Abstract
Hematopoietic disorders such as myelodysplastic syndromes (MDS) show a high frequency of methylation of tumor suppressor genes. DNA methyltransferase (DNMT) inhibitors such as azacitidine and decitabine are used to target DNA methylation in MDS patients. Combining these drugs with histone deacetylase (HDAC) inhibitors in vitro resulted in synergistic tumor suppressor gene re-expression. Several phase I trials have examined methylation, gene expression and DNA damage as markers of clinical response to DNMT and HDAC inhibitors, with conflicting results. Trials are ongoing to investigate early methylation changes and DNA damage markers to understand the mechanisms of these drugs and as potential predictors of clinical response.
Keywords: DNA methyltransferase inhibitors, Azacitidine, Histone deacetylase inhibitors, Myelodysplastic syndromes treatment, Predictors of response, Cancer epigenetics
Introduction to cancer epigenetics
Carcinogenesis results from a combination of genetic abnormalities and epigenetic modifications, which lead to dysregulation of the genes controlling cell proliferation, differentiation, and apoptosis [1,2]. Epigenetics refers to modifications of chromatin which determine the transcriptional capacity of a cell. Cancer cells have markedly different epigenomes compared with normal cellular counterparts, and epigenetic silencing of genes is at least as common as that produced by gene mutation and deletion [1,3].
DNA methylation is a good example of the important role of epigenetic modifications in cancer. This process involves the addition of a methyl group at the 5-carbon position of cytosine residue by the enzyme DNA methyltransferase (DNMT) [1,3]. DNA methylation occurs where guanine (G) follows cytosine (C) in the DNA sequence. These are called “CpG dinucleotides”. Clusters of dinucleotides (CpG islands) are often found within transcription promoter regions in DNA [1,3]. When the CpG islands are highly methylated, they bind specific proteins (called methyl-binding proteins) which recruit transcriptional co-repressors such as histone deacetylases (HDACs). Ultimately, expression of the gene is suppressed, referred to as gene silencing [1,4].
Myelodysplastic syndromes and epigenetics
Myelodysplastic syndromes (MDS) are a group of myeloid clonal hemopathies that result in peripheral cytopenias and, in many patients, eventual progression to acute myeloid leukemia (AML) [5]. The extent to which MDS represents epigenetically driven cancers is still being discussed. DNA methylation had been thought to play an important role in the disrupted hematopoiesis. A phase I study in 30 patients with MDS, chronic myelomonocytic leukemia (CMML), or high-risk AML reported a high prevalence of methylation for the tumor suppressor genes p15, CDH-1, DAP-kinase, and SOCS-1. This study showed that the frequency of methylation of these genes was 79%, 48%, 28%, and 62%, respectively [6].
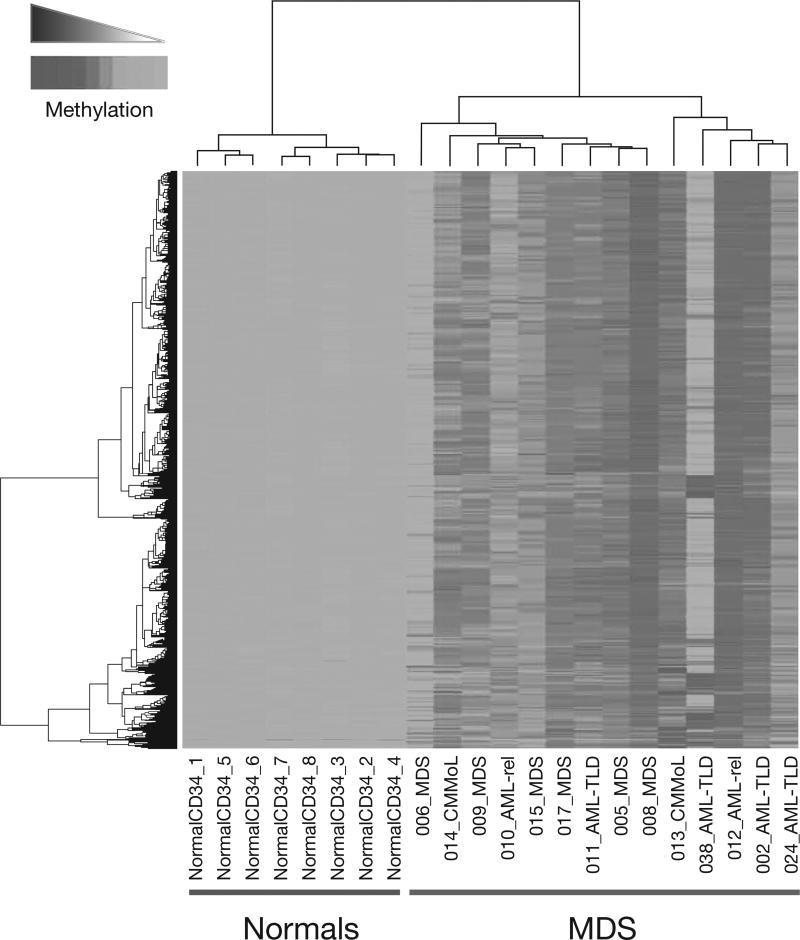
In another study, the cells from 14 patients with MDS were profiled against normal CD34+ cells from 8 control patients and cells from 15 patients with de novo AML using a genomics-based methylation assay called HELP (Hpall tiny fragment enrichment by ligand mediated polymerase chain reaction [PCR]) [7]. The HELP assay provides a quantitative representation of the methylation levels of thousands of CpG-rich sites throughout the genome [8]. Figure 1 shows that over 700 unique genes were hypermethylated in the MDS patients compared with normal CD34+ cells [7].
Fig. 1.
Hypermethylated genes in myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) compared with normal CD34+ cells. A total of 736 unique genes were hypermethylated in 14 MDS patients compared with normal CD34+ cells from 8 controls [7]. Reproduced from Figueroa ME, et al. Blood 2009; doi: 10.1182/blood-2009-01-200519 © 2009 by The American Society of Hematology. All rights reserved.
Epigenetic treatments for MDS
Methyltransferase inhibitors
DNA methylation is a reversible epigenetic process [1], which makes it an attractive potential therapeutic target. The only approved way to target DNA methylation is to inhibit the DNMT enzymes. DNMT inhibitors clinically available and indicated for the treatment of MDS include the nucleoside analogs azacitidine and 2′-deoxy-5-azacytidine, or decitabine. Decitabine differs structurally from normal deoxycytidine by the substitution of the carbon at position 5 with nitrogen [9]. These drugs inhibit DNA methylation in vitro when present during DNA replication. Once incorporated into DNA in place of cytosines, azacitidine and decitabine form irreversible adducts with DNMT, permanently inactivating the enzyme. This leads to depletion of DNMT inside the cell [1]. When DNA synthesis occurs in absence of DNMT, the cytosine residues in daughter DNA strands do not become methylated [10]. Thus, the previously methylated genes can be re-expressed in daughter cells and can potentially promote normal cellular differentiation, senescence, or apoptosis [11].
The use of azacitidine resulted in an overall response rate (complete response [CR] + partial response + hematological improvement) of approximately 44% in MDS and CMML patients, with CRs seen in 13–15% of patients [12]. The overall response rate to the Food and Drug Administration-approved dose schedule of decitabine was 30%, with 9% of patients having a CR [13]. Both azacitidine and decitabine are recommended for the treatment of patients with lower-risk MDS with clinically significant cytopenias [5]. As azacitidine led to a doubling of 2-year overall survival (OS) in higher-risk MDS patients compared to the use of best supportive care, low-dose cytarabine, or intensive cytarabine-based remission induction therapy [14], azacitidine is preferred in patients with higher-risk MDS who are not candidates for stem cell transplantation [5]. Decitabine did not improve survival compared with best supportive care in two randomized trials [13,15].
HDAC inhibitors
HDACs catalyze the removal of acetyl groups from lysine residues of histone tails. Histone deacetylation is associated with heterochromatin formation, and silencing of transcription of associated genes. In some cancer cells, there is overexpression of HDACs [1]. HDAC inhibitors enable reacetylation of the histone lysine residues that are required for transcriptionally active chromatin [1]. This class of agents was developed to induce more normal differentiation in hematopoietic cells; the only clinically available HDAC inhibitor is vorinostat, an oral drug approved for the treatment of cutaneous T-cell lymphoma [16]. Many other HDAC inhibitors are in clinical development.
The DNMT inhibitor + HDAC inhibitor combination
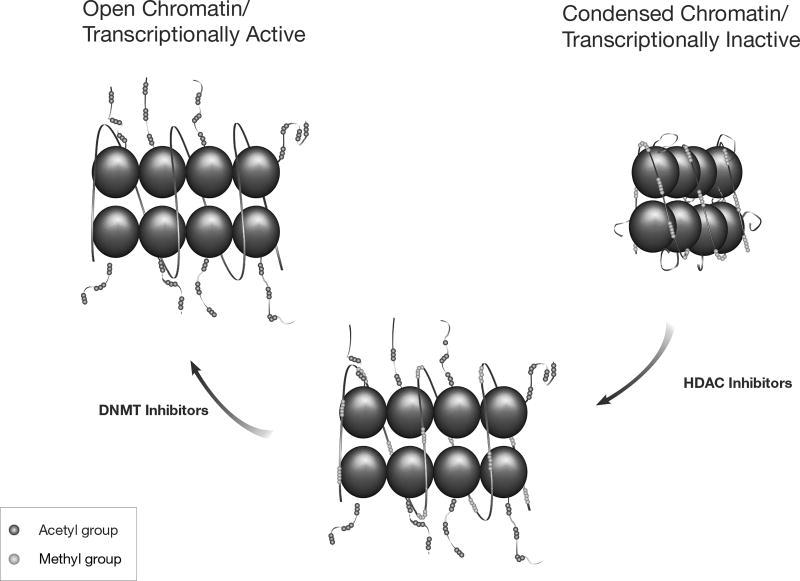
In a malignant cell, the expression of methylated genes is silenced because the lysine-rich tails of associated histones are deacetylated in response to CpG methylation. The methylated cytosines recruit methyl-binding proteins, which recruit transcriptional co-repressors including HDACs [1]. Treatment with a DNMT inhibitor, such as azacitidine, will theoretically reverse the methylation of the genes in daughter cells, causing re-expression of the gene to a small degree. If an HDAC inhibitor is then added, remaining HDACs are blocked. This further drives the chromatin into a more open, transcriptionally active form, which results in more robust gene re-expression. Figure 2 depicts the processes of epigenetic modulation of gene expression by DNA methylation and histone acetylation.
Fig. 2.
DNA methylation and histone acetylation – epigenetic modulation of gene expression. Treatment with a DNA methyltransferase (DNMT) inhibitor can reverse the DNA methylation, resulting in re-expression of the genes. When histone deacetylase (HDAC) inhibitors are added, HDACs are blocked. These processes drive the chromatin into a more open, transcriptionally active, form.
The combination of a DNMT inhibitor with an HDAC inhibitor as a treatment strategy for hematologic malignancies is interesting. This concept comes from in vitro data published by Cameron et al. in which myeloid leukemia cells with a heavily methylated genome (KG1a cells) were exposed to decitabine alone, the prototype HDAC inhibitor trichostatin A alone, and sequence of decitabine followed by the addition of trichostatin A [17]. The DNMT inhibitor alone caused the p15 gene to be re-expressed to a minor degree, the HDAC inhibitor alone did not cause gene re-expression, whereas the sequential use of both drugs together caused synergistic gene re-expression of p15 [17]. The sequence here is crucial; DNMT inhibitor exposure must be first, followed by the HDAC inhibitor.
Methylation levels, gene expression and clinical response
Although both azacitidine and decitabine show clinical response, it is unknown to what extent the clinical activity of DNMT inhibitors depends upon reversal of methylation, or if a clinical response can be predicted by reversal of methylation early in the course of treatment. The ability to predict a clinical response, based on methylation response, would be particularly helpful as currently many patients require 4–6 months of therapy before clinical benefit can be assessed. This approach could, theoretically, be used as a means to monitor therapy in patients with MDS.
Monotherapy with azacitidine
Bone marrow samples from the AZA-001 study, which randomized higher-risk MDS patients to azacitidine or one of three conventional care regimens [14], were examined for the methylation of several tumor suppressor genes [18]. The potential interaction between gene methylation, treatment arm, and OS was investigated. The OS benefit observed with azacitidine versus conventional care was independent of methylation status of the genes analyzed. However, increasing methylation was associated with poorer OS and patients with lower levels of methylation treated with azacitidine had the best OS, suggesting they may obtain greater benefit from azacitidine [18].
Monotherapy with decitabine
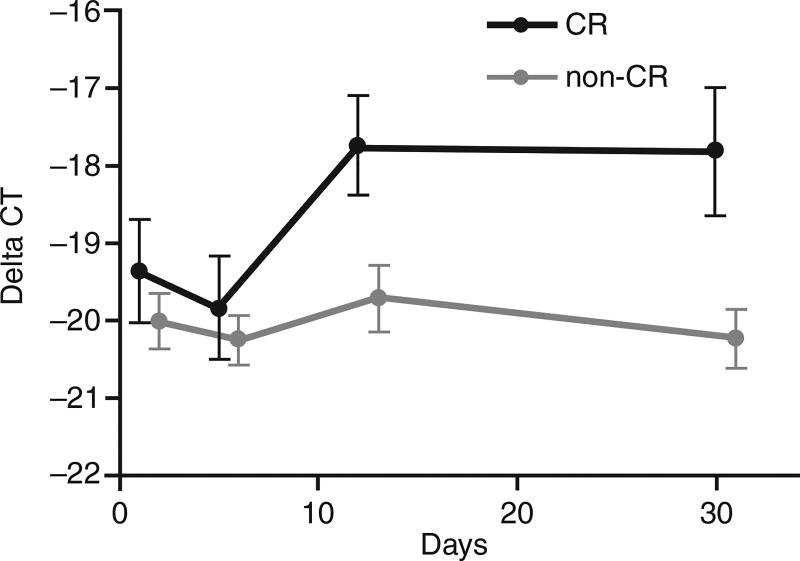
A correlation between an epigenetic target and clinical response was shown in a randomized comparison of three schedules of low-dose decitabine in patients with higher-risk MDS and CMML [19]. The overall clinical response rate reported was 73%, including 34% of patients having a CR. Quantitative real-time PCR was used to look for p15 gene expression in responders versus non-responders. The incremental increase in p15 expression in responders was higher compared with non-responders (Fig. 3) [19]. However, the p15 expression levels were very low so the true biological significance of this should be confirmed in larger studies.
Fig. 3.
Epigenetic modulation by decitabine. p15 gene expression in responders (complete response [CR]) versus non-responders (non-CR) [19]. Reproduced from Kantarjian H, et al. Blood. 2007;109:52–7 © 2007 by The American Society of Hematology. All rights reserved.
Azacitidine + sodium phenylbutyrate
A phase I dose-finding trial was designed to examine the sequential administration of a DNMT inhibitor (azacitidine) followed by an HDAC inhibitor (sodium phenylbutyrate) in patients with MDS or AML. The study end points were tolerability, response rates, and whether clinical responses were associated with reversal of hypermethylation [20]. Azacitidine was given subcutaneously at varying doses for 5, 10, or 14 days of each 28-day cycle. Sodium phenylbutyrate was given as a 7-day continuous infusion following azacitidine. Eleven of 29 evaluable patients (38%) responded to therapy. Twelve patients were analyzed for hypermethylation of p15 and/or CDH-1 using methylation-specific PCR [20]. All patients with reversed methylation of these genes showed a clinical response (n = 6), whereas the patients who did not show methylation reversal were all non-responders (n = 6). The comparison between responders and non-responders was statistically significant (p = 0.002).
Azacitidine + entinostat
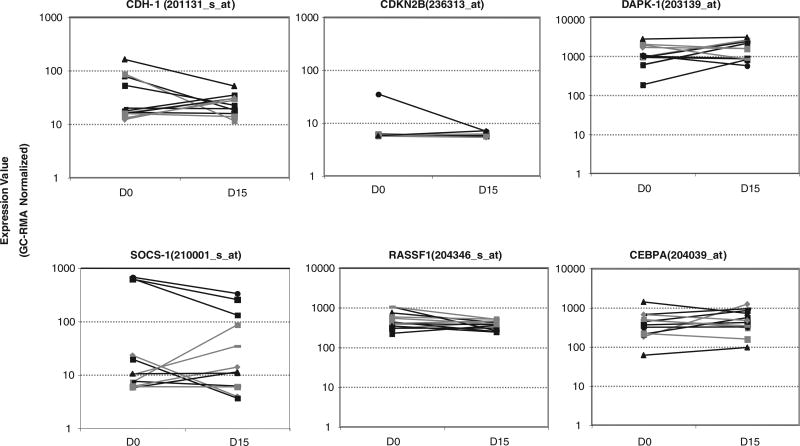
A more recent phase I trial examined the combination of azacitidine with a more potent orally bioavailable HDAC inhibitor, entinostat (MS-275), in 30 patients with MDS, CMML, or high-risk AML [6]. Azacitidine was given at varying doses for 10 days of each 28-day cycle. Entinostat was given at four different doses on days 3 and 10 only [6]. Clinical response was seen in 46% of patients, 50% of which were CRs or partial responses [6]. A correlation between a clinical response and reversal of methylation in four different tumor suppressor genes (p15, CDH-1, DAP-kinase, and SOCS-1) was not found in this study (Fig. 4) [6].
Fig. 4.
Plots of normalized gene expression in evaluable patients before and after one cycle of azacitidine and entinostat. Tested tumor suppressor genes included CDH-1, p15 (denoted CDKN2B), DAPK-1, SOCS-1, and two other genes (RASSF1 and CEBPA) frequently hypermethylated in myelodysplastic syndromes [6]. Reproduced from Fandy TE, et al. Blood. 2009;114:2764–73 © 2009 by The American Society of Hematology. All rights reserved.
Expression arrays were performed at various time points in CD34+ cells of treated patients. Focusing on genes which are frequently methylated in MDS and AML, there was no signature that differentiated responding patients from non-responding patients overall, or any expression signature at baseline predicting the response [6]. However, these arrays may not have been sufficiently sensitive to detect the small changes in gene expression [6].
It is unclear why there was such a difference in the apparent association between methylation reversal and clinical response in the two phase I trials discussed above, but it may be related to the timing and the treatment sequence of the actual HDAC inhibitor. In the first trial, phenylbutyrate was given after completion of azacitidine, whereas in the second trial, azacitidine and entinostat were given concurrently for 1 week. As DNMT inhibitors require cells to be actively dividing for activity, and HDAC inhibitors are potent inhibitors of the cell cycle, it might be that entinostat decreased incorporation of azacitidine into DNA [6].
DNA damage and clinical response
DNA damage may be a confounder when investigators study the best use of epigenetics to monitor and predict clinical responses to DNMT and HDAC inhibitors. Azacitidine and decitabine are both DNA damaging agents which act through direct incorporation into DNA [21,22]. In addition, HDAC inhibitors cause apoptosis by increasing DNA damage and causing mis-repair of double-stranded DNA breaks [23]. Gamma H2AX is a surrogate marker for DNA damage. Its expression is closely correlated with double-stranded DNA breaks. In HCT116 colon cancer cells with DNMTs knocked out, decitabine still induced DNA damage as suggested by gamma H2AX expression [24]. Decitabine also induced gamma H2AX expression in leukemia cells compared with no treatment [22]. These studies imply that the DNA damage caused by hypomethylating agents is not entirely DNMT-mediated. In the recent phase I study of azacitidine plus entinostat, gamma H2AX expression induction was used to assess the degree of DNA damage. Starting at the lowest dose of azacitidine (30mg/m2) and going up the entinostat dose–response curve, there is increasing induction of gamma H2AX. Likewise, starting at the lowest entinostat dose (2mg/m2) and going up the azacitidine dose–response curve, there is also induction of gamma H2AX [6]. As with methylation reversal, no correlation was found between DNA damage and clinical response. Thus, the precise mechanism by which these classes of drugs effect their clinical responses remains uncertain.
Future trials
Comparison of DNMT inhibitor + HDAC inhibitor combination dosing strategies
Considering the conflicting results observed in these two phase I studies combining DNMT and HDAC inhibitors, a further study is planned with azacitidine and entinostat in patients with AML to compare sequential administration of DNMT and HDAC inhibitors versus the overlapping schedule examined in the Fandy et al. study [6]. This study will test the hypothesis that sequential administration may induce a greater degree of methylation reversal, gene re-expression, and more clinical responses.
Phase II randomized trial: E1905 Intergroup
The relevance of methylation changes is also being investigated in a phase II randomized study of azacitidine (50mg/m2/day for 10 days), with or without oral entinostat on days 3 and 10, in approximately 200 patients with MDS, CMML, or AML. The goal is to double the rate of hematologic normalization from the 15% expected from azacitidine alone to 30%. A variety of molecular studies are being carried out to determine if methylation changes are important, if DNA damage is the more important marker of clinical response, or if it is a combination of both. Studies will include gene-specific methylation studies, genomic-based methylation studies, and DNA damage as assessed by gamma H2AX expression. This study has finished accrual, and data should be available early 2010 (ClinicalTrials.gov Identifier NCT00313586).
Conclusions
Epigenetic processes remain highly attractive targets for anti-cancer therapies. Drugs that target these epigenetic processes have profound anti-tumor activity in vitro. The DNMT inhibitors azacitidine and decitabine, given alone or in combination with HDAC inhibitors, have profound anti-tumor activity in vivo in the treatment of hematologic malignancies. However, the extent to which clinical activity and response depend on epigenetic activities remains unclear. Phase I studies of hypomethylation and gene re-expression in MDS, following treatment with DNMT and HDAC inhibitors, have reported conflicting results. Further studies are required to identify the optimal epigenetic drug targets, and the mechanisms underlying clinical response and survival in malignancies.
Acknowledgments
The author received editorial support from Excerpta Medica in the preparation of this manuscript, funded by Celgene Corporation. The author is fully responsible for content and editorial decisions for this manuscript.
Conflict of interest statement
The author has received consultancy fees and research funding from Celgene Corporation, and has equity ownership in Celgene Corporation.
References
- 1.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 2.Fenton RG, Longo DL. Cancer cell biology and angiogenesis. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 16. New York: McGraw-Hill; 2005. pp. 453–64. [Google Scholar]
- 3.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–59. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 4.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Myelodysplastic Syndromes. Version 2.2010. Clinical Practice Guidelines in Oncology. [Accessed October 9, 2009]. Available at: http://www.nccn.org.
- 6.Fandy TE, Herman JG, Kerns P, Jiemjit A, Sugar EA, Choi SH, et al. Early epigenetic changes and DNA damage do not predict clinical response in an overlapping schedule of 5-azacytidine and entinostat in patients with myeloid malignancies. Blood. 2009;114:2764–73. doi: 10.1182/blood-2009-02-203547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueroa ME, Skrabanek L, Li Y, Jiemjit A, Fandy TE, Paietta E, et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood. 2009 doi: 10.1182/blood-2009-01-200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa ME, Melnick A, Greally JM. Genome-wide determination of DNA methylation by Hpa II tiny fragment enrichment by ligation-mediated PCR (HELP) for the study of acute leukemias. Methods Mol Biol. 2009;538:395–407. doi: 10.1007/978-1-59745-418-6_20. [DOI] [PubMed] [Google Scholar]
- 9.Prescribing information. Dublin, CA: SuperGen, Inc.; 2008. Dacogen® (decitabine for injection) [Google Scholar]
- 10.Lu LJ, Randerath K. Long term instability and molecular mechanism of 5-azacytidine-induced DNA hypomethylation in normal and neoplastic tissues in vivo. Mol Pharmacol. 1984;26:594–603. [PubMed] [Google Scholar]
- 11.Silverman LR. Targeting hypomethylation of DNA to achieve cellular differentiation in myelodysplastic syndromes (MDS) Oncologist. 2001;6(Suppl 5):8–14. doi: 10.1634/theoncologist.6-suppl_5-8. [DOI] [PubMed] [Google Scholar]
- 12.Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Arbitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer. 2006;106:1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 14.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijermans P, Suciu S, Baila L, Platzbecker U, Giagounidis A, Selleslag D, et al. Low dose decitabine versus best supportive care in elderly patients with Intermediate or High risk MDS not eligible for intensive chemotherapy: final results of the randomized phase III study (06011) of the EORTC Leukemia and German MDS Study Groups. Blood. 2008;112 Abstract 226. [Google Scholar]
- 16.Prescribing information. Whitehouse Station, NJ: Merck & Co., Inc.; Jul, 2008. Zolinza™ (vorinostat capsules) [Google Scholar]
- 17.Cameron EE, Bachman KE, Myöhänen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–7. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 18.Herman JG, Gore SD, Mufti GJ, Fenaux P, Santini V, Silverman LR, et al. Relationship among gene methylation, azacitidine treatment, and survival in patients with higher-risk myelodysplastic syndromes (MDS): results from the AZA-001 trial. AACR. 2009 Abstract 4746. [Google Scholar]
- 19.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O’Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–7. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 20.Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–9. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 21.Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008;123:8–13. doi: 10.1002/ijc.23607. [DOI] [PubMed] [Google Scholar]
- 22.Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. DNA methylation inhibitor 5-aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. 2008;28:752–71. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosato RR, Almenara JA, Dai Y, Grant S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther. 2003;2:1273–84. [PubMed] [Google Scholar]
- 24.Jiemjit A, Fandy TE, Carraway H, Bailey KA, Baylin S, Herman JG, et al. p21(WAF1/CIP1) induction by 5-azacytosine nucleosides requires DNA damage. Oncogene. 2008;27:3615–23. doi: 10.1038/sj.onc.1211018. [DOI] [PMC free article] [PubMed] [Google Scholar]