Abstract
A quarter of ischaemic strokes are lacunar subtype, typically neurologically mild, usually resulting from intrinsic cerebral small vessel pathology, with risk factor profiles and outcome rates differing from other stroke subtypes. This European Stroke Organisation (ESO) guideline provides evidence-based recommendations to assist with clinical decisions about management of lacunar ischaemic stroke to prevent adverse clinical outcomes. The guideline was developed according to ESO standard operating procedures and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. We addressed acute treatment (including progressive lacunar stroke) and secondary prevention in lacunar ischaemic stroke, and prioritised the interventions of thrombolysis, antiplatelet drugs, blood pressure lowering, lipid lowering, lifestyle, and other interventions and their potential effects on the clinical outcomes recurrent stroke, dependency, major adverse cardiovascular events, death, cognitive decline, mobility, gait, or mood disorders. We systematically reviewed the literature, assessed the evidence and where feasible formulated evidence-based recommendations, and expert concensus statements. We found little direct evidence, mostly of low quality. We recommend that patients with suspected acute lacunar ischaemic stroke receive intravenous alteplase, antiplatelet drugs and avoid blood pressure lowering according to current acute ischaemic stroke guidelines. For secondary prevention, we recommend single antiplatelet treatment long-term, blood pressure control, and lipid lowering according to current guidelines. We recommend smoking cessation, regular exercise, other healthy lifestyle modifications, and avoid obesity for general health benefits. We cannot make any recommendation concerning progressive stroke or other drugs. Large randomised controlled trials with clinically important endpoints, including cognitive endpoints, are a priority for lacunar ischaemic stroke.
Keywords: Guideline, systematic review, stroke, small vessel disease, lacunar stroke, alteplase, thrombolysis, antiplatelet, antihypertensive, lipid lowering
Introduction
Cerebral small vessel disease (cSVD) is a common cause of stroke, lacunar stroke about 25% of all ischaemic strokes and the major cause of intracerebral haemorrhage. 1 It also causes cognitive impairment, mobility and mood disorders, or it can be covert and detected on a brain scan performed for other purposes. cSVD is due to intrinsic disease in the perforating cerebral arterioles, thought to be at least partly due to endothelial or other vascular wall dysfunction.
This guideline is the second in a series of ESO Guidelines addressing management of patients with cSVD and focuses on ischaemic stroke resulting from cSVD, that is, management of patients with clinically suspected or presumed lacunar ischaemic stroke. The first of the series addressed covert cSVD. 1
We define ‘lacunar ischaemic stroke’ as ‘a combination of clinical findings suggestive of acute stroke due to a small subcortical ischaemic lesion (which may or may not be visible on brain imaging) thought to be due to cerebral small arterial vessel disease (i.e. no obvious large artery/cardioembolic cause)’, (Panel 1). 2 All mentions of ‘lacunar ischaemic stroke’ in this guideline refer to patients presenting with a stroke where symptoms and signs are consistent in time and location with a recent small subcortical infarct. It does not refer to lacunes seen on brain imaging without clearly related acute symptoms.
Panel 1.
Common Lacunar Syndromes
|
Yes
: Pure motor stroke involving more than one body location, e.g. face, upper extremity, lower extremity Pure sensory stroke involving more than one body location, e.g. face, upper extremity, lower extremity Sensory-motor stroke, involving more than one body location, e.g. face, upper extremity, lower extremity Ataxic hemiparesis Dysarthria clumsy hand syndrome No : Monoparesis Hemispatial neglect Dysphasia Visual loss Any other higher cerebral dysfunction Isolated dysarthria Isolated ataxia Isolated vertigo Worst NIHSS >8 Headache Alteration of consciousness |

|
Lacunar ischaemic stroke has a somewhat different risk factor and outcome profile compared to other stroke subtypes. Carotid or vertebrobasilar stenosis and cardioembolic sources are unusual. 3 Lacunar stroke is associated with increased risk of recurrent stroke, death or dependency 4 and cognitive impairment, 5 in the long term.
Currently, patients with lacunar ischaemic stroke receive acute treatment (e.g. thrombolysis) and secondary prevention (i.e. antiplatelet drug(s), antihypertensive threatment, lipid lowering, and lifestyle advice) as for other types of ischaemic stroke. However, although many patients with lacunar ischaemic stroke were included in acute stroke treatment and secondary prevention trials, the results were rarely analysed separately for lacunar stroke. 6 Additionally, the only large trial to test dual versus single antiplatelet drugs long term stopped prematurely due to hazard, 7 and found that intensive versus guideline blood pressure lowering did not reduce recurrent stroke or prevent cognitive decline.8,9 Hence it is uncertain whether current treatment or prevention approaches are best suited for treatment in lacunar ischaemic stroke.
The aim of this guideline is to provide recommendations to guide cerebrovascular disease care providers to reach clinical decisions when assessing patients with suspected or presumed lacunar ischaemic stroke, along with investigation and management strategies to reduce the risk of recurrent stroke, long-term disability, cognitive, mobility, and mood disorders.
We first describe the selection of topics and some general advice on clinical assessment and diagnosis in lacunar stroke, prior to presenting the findings of each Population, Intervention, Comparator and Outcome (PICO) question.
Advice on clinical assessment and diagnostics in lacunar ischaemic stroke
The Guideline Module Working Group (MWG) was conscious that lacunar ischaemic stroke is a somewhat neglected subtype of stroke and felt that it would be useful to provide some key background in order to use this guideline effectively.
Clinical assessment : Lacunar syndromes are described in Panel 1. 2 However, these symptoms and signs may overlap with cortical stroke syndromes in 15%–20% of cases (‘clinical-imaging mismatch’) 10 and may be difficult to recognise in the acute stages. Some clinical findings increase the likelihood that an ischaemic stroke is due to lacunar infarction in the acute phase. For example, within 6 h of symptom onset, neither lacunar clinical syndromes or NIHSS alone have good sensitivity or specificity for small subcortical infarcts, but the combination of a lacunar syndrome (the five commonest being: pure motor stroke, pure sensory stroke, sensory-motor stroke, ataxic hemiparesis and dysarthria clumsy hand) and stroke severity of NIHSS <7, gives a high specificity, positive and negative predictive values to detect acute small subcortical infarction. 11 Conversely, the presence of higher cortical dysfunction such as neglect, dysphasia, visual loss, suggests a cortical or larger subcortical infarct. Pre-existing lacunes, leukoaraiosis/other neuroimaging markers of SVD, elevated BP and history of diabetes may also increase the likelihood of a new stroke being due to a small subcortical infarct (summarised in Ref. 11 ).
Underlying causes/differential diagnoses not to be missed (Panel 2): Small subcortical infarcts are not always due to sporadic intrinsic small vessel disease. It is important to avoid missing situations where an alternative guideline-based treatment should be considered. Clinical and/or imaging features that point to higher likelihood of cardio- or atherothromboembolic sources in a patient presenting with a lacunar clinical syndrome and/or a small subcortical infarct include: isolated subcortical infarct without any other signs of SVD on neuroimaging, cortical combined with subcortical infarcts; multiple contemporaneous infarcts involving more than one main arterial territory; large subcortical infarcts (as a guide>2 cm max diameter in the acute stage, but perceived size may depend on imaging modality); arterial dissection; and rare differential diagnoses of monogenic SVD (e.g. with unusually high burden of white matter hyperintensities (WMH), lacunes, microbleeds, and vascular risk factors in relation to age; lesion distribution; and family history 12 ) especially in younger people. Cardioembolic and large artery disease (i.e. atherothromboembolic sources, dissection, embolic stroke of unknown source) should be managed according to the relevant guidelines (Table 1).
Panel 2.
Alternative ischaemic cerebrovascular causes with specific treatments that are not to be missed in patients with suspected lacunar stroke, and standard investigations; note that practice varies between countries and hospitals and this is general guidance.
| Alternative cause | Clues that the cause is NOT intrinsic small vessel disease | Investigations |
|---|---|---|
| *Brain imaging, neck artery imaging, BP, routine haematology, blood glucose, lipids, coagulation, liver, kidney function tests, proteinuria | ||
| Cardioembolic: | • Clinical history, symptoms, and findings suggesting cardiac disease • Contemporaneous cortical and small subcortical infarcts • Multiple contemporaneous infarcts in different arterial territories which may include a small subcortical infarct • Large subcortical infarct on imaging (as a guide, >2 cm axial diameter in the acute stage, although cardioembolic infarcts can be smaller and perceived size may depend on the type of imaging) |
*ECG
*Cardiac echocardiography including PFO detection** Prolonged ECG monitoring |
| Large artery atherothromboembolism | • Contemporaneous cortical and small subcortical infarcts • Large subcortical infarct on imaging (>2 cm axial diameter in the acute stage) • Manifestations of atherosclerosis in other organs e.g. coronary arteries, aorta, extremities |
*Carotid/vertebral artery imaging, using ultrasound, CTA, or MRA |
| Arterial dissection | • Clinical symptoms and findings suggesting dissection • Odd-shaped subcortical infarct • Presence of cortical infarct |
*Carotid/vertebral artery imaging, using ultrasound, CTA, or MRA |
| Intracranial atheromatous stenosis | • Large subcortical infarct (>2 cm axial diameter) • Long tubular infarct (>2 cm long) extending from the inferior perforating substance superiorly into the basal ganglia |
Intracranial CT or MR angiography. Vessel wall MRI |
| Rare monogenic causes | • Worse WMH, more lacunes, perivascular spaces, and microbleeds than usual for age, and vascular risk factors • Concomitant other manifestations from other organs, for example, eye, skin, heart, ear, kidney • Cerebral lesion distribution • Young onset • Family history |
Genetic testing |
Should be routine in all ischaemic strokes including lacunar.
PFO detection usually clinically relevant only for patients under 60–65 years of age.
Table 1.
Current guidelines on acute treatment or prevention of stroke relevant to presumed lacunar ischaemic stroke.
| Name of guideline | Date pub | Key findings related to acute phase treatment of SVD related stroke | Key findings related to secondary prevention of SVD related stroke | Key findings when presence SVD influences other treatments |
|---|---|---|---|---|
| ESO Guidelines | ||||
| ESO Guideline on covert cerebral small vessel disease 1 |
2021 | Nil specific – focussed on patients with SVD and no diagnosis stroke/TIA/Cognitive imp/mood/mobility | Nil | Nil |
| European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT)Guidelines on Mechanical Thrombectomy in Acute Ischaemic Stroke 13 |
2019 | Nil | Nil | Nil |
| A European Stroke Organisation (ESO) guideline on antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation. 14 | 2019 | Nil | Recommendations are weak regarding treatment with oral anticoagulants in . . . specific patient subgroups including those with small vessel disease, because of a lack of strong evidence; Recommendations. In patients with non-valvular AF, previous ischemic stroke or TIA and SVD, we cannot make recommendations about whether non-vitamin K antagonist oral anticoagulants should be preferred over vitamin K antagonists for reducing recurrent stroke or thromboembolism: Quality of evidence – Low; Strength of recommendation: Weak |
No randomised controlled trials investigating the efficacy and safety of: antiplatelet therapy compared to no antithrombotic treatment; vitamin K antagonists versus antiplatelet therapy; or direct oral anticoagulants versus vitamin K antagonists, for prevention of recurrent stroke or other adverse outcomes in patients with non-valvular AF and SVD (WMH and CMBs). |
| European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment 15 | 2021 | Nil on treatment, focussed on post stroke cognitive impairment | Nil on treatment | WMH on MRI may predict post stroke cognitive impairment |
| European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack 16 |
2022 | Nil | Nil | Nil |
| Consensus statements and recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 11–13 November 201817 |
11–13 November 2018 | Nil | Nil | Individual decision making on OAC after ICH should consider (Grade C): quality of BP control, age, ICH location, burden of small vessel disease (cerebral microbleeds (CMBs), leukoaraiosis, cortical super- ficial siderosis, CAA), additional antiplatelet therapy. OAC in patients with evidence of CMBs should not be withheld (Grade C). |
| EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke 18 | 2020 | Nil | Nil | Nil |
| European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke 19 |
2021 | Although there are ongoing discussions about the use of IVT in patients with lacunar stroke, there is currently no strong evidence that it should be avoided |
Nil | IVT is recommended in the presence of a small-to-moderate burden of white matter lesions and suggested in the presence of a high burden of lesions When CMB burden is unknown or known to be low (e.g. <10), we suggest intravenous thrombolysis with alteplase. When CMB burden has been previously reported to be high (e.g. >10), we suggest no intravenous thrombolysis. All members suggest against screening with MRI to assess CMB burden before making a treatment decision regarding IVT |
| European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack 20 |
2021 | Nil | Low risk TIA was defined by absence of high risk features (i.e. those in whom brain-tissue damage has not been detected on diffusion-weighted imaging, with no documented stenosis in the ipsilateral cerebral artery, no major cardiac source of embolism, no small vessel disease, and an ABCD2 score of less than 4) |
|
| Other European Guidelines | ||||
| Monogenic cerebral small-vessel diseases: diagnosis and therapy. Consensus recommendations of the European Academy of Neurology 12 |
2020 | Patients with CADASIL should not receive thrombolysis for acute small-vessel ischaemic stroke (which is almost always the case) |
Anticoagulants are not recommended for stroke prophylaxis in CADASIL due to the risk of intracerebral haemorrhage, but they are not contraindicated if there is another strong indication (e.g. atrial fibrillation, pulmonary embolus) |
Nil |
| AHA/ASA | ||||
| 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack - A Guideline From the American Heart Association/American Stroke Association 21 | 2021 | Nil | In patients with ischemic stroke related to small vessel disease, the usefulness of cilostazol for secondary stroke prevention is uncertain Targeted strategies for secondary prevention after small vessel stroke that also reduce the risk of vascular dementia are lacking Studies that showed benefit from PFO closure excluded lacunar strokes |
Nil |
| Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke 22 |
2019 | Nil | For prevention of recurrent stroke, the use of MRI is reasonable in some patients with AIS to provide additional information to guide selection of appropriate secondary stroke prevention treatments. [Two studies from the 1990s evaluating repeat neuroimaging recommended repeat CT over additional MRI for most clinical situations in AIS with the exceptions of documenting lacunar and infratentorial infarcts, but they did not present evidence of a benefit on outcome for these situations] |
Administration of IV alteplase in eligible patients without first obtaining MRI to exclude cerebral microbleeds (CMBs) is recommended In otherwise eligible patients who have previously had a small number (1–10) of CMBs demonstrated on MRI, administration of IV alteplase is reasonable. In otherwise eligible patients who have previously had a high burden of CMBs (>10) demonstrated on MRI, treatment with IV alteplase may be associated with an increased risk of sICH, and the benefits of treatment are uncertain. Treatment may be reasonable if there is the potential for substantial benefit. |
| AHA/ASA Scientific Statement – Prevention of Stroke in Patients With Silent Cerebrovascular Disease 23 |
2017 | Nil | Nil | It is reasonable to provide anticoagulation therapy to patients with microbleeds when there is an indication (e.g. AF). It is reasonable to provide antiplatelet therapy to patients with microbleeds when there is an indication. It is reasonable to administer intravenous alteplase to patients with acute ischemic stroke and evidence of microbleeds if it is otherwise indicated. It is reasonable to perform endovascular thrombectomy in patients with acute ischemic stroke and evidence of microbleeds. |
| Canadian Stroke Best Practice Recommendations | ||||
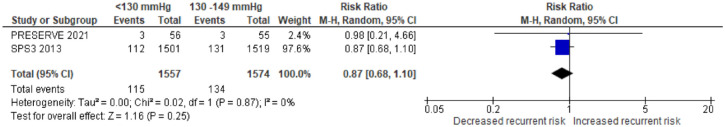
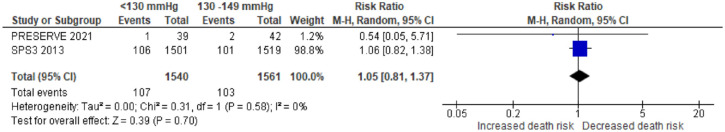
| Canadian Stroke Best Practice Recommendations – secondary prevention 24 | 2020 | Nil | Recommends aggressive blood pressure in patients with lacunar stroke (systolic target of consistently lower than 130 mmHg) | Lacunar stroke contraindicates PFO closure |
| Canadian Stroke Best Practice Recommendations – acute management 25 | 2022 | Nil | Nil | Nil |
| UK-based guidelines | ||||
| Royal College of Physicians – National clinical guideline for stroke 26 |
2016 | Nil | Mentioning the SPS3 trial that suggests targeting a systolic BP of below 130 mmHg in patients with recent lacunar stroke | Reminding that pre-existing cSVD should be assessed when evaluating the association between statin use and cerebral haemorrhage |
| NICE guideline – Stroke and transient ischaemic attack in over 16s: diagnosis and initial management; https://www.nice.org.uk/guidance/NG128 | 2019 | Nil | Nil | Nil |
| Australia/New Zealand | ||||
| Australian and New Zealand Clinical Guidelines for Stroke Management – Acute stroke management 27 | 2021 | Mentions a substudy of WAKE-UP which was able to show a very similar benefit of alteplase in the lacunar subgroup compared to non-lacunar patients, providing reassurance that lacunar stroke patients do indeed benefit from thrombolysis. | Secondary prevention with antihypertensives in patients with recent lacunar stroke is safe and effective. | CMBs at brain MRI do not contraindicate i.v. thrombolysis |
| Australian and New Zealand Clinical Guidelines for Stroke Management – Secondary prevention 27 | 2021 | Nil | Antiplatelet treatment (without specific drug preference) is effective for secondary lacunar stroke prevention | Patients with lacunar stroke should not undergo PFO closure |
| Asian countries | ||||
| Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases
28
Also see Chinese guideline for diagnosis and treatment of cerebral small vessel disease 2020. 29 |
2020 | At present, it is recommended to manage blood pressure, and use of aspirin, clopidogrel or cilostazol (class I, level of evidence B). The blood pressure of patients should be closely monitored (class IIa, level of evidence B). Control of systolic and diastolic pressure is the key factor to control the incidence and progression of cerebral small vessel disease (class IIa, level of evidence B). It is necessary to monitor the 24 hours ambulatory blood pressure in patients with cerebral small vessel disease. When conditions permit, it is best to detect changes in blood pressure during head upright tilt test (class I, level of evidence B). |
Nil | Routine use of MRI to identify intracranial microhaemorrhage, which can affect decisions to IV thrombolysis, is not recommended (class III, level of evidence B). Prethrombolytic MRI examination showed that IV thrombolysis was reasonable in patients with a number of (1–10) cerebral microbleeds (class IIa, level of evidence B). Prethrombolytic MRI examination showed that IV thrombolysis was associated with an increased risk of symptomatic intracerebral haemorrhage in patients with a number of (>10) cerebral microbleeds, and the clinical benefit is not clear. If there may be significant potential benefits, IV thrombolysis may be reasonable (class IIa, level of evidence B). |
| Japanese Stroke Guidelines 30 | 2021 | Nil | In patients who experience ischemic stroke or TIA, without bilateral severe carotid artery stenosis and occlusion of major intracranial artery, who present with lacunar infarction, or undergoing antithrombotic therapy, as lower target value of blood pressure is favourable if possible, a target value of <130/80 mmHg for antihypertensive therapy is reasonable (Grade B, LOE Moderate) | Nil |
SVD: small vessel disease; WMH: white matter hyperintensities on MRI; CMB: cerebral microbleeds; CAA: cerebral amyloid angiopathy; BP: blood pressure; OAC: oral anticoagulation; PFO: patent foramen ovale; AIS: acute ischaemic stroke; IVT: intravenous thrombolysis.
Summary of aetiologic work-up to be considered in suspected or presumed lacunar ischaemic stroke (Panel 2): All patients presenting with a suspected or presumed lacunar ischaemic stroke should undergo brain imaging, neck artery imaging (carotid ultrasound/CTA/MRA), screening for AF, and other routine stroke examinations (BP, blood glucose, lipids, markers of renal function, etc) may be considered to identify treatable causes of the stroke, relevant comorbidities and preventable causes of future stroke. Echocardiography and cerebral artery imaging may be indicated and additional tests (e.g. ambulatory ECG monitoring) further warranted depending on age and initial findings (e.g. fundus examination in diabetes or hypertension).
In the acute situation, CT or MR brain imaging is usually performed although CT does not show the acute small subcortical infarct in around 50% of cases within the first 6 h. 31 Diagnostic algorithms have been suggested. 11 CT angiography of cerebral arteries may detect atherosclerotic changes including lesions at the origin of small penetrating arteries. CT perfusion, if performed routinely, should be examined closely since it may show a perfusion defect in a relevant brain region. 32 MRI is very useful instead of or in addition to CT, when the relevant sequences are included (including DW-MRI, FLAIR, SWI, T1 and T2) since it has higher sensitivity for recent small subcortical as well as cortical infarcts and is often well tolerated in patients with lacunar ischaemic stroke. If not available acutely, then MRI is still worth performing up to a few weeks later, although the interpretation may be complicated since some small subcortical infarcts can disappear subacutely. 33
Cognitive evaluation should be considered in all patients with suspected/presumed lacunar ischaemic stroke in view of the high rate of cognitive impairment 5 and to provide a baseline. Progression of cSVD may manifest as cognitive decline rather than recurrent stroke or dependency. Practical routine screening tools can include MMSE, MoCA and Trail Making test. A more comprehensive assessment by a neuropsychologist may be needed by patients of working age, or if a specific deficit or complaint is identified. Screening for neuropsychiatric symptoms (i.e. depression, apathy) should also be considered since these symptoms are relatively common, the tests provide a baseline and cSVD progression may manifest through fluctuation in neuropsychiatric symptoms. 34
Methods
Composition and approval of the Module Working Group
These guidelines were initiated by the ESO. Two chairpersons (Arne Lindgren and Joanna Wardlaw) were selected to assemble and coordinate the Guideline Module Working Group (MWG). The final group contained 17 experts (12 senior members and 5 fellows) plus a methodologist. The ESO Guideline Board and Executive Committee reviewed the intellectual and financial disclosures of all MWG members and approved the composition of the group. The full details of all MWG members and their disclosures are included in Supplemental Table 1.
Development and approval of clinical questions
This guideline was prepared according to the ESO standard operating procedures (SOP), 35 which are based on the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework. 36 Supplemental Table 2 describes outcome grading. While recognising that many patients presenting with stroke also have pre-existing changes of cSVD on brain imaging, the MWG identified clinical lacunar ischaemic stroke as the most important topic to address in this guideline, deferring the topic of ‘other stroke subtypes with cSVD on imaging’ to a future guideline in view of the complexity of including both in one guideline. The MWG also identified that acute treatment (arbitrarily defined as ‘usually implemented within the first 24–48 h after symptom onset’), progressive lacunar stroke and secondary prevention were of great clinical interest, with some overlap between them. In the acute phase the clinical diagnosis of lacunar ischaemic stroke is often not completely clear, and we therefore used the term suspected acute lacunar ischaemic stroke for these situations. In contrast we used the term lacunar ischaemic stroke for the more long-term situations where secondary prevention becomes a priority. Interventions included thrombolysis and ‘other’ (i.e. novel) agents for acute treatment and progressive stroke; antiplatelet and antihypertensive agents for acute treatment, progressive stroke and secondary prevention; and lipid lowering and lifestyle interventions and other agents for secondary prevention. Common outcomes included recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, Major Adverse Cardiovascular Event (MACE), mobility or gait disorder, and mood disorders. Questions were formatted using the PICO approach (Population, Intervention, Comparator and Outcome), generating 10 PICO questions in total (five acute, including progressive, and five secondary prevention, as summarised in Table 8) and reviewed by two external reviewers as well as members of the ESO Guideline board and Executive Committee. The outcomes were rated by members of the MWG as: critical, important or of limited importance according to GRADE criteria. The final decision on outcomes used a Delphi approach. Results of the outcomes rating for each PICO question are included in the Supplemental Table 2.
Literature search
For each PICO question, search terms were developed by the MWG and guideline methodologist (SH). Where a validated search strategy was available, this was used or adapted. Where there was a recent relevant systematic review on the question of interest, the corresponding search strategy and results were used and updated as necessary. Search strategies are described in Supplemental Appendix.
The search was performed by the ESO Guideline methodologist (SH). The following databases: Medline, Embase and Cochrane databases were searched from inception to November 2022. Reference lists of review articles, trials papers, the authors’ personal reference libraries, conference proceedings and previous guidelines were also searched for additional relevant records.
Search results were loaded into the web-based Covidence platform (Health Innovation, Melbourne, Australia) for assessment by the MWG. Two or more MWG members were assigned to independently screen the titles and abstracts of publications registered in Covidence and then assess the full text of studies determined to be potentially relevant. All disagreements were resolved by discussion between the two reviewers or by a third MWG member.
We prioritised randomised controlled trials (RCTs) but where data were limited, we also considered health registry data analyses, large observational studies and systematic reviews or meta-analyses of observational studies. We considered only studies in humans. We excluded publications with only conference abstracts available.
All PRISMA diagrams summarising the search findings are available in the Supplement PRISMA diagrams.
Data analysis
Data extraction was performed by the MWG fellows and members (and the methodologist for PICO 3/7), using a pre-designed data extraction template. The ESO methodologist performed appropriate meta-analyses. Any discrepancy during data extraction stage was resolved by discussion. In the case that relevant data were not reported in an eligible study, we attempted to contact the corresponding author or co-authors of the study. If no answer was received, data were considered as missing.
Due to the expectation of high heterogeneity, random-effects meta-analyses were conducted using Review Manager (RevMan) software (Cochrane) version 5.4.3. Statistical heterogeneity across studies was assessed using the I 2 statistic, and classified as moderate (⩾30%), substantial (⩾50%) or considerable (⩾75%). 37
Where appropriate, we performed outcome analyses based on stroke outcome subtypes any stroke, ischaemic stroke and haemorrhagic stroke; and severity (major adverse cardiovascular events). Where suitable, we grouped the trials into those with acute phase results (approximately within 2–4 weeks after stroke onset), and those with long-term results (treatment administered for more than 4 weeks).
Where appropriate, network meta-analysis was conducted to compare multiple interventions simultaneously for each outcome. 38 Network meta-analysis was conducted only for PICO 6 which was found to be feasible based on abundance of available evidence, transitivity assumption, network connectivity, inconsistency or incoherence assessment (node-splitting approach). 39 Evidence of incoherence in the entire network was assessed using the design-by-treatment model. 40 Assuming a common heterogeneity parameter, network meta-analysis was performed with a frequentist framework using a multivariate meta-analysis estimated by restricted maximum likelihood to assess the comparative effectiveness.41,42 We considered ‘placebo’ as the reference group across the networks. Network meta-analysis was performed using STATA version 15.1.
Evaluation of the quality of evidence and formulation of recommendations
The risk of bias of each included randomised trial was assessed with the Cochrane RoB2 tool by the ESO methodologist and MWG member independently. 43 Any discrepancy or confusion in RoB judgement was discussed with a third MWG member. 44
The results of data analysis were imported into the GRADEpro Guideline Development Tool (McMaster University, 2015; developed by Evidence Prime, Inc.). For each PICO question, and each outcome, the following were considered: risk of bias based on the type of available evidence (randomised or observational studies); considerations on inconsistency of results; indirectness of evidence, imprecision of results, and other possible bias. GRADE evidence profiles/summary of findings tables were generated and used to prepare recommendations. ‘Evidence-based Recommendations’ were based on the GRADE methodology. The direction, strength and formulation of the recommendations were determined according to the GRADE evidence profiles and the ESO-SOP.35,36
Finally, Expert Consensus Statements were added particularly whenever the PICO group considered that there was insufficient evidence available to provide Evidence-based Recommendations and practical guidance is needed for routine clinical practice. The Expert Consensus Statements were based on voting by all expert MWG members (summarised in Supplemental Table 3). Importantly, these Expert Consensus Statements should not be regarded as Evidence-based Recommendations, since they only reflect the opinion of the writing group.
Drafting of the document, revision and approval
Each PICO question was addressed in distinct sections, in line with the updated ESO SOP. 35
First, ‘Analysis of current evidence’ summarised current pathophysiological considerations followed by a summary and discussion of the results of the identified RCTs and other studies.
Second, ‘Additional information’ was added when more details on the studies referred to in the first section were needed to provide information on key subgroup analyses of the included studies, on ongoing or future RCTs, and on other studies which can provide important clinical guidance on the topic.
Third, an ‘Expert Consensus Statement’ paragraph was added when the MWG considered that insufficient evidence was available to provide evidence-based recommendations for situations in which practical guidance is needed for everyday clinical practice.
The Guideline document was reviewed several times by all MWG members and modified using a Delphi approach until consensus was reached. The final submitted document was peer-reviewed by two external reviewers, two members of the ESO Guideline Board and one member of the Executive Committee.
Results
PICO 1:
In patients with suspected lacunar ischaemic stroke, does thrombolytic treatment (including at extended time window and wake-up stroke, alteplase/tenecteplase/other), compared to avoiding this intervention/other thrombolytic/dose/etc, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder and mood disorders?
Analysis of current evidence
The use of intravenous thrombolysis in patients with lacunar stroke has been debated over the years, for three reasons. 45 Firstly, lacunar strokes are mild in many instances, and this may raise the suspicion of an unclear benefit over side effects, particularly the risk of bleeding. Secondly, thrombosis in the perforating arteriole is not identified routinely in lacunar stroke because the affected vessel is too small to be visualised in vivo. Thirdly, patients with lacunar stroke usually have other features of small vessel disease (SVD) (e.g. white matter hyperintensities and microbleeds) that are associated with an increased haemorrhagic risk, whether or not linked with thrombolysis. 31 Additionally, amongst neurologically milder strokes, in a routine clinical setting, the diagnosis of the ischaemic stroke subtype can be difficult in the hyperacute or acute time window, although use of diagnostic algorithms 11 and CT perfusion 32 may help, particularly in the absence of MRI. In the first 24–48 h after stroke onset, approximately 15% of clinical lacunar syndromes are due to a cortical infarct on brain imaging and about 15%–20% of neurologically mild cortical syndromes are due to a recent small subcortical infarct. 10 A further point to note is that dependency is a less frequent outcome of lacunar 46 than of other more severe stroke subtypes and therefore functional outcome using the often dichotomised mRS 0–2 versus mRS 3-6 may not be the most sensitive measure of outcome when assessing the effects of acute treatments such as thrombolysis, and an alternative for lacunar ischaemic stroke trials may be to use mRS 0–1 versus 2–6 instead. This notwithstanding, up to 2014, observational and limited randomised trial data had suggested that thrombolysis is an effective treatment in acute lacunar stroke, and that while the presence of cSVD increases the risk of intracerebral haemorrhage during thrombolysis, it did not represent an absolute exclusion criterion. 45
Our literature search retrieved 897 papers (Supplement PRISMA diagram). 851 non-duplicate studies were screened, of which 47 were assessed for eligibility. Two trials were identified by independent searching. Finally, five RCTs were relevant to the review of which three could be meta-analysed (Supplemental Table 4).
Only one outcome, good functional outcome, that is recognised to have limitations in assessing outcome after lacunar stroke, is available for more than two trials. The risk of bias is low but the sample size is small (521 patients is smaller than the National Institute of Neurological Disorders and Stroke (NINDS) trial and all the European Cooperative Acute Stroke Study (ECASS) trials), the certainty is very low, and the confidence intervals are very wide. Data on SICH and death are extremely limited.
Three trials of intravenous alteplase versus control included patients with lacunar stroke where the outcome data for the lacunar patients could be extracted: NINDS, 47 IST-348,49 and Wake-up. 50 One trial, Enhanced Control of Hypertension and Thrombolysis Stroke Study, (ENCHANTED) tested low versus standard dose of alteplase and provided data on lacunar stroke. 51 In another RCT in patients with mild stroke, the Potential of rtPA for Ischaemic Strokes With Mild Symptoms (PRISMS) trial, 52 35% of patients had lacunar stroke but the results were not published by stroke subtype.
The original NINDS trial 47 (total n = 624) included a small subsample (n = 81) of patients with lacunar stroke, of whom 51 were randomised to alteplase and 30 to placebo within 3 h from symptoms onset.
In the IST-3, n = 3035, 168 patients with lacunar stroke within 6 h from symptoms onset were randomised to alteplase 0.9 mg/kg and 164 to placebo. 48 A secondary analysis considered patients randomised within 3 h of stroke who were NIHSS ≤5, with pretreatment blood pressure <185/110 mmHg, and no other alteplase exclusion criteria. 106/3035 met the restricted criteria in whom allocation to alteplase was associated with an increase in Oxfordfordshire Handicap Score (OHS) 0–2 (84% alteplase vs 65% control; (OR 3.31, 95% CI 1.24, 8.79) and a favourable shift in OHS distribution (OR 2.38, 95% CI 1.17, 4.85). There was no significant effect of alteplase on OHS 0–1 (60% vs 51%; OR 1.92, 95% CI 0.83, 4.43). 49
The Wake-up trial 50 enrolled patients with wake up stroke or unknown time of symptom onset with a DWI-FLAIR mismatch indicating salvageable tissue amongst which were included 108 patients with lacunar ischaemic stroke, of whom 55 were randomised to alteplase, 53 to placebo.
The ENCHANTED trial 51 enrolled a subgroup of 241 patients with lacunar stroke within 4.5 h from symptoms onset treated with alteplase 0.6 mg/kg and 249 randomised to alteplase 0.9 mg/kg.
PRISMS included 35% lacunar stroke, 29% undetermined type (some of which may have been lacunar) and 29% large artery atherosclerosis or cardioembolic. However, results were not presented by subtype and therefore cannot be included in the meta-analysis. Worth noting is the overall neutral result for all patients and the excess of haemorrhages in the alteplase group.
The duration of follow-up was 90 days for all except the IST3 trial which was 6 months.
The main drug evaluated was alteplase in a standard 0.9 mg/kg dose (in ENCHANTED a comparison of different doses was available).
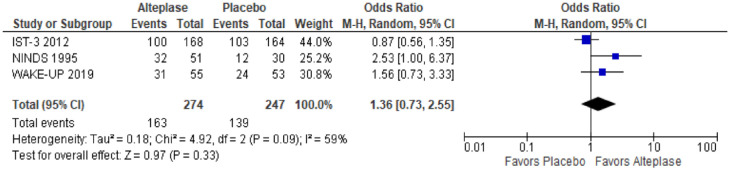
A meta-analysis of three trials (NINDS, IST-3, Wake-up) on good functional outcomes was possible (Table 2, Figure 1). Some data were available on SICH and death, but this was not meta-analysable since events were too sparse. We provide a narrative summary of available outcomes. There were no data on recurrent ischaemic stroke, MACE, cognitive impairment or dementia, mobility or mood disorders.
Table 2.
PICO 1: GRADE evidence profile table for alteplase compared to placebo for good functional outcome in patients with lacunar stroke.
| Certainty assessment | No of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Alteplase | Placebo | Relative (95% CI) | Absolute (95% CI) | ||
| Good Functional Outcome (defined as mRS scores 0–1; Oxford Handicap Score 0–2) | ||||||||||||
| 3 | Randomised controlled trials | Not serious | Serious a | Serious | Very serious b | None | 163/274 (59.5%) | 139/247 (56.3%) | OR 1.36 (0.73, 2.55) |
74 more per 1000 (from 78 fewer to 204 more) |
⨁○○○ Very Low |
CRITICAL |
CI: confidence interval; OR: odds ratio.
High heterogeneity (I 2 = 59%).
Low sample size and confidence interval crosses the clinical decision threshold.
Figure 1.
The effect of alteplase versus placebo on favourable functional outcome (OHS 0–2 in IST-3)/excellent functional outcome (mRS 0–1 in NINDS and Wake-up trials) in patients with acute lacunar ischaemic stroke.
Functional outcome
Three trials have evaluated the effect of thrombolysis with alteplase 0.9 mg/kg on functional outcome, total n = 521. Allocation to alteplase was associated with non-significantly higher odds of good functional outcome defined as mRS 0–1 in NINDS and Wake up and as Oxford Handicap Scale 0–2 in IST-3 (p = 0.33) (Figure 1).
Symptomatic intracranial haemorrhage (SICH)
In Wake-up, 1/55 lacunar stroke patients allocated alteplase had SICH compares with none of 53 patients allocated placebo. Data were not provided in SICH for lacunar stroke patients in NINDS or IST-3.
In ENCHANTED, none of 241 lacunar stroke patients allocated 0.6 mg/kg alteplase had SICH versus 1/249 patients allocated 0.9 mg/kg alteplase. For any ICH, there were 11/241 (4.6%) ICH in the low dose and 7/249 (2.8%) ICH in the standard dose groups, aOR 1.50, 95% CI 0.56, 3.99.
Death
Data amongst patients with lacunar stroke allocated alteplase versus no alteplase were available from WAKE-up: 1/55 patients allocated alteplase versus 0/53 allocated no alteplase died within 90 days.
In ENCHANTED, 1/241 patients with lacunar stroke allocated low dose alteplase and 2/249 lacunar strokes allocated standard dose alteplase died within 90 days, aOR 0.44, 95% CI 0.03, 5.71.
Additional information
In ENCHANTED, 51 490 patients with lacunar stroke were compared to 2098 patients with non-lacunar stroke. Compared with patients with non-lacunar stroke, patients with lacunar stroke had better functional outcomes on either dose of alteplase (mRS 2–6, adjusted OR 0.60, 95% CI 0.47, 0.77), presumably reflecting that outcomes are better in general after mild than more severe stroke. Outcomes with low dose versus standard dose of alteplase did not differ (mRS 2–6, aOR 1.04, 95% CI 0.87, 1.24). In general, lower dose of alteplase reduced the risk of SICH across all patients (although no significant effect was seen in the lacunar stroke, Supplemental Table 4).
The PRISMS trial 52 randomised 313 patients with NIHSS ≤5 to receive 0.9 mg/kg alteplase (n = 156) or 325 mg oral aspirin (n = 157) within 3 h of onset of whom 37% of patients had a lacunar stroke. The planned sample size was 948 but the trial stopped early due to slow enrolment. The primary outcome was favourable outcome (mRS 0–1) at 90 days, seen in 122 patients (78.2%) allocated alteplase versus 128 patients 81.5% allocated aspirin (adjusted absolute risk difference −1.1%, 95% CI −9.4%, 7.3%). There were 5 SICH (3.2%) in patients allocated alteplase versus none in patients allocated aspirin, absolute risk difference 3.3%, 95% CI 0.8%, 7.4%. Details on death as an outcome for the whole sample, as well as specific information on outcomes for patients with lacunar stroke, were not reported.
The Antiplatelet versus R-tPA for Acute Mild Ischaemic Stroke (ARAMIS) trial, published in 2023, 53 randomised 760 patients with minor non-disabling stroke to alteplase 0.9 mg/kg versus dual antiplatelet therapy (DAPT) with aspirin and clopidogrel within 4.5 h of onset, of whom 166 (21.1%) were classified as small vessel occlusion. The primary outcome was excellent functional outcome (mRS < 2) which for patients with lacunar infarct/small artery occlusion was achieved in 83/87 allocated DAPT and 73/79 allocated alteplase, risk difference 3.0%, 95% CI −4.3%, 10.3%, in favour of DAPT. The result for the whole trial was similar, 346/369 DAPT and 320/350 allocated alteplase had excellent functional outcome, an adjusted risk difference of 2.3%, 95% CI −1.6%, 6.1%, p < 0.001 for non-inferiority in favour of DAPT. In the whole trial, SICH (1 DAPT, 3 alteplase) and any bleeding (6 DAPT, 19 alteplase) were infrequent and not reported for the lacunar subgroup.
The Austrian Stroke Unit Registry 54 records patients admitted to Stroke Units in Austria. In a retrospective analysis of patients with lacunar or non lacunar stroke who received alteplase or not (401 each), matched for NIHSS, prestroke mRS and other risk factors, patients with lacunar stroke who received alteplase had better functional outcome at 3 months than patients with lacunar stroke who did not receive alteplase (p < 0.001). SICH occurred in 1% of patients who received alteplase and 0.2% of those not given alteplase.
Marcelinus et al. 55 retrospectively analysed patients with lacunar ischaemic stroke from a stroke registry of the neurology department of first Affiliated Hospital of Zhengzhou University from January 2013 to December 2020. They used propensity score matching to compare patients who received alteplase versus those who did not. The primary outcome was favourable functional outcome at 3 months after stroke onset, defined by attaining a score of ≤2 points on the modified Rankin scale (mRS). 132 of 717 patients were identified of whom 44 pairs of alteplase–no alteplase were successfully matched. After propensity matching, the patients who received alteplase were more likely to have a favourable outcome at 3-month follow-up (OR = 0.247, 95% CI 0.074, 0.830, p = 0.024). There was one case of asymptomatic ICH in alteplase-treated patients.
The available trials and sample with data on lacunar ischaemic stroke do not provide any data on risk of SICH according to cSVD burden. Data from IST3 found that SICH was increased with severe WMH and old infarcts but there was still benefit of alteplase. 31 Observational analyses also show that SICH risk is increased in patients with severe WMH and old infarcts and should be balanced against the likelihood of benefit from thrombolysis. 56
Current clinical guidelines on thrombolytic treatment 19 (Table 1) do not provide specific recommendations on subtypes of ischaemic stroke.
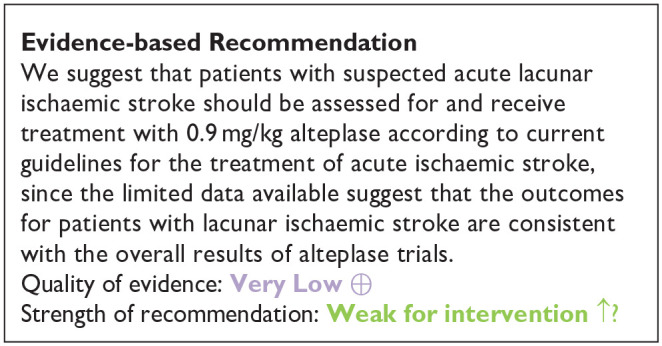
In summary, the MWG noted that the data on alteplase in lacunar ischaemic stroke was very limited, that lacunar ischaemic stroke is difficult to identify clinically or with CT brain scanning (as used in most trials) in the acute phase, that dependency (the main outcome measure in trials of thrombolysis) is infrequent after lacunar stroke giving limited power to detect effects, and that the confidence intervals overlapped the line of no effect. In general, the trend was in the direction of benefit from alteplase, and consistent with the overall results of trials of alteplase versus control across all stroke severities and subtypes. 19 For this reason, the MWG provided a cautiously worded Evidence-based Recommendation, voted on two Expert Concensus Statements concerning use of rt-PA in lacunar stroke, and concurred cautiously that patients with lacunar ischaemic stroke should be managed as per current guidelines on use of thrombolysis in ischaemic stroke. 19
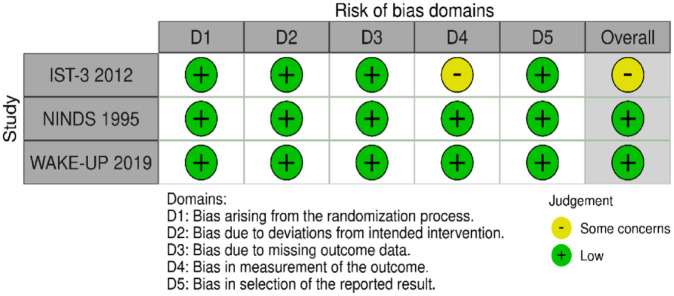
PICO 1 Risk of bias assessment for good functional outcome
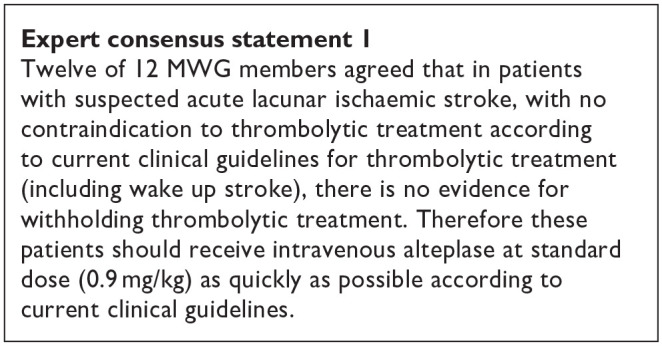
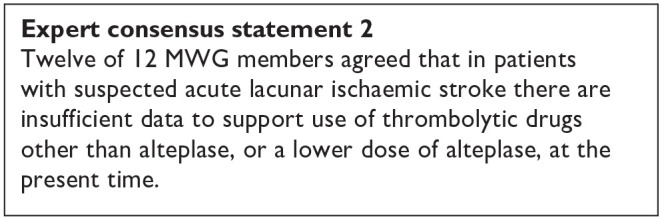
PICO 2:In patients with suspected acute lacunar ischaemic stroke, does acute treatment with antiplatelets (considering single/dual, duration, and whether any particular antiplatelet or combination of antiplatelets is better), compared to avoiding/less of/alternative antiplatelet intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders?
This PICO addresses antiplatelet treatment; anticoagulant treatment is covered in PICO 4 (progressive lacunar stroke) and PICO 5 (other treatments).
Analysis of current evidence
We performed a combined literature search for PICO 2 and PICO 6 (Supplemental Table 5; Supplement PRISMA diagrams) and identified 3 acute antiplatelet treatment trials.
These were heterogeneous regarding intervention or timing of outcome evaluation and therefore no meta-analysis was possible.
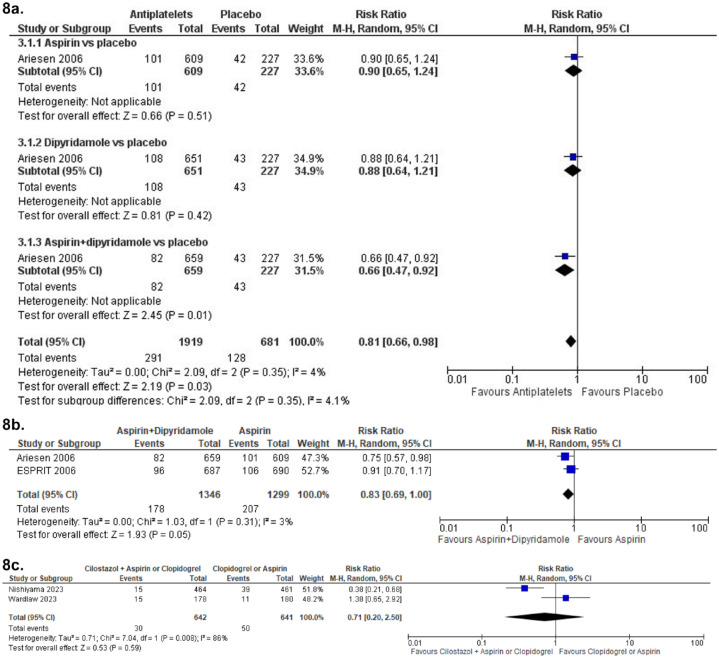
Aspirin versus placebo
The Chinese Acute Stroke Trial (CAST) included patients with ischaemic stroke within 48 h from the event onset. Patients were randomised to receive either aspirin 160 mg or placebo. 57 Patients were followed up to 4 weeks. In the subgroup of patients with lacunar stroke, aspirin was not superior to placebo in preventing a composite of any stroke, myocardial infarction and death (RR 0.89, 95% CI 0.66, 1.21). 58
The International Stroke Trial (IST) 59 used a factorial design to examine treatment with heparin 5000 IU or 12500 IU twice daily or no heparin, as well as aspirin 300 mg daily or no aspirin. There were two primary outcomes: death within 14 days, and death or dependency at 6 months. The trial found a trend towards better primary outcome in the aspirin group (death at 14 days 9.0% vs 9.4% and death or dependency at 6 months 62.2% vs 63.5%, (2p = 0.07)). When analysing additional outcomes, the authors found that death at 14 days (2.8% vs 3.9% (2p < 0.001)) as well as a reduction of death or non-fatal recurrent stroke (11.3% vs 12.4% (2p = 0.02)) were significantly better for the aspirin group and the authors concluded that their study and the Chinese Acute Stroke Trial (CAST) 57 suggest that aspirin should be started as early as possible after acute ischaemic stroke onset. 59 In a lacunar stroke subgroup analysis, there were 1112 primary events among 2308 patients in the aspirin group and 1116 primary events among 2308 patients in the control group for being dead or dependent at 6 months (corresponding to OR 0.99, 95% CI 0.88, 1.11). 59
Intensive antiplatelet therapy versus standard therapy
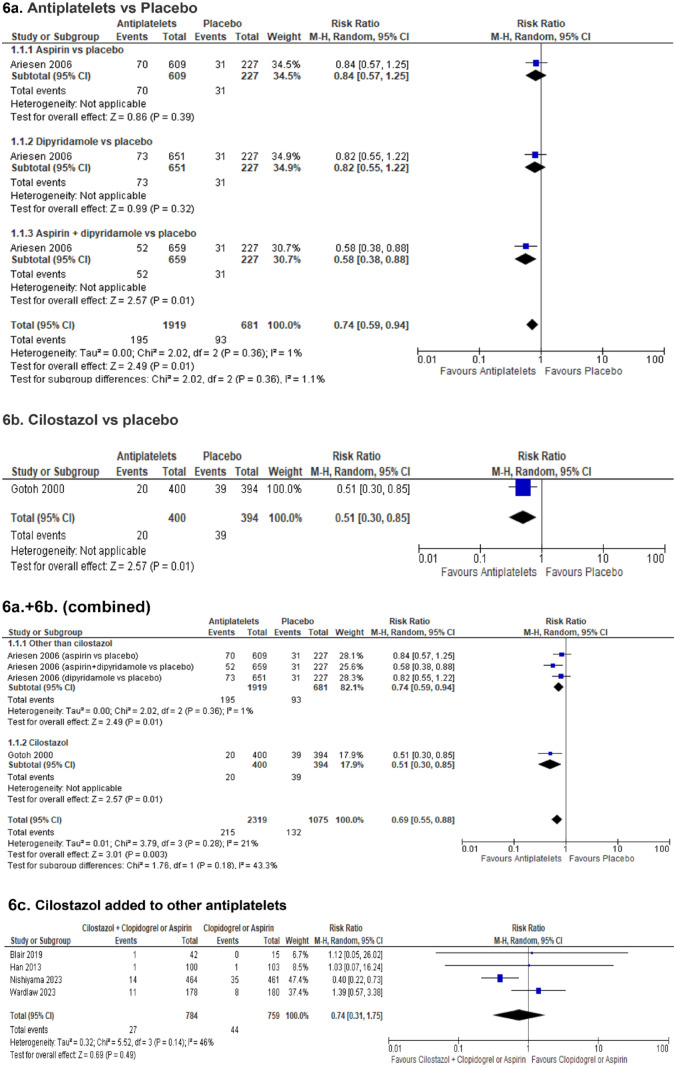
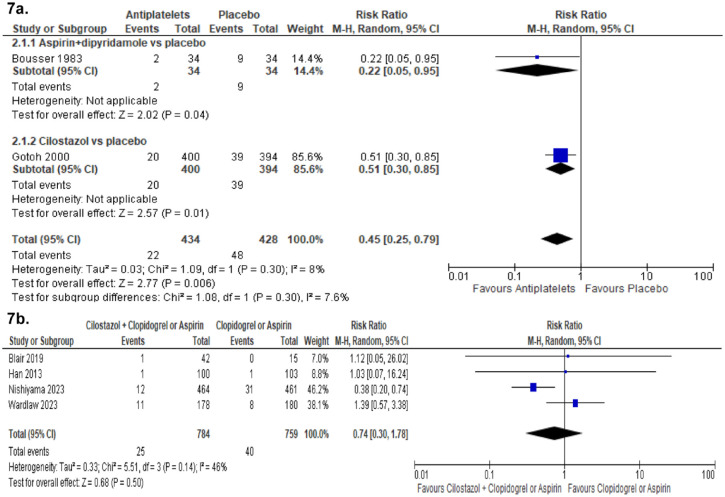
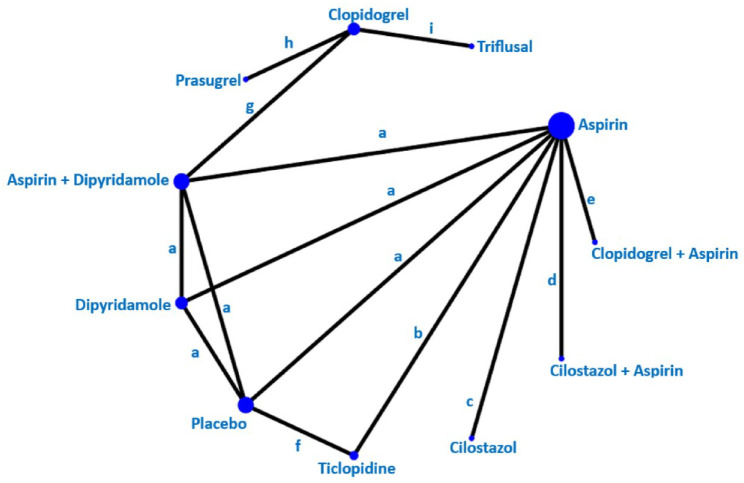
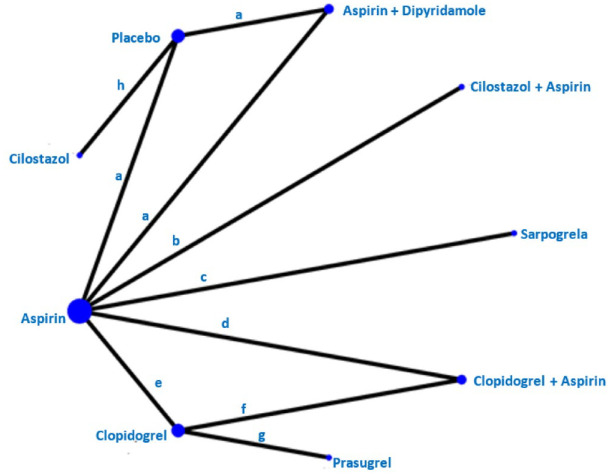
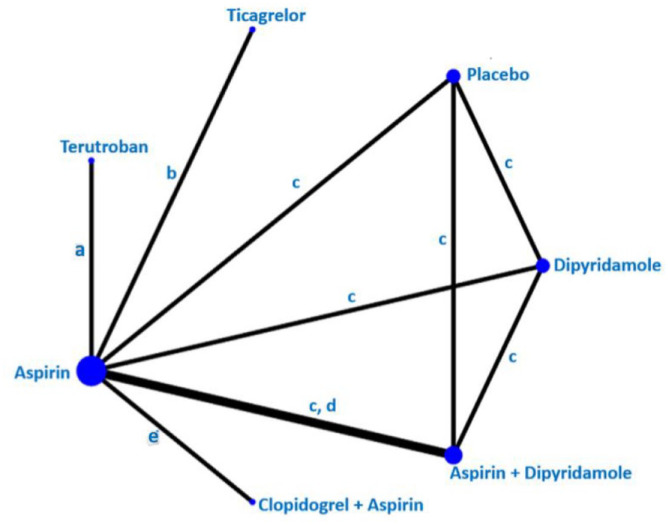
The Triple Antiplatelets for Reducing Dependency after Ischaemic Stroke (TARDIS) trial 60 compared the combined administration of three antiplatelets with either aspirin and dipyridamole in combination denoted as ‘Intensive antiplatelet therapy’, or clopidogrel alone denoted as ‘Guideline-based antiplatelet therapy’. Patients were included within 48 h of stroke/TIA onset and received one of these treatments for 30 days. Primary outcome was defined as the combined incidence and severity of any recurrent stroke (ischaemic or haemorrhagic; assessed using the modified Rankin Scale) or TIA within 90 days. The trial was stopped early because of an increase of major bleedings in the intensive treatment group without a reduction of the primary outcome. 60 Among the 1556 patients receiving intensive antiplatelet therapy, 646 (42%) had a previous lacunar ischaemic stroke and among the 1540 patients receiving Guideline therapy 642 (42%) had a previous lacunar ischaemic stroke according to the Oxfordshire Community Stroke Project (OCSP) classification. For the subgroup of patients with lacunar ischaemic stroke, there was no difference in the primary outcome at 90 days (OR 1.0, 95% CI 0.6, 1.5).
Cilostazol versus no antithrombotic treatment
No trial with specific results for patients with suspected acute lacunar ischaemic stroke was identified. Some additional information is provided in PICO 5.
Cilostazol versus other antiplatelet agents
No trial with specific results for patients with suspected acute lacunar ischaemic stroke was identified. Some additional information is provided in PICO 5.
Dual antiplatelet therapy
The CHANCE 61 and POINT 62 trials found effect but did not specifically report results for patients with lacunar ischaemic stroke in their primary reports. However, it is not uncommon that there is a substantial proportion of patients with lacunar/small vessel stroke in clinical trials.57,59,63 CHANCE subsequently reported a subgroup analysis, 64 with no detected difference in the primary efficacy outcome between single small subcortical infarction patients without or with parental artery disease (considered to be related to atherosclerosis), but this may be due to insufficient power and that the definition of small vessel disease was not equal to that of lacunar ischaemic stroke.
Additional information
The CHANCE-2 trial included patients with minor stroke or transient ischaemic attack within 24 h of symptom onset and carring CYP2C19 loss-of-function alleles, for treatment with either ticagrelor+aspirin or clopidogrel+aspirin for 90 days. 65 A recently published subgroup analysis results showed that for 1750 patients with small-vessel occlusion ischaemic stroke, the primary outcome new stroke within 90 days occurred in 5.3% overall, and was less frequent among those administered ticagrelor+aspirin (3.6%) versus 7.0% of those administered clopidogrel+aspirin (OR 0.51, 95% CI 0.33, 0.79, p = 0.002). 66 The stroke recurrence rates were higher among the 1696 participants with large artery atherosclerosis (10.2% overall) with no difference in the recurrence rate between those allocated ticagrelor-aspirin (9.8%) versus those allocated clopidogrel-aspirin (10.7%; OR 0.86, 95% CI 0.63, 1.18, p = 0.34). Based on this secondary analysis of a subgroup, it has been suggested that DAPT with ticagrelor-aspirin initiated very early and maintained for 90 days after stroke onset may perhaps be beneficial in patients with lacunar ischaemic stroke. 67
The Method Working Group discussed whether to include an Expert Consensus Statement with specific recommendations on short-term DAPT in patients with presumed lacunar ischaemic stroke. After these discussions the group reached an agreement to not include a specific Expert Consensus Statement regarding early short-term DAPT because of (1) inclusion also of TIA patients in the previous trials as shown in the Table immediately below, (2) the paucity of specific data for lacunar ischaemic stroke in previous trials shown in the Table immediately below (none of these trials reported specific outcome details on lacunar ischaemic stroke), (3) the early stroke recurrence rate is lower for lacunar ischaemic stroke than for other subtypes of ischaemic stroke, 4 and (4) because the pathogenetic mechanism in lacunar ischaemic stroke may usually be non-atherosclerotic and non-embolic, 3 antiplatelet treatment may not be as effective as in other subtypes of ischaemic stroke.
| Trial | Proportion of patients with lacunar mechanism | Proportion of patients with TIA |
|---|---|---|
| FASTER 68 | 25.3%–36.1% | At least 39% |
| CHANCE 61 | Not reported,* | 27.9% |
| POINT 62 | Not reported | 43.2% |
| THALES 69 | Not reported | 9% |
Liu et al. 70 reported that among the total of 5170 subjects in CHANCE, a subgroup of 1089 underwent MR angiography, where 56% had intracranial large vessel stenosis (ICAS) and that there was no treatment effect interaction when comparing ICAS versus other (where the majority might be suspected to be small vessel disease), (p = 0.52).
The MWG recognise that the amount and level of evidence for short-term DAPT in lacunar ischaemic stroke is rather low and new data on suspected lacunar ischaemic stroke are becoming available which may clarify the use of dual antiplatelet drugs short-term after suspected acute lacunar ischaemic stroke. For the above reasons, the Expert Consensus Statement is worded very cautiously, and presently no solid recommendations can be given.
The ESO expedited recommendation for the use of short-term dual antiplatelet therapy early after minor stroke and high-risk TIA does not specifically mention lacunar ischaemic stroke or small vessel disease, 71 but, although we found little evidence to support short-term DAPT specifically in lacunar ischaemic stroke, we also found no evidence contradicting the suggestion to follow these recommendations. It should also be noted that in the acute phase within 24 h after stroke onset it is often difficult to diagnose with certainty that the ischaemic stroke is indeed a lacunar ischaemic stroke, further adding to the support for using the previously published ESO recommendations. 71
As there are no additional data suggesting that patients with lacunar ischaemic stroke should be treated differently than the current recommendations for acute antiplatelet treatment for ischaemic stroke 71 it may therefore be reasonable to use dual antiplatelet therapy short term according to the CHANCE and POINT trials’ protocols.


PICO 3: In patients with suspected acute lacunar ischaemic stroke, does immediate antihypertensive treatment (considering agent and BP target), compared to avoiding this intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder and mood disorders?
Analysis of current evidence
Five randomised controlled trials (RCTs) have assessed the impact of immediate antihypertensive treatment compared to avoiding this intervention in patients with acute ischaemic stroke, with secondary analyses on acute lacunar ischaemic stroke patients: CATIS, 72 SCAST, 73 ENOS, 74 VENTURE 75 and ENCHANTED 76 (Table 3; Supplemental Table 6; Supplement PRISMA diagrams). While lower SBP after acute stroke has been associated with better outcome in several observational studies,77,78 and lower risk of symptomatic intracerebral haemorrhage in a trial and registry setting,79,80 an important concern is that rapid BP reductions might worsen cerebral ischaemia through hypoperfusion with compromised autoregulation and collateral flow. 81 While none of these trials was specifically addressing this question in patients with acute lacunar ischaemic stroke, all have reported some secondary analysis results in this subgroup of patients, encompassing haemorrhagic stroke, major adverse cardiovascular events (MACE), dependency, depression and death. No subgroup information is available for the other outcomes (recurrent ischaemic stroke, cognitive impairment or dementia, mobility or gait).
Table 3.
GRADE evidence profile for PICO3 – In patients with lacunar stroke, does immediate antihypertensive treatment (considering agent and BP target), compared to avoiding this intervention, reduce functional outcome, MACE and other outcomes?
| Certainty assessment | No of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsist-ency | Indirect-ness | Imprec-ision | Other consider-ations | Interv-ention | Compar-ator | Odds Ratio (95% CI) | Absolute (95% CI) | ||
| Functional Outcome | ||||||||||||
| 4 | Randomised trials | Not serious | Not serious | Not serious | Seriousa | None | NR | NR | OR 0.98 (0.74,1.28),* | 1 fewer per 1000 (from 1 fewer to 1 fewer) *** |
⨁⨁⨁○ Moderate |
CRITICAL |
| OR 0.99 (0.78, 1.27)** | ||||||||||||
| MACE | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Seriousa | None | 25/321 (7.8%) | 21/359 (5.8%) | OR 1.33 (0.73, 2.44) |
18 more per 1000 (from 15 fewer to 73 more) |
⨁⨁⨁○ Moderate |
CRITICAL |
| Shift in mRS distribution | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Seriousa | None | -/634 | -/613 | OR 0.98 (0.72, 1.34),* |
1 fewer per 1000 (from 1 fewer to 1 fewer)0 fewer per 1000 (from 0 fewer to 0 fewer) |
⨁⨁⨁○ Moderate |
CRITICAL |
| OR 0.94 (0.80, 1.10)** | ||||||||||||
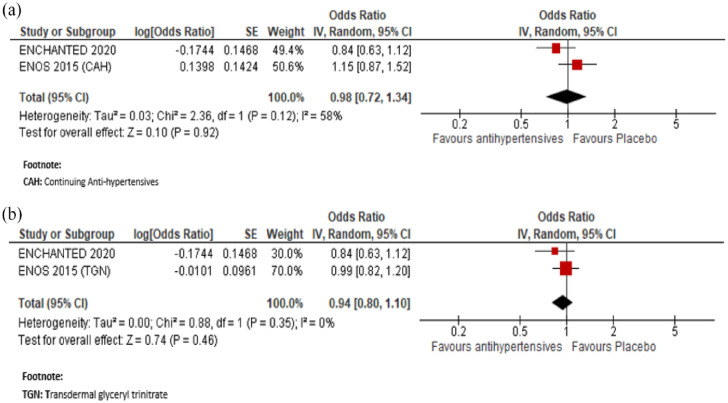
CI: confidence interval; OR: odds ratio; a Confidence interval crosses the clinical decision threshold.
Represents odds ratio for continuing anti-hypertensives (ENOS).
Represents odds ratio for transdermal glyceryl trinitrate (ENOS).
Represents identical absolute 95% CI for both * and **
The China Antihypertensive Trial in Acute Ischaemic Stroke (CATIS) trial is a single-blind, blinded end-points randomised clinical trial, conducted among 4071 patients with non-thrombolysed ischaemic stroke within 48 h of onset and elevated systolic blood pressure (SBP, 140–220 mmHg), recruited in China. 72 Patients were randomly assigned to receive antihypertensive treatment (N = 2038, aimed at lowering systolic blood pressure by 10%–25% within the first 24 h after randomisation, achieving BP less than 140/90 mmHg within 7 days, and maintaining this level during hospitalisation) or to discontinue all antihypertensive medications (control) during hospitalisation (N = 2033). Mean time from stroke onset to randomisation was 15 h. Among these patients, 417 and 385 had lacunar ischaemic stroke in the antihypertensive treatment and control arm respectively. BP lowering was achieved with either intravenous angiotensin receptor inhibitors (ACEi) (first line), oral calcium channel antagonists (CCB) (second line), or oral diuretics. Mean SBP was reduced more drastically within 24 h in the antihypertensive treatment group than in the control group (absolute difference −9.1 mmHg; p < 0.001).
Overall, the primary and secondary composite outcomes of death and major disability (mRS 3–6) at 14 days or hospital discharge and 3-month respectively did not differ between treatment groups (p = 0.98 and 0.93).
The Angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST) trial is a randomised, placebo-controlled, double-blind trial where patients with acute stroke (ischaemic or haemorrhagic) and SBP ⩾ 140 mmHg were randomised within 30 h of symptom onset to receive candesartan or placebo for 7 days, with doses increasing from 4 mg on day 1 to 16 mg on days 3 to 7. 73 Mean time from stroke onset to randomisation was 18 h. In total, 2029 patients from Northern Europe were included in the intention-to-treat analysis (1017 in the candesartan and 1012 in the placebo group), of whom 588 with lacunar ischaemic stroke (279 in the candesartan group and 309 in the placebo group), defined based on OCSP criteria. The average achieved SBP difference between randomised groups during the 7-day treatment period was of 5 mmHg.
Overall, at 6 months, the risk of the composite vascular endpoint (vascular death, myocardial infarction, or stroke) did not differ between treatment groups (p = 0.52), while analysis of functional outcome (shift in modified Rankin Scale (mRS) using ordinal logistic regression) suggested a nominally significant (p = 0.048) higher risk of poor outcome in the candesartan group (not significant after multiple testing correction at p ≤ 0.025).
The Efficacy of Nitric Oxide in Stroke (ENOS) trial is a partial-factorial trial where patients admitted to hospital with an acute ischaemic or haemorrhagic stroke and raised SBP (systolic 140–220 mmHg) were randomly assigned, within 48 h of stroke onset, to 7 days of transdermal glyceryl trinitrate (GTN, 5 mg per day) or to no GTN (control group). 74 A subset of patients who were taking antihypertensive drugs before their stroke were also randomly assigned to continue or stop taking these drugs. Mean time from stroke onset to randomisation was 26 h. In total, 4011 patients (80% from Europe, 14% from Asia) were included (2000 assigned to the GTN group and 2011 to the no GTN group), of whom 1397 with lacunar ischaemic stroke (695 in the GTN group and 702 in the no GTN group), defined based on OCSP criteria. Mean SBP was significantly reduced on day 1 in patients allocated to GTN compared with controls (difference −7.0 mmHg, p < 0.0001), and on day 7 in patients allocated to continue antihypertensive drugs compared with patients randomised to stop them (difference −9.5 mmHg, p < 0.0001).
Overall, at 3 months, the primary outcome (shift in mRS using ordinal logistic regression) did not differ in either treatment comparison. GTN had no significant effects on any of the secondary outcomes. Patients who continued their BP-lowering drugs were significantly more likely to have died in hospital, be dead or disabled (Barthel index < 60) and had significantly lower cognition scores at day 90 than were those who stopped treatment. 74
The Valsartan Efficacy oN modesT blood pressUre REduction in acute ischaemic stroke (VENTURE) study group 75 performed a randomised, open-label, blinded-end-point trial examining whether lowering BP with valsartan versus placebo could improve death or dependency after acute ischaemic stroke. Out of 393 subjects included in the trial overall, 44% of participants had lacunar ischaemic stroke. Mean time from stroke onset to randomisation was 12 h.
Overall, this trial failed to achieve the intended target in the valsartan group (defined as a 15% decrease from baseline or to 145 mmHg), (SBP 146.8 mmHg in the valsartan group vs 147.1 mmHg in the control group on the second day. The difference in primary outcome, death or dependency at 90 days, between the treatment and control groups (24.6% vs 22.6%) was not statistically significant. However, reducing BP with valsartan during this acute period was associated with an elevated risk of early neurological deterioration (END) during the first 7 days (secondary outcome).
The Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED) assessed intensive blood pressure (BP) lowering compared with guideline-recommended BP lowering, in patients treated with alteplase for acute ischaemic stroke. 76 The design was a partial-factorial, open-label, blinded-endpoint trial of thrombolysis-eligible patients (age ⩾ 18 years) with acute ischaemic stroke and SBP ⩾ 150 mmHg. Eligible patients were randomly assigned within 6 h of stroke onset to receive intensive (target SBP 130–140 mmHg within 1 h) or guideline (target SBP < 180 mmHg) BP lowering treatment over 72 h. Mean time from stroke onset to randomisation was 3 h. In total, 2196 patients (74% Asian) were included in the intention-to-treat analysis (1081 in the intensive group and 1115 in the guideline group), of whom 623 with lacunar ischaemic stroke (333 in the intensive group and 290 in the guideline group), defined based on TOAST criteria. The average achieved SBP difference between randomised groups was of 6 mmHg, much smaller than the envisaged 15 mmHg.
Overall, at 3 months, intensive BP lowering compared with current guideline-recommended BP management after IV alteplase therapy was not associated with a significant difference in functional recovery, as assessed by a shift in the distribution of mRS scores. It was associated with a significant reduction in the incidence of intracranial haemorrhage. 76
Dependency, early neurological deterioration and death
In the CATIS trial, 72 a secondary subgroup analysis for the primary outcome (combination of death and major disability (mRS 3–6) at 14 days or hospital discharge) showed a trend (p = 0.06) towards interaction with ischaemic stroke subtypes. Although not significant, lacunar stroke was the only stroke subtype for which antihypertensive treatment tended to be associated with lower risk of mRS 3–6 (Supplemental Table 6).
In the SCAST trial, 73 there was a significant trend towards a better functional outcome (mRS, ordinal logistic regression) with candesartan in patients with total anterior circulation ischaemic stroke (TACI) and partial anterior circulation ischaemic stroke (PACI), than in patients with lacunar infarction (LACI, p value for trend = 0.02). 82 The difference was no longer significant in the adjusted analysis, and, overall, there was no heterogeneity of treatment effect between the subgroups (p = 0.11) 82 (Supplemental Table 6).
In the ENOS trial, 74 the neutral effect on functional outcome (shift in 3-months mRS score distribution) of early BP lowering by transdermal glyceryl trinitrate was comparable across acute ischaemic stroke subtypes, with a neutral effect in lacunar ischaemic stroke specifically (OR 0.99, 95% CI 0.82, 1.19; Supplemental Table 6). Secondary outcomes in lacunar ischaemic stroke patients are not reported.
In the VENTURE trial, 75 the neutral efficacy outcomes did not differ in subgroups stratified by ischaemic stroke subtypes (P interaction 0.62 for death or dependency and 0.90 for MACE).
In the ENCHANTED trial, 76 in secondary analyses published as part of the main trial results, no significant heterogeneity of the treatment effect on the main outcome (shift on 3-month mRS score) was observed across ischaemic stroke subtypes classified on the basis of clinician diagnosis based on TOAST criteria (p for interaction 0.90) 76 (Supplemental Table 6). 76 Subsequently, as there was concern that this approach may have over-estimated the frequency of lacunar ischaemic stroke, classification as lacunar ischaemic stroke was reassessed by a combination of clinical and centrally adjudicated imaging findings. 83 This analysis comprised 454 patients with definite/probable lacunar ischaemic stroke (definite 155; probable 299) and 1178 patients with definite/probable non-lacunar ischaemic stroke, who all received intravenous alteplase. The overall treatment effect of intensive BP reduction versus guideline-recommended BP management on functional, safety and other clinical outcomes were comparable to the main results of the ENCHANTED trial (Supplemental Table 6). There was no heterogeneity of treatment effect on primary and other outcomes across the subgroups of lacunar and non-lacunar AIS, (all p:s for interaction ≥ 0.19).
Haemorrhagic stroke
In the ENCHANTED trial, 76 in the secondary analysis where the classification as lacunar ischaemic stroke was reassessed by a combination of clinical and centrally adjudicated imaging findings, 83 there was no significant difference on any outcomes by randomisation in the lacunar ischaemic stroke subgroup. (Supplemental Table 6). 83
MACE
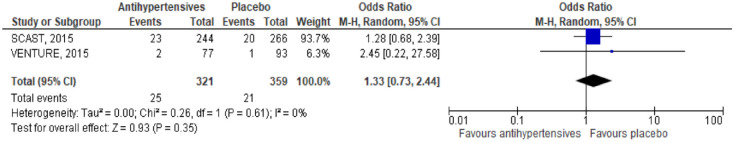
In the SCAST trial, 73 for the composite vascular endpoint (vascular death, myocardial infarction or stroke) there were no differences in treatment effect for either subgroup (p = 0.28), 82 (Supplemental Table 6).
Post-stroke depression
In the prespecified substudy 84 of the CATIS 72 trial, a total of 642 patients with acute ischaemic stroke within 48 h of onset and elevated systolic BP at 7 sites of CATIS were included. Patients were randomly assigned to receive antihypertensive treatment (n = 318) or to the control group (n = 324). The primary outcome was depression (Hamilton Rating Scale for Depression score ⩾ 8) at 3-month post-treatment follow-up. There were 102 lacunar stroke patients (defined according to TOAST criteria) in the antihypertensive group and 104 lacunar stroke patients in the control group. The number of events (Hamilton Rating Scale for Depression score ⩾ 8) was slightly higher in the antihypertensive group than in the control group (OR 1.42, 95% CI 0.82, 2.46). There was no heterogeneity of treatment effect on post-stroke depression across stroke subtypes (p = 0.07).
In summary, there is no evidence that immediate antihypertensive treatment, compared to avoiding this intervention, may be more beneficial in patients with suspected acute lacunar ischaemic stroke than in acute ischaemic stroke patients overall, in whom the absence of benefit has already been demonstrated and reflected in guidelines, with which we concur. 85
Time to treatment may have an effect and varies across RCTs (6–48 h), but in trials with longer time windows analyses stratified on time to treatment are not available in patients with lacunar ischaemic stroke.
Results of the aforementioned trials may also have been diluted by the modest difference in BP reduction between the intervention and control groups in most trials.
Most RCTs excluded patients with extremely elevated systolic blood pressure (>220 mmHg), or do not provide separate results in the subgroup in patients with lacunar ischaemic stroke and SBP >220 mmHg.
Meta-analyses
Few data were available to conduct meta-analyses.
Primary meta-analyses were conducted in acute lacunar ischaemic stroke patients without thrombolysis only. Indeed, optimal BP management may be different in patients undergoing reperfusion therapy and those who are not. Of note, we did include in this meta-analysis two studies that included a small percentage (<12%) of patients who underwent thrombolysis (ENOS, 74 SCAST 82 ), although subgroup results in lacunar ischaemic stroke without thrombolysis weren’t available.
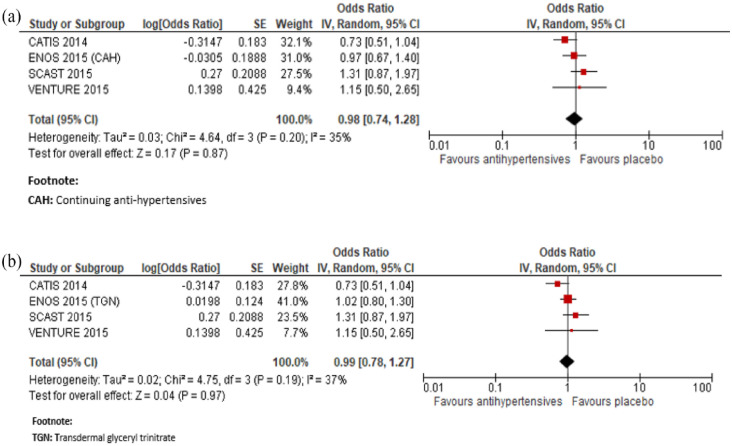
In a meta-analysis combining CATIS, 72 SCAST, 82 VENTURE 75 and ENOS immediate antihypertensive treatment (using a defined dose of Candesartan, Valsartan, transdermal glyceryl trinitrate or targeting a BP <140/90 mg with ACEi, CCB, or diuretics), compared to avoiding this intervention was not associated with a better functional outcome: OR 0.98, 95% CI 0.74, 1.28, for mRS 3–6, Figure 2.
Figure 2.
Meta-analysis of immediate antihypertensive therapy in suspected lacunar ischaemic stroke on functional outcome (mRS 3-6 vs 0-2), (a) continuing versus stopping antihypertensive treatment and (b) transdermal GTN versus no GTN.
In a meta-analysis combining SCAST 82 and VENTURE, 75 immediate antihypertensive treatment, compared to avoiding this intervention was not associated with a lower risk of MACE: HR 1.33, 95% CI 0.73, 2.44, Figure 3.
Figure 3.
Meta-analysis of immediate antihypertensive therapy in suspected lacunar ischaemic stroke on MACE.
As recanalisation may theoretically be less critical in lacunar ischaemic stroke compared to ischaemic stroke with large vessel occlusion, in particular with a lower risk of broad hypoperfusion related to BP lowering, 83 we also conducted pragmatic meta-analyses combining studies with and without thrombolysis.
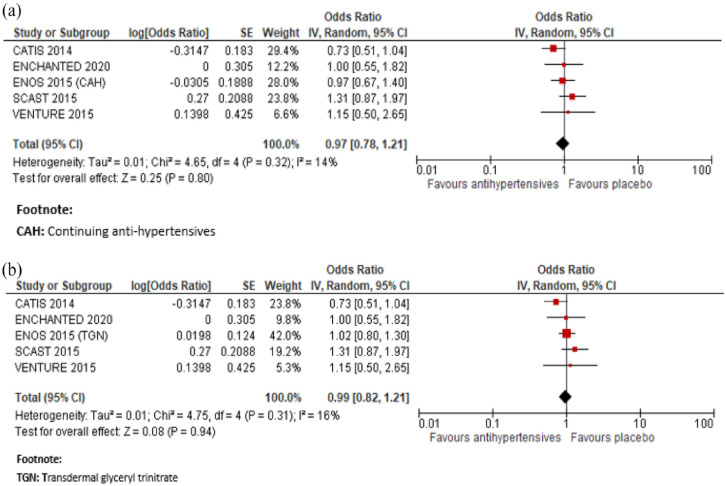
In a meta-analysis combining CATIS, 72 SCAST, 82 VENTURE, 75 ENCHANTED, 83 and ENOS 74 immediate antihypertensive treatment (using a defined dose of Candesartan, Valsartan, targeting a BP <140/90 mg or a SBP 130–140 mmHg), compared to avoiding this intervention was not associated with a better functional outcome: OR 0.99, 95% CI 0.82, 1.21, for mRS 3–6, Figure 4.
Figure 4.
Meta-analysis of immediate antihypertensive therapy in suspected lacunar ischaemic stroke, subgroup analysis comparing with versus without thrombolytic treatment on functional outcome (mRS 3-6 vs 0-2), (a) continuing versus stopping antihypertensive treatment and (b) transdermal GTN versus no GTN.
In a meta-analysis combining ENOS 74 and ENCHANTED, 76 immediate antihypertensive treatment (using transdermal glyceryl trinitrate or targeting a SBP 130–140 mmHg), compared to avoiding this intervention was also not associated with a better functional outcome measured by the shift in mRS score distribution: OR (continuing anti-hypertensives): 0.98, 95% CI 0.72, 1.34; OR (transdermal glyceryl trinitrate): 0.94, 95% CI 0.80, 1.10, for unfavourable shift (Figure 5).
Figure 5.
Meta-analysis of immediate antihypertensive therapy in suspected lacunar ischaemic stroke on functional outcome analysed using ordinal shift analysis of the mRS, (a) continuing versus stopping antihypertensive treatment and (b) transdermal GTN versus no GTN.
Additional information
Previous meta-analyses of RCTs have focused on BP lowering in acute ischaemic stroke at large only.
A meta-analysis of 18 RCTs assessing the impact of BP lowering drugs in acute ischaemic stroke on mortality, found no evidence of a beneficial effect 85 : OR 1.00, 95% CI 0.84, 1.19, p = 0.98, I 2 = 35%. Similarly, a meta-analysis of 12 RCTs assessing the impact of BP lowering drugs in acute ischaemic stroke on improved functional outcome (mRS 0–2) at 3–6 months following symptom onset found no statistically significant difference between the use of any BP lowering drugs compared with control 85 : OR 0.98, 95% CI 0.85, 1.12, p = 0.72, I 2 = 35%.
Most of these RCTs (except for those mentioned above) did not include subgroup analyses in patients with lacunar ischaemic stroke.86–97
Of note, a meta-analysis of two RCTs (RIGHT 98 and RIGHT-2 99 ) assessing the impact of hyperacute pre-hospital BP lowering with any vasodepressor drug showed no difference between transdermal GTN and placebo for the outcome of death at 3 months or functional outcome (mRS 3–6). 85 However no subtype-specific results for lacunar ischaemic stroke were reported, a challenge in the pre-hospital (pre-imaging) setting.
Other guidelines
To our knowledge, no previous guideline has focused specifically on acute BP lowering in patients with lacunar ischaemic stroke. Recent guidelines on blood pressure management in acute ischaemic stroke at large are summarised below as a point of comparison:
The previously published ESO guideline (Table 1), with which we concur, suggests against routine BP lowering in the pre-hospital setting and in the first 24 h following symptom onset in hospitalised patients with any acute ischaemic stroke and BP<220/110 mmHg not treated with intravenous thrombolysis or mechanical thrombectomy (unless necessary for a comorbid condition). In patients with any acute ischaemic stroke undergoing intravenous thrombolysis it suggests maintaining BP below 185/110 mmHg before bolus and below 180/105 mmHg after bolus, and for 24 h after alteplase infusion. The guideline also states that there is continued uncertainty over the benefits and risks (advantages/disadvantages) of continuing versus temporarily stopping previous blood pressure lowering therapy. Moreover, although the effects of blood pressure lowering in acute ischaemic stroke patients with SBP >220 mmHg are unknown, as such patients were excluded in most RCTs, careful blood pressure reduction (<15% SBP in 24 h) was deemed reasonable and likely to be safe in an Expert Consensus Statement. 85
The current US guidelines for acute ischaemic stroke regardless of stroke subtype are to allow patients to autoregulate blood pressure to maintain perfusion for 24 h, up to a SBP of 220 mmHg for patients who did not receive alteplase and 180 mmHg for those who received tPA. 100
The Chinese guidelines for BP management in the acute phase of ischaemic stroke do not provide any specific guideline for the lacunar ischaemic stroke subtype either. 28 For patients with BP <220/120 mmHg, who do not receive IV alteplase or endovascular treatment and do not have complications requiring emergency antihypertensive treatment, this guideline states that starting or restarting antihypertensive therapy within the first 48–72 h after AIS is not effective in preventing death or severe disability. For patients with BP ⩾220/120 mmHg, who do not receive IV alteplase or endovascular treatment and do not have complications requiring emergency antihypertensive treatment, the effect of starting or restarting antihypertensive therapy within the first 48–72 h after AIS is uncertain. The guideline states that it may be reasonable to reduce BP by 15% within the first 24 h after stroke. 28
Registry data
A large Korean multicentre stroke registry data on 3042 patients with acute lacunar ischaemic stroke and 97349 systolic blood pressure measurements, recently showed that the relationship of SBP levels with poor outcome (3-months mRS 2-6) after acute stroke was strongly time-dependent. 101 Elevated SBP was associated with poor outcome in patients with acute lacunar ischaemic stroke, except in the very early phase of acute stroke (first 4 h). At 1 and 4 h after stroke onset, the relationship between SBP and poor outcome showed a non-linear association. The nadir (BP value with lowest percentage of poor outcome) was 155 mmHg at 1 h and 124 mmHg at 4 h. After this time period, from 4 to 72 h after stroke onset, higher SBP was linearly associated with a poorer outcome, with the steepest slope observed at 16–24 h from onset. After that the effect of SBP on poor outcomes seemed to diminish, in line with previous reports.102, 103 These results suggest a complex time-dependent pattern of association between BP level in acute lacunar stroke patients and functional outcome. They may, at least in part, explain the lack of benefit of BP lowering in published clinical trials and could be informative for the design of future RCTs. Based on this registry data it was estimated that a 10 mmHg reduction of SBP at the timepoints of 16–72 h could improve the outcome by approximately 2%. 101
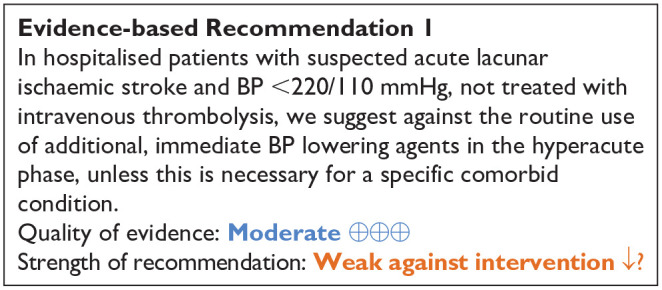



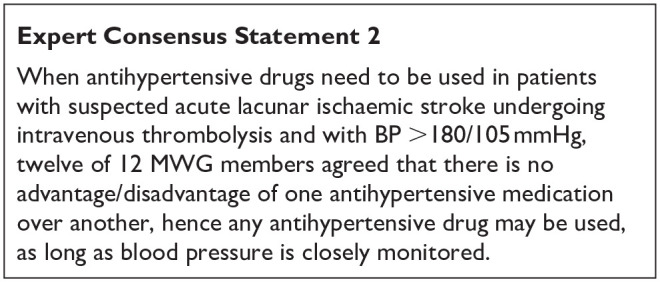

PICO 3 Risk of bias of randomised controlled trials (outcome: functional, shift in mRS score distribution)
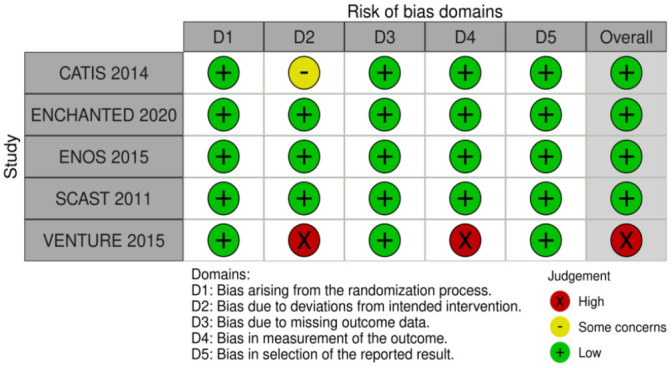
PICO 4: In patients with suspected acute lacunar ischaemic stroke and progressive symptoms, does acute treatment with antiplatelets/anticoagulants/thrombolysis/other agent, compared to less intense or avoiding this intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders?
Definition: Most lacunar strokes present with a neurological deficit which improves steadily after onset. 104 However, progressive worsening of neurological, particularly motor, symptoms in the hours or days after initial symptom onset occurs in some patients with lacunar stroke syndromes leading to unexpectedly worse disability than is typical in lacunar stroke. 105 This deterioration is referred to variously as early neurological deterioration (END), progressive stroke, progressing stroke, stuttering onset, or capsular warning syndrome (CWS); we use the term ‘progressive lacunar stroke’.
‘Progressive lacunar stroke’ is defined quite variably, including as an increase in NIHSS of ⩾2 points within 72 h106,107 or ⩾3 points in total and ⩾2 points for limb paresis within 5 days, 108 or ⩾4 in total,109, 110 or ⩾2 by 48 h 111 or the seventh day after admission, 112 or ⩾1 within 5 days of onset,113,114 or ⩾1115,116 in the NIHSS, or ‘any persistent neurological worsening’. 117 Capsular warning syndrome (‘capsular’ or ‘stroke warning syndrome’) is variously defined as ‘⩾3118–121 or ⩾2 122 stereotyped episodes of lacunar symptoms within 24, 48, or 72 h, with complete neurological resolution between episodes’. ‘Stuttering’ or ‘fluctuating’ lacunar syndrome’ is defined as ‘neurologic deficit’ with periods of improvement and worsening (with or without full resolution). 123
Progressive or stuttering stroke and capsular warning syndrome should be differentiated from early recurrent stroke occurring in the first few days since the mechanism and treatment implications may be different. Progressive stroke is a gradual deterioration whereas early recurrent stroke causes a sudden deterioration in neurological status or different neurological symptoms (indicating different brain region) to those of the initial stroke.
Frequency, risk factors, outcomes: Stuttering onset, capsular warning syndrome or progressive worsening of symptoms occur in 20%–36% of patients with lacunar clinical syndrome in the hours to days after first symptom onset.105,124 Risk factors such as motor paresis,105,108 larger infarct volume,108,124 more severe stroke at admission, 101 worse perfusion in the infarct,105,106 more proximal location in the internal capsule or basal ganglia and larger diameter107,108 seem fairly consistent between studies. Other risk factors such as male sex,105,108 or older age 108 are variable. It is not consistently associated with BP, 105 hypertension, or cardiac embolic sources104,105,124 An apparent association with D-dimer, thrombin and fibrin formation 125 may suggest a stuttering thrombotic process. Progressive stroke is associated with worse functional outcomes possibly reflecting the frequent occurrence in patients with motor symptoms.105,109,117
The systematic literature search identified 567 titles/abstracts, of which 45 were duplicates (Supplement PRISMA diagrams). Of the remaining 522, 21 were selected for full text review and of these six were selected for extraction. Some additional papers were identified during searches for the other PICOs and further articles were identified in reviews or other sources.
This provided two randomised trials both testing antiplatelet drugs (cilostazol, 109 clopidogrel 112 ), plus three prospective observational studies, 14 retrospective observational studies, two case series, one case report (Supplemental Tables 7 and 8) and one review. 105 The two randomised trials were too different to perform a meta-analysis but we were able to calculate ORs for progressive stroke and mRS.
Analysis of Current Evidence
Randomised Trials : We found two RCTs that assessed interventions to reduce progressive stroke that included patients with lacunar stroke, but unfortunately in both trials, lacunar stroke was a subset (albeit > 50%) of the population, and neither trial reported outcomes including mRS for the lacunar group alone (Supplemental Table 7, Table 4). There were therefore no published RCTs assessing directly the effect of any interventions in progressive lacunar ischaemic stroke on the stated PICO outcomes.
Table 4.
GRADE evidence profile for PICO 4.
| Certainty assessment | No of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Cilostazol + standard care treatment | No cilostazol + standard care treatment | Relative (95% CI) | Absolute (95% CI) | ||
| a) Shimizu et al. 109 | ||||||||||||
| Good outcome (mRS 0–2 at 90 days) all patients (approx 68% were lacunar stroke) | ||||||||||||
| 1 | Randomised trial | Serious a | Not serious | Serious | Serious b | None | 221/251 (88.1%) | 217/256 (84.8%) | OR 1.32 (0.79, 2.21) |
33 more per 1000 (from 33 fewer to 77 more) |
⨁○○○ Very low |
CRITICAL |
| Progressive symptoms (⩾4-point increase on NIHSS) in the subgroup (n = 343) with lacunar ischaemic stroke | ||||||||||||
| 1 | Randomised trial | Serious a | Not serious | Serious | Serious b | None | 7/154 (4.5%) | 9/173 (5.2%) | OR 0.87 (0.32, 2.39) |
6 fewer per 1000 (from 35 fewer to 64 more) |
⨁○○○ Very low |
CRITICAL |
| b) Nishi et al.
112
Good outcome (mRS 0–2 at 90 days) all patients (29 lacunar stroke, 23 BAD) | ||||||||||||
| 1 | randomised trial | Serious a | Not serious | Serious | Serious b | None | 22/28 (78%) | 17/25 (68%) | OR 1.73 (0.50, 5.92) |
106 more per 1000 (from 165 fewer to 246 more) | ⨁○○○ very low |
CRITICAL |
| Progressive symptoms (⩾2 point increase on NIHSS) all patients (29 lacunar stroke, 23 BAD) | ||||||||||||
| 1 | randomised trial | serious a | not serious | serious | serious b | none | 0/28 (0.0%) | 4/26 (15.4%) | OR 0.09 (0.00, 1.72) |
138 fewer per 1000 (from – to 84 more) |
⨁○○○ very lowow |
CRITICAL |
BAD: branch atheromatous disease; CI: confidence interval; OR: odds ratio. Unfortunately data were not available for either outcome for just the lacunar stroke subgroup.
Explanations.
Open-label trial.
Confidence interval crosses the clinical decision threshold and optimal information size not met.
One pilot multicentre RCT 109 (Table 4a) in 55 sites in Japan, randomised 510 non-cardioembolic progressive stroke patients (mean age 63) including 343 with lacunar stroke subtype, to cilostazol versus no cilostazol, open label. All patients received Japanese guideline treatment including antiplatelet or an antithrombin agent. Cilostazol was started within 24 h and continued for 3 months. The outcome was the rate of progressive stroke defined as ⩾4pt on NIHSS on day 3 and/or 5, and mRS 0–1 at 3 months, but the authors only reported the rate of progressive stroke in the lacunar subgroup, and we were unable to obtain additional information from the authors. In 343 patients with lacunar stroke, 7/154 (3.2%) allocated cilostazol and 9/175 (6.3%) allocated no cilostazol experienced progression (OR 0.869, 95% CI 0.304, 2.386, p = 0.143). mRS 0–2 was reported for all 507 patients (of which lacunar made up 68%) did not differ between cilostazol and no cilostazol (221/251, 88.1%, vs 217/256. 84.8%, OR 1.32, 95% CI 0.79, 2.21). There were several sources of bias: the method of randomisation was not given, the trial was open label, it is unclear if follow-up was blinded, and the guideline treatment was heterogeneous.
The other RCT 112 (Supplemental Table 7, Table 4b) in Japan enrolled 54 patients with lacunar infarcts (n = 29) or branch atheromatous disease (n = 23) within 48 h of stroke and randomised 28 to argatroban, aspirin and clopidogrel (AAC) and 26 to argatroban and aspirin (AA) and therefore is a trial of clopidogrel versus no clopidogrel on a background of argatroban and aspirin. The outcomes were progressive stroke, defined as worsening of ⩾ 2 NIHSS on the seventh day of admission, and mRS at 3 months, but neither outcome was reported for the lacunar stroke group alone. There were fewer progressive strokes in the AAC group than in the AA group (0 [0%] versus 4 [16%] p = 0.04) but no difference in dependency (mRS 3–6) at 3 months (AAC 6 [21%] vs AA 8 [32%], p = 0.53). Sources of bias included randomisation by sealed envelope, open label and follow-up by hospital staff (blinding not mentioned).
We found one entry in a trial registry (UMIN-CTR Clinical Trial) for a RCT of cilostazol+ aspirin versus aspirin started within 48 h of symptom onset and continued for 14 days to prevent progressive symptoms in lacunar stroke in Japan with outcomes including NIHSS and Barthel Index at 14 days (C000000454, Terayami et al, 2006), but could not find any results.
Additional information
The 19 observational studies (Supplemental Table 8) included various types of progressive lacunar stroke, were mostly retrospective, or prospective with historical controls, and tested several different interventions: antiplatelets (DAPT 3; cilostazol 2; tirofiban 2; abciximab 2), rt-PA (5), heparin (2), BP lowering (valsartan +/-indapamide 1), BP raising (phenylephrine 2), statin (1). Outcomes also varied: 11 retrospective, one prospective observational study and two case series reported outcomes of mRS, NIHSS, haemorrhagic stroke or recurrent stroke (majority used mRS); the other four retrospective and one prospective study used the outcome of progressive stroke.
The heterogeneity of patients, interventions, outcomes and the low quality of evidence provided by retrospective or prospective studies as regards interventions, makes it very difficult to summarise these data. However, considering the larger studies (n > 50), commoner interventions (antiplatelet, rt-PA, phenylephrine), outcomes (mRS, progression) and ignoring whether the studies were retro- or prospective, we derived the following summary.
DAPT versus no DAPT108,126: in 458 patients with lacunar stroke, amongst whom 130 patients developed early neurological deterioration, amongst the 97 who received DAPT, mostly for 5 days versus the 33 who did not receive DAPT, NIHSS was better at discharge than admission (68% vs 35%, p = 0.002), fluctuations were fewer (absent in 79% vs 33%, p < 0.001) but there was no difference in mRS at discharge (80% vs 73%, p = 0.46).
Cilostazol versus aspirin 111 : In 453 patients with lacunar stroke or branch atheromatous disease, amongst those who received cilostazol versus historical controls who received aspirin, there were fewer episodes of early neurological deterioration (18.5% vs 31.4%, p = 0.002), and lower mean mRS at 1 month (1.9 SD ± 1.5 vs 2.3 SD ± 1.5, p = 0.011).
Alteplase versus no alteplase117,121: In 72 patients with capsular warning syndrome, 121 IV alteplase versus no IV alteplase did not improve mRS 0–2 at 3 months (IV alteplase: 23 [85%] versus no IV alteplase: 38 [84%], p = 0.993). In 100 patients with anterior choroidal artery ischaemic stroke of whom 46 progressed, 117 12/21 who progressed, versus 9/54 who did not progress, received alteplase (p = 0.3) but patients who progressed had more severe strokes at admission.
Intensive BP lowering: One study 114 in 119 patients with lacunar stroke confirmed on MRI (<20 mm in DWI) and SBP ⩾160 mmHg, tested intensive BP lowering with valsartan+/- indapamide (to SBP goal <180 mmHg during the first 7 days after admission, <160 mmHg days 7–14, and <140 mmHg after day 14), vs historical controls with BP managed according to usual targets. BP was similar between groups in the first 14 days with no difference in progression of motor symptoms (intensive vs control: 14 [24%] vs 16 [27%], p = 0.87).
Phenylephrine to elevate BP : two studies tested phenylephrine to elevate BP in patients with lacunar stroke and progressive motor symptoms (n = 82, 115 66 116 ) on NIHSS and mRS. Amongst patients who received phenylephrine, mean NIHSS at discharge was lower (1.1 SD 1.47 vs 1.86 SD 1.92, p = 0.042 115 ; 4.4 ± 2.5 vs 6.0 ± 3.7, p = 0.036 116 ) and more patients were independent (mRS 0–2) at discharge (62%vs 50%, p = 0.044) 115 or at 3 months (18 [72%] vs 15 [36.6%], p = 0.011). 116 However many patients given phenylephrine did not achieve target BP, 115 and many patients who did not receive phenylephrine had higher systolic BPs than those who were given phenylephrine. 116
Cilostazol±edaravone versus other drugs (argatroban, ozagrelsodium, urokinase) 113 : in 218 patients with large lacunar infarcts, cilostazol+edaravone did not reduce motor progression (int. vs control: 49 [49%] vs 55 [47%], p = 0.83).
Statins 110 : (published in abstract but with details of results) amongst 277 patients with lacunar stroke of whom 24 had early neurological deterioration, in a retrospective analysis of DAPT, anticoagulation and statins, more patients without early neurological deterioration (41.9%) received early statin intervention (newly initiated, dose-escalation, or switching to a strong statin) than did patients with early neurological deterioration (20.8%), and statin use was associated with less early neurological deterioration in a multivariate model (OR 0.22, 95% CI 0.06, 0.68, p <0.01).
One ongoing multicentre trial in France, Induced hypertension in acute PRogrESsive perforating artery Stroke Using peripheral dilute norepinephrine, PHRC-21-0096 (PRESSURE, NCT06059144) in 358 patients with progressive lacunar ischaemic stroke, may provide further evidence in future on the benefit or harm of drug-induced hypertension on functional independence at 90 days.
Progressive symptoms lead to worse outcome after lacunar stroke but estimates of frequency vary, likely due to varying definitions amongst other factors.
The evidence base on interventions is very limited, mostly made up of retrospective studies or prospective studies that use historical controls.
There is no evidence to recommend any particular antiplatelet, BP management regimen (raising or lowering), rt-PA, anticoagulation, statin, or other treatment for progressive lacunar stroke that is different to treatment for lacunar stroke in the acute phase without progressive symptoms as described in PICOs 1–3 and 5.
While it is clear that more high-quality trials are needed in progressive lacunar stroke, this should not take precedence over the major need for high quality trials in all suspected acute lacunar ischaemic stroke.
We therefore suggest that patients with suspected lacunar ischaemic stroke and progressive symptoms should be included in all trials in acute lacunar ischaemic stroke but identified as a specific subgroup with prespecified planned analysis of the treatment effect in this subgroup. There is also an urgent need to agree a concensus definition for progressive lacunar ischaemic stroke.
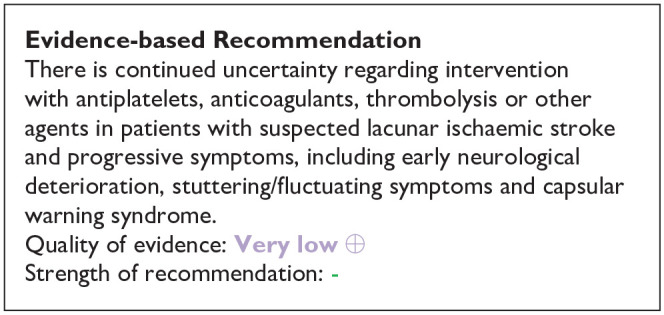
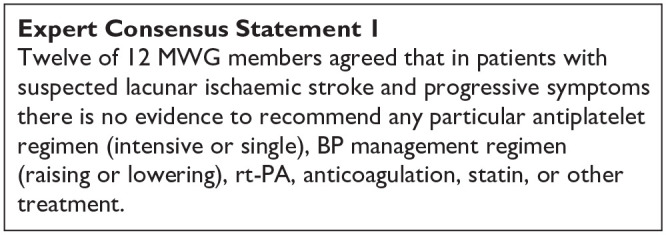
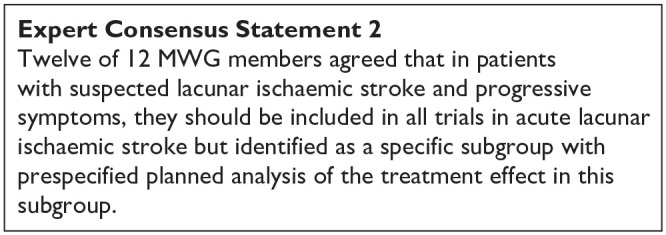

PICO 4 Risk of bias of randomised controlled trials (outcome: mRS and progressive stroke)

PICO 5: In patients with suspected acute lacunar ischaemic stroke, does acute treatment with other agents such as phosphodiesterase-3 inhibitors (e.g. cilostazol, pentoxifylline), anti-inflammatory agents (e.g. minocycline), anticoagulants, nitric oxide donors (e.g. transdermal glyceryl trinitrate), phosphodiesterase 5 inhibitors (sildenafil, tadalafil, dipyridamole), or other relevant agents not addressed in the other PICOs, compared to less intense or avoiding this intervention, reduce any recurrent stroke, recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders?
Analysis of current evidence
The systematic literature searches for PICO 5 and 10 were performed together (Supplement PRISMA diagrams). The search identified 248 titles/abstracts. Of these, 46 articles were selected for full-text review, and 8 were selected for data extraction.46,127–132 Three additional articles were identified by the searches in other PICO questions.131–135 After reference screening of reviews, we added another 2 RCTs.131,136 This delivered 13 RCTs, of which 7 were assigned to PICO 5.
For PICO 5, we ordered the text according to agent. We found two studies on intravenous Magnesium. Due to the heterogeneity of outcomes, the data were not suitable for pooling. We found one study on cilostazol, two studies on anticoagulation, one on glyceryl trinitrate and one on Xueshuantong (Chinese medicinal herb). These are summarised in Supplemental Table 9.
Magnesium
Magnesium has been studied as a neuroprotective agent, although the exact mechanism remains uncertain and may be multimodal. 137 Amongst others, magnesium acts as an NMDA glutamate receptor antagonist, blocks calcium cell entry and exhibits cardiovascular effects.
One randomised placebo-controlled, double-blinded trial (IMAGES, 2004) included 2386 previously independent patients with acute ischaemic stroke, in which study treatment could be started within 12 h of stroke onset. 138 Of these, 765 patients (32%) had a clinical lacunar stroke syndrome. Patients received intravenous magnesium (4 g bolus followed by 16 g over 24 h) or placebo. The primary outcome was a joint binary outcome of death and disability at 90 days, in which Barthel score and mRS were combined. In the whole cohort, the primary outcome was not improved by magnesium (OR 0.95, 95% CI 0.80, 1.13, p = 0.59). A post-hoc analysis identified patients with lacunar syndrome as a potential subgroup that could benefit from magnesium (common OR 0.70, 95% CI 0.53, 0.92). However, the study was performed between 1997 and 2003 and major changes in acute stroke management occurred since then, like iv-thrombolysis (proportion in the trial not mentioned), stroke unit care and early start of antiplatelet therapy. Furthermore, although the trial aimed at including ischaemic stroke, imaging was not required before trial entry resulting in 8.9% primary haemorrhage among the lacunar stroke syndrome subjects. 139 Therefore, we think the results of this study are hardly generalisable to the lacunar stroke population in current clinical practice.
A smaller trial with similar design (2013) included 107 patients with acute ischaemic stroke and symptoms less than 12h, of which 41 (38%) had a lacunar syndrome. 140 Patients were randomised between intravenous magnesium or placebo. Primary outcome was the NIHSS score at 90 days, which was different between the two groups (magnesium 4.7 ± 5.0 vs placebo 7.2 ± 5.7; p = 0.032). In patients with lacunar syndrome, NIHSS was 1.6 ± 1.4 in the Mg group versus 3.3 ± 1.9 in the placebo group (p = 0.003). The clinical relevance of this difference is uncertain. Secondary outcomes (mRS and mortality at 90 days) were not reported for the subgroup of lacunar stroke.
Cilostazol
We identified no cilostazol trials with relevant clinical outcomes that reported specific results for patients with acute lacunar ischaemic stroke. The ECLIPse trial 136 randomised 103 patients with acute lacunar stroke to either cilostazol plus aspirin or placebo plus aspirin for a duration of 90 days. 136 The median time from symptom onset to randomisation was 5 days, and 78% of patients were randomised within 7 days. The primary outcome was pulsatility index on Doppler ultrasound, a non-clinical measure, but recurrent ischaemic stroke within 90 days was reported as a secondary outcome: it occurred in one patient in both groups.
Glyceryl trinitrate
Nitric oxide (NO) donors such as Glyceryl trinitrate (GTN) are candidate treatments for acute lacunar stroke because of several effects. NO is a cerebral and systemic vasodilator that lowers blood pressure, and has antiplatelet and neuroprotective properties. 141
The Efficacy of Nitric Oxide in Stroke (ENOS) trial randomised 4011 patients with acute ischaemic or haemorrhagic stroke within 48 h of onset between transdermal GTN 5 mg or placebo during 7 days.74,142 The recruitment took over 12 years (2001–2013). 623 (15%) patients had a clinical lacunar stroke syndrome with a compatible CT or MRI scan (acute lacunar infarct or no visible lesion), and 143 had an imaging confirmed lacunar infarct. GTN had no effect on mRS score at 90 days in patients with clinical lacunar stroke syndrome and compatible scan (OR 1.09, 95% CI 0.82, 1.45) nor in those with confirmed lacunar infarction (OR 1.00, 95% CI 0.53, 1.87). The effect on cognition and mood was neutral (not published; personal communication). Of note, the ENOS trial was also included in the analysis of PICO 3 on immediate antihypertensive treatment in acute lacunar stroke.
Based on this one trial, with low risk of bias, which could not show a positive effect on functional outcome, we suggest against the use of GTN in patients with suspected acute lacunar stroke.
Anticoagulation
We found two trials on low molecular weight (LMW) heparin or heparinoid, conducted between 1990 and 2000, which presented prespecified results on acute lacunar stroke subgroups. TOAST was a randomised placebo-controlled trial of danaparoid, an LMW heparinoid given in a 7-day course, in acute ischaemic stroke of which 24% were reported to be of lacunar subtype. 133 Treatment with danaparoid was not associated with improvement in favourable functional outcome at 90 days in the total group, nor the lacunar subtype. TAIST was a randomised aspirin-controlled trial testing medium or high dose tinzaparin, an LMW heparin, within 48 h of acute ischaemic stroke and given for up to 10 days. It included 40% lacunar stroke subtypes. 134 Tinzaparin in medium or high dose did not improve functional outcome at 180 days compared with aspirin in the whole group, and this result was not different in the lacunar stroke subtype.
Nowadays, there is ample evidence to start antiplatelet therapy as soon as possible after acute ischaemic stroke, and, referring to PICO 2, consensus that this also applies to acute lacunar stroke, resulting in a recommendation against the use of therapeutic LMW heparin or heparinoid in patients with acute lacunar stroke.
Xueshuantong
Xueshuantong is an extraction from a Chinese medicinal herb with an anti-inflammatory effect, administered intravenously. One small randomised non-placebo controlled trial in elderly Chinese patients with acute lacunar stroke reported significant more reduction in NIHSS score between admission and discharge after 4 weeks of treatment with Xueshuantong compared to no treatment. 135 Exact results were not reported, but reading from the figures, the difference in NIHSS score reduction between both groups seemed less than 1. The quality of the study was low, and we found no confirmatory trials.
We found no trials on acute treatment with any other agents reporting on death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, or mood.
Additional information
Cilostazol
In addition to the earlier mentioned ECLIPse trial, that studied the effect of cilostazol in lacunar stroke patients, there were several randomised trials on cilostazol in acute non-cardioembolic stroke, including a substantial proportion of lacunar stroke patients.109,143–145 In these trials cilostazol was started within 24–48 h of stroke onset. The smallest study (76 patients, 47% small vessel occlusion) showed that neurological deterioration or stroke recurrence within 14 days was significantly lower in the cilostazol+aspirin group than in the control group (aspirin only) (6%vs 28%, RR 0.21, 95% CI 0.05, 0.87, p = 0.013). 143 However, this positive effect was not found in the other trials, although it must be noted that the comparator groups were different. In CAIST, 458 acute ischaemic stroke patients (57% lacunar) were randomly assigned to cilostazol or aspirin. 144 Functional independency (mRS 0–2) was not different between the groups at 90 days. In the study of Shimizu et al. (507 patients, two-thirds lacunar), the rate of progressive stroke on days 3 and/or 5 did not differ between the cilostazol and control (optimal medical treatment only) group, nor did mRS score 0–1 at 90 days. 109 Of note, the use of antiplatelet agents other than cilostazol was more common in the control group than in the cilostazol group (89.8%vs 30.7%). The study of Aoki et al. included 1201 patients with acute ischaemic stroke (44% lacunar stroke), randomising between dual therapy with cilostazol+aspirin versus cilostazol alone. 145 Dual therapy did not decrease the combined occurrence of neurological deterioration, stroke recurrence, and TIA events within 14 days, nor was there a difference in mRS 0–1 at 90 days.
The ECLIPse trial was not powered to find a difference in clinical outcome. Although a substantial proportion of the included patients in the abovementioned trials were of the lacunar subtype, separate results on stroke subtypes were not reported. Furthermore, the comparator groups differed between the studies, and we note some quality concerns (3 of 4 studies were non-blinded). Cilostazol has not been studied against, or on top of, clopidogrel or dual therapy with aspirin+clopidogrel. Reflecting the Asian origin of the abovementioned studies, cilostazol is included in Asian stroke guidelines: in the Japan stroke society guideline, cilostazol ‘may be considered as single antiplatelet therapy or with aspirin in patients with non-cardioembolic stroke within 48h of onset’. 30 The guideline of the Chinese stroke association gives cilostazol as an alternative if aspirin or clopidogrel is not available. 28 ESO stroke guidelines, the AHA guideline for the early management of patients with acute ischaemic stroke, the Canadian and the Australian and New Zealand stroke guidelines have no recommendation on the use of cilostazol in the acute stroke phase.24,27,71,100
Taken together, we feel we cannot recommend on the use of cilostazol for acute treatment in patients with lacunar stroke.
Ongoing trials
We are not aware of ongoing trials on acute treatments with other agents in acute lacunar stroke. Agents such as cilostazol, phosphodiesterase inhibitors and anti-inflammatory drugs are currently being investigated in secondary prevention trials but might also be interesting in the acute phase.





PICO 6:
In patients with lacunar ischaemic stroke, does long term treatment with antiplatelets (single or dual, duration and whether any particular antiplatelet or combination of antiplatelets is better), compared to avoiding/less of/alternative antiplatelet intervention reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorders, and mood disorders?
Analysis of current evidence:
We identified 22 trials9,46,127,130,131,136,146–162 (Supplemental Table 10; Supplement PRISMA diagrams), with a follow-up of clinical outcomes exceeding 4 weeks from randomisation.
For shorter periods with focus on acute phase trials – we identified 3 trials - please see PICO 2 section.
The trials examined different treatments and had various outcomes (Supplemental Table 10; Table 5). This results in a complex situation (illustrated in Figures 6–11) where it is usually not possible to compare studies directly between each other. For example, Prasugrel, Triflusal and Vorapaxar were only included in one trial each.149,159,163 Six trials included groups receiving placebo. Several other trials were non-blinded where subjects receiving treatment with the study drug were compared with subjects not receiving the study drug. The results of the individual trials are described below. Because cilostazol was studied as an add-on to usual antiplatelet treatment in most trials, and important additional non-antiplatelet effects of cilostazol have been suggested, the cilostazol trials are described in a separate section. Regarding the effect of combining cilostazol and isosorbide mononitrate – please see PICO 10.
Table 5.
GRADE evidence profile for PICO 6: long-term antiplatelet treatment (single or dual, duration, and whether any particular antiplatelet or combination of antiplatelets is better), compared to avoiding/less of/alternative antiplatelet intervention, to reduce recurrent ischaemic stroke, any stroke and MACE in patients with lacunar ischaemic stroke. Please compare with Figures 6-8.
| Certainty assessment | No of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Intervention | Comparator | Relative (95% CI) | Absolute (95% CI) | ||
| Any stroke (Antiplatelets vs placebo – other than Cilostazol) | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Not serious | Not serious | None | 195/1919 (10.2%) | 93/681 (13.7%) | RR 0.74 (0.59, 0.94) |
36 fewer per 1000 (from 56 fewer to 8 fewer) |
⨁⨁⨁⨁ High |
CRITICAL |
| Any stroke (Cilostazol vs placebo) | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Not serious | Serious a | None | 20/400 (5.0%) | 39/394 (9.9%) | RR 0.51 (0.30, 0.85) |
49 fewer per 1000 (from 69 fewer to 39 more) |
⨁⨁○○ Low |
CRITICAL |
| Any stroke (Antiplatelets vs Placebo – including Cilostazol) | ||||||||||||
| 2 | randomised trials | not serious | not serious | serious b | not serious | none | 215/2319 (9.3%) | 132/1075 (12.3%) | RR 0.69 (0.55, 0.88) |
38 fewer per 1000 (from 55 fewer to 15 fewer) |
⨁⨁⨁○ Moderate |
CRITICAL |
| Any stroke (Cilostazol added to other antiplatelets) | ||||||||||||
| 4 | Randomised trials | Not serious | Not serious | Not serious | Serious c | None | 27/784 (3.4%) | 44/759 (5.8%) | RR 0.74 (0.31, 1.75) |
15 fewer per 1000 (from 40 fewer to 43 more) |
⨁⨁⨁○ Moderate |
CRITICAL |
| Ischaemic stroke (Antiplatelets vs Placebo – including Cilostazol) | ||||||||||||
| 2 | Randomised trials | Serious d | Not serious | Serious | Not serious | None | 22/434 (5.1%) | 48/428 (11.2%) | RR 0.45 (0.25, 0.79) |
62 fewer per 1000 (from 84 fewer to 24 fewer) |
⨁⨁○○ Low |
CRITICAL |
| Ischaemic stroke (Cilostazol added to other antiplatelets) | ||||||||||||
| 4 | Randomised trials | Not serious | Not serious | Not serious | Serious c | None | 25/784 (3.2%) | 40/759 (5.3%) | RR 0.74 (0.30, 1.78) |
14 fewer per 1000 (from 37 fewer to 41 more) |
⨁⨁⨁○ Moderate |
CRITICAL |
| MACE Outcome (Antiplatelets vs placebo – other than Cilostazol) | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Not serious | Not serious | None | 291/1919 (15.2%) | 128/681 (18.8%) | RR 0.81 (0.66, 0.98) |
38 fewer per 1000 (from 64 fewer to 4 fewer) |
⨁⨁⨁⨁ High |
CRITICAL |
| MACE Outcome (Aspirin + Dipyridamole vs Aspirin) | ||||||||||||
| 2 | randomised trials | not serious | not serious | not serious | serious e | none | 178/1346 (13.2%) | 207/1299 (15.9%) | RR 0.83 (0.69, 1.00) |
27 fewer per 1000 (from 49 fewer to 0 fewer) |
⨁⨁⨁○ Moderate |
CRITICAL |
| MACE Outcome (Cilostazol added to other antiplatelets) | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Not serious | Serious f | None | 30/642 (4.7%) | 50/641 (7.8%) | RR 0.71 (0.20, 2.50) |
23 fewer per 1000 (from 62 fewer to 117 more) |
⨁⨁○○ Low |
CRITICAL |
CI: confidence interval; RR: risk ratio.
Explanations
Optimal information size not met (less event and small sample size).
Variation in interventions.
Wide confidence interval and clinical decision threshold crosses the line of no effect.
Included studies had some concern to high concern/high risk of bias.
Clinical decision threshold touches the line of no effect.
Wide confidence interval and clinical decision threshold crosses the line of no effect.
Figure 6.
PICO 6 Antiplatelet drugs in secondary prevention of any recurrent stroke in patients with lacunar ischaemic stroke: (6a.) Antiplatelets versus Placebo. (6b.) Cilostazol versus placebo. (6a. + 6b.) combined. (6c.) Cilostazol added to other antiplatelets.
Figure 7.
PICO 6 Antiplatelet drugs in secondary prevention of recurrent ischaemic stroke in patients with lacunar ischaemic stroke: (7a.) Antiplatelets versus Placebo. (7b.) Cilostazol added to other antiplatelets.
Figure 8.
PICO 6 Antiplatelet drugs in secondary prevention of MACE in patients with lacunar ischaemic stroke: (8a.) Antiplatelets versus Placebo. (8b.) Aspirin + Dipyridamole versus Aspirin. (8c.) Cilostazol added to other antiplatelets.
Figure 9.
Network Meta-analysis of antiplatelet trials including the outcome ‘Any stroke’.
a. ESPS-2 (Ariesen; 2006) 147
b. AAASPS (Gorelick; 2003) 164
c. CSPS 2 (Shinohara; 2010) 130
d. ECLIPSE (Han; 2013) 136
e. SPS3 (Benavente; 2012) 165
f. CATS (Gent; 1989, Kwok; 2015)58, 150
g. PRoFESS (Sacco; 2008) 155
h. PRASTRO-I (Kitazono; 2021) 149
i. MAESTRO (Han; 2017) 163
Figure 10.
Network Meta-analysis of antiplatelet trials including the outcome ‘Ischaemic stroke’.
a. AICLA (Bousser; 1983) 146
b. ECLIPSE (Han; 2013) 136
c. S-ACCESS (Shinohara; 2008) 148
d. SPS3 (Benavente; 2012) 165
e. CSPS.com (Nishiyama; 2023) 131
f. MATCH (Diener; 2004) 153
g. PASTRO-I (Kitazono; 2021) 149
h. CSPS (Matsumoto; 2006) 162
Figure 11.
Network Meta-analysis of antiplatelet trials including the outcome major adverse cardiovascular event (MACE) in patients with lacunar ischaemic stroke.
aPERFORM (Bousser; 2011) 156
bSOCRATES (Amarenco; 2017) 160
cESPS-2 (Ariesen; 2006) 147
dESPRIT (ESPRIT; 2006) 154
eSPS3 (Benavente 2012)165
Many trials included ‘any stroke’, ‘ischaemic stroke’, or ‘major adverse cardiovascular event (MACE)’ among outcomes. We meta-analysed each of these three outcomes where appropriate (Figures 6–8, Table 5). Because fewer trials included cognitive impairment or dementia, haemorrhagic stroke, mobility or gait disorder or mood disorders, we did not perform meta-analyses for these outcomes.
As an exploratory analysis we also performed network meta-analyses (NMA) 38 where we made indirect comparisons (also see Supplement). In the context of multiple agents for the same indication, a network meta-analysis can be a useful synthesis. We consider NMA as an exploratory rather than definitive analysis, and would urge great caution in the interpretation of these indirect network meta-analyses results as the included trials had substantial heterogeinty and there was no common comparator.
Antiplatelet trials except for Cilostazol
Antiplatelets versus placebo
Aspirin versus placebo
This was studied in two trials.57,58,146,147 The Accidents Ischémiques Cérébraux Liés a l’Atherosclerose (AICLA) trial, included 604 patients with atherothrombotic ischaemic strokes or TIA within 1 year from the index event. Patients were randomised in a double-blind fashion to three times daily receive either aspirin 330 mg, aspirin 330 mg plus dipyridamole 75 mg, or placebo, with evaluation at least every 4 months for 3 years. The trial included 16% of patients with lacunar stroke defined as ‘probable lacune’. The combination of aspirin + dipyridamole was superior to placebo in the prevention of recurrent ischaemic stroke (RR 0.22, 95% CI 0.05, 0.95); aspirin was not superior to placebo (RR 0.38, 95% CI 0.11, 1.27) and aspirin + dipyridamole was not superior to aspirin alone (RR 0.59, 95% CI 0.11, 3.29). 146 Data from the AICLA study should be taken with caution as they are based on very low numbers. Additionally, the trial did not report safety outcomes.
The second European Stroke Prevention Study (ESPS-2) trial included 6602 patients with previous minor ischaemic stroke or TIA that were randomised to aspirin (25 mg twice daily) + modified-release dipyridamole (200 mg twice daily), aspirin alone, dipyridamole alone, or placebo.58,147 There were 2600/6602 subjects with small vessel disease, defined as having one of the classical clinical lacunar syndromes. For these subjects, the combination of aspirin + dipyridamole was superior to placebo and to aspirin alone in prevention of any stroke (HR 0.56, 95% CI 0.40, 0.78, and HR 0.68, 95% CI 0.48, 0.97, respectively) and MACE (HR 0.64, 95% CI 0.48, 0.84, and HR 0.74, 95% CI 0.55, 0.99, respectively), while aspirin alone versus placebo and dipyridamole alone versus placebo were not significant.
We did not perform a meta-analysis of the AICLA and ESPS-2 trials because they reported different outcomes.
The Chinese Acute Stroke Trial (CAST) included patients within 48 h after stroke onset and compared aspirin with placebo. 57 We did not include the CAST trial into PICO 6 because the trial did not report outcome results after more than 4 weeks. Please see PICO 2 section for more information.
Ticlopidine versus placebo
The Canadian American Ticlopidine Study (CATS) randomised 1072 patients to receive 250 mg Ticlopidine twice daily or placebo entering the study 1 week - 4 months after stroke onset. Patients were followed up for a mean of 24 months. 150 The event rate for stroke, myocardial infarction or vascular death, taken together was significantly lower in the Ticlopidine group. 150 In a subgroup analysis of 274 patients with lacunar stroke reported by Kwok et al. 58 there was a lower proportion of recurrent stroke among those treated with Ticlopidine (HR 0.52, 95% CI 0.28, 0.95). 58
Comparisons between different antiplatelet treatments
Aspirin versus clopidogrel
The Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com) 130 mainly focused on cilostazol treatment and included 925 subjects with symptomatic lacunar stroke. 131 However, among these subjects not receiving cilostazol, a comparison between aspirin only versus clopidogrel only treatment was possible where 9 of 195 (4.6%) patients assigned aspirin and 22 of 265 (8.3%) patients assigned clopidogrel had primary outcome defined as first recurrence of an ischaemic stroke (RR 0.56, 95% CI 0.26, 1.18). 131
Prasugrel versus clopidogrel
The comparison of PRAsugrel and clopidogrel in Japanese patients with ischaemic STROke (PRASTRO-I) trial was a phase III randomised controlled trial which included patients with non-cardioembolic ischaemic stroke within 1–26 weeks from informed consent. 149 Patients were randomised to either prasugrel 3.75 mg daily or clopidogrel 75 mg daily. Patients were followed-up for 96 weeks. A subgroup analysis of the PRASTRO-I trial stratified the trial results according to stroke aetiology where lacunar stroke was defined according to the TOAST classification criteria. Among patients with lacunar stroke, the risks of recurrent ischaemic stroke (HR 0.81, 95% CI 0.43, 1.51), any stroke (HR 0.86, 95% CI 0.47, 1.56), or MACE (HR 0.82, 95% CI 0.45, 1.50) were not different between the prasugrel and the clopidogrel group.
Terutroban versus aspirin
The Prevention of cerebrovascular and cardiovascular Events of ischaemic origin with teRutroban in patients with a history oF ischaemic strOke or tRansient ischaeMic attack (PERFORM) trial15 6 randomised patients with ischaemic stroke in the previous 3 months or a TIA in the previous 8 days to terutroban 30 mg daily or aspirin 100 mg daily. Among the 1733 patients with lacunar stroke in the trial, no difference between the treatment arms regarding fatal or non-fatal ischaemic stroke, fatal or non-fatal myocardial infarction, or other vascular death (except haemorrhagic death) was seen, with these events occurring in 54 of 856 assigned terutroban and 61 of 877 assigned aspirin (HR 0.90 (95% CI 0.62, 1.31). 156
Ticlopidine versus aspirin
The African American Antiplatelet Stroke Prevention Study (AAASPS) trial randomised patients with ischaemic stroke occurred within 7–90 days to ticlopidine 500 mg daily versus aspirin 650 mg daily.151,164 Patients were followed up for up to 2 years. The trial found that in patients with lacunar stroke, ticlopidine was not superior to aspirin for secondary prevention of any stroke (HR 0.98, 95% CI 0.64, 1.51).
Ticlopidine versus clopidogrel
A pooled analysis of one phase IIIa trial 157 and one phase IIIb trial157,158 (no acronyms for these two trials were found) included patients with ischaemic stroke occurring >8 days before treatment who were randomised to receive either ticlopidine (200 mg once daily) or clopidogrel (75 mg once daily). 157 Patients were followed up to 52 weeks. In patients with lacunar stroke, ticlopidine was not superior to clopidogrel in the prevention of a composite of ischaemic stroke, myocardial infarction and death (RR 0.85, 95% CI 0.46, 1.55).
Triflusal versus clopidogrel
The Comparison of Triflusal and Clopidogrel Effects in Secondary Prevention of stroke based on cytochrome P450 2C19 genotyping (MAESTRO) trial included patients with ischaemic stroke within 90 days that were randomised to triflusal (300 mg twice daily) or clopidogrel (75 mg daily). 152 Patients were followed up for a median of 2.7 years. In patients with lacunar stroke, the trial found no difference between triflusal and clopidogrel in secondary prevention of any stroke (HR 0.68, 95% CI 0.20, 2.32). Notably, these trial results only include information for poor metabolisers of clopidogrel selected via genotyping. Therefore, the results of the MAESTRO trial cannot be directly applied to all patients with lacunar stroke.
Ticagrelor versus aspirin
The Acute Stroke or Transient Ischaemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes (SOCRATES) trial randomised patients with ischaemic stroke, recruited within 24 h of stroke onset, to receive either ticagrelor (90 mg twice daily) or aspirin (100 mg once daily). 160 Patients were treated for 90 days when follow-up occurred. In the subgroup of patients with lacunar stroke – defined according to the ASCOD criteria – ticagrelor was not superior to aspirin in prevention of MACE – defined as a composite of stroke, myocardial infarction, or death (HR 0.91, 95% CI 0.72, 1.14).
Sarpogrelate versus aspirin
Sarpogrelate is an antiplatelet compound inhibiting 5-hydroxytryptamine receptors and platelet aggregation. 148 In the Sarpogrelate-Aspirin Comparative Clinical study for Efficacy and Safety in Secondary prevention of cerebral infarction (S-ACCESS) trial, patients were randomised to receive sarpogrelate (100 mg three times per day) or aspirin (81 mg per day). In the subgroup of patients with lacunar infarction, no significant difference regarding the recurrent ischaemic stroke event rates were observed with 5.95% per year in the Sarpogrelate group and 4.53% per year in the aspirin group (HR1.31; 95% CI 0.84, 2.04). 148
Dual antiplatelet therapy (DAPT) or triple antiplatelet therapy versus single antiplatelet therapy (SAPT) aspirin + clopidogrel versus aspirin
The Secondary Prevention of Small Subcortical Strokes (SPS3) was specifically designed for the secondary prevention of lacunar stroke. 9 The trial included 3020 patients with symptomatic lacunar stroke experienced within 180 days. Patients were randomised after at least 2 weeks from the qualifying stroke to antihypertensive treatment (systolic BP target: <130 mmHg vs 130–149 mmHg) and to antiplatelet treatment (aspirin 325 mg daily plus clopidogrel 75 mg daily vs aspirin alone). Patients were followed up for a mean of 3.4 years. The trial showed that the combination of aspirin and clopidogrel, compared with aspirin alone, was not associated with reduced risk for recurrent ischaemic stroke (HR 0.82, 95% 0.63, 1.09), haemorrhagic stroke (HR 1.65, 95% CI 0.83, 3.31), any stroke (HR 0.92, 0.72, 1.16), or MACE (HR 0.89, 95% CI 0.72, 1.11), However, the combination of aspirin and clopidogrel increased the risk of major haemorrhage (HR 1.97, 95% CI 1.41, 2.71) or death (HR 1.52, 95% CI 1.14, 2.04).
Aspirin + clopidogrel versus clopidogrel alone
The Management of ATherothrombosis with Clopidogrel in High-risk patients (MATCH) study included patients with ischaemic stroke within 3 months. Patients were randomised to a combination of clopidogrel 75 mg + aspirin 75 mg daily or clopidogrel alone. 153 Patients were followed up to 18 months. Overall, the MATCH trial showed that after recent ischaemic stroke or transient ischaemic attack in high-risk patients, the combination of aspirin and clopidogrel did not reduce the risk of recurrent stroke compared with clopidogrel alone but instead increased the risk of life-threatening or major bleeding. 153 The original report did not present subgroup analyses for lacunar stroke, but this was done in a meta-analysis 58 that retrieved data from the MATCH trial for the subgroup of patients with lacunar stroke – according to the TOAST definition. This could not show that the combination of aspirin + clopidogrel was superior to clopidogrel alone in preventing recurrent ischaemic stroke in the long term (RR 0.97, 95% CI 0.79, 1.20) in patients with lacunar ischaemic stroke. 58
Aspirin + dipyridamole versus aspirin
The European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) trial included patients with minor ischaemic stroke or with TIA within the last 6 months. Patients were randomised to aspirin 30–325 mg daily plus dipyridamole 200 mg daily versus aspirin alone and were followed-up for a maximum of 5 years (mean 3.5 years). 154 In patients with lacunar stroke, whose subgroup results were retrieved from a meta-analysis, 58 the aspirin + dipyridamole combination was not superior to aspirin alone in the prevention of a composite of any stroke, myocardial infarction, and death (RR 0.91, 95% CI 0.70, 1.17). 58
Aspirin + dipyridamole versus clopidogrel
The Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial randomised patients with recent (<90 days) ischaemic stroke to aspirin (25 mg daily) + extended-release dipyridamole (200 mg twice daily) versus clopidogrel (75 mg daily); the trial also randomised patients to telmisartan 80 mg daily versus placebo to study effects of blood pressure treatment. 155 The patients were followed for a mean of 2.5 years. In the subgroup of patients with lacunar stroke – defined according to the TOAST classification criteria – aspirin + dipyridamole was not superior to clopidogrel for secondary prevention of any stroke (HR 0.96, 95% CI 0.84, 1.09).
Vorapaxar + standard antiplatelet therapy versus standard antiplatelet therapy
Vorapaxar is an antiplatelet drug that antagonises protease-activated receptor-1. The TRA 2°P-TIMI 50 trial was a secondary prevention RCT evaluating treatment with vorapaxar, compared with placebo, on top of standard antiplatelet therapy (54%–55% consisting of aspirin alone), among patients with stable atherosclerosis such as myocardial infarction, non-cardioembolic ischaemic stroke or peripheral arterial disease. 159 In a prespecified analysis of stroke patients, of which 47% had lacunar stroke, no significant difference between vorapaxar and placebo was found in cardiovascular death, myocardial infarction or stroke, while bleeding complications increased. In lacunar stroke HR for MACE was 0.99, 95% CI 0.75, 1.31, while there was an increased risk for bleeding complications (HR 2.85, 95% CI 1.62, 5.06). 159 Vorapaxar is now considered contraindicated in patients with a history of stroke.
Cilostazol trials
Several trials have been with subjects of Asian ethnicity but trials with subjects with European descent have also been performed. Cilostazol use may sometime be limited by side effects and some patients report to headache, diarrhoea, dizziness, or increased heart rate. 166
Cilostazol versus placebo
The Cilostazol Stroke Prevention Study (CSPS) included patients with ischaemic stroke within 1–6 months randomised to receive cilostazol 100 mg twice daily or placebo.161,162 Overall, patients included in the trial were followed up for a mean of 652 days in the cilostazol group and 570 days in the placebo group. No data was reported for the subgroup of patients with lacunar stroke in the initial report, 161 but a subsequent report on the subgroup of patients with neuroimaging-confirmed lacunar stroke found that cilostazol was superior to placebo in preventing recurrent ischaemic stroke (RR 0.51, 95% CI 0.30, 0.85). 162
Combinations of Cilostazol and other antiplatelets
Cilostazol versus aspirin
The second Cilostazol Stroke Prevention Study-2 (CSPS-2) trial randomised patients with a history of ischaemic stroke 1–6 months before treatment start to cilostazol 100 mg twice daily or aspirin 81 mg daily. 167 Patients were followed up to 29 months. In the subgroup of patients with lacunar stroke, cilostazol was not superior to aspirin for secondary prevention of any stroke (HR 0.75, 95% CI 0.54, 1.04).
Cilostazol + aspirin versus aspirin
The Effect of Cilostazol in the Acute Lacunar Infarction Based on Pulsatility Index of Transcranial Doppler (ECLIPse) trial included 203 patients with lacunar stroke – defined according to the TOAST classification – within 7 days from onset. 136 Patients were randomised to a combination of cilostazol 100 mg twice daily + aspirin 100 mg daily versus aspirin alone and followed up to 90 days. The trial found no superiority of cilostazol + aspirin over aspirin alone in the prevention of recurrent ischaemic stroke (RR 1.03, 95% CI 0.07, 16.24). The data should be interpreted with caution as only one recurrent ischaemic stroke occurred in both the intervention and control group over the short follow-up period of 90 days.
Cilostazol + aspirin/clopidogrel versus aspirin/clopidogrel
The Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com) trial included patients with lacunar stroke occurring between 8 and 180 days before treatment. 131 Patients were randomised to cilostazol 100 mg twice daily + aspirin (81–100 mg daily) (defined as DAPT) or clopidogrel (50–75 mg daily) versus aspirin or clopidogrel alone and followed up for a median of 1.4 years. The trial found that DAPT with cilostazol was superior to single antiplatelet therapy (SAPT) in preventing recurrent ischaemic stroke (adjusted HR 0.43, 95% CI 0.22, 0.85), any stroke (adjusted HR 0.45, 95% CI 0.24, 0.84) and MACE (adjusted HR 0.42, 95% CI 0.23, 0.77) with no significant increase in haemorrhagic stroke (HR 0.53, 95% 0.10, 2.88) or death (HR 3.20, 95% CI 0.33, 30.8) even though small numbers – only 6 haemorrhagic strokes (2 in the intervention group vs 4 in the comparator group) and 4 deaths (3 in the intervention group vs 1 in the comparator group) during the follow-up might affect the risk estimation. 131
Cilostazol+aspirin/clopidogrel/no other antiplatelets versus aspirin/clopidogrel/no other antiplatelets
The LACunar Intervention-1 (LACI-1) trial was a factorial trial in which patients with clinically confirmed lacunar ischaemic stroke in the past 4 years were randomised to isosorbide mononitrate (25 mg daily increased to 25 mg twice daily), cilostazol (50 mg twice daily increased to 100 mg twice daily), both, or none. 127 There were 55 participants (97%) taking clopidogrel for secondary stroke prevention and two (3%) taking aspirin. Patients were followed up for 11 weeks. During the follow-up period, one recurrent ischaemic stroke occurred in the cilostazol group, versus none in the control group and there were no significant differences regarding cognitive outcomes.
The Lacunar Intervention Trial-2 (LACI-2) recruited 363 subjects with clinical lacunar ischaemic stroke for a 2 × 2 factorial randomisation to cilostazol 200 mg daily, isosorbide mononitrate (ISMN) 40–60 mg daily, both, or neither drug on top of their standard medication that usually included antiplatelet treatment with clopidogrel as in LACI-1. 46 There were no haemorrhagic strokes registered during the 12 months follow-up period. Cilostazol versus no cilostazol treatment did not differ regarding recurrent ischaemic stroke or any stroke (adjusted OR (aOR) 1.35, 95% CI 0.51, 3.57), cognitive impairment (adjusted mean MoCA difference 0.37, 95% CI−0.37, 1.11), depression (adjusted mean Zung depression scale difference −3.34, 95% CI−6.81, 0.14, or death (aOR 0.90, 95% CI 0.08, 10.26). However, dependency was less common in the cilostazol treatment group (aOR 0.46, 95% CI 0.22, 0.95, for mRS>2). 46 For discussion on ISMN treatment results, please see PICO 10 section.
PICO 6 Risk of bias assessment of included studies using RoB 2.0 tool (outcome: Any stroke, Ischaemic stroke, MACE)
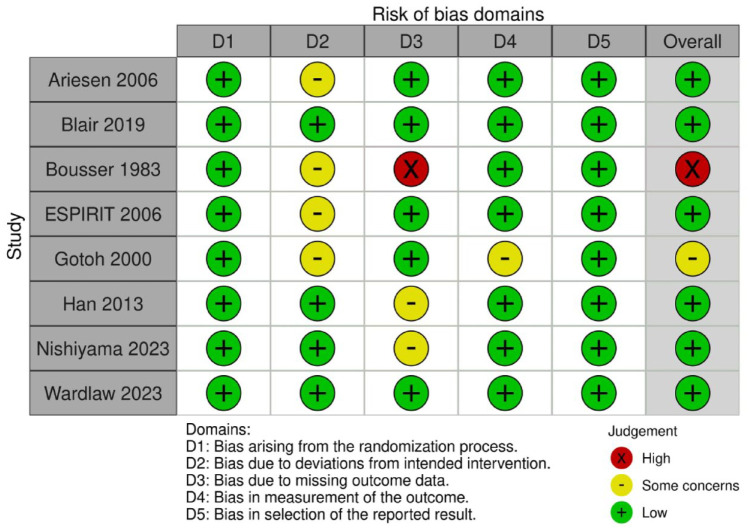
Network meta-analyses
A previous network metal-analysis (NMA) of antiplatelet treatment in lacunar stroke with a literature search up to May 2022 has been published. 168 We now performed an updated NMA, with results of indirect comparisons among the different antiplatelet agents – or combination of them – for the prevention of three specific outcomes: ‘Any stroke’ ‘Ischaemic stroke’, or ‘MACE’ are reported in Figures 9 to 11, respectively and only including randomised controlled trials. The NMA was performed with Stata 1.15 according to the methods laid down in Cochrane handbook. 38
In these Figures, the size of the dots is proportional to the number of patients included in trials, while the widths of lines are proportional to the number of trials. For the outcome ‘Any stroke’, aspirin was the drug tested in most patients, followed by aspirin + dipyridamole and by clopidogrel; for the outcome ‘Ischaemic stroke’, the most tested drug was again aspirin, followed by the aspirin-clopidogrel combination and by cilostazol. It should be noted that cilostazol was tested in several studies (wide lines) each including few patients (small dots), while the other drugs were usually the object of single trials (narrow lines).
Supplemental tables report the quantitative results of indirect comparisons. The results should be taken with extreme caution as they derive from indirect comparisons and are therefore less reliable than those of direct comparisons. This is the rationale for why we do not include the results from the network meta-analyses into our recommendations. However, the analyses indicate that there may be a need for further studies on for example, cilostazol treatment or the combination of dipyridamole and aspirin.
Any stroke
The network diagram for any stroke is presented in Figure 9. We included 9 RCTs in the analysis with 21080 participants. For details regarding detailed numerical values – please see Supplemental Table 11. No evidence of global or loop-specific incoherence was found in the network. Compared to placebo, only Aspirin + Dipyridamole (RR 0.56, 95% CI: 0.41, 0.77, low certainty evidence), Cilostazol (RR 0.55, 95% CI 0.36, 0.84, low certainty evidence) and Clopidogrel (RR 0.59, 95% CI 0.42, 0.83, low certainty evidence) reduces the risk of any stroke in patients with lacunar stroke (Supplemental Table 11).
The size of dots is proportional to the number of patients included in trials, while the widths of the lines are proportional to the number of trials.
Ischaemic stroke
The network diagram for ischaemic stroke is presented in Figure 10. We included 8 RCTs in the analysis with 9862 participants. For details regarding detailed numerical values – please see Supplemental Table 12. No evidence of global or loop-specific incoherence was found in the network. Compared to placebo, only Aspirin + Dipyridamole (RR 0.22, 95% CI 0.05, 0.95, low certainty evidence) and Cilostazol (RR 0.51, 95% CI 0.30, 0.85, low certainty evidence) reduces the risk of ischaemic stroke in patients with lacunar stroke (Supplemental Table 12).
The size of dots is proportional to the number of patients included in trials, while the widths of the lines are proportional to the number of trials.
MACE
The network diagram for major adverse cardiovascular outcome is presented in Figure 11. We included 5 RCTs in the analysis with 12569 participants. For details regarding detailed numerical values – please see Supplemental Table 13. No evidence of global or loop-specific incoherence was found in the network. Compared to placebo, only Aspirin + Dipyridamole (RR 0.70, 95% CI 0.56, 0.88, low certainty evidence) and Clopidogrel + Aspirin (RR 0.74, 95% CI 0.54, 0.99, low certainty evidence) reduces the risk of MACE in patients with lacunar stroke (Supplemental Table 13).
The size of dots is proportional to the number of patients included in trials, while the widths of the lines are proportional to the number of trials.
Additional information
Different monotherapy choices are recommended in different countries and are somewhat influenced by the findings for example, in the CAPRIE trial. 169 A subgroup analysis of the SPS3 trial considered the CYP2C19 metaboliser status in patients with lacunar stroke, comparing the CYP2C19*2 with CYP2C19*17 allele; the metaboliser status did not influence the rate of recurrent ischaemic stroke and major bleeding. 170 The recently published subgroup analysis from the CHANCE-2 trial that included patients with minor stroke or transient ischaemic attack within 24 h of symptom onset and carring CYP2C19 loss-of-function alleles, for treatment with either ticagrelor+aspirin or clopidogrel+aspirin for 90 days, 65 showed that for 1750 patients with small vessel occlusion ischaemic stroke, the primary outcome new stroke within 90 days occurred among 3.6% of those administered ticagrelor+aspirin versus 7.0% of those administered clopidogrel+aspirin (0.51, 95% CI 0.33, 0.79, p = 0.002). 66 This is also discussed in the PICO 2 section of this GL. We did not perform a systematic screen of all relevant literature concerning this topic, therefore we cannot make any specific recommendations. It should be noted that this PICO 6 addresses long term recommendations. The MWG’s recommendations on antiplatelet treatment in the acute phase after lacunar stroke are described in PICO 2, which includes an Expert consensus statement that initiation of antiplatelet therapy should be started as soon as possible after stroke onset.
Previous systematic reviews
A pooled analysis of 17 trials with 42234 subjects with lacunar stroke concluded that a single antiplatelet agent compared with placebo is adequate for secondary stroke prevention, whereas dual antiplatelet therapy should not be used for long-term treatment, 58 which is in line with our results.
A systematic review and meta-analysis on cilostazol in stroke prevention performed an exploratory sensitivity analysis of 9 trials where more than 40% (on average of the participants had lacunar stroke. 171 Cilostazol treatment was associated with a reduction of recurrent ischaemic stroke (OR 0.64, 95% CI 0.52, 0.79), 171 but the authors concluded that more evidence is needed before cilostazol is used more widely in stroke in routine practice.
Another systematic review and meta-analysis of 13 studies with 33011 subjects suggested that cilostazol may be a priority option for secondary prevention in lacunar stroke, but that this needs further study. 168
Current guidelines
We found no specific recommendations regarding long-term antiplatelet treatment in lacunar ischaemic stroke in guidelines from AHA/ASA 2021, 21 Canadian Stroke Best Practice 2020, 24 ESO 2022, 16 or Australia ( https://informme.org.au/guidelines/living-clinical-guidelines-for-stroke-management and https://app.magicapp.org/#/guideline/8L0RME/section/j1qQXj , accessed on July 7th, 2023) (Table 1).

*Defined as more than 2–4 weeks.

PICO 7:
In patients with lacunar ischaemic stroke, does antihypertensive treatment considering a particular agent or target, compared to less intense or avoiding this intervention given long term, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder and mood disorders?
Analysis of current evidence
Two RCTs have examined the impact of antihypertensive treatment compared to less intense or avoiding this intervention on risk of recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke and MACE in patients with a history of lacunar ischaemic stroke, SPS3 (primary outcomes) and How intensively should we treat blood PRESsure in established cERebral small VEssel disease? (PRESERVE) trial (secondary outcome),9,172 No data are available on the impact of antihypertensive treatment on mobility, gait disorder or mood disorders in lacunar ischaemic stroke patients. No subgroup analyses in lacunar ischaemic stroke patients are available either in large RCTs on secondary prevention of ischaemic stroke at large. Details of the trials see Supplemental Table 14; PRISMA diagram see Supplement PRISMA diagrams.
Blood-pressure targets in patients with recent lacunar stroke (SPS3) randomised trial
The SPS3 trial 9 is a randomised open-label trial, including patients living in North America, Latin America and Spain who had a recent, MRI-defined symptomatic lacunar ischaemic stroke. Patients were randomly assigned, according to a two-by-two multifactorial design, to a SBP target of 130–149 mmHg or < 130 mmHg. The primary endpoint was reduction in any stroke. Patients were included at least 2 weeks poststroke, with a median of 62 days after the event. In total, 3020 patients were enrolled, 1519 in the higher-target group and 1501 in the lower-target group and followed up for 3.7 ± 2.0 years. After 1 year, the achieved difference in mean SBP was 11 mmHg between the higher-target and the lower-target group.
Non-significant rate reductions were seen for all stroke (HR 0.81, 95% CI 0.64, 1.03, p = 0.08) and the composite MACE outcome of myocardial infarction or vascular death (HR 0.84, 95% CI 0.68, 1.04, p = 0.32) with the lower SBP target (Supplemental Table 14). The rate of ICH was significantly reduced (HR 0.37, 95% CI 0.15, 0.95, p = 0.03) (Supplemental Table 14). Changes in Cognitive Ability Screening Instrument (CASI) statistical Z scores over time and cumulative incidence of mild cognitive impairment did not differ between assigned BP target groups (p = 0.520 and p=0.555). 8
Randomised trial of intensive versus standard blood pressure control in small vessel disease (PRESERVE)
The PRESERVE trial 172 is a randomised, parallel, multicentre controlled, blinded-outcomes clinical trial where 111 participants with MRI-confirmed lacunar ischaemic stroke and confluent WMH were recruited at least 3 months poststroke and randomised to standard (SBP 130–140 mmHg) (n = 56) or intensive (SBP < 125 mmHg) (n = 55) BP targets. The primary endpoint was change in diffusion tensor imaging (DTI) white matter mean diffusivity (MD) peak height between baseline and 24 months and secondary endpoints included change in cognitive performance. Noteworthy, the initial endpoint was a global cognitive score with DTI-MRI as a secondary end point, but following the publication of the SPS3 cognition study, showing that cognitive change could not be detected over 2 years in 2916 participants with lacunar ischaemic stroke, recruitment to the cognitive only arm of PRESERVE was halted and only recruitment to the DTI-MRI arm continued (which had a smaller sample size of 180), with the primary end point of the overall study becoming DTI. Mean BP was reduced by −15.3 and −23.1 mmHg in the standard and intensive groups respectively (p < 0.001).
Over 24 months follow-up there was no difference between treatment groups for the primary endpoint of change in MD peak height on DTI (p = 0.92) or the secondary endpoint of change in cognitive performance (p > 0.33, Supplemental Table 14). Of note, in the overall sample there was a significant deterioration in white matter microstructure on multimodal diffusion tensor imaging-magnetic resonance imaging but no detectable decrease in cognition. During follow-up, there were three strokes, and one death in the intensive, and three strokes, and two deaths in the standard arms.
The number of patients with any side effect was 45 in the intensive arm and 36 in the standard arm (OR 2.48, 95% CI 0.96, 6.73, p = 0.05). There was no difference between groups in the number of falls (intensive 21, standard 14; OR 0.54, 95% CI 0.22, 1.31, p = 0.16), or postural-related dizziness (intensive 27, standard 22; OR 0.67, 95% CI 0.30, 1.52, p = 0.34).
Meta-analysis results
Risk of recurrent stroke
When combining SPS3 and PRESERVE, the lower target group (<130 mmHg) was found to be non-significantly associated with a relative risk reduction in recurrent stroke of 13% (RR 0.87, 95% CI 0.68, 1.10, p = 0.25) compared to the higher target group (130–149 mmHg) Table 6, Figure 12.
Table 6.
PICO 7 – GRADE evidence profile table. In patients with lacunar stroke, does antihypertensive treatment considering a particular agent or target, compared to less intense or avoiding this intervention, reduce recurrent stroke and risk of death?
| Certainty assessment | No of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Intervention | Comparator | Relative (95% CI) | Absolute (95% CI) | ||
| Recurrent stroke | ||||||||||||
| 2 | Randomised controlled trials | serious a | not serious | not serious | serious b | none | 115/1557 (7.4%) | 134/1574 (8.5%) | RR 0.87 (0.68, 1.10) |
11 fewer per 1000 (from 27 fewer to 9 more) |
⨁⨁○○ Low |
CRITICAL |
| Death | ||||||||||||
| 2 | Randomised controlled trials | serious a | not serious | not serious | serious b | none | 107/1540 (6.9%) | 103/1561 (6.6%) | RR 1.05 (0.81, 1.37) |
3 more per 1000 (from 13 fewer to 24 more) |
⨁⨁○○ Low |
CRITICAL |
CI: confidence interval; RR: Relative Risk.
Low to high risk of bias.
Confidence interval crosses the clinical decision threshold.
Figure 12.
PICO 7. Long term intensive versus guideline BP reduction to prevent recurrent stroke in patients with lacunar ischaemic stroke.
Risk of death
When combining SPS3 and PRESERVE, no significant association was found between the lower and higher target BP group in terms of risk of death (p = 0.70) Figure 13.
Figure 13.
PICO 7. Long term intensive versus guideline BP reduction to prevent death in patients with lacunar ischaemic stroke.
PICO 7 Risk of bias of randomised controlled trials (outcome: recurrent stroke, death)
Additional information
A large number of secondary analyses of the SPS3 trial in subgroup of patients have been conducted (Supplemental Table 14), demonstrating:
A modifying effect of WMH burden on the risk of recurrent stroke 173 : Participants with higher WMH burden appeared to experience greater benefit from intensive BP lowering in prevention of recurrent stroke (p for interaction 0.04).
A modifying effect of WMH burden on vascular outcomes amongst older SPS3 participants 174 :
Among 1263 participants aged 65 years and older a lower SBP target (<130 mmHg) appeared more beneficial among those with worse WMH burden for risk of recurrent stroke (P for interaction 0.01) and MACE (p for interaction 0.03). No significant interaction was observed for change in cognitive function.
No significant modifying effect of cerebral microbleeds on the risk of recurrent stroke and death 175: Intensive BP lowering significantly reduced risk of stroke recurrence in patients with CMB (HR 0.5, 95% CI 0.3, 0.9) but not patients without CMB (HR 0.7, 95% CI 0.4, 1.3), however, the difference in the magnitude of effect was not significant (p for interaction 0.34). No effect on all-cause mortality was detected in patients with (HR 0.9, 95% CI 0.4, 1.8) or without CMB (HR 0.8, 95% CI 0.5, 1.5, p for interaction 0.99)
Several secondary analyses have also examined the relation of blood pressure reduction with outcomes, prompting caution:
After a mean follow up of 3.7 years, there was a J-shaped association between achieved blood pressure and outcomes. 176 For example, above a SBP of 124 mmHg, 1 standard deviation higher (11.1 mmHg) was associated with increased mortality (aHR 1.9, 95% CI 1.4, 2.7), whereas below this level, this relationship was inverted (aHR 0.29, 95% CI 0.10, 0.79, p < 0.001) for interaction. Above a DBP of 67 mmHg, a 1 standard deviation higher (8.2 mmHg) was associated with an increased risk of stroke (aHR 2.2, 95% CI 1.4, 3.6), whereas below this level, the association was in the opposite direction (aHR 0.34, 95% CI 0.13, 0.89), p = 0.02 for interaction. The lowest risk of all events occurred at a nadir of ≈ 120–128 mmHg systolic blood pressure and 65–70 mmHg diastolic blood pressure.
Another study used an unsupervised cluster procedure to identify distinct patterns of BP change during the first 9 months of anti-hypertensive therapy intensification among 1331 SPS3 participants randomised to the lower BP target (SBP<130 mmHg). 177 Compared to mild reducers (mildly elevated baseline SBP and minimal visit-to-visit BP variability) moderate reducers (moderately elevated baseline SBP and moderate visit-to-visit BP variability) had a higher risk of death (aHR 1.6, 95% CI 1.0, 2.7), MACE (aHR 2.1, 95% CI 1.4, 3.2) and stroke (aHR 2.6, 95% CI 1.7, 4.1), while large reducers (very elevated baseline SBP with very large visit-to-visit BP variability) had the highest risk of death (aHR 2.3, 95% CI 1.2, 4.4). 177
Other guidelines
Few guidelines have addressed the question of BP lowering for secondary stroke prevention in lacunar ischaemic stroke patients specifically (Table 1):
Current US guidelines for the prevention, detection, evaluation and management of high blood pressure in adults suggest that tor adults with a lacunar stroke, a target SBP goal of less than130 mmHg may be reasonable (Class IIb; Level of Evidence B-randomised).178,179
A recent Chinese guideline for clinical management of cerebrovascular disorders 28 recommends to manage blood pressure in patients with lacunar ischaemic stroke (Class I, level of evidence B). As cSVD leads to a significant decrease in the adaptability of brain tissue to excessive hypertension and hypotension the blood pressure of patients should be closely monitored (Class IIa, level of evidence B). Control of systolic and diastolic pressure is described as the key factor to control the incidence and progression of cSVD (Class IIa, level of evidence B). The guideline also recommends to monitor the 24 h ambulatory blood pressure and, when possible, to detect changes in BP during head upright tilt test (Class I, level of evidence B).
Another, recent Chinese guideline (in Chinese), specifically dedicated to the diagnosis and treatment of cSVD states, first, that an SBP target of below 130 mmHg in patients with cSVD might achieve better outcomes (strength of recommendation: I/level of evidence: A); in the case of co-existing large artery atherosclerosis the magnitude and rate of blood pressure reduction should be relatively small and slow. Second, this guideline states that antihypertensive drugs that reduce blood pressure variability may be more effective in managing cSVD, highlighting that calcium channel blockers and renin angiotensin system inhibitors are more effective in stabilising blood pressure variability (strength of recommendation: II/level of evidence: B). 29
Other guidelines focus on BP lowering for secondary stroke prevention in general, with no specific recommendations for lacunar ischaemic stroke:
The latest ESO guideline on secondary stroke prevention 16 recommends BP lowering treatment in people with previous ischaemic stroke or TIA, to reduce the risk of recurrent stroke, aiming for BP <130/80 mmHg. In these people it supports the use of out of office blood pressure measurements wherever feasible, to achieve better long-term control of blood pressure. It supports initiation of a combination of two blood pressure lowering drugs to reduce the risk of recurrent stroke, with consideration of monotherapy where there are potential risks of hypotension, such as in frail, elderly people and people with borderline hypertension. The use of out of office BP measurements is supported wherever feasible.
The AHA/ASA 2021 secondary stroke prevention guideline 21 recommends an office BP goal of <130/80 mmHg in patients with hypertension who experience a stroke or TIA to reduce the risk of recurrent stroke and vascular events (Class 1; Level of Evidence B-randomised). In these patients, treatment with a thiazide diuretic, angiotensin-converting enzyme inhibitor, or angiotensin II receptor blockers is useful for lowering BP and reducing recurrent stroke risk (Class 1; Level of Evidence A); individualised drug regimens that take into account patient comorbidities, agent pharmacological class and patient preference are recommended to maximise drug efficacy (Class 1; Level of Evidence B-nonrandomised). In patients with no history of hypertension who experience a stroke or TIA and have an average office BP of ⩾130/80 mmHg, antihypertensive medication treatment can be beneficial to reduce the risk of recurrent stroke, ICH and other vascular events (Class 2a; Level of Evidence B-randomised).
Systematic reviews and meta-analyses
The largest most recent meta-analysis exploring the impact of BP-lowering treatment on clinical outcomes in patients with a history of stroke or TIA was conducted as part of the ESO secondary stroke prevention guideline, 16 but did not include any specific subgroup analyses for lacunar ischaemic stroke.
When comparing BP lowering treatment to no intervention, on meta-analysis of data from nine trials,180–188 there was a significant reduction in the odds of recurrent stroke by almost 20% (OR 0.81, 95% CI 0.71, 0.92, p = 0.002 non significant for ischaemic and haemorrhagic stroke separately), a significant reduction in MACE (OR 0.80, 95% CI 0.69, 0.94, p = 0.006), and no significant reduction in all cause death (seven trials,180,182–184,186,187 OR 0.97, 95% CI 0.90, 1.05, p = 0.51). There were insufficient data to allow analysis of the effect of antihypertensive medication on dementia and functional outcome.
When comparing intensive BP treatment with a standard BP reduction strategy, there was a significant reduction in recurrent stroke with (OR 0.79, 95% CI 0.64, 0.98, p = 0.029), significant for haemorrhagic (p = 0.033) but not ischaemic stroke (p = 0.228) and no significant difference between groups for the outcomes of major vascular events, all-cause death on meta-analysis. Functional outcome was only assessed in the SPS3 trial, 9 with no significant difference between intensive and standard BP reduction groups for poor outcome (mRS 3–6).
Regarding combined versus monotherapy, 16 trials in people with essential hypertension show that the former leads to better control of BP.189,190 A large systematic review and meta-analysis shows that the extra BP reduction from combining two drug classes is approximately five times greater than doubling the dose of one drug. 191 The European Society of Hypertension and European Society of Cardiology guidelines 192 which recommend initiation of antihypertensive treatment with combination treatment, except in people at increased risk of hypotension and those with mild hypertension and low cardiovascular risk. In the absence of alternative specific evidence for secondary prevention in stroke, and supportive evidence for the potential benefit of combination treatment in the Perindopril Protection Against Recurrent Stroke Study (PROGRESS), 180 this European guidance is therefore applicable for most people with prior stroke. No specific data is available for lacunar ischaemic stroke.
Regarding the choice of BP-lowering molecules, in primary prevention trials, calcium channel blockers (CCBs) appear to be slightly more efficacious than other classes in prevention of stroke at large,193,194 and a greater consistency of BP control and lower systolic BP variability) 193 has been observed with CCBs and thiazide-like diuretics. Based on primary prevention guidelines, plus supportive evidence from drug classes used in trials such as PROGRESS,180,195 initiation of treatment with a combination of antihypertensive medication, usually containing either a thiazide-like diuretic (such as indapamide) or a CCB (such as amlodipine or felodipine), combined with a RAS inhibitor (ACE inhibitor or angiotensin 2 receptor blocker) was considered reasonable in patients with a history of stroke or TIA. 16 While no trial data is available specifically for patients with lacunar ischaemic stroke, indirect evidence from a Mendelian randomisation study can be reported. 196 In this study genetic proxies for calcium channel blockers, but not β-blockers, were associated with lower risk of any stroke and ischaemic stroke and showed particularly strong associations with small vessel stroke and WMH, an MRI-marker of cSVD.
For patients with prior stroke or TIA, there is concern that a possible lower BP threshold may increase the risk of stroke, or a J-curve effect. In the past, post hoc analyses of RCTs, meta-analyses and population-based studies of patients with cerebrovascular disease have shown an inconsistent relationship between achieved SBP <120 mmHg and poor outcomes.195,197–200 New data from RCTs and large meta-analyses now provide compelling evidence that neurologically stable patients with cerebrovascular disease also benefit from a BP goal of <130/80 mmHg and that BP targets for stroke prevention should be more aligned with targets for prevention of other cardiovascular conditions. However, there is insufficient evidence to recommend a lower limit of BP within the normal range for patients with prior stroke.


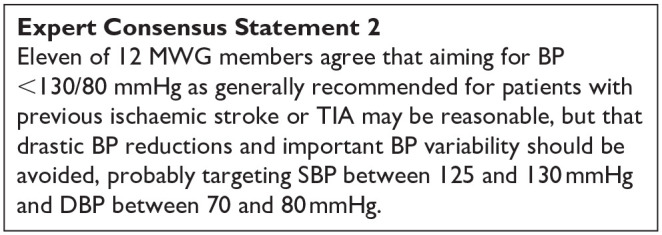
PICO 8:
In patients with lacunar ischaemic stroke, does treatment with lipid lowering agents (considering a particular agent, dose, target), compared to less intense or avoiding this intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders?
Analysis of current evidence
We identified two randomised clinical trials relevant to this PICO question,201,202 (Supplemental Table 15; Supplement PRISMA diagrams). Other RCTS and observational studies did not adequately subtype ischaemic stroke or report outcomes stratified by stroke subtype.
The main SPARCL trial randomised 4731 patients with a history of stroke, TIA 1–6 months previously and with no pre-existing indication for lipid lowering therapy to receive 80 mg atorvastatin versus placebo. 203 Patients with atrial fibrillation, peripheral vascular disease or coronary artery disease were excluded. The main results showed that over a follow up of 4.9 years atorvastatin reduced the overall incidence of stroke and cardiovascular events with no effect on mortality.
A subsequent sub-analysis of the SPARCL trial included here investigated primary end points and outcomes stratified by presenting stroke subtype (large vessel, small vessel, unknown cause and ICH). 201 There were limited numbers of patients with cardio-embolic stroke as those with AF and other heart disease were excluded. Thirty percent of participants had small vessel (referred to here as lacunar) stroke, 16% large vessel, 22% unknown, 31% TIA and 2% ICH. Of the 1409 patients with lacunar stroke at baseline the primary outcome of any stroke occurred in 93 (13.1%) of the atorvastatin treated group and 109 (15.2%) of the control group with a HR 0.85, 95% CI 0.64, 1.12, p = 0.249. The HR were similar for secondary outcomes of Stroke/TIA or MACE. The primary end point event rate of fatal or non-fatal stroke for those randomised to atorvastatin versus placebo was 13.1% versus 18.6% for patients with large vessel stroke, 11.2% versus 12.7% for unknown cause and 7.6% versus 8.8% for TIA. The trial did not find a difference in the efficacy of treatment for the primary outcome of stroke (large vessel stroke HR 0.70, 95% CI 0.49, 1.02, TIA HR 0.81, 95% CI 0.57, 1.17, SVD HR 0.85, 95% CI 0.64, 1.12, unknown cause HR 0.87, 95% CI 0.61, 1.24, haemorrhagic stroke HR 3.24, 95% CI 1.01, 10.4; P for heterogeneity = 0.421), or MACE (P for heterogeneity = 0.360) based on stroke subtype of the index event. It should be noted however that this was a post hoc subgroup analysis.
The J-STARS trial was a randomised multicentre open label parallel group study which randomised 1578 patients with non cardio-embolic ischaemic stroke and total cholesterol levels of 4.65–6.21 mmol/L to receive pravastatin 10 mg once daily versus placebo with a primary outcome of stroke/TIA over a mean follow up period of 4.9 years. 202 At baseline 1006 patients had lacunar infarcts, 401 atherothrombotic and 171 unknown aetiology. The main results for the trial showed no significant difference for the primary outcome of stroke/TIA between the groups but the rate of atherothrombotic infarction was lower in the pravastatin group compared to the control group (0.21% vs 0.64%/year, p = 0.0047, aHR 0.33, 95% CI 0.15, 0.74). Data are presented in the Supplemental material of the publication, providing outcomes stratified by baseline stroke subtype. 202 In the 1006 patients with lacunar stroke at baseline there was no significant difference in the primary outcome of TIA/stroke (49 stroke outcomes in the pravastatin arm and 47 stroke outcomes in the control arm) but the authors report a trend in reduction of incident atherothrombotic infarcts for patients with lacunar stroke at baseline albeit limited by small event rates (0 in case group, 10 in control).
A meta-analysis was performed using the data from the subgroup analysis of the SPARCL trial and the J-STARS trial to evaluate whether statins would be effective in reducing the risk of stroke/TIA in patients with lacunar ischaemic stroke.201,202 Neither Pravastatin 10 mg nor Atorvastatin 80 mg was found to be effective in reducing the risk of stroke/TIA in patients with lacunar ischaemic stroke (Figure 14). Furthermore, combining the results of the 2 trials, statin use was not associated with a significant reduction of the risk of stroke/TIA (RR 0.93, 95% CI 0.77, 1.12) (Figure 14). Taken together, since neither trial focused on lacunar ischaemic stroke and the meta-analysis was inconclusive, we feel that the evidence is insufficient to make a recommendation (Table 7) and therefore the Evidence-based Recommendation reflects the continued uncertainty over lipid lowering specifically in lacunar ischaemic stroke.
Figure 14.
PICO 8. Effect of lipid lowering on recurrent stroke or TIA in patients with lacunar ischaemic stroke.
Table 7.
GRADE evidence profile for PICO 8 long-term lipid lowering agents (considering a particular agent, dose, target), compared to less intense or avoiding this intervention, to reduce stroke or TIA in patients with lacunar ischaemic stroke.
| Certainty assessment | No of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Incon-sistency | Indirect-ness | Impre-cision | Other considerations | Statins | Placebo | Relative (95% CI) | Absolute (95% CI) | ||
| Stroke/TIA | ||||||||||||
| 2 | randomised trials | serious | not serious | not serious | serious a | none | 173/1210 (14.3%) | 185/1205 (15.4%) | RR 0.93 (0.77, 1.12) |
11 fewer per 1000 (from 35 fewer to 18 more) |
⨁⨁○○ Low |
CRITICAL |
CI: confidence interval; RR: risk ratio; TIA: Transient Ischaemic Attack.
Explanations
Confidence interval crosses the clinical decision threshold.
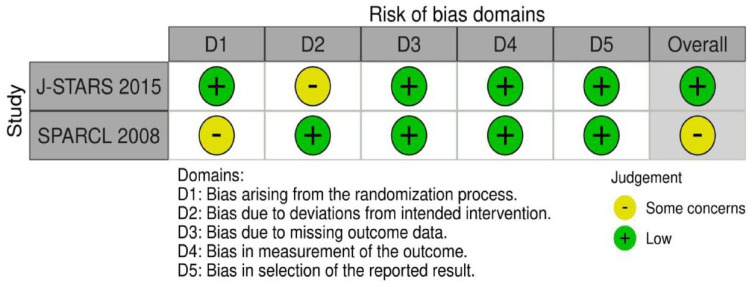
PICO 8 Risk of bias of randomised controlled trials (outcome: recurrent stroke)
Additional information
The Heart Protection Study included 3280 patients with cerebrovascular disease (defined as non-disabling stroke, TIA or having had a carotid endarterectomy) in whom treatments with simvastatin 40 mg versus placebo reduced subsequent major vascular events over 5 years follow up n = 406 (24.7%) versus 488 (29.8%); p=0.001. 204 Although patients with lacunar stroke are likely to have been recruited to this study there is no mention of the proportion with lacunar stroke.
Similarly, in the Treat Stroke to Target Trial 2860 patients with recent stroke or TIA and ‘atherosclerotic disease’ were randomised in France and South Korea to receive lipid lowering medication (statin, ezetimibe or both) with a lower target (LDL-C <1.8 mmol/L (<70 mg/dL)) or higher target (2.3–2.8 mmol/L (90–110 mg/dL)). 205 The composite primary end point (MACE) occurred in 121 patients (8.5%) in the lower-target group and in 156 (10.9%) in the higher-target group (aHR 0.78, 95% CI 0.61, 0.98; p = 0.04) suggesting benefit to a lower target. Again, stroke was not subtyped.
International US, 21 European16,206 and local guidelines (UK/Ireland https://www.strokeguideline.org/, Sweden https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-riktlinjer/2020-1-6545-kunskapsunderlag-2020.pdf) recommend intensive LDL-C lowering therapy in patients following ischaemic stroke with the AHA and UK guidance specifying atorvastatin 80 mg od and to consider other agents based upon co-morbidities with the AHA recommending a target level of LDL-C <1.8 mmol/L (<70 mg/dL) (Table 1). Where documented, there was good representation of patients with lacunar stroke in the background trials and there is no convincing evidence that patient with lacunar stroke subtypes should be treated differently from other ischaemic stroke subtypes with regards to management of lipid profiles.
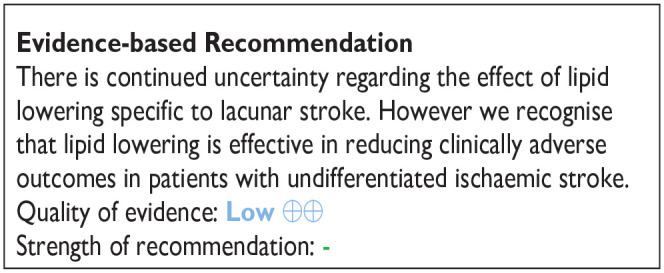

PICO 9:
In patients with lacunar ischaemic stroke, does treatment with lifestyle interventions (e.g. smoking cessation, dietary interventions, weight reduction, physical exercise, cognitive/behavioural or social interventions, sleep disorder/sleep apnoea interventions, or a mixture of these), compared to less intense or avoiding this intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders?
Lifestyle factors such as smoking, physical inactivity, diet and sodium intake are well-known modifiable risk factors for stroke21,207–209 and have been associated with the severity of SVD brain changes in observational studies.210–212 Rigorous management of lifestyle factors has been recommended alongside evidence-based medications. 211 However, trials investigating the impact of lifestyle and behavioural interventions in SVD are still scarce. The ESO Guideline on covert cerebral SVD in 2021 gave no recommendation for any specific lifestyle intervention due to insufficient direct evidence but stated that it is reasonable to promote healthy lifestyle interventions as recommended in primary prevention for vascular disease. 1 None of the previous stroke prevention guidelines have explicitly referred to stroke subtype when discussing lifestyle modifications (Table 1).1,24,213–215 Here, we evaluate the evidence for different lifestyle interventions on adverse outcomes in patients with clinically evident lacunar stroke.
Analysis of current evidence
The literature search identified two RCTs addressing the effect of lifestyle interventions in reducing adverse clinical outcomes in patients with lacunar stroke (Supplement PRISMA diagram), one on physical exercise and the other on a dietary intervention (nutritional supplement). As it was not possible to pool the studies for meta-analysis, we describe the results narratively. Summary of the main findings is given in Supplemental Table 16.
Physical exercise
The HITPALS study included patients with acute/postacute lacunar stroke or TIA with MRI-confirmed signs of a previous lacunar stroke.208,216 The patients were randomised within 3 weeks after the symptom onset to 3-month home-based high-intensity interval training (15 min a day, 5 days per week, with weekly telephone calls to ensure compliance) or usual care including encouragement to lifestyle changes (baseline n = 31+32). The primary outcome was cardiorespiratory fitness (Graded Cycling Test with Talk Test), while the secondary outcomes included measures of post-stroke fatigue, depression, mental well-being, chronic stress, cognition (Montreal Cognitive Assessment), blood pressure, BMI and physical activity. The home-based training protocol was safe and well-received by the patients. In the intervention group, vigorous physical activity initially increased more than in the control group; however, the difference was not sustained over time. Regarding the primary outcome, no significant difference in cardiorespiratory fitness was found between the groups after the treatment or in 12-month follow-up. Secondary outcomes improved in a similar way in both study groups. A possible interpretation of the findings is that adherence to physical activity programmes post-stroke is poor in the long term. Both groups – even the control group – might have enhanced their physical activity or increased their awareness of the importance of physical activity, which could explain the improved outcomes of both study groups. The study was discussed to be limited by the small sample size, and possibly insufficient intensity of exercise or a bias in selection of patients.
Dietary interventions
A substudy of the VITATOPS trial reported data on a subgroup of patients with recent lacunar stroke and cognitive impairment but no dementia. 217 The trial was a randomised, double-blind, placebo-controlled trial of a daily administration of B-vitamins (2 mg folic acid, 25 mg vitamin B6 and 0.5 mg vitamin B12) versus placebo (baseline n = 118+112). The outcome of the study was defined as the change in cognitive test scores over 5 years. Overall, the trial failed to demonstrate any significant effect of the vitamin B complex on cognitive outcomes. The results do not support a routine administration of vitamin B to reduce cognitive decline in patients with lacunar stroke and cognitive impairment no dementia.
Other lifestyle interventions
The literature search found no RCTs on the effect of other lifestyle interventions such as smoking cessation, weight reduction, cognitive/behavioural or social interventions or sleep disorder/sleep apnoea interventions in reducing adverse outcomes in patients with lacunar stroke.
Additional information
Given the small number of trials providing direct evidence for the effectiveness of lifestyle interventions in preventing adverse clinical outcomes in patients with lacunar stroke, we also reviewed indirect evidence from relevant observational studies and RCTs with related patient groups.
A secondary analysis of the SPS3 trial assessed the association of smoking status on major cardiovascular outcomes in patients with MRI-confirmed symptomatic lacunar stroke. 218 The patients were classified as never smokers, prior smokers, current smokers who quit within 3 months of the index event, and persistent smokers. Persistent smokers had an increased risk of composite outcome of death, stroke and myocardial infarction, compared with never smokers. Notably, there was no difference between never smokers, former smokers and those who quit smoking suggesting that smoking cessation after stroke may have beneficial effects in reducing the risk of death and cardiovascular events.
The Impact of physical activity in vascular cognitive impairment (AFIVASC) study is an ongoing trial including community dwellers with either probable mild vascular cognitive impairment (VCI) or previous stroke (>6 months before) or TIA (>1 month before), who have no dementia or significant functional impairment. 219 Patients are randomised to usual care or a structured physical activity intervention (three sessions a week). The primary outcome is decline in cognitive status, whereas secondary outcomes include changes in cognitive measures, quality of life, functional and motor status as well as radiological markers of SVD progression on brain MRI. The trial has not planned subgroup analyses for patients with lacunar strokes. The first cross-sectional observational data (prior to randomisation) have suggested that objective measurement (accelerometry) is more realiable indicator of physical activity as compared to self-reports, and that physical exercise as performed at least according to WHO recommendations is significantly associated with higher global cognition, attention and processing speed. 220
Excessive salt intake is as an important risk factor for cardiovascular disease and stroke. It has been estimated that even a modest reduction in salt consumption (by 1 g/day) would result in a considerable decrease in adverse outcomes. 221 In patients with minor stroke (lacunar or cortical), dietary salt intake has been cross-sectionally associated with lacunar stroke type as well as more severe WMH and other SVD brain changes.222,223 Both animal and human studies have demonstrated several adverse effects of high salt intake on small and large arteries beyond increased blood pressure, including impaired endothelial function and increased arterial stiffness. 224 Besides the effects of high Na+ on cerebral vascular dysfunction via excess inflammation 225 and oxidative stress, 226 there is also evidence of sensitisation of central sympathetic circuits by central Na+ sensing, which occurs in the circumventricular organs leading to excessive sympathetic outflow. 227 In addition, this augmented sympathetic vascular transduction can be enhanced with an increase of potent circulating vasoconstrictors, such as endothelin-1 and arginine vasopressin, associated with dietary salt. 224
In recent years, increasing interest has focused on multidomain interventions to target several life-style factors simultaneously in the attempt of preventing cognitive impairment and dementia (reviewed in 228 ). The FINGER study was among the first trials to demonstrate beneficial effects of a 2-year multidomain intervention (diet, physical exercise, cognitive training and vascular risk monitoring) on cognitive functions in older individuals with existing dementia-related risk factors. 229 The intervention was effective in reducing incident cerebrovascular events and also total cardiovascular events (coronary event, stroke, TIA) among those with a history of cardiovascular disease. 230 As the aetiology of dementia is multifactorial and the risk factor profiles differ between individuals, preventive interventions are likely enhanced by personalised multidomain approaches and could be facilitated by digital health solutions. 228
In regard to secondary stroke prevention, most current guidelines and recommendations address lifestyle modifications but not in the light of specific stroke subtypes (Table 1).
The AHA/ASA 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischaemic Attack identifies medication adherence and a healthy lifestyle as key components of secondary stroke prevention, with the highest class of recommendation for smoking cessation (Class 1; Level of Evidence A, B-nonrandomised) and substance use (Class 1; Levels of Evidence B-nonrandomised, C-expert opinion), followed by physical activity (Class 1–2b; Levels of Evidence C-limited data, B-randomised, C-expert opinion, B-nonrandomised) and nutrition (Class 2a; Level of Evidence B-randomised). 21 AHA/ASA published 2014 a scientific statement on Physical Activity and Exercise Recommendations for Stroke Survivors, recognising that physical inactivity after stroke is highly prevalent and listing evidence supporting the use of exercise training, both aerobic and strength training, for stroke survivors that need to be customised for the individual to maximise long-term adherence. 213
Canadian Stroke Best Practice Recommendations: Secondary Prevention of Stroke Update 2020 gives recommendations on preferable lifestyle behaviours for reducing the risk of an initial stroke and the risk of a subsequent stroke for patients with a prior history of stroke, which include a healthy balanced diet, low sodium intake, physical activity, weight management, moderate alcohol consumption, avoiding recreational drug use, smoking cessation including the use of e-cigarettes as well as controlling emerging risk factors such as air pollution. 24
Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders from 2020 comprise a section on Cerebral small vessel disease management, which did not address lifestyle modifications. 28
Australian and New Zealand Clinical Guidelines for Stroke Management published in 2021 emphasised that lifestyle modifications require multimodal interventions, including education, a counselling approach and supervised/active exercise, commenced early after hospital discharge through organised processes and considering the patient’s living circumstances. 27
Recommendations of the Spanish Society of Neurology for the prevention of stroke. Interventions on lifestyle and air pollution specifically addressed lifestyle modification. They provided recommendations on common factors such as smoking, alcohol, obesity, diet and nutrition and sedentary lifestyle, as well as stress and air pollution, without addressing stroke subtypes. 215
Recently, World Stroke Organization (WSO) Guideline committee reviewed the available guidelines to summarise the existing recommendations of stroke management globally. Areas of strong agreement across guidelines were identified. For secondary prevention, lifestyle modifications were recommended (with referral to specialised services where necessary) including weight loss, regular physical exercise, smoking cessation, moderate alcohol intake, avoiding recreational drug use and reduction of salt intake. 231 According to WSO and the Global Burden of Disease research group, behavioural factors such as smoking, poor diet and low physical activity account for 47% of stroke burden, which highlights the importance of promoting stroke awareness and developing novel risk prevention strategies. 232
We also reviewed the recommendations and guidelines that referred to lifestyle modifications in the prevention of post-stroke VCI.
Regarding the effect of lifestyle-based interventions (exercise, dietary change, alcohol moderation, weight loss, smoking cessation) on post-stroke cognitive impairment, ESO-EAN joint guidelines did not make recommendations due to the very low quality of evidence. The Guidelines’ Expert Consensus Statement emphasised that lifestyle interventions, alone or in combination, should not be used solely for the prevention of post-stroke cognitive decline or dementia and recognised a need for further, adequately powered trials that assess the effect of monitored lifestyle interventions on cognitive decline following stroke. Stroke subtypes were not explicitly addressed. 15
Recommendations of the fifth Canadian Consensus Conference on the diagnosis and treatment of dementia (CCCDTD5) from 2020 and 2022 recommended physical activity but not other lifestyle interventions to reduce the risk of dementia, including Alzheimer’s and vascular dementia.233,234 The section of the guidelines dedicated to VCI, Canadian Consensus Conference on Diagnosis and Treatment of Dementia (CCCDTD5): Guidelines for management of vascular cognitive impairment from 2020 focused on medical management and did not address lifestyle modifications. 235
Clinical practice guideline for cognitive impairment of cerebral small vessel disease by the Geriatric Neurology Group of the Chinese Society of Geriatrics in 2019 recognised the beneficial effects of physical exercise on pathological processes involved in cognitive impairment and concluded that it could reduce the risk of vascular dementia. 236
The 2011 AHA/ASA Statement on Vascular Contributions to Cognitive Impairment and Dementia summarised previously published systematic literature reviews, guidelines, personal files and expert opinions and addressed in detail lifestyle modifications to prevent VCI. They recommended that in people at risk for VCI, smoking cessation is reasonable (Class IIa; Level of Evidence A), the moderation of alcohol intake, weight control and physical activity (for all: Class IIb; Level of Evidence B) may be reasonable lifestyle interventions, and that the use of antioxidants and B vitamins is not beneficial (Class III; Level of Evidence A). 237 Stroke subtypes associated with cognitive impairment were not addressed specifically.

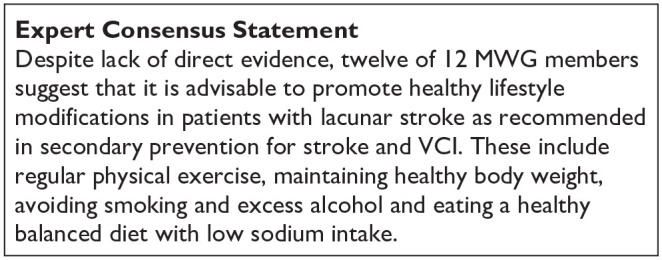
PICO 10:
In patients with lacunar ischaemic stroke, do other treatments as secondary prevention, such as phosphodiesterase-3 inhibitors (e.g. cilostazol, pentoxifylline), anti-inflammatory agents (e.g. minocycline), anticoagulants, nitric oxide donors (e.g. transdermal glyceryl trinitrate), phosphodiesterase-5 inhibitors (sildenafil, tadalafil, dipyridamole), or other relevant agents not addressed in the other PICOs, compared to less intense or avoiding this intervention, reduce any recurrent stroke, recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders?
Analysis of current evidence
The systematic literature searches for PICO 5 and 10 were performed together. The search identified 248 titles/abstracts. Of these, 46 articles were selected for full-text review, and 7 of these were selected for data extraction.46,127,130,131 Three additional articles were identified by the searches in other PICO questions,133–135 After reference screening of reviews, we added another 2 RCTs.131,136 This delivered 12 RCTs, of which 5 were assigned to PICO 10 (Supplemental Table 17, Supplement PRISMA diagrams).
For PICO 10, we found 5 studies on cilostazol and 1 study on isosorbide mononitrate.
Cilostazol
Cilostazol, a phosphodiesterase-3 inhibitor, not only inhibits platelet aggregation but also has vasodilating, endothelial protecting and anti-inflammatory effects, making it a promising agent in small vessel disease management. It is commonly used for peripheral vascular disease and in Asia-Pacific countries also for secondary stroke prevention.
The CSPS (2000) and CSPS-2 (2010) randomised-controlled trials both included a large proportion of lacunar stroke patients.129,130,161,162 Patients were randomised to receive cilostazol 200 mg/daily versus placebo (CSPS) or versus aspirin (CSPS-2). Both trials are covered in PICO 6 on antiplatelet treatment.
CSPS.com (2019) studied cilostazol on top of modern recommended antiplatelet therapy, and is therefore covered in this PICO. CSPS.com was a non-blinded RCT comparing dual therapy with cilostazol plus either aspirin or clopidogrel, with monotherapy aspirin or clopidogrel in high-risk ischaemic stroke patients. 162 Clopidogrel was used in 55% of patients and aspirin in 45%. The trial stopped before reaching half of the planned sample size at 1879 patients (49% lacunar stroke subtype) because of slow recruitment. Median follow-up time was 1.4 year. In lacunar stroke subtype, 131 the ischaemic stroke recurrence risk was significantly lower in the dual therapy group (HR 0.41, 95% CI 0.21, 0.81), without increasing the risk of severe or life-threatening bleeding (0.36, 95% CI 0.07, 1.81). Furthermore, composite vascular events was significantly lower in the dual therapy group (HR 0.41, 95% CI 0.23, 0.75), whereas the risk of haemorrhagic stroke and death from any cause did not differ significantly between the two groups (HR 0.47, 95% CI 0.08, 2.64; and HR 3.20, 95% CI 0.33, 30.8). To be noted, discontinuation of trial medication in the cilostazol group was three times as frequently as in the control group.
These CSPS studies are from Asia-Pacific countries where epidemiology and risk factors may differ from Western/European populations, and were not primarily focused on lacunar stroke subtype. Reflecting the Asian origin of these trials, cilostazol is included in Asian stroke guidelines: the Japan Stroke Society Guideline (2021) gives a high level of evidence for the effectiveness of cilostazol for prevention in non-cardioembolic stroke, and also recommends to give dual antiplatelet therapy with cilostazol and aspirin in non-cardio-embolic stroke with multiple vascular risk factors. 30 The guideline of the Chinese stroke association (2019) recommends the use of aspirin, clopidogrel, or cilostazol in ischaemic stroke caused by small vessel disease. 28 According to the AHA guideline (2021), the role of cilostazol in secondary prevention after stroke related to small vessel disease is uncertain. 21 The ESO guideline on secondary prevention (2022) does not give a recommendation on cilostazol. 16
LACI-1 was a European phase IIa, dose-tolerability, open-label trial, randomising 57 patients with lacunar stroke to ISMN 50 mg/daily, cilostazol 200 mg/daily, both ISMN and cilostazol started immediately, or both with delayed start, all in addition to clopidogrel or aspirin prescribed as standard care. 134 The trial confirmed tolerability and safety after a short-term treatment of 8 weeks. The (secondary) clinical outcomes can only be considered preliminary. The trial reported one recurrent ischaemic stroke in the ‘both drugs immediate’ group. There were no deaths or major haemorrhages. Cognition, tested with Trail making test A and B, was not different in cilostazol versus no cilostazol.
The subsequent LACI-2 trial was a 2 × 2 factorial design, open label, phase II trial. It included 363 patients with lacunar stroke and aimed to determine if cilostazol and ISMN are tolerated at the target dose for a year. 46 The study drugs were given on top of regular secondary prevention. Although the trial was not powered for efficacy, several clinical outcomes were reported: cilostazol did not reduce the composite outcome of recurrent stroke, TIA, myocardial infarction, dependence, any cognitive impairment, and death, nor did it reduce recurrent stroke or TIA. Cilostazol reduced dependency (mRS score of 3–6: 11.5% with cilostazol vs 17.5% without; aOR 0.46, 95% CI 0.22, 0.95). Cilostazol did not reduce cognitive impairment but tended to improve mood. The trial confirmed tolerability and safety and supports the feasibility of a large phase III trial.
For additional information on Cilostazol, please see PICO 6 section above.
Isosorbide mononitrate (ISMN)
ISMN is an NO donor. NO has multiple potential beneficial effects such as improvement of blood–brain barrier integrity, vasodilation, reduced inflammation and neuroprotection.
The previously mentioned LACI-1 trial studied the tolerability and safety of an 8-week course of ISMN 50 mg/daily (and cilostazol in a 2 × 2 factorial design) in 57 patients with lacunar stroke. 127 There was one recurrent ischaemic stroke in the study group that received both ISMN and cilostazol. There were no deaths or major haemorrhages. Cognition was tested with Trail making test A and B. Although time did not differ, the group on ISMN achieved more points on TMT part A (MD 2.6, 95% CI 0.1, 5.1, p = 0.05). The subsequent similarly designed LACI-2 trial included 363 patients with lacunar stroke and aimed to determine if the trial design was feasible and if ISMN and cilostazol were safe and tolerated at the target dose for a year. 46 The trial reported clinical outcomes although it was not powered for efficacy. ISMN appeared well tolerated and safe, and in addition, ISMN reduced recurrent stroke or TIA (adjusted OR 0.23, 95% CI 0.07, 0.74). The absolute reduction in participants with any cognitive impairment was 10.4% (54.4% with ISMN and 64.8% without ISMN), and on a 7-level ordinal scale cognitive impairment was reduced aOR 0.55, 95% CI 0.36, 0.86).
The combination of ISMN and cilostazol versus none of these in LACI-2 showed reduced dependency (mRS 3–6: aOR 0.27, 95% CI 0.09, 0.82) but no effect on recurrent stroke. Combination ISMN-cilostazol reduced cognitive impairment (7-level ordinal scale aOR 0.44, 95% CI 0.23, 0.85) and reduced low mood (Zung depression scale: adjusted mean difference −5.98, 95% CI −10.77, −1.20, p = 0.01).
Additional information
Anticoagulation
We did not find any studies on anticoagulation as secondary prevention specifically in patients with lacunar stroke without atrial fibrillation (AF). However, there are some studies that can provide indirect evidence as they included a substantial proportion of lacunar stroke subtype. These trials, such as WARSS (56% lacunar stroke) 238 and ESPRIT (48% small vessel stroke) 239 found no evidence that warfarin is more efficacious than antiplatelet therapy in non-cardioembolic stroke patients. Moreover, in the SPIRIT trial, warfarin was associated with increased risk of intracerebral haemorrhage, particularly in patients with leukoaraiosis. 240
We also did not find results on anticoagulation as secondary prevention in lacunar stroke subtype with AF. A lacunar stroke in a patient with AF most probably results from small vessel disease but it is challenging to rule out an embolic aetiology in clinical practice. In general, anticoagulant therapy is strongly superior to antiplatelet therapy for the prevention of recurrent stroke in ischaemic stroke patients with AF.241,242 Existing guidelines for anticoagulation in AF do not distinguish between different stroke subtypes. 14 However, a 2-year observational study in 288 stroke patients (26% lacunar subtype) with AF who were receiving anticoagulation therapy showed that the stroke recurrence rate was almost twice as high in patients with lacunar stroke subtype compared to those with cardioembolic subtype despite being on anticoagulation (8.5% vs 4.6%, p = 0.067), and the majority of recurrent events were of the lacunar subtype. 243 Furthermore, anticoagulation in patients with lacunar stroke is not without risk, as most intracerebral haemorrhages in older people result from small vessel disease. A lacunar ischaemic stroke increases the risk of both future ischaemic stroke and ICH and this may change the risk-benefit ratio of anticoagulation, especially when extensive covert lesions are present, such as microbleeds and leukoaraiosis. In general, Guidelines on prevention of recurrent stroke in patients with AF recommend DOACs over vitamin K antagonists. 14 However, the ESO guideline on secondary prevention in patients with AF 14 did not find any data on DOAC versus vitamin K antagonists in lacunar stroke, nor in patients with any previous stroke or TIA subtype and severe SVD, and concluded that ‘theoretically, DOACs might be preferable to vitamin K antagonists in patients with severe SVD, but there are no current data to support this hypothesis’. 14 Therefore the Method Working Group agreed that patients with lacunar ischaemic stroke and AF should receive anticoagulant treatment as advised in current Guidelines on anticoagulation in patients with AF. 14
Ongoing trials
Several trials in lacunar stroke patients are ongoing but few have clinical endpoints. The use of PDE-5 inhibitors might be of interest in small vessel disease by their effect on cerebral blood flow and endothelial function. A small trial including 20 lacunar stroke cases showed that a single dose of tadalafil increased blood oxygen saturation in the microvasculature at 180 min post-administration, indicating improved perfusion in the cerebral microvasculature. 244 A larger RCT (NCT05173896) is now investigating if 3 months daily intake of tadalafil is feasible in lacunar stroke patients and if it alters cerebral perfusion, neurovascular reactivity, and cognition. Sildenafil is investigated in the OxHARP study, however, the primary outcome is cerebrovascular pulsatility and reactivity and the study will not have any relevant clinical outcome measures. 245
The effect of minocycline, an antibiotic with anti-inflammatory properties, on microglial activation and blood-brain permeability in patients with lacunar stroke is investigated in the MINERVA trial. 246 However there will be no clinical outcomes.


Discussion
This guideline document was developed following the GRADE methodology and aims to assist physicians in decision-making regarding suspected lacunar ischaemic stroke. All recommendations and Expert Consensus Statements are summarised in Table 8.
Table 8.
Synoptic table of all Evidence Based Recommendations and Expert Consensus Statements.
| Evidence Based Recommendation | Expert consensus statement |
|---|---|
| PICO 1: In patients with suspected lacunar ischaemic stroke, does thrombolytic treatment (including at extended time window and wake-up stroke, alteplase/tenecteplase/other), compared to avoiding this intervention/other thrombolytic/dose/etc, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| We suggest that patients with suspected acute lacunar ischaemic stroke should be assessed for and receive treatment with 0.9 mg/kg alteplase according to current guidelines for the treatment of acute ischaemic stroke, since the limited data available suggest that the outcomes for patients with lacunar ischaemic stroke are consistent with the overall results of alteplase trials. Quality of evidence: Very Low ⊕ Strength of recommendation: Weak for intervention ↑? |
1. Twelve of twelve MWG members agreed that in patients with suspected acute lacunar ischaemic stroke, with no contraindication to thrombolytic treatment according to current clinical guidelines for thrombolytic treatment (including wake up stroke), there is no evidence for withholding thrombolytic treatment. Therefore these patients should receive intravenous alteplase at standard dose (0.9 mg/kg) as quickly as possible according to current clinical guidelines. 2. Twelve of 12 MWG members agreed that in patients with suspected acute lacunar ischaemic stroke there are insufficient data to support use of thrombolytic drugs other than alteplase, or a lower dose of alteplase, at the present time. |
| PICO 2: In patients with suspected acute lacunar ischaemic stroke, does acute treatment with antiplatelets (considering single/dual, duration, and whether any particular antiplatelet or combination of antiplatelets is better), compared to avoiding/less of/alternative antiplatelet intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| In patients suspected acute lacunar ischaemic stroke, there is continued uncertainty about a specific combination of antiplatelet therapy over monotherapy. Quality of evidence: Very low ⊕ Strength of recommendation: - |
1. Twelve of 12 experts agree to the statement that in patients with suspected lacunar ischaemic stroke, initiation of antiplatelet therapy should be started as soon as possible after stroke onset. |
| PICO 3: In patients with suspected acute lacunar ischaemic stroke, does immediate antihypertensive treatment (considering agent and BP target), compared to avoiding this intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| 1. In hospitalised patients with suspected acute lacunar ischaemic stroke and BP <220/110 mmHg, not treated with intravenous thrombolysis, we suggest against the routine use of blood pressure BP lowering agents in the hyperacute phase, unless this is necessary for a specific comorbid condition. Quality of evidence: Moderate ⊕⊕⊕ Strength of recommendation: Weak against intervention ↓? 2. In patients with suspected acute lacunar ischaemic stroke undergoing intravenous thrombolysis we suggest following the same guideline as in acute ischaemic stroke at large, that is, maintaining BP below 185/110 mmHg before bolus and below 180/105 mmHg after bolus, and for 24 hours after alteplase infusion. Quality of evidence: Very Low ⊕ Strength of recommendation: Weak for intervention ↑? 3. In patients with suspected acute lacunar ischaemic stroke there is continued uncertainty over the benefits and risks of temporarily stopping versus continuing previous BP lowering therapy. Quality of evidence: Very Low ⊕ Strength of recommendation: - |
1. Twelve of twelve MWG members agreed that there is insufficient evidence at present to provide a precise timeframe during which BP lowering agents should be avoided in patients with suspected acute lacunar ischaemic stroke. Based on current limited evidence, blood pressure lowering therapy should be avoided for at least 24 hours after symptom onset. 2. When antihypertensive drugs need to be used in patients with suspected acute lacunar ischaemic stroke undergoing intravenous thrombolysis and with BP >180/105 mmHg, twelve of 12 MWG members agreed that there is no advantage/disadvantage of one antihypertensive medication over another, hence any antihypertensive drug may be used, as long as blood pressure is closely monitored. 3. Eleven of twelve MWG members agreed that in patients with suspected acute lacunar ischaemic stroke not treated with intravenous thrombolysis and blood pressure >220/120 mmHg, careful blood pressure reduction (<15% systolic blood reduction in 24 hours) is reasonable. No specific blood pressure lowering agent can be recommended. |
| PICO 4: In patients with suspected acute lacunar ischaemic stroke and progressive symptoms, does acute treatment with antiplatelets/anticoagulants/thrombolysis/other agent, compared to less intense or avoiding this intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| There is continued uncertainty regarding intervention with antiplatelets, anticoagulants, thrombolysis or other agents in patients with suspected lacunar ischaemic stroke and progressive symptoms, including early neurological deterioration, stuttering/fluctuating symptoms and capsular warning syndrome. Quality of evidence: Very Low ⊕ Strength of recommendation: - |
1. Twelve of 12 MWG members agreed that in patients with suspected lacunar ischaemic stroke and progressive symptoms, there is no evidence to recommend any particular antiplatelet regimen (intensive or single), BP management regimen (raising or lowering), rt-PA, anticoagulation, statin, or other treatment. 2. Twelve of 12 MWG members agreed that in patients with suspected lacunar ischaemic stroke and progressive symptoms, they should be included in all trials in acute lacunar ischaemic stroke but identified as a specific subgroup with prespecified planned analysis of the treatment effect in this subgroup. 3. Twelve of 12 MWG members agreed that in patients with suspected lacunar ischaemic stroke and progressive symptoms, there is an urgent need to agree a consensus definition for progressive symptoms. |
| PICO 5: In patients with suspected acute lacunar ischaemic stroke, does acute treatment with other agents such as phosphodiesterase-3 inhibitors (e.g. cilostazol, pentoxifylline), anti-inflammatory agents (e.g. minocycline), anticoagulants, nitric oxide donors (e.g. transdermal glyceryl trinitrate), phosphodiesterase-5 inhibitors (sildenafil, tadalafil, dipyridamole), or other relevant agents not addressed in the other PICOs, compared to less intense or avoiding this intervention, reduce any recurrent stroke, recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| 1. In patients with suspected acute lacunar ischaemic stroke, there is continued uncertainty over the benefits and risks of magnesium for acute treatment. Quality of evidence: Low ⊕⊕ Strength of recommendation: - 2. In patients with suspected acute lacunar ischaemic stroke, there is continued uncertainty over the benefits and risks of cilostazol for acute treatment. Quality of evidence: Low ⊕⊕ Strength of recommendation: - 3. In patients with acute lacunar ischaemic stroke, we suggest against the use of glyceryl trinitrate to reduce dependency. Quality of evidence: Moderate ⊕⊕⊕ Strength of recommendation: Weak against intervention ↓? 4. In patients with acute lacunar ischaemic stroke, we recommend against the use of therapeutic LMW heparin/heparinoid to reduce dependency. Quality of evidence: Moderate ⊕⊕⊕ Strength of recommendation: Weak against intervention ↓? 5. In patients with acute lacunar ischaemic stroke, there is continued uncertainty over the benefits and risks of Xueshuantong to reduce dependency. Quality of evidence: Very low ⊕ Strength of recommendation: - 6. In the absence of RCTs, we cannot make recommendations on the use of any other agents, such as phosphodiesterase-3 inhibitors, anti-inflammatory agents, anticoagulants, nitric oxide donors, phosphodiesterase-5 inhibitors or otherwise not mentioned in PICO 1-4, for acute treatment in patients with lacunar ischaemic stroke, to reduce any recurrent stroke, recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders. Quality of evidence: - Strength of recommendation: - |
|
| PICO 6: In patients with lacunar ischaemic stroke, does long term treatment with antiplatelets (single or dual, duration, and whether any particular antiplatelet or combination of antiplatelets is better), compared to avoiding/less of/alternative antiplatelet intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| In patients with suspected lacunar ischaemic stroke, for secondary prevention of long-term adverse outcomes, we recommend long term single antiplatelet therapy with aspirin or clopidogrel from 2-4 weeks after stroke onset. Quality of evidence: Moderate ⊕⊕⊕ Strength of recommendation: Weak for intervention ↑? |
1. In patients with suspected lacunar ischaemic stroke twelve of 12 MWG members recommend against the use of long-term* dual or triple antiplatelet therapy. Instead, single antiplatelet therapy should be used as per the Evidence Based Recommendation, unless other conditions warrant a combination of these medications. *Defined as more than 2–4 weeks 2. In patients with suspected lacunar ischaemic stroke, eleven of 12 MWG members agreed that the current evidence was inadequate to recommend routine use of cilostazol to prevent adverse long term outcomes. |
| PICO 7: In patients with lacunar ischaemic stroke, does antihypertensive treatment considering a particular agent or target, compared to less intense or avoiding this intervention given long term, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| In patients with suspected lacunar ischaemic stroke we recommend the use of antihypertensive treatment to prevent recurrent stroke and MACE. Quality of evidence: Low ⊕⊕ Strength of recommendation: Strong for intervention ↑↑ |
1. Twelve of twelve MWG members suggest that: BP should be appropriately monitored and well controlled, when possible through use of out of office blood pressure measurements. We cannot advise any specific antihypertensive treatment. 2. Eleven of twelve MWG members agree that aiming for BP <130/80 mmHg as generally recommended for patients with previous ischaemic stroke or TIA may be reasonable, but that drastic BP reductions and important BP variability should be avoided, probably targeting SBP between 125 and 130 mmHg and DBP between 70 and 80 mmHg. |
| PICO 8: In patients with lacunar ischaemic stroke, does treatment with lipid lowering agents (considering a particular agent, dose, target), compared to less intense or avoiding this intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| There is continued uncertainty regarding the effect of lipid lowering specific to lacunar stroke. However we recognise that lipid lowering is effective in reducing clinically adverse outcomes in patients with undifferentiated ischaemic stroke. Quality of evidence: Low ⊕⊕ Strength of recommendation: - |
1. Twelve of 12 MWG members agreed that patients with lacunar ischaemic stroke should receive lipid lowering therapy given there is some evidence of benefit and no clear evidence of harm. |
| PICO 9: In patients with lacunar ischaemic stroke, does treatment with lifestyle interventions (e.g. smoking cessation, dietary interventions, weight reduction, physical exercise, cognitive/behavioural or social interventions, sleep/CPAP, or a mixture of these), compared to less intense or avoiding this intervention, reduce recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| In patients with lacunar stroke, there is continued uncertainty to indicate that any specific lifestyle interventions prevent adverse clinical outcomes. Quality of evidence: Very low ⊕ Strength of recommendation: - |
1. Despite lack of direct evidence, twelve of 12 MWG members suggest that it is advisable to promote healthy lifestyle modifications in patients with lacunar stroke as recommended in secondary prevention for stroke and VCI. These include regular physical exercise, maintaining healthy body weight, avoiding smoking and excess alcohol, and eating a healthy balanced diet with low sodium intake. |
| PICO 10: In patients with lacunar ischaemic stroke, do other treatments as secondary prevention, such as phosphodiesterase-3 inhibitors (e.g. cilostazol, pentoxifylline), anti-inflammatory agents (e.g. minocycline), anticoagulants, nitric oxide donors (e.g. transdermal glyceryl trinitrate), phosphodiesterase-5 inhibitors (sildenafil, tadalafil, dipyridamole), or other relevant agents not addressed in the other PICOs, compared to less intense or avoiding this intervention, reduce any recurrent stroke, recurrent ischaemic stroke, dependency, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders? | |
| There is continued uncertainty on the use of any other agents, such as phosphodiesterase-3 inhibitors, anti-inflammatory agents, anticoagulants, nitric oxide donors, phosphodiesterase-5 inhibitors or otherwise not mentioned in PICO 6-8, for secondary prevention in patients with lacunar ischaemic stroke, to reduce recurrent ischaemic stroke, death, cognitive impairment or dementia, haemorrhagic stroke, MACE, mobility or gait disorder, and mood disorders. Quality of evidence: Low ⊕⊕ Strength of recommendation: - |
1. In patients with lacunar ischaemic stroke without AF, twelve of 12 MWG members recommend against the use of anticoagulation for secondary prevention, if there is no other indication. 2. In patients with lacunar ischaemic stroke and AF, twelve of 12 MWG members recommend the use of anticoagulation for secondary prevention. The evidence for efficacy of anticoagulants over antiplatelet is strong in patients with AF, overruling stroke subtype. However, since the risk of ICH is increased in patients with lacunar stroke and severe SVD, we recommend strict risk factors control. |
We noted that a considerable proportion of articles were not found by the literature search and that a substantial number of additional articles were detected through other methods such as checking reference lists. This indicates that detection of relevant trials may be especially difficult for lacunar ischaemic stroke.
We considered acute treatment and secondary prevention in lacunar ischaemic stroke. We tried to reflect diagnoses, timings and practical clinical aspects of the knowledge base that will inform the management of patients with suspected lacunar ischaemic stroke. Disappointingly, there is a dearth of data on cognitive and mood outcomes despite cognitive impairment being the commonest problem after lacunar stroke and a main concern to patients. Since most data were from subgroups of trials that included all stroke or all ischaemic stroke, the most commonly available outcomes were dependency (which is less common in lacunar stroke than in other ischaemic stroke subtypes) and recurrent stroke (which in general is less common early after lacunar stroke than with large artery or cardioembolic stroke). 4
The strengths of this work include the use of the GRADE approach as mandated by ESO. The guideline builds on the Guideline for covert SVD published in 2021. 1 We have endeavoured to amass all relevant data. While we hope we have not missed any major trials we acknowledge that it is difficult to search for trials in lacunar ischaemic stroke. We contacted authors for additional data, and wherever feasible we undertook several new meta-analyses, including a network meta-analysis on antiplatelet drugs in secondary prevention.
We also acknowledge limitations. Limitations of the data reflect the dearth of any data on many of the outcomes of interest and many interventions of interest in lacunar ischaemic stroke. There were essentially no data on cognition despite cognitive impairment being common and a main concern of patients with lacunar ischaemic stroke. Most data came from a lacunar subgroup of a larger trial, which may be limited by definition, chance, small sample and often only reporting one outcome in the subgroups. Very few trials focused on lacunar ischaemic stroke. Although many trials must have included patients with lacunar ischaemic stroke, many did not distinguish lacunar from other subtypes at all, or if they did, then often did not report any results for the lacunar subgroup, or only reported a few outcomes for the lacunar stroke patients separately. Lack of long-term follow-up in many secondary prevention trials mean that effects of interventions in lacunar stroke may not have become fully apparent, since recurrent stroke, dependency, or death typically occur late after lacunar ischaemic stroke, compared with other stroke sybtypes. Finally, time constraints meant that we were not able to obtain stakeholder feedback on the guideline prior to publication, but we welcome feedback from people affected by lacunar ischaemic stroke and other forms of cSVD to help inform future guidelines, research and practice.
The Guideline is part of a series of Guidelines on the clinical management of cerebral small vessel disease: Part 1, covert cSVD, Part 2, lacunar ischaemic stroke, other Parts under consideration include 3, other stroke subtypes with a high cSVD burden, 4, cognitive, 5, mobility, and 6, mood presentations of cSVD. The Guideline Parts published so far will be updated within 5 years of publication or sooner if important new information becomes available.
Implications for clinical practice
For acute interventions, specific data on lacunar ischaemic stroke were sparse for most interventions, and the quality of evidence low or very low, but in general, the findings are within the envelope of expected effects in other ischaemic stroke subtypes, are consistent with other ESO guidelines on these treatments in ischaemic stroke in general (Table 1) as follows, with caveats:
Thrombolysis: dependency (the main outcome measure used) is less frequent after lacunar ischaemic stroke and so is not a good measure of outcome. Thus, there is a trend in the direction of benefit with alteplase, but the available data do not show a conventionally significant effect despite our meta-analyses having nearly as much data as some individual pivotal trials such as the NINDS trial.
Antiplatelet drugs: No meta-analysis was possible, but considering the individual trials’ data, consistent with other stroke subtypes, short-term dual (not triple) antiplatelet drugs in the acute phase may reduce recurrent stroke with little evidence of harm.
Blood Pressure lowering: here there were more data with consistent findings indicating that intensive BP lowering appeared harmful and should be avoided in acute lacunar ischaemic stroke unless there is another good reason to do so.
Other agents and progressive lacunar stroke: there were no data to suggest that any other agents were beneficial. The data for progressive stroke are very limited with very few trials, inconsistent definitions and outcomes. The current data on a range of potential agents are provided in text and supplement. Given that progressive stroke is common after lacunar stroke, this should be a major focus of future acute stroke treatment trials.
For secondary prevention, in general the data were also very limited, mostly reliant on subgroups, mostly of moderate to very low quality, with few trials specifically in lacunar ischaemic stroke. However, for all interventions, the general findings were similar to those for ischaemic stroke in general:
Antiplatelet drugs: A comprehensive assessment of data on antiplatelet agents, mostly as subgroups of larger trials, identified benefit for reduction in recurrent stroke and MACE with a continuous single (not long-term dual or triple) antiplatelet drug with most evidence for aspirin or clopidogrel.
Blood Pressure lowering: Data on over 3000 patients in two trials specifically in lacunar ischaemic stroke which compared intensive with less intensive BP targets did not show conventional significance for preventing recurrent stroke, no effect on death, cognitive decline or dependency. Nonetheless, BP lowering is advised.
Lipid lowering: data were particularly sparse and limited to subgroup analyses of old trials and did not show conventional significance of benefit, but were consistent with statins’ effects in any ischaemic stroke with no evidence of harm.
Lifestyle interventions: there was little direct evidence of any intervention in lacunar ischaemic stroke, although lifestyle interventions are common sense with little evidence of harm.
Other interventions: A number of mostly small trials did not identify definite benefit with any other agent, although there may be hints of benefits with some agents that may improve endothelial function, but larger trials are needed.
Recommendations for future research
We suggest several actions that would help to improve the quantity and quality of evidence for interventions to guide management in patients with lacunar ischaemic stroke, in future.
First, trials in stroke that plan to include also patients with lacunar ischaemic stroke, should subtype lacunar and other stroke subtypes at baseline so that the lacunar strokes can be identified in future analyses. The subtyping should use currently used definitions such as the Oxfordshire Community Stroke Project (OCSP) 2 definition based on symptoms and signs, or the TOAST classification (although caution is required to avoid bias due to presumed causation) 133 and if possible, even more detailed information and nomenclature as proposed by Standards for Reporting Vascular Changes on Neuroimaging −2 (STRIVE-2) collaboration. 33 Possibly, in view of differences in outcome rates between lacunar versus non-lacunar strokes, the stroke subtype should even be included in any minimisation algorithm to ensure that treatment allocation is well balanced for baseline characteristics including stroke subtype. These trials that include patients with lacunar stroke should present as many results as possibly by stroke subtype, albeit in secondary analyses. Nonetheless, if previous trials had provided data on outcomes in lacunar stroke by treatment allocation more systematically this would, by now, have resulted in a much stronger evidence base for many basic routine stroke interventions than is currently the case.
Second, there is an urgent need for more trials dedicated to lacunar stroke, in order to improve the current clinical management. Amongst the conventional routine acute stroke treatments or secondary preventions, few showed much slight benefit (acute short term antiplatelets, long-term antiplatelets); several showed no definite benefit, with confidence intervals overlapping no effect, but ‘taken on trust’, the direction of effect is in the right direction (thrombolysis, acute phase DAPT, long-term BP lowering, long-term statins, lifestyle); and one showed harm (acute BP lowering). These findings are consistent with the data in the covert cSVD Guideline, although in the case of antiplatelet drugs in covert cSVD there was evidence of harm. Of the ‘other’ interventions and in progressive lacunar stroke, there were mostly small inconclusive trials. Furthermore, the outcomes provided in the conventional stroke interventions focused on early stroke recurrence or dependency, with little to no data on cognitive impairment, a frequent consequence and major concern to patients with lacunar stroke. Trials dedicated to lacunar stroke, which does after all account for a quarter of ischaemic strokes, could focus on recruitment practices, trial designs and outcomes (especially cognition, mood) that are more relevant to lacunar stroke, critically important to assess, and where an effective intervention is more likely to impact clinical practice.
Third, the data on aspirin and clopidogrel (conventional ‘strong’ antiplatelet drugs), antihypertensives and statins indicate somewhat limited effectiveness and suggest that there is little point in attempting further trials of these agents, although the combination of aspirin and dipyridamole or aspirin and ticagrelor in acute lacunar ischaemic stroke could be of interest. This is consistent with the intrinsic nature of the underlying non-atheromatous, non-cardioembolic SVD pathology. Rather, it could be more informative to focus on emerging, newer, or novel agents with relevant modes of action to try and find more effective interventions that tackle the intrinsic pathology.
Fourth, a major issue was the difficulty in identifying the relevant literature, due to the wide range of terms used in lacunar stroke and SVD and the lack of indexing on these terms. This problem was what led to the STRIVE-1 initiative focused on SVD features on neuroimaging,
which has improved the consistency of some SVD terminology already, and has now been updated with new STRIVE recommendations. 33 Other manifestations of SVD such as WMH, silent lacunar infarcts and enlarged perivascular spaces should also whenever possible be evaluated in treatment trials.
In conclusion, there is clearly a large problem for clinical aspects of lacunar stroke and SVD still to be addressed.
Plain language summary
This guideline offers recommendations for the treatment of lacunar stroke. An ischaemic stroke happens when the blood flow to a part of the brain is interrupted causing damage to the brain. This damage causes common symptoms which can include weakness and speech. Lacunar strokes occur following damage to the small blood vessels of the brain (small vessel disease) and are common, accounting for about a quarter of all stroke cases. There is a suggestion that the treatment of patients with lacunar stroke may differ from the treatment of other types of stroke that are caused by clots going from the heart or from disease in the large blood vessels.
This guideline was written by the European Stroke Organisation who gathered an international group of experts in the area. We identified 10 key areas of uncertainty (or questions) in the treatment of lacunar stroke and then searched all of the published articles on treatment trials to try and answer these questions. For each area of uncertainty/question we assessed the quality of the research studies providing information on the topic. To make recommendations we needed to be certain that the trials were of high enough quality. If there was enough evidence to provide a recommendation we did so (all the time indicating how certain we were). If there was not enough evidence of high enough quality then we made a statement based upon our expert consensus opinion. We were keen that proposed treatments should benefit to patients and families (and not just improvement in a brain scan appearance or blood test). So for each possible treatment we looked at whether it reduced key clinical outcomes for example recurrent stroke, death, onset of memory and thinking problems or dementia, onset of reliance on others, heart attacks or other features of small vessel disease such as mobility and mood disorders.
We hope that these guidelines will help healthcare professionals and patients decide how to treat patients with lacunar stroke but also highlight where evidence is lacking and where research should focus in the future.
The questions we asked along with the answers are below:
1. Should patients with lacunar (small vessel disease) stroke receive thrombolysis (a clot busting drug in the first few hours after a stroke happens).
ANSWER: Yes – patients with lacunar stroke should receive thrombolysis if necessary (as for other types of ischaemic stroke)
2. Should patients with lacunar stroke receive antiplatelet drugs in the first few hours after the stroke happens – these act to thin the blood and are commonly used after stroke or heart attack for example, aspirin?
ANSWER: Yes - we recommend that these drugs should be started as soon as possible.
3. Should patients with lacunar stroke receive drugs to lower high blood pressure in the first 24 h after a stroke happens?
ANSWER: We recommend against lowering BP drastically in the first 24 h (unless this is needed for another reason).
4. Should patients who have a lacunar stroke and then experience a worsening of their symptoms be treated with specific drugs other than normal stroke treatments?
ANSWER: No –at the moment there is no research to guide recommendations here (nor is it clear exactly how to define this group).
5. Should patients with lacunar stroke receive any other drugs in the first few hours following a stroke?
ANSWER: We do not know if magnesium or cilostazol or the Chinese medicinal herb Xueshuantong are of benefit, we recommend against using nitrates and against using heparin in acute lacunar ischaemic stroke.
6. Should patients with lacunar stroke receive antiplatelet drugs (blood thinning drugs like aspirin) in the long term?
ANSWER: Yes - we recommend one antiplatelet drug long term (aspirin or clopidogrel) (often patients will receive both aspirin and clopidogrel for the first weeks after a stroke). There is not enough research evidence to recommend cilostazol.
7. Should patients with lacunar stroke receive drugs to lower blood pressure in the long term?
ANSWER: Yes – we recommend treating blood pressure, aiming for a level of 130/80 but avoiding drastic changes in blood pressure level.
8. Should patients with lacunar stroke receive drugs to lower cholesterol levels? ANSWER: Yes –there is general benefit for most patients following an ischaemic stroke and we recommend taking drugs to lower cholesterol.
9. Should patients with lacunar stroke adopt lifestyle measures to benefit their health?
ANSWER: Yes - Although there is a lack of direct evidence we recommend general health lifestyle modifications as recommended in stroke and dementia guidelines. These include (but are not limited to) regular physical exercise, maintaining healthy body weight, avoiding smoking and excess alcohol and eating a healthy balanced diet with low sodium (dietary salt) intake.
10. Should patients with lacunar stroke receive any other long-term treatments not mentioned above?
ANSWER– We do not know whether to use cilostazol or nitrates or not. In patients with an irregular heart beat (Atrial Fibrillation) and lacunar stroke we recommend using blood thinning drugs for example, warfarin or equivalent as for patients with other types of stroke who have atrial fibrillation, as well as treating risk factors for small vessel disease.
These recommendations will help health care professionals and patients in choosing the best treatments and also highlight where more research is needed.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231219416 for European stroke organisation (ESO) guideline on cerebral small vessel disease, part 2, lacunar ischaemic stroke by Joanna M Wardlaw, Hugues Chabriat, Frank-Erik de Leeuw, Stéphanie Debette, Martin Dichgans, Fergus Doubal, Hanna Jokinen, Aristeidis H Katsanos, Raffaele Ornello, Leonardo Pantoni, Marco Pasi, Aleksandra M Pavlovic, Salvatore Rudilosso, Reinhold Schmidt, Julie Staals, Martin Taylor-Rowan, Salman Hussain and Arne G Lindgren in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231219416 for European stroke organisation (ESO) guideline on cerebral small vessel disease, part 2, lacunar ischaemic stroke by Joanna M Wardlaw, Hugues Chabriat, Frank-Erik de Leeuw, Stéphanie Debette, Martin Dichgans, Fergus Doubal, Hanna Jokinen, Aristeidis H Katsanos, Raffaele Ornello, Leonardo Pantoni, Marco Pasi, Aleksandra M Pavlovic, Salvatore Rudilosso, Reinhold Schmidt, Julie Staals, Martin Taylor-Rowan, Salman Hussain and Arne G Lindgren in European Stroke Journal
Supplemental material, sj-docx-3-eso-10.1177_23969873231219416 for European stroke organisation (ESO) guideline on cerebral small vessel disease, part 2, lacunar ischaemic stroke by Joanna M Wardlaw, Hugues Chabriat, Frank-Erik de Leeuw, Stéphanie Debette, Martin Dichgans, Fergus Doubal, Hanna Jokinen, Aristeidis H Katsanos, Raffaele Ornello, Leonardo Pantoni, Marco Pasi, Aleksandra M Pavlovic, Salvatore Rudilosso, Reinhold Schmidt, Julie Staals, Martin Taylor-Rowan, Salman Hussain and Arne G Lindgren in European Stroke Journal
Acknowledgments
The authors would like to thank Yvonne Brüchert for assistance with meetings, referencing and organisation, and Prof Terence Quinn for methodological guidance, Dr Yajun Cheng for translating the Chinese SVD Guidelines, Prof Philip Bath and Dr Lisa Woodhouse for providing additional unpublished data from the ENOS trial, the chairs of the ESO GL Board for general guidance and the reviewers for their time and helpful insights. We thank authors of some original papers for providing additional unpublished information.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. For details, please see Supplemental Table 1.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the development of these guidelines was provided by the European Stroke Organisation, Basel, Switzerland. The author(s) did not receive financial support for the development, writing and/or publication of this guideline. Declarations of funding are provided in Supplemental Table 1
Informed consent: Not applicable
Ethical approval: Ethical approval was not necessary for the work described in this paper.
Guarantor: Arne Lindgren, Joanna Wardlaw
Contributorship: The PICO groups drafted the PICOs which were merged. The chairs drafted the introduction, methods and discussion based on points raised in discussion by the MWG members. Salman Hussain, Methodologist, conducted the GRADE and statistical analyses. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs: Joanna M Wardlaw  https://orcid.org/0000-0002-9812-6642
https://orcid.org/0000-0002-9812-6642
Hugues Chabriat  https://orcid.org/0000-0001-8436-6074
https://orcid.org/0000-0001-8436-6074
Stéphanie Debette  https://orcid.org/0000-0001-8675-7968
https://orcid.org/0000-0001-8675-7968
Martin Dichgans  https://orcid.org/0000-0002-0654-387X
https://orcid.org/0000-0002-0654-387X
Fergus Doubal  https://orcid.org/0000-0002-2769-3148
https://orcid.org/0000-0002-2769-3148
Hanna Jokinen  https://orcid.org/0000-0001-7889-1120
https://orcid.org/0000-0001-7889-1120
Aristeidis H Katsanos  https://orcid.org/0000-0002-6359-0023
https://orcid.org/0000-0002-6359-0023
Raffaele Ornello  https://orcid.org/0000-0001-9501-4031
https://orcid.org/0000-0001-9501-4031
Leonardo Pantoni  https://orcid.org/0000-0001-7357-8530
https://orcid.org/0000-0001-7357-8530
Marco Pasi  https://orcid.org/0000-0001-9976-2459
https://orcid.org/0000-0001-9976-2459
Aleksandra M Pavlovic  https://orcid.org/0000-0002-5987-9828
https://orcid.org/0000-0002-5987-9828
Salvatore Rudilosso  https://orcid.org/0000-0002-8775-6884
https://orcid.org/0000-0002-8775-6884
Julie Staals  https://orcid.org/0000-0002-9502-6937
https://orcid.org/0000-0002-9502-6937
Martin Taylor-Rowan  https://orcid.org/0000-0002-3027-5369
https://orcid.org/0000-0002-3027-5369
Salman Hussain  https://orcid.org/0000-0002-1691-8428
https://orcid.org/0000-0002-1691-8428
Arne G Lindgren  https://orcid.org/0000-0003-1942-7330
https://orcid.org/0000-0003-1942-7330
Supplemental material: Supplemental material for this article is available online.
References
- 1. Wardlaw JM, Debette S, Jokinen H, et al. ESO guideline on covert cerebral small vessel disease. Eur Stroke J 2021; 6: CXI–CLXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bamford J. Clinical examination in diagnosis and subclassification of stroke. Lancet 1992; 339: 400–402. [DOI] [PubMed] [Google Scholar]
- 3. Jackson CA, Hutchison A, Dennis MS, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke 2010; 41: 624–629. [DOI] [PubMed] [Google Scholar]
- 4. Jackson CA, Hutchison A, Dennis MS, et al. Differences between ischemic stroke subtypes in vascular outcomes support a distinct lacunar ischemic stroke arteriopathy. A prospective, hospital-based study. Stroke 2009; 40: 3679–3684. [DOI] [PubMed] [Google Scholar]
- 5. Makin SDJ, S Turpin, Dennis MS, et al. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry 2013; 84: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith EE, Markus HS. New treatment approaches to modify the course of cerebral small vessel diseases. Stroke 2020; 51: 38–46. [DOI] [PubMed] [Google Scholar]
- 7. Palacio S, Hart RG, Pearce LA, et al. Effect of addition of clopidogrel to aspirin on mortality: systematic review of randomized trials. Stroke 2012; 43: 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pearce LA, McClure LA, Anderson DC, et al.; SPS3 Investigators. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol 2014; 13: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Secondary Prevention of Small Subcortical Stroke Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Potter G, Doubal F, Jackson C, et al. Associations of clinical stroke misclassification (‘clinical-imaging dissociation’) in acute ischemic stroke. Cerebrovasc Dis 2010; 29: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arba F, Mair G, Phillips S, et al.; Third International Stroke Trial Collaborators. Improving clinical detection of acute lacunar stroke: analysis from the IST-3. Stroke 2020; 51: 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mancuso M, Arnold M, Bersano A, et al. Monogenic cerebral small-vessel diseases: diagnosis and therapy. Consensus recommendations of the European Academy of Neurology. Eur J Neurol 2020; 27: 909–927. [DOI] [PubMed] [Google Scholar]
- 13. Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) – European Society for minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J 2019; 4: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European Stroke Organisation guideline. Eur Stroke J 2019; 4: 198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quinn TJ, Richard E, Teuschl Y, et al. European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment. Eur Stroke J 2021; 6: I–XXXVIII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dawson J, Béjot Y, Christensen LM, et al. European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack. Eur Stroke J 2022; 7: I–II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed N, Audebert H, Turc G, et al. Consensus statements and recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 11–13 November 2018. Eur Stroke J 2019; 4: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bassetti CLA, Randerath W, Vignatelli L, et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur J Neurol 2020; 27: 1117–1136. [DOI] [PubMed] [Google Scholar]
- 19. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021; 6: I–LXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fonseca AC, Merwick Dennis M, et al. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Eur Stroke J 2021; 6: Clxiii–clxxxvi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke 2021; 52: e364–e467. [DOI] [PubMed] [Google Scholar]
- 22. Powers WJ, Rabinstein AA, Ackerson T. Correction to: guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 23. Smith EE, Saposnik G, Biessels GJ, et al.; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Functional Genomics and Translational Biology; and Council on Hypertension. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e44–e71. [DOI] [PubMed] [Google Scholar]
- 24. Gladstone DJ, Lindsay MP, Douketis J, et al.; Canadian Stroke Consortium. Canadian stroke best practice recommendations: secondary prevention of stroke update 2020. Can J Neurol Sci 2022; 49: 315–337. [DOI] [PubMed] [Google Scholar]
- 25. Heran M, Lindsay P, Gubitz G, et al. Canadian stroke best practice recommendations: acute Stroke Management, 7sEdition Practice Guidelines Update, 2022. Can J Neurol Sci 2022; 1–31. [DOI] [PubMed] [Google Scholar]
- 26. Royal College of Physicians of London Intercollegiate Stroke Working Party. National clinical guidelines for stroke. Report no. 5th, London, 2016. [Google Scholar]
- 27. Stroke Foundation. Australian and New Zealand Clinical Guidelines for Stroke Management. https://informme.org.au/guidelines/living-clinical-guidelines-for-stroke-management (2021, accessed 30 March 2023).
- 28. Liu L, Chen W, Zhou H, et al.; Chinese Stroke Association Stroke Council Guideline Writing Committee. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol 2020; 5: 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chinese Society of Neurology; Chinese Stroke Society. Chinese guideline for diagnosis and treatment of cerebral small vessel disease 2020. Chin J Neurol 2022; 55: 807–818. [Google Scholar]
- 30. Miyamoto S, Ogasawara K, Kuroda S, et al.; Committee for Stroke Guideline 2021, the Japan Stroke Society. Japan Stroke Society guideline 2021 for the treatment of stroke. Int J Stroke 2022; 17: 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. IST-3 collaborative group. Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischaemic stroke in the third International Stroke Trial (IST-3): secondary analysis of a randomised controlled trial. Lancet Neurol 2015; 14: 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rudilosso S, Urra X, San Román L, et al. Perfusion deficits and mismatch in patients with acute lacunar infarcts studied with whole-brain CT perfusion. Am J Neuroradiol 2015; 36: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duering M, Biessels GJ, Brodtmann A, et al. Neuroimaging standards for research into small vessel disease—advances since 2013. Lancet Neurol 2023; 22: 602–618. [DOI] [PubMed] [Google Scholar]
- 34. Clancy U, Gilmartin D, Jochems ACC, et al. Neuropsychiatric symptoms associated with cerebral small vessel disease: a systematic review and meta-analysis. Lancet Psychiatry 2021; 8: 225–236. [DOI] [PubMed] [Google Scholar]
- 35. Steiner T, Dichgans M, Norrving B, et al. European Stroke Organisation (ESO) standard operating procedure for the preparation and publishing of guidelines. Eur Stroke J 2021; 6: CXXII–CXXXIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 37. Higgins JPT, Thomas J, Chandler J, et al. (eds.). Cochrane handbook for systematic reviews of interventions. 64th ed. Chichester, UK: John Wiley & Sons, 2023. Available from www.training.cochrane.org/handbook [Google Scholar]
- 38. Chaimani A, Caldwell DM, Li T, et al. Undertaking network meta-analyses. In: Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, Inc., 2019, 285–320. [Google Scholar]
- 39. Donegan S, Williamson P, D'Alessandro U, et al. Assessing key assumptions of network meta-analysis: a review of methods. Res Synth Methods 2013; 4: 291–323. [DOI] [PubMed] [Google Scholar]
- 40. Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006; 101: 447–459. [Google Scholar]
- 41. White IR. Network meta-analysis. Stata J 2015; 15: 951–985. [Google Scholar]
- 42. White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012; 3: 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: 14898. [DOI] [PubMed] [Google Scholar]
- 44. Harrison JK, Reid J, Quinn TJ, et al. Using quality assessment tools to critically appraise ageing research: a guide for clinicians. Age Ageing 2017; 46: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pantoni L, Fierini F, Poggesi A. Thrombolysis in acute stroke patients with cerebral small vessel disease. Cerebrovasc Dis 2014; 37: 5–13. [DOI] [PubMed] [Google Scholar]
- 46. Wardlaw JM, Woodhouse LJ, Mhlanga, et al.; Lacunar Intervention Trial-2 (LACI-2) Investigator Group. Isosorbide mononitrate and cilostazol treatment in patients with symptomatic cerebral small vessel disease: the lacunar intervention trial-2 (LACI-2) randomized clinical trial. JAMA Neurol 2023; 80: 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group, Caplan LR. Tissue plasminogen activator for acute ischemic stroke. New Engl J Med 1999; 341: 1240–1241. [DOI] [PubMed] [Google Scholar]
- 48. The IST-3 Collaborative Group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 2012; 379: 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khatri P, Tayama D, Cohen G, et al.; PRISMS and IST-3 Collaborative Groups. Effect of intravenous recombinant tissue-type plasminogen activator in patients with mild stroke in the Third International Stroke Trial-3: post hoc analysis. Stroke 2015; 46: 2325–2327. [DOI] [PubMed] [Google Scholar]
- 50. Barow E, Boutitie F, Cheng B, et al.; WAKE-UP Investigators. Functional outcome of intravenous thrombolysis in patients with lacunar infarcts in the WAKE-UP trial. JAMA Neurol 2019; 76: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou Z, Delcourt C, Xia C, et al. Low-Dose vs standard-dose alteplase in acute lacunar ischemic stroke: the ENCHANTED trial. Neurology 2021; 96: e1512–e1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khatri P, Kleindorfer DO, Devlin T, et al.; PRISMS Investigators. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA 2018; 320: 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen H-S, Cui Y, Zhou Z-H, et al.; ARAMIS Investigators. Dual antiplatelet therapy vs alteplase for patients with minor nondisabling acute ischemic stroke: the ARAMIS Randomized Clinical Trial. JAMA 2023; 329: 2135–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eggers CCJ, Bocksrucker C, Seyfang L; Austrian Stroke Unit Registry Collaborators. The efficacy of thrombolysis in lacunar stroke - evidence from the Austrian stroke unit registry. Eur J Neurol 2017; 24: 780–787. [DOI] [PubMed] [Google Scholar]
- 55. Marcelinus K, Liu H, Zhang K, et al. Efficacy and safety of alteplase on treatment of acute single small subcortical infarction. Curr Neurovasc Res 2022; 19: 255–266. [DOI] [PubMed] [Google Scholar]
- 56. Whiteley WN, Slot KB, Fernandes P, et al. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke 2012; 43: 2904–2909. [DOI] [PubMed] [Google Scholar]
- 57. CAST (Chinese Acute Stroke Trial) Collaborative Group, Chen ZM. CAST: randomised placebo-controlled trial of early aspirin use in 20 000 patients with acute ischaemic stroke. Lancet 1997; 349: 1641–1649. [PubMed] [Google Scholar]
- 58. Kwok CS, Shoamanesh A, Copley HC, et al. Efficacy of antiplatelet therapy in secondary prevention following lacunar stroke: pooled analysis of randomized trials. Stroke 2015; 46: 1014–1023. [DOI] [PubMed] [Google Scholar]
- 59. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997; 349: 1569–1581. [PubMed] [Google Scholar]
- 60. Bath PM, Woodhouse LJ, Appleton JP, et al.; TARDIS Investigators. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet 2018; 391: 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, Wang Y, Zhao X, et al.; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med 2013; 369: 11–19. [DOI] [PubMed] [Google Scholar]
- 62. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. New Engl J Med 2018; 379: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shoamanesh A, Mundl H, Smith EE, et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet 2022; 400: 997–1007. [DOI] [PubMed] [Google Scholar]
- 64. Wang G, Yang X, Jing J, et al. Clopidogrel plus aspirin in patients with different types of single small subcortical infarction. Front Neurol 2021; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Y, Meng X, Wang A, et al.; CHANCE-2 Investigators. Ticagrelor versus clopidogrel in CYP2C19 loss-of-function carriers with stroke or TIA. New Engl J Med 2021; 385: 2520–2530. [DOI] [PubMed] [Google Scholar]
- 66. Xie X, Jing J, Meng X, et al.; CHANCE-2 Investigators. Dual antiplatelet therapies and causes in minor stroke or transient ischemic attack: a prespecified analysis in the CHANCE-2 Trial. Stroke 2023; 54: 2241–2250. [DOI] [PubMed] [Google Scholar]
- 67. Shoamanesh A. Is there still a role for dual antiplatelet therapy in the secondary prevention of lacunar stroke? Stroke 2023; 54: 2251–2253. [DOI] [PubMed] [Google Scholar]
- 68. Kennedy J, Hill MD, Ryckborst KJ, et al.; FASTER Investigators. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007; 6: 961–969. [DOI] [PubMed] [Google Scholar]
- 69. Johnston SC, Amarenco P, Denison H, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. New Engl J Med 2020; 383: 207–217. [DOI] [PubMed] [Google Scholar]
- 70. Liu L, Wong KS, Leng X, et al.; CHANCE Investigators. Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of CHANCE. Neurology 2015; 85: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dawson J, Merwick Webb A, et al.; European Stroke Organisation. European stroke organisation expedited recommendation for the use of short-term dual antiplatelet therapy early after minor stroke and high-risk TIA. Eur Stroke J 2021; 6: CLXXXVII–CXCI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. He J, Zhang Y, Xu T, et al.; CATIS Investigators. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the Catis randomized clinical trial. JAMA 2014; 311: 479–489. [DOI] [PubMed] [Google Scholar]
- 73. Sandset EC, Bath PM, Boysen G, et al.; SCAST Study Group. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet 2011; 377: 741–750. [DOI] [PubMed] [Google Scholar]
- 74. ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet 2015; 385: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oh MS, Yu KH, Hong KS, et al.; Valsartan Efficacy oN modesT blood pressUre REduction in acute ischemic stroke (VENTURE) study group. Modest blood pressure reduction with valsartan in acute ischemic stroke: a prospective, randomized, open-label, blinded-end-point trial. Int J Stroke 2015; 10: 745–751. [DOI] [PubMed] [Google Scholar]
- 76. Anderson C, Huang Y, Lindley R, et al. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet 2019; 393: 877–888. [DOI] [PubMed] [Google Scholar]
- 77. Geeganage CM, Bath PM. Relationship between therapeutic changes in blood pressure and outcomes in acute stroke: a metaregression. Hypertension 2009; 54: 775–781. [DOI] [PubMed] [Google Scholar]
- 78. Tikhonoff V, Zhang H, Richart T, et al. Blood pressure as a prognostic factor after acute stroke. Lancet Neurol 2009; 8: 938–948. [DOI] [PubMed] [Google Scholar]
- 79. Berge E, Cohen G, Lindley RI, et al. Effects of blood pressure and blood pressure-lowering treatment during the first 24 hours among patients in the Third International Stroke Trial of thrombolytic treatment for acute ischemic stroke. Stroke 2015; 46: 3362–3369. [DOI] [PubMed] [Google Scholar]
- 80. Ahmed N, Wahlgren N, Brainin M, et al.; SITS Investigators. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from safe implementation of thrombolysis in stroke-international stroke thrombolysis register (SITS-ISTR). Stroke 2009; 40: 2442–2449. [DOI] [PubMed] [Google Scholar]
- 81. Aries MJ, Elting JW, De Keyser J, et al. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke 2010; 41: 2697–2704. [DOI] [PubMed] [Google Scholar]
- 82. Sandset EC, Jusufovic M, Sandset PM, et al.; SCAST Study Group. Effects of blood pressure-lowering treatment in different subtypes of acute ischemic stroke. Stroke 2015; 46: 877–879. [DOI] [PubMed] [Google Scholar]
- 83. Zhou Z, Xia C, Carcel C, et al. Intensive versus guideline-recommended blood pressure reduction in acute lacunar stroke with intravenous thrombolysis therapy: the ENCHANTED trial. Eur J Neurol 2021; 28: 783–793. [DOI] [PubMed] [Google Scholar]
- 84. Zhu Z, Guo D, Shi M, et al. Effect of immediate blood pressure reduction on post-stroke depression in ischemic stroke patients: a substudy of Catis trial. J Affect Disord 2022; 300: 195–202. [DOI] [PubMed] [Google Scholar]
- 85. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J 2021; 6: Xlviii–lxxxix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Potter JF, Robinson TG, Ford GA, et al. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol 2009; 8: 48–56. [DOI] [PubMed] [Google Scholar]
- 87. Yuan F, Yang F, Zhao J, et al. Controlling hypertension after severe cerebrovascular event (CHASE): a randomized, multicenter, controlled study. Int J Stroke 2021; 16: 456–465. [DOI] [PubMed] [Google Scholar]
- 88. Muir KW, Lees KR, Ford I, et al.; Intravenous Magnesium Efficacy in Stroke (IMAGES) Study Investigators. Magnesium for acute stroke (Intravenous Magnesium efficacy in stroke trial): randomised controlled trial. Lancet 2004; 363: 439–445. [DOI] [PubMed] [Google Scholar]
- 89. Schrader J, Lüders S, Kulschewski A, et al.; Acute Candesartan Cilexetil Therapy in Stroke Survivors Study Group. The ACCESS Study: evaluation of Acute candesartan Cilexetil Therapy in stroke survivors. Stroke 2003; 34: 1699–1703. [DOI] [PubMed] [Google Scholar]
- 90. Squire IB, Lees KR, Pryse-Phillips W, et al. The effects of lifarizine in acute cerebral infarction: a pilot safety study. Cerebrovasc Dis 1996; 6: 156–160. [Google Scholar]
- 91. Horn J, de Haan RJ, Vermeulen M, et al. Very early nimodipine use in stroke (VENUS): a randomized, double-blind, placebo-controlled trial. Stroke 2001; 32: 461–465. [DOI] [PubMed] [Google Scholar]
- 92. Kaste M, Fogelholm R, Erilä T, et al. A randomized, double-blind, placebo-controlled trial of nimodipine in acute ischemic hemispheric stroke. Stroke 1994; 25: 1348–1353. [DOI] [PubMed] [Google Scholar]
- 93. Norris JW, Le Brun LH, Anderson BA. Intravenous nimodipine in acute ischaemic stroke. Cerebrovasc Dis 1994; 4: 194–196. [Google Scholar]
- 94. Azcona A, Lataste X. Isradipine in patients with acute ischaemic cerebral infarction. An overview of the Asclepios Programme. Drugs 1990; 40 Suppl 2: 52–57. [DOI] [PubMed] [Google Scholar]
- 95. Barer DH, Cruickshank JM, Ebrahim SB, et al. Low dose beta blockade in acute stroke ("BEST" trial): an evaluation. Br Med J 1988; 296: 737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ahmed N, Wahlgren NG. Effects of blood pressure lowering in the acute phase of total anterior circulation infarcts and other stroke subtypes. Cerebrovasc Dis 2003; 15: 235–243. [DOI] [PubMed] [Google Scholar]
- 97. Ahmed N, Näsman P, Wahlgren NG. Effect of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke 2000; 31: 1250–1255. [DOI] [PubMed] [Google Scholar]
- 98. Ankolekar S, Fuller M, Cross I, et al. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: the rapid intervention with glyceryl trinitrate in hypertensive stroke trial (RIGHT, ISRCTN66434824). Stroke 2013; 44: 3120–3128. [DOI] [PubMed] [Google Scholar]
- 99. Bath PM, Scutt P, Anderson CS.; RIGHT-2 Investigators Prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): an ambulance-based, randomised, sham-controlled, blinded, phase 3 trial. Lancet 2019; 393: 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Powers WJ, Rabinstein AA, Ackerson T, et al.; American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 101. Shin DW, Gorelick PB, Bae HJ ; Clinical Research Collaboration for Stroke in Korea (CRCS-K) Investigators. Time-dependent shift of the relationship between systolic blood pressure and clinical outcome in acute lacunar stroke. Int J Stroke 2022; 17: 400–406. [DOI] [PubMed] [Google Scholar]
- 102. Shin JA, Lee KJ, Lee JS, et al. Relationship between blood pressure and outcome changes over time in acute ischemic stroke. Neurology 2020; 95: e1362–e1371. [DOI] [PubMed] [Google Scholar]
- 103. Sare GM, Ali M, Shuaib A, et al.; VISTA Collaboration. Relationship between hyperacute blood pressure and outcome after ischemic stroke: data from the VISTA collaboration. Stroke 2009; 40: 2098–2103. [DOI] [PubMed] [Google Scholar]
- 104. Libman RB, Sacco RL, Shi T, et al. Neurologic improvement in pure motor hemiparesis. Implications for clinical trials. Neurology 1992; 42: 1713–1716. [DOI] [PubMed] [Google Scholar]
- 105. Del Bene A, Palumbo V, Lamassa M, et al. Progressive lacunar stroke: review of mechanisms, prognostic features, and putative treatments. Int J Stroke 2012; 7: 321–329. [DOI] [PubMed] [Google Scholar]
- 106. Huang YC, Tsai YH, Lee JD, et al. Hemodynamic factors may play a critical role in neurological deterioration occurring within 72 hrs after lacunar stroke. PLOS ONE 9(10): e108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Duan Z, Sun W, Liu W, et al. Acute diffusion-weighted imaging lesion patterns predict progressive small subcortical infarct in the perforator territory of the middle cerebral artery. Int J Stroke 2015; 10: 207–212. [DOI] [PubMed] [Google Scholar]
- 108. Berberich A, Schneider C, Herweh C, et al. Risk factors associated with progressive lacunar strokes and benefit from dual antiplatelet therapy. Eur J Neurol 2020; 27: 817–824. [DOI] [PubMed] [Google Scholar]
- 109. Shimizu H, Tominaga T, Ogawa A, et al.; Tohoku Acute Stroke Progressing Stroke Study Group. Cilostazol for the prevention of acute progressing stroke: a multicenter, randomized controlled trial. J Stroke Cerebrovasc Dis 2013; 22: 449–456. [DOI] [PubMed] [Google Scholar]
- 110. Fukuma K. Abstract T P65: early statin intervention can reduce the early neurological deterioration and recurrence in acute lacunar stroke. Abstract. Stroke 2015; 46. [Google Scholar]
- 111. Nakase T, Sasaki M, Suzuki A. The effect of acute medication with cilostazol, an anti-platelet drug, on the outcome of small vessel brain infarction. J Stroke Cerebrovasc Dis 2014; 23: 1409–1415. [DOI] [PubMed] [Google Scholar]
- 112. Nishi R, Mano T, Kobayashi Y, et al. Argatroban, aspirin, and clopidogrel combination therapy for acute penetrating artery infarction: a pilot study]. Brain Nerve 2016; 68: 181–189. [DOI] [PubMed] [Google Scholar]
- 113. Yamamoto Y, Ohara T, Ishii R, et al. A combined treatment for acute larger lacunar-type infarction. J Stroke Cerebrovasc Dis 2011; 20: 387–394. [DOI] [PubMed] [Google Scholar]
- 114. Yamamoto Y, Nagakane Y, Ohara T, et al. Intensive blood pressure-lowering treatment in patients with acute lacunar infarction. J Stroke Cerebrovasc Dis 2013; 22: 1273–1278. [DOI] [PubMed] [Google Scholar]
- 115. Lim TS, Hong JM, Lee JS, et al. Induced-hypertension in progressing lacunar infarction. J Neurol Sci 2011; 308: 72–76. [DOI] [PubMed] [Google Scholar]
- 116. Kang MJ, Yang JW, Lee YB, et al. The role of phenylephrine in patients with small deep subcortical infarct and progressive weakness. J Neurol Sci 2017; 377: 107–111. [DOI] [PubMed] [Google Scholar]
- 117. Chausson N, Joux J, Saint-Vil M, et al. Infarction in the anterior choroidal artery territory: clinical progression and prognosis factors. J Stroke Cerebrovasc Dis 2014; 23: 2012–2017. [DOI] [PubMed] [Google Scholar]
- 118. Foschi M, Pavolucci L, Rondelli F, et al.; On behalf of the Bologna TIA Study Group. Capsular warning syndrome: features, risk profile, and prognosis in a large prospective TIA cohort. Cerebrovasc Dis 2023; 52: 218–225. [DOI] [PubMed] [Google Scholar]
- 119. Camps-Renom P, Delgado-Mederos R, Martínez-Domeño A, et al. Clinical characteristics and outcome of the capsular warning syndrome: a multicenter study. Int J Stroke 2015; 10: 571–575. [DOI] [PubMed] [Google Scholar]
- 120. Li W, Wu Y, Li XS. Correction: intravenous tirofiban therapy for patients with capsular warning syndrome. Stroke Vasc Neurol 2019; 4: 108–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. He L, Xu R, Wang J, et al. Capsular warning syndrome: clinical analysis and treatment. BMC Neurol 2019; 19: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tassi R, Cerase A, Acampa M, et al. Stroke warning syndrome: 18 new cases. J Neurol Sci 2013; 331: 168–171. [DOI] [PubMed] [Google Scholar]
- 123. Hawkes MA, Braksick SA, Zhang W, et al. Can we stop the stuttering in stroke? Interventions in 40 patients with acute lacunes. J Neurol Sci 2019; 401: 1–4. [DOI] [PubMed] [Google Scholar]
- 124. Lodder J, Gorsselink EL. Progressive stroke caused by CT-verified small deep infarcts; relation with the size of the infarct and clinical outcome. Acta Neurol Scand 1985; 71: 328–330. [DOI] [PubMed] [Google Scholar]
- 125. Yamamoto Y, Oiwa K, Hayashi M, et al. [Coagulation and fibrinolytic activation in lacunar infarct patients]. Rinsho Shinkeigaku 1999; 39: 1104–1108. [PubMed] [Google Scholar]
- 126. Berberich A, Schneider C, Reiff T, et al. Dual antiplatelet therapy improves functional outcome in patients with progressive lacunar strokes. Stroke 2019; 50: 1007–1009. [DOI] [PubMed] [Google Scholar]
- 127. Blair GW, Appleton JP, Flaherty K, et al. Tolerability, safety and intermediary pharmacological effects of cilostazol and isosorbide mononitrate, alone and combined, in patients with lacunar ischaemic stroke: the LA Cunar Intervention-1 (LACI-1) trial, a randomised clinical trial. -med 2019; 11: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bath PM, Mhlanga I, Woodhouse LJ, et al. Cilostazol and isosorbide mononitrate for the prevention of progression of cerebral small vessel disease: baseline data and statistical analysis plan for the Lacunar Intervention Trial-2 (LACI-2) (ISRCTN14911850). Stroke Vasc Neurol 2022; 8: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Uchiyama S, Shinohara Y, Katayama Y, et al. Benefit of cilostazol in patients with high risk of bleeding: subanalysis of cilostazol stroke prevention study 2. Cerebrovasc Dis 2014; 37: 296–303. [DOI] [PubMed] [Google Scholar]
- 130. Shinohara Y, Katayama Y, Uchiyama S, et al.; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010; 9: 959–968. [DOI] [PubMed] [Google Scholar]
- 131. Nishiyama Y, Kimura K, Otsuka T, et al.; CSPS.com Trial Investigators. Dual antiplatelet therapy with cilostazol for secondary prevention in lacunar stroke: subanalysis of the CSPS.com Trial. Stroke 2023; 54: 697–705. [DOI] [PubMed] [Google Scholar]
- 132. Uchiyama S; for the CSPS 2 Group. Abstract 2559: subgroup analysis of the cilostazol stroke prevention study 2 (CSPS 2). Abstract. Stroke 2012; 43. [Google Scholar]
- 133. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. The Publications Committee for the trial of ORG 10172 in acute stroke treatment (TOAST) investigators. JAMA 1998; 279: 1265–1272. [PubMed] [Google Scholar]
- 134. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet 2001; 358: 702–710. [DOI] [PubMed] [Google Scholar]
- 135. Gui Q, Yang Y, Ying S, et al. Xueshuantong improves cerebral blood perfusion in elderly patients with lacunar infarction. Neural Regen Res 2013; 8: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Han SW, Lee SS, Kim SH, et al. Effect of cilostazol in acute lacunar infarction based on pulsatility index of transcranial Doppler (ECLIPse): a multicenter, randomized, double-blind, placebo-controlled trial. Eur Neurol 2013; 69: 33–40. [DOI] [PubMed] [Google Scholar]
- 137. Bradford A, Lees K. Design of the intravenous magnesium efficacy in acute stroke (IMAGES) trial. Curr Control Trials Cardiovasc Med 2000; 1: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Muir KW, Lees KR, Ford I, et al.; Intravenous Magnesium Efficacy in Stroke (IMAGES) Study Investigators. Magnesium for acute stroke (Intravenous Magnesium efficacy in stroke trial): randomised controlled trial. Lancet 2004; 363: 439–445. [DOI] [PubMed] [Google Scholar]
- 139. Aslanyan S, Weir CJ, Muir KW, et al.; IMAGES Study Investigators. Magnesium for treatment of acute lacunar stroke syndromes: further analysis of the IMAGES trial. Stroke 2007; 38: 1269–1273. [DOI] [PubMed] [Google Scholar]
- 140. Afshari D, Moradian N, Rezaei M. Evaluation of the intravenous magnesium sulfate effect in clinical improvement of patients with acute ischemic stroke. Clin Neurol Neurosurg 2013; 115: 400–404. [DOI] [PubMed] [Google Scholar]
- 141. Willmot MR, Bath PM. The potential of nitric oxide therapeutics in stroke. Expert Opin Investig Drugs 2003; 12: 455–470. [DOI] [PubMed] [Google Scholar]
- 142. Appleton JP, Woodhouse LJ, Adami A, et al.; ENOS Investigators. Imaging markers of small vessel disease and brain frailty, and outcomes in acute stroke. Neurology 2020; 94: e439–e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nakamura T, Tsuruta S, Uchiyama S. Cilostazol combined with aspirin prevents early neurological deterioration in patients with acute ischemic stroke: a pilot study. J Neurol Sci 2012; 313: 22–26. [DOI] [PubMed] [Google Scholar]
- 144. Lee YS, Bae HJ, Kang DW, et al. Cilostazol in acute ischemic stroke treatment (CAIST trial): a randomized double-blind non-inferiority trial. Cerebrovasc Dis 2011; 32: 65–71. [DOI] [PubMed] [Google Scholar]
- 145. Aoki J, Iguchi Y, Urabe T, et al.; ADS Investigators. Acute aspirin plus cilostazol dual therapy for noncardioembolic stroke patients within 48 hours of symptom onset. J Am Heart Assoc 2019; 8: e012652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bousser MG, Eschwege E, Haguenau M, et al. “Aicla” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke 1983; 14: 5–14. [DOI] [PubMed] [Google Scholar]
- 147. Ariesen MJ, Algra A, Kappelle LJ. Antiplatelet drugs in the secondary prevention after stroke: differential efficacy in large versus small vessel disease? A subgroup analysis from ESPS-2. Stroke 2006; 37: 134–138. [DOI] [PubMed] [Google Scholar]
- 148. Shinohara Y, Nishimaru K, Sawada T, et al.; S-ACCESS Study Group. Sarpogrelate-aspirin comparative clinical study for efficacy and safety in secondary prevention of cerebral infarction (S-ACCESS): a randomized, double-blind, aspirin-controlled trial. Stroke 2008; 39: 1827–1833. [DOI] [PubMed] [Google Scholar]
- 149. Kitazono T, Toyoda K, Kitagawa K, et al. Efficacy and safety of prasugrel by stroke subtype: a sub-analysis of the prastro-i randomized controlled trial. J Atheroscler Thromb 2021; 28: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gent M, Blakely JA, Easton JD, et al. The Canadian American ticlopidine Study (CATS) in thromboembolic stroke. Lancet 1989; 1: 1215–1220. [DOI] [PubMed] [Google Scholar]
- 151. Gorelick PB, Leurgans S, Richardson D, et al. African American antiplatelet stroke prevention study: clinical trial design. J Stroke Cerebrovasc Dis 1998; 7: 426–434. [DOI] [PubMed] [Google Scholar]
- 152. Han SW, Kim Y-J, Ahn SH, et al.; MAESTRO Study Investigators. Protocol for the comparison of triflusal and clopidogrel in secondary prevention of stroke based on cytochrome P450 2C19 genotyping (MASETRO study): a multicenter, randomized, open-label, parallel-group trial. Int J Stroke 2016; 11: 485–491. [DOI] [PubMed] [Google Scholar]
- 153. Diener HC, Bogousslavsky J, Brass LM, et al.; MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004; 364: 331–337. [DOI] [PubMed] [Google Scholar]
- 154. ESPRIT Study Group,et al.Halkes PH, van Gijn J, Kappelle LJ. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006; 367: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 155. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. New Engl J Med 2008; 359: 1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Bousser MG, Amarenco P, Chamorro A, et al.; PERFORM Study Investigators. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet 2011; 377: 2013–2022. [DOI] [PubMed] [Google Scholar]
- 157. Uchiyama S, Fukuuchi Y, Yamaguchi T. The safety and efficacy of clopidogrel versus ticlopidine in Japanese stroke patients: combined results of two Phase III, multicenter, randomized clinical trials. J Neurol 2009; 256: 888–897. [DOI] [PubMed] [Google Scholar]
- 158. Fukuuchi Y, Tohgi H, Okudera T, et al. A randomized, double-blind study comparing the safety and efficacy of clopidogrel versus ticlopidine in Japanese patients with noncardioembolic cerebral infarction. Cerebrovasc Dis 2008; 25: 40–49. [DOI] [PubMed] [Google Scholar]
- 159. Morrow DA, Alberts MJ, Mohr JP, et al.; Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events–TIMI 50 Steering Committee and Investigators. Efficacy and safety of vorapaxar in patients with prior ischemic stroke. Stroke 2013; 44: 691–698. [DOI] [PubMed] [Google Scholar]
- 160. Amarenco P, Albers GW, Denison H, et al.; SOCRATES Steering Committee and Investigators. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol 2017; 16: 301–310. [DOI] [PubMed] [Google Scholar]
- 161. Gotoh F, Tohgi H, Hirai S, et al. Cilostazol stroke prevention study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis 2000; 9: 147–157. [DOI] [PubMed] [Google Scholar]
- 162. Matsumoto M. Cilostazol in secondary prevention of stroke: impact of the Cilostazol Stroke Prevention Study. Atheroscler Suppl 2005; 6: 33–40. [DOI] [PubMed] [Google Scholar]
- 163. Han SW, Kim YJ, Ahn SH, et al. Effects of triflusal and clopidogrel on the secondary prevention of stroke based on cytochrome p450 2c19 genotyping. Stroke 2017; 19: 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Gorelick PB, Richardson D, Kelly M, et al.; African American Antiplatelet Stroke Prevention Study Investigators. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial. JAMA 2003; 289: 2947–2957. [DOI] [PubMed] [Google Scholar]
- 165. Benavente OR, Hart RG, McClure LA, et al.; SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke: the Secondary Prevention of Small Subcortical Stroke (SPS3) Trial. New Engl J Med 2012; 367: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Rogers KC, Oliphant CS, Finks SW. Clinical efficacy and safety of cilostazol: a critical review of the literature. Drugs 2015; 75: 377–395. [DOI] [PubMed] [Google Scholar]
- 167. Shinohara Y, Gotoh F, Tohgi H, et al. Antiplatelet cilostazol is beneficial in diabetic and/or hypertensive ischemic stroke patients. Subgroup analysis of the cilostazol stroke prevention study. Cerebrovasc Dis 2008; 26: 63–70. [DOI] [PubMed] [Google Scholar]
- 168. Hou X, Cen K, Cui Y, et al. Antiplatelet therapy for secondary prevention of lacunar stroke: a systematic review and network meta-analysis. Eur J Clin Pharmacol 2023; 79: 63–70. [DOI] [PubMed] [Google Scholar]
- 169. CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348: 1329–1339. [DOI] [PubMed] [Google Scholar]
- 170. McDonough CW, McClure LA, Mitchell BD, et al. CYP2C19 metabolizer status and clopidogrel efficacy in the secondary prevention of small subcortical strokes (SPS3) study. J Am Heart Assoc 2015; 4: e001652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. McHutchison C, Blair GW, Appleton JP, et al. Cilostazol for secondary prevention of stroke and cognitive decline: systematic review and meta-analysis. Stroke 2020; 51: 2374–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Markus HS, Egle M, Croall ID, et al.; PRESERVE Study Team. PRESERVE: randomized trial of intensive versus standard blood pressure control in small vessel disease. Stroke 2021; 52: 2484–2493. [DOI] [PubMed] [Google Scholar]
- 173. Ikeme JC, Pergola PE, Scherzer R, et al. Cerebral white matter hyperintensities, kidney function decline, and recurrent stroke after intensive blood pressure lowering: results from the secondary prevention of small subcortical strokes (SPS3) trial. J Am Heart Assoc 2019; 8: e010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Blum MR, Scherzer R, Ikeme JC, et al. Functional health and white matter hyperintensities as effect modifiers of blood pressure-lowering on cognitive function and vascular events in older secondary prevention of small subcortical strokes trial participants. J Hypertens 2020; 38: 1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Shoamanesh A, Pearce LA, Bazan C, et al.; SPS3 Trial Investigators. Microbleeds in the secondary prevention of small subcortical strokes trial: stroke, mortality, and treatment interactions. Ann Neurol 2017; 82: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Odden MC, McClure LA, Sawaya BP, et al. Achieved blood pressure and outcomes in the secondary prevention of small subcortical strokes trial. Hypertension 2016; 67: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ku E, Scherzer R, Odden MC, et al. Patterns of blood pressure response during intensive BP lowering and clinical events: results from the secondary prevention of small subcortical strokes trial. Blood Press 2018; 27: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Whelton P, Carey R, Aronow W, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 138: e426–e483. [DOI] [PubMed] [Google Scholar]
- 179. Kernan WN, Ovbiagele B, Black HR, et al.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 180. PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 181. Yusuf S, Sleight P, Pogue J, et al.; Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. New Engl J Med 2000; 342: 145–153. [DOI] [PubMed] [Google Scholar]
- 182. Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. New Engl J Med 2008; 359: 1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Hypertension-Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. Hypertension-Stroke Cooperative Study Group. JAMA 1974; 229: 409–418. [DOI] [PubMed] [Google Scholar]
- 184. PATS Collaborating Group. Post-stroke antihypertensive treatment study. A preliminary result. Chin Med J 1995; 108: 710–717. [PubMed] [Google Scholar]
- 185. Gayet JL. The study on cognition and prognosis in the elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21: 1771. [DOI] [PubMed] [Google Scholar]
- 186. The Dutch TIA Trial Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke. The Dutch TIA Trial Study Group. Stroke 1993; 24: 543–548. [DOI] [PubMed] [Google Scholar]
- 187. Eriksson S, Olofsson BO, Wester PO. Atenolol in secondary prevention after stroke. Cerebrovasc Dis 1995; 5: 21–25. [Google Scholar]
- 188. Liu L, Zhang Y, Liu G, et al.; FEVER Study Group. The felodipine event reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23: 2157–2172. [DOI] [PubMed] [Google Scholar]
- 189. MacDonald TM, Williams B, Webb DJ, et al.; British Hypertension Society Programme of Prevention And Treatment of Hypertension With Algorithm-based Therapy (PATHWAY). Combination therapy is superior to sequential monotherapy for the initial treatment of hypertension: a double-blind randomized controlled trial. J Am Heart Assoc 2017; 6: e006986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Brown MJ, McInnes GT, Papst CC, et al. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel-group trial. Lancet 2011; 377: 312–320. [DOI] [PubMed] [Google Scholar]
- 191. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med 2009; 122: 290–300. [DOI] [PubMed] [Google Scholar]
- 192. Williams B, Mancia G, Spiering W, et al.; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 193. Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375: 906–915. [DOI] [PubMed] [Google Scholar]
- 194. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338: b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Arima H, Chalmers J, Woodward M, et al. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens 2006; 24: 1201–1208. [DOI] [PubMed] [Google Scholar]
- 196. Georgakis MK, Gill D, Webb AJS, et al. Genetically determined blood pressure, antihypertensive drug classes, and risk of stroke subtypes. Neurology 2020; 95: e353–e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Ovbiagele B, Diener HC, Yusuf S, et al.; PROFESS Investigators. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA 2011; 306: 2137–2144. [DOI] [PubMed] [Google Scholar]
- 198. Ovbiagele B. Low-normal systolic blood pressure and secondary stroke risk. J Stroke Cerebrovasc Dis 2013; 22: 633–638. [DOI] [PubMed] [Google Scholar]
- 199. Lin MP, Ovbiagele B, Markovic D, et al. Systolic blood pressure and mortality after stroke: too low, no go? Stroke 2015; 46: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 200. Kim J, Gall SL, Nelson MR, et al. Lower systolic blood pressure is associated with poorer survival in long-term survivors of stroke. J Hypertens 2014; 32: 904–911. [DOI] [PubMed] [Google Scholar]
- 201. Amarenco P, Benavente O, Goldstein LB, et al.; Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. Results of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial by stroke subtypes. Stroke 2009; 40: 1405–1409. [DOI] [PubMed] [Google Scholar]
- 202. Hosomi N, Nagai Y, Kohriyama T, et al.; J-STARS Collaborators. The Japan statin treatment against recurrent stroke (J-STARS): a multicenter, randomized, open-label, parallel-group Study. EBioMedicine 2015; 2: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al.; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. New Engl J Med 2006; 355: 549–559. [DOI] [PubMed] [Google Scholar]
- 204. Collins R, Armitage J, Parish S, et al.; Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004; 363: 757–767. [DOI] [PubMed] [Google Scholar]
- 205. Amarenco P, Kim JS, Labreuche J, et al. A comparison of two LDL cholesterol targets after ischemic stroke. New Engl J Med 2020; 382: 9–19. [DOI] [PubMed] [Google Scholar]
- 206. Authors/Task Force, Members ESC, Committee for Practice Guidelines ESC, National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019; 290: 140–205. [DOI] [PubMed] [Google Scholar]
- 207. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res 2017; 120: 472–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Rutten-Jacobs LC, Larsson SC, Malik R, et al.; MEGASTROKE consortium and International Stroke Genetics Consortium. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: cohort study of 306 473 UK Biobank participants. BMJ 2018; 363: k4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Krawcyk RS, Vinther A, Petersen NC, et al. High-intensity training in patients with lacunar stroke: a one-year follow-up. J Stroke Cerebrovasc Dis 2023; 32: 106973. [DOI] [PubMed] [Google Scholar]
- 210. van Dijk EJ, Prins ND, Vrooman HA, et al. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam scan study. Stroke 2008; 39: 2712–2719. [DOI] [PubMed] [Google Scholar]
- 211. Clancy U, Appleton JP, Arteaga C, et al. Clinical management of cerebral small vessel disease: a call for a holistic approach. Chin Med J 2021; 134: 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Liu D, Cai X, Yang Y, et al. Associations of life's simple 7 with cerebral small vessel disease. Stroke 2022; 53: 2859–2867. [DOI] [PubMed] [Google Scholar]
- 213. Billinger SA, Arena R, Bernhardt J, et al.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention and Council on Clinical Cardiology. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2532–2553. [DOI] [PubMed] [Google Scholar]
- 214. Liu L, Chen W, Zhou H, et al.; Chinese Stroke Association Stroke Council Guideline Writing Committee. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol 2020; 5: 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. García Pastor A, López-Cancio Martínez E, Rodríguez-Yáñez M, et al.; Ad hoc committee of the Spanish Society of Neurology's Cerebrovascular Disease Study Group. Recommendations of the Spanish Society of Neurology for the prevention of stroke. Interventions on lifestyle and air pollution. Neurologia 2021; 36: 377–387. [DOI] [PubMed] [Google Scholar]
- 216. Steen Krawcyk R, Vinther A, Petersen NC, et al. Effect of home-based high-intensity interval training in patients with Lacunar Stroke: a randomized controlled trial. Front Neurol 2019; 10: 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Ting SKS, Earnest A, Li H, et al. B vitamins and cognition in subjects with small vessel disease: a substudy of VITATOPS, a randomized, placebo-controlled trial. J Neurol Sci 2017; 379: 124–126. [DOI] [PubMed] [Google Scholar]
- 218. Anadani M, Turan TN, Yaghi S, et al. Change in smoking behavior and outcome after ischemic stroke: post-hoc analysis of the SPS3 trial. Stroke 2023; 54: 921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Verdelho A, Madureira S, Correia M, et al. Impact of physical activity in vascular cognitive impairment (AFIVASC): study protocol for a randomised controlled trial. Trials 2019; 20: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Verdelho A, Correia M, Ferro JM, et al. Physical activity self-report is not reliable among subjects with mild vascular cognitive impairment: the AFIVASC study. J Alzheimers Dis 2022; 87: 405–414. [DOI] [PubMed] [Google Scholar]
- 221. Tan M, He F, Morris JK, et al. Reducing daily salt intake in China by 1 g could prevent almost 9 million cardiovascular events by 2030: a modelling study. BMJ Nutr Prev Heal 2022; 5: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Heye AK, Thrippleton MJ, Chappell FM, et al. Blood pressure and sodium: association with MRI markers in cerebral small vessel disease. J Cereb Blood Flow Metab 2016; 36: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Makin SDJ, Mubki GF, Doubal FN, et al. Small vessel disease and dietary salt intake: cross-sectional study and systematic review. J Stroke Cerebrovasc Dis 2017; 26: 3020–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Robinson AT, Edwards DG, Farquhar WB. The influence of dietary salt beyond blood pressure. Curr Hypertens Rep 2019; 21: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Zhang WC, Zheng XJ, Du LJ, et al. High salt primes a specific activation state of macrophages, M(Na). Cell Res 2015; 25: 893–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 2009; 89: 485–490. [DOI] [PubMed] [Google Scholar]
- 227. Stocker SD, Lang SM, Simmonds SS, et al. Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension 2015; 66: 1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kivipelto M, Palmer K, Hoang TD, et al. Trials and treatments for vascular brain health: risk factor modification and cognitive outcomes. Stroke 2022; 53: 444–456. [DOI] [PubMed] [Google Scholar]
- 229. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385: 2255–2263. [DOI] [PubMed] [Google Scholar]
- 230. Lehtisalo J, Rusanen M, Solomon A, et al. Effect of a multi-domain lifestyle intervention on cardiovascular risk in older people: the FINGER trial. Eur Heart J 2022; 43: 2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Mead GE, Sposato LA, Sampaio Silva G, et al. A systematic review and synthesis of global stroke guidelines on behalf of the World Stroke Organization. Int J Stroke 2023; 18: 499–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int J Stroke 2022; 17: 18–29. [DOI] [PubMed] [Google Scholar]
- 233. Ismail Z, Black SE, Camicioli R, et al.; CCCDTD5 participants. Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimers Dement 2020; 16: 1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Rojas-Rozo L, Vedel I, Sivananthan S, et al. Latest Canadian consensus conference on the diagnosis and treatment of dementia for primary care clinicians. Ann Fam Med 2022; 20 (Supplement 1): 2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Smith EE, Barber P, Field TS, et al. Canadian consensus conference on diagnosis and treatment of dementia (CCCDTD)5: guidelines for management of vascular cognitive impairment. Alzheimers Dement 2020; 6(1): e12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Peng D; Geriatric Neurology Group, Chinese Society of Geriatrics and Clinical Practice Guideline for Cognitive Impairment of Cerebral Small Vessel Disease Writing Group. Clinical practice guideline for cognitive impairment of cerebral small vessel disease. Aging Med 2019; 2: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Gorelick PB, Scuteri A, Black SE, et al.; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Mohr JP, Thompson JL, Lazar RM, et al.; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. New Engl J Med 2001; 345: 1444–1451. [DOI] [PubMed] [Google Scholar]
- 239. ESPRIT Study Group Halkes PH, van Gijn J. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurol 2007; 6: 115–124. [DOI] [PubMed] [Google Scholar]
- 240. The Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group. A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin. The stroke prevention in reversible ischemia trial (SPIRIT) Study Group. Ann Neurol 1997; 42: 857–865. [DOI] [PubMed] [Google Scholar]
- 241. Saxena R, Koudstaal PJ. Anticoagulants for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischaemic attack. Cochrane Database Syst Rev 2004; CD000185. [DOI] [PubMed] [Google Scholar]
- 242. Saxena R, Koudstaal P. Anticoagulants versus antiplatelet therapy for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischemic attack. Cochrane Database Syst Rev 2004; CD000187. [DOI] [PubMed] [Google Scholar]
- 243. Evans A, Perez I, Yu G, et al. Secondary stroke prevention in atrial fibrillation: lessons from clinical practice. Stroke 2000; 31: 2106–2111. [DOI] [PubMed] [Google Scholar]
- 244. Ölmestig J, Marlet IR, Hansen RH, et al. Tadalafil may improve cerebral perfusion in small-vessel occlusion stroke-a pilot study. Brain Commun 2020; 2: fcaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Webb A, Werring D, Dawson J, et al. Design of a randomised, double-blind, crossover, placebo-controlled trial of effects of sildenafil on cerebrovascular function in small vessel disease: Oxford haemodynamic adaptation to reduce pulsatility trial (OxHARP). Eur Stroke J 2021; 6: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Brown RB, Tozer DJ, Loubière L, et al. MINocyclinE to reduce inflammation and blood brain barrier leakage in small vessel diseAse (MINERVA) trial study protocol. Eur Stroke J 2022; 7: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231219416 for European stroke organisation (ESO) guideline on cerebral small vessel disease, part 2, lacunar ischaemic stroke by Joanna M Wardlaw, Hugues Chabriat, Frank-Erik de Leeuw, Stéphanie Debette, Martin Dichgans, Fergus Doubal, Hanna Jokinen, Aristeidis H Katsanos, Raffaele Ornello, Leonardo Pantoni, Marco Pasi, Aleksandra M Pavlovic, Salvatore Rudilosso, Reinhold Schmidt, Julie Staals, Martin Taylor-Rowan, Salman Hussain and Arne G Lindgren in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231219416 for European stroke organisation (ESO) guideline on cerebral small vessel disease, part 2, lacunar ischaemic stroke by Joanna M Wardlaw, Hugues Chabriat, Frank-Erik de Leeuw, Stéphanie Debette, Martin Dichgans, Fergus Doubal, Hanna Jokinen, Aristeidis H Katsanos, Raffaele Ornello, Leonardo Pantoni, Marco Pasi, Aleksandra M Pavlovic, Salvatore Rudilosso, Reinhold Schmidt, Julie Staals, Martin Taylor-Rowan, Salman Hussain and Arne G Lindgren in European Stroke Journal
Supplemental material, sj-docx-3-eso-10.1177_23969873231219416 for European stroke organisation (ESO) guideline on cerebral small vessel disease, part 2, lacunar ischaemic stroke by Joanna M Wardlaw, Hugues Chabriat, Frank-Erik de Leeuw, Stéphanie Debette, Martin Dichgans, Fergus Doubal, Hanna Jokinen, Aristeidis H Katsanos, Raffaele Ornello, Leonardo Pantoni, Marco Pasi, Aleksandra M Pavlovic, Salvatore Rudilosso, Reinhold Schmidt, Julie Staals, Martin Taylor-Rowan, Salman Hussain and Arne G Lindgren in European Stroke Journal