Abstract
Introduction:
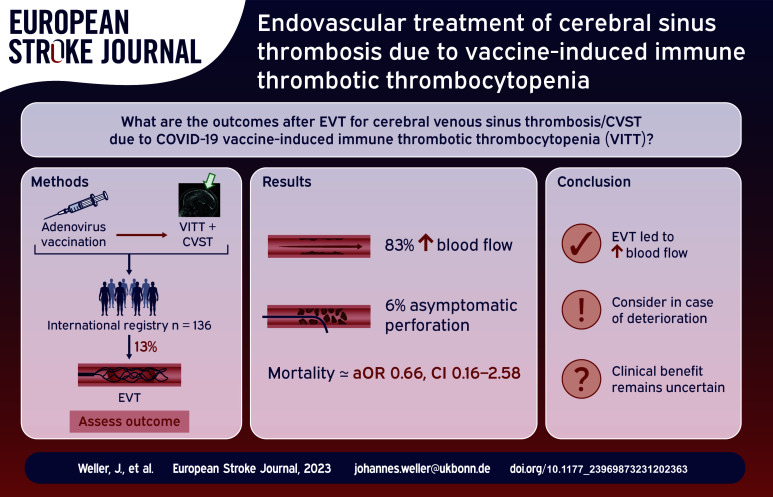
There is little data on the role of endovascular treatment (EVT) of cerebral venous sinus thrombosis (CVST) due to vaccine-induced immune thrombotic thrombocytopenia (VITT). Here, we describe clinical characteristics and outcomes of CVST-VITT patients who were treated with EVT.
Patients and methods:
We report data from an international registry of patients who developed CVST within 28 days of SARS-CoV-2 vaccination, reported between 29 March 2021 and 6 March 2023. VITT was defined according to the Pavord criteria.
Results:
EVT was performed in 18/136 (13%) patients with CVST-VITT (92% aspiration and/or stent retrieval, 8% local thrombolysis). Most common indications were extensive thrombosis and clinical or radiological deterioration. Compared to non-EVT patients, those receiving EVT had a higher median thrombus load (4.5 vs 3). Following EVT, local blood flow was improved in 83% (10/12, 95% confidence interval [CI] 54–96). One (6%) asymptomatic sinus perforation occurred. Eight (44%) patients treated with EVT also underwent decompressive surgery. Mortality was 50% (9/18, 95% CI 29–71) and 88% (8/9, 95% CI 25–66) of surviving EVT patients achieved functional independence with a modified Rankin Scale score of 0–2 at follow-up. In multivariable analysis, EVT was not associated with increased mortality (adjusted odds ratio, 0.66, 95% CI 0.16–2.58).
Discussion and conclusion:
We describe the largest cohort of CVST-VITT patients receiving EVT. Half of the patients receiving EVT died during hospital admission, but most survivors achieved functional independence.
Keywords: Intracranial thrombosis, thrombectomy, thrombocytopenia, vaccination, venous thrombosis, COVID-19
Graphical abstract.
Introduction
Cerebral venous sinus thrombosis (CVST) due to vaccine-induced immune thrombotic thrombocytopenia (VITT) is a severe adverse event after adenovirus-based SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) vaccination.1–3 VITT is caused by a pathologic autoimmune response resulting in production of antibodies against platelet factor 4, which causes a highly thrombogenic state. As a consequence, CVST-VITT patients experience severe thrombosis, often at multiple sites, 2 and have a worse prognosis compared to patients with CVST unrelated to SARS-CoV-2 vaccination. 3
In CVST unrelated to SARS-CoV-2 vaccination, the role of endovascular treatment (EVT) is uncertain. Although it seems to increase recanalization, it does not improve functional outcome and is generally not recommended as a standard treatment. Nevertheless, it is sometimes performed in severe CVST cases that do not respond to anticoagulation, and is therefore suggested as a potentially viable treatment option for a selected subgroup of CVST patients.4–8
Recommended treatment for CVST-VITT consists of non-heparin anticoagulants, immunotherapy and avoidance of platelet transfusions. 9 Nevertheless, mortality rates in CVST-VITT patients remain high.10,11 While the current literature suggests that EVT in CVST-VITT patients may be a safe and effective treatment option, the available evidence is anecdotal.12–16
According to the literature, up to one-fifth of CVST-VITT patients undergo EVT.3,17 We hypothesize that because CVST-VITT is a distinct clinical entity with a significantly higher mortality and morbidity, the potential benefit of EVT might be more pronounced in this disease compared to non-VITT CVST.2,3,11,18 In this study, we aim to provide a descriptive analysis of clinical, laboratory, and imaging characteristics and outcomes of patients with CVST-VITT treated with EVT.
Material and methods
We used data collected between March 29, 2021 and March 6, 2023 from an international registry on patients diagnosed with CVST after vaccination with any SARS-CoV-2 vaccine. 3 Formal approval was waived by the ethics committee of Amsterdam UMC and written informed consent for the use of pseudonymized care data was obtained by the participating centers from all included subjects if required by national law and hospital regulation. Inclusion criteria for this study were radiologically or autopsy-confirmed CVST with symptom onset within 28 days after SARS-CoV-2 vaccination, and definite, probable or possible VITT according to the Pavord et al. criteria. 2
We used descriptive statistics for baseline characteristics, complications and outcomes of patients treated with or without EVT. Functional outcome was rated with the modified Rankin Scale (mRS). Primary outcomes include the rate of in-hospital mortality, functional independence at follow-up (mRS 0–2) and improved blood flow of at least one treated vessel.
Thrombus load was defined as the number of affected cerebral sinus or veins. 19 Improved blood flow was defined as at least partial recanalization in one or more occluded sinus/veins according to assessment of the local investigator. The indication for EVT was provided by multiple selection from one of the following reasons: widespread thrombosis, progressive thrombosis despite conventional therapy, routine procedure, and “other.” Progressive thrombosis was favored over clinical deterioration, as the latter can have multiple causes including for example, herniation, new or enlarged lesions, seizures or systemic medical complications.
Wilson’s method was used to calculate 95% confidence intervals (CI) for main outcomes. Odds ratios (ORs) for in-hospital mortality per EVT were calculated using uni- and multivariable logistic regression models adjusted for the following pre-specified confounders in CVST-VITT: coma at presentation, intracranial hemorrhage (ICH) at presentation, baseline platelet count, immunomodulation with intravenous immunoglobulins or plasma exchange and baseline thrombus load.2,3,10 Confounders were chosen based on clinical plausibility. A two-sided probability value <0.05 was considered as statistically significant. Analyses were performed with IBM SPSS statistics (version 28.0.1.0) and R (Version 4.2.1).
Results
Among 136 CVST-VITT cases reported in the registry, 18 (13%) were treated with EVT. Median age of patients receiving EVT was 39 years (interquartile range [IQR] 28–45) and 15 (83%) were female. Baseline thrombus load was higher among patients receiving EVT with a median of 4.5 sinuses affected (IQR, 3–5.75) compared to 3 (IQR 1.25–3) in those who were not treated with EVT. Further baseline characteristics are presented in Table 1.
Table 1.
CVST-VITT patients treated with endovascular treatment.
| EVT (n = 18) | Missing n (%) | No EVT (n = 118) | |
|---|---|---|---|
| Demographic characteristics | |||
| Women, n (%) | 15 (83) | - | 87 (74) |
| Age, in years, median (IQR) | 39 (28–45) | - | 45 (28–56) |
| SARS-CoV-2 vaccination details | - | ||
| ChAdOx1 nCoV-19, n (%) | 17 (94) | 92 (78) | |
| Sinovac, n (%) | 1 (6) | 3 (2.5) | |
| Other, n (%) | 0 | 12 (10) | |
| Days from vaccination to CVST symptom onset, median (IQR) | 8 (7–10) | - | 9 (7–11) |
| Clinical characteristics at presentation | |||
| Headache, n (%) | 18 (100) | - | 108 (92) |
| Focal neurological deficits, n (%) | 14 (78) | - | 62 (54) |
| Seizure, n (%) | 5 (28) | 16 (14) | |
| Coma, n (%) | 5 (35) | 1 (6) | 22 (20) |
| Second VTE at presentation | 4 (27) | 3 (17) | 24 (21) |
| Laboratory values at presentation | |||
| Platelet count (×103/μL), median (IQR) | 42 (28–76) | - | 55 (30–84) |
| D-dimer (mg FEU/L), median (IQR) | 20 (11–35) | 2 (11) | 20 (8–28) |
| Fibrinogen (g/L), median (IQR) | 1.5 (0.8–2.4) | 2 (11) | 2.1 (1.3–2.8) |
| Platelet Factor 4 antibodies, n (%) | 13 (87) | 3 (17) | 83 (91) |
| Imaging at presentation | |||
| Non-hemorrhagic lesion, n (%) | 7 (39) | - | 29 (26) |
| Hemorrhagic lesion, n (%) | 15 (88) | 1 (6) | 74 (64) |
| Thrombus load, median (IQR) | 4.5 (3–5.75) | - | 3 (1.25–3) |
CVST: cerebral venous sinus thrombosis; EVT: endovascular treatment; FEU: fibrinogen equivalent units; IQR: interquartile range; VITT: vaccine-induced immune thrombotic thrombocytopenia; VTE: venous thromboembolism.
Sixteen of 18 (89%) EVT patients had cerebral hemorrhagic lesions on pre-intervention neuroimaging and EVT was performed at a median of 18 h (IQR 7–54) after diagnosis of CVT. Prior to EVT, 10/15 (67%) were already sedated or intubated (Table 2). The most common indications for EVT were extensive thrombosis and clinical or radiological deterioration, accounting together for 17/18 (94%) cases. More specifically, the indications included extensive thrombosis in 57%, progressive thrombosis despite conventional therapy in 50%, and other reasons include clinical worsening, progressive edema and progressive hemorrhagic lesions in 7% each (Table 3). EVT was performed with direct aspiration in 10/13 (77%) and stent retrieval in 9/13 (69%), both techniques were performed in 7/13 (54%, see Figure 1 for example), while local thrombolysis was performed in 1/13 (8%). The superior sagittal sinus, transverse and sigmoid sinus were the most frequent target vessels. Concomitant treatment included anticoagulation in 17/18 (94%) and immunoglobulins in 12/18 (67%) patients. Eight (44%) patients were treated with decompressive surgery before or after EVT (Table 3).
Table 2.
Pre-intervention status in EVT CVST-VITT patients.
| EVT CVST-VITT (n = 18) | Missing, n (%) | |
|---|---|---|
| Last pre-intervention clinical status | 5 (28) | |
| GCS <9, n (%) | 4 (31) | |
| GCS 9–12, n (%) | 4 (31) | |
| GCS 13–15, n (%) | 5 (38) | |
| ICU admission prior to EVT, n (%) | 11 (73) | 3 (17) |
| Sedation/intubation prior to EVT, n (%) | 10 (67) | 3 (17) |
| Last pre-intervention imaging | ||
| Hemorrhagic lesion, n (%) a | 16 (89) | - |
| Intraparenchymal, n (%) | 12 (80) | 3 (17) |
| Subarachnoid, n (%) | 6 (46) | 3 (17) |
| Subdural, n (%) | 2 (13) | 3 (17) |
| Epidural, n (%) | 0 | 3 (17) |
| Cerebral edema, n (%) | 6 (40) | 3 (17) |
| Midline shift, n (%) | 7 (47) | 3 (17) |
| Transtentorial herniation, n (%) | 3 (20) | 3 (17) |
| Last lab values a | ||
| Platelet count (×103/μL), median (IQR) | 67 (30–84) | - |
| D-dimer (mg FEU/L), median (IQR) | 20 (7–69) | 2 (11) |
| Fibrinogen (g/L), median (IQR) | 2.2 (1.8–3.6) | 4 (22) |
CVST: cerebral venous sinus thrombosis; EVT: endovascular treatment; FEU: fibrinogen equivalent units; IQR: interquartile range; VITT: vaccine-induced immune thrombotic thrombocytopenia.
If not available carried forward from admission (n = 3 for hemorrhagic lesion, D-Dimer and fibrinogen, n = 5 for platelet count).
Table 3.
Endovascular treatment details in CVST-VITT patients.
| EVT CVST-VITT (n = 18) | Missing n (%) | |
|---|---|---|
| Hours between diagnosis and EVT, median (IQR) | 18 (7–54) | 4 (22) |
| Indication for EVT (more than 1 possible) | 4 (22) | |
| Routine for CVST, n (%) | 1 (7) | |
| Extensive thrombosis, n (%) | 8 (57) | |
| Progressive thrombosis despite conventional therapy, n (%) | 7 (50) | |
| Other, n (%) | 3 (21) a | |
| Location of endovascular treatment | 4 (22) | |
| Superior sagittal sinus, n (%) | 9 (64) | |
| Transverse sinus, n (%) | 12 (86) b | |
| Sigmoid sinus, n (%) | 10 (71) | |
| Straight sinus, n (%) | 1 (6) | |
| Jugular vein, n (%) | 6 (43) | |
| Type of endovascular treatment (more than 1 possible) | 5 (28) | |
| Aspiration, n (%) | 10 (77) | |
| Stent retriever, n (%) | 9 (69) | |
| Local thrombolysis, n (%) | 1 (8) | |
| Concomitant treatment | ||
| Anticoagulation initiated, n (%) | 17 (94) | - |
| Pre-EVT, n (%) | 11/17 (65) | |
| Post-EVT, n (%) | 2/17 (12) | |
| Unknown sequence, n (%) | 4/17 (24) | |
| Intravenous immunoglobulins, n (%) | 12 (67) | - |
| Pre-EVT, n (%) | 6/12 (50) | |
| Post-EVT, n (%) | 5/12 (42) | |
| Unknown sequence, n (%) | 1/12 (8) | |
| Decompressive surgery, n (%) | 8 (44) | - |
| Pre-EVT, n (%) | 2/8 (25) | |
| Post-EVT, n (%) | 5/8 (68) | |
| Unknown sequence, n (%) | 1/8 (13) | |
| Platelet transfusions, n (%) | 3 (17) | - |
| Complication within 72 h | 7 (47) | 3 (17) |
| Perforation of vein/sinus, n (%) | 1 (6) | - |
| Thromboembolic complication, n (%) | 0 | |
| Brain herniation, n (%) | 5 (33) | - |
| Imaging repeated post-intervention, n (%) | 14 (93) | 3 (17) |
| Increased thrombosis, n (%) | 0 | 4 (22) |
| Improved blood flow, n (%) | 10 (83) | 6 (33) |
| Improved blood flow according to localization | ||
| Superior sagittal sinus, n (%) | 8/9 (89) | - |
| Transverse sinus, n (%) | 8/12 (67) | 2 (14) |
| Sigmoid sinus, n (%) | 6/9 (67) | 1 (10) |
| Straight sinus, n (%) | 1/1 (100) | - |
| Jugular vein, n (%) | 2/4 (50) | 2 (33) |
| Enlarged or new hemorrhagic lesions on first imaging after EVT | 9 (64) | 4 (22) |
CVST: cerebral venous sinus thrombosis; EVT: endovascular treatment; IQR: interquartile range; VITT: vaccine-induced immune thrombotic thrombocytopenia.
Clinical worsening (n = 1), progressive edema (n = 1), progressive hemorrhagic lesions (n = 1).
Bilateral involvement in two cases (14%).
Figure 1.
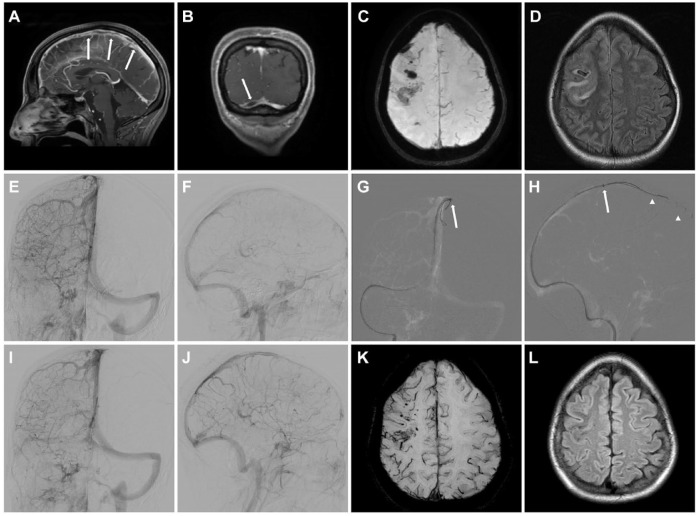
Presentation of an illustrative case of EVT in CVST-VITT. Female patient with extensive thrombosis of the superior sagittal sinus (SSS; arrows in (a)) and the right transverse sinus (TS; arrow in (b)) with microhemorrhages and focal edema (c and d). Digital subtraction angiography with injection of the right internal carotid artery (ICA; (e) posterior-anterior view, (f) lateral view) confirmed extensive occlusion of the SSS and right TS with missing opacification of the cortical veins. (g and h) EVT was performed by aspiration with a large-bore aspiration catheter (arrow: tip of the 6F Sofia plus, Microvention) in the SSS and simultaneous use of two stent retrievers (arrowheads: Solitaire 6/40 mm, Medtronic). Injection of the right ICA after three passes demonstrated recanalization of the SSS and right TS (i and j). MRI at 3-month follow-up showed edema resolution (k and l).
Asymptomatic sinus perforation occurred in one case (6%), and no thromboembolic complications were observed. Repeated imaging after the intervention showed improved blood flow in at least one location in 10/12 cases (83%, 95% CI 54–96), while enlarged or new hemorrhagic lesions were detected in 9/14 (64%, Table 3).
Mortality among EVT patients was high with 9/18 (50%, 95% CI 29–71) and the median mRS at discharge was 4 (IQR 4–6), which was higher than in non-EVT patients (mRS 3, IQR 1–6, p = 0.015). All but one of the surviving EVT patients (in total 8/18, 44%, 95% CI 25–66) achieved functional independence at a median follow-up of 2.5 months (IQR 1–6 months, Figure 2). In univariable logistic regression analysis, EVT was not associated with in-hospital mortality (OR 1.74, 95% CI 0.63–4.79), which was confirmed in multivariable analysis adjusted for pre-specified prognostic factors (adjusted OR 0.66, 95% CI 0.16–2.58, Table 4).
Figure 2.
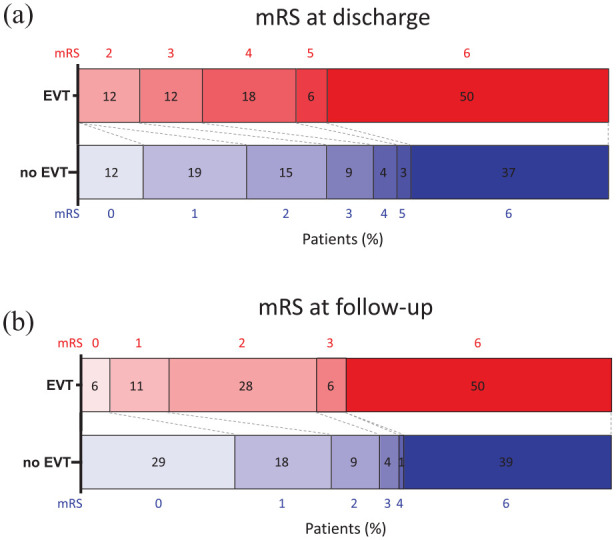
Functional outcome of CVST-VITT patients with versus without EVT. (a and b) Functional outcome (modified Rankin Scale, mRS) of CVST-VITT patients with versus without endovascular treatment (EVT).
Table 4.
Odds ratios for in-hospital mortality in uni- and multivariable regression models.
| Univariable OR | p | Multivariable OR | P | |
|---|---|---|---|---|
| EVT performed | 1.74 (0.63–4.79) | 0.28 | 0.66 (0.16–2.58) | 0.48 |
| Hemorrhagic lesion | 6.77 (2.75–19.33) | <0.001 | 5.42 (1.85–18.61) | 0.004 |
| Coma | 9.56 (3.7–28.26) | <0.001 | 4.93 (1.59–17.15) | 0.008 |
| Platelet count (×103/μL) | 0.97 (0.95–0.98) | <0.001 | 0.98 (0.96–0.99) | 0.004 |
| Immunomodulation | 0.44 (0.21–0.91) | 0.027 | 0.29 (0.10–0.76) | 0.014 |
| Thrombus load | 1.18 (0.96–1.47) | 0.13 | 1.17 (0.86–1.59) | 0.32 |
EVT: endovascular treatment; OR: odds ratio.
Discussion
In our study, 13% of CVST-VITT patients underwent EVT. This rate is in line with a British cohort study also in which 13% patients were treated with EVT, 17 but it is higher than in the study of Pavord et al. in which 7% were treated with EVT (personal communication). 2 Compared to previous anecdotal reports, the rates of technical success, complications and favorable outcomes were similar, while mortality was higher in our cohort (50% vs 27%, Supplemental Table). However, the mortality rate of 27% is lower than in CVST-VITT in general (40%) 20 and might be subject to reporting bias, as it is based on single cases and small cohorts up to n = 6.
The most common indications for EVT in this cohort were extensive thrombosis and clinical or radiological deterioration. Therefore, it is not surprising that patients treated with EVT had a higher thrombus load compared to those who were not treated with EVT. Moreover, a median of 18 h between the diagnosis and treatment suggests that EVT was not performed as a first-line treatment but rather as an adjuvant or escape treatment in deteriorating patients. This approach is consistent with the available literature: in some cases, following initial experiences with CVST-VITT patients who acutely deteriorated and died, there was a lower threshold for proceeding quickly to EVT for subsequent CVST-VITT patients. 13
Interestingly, we observed a shift in the employed recanalization techniques compared to those used in the Thrombolysis or Anticoagulation for Cerebral Venous Thrombosis trial (TO-ACT), where thrombectomy was performed in 91% and 52% received local thrombolysis. 4 In our cohort, thrombectomy was also performed in 92%, but local thrombolysis in only 8%. While the optimal endovascular treatment approach is unknown, the observed shift might be in response to the assumed high bleeding risk as CVST-VITT had high rates (89%) of hemorrhagic lesions and low platelet counts. 5 However, while TO-ACT recruited from 2011 to 2016, increasing experience among neurointerventionalists with thrombectomy techniques and improvement of devices following the publication of the 2015 stroke trials might also contribute to the observed preference.4,21
On repeated imaging, 83% of CVST-VITT patients treated with EVT had recanalization of at least one treated vessel. While characterization of venous recanalization remains challenging to measure, this outcome seems comparable to the one achieved in studies of EVT for CVST before the pandemic. 6 Unfortunately, there are no previous studies on early recanalization in CVST-VITT treated with anticoagulation and we did not gather recanalization data in non-EVT CVST-VITT patients.
Post-EVT imaging revealed a subdural hematoma in one patient, presumably due to sinus perforation and without clinical deterioration or need for surgical intervention. This low periprocedural complication rate is in line with the findings of the TO-ACT trial, confirming the safety of the procedure. 4 Nevertheless, in our cohort, almost two-thirds had a new or enlarged hemorrhagic lesion after EVT. In the majority of the cases, these were assessed as being unrelated to the procedure. The rate of new/enlarged hemorrhagic lesions in CVST-VITT patients not treated with EVT was also high (31/112), and extensive thrombosis increases the risk of hemorrhage. Still, an association between EVT and new/enlarged hemorrhagic lesions cannot be precluded.
In those who survived, functional outcomes of CVST-VITT patients treated with EVT were favorable. Almost 90% achieved functional independence, as was reported for CVST-VITT irrespective of EVT. 20 Nevertheless, half of the CVST-VITT patients treated with EVT died during the initial admission, which is similar to the mortality in patients without EVT, but significantly higher than in the EVT studies of CVST unrelated to vaccination (12%).4,6 This is most likely due to the greater severity of CVST-VITT in general. The worse severity of CVST-VITT compared to usual CVST can also be ascertained by comparing rates of decompressive surgery − 44% in this study versus 9% in TO-ACT. 4
As baseline thrombus load was higher in EVT patients and EVT was mostly indicated due to refractory disease, confounding by indication contributed to the numerically higher mortality on the unadjusted Grotta bar. In univariable as well as in multivariable analysis adjusted for pre-specified severity markers, there was no association between EVT and mortality, and the aHR of 0.66 even suggests a potential survival benefit associated with EVT in CVST-VITT. However, these observations were not statistically significant, thus limiting conclusions.
In addition to the inherent constraints of an observational registry, this study has several limitations. First, some data were collected retrospectively and consequently there are variables with high rates of missing data. Second, due to restricted sample size, generalizability might be reduced and we were not able to eliminate all confounders. Third, there was no central adjudication of clinical and radiological outcomes, as the data were collected in routine clinical care.
In conclusion, we provide a descriptive analysis of CVST-VITT patients to inform clinical management of this rare disease, which might also be helpful for related conditions with thrombotic thrombocytopenia and CVST. EVT in CVST-VITT may be safe and lead to local improvement of blood flow in most cases, but the clinical benefit seems uncertain.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231202363 for Endovascular treatment of cerebral sinus thrombosis due to vaccine-induced immune thrombotic thrombocytopenia by Johannes Weller, Katarzyna Krzywicka, Anita van de Munckhof, Franziska Dorn, Katharina Althaus, Felix J Bode, Monica Bandettini di Poggio, Brian Buck, Timothy Kleinig, Charlotte Cordonnier, Vanessa Dizonno, Jiangang Duan, Ahmed Elkady, Beng Lim Alvin Chew, Carlos Garcia-Esperon, Thalia S Field, Catherine Legault, Mar Morin Martin, Dominik Michalski, Johann Pelz, Silvia Schoenenberger, Simon Nagel, Marco Petruzzellis, Nicolas Raposo, Mona Skjelland, Domenico Sergio Zimatore, Sanjith Aaron, Mayte Sanchez van Kammen, Diana Aguiar de Sousa, Erik Lindgren, Katarina Jood, Adrian Scutelnic, Mirjam R Heldner, Sven Poli, Antonio Arauz, Adriana B Conforto, Jukka Putaala, Turgut Tatlisumak, Marcel Arnold, Jonathan M Coutinho, Albrecht Günther, Julian Zimmermann and José M Ferro in European Stroke Journal
Acknowledgments
The authors would like to thank all patients and their families who agreed for use of their data for research purposes.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AA serves as an advisory board member for Bayer and Bristol Myers Squibb and consulting fees for Boheringer Ingelheim. ABC received consulting fees from Boehringer Ingelheim. SM reports grants from Bayer, Pfizer, Boehringer Ingelheim and Daiichi Sankyo paid to her institution, and personal fees from Bayer, BMS/Pfizer, Boehringer Ingelheim, Abbvie, Portola/Alexion and Daiichi Sankyo paid to her institution. AG received speaker’s honoraria from Boehringer Ingelheim, Daichii Sankyo, Pfizer, Occlutech, Merz, and Ipsen. CGE received funding to attend a conference from Boehringer Ingelheim and Bayer and speaker honoraria from the AAN. AS has received a grant from Swiss Heart Foundation. CC received speaker honoraria from Boehringer Ingelheim, personal fees for advisory board participation from AstraZeneca and Biogen, and personal fees from Biogen and Bristol Myers Squibb. CGE received travel funding from Boehringer Ingelheim and Bayer and speaker honoraria from the AAN. DAS reports travel support from Boehringer Ingelheim, DSMB participation for the SECRET trial, advisory board participation for AstraZeneca and membership on the ESO Executive Committee. EL received grants from the Swedish State, Swedish Neurologic Society, Elsa and Gustav Lindh’s Foundation, P-O Ahl’s Foundation and Rune and Ulla Amlöv’s Foundation. FD is a consultant/proctor for Cerenovus/Johnson&Johnson, Balt, Cerus Endovascular and Phenox and received speaker’s honoraria from Acandis, Stryker, Cerenovus/Johnson&Johnson, Asahi and research support from Cerenovus/Johnson&Johnson. JMC received grants paid to his institution from Boehringer Ingelheim and Bayer for DSMB participation by Bayer. JMF reports fees and DSMB or Advisory Board participation for Boehringer Ingelheim and consulting fees from Bayer. JPa received personal fees from Boehringer Ingelheim, Bayer, Herantis Pharma and Abbott and stock ownership in Vital Signum. MA reports compensation from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Covidien, Daiichi Sankyo, Novartis, Sanofi, Pfizer, Medtronic and research grants from the Swiss National Science Foundation and the Swiss Heart Foundation. MP received personal fees for advisory board participation from Alexion. MRH reports grants from the Swiss Heart Foundation, the Bangerter Foundation, Swiss National Science Foundation, and SITEM Research Funds, and Advisory Board participation for Amgen. NR received research grants from Fulbright, Harvard University and Philippe Foundation. RL reports fees paid to his institution by Boehringer Ingelheim, Genentech, Ischemaview, Medtronic, and Medpass. SN has received consulting fees from Brainomix and lecture fees from Boehringer Ingelheim and BMS-Pfizer. SP received research support from BMS/Pfizer, Boehringer-Ingelheim, Daiichi Sankyo, European Union, German Federal Joint Committee Innovation Fund, and German Federal Ministry of Education and Research, Helena Laboratories and Werfen and speakers’ honoraria/consulting fees from Alexion, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS/Pfizer, Daiichi Sankyo, Portola, and Werfen. TK received personal fees from Boehringer Ingelheim. TSF received study medication from Bayer Canada and personal fees from HLS Therapeutics. TT has received personal fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Inventiva, and Portola Pharma. All other authors declare that there is no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Formal approval was waived by the ethical review committee Amsterdam UMC for this observational study. This study was completed in accordance with the Helsinki Declaration as revised in 2013.
Informed consent: Written informed consent for the use of pseudonymized care data was obtained by the participating centers from all included subjects if required by national law and hospital regulation.
Guarantor: JMF
Contributorship: JW, KK, JZ, and JMF designed the study. JW and KK analyzed the data and wrote the first draft. JZ and JMF supervised the study. All authors were involved in patient recruitment and reviewed and edited the manuscript.
ORCID iDs: Johannes Weller  https://orcid.org/0000-0001-5818-5392
https://orcid.org/0000-0001-5818-5392
Monica Bandettini di Poggio  https://orcid.org/0000-0001-8871-1103
https://orcid.org/0000-0001-8871-1103
Timothy Kleinig  https://orcid.org/0000-0003-4430-3276
https://orcid.org/0000-0003-4430-3276
Dominik Michalski  https://orcid.org/0000-0002-0206-5380
https://orcid.org/0000-0002-0206-5380
Nicolas Raposo  https://orcid.org/0000-0002-9152-4445
https://orcid.org/0000-0002-9152-4445
Diana Aguiar de Sousa  https://orcid.org/0000-0002-6702-7924
https://orcid.org/0000-0002-6702-7924
Adrian Scutelnic  https://orcid.org/0000-0001-9053-584X
https://orcid.org/0000-0001-9053-584X
Julian Zimmermann  https://orcid.org/0000-0003-1964-0078
https://orcid.org/0000-0003-1964-0078
Supplemental material: Supplemental material for this article is available online.
References
- 1. Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021; 384: 2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. New Engl J Med 2021; 385: 1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sánchez van Kammen M, Aguiar de Sousa D, Poli S, et al. Characteristics and outcomes of patients with cerebral venous sinus thrombosis in SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. JAMA Neurol 2021; 78: 1314–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coutinho JM, Zuurbier SM, Bousser M-G, et al. Effect of endovascular treatment with medical management vs standard care on severe cerebral venous thrombosis: the TO-ACT randomized clinical trial. JAMA Neurol 2020; 77: 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goyal M, Fladt J, Coutinho JM, et al. Endovascular treatment for cerebral venous thrombosis: current status, challenges, and opportunities. J Neurointerv Surg 2022; 14: 788–793. [DOI] [PubMed] [Google Scholar]
- 6. Siddiqui FM, Dandapat S, Banerjee C, et al. Mechanical thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke 2015; 46: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 7. Ferro JM, Bousser M-, Canhão P, et al. European stroke organization guideline for the diagnosis and treatment of cerebral venous thrombosis – endorsed by the European Academy of Neurology. Eur J Neurol 2017; 24: 1203–1213. [DOI] [PubMed] [Google Scholar]
- 8. Saposnik G, Barinagarrementeria F, Brown RD, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 1158–1192. [DOI] [PubMed] [Google Scholar]
- 9. Furie KL, Cushman M, Elkind MSV, et al. Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke 2021; 52: 2478–2482. [DOI] [PubMed] [Google Scholar]
- 10. Scutelnic A, Krzywicka K, Mbroh J, et al. Management of cerebral venous thrombosis due to adenoviral COVID-19 vaccination. Ann Neurol 2022; 92: 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krzywicka K, Aguiar de Sousa D, Cordonnier C, et al. Decompressive surgery in cerebral venous sinus thrombosis due to vaccine-induced immune thrombotic thrombocytopenia. Eur J Neurol 2023; 30: 1335–1345. [DOI] [PubMed] [Google Scholar]
- 12. Chew HS, Al-Ali S, Butler B, et al. Mechanical thrombectomy for treatment of cerebral venous sinus thrombosis in vaccine-induced immune thrombotic thrombocytopenia. AJNR Am J Neuroradiol 2022; 43: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cleaver J, Ibitoye R, Morrison H, et al. Endovascular treatment for vaccine-induced cerebral venous sinus thrombosis and thrombocytopenia following ChAdOx1 nCoV-19 vaccination: a report of three cases. J Neurointerv Surg 2022; 14: 853–857. [DOI] [PubMed] [Google Scholar]
- 14. Mahajan A, Hirsch JA. Cerebral venous thrombosis after COVID-19 vaccination: the role for endovascular treatment. J Neurointerv Surg 2022; 14: 849–850. [DOI] [PubMed] [Google Scholar]
- 15. Wiedmann M, Skattør T, Stray-Pedersen A, et al. Vaccine induced immune thrombotic thrombocytopenia causing a severe form of cerebral venous thrombosis with high fatality rate: a case series. Front Neurol 2021; 12: 721146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mirandola L, Arena G, Pagliaro M, et al. Massive cerebral venous sinus thrombosis in vaccine-induced immune thrombotic thrombocytopenia after ChAdOx1 nCoV-19 serum: case report of a successful multidisciplinary approach. Neurol Sci 2022; 43: 1499–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perry RJ, Tamborska A, Singh B, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet 2021; 398: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferro JM, Canhão P, Stam J, et al. Prognosis of cerebral vein and dural sinus thrombosis: results of the international study on cerebral vein and dural sinus thrombosis (ISCVT). Stroke 2004; 35: 664–670. [DOI] [PubMed] [Google Scholar]
- 19. Aguiar de Sousa D, Lucas Neto L, Arauz A, et al. Early recanalization in patients with cerebral venous thrombosis treated with anticoagulation. Stroke 2020; 51: 1174–1181. [DOI] [PubMed] [Google Scholar]
- 20. van de Munckhof A, Lindgren E, Kleinig TJ, et al. Outcomes of cerebral venous thrombosis due to vaccine-induced immune thrombotic thrombocytopenia after the acute phase. Stroke 2022; 53: 3206–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231202363 for Endovascular treatment of cerebral sinus thrombosis due to vaccine-induced immune thrombotic thrombocytopenia by Johannes Weller, Katarzyna Krzywicka, Anita van de Munckhof, Franziska Dorn, Katharina Althaus, Felix J Bode, Monica Bandettini di Poggio, Brian Buck, Timothy Kleinig, Charlotte Cordonnier, Vanessa Dizonno, Jiangang Duan, Ahmed Elkady, Beng Lim Alvin Chew, Carlos Garcia-Esperon, Thalia S Field, Catherine Legault, Mar Morin Martin, Dominik Michalski, Johann Pelz, Silvia Schoenenberger, Simon Nagel, Marco Petruzzellis, Nicolas Raposo, Mona Skjelland, Domenico Sergio Zimatore, Sanjith Aaron, Mayte Sanchez van Kammen, Diana Aguiar de Sousa, Erik Lindgren, Katarina Jood, Adrian Scutelnic, Mirjam R Heldner, Sven Poli, Antonio Arauz, Adriana B Conforto, Jukka Putaala, Turgut Tatlisumak, Marcel Arnold, Jonathan M Coutinho, Albrecht Günther, Julian Zimmermann and José M Ferro in European Stroke Journal