Abstract
Critical events in the life cycle of herpes simplex virus (HSV) are the binding of cytoplasmic capsids to cellular organelles and subsequent envelopment. Work from several laboratories suggests that these events occur as a result of a network of partially redundant interactions among the capsid surface, tegument components, and cytoplasmic tails of virally encoded glycoproteins. Consistent with this model, we previously showed that tegument protein VP16 can specifically interact with the cytoplasmic tail of envelope protein gH in vitro and in vivo when fused to glutathione S-transferase and to green fluorescent protein, respectively. In both instances, this association was strikingly temperature dependent: binding occurred only at 37°C and not at lower temperatures. Here we demonstrate that virally expressed full-length gH and VP16 can be coimmunoprecipitated from HSV-infected cells and that this association is also critically dependent upon the physiological temperature. To investigate the basis of this temperature requirement, we performed one- and two-dimensional nuclear magnetic resonance spectroscopy on peptides with the sequence of the gH tail. We found that the gH tail is disorganized at temperatures permissive for binding but becomes structured at lower temperatures. Furthermore, a mutated tail unable to adopt this rigid conformation binds VP16 even at 4°C. We hypothesize that the gH tail is unstructured under physiological conditions in order to maximize the number of potential tegument partners with which it may associate. Being initially disordered, the gH tail may adopt one of several induced conformations as it associates with VP16 or alternative components of the tegument, maximizing redundancy during particle assembly.
Herpes simplex virus (HSV) type 1 (HSV-1) is an alphaherpesvirus with a 150-kb double-stranded genome packaged within an icosahedral capsid. The capsid is surrounded by an amorphous protein layer known as the tegument, which is in turn surrounded by an envelope derived from host cell organelle membranes. Within the envelope are multiple virally encoded glycoproteins, which function in many aspects of the viral life cycle, such as host membrane attachment and fusion, immune evasion, and prevention of apoptosis (23, 28). The tegument is composed of at least 19 known viral polypeptide species, which perform multiple functions during the life cycle of the virus (28).
Several studies have indicated that viral envelopment and tegumentation occur at late Golgi or post-Golgi compartments, such as the trans-Golgi network and endosomes (4, 6, 15, 16, 23, 25, 31, 33). However, the molecular details that drive envelopment are still poorly understood. It is thought that a network of interactions among viral glycoproteins present in the host organelle membranes, tegument proteins, and capsid proteins is critical for this process. In this model, tegument proteins serve as a bridge, binding to the cytoplasmic tails of viral glycoproteins, each other, and capsid proteins, thus recruiting capsids to the site of envelopment and allowing capsids to bud into organelles. In support of this hypothesis, several interactions were recently shown or suggested among various tegument proteins (9, 10, 20, 22, 32), between the tegument and glycoproteins (12, 36), and between the capsid and the tegument (24, 35).
It appears that the web of interactions among the tegument and glycoproteins is very redundant. For example, VP16 is known to interact with VP22 (10) and Vhs (29, 32) as well as gH (13, 36) and possibly gD and gB (36). Also, in pseudorabies virus, tegument protein VP22 binds to both gM and gE (12), and simultaneous deletion of both glycoproteins is required to abrogate envelopment and prevent incorporation of VP22 into mature viral particles (3). In HSV, simultaneous deletion of several glycoprotein tails, such as gE, gI, and gD, is required in order to appreciably affect viral egress and inhibit viral maturation and envelopment (5, 11).
Previous work from our laboratory showed that VP16 interacts with the cytoplasmic tail of gH both in vitro and in vivo (13). In these experiments, VP16 could be specifically recovered from infected cell extracts by using a glutathione S-transferase-gH tail fusion protein. Additionally, in cells transfected with a construct expressing green fluorescent protein (GFP) fused to the gH tail and infected with wild-type HSV, anti-GFP antibodies could be used to coimmunoprecipitate VP16. These experiments also revealed that the interaction between VP16 and the gH carboxy terminus is temperature dependent, in that binding took place only at the physiological temperature and not at lower temperatures. Alanine scanning mutagenesis of the gH tail revealed that replacing a single proline residue at the center of the tail with an alanine stimulated the binding of VP16 to gH at 37°C and also relieved the temperature constraints of binding, allowing gH to interact with VP16 even at 4°C (13).
Here we report that virally expressed gH and VP16 can be coimmunoprecipitated from HSV-infected cells, confirming our previous in vitro and in vivo data. As was found in our earlier studies, this interaction is strikingly temperature dependent, occurring only at 37°C. Furthermore, two-dimensional (2D) nuclear magnetic resonance (NMR) analyses of synthetic peptides with the sequence of the gH cytoplasmic tail reveal that the temperature dependence of binding can be explained by conformational changes in the tail. We propose that the gH tail has evolved to be unstructured at a physiologically relevant temperature in order to interact with multiple possible tegument partners during HSV assembly.
MATERIALS AND METHODS
Cells and viruses.
COS7 or Vero cells were grown in Dulbecco's modified Eagle's medium supplemented with 1% penicillin-streptomycin and 10% fetal or newborn calf serum (GIBCO Laboratories), respectively. HSV strain SC16 was prepared as previously described (7). gH-negative HSV-1 SC16 (SCgHZ) was grown by infecting F6 complementing cell lines (stably expressing gH) (34) at a multiplicity of 0.01 for 65 h at 37°C. Media and cells were harvested, concentrated, sonicated, divided into aliquots, and frozen. Virus was titrated on F6 cells.
Antibodies.
Anti-VP16 monoclonal antibody 14-5 and goat anti-VP16 polyclonal antibody v-20 were obtained from Santa Cruz Biotechnology. Rabbit anti-goat antibody (A-5420) and goat anti-rabbit immunoglobulin G (A-6154) were obtained from Sigma.
Coimmunoprecipitation of full-length gH and VP16.
Cells were infected with HSV-1 strain SC16 or SCgHZ at a multiplicity of 10 or left uninfected. At 18 h postinfection, postnuclear supernatants (PNS) were prepared as previously described (15). Lysis buffer was added to each sample of PNS to final concentrations of 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 0.5% NP-40, 0.5% EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 5 μg/ml of leupeptin, 5 μg/ml antipain, 2 mM dithiothreitol, and 200 μg/ml of bovine serum albumin, and the mixtures were incubated for 30 min on ice. The resulting lysates were centrifuged for 15 min at 15,000 × g and precleared by incubation with prewashed protein G-agarose beads for 20 min at the temperature subsequently used for immunoprecipitation. After the beads were pelleted for 20 s at 15,000 × g, the supernatants were collected and mixed with new protein G beads that had been preincubated with mouse anti-VP16 monoclonal antibody for 1 h at 4°C. Incubation was performed for 30 min at the appropriate temperature. After the beads were washed five times with lysis buffer, bead-bound and unbound materials were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotted with goat anti-VP16 and rabbit anti-gH antibodies as previously described (13).
Synthesis of peptides with the sequences of the wild-type and proline-to-alanine mutant gH tails.
The sequences of the synthesized peptides were KVLRTSVPFFWRRE, corresponding to the wild-type gH cytoplasmic tail, and KVLRTSVAFFWRRE, corresponding to the proline-to-alanine (PA) mutant tail (13). Peptides were synthesized on a 0.1-mmol scale by solid-phase peptide synthesis with an ABI433A peptide synthesizer and a standard protocol (9-fluorenylmethoxy carbonyl chemistry). Preloaded resin reagents piperidine, diisopropylethylamine, N-methylpyrrolidone, dichloromethane, and 9-fluorenylmethoxy carbonyl amino acids and an hydroxybenzotriazole activation kit were purchased from Applied Biosystems. Peptide resins were cleaved and deprotected by using a mixture of trifluoroacetic acid, thioamisole, ethanedithiol, phenol, and water for 2 h. Peptides were precipitated with ether and washed.
Peptides were characterized by matrix-assisted laser desorption ionization-time-of-flight mass spectroscopy with a Perspective Biosystems Voyager DE as well as by analytical high-pressure liquid chromatography (HPLC) with a C8 reverse-phase column (2.1 by 250 mm) on a Hewlett-Packard HPLC 1090 instrument. Amino acid analysis was performed with a Hewlett-Packard Amino Quant HPLC system. Peptides were purified by HPLC with 1-cm C18 reverse-phase columns and dissolved in phosphate buffer to final concentrations of 2.7 mM peptide in 15 mM phosphate buffer at pH 6.1 (for the wild-type gH tail peptide) and 0.14 mM peptide in 20 mM phosphate buffer at pH 6.0 (for the PA mutant gH tail peptide).
NMR spectroscopy of wild-type and mutant gH peptides.
All NMR spectra were recorded at 600 MHz on a Bruker DRX600 spectrometer. 1H chemical shifts were referenced to an internal 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid standard at 0 ppm. Standard methods were used to obtain homonuclear DQF-COSY (30), TOCSY (2, 27), and NOESY (17) spectra for wild-type gH and PA peptides at 10°C and 37°C in 90% H2O and 10% D2O. The TOCSY spectra were acquired with spin-lock periods of 42 and 100 ms, and the NOESY spectra were obtained with 300-ms mixing times. For all 2D spectra, 32 scans were collected. The spectra were acquired with 4,096 complex data points in the direct dimension and 680 points in the indirect dimension, with a spectral width of 14 ppm (8,389 Hz) in both dimensions.
The one-dimensional (1D) spectra of the wild-type gH and PA peptides were collected at a series of temperatures ranging from 2 to 45°C in approximately 5° increments. 1D spectra for the wild-type gH peptide were acquired with 64 scans, for the PA peptide 1,000 scans were collected, and for both wild-type gH and PA peptides spectra were obtained with 2,048 complex data points. The temperature dependence of every identifiable resonance was monitored.
The spectra were processed by using NMRPipe (8) and analyzed by using the NMRView program (18). All structural calculations were performed by using DYANA version 1.5 (14) and MOLMOL (21). Coupling constants were measured by using the XEASY computer program (1). The Karplus equation (19) was used to derive φ angle constraints from the measured coupling constants. Distance constraints were derived from NOE cross-peak intensities within NMRView and corrected for pseudoatoms and methyl intensities within DYANA.
RESULTS
Coimmunoprecipitation of full-length gH and VP16.
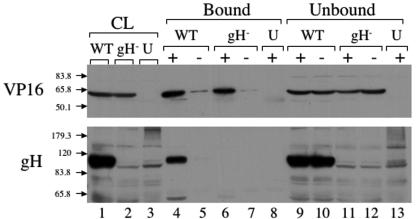
In our previous studies, we were able to show that VP16 and the gH tail interact in vivo, using a fusion protein between GFP and the gH tail (13). To definitively establish that VP16 and gH interact in the context of an authentic in vivo infection, we attempted to coimmunoprecipitate full-length VP16 and gH from infected cells. We prepared PNS from cells infected with either SC16 (a wild-type strain of HSV) or SCgHZ (a gH-negative strain of HSV) or from cells that were left uninfected. The PNS were treated with salt and detergent as outlined in the Materials and Methods to release gH and VP16 from membranes and virus particles. The lysates were then cleared by centrifugation, and incubated with prewashed Protein G beads to minimize background binding. These precleared lysates were then incubated with fresh Protein G beads that were either untreated or precoated with a monoclonal antibody directed against VP16. Immunoprecipitation was then allowed to occur at 37°C or other temperatures. Material that was immunoprecipitated with the beads as well as that remaining unbound was subjected to SDS-PAGE, and then Western blotted with polyclonal anti-VP16 or anti-gH antibodies (Fig. 1).
FIG. 1.
Coimmunoprecipitation of gH and VP16 from infected cells. Cell lysates were prepared from cells infected with HSV (wild type [WT]) or gH-null HSV (gH−) or from uninfected cells (U). Lysates were incubated (+) or not incubated (−) with mouse anti-VP16 monoclonal antibody at 37°C. Total cell lysates (CL: lanes 1 to 3), immunoprecipitated (bound) material (lanes 4 to 8), and unbound material (lanes 9 to 13) were subjected to SDS-PAGE, Western blotted, and probed with anti-gH and anti-VP16 antisera as indicated at the left. Positions and masses (in kilodaltons) of molecular mass markers are indicated by arrows. Note that 1/10 the total unbound material was run on the gels.
VP16 was immunoprecipitated from the PNS lysates when incubated with beads that were coated with anti-VP16 antibody (Fig. 1, top panel, lanes 4 and 6), but not with untreated control beads (lanes 5 and 7), nor from mock-infected cell extracts (lane 8). The lower panel in Fig. 1 shows the Western blot with anti-gH antibody. In lane 4, which corresponds to material immunoprecipitated from wild-type HSV-infected cell lysates using anti-VP16 antibody, a band was visible at ∼110 kDa, corresponding to the molecular weight of gH. This band disappeared when anti-VP16 antibodies were omitted (Fig. 1, lane 5, lower panel) or when the infecting virus was a gH-null strain (lanes 6 and 7, lower panel). These data imply that full-length gH can be coimmunoprecipitated with VP16, suggesting the two polypeptides interact in infected cells, consistent with our findings (13) and earlier cross-linking studies (36). In control experiments, gC was found to not specifically coimmunoprecipitate with anti-VP16 antibodies, showing that gH is not recovered as part of a “bulk” incompletely solubilized envelope fraction. Nevertheless, we cannot discount the possibility that other envelope and tegument components may be specifically associated with the VP16/gH complex, and we are currently examining this possibility.
Temperature dependence of VP16/gH interaction.
In our previous studies we found that binding of gH to VP16 was temperature dependent -binding was detectable within 1 h when incubations were conducted at 37°C, but not at room temperature or 4°C (13). We wanted to test whether the in vivo binding of full-length gH and VP16 was similarly dependent on temperature. Figure 2 shows a series of immunoprecipitation reactions incubated at different temperatures with lysates from SC16-infected cells in the presence (lanes 2, 4, 6, 8, 10, and 12) or absence (lanes 3, 5, 7, 9, 11, and 13) of VP16 antibody. As with the previous experiment, material that was bound (Fig. 2, lanes 2 to 7) or unbound (lanes 8 to 13) to the beads, and the initial cell lysate (lane 1) were subjected to SDS-PAGE and Western blotted for VP16 and gH. Consistent with previous in vitro and in vivo findings, full-length gH only coimmunoprecipitated with VP16 at 37°C but not at room temperature or 4°C.
FIG. 2.
Temperature dependence of VP16/gH coimmunoprecipitation. Coimmunoprecipitation experiments were performed as described in the legend to Fig. 1, except that incubation was done at 37°C, room temperature (RT), or 4°C. Western blotting was performed with total cell lysates (CL: lane 1) as well as bound (lanes 2 to 7) and unbound (lanes 8 to 13) material as described in the legend to Fig. 1. Note that 1/10 the total unbound material was run on the gels.
The striking temperature dependence of VP16/gH association, suggested the possibility that some temperature-dependent biochemical modification of VP16, or of other polypeptides in the extract, may be a prerequisite for interaction with the gH tail. However, preincubation of infected cell extracts at 37°C did not permit subsequent binding of VP16 to glutathione S-transferase-gH at 4°C (data not shown), implying that if such modifications occur, they are reversed at 4°C or require the simultaneous presence of the gH tail.
As an alternative, we considered the possibility that the temperature dependence of binding might result from conformational changes in the gH tail. In this model, the conformation of gH at 37°C is compatible with VP16 binding, but at reduced temperatures the tail undergoes conformational changes which preclude its association with VP16. Since our previous studies have shown that mutation of the proline residue in the center of the tail permits VP16 binding even at 4°C (13), this hypothesis predicts that the mutant tail would be in the binding-permissive conformation at all temperatures. To test our hypothesis, we examined the structural properties of peptides corresponding to the cytoplasmic tails of wild-type gH and the PA mutant.
Conformation of gH C-terminal peptides.
We used 1D and 2D NMR spectroscopy to characterize the temperature-dependent conformations of peptides corresponding to the C-terminal segments of wild-type (KVLRTSVPFFWRRE) and PA mutant gH (KVLRTSVAFFWRRE), beginning with the wild-type sequence at low temperature. 2D homonuclear DQF-COSY, TOCSY, and NOESY NMR spectra were used to assign the resonances of the peptide at 10°C. Figure 3A shows the fingerprint region of a 2D TOCSY spectrum. This experiment allows one to correlate, through scalar coupling, most or all of the protons in the spin system of a given residue. This region of the spectrum is particularly useful because the direct dimension represents the amide proton chemical shift, and the indirect dimension shows a set of cross-peaks to all other scalar-coupled protons in the side chain. One observes a single set of correlations for each amino acid except proline. Using these data, we were able to assign a residue type to each amide, and hence identify all the amino acid types in the peptide. A few cross-peaks in the TOCSY spectrum remained unassigned; these cross-peaks likely arise from contaminants from the peptide synthesis, and did not affect the analysis. A 2D DQF-COSY, which exclusively correlates protons on neighboring carbon or nitrogen nuclei via 3JH-H scalar coupling, was used to unambiguously assign all side chain protons, including those of Pro8 (the single proline in the tail, lying close to the center of the sequence) which do not appear in the fingerprint region of the TOCSY spectrum.
FIG. 3.
2D NMR spectra of wild-type gH peptide. (A) 2D TOCSY spectrum highlighting the amide side-chain correlations. Assignments of residue and atom types are shown. (B) 1HN-1Hα region of the NOESY spectrum. The sequence-specific connections are indicated by red lines.
A 2D NOESY experiment was used to make sequence-specific assignments. Figure 3B shows the 1HN-1Hα region of the NOESY spectrum. Each cross-peak occurs at either the chemical shift of the 1HNi-1Hαi or the 1HNi-1HαI−1, where i represents an amino acid residue in the sequence. The 1HN-1Hβ chemical shifts of Thr5 and Ser6 are also seen in this region, but are not used in the assignment process. Connections between amino acids that are adjacent in sequence are indicated in Fig. 3B. Several resonances were overlapped, including Leu3 and Arg4, Val2 and Arg13, and Thr5, and Val7 and Phe9, but it was still possible to make connections between resonances, and hence the sequence-specific assignments.
Most observed NOEs were intraresidue or short-range between adjacent residues. However, a significant number of longer medium-range NOEs were observed in the central region of the peptide, between protons in residues 5 through 10 (Fig. 4). Figure 4A illustrates NOEs between residues 8 and 5 and residues 10 and 7, with additional NOEs between residues 10 and 7 and residues 9 and 7 visible in Fig. 4B and C. A total of 113 intraresidue, 47 interresidue, and 21 medium-range NOEs were used to derive distance constraints for structure calculations. 3JN − α coupling constants were measured and used to define phi angle constraints for residues 2, 4 to 7, 9, and 11 to 14. For the structure calculation, these angles were loosely defined as falling in the range of −35° to −175°.
FIG. 4.
Expansions of the NOESY spectrum of wild-type gH peptide showing medium-range NOEs. The regions shown are NOEs between the side chains of Thr5 and Pro8 and of Val7 and Phe10 (A), the amide of Phe10 and the side chain of Val7 (B), and ring protons of Phe10 and the side chain of Val7 (C).
NMR structural models for the gH peptide at 10°C were calculated by using the program DYANA (14). Residues 4 through 10 of the nine lowest-energy structures are shown in Fig. 5. All distance constraints were satisfied to within 0.8 Å, and all angle constraints were satisfied to within 5°. The root mean square deviations between backbone atoms for this segment was 0.9 Å. Residues 1 to 3 and residues 11 to 14 appeared highly disordered, as expected for the ends of a peptide, and hence are not shown. The side chains of residues 7, 8, and 10 are highlighted in green in Fig. 5. Several NOEs were observed between residues 7 and 10 (Fig. 4). It is noteworthy that this small region of stable structure is centered around Pro8, the residue whose mutation to alanine eliminates the temperature dependence of peptide binding to VP16 (13).
FIG. 5.
Structural model of wild-type gH tail peptide at 10°C. The nine lowest-energy structures are superimposed. Residues 1 to 3 and residues 11 to 14 are highly disordered and were omitted for clarity. The backbone is colored blue, and the side chains are colored red, with the exception of those of Val7, Pro8, and Phe10, which are highlighted in green.
Temperature dependence of wild-type and mutant gH peptide conformations.
We next investigated whether any temperature-dependent structural differences existed between the two peptides. We were unable to determine a structure of the wild-type peptide at 37°C due to the scarcity of NOEs (indicating a lack of structure), and the low solubility of the PA mutant precluded 2D NMR measurements at any temperature. We therefore examined the temperature dependence of the 1D 1H NMR spectra for both peptides. Figure 6 shows a region of the 1D NMR spectrum containing aliphatic proton resonances for both peptides at a series of temperatures. In the wild-type peptide, a number of resonances shift with temperature (Fig. 6A). Figure 6C shows the temperature profiles of side chain resonances for residues 4, 8, and 10. The chemical shift changes with respect to temperature were sigmoidal, suggesting a cooperative thermal transition that corresponds to a loss of stable structure in the wild-type peptide. Interestingly, there was very little to no temperature dependence of the chemical shifts in the PA mutant (Fig. 6B), suggesting that the PA mutant has no stable structure at any temperature examined. Note that the profile of the (unstructured) wild-type peptide at the higher temperatures is expected to be similar, but not identical, to that of the mutant peptide. This is because in the PA mutant one loses the signals for the proline, gains signals from the new alanine, and the signals of the two amino acids surrounding the mutation (one on each side) will shift at least slightly as a result of being chemically bonded to a different residue type.
FIG. 6.
Temperature dependence of aliphatic proton NMR resonances in wild-type and mutant gH peptides. (A) 1D 1H NMR spectra of wild-type (WT) gH peptide recorded from 2°C (bottom) to 45°C (top). The red lines indicate a significant change in chemical shift with temperature. (B) 1D 1H NMR spectra of gH PA mutant peptide recorded from 2 to 40°C. The red lines indicate little to no change in chemical shift with temperature. (C) Temperature profiles of three individual resonances (Arg4, Pro8, and Phe10, indicated in the single-letter amino acid code) of the wild-type gH peptide indicating cooperative changes with temperature.
DISCUSSION
In our previous studies we have shown that VP16 and the gH cytoplasmic tail interact, and that the binding was strikingly affected by temperature, both in vitro and in vivo (13). Here we have confirmed the in vivo binding of full-length gH and VP16, and shown that it exhibits similar temperature dependence to our in vitro assay system. The observation that mutation of the central proline residue (Pro8) to alanine relieved this temperature dependence prompted us to test the effects of temperature on the conformation of synthetic peptides with the sequence of the gH cytoplasmic tail.
The solution conformations of the gH peptide exhibited several interesting features. We first attempted to determine the structure at 37°C, but very few NOEs were observed in either NOESY or ROESY spectra, indicating that the peptide is dynamic and unstructured at this temperature. We were able to determine the structure at 10°C. A number of obvious medium-range NOEs were observed between protons of residues 5 through 10, including several between protons of Val7 and Phe10. These two residues are in close van der Waals contact in the resulting structural models. This results in a tight turn of the peptide backbone, facilitated by Pro8. There is also a distinct NOE between the δ1 proton of Pro8 and the γ proton of Thr5. The absence of a NOE involving the δ2 proton suggests that the backbone is in a specific, stable structure. We propose that this small amount of stable structure is what inhibits binding to VP16 at temperatures lower that 37°C. At 37°C, the energy of thermal fluctuations is greater than the energy of the intramolecular interactions, and therefore the structure is destabilized. This is consistent with an induced fit mechanism in which the peptide must be unstructured in order to bind VP16. Any preformed structure inhibits binding.
This idea is supported by the fact that the PA mutant can bind at any temperature studied. A proline can restrict the conformation of a peptide. This in turn would reduce the conformational entropy contribution to the Gibbs free energy, hence allowing the enthalpy term to make a greater contribution. Thus, a few relatively weak interactions could result in the stabilization of a structure when a proline is present. Conversely, as in this situation, substitution of a proline with alanine increases the entropy and destabilizes the peptide structure. We attempted to determine the structure of the PA mutant at 10°C but were unsuccessful. The PA mutant is much less soluble than the wild-type peptide and yielded very weak NMR signals. Hence, we carried out a series of 1D 1H NMR experiments at temperatures varying from 2 to 40°C (Fig. 6). If one looks carefully at the wild-type peptide data, it is apparent that several resonances shift with temperature. Closer inspection reveals that the temperature profiles of individual resonances have a sigmoidal shape, typical of a cooperative denaturation curve. On the contrary, in the PA NMR spectrum, it is apparent that the resonances in the same spectral region have very little to no temperature dependence. This suggests that the mutant peptide does not contain the structure that is present in the wild-type peptide.
In summary, we have demonstrated that at 10°C a small segment of the gH peptide is structured. As the temperature is increased, this structure denatures. It is the loss of this structure that facilitates the peptide binding to VP16. The presence of the proline at position eight is a major contributor to the stability of this structure, and hence, if one mutates this proline to alanine, the structure is completely destabilized and the peptide binds freely at all temperatures.
The residues involved in the conformational transitions, as identified by sequential 1D NMR spectra, are remarkably consistent with the findings of our previous mutagenesis study (13). These residues include the leucine at position 3, the proline at position 8, the phenylalanine at position 10, the tryptophan at position 11, the glutamic acid residue at position 14, and to a more limited extent the arginine at position 13. These residues have all been shown to be involved in VP16 binding, either by promoting binding (Leu3, Phe10, Trp11, and Arg13) or by interfering with it (Pro8 and Glu14) (13).
It is becoming increasingly clear that HSV particle assembly is a process with a great deal of inherent redundancy (3, 11, 12, 26). The fact that the gH cytoplasmic tail must be unstructured in order to bind VP16 suggests one mechanism by which such redundancy may be achieved. We hypothesize that gH may bind VP16 via an “induced-fit” mechanism, wherein the tail must be malleable and unstructured before binding in order to assume a specific conformation upon interaction with VP16 but perhaps an alternative conformation when binding other tegument components. In this way, the gH tail can select from an array of possible binding partners, Such flexibility would maximize the efficiency with which viral structural components are assembled into the maturing particle.
Acknowledgments
This work was supported by NIH grants AI38265 (to D.W.W.) and GM55371 (to M.E.G.), by fellowship PF-02-014-01-GMC from the American Cancer Society (to D.E.K.), and by NIH training grant T32 GM07491 (to S.T.G.). Core support was provided by NIH cancer center grant P30-CA13330.
Rabbit anti-gH antisera were generously provided by A. C. Minson. We thank Ruth Angeletti and Lisa Mints in the Laboratory for Macromolecular Analysis of the Albert Einstein College of Medicine for peptide synthesis and helpful advice.
REFERENCES
- 1.Bartels, C., T. Xia, M. Billeter, P. Güntert, and K. Wüthrich. 1995. The program XEASY for computer supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR 6:1-10. [DOI] [PubMed] [Google Scholar]
- 2.Bax, A., and D. G. Davis. 1985. MLEV-17-based two-dimentional homonuclear magnetization transfer spectroscopy. J. Magn. Reson. 65:355-360. [Google Scholar]
- 3.Brack, A. R., J. M. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browne, H., S. Bell, T. Minson, and D. W. Wilson. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browne, H. M., B. C. Bruun, and A. C. Minson. 1996. Characterization of herpes simplex virus type 1 recombinants with mutations in the cytoplasmic tail of glycoprotein H. J. Gen. Virol. 77:2569-2573. [DOI] [PubMed] [Google Scholar]
- 6.Brunetti, C. R., K. S. Dingwell, C. Wale, F. L. Graham, and D. C. Johnson. 1998. Herpes simplex virus gD and virions accumulate in endosomes by mannose 6-phosphate-dependent and -independent mechanisms. J. Virol. 72:3330-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Church, G. A., and D. W. Wilson. 1997. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 71:3603-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaglio, F., S. Grzesiek, G. W. Vuister, G. Zhu, J. Pfeifer, and A. Bax. 1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6:277-293. [DOI] [PubMed] [Google Scholar]
- 9.Desai, P., G. L. Sexton, J. M. McCaffery, and S. Person. 2001. A null mutation in the gene encoding the herpes simplex virus type 1 UL37 polypeptide abrogates virus maturation. J. Virol. 75:10259-10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott, G., G. Mouzakitis, and P. O'Hare. 1995. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J. Virol. 69:7932-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farnsworth, A., K. Goldsmith, and D. C. Johnson. 2003. Herpes simplex virus glycoproteins gD and gE/gI serve essential but redundant functions during acquisition of the virion envelope in the cytoplasm. J. Virol. 77:8481-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs, W., B. G. Klupp, H. Granzow, C. Hengartner, A. Brack, A. Mundt, L. W. Enquist, and T. C. Mettenleiter. 2002. Physical interaction between envelope glycoproteins E and M of pseudorabies virus and the major tegument protein UL49. J. Virol. 76:8208-8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross, S. T., C. A. Harley, and D. W. Wilson. 2003. The cytoplasmic tail of Herpes simplex virus glycoprotein H binds to the tegument protein VP16 in vitro and in vivo. Virology 317:1-12. [DOI] [PubMed] [Google Scholar]
- 14.Guntert, P., C. Mumenthaler, and K. Wuthrich. 1997. Torsion angle dynamics for NMR structure calculation with the new program DYANA. J. Mol. Biol. 273:283-298. [DOI] [PubMed] [Google Scholar]
- 15.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harman, A., H. Browne, and T. Minson. 2002. The transmembrane domain and cytoplasmic tail of herpes simplex virus type 1 glycoprotein H play a role in membrane fusion. J. Virol. 76:10708-10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeener, J. 1979. Investigation of exchange processes by two-dimensional NMR spectroscopy. J. Chem. Phys. 71:4546-4553. [Google Scholar]
- 18.Johnson, B. A., and R. A. Blevins. 1994. NMRView: a computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4:603-614. [DOI] [PubMed] [Google Scholar]
- 19.Karplus, M. 1959. Contact electron-spin coupling of nuclear magnetic moments. J. Chem. Phys. 30:11-18. [Google Scholar]
- 20.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koradi, R., M. Billeter, and K. Wuthrich. 1996. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14:51-55. [DOI] [PubMed] [Google Scholar]
- 22.Loomis, J. S., R. J. Courtney, and J. W. Wills. 2003. Binding partners for the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 77:11417-11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMillan, T. N., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNabb, D. S., and R. J. Courtney. 1992. Analysis of the UL36 open reading frame encoding the large tegument protein (ICP1/2) of herpes simplex virus type 1. J. Virol. 66:7581-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miranda-Saksena, M., R. A. Boadle, P. Armati, and A. L. Cunningham. 2002. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J. Virol. 76:9934-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mossman, K. L., R. Sherburne, C. Lavery, J. Duncan, and J. R. Smiley. 2000. Evidence that herpes simplex virus VP16 is required for viral egress downstream of the initial envelopment event. J. Virol. 74:6287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rance, M. 1987. Improved techniques for homonuclear rotating-frame and isotropic mixing experiments. J. Magn. Reson. 74:557-564. [Google Scholar]
- 28.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 29.Schmelter, J., J. Knez, J. R. Smiley, and J. P. Capone. 1996. Identification and characterization of a small modular domain in the herpes simplex virus host shutoff protein sufficient for interaction with VP16. J. Virol. 70:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaka, A. J., and R. Freeman. 1983. Simplification of NMR spectra by filtration through multiple-quantum coherence. J. Magn. Reson. 51:169-173. [Google Scholar]
- 31.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smibert, C. A., B. Popova, P. Xiao, J. P. Capone, and J. R. Smiley. 1994. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein Vhs. J. Virol. 68:2339-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson, D. W., N. Davis-Poynter, and A. C. Minson. 1994. Mutations in the cytoplasmic tail of herpes simplex virus glycoprotein H suppress cell fusion by a syncytial strain. J. Virol. 68:6985-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou, Z. H., D. H. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu, Q., and R. J. Courtney. 1994. Chemical cross-linking of virion envelope and tegument proteins of herpes simplex virus type 1. Virology 204:590-599. [DOI] [PubMed] [Google Scholar]