.30 ± 0.10** (6.92%) | 2.48 ± 0.24 (0.40%) | 2.45 ± 0.21 (0.16%) |
| Root | 200 | 2.43 ± 0.14 | 2.50 ± 0.10 (0.56%) | 2.56 ± 0.10 (1.03%) | 2.49 ± 0.13 (0.48%) | 2.45 ± 0.12 (0.16%) |
| Root | 400 | 2.38 ± 0.01 | 2.48 ± 0.11 (0.79%) | 3.49 ± 0.11*** (8.80%) | 2.91 ± 0.35 (4.20%) | 2.51 ± 0.08 (1.03%) |
Note: All values are Mean ± SEM and statistically analyzed using One‐Way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison test, n = 5. *p < .05, **p < .01, ***p < .001 when compared with control.
TABLE 4b.
Effect of Coelogyne suaveolens bulb and root extract on %elongation of latency time.
| Test samples | Dose (mg/kg) | %elongation of latency time | |||
|---|---|---|---|---|---|
| 30 min (%) | 60 min (%) | 90 min (%) | 120 min (%) | ||
| Standard | 50 | 61.23 | 69.10 | 69.12 | 68.80 |
| Bulb | 200 | 2.78 | 1.93 | 0.80 | 0.81 |
| Bulb | 400 | 32.13 | 23.03 | 0 | 0.41 |
| Root | 200 | 2.00 | 0.78 | 0.40 | 0.41 |
| Root | 400 | 1.21 | 27.22 | 14.78 | 2.79 |
4.4.3. Hot plate method
C. suaveolens bulb and root extract at doses of 200 mg/kg and 400 mg/kg showed analgesic activity by the hot plate method. The bulb extract at 400 mg/kg showed significant activity (p < .01) at 30 min and (p < .001) at 60 min when compared to the control. Additionally, root extract at 400 mg/kg showed significant analgesic activity (p < .05) at 60 min. Among the two doses of C. suaveolens, the 200 mg/kg dose failed to show statistically significant analgesic activity when compared with the control. Furthermore, bulb extract at a dose of 400 mg/kg showed a 20.32%, 27.39%, and 2.56% increase in reaction time at 30, 60, and 90 min, respectively, whereas for root extract, the values are 10.92%, 9.96%, and −2.32%, which are demonstrated in Table 5.
TABLE 5.
Changes in reaction time produced by Coelogyne suaveolens using the hot plate method.
| Groups | Before treatment | After treatment | % increase in reaction time | ||||
|---|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 30 min | 60 min | 90 min | ||
| Control | 4.168 ± 0.499 | 4.165 ± 0.053 | 4.155 ± 0.043 | 4.14 ± 0.046 | – | – | – |
| Standard | 4.21 ± 0.057 | 6.943 ± 0.32*** | 7.29 ± 0.031*** | 7.0 ± 0.045*** | 64.917 | 73.159 | 66.271 |
| Bulb200 | 4.17 ± 0.051 | 4.156 ± 0.052 | 4.20 ± 0.023 | 3.998 ± 0.088 | −0.336 | 0.719 | −4.125 |
| Bulb400 | 4.168 ± 0.048 | 5.015 ± 0.0096** | 5.31 ± 0.015*** | 4.275 ± 0.2604 | 20.321 | 27.399 | 2.567 |
| Root200 | 4.185 ± 0.023 | 4.103 ± 0.0665 | 4.123 ± 0.036 | 4.088 ± 0.046 | −1.959 | −1.481 | −2.318 |
| Root400 | 4.165 ± 0.44 | 4.62 ± 0.091 | 4.58 ± 0.206* | 4.068 ± 0.122 | 10.924 | 9.964 | −2.329 |
Note: All values are Mean ± SEM and statistically analyzed using One‐Way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison test, n = 5. *p < .05, **p < .01, ***p < .001 when compared with control.
4.5. Anxiolytic profiling
4.5.1. Elevated plus maze method
EPM or elevated plus maze method is a well‐established test for evaluating the anxiolytic activity of compounds; it measures exploratory behavior in an anxiety‐provoking situation and is used to assess psychomotor capabilities and emotional aspects in rodents. This study examined the anxiolytic activity of the ethyl acetate fraction of the acetonic extract of C. suaveolens bulb and root. The results in Table 6 showed that the ethyl acetate fraction of bulb and root at a dose of 400 mg/kg body weight spent 5.00 and 5.20 s, respectively, in the open arm of the EPM apparatus, whereas in the standard it was 57.72 s, but in the control group it was 0.50 s. Again, it spent more time in the closed arm (279 and 268.8 s) in comparison to the standard (214 s). The other two doses (200 mg/kg) of bulb and root did not show any significant results. Moreover, all the extracts spent more time in the closed arm and less time in the open arm of the EPM apparatus in comparison to the standard, but in comparison to the control, they spent more time in the open arm and less time in the closed arm. From the observed result, it could be concluded that bulb 400 and root 400 have moderate anxiolytic activity.
TABLE 6.
Evaluation of anxiolytic activity of Coelogyne suaveolens bulb and root by using the Elevated Plus Maze Method.
| Test samples | Dose (mg/kg) | Open arm (OA) | Closed arm (CA) | ||
|---|---|---|---|---|---|
| Time spent (sec) | No. of entries | Time spent (sec) | No. of entries | ||
| Control | 10 mL/kg | 0.50 ± 0.32 | 0.80 ± 0.37 | 296 ± 1.87 | 14.60 ± 1.44 |
| Standard | 1 | 52.72 ± 0.86*** | 8.60 ± 0.51*** | 214 ± 1.08*** | 2.80 ± 0.37*** |
| Bulb | 200 | 2.40 ± 1.29 | 0.80 ± 0.37 | 294 ± 2.08 | 10.20 ± 1.20* |
| Bulb | 400 | 5.00 ± 2.07* | 1.00 ± 0.32 | 279 ± 2.41*** | 8.00 ± 0.84*** |
| Root | 200 | 2.80 ± 1.16 | 1.00 ± 0.32 | 292 ± 2.43 | 7.20 ± 0.86*** |
| Root | 400 | 5.20 ± 0.84* | 2.40 ± 0.25 | 268.8 ± 1.88*** | 5.40 ± 0.51*** |
Note: All values are Mean ± SEM and statistically analyzed using One‐Way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison test, n = 5. *p < .05, **p < .01, ***p < .001 when compared with control.
4.5.2. Hole‐board test
In the hole board test, it has been observed that the ethyl acetate fraction of the root extract of C. suaveolens at doses of 400 mg/kg showed highly significant (p < .001) anxiolytic activity (41.40 head dipping) compared to the control. On the other hand, the number of heads dipped in the positive control group was 42.20, as depicted in Table 7. The other doses of the extract did not show any significant change compared to the control. This indicates that the root extract of C. suaveolens at doses of 400 mg/kg may have a more selective anxiolytic effect on certain aspects of behavior.
TABLE 7.
Effect of Coelogyne suaveolens bulb and root extract on anxiolytic profiling using the Hole‐board test method.
| Group | Dose (mg/kg) | Frequency of head‐dipping | Mean ± SEM | ||||
|---|---|---|---|---|---|---|---|
| M‐1 | M‐2 | M‐3 | M‐4 | M‐5 | |||
| Control | 10 mL/kg | 32 | 23 | 33 | 25 | 20 | 26.60 ± 2.54 |
| Standard | 1 | 45 | 39 | 46 | 40 | 41 | 42.20 ± 1.39*** |
| Bulb | 200 | 27 | 32 | 19 | 25 | 27 | 26.00 ± 2.10 |
| Bulb | 400 | 37 | 24 | 38 | 27 | 28 | 30.80 ± 2.82 |
| Root | 200 | 28 | 39 | 24 | 34 | 28 | 30.60 ± 2.68 |
| Root | 400 | 46 | 46 | 32 | 40 | 43 | 41.40 ± 2.60*** |
Note: All values are Mean ± SEM and statistically analyzed using One‐Way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison test, n = 5. *p < .05, **p < .01, ***p < .001 when compared with control.
4.6. Sedative profiling
4.6.1. Open‐field test
Based on Open Field data, it appears that the ethyl acetate fraction of bulb and root at the dose of 400 mg/kg at 30 min (71.4 and 58.2 no. of movements, respectively) (p < .001) when compared to the control group (112.2 no. of movements) and the values are very close to the standard (73 no. of movements), suggesting that the extract might have a potential sedative effect. The other two doses of 200 mg/kg of bulb and root extracts of the plant also showed reduced numbers of movements at 30 min (79.6 no. of movements) (p < .01) and (68.2 no. of movements) (p < .001), respectively. The extract may have a sedative effect, as the open field test showed a decline in locomotor activity and rearing behavior, as shown in Table 8.
TABLE 8.
Effect of Coelogyne suaveolens bulb and root extract on the number of movements using the open field method.
| Test samples | Dose (mg/kg) | Number of movements | ||
|---|---|---|---|---|
| 0 min | 30 min | 60 min | ||
| Control | 10 mL/kg | 100.2 ± 13.56 | 112.2 ± 5.83 | 45.2 ± 8.53 |
| Diazepam | 1 | 86.4 ± 5.12 | 73 ± 2.92*** | 38 ± 1.95 |
| Bulb | 200 | 78.4 ± 13.7 | 79.6 ± 11.42** | 60 ± 12.01 |
| Bulb | 400 | 72.2 ± 6.16 | 71.4 ± 5.33*** | 58.2 ± 7.55 |
| Root | 200 | 72.6 ± 4.86 | 68.2 ± 1.11*** | 39.4 ± 3.88 |
| Root | 400 | 72 ± 9.6 | 58.2 ± 5.83*** | 58 ± 3.44 |
Note: All values are Mean ± SEM and statistically analyzed using One‐Way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison test, n = 5. *p < .05, **p < .01, ***p < .001 when compared with control.
4.6.2. Hole cross method
During the hole cross test, there was a progressive decrease in the frequency with which the mice traversed between the chambers over a span of 2 h, as indicated in Table 9. In the hole cross method, it has been observed that in the ethyl acetate fraction of bulb and root at doses of 200 and 400 mg/kg body weight, the decreased locomotor activity of the experimented animals occurred. Among these samples, the ethyl acetate fraction of the root of C. suaveolens at doses of 200 and 400 mg/kg body weight mostly decreased the frequency of movement of the mice through the hole at 30 min (2.60 and 2.00) (p < .001) compared to standard (6.00) at 30 min. It was also observed that the ethyl acetate fraction of the bulb of the plant at a dose of 400 mg/kg body weight decreased the locomotor activity of the test animals at 30 min (5.60; p < .05). This result indicates that the extracts can also decrease exploratory behavior, which suggests a sedative effect.
TABLE 9.
Effect of Coelogyne suaveolens bulb and root extract on the hole cross test.
| Test samples | Dose (mg/kg) | Number of movements | ||
|---|---|---|---|---|
| 0 min | 30 min | 60 min | ||
| Control | 10 mL/kg | 11.00 ± 0.44 | 8.40 ± 0.24 | 4.40 ± 0.81 |
| Standard | 1 | 7.60 ± 0.40* | 6.00 ± 0.32 | 2.80 ± 0.58 |
| Bulb | 200 | 10.40 ± 1.81 | 7.60 ± 0.24 | 8.40 ± 2.11 |
| Bulb | 400 | 8.20 ± 0.49 | 5.60 ± 0.51* | 7.80 ± 0.37 |
| Root | 200 | 8.00 ± 0.71 | 2.60 ± 1.21*** | 5.80 ± 0.37 |
| Root | 400 | 8.40 ± 0.51 | 2.00 ± 0.77*** | 5.60 ± 0.81 |
Note: All values are Mean ± SEM and statistically analyzed using One‐Way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison test, n = 5. *p < .05, **p < .01, ***p < .001 when compared with control.
4.6.3. Rotarod method
In the rotarod test, root extract at 400 mg/kg showed a significant decrease in the time spent on the rod (130 s at 30 min, 126 s at 60 min, and 134.60 s at 90 min), which, when compared to the control, was statistically significant (p < .001), as shown in Table 10. Again, bulb extract at 400 mg/kg showed a significant decrease in time spent on the rod (154 s at 30 min, 150 s at 60 min, and 157.4 s at 90 min); this also differed from the control in a statistically significant way (p < .001). The other two doses (200 mg/kg) of the root and bulb of the plant did not show any significant results. The rotarod test revealed a decrease in motor coordination and balance, which is a common effect of CNS depressants. Diazepam, on the other hand, was discovered to be a more effective muscle relaxant than the extracts.
TABLE 10.
Effect of Coelogyne suaveolens bulb and root extract on motor coordination using the rota‐rod method.
| Test samples | Dose (mg/kg) | Time (sec) of animals remained without falling from the rod at 24 rpm | ||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | ||
| Control | 10 mL/kg | 179.20 ± 0.80 | 180 | 180 |
| Standard | 1 | 76.40 ± 2.09*** | 115.4 ± 2.06*** | 156.4 ± 1.57*** |
| Bulb | 200 | 175.60 ± 2.04 | 174.4 ± 1.86 | 177.4 ± 1.67 |
| Bulb | 400 | 154 ± 3.05*** | 150 ± 3.91*** | 157.4 ± 2.54*** |
| Root | 200 | 176 ± 2.48 | 173.8 ± 1.96 | 176 ± 1.82 |
| Root | 400 | 130 ± 7.20*** | 126 ± 2.72*** | 134.6 ± 6.71*** |
Note: All values are Mean ± SEM and statistically analyzed using One‐Way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison test, n = 5. *p < .05, **p < .01, ***p < .001 when compared with control.
4.7. Molecular docking analysis
Tables 11a and 11b as well as Figures 3a and 3b display the docking score and the non‐bond interactions of the docked compounds for analgesic activity. Our molecular docking analysis showed that every compound examined interacted with each of the target proteins. In terms of binding affinity toward COX‐1 (PDB ID: 5wbe), alpha‐linolenic acid (−5.9 kcal/mol), {bicyclo[3.1.1]heptan‐3‐one, 2,6,6‐trimethyl‐, (1.alpha., 2.beta., 5.alpha.)‐} (−5.8 kcal/mol), and geranyl acetate (−5.7 kcal/mol) were shown to be the strongest. The maximum binding affinity was shown by 3,6‐dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran with human COX‐2 (PDB ID: 5ikq). Several other compounds also exhibited strong binding affinity toward the target protein, including {bicyclo[3.1.1]heptan‐3‐one,2,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐} (−6.2 kcal/mol), geranyl acetate (−6 kcal/mol), and alpha‐linolenic acid (−6 Kcal/mol).
TABLE 11a.
Binding affinity and interaction of the selected compounds with the amino acid residue of cyclooxygenase‐1 (PDB ID: 5wbe).
| Compound name | Binding affinity (kcal/Mol) | Hydrogen bonds | Hydrophobic bonds | ||
|---|---|---|---|---|---|
| Conventional | Carbon‐hydrogen | Pi‐alkyl | Alkyl | ||
| 1,6‐Octadien‐3‐ol,3,7‐dimethyl‐ | −4.8 | TRP100 | ARG120, LEU112 (2), VAL119 (2), VAL116 (2), LEU357, LEU93 (2), LEU115 (2), ILE89 | ||
| Bicyclo[3.1.1]heptan‐3‐one,2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐ | −5.8 | ALA527 | ILE523, GLY526 | TYR355 | LEU352 (2), ALA527 (3), VAL349, ILE523, VAL349 (2) |
| Isopulegol | −5.1 | ARG120 | ILE89 (3), VAL119 (2), LEU115, VAL116, PRO86 (2) | ||
| 3,6‐Dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran | −5.2 | ARG120 (2) | ILE89, LEU93 (2), LEU112 (2), LEU115 (3), VAL116 (3), VAL119, LEU357 | ||
| Geranyl acetate | −5.7 | ARG120 | VAL116 | TRP100 | ILE89 (2), LEU115, LEU112, LEU92 (2), LEU93 (2) |
| 9,12‐Octadecadienoic acid (Z,Z)‐ | −5.1 | ARG83 | VAL116 (2), VAL119 (2), LEU115 (3), ILE89 (2), LEU93, LEU112 (2) | ||
| Alpha‐linolenic acid | −5.9 | ARG120 (2) | TRP100 (2) | VAL116, VAL119, LEU115 (2), LEU93 (2), LEU112 (2), LEU92, VAL103 | |
Note: Bold letters indicate the best binding score.
TABLE 11b.
Binding affinity and interaction of the selected compounds with the amino acid residue of human cyclooxygenase‐2 (PDB ID: 5ikq).
| Compound name | Binding affinity (kcal/Mol) | Hydrogen bonds | Hydrophobic bonds | ||
|---|---|---|---|---|---|
| Conventional | Carbon‐hydrogen | Pi‐alkyl | Alkyl | ||
| 1,6‐Octadien‐3‐ol,3,7‐dimethyl‐ | −5.8 | TYR385 | PHE381, TYR385, PHE518 | VAL523, ALA527, LEU384, LEU352 (3), VAL523 (2), VAL349 | |
| Bicyclo[3.1.1]heptan‐3‐one,2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐ | −6.2 | SER530 | SER530 | VAL349, ALA527, VAL523 (2), LEU352 (3) | |
| Isopulegol | −5.6 | ALA199 | HIS207, TYR385 (2), TRP387 (2) | ALA202, LEU391, LEU390 | |
| 3,6‐Dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran | −6.8 | TYR385, PHE518 | VAL349 (2), LEU352 (3), VAL523 (2), ALA527 (2) | ||
| Geranyl acetate | −6 | HIS386 | TYR385, HIS207, PHE200 | LEU391 (2), LEU390, ALA202, ALA199 (2) | |
| 9,12‐Octadecadienoic acid (Z,Z)‐ | −5.6 | HIS207 | ALA202, VAL444, VAL447, LEU294, LEU391 (2), LEU390 | ||
| Alpha‐linolenic acid | −6 | HIS207 | HIS388, PHE395, TYR404 | ALA202, VAL444, VAL447 (2), LEU391 (3), LEU390, VAL295 | |
Note: Bold letters indicate the best binding score.
FIGURE 3a.
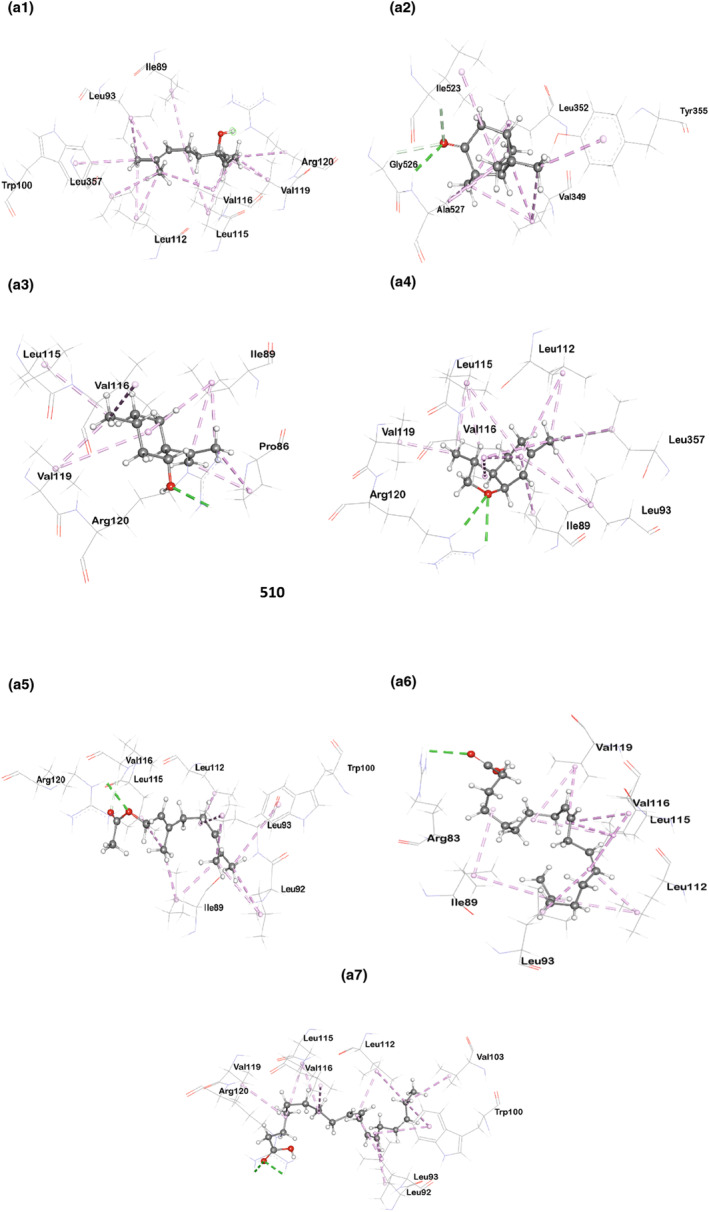
Molecular docking interaction of compounds against Cyclooxygenase‐1 (PDB ID: 5wbe): (a1) 1,6‐Octadien‐3‐ol,3,7‐dimethyl‐, (a2) Bicyclo[3.1.1]heptan‐3‐one,2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐, (a3) Isopulegol, (a4) 3,6‐Dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran, (a5) Geranyl Acetate, (a6) 9,12‐Octadecadienoic acid (Z,Z)‐, and (a7) Alpha Linolenic Acid.
FIGURE 3b.

Molecular docking interaction of compounds against Cyclooxygenase‐2 (PDB ID: 5ikq): (b1) 1,6‐Octadien‐3‐ol,3,7‐dimethyl‐, (b2) Bicyclo[3.1.1]heptan‐3‐one,2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐, (b3) Isopulegol, (b4) 3,6‐Dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran, (b5) Geranyl Acetate, (b6) 9,12‐Octadecadienoic acid (Z,Z)‐, and (b7) Alpha Linolenic Acid.
5. DISCUSSION
In this study, we have tested C. suaveolens bulb and root extract for identifying bioactive compounds and tested rodents for CNS, analgesic, and sedative profiling.
The acetic acid induction test is widely used to determine peripheral analgesic efficacy (Trongsakul et al., 2003). The pain induced by acetic acid occurs through an indirect mechanism involving the elevation of PG2 and PG2α levels at receptor sites within organ cavities. This suggests that carboxylic acid functions by indirectly enhancing the release of endogenous mediators (Ezeja et al., 2011; Lee & Choi, 2008). Acetic acid causes squirming in experimental animals via a chemosensitive nociceptor (Onasanwo & Elegbe, 2006). Nonsteroidal anti‐inflammatory medications work by inhibiting sensory neuron activation in response to inflammatory mediators (Akindele et al., 2011). Furthermore, the percentage reduction in the number of stomach squirms might indicate the extent of analgesia (Marchioro et al., 2005). The bulb and root extract, administered at 200 and 400 mg/kg doses, significantly reduced the average number of writhes, similar to the effect of standard aceclofenac. The idea behind the tail immersion method is that substances resembling morphine can specifically prolong the time it takes for the tail withdrawal reflex to occur in mice. The effectiveness of the extract's analgesic properties is gauged by the extension of the initial latency period (Elisabetsky et al., 1995; Pal et al., 1999). Both the root and bulb extracts, specifically at a 400 mg/kg dose, increased the reaction time, % MPE (maximum possible effect), and basal latency. The effect of the bulb extract at this dose remained stable between 30 and 60 min. The delayed onset of action of the root extract, as compared to the bulb extract, might be attributed to differences in active compounds or their pharmacokinetics.
The fact that the extracts enhance basal latency suggests that they may activate a centrally mediated analgesic mechanism (Bachhav et al., 2009). Sensory nerves sensitize the nociceptors in this approach, and the participation of endogenous chemicals such as prostaglandins is minimized (Uche & Aprioku, 2008). The hot plate test is commonly used for evaluating centrally acting analgesics, but it may not effectively assess peripherally acting analgesics. It has limitations, as sedatives, muscle relaxants, and psychotomimetics can produce false positives, and mixed opiate agonists‐antagonists yield unreliable results. However, C. suaveolens bulb extract at 400 mg/kg demonstrated significant activity at 30 min (p < .01) and 60 min (p < .001) compared to the control. This suggests that C. suaveolens exhibits both central and peripheral analgesic activity, as indicated by the results from these three methods.
The EPM is an in vivo method used to evaluate the potential for anxiolytic activity in experimental animals. In this test, animals typically exhibit a preference for the enclosed areas of the maze and may avoid open segments that lack protective walls, which are considered less favorable (Collimore & Rector, 2014).
The manifestation of notable alterations in the open‐arm behavior of animals subjected to experimental plant extracts can be interpreted as indicative of the anxiolytic efficacy of these extracts. An established anxiolytic drug, like benzodiazepine, is commonly used for this purpose. In the CNS (central nervous system), GABA (gamma‐aminobutyric acid) plays a crucial role as a major neurochemical. Plant extracts may exert their effects by enhancing GABAergic inhibition in the CNS, leading to hyperpolarization and a reduced firing rate of vital neurons in the brain. Alternatively, they may directly activate GABA receptors (Verma et al., 2010). Previous research suggests that plants containing saponins, tannins, and flavonoids may have an impact on various CNS disorders (Yadav et al., 2013). Initial phytochemical analyses and evaluations of plant extracts have suggested that neuroactive steroids and flavonoids might operate as agents binding to GABA receptors within the central nervous system, akin to molecules resembling benzodiazepines (Verma et al., 2010). When compared to the control group in the current investigation, the bulb and root extracts at 400 mg/kg showed mild anxiolytic efficacy.
The hole board test is an alternative method for assessing anxiolytic activity. It depends on tracking experimental animals' head‐dipping behavior while utilizing the hole‐board instrument. If the animals are sensitive to changes in their emotional or anxiolytic state, an increase in head‐dipping behavior might be observed (Foyet et al., 2012; Wei et al., 2007). Another crucial aspect of assessing a drug's impact on the central nervous system (CNS) is to observe its effect on the locomotor activity of the test animals. Locomotor activity can indicate CNS stimulation, where increased alertness leads to heightened motion activity, or it can imply a sedative effect when motion activity is reduced (Bhattacharya & Satyan, 1997). The level of CNS excitement can be gauged from locomotor activity, and a decrease in this activity is closely associated with CNS depression‐induced sedation (Islam et al., 2015). In this study, the CNS depressant activity of the ethyl acetate fraction of acetonic extract from C. suaveolens bulb and root was evaluated using three behavioral methods. The open field method measured the animals’ locomotor activity, providing insights into the general level of CNS activity. The hole cross method assessed exploratory behavior, which is also related to CNS activity. Lastly, the Rotarod method evaluated the extract's ability to impair motor coordination and balance in animals. All three methods indicated that the extract may possess potent sedative activity.
Molecular docking is commonly used to predict the binding orientation of small molecules to enzymes and receptors (Gohlke et al., 2000). Cyclooxygenase‐1 (COX‐1) and cyclooxygenase‐2 (COX‐2) enzymes play pivotal roles in prostaglandin synthesis and have been identified as potential therapeutic targets for various inflammatory and pain‐related conditions. Our analysis revealed significant insights into the binding interactions of the compounds with COX‐1 (PDB ID: 5wbe) and COX‐2 (PDB ID: 5ikq). Among the compounds investigated, some of them, like {1,6‐octadien‐3‐ol, 3,7‐dimethyl‐}, isopulegol, and geranyl acetate, revealed analgesic behaviors (Nazimova et al., 2016; Peana et al., 2003 Quintans‐Júnior et al., 2013), while alpha‐linolenic acid, {bicyclo[3.1.1]heptan‐3‐one, 2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐}, and {3,6‐dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran} have been found to possess anti‐inflammatory activity from previous studies (Chou et al., 2018; Miao et al., 2019; Zhao et al., 2005).
In our present study, all the compounds showed a favorable docking score toward both cyclooxygenase‐1 (PDB ID: 5wbe) and human cyclooxygenase‐2 (PDB ID: 5ikq), with alpha‐linolenic acid being the highest (binding affinity: −5.9) for cyclooxygenase‐1 and 3,6‐dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran (binding affinity: −6.8) for cyclooxygenase‐2. Besides, bicyclo[3.1.1]heptan‐3‐one,2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐, and geranyl acetate also displayed a noteworthy affinity for cyclooxygenase‐1, with docking scores of −5.8 and −5.7, respectively, where it is −6.2 and −6 for cyclooxygenase‐2, respectively. These high docking scores suggest strong binding interactions between these compounds and the respective enzymes, indicating their potential as potent inhibitors. Interestingly, against Cyclooxenase‐1, all the examined compounds interacted with ARG120 residue either by forming hydrogen or hydrophobic bonds except {bicyclo[3.1.1]heptan‐3‐one, 2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐} and {9,12‐octadecadienoic acid (Z,Z)‐}. However, {bicyclo[3.1.1]heptan‐3‐one, 2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐} formed a hydrophobic bond with TYR355 residue. These interactions with ARG120 and TYR355 are notable, as both residues play a significant role in regulating the entry of substrates or inhibitors into the long hydrophobic channel of cyclooxygenases (Cingolani et al., 2017).
As for Cyclooxygenase‐2, {3,6‐dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran}, geranyl acetate, isopulegol, and {1,6‐octadien‐3‐ol,3,7‐dimethyl‐} showed hydrophobic interactions with TYR385 residue. {1,6‐Octadien‐3‐ol,3,7‐dimethyl‐} also formed a conventional hydrogen bond with the TYR385 residue. This interaction is particularly significant due to the fact that TYR385 is known to play a vital role in the transformation of arachidonic acid into prostaglandins. TYR385 is responsible for commencing the oxygenation of arachidonic acid in cyclooxygenases by abstracting the pro‐S hydrogen at the C‐13 position, which is an important step in the conversion (Schneider & Brash, 2000). Again, {bicyclo[3.1.1]heptan‐3‐one, 2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)‐} forms two hydrogen bonds (conventional and carbon‐hydrogen) with SER530, which is also significant as binding with SER530 may change the catalytic activity of Cyclooxygenase‐2 (Giménez‐Bastida et al., 2019).
These findings suggest that our compounds effectively target the active site residues of Cyclooxygenase‐1 and Cyclooxygenase‐2, suggesting their potential to disrupt the enzymatic activity and inhibit prostaglandin synthesis.
6. CONCLUSION
These investigations unveil promising pharmacological possibilities that could contribute to the advancement of modern medicine in deriving drugs from plant origins, offering diverse therapeutic potentials with minimal adverse effects. C. suaveolens emerges as a potential remedy for certain neuropharmacological conditions and pain relief. This research holds the potential to shed light on crucial domains within biomedical science that have yet to be extensively explored by researchers. In addition, seven compounds’ binding orientations to the cyclooxygenase‐1 and cyclooxygenase‐2 enzymes were explored in our molecular docking investigation. Alpha‐linolenic acid, 3,6‐dimethyl‐2,3,3a,4,5,7a‐hexahydrobenzofuran, {Bicyclo[3.1.1]heptan‐3‐one,2,6,6‐trimethyl‐, (1.alpha.,2.beta.,5.alpha.)}, and geranyl acetate were among the compounds tested, and their promising docking scores for both enzymes suggested that they could be able to block prostaglandin synthesis. The compounds’ capacity to target key regions involved in the enzymatic activity is further demonstrated by their interactions with important residues, such as ARG120 and TYR355 in cyclooxygenase‐1 and TYR385 and SER530 in cyclooxygenase‐2. These results provide further evidence that the compounds under study have the potential to operate as analgesic drugs by inhibiting the enzymatic activity of cyclooxygenases 1 and 2. In a nutshell, the investigation into the medicinal potential of C. suaveolens has yielded promising results, but several uncertainties warrant consideration in future research. First, small sample sizes and replication across experiments raise concerns about statistical robustness. Future studies should incorporate larger sample sizes and multiple replicates to enhance the validity of results. Additionally, while dose‐dependent effects were noted in some experiments, a more comprehensive dose–response analysis is needed to establish the optimal therapeutic dosage. Furthermore, it is crucial to acknowledge that the observed effects in Swiss albino mice may not directly translate to human responses, necessitating further research using diverse animal models and, ultimately, clinical trials. Lastly, ongoing efforts to identify and characterize the exact bioactive compounds causing the therapeutic potential in the extract's ethyl acetate fraction should be pursued to provide a more comprehensive understanding of the plant's therapeutic potential.
AUTHOR CONTRIBUTIONS
Taslima Akter Eva: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); writing – original draft (lead); writing – review and editing (equal). Husnum Mamurat: Conceptualization (equal); data curation (equal); formal analysis (equal); software (equal); writing – review and editing (equal). Md. Habibul Hasan Rahat: Data curation (supporting); formal analysis (supporting); writing – original draft (supporting); writing – review and editing (supporting). S. M. Moazzem Hossen: Conceptualization (equal); data curation (equal); methodology (equal); project administration (equal); validation (lead).
CONFLICT OF INTEREST STATEMENT
The authors state that the publication of this article does not present any conflicts of interest for them.
INSTITUTIONAL REVIEW BOARD STATEMENT
The study protocol was authorized in accordance with government directives under Pharm/P&D/CUDP‐16,2023:10 by the Department of Pharmacy, University of Chittagong, Chittagong, Bangladesh.
ACKNOWLEDGMENTS
We appreciate and acknowledge Research and Publication Cell, University of Chittagong and as well as the Pharmacy Department, University of Chittagong.
Eva, T. A. , Mamurat, H. , Rahat, M. H. H. , & Hossen, S. M. M. (2024). Unveiling the pharmacological potential of Coelogyne suaveolens: An investigation of its diverse pharmacological activities by in vivo and computational studies. Food Science & Nutrition, 12, 1749–1767. 10.1002/fsn3.3867
DATA AVAILABILITY STATEMENT
Upon reasonable request, the corresponding author will provide the data that back up the study's conclusions.
REFERENCES
- Afrin, S. R. , Islam, M. R. , Khanam, B. H. , Proma, N. M. , Didari, S. S. , Jannat, S. W. , & Hossain, M. K. (2021). Phytochemical and pharmacological investigations of different extracts of leaves and stem barks of Macropanax dispermus (Araliaceae): A promising ethnomedicinal plant. Future Journal of Pharmaceutical Sciences, 7(1), 1–13. [Google Scholar]
- Akindele, A. J. , Ibe, I. F. , & Adeyemi, O. O. (2011). Analgesic and antipyretic activities of Drymaria cordata (Linn.) Willd (Caryophyllaceae) extract. African Journal of Traditional, Complementary, and Alternative Medicines, 9(1), 25–35. 10.4314/ajtcam.v9i1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Nuri, M. , Abu‐Reidah, I. M. , Alhajeh, A. A. , Omar, G. , Adwan, G. , & Warad, I. (2022). GC–MS‐based metabolites profiling, in vitro antioxidant, anticancer, and antimicrobial properties of different solvent extracts from the botanical parts of Micromeria fruticosa (Lamiaceae). Processes, 10(5), 1016. [Google Scholar]
- Arunkumar, G. , Anbarasan, B. , Murugavel, R. , Uthrapathi, S. , & Visweswaran, S. (2019). Evaluation of analgesic activity of Gandhaga Sarkkarai in Swiss Albino mice by Eddy's hot plate method. 10.20959/wjpr20201-16141 [DOI]
- Bachhav, R. S. , Gulecha, V. S. , & Upasani, C. D. (2009). Analgesic and anti‐inflammatory activity of Argyreia speciosa root. Indian Journal of Pharmacology, 41(4), 158–161. 10.4103/0253-7613.56066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow, B. , & Michaelis, S. (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17(3), 327–335. 10.31887/DCNS.2015.17.3/bbandelow [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman, H. M. , Westbrook, J. , Feng, Z. , Gilliland, G. , Bhat, T. N. , Weissig, H. , Shindyalov, I. N. , & Bourne, P. E. (2000). The protein data bank. Nucleic Acids Research, 28(1), 235–242. 10.1093/nar/28.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya, S. K. , & Satyan, K. S. (1997). Experimental methods for evaluation of psychotropic agents in rodents: I—Anti‐anxiety agents. Indian Journal of Experimental Biology, 35(6), 565–575. [PubMed] [Google Scholar]
- Chou, S. T. , Lai, C. C. , Lai, C. P. , & Chao, W. W. (2018). Chemical composition, antioxidant, anti‐melanogenic and anti‐inflammatory activities of Glechoma hederacea (Lamiaceae) essential oil. Industrial Crops and Products, 122, 675–685. 10.1016/j.indcrop.2018.06.032 [DOI] [Google Scholar]
- Cingolani, G. , Panella, A. , Perrone, M. G. , Vitale, P. , Di Mauro, G. , Fortuna, C. G. , Armen, R. S. , Ferorelli, S. , Smith, W. L. , & Scilimati, A. (2017). Structural basis for selective inhibition of Cyclooxygenase‐1 (COX‐1) by diarylisoxazoles mofezolac and 3‐(5‐chlorofuran‐2‐yl)‐5‐methyl‐4‐phenylisoxazole (P6). European Journal of Medicinal Chemistry, 138, 661–668. 10.1016/j.ejmech.2017.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collimore, K. C. , & Rector, N. A. (2014). Treatment of anxiety disorders with comorbid depression: A survey of expert CBT clinicians. Cognitive and Behavioral Practice, 21(4), 485–493. 10.1016/j.cbpra.2014.01.007 [DOI] [Google Scholar]
- Cragg, G. M. , & Newman, D. J. (2005). Plants as a source of anti‐cancer agents. Journal of Ethnopharmacology, 100(1–2), 72–79. 10.1016/j.jep.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Crawley, J. N. (1985). Exploratory behavior models of anxiety in mice. Neuroscience and Biobehavioral Reviews, 9(1), 37–44. 10.1016/0149-7634(85)90030-2 [DOI] [PubMed] [Google Scholar]
- Dambisya, Y. M. , Lee, T. L. , Sathivulu, V. , & Mat Jais, A. (1999). Influence of temperature, pH and naloxone on the antinociceptive activity of Channa striatus (haruan) extracts in mice. Journal of Ethnopharmacology, 66(2), 181–186. 10.1016/s0378-8741(98)00169-x [DOI] [PubMed] [Google Scholar]
- De Cosmo, G. , & Congedo, E. (2015). The use of NSAID, s in the postoperative period: Advantage and sdisfantages. Journal of Anesthesia & Critical Care, 3(November, 1–11. [Google Scholar]
- di Stasi, L. C. , Costa, M. , Mendaçolli, S. L. , Kirizawa, M. , Gomes, C. , & Trolin, G. (1988). Screening in mice of some medicinal plants used for analgesic purposes in the state of São Paulo. Journal of Ethnopharmacology, 24(2–3), 205–211. 10.1016/0378-8741(88)90153-5 [DOI] [PubMed] [Google Scholar]
- Dou, M. M. , Zhang, Z. H. , Li, Z. B. , Zhang, J. , & Zhao, X. Y. (2016). Cardioprotective potential of Dendrobium officinale Kimura et Migo against myocardial ischemia in mice. Molecular Medicine Reports, 14(5), 4407–4414. 10.3892/mmr.2016.5789 [DOI] [PubMed] [Google Scholar]
- Doukkali, Z. , Taghzouti, K. , Bouidida, E. L. , Nadjmouddine, M. , Cherrah, Y. , & Alaoui, K. (2015). Evaluation of anxiolytic activity of methanolic extract of Urtica urens in a mice model. Behavioral and Brain Functions, 11, 19. 10.1186/s12993-015-0063-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisabetsky, E. , Amador, T. A. , Albuquerque, R. R. , Nunes, D. S. , & Carvalho, A. d C. (1995). Analgesic activity of Psychotria colorata (Willd. ex R. & S.) Muell. Arg. alkaloids. Journal of Ethnopharmacology, 48(2), 77–83. 10.1016/0378-8741(95)01287-n [DOI] [PubMed] [Google Scholar]
- Emamghoreishi, M. , Khasaki, M. , & Aazam, M. F. (2005). Coriandrum sativum: Evaluation of its anxiolytic effect in the elevated plus‐maze. Journal of Ethnopharmacology, 96(3), 365–370. 10.1016/j.jep.2004.06.022 [DOI] [PubMed] [Google Scholar]
- Ezeja, M. , Omeh, Y. , Ezeigbo, I. , & Ekechukwu, A. (2011). Evaluation of the analgesic activity of the methanolic stem bark extract of Dialium guineense (wild). Annals of Medical and Health Sciences Research, 1(1), 55–62. [PMC free article] [PubMed] [Google Scholar]
- Fan, S. H. , Ali, N. A. , & Basri, D. F. (2014). Evaluation of analgesic activity of the methanol extract from the galls of Quercus infectoria (Olivier) in rats. Evidence‐Based Complementary and Alternative Medicine, 2014, 976764. 10.1155/2014/976764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- File, S. E. , & Wardill, A. G. (1975). Validity of head‐dipping as a measure of exploration in a modified hole‐board. Psychopharmacologia, 44(1), 53–59. 10.1007/BF00421184 [DOI] [PubMed] [Google Scholar]
- Foyet, H. S. , Tsala, D. E. , Bouba, A. A. , & Hritcu, L. (2012). Anxiolytic and antidepressant‐like effects of the aqueous extract of Alafia multiflora stem barks in rodents. Advances in Pharmacological Sciences, 2012, 912041. 10.1155/2012/912041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesner, R. A. , Banks, J. L. , Murphy, R. B. , Halgren, T. A. , Klicic, J. J. , Mainz, D. T. , Repasky, M. P. , Knoll, E. H. , Shelley, M. , Perry, J. K. , Shaw, D. E. , Francis, P. , & Shenkin, P. S. (2004). Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. Journal of Medicinal Chemistry, 47(7), 1739–1749. 10.1021/jm0306430 [DOI] [PubMed] [Google Scholar]
- Giménez‐Bastida, J. A. , Boeglin, W. E. , Boutaud, O. , Malkowski, M. G. , & Schneider, C. (2019). Residual cyclooxygenase activity of aspirin‐acetylated COX‐2 forms 15 R‐prostaglandins that inhibit platelet aggregation. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 33(1), 1033–1041. 10.1096/fj.201801018R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohlke, H. , Hendlich, M. , & Klebe, G. (2000). Knowledge‐based scoring function to predict protein‐ligand interactions. Journal of Molecular Biology, 295(2), 337–356. 10.1006/jmbi.1999.3371 [DOI] [PubMed] [Google Scholar]
- Gunaydin, C. , & Bilge, S. S. (2018). Effects of nonsteroidal anti‐inflammatory drugs at the molecular level. The Eurasian Journal of Medicine, 50(2), 116–121. 10.5152/eurasianjmed.2018.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, B. D. , Dandiya, P. C. , & Gupta, M. L. (1971). A psycho‐pharmacological analysis of behaviour in rats. Japanese Journal of Pharmacology, 21(3), 293–298. 10.1254/jjp.21.293 [DOI] [PubMed] [Google Scholar]
- He, T. B. , Huang, Y. P. , Yang, L. , Liu, T. T. , Gong, W. Y. , Wang, X. J. , Sheng, J. , & Hu, J. M. (2016). Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale . International Journal of Biological Macromolecules, 83, 34–41. 10.1016/j.ijbiomac.2015.11.038 [DOI] [PubMed] [Google Scholar]
- Hijazi, M. A. , El‐Mallah, A. , Aboul‐Ela, M. , & Ellakany, A. (2017). Evaluation of analgesic activity of Papaver libanoticum extract in mice: Involvement of opioids receptors. Evidence‐Based Complementary and Alternative Medicine, 2017, 8935085. 10.1155/2017/8935085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, M. R. , Alam, R. , Chung, H. J. , Eva, T. A. , Kabir, M. F. , Mamurat, H. , Hong, S. T. , Hafiz, M. A. , & Hossen, S. M. (2023). In vivo, in vitro and in silico study of Cucurbita moschata flower extract: A promising source of natural analgesic, anti‐inflammatory, and antibacterial agents. Molecules, 28(18), 6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen, S. M. M. , Yusuf, A. T. M. , Emon, N. U. , Alam, N. , Sami, S. A. , Polash, S. H. , Nur, M. A. , Mitra, S. , Uddin, M. H. , & Emran, T. B. (2022). Biochemical and pharmacological aspects of Ganoderma lucidum: Exponent from the in vivo and computational investigations. Biochemistry and Biophysics Reports, 32, 101371. 10.1016/j.bbrep.2022.101371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda, M. K. , Hoque, M. M. , & Alam, M. D. O. (2021). New report of three orchid species to the flora of Bangladesh. Indian For, 147(5), 508–511. [Google Scholar]
- Islam, N. U. , Khan, I. , Rauf, A. , Muhammad, N. , Shahid, M. , & Shah, M. R. (2015). Antinociceptive, muscle relaxant and sedative activities of gold nanoparticles generated by methanolic extract of Euphorbia milii . BMC Complementary and Alternative Medicine, 15, 160. 10.1186/s12906-015-0691-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes, C. L. , & Crabbe, J. C. (2006). Pharmacological and genetic influences on hole‐board behaviors in mice. Pharmacology, Biochemistry, and Behavior, 85(1), 57–65. 10.1016/j.pbb.2006.07.007 [DOI] [PubMed] [Google Scholar]
- Koster, R. , Anderson, M. , & De Beer, E. J. (1959). Acetic acid for analgesic screening. Federation Proceedings, 18, 412–417. [Google Scholar]
- Kumar Paliwal, S. , Sati, B. , Faujdar, S. , & Sharma, S. (2016). Studies on analgesic, anti‐inflammatory activities of stem and roots of Inula cuspidata C.B Clarke. Journal of Traditional and Complementary Medicine, 7(4), 532–537. 10.1016/j.jtcme.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Ali, I. , Abbas, F. , Khan, N. , Gupta, M. K. , Garg, M. , Kumar, S. , & Kumar, D. (2023). In‐silico identification of small molecule benzofuran‐1, 2, 3‐triazole hybrids as potential inhibitors targeting EGFR in lung cancer via ligand‐based pharmacophore modeling and molecular docking studies. In Silico Pharmacology, 11(1), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumawat, R. K. , Kumar, S. , & Sharma, S. (2012). Evaluation of analgesic activity of various extracts of Sida tiagii Bhandari. Acta Poloniae Pharmaceutica, 69(6), 1103–1109. [PubMed] [Google Scholar]
- Lee, K. H. , & Choi, E. M. (2008). Analgesic and anti‐inflammatory effects of Ligularia fischeri leaves in experimental animals. Journal of Ethnopharmacology, 120(1), 103–107. 10.1016/j.jep.2008.07.038 [DOI] [PubMed] [Google Scholar]
- Ljungberg, T. , & Ungerstedt, U. (1976). Automatic registration of behaviour related to dopamine and noradrenaline transmission. European Journal of Pharmacology, 36(1), 181–188. 10.1016/0014-2999(76)90270-3 [DOI] [PubMed] [Google Scholar]
- Luo, Q. L. , Tang, Z. H. , Zhang, X. F. , Zhong, Y. H. , Yao, S. Z. , Wang, L. S. , Lin, C. W. , & Luo, X. (2016). Chemical properties and antioxidant activity of a water‐soluble polysaccharide from Dendrobium officinale . International Journal of Biological Macromolecules, 89, 219–227. 10.1016/j.ijbiomac.2016.04.067 [DOI] [PubMed] [Google Scholar]
- Madden, J. C. , Enoch, S. J. , Paini, A. , & Cronin, M. T. (2020). A review of in silico tools as alternatives to animal testing: Principles, resources and applications. Alternatives to Laboratory Animals, 48(4), 146–172. [DOI] [PubMed] [Google Scholar]
- Mahanur, V. B. , Rajge, R. R. , Pal, R. S. , Chaitanya, M. V. N. L. , Vishwas, S. , Gupta, S. , Gupta, G. , Kumar, D. , Oguntibeju, O. O. , ur Rehman, Z. , Aba Alkhayl, F. F. , Thakur, V. , Pandey, P. , Mazumder, A. , Adams, J. , Dua, K. , & Singh, S. K. (2023). Harnessing unexplored medicinal values of red listed South African weed Erigeron bonariensis: From ethnobotany to biomedicine. South African Journal of Botany, 160, 535–546. 10.1016/j.sajb.2023.07.031 [DOI] [Google Scholar]
- Malairajan, P. , Gopalakrishnan, G. , Narasimhan, S. , & Veni, K. J. K. (2006). Analgesic activity of some Indian medicinal plants. Journal of Ethnopharmacology, 106(3), 425–428. 10.1016/j.jep.2006.03.015 [DOI] [PubMed] [Google Scholar]
- Marchioro, M. , Blank, M.d F. , Mourão, R. H. , & Antoniolli, A. R. (2005). Anti‐nociceptive activity of the aqueous extract of Erythrina velutina leaves. Fitoterapia, 76(7–8), 637–642. 10.1016/j.fitote.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Miao, Y. H. , Hu, Y. H. , Yang, J. , Liu, T. , Sun, J. , & Wang, X. J. (2019). Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Advances, 9(47), 27510–27540. 10.1039/C9RA04917G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, S. E. E. , Nicolson, K. P. , & Smith, B. H. (2019). Chronic pain: A review of its epidemiology and associated factors in population‐based studies. British Journal of Anaesthesia, 123(2), e273–e283. 10.1016/j.bja.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa, T. , Yagi, T. , Kagiyama, A. , & Niki, H. (1996). Radial maze performance, open‐field and elevated plus‐maze behaviors in Fyn‐kinase deficient mice: Further evidence for increased fearfulness. Brain Research. Molecular Brain Research, 37(1–2), 145–150. 10.1016/0169-328x(95)00300-h [DOI] [PubMed] [Google Scholar]
- Morris, S. A. , Hatcher, H. F. , & Reddy, D. K. (2006). Digoxin therapy for heart failure: An update. American Family Physician, 74(4), 613–618. [PubMed] [Google Scholar]
- Nasrin, S. , Islam, M. N. , Tayab, M. A. , Nasrin, M. S. , Siddique, M. A. B. , Emran, T. B. , & Reza, A. S. M. A. (2022). Chemical profiles and pharmacological insights of Anisomeles indica Kuntze: An experimental chemico‐biological interaction. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 149, 112842. 10.1016/j.biopha.2022.112842 [DOI] [PubMed] [Google Scholar]
- Nazimova, E. , Pavlova, A. , Mikhalchenko, O. , Il'ina, I. , Korchagina, D. , Tolstikova, T. Y. , Volcho, K. , & Salakhutdinov, N. (2016). Discovery of highly potent analgesic activity of isopulegol‐derived (2 R, 4a R, 7 R, 8a R)‐4, 7‐dimethyl‐2‐(thiophen‐2‐yl) octahydro‐2 H‐chromen‐4‐ol. Medicinal Chemistry Research, 25, 1369–1383. 10.1007/s00044-016-1573-3 [DOI] [Google Scholar]
- Onasanwo, S. A. , & Elegbe, R. A. (2006). Anti‐nociceptive and anti‐inflammatory properties of the leaf extracts of Hedranthera barteri in rats and mice. African Journal of Biomedical Research, 9(2), 109–117. 10.4314/ajbr.v9i2.48891 [DOI] [Google Scholar]
- Orlando, B. J. , & Malkowski, M. G. (2016). Substrate‐selective inhibition of cyclooxygeanse‐2 by fenamic acid derivatives is dependent on peroxide tone. Journal of Biological Chemistry, 291(29), 15069–15081. 10.1074/jbc.M116.725713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, S. , Sen, T. , & Chaudhuri, A. K. (1999). Neuropsychopharmacological profile of the methanolic fraction of Bryophyllum pinnatum leaf extract. The Journal of Pharmacy and Pharmacology, 51(3), 313–318. 10.1211/0022357991772312 [DOI] [PubMed] [Google Scholar]
- Pan, L. H. , Li, X. F. , Wang, M. N. , Zha, X. Q. , Yang, X. F. , Liu, Z. J. , Luo, Y. B. , & Luo, J. P. (2014). Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. International Journal of Biological Macromolecules, 64, 420–427. 10.1016/j.ijbiomac.2013.12.024 [DOI] [PubMed] [Google Scholar]
- Peana, A. T. , Paolo, S. D. , Chessa, M. L. , Moretti, M. D. , Serra, G. , & Pippia, P. (2003). (−)‐Linalool produces antinociception in two experimental models of pain. European Journal of Pharmacology, 460(1), 37–41. doi: 10.1016/S0014-2999(02)02856-X [DOI] [PubMed] [Google Scholar]
- Pellow, S. , Chopin, P. , File, S. E. , & Briley, M. (1985). Validation of open:Closed arm entries in an elevated plus‐maze as a measure of anxiety in the rat. Journal of Neuroscience Methods, 14(3), 149–167. 10.1016/0165-0270(85)90031-7 [DOI] [PubMed] [Google Scholar]
- Phillipson, J. D. (2001). Phytochemistry and medicinal plants. Phytochemistry, 56(3), 237–243. 10.1016/s0031-9422(00)00456-8 [DOI] [PubMed] [Google Scholar]
- Queiroz, E. F. , Wolfender, J. L. , & Hostettmann, K. (2009). Modern approaches in the search for new lead antiparasitic compounds from higher plants. Current Drug Targets, 10(3), 202–211. 10.2174/138945009787581113 [DOI] [PubMed] [Google Scholar]
- Quintans‐Júnior, L. , Moreira, J. C. , Pasquali, M. A. , Rabie, S. , Pires, A. S. , Schröder, R. , Rabelo, T. K. , Santos, J. P. A. , Lima, P. S. S. , Cavalcanti, S. C. H. , Araújo, A. A. S. , Quintans, J. S. S. , & Gelain, D. P. (2013). Antinociceptive activity and redox profile of the monoterpenes. International Scholarly Research Notices, 2013, 1–11. 10.1155/2013/459530 [DOI] [Google Scholar]
- Rates, S. M. (2001). Plants as source of drugs. Toxicon: Official Journal of the International Society on Toxinology, 39(5), 603–613. 10.1016/s0041-0101(00)00154-9 [DOI] [PubMed] [Google Scholar]
- Rosenblum, A. , Marsch, L. A. , Joseph, H. , & Portenoy, R. K. (2008). Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Experimental and Clinical Psychopharmacology, 16(5), 405–416. 10.1037/a0013628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma, M. K. , Saha, D. , Das, B. K. , Das, T. , Azizov, S. , & Kumar, D. (2023). A delve into the pharmacological targets and biological mechanisms of Paederia foetida Linn.: A rather invaluable traditional medicinal plant. Naunyn‐Schmiedeberg's Archives of Pharmacology, 396, 2217–2240. 10.1007/s00210-023-02496-4 [DOI] [PubMed] [Google Scholar]
- Schneider, C. , & Brash, A. R. (2000). Stereospecificity of hydrogen abstraction in the conversion of arachidonic acid to 15R‐HETE by aspirin‐treated cyclooxygenase‐2: Implications for the alignment of substrate in the active site. Journal of Biological Chemistry, 275(7), 4743–4746. 10.1074/jbc.275.7.4743 [DOI] [PubMed] [Google Scholar]
- Singh, M. , Kumar, R. , Sharma, S. , Kumar, L. , Kumar, S. , Gupta, G. , Dua, K. , & Kumar, D. (2023). Hedychium spicatum: A comprehensive insight into its ethnobotany, phytochemistry, pharmacological and therapeutic attributes. South African Journal of Botany, 161, 638–647. 10.1016/j.sajb.2023.08.046 [DOI] [Google Scholar]
- Sofowora, A. (1996). Research on medicinal plants and traditional medicine in Africa. Journal of Alternative and Complementary Medicine (New York, N.Y.), 2(3), 365–372. 10.1089/acm.1996.2.365 [DOI] [PubMed] [Google Scholar]
- Sunanda, B. P. , Latha, K. , Rammohan, B. , Uma Maheswari, M. S. , & Surapaneni, K. M. (2014). Evaluation of the neuroprotective effects of curcumin (turmeric) against scopolamine induced cognitive impairment in mice. International Journal of Pharmaceutical and Phytopharmacological Research, 6, 133–136. [Google Scholar]
- Tang, H. , Zhao, T. , Sheng, Y. , Zheng, T. , Fu, L. , & Zhang, Y. (2017). Dendrobium officinale Kimura et Migo: A review on its ethnopharmacology, phytochemistry, pharmacology, and industrialization. Evidence‐Based Complementary and Alternative Medicine, 2017, 7436259. 10.1155/2017/7436259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trongsakul, S. , Panthong, A. , Kanjanapothi, D. , & Taesotikul, T. (2003). The analgesic, antipyretic and anti‐inflammatory activity of Diospyros variegata Kruz. Journal of Ethnopharmacology, 85(2–3), 221–225. 10.1016/s0378-8741(03)00020-5 [DOI] [PubMed] [Google Scholar]
- Trott, O. , & Olson, A. J. (2009). Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uche, F. I. , & Aprioku, J. S. (2008). The phytochemical constituents, analgesic and anti‐inflammatory effects of methanol extract of Jatropha curcas leaves in Mice and Wister albino rats. Journal of Applied Sciences and Environmental Management, 12(4), 99–102. 10.4314/jasem.v12i4.55247 [DOI] [Google Scholar]
- Van Rensburg, R. , & Reuter, H. (2019). An overview of analgesics: NSAIDs, paracetamol, and topical analgesics part 1. South African Family Practice., 61, S4–S10. 10.1080/20786190.2019.1610228 [DOI] [Google Scholar]
- VanWagenen, B. C. , Larsen, R. , Cardellina, J. H. , Randazzo, D. , Lidert, Z. C. , & Swithenbank, C. (1993). Ulosantoin, a potent insecticide from the sponge Ulosa ruetzleri. The Journal of Organic Chemistry, 58(2), 335–337. 10.1021/jo00054a013 [DOI] [Google Scholar]
- Verma, A. , Jana, G. K. , Sen, S. , Chakraborty, R. , Sachan, S. , & Mishra, A. (2010). Pharmacological evaluation of Saraca indica leaves for central nervous system depressant activity in mice. Journal of Pharmaceutical Sciences and Research, 2(6), 338–343. [Google Scholar]
- Vogel, H. G. , Maas, J. , & Gebauer, A. (Eds.). (2010). Drug discovery and evaluation: Methods in clinical pharmacology. Springer Science & Business Media. [Google Scholar]
- Wei, X. Y. , Yang, J. Y. , Wang, J. H. , & Wu, C. F. (2007). Anxiolytic effect of saponins from Panax quinquefolium in mice. Journal of Ethnopharmacology, 111(3), 613–618. 10.1016/j.jep.2007.01.009 [DOI] [PubMed] [Google Scholar]
- Yadav, G. , Garg, V. K. , Thakur, N. , & Khare, P. (2013). Locomotor activity of methanolic extract of Saraca indica bark. Advances in Biology Research, 7, 1–3. [Google Scholar]
- Zarghi, A. , & Arfaei, S. (2011). Selective COX‐2 inhibitors: A review of their structure‐activity relationships. Iranian Journal of Pharmaceutical Research, 10(4), 655–683. [PMC free article] [PubMed] [Google Scholar]
- Zhao, G. , Etherton, T. D. , Martin, K. R. , Heuvel, J. P. V. , Gillies, P. J. , West, S. G. , & Kris‐Etherton, P. M. (2005). Anti‐inflammatory effects of polyunsaturated fatty acids in THP‐1 cells. Biochemical and Biophysical Research Communications, 336(3), 909–917. 10.1016/j.bbrc.2005.08.204 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon reasonable request, the corresponding author will provide the data that back up the study's conclusions.


