Abstract
We have previously isolated mutants of the major-group human rhinovirus type 89 that grow in cells deficient in intercellular adhesion molecule 1 (ICAM-1), the receptor used by the wild-type virus for cell entry [A. Reischl, M. Reithmayer, G. Winsauer, R. Moser, I. Goesler, and D. Blaas., J. Virol. 75:9312-9319, 2001]. We now demonstrate that one of these variants utilizes heparan sulfate proteoglycan (HSPG) as a cellular receptor. Adaptation to ICAM-1-deficient cells not only resulted in the newly acquired receptor specificity but also rendered the virus less stable at low pH and at elevated temperatures. This instability might compensate for the absence of the uncoating activity of ICAM-1. Whereas wild-type virus infection via ICAM-1 proceeded in the presence of the vesicular H+-ATPase inhibitor bafilomycin A1, infection by the mutant via HSPG was prevented by the drug. This suggests that the low pH prevailing in endosomal compartments is required for uncoating in the absence of the catalytic activity of ICAM-1.
Human rhinoviruses (HRVs), the main causative agents of common cold infections and members of the picornavirus family, are small (∼30-nm diameter) icosahedral particles composed of 60 copies of each of the four viral capsid proteins VP1 through VP4. The capsid encases a single-stranded RNA molecule of positive (messenger sense) polarity encoding a polyprotein, which is cotranslationally and autocatalytically processed by the viral proteinases 2Apro and 3Cpro, giving rise to the structural and nonstructural proteins (for a review, see reference 47). One of the means of classification of a respiratory virus as an HRV is its instability at low pH; incubation at acid pH quickly induces a conformational modification of the capsid, giving rise to noninfectious subviral particles (52, 57). Particles obtained by exposure to low pH or elevated temperature in vitro are structurally similar to those produced in vivo during the course of infection (29). For some HRVs, the low-pH environment encountered in the endosomal system is required for the release of the genomic RNA into the cytosol, where translation and replication take place.
More than 100 HRV serotypes have been characterized, and based on phylogeny, they are divided into genera A and B (30, 44). Some genus A and all genus B HRVs (the 89 major receptor group HRVs) gain access to the cell via human intercellular adhesion molecule 1 (ICAM-1), whereas 10 minor receptor group HRVs (all belonging to genus A) use members of the low-density lipoprotein receptor (LDLR) family for cell entry (58). These include the LDLR proper, the very-low-density lipoprotein receptor, and LRP, the LDLR-related protein (22, 34). HRV type 87 (HRV87) has been shown to utilize a sialylated glycoprotein as a cellular receptor (58) and was recently reclassified as an acid-sensitive enterovirus, EV68, that utilizes decay-accelerating factor for cell entry (6). The principles underlying receptor discrimination are only partly understood, and on the basis of the amino acid sequences of the capsid proteins and the three-dimensional structure of the viral shell at atomic resolution, it is currently not possible to classify a given serotype as a minor- or a major-group HRV (59, 60). Switching from one receptor specificity to the other has not been observed, and HeLa cells were not infected by the major-group virus HRV14 at a high multiplicity of infection when ICAM-1 on the plasma membrane was blocked with a monoclonal antibody (11). This suggests that at least for HRV14, single-amino-acid changes that occur with a frequency of 10−5 to 10−4 (49) do not suffice to acquire binding affinity for the receptor of the other group.
ICAM-1 is not only responsible for cell attachment of major-group HRVs but also facilitates the release of the genomic RNA, as demonstrated in vitro using soluble recombinant fragments of this protein (9, 19, 23). Apparently, interaction of the N-terminal immunoglobulin-like domain of ICAM-1 with amino acid residues within the viral canyon, a cleft encircling the fivefold axes of icosahedral symmetry, lowers the energy barrier of the conversion reaction from native virus to subviral particles (39), as has also been shown for poliovirus receptor and poliovirus (55); as opposed to native virus, which has a sedimentation constant of 150S, subviral A particles sediment at 135S and are believed to be an intermediate, and B particles, sedimenting at 80S, are believed to be the end product of the uncoating reaction (29, 32, 38). The former have shed the innermost capsid protein, VP4, but still contain the genomic RNA; B particles have also released the RNA.
Infection by HRV14, a major-group HRV, is only partially inhibited by the macrolide antibiotic bafilomycin A1, a specific inhibitor of the vesicular proton motive ATPase. From this, it was concluded that ICAM-1 is by itself capable of catalyzing viral uncoating in vivo, even at neutral pH (2). This is in contrast to LDLR, which only acts as a vehicle for cell entry but does not take part in the release of the RNA. Consequently, uncoating of HRV2, a prototype minor-group virus, does not occur at neutral pH, as the carboxylic ionophore monensin (37) and bafilomycin A1 completely abrogate infection (3, 40, 41, 46). This most probably applies also to the other minor-group HRVs.
Based on the relatively high sequence similarity of the capsid proteins of HRV2 and HRV89, between 62% (VP1) and 94% (VP4), we reasoned that only a small number of mutations might be necessary to change the receptor specificity of the latter serotype from ICAM-1 to LDLR. We further thought that the presence of a low concentration of ICAM-1 would facilitate attachment and replication of variants which had not yet acquired strong binding affinity for a putative new receptor but still bind ICAM-1. Using selection on HEp-2 cells, which express only low levels of ICAM-1 compared to HeLa cells, we indeed succeeded in isolating variants of HRV89 with affinity for another receptor (42). However, these isolates failed to attach to and were not neutralized by recombinant fragments of very-low-density lipoprotein receptor and thus had not switched to a minor-group receptor. The current paper reports on the further characterization of some of the HEp-2 cell-adapted mutants and the identification of the receptor used for cell entry. Most experiments were carried out with isolate 15 (42). However, we assume that the results can be extrapolated to the other variants obtained in the selection process; below, we shall refer to the number of the clone described by Reischl and colleagues with a subscript (i.e., clone 15 will be termed “HRV8915”).
MATERIALS AND METHODS
Materials.
Heparinase I (catalogue no. H2519), phosphatidylinositol-specific phospholipase C (from Bacillus cereus; catalogue no. P5542), and chondroitinase (catalogue no. C2905) were from Sigma-Aldrich GmbH, Vienna, Austria; neuraminidase was from Behring, Marburg, Germany. Proteoglycans were from Polygono Industrial, Barcelona, Spain; HRV89 was originally obtained from the American Type Culture Collection (LGC Promochem, Teddigton, Middlesex, United Kingdom). COS-7 cells were from LGC; as verified by fluorescence-activated cell sorter analysis, they are negative for ICAM-1 expression (M. Reithmayer, unpublished data). HeLa-H1 cells (Flow Laboratories) were grown in minimal essential medium, and COS-7 cells in Dulbecco's modified Eagle medium. The media were supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. For infection, the respective growth media were reduced to 2% FCS, and 30 mM MgCl2 was added (infection medium). All tissue culture plasticware was from Corning GmbH, Wiesbaden, Germany.
Metabolic labeling of the virus.
HeLa cells were grown in six-well plates to about 80% confluency. The cells were washed with phosphate-buffered saline (PBS) and infected with 300 μl virus suspension in PBS at 108 50% tissue culture infective doses (TCID50) for 1 h at 34°C with rocking. Five hundred microliters of infection medium was added, and incubation was continued for a further 3 h at 34°C. The cells were washed with PBS and further incubated for 3 h in 800 μl of methionine-cysteine-free infection medium containing 2% dialyzed FCS. [32S]methionine-cysteine (0.1 mCi) (Hartmann Analytic GmbH, Braunschweig, Germany) was added per well; when more than 80% of the cells showed cytopathic effect, they were subjected to three freeze-thaw cycles. Cell debris was removed by centrifugation for 30 min in an Eppendorf centrifuge at full speed at 4°C. Virus in the supernatant was pelleted at 70,000 rpm for 1 h in a tabletop ultracentrifuge (rotor TLA 100.3; Beckman Instruments Inc., Fullerton, CA) and resuspended in 50 μl of Tris-buffered saline overnight. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by autoradiography showed only the viral capsid proteins.
Enzymatic treatment of COS-7 cells and virus binding assays.
Cells were grown in 24-well plates until ∼80% confluent, washed with PBS containing 1 mM MgCl2 and 1 mM CaCl2 (PBS++), and incubated for 2 h at 37°C with 2 U/ml of the specified enzymes in PBS++ or in buffer alone. Neuraminidase was used at 0.2 U/ml. The cells were washed with cold PBS, virus at 105 TCID50 was added, and incubation continued for 1 h at 4°C. Unbound virus was washed off with cold PBS, the cells were subjected to three freeze-thaw cycles in 200 μl of infection medium, and virus that had associated with the cells was determined by end point dilution.
Inhibition of cell attachment with proteoglycans.
Cells grown in 24-well plates to ∼80% confluency were washed with PBS, and 200 μl of infection medium containing 2 mg/ml of heparin, heparan sulfate (HS), dermatan sulfate, or chondroitin sulfate was added. Radiolabeled virus (∼18,000 cpm/well) was added, and incubation continued for 1 h at 34°C. After being washed with PBS, the cells were detached with trypsin, and cell-associated radioactivity was determined by liquid scintillation counting.
Inhibition of proteoglycan sulfation with chlorate.
Incorporation of sulfate into the proteoglycans was inhibited with chlorate as described previously (48). Briefly, cells were grown in medium containing 50 mM NaClO3 for 3 days in 24-well plates to about 80% confluency. After being washed with PBS, 200 μl infection medium containing or not containing 2 mg/ml heparin was added and the cells were challenged with ∼6,000 cpm/well of radiolabeled virus for 1 h at 34°C. The cells were then washed with PBS and detached with trypsin, and the radioactivity was determined.
Virus inactivation by exposure to low pH or incubation at 47°C.
Virus (106 TCID50 in 200 μl PBS) was adjusted to pH 4.5 by addition of 6.8 μl 0.5 M acetate buffer, pH 4; incubated for 15 and 30 min; and neutralized with 6.6 μl 0.5 M Na3PO4. Alternatively, virus was adjusted to pH 5.6 by addition of 2.7 μl of acetate buffer, incubated at 34°C, and reneutralized by addition of 2.3 μl of 0.5 M Na3PO4. For heat inactivation, identical samples were incubated in a water bath at 47°C for 15 and 30 min and immediately transferred to 4°C. Infectivity was determined by end point dilution.
Inhibition of endosomal acidification by bafilomycin A1.
Cells were grown in 24-well plates to about 80% confluency. The cells were washed with PBS, and then 200 μl of 200 nM bafilomycin A1 (Eubio, Vienna, Austria) in infection medium and/or 10 μg of antibody R6.5 (51) was added and preincubated for 1 h at 37°C. Two hundred microliters of virus (106 TCID50) was added, and incubation continued for 1 h at 34°C. The cells were then washed twice with ice-cold PBS, and 200 μl of fresh medium containing 100 nM bafilomycin A1 and/or 10 μg of R6.5 was added and incubated for a further 2, 11, or 23 h. The samples were then subjected to three freeze-thaw cycles. Infectivity was determined by end point dilution.
Inhibition of viral infection with proteoglycans.
COS-7 cells in 96-well plates were challenged with virus at 107 TCID50/well in 100 μl of infection medium with various concentrations of heparin. After incubation for up to 2 days at 34°C, metabolically active cells were determined by addition of CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay reagent (Promega Corporation, Madison, WI) for 12 h at 34°C, followed by measurement of A495 in a microplate reader.
Virus binding to cells deficient in heparan sulfate proteoglycan (HSPG) synthesis.
CHO-K1 (wild type [wt]), pgsA-745 (deficient in xylosyltransferase), and pgsD-677 (doubly deficient in N-acetylglucosaminyltransferase and glucuronyltransferase) cells were grown in 24-well plates to about 80% confluency. After being washed with PBS, the cells were incubated with ∼20,000 cpm/well of radiolabeled virus in 200 μl infection medium for 1 h at 4°C. The cells were washed with PBS and detached with trypsin, and the radioactivity was determined by liquid scintillation counting.
RESULTS
HRV8915 binding to COS-7 cells is inhibited by proteoglycans.
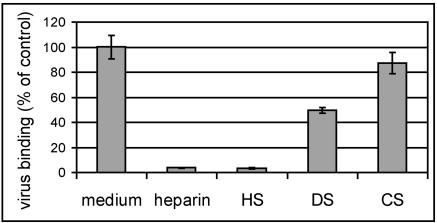
Recently, many viruses have been found to use HS as a receptor or coreceptor (8, 10, 12, 14, 15, 18, 25, 45, 53, 61, 63), or they can adapt to bind HS (4, 24, 28, 50, 64; reviewed in reference 31). HS is a heterogeneous, highly sulfated polysaccharide with repeating units that are predominantly composed of N-acetylglucosamine and iduronic acid (56). Although mostly ionic in character, interactions between HS and its ligands are relatively specific. Therefore, in a first attempt at the identification of the receptor used by the HEp-2 cell-adapted HRV89 mutants, we carried out competition experiments with various proteoglycans. COS-7 cells grown in 24-well plates were incubated with radiolabeled HRV8915 in the presence of 2 mg/ml of heparin, heparan sulfate, chondroitin sulfate, and dermatan sulfate. The cells were washed, and cell-associated virus was quantified (Fig. 1). Binding was greatly reduced by heparin and by heparan sulfate. Chondroitin sulfate and dermatan sulfate reduced binding to a minor extent. In agreement with earlier results (42), wt HRV89 showed only background binding to COS-7 cells (not shown).
FIG. 1.
Binding of HRV8915 to COS-7 cells is inhibited by sulfated glycans. COS-7 cells grown in 24-well plates were incubated for 1 h at 37°C with radiolabeled HRV8915 in the presence of 2 mg/ml of the proteoglycans indicated (DS, dermatan sulfate; CS, chondroitin sulfate). The supernatant was removed, the cells were washed, and cell-associated radioactivity versus total radioactivity was determined by liquid scintillation counting. Since the absolute values obtained differed somewhat from experiment to experiment, virus bound in plain medium (control) was set to 100% in each experiment and the other values were normalized accordingly. The mean ± standard deviation of three experiments carried out in parallel is shown.
To investigate whether heparin also protected the cells from viral damage, infection was carried out in the presence of various concentrations of heparin and cell viability was assessed by monitoring their metabolic activity. This method was chosen, since COS-7 cells, unlike HeLa cells, only incompletely detach from the plastic upon infection, and therefore, cell damage is somewhat difficult to monitor macroscopically. In agreement with the previous data, high concentrations of heparin inhibited infection. However, at low heparin concentrations, the cytopathic effect following infection was increased (see below).
Heparinase treatment of COS-7 cells reduces HRV8915 attachment.
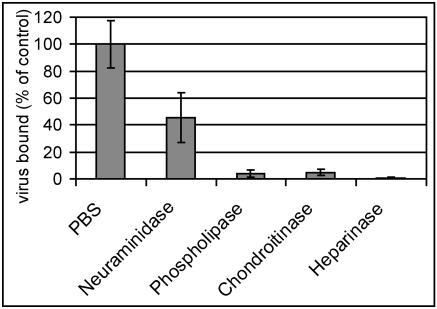
In order to confirm the involvement of heparan sulfate in attachment of HRV8915 to COS-7 cells, enzymatic treatments were carried out. Cells grown in 24-well plates were incubated with phosphatidylinositol-specific phospholipase C, neuraminidase, heparinase I, and chondroitinase; released material was washed away; and the cells were incubated with HRV8915 at 4°C for 60 min to allow attachment but to prevent internalization and uncoating. The cells were washed, and plasma membrane-attached virus was recovered by three freeze-thaw cycles. After removal of cell debris by low-speed centrifugation, the titer of the virus in the supernatant was determined by end point dilution (Fig. 2). Compared with the cells incubated in the absence of any enzymes (control), it can be seen that treatment with heparinase reduced viral binding by more than 99%. Treatment with phospholipase C or with chondroitinase also resulted in substantial reduction of binding, whereas digestion with neuraminidase diminished the binding to about 50% of the control. Some HSPGs, such as glypican (13), are attached to the membrane via glycosyl phosphatidylinositol anchors (5, 62) and might thus be removed by the phospholipase treatment. The reduction of binding by the treatment with neuraminidase suggests that sialic acid residues or simply negative charges are important for the interaction.
FIG. 2.
Binding of HRV8915 is diminished upon treatment of COS-7 cells with enzymes degrading proteoglycans. COS-7 cells grown in 24-well plates were incubated for 2 h at 37°C with 0.2 U/ml neuraminidase and 2 U/ml other enzymes in PBS++ as indicated. The cells were washed with cold PBS, and 105 TCID50 of HRV8915 in 200 μl cold medium was added. After incubation for 1 h at 4°C, the supernatant was removed, the cells were washed with cold PBS, 200 μl infection medium was added, and plasma membrane bound virus was released by three freeze-thaw cycles. Cell debris was removed by low-speed centrifugation, and virus present in the supernatant was determined by end point dilution and is given as a percentage of the control (incubation in PBS without any addition). The mean ± standard deviation of three independent experiments is shown.
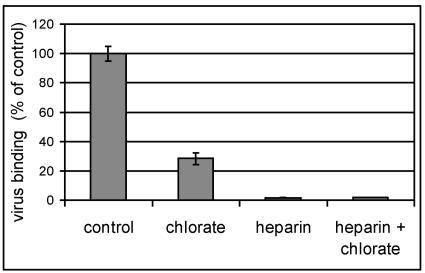
COS-7 cells grown in the presence of chlorate exhibit a strongly reduced virus binding capacity.
Further evidence for involvement of sulfated proteoglycans in viral binding was obtained by inhibition of sulfate incorporation by chlorate (21). Cells were grown in medium containing 50 mM NaClO3 and infected with radiolabeled virus. As seen in Fig. 3, growth of the cells in the presence of chlorate strongly reduced virus binding. If heparin was present during the attachment period, binding was even more reduced, indicating that synthesis of the glycans was not completely prevented by the chlorate.
FIG. 3.
Growth of COS-7 cells in the presence of chlorate reduces viral binding. Cells were grown for 3 days in medium containing 50 mM NaClO3, washed, incubated with radiolabeled HRV8915 for 1 h, and washed again, and cell-associated radioactivity was determined by liquid scintillation counting. Values are given as percentages of virus binding to cells grown in the absence of chlorate (control). The mean ± standard deviation of three experiments carried out in parallel is shown.
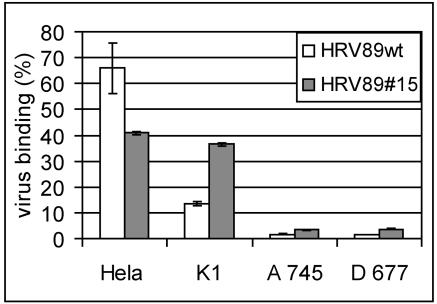
CHO cell mutants deficient in proteoglycan synthesis fail to bind HRV8915.
CHO cells with defects in the synthesis of HSPG have been extensively used to demonstrate the involvement of HS in binding of various viruses, such as respiratory syncytial virus (36), human papillomavirus (18), Dengue virus (10), tick-borne encephalitis virus (33), and foot-and-mouth disease virus (FMDV) (25), among others. CHO pgsA-745 cells are deficient in xylosyltransferase and fail to produce any glucosaminoglycans; pgsD-677 cells are doubly deficient in N-acetylglucosaminyltransferase and glucuronyltransferase and therefore lack HS but synthesize threefold-higher levels of CS than the wt (16). These cells, as well as control wt CHO-K1 cells, were grown in 24-well plates and challenged with 35S-labeled wt HRV89 and HRV8915, respectively. The supernatant containing unattached virus was collected, the cells were dislodged by trypsinization, and radioactivity in the supernatant and in the pellet was determined by liquid scintillation counting. As seen from the percent radioactivity in the cell pellet (Fig. 4), binding of wt as well as mutant virus to both CHO cell lines deficient in heparan sulfate synthesis was low, whereas the mutant virus attached well to CHO-K1 cells. It is noticeable, however, that wt virus also attached to CHO-K1 cells, although to a considerably lower extent (13% binding compared to 36% binding of the mutant virus). This finding agrees with results of experiments in which binding of wt HRV89 to HeLa cells was also somewhat reduced by heparin (data not shown) and indicates that wt HRV89 might exhibit low affinity for heparin.
FIG. 4.
Binding of HRV8915 to cells deficient in HSPG synthesis is reduced. CHO-K1 (K1), wt; pgsA-745 (A 745), deficient in xylosyltransferase; pgsD-677 (D 677), doubly deficient in N-acetylglucosaminyltransferase and glucuronyltransferase. Cells were grown in 24-well plates and challenged with about 20,000 cpm of virus for 60 min at 4°C. Cell-associated radioactivity was determined by scintillation counting. Virus bound to the cells is expressed as a percentage of total radioactivity added. Wild-type HRV89 was included as a control. The mean ± standard deviation of three experiments carried out in parallel is shown.
Hep-2 cell-adapted mutants are less stable.
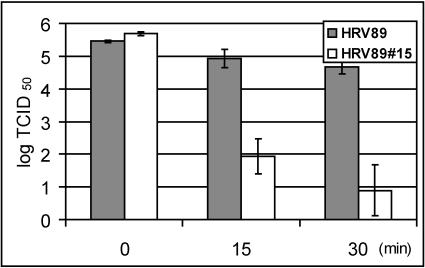
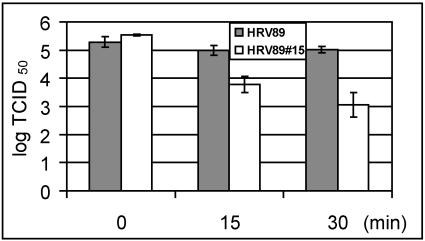
The HRV89 mutants have been shown to be more easily inactivated by soluble recombinant ICAM-1 in vitro than the wild type (42). This might indicate that the mutations were associated with decreased stability of the viral capsid. We therefore wondered whether the HRV89 mutants were more readily inactivated at elevated temperature and at low pH in vitro. Wild-type and mutant viruses were incubated at 50°C, and infectivity was determined after 15 and 30 min of incubation time. The mutant had completely lost its infectivity after 15 min, whereas the infectivity of the wt was reduced by 5 log units at 15 min and by 6 log units at 30 min of incubation time (not shown). In order to conserve some infectivity of the mutant, the same experiment was then repeated at 47°C (Fig. 5). At this temperature, the infectivity of the mutant was reduced by 4 log units at 15 min and by 5 log units at 30 min, which again clearly exceeded the inactivation of the wt virus. The same experiments were also carried out with the mutants HRV8927, HRV8934, HRV8944, and HRV8979 (42); all of them were more readily inactivated by the heat treatment than the wild type (data not shown).
FIG. 5.
Thermal inactivation of HRV8915 compared to wt HRV89. Virus was incubated at 47°C in PBS, and viral infectivity was determined by end point dilution assays at the indicated time points. The mean of three independent experiments ± standard deviation is shown.
Major-group viruses not only attach to human ICAM-1 but, as explicitly demonstrated for HRV3, HRV14, and HRV16, the receptor also aids uncoating (9, 19, 23). As shown for the minor-group virus HRV2, at least LDLR has no such activity in vitro (35) and LDL receptors only function as vehicles for virus internalization but do not actively take part in the uncoating reaction (7). For the minor receptor group viruses, uncoating is thus rather mediated by the low-pH environment prevailing in the endosomal system (37, 40, 46). In accordance with these data, HRV14 infects HeLa cells, although with lower efficiency, in the presence of the specific proton ATPase inhibitor bafilomycin A1, which raises the vesicular pH to neutrality. Conversely, HRV2 infection is prevented by the drug (2). We thus wondered whether, at low pH, the mutants would be more readily inactivated than the wild type. Virus was incubated in Na-acetate buffer (to adjust the solution to pH 4.5) for 15 and 30 min at room temperature, the pH was brought back to neutrality, and virus survival was compared to that at time zero. As seen in Fig. 6, the mutant was much less stable at low pH than the wild type. The decreased stability might be necessary to facilitate uncoating in the absence of the catalytic action of ICAM-1.
FIG. 6.
Sensitivity of mutant HRV89 to low pH compared to wt HRV89. Virus was incubated in Na+-acetate buffer of pH 4.5 for the times indicated at room temperature, and residual viral infectivity was determined by end point dilution assay. The mean ± standard deviation of three independent experiments is shown.
In COS-7 cells, HRV8915 uncoating appears to depend on low endosomal pH.
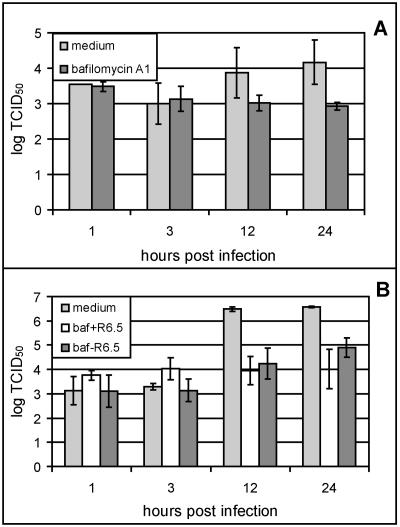
Whereas the minor-group virus HRV2 is strictly dependent on low endosomal pH and cannot infect the host cell in the presence of bafilomycin A1, an inhibitor of the vesicular H+ ATPases (40), infection of HeLa cells by the major-group virus HRV14 proceeds in the presence of the drug, although with reduced efficiency (2). This was taken to indicate that ICAM-1 also catalyzes uncoating of HRV14 in vivo. We therefore asked whether wt HRV89 was similarly able to initiate infection in the presence of the drug, whereas HRV8915 would depend on the low endosomal pH in the absence of ICAM-1. Since the mutants also bind ICAM-1 (42), the experiment could not be carried out in HeLa cells and COS-7 cells were used instead. As seen in Fig. 7A, the titer of HRV8915 slowly decreased in COS-7 cells in the presence of bafilomycin A1, and no replication was apparent. HRV2 remained equally infectious over more than 20 h within cells infected in the presence of the drug (2). The decrease in infectivity of HRV8915 is in line with its lower stability. On the other hand, when HeLa cells were infected with the mutant virus and ICAM-1 was blocked by the R6.5 antibody (Fig. 7B), some de novo viral synthesis was seen even in the presence of bafilomycin. This is most probably due to incomplete blockage of the receptor within the endosomes. Some free ICAM-1 might be sufficient to uncoat the labile HRV8915 variant (42). Similarly to HRV14, wild-type HRV89 replicated in the presence of the drug with reduced efficiency (data not shown). Taken together, these data suggest that HRV8915 might be dependent on the low-pH environment in COS-7 cells lacking the catalytic virus-uncoating activity of ICAM-1.
FIG. 7.
HRV8915 is dependent on low endosomal pH for infection. COS-7 cells (A) and HeLa cells (B) grown in 24-well plates were preincubated with and without 200 nM bafilomycin (baf) A1 for 60 min at 34°C as indicated, challenged with HRV8915, and further incubated at 34°C in the presence of 100 nM of the drug. At the times indicated, the plates were subjected to three freeze-thaw cycles, cell debris was removed by low-speed centrifugation, and the viral titer in the supernatant was determined. Where indicated, bafilomycin and/or the ICAM-1 blocking antibody R6.5 was present during the whole experiment. The mean of four independent experiments ± standard deviation is shown.
At low concentrations, heparin stimulates viral infection.
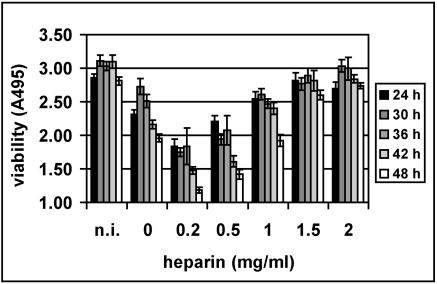
For some heparin-binding viruses, e.g., a particular strain of FMDV (25), another picornavirus, and adeno-associated virus type 2 (53), infection is inhibited at μg/ml concentrations of heparin. Therefore, we infected COS-7 cells with HRV8915 in the presence of various concentrations of heparin to determine the lowest concentration resulting in viral inhibition. Whereas the cells were fully protected against infection between 1,500 and 2,000 μg/ml (Fig. 8; also compare Fig. 1), cell damage was clearly enhanced at lower heparin concentrations. This was most pronounced at 48 h p.i. Control experiments showed that the heparin itself is not toxic to the cells at any of the concentrations used. Heparan sulfate had similar effects, with low concentrations enhancing and higher concentrations inhibiting virus-induced cell damage (data not shown). Viability of COS-7 cells was not reduced when challenged with HRV16 or wt HRV89 in the presence of any concentration of heparin.
FIG. 8.
Low concentrations of heparin stimulate infection. COS-7 cells grown in 96-well plates were challenged with HRV8915 at 107 TCID50/well in the presence of the indicated concentrations of heparin. After incubation at 34°C for the times indicated, cell viability was assessed. Control, noninfected cells (n.i.). Viability was determined in a microplate reader at 495 nm. A typical experiment out of four with the mean of four parallel determinations ± standard deviation is shown.
DISCUSSION
In this report, we demonstrate that a variant of the major-group HRV89, adapted to grow in COS-7 cells that are devoid of the viral receptor ICAM-1, uses proteoglycans, preferentially heparan sulfate, for infection. This is substantiated (i) by inhibition of viral binding by heparin and heparan sulfate (Fig. 1), (ii) by loss of binding upon treatment of the cells with heparinase (Fig. 2), (iii) by reduction of virus binding to cells grown in the presence of the sulfation inhibitor NaClO3 (Fig. 3), and (iv) by substantially less viral binding to CHO cell mutants defective in heparan sulfate synthesis than to wt CHO-K1 cells (Fig. 4). The binding seems to be of moderate specificity, since some inhibitory effect was also noted for other glycans, such as dermatan sulfate and chondroitin sulfate (Fig. 1), and neuraminidase treatment of the cells reduced viral attachment to a small extent (Fig. 2). The glycan serving as a receptor appears to be anchored in the plasma membrane via phosphatidylinositol, since it is released upon treatment with phosphatidylinositol-specific phospholipase (Fig. 2). The interaction between virus and this receptor must be ionic in nature, which is also supported by the finding that the HRV89 variants adapted to grow in HEp2 cells have mutations at their surfaces that render them more basic. However, similarly to heparin-binding FMDV (17), our viral isolates lack a particular heparin recognition sequence, such as BBXB or BBBXXB, where B is a basic amino acid residue (54).
It has been observed that a number of viruses acquire affinity for heparin upon propagation in tissue culture without any selection pressure (28, 43, 50). However, we selected for variants that had become independent from ICAM-1 (42). This might have accelerated a process that occurs anyway in tissue culture. Indeed, in accordance with the literature cited above, we observed that wt HRV89 exhibited some low proteoglycan binding affinity that was somewhat increased upon extended serial passaging, even in HeLa cells that possess ICAM-1 (data not shown). It is also noteworthy that in these cells, all the variants replicated slightly more rapidly than the wt.
In addition to the mutations responsible for heparin binding, the other mutations that are clustered at protomer interfaces appear to be responsible for the decrease in stability of the viral capsid. This is seen from the much lower concentrations of soluble ICAM-1 required for inactivation (42), and as shown in our present report, from the mutants being more easily inactivated at elevated temperature (Fig. 5) and at low pH (Fig. 6). This is in line with inhibition of infection by the vesicular proton-ATPase inhibitor bafilomycin A1 in COS-7 cells (Fig. 7A). Since a pH of 4.5, as used in the inactivation experiment (Fig. 6), is normally not attained in endosomes, we repeated the experiment at pH 5.6 and used an incubation temperature of 34°C to match physiologic conditions; e.g., HRV2, which strictly depends on low pH for uncoating, undergoes a structural modification at pH 5.6 (7, 20). Under these circumstances, after 30 min, the titer of wt virus decreased by 0.2 log units and that of the mutant by 0.7 log units (data not shown), underscoring that the inferior pH stability of the mutant might be physiologically meaningful, allowing for easier uncoating. Therefore, we believe that in the absence of ICAM-1, the capsid needs to be less stable because uncoating can only be achieved by the low-pH environment in the endosomal lumen.
We observed that cell damage caused by the virus was prevented in the presence of 1.5- to 2-mg/ml concentrations of heparin and heparan sulfate but was rather stimulated by ∼10-fold-lower concentrations of the inhibitors (Fig. 8). This stimulation was seen down to a concentration of 2 μg/ml of heparin (not shown). A similar effect has been described by Jinno-Oue and colleagues for murine leukemia virus PVC-211 (26). In this case, heparin was found to augment infection at concentrations between 1 and 50 μg/ml, whereas it was inhibitory at higher concentrations. The authors hypothesized that heparin bridged the virus with a putative heparin receptor at low concentrations but saturated the binding sites at high concentrations. In the case of HRV8915, we observed aggregation of the virus by 2-mg/ml concentrations of heparin, since it was pelleted by low-speed centrifugation in an Eppendorf centrifuge (not shown). This, together with the results discussed above, explicitly demonstrates the affinity of the virus for heparin but does not explain the stimulatory effect. When the cells were preincubated with heparin, washed, and challenged with virus, no stimulation of infection was noted (data not shown). This excludes “bridging” but suggests that virus carrying a small number of attached heparin molecules in some way either attaches to or enters the cells more efficiently; it is also conceivable that virus-attached heparin aids uncoating. This might possibly be brought about by a slight decrease in intraendosomal pH, as was observed for macrophages incubated with the polyanion dextran sulfate (27). In this case, dextran sulfate was taken up complexed to serum lipoproteins and decreased the pH of lysosomes by between 0.3 and 0.4 units.
In summary, we have demonstrated that a major-group rhinovirus can adapt to use a sulfated proteoglycan as a receptor for cell entry and infection. This underscores the great plasticity of the picornavirus family, whose members not only use a large number of structurally and functionally different receptors despite similar structure but, due to their high mutation rate of between 10−5 and 10−4 nucleotides per generation (1), these viruses relatively easily adapt to other receptors, such as heparan sulfate. In the case of HRV89, the adaptation is correlated with loss of stability that most probably facilitates uncoating in the absence of the catalytically active ICAM-1.
Acknowledgments
This work was funded by grant P14503-B09 from the Austrian Science Foundation.
We thank Renate Fuchs for discussions and critically reading the manuscript.
REFERENCES
- 1.Baranowski, E., C. M. Ruiz-Jarabo, and E. Domingo. 2001. Evolution of cell recognition by viruses. Science 292:1102-1105. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, N., E. Prchla, M. Schwab, D. Blaas, and R. Fuchs. 1999. Human rhinovirus HRV14 uncoats from early endosomes in the presence of bafilomycin. FEBS Lett. 463:175-178. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard, K. A., W. B. Klimstra, and R. E. Johnston. 2000. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276:93-103. [DOI] [PubMed] [Google Scholar]
- 5.Bernfield, M., R. Kokenyesi, M. Kato, M. T. Hinkes, J. Spring, R. L. Gallo, and E. J. Lose. 1992. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 8:365-393. [DOI] [PubMed] [Google Scholar]
- 6.Blomqvist, S., C. Savolainen, L. Raman, M. Roivainen, and T. Hovi. 2002. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J. Clin. Microbiol. 40:4218-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brabec, M., G. Baravalle, D. Blaas, and R. Fuchs. 2003. Conformational changes, plasma membrane penetration, and infection by human rhinovirus type 2: role of receptors and low pH. J. Virol. 77:5370-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brynes, A. P., and D. E. Griffin. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72:7349-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casasnovas, J. M., and T. A. Springer. 1994. Pathway of rhinovirus disruption by soluble intercellular adhesion molecule 1 (ICAM-1): an intermediate in which ICAM-1 is bound and RNA is released. J. Virol. 68:5882-5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y. P., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866-871. [DOI] [PubMed] [Google Scholar]
- 11.Colonno, R. J., P. L. Callahan, and W. J. Long. 1986. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J. Virol. 57:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compton, T., D. M. Nowlin, and N. R. Cooper. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834-841. [DOI] [PubMed] [Google Scholar]
- 13.David, G., V. Lories, B. Decock, P. Marynen, J. J. Cassiman, and H. van den Berghe. 1990. Molecular cloning of a phosphatidylinositol-anchored membrane heparan sulfate proteoglycan from human lung fibroblasts. J. Cell Biol. 111:3165-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268:382-390. [DOI] [PubMed] [Google Scholar]
- 15.Escribano-Romero, E., M. A. Jimenez-Clavero, P. Gomes, J. A. Garcia-Ranea, and V. Ley. 2004. Heparan sulphate mediates swine vesicular disease virus attachment to the host cell. J. Gen. Virol. 85:653-663. [DOI] [PubMed] [Google Scholar]
- 16.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fry, E. E., S. M. Lea, T. Jackson, J. W. I. Newman, F. M. Ellard, W. E. Blakemore, R. Abu Ghazaleh, A. Samuel, A. M. Q. King, and D. I. Stuart. 1999. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. EMBO J. 18:543-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giroglou, T., L. Florin, F. Schafer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greve, J. M., C. P. Forte, C. W. Marlor, A. M. Meyer, H. Hooverlitty, D. Wunderlich, and A. McClelland. 1991. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J. Virol. 65:6015-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruenberger, M., D. Pevear, G. D. Diana, E. Kuechler, and D. Blaas. 1991. Stabilization of human rhinovirus serotype-2 against pH-induced conformational change by antiviral compounds. J. Gen. Virol. 72:431-433. [DOI] [PubMed] [Google Scholar]
- 21.Hallak, L. K., D. Spillmann, P. L. Collins, and M. E. Peeples. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 74:10508-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofer, F., M. Gruenberger, H. Kowalski, H. Machat, M. Huettinger, E. Kuechler, and D. Blaas. 1994. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. USA 91:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooverlitty, H., and J. M. Greve. 1993. Formation of rhinovirus-soluble ICAM-1 complexes and conformational changes in the virion. J. Virol. 67:390-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulst, M. M., H. G. van Gennip, and R. J. Moormann. 2000. Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparan sulfate due to a single amino acid change in envelope protein E(rns). J. Virol. 74:9553-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. Q. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jinno-Oue, A., M. Oue, and S. K. Ruscetti. 2001. A unique heparin-binding domain in the envelope protein of the neuropathogenic PVC-211 murine leukemia virus may contribute to its brain capillary endothelial cell tropism. J. Virol. 75:12439-12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kielian, M. C., and Z. A. Cohn. 1982. Intralysosomal accumulation of polyanions. II. Polyanion internalization and its influence on lysosomal pH and membrane fluidity. J. Cell Biol. 93:875-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korant, B. D., K. Lonberg Holm, J. Noble, and J. T. Stasny. 1972. Naturally occurring and artificially produced components of three rhinoviruses. Virology 48:71-86. [DOI] [PubMed] [Google Scholar]
- 30.Ledford, R. M., N. R. Patel, T. M. Demenczuk, A. Watanyar, T. Herbertz, M. S. Collett, and D. C. Pevear. 2004. VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 78:3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, J., and S. C. Thorp. 2002. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 22:1-25. [DOI] [PubMed] [Google Scholar]
- 32.Lonberg Holm, K., and J. Noble Harvey. 1973. Comparison of in vitro and cell-mediated alteration of a human rhinovirus and its inhibition by sodium dodecyl sulfate. J. Virol. 12:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandl, C. W., H. Kroschewski, S. L. Allison, R. Kofler, H. Holzmann, T. Meixner, and F. X. Heinz. 2001. Adaptation of tick-borne encephalitis virus to BHK-21 cells results in the formation of multiple heparan sulfate binding sites in the envelope protein and attenuation in vivo. J. Virol. 75:5627-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marlovits, T. C., C. Abrahamsberg, and D. Blaas. 1998. Very-low-density lipoprotein receptor fragment shed from HeLa cells inhibits human rhinovirus infection. J. Virol. 72:10246-10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marlovits, T. C., T. Zechmeister, M. Gruenberger, B. Ronacher, H. Schwihla, and D. Blaas. 1998. Recombinant soluble low density lipoprotein receptor fragment inhibits minor group rhinovirus infection in vitro. FASEB J. 12:695-703. [DOI] [PubMed] [Google Scholar]
- 36.Martinez, I., and J. A. Melero. 2000. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J. Gen. Virol. 81:2715-2722. [DOI] [PubMed] [Google Scholar]
- 37.Neubauer, C., L. Frasel, E. Kuechler, and D. Blaas. 1987. Mechanism of entry of human rhinovirus 2 into HeLa cells. Virology 158:255-258. [DOI] [PubMed] [Google Scholar]
- 38.Noble Harvey, J., and K. Lonberg Holm. 1974. Sequential steps in attachment of human rhinovirus type 2 to HeLa cells. J. Gen. Virol. 25:83-91. [DOI] [PubMed] [Google Scholar]
- 39.Nurani, G., B. Lindqvist, and J. M. Casasnovas. 2003. Receptor priming of major group human rhinoviruses for uncoating and entry at mild low-pH environments. J. Virol. 77:11985-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prchla, E., E. Kuechler, D. Blaas, and R. Fuchs. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prchla, E., C. Plank, E. Wagner, D. Blaas, and R. Fuchs. 1995. Virus-mediated release of endosomal content in vitro: different behavior of adenovirus and rhinovirus serotype 2. J. Cell Biol. 131:111-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reischl, A., M. Reithmayer, G. Winsauer, R. Moser, I. Gosler, and D. Blaas. 2001. Viral evolution toward change in receptor usage: adaptation of a major group human rhinovirus to grow in ICAM-1-negative cells. J. Virol. 75:9312-9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.SaCarvalho, D., E. Rieder, B. Baxt, R. Rodarte, A. Tanuri, and P. W. Mason. 1997. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 71:5115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi. 2002. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 83:333-340. [DOI] [PubMed] [Google Scholar]
- 45.Sawitzky, D., H. Hampl, and K. O. Habermehl. 1990. Comparison of heparin-sensitive attachment of pseudorabies virus (PRV) and herpes simplex virus type 1 and identification of heparin-binding PRV glycoproteins. J. Gen. Virol. 71:1221-1225. [DOI] [PubMed] [Google Scholar]
- 46.Schober, D., P. Kronenberger, E. Prchla, D. Blaas, and R. Fuchs. 1998. Major and minor-receptor group human rhinoviruses penetrate from endosomes by different mechanisms. J. Virol. 72:1354-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semler, B. L., and E. Wimmer. 2002. Molecular biology of picornaviruses. ASM Press, Washington, D.C.
- 48.Seo, T., and R. W. St. Clair. 1997. Heparan sulfate proteoglycans mediate internalization and degradation of beta-VLDL and promote cholesterol accumulation by pigeon macrophages. J. Lipid Res. 38:765-779. [PubMed] [Google Scholar]
- 49.Sherry, B., A. G. Mosser, J. Colonno, and R. R. Rueckert. 1985. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J. Virol. 57:246-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smit, J. M., B. L. Waarts, K. Kimata, W. B. Klimstra, R. Bittman, and J. Wilschut. 2002. Adaptation of alphaviruses to heparan sulfate: interaction of Sindbis and Semliki Forest viruses with liposomes containing lipid-conjugated heparin. J. Virol. 76:10128-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith, C. W., R. Rothlein, B. J. Hughes, M. M. Mariscalco, H. E. Rudloff, F. C. Schmalstieg, and D. C. Anderson. 1988. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J. Clin. Investig. 82:1746-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stott, E. J., and R. A. Killington. 1972. Rhinoviruses. Annu. Rev. Microbiol. 26:503-524. [DOI] [PubMed] [Google Scholar]
- 53.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Templeton, D. M. 1992. Proteoglycans in cell regulation. Crit. Rev. Clin. Lab. Sci. 29:141-184. [DOI] [PubMed] [Google Scholar]
- 55.Tsang, S. K., B. M. McDermott, V. R. Racaniello, and J. M. Hogle. 2001. Kinetic analysis of the effect of poliovirus receptor on viral uncoating: the receptor as a catalyst. J. Virol. 75:4984-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turnbull, J., A. Powell, and S. Guimond. 2001. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 11:75-82. [DOI] [PubMed] [Google Scholar]
- 57.Tyrrell, D. A., and R. Parsons. 1960. Some virus isolations from common colds. III. Cytopathic effects in tissue cultures. Lancet i:239-242. [DOI] [PubMed] [Google Scholar]
- 58.Uncapher, C. R., C. M. Dewitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]
- 59.Verdaguer, N., I. Fita, M. Reithmayer, R. Moser, and D. Blaas. 2004. X-ray structure of a minor group human rhinovirus bound to a fragment of its cellular receptor protein. Nat. Struct. Mol. Biol. 11:429-434. [DOI] [PubMed] [Google Scholar]
- 60.Vlasak, M., S. Blomqvist, T. Hovi, E. Hewat, and D. Blaas. 2003. Sequence and structure of human rhinoviruses reveal the basis of receptor discrimination. J. Virol. 77:6923-6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yanagishita, M., and V. C. Hascall. 1992. Cell surface heparan sulfate proteoglycans. J. Biol. Chem. 267:9451-9454. [PubMed] [Google Scholar]
- 63.Zautner, A. E., U. Korner, A. Henke, C. Badorff, and M. Schmidtke. 2003. Heparan sulfates and coxsackievirus-adenovirus receptor: each one mediates coxsackievirus B3 PD infection. J. Virol. 77:10071-10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao, Q., J. M. Pacheco, and P. W. Mason. 2003. Evaluation of genetically engineered derivatives of a Chinese strain of foot-and-mouth disease virus reveals a novel cell-binding site which functions in cell culture and in animals. J. Virol. 77:3269-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]